Abstract
Optical coherence tomography (OCT) is a useful adjunct imaging technique for retinal and optic nerve pathology. Advances in OCT have made it a useful diagnostic tool in ethambutol toxicity, hydroxychloroquine retinopathy, optic nerve head drusen, glaucoma, idiopathic intracranial hypertension, and multiple sclerosis, to name a few conditions. Care should be taken when interpreting OCT data, as errors and artifacts can occur. However, OCT has revolutionized ophthalmology and will continue to shape the ophthalmic field as new advances develop.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Optical coherence tomography
- Normal anatomy
- Artifacts
- Multicolor
- Ethambutol
- Hydroxychloroquine
- Optic nerve head drusen
- Glaucoma
- Idiopathic intracranial hypertension
- Multiple sclerosis
History of Optical Coherence Tomography
Imaging techniques have gained popularity as a useful adjunctive technology to diagnosis and management in ophthalmology. Techniques such as ultrasound imaging, computed tomography, and magnetic resonance imaging have been useful adjuncts, but all are limited for fine intraocular evaluation. However, in 1991, a technique called optical coherence tomography (OCT) was first demonstrated [1] and became commercially available in 1996 (Humphrey Systems, Dublin, CA) [2]. OCT is a noninvasive medical imaging technique that utilizes the principle of optical scattering to reconstruct intraocular images. An optical signal is either transmitted through or reflected by a tissue, and these different signals are used to reconstruct a spatial picture of the tissue being imaged [1].
Advances in OCT technology have quickly transformed it into one of the indispensable techniques for ophthalmology and arguably has transformed it into the most utilized imaging technology. Refinements such as higher acquisition rates of images, higher resolution of images, and increasing ability to image transparent tissues in the anterior segment have been indispensable. Most recently, advances such as swept-source OCT (SS-OCT) and OCT-angiography (OCT-A) have even given the ability to reconstruct an angiographic image and utilize a multilayered assessment to retinal pathology [3]. Another imaging technique, multicolor OCT, has been developed for spectral-domain OCT (SD-OCT, Heidelberg Engineering, Heidelberg, Germany), in which three simultaneously acquired reflective images are created using three laser wavelengths of blue, green, and infrared, as another means of cross-sectional imaging [4].
Normal Anatomy
Advances in resolution of OCT have made ultrafine structural detail possible. The anatomic correlates have been increasingly speculated, but a recent international committee joined to create a consensus nomenclature for OCT [5].
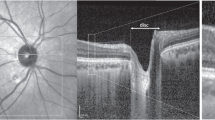
The consensus nomenclature helps clinicians by defining structures on OCT, therefore making standardized interpretation possible. Starting from the inner retina, the first hyperreflective band that is occasionally evident is the posterior cortical vitreous, and analysis of this line can be helpful for conditions such as posterior vitreous detachment or vitreomacular traction. After this, a hyporeflective preretinal space may be evident. Next, the hyperreflective nerve fiber layer is present, often attenuated in conditions such as glaucomatous optic neuropathy. The next series of alternating hypo- and hyperreflective bands correspond to the consecutive retinal layers, as is seen in Fig. 1.1. Close analysis of these layers can give evidence as to varying pathology. For example, hyperreflective areas in the outer plexiform layer could suggest exudates from diabetic retinopathy, whereas atrophy of the inner retinal layers could suggest a central retinal artery occlusion. Closer to the inner retina, the ellipsoid zone, or the hyperreflective region of the photoreceptor with densely packed mitochondria, has gained scrutiny recently with the advent of ocriplasmin injections and is thought to account for a large part of vision [6, 7]. The ellipsoid zone is also examined in any patient complaining of unexplained visual loss, to see if disruption of the photoreceptors could account for their visual symptoms.
Normal anatomic landmarks as defined by the IN OCT Consensus [5], on a representative patient’s OCT
OCT can also be helpful for imaging the choroid, although the choriocapillaris can be imaged with more clarity using enhanced-depth imaging OCT (EDI-OCT) of the choroid.
Conditions in Which OCT Is Helpful
Medication Toxicity
Ethambutol
Ethambutol optic neuropathy is a type of toxic optic neuropathy characterized by bilateral, painless vision loss that progresses with continued intake of the medication [8]. Classically, ethambutol-associated optic neuropathy has been monitored with the use of Ishihara pseudoisochromatic color plates, visual field testing, and serial best-corrected visual acuities. However, OCT could be a helpful adjunctive technique, as it can demonstrate early retinal ganglion layer loss with preferential papillomacular bundle loss that may precede other clinical signs of toxic neuropathy [9]. Early detection of ethambutol optic neuropathy can be helpful, as the vision and structural loss are largely reversible in many cases.
Hydroxychloroquine
Hydroxychloroquine is a disease-modifying antirheumatic drug that is used for the chronic treatment of certain rheumatologic and dermatologic conditions. Retinal toxicity remains a well-known side effect of chronic use, with the risk increasing with higher cumulative doses over 1,000 g or daily intake of more than 5–6.5 mg/kg/day. Early detection of retinal toxicity is imperative, as it is often irreversible and can progress even if therapy has been discontinued [10].
The current screening protocol for hydroxychloroquine toxicity includes 10-2 Humphrey Visual Fields (HVFs) along with SD-OCT to detect early perifoveal ellipsoid changes in a “flying saucer sign” [11].
Optic Nerve Head Drusen (ONHD)
The presence of optic disk obscuration on clinical exam can be the initial trigger that leads to an extensive evaluation for optic disk edema, including magnetic resonance imaging (MRI) and magnetic resonance venography (MRV). However, in certain subsets of individuals, the presence of optic nerve head drusen (ONHD) can create the clinical appearance of disk elevation, due to congenital buried foci of calcium. ONHD classically appear on B-scan ultrasonography as hyperreflective elements that persist at low gain, but small-buried ONHD can sometimes be difficult to visualize.
OCT can show optically empty cavities posterior to disk elevation, which correspond to buried drusen. Additionally, OCT visualization of the peripapillary retinal nerve fiber layer (RNFL) with SD-OCT can be useful to differentiate optic disk edema from ONHD [12]. Recent reports have also noted that enhanced-depth imaging OCT (EDI-OCT) and SS-OCT allow better detection of ONHD and help to correlate drusen with associated visual field changes [13].
Glaucoma
Glaucoma is a progressive loss of peripheral nerve fiber layers due to many underlying risk factors, the most well known of which is intraocular pressure. Classically, peripheral visual field loss on HVF has been a sign of glaucoma, and visual field defects are followed over time to detect progression. However, often visual fields are unreliable or change significantly from visit to visit, depending on patient attention and fatiguability. Therefore, OCT can serve as a useful adjunct to follow glaucomatous changes over time in patients who do not test well with HVF [14, 15] (Fig. 1.2).
Idiopathic Intracranial Hypertension
Patients with idiopathic intracranial hypertension (IIH) often present with bilateral optic disk edema from elevated intracranial pressure, which commonly responds to acetazolamide. If responding well to therapy, optic disk edema should decrease or stay stable over time. Classically, response to acetazolamide was measured by serial repeat lumbar punctures to measure opening pressure of the cerebrospinal fluid, to guide titration of acetazolamide along with assessment of the need for surgical intervention. However, with the onset of OCT, quantitative measurements of optic disk edema can help guide response therapy, thereby replacing the need for repeated lumbar punctures [16] (Fig. 1.3).
Multiple Sclerosis
Episodes of optic neuritis often show up on OCT as optic disk edema. As optic neuritis resolves and turns into optic pallor, this can also be seen on OCT as a relative loss of the nerve fiber layer.
OCT can be helpful in patients with remitting relapsing multiple sclerosis to detect new bouts of optic neuritis. This quantitative measure of optic nerve swelling can be especially useful in patients with concurrent ocular disorders that can contribute to decreased vision (Figs. 1.4 and 1.5).
Common Artifacts and Errors
-
1.
Centering of the optic nerve around the scanner image
RNFL thickness depends upon centering of the optic nerve, in order to achieve an accurate assessment of RNFL thickness. Lack of centering contributes to inaccurate overall RNFL measurements. Typically, the RNFL becomes falsely thicker in the quadrant in which the scan is closer to the disk and thinner in the quadrant in which the scan is displaced further from the disk [17]. Additionally, improper centering makes the reproducibility of scans more variable.
-
2.
RNFL thickness in hypoplastic optic disks, enlarged optic disks, and myopic optic disks
RNFL algorithms also depend on the presence of a relatively “normal” sized optic disk. With smaller optic disks, the RNFL thickness measurement typically declines and can create the appearance of thinner overall RNFL measurements. With larger optic disks, the RNFL thickness measurements increase, perhaps due to shorter distance between the circular scan and the optic disk edge [18]. With myopic disks, RNFL thickness can also be thinner and variable, resulting in a higher amount of false positives with routine glaucoma screening [19]. This important concept should be kept in mind when interpreting any OCT scan.
-
3.
Algorithm error
RNFL thickness is based on an algorithm that calculates the anterior border of the RNFL. However, occasionally, this algorithm can pick up incorrect or inaccurate RNFL parameters, whether due to marked RNFL edema that distorts the underlying retina and choroid, or due to vitreous overlying the RNFL. A tip-off to an algorithm error is when the RNFL graph goes out of range (red arrow) or to zero baseline (blue arrow) (Fig. 1.6).
-
4.
RNFL measurements that exceed the software of the OCT device
Rarely, as a disease process progresses, it outgrows the ability to detect change on OCT. This typically happens at very low measurements (extreme atrophy of RNFL) or very high measurements (extreme edema and swelling of the RNFL). At these very high or very low measurements, there is less validity of changes in the RNFL.
-
5.
Maculopathies and neuropathies affecting macular thickness scans and nerve fiber layer measurements
An important point to keep in mind is that a maculopathy that affects the retina and causes an overall decrease in the nerve fiber layer and ganglion cell layer will also decrease RNFL measurements at the optic nerve. Similarly, a condition which causes atrophy of RNFL at the optic nerve will also cause a thinning of the outer retinal layers throughout the macula.
Introduction to Multicolor OCT
Multicolor OCT is increasingly gaining interest among ophthalmologists. Confocal scanning laser ophthalmoscopy is utilized at three different wavelengths: blue (486 nm), green (518 nm), and infrared (IR; 815 nm) to reconstruct a series of images of varying retinal depth [4]. The different colored wavelengths penetrate at different levels to create detailed images of retinal layers. The blue light provides detailed images of superficial retina, including the retinal nerve fiber layer, ganglion cell layer, and epiretinal membranes. The green light penetrates deeper and is strongly absorbed by hemoglobin and therefore provides detailed images of midretinal layers, blood vessels, hemorrhage, and exudates. Finally, red light provides the most depth of penetration and provides images of the choroid, retinal pigment epithelium, and photoreceptors (Fig. 1.7).
References
Huang D, Swanson EA, Lin CP, Schuman JS, Stinson WG, Chang W, et al. Optical coherence tomography. Science. 1991;254(5036):1178–81.
Fujimoto JG, Pitris C, Boppart SA, Brezinkski ME. Optical coherence tomography: an emerging technology for biomedical imaging and optical biopsy. Neoplasia. 2000;2(1–2):9–25.
Ishibazawa A, Nagaoka T, Takahashi A, Omae T, Tani T, Sogawa K, et al. Optical coherence tomography angiography in diabetic retinopathy: a prospective pilot study. Am J Ophthalmol. 2015;160(1):35–44.
Moussa NB, Georges A, Capuano V, Merle B, Souied EH, Querques G. Multicolor imaging in the evaluation of geographic atrophy due to age-related macular degeneration. Br J Ophthalmol. 2015;99(6):842–7.
Staurenghi G, Sadda S, Chakravarthy U, Spaide RF, International Nomenclature for Optical Coherence Tomography Panel. Proposed lexicon for anatomic landmarks in normal posterior segment spectral-domain optical coherence tomography. The IN OCT Consensus. Ophthalmology. 2014;121(8):1572–8.
Tibbets MD, Reichel E, Witkin AJ. Vision loss after intravitreal ocriplasmin: correlation of spectral-domain optical coherence tomography and electroretinography. JAMA Ophthalmol. 2014;132(4):487–90.
Sharma P, Juhn A, Houston SK, Fineman M, Chiang A, Ho A, et al. Efficacy of ocriplasmin for vitreomacular traction and full-thickness macular hole. Am J Ophthalmol. 2015;159(5):861–7.
Kerrison JB. Optic neuropathies caused by toxins and adverse drug reactions. Ophthalmol Clin North Am. 2004;17:481–8.
Vieira LM, Silva NF, Dias Dos Santos AM, Anjos RS, Abegao Pinto LA, Vincente AR, et al. Retinal ganglion cell layer analysis by optical coherence tomography in toxic and nutritional optic neuropathy. J Neuroophthalmol. 2015;3:242–5.
Marmor MF, Kellner U, Lai TYY, Lyons JS, Mieler WF, American Academy of Ophthalmology. Revised recommendations on screening for chloroquine and hydroxychloroquine retinopathy. Ophthalmology. 2011;118:415–22.
Chen E, Brown DM, Benz MS, Fish RH, Wong TP, Kim RY, et al. Spectral domain optical coherence tomography as an effective screening test for hydroxychloroquine retinopathy (the “flying saucer” sign). Clin Ophthalmol. 2010;4:1151–8.
Sarac O, Tasci YY, Gurdal C, Can I. Differentiation of optic disc edema from optic nerve head drusen with spectral-domain optical coherence tomography. J Neuroophthalmol. 2012;32(3):207–11.
Silverman AL, Tatham AJ, Medeiros FA, Weinreb RN. Assessment of optic nerve head drusen using enhanced depth imaging and swept source optical coherence tomography. J Neuroophthalmol. 2014;34(2):198–205.
Wu Z, Xu G, Weinreb RN, Yu M, Leung CK. Optic nerve head deformation in glaucoma: a prospective analysis of optic nerve head surface and lamina cribrosa surface displacement. Ophthalmology. 2015;122(7):1317–29.
Bussel II, Wollstein G, Schuman JS. OCT for glaucoma diagnosis, screening and detection of glaucoma progression. Br J Ophthalmol. 2014;98 Suppl 2:ii15–9.
Kaufhold F, Kadas EM, Schmidt C, Kunte H, Hoffmann J, Zimmermann H, et al. Optic nerve head quantification in idiopathic intracranial hypertension by spectral domain OCT. PLoS One. 2012;7(5):e36965.
Vizzeri G, Bowd C, Medeiros FA, Weinreb RN, Zangwill LM. Effect of improper scan alignment on retinal nerve fiber layer measurements using Stratus optical coherence tomograph. J Glaucoma. 2008;17(5):341–9.
Savini G, Zanini M, Carelli V, Sadun AA, Ross-Cisneros FN, Barboni P. Correlation between retinal nerve fibre layer thickness and optic nerve head size: an optical coherence tomography study. Br J Ophthalmol. 2005;89(4):489–92.
Vernon SA, Rotchford AP, Negi A, Ryatt S, Tattersal C. Peripapillary retinal nerve fibre layer thickness in highly myopic caucasians as measured by Stratus optical coherence tomography. Br J Ophthalmol. 2008;92(8):1076–80.
Disclosure
Dr. Sergott is a paid consultant to Heidelberg Engineering as well as the recipient of research funding. He has participated in the development process of several optic nerve and retinal imaging technologies. He does not hold any patents and receives no royalties from Heidelberg Engineering.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Sharma, P., Sergott, R.C. (2016). Guide to OCT Image Interpretation with Normal and Anatomic Variants. In: Girach, A., Sergott, R. (eds) Optical Coherence Tomography. Springer, Cham. https://doi.org/10.1007/978-3-319-24817-2_1
Download citation
DOI: https://doi.org/10.1007/978-3-319-24817-2_1
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-24815-8
Online ISBN: 978-3-319-24817-2
eBook Packages: MedicineMedicine (R0)