Abstract
Cacao (Theobroma cacao L.) is native to tropical South America, but as the unique source of cocoa butter and powder for the 200 billion USD global confectionery market, it is cultivated globally. Despite its economic importance, cocoa was, and continues to be, predominantly produced in low-input and low-output systems. Production constraints, including depletion of soil fertility on cacao farms, increasing damage due to diseases and pests, and expanding labor costs, limit cacao sustainability. Therefore, instead of increasing yields, the predominant contributing factor that keeps up with the rising demand for cocoa products has been expansion to new production regions. The future of the world’s cocoa economy depends significantly upon using germplasm with a broad genetic base to breed new varieties with disease and pest resistance, desirable quality traits, and the ability to adapt to changing environments. Cacao differs from major field crops with regard to the untapped wild populations, which are still abundant in the Amazon region where they are coevolving with the pathogens. Moreover, in the absence of reproductive barriers, these wild populations could be readily crossed with cultivated crops. Yet only a very small fraction of the wild germplasm, mostly represented by a small number of clones in the so-called Pound collection, has been used for breeding since the 1940s. Contributions from this small set of clones have made tremendous impacts in disease resistance and adaptability. However, breeding efforts in the past 70 years have been reshuffling this small fraction of genetic diversity, with little addition of new variation. The on-farm genetic diversity in Southeast Asia and West Africa is low and cannot meet the challenge of the mounting pressure from diseases and pests. New breeding strategies are needed to combine more disease resistance genes/alleles from untapped wild germplasm and provide farmers with enhanced genetic diversity.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Theobroma cacao L. is an important tropical rainforest tree, previously classified in the Sterculiaceae and presently recognized as a member of the family Malvaceae (Bayer and Kubitzki 2003) that originated in tropical South America (Bartley 2005; Cheesman 1944; Wood and Lass 2001). The tree is commonly known as cacao, while the term cocoa is reserved for the products made from the dried and fermented seeds. T. cacao encompasses many morphologically variable populations with a marked potential for inter- and intra-matings (Bartley 2005; Cheesman 1944). Although self-fertilization is possible in self-compatible plants, outcrossing is the predominant strategy.
Early use of the plant concentrated on the pulp and beans , and the former may have been the first factor that led to cacao farming. Archeological studies, in the Ulua Valley in Honduras, showed that the Olmecs fermented the sweet cacao pulp to make an alcoholic drink at least 3000 years ago, well before the practice of grinding the bitter seeds to produce a chocolate drink (Henderson et al. 2007; Powis et al. 2011). Since the cacao bean was additionally used for monetary, cultural, and political reasons, the tree was widely grown in Mesoamerica before the Spanish arrived (Bergmann 1969; Young 1994). However, the earliest people carried only a single strain of cacao out of the Amazon into Mesoamerica, where cacao was cultivated deliberately. Later on, the European colonists introduced a couple of other strains from the Amazon and transferred these traditional varieties to their newly won lands and colonies. Therefore, the cacao economy in the colonial time was based on a very narrow genetic background (Dias 2001a; Bartley 2005). On the other hand, little difference exists between cultigens and wild cacao in terms of their morphological characteristics and agronomic traits. It is still common today for the Amerindian to directly take native trees found in nature and adopt them as a crop, with little deliberate changes to their phenotypic features. Therefore, unlike many other domesticated field crops, cacao has the advantage that wild germplasm can be directly used in breeding or commercial production (Bartley 2005; Dias and Resende 2001; Eskes and Efron 2006).
The “wild relatives of cacao” include two types of germplasm . The first type is the large spectrum of wild populations that spontaneously grow in the Amazonian rainforest, from French Guiana to Bolivia. The second type of germplasm refers to the 22 related Theobroma species (Cuatrecasas 1964; Zhang et al. 2011), which have made negligible contribution to cacao improvement, due to interspecific crossing barriers. So far, conservation efforts have focused on the wild T. cacao populations. The main exception is T. grandiflorum (cupuassu) , which is considered an important fruit crop in various Amazonian countries. Research studies on cupuassu have included breeding and germplasm collection (Alves, et al. 2007); germplasm characterization, interspecific hybridization, and product development (Silva et al. 2001); and phylogenetic studies (Silva et al. 2004; Silva and Figueira 2005).
Today, cacao is cultivated extensively as the unique source of cocoa butter and powder for the confectionery industry. According to the World Cocoa Foundation (WCF) , the production of cacao takes place mainly on small-scale farms in developing countries across Africa, Asia, and Latin America. The number of cacao farmers, worldwide, is 5–6 million, and the number of people who depend upon cacao for their livelihood is 40–50 million worldwide (World Cocoa Foundation 2012). The majority of cacao farmers employ a low-technology and low-finance approach, bordering on subsistence agriculture.
The annual worldwide production of cacao is estimated at 4.3 million tons for 2010–2011 (International Cocoa Organization 2012). During the past 100 years, there has been an average increase in demand of 3 % per year. The last 10 years have witnessed an increasing geographical concentration in cacao growing, with the African region now firmly established as the top supplier (Fig. 1.1). Increased demand has been met by expansion in production, mainly in the major West African cacao-producing countries. The demand for cacao is estimated to exceed supply with cocoa consumption increasing in emerging middle-income countries, including Brazil, China, Eastern Europe, India, Mexico, and Russia. Revenue derived from the sale and export of cacao provides crucial support to livelihoods of farmers and landholders throughout the tropics.
Top 20 cacao-producing countries with a total output of 4,309,000 tons of cacao beans in 2010–2011 (ICCO, 2012). http://www.icco.org/about-us/international-cocoa-agreements/cat_view/30-related-documents/45-statistics-other-statistics.html
2 Agronomy of Cacao
2.1 Cultivation
Cacao is cultivated within 20° of the equator (Toxopeus 1985) with the major producers having easy access to supplies of low-cost labor and forest land (Woods 2003). Irrigation is rarely applied, but may be undertaken in countries with a dry season (rainfall less than 100 mm/month) to prevent drought stress . Cacao can be propagated from seeds, rooted cuttings, or grafted or budded plants. Many cacao farmers have seedling trees on their farms. Seeds can be obtained from open-pollinated or biparental crosses (sometimes called “hybrid” crosses) carried out in seed gardens or from their own farm or local community. Vegetative (or clone) propagation, by budding or grafting onto rootstocks or by cuttings, is increasingly practiced in Asia and Latin America (Maximova et al. 2005; Wood and Lass 2008). Large-scale vegetative propagation of planting materials by somatic embryogenesis is being undertaken in Indonesia and Côte d’Ivoire. Clones propagated by orthotropic rooted cuttings of somatic embryo-derived plants are not different from clones propagated by grafting in performance and bean quality traits (Goenaga et al. 2015).
In most countries, the average planting density is 1000–1200 trees ha−1 with a minimum of 600 trees ha−1. In the high-yielding and high-input system in Malaysia, the planting density can reach 3333 trees ha−1 and optimal planting den sities are highly variable for different clones (Lockwood and Pang 1996).
Pruning increases the productivity of a tree because it can optimize the structure of tree canopy, improve photosynthesis activity, facilitate pollination of the flowers and strengthen the formation of new leaves and growth of the pods. Pruning is also an effective measure to control cacao diseases and insect pests. All dead branches and chupons (new branches that grow upward out of the trunk) need to be removed. As they grow, trees should be pruned to control both height (3–4 m is ideal) and shape of the tree, which expedites maintenance and harvesting (Wood and Lass 2008).
Phytosanitation is one of the most cost-effective method for reducing pests and disease for small-holder farmers. It refers to the removal and burial of diseased cacao pods, branches, leaves, and weeds. Field trials in Peru found that weekly removal of pods infected with black pod reduced incidence of the disease by 35–66 % and improved yield by 26–36 % (Soberanis et al. 1999).
Cacao farms can become significantly depleted of nutrients, due to the many years of low or no fertilization input (Baligar and Fageria 2014; Wood and Lass 2008). Soil nutrition deficits are a critical hindrance to cacao productivity in most areas. The current level of soil fertility on cacao farms in West Africa averages less than 10 % of what is necessary for productive crops and soil (Cocoa Fertilizer Initiative, http://www.idhsustainabletrade.com/Fertilzer). Cacao responds well to fertilization, especially on farms where harvested or pruned plant material is not left in the field to decompose (Baligar and Fageria 2014). For each 1000 kg of dry beans harvested, about 20 kg N, 4 kg P, and 10 kg K is removed from the soil. If pod husks are also removed from the field, the amount of K removed increases to about 50 kg (Puentes–Páramo et al. 2014). About 200 kg N, 25 kg P, 300 kg K, and 140 kg Ca are needed per hectare to grow the trees prior to pod production (Moriarty et al. 2014). Fertilization is also believed to extend the productive life of trees. It is estimated that fertilizer alone may be sufficient to increase yield by 500 kg to 1 ton/ha (Moriarty et al. 2014). Nonetheless, few smallholders use agrochemicals because they lack the funds to purchase them at the time they need to be applied (Cocoa Fertilizer Initiative, http://www.idhsustainabletrade.com/Fertilzer).
2.2 Fruit and Harvest
Cacao trees require approximately 3–5 years to bear their first fruits, commonly known as “pods ,” and can remain productive for several decades. The cacao flowers develop as compact inflorescences, directly on the woody tissue of suitable physiological age, throughout the trunk and canopy of the tree, and are pollinated by small flying insects. The pods take approximately 5–6 months to develop and mature, and once ripe, each consists of a thick husk enclosing some 40–50 seeds that are surrounded by a semisweet acidic pulp. The average yield is 450 kg ha−1 (Food and Agricultural Organization 2014), but yields of up to 3000 kg ha−1 are possible under good agricultural practices that combine management practices, pest control, improved plant material, and appropriate fertilizer application (Maharaj et al. 2005; Pang 2006). Low farm gate prices, lack of access to farming inputs/fertilizers, and finance are the main barriers to high-yielding cacao production. In many cases, farmers have limited knowledge of improved production techniques and farm management skills. The participation of new or traditional farmers who use suboptimal farming practices also contributes to poor production and a low-grade product.
Harvesting pods from cacao trees is labor-intensive and occurs within a short season. Harvesting varies by area and climatic conditions with the first harvest typically falling between April and June and a second harvest around October. Harvested pods are cracked open by hand, and the pulp and seeds are manually separated from the husks. The bulked cacao seed mass is generally fermented in simple heaps covered by banana leaves, resulting in variable cacao bean quality. However, a better practice is fermentation in wooden boxes fitted with drainage holes. This is increasingly present in central facilities, cooperatives, or on large farms. The fermentation period is variable and depends primarily on the type of cacao, generally taking 3–8 days. Cacao with high Criollo ancestry typically has a shorter fermentation period than Forastero or Trinitario cacao. A critical mass of fermenting beans is required to achieve the temperatures necessary for ideal fermentation. After fermentation, the beans are commonly sun-dried to reduce the moisture content, ideally to 7.5 %. The fermentation process initiates the formation of flavor precursors which are only fully developed following drying and roasting. The dried cacao beans are usually bagged on farm and transported to the ports for export or local processors. Before making cocoa and chocolate products, the beans are roasted, usually by the manufacturer, to develop the final chocolate flavor. Then the shells are removed from the roasted beans and the cocoa nibs are treated with alkalizing agent (usually potassium carbonate), to modify the flavor and color. The nibs are then further milled to create cocoa liquor, which is used to make chocolate paste, cocoa, cocoa butter, and chocolate (International Cocoa Organization 2013).
3 History of Cacao
3.1 Ancient Cacao Agriculture and Traditional Variety Names
Cultivation of cacao started in Mesoamerica , where cultural elaboration and use of cacao can be traced back more than 3000 years (Gómez-Pompa et al. 1990; Henderson et al. 2007; Powis et al. 2011). The ancient cultigens that were deliberately planted and utilized by Amerindian civilizations including the Mayas (300–900 AD) and the Olmecs (400–1200 BC) (Henderson et al. 2007) became known as Criollo cacao (“Creole” in Spanish). Cacao depictions in Mayan artifacts provide supporting evidence for the deliberate planting by early peoples in Costa Rica, Belize, El Salvador, Guatemala, Honduras, Mexico, and Nicaragua (Wood 1985a; Coe and Coe 1996; Dias 2001a).
“Criollo” is frequently used in contrast to the later introductions called “Forastero ” in the literature. Preuss (1901) and Bartley (2005) indicated that the word Criollo means native or first variety cultivated outside the indigenous range of the species, thus distinguishing it from later introductions, whereas Forastero or Forestero means “of the forest” or foreign, i.e., not among the first cultivated or indigenous variety of a region. The names applied to local variations in the Criollo group are often of a descriptive nature, usually referring to fruit characters, including Porcelana, Pentagona, Angoleta, and Cundeamor. In spite of the morphological variations, Criollo cacao is self-compatible and is nearly fully homozygous as revealed by SSR and SNP markers (Motilal et al. 2010; Ji et al. 2013).
In the Amazonian rainforest, the evidence of cacao cultivation by different indigenous groups has been minimal (Bartley 2005; Dias 2001a; Sánchez et al. 1989). It was suggested that Amazonian tribes might not have had the need to formally cultivate a tree that occurred in abundance (Bartley 2005; Dias 2001a). Clement (1999), based on the ease with which cacao survives in abandoned humid forest ecosystems, classified cacao as a crop with semidomesticated populations. Furthermore, cacao was probably not grown in the Caribbean islands during pre-Columbian times (Wood 1985a).
In addition to Criollo, the Nacional and Amelonado groups were classed in the category “traditional cultivars,” which was interpreted to represent some degree of domestication (Clement et al. 2010). These authors indicated that the results of Motamayor et al. (2008) show that Criollo and Nacional cacaos group together as an Ecuadorian assemblage of western Amazonia, whereas Amelonado groups with French Guiana cacao, indicating a possible eastern Amazonian origin. Bartley (2005) opined that it was likely that the Nacional cacao of Ecuador existed for several centuries prior to the arrival of the Europeans. However, Loor Solorzano et al. (2012) suggested that T. cacao and its products were part of the pre-Columbian culture around 2000 BC, a controversy that was not resolved, although the center of origin of Nacional cacao was suggested, based on microsatellite evidence, to be in the southern Amazonian area of Ecuador. Patiño (2002 as cited in Clement et al. 2010) argued that the Amelonado cacao in eastern Pará of Brazil was from ancient cultivation. Similarly, Barrau (1979 as cited in Clement et al. 2010) suggested, based on ethnographic observations, that cacao had long been cultivated in French Guiana by the native peoples. Drawing on the opposite extremes of cacao distribution in the Americas for Criollo and Amelonado, and the low number of private alleles, Clement et al. (2010) reasoned that Amelonado cacao should be considered to be at least incipiently domesticated in eastern Amazonia.
Amelonado cacao, so called because of the fruit shape (Spanish for “melon shaped”), is another ancient variety. Based on the pod characteristics , this variety has been called Indio, Amelonado, Calabacillo, Matina, Común, Catongo, and Pará (Bartley 2005; van Hall 1932). The Amelonado cacao is more widespread than Criollo in Mesoamerica and the Caribbean, has better adaptability than the ancient Criollo cacao, and, thus, is either replaced or hybridized with Criollo in many places. The earliest time period for cultivating Amelonado (Lower Amazon Forastero cacao) in Mesoamerica and the Caribbean is not clear.
3.2 Colonial History
The cata strophes (Table 1.1) of cacao production in the last four centuries, often caused by diseases and pests, were the main force driving cacao dispersal and the shift of production centers. Severe cacao disasters, generally known as blasts or blights, occurred in the early colonial plantations in Latin America and the Caribbean (Motilal and Sreenivasan 2012 and references therein). Historical records have shown that the “blasts” that occurred in several Caribbean countries had different origins (Motilal and Sreenivasan 2012). Cacao production in Martinique experienced severe disasters in 1671 and 1727 (Quesnel 1967), which decimated entire plantations and almost ruined the cacao industry of the island (Kimber 1988). However, the Amelonado cacao that survived was likely transferred to surrounding islands, which had undergone similar devastation events. Direct introduction from South America into cacao plantations of the Caribbean islands also occurred (Preuss 1901; van Hall 1932; Bartley 2005).
During the Spanish colonial rule, Trinidad cacao planters grew mostly Criollo cacao. Prior to, and even more so after, the 1725 destruction of the majority of the cacao crop by a trifecta of climatic, agronomic, and genetic causes (Motilal and Sreenivasan 2012), Forastero material was introduced, most likely from Brazil (Shephard 1932; Joseph [1838] 1970), Hispaniola (Bartley 2005), and Venezuela (Bartley 2005). Several resultant natural hybridization events (Motilal et al. 2010; Motilal and Sreenivasan 2012; Yang et al. 2013) led to the Trinitario germplasm, which is noted for its fine flavor (Toxopeus 1985). Heterosis (hybrid vigor) resulted in vigorous planting material, which was then reintroduced to Venezuela. The term Trinitario probably accompanied the transferred germplasm and has since been used to describe these cacao types, arising as products of hybridization and recombination through various generations, which are now known in the trade for their floral/fruity flavors. Much later, at the then Imperial College of Tropical Agriculture in Trinidad, an extensive survey of the Trinitario population was conducted, resulting in the selection of approximately 100 Imperial College Selections (ICS) clones, which were selected princip ally for yield characteristics (Pound 1932, 1934).
3.3 Ancestry of Trinitario
The genetic composition of Trinitario cacao has been further dissected to clarify whether their ancestry includes only Criollo and Amelonado (Johnson et al. 2009; Motilal et al. 2010; Yang et al. 2013). Recent SSR analysis showed that the genesis of Trinitario cacao was when a limited population of Criollo × Forastero hybrids emanated from the introduced Forastero population of Trinidad (Motilal et al. 2010). The multi-lineage origin of modern Trinitario is also supported by analysis based on plastidic single nucleotide polymorphisms (cpSNPs) and polymorphic simple sequence repeats in plastids (cpSSRs) (Yang et al. 2013). Three cpSNP haplotypes were revealed in the Trinitario cultivars sampled in Trinidad, each highly distinctive and corresponding to reference genotypes for the Criollo, Upper Amazon Forastero, and Lower Amazon Forastero varietal groups. These three cpSNP haplotypes likely represent the founding lineages of cacao in Trinidad and Tobago. The cpSSRs were more variable with eight haplotypes, but these clustered into three groups corresponding to the three cpSNP haplotypes. The most common haplotype found in farms of Trinidad and Tobago was Amelonado, followed by Upper Amazon Forastero and then Criollo. The authors concluded that the Trinitario cultivar group was of complex hybrid origin and was de rived from at least three original introduction events.
3.4 Out of America: Dispersal of Cacao to the Old World
In spite of the complex requirement of planting materials, environmental factors, and management practices, cacao was, and continues to be, dominantly produced in low-input, low-output, and high-risk systems. Sustainability is limited by many factors, which constrain production, including the depletion of soil fertility on farms and the legions of pests causing damage and diseases . Cacao agriculture provides a prime example of the continued confrontation of crop production with new and recurrent epidemics (Table 1.1). As the center of cacao production shifted from place to place, a small fraction of the cultigens were transported to the new production sites. Genetic diversity represented in these cultigens is actually a tiny fraction of available genetic diversity in the primary gene pool of cacao in South America. The low level of genetic diversity in cacao farms could not meet the challenge of mounting pressure of diseases and pests, and expansion to new production regions has been essential to keep up with the world demand for cocoa products.
The first contact that Europeans had with the crop was attributed [by Oviedo y Valdez (1855) as cited in Bartley (2005)] to Alonso Pinzón in 1510 in southern Yucatan. In the sixteenth century, the Europeans started to cultivate cacao in Asia and Africa where the Criollo, Amelonado, and the Trinitario hybrids started their route of dispersal from the Americas to the old world.
The first shipment of cacao germplasm to Southeast Asia was recorded in 1560, when the Dutch introduced cacao that was believed to be the fine flavor variety “Venezuelan Criollo ” into Celebes, Indonesia (van Hall 1932). Cacao production started in northern Sulawesi where cacao was processed and consumed only locally (van Hall 1932). Another introduction to this region in 1670, believed to be a Criollo variety from Mexico, was via the Acapulco-Manila galleons (Bartley 2005). Around 1770, the Dutch introduced cacao to Peninsular Malaysia (Thong et al. 1992), and fruiting cacao was subsequently found in Malacca [Koenig (1894) as cited in Thong et al. (1992)]. In 1798, the British took cacao to Madras, India, from the island of Amboina, and it was introduced into Ceylon (now Sri Lanka) from Trinidad at about the same time (Ratnam 1961; Wood 1991). From Ceylon, cacao was subsequently transferred to Singapore and Fiji (1880), Samoa (1883), Queensland (1886), and Bombay and Zanzibar (1887). Cacao was also grown in Malaysia as early as in 1778 and in Hawaii by 1831 (Bartley 2005). Remnants of the ancient Criollo, Amelonado, and Trinitario populations can still be found in Asia and Pacific regions, such as Indonesia (Susilo et al. 2011), South Pacific (Fiji and Samoa), and Madagascar (Zhang et al., unpublished data).
With the establishment of chocolate manufacturing in Europe in the second half of the eighteenth century and the increase in chocolate consumption in North America, there was an explosion in demand, requiring yet more cacao to be produced. Commercial cultivation started in Africa after the Portuguese introduced Amelonado cacao into Principe in 1822. By the 1850s, cultivation of cacao spread to the main island of Sao Tome, where the Amelonado cacao (“Común” in Bahia, Brazil) became known as Sao Tome “Creoulo .” This self-compatible variety was then brought by Spaniards into the island of Fernando Po (now Bioko), Equatorial Guinea, and repeatedly introduced into the mainland West Africa (Bartley 2005). The limited genetic diversity within the initial foundation of West Africa Amelonado was also mentioned by van Hall (1932). During the late nineteenth century, the colonial administration also introduced some red-pod cacao materials from British West Indies into botanical gardens established in Aburi (Ghana) and Lagos (Nigeria) (Toxopeus 1964). Consequently, the bulk of cacao grown on farmers’ plantation must have consisted of a mixture of these earlier varieties, with the self-compatible “West African Amelonado” type dominating the production at the beginning of the twentieth century.
The shift in the world’s center of cacao production followed a boom-and-bust pattern, from Mesoamerica to Venezuela, from Venezuela to Ecuador, from Ecuador to Brazil, and from Brazil to West Africa (Ruf and Schroth 2004). As new countries/regions adopted the crop, the previous production centers collapsed. Production shifts from one country to the next were reproduced by similar cycles on a subnational scale (Ruf and Schroth 2004). Among the many factors contributing to this boom-and-bust cycle, the impact of biotic constraints, due to the limited on-farm genetic diversity, apparently played a key role. Subsequently, it was only a matter of time before coevolved fungal pathogens moved—naturally or human assisted—from their forest hosts into the cacao plantations. The various catastrophic “blasts” that occurred in the last 400–500 years suggest that disease was the main force that drove cacao dispersal and the shifting of production centers. Therefore, a brief review of the cacao primary gene pool is essential for improving our understanding about future sustainability of cacao production.
4 Upper Amazon: Cacao’s Primary Gene Pool
The term “Upper Amazon” has been used to describe the location of most of the known wild cacao populations from the “Alto Amazonas ,” a region from the start of the Marañón River in Peru to the frontier of Brazil. In this region, a series of major river systems in Peru , Ecuador, Colombia, and Brazil flow into the Marañón and Amazon rivers. Wild cacao populations are found in these river basins in both spontaneous (without human interference) and subspontaneous forms (wild cacao trees exploited by man) prior to European occupation (Almeida 2001; Bartley 2005). Wild cacao germplasm samples from the expeditions in the Amazon were predominantly collected along the banks of navigable rivers (Pound 1938; Lachenaud and Sallée 1993; Lachenaud et al. 1997; Almeida 2001). Each natural cacao population has a narrow genetic base and is thought to have been founded by a limited amount of reproductive materials (Pound 1945; Bartley 2005).
Genetic diversity of natural cacao populations is generally stratified by the major river systems in the Amazon (Pound 1938; Almeida 2001; Bartley 2005). Within each river basin, wild cacao is usually grouped in patches and separated by large spatial distances between patches. It is hypothesized that gene flow in cacao is limited and mating is likely confined within patches (Chapman and Soria 1983), due to the short distance of seed dispersal by rodents and monkeys and short-distance pollen dispersal by insects, including midge species ( Forcipomyia spp.) as well as other insect vectors.
A significant departure from the Hardy-Weinberg equilibrium (HWE) was detected in the French Guiana wild populations (Lachenaud and Zhang 2008). In addition to the likely short-distance gene dispersal, cacao has a gameto-sporophytic self-incompatibility system (Cope 1962), which works in a quantitative manner (Lanaud et al. 1987). Self-compatibility in some genotypes is partially responsible for the high fixation index in several natural populations. Indeed, fully homozygous genotypes were frequently found in the populations from French Guiana (Lachenaud and Zhang 2008). Some wild cacao trees are found in the form of single plants, but the majority will form a “clump” (several trunks at different development stages and overlapping generations at one growing site). This apparent generation overlap within a patch is another likely factor contributing to mating between relatives, thus increasing the level of inbreeding. The multiple trunks can also come from self-propagation by chupon production , which increases the chance of inbreeding by self-mating as opposed to inbreeding by mating between family members.
Despite the commonly perceived short-distance gene flow and limitation in effective population size in wild cacao, isolation by distance was detected only over a long geographical range (e.g., a few hundred kilometers) and not in a local basin or short distance (Zhang, et al. 2006). Sereno et al. (2006) reported that in the natural or seminatural populations sampled in four regions of Brazil (Acre, Rondonia, Lower Amazon, and Upper Amazon), most of the genetic diversity was allocated within populations rather than between populations, indicating a typically high gene flow . Therefore, some of the apparently isolated populations may actually belong to the same metapopulation in terms of gene dispersal, which impacted their genetic differentiation. A study on the cacao mating system at the hierarchical levels of fruits and individuals showed that the cacao population was spatially aggregated, with significant spatial genetic structure up to 15 m. Mating was correlated within, rather than among, the fruits, suggesting that a small number of pollen donors fertilized each fruit (Silva et al. 2011). A similar study in the northeast lowlands of Bolivia revealed 7–14 % self-pollination in wild cacao populations. Cacao pollen was transported up to 3 km, with an average of 922 m, suggesting pollination distances larger than those typically reported in tropical understory tree species (de Schawe et al. 2013).
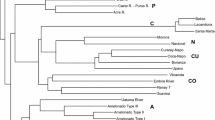
Using SSR markers , Motamayor et al. (2008) genotyped 1241 cacao accessions existing in most of the ex situ germplasm collections in Latin America. The result led to the identification of 10 genetic clusters in the Forastero cacao, which was proposed as a new classification of the cacao germplasm (Fig. 1.2).
Neighbor joining tree from Cavalli-Sforza and Edwards genetic distance [16] matrix among the 36 subclusters identified using structure (559 clones). Motamayor, et al. (2008) Geographic and Genetic Population Differentiation of the Amazonian Chocolate Tree ( Theobroma cacao L ). PLoS One 3(10): e3311. doi:10.1371/journal.pone.0003311
Thomas et al. (2012), using the same SSR data set, reanalyzed the spatial pattern of intraspecific diversity of cacao in Latin America. Grid-based calculations of allelic richness, Shannon diversity, and Nei’s gene diversity and spatial cluster analysis suggested the highest levels of genetic diversity were observed in the Upper Amazon areas from southern Peru to the Ecuadorian Amazon and the border areas between Colombia, Peru , and Brazil (Fig. 1.3). Simulation modeling suggests that cacao was already widely distributed in the western Amazon before the onset of glaciations. During glaciations, cacao populations were likely to have been restricted to several refugia where they presumably underwent genetic differentiation, resulting in a number of genetic clusters which are representative of, or closely related to, the original wild cacao populations. The analyses also suggested that genetic differentiation and geographical distribution of a number of other clusters seem to have been significantly affected by processes of human management and accompanying genetic bottlenecks.
Spatial variation of different genetic parameters, represented at a resolution of ten-minute grid cells and a circular neighborhood of 1 degree. Highest values are consistently observed in the extensive bean-shaped Amazonian area covering both the Peruvian-Brazilian border and the southern part of the Colombian-Brazilian border, as well as Amazonian Ecuador. Thomas et al. (2012). doi:10.1371/journal.pone.0047676.g001
Since 2008, a series of collecting expeditions have been launched to survey the full cacao genetic diversity in the Peruvian Amazon (Fig. 1.4a). The expeditions were aimed at areas lacking representation in the ex situ cacao germplasm collections. The first geographical focus was the major tributaries of Rio Marañón, including Rio Santiago, Rio Pastaza, Rio Nucuray, Rio Urituyacu, Rio Tigre, Rio Ucayali, Rio Madre de Dios, and Rio Putumayo. Within each subbasin, the identification of collecting sites was assisted by GPS mapping tools. Habitat descriptions were examined and the target area was chosen based on the potential for complementary diversity. The expeditions were supported by the Peruvian government and by the USDA. To date, a total of 520 wild trees have been collected, representing 19 river basins. Preliminary characterization using SNP markers showed a significant amount of diversity complementing the existing national and international ex situ collections. The living trees were propagated in the facilities of Tropical Crop Institute (ICT—Spanish acronym) in Tarapoto, Peru (Fig. 1.4b). These trees are currently being evaluated for agronomic traits with the emphasis on resistance to diseases and bean quality and flavor. Next-generation sequencing (NGS) genotyping of these trees is being planned, together with all the wild trees from the International Cocoa Genebank, Trinidad (ICG, T), and other major ex situ collections, to provide a comprehensive overview of the diversity distribution in the cacao primary gene pool.
5 The “Pound Collection”
5.1 Collection from Wild Cacao Germplasm
The outbreak of witches’ broom disease (WBD) in Trinidad in the late 1920s led to the search for genetic resistance in the Upper Amazonian region. During the 1930s–1940s, wild germplasm was collected from the Upper Amazon basin of Ecuador and Peru (Pound 1938, 1945; Wood and Lass 2008). To date, the germplasm that has made the most fundamental contribution to the modern cacao breeding programs is, by far, the Pound collection (named after the collector F. J. Pound). This collection results from the first cacao germplasm collecting expedition into the Upper Amazon, and the collecting sites included part of the tributaries of Rio Ucayali, Rio Morona, and Rio Marañón (Pound 1938, 1945; Bartley 2005; Zhang et al. 2009; Fig. 1.5).
This led to the establishment of the “Pound collection” in Iquitos, Peru. Pound’s expeditions were aimed at searching for genotypes resistant to WBD, caused by the fungus Moniliophthora perniciosa (Stahel) (Aime and Phillips-Mora 2005), after the outbreak of witches’ broom disease (WBD) in Trinidad in the late 1920s. These germplasm accessions have henceforth served as the foundation for breeding programs around the world for resistance to WBD. The Pound collection was primarily comprised of five germplasm groups : “Iquitos Mixed Calabacillo” (IMC), “Morona” (MO), “Nanay” (NA), “Parinari” (PA), and “Scavina” (SCA) (Pound 1938, 1943, 1945). An unknown number of pods (fruits) were collected from trees without any symptoms of WBD. The 25 half-sib families yielded 250 fruits (Lockwood and End 1993; Motilal and Butler 2003). The seeds were then bulked and sent to Barbados where approximately 2500 seedlings were raised (Toxopeus and Kennedy 1984). These germplasms were transferred to Trinidad in the form of bud wood and vegetatively propagated onto rootstock at Marper Estate in Plum Mitan, Manzanilla, Trinidad, as 486 accessions (Motilal and Butler 2003). In addition to the 25 half-sib families from the five accessions groups, Pound’s collection also includes 32 clones, which Pound collected in 1943 when he revisited the same sites where the NA, IMC, and SCA were previously collected. These accessions were collected as bud woods and were referred as “Pound clones” or “P clones .”
In addition to these five groups collected from Peru from 1938 to 1943, Pound also collected 80 half-sib families from western Ecuador. The 80 half-sib families yielded 1185 Ecuadorian Refractario that are in the ICG, T (Fig. 1.6a, b) and were the result of the first expedition (Lockwood and End 1993).
(a) ICGT looking north, Field 5B on left, Field 6B on right, Field 5A at sign on upper left, and Field 6A at sign on upper right. Observer has walked away from southern T junction between 5B and 6B of Google map. Photo courtesy Lambert A. Motilal. (b) Aerial view of ICGT from Google Earth. ICGT bordered on north by Caroni River. Field to right of 6B is Field 7 with individuals from recent crosses
Among the 80 or so different germplasm groups held in the ICG, T, those in the Pound collection are among the most widely distributed germplasm, due to their valuable agronomic traits and their potential for resistance to WBD (Lockwood and End 1993; International Cacao Germplasm Database, http://www.icgd.reading.ac.uk/) (Table 1.2). In many cacao-producing regions around the world, the Pound selections of Upper Amazonian cacao are either adopted directly as clones or used as parents for the production of seed families. This collection is, by far, the most widely used germplasm for cacao breeding in the world (Bartley 1994, 2005; International Cacao Germplasm Database, http://www.icgd.reading.ac.uk; Posnette 1986).
Numerous additional collecting expeditions took place in the Amazon rainforest after Pound. Among these expeditions, Table 1.3 lists the significant ones for their geographical location, genetic diversity, and potential breeding value for cacao improvement. Nonetheless, a majority of these collections are either not in the international collections or were not well distributed internationally for the time being.
Although the international and national collections in total contain a substantial amount of genetic diversity, recent expeditions have discovered novel variability not contained within existing collections. This points to the importance of filling the diversity gaps in the ex situ collections and the need to systematically sample areas, with the objective of capturing novel variability existing in the primary gene pool. A number of expeditions to sample the variability within the Amazonian home of cacao are presently underway in Brazil, Ecuador, French Guiana, and Peru.
5.2 Impact of “Pound Collection” in Cacao Breeding
Breeding programs started in the 1920s in the major cacao-growing countries with phenotypic selection of locally available germplasm, but the large genetic diversity present in the wild Amazonian populations is yet to be widely exploited and utilized in cultivated varieties (Bartley 2005). Among the wide range of Trinitario varieties, the group of clones that has most influenced cacao breeding undoubtedly came from Trinidad. These ICS accessions were used at different times in various countries.
The WBD outbreak in Trinidad in the late 1920s exposed the deficiency in the ICS clonal selection, which was primarily based on productivity rather than disease resistance. The agronomically important TSH hybrids, developed in Trinidad over 60 years of breeding, were mostly based on four clones: SCA 6, IMC 67, POUND 18 and ICS 1 (Gonsalves 1996), of which ICS 1 was a descendant of the hybridization that occurred in Trinidad post-1725.
The outbreak of cocoa swollen shoot disease in the 1930s in Ghana, Togo, and Nigeria almost destroyed the cacao industry due to the lack of resistance in the West Africa Amelonado germplasm. The demand for new genetic variation resulted in the first large-scale dissemination of UAF germplasm when Dr. A. F. Posnette visited Trinidad in the early 1940s. Posnette studied the incompatibility reactions with the newly arrived germplasm collected by Pound and produced seed progenies. A total of 121 cacao pods were introduced from Trinidad. After being quarantined in Accra, Ghana, the shipment reached the West African Cocoa Research Institute headquarters in Tafo, Ghana, and Ibadan in Nigeria (Toxopeus 1964). Each pod was numbered serially with the prefix T (Trinidad), resulting from either open pollination (T1–T59) or from open pollination (T60–T121) for which both parents are known (Table 1.4).
The resultant plants (about 3000) were planted out at Tafo, Ghana, in 1945. In 1948, the precocity and generally superior performance of these progenies of Upper Amazon parentage were definitely acknowledged. For the purpose of breeding for resistance to cocoa swollen shoot virus (CSSV) disease, cuttings of the parental clones have been introduced since the 1950s. Out of the progenies, a small fraction of the elite selections were used to produce second and third generations of Amazon known as “F3 Amazon” or “Mixed Amazon” in Ghana (Aikpokpodion 2012). By early 1960, the “F3 Amazon” or “Mixed Amazon” had been widely distributed to farmers in Ghana and Nigeria to cope with CSSV. In Ghana, seed production plots planted with selected Amazon × Amelonado hybrids and Inter-Amazon hybrids are the main sources of materials for farmers. In a recent survey, Edwin and Masters (2005) showed that released hybrids accounted for 42 % higher yields obtained by Ghanaian farmers. In Cote d’Ivoire, the 12 selected Upper Amazon-derived hybrids (Besse 1975) played a very significant role in the phenomenal increase in cacao production. In Nigeria, the Old Western Region, comprised of the present Ondo, Oyo, Ogun, Ekiti, and Osun states, had functional Cocoa Development Units (CDU) or Tree Crop Unit (TCU), responsible for distributing seeds of the “F3 Amazon” and the WACRI Series II varieties from seed gardens (Toxopeus 1964; Aikpokpodion et al. 2009).
6 Current On-Farm Diversity in West Africa and Southeast Asia
Surveys of planting materials in West Africa have shown that a mixture of Amelonado landraces and Upper Amazon-derived cacao hybrids distributed by the government are present in farmers’ fields (Aikpokpodion et al. 2009, 2012; Edwin and Masters 2005). Among the parental germplasm groups from the Pound collections , the Parinari group had the greatest impact on the farmer’s selections (Aikpokpodion et al., 2012). Unlike the traditional local Amelonado, which has only one peak of fruit production, this Amazon cacao has year-round production, high-yielding potential, and a shorter juvenile period. These are important criteria used by local farmers for selecting “improved varieties.” The traditional West African Amelonado, on the other hand, are appreciated for their low vigor, high bean-to-pod volume ratio (less mucilage and placenta content), and less susceptibility to black pod disease (Aikpokpodion et al. 2012).
Farmers in Côte d’Ivoire largely used their own planting materials to establish new plantations in new cacao-growing regions . However, many of the planting materials were originally introduced by farmers from Ghana (Pokou et al. 2009). Assessment of on-farm genetic diversity in six producing regions of Côte d’Ivoire was reported by Pokou et al. (2009). Based on farmer selections sampled from 280 farms, open-pollinated seed progenies were collected from 561 trees. Twelve microsatellite markers were used to assess parentage and genetic diversity. Most of the farm accessions appeared to be hybrids between Upper Amazon (UA) and Lower Amazon (LA, Amelonado) or African Trinitario parental genotypes. However, a certain percentage of accessions appeared to be fairly pure UA or LA types. The best accessions for black pod resistance appear to be mostly hybrids between Upper Amazon and Amelonado.
A survey of 400 farm accessions in Cameroon, based on 12 microsatellite loci, suggests that 25.5 % of the farm accessions are still closely related to the traditional Amelonado variety called “German cocoa” by the farmers (Efombagn et al. 2006, 2008). Another 46.3 % of the farm accessions were found to be direct descendants (20.8 % first-generation (F1) hybrids and 25.5 % selfed genotypes) from 24 parental clones used in bi-clonal seed gardens (BSGs) established in the 1970s in southern and western Cameroon. Furthermore, 28.3 % of farm accessions appeared to descend from uncontrolled pollination events in cacao farms, which could be related to a common practice of cacao growers to use seeds collected from their own farm for new plantings.
A survey of ancestry and parentage in farmers’ selections in Sulawesi, Indonesia, using SSR markers generated similar result as those observed in West Africa. Sulawesi farmer selections are mainly comprised of hybrids derived from Trinitario and two Upper Amazon Forastero groups. Trinitario made the largest ancestral contribution to these farmer selections, with an average population membership (Q value) of 51.0 %. The second largest contributor was from the Parinari group, which explained 27.5 % of the ancestry. The Nanay group accounted for 12.6 % of the admixture, and the group of Morona/Scavina and Iquitos Mixed Calabacillo only explained 4.3 and 4.7 % of the assigned membership of the tested farmer selections (Diny Dinarti and Dapeng Zhang, unpublished data).
The low level of on-farm diversity in Asia and Africa is also demonstrated in the seed gardens that provide planting materials for the next several decades in West Africa. Cacao seed gardens are considered an efficient and dynamic seed production and distribution system in West Africa, because they play a significant role in replacing the aging cacao trees in this region. There are a total of 47 seed gardens in Cameroon (3), Ghana (26), and Nigeria (18), which are predominantly run by the state at subsidized prices. These seed gardens produced approximately five to six million hybrid pods in 2008 (Asare et al. 2010). However, parental clones used in these seed gardens reflect a narrow genetic background. The total number of parental clones currently used in seed gardens for production of hybrid pods is approximately 50 (Table 1.5). However, pedigrees from the Upper Amazon Forastero germplasm can be traced back to no more than 10 clones from the Pound collection (e.g., NA 32, PA 7, PA 35, SCA 6, SCA 12). This is the same small set of Upper Amazon Forastero that was introduced into Africa 70 years ago. Since then, no new genes or alleles have been added to the breeding pool .
The series of on-farm diversity surveys in West Africa and Southeast Asia, as mentioned above, showed that the current level of functional allelic diversity is low in these major cacao-producing countries. The available resistances to cacao disease and pests in the major producing countries have been dominantly based on no more than 10 mother trees from Pound collection, which were introduced from Trinidad in the mid-1940s. As a result, the breeding efforts since then have been reshuffling these limited genetic variations, despite the superficially high level of heterozygosity and gene diversity (typical of an outcrossing species) in farmer selections and breeding lines. This small set of Pound accessions, which form the foundation for current on-farm diversity in Asia and Africa (and to large extent in Latin America as well), represents only a tiny fraction of the existing genetic diver sity in the primary gene pool of cacao. Cacao is very different from any other major field crop in terms of the amount of available wild populations, which still exist in the Amazon and are coevolving with the pathogens. Moreover, these wild populations can be readily crossed with cultivated varieties without reproductive barriers. Recent QTL and functional diversity studies have showed that different germplasm groups harbor different genes/alleles of disease resistance, as reviewed by Lanaud et al. (2009). A major change must be taken in cacao breeding in order to cope with these threats. New breeding approaches should take full advantage of the available diversity in wild populations. An important strategy is long-term germplasm enhancement through pre-breeding, i.e., introgression of exotic germplasm to improve population diversity, which has generally been recommended for most domesticated crops (Stander 1993), as well as for cacao in particular (Dias 2001b; Eskes 2011; Surujdeo-Maharaj et al. 2001; Tahi et al. 2000). Pre-breeding programs allow accumulation of resistance genes/alleles from different origins, thus increasing future on-farm genetic diversity.
References
Aikpokpodion, P. (2012). Defining genetic diversity in the chocolate tree, Theobroma cacao L. grown in West and Central Africa. In M. Çalişkan (Ed.), Genetic diversity in plants, Chap. 10. InTech. doi:10.5772/33101. Available from http://www.intechopen.com/books/genetic-diversity-in-plants/defining-genetic-diversity-in-the-chocolate-tree-theobroma-cacao-l-grown-in-west-and-central-africa
Aikpokpodion, P. O., Motamayor, J. C., Adetimirin, V. O., Adu-Ampomah, Y., Ingelbrecht, I., Eskes, A. B., Schnell, R. J., Kolesnikova-Allen, M. (2009). Genetic diversity assessment of sub-samples of cacao, Theobroma cacao L. collections in West Africa using simple sequence repeats marker. Tree Genetics and Genomes, 5, 699–711.
Aime, M. C., & Phillips-Mora, W. (2005). The causal agents of witches’ broom and frosty pod rot of cacao (chocolate, Theobroma cacao) form a new lineage of Marasmiaceae. Mycologia, 97, 1012–1022.
Almeida, C. M. V. C. (2001). Ecology of natural populations. In L. A. S. Dias (Ed.), Genetic improvement of cacao, Chap. 4 (EcoPort version by P. Griffee, FAO). Available at http://ecoport.org/ep?SearchType=earticleView&earticleId=197&page=-2#section2690
Alves, R. M., Sebbenn, A. M., Artero, A. S., Clement, C., & Figueira, A. (2007). High levels of genetic divergence and inbreeding in populations of cupuassu (Theobroma grandiflorum). Tree Genetics and Genomes, 3, 289–298.
Asare, R., Afari-Sefa, V., Gyamfi, I., Okafor, C., & Mva Mva, J. (2010). Cocoa seed multiplication: An assessment of seed gardens in Cameroon, Ghana and Nigeria (STCP Working Paper Series, Issue 11). Accra: Sustainable Tree Crops Program (STCP), International Institute of Tropical Agriculture.
Baligar, V. C., & Fageria, N. K. (2014). Nutrient use efficiency in plants: An overview. In A. Rakshit, H. B. Singh, & A. Sen (Eds.), Nutrient use efficiency: From basics to advances (pp. 1–14). New Delhi: Springer.
Barros, O. (1981). Avances en la represión de la moniliasis del cacao. In Proceedings of the Eighth International Cocoa Research Conference. Cargagena: Cocoa Producers’ Alliance.
Bartley, B. G. D. (1994). A review of cacao improvement: Fundamentals, methods and results. In M. J. End, A. B. Eskes, M. T. Lee, & G. Lockwood (Eds.), Proceedings of the International Workshop Cocoa Breeding Strategies (pp. 3–16). Kuala Lumpur: INGENIC. Available at http://www.incocoa.org/data/ingenic_workshop_1_proceedings_1994.pdf.
Bartley, B. G. D. (2005). The genetic diversity of Cacao and its utilization. Wallingford: CABI Publishing.
Bayer, C., & Kubitzki, K. (2003). Malvaceae. In K. Kubitzki (Ed.), The families and genera of vascular plants. Dicotyledons: Malvales, Capparales and non-betalain Caryophyllales (Vol. 5, pp. 225–311). Berlin: Springer.
Bergmann, J. F. (1969). The distribution of cacao cultivation in pre-Columbian America. Annals of the Association of American Geographers, 59, 85–96. doi:10.1111/j.1467-8306.1969.tb00659.x.
Besse, J. (1975). La selection generative du cacaoyer en Cote d’Ivoire: bilan et orientation des recherches en 1975. In Proceedings of the Fifth International Cocoa Research Conference. Ibadan: Cocoa Producers’ Alliance.
Chan, C. L., & Syed, K. S. W. (1976). Vascular-streak dieback of cocoa in Peninsula Malaysia. In Proceedings of the Cocoa Coconut Seminar (pp. 134–144). Tawau: East Malaysia Planters’ Association.
Chapman, R. K., & Soria, S. J. (1983). Comparative Forcipomyia (Diptera, Ceratopogonidae) pollination of cacao in Central America and Southern Mexico. Revista Theobroma (Brasil), 13, 129–139.
Cheesman, E. (1944). Notes on the nomenclature, classification and possible relationships of cocoa populations. Tropical Agriculture, 21, 145–146.
Clement, C. R. (1999). 1492 and the loss of Amazonian crop genetic resources. I. The relation between domestication and human population decline. Economic Botany, 53, 188–202.
Clement, C. R., de Cristo-Araújo, M., d’Eeckenbrugge, G. C., Pereira, A. A., & Picanço-Rodrigues, D. (2010). Origin and domestication of native Amazonian crops. Diversity, 2, 72–106. doi:10.3390/d2010072.
Coe, S. D., & Coe, M. D. (1996). The true history of chocolate. London: Thames and Hudson.
Cope, F. W. (1962). The mechanism of pollen incompatibility in Theobroma cacao L. Economic Botany, 17, 157–182.
Cuatrecasas, J. (1964). Cacao and its allies, a taxonomic revision of the genus Theobroma. In Contributions from the United States National Herbarium (Vol. 35, pp. 379–614). Washington, DC: Smithsonian Institution.
de Schawe, C. C., Durka, W., Tscharntke, T., Hensen, I., & Kessler, M. (2013). Gene flow and genetic diversity in cultivated and wild cacao (Theobroma cacao) in Bolivia. American Journal of Botany, 100, 2271–2279.
Dias, L. A. S. (2001a). Origin and distribution of Theobroma cacao L: A new scenario. In L. A. S. Dias (Ed.), Genetic improvement of cacao, Chap. 3 (EcoPort version by P. Griffee, FAO). Available at http://ecoport.org/ep?SearchType=earticleView&earticleId=197&page=-2#section2683
Dias, L. A. S. (2001b). The contributions of breeding. In L. A. S. Dias (Ed.), Genetic improvement of cacao, Chap. 12 (EcoPort version by P. Griffee, FAO). Available at http://ecoport.org/ep?SearchType=earticleView&earticleId=197&page=-2#section2737
Dias, L. A. S., & Resende, M. D. V. (2001). Selection strategies and methods. In L. A. S. Dias (Ed.), Genetic improvement of cacao, Chap. 6 (EcoPort version by P. Griffee, FAO). Available at http://ecoport.org/ep?SearchType=earticleView&earticleId=197&page=-2#section2701
Edwin, J., & Masters, W. A. (2005). Genetic improvement and cocoa yields in Ghana. Experimental Agriculture, 41, 491–503.
Efombagn, M. I. B., Motamayor, J. C., Sounigo, O., Eskes, A. B., Nyassé, S., Cilas, C., et al. (2008). Genetic diversity and structure of farm and genebank accessions of cacao (Theobroma cacao L.) in Cameroon revealed by microsatellite markers. Tree Genetics and Genomes, 4, 821–831.
Efombagn, M. I. B., Sounigo, O., Nyasse, S., Manzanares-Dauleux, M., Cilas, C. B., Eskes, A. B., et al. (2006). Genetic diversity in cocoa germplasm of southern Cameroon revealed by simple sequences repeat (SSRs) markers. African Journal of Biotechnology, 5, 1441–1449.
Eskes, A. B. (Ed). (2011). Collaborative and participatory approaches to cocoa variety improvement. Final report of the CFC/ICCO/Bioversity project on “Cocoa productivity and quality improvement: A participatory approach” (2004–2010). Amsterdam, London and Rome: CFC, ICCO, and Bioversity International. Available at http://www.bioversityinternational.org/e-library/publications/detail/collaborative-and-participatory-approaches-to-cocoa-variety-improvement/
Eskes, A. B., & Efron, Y. (Eds). (2006). Global approaches to cocoa germplasm utilization and conservation. Final report of the CFC/ICCO/IPGRI project on “Cocoa germplasm utilization and conservation: A global approach” (1998–2004). London and Rome: ICCO and IPGRI. Available at http://www.bioversityinternational.org/e-library/publications/
Ferry, R. J. (1989). The colonial elite of Caracas: Formation and crisis 1567-1767. Berkeley: University of California Press. Available at http://publishing.cdlib.org/ucpressebooks/view?docId=ft5r29n9wb&chunk.id=d0e167&toc.depth=1&toc.id=d0e167&brand=ucpress.
Firman, I. D., & Vernon, A. J. (1970). Cocoa canker caused by Phytophthora palmivora. Annals of Applied Biology, 65, 65–73.
Food and Agricultural Organization. (2014). FAOSTAT. Food and Agricultural Commodities Production. Available at http://faostat.fao.org/site/339/default.aspx
Goenaga, R. J., Guiltinan, M., Maximova, S., Seguine, E., & Irrizary, H. (2015). Yield performance and bean quality traits of cacao propagated by grafting and somatic embryo-derived cuttings. HortScience, 50, 358–362.
Gómez-Pompa, A., Flores, J. S., & Fernandez, M. A. (1990). The sacred cacao groves of the Maya. Latin American Antiquity, 1, 247–257. doi:10.2307/972163. Available at http://www.jstor.org/stable/972163?seq=1#page_scan_tab_contents.
Gonsalves, C. (1996). History of cocoa breeding in the Ministry of Agriculture, Trinidad. Cocoa Research Unit Newsletter, 3, 4–6.
Henderson, J. S., Joyce, R. A., Hall, G. R., Hurst, W. J., & McGovern, P. E. (2007). Chemical and archaeological evidence for the earliest cacao beverages. Proceedings of the National Academy of Sciences USA, 104, 18937–18940.
Historicus. (1896). Cocoa: All about it. London: Sampson Low Marston (Reproduction 2013, HardPress Publishing).
International Cocoa Organization. (2012). The World Cocoa Economy: Past and present. London: ICCO. Available at http://www.icco.org/about-us/international-cocoa-agreements/cat_view/30-related-documents/45-statistics-other-statistics.html.
International Cocoa Organization. (2013). Processing cocoa: Summary of the process of transforming cocoa beans into chocolate. Available at http://www.icco.org/about-cocoa/processing-cocoa.html
Ji, K., Zhang, D., Motilal, L. A., Boccara, M., Lachenaud, P., & Meinhardt, L. W. (2013). Genetic diversity and parentage in farmer varieties of cacao (Theobroma cacao) L. from Honduras and Nicaragua as revealed by single nucleotide polymorphism (SNP) markers. Genetic Resources and Crop Evolution, 60, 441–453.
Johnson, W. H. (1912). Cocoa: Its cultivation and preparation. London: John Murray. Available at http://ia902608.us.archive.org/1/items/cocoaitscultivat00johnrich/cocoaitscultivat00johnrich.pdf.
Johnson, E. S., Bekele, F. B., Brown, S. J., Song, Q., Zhang, D., Meinhardt, L. W., et al. (2009). Population structure and genetic diversity of the Trinitario cacao (Theobroma cacao L.) from Trinidad and Tobago. Crop Science, 49, 564–572.
Joseph, E. L. ([1838] 1970). History of Trinidad (Cass Library of West Indian Studies No. 13). London: Frank Cass and Co. Ltd (Reprinted).
Kimber, C. T. (1988). Martinique revisited. The changing plant geographies of a West Indian Island. College Station, TX: Texas A&M University Press.
Lachenaud, P., Mooleedhar, V., & Couturier, C. (1997). Les cacaoyers spontanés de Guyane: Nouvelles prospections (Wild cocoa trees in French Guiana. New Surveys). Plantations, Recherche, Développement, 4, 25–32.
Lachenaud, P., & Sallée, B. (1993). Les cacaoyers spontanés de Guyane. Localisation, écologie, morphologie. Café, Cacao, Thé (Paris), 37, 101–114.
Lachenaud, P., & Zhang, D. P. (2008). Genetic diversity and population structure in wild stands of cacao trees (Theobroma cacao L.) in French Guiana. Annals of Forest Science, 65, 310–317.
Lanaud, C., Fouet, O., Clément, D., Boccara, M., Risterucci, A. M., Surujdeo-Maharaj, S., et al. (2009). A meta-QTL analysis of disease resistance traits of Theobroma cacao L. Molecular Breeding, 24, 361–374.
Lanaud, C., Sounigo, O., Amefia, Y. K., Paulin, D., Lachenaud, P., & Clément, D. (1987). Nouvelles donnés sur le fonctionnement du système d’incompatibilitè du cacaoyer et ses conséquences pour la sélection. Café, Cacao, Thé (Paris), 31, 267–277.
Lass, R. A. (1985). Diseases. In G. A. R. Wood & R. A. Lass (Eds.), Cocoa (4th ed., pp. 265–365). Oxford: Blackwell Science.
Lockwood, G., & End, M. (1993). History, technique and future needs for cacao collection. In Proceedings of International Workshop on Conservation and Utilization of Cocoa Genetic Resources in the 21st Century, 1992 (pp. 1–14). Port of Spain: Cocoa Research Unit, The University of the West Indies.
Lockwood, G., & Pang, J. T. Y. (1996). Yields of cocoa clones in response to planting density in Malaysia. Experimental Agriculture, 32, 41–47.
Loor Solorzano, R. G., Fouet, O., Lemainque, A., Pavek, S., Boccara, M., Argout, X., Amores, F., Courtois, B., Risterucci, A. M., & Lanaud, C. (2012). Insight into the wild origin, migration and domestication history of the fine flavour Nacional Theobroma cacao L variety from Ecuador. PLoS One, 7, e48438. doi:10.1371/journalpone0048438.
Maharaj, K., Indalsingh, T., Ramnath, D., & Cumberbatch, A. (2005). High density planting of cacao: The Trinidad and Tobago experience. In F. Bekele, M. End, & A. B. Eskes (Eds.), Proceedings of the International Workshop on Cocoa Breeding for Improved Production Systems (pp. 171–182). Accra: INGENIC and the Ghana Cocoa Board.
Maximova, S. N., Young, A., Pishak, S., Miller, C., Traore, A., & Guiltinan, M. J. (2005). Integrated system for propagation of Theobroma cacao L. In S. M. Jain & P. K. Gupta (Eds.), Protocols for somatic embryogenesis in woody plants (pp. 209–229). Dordrecht: Springer.
Moriarty, K., Elchinger, M., Hill, G., Katz, J., & Barnett, J. (2014). Cacao intensification in Sulawesi: A green prosperity model project (NREL Work for Others Report: NREL/TP-5400-62434). Golden, CO: National Renewable Energy Laboratory. Available at http://www.nrel.gov/docs/fy14osti/62434.pdf.
Motamayor, J. C., Lachenaud, P., da Silva e Mota, J. W., Loor, R., Kuhn, D. N., Brown, J. S., et al. (2008). Geographic and genetic population differentiation of the Amazonian chocolate tree (Theobroma cacao L). PLoS One, 3, e3311. doi:10.1371/journal.pone.0003311.
Motilal, L., & Butler, D. (2003). Verification of identities in global cacao germplasm collections. Genetic Resources and Crop Evolution, 50, 799–807.
Motilal, L. A., & Sreenivasan, T. N. (2012). Revisiting 1727: Crop failure leads to the birth of Trinitario cacao. Journal of Crop Improvement, 26, 599–626.
Motilal, L., Zhang, D., Umaharan, P., Mischke, S., Mooleedhar, V., & Meinhardt, L. W. (2010). The relic Criollo cacao in Belize-genetic diversity and relationship with Trinitario and other cacao clones held in the International Cocoa Genebank, Trinidad. Plant Genetic Resources: Characterization and Utilization, 8, 106–115. doi:10.1017/S1479262109990232. Available at http://dx.doi.org/doi:10.1017/S1479262109990232.
Pang, J. T. Y. (2006). Yield efficiency in progeny trials with cocoa. Experimental Agriculture, 42, 289–299.
Pereira, J. L., Pizzigatti, R., & Mandarino, E. P. (1980). Levantamento fitosanitário das áreas afectadas pelo cancro de Phytophthora do cacaueiro nos estados da Bahia e Espirito Santo. Boletim Técnico, 72. Itabuna: CEPLAC. Available at http://www.ceplac.gov.br/paginas/publicacoes/paginas/boletim_tecnico/cartilhas/BOLETIM%20TÉC.%20N°%2072.pdf
Pokou, N. D., N’Goran, J. A. K., Lachenaud, P., Eskes, A. B., Motamayor, J. C., Schnell, R., et al. (2009). Recurrent selection of cocoa populations in Côte d’Ivoire: Comparative genetic diversity between the first and second cycles. Plant Breeding, 128, 514–520. doi:10.1111/j.1439-0523.2008.01582.x.
Posnette, A. F. (1986). Fifty years of cocoa research in Trinidad and Tobago. St. Augustine: Cocoa Research Unit, University of the West Indies.
Pound, F. J. (1932). The genetic constitution of the cacao crop. In F. Annual (Ed.), Report on cacao research (Vol. 193, pp. 10–24). Port of Spain: GPO.
Pound, F. J. (1934). The progress of selection. In Third Annual Report on Cacao Research, 1933 (Vol. 25–28). Port of Spain: Government Printing Office.
Pound, F. J. (1938). Cacao and Witches’ broom disease (Marasmius perniciosus) of South America with notes on other species of Theobroma. Port of Spain: Yuille’s Printery.
Pound, F. J. (1943). Cacao and Witches’ broom disease (Marasmius perniciosus). Port of Spain: Yuille’s Printery.
Pound, F. J. (1945). A note on the cacao population of South America. In Report and Proceedings of the Cocoa Research Conference held at Colonial Office, May-June 1945 (pp. 131–133). London: His Majesty’s Stationery Office.
Powis, T. G., Cyphers, A., Gaikwad, N. W., Grivetti, L., & Cheong, K. (2011). Cacao use and the San Lorenzo Olmec. Proceedings of the National Academy of Sciences USA, 108, 8595–8600.
Preuss, P. (1901). Der Kakao, seine Kultur und Aufbereitung [Cocoa, its cultivation and preparation]. In Expedition Nach Central- Und Südamerika (Chap. 13, pp. 167–277). Berlin: Colonial Economic Committee. Reprinted 1985: Archives of Cocoa Research 3, 15–115, Brussels: International Office of Cocoa and Chocolate.
Puentes–Páramo, Y. J., Menjivar–Flores, J. C., Gómez–Carabalí, A., & Aranzazu–Hernández, F. (2014). Absorción y distribución de nutrientes en clones de cacao y sus efectos en el rendimiento [Absorption and distribution of nutrients in cocoa and its effect on yield]. Acta Agronómica, 63, 145–152.
Quesnel, V. C. (1967). A short history of cacao and chocolate: 1. Historical. Journal of the Agricultural Society of Trinidad and Tobago, 67, 19–24.
Ratnam, R. (1961). Introduction of Criollo cacao into Madras state. South Indian Horticulture, 9, 24–29.
Rocha, H. M., & Ram, C. (1971). Cancro em cacaueiros na Bahia. Revista Theobroma (Brasil), 1, 44–47.
Ruf, F., & Schroth, G. (2004). Chocolate forests and monocultures: An historical review of cocoa growing and its conflicting role in tropical deforestation and forest conservation. In G. Schroth, G. A. B. Fonseca, C. A. Harvey, C. Gascon, H. L. Vasconcelos, & A. M. N. Izac (Eds.), Agroforestry and biodiversity conservation in tropical landscapes (pp. 107–134). Washington: Island Press.
Sánchez, P. A., Jaffé, K., & Muller, M. C. (1989). El género Theobroma en el Territorio Federal Amazonas (Venezuela). I. Notas etnobotánicas y consideraciones agronómicas. Turrialba, 39, 440–446. Available at http://atta.labb.usb.ve/Klaus/art48.pdf.
Sereno, M. L., Albuquerque, P. S. B., Vencovsky, R., & Figueira, A. (2006). Genetic diversity and natural population structure of cacao (Theobroma cacao L) from the Brazilian Amazon evaluated by microsatellite markers. Conservation Genetics, 7, 13–24.
Shephard, C. Y. (1932). The cacao industry of Trinidad: Some economic aspects. Part III–History of the industry up to 1870. Port of Spain: Government Printing Office.
Shephard, C. ([1831] 1971). An historical account of the Island of Saint Vincent. London: Frank Cass and Co. Ltd (Published 1971).
Silva, C. R. S., Albuquerque, P. B. S., Ervedosa, F. R., Mota, J. W. S., Figueira, A., & Sebbenn, A. M. (2011). Understanding the genetic diversity, spatial genetic structure and mating system at the hierarchical levels of fruits and individuals of a continuous Theobroma cacao population from the Brazilian Amazon. Heredity, 106, 973–985.
Silva, C. R. S., & Figueira, A. (2005). Phylogenetic analysis of Theobroma (Sterculiaceae) based on Kunitz-like trypsin inhibitor sequences. Plant Systematics and Evolution, 250, 93–104.
Silva, C. R. S., Figueira, A. V. O., & Souza, C. A. S. (2001). Diversity in the genus Theobroma. In L. A. S. Dias (Ed.), Genetic improvement of cacao, Chap. 2 (EcoPort version by P. Griffee, FAO). Available at http://ecoport.org/ep?SearchType=earticleView&earticleId=197&page=2679
Silva, C. R. S., Venturieri, G. A., & Figueira, A. (2004). Description of Amazonian Theobroma L. collections, species identification, and characterization of interspecific hybrids. Acta Botanica Brasilica, 18, 333–341.
Soberanis, W., Rios, R., Arevalo, E., Zuniga, L., Cabezas, O., & Krauss, U. (1999). Increased frequency of phytosanitary pod removal in cacao (Theobroma cacao) increases yield economically in eastern Peru. Crop Protection, 10, 677–685.
Southey, T. ([1827] 1968). Chronological history of the West Indies (Vol. II). London: Frank Cass and Co. Ltd (Reprinted).
Stander, J. R. (1993). Pre-breeding from the perspective of the private plant breeder. Journal of Sugar Beet Research, 30, 197–207.
Surujdeo-Maharaj, S., Umaharan, P., & Iwaro, A. D. (2001). A study of genotype-isolate interaction in cacao (Theobroma cacao L): Resistance of cacao genotypes to isolates of Phytophthora palmivora. Euphytica, 118, 295–303.
Susilo, A., Zhang, D., Motilal, L., & Meinhardt, L. W. (2011). Assessing genetic diversity in java fine-flavor cocoa (Theobroma cacao L.) germplasm by simple sequence repeat (SSR) markers. Tropical Agriculture, 55, 84–92.
Tahi, G. M., Kebe, B. I., Eskes, A. B., Ouattara, S., Sangare, A., & Mondeil, F. (2000). Rapid screening of cacao genotypes for field resistance to Phytophthora palmivora using leaves, twigs and roots. European Journal of Plant Pathology, 106, 87–94.
Thomas, E., van Zonneveld, M., Loo, J., Hodgkin, T., Galluzzi, G., & van Etten, J. (2012). Present spatial diversity patterns of Theobroma cacao L. in the neotropics reflect genetic differentiation in Pleistocene refugia followed by human-influenced dispersal. PLoS One, 7, e47676. doi:10.1371/journal.pone.0047676.
Thong, K. C., Ng, S. K., Ooi, H. S. H., & Leng, K. Y. (1992). Cocoa in Peninsular Malaysia I: The early history. Cocoa Growers’ Bulletin, 45, 7–25.
Toxopeus, H. (1964). F3 Amazon in Nigeria. In Annual Report of the Cocoa Research Institute of Nigeria, Ibadan, 1963/64, 13–23. Reprinted 1982: Archives of Cocoa Research, 1, 179–191.
Toxopeus, H. (1985). Botany, types and populations. In G. A. R. Wood & R. A. Lass (Eds.), Cocoa (4th ed., pp. 11–37). Oxford: Blackwell Science.
Toxopeus, H., & Kennedy, A. J. (1984). A review of the Cocoa Research Unit programme. In C. Research (Ed.), Unit report for 1981-1983 (pp. 8–12). St. Augustine: The University of the West Indies.
Van Hall, C. J. J. (1932). Cacao (2nd ed.). London: Macmillan and Co., Limited.
Woods, D. (2003). The tragedy of the cocoa pod: Rent-seeking, land and ethnic conflict in Ivory Coast. The Journal of Modern African Studies, 41, 641–655.
Wood, G. A. R. (1985a). History and development. In G. A. R. Wood & R. A. Lass (Eds.), Cocoa (4th ed., pp. 1–10). Oxford: Blackwell Science.
Wood, G. A. R. (1985b). Production. In G. A. R. Wood & R. A. Lass (Eds.), Cocoa (4th ed., pp. 543–586). Oxford: Blackwell Science.
Wood, G. A. R. (1991). A history of early cocoa introductions. Cocoa Growers’ Bulletin, 44, 7–12.
Wood, G. A. R., & Lass, R. A. (Eds.). (2001). Cocoa (4th ed.). Oxford: Blackwell Science.
Wood, G. A. R., & Lass, R. A. (Eds.). (2008). Cocoa (4th ed.). Hoboken, NJ: Wiley Online Library. doi:10.1002/9780470698983. Available at http://onlinelibrary.wiley.com/book/10.1002/9780470698983
World Cocoa Foundation. (2012). Cocoa market update. Available at http://worldcocoafoundation.org/wp-content/uploads/Cocoa-Market-Update-as-of-3.20.2012.pdf
Yang, J. Y., Scascitelli, M., Motilal, L. A., Sveinsson, S., Engels, J. M. M., Kane, N., et al. (2013). Complex origin of Trinitario-type Theobroma cacao (Malvaceae) from Trinidad and Tobago revealed using plastid genomics. Tree Genetics and Genomes, 9, 829–840. doi:10.1007/s11295-013-0601-4.
Young, A. M. (1994). The chocolate tree: A natural history of cacao. Washington, DC: Smithsonian Nature Books.
Zhang, D. P., Arevalo-Gardini, E., Mischke, S., Zúñiga-Cernades, L., Barreto-Chavez, A., & Adriazola del Aguila, J. (2006). Genetic diversity and structure of managed and semi-natural populations of cacao (Theobroma cacao) in the Huallaga and Ucayali valleys of Peru. Annals of Botany, 98, 647–655.
Zhang, D. P., Boccara, M., Motilal, L., Mischke, S., Johnson, E. S., Butler, D. R., et al. (2009). Molecular characterization of an earliest cacao (Theobroma cacao L) collection from Peruvian Amazon using microsatellite DNA markers. Tree Genetics and Genomes, 5, 595–607. doi:10.1007/s11295-009-0212-2.
Zhang, D. P., Figueira, A., Motilal, L., Lachenaud, P., & Meinhardt, L. W. (2011). Theobroma. In C. Kole (Ed.), Wild crop relatives: Genomic and breeding resources (pp. 277–296). Berlin: Springer.
Acknowledgments
We wish to thank Sue Mischke and Mary Strem, SPCL, Beltsville Agricultural Research Center, USDA/ARS, for their review and editing of the manuscript. Mention of a trademark or proprietary product does not constitute a guarantee or warranty of the product by the US Department of Agriculture and does not imply its approval to the exclusion of other products that may also be suitable. USDA is an equal opportunity provider and employer.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Zhang, D., Motilal, L. (2016). Origin, Dispersal, and Current Global Distribution of Cacao Genetic Diversity. In: Bailey, B., Meinhardt, L. (eds) Cacao Diseases. Springer, Cham. https://doi.org/10.1007/978-3-319-24789-2_1
Download citation
DOI: https://doi.org/10.1007/978-3-319-24789-2_1
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-24787-8
Online ISBN: 978-3-319-24789-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)