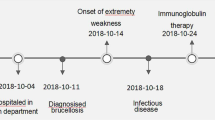
Abstract
Brucella polyradiculopathy is a rare manifestation of neurobrucellosis. Polyradiculopathy usually gradually progress and cause numbness and muscle weakness in extremities. Patients may present with pain, sensory abnormality, weakness of the extremities, and gate abnormality. Neurobrucellosis is diagnosed according to signs and symptoms, using laboratory tests including serology and imaging. Magnetic resonance imaging with gadolinium injection usually shows enhancement of lumbar nerve roots in Brucella polyradiculopathy. For confirmation of polyradiculopathy, nerve conduction studies and electromyography examination are used. Treatment of Brucella polyradiculopathy is not different from that of neurobrucellosis. The most common medications used for the treatment are rifampicin, trimethoprim-sulfamethoxazole, doxycycline, ceftriaxone, ciprofloxacin, and streptomycin. Clinical features of the patients guide the physician for duration of treatment. Prompt diagnosis without delay in treatment is associated with fewer sequelae and excellent prognosis.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Brucella infection rarely involves the central nervous system (CNS) with different clinical manifestations [2, 16, 21, 30]. Presentation may be with depression, encephalitis, meningitis, brain abscess, convulsion, cranial nerve palsy, subarachnoid hemorrhage, and decreased level of consciousness [19]. Brucellosis may affect the peripheral nervous system (PNS) with a variety of signs and symptoms [27]. Neurologic involvement due to brucellosis was reported in 1.7–10 % of the patients with brucellosis [8, 11, 25].
Complications of neurobrucellosis may be due to the acute-febrile phase of the diseases or may be due to the toxic-febrile phase, i.e., those related to primary involvement of CNS or PNS by the Brucella bacteria. Involvement of the CNS is commonly acute and presents as meningitis or encephalitis. Involvement of the PNS mainly present as polyradiculoneuritis [6]. Neurobrucellosis may affect any part of the CNS and PNS and present as any neurological disease [18]. Neurobrucellosis presentations are myelitis, myelopathy, paraplegia, radiculoneuritis, intracerebral abscess, epidural abscess, intradural abscess, demyelination, Guillain-Barré syndrome, polyneuritis, and cranial nerve palsy [7, 8, 10, 11, 16, 30]. Shehata et al. [26] demonstrated that CNS involvement was detected in 33 % of patients and PNS involvement was seen in 22 % of patients. In some cases, however, combined involvement of both CNS and PNS may occur simultaneously [4].
2 Clinical Manifestations
Clinical manifestations of neurobrucellosis are categorized as central and peripheral involvement. Peripheral involvements include neuropathy or radiculopathy. Central involvements include meningitis, cranial nerve palsy, and encephalitis [24].
Clinical manifestation of neurobrucellosis may be due to direct effect of microorganism on the nervous system or may be due to indirect effect with immune mechanisms that eventually lead to neuropathy. In animal model, anti-GM1 ganglioside antibodies are found to be produced in the response to ganglioside-like molecules of Brucella. These antibodies can cause flaccid weakness and ataxia-like symptoms.
Neurobrucellosis may present as meningoencephalitis, polyradiculoneuropathy, or both. The main presentation of the peripheral form is polyradiculoneuropathy and the main presentation of the central form is meningoencephalitis [17]. Brucella involvement of the spine has no specific manifestation. It may be due to an abscess or granuloma with compression effect on the spinal cord [24]. Polyradiculopathy is one of the rare clinical manifestations of brucellosis.
Brucellosis may affect the CNS and PNS via direct effects of microorganisms or indirect effect by toxin or cytokines. So the presentation may differ ranging from acute illness to chronic form [13]. Polyradiculopathy usually gradually progress and cause sensory and motor weakness in upper and lower limbs [20]. Patients may present with pain, sensory loss and weakness of the limbs, and difficulty of walking [13] but usually present with weakness of the lower extremities [5]. Spinal root involvement presents as lower motor neuron diseases [3]. Magnetic resonance imaging (MRI) is a useful imaging modality for the diagnosis of spinal root involvement. Spinal root involvement and subsequence polyradiculopathy are similar to other neurological diseases [17]. Radiculopathy is probably due to inflammation of the meninges and the intrathecal portion of the roots. The pathogenesis of myelopathy may involve demyelination as spasticity persists or worsens after radiculopathy improves [5]. Neurobrucellosis may present as Guillain-Barré-like syndrome with bilateral polyradiculopathy without sensory involvement [3]. Neurobrucellosis may be manifest as recurrent transverse myelitis. Immune system response and recurrent transverse myelitis due to neurobrucellosis has been reported. Krishnan et al. [22] reported a patient with four episodes of transverse myelitis that lead to quadriplegia. Brucella antibody was detected from his cerebrospinal fluid (CSF). High level of interleukin-6, IL-8, and macrophage chemoattractant protein-1 in CSF was detected. The patient responded to intravenous cyclophosphamide and plasma exchange [22]. Neurobrucellosis symptoms may last 8 weeks before diagnosis, ranging from 1 week to 4 months [6]. The recovery from neurobrucellosis may be accompanied by some sequelae. Paraparesis, dementia, sphincter dysfunction, and peripheral facial paralysis may occur [10].
3 Diagnosis
Brucellosis polyradiculopathy is often diagnosed by its neurological manifestations, laboratory tests, and imaging [9]. Neurobrucellosis is a treatable disease. Most of the diagnostic methods may reveal negative results. That is why high index of suspicion is necessary for the diagnosis of neurobrucellosis especially in endemic areas [1]. Clinical suspicion and accurate evaluation of patients with polyradiculoneuritis is the most important clue in diagnosis and treatment [14]. Early diagnosis is essential to prevent severe and permanent complications and sequelae of nerve roots [2, 8, 24]. Brucella tube agglutination with Coombs test is sensitive and specific. Enzyme-linked immunosorbent assay is considered superior to the agglutination tests [9]. Blood culture is not a useful laboratory test for diagnosis of Brucella polyradiculopathy. It takes a long time for results; moreover the results are usually negative [28]. In Erdem et al.’s study [12], blood culture was positive for brucellosis in 37 % by automated method and CSF culture was positive in 25 % and 9 % by automated and conventional CSF culture, respectively. Clinical and radiologic correlations are necessary. It may have a range from a normal imaging of a patient with severe clinical manifestation to imaging abnormality with or without significant clinical finding (Figs. 17.1 and 17.2) [4]. MRI of the spine may reveal swelling of the nerve root that may be enhanced by the use of gadolinium [20]. Diagnosis of Brucella polyradiculopathy or polyradiculoneuritis can be achieved by CSF analysis, laboratory tests including serology, electromyography (EMG) and nerve conduction velocity (NCV) examinations, and correlation with clinical manifestations [13]. MRI with gadolinium injection usually shows enhancement of the lumbar nerve root [5]. For confirmation of polyradiculopathy, NCV studies and EMG examination are used. Prolonged F waves, decreased NCVs and amplitude, and paraspinal muscle denervation potentials may be seen by NCV and EMG study [13].
A 35-year-old woman with numbness of lower extremity. (a) Sagittal T2-weighted MRI scan shows loss of low signal intensity of the cortical end plates and high signal in disk space and herniated disk. (b) T1-weighted MRI depicts loss of low signal intensity of the cortical end plates, destruction of the intervertebral disk space and end plates and decreased signal intensity in L1–L2 vertebral bodies. (c) Axial T1-weighted MRI shows end plates with decreased signal intensity and high signal intensity in paravertebral soft tissues
A 41-year-old woman with pain and weakness of lower extremity. (a) Sagittal T2-weighted MRI shows intervertebral disk with decreased signal intensity. (b) T1-weighted MRI reveals destruction of the intervertebral disk space and end plates and decreased signal intensity in L2–L3 vertebral bodies. (c) Axial T1-weighted MRI depicts end plates with decreased signal intensity
4 Treatment
As polyradiculoneuritis is one manifestation of neurobrucellosis, the treatment is the same as neurobrucellosis. Polyradiculoneuritis should be diagnosed and managed promptly. It may cause permanent and irreversible sequelae with diagnosis or treatment delay [29]. Treatment of polyradiculoneuritis may be a combination of doxycycline, rifampicin, trimethoprim/sulfamethoxazole, ciprofloxacin, ceftriaxone, and streptomycin. Duration of therapy varies in different studies, most of them recommend at least 3 months treatment [5, 23]. Duration of treatment ranging from several weeks to several months depends on patient’s condition; it may be shortened to 12 weeks if rapid improvement occurs. According to Gül et al.’s study [18], duration of therapy is 6 months with combination antibiotic therapy, in spite of the fact that the therapy should be individualized. In contrast in Asadipouya et al.’s study from Iran [6], duration of treatment was as short as 8 weeks in about half of the patients. Corticosteroids are not proven to be useful on localized brucellosis [13]. Moreover, rehabilitation should also be a part of the treatment in brucellosis polyradiculopathy [15].
Conclusion
Diagnosis of Brucella polyradiculoneuritis needs a high index of suspicion [9]. In patients with polyradiculoneuritis, brucellosis should be considered in the differential diagnosis especially in endemic areas. That may lead to early diagnosis and treatment [13], and in this situation the prognosis will be excellent. Some items will correlate closely with prognosis that includes duration of illness, Brucella spp. virulence, timing between diagnosis and starting antibiotic, and duration of antibiotic therapy [6].
Abbreviations
- CNS:
-
Central nervous system
- CSF:
-
Cerebrospinal fluid
- EMG:
-
Electromyography
- MRI:
-
Magnetic resonance imaging
- NCV:
-
Nerve conduction velocity
- PNS:
-
Peripheral nervous system
References
Adeva-Bartolomé MT, Montes-Martínez I, Castellanos-Pinedo F, Zurdo-Hernández JM, de Castro-García FJ (2005) Neurobrucellosis: four case reports. Rev Neurol 41:664–666
Ahmed R, Patil BS (2009) Neurobrucellosis: a rare cause for spastic paraparesis. Braz J Infect Dis 13:245
Alshareef AA (2009) Case report of polyradiclopathy, hearing loss, and ataxia as presentation of neurobrucellosis. JKAU Med Sci 16:85–92
Al-Sous MW, Bohlega S, Al-Kawi MZ, Alwatban J, McLean DR (2004) Neurobrucellosis: clinical and neuroimaging correlation. AJNR Am J Neuroradiol 25:395–401
Al-Sous MW, Bohlega SA, Al-Kawi MZ, McLean DR, Ghaus SN (2003) Polyradiculopathy. A rare complication of neurobrucellosis. Neurosciences (Riyadh) 8:46–49
Asadipooya K, Dehghanian A, Omrani GH, Abbasi F (2011) Short-course treatment in neurobrucellosis: a study in Iran. Neurol India 59:101–103
Babamahmoodi F, Babamahmoodi A (2011) Brucellosis, presenting with Guillain-Barré syndrome. J Glob Infect Dis 3:390–392
Baykal T, Baygutalp F, Senel K, Levent A, Erdal A, Ugur M, Ozgocmen S (2012) Spastic paraparesis and sensorineural hearing loss in a patient with neurobrucellosis. J Back Musculoskelet Rehabil 25:157–159
Budnik I, Fuchs I, Shelef I, Krymko H, Greenberg D (2012) Unusual presentations of pediatric neurobrucellosis. Am J Trop Med Hyg 86:258–260
Demiroğlu YZ, Turunç T, Karaca S, Arlıer Z, Alışkan H, Colakoğlu S, Arslan H (2011) Neurological involvement in brucellosis; clinical classification, treatment and results. Mikrobiyol Bul 45:401–410
Elzein FE, Mursi M (2014) Case report: Brucella induced Guillain-Barré syndrome. Am J Trop Med Hyg 91:1179–1180
Erdem H, Kilic S, Sener B, Acikel C, Alp E, Karahocagil M, Yetkin F, Inan A, Kecik Bosnak V, Gul HC, Tekin-Koruk S, Ceran N, Demirdal T, Yilmaz G, Ulu-Kilic A, Ceylan B, Dogan-Celik A, Nayman-Alpat S, Tekin R, Yalci A, Turhan V, Karaoglan I, Yilmaz H, Mete B, Batirel A, Ulcay A, Dayan S, Seza Inal A, Ahmed SS, Tufan ZK, Karakas A, Teker B, Namiduru M, Savasci U, Pappas G (2013) Diagnosis of chronic brucellar meningitis and meningoencephalitis: the results of the Istanbul-2 study. Clin Microbiol Infect 19:E80–E86
Ertem G, Kutlu G, Hatipoglu ÇA, Bulut C, Demirz AP (2012) A rare presentation of brucellosis: polyradiculopathy and peripheral neuritis. Turk J Med Sci 42:359–364
Faraji F, Didgar F, Talaie-Zanjani A, Mohammadbeigi A (2013) Uncontrolled seizures resulting from cerebral venous sinus thrombosis complicating neurobrucellosis. J Neurosci Rural Pract 4:313–316
Goktepe AS, Alaca R, Mohur H, Coskun U (2003) Neurobrucellosis and a demonstration of its involvement in spinal roots via magnetic resonance imaging. Spinal Cord 41:574–576
Guenifi W, Rais M, Gasmi A, Ouyahia A, Boukhrissa H, Mechakra S, Houari M, Nouasria B, Lacheheb A (2010) Neurobrucellosis: description of 5 cases in Setif Hospital Algeria. Med Trop 70:309–310
Gul HC, Erdem H, Bek S (2009) Overview of neurobrucellosis: a pooled analysis of 187 cases. Int J Infect Dis 13:e339–e343
Gul HC, Erdem H, Gorenek L, Ozdag MF, Kalpakci Y, Avci IY, Besirbellioglu BA, Eyigun CP (2008) Management of neurobrucellosis: an assessment of 11 cases. Intern Med 47:995–1001
Hadda V, Khilnani GC, Kedia S (2009) Brucellosis presenting as pyrexia of unknown origin in an international traveller: a case report. Cases J 2:7969
Ishibashi M, Kimura N, Takahashi Y, Kimura Y, Hazama Y, Kumamoto T (2011) A case of neurosarcoidosis with swelling and gadolinium enhancement of spinal nerve roots on magnetic resonance imaging. Rinsho Shinkeigaku 51:483–486
Kanik-Yǖksek S, Gülhan B, Ozkaya-Parlakay A, Tezer H (2014) A case of childhood brucellosis with neurological involvement and epididymo-orchitis. J Infect Dev Ctries 8:1636–1638
Krishnan C, Kaplin AI, Graber JS, Darman JS, Kerr DA (2005) Recurrent transverse myelitis following neurobrucellosis: immunologic features and beneficial response to immunosuppression. J Neurovirol 11:225–231
Koussa S, Tohmé A, Ghayad E, Nasnas R, El Kallab K, Chemaly R (2003) Neurobrucellosis: clinical features and therapeutic responses in 15 patients. Rev Neurol (Paris) 159:1148–1155
Nas K, Tasdemir N, Cakmak E, Kemaloglu MS, Bukte Y, Geyik MF (2007) Cervical intramedullary granuloma of Brucella: a case report and review of the literature. Eur Spine J 16(Suppl 3):S255–S259
Ozkavukcu E, Tuncay Z, Selçuk F, Erden I (2009) An unusual case of neurobrucellosis presenting with unilateral abducens nerve palsy: clinical and MRI findings. Diagn Interv Radiol 15:236–238
Shehata GA, Abdel-Baky L, Rashed H, Elamin H (2010) Neuropsychiatric evaluation of patients with brucellosis. J Neurovirol 16:48–55
Tajdini M, Akbarloo S, Hosseini SM, Parvizi B, Baghani S, Aghamollaii V, Tafakhori A (2014) From a simple chronic headache to neurobrucellosis: a case report. Med J Islam Repub Iran 28:12
Trifiletti RR, Restivo DA, Pavone P, Giuffrida S, Parano E (2000) Diabetes insipidus in neurobrucellosis. Clin Neurol Neurosurg 102:163–165
Ulu-Kilic A, Karakas A, Erdem H, Turker T, Inal AS, Ak O, Turan H, Kazak E, Inan A, Duygu F, Demiraslan H, Kader C, Sener A, Dayan S, Deveci O, Tekin R, Saltoglu N, Aydın M, Horasan ES, Gul HC, Ceylan B, Kadanalı A, Karabay O, Karagoz G, Kayabas U, Turhan V, Engin D, Gulsun S, Elaldı N, Alabay S (2014) Update on treatment options for spinal brucellosis. Clin Microbiol Infect 20:75–82
Vajramani GV, Nagmoti MB, Patil CS (2005) Neurobrucellosis presenting as an intramedullary spinal cord abscess. Ann Clin Microbiol Antimicrob 4:14
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Abbasi, F., Korooni, S., Turgut, A.T. (2016). Brucella Polyradiculoneuritis. In: Turgut, M., Haddad, F., de Divitiis, O. (eds) Neurobrucellosis. Springer, Cham. https://doi.org/10.1007/978-3-319-24639-0_17
Download citation
DOI: https://doi.org/10.1007/978-3-319-24639-0_17
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-24637-6
Online ISBN: 978-3-319-24639-0
eBook Packages: MedicineMedicine (R0)