Abstract
This chapter reviews the use of plant growth regulator for fresh vegetable and fruit quality. Endogenous plant growth regulators, also named phytohormones, are important regulator of many functions in plant development and physiology. First, this chapter describes the five major classes of PGR (auxins, gibberellins, cytokinins, abscisic acid, and ethylene) including their nature, physiological functions, and horticultural practices. Some synthetic PGRs are used extensively to control sensorial and nutritional quality and ripening or senescence processes. So we focus this chapter on the effects of exogenously PGR applied in pre- and postharvest to maintain the quality of fresh produce.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
Plant growth and development are regulated not only by external factors, as light and temperature, but mainly by internal factors, as its genetic factors and endogenous “phytohormones” also named plant growth regulators (PGRs). The PGRs are defined as organic compounds, naturals, or synthetics, controlling (stimulation or inhibition) plant growth and development functions, and this at very low concentration (nM–pM). In particular, PGRs are involved in the regulation of cell division, cell enlargement, and cell differentiation. Like that, they control organogenesis, senescence, and dormancy from germination stage to fructification. They also play a major role in regulating physiological functions as stomata aperture control, sugar metabolism, and plant stress responses.
The most important natural PGRs belong to one of the five major hormone classes : auxins, gibberellins (GAs), cytokinins (CKs), ethylene (C2H4), and abscisic acid (ABA). More recently, other PGRs have been discovered: brassinolide group (BRs), salicylates (SA), jasmonate (JA), and derivatives and polyamines (PAs). Some authors also consider that nitric oxide (NO) is a phytohormone that is also used in postharvest treatment (Lichanporn and Techavuthiporn 2013; Manjunatha et al. 2010).
In plants, PGRs have multiple functions depending on their concentration or their localization and often act in synergy or antagonism with another PGRs. In addition, any given PGR may affect the biosynthesis of another. So, the balance between endogenous hormones has important consequences on physiological responses in fruit maturation and ripening (McAtee et al. 2013). As this equilibrium could be modified by exogenous applications of PGRs, one can change plant physiology and the developmental program to obtain positive effects on both cultural and postharvest behavior. Nowadays, numerous PGRs are produce synthetically and are used in agriculture to optimize the production and quality of plants, fruits, and vegetables. The application of PGR can provide important economic advantages to fruit farmers in stimulating specific responses such as increase in fruit size and delay or enhance maturity or senescence of orchard and vegetables. Sometimes, preharvest application may have beneficial carryover effects on postharvest quality. Finally, PGR could also be applied after harvest to improve storage, and induce or delay ripening and control quality of fresh produces, of course if its effects are not harmful to the consumer.
The objective of this chapter is to give an overview of properties of PGRs and some recent developments in plant growth regulator efficiencies to improve and maintain fresh product quality.
The Five Major Classes of Plant Growth Regulators
Auxins
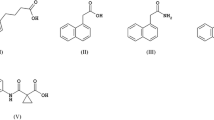
Auxin is the first identified plant hormone and whose effects on cell elongation and division have been shown by Frits Went in 1926. In plants, the principal auxin is the indole-3-acetic acid (IAA) synthetized by tryptophan-dependent or tryptophan-independent pathways in meristems and developing organs (Mano and Nemoto 2012; Korasick et al. 2013; Lehmann et al. 2010; Tivendale et al. 2014). Natural auxins, such as phenylacetic acid (PAA), 4-chloro-3-acetic acid (4-Cl-IAA), and indole-3-butyric acid (IBA), also regulate cell division, cell growth, ethylene biosynthesis, root development, leaf formation, stem elongation, apical dominance, and differentiation of vascular tissues and fruit setting (Woodward and Bartel 2005; Simon and Petrášek 2011). However, depending on auxin levels the response to auxin application could be opposed. The structures of most important natural and synthetic auxins are shown in Fig. 11.1.
Synthetic auxins such as α-naphthaleneacetic acid (NAA), and 2,4-dichlorophenoxyacetic acid, better known under the name of 2,4-D, induce similar physiological responses as natural auxins. Due to their properties on organogenesis, NAA and IBA are routinely used to stimulate root initiation and differentiation for the vegetative propagation of plants from stem and leaf cutting. It must not be forgotten that, usually, the synthetic auxins, such as 2,4-D, Picloram®, and Dicamba®, are mainly used as selective herbicide. In fact, used in high concentration, auxin stimulates the production of ethylene that inhibits elongation growth, causes leaf abscission, and finally destroys the plant.
Synthetic auxins were widely used in arboriculture and horticulture for:
-
Induction of parthenocarpic fruits in tomato and grapes to obtain seedless fruits appreciated by consumer.
-
Inhibition of preharvest fruit drop: application of NAA 10–50 ppm in mango, citrus, and chilies reduces fruit drop by preventing formation of abscission layer.
-
Induction of flower and fruit thinning to avoid the biennial bearing in tree fruit and produce higher fruit size (Stern et al. 2007a, b; Schröder et al. 2013).
-
Promotion of fruit growth and advance maturity in loquat and Satsuma mandarin fruits (Amorós et al. 2004; Ortolá et al. 1991).
Postharvest treatments with synthetic auxins have been also investigated. Auxin application is well documented in citrus fruits. Postharvest application of 2,4-D has been used to retard calyx abscission, and water loss, which occurs as the consequence of degreening induced by ethylene (Salvador et al. 2010; (Sdiri et al. 2013; Sdiri et al. 2012)). Auxins have also been used to affect biotic stresses of harvested fruits and vegetables. In mango, application of 2,4-D in wax combined with hot water brushing treatment improved quality during storage by limiting side rots caused by Alternaria alternata (Kobiler et al. 2001). In addition, Wang et al. (2008) have shown that 2,4-D reduced chilling injury in mango through an increase of ABA and GA levels and an augmentation of antioxidant defenses. In Chilean strawberry, NAA postharvest treatment delays fruit ripening without effect on firmness (Figueroa et al. 2012). The methyl ester of NAA is used to prevent the sprouting of potato tubers and hence increases storage life (Suttle 2003).
In recent years, European Union legislation has restricted the use of 2,4-D even as preharvest application. The level of 2,2-D residue should be below 0.5 ppm on food. Thus, it is absolutely necessary to test safely and environmental friendly new auxins in order to maintain the quality of fresh produce.
Gibberellins
Gibberellins (GAs) are the second most important family of PGR. GAs belong to the group of tetracyclic diterpenoid acids (Fig. 11.2). The ent-gibberelane skeleton (ent-kaurene) of GAs is synthesized from isopentenyl diphosphate (IPP) via the mevalonate or the methyl erythro 4-phosphate (MEP) pathways to give GA12 and GA53. The conversion between the different forms of GAs is provided by gibberellin oxidases. Many advances have been made in understanding the regulation of GA biosynthesis, transport, and signaling (see review of Thomas et al. 2005). Today, over 136 GAs have been identified in plants and fungi. As other hormones, gibberellins play many roles in plant development processes. GAs are implicated in cell elongation and division, stem elongation, breaking dormancy, and the vegetative phase to flowering transition. The first GA identified is the gibberellic acid (GA3), found in Gibberella fujikuroï that is responsible of bakanae disease whose symptoms are chlorotic and abnormally elongated leaves. Consequently, GA3 is the most described in literature; however, the bioactive forms of GAs are GA1 and GA4 for stem elongation. The predominant forms of GAs applied are GA3, GA4, GA7, or a mixture of GA4 and GA7.
Synthetic GAs were widely used in arboriculture and horticulture with consequence for postharvest quality (Serrano et al. 2004):
-
In table grapes, GA3 increases fruit size and reduce rachis browning and extension of shelf-life (Raban et al. 2013).
-
In citrus, pineapple, cherries, and peach, GAs are known to maintain and/or increase color and firmness.
-
In cherry, GA3 delays fruit ripening (Zhang and Whiting 2011) and extend crown life of pineapple.
-
GA can delay storage disorders such as internal browning (Lurie and Crisosto 2005).
-
Preharvest GA treatment can control disease development in persimmon fruit during its storage (Biton et al. 2014). Nevertheless, GA3 application in seedless table grapes could predispose to gray mold caused by botrytis cinerea (Zoffoli et al. 2009).
-
In leafy vegetable, GA3 treatment during production delays the senescence of product and maintains chlorophyll content during storage (Lers et al. 1998).
Gibberellins are an excellent PGR for postharvest application. Indeed, some GA as GA3 and GA4 are natural hormones and by consequence this forms could be used with any restriction in most part of world. Post harvest applications of GA allow the maintenance of firmness and the enhancement of storage of fruits and vegetables. In order to extend the green life of bananas after the harvest, some growers apply gibberellic acid directly to the bananas’ hands. Subsequently, GA3 treatment also maintains fruit quality longer, ensuring that the bananas will get to their destination in optimum condition (Vargas and Lopez 2011). Postharvest GA3 treatment also induces antioxidant defenses and so reduces chilling injury during storage in tomato (Ding et al. 2015) and delays ripening and maintains green peel color longer in orange (Gambetta et al. 2014) and in papaya (Ramakrishna et al. 2002) and with higher level of ascorbic acid in mango (Khader et al. 1988; Khader 1991).
Cytokinins
Cytokinins (CKs) are PGRs that induce cell division, delay senescence, and regulate pathogen resistance. Cytokinins are N6-adenine derivates discovered for the first time in coconut (Cocos nucifera) milk by Johannes van Overbeek in 1941 (for review, Sakakibara 2006). Zeatin (cis and trans isomers) is the most common natural CK. Dihydrozeatin, isopentenyl adenine (iPA), and zeatin riboside naturally occur in plants (Fig. 11.3). CK main application comes from their ability to stimulate the growth and formation of new roots and buds in in vitro cultures and therefore control the regeneration and the growth for the plant micropropagation purpose. For these applications, synthetic cytokinin analogs have been developed from purines (6-benzyladenine (6-BA), tetra hydro pyrane benzyladenine (PBA), and phenylurea (Forchlorfenuron (CPPU)). These analogs could also be used in arboriculture for thinning in apples and pears and so, adjusts crop load, resulting in the best crop yield and guaranteeing return bloom (Flaishman et al. 2001; Hayata et al. 2000). In fruits, synthetic CK acts synergistically with natural auxins to stimulate cell division, resulting in increased fruit size at harvest in cherry (Zhang and Whiting 2011). Moreover, mixture of 6-BA and GA applied during culture improved persimmon quality during storage and enhances tolerance to Alternaria black spot (Biton et al. 2014). In the same way, CPPU applied during fruit development improves cold storage in grapes and kiwifruits (Kim et al. 2006; Marzouk and Kassem 2011). However, no effect of CPPU, applied alone, has been observed by Raban et al. (2013).
Due to its inhibition of senescence, harvest treatment with 6-BA improves appearance of green asparagus spears, rocket, broccoli, and cucumber and positively affect firmness and chlorophyll and ascorbic acid content (An et al. 2006; Koukounaras et al. 2010; Costa et al. 2005; Chen and Yang 2013). Postharvest CK treatment has also effects on fruit texture. So, summer squash sprayed with 6-BA reduces pectin solubilization and later prevents texture deterioration during cold storage. The CPPU is also used combined with GA treatment and the mixture can also delay ripening and increase fungal tolerance in banana and broccoli (Huang and Jiang 2012).
Abscisic Acid
Abscisic acid (ABA) is a 15-carbon sesquiterpene synthetized from carotenoids in chloroplasts and other plastids. The 9-cis-epoxycarotenoid dioxygenase (NCED) is the key enzyme for ABA biosynthesis (Seo and Koshiba 2002). ABA is involved in inhibition of plant growth; it promotes senescence and abscission of leaves and it controls stomatal closure (Garcia-Mata and Lamattina 2007). Dormancy and induction of buds and seeds are also regulated by ABA. This PGR plays a major role in fruit ripening in particular in tomato and mango where ABA controls, via ethylene production, cell wall catabolism leading to a modification of texture and to a diminution of shelf-life (Leng et al. 2014; Zaharah et al. 2013). Therefore, exogenous ABA application enhances the color and maintains postharvest quality of “Crimson Seedless” grapes (Cantín et al. 2007; Ferrara et al. 2013) and induces anthocyanin synthesis in litchi (Singh et al. 2014). In addition, the production of ABA is emphasized by stress, including water loss or freezing temperature during fruit and vegetable storage (Romero et al. 2013; Lafuente and Sala 2002). However, agricultural use of ABA is limited by its susceptibility to light and by the high cost of its production (Gianfagna 1995; Abrams et al. 1997). Therefore, analogs or not and agonist of ABA have been developed that mimic ABA but are more resistant to degradation and less costly to synthesize (Grossmann and Jung 1984; Schubert et al. 1990) (Fig.11.4). So, long-lasting synthetic analogs of ABA, 8’ methylene methyl-ester ABA (PBI 365) and 8’ acetylene methyl-ester ABA (PBI 429), have been developed and positively checked for maintain quality of cut Baccara roses (Pompodakis and Joyce 2003). Pyrabactin (4-bromo-N-(2-pyridinylmethyl)-1-naphthalenesulfonamide) is the first agonist of ABA that is not an analog. Pyrabactin acts as an activator of abscisic acid receptors and activates ABA pathway in a manner very similar to ABA (Puli and Raghavendra 2012). When it is sprayed onto plants it acts in the same way as ABA, and helps them survive with less water. Sean Cutler of the university of California-Riverside developed another molecule, the quinabactin. It mimics also the functions of ABA that makes plants more tolerant to water deficit (Okamoto et al. 2013). However, the unfairness of these products must be demonstrated before use in pre- and postharvest.
Ethylene
Ethylene (C2H4) is the first gaseous hormone identified in plant. Ethylene synthesized from methionine by a well-defined pathway in which 1-aminocyclopropane-1-carboxylic acid (ACC) synthase and ACC oxidase catalyze the reactions from S-adenosylmethionine to ACC and ACC to ethylene, respectively (Yang and Hoffman 1984). C2H4is produced during fruit ripening (see review of Alexander and Grierson 2002; Liu et al. 1999; Mworia et al. 2010), especially in climacteric fruits such as bananas, apples, pears, mangos, and kiwis, and it is involved in senescence and abscission of leaves and flowers. Ethylene synthesis is also induced in response to drought, wounding, chilling injury, and pathogen infection. Climacteric fruits such as in bananas, mangos, tomatoes, and avocados are often harvested at a physiological stage that is considered “commercial maturity” and corresponding to green mature stage just before ripening has initiated. Ripening to obtain “ready-to-eat” fruits can then be conducted under controlled conditions and ethylene fumigation to achieve uniform appearance and quality of ripe fruit. When fruit are exposed to ethylene under these controlled conditions they initiate their respiratory climacteric pattern, induce endogenous ethylene biosynthesis and ripen. In leafy and florets vegetables as broccoli, ethylene application provokes senescence (Costa et al. 2005). Usually, treatments to induce ripening are achieved with ethylene-releasing compounds such as 2-chloroethylphosphonic acid (ethephon) in banana and citrus. However, ethylene promotes calyx abscission in citrus and auxin treatment must be used to avoid this effect. In addition, fast ripening induced by ethylene treatment can affect fruit quality if the concentration and the timing of application are not adequate.
Other PGRs
Salicylic acid (SA) is a phenolic compound similar to aspirin (acetylsalicylic acid) has been involved in defense responses to several abiotic stresses and to plant pathogens. The most important effect of SA is the induction of systemic resistance acquired (SAR). SA is synthesized from phenylalanine and this synthesis involved the phenylalanine ammonia lyase (PAL) and the benzoic acid 2 hydroxylase (BA2H). SA could be found as free or conjugated compound (Fig. 11.5). It has been shown that exogenous application of salicylic acid led to the accumulation of proteins linked to the resistance and thus significantly reduces the extent of damage caused by pathogens. SA can also inhibit ethylene biosynthesis. SA presents several advantages in postharvest application due their effect on fruit ripening, firmness (Marzouk and Kassem 2011; Ranjbaran et al. 2011), antioxidants (Bal and Celik 2010; Chen et al. 2006; Divya et al. 2014; Huang et al. 2008), and disease resistance (Asghari and Aghdam 2010). Chapter 5 describes the impact of SA postharvest treatments on fresh produce quality.
Jasmonic acid (JA) and its derivates as methyl jasmonate (Me-JA) are volatile fatty compounds, derived from the family of octadecanoic fatty acids, and synthesized from linolenic acid membrane of chloroplast (Fig. 11.5). In the chloroplast, linolenic acid, after the action of lipoxygenase, is converted to a cyclized intermediate 12-keto-phytodienoic acid, which, after transport in the peroxisome, is first reduced and then converted after β -oxidation in jasmonic acid (Fig. 11.6). The exogenous jasmonic acid inhibits plant growth and stimulates various processes related to senescence, ripening, and antioxidant defenses (Kucuker et al. 2014; Flores et al. 2015; Concha et al. 2013). It is also known to induce transcription of genes involved in the synthesis of plant defense proteins in response to biotic stress (Wasternack 2014). So, Me-JA postharvest treatment has been shown to reduce Alternaria alternata development in tomato (Chen et al. 2014) and Penicillium citrinum in Chinese bayberry (Wang et al. 2014). See also Chap. 6.
Brassinolides or Brassinosteroids (BR) are steroids compounds discovered for the first time in the pollen of oilseed rape (Brassica napus) and brassicaceae family (Fig. 11.5). It was subsequently shown that these compounds are ubiquitous among plant species and BRs are present in all parts of the plant but their levels are higher in pollen and seeds. As other PGRs, BRs are involved in many mechanisms of development, photomorphogenesis, leaf senescence, and resistance to stress. BRs have the ability to regulate cell cycle and consequently cell division. BRs have sometimes been used in agriculture to increase production of crops, but up to now, theirs applications to fields is poorly described. BR postharvest treatment induces tomato fruit ripening through stimulation of ethylene biosynthesis (Zhu et al. 2015a, b).
Conclusion
In postharvest, PGR may be used to extend shelf-life, to delay senescence, to control ripening, and to limit disease development. These PGRs have also been found to decrease fungal infections, altering or not the quality of the fresh product. The effects of any given PGR depend on many factors as concentration, levels of other endogenous PGR, environmental conditions, signaling factors, or sensitivity of each plant species or cultivar. Therefore, it is difficult to predict the action of exogenous application of PGR and several researches could be realized before extend the use of the molecules.
The environmental and health effects of PGR used for food production are a problem today. For example, in France, ethylene is the only authorized PGR for postharvest application and only banana and citrus. Thus, it is absolutely necessary to develop environmentally friendly new PGR accepted by the consumer for maintain and/or improve quality of fresh product. Gibberellins and salicylic acid and derivate might be good candidates for postharvest treatments.
Nevertheless, different approaches could be developed to manipulate endogenous hormone balance in the good way for the quality such as environmental factors and in particular using light-emitting diode (LED) irradiation technology.
I should like to recommend the excellent book entitled “Plants hormones: biosynthesis, signal transduction and Action!” by Davies (2010).
References
Abrams, S. R., P. A. Rose, A. J. Cutler, J. J. Balsevich, and M. K. Walker-Simmons (1997) 8 Methylene ABA: An effective and persistent analog of abscisic acid. Plant Physiology 144:89–97.
Alexander, L., & Grierson, D. (2002). Ethylene biosynthesis and action in tomato: a model for climacteric fruit ripening. Journal of Experimental Botany, 53(377), 2039–2055. doi:10.1093/jxb/erf072.
Amorós, A., Zapata, P., Pretel, M. T., Botella, M. A., Almansa, M. S., & Serrano, M. (2004). Role of naphthalene acetic acid and phenothiol treatments on increasing fruit size and advancing fruit maturity in loquat. Scientia Horticulturae, 101(4), 387–398. doi:10.1016/j.scienta.2003.11.010. doi:http://dx.doi.org/.
An, J., Zhang, M., Lu, Q., & Zhang, Z. (2006). Effect of a prestorage treatment with 6-benzylaminopurine and modified atmosphere packaging storage on the respiration and quality of green asparagus spears. Journal of Food Engineering, 77(4), 951–957. doi:10.1016/j.jfoodeng.2005.08.024. http://dx.doi.org/.
Asghari, M., & Aghdam, M. S. (2010). Impact of salicylic acid on post-harvest physiology of horticultural crops. Trends in Food Science and Technology, 21(10), 502–509. doi:10.1016/j.tifs.2010.07.009. doi:http://dx.doi.org/.
Bal, E., & Celik, S. (2010). The effects of postharvest treatments of salicylic acid and potassium permanga- nate on the storage of kiwifruit. Bulgarian Journal of Agricultural Science, 16, 576–584.
Biton, E., Kobiler, I., Feygenberg, O., Yaari, M., Friedman, H., & Prusky, D. (2014). Control of alternaria black spot in persimmon fruit by a mixture of gibberellin and benzyl adenine, and its mode of action. Postharvest Biology and Technology, 94, 82–88. doi:10.1016/j.postharvbio.2014.03.009. http://dx.doi.org/.
Cantín, C. M., Fidelibus, M. W., & Crisosto, C. H. (2007). Application of abscisic acid (ABA) at veraison advanced red color development and maintained postharvest quality of ‘Crimson Seedless’ grapes. Postharvest Biology and Technology, 46(3), 237–241. doi:10.1016/j.postharvbio.2007.05.017. http://dx.doi.org/.
Chen, J.-Y., Wen, P.-F., Kong, W.-F., Pan, Q.-H., Zhan, J.-C., Li, J.-M., et al. (2006). Effect of salicylic acid on phenylpropanoids and phenylalanine ammonia-lyase in harvested grape berries. Postharvest Biology and Technology, 40(1), 64–72. doi:10.1016/j.postharvbio.2005.12.017. http://dx.doi.org/.
Chen, B., & Yang, H. (2013). 6-Benzylaminopurine alleviates chilling injury of postharvest cucumber fruit through modulating antioxidant system and energy status. Journal of the Science of Food and Agriculture, 93, 1915–1921. doi:10.1002/jsfa.5990.
Chen, J., Zou, X., Liu, Q., Wang, F., Feng, W., & Wan, N. (2014). Combination effect of chitosan and methyl jasmonate on controlling Alternaria alternata and enhancing activity of cherry tomato fruit defense mechanisms. Crop Protection, 56, 31–36. doi:10.1016/j.cropro.2013.10.007. http://dx.doi.org/.
Concha, C. M., Figueroa, N. E., Poblete, L. A., Oñate, F. A., Schwab, W., & Figueroa, C. R. (2013). Methyl jasmonate treatment induces changes in fruit ripening by modifying the expression of several ripening genes in Fragaria chiloensis fruit. Plant Physiology and Biochemistry, 70, 433–444. doi:10.1016/j.plaphy.2013.06.008. http://dx.doi.org/.
Costa, M. L., Civello, P. M., Chaves, A. R., & Martínez, G. A. (2005). Effect of ethephon and 6-benzylaminopurine on chlorophyll degrading enzymes and a peroxidase-linked chlorophyll bleaching during post-harvest senescence of broccoli (Brassica oleracea L.) at 20 °C. Postharvest Biology and Technology, 35(2), 191–199. doi:10.1016/j.postharvbio.2004.07.007. http://dx.doi.org/.
Davies, P. J. (2010) Plant Hormones: Biosynthesis, Signal Transduction, Action!. p. 801pp Springer, Dordrecht, The Netherlands
Ding, Y., Sheng, J., Li, S., Nie, Y., Zhao, J., Zhu, Z., et al. (2015). The role of gibberellins in the mitigation of chilling injury in cherry tomato (Solanum lycopersicum L.) fruit. Postharvest Biology and Technology, 101, 88–95. doi:10.1016/j.postharvbio.2014.12.001. http://dx.doi.org/.
Divya, P., Puthusseri, B., & Neelwarne, B. (2014). The effect of plant regulators on the concentration of carotenoids and phenolic compounds in foliage of coriander. LWT - Food Science and Technology, 56(1), 101–110. doi:10.1016/j.lwt.2013.11.012. http://dx.doi.org/.
Ferrara, G., Mazzeo, A., Matarrese, A., Pacucci, C., Pacifico, A., Gambacorta, G., et al. (2013). Application of Abscisic Acid (S-ABA) to ‘Crimson Seedless’ Grape Berries in a Mediterranean Climate: Effects on Color, Chemical Characteristics, Metabolic Profile, and S-ABA Concentration. Journal of Plant Growth Regulation, 32(3), 491–505. doi:10.1007/s00344-012-9316-2.
Figueroa, C. R., Opazo, M. C., Vera, P., Arriagada, O., Díaz, M., & Moya-León, M. A. (2012). Effect of postharvest treatment of calcium and auxin on cell wall composition and expression of cell wall-modifying genes in the Chilean strawberry (Fragaria chiloensis) fruit. Food Chemistry, 132(4), 2014–2022. doi:10.1016/j.foodchem.2011.12.041.
Flaishman, M. A., Shargal, A., & Stern, R. A. (2001). The synthetic cytokinin CPPU increases fruit size and yield of ‘Spadona’ and ‘Costia’ pear (Pyrus communis L.). Journal of Horticultural Science and Biotechnology, 76, 145–149.
Flores, G., Blanch, G.P., Ruiz del Castillo, M.L. (2015). Postharvest treatment with (−) and (+)-methyl jasmonate stimulates anthocyanin accumulation in grapes. LWT: Food Science and Technology. (0) doi:http://dx.doi.org/10.1016/j.lwt.2014.12.033.
Gambetta, G., Mesejo, C., Martínez-Fuentes, A., Reig, C., Gravina, A., & Agustí, M. (2014). Gibberellic acid and norflurazon affecting the time-course of flavedo pigment and abscisic acid content in ‘Valencia’ sweet orange. Scientia Horticulturae, 180, 94–101. doi:10.1016/j.scienta.2014.10.021. http://dx.doi.org/.
Garcia-Mata, C., & Lamattina, L. (2007). Abscisic acid (ABA) inhibits light-induced stomatal opening through calcium- and nitric oxide-mediated signaling pathways. Nitric Oxide, 17(3–4), 143–151.
Gianfagna, T.J. (1995) Use of natural and synthetic growth regulators, In: P.J. Davis, ed, Plant hormones: Physiology, biochemistry, and molecular biology. Kluwer Academic Publishers, Boston, 762–766.
Grossmann, K. and J. Jung. (1984) Influence of terpenoid analogues of abscisic acid on stomatal movement and leaf senescence. Journal of Agronomy and Crop Science 153:14–22.
Hayata, Y., Niimi, Y., Inoue, K., & Kondo, S. (2000). CPPU and BA, with and without pollination, affect set, growth, and quality of muskmelon fruit. HortScience, 35, 868–870.
Huang, H., & Jiang, Y. (2012). Effect of plant growth regulators on banana fruit and broccoli during storage. Scientia Horticulturae, 145, 62–67. doi:10.1016/j.scienta.2012.07.025. http://dx.doi.org/.
Huang, R.-H., Liu, J.-H., Lu, Y.-M., & Xia, R.-X. (2008). Effect of salicylic acid on the antioxidant system in the pulp of ‘Cara cara’ navel orange (Citrus sinensis L. Osbeck) at different storage temperatures. Postharvest Biology and Technology, 47(2), 168–175. doi:10.1016/j.postharvbio.2007.06.018. http://dx.doi.org/.
Khader, S. E. S. A. (1991). Effect of preharvest application of GA3 on postharvest behaviour of mango fruits. Scientia Horticulturae, 47(3–4), 317–321. doi:10.1016/0304-4238(91)90014-P. http://dx.doi.org/.
Khader, S. E. S. A., Singh, B. P., & Khan, S. A. (1988). Effect of GA3 as a post-harvest treatment of mango fruit on ripening, amylase and peroxidase activity and quality during storage. Scientia Horticulturae, 36(3–4), 261–266. doi:10.1016/0304-4238(88)90060-X. http://dx.doi.org/.
Kim, J. G., Takami, Y., Mizugami, T., Beppu, K., Fukuda, T., & Kataoka, I. (2006). CPPU application on size and quality of hardy kiwifruit. Scientia Horticulturae, 110, 219–222.
Kobiler, I., Shalom, Y., Roth, I., Akerman, M., Vinokur, Y., Fuchs, Y., et al. (2001). Effect of 2,4-dichlorophenoxyacetic acid on the incidence of side and stem end rots in mango fruits. Postharvest Biology and Technology, 23(1), 23–32. doi:10.1016/S0925-5214(01)00092-8. http://dx.doi.org/.
Korasick, D. A., Enders, T. A., & Strader, L. C. (2013). Auxin biosynthesis and storage forms. Journal of Experimental Botany, 64(9), 2541–2555. doi:10.1093/jxb/ert080.
Koukounaras, A., Siomos, A. S., & Sfakiotatis, E. (2010). Effects of 6-BA treatmentson yellowingandquality of storedrocket (Erucasativamill.) leaves. Journal of Food Quality, 33, 768–779. doi:10.1111/j.1745-4557.2010.00354.x.
Kucuker, E., Ozturk, B., Celik, S. M., & Aksit, H. (2014). Pre-harvest spray application of methyl jasmonate plays an important role in fruit ripening, fruit quality and bioactive compounds of Japanese plums. Scientia Horticulturae, 176, 162–169. doi:10.1016/j.scienta.2014.07.007. http://dx.doi.org/.
Lafuente, M. T., & Sala, J. M. (2002). Abscisic acid levels and the influence of ethylene, humidity and storage temperature on the incidence of postharvest rindstaning of ‘Navelina’ orange (Citrus sinensis L. Osbeck) fruit. Postharvest Biology and Technology, 25(1), 49–57. doi:10.1016/S0925-5214(01)00162-4. http://dx.doi.org/.
Lehmann, T., Hoffmann, M., Hentrich, M., & Pollmann, S. (2010). Indole-3-acetamide-dependent auxin biosynthesis: A widely distributed way of indole-3-acetic acid production? European Journal of Cell Biology, 89(12), 895–905. doi:10.1016/j.ejcb.2010.06.021. http://dx.doi.org/.
Leng, P., Yuan, B., & Guo, Y. (2014). The role of abscisic acid in fruit ripening and responses to abiotic stress. Journal of Experimental Botany, 65(16), 4577–4588. doi:10.1093/jxb/eru204.
Lers, A., Jiang, W. B., Lomanies, E., & Aharoni, N. (1998). Gibberellic acid and CO2 additive effect in retarding postharvest senescence of parsley, J. Food Science, 63, 66–68.
Lichanporn, I., & Techavuthiporn, C. (2013). The effects of nitric oxide and nitrous oxide on enzymatic browning in longkong (Aglaia dookkoo Griff.). Postharvest Biology and Technology, 86, 62–65. doi:10.1016/j.postharvbio.2013.06.021. http://dx.doi.org/.
Liu, X., Shiomi, S., Nakatsuka, A., Kubo, Y., Nakamura, R., & Inaba, A. (1999). Characterization of Ethylene Biosynthesis Associated with Ripening in Banana Fruit. Plant Physiology, 121(4), 1257–1265. doi:10.1104/pp. 121.4.1257.
Lurie, S., & Crisosto, C. H. (2005). Chilling injury in peach and nectarine. Postharvest Biology and Technology, 37(3), 195–208. doi:10.1016/j.postharvbio.2005.04.012. http://dx.doi.org/.
Manjunatha, G., Lokesh, V., & Neelwarne, B. (2010). Nitric oxide in fruit ripening: Trends and opportunities. Biotechnology Advances, 28(4), 489–499. doi:10.1016/j.biotechadv.2010.03.001.
Mano, Y., & Nemoto, K. (2012). The pathway of auxin biosynthesis in plants. Journal of Experimental Botany. doi:10.1093/jxb/ers091.
Marzouk, H. A., & Kassem, H. A. (2011). Improving yield, quality, and shelf life of Thompson seedless grapevine by preharvest foliar applications. Scientia Horticulturae, 130(2), 425–430. doi:10.1016/j.scienta.2011.07.013. http://dx.doi.org/.
McAtee, P., Karim, S., Schaffer, R., & David, K. (2013). A dynamic interplay between phytohormones is required for fruit development, maturation, and ripening. Frontiers in Plant Science, 4, 79. doi:10.3389/fpls.2013.00079.
Mworia, E. G., Yoshikawa, T., Yokotani, N., Fukuda, T., Suezawa, K., Ushijima, K., et al. (2010). Characterization of ethylene biosynthesis and its regulation during fruit ripening in kiwifruit, Actinidia chinensis ‘Sanuki Gold’. Postharvest Biology and Technology, 55(2), 108–113. doi:10.1016/j.postharvbio.2009.08.007. http://dx.doi.org/.
Okamoto, M., Peterson, F. C., Defries, A., Park, S.-Y., Endo, A., Nambara, E., et al. (2013). Activation of dimeric ABA receptors elicits guard cell closure, ABA-regulated gene expression, and drought tolerance. Proceedings of the National Academy of Sciences, 110(29), 12132–12137. doi:10.1073/pnas.1305919110.
Ortolá, A. G., Monerri, C., & Guardiola, J. L. (1991). The use of naphthalene acetic acid as a fruit growth enhancer in Satsuma mandarin: a comparison with the fruit thinning effect. Scientia Horticulturae, 47(1–2), 15–25. doi:10.1016/0304-4238(91)90023-R. http://dx.doi.org/.
Pompodakis, N. E., & Joyce, D. C. (2003). Abscisic acid analogue effects on the vase life and leaf crisping of cut Baccara roses. Australian Journal of Experimental Agriculture, 43(4), 425–428. doi:10.1071/EA02036. http://dx.doi.org/.
Puli, M. R., & Raghavendra, A. S. (2012). Pyrabactin, an ABA agonist, induced stomatal closure and changes in signalling components of guard cells in abaxial epidermis of Pisum sativum. Journal of Experimental Botany, 63(3), 1349–1356. doi:10.1093/jxb/err364.
Raban, E., Kaplunov, T., Zutahy, Y., Daus, A., Alchanatis, V., Ostrovsky, V., et al. (2013). Rachis browning in four table grape cultivars as affected by growth regulators or packaging. Postharvest Biology and Technology, 84, 88–95. doi:10.1016/j.postharvbio.2013.03.021. http://dx.doi.org/.
Ramakrishna, M., Haribabu, K., & Purushotham, K. (2002). Effect of post-harvest application of growth regulators on storage behavior of papaya (Carica papaya L.). Journal of Food Science and Technology, 39(6), 657–659.
Ranjbaran, E., Sarikhani, H., Bakhshi, D., & Pouya, M. (2011). Investigation of Salicylic Acid Application to Reduce Postharvest Losses in Stored ‘Bidaneh Ghermez’ Table Grapes. International Journal of Fruit Science, 11(4), 430–439. doi:10.1080/15538362.2011.630591.
Romero, P., Rodrigo, M. J., & Lafuente, M. T. (2013). Differential expression of the Citrus sinensis ABA perception system genes during postharvest fruit dehydration. Postharvest Biology and Technology, 76, 65–73. doi:10.1016/j.postharvbio.2012.09.010. http://dx.doi.org/.
Sakakibara, H. (2006). Cytokinins: activity, biosynthesis, and translocation. Annual Review of Plant Biology, 57, 431–449.
Salvador, A., Sdiri, S., Navarro, P., Monterde, A., & Martínez-Jávega, J. M. (2010). The use of auxins to maintain postharvest quality of citrus fruit. Acta Horticulturae, 877, 671–677.
Schröder, M., Link, H., & Bangerth, K. F. (2013). Correlative polar auxin transport to explain the thinning mode of action of benzyladenine on apple. Scientia Horticulturae, 153, 84–92. doi:10.1016/j.scienta.2013.02.001. http://dx.doi.org/.
Schubert, J., K. Roser, K. Grossmann, H. Sauter, and J. Jung. (1990) Transpiration-inhibiting abscisic acid analogs. Journal of Plant Growth Regulation 10:27–32.
Sdiri, S., Navarro, P., Monterde, A., Benabda, J., & Salvador, A. (2012). New degreening treatments to improve the quality of citrus fruit combining different periods with and without ethylene exposure. Postharvest Biology and Technology, 63(1), 25–32. doi:10.1016/j.postharvbio.2011.08.005. http://dx.doi.org/.
Sdiri, S., Navarro, P., & Salvador, A. (2013). Postharvest application of a new growth regulator reduces calyx alterations of citrus fruit induced by degreening treatment. Postharvest Biology and Technology, 75, 68–74. doi:10.1016/j.postharvbio.2012.08.004. http://dx.doi.org/.
Seo, M., & Koshiba, T. (2002). Complex regulation of ABA biosynthesis in plants. Trends in Plant Science, 7(1), 41–48.
Serrano, M., Martínez-Romero, D., Zuzunaga, M., Riquelme, F., & Valero, D. (2004). Calcium, Polyamine and Gibberellin Treatments to Improve Postharvest Fruit Quality. In S. Jain (Ed.), Dris R (pp. 55–68). Springer Netherlands: Production Practices and Quality Assessment of Food Crops. doi:10.1007/1-4020-2535-1_3.
Simon, S., & Petrášek, J. (2011). Why plants need more than one type of auxin. Plant Science, 180(3), 454–460. doi:10.1016/j.plantsci.2010.12.007. http://dx.doi.org/.
Singh, S. P., Saini, M. K., Singh, J., Pongener, A., & Sidhu, G. S. (2014). Preharvest application of abscisic acid promotes anthocyanins accumulation in pericarp of litchi fruit without adversely affecting postharvest quality. Postharvest Biology and Technology, 96, 14–22. doi:10.1016/j.postharvbio.2014.05.005. http://dx.doi.org/.
Stern, R. A., Flaishman, M., Applebaum, S., & Ben-Arie, R. (2007a). Effect of synthetic auxins on fruit development of ‘Bing’ cherry (Prunus avium L.). Scientia Horticulturae, 114(4), 275–280. doi:10.1016/j.scienta.2007.07.010. http://dx.doi.org/.
Stern, R. A., Flaishman, M., & Ben-Arie, R. (2007b). Effect of synthetic auxins on fruit size of five cultivars of Japanese plum (Prunus salicina Lindl.). Scientia Horticulturae, 112(3), 304–309. doi:10.1016/j.scienta.2006.12.032. http://dx.doi.org/.
Suttle, J. (2003). Auxin-induced sprout growth inhibition: Role of endogenous ethylene. American Journal of Potato Research, 80(5), 303–309. doi:10.1007/BF02854314.
Thomas, S. G., Rieu, I., & Steber, C. M. (2005). Gibberellin metabolism and signaling. In G. Litwack (Ed.), Vitamins and Hormones (pp. 289–337). London: Elsevier.
Tivendale, N. D., Ross, J. J., & Cohen, J. D. (2014). The shifting paradigms of auxin biosynthesis. Trends in Plant Science, 19(1), 44–51. doi:10.1016/j.tplants.2013.09.012. http://dx.doi.org/.
Vargas, A., & Lopez, J. A. (2011). Effect of dose rate, application method and commercial formulations of GA3 on banana (Musa AAA)fruit greenlife. Global Science Books. Fresh Produce, 5(1), 51–55.
Wang, K., Jin, P., Han, L., Shang, H., Tang, S., Rui, H., et al. (2014). Methyl jasmonate induces resistance against Penicillium citrinum in Chinese bayberry by priming of defense responses. Postharvest Biology and Technology, 98, 90–97. doi:10.1016/j.postharvbio.2014.07.009. http://dx.doi.org/.
Wang, B., Wang, J., Liang, H., Yi, J., Zhang, J., Lin, L., et al. (2008). Reduced chilling injury in mango fruit by 2,4-dichlorophenoxyacetic acid and the antioxidant response. Postharvest Biology and Technology, 48(2), 172–181. doi:10.1016/j.postharvbio.2007.10.005. http://dx.doi.org/.
Wasternack, C. (2014). Action of jasmonates in plant stress responses and development: Applied aspects. Biotechnology Advances, 32(1), 31–39. doi:10.1016/j.biotechadv.2013.09.009. http://dx.doi.org/.
Woodward, A. W., & Bartel, B. (2005). Auxin: Regulation, Action, and Interaction. Annals of Botany, 95(5), 707–735. doi:10.1093/aob/mci083.
Yang, S. F., & Hoffman, N. E. (1984). Ethylene biosynthesis and its regulation in higher plants. Annual Review of Plant Physiology, 35, 155–189.
Zaharah, S. S., Singh, Z., Symons, G. M., & Reid, J. B. (2013). Mode of action of abscisic acid in triggering ethylene biosynthesis and softening during ripening in mango fruit. Postharvest Biology and Technology, 75, 37–44. doi:10.1016/j.postharvbio.2012.07.009. http://dx.doi.org/.
Zhang, C., & Whiting, M. D. (2011). Improving ‘Bing’ sweet cherry fruit quality with plant growth regulators. Scientia Horticulturae, 127(3), 341–346. doi:10.1016/j.scienta.2010.11.006. http://dx.doi.org/.
Zhu, T., Tan, W.-R., Deng, X.-G., Zheng, T., Zhang, D.-W., & Lin, H.-H. (2015a). Effects of brassinosteroids on quality attributes and ethylene synthesis in postharvest tomato fruit. Postharvest Biology and Technology, 100, 196–204. doi:10.1016/j.postharvbio.2014.09.016. http://dx.doi.org/.
Zhu, F., Yun, Z., Ma, Q., Gong, Q., Zeng, Y., Xu, J., et al. (2015b). Effects of exogenous 24-epibrassinolide treatment on postharvest quality and resistance of Satsuma mandarin (Citrus unshiu). Postharvest Biology and Technology, 100, 8–15. doi:10.1016/j.postharvbio.2014.09.014. http://dx.doi.org/.
Zoffoli, J. P., Latorre, B. A., & Naranjo, P. (2009). Preharvest applications of growth regulators and their effect on postharvest quality of table grapes during cold storage. Postharvest Biology and Technology, 51(2), 183–192. doi:10.1016/j.postharvbio.2008.06.013. http://dx.doi.org/.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
LOPEZ-LAURI, F. (2016). Plant Growth Regulators. In: Siddiqui, M., Ayala Zavala, J., Hwang, CA. (eds) Postharvest Management Approaches for Maintaining Quality of Fresh Produce. Springer, Cham. https://doi.org/10.1007/978-3-319-23582-0_8
Download citation
DOI: https://doi.org/10.1007/978-3-319-23582-0_8
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-23581-3
Online ISBN: 978-3-319-23582-0
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)