Abstract
Arbuscular mycorrhizal fungi (AMF) are important soil organisms that assist the tropical plant species for establishment, survival, and growth. The plant response to AMF displays great variations among plant species belonging to different ecological groups of succession. Seedlings of the native heliophilous herbaceous display low growth response when inoculated with AMF in fertile soil, but the intensity of the AMF root colonization is very high, influencing differentially the survival, growth, and flowering. Seedlings of the woody species of the early stages of succession are highly responsive to inoculation and show a higher degree of AMF root colonization than seedlings of late stages of succession. In the same way, field soils from early-successional stages such as heliophilous herbaceous vegetation and young secondary forest display fine roots with higher AMF colonization, higher spore abundance, and higher inoculum potential than soils from late stages of succession. As a consequence, the AMF association emerges as an important tool among plant species of the early-successional phases toward initial tropical forest formation and structuring. In later stages of succession, the woody species display reduced use of the AMF and consequently soils of the mature forests present low AMF inoculum potential. In this study, the possible causes for decreasing the AMF association and soil inoculum potential during tropical succession and the importance of the AMF for plants involved in secondary forest formation are discussed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Carbon economy
- Inoculum potential
- Nutrient acquisition
- Plant functional groups
- Plant response to AMF
- Root colonization
- Soil fertility
- Spore density
- Successional continuum
- Symbioses
- Tropical forest
5.1 Introduction
During tropical succession, light-demanding pioneer and early-secondary tree species, which have very fast growth rates, replace the early-successional plant communities such as grasses, shrubs, and forbs. At later stages of succession, pioneer and early-secondary trees species, which are plants incapable of growing and reproducing under their own shadow (Saldarriaga et al. 1988), are replaced by late-secondary and climax tree species, which predominate in closed canopies and display intrinsic slow growth rates and tolerance to shading (Denslow and Guzman 2000; Guariguata and Ostertag 2001; Zangaro et al. 2003).
Many plant species belonging to different ecological groups of succession in tropical ecosystems rely on arbuscular mycorrhizal fungi (AMF ) for water and nutrients uptake. The AMF external hyphae increase the volume of soil that can be explored beyond the depletion zone formed around the absorbing roots (Smith and Read 2008), mainly in relation to P, and receive carbohydrates from the host plant. The low diameter of AMF hyphae can uptake P from small sites that cannot be accessed by root hairs (Jakobsen 1995). AMF make symbiosis with many plant species, including herbaceous and woody (Janos 1983; Sanders et al. 1996; Zangaro et al. 2003). This symbiosis plays an important role in plant nutrient acquisition, growth, and survival (Smith and Read 2008), besides increasing the photosynthetic rate and roots longevity (Linderman 1988; Comas et al. 2002). The AMF association is important for rehabilitation of degraded lands and is highly promising for inoculation of native woody species, especially in low-fertility soils (Perry et al. 1987; Zangaro et al. 2000). In this chapter, we will discuss differences in the relationships among distinct plant functional groups and AMF, as well as the implications of these relations for tropical ecological succession.
5.2 Differential Response of Tropical Herbaceous and Woody Species to AMF
AMF are an important biotic factor that influences differentially the establishment, survivor, growth, and reproduction of herbaceous and shrubby species of early phases of tropical succession. Rondina et al. (2014) assessed the effect of AMF on seedling development of 27 heliophilous herbaceous and shrubby tropical species grown in low- and high-fertility soils for 100 days. As shown in Fig. 5.1, most species grown in both soil types exhibited high AMF root colonization (80 %). In the low-fertility soil and non-AMF inoculation, the individuals of most plant species died between 50 and 70 days after the experiment installation. The individuals of the few plant species that survived in this condition grew little and displayed about 88 % less shoot dry mass than plants inoculated with AMF (Fig. 5.1). In the low-fertility soil, only six species flowered and flowering increased with AMF in one plant species and four species only flowered in the presence of AMF (Fig. 5.2). In the high-fertility soil, plant species non-inoculated with AMF exhibited seedlings about 13 % less shoot dry mass (Fig. 5.1), lower total leaf area, leaf area expansion, total root length, and nutrient concentrations in shoots than seedlings inoculated with AMF. Sixteen plant species flowered in the high-fertility soil, but 11 species displayed earlier flowering and 10 species exhibited more abundant flowering when grown with AMF (Fig. 5.2). Rondina et al. (2014) attributed the better flowering of mycorrhizal plants to the higher nutrient concentration in shoots (especially P and N) when compared with non-mycorrhizal plants. It possibly allowed more nutrients mobilized to the production of flowers. Furthermore, more nutrients in combination with the increase of total leaf area and leaf area expansion, also displayed by mycorrhizal plants, may enhance photosynthesis and C availability to flowering. The early and more abundant flowering exhibited by mycorrhizal plants may be very advantageous for these plants, which have short life span and are commonly highly prolific, in competition at the beginning of tropical succession.
Shoot biomass response to AMF (a) and mycorrhizal colonization (b) of herbaceous and shrubby plant species of early stages of tropical succession grown in low- and high-fertility soils. Vertical bars indicate the standard error (n = 5). Dashed lines represent the average of plants’ response to AMF and mycorrhizal infection intensity considering all species in the low-fertility soil (n = 26); dotted lines represent the same in the high-fertility soil (n = 27). Means followed by the same letter do not differ from each other by Student’s t test at P < 0.05. *Indicate significant differences in mycorrhizal infection intensity between soil fertilities within species by Student’s t test (*P < 0.05; **P < 0.01). Dagger represents death of the plants in the treatment. Species: AH = Amaranthus hybridus, AC = Asclepias curassavica, BC = Baccharis sp., HB = Hypochaeris brasiliensis, PR = Porophyllum ruderale, VC = Vernonia cognata, VP = Vernonia polyanthes, TS = Tecoma stans, MC = Momordica charantia, CN = Chamaecrista nictitans, CI = Crotalaria incana, IH = Indigofera hirsuta, MI = Mimosa invisa, SO = Senna obtusifolia, HS = Hyptis spicigera, LN = Leonotis nepetifolia, LS = Leonurus sibiricus, SM = Sidastrum micranthum, CE = Cenchrus echinatus, CH = Chloris elata, DI = Digitaria insularis, EP = Eragrostis pilosa, MM = Melinis minutiflora, MR = Melinis repens, PP = Pennisetum purpureum, SA = Sorghum arundinaceum, SV = Solanum viarum. Data from Rondina et al. (2014) with changes
Number of flowers per plant and days elapsed for appearance of the first flower buds after plant emergence (number in brackets) of herbaceous and shrubby plant species of early stages of tropical succession grown in low- and high-fertility soils, with or without AMF. Vertical bars indicate the standard error (n = 5). Means followed by the same lowercase (low-fertility soil) or uppercase (high-fertility soil) letters do not differ by Student’s t-test at P < 0.05. Species: Amaranthus hybridus (a), Hypochaeris brasiliensis (b), Porophyllum ruderale (c), Momordica charantia (d), Chamaecrista nictitans (e), Crotalaria incana (f), Indigofera hirsuta (g), Senna obtusifolia (h), Hyptis spicigera (i), Leonotis nepetifolia (j), Leonurus sibiricus (k), Cenchrus echinatus (l), Digitaria insularis (m), Eragrostis pilosa (n), Melinis repens (o), Sorghum arundinaceum (p). Data from Rondina et al. (2014)
Thus, AMF have different influences on the development of herbaceous and shrubby tropical species, depending on soil fertility : in low-fertility soil, AMF especially affect the survival, growth, and flowering. But in high-fertility soil, although mycorrhizal symbiosis did not provide large plant biomass accumulation, AMF still have a very important role in shoot nutrient concentrations and flowering. Some studies have found similar results for herbaceous species of other South America ecosystems. Urcelay et al. (2012) showed that in three typical Asteraceae species of Chaquean region (Bidens pilosa, Tagetes minuta, and Zinnia peruviana), mycorrhizal plants had lower shoot dry mass than non-mycorrhizal plants. Grilli et al. (2014) reported that the mycorrhizal colonization of Euphorbia acerensis and Euphorbia dentata can be high (80 %), and plants associated with AMF exhibited lower or not different dry mass than plants without AMF. However, the high mycorrhizal colonization influenced negatively the reproduction traits of these Euphorbiaceae species, like inflorescence and fruits production.
The seedling growth response to AMF of approximately 150 native woody species belonging to different ecological successional groups from Brazilian tropical forests, studied by Carneiro et al. (1996), Siqueira et al. (1998), Zangaro et al. (2000, 2002, 2003, 2005, 2007), Pouyú-Rojas and Siqueira (2000), Siqueira and Saggin-Júnior (2001), Zangaro and Andrade (2002), Matsumoto et al. (2005), Patreze and Cordeiro (2005), Pasqualini et al. (2007), and Vandresen et al. (2007), are shown in Fig. 5.3. Plant woody species were classified into different ecological successional groups such as pioneer, early-secondary, late-secondary, and climax. The response to AMF inoculation and the intensity of AMF root colonization of the native woody species decreased with the advance among successional ecological groups. Plant biomass response to AMF and AMF root colonization was very high among early-successional woody species, revealing the importance of arbuscular mycorrhizal association for the initial growth of this woody species, which are involved in the initial tropical forest structuring. In contrast, plant species belonging to late stages of succession that dominates in the mature forests showed low AMF root colonization and biomass response. These woody species display limited use of the AMF as tool for mineral acquisition during seedling stages.
Plant response to AMF inoculation (a) and AMF root colonization (b) of native woody species belonging to different successional stages. Means followed by same letter are not different by Tukey–Kramer HSD test at 0.05 level. Data from 93 plant species for response to inoculation and 121 plant species for root colonization. Data from Zangaro (2012)
Pioneer and early-secondary woody species when grown in the absence of AMF presented lower nutrient concentrations in leaves. These concentrations increased strongly when the same plant species was grown with arbuscular mycorrhizas (Zangaro et al. 2003), indicating that the pioneer and early-secondary species are not able to acquire nutrients from a low-fertility soil when AMF are not present. Pioneer and early-secondary species show inherent high growth rate and their high nutrient accumulation when grown with AMF, suggesting that the high demand for nutrients can be reached only in the presence of AMF association. For late-secondary and climax species there was no alteration in nutrient concentration and accumulation on leaves in the presence or absence of AMF, which probably led to the absence of plant biomass response to AMF in both high- and low-fertility soils (Zangaro et al. 2007).
5.3 Fine Root Traits and AMF Colonization Intensity for Nutrient Acquisition
It has been widely accepted from research in temperate regions that the plant root architecture controlled mycorrhizal benefit. Plant species that explore large soil volume display long fine roots, highly branched, with low diameter, covered with numerous root hairs, and are expected to exhibit low levels of AMF root colonization (Manjunath and Habte 1991; Schweiger et al. 1995; Brundrett 2002). On the other hand, plant species with coarse root systems and few root hairs tend to have high AMF colonization (Baylis 1975; Graham and Syvertsen 1985; Hetrick et al. 1992; Manjunath and Habte 1991; Reinhardt and Miller 1990; Schweiger et al. 1995). The results of Rondina et al. (2014) and Zangaro et al. (2005, 2007), for seedlings grown in greenhouse, and Zangaro et al. (2008, 2012a, b, 2013, 2014), for field results from tropical herbaceous and native woody species, do not support this hypothesis. The AMF root colonization and spore production among tropical native woody species belonging to different phases of succession display relations with the morphological root characteristics and plant metabolic demand. Generally, plant species belonging to early-successional stages with fine roots and abundant and long root hairs displayed high AMF colonization and sporulation (Table 5.1). By contrast, late-successional woody species with coarse roots and few root hairs displayed low AMF colonization and spore production (Zangaro et al. 2005, 2007).
In tropical soils, which generally have low P available, the early-successional woody species with apparent root morphology for high uptake capacity are not able to ensure adequate nutrition for maintaining their inherent fast growth rate, becoming arbuscular mycorrhizas essential for their nutrient acquisition. Zangaro et al. (2005, 2007, 2008, 2014) suggested that, in addition to high carbon allocation to fine root construction, early-successional species maintain more AMF because they present morphological root traits (high root length, high specific root length, low diameter, long and dense root hairs) with high interface, which favor the contact with mycorrhizal propagules in the soil. Indeed, the high nutrient accumulation exhibited for early-successional species expresses its high external demand for nutrients. Thus, fast-growing woody species display inherent intense metabolism that demands high amounts of nutrients to support the high growth rates and, therefore, maintain more AMF in root and soil to supply their nutritional needs. The relatively higher investment in leaves by the early-successional species increases the amount of photoassimilates that can be allocated to roots (Nielsen et al. 1998; Lynch and Ho 2005) and more carbohydrates may be provided to AMF in roots.
By contrast, plants from mature forest present less AMF colonization in fine roots in addition to less sporulation in soil. Several features such as shading, low growth rates, and metabolic demands may result in a reduced availability of carbohydrates to AMF in roots and consequently less mycorrhizal root colonization and sporulation in the mature forest (Zangaro et al. 2005, 2007, 2012a, 2014). Late-successional woody species display root morphology for low nutrient acquisition capacities (low root length, low specific root length, short and sparse root hairs) and are able to maintain their growth in the absence of AMF, even in deficient P soils (Zangaro et al. 2007). Therefore, the fine roots alone may be responsible for the nutrient acquisition among late-successional trees species. This indicates that slow-growing species may exhibit other strategies for nutrient acquisition instead of AMF association, as an additional enzymatic nutrient acquisition mechanisms (Chapin 1980), nutrient use efficiency (Manjunath and Habte 1991; Koide 1991), low requirement due to both low growth rate and metabolic demand (Zangaro et al. 2007), and high seed reserves for seedlings growth (Siqueira et al. 1998; Zangaro et al. 2000; Pasqualini et al. 2007).
5.4 Abundance of AMF in Different Stages of Succession
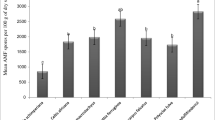
Mycorrhizal variables such as AMF root colonization and AMF spores density in soil were accessed over several years from sites covered with grasses, secondary forests, and mature forests in Atlantic, Araucaria, and Pantanal ecosystems in Brazil (Fig. 5.4). Mycorrhizal root colonization and mycorrhizal spore number over several months were also accessed from grassland, scrub, secondary forest, and mature forest of the Atlantic rainforest biome, located at Londrina municipality, Paraná state, Southern Brazil (Fig. 5.5). The AMF root colonization and the AMF spore density in soil decreased over succession, independently of studied biome. A positive correlation between spore numbers and mycorrhizal colonization was found, and these AMF variables can be considered as indicators of mycorrhizal incidence in soil (Picone 2000; Cardoso et al. 2003). The high and positive correlations found between AMF root colonization and AMF spore density may indicate the mycelial biomass of AMF in the soil, the AMF inoculum potential in the field, and the potential of plant species from different functional ecological groups to support AMF association. Mycorrhizal colonization and AMF spores in soils showed a high close correlation with parameters of fine root morphology (Table 5.1), but low correlation with root dry mass, suggesting that the morphological root characteristics are more important to the symbiosis than the fine root mass. These results toward plant investment in fine root morphology and AMF root colonization as an important way for plant nutrient acquisition.
Mycorrhizal root infection intensity and mycorrhizal spore number over several years from Araucaria ecosystem (a, b), Atlantic ecosystem (c, d), and Pantanal ecosystem (e, f) in Brazil. In Araucaria ecosystem Grass (grassland site), Sec 15 (15-year-old secondary forest), Sec 30 (30-year-old secondary forest), Sec 50 (50-year-old secondary forest), and Mature (mature forest). In Atlantic ecosystem Grass (grassland site), Scrub (5-year-old scrub vegetation), Secondary (20-year-old secondary forest), and Mature (mature forest). In Pantanal ecosystem Grass (grassland site), Secondary (15-year-old secondary forest), and Mature (mature forest). Error bars are + SE of the means (n = 15). Means followed by the same letter among successional sites are not different by Tukey’s test at 0.05 level. Data from Zangaro et al. (2012a)
Mycorrhizal root colonization (a) and mycorrhizal spore number (b) over several months from grassland, scrub, secondary forest, and mature forest of the Atlantic rainforest biome, located at Londrina municipality, Paraná state, Southern Brazil. Soils and fine root samples were assessed at 0–5 cm depth from October 2006 until November 2007. Error bars are ± 1 SE. Means followed by the same letter are not different by Tukey’s test at 0.05 level. Small letters compare means within a same successional site. Capital letters compare among successional sites (n = 13, P < 0.001 for AMF root colonization and n = 13, P < 0.001 for AMF spore number). Data from Zangaro et al. (2013)
The AMF root colonization and AMF spores density in soil exhibited strong reduction during succession progress in all areas studied. These results reflect the greater investment in arbuscular mycorrhizal symbiosis by host plants of the early-successional phases than native woody species of mature forest. The higher AMF fungi variables in early-successional stages than in mature forests comply with the higher AMF spores density found in pasture or natural grasses sites than in forests in Australia (Jasper et al. 1991), in addition to humid secondary forest (Fischer et al. 1994) and mature forest (Johnson and Wedin 1997) in Costa Rica, dry forest in Mexico (Allen et al. 1998), natural sites in Venezuela (Cuenca et al. 1998), lowland evergreen forests in Nicaragua and Costa Rica (Picone 2000), tropical forest in southern Brazil (Zangaro and Andrade 2002), and low-fertility soil at mature forest in Costa Rica (Lovelock et al. 2003). Besides, Zangaro et al. (2000) found low density of AMF spores and root colonization in plants from mature forest in southern Brazil and suggested that slow-growing species are less able to keep AMF due to growth in relative high soil fertility and shaded environments and exhibit low metabolic activity. Aidar et al. (2004) verified that AMF root colonization and AMF spores density decreased with increasing soil fertility in a chronosequence of an Atlantic forest in southeast Brazil. Powers et al. (2005) related that the amount of AMF hyphae in the soils of four tropical forests in Central and South America was unexpectedly quite low and suggested that plants of mature forests must rely on their fine roots instead of AMF for nutrient uptake. In an analysis in 15 published papers, Zangaro and Moreira (2010) verified that the amount of AMF spores in soils from mature forests and old secondary forests of the Brazilian Atlantic forest biome was lower than in recent secondary forests and open areas.
5.5 Inoculum of AMF Available in Soil
The soil inoculum potential of AMF decreased strongly over succession in the soils from the mature tropical forest, in a gap in the same mature forest, and in a recent secondary forest (Zangaro et al. 2000). The inoculum potential of AMF in the soil of the secondary forest was approximately five times greater than in the mature forest and in its gap. In another experiment (Zangaro et al. 2012a) the response to inoculation and the root colonization were accessed in seedlings of the woody mycotrophic species Heliocarpus popayanensis (Malvaceae) grown in 15 soils classes for 40 days and subsequently planted in infertile clay soil containing 1.66 mg P dm−3, 1.24 mg N dm−3, and 2.82 g C dm−3 (Fig. 5.6). Seedlings grown in soils from early stages of succession and secondary forests displayed higher AMF root colonization and biomass response to AMF than seedlings grown in mature forests soils. These results emphasize the high AMF inoculum potential in the soils from early stages of tropical succession and the young secondary forests and the low potential in mature forest conditions. Thus, the herbaceous plant from open environments and pioneer and early-secondary woody species may be able to multiply the AMF in large amounts (Zangaro et al. 2013, 2014), allowing high inoculum potential for their offsprings (Rondina et al. 2014). On the other hand, in the mature forest, the inoculum potential was very low, indicating that the late-secondary and climax species that compose the most part of the vegetation in a mature forest have weak mycotrophy and, as a consequence, the potential of AMF inoculum is low in mature forest conditions.
Shoot dry matter (a) and root colonization (b) of the tropical native woody Heliocarpus popayanensis grown in infertile soil. AM fungi inoculums are from five early-successional areas (E), five secondary forests (S), and five mature forests (M). Error bars represent ± 1 SE. Means followed by same letter are not different by Tukey test at 0.05 level
5.6 Implication of the AMF Inoculum Potential for Secondary Forest Formation
The high mycorrhizal colonization and plant biomass response to AMF among early-successional woody species grown in greenhouse, in addition to high AMF root colonization and AMF spore abundance in soils from sites covered with grasses and young secondary forests, reflect the host plant potential for multiplying the AMF among heliophilous herbaceous plants and fast-growing woody species. The intense host investment to maintain high AMF soil inoculum potential toward large density of AMF propagules production, which may be favorable for the plant installation and recruitment of fast-growing species. The early-successional woody species are highly responsive to AMF colonization, regardless of soil fertility level (Siqueira et al. 1998; Zangaro et al. 2007), and exhibit great aggressiveness during establishment in open and disturbed areas (Zangaro et al. 2003). Thus, AMF may be the main biotic factor for the establishment and growth acceleration of the native woody species that lead for initial tropical forest structuring (Zangaro et al. 2000). In the later stages of succession, the light limitation increases and early-successional woody species with typical fast growth rate and shade intolerance has difficulty to grow, reproduce, and maintain AMF association, attributed to low irradiance and its effect on fine roots carbohydrate availability to these fungi. The reduction of AMF in later stages of succession under light limitation is connected with concomitant decrease of the shade-intolerant host plant species. These declines have important implications for tropical forest succession, because the incapacity of the early-successional woody species to maintain AMF associations limits the acquisition of water and nutrient reducing their regeneration potential, recruitment, and competitive ability in later stages of succession (Zangaro et al. 2012a).
During succession progress, the fast growth and turnover of these shade-intolerant woody species provides a continuous soil organic enrichment and improves the soil structure (Uhl et al. 1982; Zangaro et al. 2003, 2009). The increase of soil surface fertility along the succession progress is attributable to the biomass accumulation and decomposition as the vegetation develops along the time (Silver et al. 1996; Guariguata and Ostertag 2001; Lugo and Helmer 2004; Boeger et al. 2005). Decomposition of soil organic matter produced by fast-growing woody species is important because of its critical role in the cycling of essential plant nutrients (Degens et al. 2000). The transformations above- and belowground during initial forest structuring allow the posterior establishment and growth of slow-growing woody species, which are dominant in mature forests. Therefore, the adult native woody species that dominate the late succession phases of the tropical forests maintain low amount of AMF due to low AMF association requirements. Plants in mature forests, under low light intensity, can be more limited by carbon than nutrient availability in soils, restricting the carbohydrate allocation to AMF and decreasing the root colonization, which suggests that the low levels of AMF in mature forests could be due to high carbon cost for maintaining the arbuscular mycorrhizal symbiosis in soils containing sufficient nutrient amounts (Zangaro 2012; Zangaro et al. 2014). Therefore, the low plant metabolic demand and the light availability appear to be important factors that determine AMF colonization and sporulation in mature forests. As large amount of plant photosynthetic products can be drained by AMF (Nielsen et al. 1998; Lynch and Ho 2005), the cost for maintaining high level of AMF can be significant for plant species in mature forests. Thus, the symbiotic limitations are an important means for plant energy conservation in mature forests (Zangaro et al. 2012b). Besides, lipid-rich spores and fungal hyphae are subject to predation and parasitism, since they serve as a food source for a wide range of soil animals (Rabatin and Stinner 1988; Stürmer et al. 2006). The soil organisms increase during succession (Coleman et al. 2004), and the competition with soil organisms and the hyphae and spores predation may be other important aspects that contribute to the decrease of the AMF in soils of mature forests.
References
Aidar MPM, Carrenho R, Joly CA (2004) Aspects of arbuscular mycorrhizal fungi in an Atlantic Forest chronosequence. Biota Neotropica 4:1–15
Allen EB, Rincon E, Allen MF, Perez-Jimenez A, Huante P (1998) Disturbance and seasonal dynamics of mycorrhizae in a tropical deciduous forest in Mexico. Biotropica 30:261–274
Baylis GTS (1975) The magnolioid mycorrhiza and mycotrophy in root systems derived from it. In: Sanders FE, Mosse B, Tinker PB (eds) Endomycorrhizas. Academic, New York, pp 373–389
Boeger MRT, Wisniewski C, Reissmann CB (2005) Nutrientes foliares de espécies arbóreas de três estádios sucessionais de floresta ombrófila densa no sul do Brasil. Acta Bot Bras 19:167–181
Brundrett MC (2002) Coevolution of roots and mycorrhizas of land plants. New Phytol 154:275–304
Cardoso IM, Boddington CL, Janssen BH, Oenema O, Kuyper TW (2003) Distribution of mycorrhizal fungal spores in soils under agroforestry and monocultural coffee systems in Brazil. Agrofor Syst 58:33–43
Carneiro MAC, Siqueira JO, Davide AC, Gomes LJ, Curi N, Vale FR (1996) Fungo micorrízico e superfosfato no crescimento de espécies arbóreas tropicais. Scientia Florestalis 50:21–36
Chapin FS (1980) The mineral nutrition of wild plants. Ann Rev Ecol Syst 11:233–260
Coleman DC, Crossley DA Jr, Hendrix PF (2004) Fundamentals of soil ecology. Elsevier Academic, San Diego
Comas LH, Bouma TJ, Eissenstat DM (2002) Linking root traits to potential growth rate in six temperate tree species. Oecologia 132:34–43
Cuenca G, Andrade Z, Escalante G (1998) Diversity of glomalean spores from natural, disturbed and revegetated communities growing on nutrient-poor tropical soils. Soil Biol Biochem 30:711–719
Degens BP, Schipper LA, Sparling GP, Vojvodic–Vukovic M (2000) Decreases in organic C reserves in soils can reduce the catabolic diversity of soil microbial communities. Soil Biol Biochem 32:189–196
Denslow JS, Guzman S (2000) Variation in stand structure, light, and seedling abundance across a tropical moist forest chronosequence, Panama. J Veg Sci 11:201–212
Fischer CR, Janos DP, Perry DA, Linderman RG (1994) Mycorrhiza inoculum potentials in tropical secondary succession. Biotropica 26:369–377
Graham JH, Syvertsen JP (1985) Host determinants of mycorrhizal dependency of citrus rootstock seedlings. New Phytol 101:667–676
Grilli G, Urcelay C, Longo MS, Galetto L (2014) Mycorrhizal fungi affect plant growth: experimental evidence comparing native and invasive hosts in the context of forest fragmentation. Plant Ecol 215:1513–1525
Guariguata MR, Ostertag R (2001) Neotropical secondary forest succession: changes in structural and functional characteristics. For Ecol Manage 148:185–206
Hetrick BAD, Wilson GWT, Todd TC (1992) Relationships of mycorrhizal symbiosis, root strategy, and phenology among tallgrass prairie forbs. Can J Bot 70:1521–1528
Jakobsen I (1995) Transport of phosphorus and carbon in VA Mycorrhizas. In: Varma A, Hock B (eds) Mycorrhiza: structure, function, molecular biology and biotechnology. Springer, Berlin, pp 297–324
Janos DP (1983) Tropical mycorrhizas, nutrient cycles and plant growth. In: Sutton SL, Whitmore TC, Chadwick AC (eds) Tropical rain forest: ecology and management. Blackwell Scientific Publication, Oxford, pp 327–345
Jasper DA, Abbott LK, Robson AD (1991) The effect of soil disturbance on vesicular-arbuscular mycorrhizal fungi in soils from different vegetation types. New Phytol 118:471–476
Johnson NC, Wedin DA (1997) Soil carbon, nutrients, and mycorrhizae during conversion of dry tropical forest to grassland. Ecol Appl 7:171–182
Koide RT (1991) Nutrient supply, nutrient demand and plant response to mycorrhizal infection. New Phytol 117:365–386
Linderman RG (1988) VA (vesicular-arbuscular) mycorrhizal simbiosis. Atlas Sci Anim Plant Sci 1:183–188
Lovelock CE, Andersen K, Morton JB (2003) Influence of host tree species and environmental variables on arbuscular mycorrhizal communities in tropical forests. Oecologia 135:268–279
Lugo AE, Helmer E (2004) Emerging forest on abandoned land: Puerto Rico’s new forests. For Ecol Manage 190:145–161
Lynch JP, Ho MD (2005) Rhizoeconomics: carbon costs of phosphorus acquisition. Plant Soil 269:45–56
Manjunath A, Habte M (1991) Root morphological characteristics of host species having distinct mycorrhizal dependency. Can J Bot 69:671–676
Matsumoto LS, Martines AM, Avanzi MA, Albino UB, Brasil CB, Saridakis DP, Rampazo LGL, Zangaro W, Andrade G (2005) Interactions among functional groups in the cycling of, carbon, nitrogen and phosphorus in the rhizosphere of three successional species of tropical woody trees. Appl Soil Ecol 28:57–65
Nielsen KL, Bouma TJ, Lynch JP, Eissenstat DM (1998) Effects of phosphorus availability and vesicular-arbuscular mycorrhizas on the carbon budget of common bean (Phaseolus vulgaris). New Phytol 139:647–656
Pasqualini D, Uhlmann A, Stürmer SL (2007) Arbuscular mycorrhizal fungal communities influence growth and phosphorus concentration of woody plants species from the Atlantic rain forest in South Brazil. For Ecol Manage 245:148–155
Patreze CM, Cordeiro L (2005) Nodulation, arbuscular mycorrhizal colonization and growth of some legumes native from Brazil. Acta Bot Bras 19:527–537
Perry DL, Molina R, Amaranthus MP (1987) Mycorrhizae, mycorrhizospheres and reforestation: current knowledge and research needs. Can J Forest Res 17:929–940
Picone C (2000) Diversity and abundance of arbuscular-mycorrhizal fungus spores in tropical forest and pasture. Biotropica 32:734–750
Pouyú-Rojas E, Siqueira JO (2000) Micorriza arbuscular e fertilização do solo no desenvolvimento pós-transplante de mudas de sete espécies florestais. Pesq Agrop Brasileira 35:103–114
Powers JS, Treseder KK, Lerdau MT (2005) Fine roots, arbuscular mycorrhizal hyphae and soil nutrients in four neotropical rain forests: patterns across large geographic distance. New Phytol 165:913–921
Rabatin SC, Stinner BR (1988) Indirect effects of interactions between VAM fungi and soil-inhabiting invertebrates on plant processes. Agric Ecosyst Environ 24:135–146
Reinhardt DR, Miller RM (1990) Size classes of root diameter and mycorrhizal fungal colonization in two temperate grassland communities. New Phytol 116:129–136
Rondina ABL, Lescano LEAM, Alves RA, Matsuura EM, Nogueira MA, Zangaro W (2014) Arbuscular mycorrhizas increase survival, precocity and flowering of herbaceous and shrubby species of early stages of tropical succession in pot cultivation. J Trop Ecol 30:599–614
Saldarriaga JG, West DC, Tharp ML, Uhl C (1988) Long-term chronosequence of forest succession in the upper Rio Negro of Colombia and Venezuela. J Ecol 76:938–958
Sanders IR, Clapp JP, Wiemken A (1996) The genetic diversity of arbuscular mycorrhizal fungi in natural ecosystems—a key to understanding the ecology and functioning of the mycorrhizal symbiosis. New Phytol 133:123–134
Schweiger PF, Robson AD, Barrow N (1995) Root hair length determines beneficial effect of a Glomus species on shoot growth of some pasture species. New Phytol 131:247–254
Silver WL, Scatena FN, Johnson AH, Siccama TG, Watt F (1996) At what temporal scales does disturbance affect below-ground nutrient pools? Biotropica 28:441–457
Siqueira JO, Saggin-Júnior OJ (2001) Dependency on arbuscular mycorrhizal fungi and responsiveness of some Brazilian native woody species. Mycorrhiza 11:245–255
Siqueira JO, Carneiro MAC, Curi N, Rosado SCS, Davide AC (1998) Mycorrhizal colonization and mycotrophic growth of native woody species as related to successional groups in southeastern Brazil. For Ecol Manage 107:241–252
Smith SE, Read DJ (2008) Mycorrhizal symbiosis, 3rd edn. Academic, London
Stürmer SL, Klauberg Filho O, Queiroz MH, Mendonça MM (2006) Occurrence of arbuscular mycorrhizal fungi in soils of early stages of a secondary succession of Atlantic Forest in South Brazil. Acta Bot Bras 20:513–521
Uhl C, Jordan C, Clark K, Herrera R (1982) Ecosystem recovery in Amazon caatinga forest after cutting and burning, and bulldozer clearing treatment. Oikos 38:313–320
Urcelay C, Tecco PA, Pérez M, Grilli G, Longo MS, Battistella R (2012) Mycorrhizal status and responsiveness of early successional communities from Chaquean Region in Central Argentina. In: Pagano M (ed) Mycorrhiza: occurrence in Natural and Restored Environments. Nova, New York, pp 147–163
Vandresen J, Nishidate FR, Torezan JMD, Zangaro W (2007) Inoculação de fungos micorrízicos arbusculares e adubação na formação e pós-transplante de mudas de cinco espécies arbóreas nativas do sul do Brasil. Acta Bot Bras 21:753–765
Zangaro W (2012) Arbuscular mycorrhizas and their importance for tropical forest formation in Brazil. In: Pagano M (ed) Mycorrhiza: occurrence in natural and restored environments. Nova, New York, pp 40–73
Zangaro W, Andrade G (2002) Micorrizas arbusculares em espécies arbóreas nativas da bacia do rio Tibagi. In: Medri ME, Bianchini E, Pimenta JA, Shibata O (eds) A bacia do rio Tibagi. Edição dos editores, Londrina, pp 171–210
Zangaro W, Moreira M (2010) Micorrizas arbusculares nos biomas floresta atlântica e floresta de araucária. In: Siqueira JO, Souza FA, Cardoso EJBN, Tsai SM (eds) Micorrizas: trinta anos de pesquisa no Brasil. Editora UFLA, Brasília, pp 279–310
Zangaro W, Bononi VLR, Trufen SB (2000) Mycorrhizal dependency, inoculum potential and habitat preference of native woody species in South Brazil. J Trop Ecol 16:603–622
Zangaro W, Nisizaki SMA, Domingos JCB, Nakano EM (2002) Micorrizas arbusculares em espécies arbóreas da bacia do rio Tibagi, Paraná. Cerne 8:77–87
Zangaro W, Nisizaki SMA, Domingos JCB, Nakano EM (2003) Mycorrhizal response and sucessional status in 80 woody species from south Brazil. J Trop Ecol 19:315–324
Zangaro W, Nishidate FR, Camargo FRS, Romagnoli GG, Vandresen J (2005) Relationships among arbuscular mycorrhizas, root morphology and seedling growth of tropical native woody species in southern Brazil. J Trop Ecol 21:529–540
Zangaro W, Nishidate FR, Vandresen J, Andrade G, Nogueira MA (2007) Root mycorrhizal colonization and plant responsiveness are related to root plasticity, soil fertility and successional status of native woody species in southern Brazil. J Trop Ecol 23:53–62
Zangaro W, Assis RL, Motta AM, Rostirola LV, Souza PB, Gonçalves MC, Andrade G, Nogueira MA (2008) Arbuscular mycorrhizal association and fine root traits changes during succession in southern Brazil. Mycorrhiza 19:37–45
Zangaro W, Nogueira MA, Andrade G (2009) Arbuscular mycorrhizal fungi used as biofertilizers in revegetation programmes. In: Rai M (ed) Advances in fungal biotechnology. I.K. International Publishing House, New Delhi, pp 351–378
Zangaro W, Ansanelo AP, Lescano LEAM, Alves RA, Rondina ABL, Nogueira MA (2012a) Infection intensity, spore density and inoculum potential of arbuscular mycorrhizal fungi decrease during secondary succession in tropical Brazilian ecosystems. J Trop Ecol 28:453–462
Zangaro W, Alves RA, Lescano LEAM, Ansanelo AP, Nogueira MA (2012b) Investment in fine roots and arbuscular mycorrhizal fungi decrease during succession in three Brazilian ecosystems. Biotropica 44:141–150
Zangaro W, Rostirola LV, Souza PB, Alves RA, Lescano LEAM, Rondina ABL, Nogueira MA, Carrenho R (2013) Root colonization and spore abundance of arbuscular mycorrhizal fungi in distinct successional stages from an Atlantic rainforest biome in southern Brazil. Mycorrhiza 23:221–233
Zangaro W, Alves RA, Souza PB, Rostirola LV, Lescano LEAM, Rondina ABL, Nogueira MA (2014) Succession and environmental variation influence soil exploration potential by fine roots and mycorrhizal fungi in an Atlantic ecosystem in southern Brazil. J Trop Ecol 30:237–248
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Zangaro, W., Rondina, A.B.L. (2016). Arbuscular Mycorrhizas in Different Successional Stages in Some Brazilian Ecosystems. In: Pagano, M. (eds) Recent Advances on Mycorrhizal Fungi. Fungal Biology. Springer, Cham. https://doi.org/10.1007/978-3-319-24355-9_5
Download citation
DOI: https://doi.org/10.1007/978-3-319-24355-9_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-24353-5
Online ISBN: 978-3-319-24355-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)