Abstract
This chapter reviews the sterol compositions reported for the different classes of microalgae. For a few major phyla such as the green microalgae, diatoms, dinoflagellates and haptophytes a sufficiently large number of species (over 100 in the case of diatoms) have been analysed for sterols allowing some generalisations to be made and characteristic features to be identified. In many other algal classes there is a paucity of sterol data available as only a few species have been analysed. These compositions are discussed in terms of taxonomic groupings and from the viewpoint of the various steps involved in sterol biosynthesis that introduce specific double bonds or alkylate the sterol side-chain. Green microalgae are shown to have a variety of compositions including some with simple distributions of sterols dominated by the C29 sterol 24-ethylcholest-5-en-3β-ol (sitosterol) more commonly associated with higher plants. In other green algae a predominance of Δ7-unsaturated sterols is found dominated by 24-methylcholesta-5,7,22E-dien-3β-ol (ergosterol). Diatoms contain a surprising diversity of sterol distributions with over 40 sterols identified. Common sterols include 24-methylcholesta-5,22E-dien-3β-ol, 24-methylenecholesterol and cholesterol. Some diatom genera display distinctive distributions such as Amphora which contains high contents of the C29 sterol 24-ethylcholesta-5,22E-dien-3β-ol. A number of unusual sterols have been found in smaller amounts in a few species including 4-methylsterols and the C30 sterol gorgosterol. Sterols with a methyl group at C-23 are also surprisingly common showing that this feature is not unique to dinoflagellate sterols. Many dinoflagellates contain mixtures of 4-methylsterols including the C30 sterol 4α,23,24-trimethyl-5α-cholest-22E-en-3β-ol (dinosterol), as well as related 5α(H)-stanols. A few genera contain unusual sterols such as Amphidinium and Karenia species which contain Δ8(14)-unsaturated sterols. Haptophytes, in contrast, usually have simple sterol distributions, often dominated by 24-methylcholesta-5,22E-dien-3β-ol also found in diatoms.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Sterols are tetracyclic triterpenoids biosynthesized by all eukaryotic organisms (Desmond and Gribaldo 2009). A generalized structure with carbon atoms numbered is shown in Fig. 1. Sterol distributions in microalgae display a wide range of structures reflecting subtle differences in the sterol biosynthetic pathway used by different organisms (e.g. Goad et al. 1974; Volkman 1986, 2003, 2005; Volkman et al. 1998; Nes 2011). In some cases the presence of specific sterols follows taxonomic classifications quite closely. In other classes, a wide range of structures can be found (Table 1) either because the taxonomic grouping is actually polyphyletic or the alga accumulates sterols produced at different stages in the biosynthetic pathway perhaps due to a defect in later biosynthetic steps.
Sterol biosynthesis occurred very early in the history of life on Earth (Kodner et al. 2008), and the presumed last common ancestor of the eukaryotes likely had a large panel of enzymes for sterol biosynthesis (Desmond and Gribaldo 2009). These biosynthetic pathways have continued to evolve over geological time and thus one might expect to see certain “unusual” or rare sterols in particular algal classes due to specific changes in one or more of the many genes needed for sterol biosynthesis.
This review provides characteristic features of the sterol distributions in each of the major microalgal classes and builds on previous reviews by this author (Volkman 1986, 2003, 2005; Volkman et al. 1998). However, a difficulty immediately arises as to which taxonomic scheme to use. Ideally this should reflect the evolution of the different algal classes, but a consensus is not yet available. There are 15 traditionally recognized phyla in microalgae and most of the papers cited in this review have assigned species based on this classification. These 15 phyla are: Cyanophyta (cyanobacteria, also referred to as blue-green algae in the older literature), Rhodophyta (red algae), Euglenophyta (euglenoids), Cryptophyta (cryptomonads), Pyrrophyta (dinoflagellates), Raphidophyta (raphidophytes), Chrysophyta (chrysophytes, golden-brown algae), Xanthophyta (=Tribophyta, yellow-green algae), Chlorophyta (green algae), Eustigmatophyta (eustigmatophytes), Phaeophyta (brown algae), Prasinophyta (prasinophytes), Bacillariophyta (diatoms), Glaucophyta (glaucophytes).
Undoubtedly these classification schemes will continue to be refined as new gene sequence data become available. While it would have been more satisfying to structure the paper around an agreed taxonomic scheme that reflects the evolution of microalgal classes, this has not been possible. The taxonomy of many microalgal groups is in a state of flux and it is not uncommon to see some species transferred to completely different classes and species names being changed several times. Accordingly the data are structured according to the major phyla and where possible assigned to different algal classes within each phylum. Revised taxonomic assignments have been used based on those presented in AlgaeBase (Guiry and Guiry 2015), with a reference back to the original classification used in the paper cited where there has been a revision. The chapter “Systematics, Taxonomy and Species Names: Do They Matter?” of this book (Borowitzka 2016) also provides the names and current taxonomically accepted affiliation of all taxa mentioned in the present chapter.
2 Sterol Biosynthesis
Sterols are an end-product of the cyclization of the C30 isoprenoid 2,3-oxidosqualene.
Cyclisation either produces lanosterol as in animals, fungi, and some algae and bacteria or cycloartenol as in higher plants and most algae (Fig. 2). Many plants also convert 2,3-oxidosqualene to pentacyclic triterpenes such as α- and β-amyrin while bacteria generally synthesize cyclic triterpenes such as hopenes by cyclization of squalene rather than oxidosqualene (Buntel and Griffin 1994; Volkman 2005). For a comprehensive review of the biosynthesis of cholesterol and other sterols, primarily in higher plants and fungi, and the enzymes involved the reader is referred to the review by Nes (2011).
In microalgae, sterol biosynthesis can give rise to sterols having C26 to C31 carbon atoms and structural variations including 4,4-dimethyl substituents, a 4-methyl substituent or no methyl group at C-4 (so-called 4-desmethylsterols that comprise the majority of sterols in microalgae) (reviewed by Volkman 2003, 2005). Addition of one to four methyl substituents to the side-chain provides a wide variety of structures including 24-methyl, 24-methylene, 24-ethyl, 24-ethylidene, 24-n-propyl, 24-isopropyl, 23,24-dimethyl, 22,23-methylene (cyclopropyl) and 23-methyl sterols (Fig. 3).
Further variety is provided by the different locations for the double bonds including Δ5, Δ7, Δ8(9), Δ8(14), Δ22, Δ5,7, Δ5,22, Δ5,24, Δ7,22, Δ5,24(28), Δ8(14),24(28) and Δ5,7,9(11) amongst others. Structures of the main variants are shown in Fig. 3. Double bonds are generated by hydrogen transfers to produce different isomers. Early in the sterol biosynthesis pathway the double bond position is at Δ8, which is subsequently isomerized to Δ7. Introduction of a second double bond at C-5 leads to Δ5,7 sterols and reduction of the Δ7 double bond gives rise to the Δ5 sterols (Dempsey 1965; Doyle et al. 1972) (Fig. 4), which dominate the sterol distributions in most microalgae. Side-chain alkylation can occur at any of these stages giving rise to C-24 alkylated sterols with unusual double bond positions in the ring system.
The presence of particular sterols in an alga can often be understood by considering them as products of the many different steps involved in sterol biosynthesis. The biosynthesis of sterols will not be covered in detail here and the reader is referred to other papers outlining the key steps (e.g. Goad et al. 1974; Chappell 2002; Nes 2011; Miller et al. 2012; Xue et al. 2012). While sterol synthesis is undoubtedly very ancient, some steps may have evolved more recently.
2.1 Side-Chain Biosynthesis
The sterol distributions of most microalgae are characterized by a high proportion of sterols that are alkylated in the side-chain with either a methyl group, ethyl group (two methyl transfers), or rarely a propyl group at C-24 (e.g. Goad et al. 1974; Raederstorff and Rohmer 1984; Patterson 1994). Other variants include the 23,24-dimethyl side-chains found in the 4-methylsterols of dinoflagellates (e.g. Withers 1987) and in the 4-desmethylsterols of some diatoms and haptophytes (e.g. Volkman et al. 1993; Rampen et al. 2009b). These alkylations are brought about by S-adenosyl-L-methionine (AdoMet = SAM) sterol methyltransferases (SMTs: e.g. Nes 2003; Nes et al. 2003). SMTs do not occur in animal systems (Nes 2000). Goad et al. (1974) describe six different mechanisms by which alkyl groups might arise in the side-chains of sterols. Nes and co-workers have proposed an active-site model, which they term the “steric-electric plug” model to describe C-24 alkylation (reviewed by Nes 2003; Nes et al. 2003). These pathways are complex and show phylogenetic groupings.
Work by Dennis and Nes (2002) showed that the SMT from Glycine max expressed in Escherichia coli cells catalyses the step-wise conversion of cycloartenol to 24(28)-methylenecycloartanol and then to a mixture of stereochemically related Δ24(28)Z-ethylidene-, Δ24(28)E-ethylidene- and Δ25(27)-24β-ethylcyclosterols. Campesterol (24α-methylcholesterol) and dihydrobrassicasterol (24β-methylcholesterol), typical C28 sterols in higher plants, are biosynthesized from desmosterol via 24-methylenecholesterol and 24-methyldesmosterol. The typical plant C29-sterol, sitosterol (24α-ethylcholesterol), is produced from 24-methylenecholesterol via isofucosterol and 24-ethyldesmosterol.
2.2 C-24 Stereochemistry
The stereochemistry of the alkyl substituents in the side-chain can be α- or β- oriented depending on the biosynthetic pathways by which the side-chain is alkylated. Different algal classes usually produce sterols with either 24α or 24β stereochemistry, but only rarely do both occur (e.g. in some diatoms; Gladu et al. 1991b). For example, the C28 and C29 Δ5 unsaturated sterols of vascular plants generally have the 24α configuration as do the C28 sterols of most diatoms (Maxwell et al. 1980; Nes and Nes 1980; Gladu et al. 1991b). In contrast, green algae and dinoflagellates biosynthesize sterols with the 24β configuration (Goad et al. 1974; Bohlin et al. 1981; Goad and Withers 1982; Miller et al. 2012).
Note that due to the IUPAC-IUB JCBN sequence rules, 24α corresponds to 24R when the side-chain is saturated, but it becomes 24S when a Δ22 double bond is present (Fig. 1). The side-chain α- and β- nomenclature is not related to that which is used to describe the orientation of methyl substituents on the ring system (see Goad and Akihisa 1997 for further explanation). 24β-Sterols (which are common in green algae) arise from Δ25(27) sterols. Reduction of Δ24(28) sterols can give rise to either 24α or 24β sterols (Goad et al. 1974). Fungi apparently use this pathway exclusively for the production of 24β epimers.
The majority of published data on algal sterols has been obtained by GC–MS analysis with reliance on relative retention data (e.g. Itoh et al. 1982) and reference mass spectra to identify specific isomers (e.g. de Leeuw et al. 1983; Jones et al. 1994; Gerst et al. 1997; Volkman et al. 1997). While GC–MS is a sensitive technique and very useful for identifying small amounts of sterols in complex mixtures it does not provide information on the configuration at C-24 (Gerst et al. 1997; Giner et al. 2008). The epimers can be separated on very long, polar glass capillary columns (Maxwell et al. 1980; Thompson et al. 1981; Ikekawa et al. 1989), but analysis times are very long and to date the method has not been widely exploited. HPLC techniques are also now available (Chitwood and Patterson 1991), but are also rarely utilized. Unambiguous assignment of C-24 stereochemistry can be obtained by NMR (e.g. Wright et al. 1978; Chui and Patterson 1981; Goad and Akihisa 1997; Miller et al. 2012), but this requires isolation and purification of each sterol within the complex mixtures found in most microalgae.
Identification of the stereochemistry at C-24 can indicate which of the many sterol pathways operates in that alga. Such data combined with information from incubation with [CD3]-methionine and labeled mevalonate can be powerful tools for studying sterol biosynthesis in microalgae (Goad et al. 1974). Several algal species, including a diatom, two prymnesiophytes and two cryptophytes, have been shown to produce 24α-methylcholesta-5,22E-dien-3β-ol (epibrassicasterol; Rubinstein and Goad 1974; Maxwell et al. 1980; Goad et al. 1983). Green algae on the other hand mainly contain 24β sterols. In some cases both epimers can be found (Raederstorff and Rohmer 1984) as in an unidentified alga believed to be a chrysophyte (Kokke et al. 1984); 24α-ethylcholest-5-en-3β-ol comprised 10.2 % of 4-desmethyl sterols compared with 3.0 % for the 24β epimer.
C-24 stereochemistry is not always consistent within a single taxonomic grouping. For example, Gladu et al. (1990) examined nine strains of phytoplankton which contain 24-methylcholesta-5,22E-dien-3β-ol as their principal sterol. The two strains provisionally identified as lsochrysis (a haptophyte) contained brassicasterol (24β-methylcholesta-5,22E-dien-3β-ol); whereas all other species examined (two haptophytes, two rhodophytes and two diatoms) contained primarily epibrassicasterol 24α-methylcholesta-5,22E-dien-3β-ol).
2.3 Biochemical Forms of Sterols
The methods used in most studies of sterols in microalgae do not provide information about the chemical form in which the sterol exists since the extracts are usually saponified and any steryl esters are converted to the free sterols. In most microalgae, the sterols mainly exist in a non-esterified (i.e. free) form but this is not always the case. For example, Véron et al. (1996) studied the sterols of seven unicellular algae including representatives from the Prasinophyceae, Haptophyceae, Eustigmatophyceae, and Bacillariophyceae (as then defined). All synthesized free sterols and esterified forms (steryl esters, acyl steryl glycosides, and steryl glycosides), but free sterols predominated in five of the species. In contrast, the eustigmatophyte Nannochloropsis oculata and the diatom Thalassiosira pseudonana, contained mostly esterified sterols whereas in the diatom Chaetoceros calcitrans glycosylated forms represented over 60 % of total sterols. The pennate diatom Haslea ostrearia synthesized large amounts of steryl glycosides consisting mainly of the uncommon sterol 23,24-dimethylcholest-5-en-3β-ol.
2.4 Effects of Environmental Condition on Sterol Compositions
Minor differences in the relative proportions of sterols with culture age and growth state have been observed (Ballantine et al. 1979), but it comparatively rare for sterol distributions to change dramatically with changes in environmental conditions in contrast to fatty acid compositions. Sterol compositions thus are usually good chemotaxonomic tools for studying microalgal relationships and biochemistry. Sterol concentrations per cell or dry weight, however, can be affected. For example, Mercer et al. (1974) found no change in sterol composition with culture age in three species of xanthophytes, but noted that aeration increased the cholesterol content. A detailed review of this topic is not possible here, but some leading references are provided to guide the reader.
Piepho et al. (2010) studied the sterol content in freshwater green algae Desomodesmus communis (as S. quadricauda) and Chlamydomonas, the cryptophyte Cryptomonas ovata and the diatom Cyclotella meneghiniana, and found that sterol contents increased significantly with increasing light in three out of four species and that sterol content decreased with increasing light at low phosphorus supply. Piepho et al. (2012) observed that sterol concentrations were higher at 25 °C than at 10 °C in D. quadricauda and C. meneghiniana, but were not affected by temperature in C. ovata. Temperature and phosphorus supply interacted to affect sterol concentrations in C. meneghiniana presumably due to the bioconversion of 24-methylene-cholesterol to 24-methylcholesta-5,22E-dien-3β-ol. Jo et al. (2004) found the same major sterols in autotrophically grown and heterotrophically grown Tetraselmis suecica, but the proportions of these and total amounts varied with culture age.
3 Sterols in Cyanobacteria
The suggestion that cyanobacteria (blue-green algae) contain sterols has been much debated. Some early studies suggested that their presence was due to contamination since on repeated purification the amount of sterols declined (Ourisson et al. 1987) and others have obtained similar data (Volkman, unpublished data; Summons et al. 2001). However, there are more than a dozen reports of sterols being found in cyanobacteria (summarized by Volkman 1986, 2003). These include simple mixtures in which cholesterol and 24-ethylcholest-5-en-3β-ol predominate, but Δ7 and Δ5,7-sterols including ergosterol have also been reported (De Souza and Nes 1968). In almost all cases the amounts of sterols isolated are quite small (Hai et al. 1996). Summons et al. (2001) suggested that the sterols present in cyanobacterial cultures are derived from contaminating yeasts and fungi. The balance of evidence seems to indicate that cyanobacteria do not synthesize sterols although a few eubacteria can synthesize sterols that are not alkylated in the side-chain (reviewed by Volkman 2005).
4 Sterols in the Phylum Rhodophyta
The division or subphylum Rhodophyta contains both microalgae and macroalgae, generally termed red algae. Most members are marine macroalgae, but unicellular marine coccids are known including Porphyridium, Rhodosorus and Rhodella. Unicellular red algae that form mucilaginous colonies are considered primitive. The taxonomy has been through a number of changes. The Bangiophyceae, as defined traditionally, is paraphyletic and taxonomic identification of species has been difficult because of a lack of distinct morphological features, and the presumed morphological plasticity of the species. It has since been merged with the Floridophyceae to form the Rhodophyceae.
Most macrophyte red algae contain primarily C27 sterols with cholesterol predominant (e.g. Palmerno et al. 1984), although several species contain large amounts of cholesta-5,24-dien-3β-ol (desmosterol) (Gibbons et al. 1967). Only a few red macroalgae contain traces of C28 (e.g. 24-methylcholesta-5,22E-dien-3β-ol) and very rarely C29 sterols. This is in sharp contrast to the sterol distributions found in microscopic red algae which, while often dominated by cholesterol, can also show high contents of C28 4-desmethyl sterols and occasionally biosynthetically “primitive” 4-methyl sterols.
4.1 Class Bangiophyceae
The sterols of the macroalgae Pyropia/Porphyra are dominated by cholesterol (60 %) whereas Stylonema alsidii (as Goniotrichum elegans) contains both 24-methylcholesta-5,22E-dien-3β-ol and cholesterol (Brothers and Dickson 1980). Sterol analyses of microalgae in this class appear not to have been reported.
4.2 Class Porphyridiophyceae
The microalga Porphyridium cruentum contains 24-methylcholesta-5,7,22-trien-3β-ol (ergosterol) and cholesta-5,22-dien-3β-ol (Beastall et al. 1971). In contrast, other species of Porphyridinium contain unusual 4-methyl Δ8-unsaturated sterols including 4α-methyl-5α-cholesta-8,22-dien-3β-ol, 4α,24-dimethyl-5α-cholesta-8,22-dien-3β-ol, 4-methylcholest-8-en-3β-ol and 4,24-dimethylcholest-8-en-3β-ol (Beastall et al. 1974), perhaps due to the lack of a Δ8 to Δ7 isomerase. Duperon et al. (1983) showed that Porphyridinium sp. contains free sterols, steryl glycosides and acylated steryl glycosides.
4.3 Class Florideophyceae
Desmosterol was the major sterol found in three samples of the macroalga Palmaria (Rhodymenia) palmata (82 %, 97.2 % and 60.4 %) while cholesterol (92.3 %) predominated in a fourth (Idler et al. 1968). There was also significant variation in the principal sterol of two samples of the macroalga Devaleraea ramentacea (as Halosaccion ramentaceum). One contained predominantly cholesterol (85.4 %), the other desmosterol (81.8 %).
4.4 Class Stylonematophyceae
The major sterol in the microalgae Rhodosorus sp. (CS-210) and Rhodosorus lens (= R. salina?) is the C28 sterol 24-methylcholesta-5,22E-dien-3β-ol (Gladu et al. 1990; Dunstan et al. 2005) as found in some cryptomonads, diatoms and haptophytes. Rhodosorus sp. (CS-210) contains in addition small amounts of cholesterol (2 %) and various Δ7-sterols including the rare 4-methyl-5α-cholest-7-en-3β-ol (5.8 %) and 4-methyl-5α-cholesta-7,22-dien-3β-ol (12.3 %).
5 Sterols in the Phylum Chlorophyta
The Viridiplantae comprises the green algae and their descendants the land plants. One clade, the Chlorophyta, comprises the early diverging prasinophytes, which gave rise to the core chlorophytes. The other clade, the Streptophyta, includes the charophyte green algae from which land plants evolved. Many uncertainties about the evolution of this phylum persist, including the branching orders of the prasinophyte lineages, the relationships among core chlorophyte clades (Chlorodendrophyceae, Ulvophyceae, Trebouxiophyceae and Chlorophyceae), and the relationships among the streptophytes (Leliaert et al. 2012). As a consequence, it is difficult to classify some of the reported sterol distributions of various species due to changes in their taxonomic assignments.
The Chlorophyta contain chlorophylls a and b and store starch inside the chloroplast. There are thought to be between 9,000 and 12,000 species. Green algae became ecologically important about 600–800 Ma ago (Knoll et al. 2007; Kodner et al. 2008), possibly in response to increases in Fe content in the ocean (Canfield et al. 2008). Morphological analogues of the Ulvaphyceae have been found in Spitsbergen sediments 700–750 Ma (Butterfield et al. 1994). Green algae are thought to be responsible for the high abundance of C29 steranes seen in some Cambrian sediments and crude oils (e.g. Kodner et al. 2008). However, when one examines the sterols of modern groups of green alga the situation is much more complex. Many chlorophytes contain mixtures of Δ7, Δ5,7 and Δ7,22 sterols (Holden and Patterson 1982), but a few contain mainly Δ5-unsaturated sterols. In the latter group, 24-methylcholest-5-en-3β-ol and 24-ethyl-cholesta-5,22E-dien-3β-ol usually predominate with moderate amounts of 24-ethylcholest-5-en-3β-ol.
5.1 Class Chlorophyceae
This class is primarily freshwater and includes genera such as Chlamydomonas, Pyramimonas, Scenesdemus and Oedogonium. Chlamydomonas contains more than 600 species and has been shown to be polyphyletic (Proschold et al. 2001) which makes assessment of the early sterol literature difficult. It has been proposed that species in clades other than that containing C. reinhardtii must be transferred to other genera (Proschold et al. 2001).
Miller et al. (2012) have shown that the green alga Chlamydomonas reinhardtii synthesizes cycloartenol and converts it to ergosterol and 24-ethyl-5α-cholesta-7,22-dien-3β-ol (7-dehydroporiferasterol) (both having a C24 β-alkyl group) through a highly conserved sterol C24-methylation-C25-reduction pathway that is distinct from the acetate-mevalonate pathway that produces fungal lanosterol and thence to ergosterol by the Δ24(28)-olefin pathway.
Volkman et al. (1994) identified the major sterols in Pyramimonas cordata as 24-ethyl-cholesta-5,24(28)Z-dien-3β-ol (88.5 %) with a small amount of the 24(28)E isomer (0.8 %) and an unusual dihydroxylated C29 sterol 24-ethylcholesta-5,28(29)-dien-3β,24-diol (saringosterol) probably formed by oxidation of the major sterol.
Dunaliella minuta is unusual in that under stationary phase conditions it produces mainly C27 sterols (Ballantine et al. 1979). Dunaliella salina has been reported to produce C27 sterols at high salinities (4 M) (Kelly 2009) although C29 sterols are more commonly associated with this species (Peeler et al. 1989).
The freshwater species Desmodesmus communis (as Scenedesmus quadricauda) contains only Δ7 sterols 24-methyl-5α-cholest-7-en-3β-ol, 24-ethyl-5α-cholesta-7,22-dien-3β-ol and 24-ethyl-5α-cholest-7-en-3β-ol (Cranwell et al. 1990). In contrast, freshwater Eudorina unicocca contains 5 sterols: 24-methylcholesterol (32 %), cholesterol (27 %), 24-ethylcholesta-5,22E-dien-3β-ol (22 %), 24-ethyl-5α-cholest-7-en-3β-ol and 24-methylcholesterol (4 %) (Cranwell et al. 1990). This species is unusual in that it contains both Δ5 and Δ7 sterols.
5.2 Class Ulvophyceae
Very few data are available for microalgal members of this class of green algae. The freshwater species Ulothrix zonata contains a complex mix of sterols with 24-methylenecholesterol (41 %), 24-methylcholesterol (23 %), 24-ethylcholesta-5,24(28)Z-dien-3β-ol (16 %) and 24-ethyl-cholesterol (12 %) as major sterols (Cranwell et al. 1990).
5.3 Class Mamiellophyceae
This class is primarily marine and includes the smallest eukaryotic algal genus Micromonas which contains a single species, M. pusilla, which is the dominant photosynthetic picoeukaryote in some marine ecosystems (e.g. Throndsen 1976). Unlike many marine algae, it is distributed widely in both warm and cold waters. Under the light microscope it is easily mistaken for a rapidly swimming bacterium. Based on pigment analysis, Micromonas shows affinities with the Mamiellales group of the Prasinophyceae, but it lacks the characteristic scales of this group. Note that some still classify this genus in the Prasinophyceae. The sterols of Micromonas pusilla (strain CS-98) and a tropical strain Micromonas aff. pusilla (CS-170) were reported by Volkman et al. (1994) who found the same suite of 4 major sterols, but in very different proportions. The major sterol in CS-98 was isofucosterol (71.7 %), but in CS-170 this was only 19.4 % of total sterols. The second most abundant sterol in CS-98 was 24-methylenecholesterol (15.9 %), but this was the major sterol in CS-170 (54.4 %). This difference seems to reflect a greater extent of alkylation of the 24(28) double bond in CS-98. Both strains contained an unusual dihydroxylated C29 sterol 24-ethylcholesta-5,28(29)-dien-3β,24-diol (saringosterol; 3.1 % of total sterols in CS-98 and 14.2 % in CS-170). It seems likely that saringosterol is formed from enzymatic oxidation of 28-isofucosterol.
5.4 Class Chlorodendrophyceae
This class includes freshwater and marine species and now includes the marine flagellate Tetraselmis (previously assigned to the Prasinophyceae). Patterson et al. (1993b) examined 11 isolates of Tetraselmis and found only 3 sterols. The principal sterol in eight isolates was either 24-methylenecholesterol or 24-methylcholesterol, both C28 sterols, with the latter identified as the 24α-isomer campesterol in each case. In the other three isolates, cholesterol was the principal sterol, which is highly unusual for a green alga, together with smaller amounts of 24-methylene-cholesterol and campesterol.
Volkman (1986) and Volkman et al. (1994) reported the sterol composition for Tetraselmis chui and found a single major sterol (>96 %) identified as 24-methylcholesterol. In contrast, Ballantine et al. (1979) found a much greater variety of sterols in Tetraselmis tetrathele which included 24-methylcholesterol (34 %) and 24-methylcholesta-5,22-dien-3β-ol (57.7 %). Similar data were reported by Lin et al. (1982) for another species Tetraselmis suecica where the proportions of these two sterols were 48.1 % and 50.9 % respectively.
Jo et al. (2004) examined the sterol dynamics of a strain of Tetraselmis suecica grown both autotrophically and heterotrophically. Six major sterols were found in the photoautotrophically grown cells: cholesta-5,22-dien-3β-ol, 24-methylcholesterol, cholesterol, 24-methyl-cholesta-5,22-dien-3β-ol, 24-methylcholesta-5,24-dien-3β-ol, and 24-ethylchlolesta-5,24-dien-3β-ol in decreasing order of abundance. The total amounts of sterols after 1 week of culture were quite similar as was the composition, but total amounts declined in weeks 2 and 3 and 24-methylcholesterol became the major sterol. Similar changes were found when the cells were grown heterotrophically.
The effect of culture renewal rate (RR) on sterol amounts in T. suecica was studied by Fabrégas et al. (1997). The major sterol 24-methylcholesterol ranged from 137 fg cell−1 with a 10 % RR to 40 fg cell−1 with a 40 % RR and 24-methylenecholesterol ranged from 403 fg cell−1 with a 10 % RR to 80 fg cell−1 with a renewal rate of 50 %.
5.5 Class Trebouxiophyceae
The Trebouxiophyceae contains the three orders: Chlorellales, Prasiolales and Trebouxiales. Botryococcus braunii is perhaps the best known member of this class of microalgae. Chlorella was formerly assigned to the Chlorophyceae but most species have now been assigned to the Trebouxiophyceae, although Huss et al. (1999) have proposed that only four species should be kept in the genus Chlorella within the Trebouxiophyceae, i.e. C. vulgaris, C. lobophora, C. sorokiniana and C. kessleri (= Parachlorella kessleri). Since the description of the type species Chlorella vulgaris, more than a hundred “Chlorella” species have been established in the literature (Goers et al. 2010), but most have been assigned to other genera.
Extensive data on the sterols in Chlorella species have been published by Patterson and coworkers (Patterson 1967, 1969, 1974; Dickson et al. 1972; Dickson and Patterson 1973; Patterson et al. 1974, 1992). These data were collated and expanded by Holden and Patterson (1982) who analysed the sterols in 35 species. From these data they were able to group the algae into six categories according to their sterol distribution. Group Ia contained Δ5 sterols including cholesterol, 24β-ethylcholesta-5,22-dien-3β-ol (poriferasterol), 24β-methylcholesterol (5-ergostenol) and 24β-ethylcholesterol (clionasterol), all with 24β stereochemistry. The two isolates in Group 1B contained a related composition, but the major product was 24β-methylcholesterol (69–73 % of total sterol) and significant amounts of the C29 homolog of ergosterol, 24β-ethylcholesta-5,7,22-trien-3β-ol (7-dehydroporiferasterol), was also found. Group II contained Δ7 sterols presumably due to the lack of genes needed for the introduction of the Δ5 double bond. The major sterol was the C29 sterol, 24β-ethylcholesta-7,22-dien-3β-ol (chondrillasterol). Group IIIa contained double bonds at both Δ5 and Δ7 presumably due to the lack of a Δ7 reductase in some species. These isolates lack the ability to introduce a second alkyl group at C-24 resulting in the production, exclusively, of the C28 sterols ergosterol with lesser amounts of its mono- and diunsaturated derivatives. Group IIIb was similar, but these species are able to introduce a second alkyl group at C-24, producing the C29 homologue of ergosterol, 24β-ethylcholesta-5,7,22-trien-3β-ol, plus 24β-ethyl-5α-cholesta-7,22-dien-3β-ol and 24β-ethyl-5α-cholest-7-en-3β-ol (7-chondrillastenol). The presence of C29 sterols in ergosterol-synthesizing organisms is a rare occurrence. The last type of sterol biosynthetic pattern, Group IIIc, was encountered in only one strain of Chlorella. This contained unique Δ8 and Δ8,22 sterols along with ergosterol, 24β-methylcholesta-5,7-dien-3β-ol (5,7-ergostadienol), and 24β-methyl-5α-cholest-7-en-3β-ol (7-ergostenol) as originally published by Patterson et al. (1974). These grouping align well with the various stages in the sterol biosynthetic pathway and this would seem to be a useful way to categorize sterol patterns. However, the taxonomic status of some of these strains is uncertain and it remains to be seen whether they match closely with modern views on Chlorella taxonomy.
Chlorella autotrophica (now Chlorella vulgaris var. autotrophica) contains a complex mixture of C28 and C29 sterols with Δ7, Δ5,7 and Δ5,7,9(11) nuclear double bond systems (Patterson et al. 1992). This alga also contained the rare tetraunsaturated sterols, 24-methylcholesta-5,7,9(11),22-tetraen-3β-ol and 24-ethylcholesta-5,7,9(11),22-tetraen-3β-ol.
Akihisa et al. (1992) identified ergosterol (51 %) and 7-dehydroporiferasterol (30 %), as the principal sterols of Chlorella vulgaris. They also identified the unusual 24β-methyl-Δ9(11)-sterols, 24β-methyl-5α-cholest-9(11)-en-3β-ol and 14α,24β-dimethyl-5α-cholest-9(11)-en-3β-ol, and the same two 24β-alkyl-Δ5,7,9(11),22-sterols, 24β-methylcholesta-5,7,9(11),22E-tetraen-3β-ol (9(11)-dehydroergosterol) and 24β-ethylcholesta-5,7,9(11),22E-tetraen-3β-ol found in C. autotrophica.
More recent work by Goers et al. (2010) has confirmed that sterol composition is a reliable chemotaxonomic marker within several groups of Chlorella and found high contents of ergosterol in nine species, all from the Chlorellaceae. More distant relatives within the Trebouxiophyceae or representatives of the Chlorophyceae did not contain ergosterol. The sterols in Mucidosphaerium (Dictyosphaerium) pulchellum were reported by Cranwell et al. (1990). The major sterol was cholesterol (90 %) with a small quantity of 24-ethylcholesterol (8 %).
5.6 Class Prasinophyceae
Prasinophytes can be important constituents of the phytoplankton in oceanic waters. Volkman et al. (1994) reported sterol compositions for Pyramimonas cordata, and Pycnococcus provasolii and found relatively simple distributions of Δ5-sterols. The major sterols were 24-methylenecholesterol (which also occurs in diatoms), 24-methylcholesterol and 24-ethylcholesta-5,24(28)Z-dien-3β-ol (28-isofucosterol). They proposed that 24-methylcholesterol may be a useful marker for these microalgae. Minor amounts of C30 n-propylcholestane were produced after hydrogenation of the sterol mixture indicating the presence of C30 sterols that were not characterized.
Patterson et al. (1992) found that an un-named strain of Pyramimonas contained only 24-methylenecholesterol as a major sterol component (99 %) together with a trace of cholesterol. In marked contrast, Pyramimonas grossii contained a complex mixture of C28 and C29 sterols with Δ7, Δ5,7 and Δ5,7,9(11) nuclear double bond systems. Sterols were found both with and without the C-22 side chain double bond; ergosterol and 24β-ethylcholesta-5,7,22-trien-3β-ol (7-dehydroporiferasterol) were the principal sterols. Rare tetraene sterols, 24-methylcholesta-5,7,9(11),22-tetraen-3β-ol and 24-ethylcholesta-5,7,9(11),22-tetraen-3β-ol were found in P. grossi as in Chlorella vulgaris var. autotrophica and Dunaliella tertiolecta (Patterson et al. 1992).
6 Sterols in the Phylum Charophyta
6.1 Class Conjugatophyceae
Few data are available for this class. Cranwell et al. (1990) analysed the sterols in the two desmids Cosmarium bioculatum and Xanthidium subhastiferum. Both contained three main sterols: 24-methylcholesterol, 24-ethylcholesterol and 24-ethylcholesta-5,22E-dien-3β-ol, which are the same three sterols found in higher plants.
7 Sterols in the Phylum Dinophyta
7.1 Class Dinophyceae
Dinoflagellates (Dinophyceae) are generally considered to be the major source of 4-methyl sterols in aquatic environments (e.g. de Leeuw et al. 1983; Volkman 2003 and refs therein), but they are also potential contributors of 4-desmethyl sterols. In most species, 4-methyl sterols predominate (e.g. Leblond and Chapman 2002), but exceptions are known (e.g. Teshima et al. 1980). Complex mixtures of sterols are usually found (Withers et al. 1979; Wengrovitz et al. 1981; Piretti et al. 1997; Amo et al. 2010), and many species contain unusual sterols having Δ7 or Δ8 double bonds (e.g. Hallegraeff et al. 1991) and unusual patterns of side-chain alkylation such as 23,24-dimethyl substitution (Leblond and Lasiter 2012 and refs therein).
The major sterol in many dinoflagellates is the 4-methyl sterol dinosterol (4α,23,24-trimethyl-5α-cholest-22E-en-3β-ol; Fig. 5) which has no double bond in the ring system and an unusual 23,24-dimethyl side-chain alkylation (Shimizu et al. 1976; Boon et al. 1979). Because of its specificity as a dinoflagellate marker it has been widely used for paleoclimate studies (e.g. Boon et al. 1979; Makou et al. 2010; Castaneda et al. 2011). Methods to isolate purified dinosterol and other sterols from dinoflagellates for structural or isotopic analysis are now available using reverse phase and normal phase HPLC (Atwood and Sachs 2012). Although dinosterol appears to be a reliable biomarker for dinoflagellates, it should be noted that some dinoflagellates do not contain this sterol at all (Teshima et al. 1980; Kokke et al. 1981; Goad and Withers 1982).
Comparison of structures of two C30 sterols: (a) dinosterol (4α,23,24-trimethyl-5α-cholest-22E-en-3β-ol) as found in many dinoflagellates and structurally isomeric 4α-methyl,24-ethyl-5α-cholest-22E-en-3β-ol (b) as found in haptophytes from the Pavlovophyceae . Also shown is the structure of a dihydroxylated 4-methyl sterol termed a pavlovol (c) that appear to be unique to the Pavlovophyceae
Recently Leblond et al. (2010) published a review of the sterols and steroid ketones in dinoflagellates and related these to taxonomic assignments based on 18 rDNA phylogeny. These authors compiled a database from 102 published analyses of dinoflagellates which contained 58 distinct identified sterols and steroid ketones. Data on both sterols and 18S rDNA were available for 82 of the 102 strains. These data were clustered into six groups:
-
1.
Mainly Karenia and Karlodinium species containing 4-desmethyl sterols with Δ8(14),22 diunsaturation such as 4α,24-dimethyl-5α-cholesta-8(14),22-dien-3β-ol and 27-nor-4α,24-dimethyl-5α-cholesta-8(14),22-dien-3β-ol;
-
2.
Mainly Amphidium species containing 4α-methyl-5α-cholest-8(14)-en-3β-ol and 4α,24-dimethylcholesta-8(14),24(28)-dien-3β-ol. Note that both groups 1 and 2 are distinguished by sterols having a Δ8(14) double bond which is uncommon in other dinoflagellates;
-
3.
This grouping contained Polarella glacialis, Protoceratium reticulatum, Lingulodinium polyedra and Gymnodinium simplex. Major sterols were cholesta-5,22Z-dien-3β-ol, 24-methylcholesta-5,22E-dien-3β-ol, and 4α,24-dimethyl-5α-cholestan-3β-ol;
-
4.
This contained Akashiwo sanguinea and prominent sterols 24-methylcholest-5α-cholest-22E-en-3β-ol, 23,24-dimethyl-5α-cholest-22E-en-3β-ol and 4α,24-dimethyl-5α-cholestan-3β-ol;
-
5.
This contained genera such as Alexandrium, Prorocentrum, and Symbiodinium. Major sterols were cholesterol and 4α,23,24-trimethyl-5α-cholest-22E-en-3β-ol (dinosterol);
-
6.
This contained Alexandrium, Gymnodinium, Heterocapsa, Pfiesteria, Pyrocystis and Thoracosphaera. Prominent sterols were 4α,24-dimethyl-5α-cholestan-3β-ol, 4α,23,24-trimethyl-5α-cholestan-3β-ol (dinostanol) and dinosterol.
Several of these groupings matched well with DNA-based phylogeny, but note that some genera occur in two groups as do some sterols. Volkman et al. (1999b) examined the sterols of four species of the dinoflagellate genus Prorocentrum and found over 20 sterols which varied considerably in abundance between the species. LeBlond et al. (2010) found that all Prorocentrum species were closed related by 18S rDNA analysis, but based on their sterols they were distributed in both clusters 5 and 6. All contained 23,24-dimethylcholesta-5,22E-dien-3β-ol, dinosterol and dinostanol. Those in cluster 5 also produced cholesterol not found in cluster 6 while those in cluster 6 produced 24-methylenecholesterol not found in cluster 5. Similarly, Pyrocystis lunula and Pyrocystis noctiluca (=Pyrocystis pseudonoctiluca), although closely related by 18 S rDNA analysis had quite different sterol distributions with dinosterol abundant in P. noctiluca but absent from P. lunula (Dahmen and Leblond 2011).
Sterols containing a cyclopropyl ring in the side-chain such as gorgosterol are known from various marine animals, particularly coelenterates and a few dinoflagellates (Withers 1987). Some species of Gonyaulax contain large amounts of cholesterol but there does not appear to be a single distribution of 4-desmethyl sterols which characterizes these algae.
Karenia brevis has been shown (Leblond and Chapman 2002) to possess two major sterols, (24S)-4α,24-dimethyl-5α-cholesta-8(14),22-dien-3β-ol (abbreviated ED in their work) and its 27-nor derivative (NED). These novel structures are also found in Karenia mikimotoi and Karlodinium micrum (=Karlodinium veneficum), two dinoflagellates closely related to K. brevis (Leblond and Chapman 2002). They are also found as minor components of the more complex sterol profiles of other members of the Gymnodinium/Peridinium/Prorocentrum (GPP) taxonomic group (Leblond and Chapman 2002).
A predominance of the 4-methyl and 4-desmethyl Δ8(14) sterols and a lack of dinosterol was reported by Mooney et al. (2007) for species in the Karenaceae. Unusual sterols included 23-methyl-27-nor-24-methylcholesta-8(14),22-dien-3β-ol (Karenia papilionacea, 59–66 %); 27-nor-(24R)-4α,24-dimethyl-5α-cholesta-8(14),22-dien-3β-ol (brevesterol; Takayama tasmanica 84 %, Takayama helix 71 %, Karenia brevis 45 %, Karlodinium sp.? 40 %, Karenia mikimotoi 38 %); and 4α,24-dimethyl-5α-cholesta-8(14),22-dien-3β-ol (gymnodinosterol; K. mikimotoi 48 %, Karenia umbella 59 %, Karlodinium veneficum 71–83 %). In Takayama species, five steroid ketones were identified, including for the first time the 3-keto form of brevesterol and gymnodinosterol. Steroid ketones have also been reported in Scrippsiella trochoidea (Harvey et al. 1988), Prorocentrum spp. (Volkman et al. 1999b) and Crypthecodinium cohnii (Withers et al. 1978).
8 Sterols in the Phylum Haptophyta
Haptophytes usually contain from one to five major sterols, and commonly cholesterol or 24-methylcholesta-5,22E-dien-3β-ol predominates (Volkman et al. 1981). Moderate amounts of the C29 sterols 24-ethylcholesterol and 24-ethylcholesta-5,22E-dien-3β-ol are found in several species (Conte et al. 1994).
8.1 Class Coccolithophyceae
Well known examples of this class include the coccolithophorids Emiliania huxleyi and Gephyrocapsa oceanica. Both are major sources of organic matter in marine ecosystems and can form large blooms easily visible to satellites due to shedding of their coccolith scales. The sterol distributions of both species are very simple and are dominated (>90 %) by 24-methylcholesta-5,22E-dien-3β-ol (Volkman et al. 1980b, 1995). The principal sterol in Pleurochrysis carterae and an unidentified haptophyte strain CCMP1215 was also shown to be 24-methylcholesta-5,22E-dien-3β-ol by Ghosh et al. (1998). In E. huxleyi, the stereochemistry at C-24 has been shown to be 24α (Maxwell et al. 1980) as found also in the sterols of Pleurochrysis carterae which contains stigmasterol (24α-ethylcholesta-5,22E-dien-3β-ol) and epibrassicasterol (Gladu et al. 1990). Chrysotila lamellosa (= Ruttnera lamellosa) also contains 24α-methylcholesta-5,22E-dien-3β-ol (epibrassicasterol) as well as significant amounts of Δ5- and Δ5,22-C29 sterols (Rontani et al. 2004). Goad et al. (1983) identified 24α-methylcholesta-5,22E-dien-3β-ol as the major sterol of the marine haptophyte Isochrysis galbana.
Phaeocystis pouchetii is a major phytoplankton species in polar oceans and a major food source for krill (Hamm et al. 2001). Its dominant sterol (93–100 %) is 24-methylcholesta-5,22E-dien-3β-ol irrespective of culture age or life stage (Nichols et al. 1991). Small amounts of cholesterol (8 %) were found in one strain (A1-3) and strain DE10 contained 24-methylenecholesterol (Nichols et al. 1991). In marked contrast to the above results, Ghosh et al. (1998) showed that Prymnesium parvum contained only small amounts of sterols consisting solely of cholesterol.
Hymenosulphate, a novel sterol sulphate with Ca-releasing activity has been isolated from the cultured marine haptophyte Hymenomonas sp. by Kobayashi (1989).
8.2 Class Pavlovophyceae
A number of papers have reported the unusual sterol compositions of species in the genus Pavlova. Volkman et al. (1990) reported the presence of 4-methylsterols, 5α(H)-stanols, 4-desmethyl sterols and unusual dihydroxylated sterols called pavlovols (Fig. 5). The major 4-desmethyl sterol in each of the species analysed by these authors was 24-ethylcholesta-5,22E-dien-3β-ol which occurred with smaller amounts of 24-ethylcholesterol and in some species cholesterol. Two species also contained significant amounts of the 5α(H)-stanol 24-ethyl-5α-cholest-22E-en-3β-ol. The major 4-methyl sterol was a C30 sterol identified as 4α-methyl-24-ethyl-5α-cholest-22E-en-3β-ol. This sterol has a similar structure to dinosterol, which occurs in dinoflagellates, except that the side-chain contains a 24-ethyl group rather than 23,24-dimethyl substitution (Fig. 5). Minor 4-methylsterols included 4,24-dimethyl-5α-cholest-22E-en-3β-ol and the fully saturated stanol 4,24-dimethylcholestanol.
Pavlovols have a second hydroxyl group at C-4 and a C-4 methyl group (Fig. 5) in the sterol ring system (e.g. Volkman et al. 1990; Gladu et al. 1991a; Patterson et al. 1992; Véron et al. 1996; Rauter et al. 2005) and have been proposed as a chemotaxonomic marker for this subgroup of haptophytes (Volkman et al. 1997). Mass spectra of the TMSi-ether derivatives of 4α,24-dimethyl-5α-cholestan-3β,4β-diol and 4α-methyl,24-ethyl-5α-cholestan-3β,4β-diol found in Pavlova pinguis and 4α,24-dimethyl-5α-cholest-22E-en-3β,4β-diol found in Diacronema vlkianum can be found in Volkman et al. (1997).
9 Sterols in the Phylum Euglenophyta
9.1 Class Euglenophyceae
This genus was established by Ehrenberg to accommodate those euglenoid organisms that have eyespots. Euglena (Astasia) longa, a natural mutant of Euglena that has lost all potential for photosynthesis contains cycloartenol metabolites indicative of biosynthesis by a plant-type mechanism (Anding et al. 1971; Rohmer and Brandt 1973) since lanosterol was not detected (Anding et al. 1971). Anding and Ourisson (1973) reported the presence of ergosterol in both light-grown and dark-grown Euglena gracilis and an unusual 4-methylsterol 4α,24-dimethyl-5α-cholest-8(9)-en-3β-ol has also been reported (Anding et al. 1971).
Brandt et al. (1970) found that free sterols predominate over bound sterols in light-grown green-coloured E. gracilis whereas in dark-grown white cells the reverse is true. The free sterols of green cells consist almost exclusively of Δ7-sterols (98 %) while in white cells Δ5-sterols make up 31 % of the sterols.
Zielinski et al. (1982) reported that the freshwater Eutreptia viridis contained 18 different sterols including a novel sterol with the rare Δ23-unsaturation 24-ethylcholesta-5,7,23Z-trien-3β-ol. The free sterols were dominated by Δ5,7-diunsaturated sterols (ca. 80 %).
10 Sterols in the Phylum Cryptophyta
10.1 Class Cryptophyceae
The Cryptophyceae is a class of algae within the Pyrrhophyta in some systems of classification. Cryptomonads are aquatic unicellular eukaryotes that inhabit both marine and freshwater environments. Most cryptomonads are photosynthetic (and are thus also referred to as cryptophytes) and possess plastids that are very diverse in pigmentation. Cyptomonads are common in freshwater systems but can also be found in marine and brackish habitats. Each cell is around 10–50 μm in size and flattened in shape, with typically two slightly unequal flagella.
Dunstan et al. (2005) examined the sterols of seven cryptophytes. The major sterol in Rhodomonas spp. (CS-215, CS-694), Rhodomonas salina (CS-174, CS-24), and Proteomonas sulcata (CS-412) was 24-methylcholesta-5,22E-dien-3β-ol (91–99 % of total sterols) together with small amounts of cholesterol (1–2.7 %). Rhodomonas maculata (CS-85) had the same two sterols, but cholesterol was more abundant (17.7 %). Chroomonas placoidea (CS-200) contained in addition the C29 sterol 24-ethylcholesta-5,22E-dien-3β-ol (35.5 %).
11 Sterols in the Phylum Glaucophyta
11.1 Class Glaucophyceae
Glaucophytes (or Glaucocystophytes) are freshwater algae that have an almost intact cyanobacterium, referred to as a cyanelle, as the photosynthetic organelle. Heimann et al. (1997) reported the presence of “sitosterol” and an identified sterol in Cyanophora paradoxa. A more recent analysis by Leblond et al. (2011) found that C. paradoxa and Glaucocystis nostochinearum contained very simple sterol distributions consisting of sterols more typically found in higher plants: 24-methylcholesterol, 24-ethylcholesta-5,22E-dien-3β-ol, and 24-ethylcholesterol.
12 Sterols in the Phylum Picozoa
Picoeukaryotes (defined as cells <3 μm) are now known to be ubiquitous in surface waters of all oceans and are likely to be the most abundant eukaryotes in the sea. Most are phototrophic, but some are heterotrophic, especially in oligotrophic coastal sites. In 2007, a novel and widespread picoeukaryotic lineage with affinities to cryptophytes and katablepharids, the “picobiliphytes” was reported from 18S environmental clone library sequences (Not et al. 2007). Until the work of Seenivasan et al. (2013) these heterotrophs (which may be related to glaucocystophytes) had remained uncultured. These authors described Picomonas judraskeda gen. et sp. nov., from marine coastal surface waters, and established a new phylum, Picozoa. No sterol data are available as yet from cultures of these ecologically important organisms.
13 Sterols in the Phylum Cercozoa
13.1 Class Chlorarachniophyceae
Chlorarachniophytes are marine unicellular algae that possess secondary plastids of green algal origin. Although chlorarachniophytes are a small group (the phylum of Chlorarachniophyta contains 14 species in 8 genera), they have variable and complex life cycles that include amoeboid, coccoid, and/or flagellate cells (Hirakawa et al. 2011). They are typically mixotrophic and photosynthetic and have the form of small amoebae, with branching cytoplasmic extensions that capture prey and connect the cells together. The amoeboid morphology may be the result of secondary endosymbiosis of a green alga by a nonphotosynthetic amoeba or amoeboflagellate. The only sterols present in genera Bigelowiella, Gymnochlora, and Lotharella were identified as 24α-methylcholesta-5,22E-dien-3β-ol) and one of the epimeric pair poriferasterol/stigmasterol (24-ethylcholesta-5,22E-dien-3β-ol) (Leblond et al. 2005).
14 Sterols in the Phylum Heterokontophyta (Ochrophyta)
The heterokonts (or stramenopiles) are chromists with chloroplasts surrounded by four membranes. There are more than 100,000 known species. Given the diversity of species present and wide variety of evolutionary paths involved (Leipe et al. 1994) it is not surprising that a great diversity of sterol patterns exist for this Phylum.
14.1 Diatom Classes
Diatoms are photosynthetic secondary endosymbionts found throughout marine and freshwater environments, and are believed to be responsible for around one-fifth of the primary productivity on Earth (Bowler et al. 2008 and refs therein). In spite of the fact that the pennate and centric lineages have only been diverging for 90 million years, their genome structures are dramatically different and a substantial fraction of genes (ca. 40 %) are not shared.
The sterols of diatoms have been the best studied of all the algal classes with data available for more than 100 species (e.g. Orcutt and Patterson 1975; Volkman 1986; Barrett et al. 1995; Rampen et al. 2009a, b, c, 2010). Rampen et al. (2010) analysed the sterols of over 100 diatom strains and detected 44 different sterols of which 11 were considered to be major sterols (i.e. >10 % of total sterols). Two-thirds of the species contained 24-methylenecholesterol, but 24α-methylcholesta-5,22E-dien-3β-ol (epibrassicasterol) which is often used as a diatom marker was only the fifth most common sterol. Cholesterol was abundant in a few species, but in Amphora species 24-ethylcholesta-5,22E-dien-3β-ol predominates (Gladu et al. 1991b).
Some diatoms have been reported to contain large amounts of Δ7-unsaturated sterols (e.g. Thassiosira pseudonana, Odontella (Biddulphia) aurita and Fragilaria sp.; Orcutt and Patterson 1975), but this seems to be rare. Giner and Wikfors (2011) have recently reported Δ7 sterols in Ditylum brightwellii.
A few diatoms lack appreciable amounts of C-24 alkylated sterols. One example is Biddulphia sinensis (now Odontella sinensis) which contains mostly cholesta-5,22E-dien-3β-ol (Volkman et al. 1980a). In contrast Biddulphia aurita (now Odontella aurita) analysed by Orcutt and Patterson (1975) contained none of this sterol and among the six identified sterols were the uncommon sterols 24-methylcholest-8(9)-en-3β-ol (22.8 %) and 24-methyl-5α-cholesta-7,22E-dien-3β-ol (18 %).
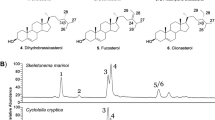
One interesting report is the presence of the C30 sterol gorgosterol in species from the genus Delphineis (Rampen et al. 2009c). Figure 6 shows a chromatogram of the sterols found in this alga. This sterol contains a cyclopropyl group in the side-chain which is more commonly associated with the sterols of jellyfish and highlights the diversity of sterol biosynthesis pathways developed in these microalgae. Giner and Wikfors (2011) have confirmed using NMR that the pennate diatom Delphineis sp. (CCMP 1095) contains gorgosterol, as well as the 27-nor C27 sterol occelasterol.
Chromatogram showing the main sterols present in Delphineis sp. CCMP 1095. (I) Cholesta-5,22E-dien-3β-ol; (II) 24-Methylcholesta-5,22E-dien-3β-ol; (III) 23,24-Dimethylcholesta-5,22E-dien-3β-ol; (IV) 22,23-Methylene-23,24-dimethylcholest-5-en-3β-ol (gorgosterol) with structures of the major sterols (From Rampen et al., 2009c)
Volkman et al. (1993) showed that a strain of Navicula sp. (CS-146) contained small amounts of the dinoflagellate marker sterol dinosterol plus other 4-methyl sterols including 4α,24-dimethyl-5α-cholest-22E-en-3β-ol (9.7–12.6 %), 4α,24-dimethyl-5α-cholestan-3β-ol (2.0–3.4 %) and 4α,23,24-trimethyl-5α-cholestan-3β-ol (dinostanol or its C-23/C-24 epimer; 0.3–0.6 %). The 4-desmethyl sterol fraction included the common diatom sterol 24-methylcholesta-5,22E-dien-3β-ol (20.2–30.5 %), but the major sterol was 24-ethylcholesterol (31.0–38.6 %) more usually associated with higher plants.
Sterols containing 23-methyl group rather than the more usual 24-methyl group are also found in diatoms. 23-Methylcholesta-5,22E-dien-3β-ol was found in 14 out of 106 diatom cultures studied by Rampen et al. (2009a) thus confirming earlier reports of such sterols in diatoms (Barrett et al. 1995). These data confirm that this unusual pattern of side-chain alkylation is not restricted to the dinoflagellates. Rampen et al. (2009b) further showed that diatoms are a likely source of steroidal hydrocarbons containing 23,24-dimethyl alkylation in sediments and petroleum since their precursor 4-desmethyl-23,24-dimethyl sterols were present in 22 of the cultures they studied.
Giner and Wikfors (2011) re-examined the occurrence of sterols with 23,24-dimethyl side-chains in diatoms. The centric diatom Triceratium dubium (= Biddulphia sp. CCMP 147) contained a high proportion of 23-methylated sterols of which 23,24-dimethylcholesta-5,22E-dien-3β-ol was 37.2 % of total sterols. They also showed that the sterol composition of Ditylum brightwellii (CCMP 358) is very complex and includes 5α(H)-stanols and Δ7-sterols, in addition to the predominant Δ5-sterols. A pair of previously unknown sterols, 24-ethylcholesta-5,24,28-trien-3β-ol and 24-ethylcholesta-24,28-dien-3β-ol, were also detected and their structures determined by NMR and by synthesis of the former sterol derived from saringosterol. Also detected in D. brightwellii was the previously unknown 23-methyl-5α-cholesta-7,22-dien-3β-ol.
14.2 Class Eustigmatophyceae
Eustigmatophytes are a small group of coccoid microalgae. Most are freshwater or live in soils, with the main marine species represented by the genus Nannochloropsis. Their colour is distinctive due to the presence of the accessory pigments violaxanthin and β-carotene. Eustigmatophytes contain unusual long chain C28–C32 n-alkyl-1,15-diols, and the corresponding hydroxy ketones (Volkman et al. 1992, 1999a; Méjanelle et al. 2003).
Sterol data for this algal class are rather limited. Volkman et al. (1999a) analysed three freshwater species Eustigmatos vischeri, Vischeria helvetica and Vischeria punctata and demonstrated that the sterol distributions consisted predominantly of 24-ethylcholesterol with small amounts of cholesterol, 24-methylcholesterol, 24-ethylcholesta-5,22E-dien-3β-ol and isofucosterol.
The high proportion of C29 sterols in freshwater species is in marked contrast to marine species from the genus Nannochloropsis which contain a dominance of cholesterol (Volkman et al. 1992; Patterson et al. 1994; Méjanelle et al. 2003). Volkman et al. (1992) studied the lipids of N. oculata, N. salina and an un-named species and found simple distributions of sterols dominated by cholesterol (>75 %) together with small amounts of the C29 sterols 24-ethylcholesta-5,24(28)E-dien-3β-ol (fucosterol) and 24-ethylcholesta-5,24(28)Z-dien-3β-ol (isofucosterol). N. salina also contained 24-ethylcholesterol. The sterol composition of N. gaditana is similar with a predominance of cholesterol and lesser amounts of 24-ethylcholesterol (Méjanelle et al. 2003). Véron et al. (1996) showed that the sterols in N. oculata were mostly esterified rather than being present as free sterols.
The value of sterols as a chemotaxonomic tool was demonstrated by Gladu et al. (1995) who studied strain UTEX 2341 which had previously been identified as Chlorella minutissima. This strain contained cholesterol as the principal sterol along with 24-methylenecholesterol, fucosterol, and isofucosterol which was inconsistent with any of 35 Chlorella strains analyzed at that time. This finding and other data showed that the strain was actually a eustigmatophyte.
14.3 Class Synurophyceae
The Synurophyceae are keterokonts closely related to the Chrysophyceae. Both have a long “flimmer” flagellum and a short “whiplash” flagellum. Fucoxanthin is the main pigment responsible for their “golden brown” colouration. Synurophytes have bristles and scales which are taxon-specific. Silicified resting stages called stomatocysts are unique to the Chrysophyceae and Synurophyceae and may be preserved in sediments facilitating their use as palaeoenvironmental indicators. The oldest silicified scales and bristles are found in Middle Eocene freshwater sediments from northwest Canada, but the oldest stomatocysts have been found in Early Cretaceous marine sediments from the Southern Ocean.
An early study by Collins and Kalnins (1969) of the sterols in Synura petersenii (then assigned to the Chrysophyta) identified only two sterols identified as cholesterol and sitosterol.
14.4 Class Chrysophyceae
Chrysophytes have been subject to considerable revision with many species moved to other classes such as the closely related Synurophyceae and Pelagophyceae (Jordan and Iwataki 2012). One of the first lipid studies of Ochromonas danica was by Halevy et al. (1966) who found four sterols and identified the C28 sterol ergosterol and C29 sterol 24-ethylcholesta-5,22E-dien-3β-ol (reported as the 24α isomer stigmasterol). Further work on Ochromonas danica and Poteriochromonas (Ochromonas) malhamensis was carried out by Gershengorn et al. (1968) who found cycloartenol and 24-methylenecycloartanol but no lanosterol thus providing evidence of the plant-type biosynthetic pathway used by these algae (Fig. 2). The C29 sterol 24β-ethylcholesta-5,22E-dien-3β-ol (poriferasterol) was identified as the major sterol. Melting points were used to define the stereochemistry as 24β which is opposite to the 24α stereochemistry implied by the identification of stigmasterol by Halevy et al. (1966). The C28 sterol 24β-methylcholesta-5,22E-dien-3β-ol (brassicasterol) has been identified in an unidentified species assigned to the order Sarcinochrysidales (Chrysophyceae) (Rohmer et al. 1980; Kokke et al. 1984).
The sterols of Ochromonas danica have been identified as ergosterol, brassicasterol, 22-dihydrobrassicasterol, clionasterol, poriferasterol, and probably 7-dehydroporiferasterol. By contrast Poteriochromonas malhamensis contains only poriferasterol as the major sterol component.
Poteriochromonas (Ochromonas) malhamensis was one of the first species used to probe the biosynthetic pathways occurring in microalgae. For example, Knapp et al. (1971) were able to show that the three 24-ethylidene sterols fucosterol, iso-fucosterol and 24-ethyl-5α-cholesta-7,24(28)-dien-3β-ol could all be transformed to poriferasterol, by P. malhamensis, but with varying degrees of efficiency,
Billard et al. (1990) showed that the C29 sterols 24-ethylcholesta-5,22E-dien-3β-ol, 24-ethylcholesterol and fucosterol predominated in the genera Chrydoderma, Chrysowaernella, Chrysomeris and Giraudyopsis. The status of the first genus is uncertain, but the latter three are now classified in the class Chrysomerophyceae. This is in marked contrast to the sterols found in Sarcinochrysis and Nematochrysopsis which contain the C30 sterol 24-n-propylidenecholesterol (previously assigned to the Chrysophyceae, but now considered to be part of the Pelagophyceae).
A paleoenvironmental application by Soma et al. (2007) linked the profile of 24-ethylcholesta-5,22E-dien-3β-ol in steryl chlorin esters (SCEs) in sediments of Lake Baikal over the past 28,000 years with the reported distribution of chrysophyte cysts during the Holocene.
14.5 Class Chrysomerophyceae
Chrysomeris ramosa contains 24-ethylcholesterol as the major sterol together with 24-ethylcholesta-5,22E-dien-3β-ol, 24-methylcholesterol and cholesterol (Billard et al. 1990), but percentage data were not provided. Giraudyopsis stellifer also contained 24-ethylcholesterol as the major sterol, but other sterols included fucosterol, 24-ethylcholesta-5,22E-dien-3β-ol, 24-methylenecholesterol, 24-methylcholesta-5,22E-dien-3β-ol and cholesterol (Billard et al. 1990).
14.6 Class Pelagophyceae
These small unicellular algae were formerly grouped with Chrysophyceae and are classified into two orders: Pelagomonadales and Sarcinochrysidales (Giner et al. 2009). These are the only algae known to date that synthesize the C30 sterol 24-propylidene cholesterol. In a comprehensive study, Giner et al. (2009) analysed the sterol compositions of 42 strains of pelagophyte algae including 17 strains of Aureococcus anophagefferens using a combination of GC and HPLC techniques. 1H-NMR data were obtained for 17 strains. All strains analyzed contained 24-propylidenecholesterol. All strains from the order Sarcinochrysidales contained the (24E)-isomer, while all strains in the order Pelagomonadales contained the (24Z)-isomer, either alone or together with the (24E)-isomer. The occurrence of Δ22 and 24α-sterols was limited to the Sarcinochrysidales. The first occurrence of 24-n-propylcholesta-5,22E-dien-3β-ol in an alga, CCMP 1410, was reported. Traces of the rare sterol 26,26-dimethyl-24-methylenecholesterol were detected in Aureococcus anophagefferens, and the (25R)-configuration was proposed, based on biosynthetic considerations. Traces of a novel sterol, 24-propylidenecholesta-5,25-dien-3β-ol, were detected in several species. Raederstorff and Rohmer (1984) analysed the sterols of Nematochrvsopsis roscoffensis (=N. marina) and also found the C30 sterols 24(E)-24-n-propylidene-cholesterol and 24-n-propylcholesterol.
14.7 Class Xanthophyceae
There are about 600 species and 100 families of Xanthophyceae, but most are very rare. Common genera include Botrydium, Tribonema and Vaucheria. These yellow-green microalgae are mostly found in fresh water or wet soil, but a few are marine. They can be confused with green algae because of their pigmentation, but they are actually secondary endosymbionts that evolved from protists that engulfed a microalga and assimilated its chloroplasts. Most xanthophytes are coccoid or filamentous, but some are siphonous. 18S ribosomal RNA gene analysis indicates that the Xanthophyceae is most closely related to the Phaeophyceae and that the class may be paraphyletic (Potter et al. 1997).
Mercer et al. (1974) studied the sterols of Botrydium granulatum, Tribonema aequale and Monodus subterraneus (= Monodus subterranea). In each case, the major sterols were cholesterol and 24β-ethylcholesterol (clionasterol), and their proportions did not vary with age of the cultures. Small amounts of cycloartenol and 24-methylenecycloartanol were also found in all three algae. The biosynthesis of clionasterol in M. subterranea was studied by Mercer and Harries (1975). Clionasterol grown in the presence of labelled methionine showed the participation of a 24-ethylidene sterol intermediate in its biosynthesis. Data from cells incubated with labelled mevalonic acid showed that the 24-ethylidene sterol intermediate is reduced directly to clionasterol and not isomerized to a Δ24-sterol which is then reduced.
14.8 Class Dictyochophyceae
Dictyocha is the generic name for all extant silicoflagellates, or for species in which the basket-shaped skeleton consists of a single ring with several connecting bars (Kristiansen and Preisig 2001). Few data are available for these microalgae. Patterson and Van Valkenburg (1990) analysed the sterols from cultured Dictyocha fibula and found only 4-methyl-cholesta-5,22E-dien-3β-ol and 24-methylenecholesterol.
14.9 Class Raphidophyceae
Marine and freshwater raphidophytes form a monophyletic group and 18S ribosomal RNA gene sequences suggest that the Raphidophyceae is a sister taxon to the Phaeophyceae-Xanthophyceae clades, but the bootstrap value was only 40 % (Potter et al. 1997). These microalgae are of interest for their propensity to form harmful algal blooms in both marine (brown pigmented species) and freshwater (green pigmented species) environments. The lipids of Raphidophyceae microalgae still remain understudied and the taxonomy is not clearly established. Fatty acids and pigments have been used as chemotaxonomic markers for these algae (Mostaert et al. 1998) as well as sterols (Nichols et al. 1987; Patterson and Van Valkenburg 1990; Marshall et al. 2002; Giner et al. 2008; Leblond et al. 2013).
Nichols et al. (1987) reported data on the sterols and fatty acids of the red tide flagellates Heterosigma akashiwo and Chattonella antiqua (=Chattonella marina var. antiqua) and this work was expanded by Marshall et al. (2002) who reported the sterols and fatty acids in six marine raphidophyte algae. The dominant sterol in Chattonella spp., H. akashiwo and Fibrocapsa japonica was identified as 24-ethylcholesterol, but the configuration at C-24 was not specified at that time (Nichols et al. 1987; Marshall et al. 2002); a reinvestigation of the sterols of C. marina using 1H NMR spectrometry indicated the presence of the 24α-configuration (sitosterol) (Giner et al. 2008). The Chattonella species also contained small amounts of the uncommon sterol cholest-8(9)-en-3β-ol (1.8–5 %). F. japonica contained the highest proportion of 24-ethylcholesterol (84–92 %) with small amounts of cholesterol (5–11 %) and isofucosterol (24-ethylcholesta-5,24(28)Z-dien-3β-ol; ca. 2 %; Marshall et al. 2002).
Giner et al. (2008) also analysed the sterols and fatty acid compositions of three harmful algal species previously classified in the genus Chattonella (Raphidophyceae). These were “Chloromorum toxicum” (ex a North American strain identified originally as Chattonella cf. verruculosa, and probably a member of the Raphidophyceae; Edvardsen et al 2007), Verrucophora farcimen (= Pseudochattonella farcimen; Dictyochophyceae), and Verrucophora verruculosa (= Pseudochattonella verruculosa; Dictyochophyceae). C. toxicum contained the 24β-ethyl sterols, poriferasterol (24β-ethylcholesta-5,22E-dien-3β-ol) and clionasterol (24β-ethylcholesterol), as its major sterols. In contrast, the stereochemistry of the 24-ethyl sterols of Chattonella marina and Heterosigma akashiwo, was determined to be 24α and 24β, respectively (Giner et al. 2008). Both Pseudochattonella (Verrucophora) strains contained the 27-nor C27 sterol occelasterol as the only detected sterol.
Patterson and Van Valkenburg (1990) reported that Olisthodiscus luteus contained 24-ethylcholesterol, 24-ethylcholestanol, 24-methylcholesterol and cholesterol. The presence of a fully saturated stanol in microalgae is unusual. The only sterol analysis of a freshwater species is by Leblond et al. (2013) who reported sterol compositions for 21 isolates of the green-pigmented, raphidophyte Gonyostomum semen from Scandinavian lakes. All contained the C29 sterols 24-ethylcholesta-5,22E-dien-3β-ol and 24-ethylcholesterol as major components together with smaller amounts of 24-methylcholesterol. The same three sterols occur in higher plants.
15 Conclusions
The published literature on the sterol compositions of microalgae continues to increase but it is still far from comprehensive. Extensive data are now available for green algae, diatoms and dinoflagellates from which it has been possible to assemble groupings of species containing common sterol distributions. In some cases these align with taxonomic groupings but in others species that are only distantly related can have similar compositions. Unfortunately, sterol data for some algal classes are still restricted to just a handful of species and some supposed characteristics may be challenged as additional compositional data are obtained. It is apparent that some earlier sterol identifications are now known to be in error (particularly with regard to C-24 stereochemistry) and many sterols are more widely distributed than had been thought. This should not be a surprise since sterol biosynthesis is an ancient microalgal trait and variations in biosynthesis are to be expected over such geologically long time periods. As sequence data become more readily available it will be useful to test these sterol groupings against taxonomies defined from genetic data and to discover what changes in genetic makeup are responsible for variations in sterol compositions.
References
Akihisa T, Hori T, Suzuki H, Sakoh T, Yokota T, Tamura T (1992) 24β-Methyl-5α-cholest-9(11)-en-3β-ol, two 24β-alkyl-Δ5,7,9(11)-sterols and other 24β-alkylsterols from Chlorella vulgaris. Phytochemistry 31:1769–1772
Amo M, Suzuki N, Kawamura H, Yamaguchi A, Takano Y, Horiguchi T (2010) Sterol composition of dinoflagellates: different abundance and composition in heterotrophic species and resting cysts. Geochem J 44:225–231
Anding C, Ourisson G (1973) Presence of ergosterol in light-grown and dark-grown Euglena gracilis Z. Eur J Biochem 34:345–346
Anding C, Brandt RD, Ourisson G (1971) Sterol biosynthesis in Euglena gracilis Z. Sterol precursors in light-grown and dark-grown Euglena gracilis Z. Eur J Biochem 24:259–263
Atwood AR, Sachs JP (2012) Purification of dinosterol from complex mixtures of sedimentary lipids for hydrogen isotope analysis. Org Geochem 48:37–46
Ballantine JA, Lavis A, Morris RJ (1979) Sterols of the phytoplankton-effects of illumination and growth stage. Phytochemistry 18:1459–1466
Barrett SM, Volkman JK, Dunstan GA, LeRoi JM (1995) Sterols of 14 species of marine diatoms (Bacillariophyta). J Phycol 31:360–369
Beastall GH, Rees HH, Goodwin TW (1971) Sterols in Porphyridium cruentum. Tetrahedon Lett 52:4935–4938
Beastall GH, Tyndall AM, Rees HH, Goodwin TW (1974) Sterols in Porphyridium series. 4α-Methyl-5α-cholesta-8,22-dien-3β-ol and 4α,24-dimethyl-5α-cholesta-8,22-dien-3β-ol: two novel sterols from Porphyridium cruentum. Eur J Biochem 41:301–309
Billard C, Daguet JC, Maume D, Bert M (1990) Sterols and chemotaxonomy of marine Chrysophyceae. Bot Mar 33:225–228
Bohlin L, Kokke WCMC, Fenical W, Djerassi C (1981) 4α-Methyl-24S-ethyl-5α-cholestanol-3β-ol and 4α-methyl-24S-ethyl-5α-cholest-8(14)-en-3β-ol, two new sterols from a cultured marine dinoflagellate. Phytochemistry 20:2397–2401
Boon JJ, Rijpstra WIC, de Lange F, de Leeuw JW, Yoshioka M, Shimizu Y (1979) Black Sea sterol – a molecular fossil for dinoflagellate blooms. Nature 277:125–127
Borowitzka MA (2016) Systematics, taxonomy and species names: do they matter? In: Borowitzka MA, Beardall J, Raven JA (eds) The physiology of microalgae. Springer, Dordrecht, pp 655–681
Bowler C et al (2008) The Phaeodactylum genome reveals the evolutionary history of diatom genomes. Nature 456:239–244
Brandt RD, Pryce RJ, Anding C, Ourisson G (1970) Sterol biosynthesis in Euglena gracilis Z. Comparative study of free and bound sterols in light and dark grown Euglena gracilis Z. Eur J Biochem 17:344–349
Brothers SL, Dickson LG (1980) Sterols of Goniotrichum elegans. Phytochemistry 19:2357–2358
Buntel CJ, Griffin JH (1994) Evolution of sterol and triterpene cyclases. In: Nes WD (ed) Isopentenoids and other natural products – evolution and function, ACS Symposium Series No 562. American Chemical Society, Washington, pp 44–54
Butterfield NJ, Knoll AH, Swett K (1994) Paleobiology of the upper proterozoic Svanbergfjellet formation, Spitsbergen. Fossils Strata 34:1–84
Canfield DE, Poulton SW, Knoll AH, Narbonne GM, Ross G, Goldberg T, Strauss H (2008) Ferruginous conditions dominated later Neoproterozoic deep-water chemistry. Science 321:949–952
Castaneda IS, Werne JP, Johnson TC, Powers LA (2011) Organic geochemical records from Lake Malawi (East Africa) of the last 700 years, part II: biomarker evidence for recent changes in primary productivity. Palaeogeogr Palaeoclimatol Palaeoecol 303:140–154
Chappell J (2002) The genetics and molecular genetics of terpene and sterol origami. Curr Opin Plant Biol 5:151–157
Chitwood DJ, Patterson GW (1991) Separation of epimeric pairs of C-24 alkylsterols by reversed-phase high performance liquid chromatography of the free sterols at subambient temperature. J Liq Chromatogr 14:151–163
Chiu PL, Patterson GW (1981) Quantitative estimation of C-24 epimeric sterol mixtures by 220 MHz nuclear magnetic resonance spectroscopy. Lipids 16:203–206
Collins RP, Kalnins K (1969) Sterols produced by Synura petersenii (Chrysophyta). Comp Biochem Physiol 30:779–782
Conte MH, Volkman JK, Eglinton G (1994) Lipid biomarkers of the Haptophyta. In: Green JC, Leadbeater BSC (eds) The haptophyte algae. Clarendon Press, Oxford, pp 351–377
Cranwell PA, Jaworski GHM, Bickley HM (1990) Hydrocarbons, sterols, esters and fatty acids in six freshwater chlorophytes. Phytochemistry 29:145–151
Dahmen JL, Leblond JD (2011) Free sterol composition of species in the dinoflagellate genus Pyrocystis: a spectrum of sterol diversity. J Eukaryot Microbiol 58:475–479
de Leeuw JW, Rijpstra WIC, Schenck PA, Volkman JK (1983) Free, esterified and residual bound sterols in Black Sea Unit I sediments. Geochim Cosmochim Acta 47:455–465
De Souza NJ, Nes WR (1968) Sterols: isolation from a blue-green alga. Science 162:363
Dempsey ME (1965) Pathways of enzymic synthesis and conversion to cholesterol of Δ5,7,24-cholestatrien-3β-o1 and other naturally occurring sterols. J Biol Chem 240:4176–4177
Dennis AL, Nes WD (2002) Sterol methyl transferase. Evidence for successive C-methyl transfer reactions generating Δ24(28)- and Δ25(27)-olefins by a single plant enzyme. Tetrahedon Lett 43:7017–7021
Desmond E, Gribaldo S (2009) Phylogenomics of sterol synthesis: insights into the origin, evolution, and diversity of a key eukaryotic feature. Genome Biol Evol 1:364–381
Dickson LG, Patterson GW (1973) Inhibition of sterol biosynthesis in Chlorella ellipsoidea by AY-9944. Lipids 7:635–643
Dickson LG, Patterson GW, Cohen CF, Dutky SR (1972) Two novel sterols from inhibited Chlorella ellipsoidea. Phytochemistry 11:3473–3477
Doyle PJ, Patterson GW, Dutky SR, Thompson MJ (1972) Triparanol inhibition of sterol biosynthesis in Chlorella emersonii. Phytochemistry 11:1951–1960
Dunstan GA, Brown MR, Volkman JK (2005) Cryptophyceae and Rhodophyceae; chemotaxonomy, phylogeny, and application. Phytochemistry 66:2557–2570
Duperon R, Thiersault M, Duperon P (1983) Occurrence of steryl glycosides and acylated steryl glycosides in some marine algae. Phytochemistry 22:535–538
Edvardsen B, Eikrem W, Shalchian-Tabrizi K, Riisberg I, Johnsen G, Naustvoll L, Throndsen J (2007) Verrucophora farcimen gen. et sp. nov. (Dictyochophyceae, Heterokontophyta) – a bloom-forming ichtyotoxic flagellate from the Skagerrak, Norway. J Phycol 43:1054–1070
Fabrégas J, Aran J, Morales ED, Lamela T, Otero A (1997) Modification of sterol concentration in marine microalgae. Phytochemistry 46:1189–1191
Gershengorn MC, Smith ARH, Goulston G, Goad LJ, Goodwin TW, Haines TH (1968) The sterols of Ochromonas danica and Ochromonas malhamensis. Biochemistry 7:1698–1706
Gerst N, Ruan B, Pang J, Wilson WK, Schroepfer GJJ (1997) An updated look at the analysis of unsaturated C27 sterols by gas chromatography and mass spectrometry. J Lipid Res 38:1685–1701
Ghosh P, Patterson GW, Wikfors GH (1998) Sterols in some marine Prymnesiophyceae. J Phycol 34:511–514
Gibbons GF, Goad LJ, Goodwin TW (1967) The sterols of some marine red algae. Phytochemistry 6:677–683
Giner J-L, Wikfors GH (2011) “Dinoflagellate sterols” in marine diatoms. Phytochemistry 72:1896–1901
Giner J-L, Zhao H, Tomas C (2008) Sterols and fatty acids of three harmful algae previously assigned as Chattonella. Phytochemistry 69:2167–2171
Giner J-L, Zhao H, Boyer GL, Satchwell MF, Andersen RA (2009) Sterol chemotaxonomy of marine pelagophyte algae. Chem Biodivers 6:1111–1130
Gladu PK, Patterson GW, Wikfors GW, Chitwood DJ, Lusby WR (1990) The occurrence of brassicasterol and epibrassicasterol in the Chromophycota. Comp Biochem Physiol 97B:491–494
Gladu PK, Patterson GW, Wikfors GH, Lusby WR (1991a) Free and combined sterols of Pavlova gyrans. Lipids 26:656–659
Gladu PK, Patterson GW, Wikfors GW, Chitwood DJ, Lusby WR (1991b) Sterols of some diatoms. Phytochemistry 30:2301–2303
Gladu PK, Patterson GW, Wikfors GH, Smith BC (1995) Sterol, fatty acid, and pigment characteristics of UTEX 2341, a marine eustigmatophyte identified previously as Chlorella minutissima (Chlorophyceae). J Phycol 31:774–777
Goad LJ, Akihisa T (1997) Analysis of sterols. Blackie Academic, London, pp 1–437
Goad LJ, Withers N (1982) Identification of 27-nor-(24R)-24-methylcholesta-5,22-dien-3β-ol and brassicasterol as the major sterols of the marine dinoflagellate Gymnodinium simplex. Lipids 17:853–858
Goad LJ, Lenton JR, Knapp FF, Goodwin TW (1974) Phytosterol side chain biosynthesis. Lipids 9:582–595
Goad LJ, Holz GGJ, Beach DH (1983) Identification of (24S)-24-methylcholesta-5,22-dien-3β-ol as the major sterol of a marine cryptophyte and a marine prymnesiophyte. Phytochemistry 22:475–476
Goers M, Schumann R, Gustavs L, Karsten U (2010) The potential of ergosterol as chemotaxonomic marker to differentiate between “Chlorella” species (Chlorophyta). J Phycol 46:1296–1300
Guiry MD, Guiry GM (2015) AlgaeBase. World-wide electronic publication, National University of Ireland, Galway. http://www.algaebase.org
Hai T, Schneider B, Schmidt J, Adam G (1996) Sterols and triterpenoids from the cyanobacterium Anabaena hallensis. Phytochemistry 41:1083–1084
Halevy S, Avivi L, Katan H (1966) Sterols of soil amoebas and Ochromonas danica: phylogenetic approach. J Protozool 12:293–296
Hallegraeff GM, Nichols PD, Volkman JK, Blackburn SI, Everitt DA (1991) Pigments, fatty acids, and sterols of the toxic dinoflagellate Gymnodinium catenatum. J Phycol 27:591–599
Hamm C, Reigstad M, Riser CW, Mühlebach A, Wassmann P (2001) On the trophic fate of Phaeocystis pouchetii. VII. Sterols and fatty acids reveal sedimentation of P. pouchetii-derived organic matter via krill fecal strings. Mar Ecol Prog Ser 209:55–69
Harvey HR, Bradshaw SA, O’Hara SCM, Eglinton G, Corner EDS (1988) Lipid composition of the marine dinoflagellate Scrippsiella trochoidea. Phytochemistry 27:1723–1729
Heimann K, Becker B, Harnisch H, Mukherjee KD, Melkonian M (1997) Biochemical characterization of plasma membrane vesicles of Cyanophora paradoxa. Bot Acta 110:401–410
Hirakawa Y, Howe A, James ER, Keeling PJ (2011) Morphological diversity between culture strains of a chlorarachniophyte, Lotharella globosa. PLoS One 6:e23193
Holden MJ, Patterson GW (1982) Taxonomic implication of sterol composition in the genus Chlorella. Lipids 17:215–219
Huss VAR, Frank C, Hartmann EC, Hirmer M, Kloboucek A, Seidel BM, Wenzeler P, Kessler E (1999) Biochemical taxonomy and molecular phylogeny of the genus Chlorella sensu lato (Chlorophyta). J Phycol 35:587–598
Idler DR, Saito A, Wiseman P (1968) Sterols in red algae (Rhodophyceae). Steroids 11:465–473
Ikekawa N, Fujimoto Y, Kadota S, Kikuchi T (1989) Effective separation of sterol C-24 epimers. J Chromatogr 468:91–98
Itoh T, Tani H, Fukishima K, Tamura T, Matsumoto T (1982) Structure-retention relationship of sterols and triterpene alcohols in gas chromatography on a glass capillary column. J Chromatogr 234:65–76
Jo Q, Choy EJ, Park DW, Véron B (2004) Sterol dynamics of heterotrophic Tetraselmis suecica and its nutritional implication in the bivalve aquaculture. Aquat Res 35:371–377
Jones GJ, Nichols PD, Shaw PM (1994) Analysis of microbial sterols and hopanoids. In: Goodfellow M, O’Donnell AG (eds) Chemical methods in prokaryotic systematics. Wiley, Chichester, pp 163–195
Jordan RW, Iwataki M (2012) Chrysophyceae and Synurophyceae. In: eLS. Wiley, Chichester. doi:10.1002/9780470015902.a0023690
Kelly AE (2009) Hydrocarbon biomarkers for biotic and environmental evolution through the Neoproterozoic-Cambrian transition. PhD thesis, Massachusetts Institute of Technology, Boston
Knapp FF, Greig JB, Goad LJ, Goodwin TW (1971) Conversion of 24-ethylidene-sterols into poriferasterol by Ochromonas malhamensis. J Chem Soc D Chem Commun:707–709
Knoll AH, Summons RE, Waldbauer JR, Zumberge J (2007) The geological succession of primary producers in the oceans. In: Falkowski P, Knoll AH (eds) The evolution of photosynthetic organisms in the oceans. Elsevier Academic Press, Burlington, pp 133–163
Kobayashi J (1989) Hymenosulphate, a novel sterol sulphate with Ca-releasing activity from the cultured marine haptophyte Hymenomonas sp. Perkin Trans 1:101–103
Kodner RB, Pearson A, Summons RE, Knoll AH (2008) Sterols in red and green algae: quantification, phylogeny, and relevance for the interpretation of geologic steranes. Geobiology 6:411–420
Kokke WCMC, Fenical W, Djerassi C (1981) Sterols with unusual nuclear unsaturation from three cultured marine dinoflagellates. Phytochemistry 20:127–134
Kokke WCMC, Shoolery JN, Fenical W, Djerassi C (1984) Biosynthetic studies of marine lipids. 4. Mechanism of side chain alkylation in (E)-24-propylidenecholesterol by a chrysophyte alga. J Org Chem 49:3742–3752
Kristiansen J, Preisig, HR (eds) (2001) Encyclopedia of chrysophyte genera. Bibliotheca Phycologia, Band 110. J Cramer, Berlin, 260 pp
Leblond JD, Chapman PJ (2002) A survey of the sterol composition of the marine dinoflagellates Karenia brevis, Karenia mikimotoi, and Karlodinium micrum: distribution of sterols within other members of the class Dinophyceae. J Phycol 38:670–682
Leblond JD, Lasiter AD (2012) Sterols of the green-pigmented, aberrant plastid dinoflagellate, Lepidodinium chlorophorum (Dinophyceae). Protist 163:38–46
Leblond JD, Dahmen JL, Seipelt RL, Elrod-Erickson MJ, Kincaid R, Howard JC, Evens TJ, Chapman PJ (2005) Lipid composition of Chlorarachniophytes (Chlorarachniophyceae) from the genera Bigelowiella, Gymnochlora, and Lotharella. J Phycol 41:311–321
Leblond JD, Lasiter AD, Li C, Logares R, Rengefors K, Evens TJ (2010) A data mining approach to dinoflagellate clustering according to sterol composition: Correlations with evolutionary history. Int J Data Min Bioinforma 4:431–451
Leblond JD, Timofte HI, Roche SA, Porter NM (2011) Sterols of glaucocystophytes. Phycol Res 59:129–134
Leblond JD, Dahmen AS, Lebret K, Rengefors K (2013) Sterols of the green-pigmented, freshwater raphidophyte, Gonyostomum semen, from Scandinavian lakes. J Eukaryot Microbiol 60:399–405
Leipe DD, Wainright PO, Gunderson JH, Porter D, Patterson DJ, Valois F, Himmerich S, Sogin ML (1994) The stramenopiles from a molecular perspective – 16S-like ribosomal-RNA sequences from Labyrinthuloides minuta and Cafeteria roenbergensis. Phycologia 33:369–377
Leliaert F, Smith DR, Moreau H, Herron MD, Verbruggen H, Delwiche CF, De Clerck O (2012) Phylogeny and molecular evolution of the green algae. Crit Rev Plant Sci 31:1–46
Lin DS, Ilias AM, Conner WE, Caldwell RS, Cory HT, Daves GDJ (1982) Composition and biosynthesis of sterols in selected marine phytoplankton. Lipids 17:818–824
Makou MC, Eglinton TI, Oppo DW, Hughen KA (2010) Postglacial changes in El Niño and La Niña behavior. Geology 38:43–46
Marshall J-A, Nichols PD, Hallegraeff GM (2002) Chemotaxonomic survey of sterols and fatty acids in six marine raphidophyte algae. J Appl Phycol 14:255–265
Maxwell JR, Mackenzie AS, Volkman JK (1980) Configuration at C-24 in steranes and sterols. Nature 286:694–697
Méjanelle L, Sanchez-Gargallo A, Bentaleb I, Grimalt JO (2003) Long chain n-alkyl diols, hydroxy ketones and sterols in a marine eustigmatophyte, Nannochloropsis gaditana, and in Brachionus plicatilis feeding on the algae. Org Geochem 34:527–538
Mercer EI, Harries WB (1975) The mechanism of alkylation at C-24 during clionasterol biosynthesis in Monodus subterraneus. Phytochemistry 14:439–443
Mercer EI, London RA, Kent ISA, Taylor AJ (1974) Sterols, sterol esters and fatty acids of Botrydium granulatum, Tribonema aequale and Monodus subterraneus. Phytochemistry 13:845–852
Miller MB, Haubrich BA, Wang Q, Snell WJ, Nes WD (2012) Evolutionarily conserved Δ25(27)-olefin ergosterol biosynthesis pathway in the alga Chlamydomonas reinhardtii. J Lipid Res 53:1636–1645
Mooney BD, Nichols PD, De Salas MF, Hallegraeff GM (2007) Lipid, fatty acid, and sterol composition of eight species of Kareniaceae (Dinophyta): chemotaxonomy and putative lipid phycotoxins. J Phycol 43:101–111
Mostaert AS, Karsten U, Hara Y, Watanabe MM (1998) Pigments and fatty acids of marine raphidophytes: a chemotaxonomic re-evaluation. Phycol Res 46:213–220
Nes WD (2000) Sterol methyl transferase: enzymology and inhibition. Biochim Biophys Acta Mol Cell Biol Lipids 1529:63–88
Nes WD (2003) Enzyme mechanisms for sterol C-methylations. Phytochemistry 64:75–95
Nes WD (2011) Biosynthesis of cholesterol and other sterols. Chem Rev 111:6423–6451
Nes WR, Nes WD (1980) Lipids in evolution. Plenum Press, London, 244 pp
Nes WD, Song ZH, Dennis AL, Zhou WX, Nam J, Miller MB (2003) Biosynthesis of phytosterols. Kinetic mechanism for the enzymatic C-methylation of sterols. J Biol Chem 278:34505–34516
Nichols PD, Volkman JK, Hallegraeff GM, Blackburn SI (1987) Sterols and fatty acids of the red tide flagellates Heterosigma akashiwo and Chattonella antiqua (Raphidophyceae). Phytochemistry 26:2537–2541
Nichols PD, Skerratt JH, Davidson A, Burton H, McMeekin TA (1991) Lipids of cultured Phaeocystis pouchetii: signatures for food-web, biogeochemical and environmental studies in Antarctica and the Southern Ocean. Phytochemistry 30:3209–3214
Not F, Valentin K, Romari K, Lovejoy C, Massana R et al (2007) Picobiliphytes: a marine picoplanktonic algal group with unknown affinities to other eukaryotes. Science 315:253–255
Orcutt DM, Patterson GW (1975) Sterol, fatty acid and elemental composition of diatoms grown in chemically defined media. Comp Biochem Physiol 50B:579–583
Ourisson G, Rohmer M, Poralla K (1987) Prokaryotic hopanoids and other polyterpenoid sterol surrogates. Ann Rev Microbiol 41:301–333
Palermo JA, Seldes AM, Gros EG (1984) Free sterols of the red alga Gigartina skottsbergii. Phytochemistry 23:2688–2689
Patterson GW (1967) Sterols of Chlorella. II. The occurrence of an unusual sterol mixture in Chlorella vulgaris. Plant Physiol 42:1457–1459
Patterson GW (1969) Sterols of Chlorella. III. Species containing ergosterol. Comp Biochem Physiol 31:391–394
Patterson GW (1974) Sterols of some green algae. Comp Biochem Physiol B 47:453–457
Patterson GW (1994) Phylogenetic distribution of sterols. In: Nes WD (ed) Isopentenoids and other natural products. Evolution and function, ACS Symposium Series No. 562. American Chemical Society, Washington, pp 90–108
Patterson GW, Van Valkenburg SD (1990) Sterols of Dictyocha fibula (Chrysophyceae) and Olisthodiscus luteus (Raphidophyceae). J Phycol 26:484–489
Patterson GW, Thompson MJ, Dutky SR (1974) Two new sterols from Chlorella ellipsoidea. Phytochemistry 13:191–194
Patterson GW, Gladu PK, Wikfors GH, Lusby WR (1992) Unusual tetraene sterols in some phytoplankton. Lipids 27:154–156
Patterson GW, Gladu PK, Wikfors GH, Parish EJ, Livant PD, Lusby WR (1993a) Identification of two novel dihydroxysterols from Pavlova. Lipids 28:771–773
Patterson GW, Tsitsa-Tzardis E, Wikfors GH, Gladu PK, Chitwood DJ, Harrison D (1993b) Sterols of Tetraselmis (Prasinophyceae). Comp Biochem Physiol B 105:253–256
Patterson GW, Tsitsa-Tzardis E, Wikfors GH, Ghosh P, Smith BC, Gladu PK (1994) Sterols of eustigmatophytes. Lipids 29:661–664
Peeler TC, Stephenson MB, Einspahr KJ, Thompson GA (1989) Lipid characterization of an enriched plasma-membrane fraction of Dunaliella salina grown in media of varying salinity. Plant Physiol 89:970–976
Piepho M, Martin-Creuzburg D, Wacker A (2010) Simultaneous effects of light intensity and phosphorus supply on the sterol content of phytoplankton. PLoS One 5(12):e15828. doi:10.1371/journal.pone.0015828
Piepho M, Martin-Creuzburg D, Wacker A (2012) Phytoplankton sterol contents vary with temperature, phosphorus and silicate supply: a study on three freshwater species. Eur J Phycol 47:138–145
Piretti MV, Pagliuca G, Boni L, Pistocchi R, Diamante M, Gazzotti T (1997) Investigation of 4-methyl sterols from cultured dinoflagellate algal strains. J Phycol 33:61–67
Potter D, Saunders GW, Andersen RA (1997) Phylogenetic relationships of the Raphidophyceae and Xanthophyceae as inferred from nucleotide sequences of the 18S ribosomal RNA gene. Am J Bot 84:966–972
Proschold T, Marin B, Schlosser UG, Melkonian M (2001) Molecular phylogeny and taxonomic revision of Chlamydomonas (Chlorophyta). I. Emendation of Chlamydomonas Ehrenberg and Chloromonas Gobi, and description of Oogamochlamys gen. nov. and Lobochlamys gen. nov. Protist 152:265–300
Raederstorff D, Rohmer M (1984) Sterols of the unicellular algae Nematochrysopsis roscoffensis and Chrysotilla lamellosa: isolation of 24(E)-24-n-propylidenecholesterol and 24-n-propylcholesterol. Phytochemistry 23:2835–2838
Rampen SW, Schouten S, Hopmans EC, Abbas B, Noordeloos AAM, Van Bleijswijk JDL, Geenevasen JAJ, Sinninghe Damsté JS (2009a) Diatoms as a source for 4-desmethyl-23,24-dimethyl steroids in sediments and petroleum. Geochim Cosmochim Acta 73:377–387
Rampen SW, Schouten S, Hopmans EC, Abbas B, Noordeloos AAM, Geenevasen JAJ, Moldowan JM, Denisevich P, Sinninghe Damsté JS (2009b) Occurrence and biomarker potential of 23-methyl steroids in diatoms and sediments. Org Geochem 40:219–228
Rampen SW, Volkman JK, Hur SB, Abbas BA, Schouten S, Jameson ID, Holdsworth DG, Bae JH, Sinninghe Damsté JS (2009c) Occurrence of gorgosterol in diatoms of the genus Delphineis. Org Geochem 40:144–147
Rampen SW, Abbas BA, Schouten S, Sinninghe Damsté JS (2010) A comprehensive study of sterols in marine diatoms (Bacillariophyta): implications for their use as tracers for diatom productivity. Limnol Oceanogr 55:91–105
Rauter AP, Filipe MM, Prata C, Noronha JP, Sampayo MAM, Justino J, Bermejo J (2005) A new dihydroxysterol from the marine phytoplankton Diacronema sp. Fitoterapia 76:433–438
Rohmer M, Brandt RD (1973) Les stérols et leur précurseurs chez Astasia longa Pringsheim. Eur J Biochem 36:446–454
Rohmer M, Kokke WCMC, Fenical W, Djerassi C (1980) Isolation of two new C30 sterols, 24(E)-n-propylidenecholesterol and 24-n-propylcholesterol, from a cultured chrysophyte. Steroids 35:219–231
Rontani JF, Beker B, Volkman JK (2004) Long-chain alkenones and related compounds in the benthic haptophyte Chrysotila lamellosa Anand HAP 17. Phytochemistry 65:117–126
Rubinstein I, Goad LJ (1974) Occurrence of (24S)-24-methylcholesta-5,22E-dien-3β-ol in the diatom Phaeodactylum tricornutum. Phytochemistry 13:485–487
Seenivasan R, Sausen N, Medlin LK, Melkonian M (2013) Picomonas judraskeda gen. et sp. nov.: the first identified member of the Picozoa phylum nov., a widespread group of picoeukaryotes, formerly known as ‘picobiliphytes’. PLoS One 8(3):e59565
Shimizu Y, Alam M, Kobayashi A (1976) Dinosterol, the major sterol with a unique side-chain in the toxic dinoflagellate, Gonyaulax tamarensis. J Am Chem Soc 98:1059–1060
Soma Y, Tani Y, Soma M, Mitake H, Kurihara R, Hashomoto S, Watanabe T, Nakamura T (2007) Sedimentary steryl chlorin esters (SCEs) and other photosynthetic pigments as indicators of paleolimnological change over the last 28,000 years from the Buguldeika saddle of Lake Baikal. J Paleolimnol 37:163–175
Summons RE, Jahnke LL, Cullings KW, Logan GA (2001) Cyanobacterial biomarkers: triterpenoids plus steroids? Eos Trans AGU 82(47), Fall Meeting Supplement, Abstract B22D-0184
Teshima SI, Kanazawa A, Tago A (1980) Sterols of the dinoflagellate, Noctiluca milialis. Mem Fac Fish Hokkaido Univ 29:319–326
Thompson RH, Patterson G, Thompson MJ, Slover HT (1981) Separation of pairs of C-24 epimeric sterols by glass capillary gas liquid chromatography. Lipids 16:694–699
Throndsen J (1976) Occurrence and productivity of small marine flagellates. Nor J Bot 23:269–193
Véron B, Dauguet J-C, Billard C (1996) Sterolic biomarkers in marine phytoplankton. II. Free and conjugated sterols of seven species used in mariculture. J Phycol 34:273–279
Volkman JK (1986) A review of sterol markers for marine and terrigenous organic matter. Org Geochem 9:83–99
Volkman JK (2003) Sterols in microorganisms. Appl Microbiol Biotechnol 60:495–506
Volkman JK (2005) Sterols and other triterpenoids: source specificity and evolution of biosynthetic pathways. Org Geochem 36:139–159
Volkman JK, Eglinton G, Corner EDS (1980a) Sterols and fatty acids of the marine diatom Biddulphia sinensis. Phytochemistry 19:1809–1813
Volkman JK, Eglinton G, Corner EDS, Sargent JR (1980b) Novel unsaturated straight-chain C37-C39 methyl and ethyl ketones in marine sediments and a coccolithophorid Emiliania huxleyi. In: Douglas AG, Maxwell JR (eds) Advances in organic geochemistry 1979. Pergamon Press, Oxford, pp 219–227
Volkman JK, Smith DJ, Eglinton G, Forsberg TE, Corner EDS (1981) Sterol and fatty acid composition of four marine haptophycean algae. J Mar Biol Assoc UK 61:509–527
Volkman JK, Kearney P, Jeffrey SW (1990) A new source of 4-methyl sterols and 5α(H)-stanols in sediments: prymnesiophyte microalgae of the genus Pavlova. Org Geochem 15:489–497
Volkman JK, Barrett SM, Dunstan GA, Jeffrey SW (1992) C30-C32 alkyl diols and unsaturated alcohols in microalgae of the class Eustigmatophyceae. Org Geochem 18:131–138
Volkman JK, Barrett SM, Dunstan GA, Jeffrey SW (1993) Geochemical significance of the occurrence of dinosterol and other 4-methyl sterols in a marine diatom. Org Geochem 20:7–15
Volkman JK, Barrett SM, Dunstan GA, Jeffrey SW (1994) Sterol biomarkers for microalgae from the green algal class Prasinophyceae. Org Geochem 21:1211–1218
Volkman JK, Barrett SM, Blackburn SI, Sikes EL (1995) Alkenones in Gephyrocapsa oceanica: implications for studies of paleoclimate. Geochim Cosmochim Acta 59:513–520
Volkman JK, Farmer CL, Barrett SM, Sikes EL (1997) Unusual dihydroxysterols as chemotaxonomic markers for microalgae from the order Pavlovales (Haptophyceae). J Phycol 33:1016–1023
Volkman JK, Barrett SM, Blackburn SI, Mansour MP, Sikes EL, Gelin F (1998) Microalgal biomarkers: a review of recent research developments. Org Geochem 29:1163–1179
Volkman JK, Barrett SM, Blackburn SI (1999a) Eustigmatophyte microalgae are potential sources of C29 sterols, C22-C28 n-alcohols and C28-C32 n-alkyl diols in freshwater environments. Org Geochem 30:307–318
Volkman JK, Rijpstra WIC, de Leeuw JW, Mansour MP, Jackson AE, Blackburn SI (1999b) Sterols of four dinoflagellates from the genus Prorocentrum. Phytochemistry 52:659–668
Wengrovitz PS, Sanduja R, Alam M (1981) Dinoflagellate sterols. 3. Sterol composition of the dinoflagellate Gonyaulax monilata. Comp Biochem Physiol B 69:535–539
Withers N (1987) Dinoflagellate sterols. In: Taylor FJR (ed) The biology of dinoflagellates. Blackwell Scientific, Oxford, pp 316–359
Withers NW, Tuttle RC, Holz GG, Beach DH, Goad LJ, Goodwin TW (1978) Dehydrodinosterol, dinosterone and related sterols of a non-photosynthetic dinoflagellate Crypthecodinium cohnii. Phytochemistry 17:1987–1989
Withers NW, Kokke WCMC, Rohmer M, Fenical WH, Djerassi C (1979) Isolation of sterols with cyclopropyl-containing side chains from the cultured marine alga Peridinium foliaceum. Tetrahedon Lett 38:3605–3608
Wright JLC, McInnes AG, Shimizu S, Smith DG, Walte JA, Idler D, Khalil W (1978) Identification of C-24 alkyl epimers of marine sterols by 13C nuclear magnetic resonance spectroscopy. Can J Chem 56:1898–1903
Xue ZY, Duan LX, Liu D, Guo J, Ge S, Dicks JO, Omaille P, Osbourn A, Qi XQ (2012) Divergent evolution of oxidosqualene cyclases in plants. New Phytol 193:1022–1038
Zielinski J, Kokke WCMC, Fenical W, Djerassi C (1982) Sterols of the cultured euglenoid Eutreptia viridis: a novel Δ23-unsaturated sterol. Steroids 40:403–411
Acknowledgements
I thank Dr Susan Blackburn, Ian Jameson and staff of the Australian National Algae Culture Collection at CSIRO in Hobart for providing algal cultures over many years that have led to many new findings about the lipids in microalgae. I also thank Dan Holdsworth, Graeme Dunstan and Stephanie Barrett for their analytical skills and Dr Roger Summons, Dr Shirley Jeffrey (deceased), Dr Jan de Leeuw, Dr Jaap Sinninghe Damsté, Dr Sebastiaan Rampen, Dr G.W. Patterson and Dr L.J. Goad for helpful discussions over the years.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Volkman, J.K. (2016). Sterols in Microalgae. In: Borowitzka, M., Beardall, J., Raven, J. (eds) The Physiology of Microalgae. Developments in Applied Phycology, vol 6. Springer, Cham. https://doi.org/10.1007/978-3-319-24945-2_19
Download citation
DOI: https://doi.org/10.1007/978-3-319-24945-2_19
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-24943-8
Online ISBN: 978-3-319-24945-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)