Abstract
Frontotemporal dementia (FTD) is a clinical term that encompasses a heterogeneous group of disorders that affect predominantly behaviour and language to varying degrees. FTD is the most common form of young onset dementia. In this chapter, we discuss the main clinical and neuropsychological, and radiological characteristics of the FTD spectrum. We also summarise the advances in fields of FTD neuropathology, neuroimaging and proteomic biomarker technology. We outline current treatment options and discuss potential future therapies currently under investigation in the context of our rapidly increasing understanding of underlying disease patho-mechanisms in this disorder.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Frontotemporal dementia
- Behavioural variant
- Primary progressive aphasia
- Semantic variant
- Non-fluent variant
7.1 Introduction
The terms “Frontotemporal dementia” or “Frontotemporal lobar degeneration” are often used interchangeably to describe a clinically and pathologically heterogeneous group of disorders characterized by degeneration of the frontal and temporal lobes. This disorder is comprised of multiple clinical variants. These include the “frontal” or “behavioural” variant which is characterised by decline in behaviour and several language variants where the clinical presentations is with language difficulties. Neary et al. use the umbrella term Frontotemporal Lobar Degeneration (FTLD) for all of the above and reserved the term “Frontotemporal dementia” to refer specifically to the “frontal” / “behavioural” variant. In contrast, McKhann et al. 2001 used the term Frontotemporal dementia (FTD) to refer to all of the above presentations. For the purposes of this chapter we use the term Frontotemporal dementia (FTD) in the general sense of McKhann et al. In addition, the term “behavioural” variant FTD is currently preferred to the tem “frontal” as it addresses the behavioural syndrome rather the presumed anatomical localisation and is thus more comparable to the terms used for the language variants. The description of the FTD language variants in this chapter is guided the most recent classification of primary progressive aphasia (PPA) syndromes and includes progressive non-fluent aphasia or nfvPPA (previously PNFA), the semantic variant or svPPA (previously called semantic dementia) and the recently described logopenic variant or lvPPA.
7.2 History
Arnold Pick (1892) is credited with the first description of a progressive disorder of behaviour and language associated with circumscribed atrophy of the frontal and temporal lobes. Later, Alois Alzheimer (1911) described the classical histological changes associated with “Pick’s disease” that of inter-neuronal inclusions and ballooned neurons. In the 1980’s, two groups in Lund, Sweden and Manchester, United Kingdom published separately large series of patients with frontotemporal atrophy and dementia with prominent behaviour and language difficulties. They noted that Pick-type histology was only one of three main histological changes seen and they came up with the first consensus criteria for frontotemporal dementia. At the same time Mesulam described a series of patients with a progressive language disorder with sparing of other cognitive domains and non-Alzheimer’s pathology, which he termed primary progressive aphasia (PPA).
Over the subsequent decade further clinical, imaging and pathological studies prompted the consensus group to refine the criteria in 1998. They separated the language variants from the behavioural disorder. The separation of these syndromes has led research focused on different clinical variants. This has resulted in significant advances in our understanding of the genetics and molecular pathology underlying these variants. However, overlap between the clinical syndromes is still apparent. Indeed, in familial FTD each of the different clinical syndromes can be seen in the same kindred. Also, in later stages of each syndrome, patients will often have a mixed clinical picture of behaviour and language difficulties.
7.3 Epidemiology and Demographics
Age of onset of FTD is typically younger than other forms of dementia being between 45 and 65 years, though cases are reported outside this range. FTD is the third most common form of cortical dementia after Alzheimer’s disease (AD) and Dementia with Lewy Bodies (DLB) and the second most common cause of young-onset dementia after AD.
Studies in the UK and U.S.A suggest that the prevalence of FTD in 45–64 age group is 15–22 per 100,000 with incidence estimates for the same age group ranging from 2.7 to 4.1 per 100,000 person-years. A population-based study in the Netherlands reported lower prevalence rates of 3–4 per 100,000 in the 45–64 age group, 9.4 per 100,000 for the 60–69 age group, and 3.8 per 100,000 at 70–79 age group. Although this supports that concept that FTD is not a dementia of old age, a recent study based on the Swedish Registry for Dementia, reported that 70 % of FTD cases were 65 years or older at the time of diagnosis. Both FTD and AD displayed an increased incidence with age in that study, with FTD incidence in the 80–84 years reaching 6.04 per 100,000 person-years.
Data from UK registry suggest a male predominance (14:3), several studies from Italy suggest a female predominance (1: 1.3–2), while other reports suggested equal sex distribution.
The duration of illness from onset to death has a range from 2 to 20 years with a mean of 6 to 8 years. Behavioural variant FTD (bvFTD) is the most common FTD syndrome affecting 55 % of all cases, while non-fluent progressive aphasia (nfvPPA) accounts for 25 %, and the semantic variant (svPPA) for 20 %. Demography does differ between the syndromes: For example, svPPA has a later age of onset (though still younger than typical AD patients) and a slower rate of progression with a median survival of about 12 years. Patients with bvFTD have the earliest age of onset and generally more rapid progression with a survival of about 9 years. The presence of co-morbid motor involvement in the form of amyotrophic lateral scleroses (FTD with co-morbid ALS or FTD-ALS) is associated with shortest survival (2–5 years).
7.4 Clinical Features
As with other forms of cortical dementia, symptoms are gradual in onset and progressive over time. Below is a description of the most common FTD variants.
7.4.1 Behavioural Variant FTD (bv-FTD)
The hallmark of bvFTD is gradual onset deterioration in behaviour and inter-personal conduct. Patients often lack insight into their abnormal behaviour. This often leads to delay in seeking medical attention. Thus detailed semi-structured interviews with family members focused on behavioural changes are an essential component of evaluating patients with suspected bvFTD. It is important to note that the patient’s family and friends often excuse some or all the personality changes, at least initially, as part of “mid-life crisis”. Thus, the interviews should include a mixture of open questions (e.g. “have you noticed any change in personality/behaviour?”) and direct questions about the specific behavioural changes that can be encountered in this condition.
It is usually socially inappropriate interpersonal behaviour that is noted initially due to impulsivity and disinhibition of verbal, physical or sexual impulses. Difficulties with interpersonal conduct are not only due to disinhibition but also due to deficits in emotional processing, social awareness and social cognitive skills. These deficits can lead to inability to express and recognize facial or vocal expressions of emotion and/or inferring the mental states and intentions of others from social cues. These abilities form part of the so called “theory of mind”. This results in problematic social interactions, loss of empathy and a reduced concern for others. These behaviours are often perceived by the caregiver as ‘out of character’ and constitute a significant change in personality. It is important to note that for these reasons distress is common among the caregivers of bvFTD patients, compared with caregivers of other forms of cortical dementia.
In tandem with the decline in interpersonal conduct, there is often a change in personal conduct, usually in form of loss of drive/motivation or apathy and rarely hyperactivity. Apathy is often noted by caregivers and can be mistaken for depression. It is one of the factors that contribute to the loss in previous hobbies and social activities and the decline in personal hygiene and grooming that is frequently reported.
Other behavioural symptoms that have been reported in bvFTD patients include perseverative and stereotyped (ritualistic/compulsive) behaviour and speech patterns. This can include simple behaviours such as humming, or tapping, as well as complex behaviours such as constant checking of door locks or light switches. Preservative speech patterns includes repeating the same catchphrases and repeating what others say (echoclalia). Patients may also develop hyper-orality, over-eating or bingeing, and change in food preference (usually developing a “sweet tooth”). Utilization behaviour, the act of compulsively grasping and using objects within one’s visual field, has also been described and seems to be particularity frequent in bvFTD patients from Asia.
The cognitive symptoms experienced are due to executive dysfunction – therefore patients have difficulties in planning, problem solving, organization, attention and mental flexibility. These are symptoms that are not easily identified either by the caregiver or history taker but important clues include decline in performance at work and home (“activities of daily living”) and adherence to a rigid routine or way of doing things. Notably, cognitive function can be intact early in the disease even in the context of marked behavioural changes.
Cummings et al. have suggested three distinct bvFTD syndromes based on the predominance of disinhibition, apathy or dysexecutive behaviour. In contrast, Snowden et al. suggested that bvFTD can be subdivided into apathetic variant, disinhibited variant and a variant of stereotyped-compulsive syndromes. These syndromic sub-classifications are supported by anatomic and radiological correlations. Apathetic behaviour has been associated with changes in medial frontal cortex, anterior cingulate and superior frontal gyrus, particularly on the right. Disorders of self-regulation and disinhibition as associated with changes in the ventromedial prefrontal cortex (VMPC), the anterior temporal lobe, the amygdala, and the orbito-frontal cortical subcortical circuit. Dorsolateral prefrontal (DLPC) changes are linked to a dysexecutive syndrome. Overall involvement of the right hemisphere is associated with more severe behavioural changes. However, in practice these symptoms frequently co-occur, and the clinical usefulness of these distinctions is limited.
7.4.2 Language Variants of bv-FTD
Patients with the language variants of FTD are generally more aware of their deficits compared to bvFTD patients and they will frequently complain, “I’ve forgotten the words for things”. However, the presentation can still be delayed as their difficulties are often put down as normal aging.
In progressive non-fluent aphasia (nfvPPA) there is breakdown in spontaneous speech. The core changes in nfvPPA are reduced fluency leading to effortful halting speech and deficits in grammar. The later deficits often lead to simplification, distorted word order as well as omissions of function words such articles and propositions (see Table 7.1 for examples).
Anomia is common due to difficulties in word retrieval but object knowledge remains intact. Thus although the patient cannot name an object, they may be able to pick the correct name from choice and will be able to match the object with other objects that semantically linked to it.
Comprehension for single words in usually intact but impaired comprehension of syntactically complex sentences is often an early feature. In addition, these patients have difficulties in speech production due to speech apraxia leading to word sound distortions.
The semantic variant (svPPA, previously semantic dementia) is the second FTD language variant. The hallmark of svPPA is loss of semantic knowledge. This manifests as severe naming and word comprehension impairment in the context of fluent, effortless, and grammatically correct speech output. Semantic paraphasias are common. Words are often substituted by a semantically related but more frequent object (e.g. “horse” for “zebra”) or a more general categorical term (e.g. “animal” for “cow”).
Due to the loss of semantic knowledge, patients have difficulties in single word comprehension and are unable to match the object with semantically similar objects or pick an object from description of its use. Knowledge loss affects initially the less frequent or atypical exemplars of category (e.g. first “hamster”, then ‘rabbit’, then ‘animal’). Knowledge of more personally relevant objects is more resistant. Of note, episodic memory remains intact and patients often remain oriented, are able to keep appointments, and to learn and recall visuospatial information.
Repetition is not impaired in svPPA and patients can repeat multi-syllable words without difficulty. In contrast writing and reading relies exclusively on phonological (letter by letter) reading/writing as patients have no access to previously learned word knowledge, which includes the correct way to read irregular words (such as “yacht” or “cough”). This leads regularisation errors when reading or writing. This is termed as “surface alexia/dyslexia” and “surface agraphia/dysgraphia” respectively.
The above described svPPA phenotype is usually associated with predominant involvement of the dominant hemisphere. A rarer form of svPPA is seen where the disease process affects mainly the non-dominant hemisphere. The phenotype in this form usually includes agnosia (a failure to recognise objects or people) and more prominent behavioural changes.
The logopenic variant of progressive aphasia (lvPPA) is the third type of primary progressive aphasia syndrome. This variant has been recognised only recently and its exact syndromic classification remains controversial. Unlike the other language variants of PPA, the most common pathology underlying this disorder is that of Alzheimer’s type pathology. However, recent evidence suggests that 23 % of lvPPA patient have FTD pathology on autopsy.
lvPPA is characterised by fluctuating interruptions of fluency due to word finding difficulties, with intact grammar and sound production. There is often impaired repetition and comprehension, particularly for long improbable phrases, an observation in line with the hypotheses that episodic memory impairment plays a central role in this condition.
Finally, it is worth noting that while the most salient complaints in language variants of FTD are by definition language related, there are often associated behavioural changes, particularly in svPPA patients. The pattern of behavioural changes in language variant FTD may be different from that of bvFTD. Some reports suggest that apathy, lack of empathy and hyper-orality are more common in bvFTD while compulsive and complex stereotypic behaviours, mental rigidity anxiety, repetitive themes, and sleep disorders may be more common in svPPA.
7.5 Associated Syndromes
7.5.1 Syndromes That Overlap and Mimic FTD
Research has shown the significant pathological and molecular overlap between FTD and other neurodegenerative disorders and hence the crossover in clinical features. Table 7.2 summarizes the syndrome that overlap.
On the other hand, there are a range of clinical disorders that have no pathological overlap but whose clinical phenotype can mimic those of FTD. Table 7.3 summarises the syndrome that mimic FTD.
7.6 Clinical Assessment
The first part of the clinical assessment in FTD patients is obtaining a detailed history, emphasising any changes in behaviour, language and other cognitive domains and their impact on the patient’s ability to function at home and at work and to maintain inter-personal relationships. The history should also include a detailed family history and clues suggesting overlap or mimic syndromes (see Table 7.2 and Fig. 7.1). The examination should cover both cognitive functions and motor signs.
Cognitive assessment should focus on identifying the cognitive deficits specific to each syndrome in addition to noting which cognitive domains are spared. The most affected domains are executive functions and (in the language variants) language function. In contrast, orientation, calculation, visuospatial skills should be relatively well preserved early in FTD. Although early memory impairment is generally considered to exclude a diagnoses of FTD, recent evidence suggest that memory changes in FTD are common, especially in younger patients. Occasionally, memory dysfunction occasionally can be as severe as that observed in AD patients. In fact, severe amnesia can be the presenting feature in some patients. However, typically memory function in FTD affects free recall to a higher degree than recognition or cued recall while in AD the impairment extends to all three aspects of episodic memory.
A variety of rapid screening tests have been designed specifically for the purpose of discriminating FTD from non-FTD dementias such as the third version Addenbrooke’s Cognitive Examination (ACE-III, Hsieh 2013) which is the most updated version of the ACE (Mathuranath 2000), the Executive Interview (Royall, Mahurin RK, and Gray, 1992), and the Frontal Assessment Battery (FAB, Dubois, Slachevsky, Litvan and Pillon B, 2000), and The Montreal Cognitive Assessment (MoCA, Nasreddine et al. 2005). If feasible, the patient can be referred to a clinical psychologist for a more detailed neuropsychological evaluation.
The motor examination should focus on the presence of motor signs suggesting disease mimics (e.g. stroke) and disorders that overlap with FTD such as corticobasal syndrome (CBS), Progressive Supranuclear Palsy (PSP), and amyotrophic form of Motor Neuron Disease (FTD-ALS). Thus presence of extra-pyramidal signs, muscle wasting, fasciculations or unilateral apraxia add to the behavioural and cognitive profile in characterizing the syndrome (Fig. 7.1).
7.7 Neuropsychological Assessment
Given the wide variability of presentations in FTD and the fact that there are a number of other overlapping conditions, a comprehensive neuropsychological assessment can greatly contribute to differential diagnosis. However, as FTD can frequently present with alterations in behaviour and personality one should not over-rely on cognitive testing per se. Depending on the locus of pathology patients may do extremely poorly on conventional “frontal lobe” tests (such as the Wisconsin Card Sorting Test, Trail Making Test, verbal fluency etc.), or they may in fact perform normally, yet still show major impairment in self- and social regulation. Therefore it is essential to conduct a detailed evaluation of behaviour.
Behavioural evaluation should include direct observation of the patient as well as collateral information from informants using semi-structured interviews and preferably a suitably designed behavioural instrument. One such instrument is the Frontal Systems Behaviour Scale (FrSBe, Grace & Molloy 2001) which was designed based on Cummings’ neuroanatomical model to provide self and informant based ratings of apathy, disinhibition, and executive dysfunction both pre-morbidly and currently. In addition to permitting age and gender graded interpretation of behaviour change, the self- and informant-based ratings can be compared to evaluate the degree of insight or lack thereof. Other commonly used behavioural instruments include the Cambridge Behavioural Inventory (CBI, Wedderburn et al. 2008) and the Neuropsychiatric Inventory Questionnaire (NPI-Q, Kaufer et al. 2000).
The typical pattern of cognitive change reported in FTD patients is impairment of executive functions. There are purpose designed comprehensive batteries to evaluate a broad range of cognitive processes encompassed within the term executive function, the majority of which are dependent on the integrity of the frontal lobes. Notable examples include the Delis-Kaplan Executive Function System (D-KEFS, Delis, Kaplan, & Kramer, 2001) and Behavioural Assessment of the Dysexecutive Syndrome (BADS, Wilson, Alderman, Burgess, Emslie, & Evans, 1996), (see Chap. 3 for a more detailed discussion of tasks of executive functions).
As noted above, the patient may or may not have associated significant memory impairment. Attention, orientation and visuo-spatial functions are typically preserved in FTD patients. However, it is worth noting that qualitative cognitive analysis is as important as the patient’s quantitative performance on the different neuropsychological tests. Patients can fail the same tests for different reasons. For example, an AD patient could fail visuo-construction tasks such as copy trial of the Rey Osterrieth Complex Figure or Block Design because of memory problems and/or poor visuo-spatial functions while FTD patients might have difficulties with the same task due to constructional praxis and planning problems.
Patients with an early disease focused in the orbitofrontal-ventromedial prefrontal cortex, may perform normally on tests traditionally considered sensitive to frontal lobe/executive dysfunction but have a profound deficit in everyday decision making and social regulation. To address this issue novel batteries have been developed to focus on emotional decision making and social cognitive skills. Many tasks intend to mimic every day functioning and as such maybe more sensitive to cognitive changes in early bv-FTD.
One such battery is the Executive and Social Cognitive Battery (ESCB, Torralva 2009) which includes the Iowa Gambling Task (IGT, Bechara et al. 1994), the Multiple Errands Task (MET, Shallice & Burgess, 1991), the Hotel task (HOT, Manly et al. 2002), the Mind in the Eyes task (Baron-Cohen et al. 2001), and the Faux Pas test (Stone et al. 1998). The IGT evaluates decision making and learning in high and low risk situations. During the task healthy individuals learn to avoid the risky choices while those with FTD continue to make high risk choices which results in an overall net loss. The MET and the HOT are both designed to mirror everyday tasks. The MET involves the participant running “real life” errands such as purchasing three items (a soda, a postcard and a letter), using the internal phone, and obtaining and writing down pieces of information such as the area code of a city. The Hotel task involves activities that are often undertaken as part of running a hotel such as sorting bills by client name. The Mind in the Eyes task requires the participant to choose from four options the word that most accurately describes how a set of individuals are feeling based on photographs of their eye region. The Faux Pas test is based on “theory of mind” (the ability to infer another’s thoughts and feelings based on social cues). Changes in this ability may underlie some of the changes in personality and social functioning frequently seen in FTD patients. The task entails hearing 20 short stories, 10 of which contain a social faux pas and 10 of which are neutral. Following each story, questions are asked to evaluate the patient’s social awareness and understanding. Patients with bvFTD, but not patients with AD do poorly on this test.
7.8 Neuropathology
The neuropathology associated with the clinical entities of FTD is heterogeneous with the unifying feature being a relatively selective and impressive atrophy of the frontal and/or temporal lobes on macroscopic examination of the brain. The pattern of atrophy in FTD is linked to the clinical phenotype: mainly left hemispheric atrophy in nfvPPA, bilateral (often asymmetrical) atrophy of the middle and inferior temporal lobes in svPPA, while in bv-FTD there is usually early bilateral orbito-mesial atrophy, followed by atrophy of the dorsolateral frontal cortex, temporal pole, hippocampal formation, and the basal ganglia.
Importantly, the lack of macroscopic atrophy does not exclude microscopic evidence of FTD pathology. Crucial also is the observation that microscopic neuropathological changes in FTD do not map onto clinical features in a one-to-one manner. The association of a highly specific constellation of symptoms and signs with more than one neuropathological signature may seem paradoxical but it may be understood in terms of systems neurodegeneration i.e. degeneration of neuronal populations that are connected structurally and functionally.
The microscopic appearance of the cortex in FTD patients includes neuronal loss, widespread spongiosis and astrocytosis obscuring normal pathology. In addition, as in other neurodegenerative conditions, FTD is characterised by specific kinds of intracellular protein inclusions. In 1911 Alois Alzheimer described round silver-impregnated inclusions and swollen neuronal perikarya (cell bodies) in cases of dementia with prominent language and behavioural symptoms, first described clinically by Arnold Pick in 1892. The inclusions would become known as “Pick Bodies”, the defining histopathological lesion of Pick’s disease.
In the last 30 years there has been significant progress in the neuropathology of FTD. This prompted the Midwest Consortium for Frontotemporal (Lobar) Degeneration and other groups to review the existing neuropathological diagnostic criteria for FTD. Currently, the heterogeneous group of disorders referred to under the umbrella term of FTD are categorized pathologically based on the biochemical composition of the observed protein inclusions.
7.8.1 Tau
Tau is a phosphorylated protein expressed predominantly in neurons. The phosphorylation of tau is believed to be critical to its ability to bind and stabilize neuronal microtubules which are cytoskeletal structures of axonal transport.
Tauopathies are characterized by deposits of tau protein and represent approximately 40–50 % of sporadic FTD cases. A tauopathy is almost always observed in post mortem studies of patients with nfvPPA, FTD with Pick bodies (Pick’s disease), and patients with BVFTD and parkinsonism linked to mutations in the microtubule-associated protein tau (MAPT) on chromosome 17 (FTDP17, see Sect. 7.9 below). Behavioural or language variant FTD patients who exhibit extrapyramidal signs often have tau pathology. Other tauopathies include cortico-basal degeneration (CBD), progressive supranuclear palsy (PSP), argyrophilic grain disease, and neurofibrillary tangle dementia (Tangle-only dementia). Although tau pathology is also present in AD, this disease is usually not referred to “tauopathy” since it always associated with co-existing amyloid pathology.
Pick bodies as originally described by Alzheimer are now known to be spherical cytoplasmic inclusions that are tau positive. Pick bodies are typically found in the cingulated gyrus, insula, inferior parietal lobule and inferior temporal gyri. They are also found in the mesial structures particularly the granule cells of the dentate fascia. White matter pick bodies are more common in CBD and PSP but can be found in FTD. Pick bodies may also be found in the basal ganglia and substantia nigra.
7.8.2 TDP-43
Many FTD cases are tau negative. Until recently, ubiquitin immunohistochemistry was the only method to detect the abnormal protein inclusions in the vast majority of these cases, prompting the term frontotemporal dementias with ubiquitin inclusions (FTLD-U), which is thought to represent about 60 % of FTD cases.
A major discovery in 2006 by Neumann and colleagues revealed that the main constituent of ubiquitin inclusions in up to 90 % of FTLD-U cases is a protein called trans-activating responsive DNA-binding protein 43 (TDP-43). It is now recognized that almost all svPPA and FTD-ALS patients as well as 50–60 % of bvFTD patients are TDP-43 positive. Of note, the vast majority of patients with sporadic amyotrophic lateral scleroses (ALS) and some familial ALS patients are also positive for TDP-43 pathology. This discovery was a major milestone in understanding the degree of overlap between ALS and FTD and led to the emergence of a new family of neurodegenerative disorders, the so called TDP-43 proteinopathies, which includes ALS as well as FTLD-U with and without ALS.
TDP-43 is a ubiquitously expressed protein encoded by the TARDBP gene on chromosome one. It is a nuclear protein, but it shuttles between the nucleus and the cytoplasm with normally low concentrations in the cytoplasm. Both the structure and cellular distribution of TDP-43 is abnormal in TDP-43 proteinopathies. TDP-43 loses its nuclear position and aggregates mainly in cytoplasm. The TDP-43 protein itself is truncated at the N-terminus while the remaining C-terminus fragment is abnormally phosphorylated to form insoluble cytoplasmic protein deposits observed in the brain and spinal cord in FTLD-U and sporadic ALS.
Brains with TDP-43 pathology can display neuronal cytoplasmic inclusions (NCIs), neuronal nuclear inclusions (NNIs) and/or dystrophic neurites (DNs). These changes have been described in the neocortex and hippocampus and the lower motor neurons in both FTLD-U and ALS. On other hand, TDP-43 positive glial inclusions (GI) were mainly considered a feature of ALS, but have been described in FTLD-U.
There is pathological heterogeneity within the TDP-43 proteinopathy family with more than one classification proposed. The harmonized classification system, recently published by Mackenzie et al. in 2011, described four main subtypes of TDP-43 pathology (see Table 7.4).
The pathological subtypes summarized in Table 7.4 have distinct clinical and genetic correlations. In subtype A, the clinical presentation is usually bvFTD or nfvPPA (rarely svPPA) with high prevalence (up to 50 %) of familial disease. This subtype is consistently reported in patients with GRN gene mutations and has recently been reported in C9orf72 gene mutations.
Subtype B is associated a clinical picture of FTD-ALS or bvFTD and is associated with generally poor prognoses. Genetically, subtype B is linked to mutations in the C9orf72 gene.
Subtype C is associated with the svPPA variant of FTD and less commonly bvFTD but there are no genetic associations to date.
Subtype D is associated with familial Inclusion body myopathy with Paget Disease of Bone and frontotemporal dementia (IBMPFD) which is due to mutations in the valosin-containing protein (VCP) gene.
Detailed discussions of these gene mutations are included in the Sect. 7.9 below.
Of note, the authors of the new classification highlighted its limitations which include failure to incorporate sub-cortical changes or describe the TDP-43 pathology recently reported in normal aging population, in the Guam Parkinson Dementia Complex patients, in about 20 % of AD patients, and up to 70 % of patients with hippocampal scleroses with and without AD as demonstrated by Amador-Ortiz et al. (2007).
7.8.3 FUS
Up to 10 % of FTLD-U patients are not only tau negative but also TDP43 negative. In addition, 15 % of ALS-FTD cases do not belong to the FTLD-U subtype and do not stain with ubiquitin or TDP-43. Interestingly, most cases of familial ALS associated with mutations in the superoxide mutase gene are also TDP-43 negative.
In 2009, the inclusions in some TDP-43 negative FTLD-U cases and some familial ALS cases are found to be positive for Fused in Sarcoma (FUS) protein. FTLD-U patients who are positive for FUS protein are termed FTD-FUS.
FUS is a protein involved in RNA processing and is strikingly similar to TDP43 in in structure and function. Nuclear FUS is believed to play an essential role in regulation of transcription and pre-mRNA splicing while in the cytoplasmic FUS is likely to be involved mRNA transport.
All reported cases of FTLD-FUS have been associated with a clinical diagnosis of bvFTD (with or without the signs of MND).
7.8.4 Rare Subtypes
A small number of tau negative FTLD-U cases are negative for TDP-43 and FUS. These are referred to as FTLD- ubiquitin proteasome system (FTLD-UPS).
Other rare types of FTD pathology include basophilic inclusion body and neuronal intermediate filament inclusion disease.
There are also FTD cases that are negative for tau as well as ubiquitin. They form a rare and controversial entity referred to a Dementia lacking distinctive histopathology (DLDH). This previously large group of identified pathology has been gradually replaced by newly identified pathological variants (Fig. 7.2).
7.8.5 Clinical Phenotype and Neuropathology
As noted in the beginning of this section, the clinical presentation in FTD does not map in a one-to-one manner with the clinical phenotypes. The exceptions to this general rule are FTD-ALS which is generally positive for TDP-43. In a recent study by Chare and colleagues (2014) the newly proposed clinical classifications of FTD were retrospectively applied to 135 autopsy ascertained FTD cases from the Cambridge Brain Bank (UK) and the Sydney Brain Bank (Australia). The brain pathology underlying different FTD syndromes in that cohort was as follows:
-
bvFTD patients :42 % FTLD-Tauopathy, 30 % FTLD-TDP-43 proteinopathy, 13 % FTLD-FUS, 9 % Alzheimer’s type pathology, and 6 % other rare FTLD pathologies;
-
svFTD : 68 % FTLD-TDP-43 proteinopathy while both FTLD-tauopathy and AD type pathology were responsible for 16 % of cases each;
-
nfvPPA: 50 % FTLD-tauopathy, 31 % AD type pathology, and 19 % TDP43-proteinopathy; and
-
lvPPA: 77 % AD type pathology, 14 % TDP-43 proteinopathy and 9 % FTLD-tauopathy.
7.9 Genetics
The progress in this area, moribund for decades despite the recognition of the important of heritability since the 1920s, has been rapid and ever-expanding in the last two decades. The traditional disease dichotomy of ‘sporadic’ versus ‘genetic’ is still used but is increasingly difficult to support, due to the likely polygenic factors influencing sporadic FTD. Nevertheless, the first step in determining whether there is a genetic influence in a disorder is to establish the frequency of a family history of the disorder.
The earliest well documented large pedigree was first reported in 1939 by Sanders and Colleagues who described an autosomal dominant dementing disorder with behavioural and cognitive disturbances with relatively preserved memory affecting a Dutch Kindred. However, the earliest estimates of the frequency of family history came from the Lund and Manchester clinico-pathological series, which estimated that up to 50 % of patients with FTD had a first degree relative with dementia. More recent studies have confirmed that about 30–50 % of FTD patients have family histories of dementia. However, the accuracy of ascertainment of familial disease is confounded by informant reliability, the late onset of the disease, the possibility of death before disease expression, and the fact that informants often report a vague history of “dementia” of unknown type in elderly relatives. It is also worth noting that the variable phenotype of FTD seems to have a direct effect of reported rates of familial disease with highest rates reported in FTD with co-morbid ALS (up to 60 %) and lowest rates reported in svPPA variants of FTD (20 % or less).
The main genes are associated with familial FTD are listed here in chronological order of discovery
-
1.
MAPT gene on chromosome 17, (1998),
-
2.
VCP gene on chromosome 9, (2004),
-
3.
CHMP2B gene on chromosome 3, (2005),
-
4.
GRN (or PGRN) gene on chromosome 17 (2006),
-
5.
TARDBP gene on chromosome 1, (2008),
-
6.
C9orf72 gene on chromosome 17, (2011),
-
7.
SQSTM1 gene on chromosome 5 (2012).
Mutations in GRN, MAPT and C9ORF72 together account for the majority of cases, with the C9orf72 repeat expansion reported to account for up to half familial cases in some populations. On the other hand, mutations in VCP, CHMP2B, SQSTM1 and TARDBP genes are rare, each explaining less than 1–5 % of the familial FTLD. We provide below a brief summary of the phenotypic and neuropathological profile reported with each of these gene mutations.
7.9.1 MAPT Gene Mutations
In 1994, Wilhelmson et al. described a large Irish and American Kindred with a genetic locus linked to 17q21-22 where the tau gene is located. The disease, originally known as Disinhibition-Dementia-Parkinsonism-Amyotrophy Complex or DDPAC, was reported to have a highly variable phenotype. In 1998, the MAPT gene was identified and the disease was subsequently termed FTD with Parkinsonism linked to chromosome 17 or “FTDP-17”.
The age of onset in patients with this disease is between 30 and 65 years. The clinical spectrum encompasses behavioural and cognitive changes typical of bvFTD with memory and praxis relatively preserved. By definition most patients have prominent parkinsonian features while amyotrophy is less common. Most recently, it has become clear that such widely varying clinical syndromes such as PSP, CBS and FTD can co-exist within families with MAPT mutations. At autopsy MAPT positive patients are usually positive for the microtubule-associated protein Tau.
Tau has six known isoforms of tau protein which are produced by alternative splicing of exons 2, 3, and 10. Alternative splicing of exon 10, results in tau isoforms with either three or four amino acids repeats in the C-terminus microtubule binding domain (3R and 4R isoforms respectively). More or less equal amounts of the 3R and 4R isoforms are produced in normal circumstances. Pathogenic MAPT mutations are thought to alter the splicing processes in a manner that promote an increase in the production of 4R isoforms. The proposed effects of this change are reduced binding to microtubules, formation of neurotoxic aggregates and abnormal axonal transport.
7.9.2 VCP Gene Mutation
In 2004, mutations in the valosin-containing protein (VCP) gene located on chromosome 9p13.3 were identified. The protein product of this gene is a ubiquitously expressed, highly abundant, multi-function ATPase which has been reported to play a role in the assembly of the endoplasmic reticulum and Golgi body, protein breakdown, inhibition of protein aggregation as well as DNA replication. Pathogenic mutations are believed to cause loss of function leading to disruption of the ubiquitin-proteasome mediated protein degradation system, autophagy, and/or ATP production within the cell.
Clinically VCP mutations are associated with inclusion body myopathy with Paget’s disease of bone and frontotemporal dementia (IBMPFD). This condition usually presents with a disabling myopathy starting in the proximal muscles then spreading to involve the distal muscles, as well as the heart and respiratory muscles. Muscle biopsy show sarcoplasmic inclusion bodies reactive with ubiquitin and TDP-43. Osteolytic bone lesions and FTD usually emerge later in the course of the disease. On autopsy, brain pathology is usually consistent with FTLD-TDP subtype D (see the Sect. 7.8).
7.9.3 CHMP2B Gene Mutation
In 1984 a researcher came across a very large family in Jutland in Denmark with an unusual dementia. There were over 27 affected individuals with a very wide range of clinical variability. In 1995 genetic linkage to chromosome 3 was established. In 2005 mutations in the gene encoding the chromatin modifying protein 2B (CHMP2B) at chromosome 3p11.2 was identified.
The CHMP2B gene protein product is a heteromeric ESCRT-III complex expressed by neurons and is believed to play an essential role in endosomal-lysosomal function, protein breakdown, and neuronal survival. Mutations usually result in aberrant splicing affecting the C-terminus of the protein.
To date, CHMP2B gene mutations have only been reported in Danish Kindreds manifesting clinically as familial FTD with autosomal dominant mode of inheritance. Pathology is usually ubiquitin positive FTD (FTLD-U) that is not only tau negative but also TDP-43 negative.
7.9.4 GRN Gene Mutations
In 2006, only 1.7 M nucleotides centromeric to the MAPT gene, a mutated gene located on chromosome 17q21.31 was identified. The GRN gene codes for progranulin, a precursor of granulin, a ubiquitously expressed growth factor. Following translation, splicing and processing in the Golgi body and endoplasmic reticulum (including aspargine-linked glycosylation and enzymatic cleavage) the mature glycoprotein (granulin) is secreted. Granulin is believed to be a neurotropic factor involved in a wide range of functions including tissue repair, inflammation, neuronal growth, and tumorigenesis. The vast majority of reported pathogenic mutations reported to date cause reduced protein expression by disrupting either translation or splicing.
GRN gene mutations are responsible for 10–20 % of familial FTD and are associated with an autosomal dominant mode of inheritance. Pathologically GRN mutations have been linked to often to severe, often asymmetrical, frontal atrophy that can extend to the basal ganglia with Mackenzie et al. in 2006 reporting changes in the caudate and substantia nigra. Microscopically GRN mutations are consistently associated with FTD-TDP43 Type A pathology (see Sect. 7.8 above).
Phenotypic expression is less consistent. The clinical presentation is predominantly bvFTD but nfvPPA and rarely ALS-FTD kindreds have been reported. A possible explanation for this heterogeneity in phenotypic expression was proposed by Rogalski et al. The authors observed that dyslexia was overrepresented in patients with PPA and their first degree relatives when compared to controls and AD patients. This led to the interesting proposal that the phenotypic expression may be a function of a latent vulnerability within the kindred as it becomes “the locus of least resistance”.
7.9.5 TARDBP Gene Mutations
In 2006 TDP-43 was identified as the major constituent of vast majority of ubiquitin positive inclusions in both FTD and ALS. In 2008 mutation in the transactive response-DNA binding protein (TARDBP) gene on chromosome 1 was identified as a cause of ALS. Since then more than 30 mutations in the TARDBP gene have been reported. Almost all reported TARDP mutations are in exon 6 affecting the highly conserved C-terminus of the TDP-43 protein known to be involved in RNA recognition.
Mutations have been reported primarily in familial and occasionally sporadic ALS or ALS-FTD, but recently the phenotype has been extended to include patients with bvFTD without motor involvement. Yet the current literature suggests that TARDBP mutations are rare, accounting for less than 1 % of FTD cases. A recent Italian study suggested that TARDBP related FTD may be associated with a predominance of svPPA variants and temporal atrophy on imaging.
7.9.6 C9ORF72 Hexanucleotide Gene Mutations
Since 1991, linkage studies of ALS-FTD and FTD kindreds suggested a locus on chromosome 9p.21. In 2011 two different groups identified the genetic mutation at that locus as a substantial expansion of GGGGCC hexanucleotide repeats in a non-coding region of the C9orf72 gene (DeJesus-Hernandez et al. 2011; Renton et al. 2011). Affected individuals had 60–1600 repeats, whereas normal individuals have less than 23–25 repeats.
The gene is known to have three transcripts. The function of the final protein product is not confirmed but there is evidence to support a role in in endocytic and autophagic pathways as well as motor function. Some evidence supports loss of function as the main pathomechanism underlying the C9orf72 gene mutation as it is associated with reduced expression of gene’s three major transcripts. However, toxic gain of function is also possible in the context of the presence of brain aggregates of both aberrant protein and abnormal repeat RNA. The latter aggregates are termed RNA foci and form core of the ‘toxic RNA’ hypothesis.
The current data suggests that the C9orf72 repeat expansion accounts for 40–50 % of cases of familial ALS, 20–25 % of FTD cases, and 0–7 % of the sporadic cases in white Americans, Europeans and Australians, with recent evidence raising the possibility of a single founder in Europe. Reported rates are much lower in non-white populations including the Chinese and the Japanese populations. Inheritance follows an autosomal dominant pattern with incomplete penetrance.
Studies published to date confirm that the most common clinical presentation associated with the C9orf72 repeat expansion is behavioural variant FTD and/or ALS. Other characteristics, including younger age at onset, florid psychotic symptoms, anxiety, poor memory, and poor outcome have been reported but are not consistent Clinical observations predating the discovery of the C9orf72 gene mutation suggested the presence of delusional ideation in younger bvFTD patients with tau negative pathology, though the anatomic substrate was not fully understood. It is conceivable that these patients might have been positive for the C9orf72 repeat expansion.
C9orf72 associated disease also has a distinct radiological and pathological signature. There is symmetrical fronto-temporal atrophy but changes often extend to involve the parieto-occiptal cortex and the cerebellum. The pathological hallmark of C9orf72 related disease is TDP-43 inclusions (predominantely subtype B) and ubiquitin-binding protein p62/sequestosome 1 inclusions. The latter inclusions are considered highly specific to the C9orf72 gene mutation as they are rare in non-C9orf72 FTD. The p62/sequestosome 1 inclusions can occur with and without co-existing TDP-43 and have been observed in the frontal neocortex, cerebellum and hippocampus.
It is important to note that C9orf72 gene mutations have been reported in patients presenting with the clinical picture of AD, cortico-basal syndrome (CBS), dementia with lewy bodies (DLB), and Huntington disease (HD).
7.9.7 SQSTM1 Gene
The sequestosome 1 (SQSTM1) gene, located on 5q35, encodes p62 protein adapter protein which is involved in multiple functions including autophagy, oxidative stress response, and cell signalling. As noted above, neuronal p62-positive inclusions have been shown to be abundant in both FTD and ALS patients, particularly disease associated with the C9orf72 gene mutation. In addition, an increase in p62 immuno-reactivity has also been reported in AD, DLB, Parkinson’s disease and HD.
Mutations in the SQSTM1 gene were initially thought to cause only Paget’s disease of the bone, but in 2011 mutations in this gene were also reported in patients with ALS. In 2012, Rubino and colleagues from the TODEM study group screened for this mutations 170 unrelated FTD patients (138 bvFTD, 6 svPPA, and 19 nfvPPA), 124 ALS patients, 288 patients with Paget’s disease and 145 healthy controls. Noncoding SQSTM1 mutations were found in three patients presenting with bvFTD. Aggressiveness, mood lability, and social withdrawal were prominent behaviours in these patients. In the same study mutations were also found in three patients presenting with bulbar ALS but not in patients with Paget’s disease or healthy controls.
A recent French series reported SQSTM1 missense mutations in 4 unrelated families with FTD out of 188 suggesting that this mutation is responsible for only about 2 % of familial cases of FTD. Of note, co-morbid parkinsonian signs were observed in 2 families, 1 family had co-existing clinical symptoms of Paget disease of bone, and 1 family had clinical symptoms of FTD-ALS.
7.9.8 Other Mutations
As discussed is the Pathology section FUS protein was identified in many TDP-43 negative FTLD-U cases. Recently, mutations in the Fused in Sarcoma (FUS) gene have been reported. Although the clinical phenotype associated with FTLD-FUS pathology is invariably FTD (with or without MND), the vast majority of cases of reported FUS gene mutations had ALS or ALS-FTD with very rare reports of pure FTD (Van Langenhove et al. 2010).
A few families with FTD have been shown to have mutations in Presenilin1 a gene usually associated with familial AD. This finding confirms the notion of convergence amongst mechanisms of neurodegeneration and is reciprocal to recent finding of MAPT polymorphisms in large AD cohorts. The exact role of presenilin in FTD is unclear but the mutations appear to be novel.
Prion protein (PRNP) gene mutations have also recently been associated with clinical pictures resembling FTD. In addition, there is a single reported case of FTD related to UBQLN2 gene mutation which is a recognized but rare cause of ALS.
7.10 Biomarkers
In FTD, a number of biomarkers are being used clinically and in a research capacity including brain imaging, neurophysiology and biological markers.
7.10.1 Brain Imaging
Brain imaging is an essential and routine examination in any dementia to exclude non-neurodegenerative (e.g. neoplastic or vascular) pathology and aid in the diagnostic process. Advances in brain imaging have grown at a remarkable pace in the last 10 years. Specific patterns of lobar atrophy can now be reliably mapped to clinical phenotypes. In this section, imaging in FTD will be discussed in the context of MRI-based techniques, non-MRI based functional imaging and other techniques worth noting including those that are in the experimental phase.
7.10.1.1 MRI-Based Techniques
7.10.1.1.1 Structural MRI
T1 weighted MRI is the method of choice for evaluation of structural changes in the brain. In particular, the addition of coronal imaging to the standard axial slicing allows for the detection of visually obvious atrophy in frontal and temporal regions. T2 weighted imaging usually using fluid attenuated sequences (FLAIR) allows for the evaluation pathology that might exclude FTD or point to a mimic syndrome such as vascular related white matter pathology.
Quantitative MRI remains generally a research tool and embodies three main techniques; volumetric analysis of specific brain regions, Voxel Based Morphometry (VBM), and serial co-registration.
The aim of structural MRI brain imaging in FTD is to exclude other pathologies and to document the presence and pattern of brain atrophy. Brain atrophy is one of the cardinal features of all neurodegenerative processes, even if it occurs at variable rates and by vastly different and sometimes convoluted processes. The most convincing hypothesis underlying the atrophic process suggests that we can no longer think of this as a generalized shrinkage but more along the lines of a Wallerian degeneration constrained by neuronal and functional networks. The process often starts focally and spreads along these networks whose predictability gives us the clinical phenotypes we know.
Brain atrophy on structural MR imaging is the most reproducible feature of all FTD subtypes. The presence of true focal atrophy has a high positive predicative value for clinical dementia. On the other hand the absence of atrophy has been noted increasingly in cases deemed to have all clinical, behavioural and neuropsychological features of bvFTD. This raises the question of either a behavioural phenocopy of FTD or else cases where atrophy is either negligible or will occur later in the disorder.
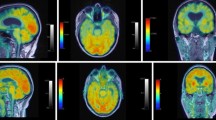
The pattern of brain atrophy on standard MRI predicts FTD clinical subtype. Focal asymmetrical frontal lobe atrophy with or without temporal atrophy is the best predictor of the bvFTD subtype (see Fig. 7.3a). Recent research indicates that the process starts in the orbitofrontal and cingulate regions and spreads to insular cortex and thence to the basal ganglia.
(a) Behavioural variant FTD. Note generalized atrophy in frontal and temporal regions (arrows) with slight asymmetry favouring more atrophy on the left. (b) Semantic dementia. Note anterior temporal tip atrophy bilaterally (arrows). (c) Progressive non-fluent aphasia. Note asymmetric atrophy favouring the left temporal lobe (arrows)
Recent VBM data suggests that even within the bvFTD group there are several distinct subgroups. One study described four subgroups based on patterns of grey matter loss. Two of these displayed temporal lobe atrophy, either in isolation (“temporal-dominant subtype”) or in combination with frontal and parietal lobe involvement (“temporo-fronto-parietal subtype”). The other two subtypes were characterized by predominance of frontal lobe atrophy with or without temporal lobe involvement (“fronto-temporal subtype” and “frontal-dominant subtype” respectively). Interestingly, while these radiological subtypes did not differ with regard to the severity of behavioural impairment, they did display significant correlations with cognitive performance as well as genetic and pathological diagnoses (which were available in about half the cohort).
Other VBM studies attempted to correlate patterns of atrophy in bvFTD to specific behavioural manifestations and reported correlations include those with apathy (frontal pole), disinhibition (subcallosal region), and abnormal motor control (dorsal medial atrophy).
MRI findings in svFTD tend to be more consistent showing atrophy of the polar regions of the temporal lobe along with the fusiform gyrus (see Fig. 7.3b). Patients typically have focal anterior temporal pole atrophy with involvement of the inferior surface (especially the fusiform gyrus) more than superior. The atrophy is usually bilateral but asymmetrical, with predominant left-sided atrophy being more common than predominant right-sided atrophy. There is still a considerable debate whether this left sided dominance of svFTD reflects biological differences or a differential pattern of referrals in which patients with a predominantly right sided atrophy presenting with a clinical picture dominated by behavioural changes are referred to psychiatric rather than neurological services.
A variable amount of frontal atrophy is almost always found in svFTD. It is clear that while atrophy maybe wide spread in both frontal and temporal lobes as in other cases of FTD, it is the predominance of the anterior and inferior temporal lobe atrophy that appear to correlate with the main clinical findings in svFTD. Loss of ability to form semantic word associations correlates most strongly with damage in Brodmann’s areas 37 and 38 which include the fusiform gyrus and inferior temporal gyrus and the anterior temporal pole respectively (for a more detailed discussion refer to the Chap. 3).
Imaging findings in nfvFTD and lvFTD are less reliable than in either bvFTD or svFTD. However, most MRI studies report predominant involvement of the left hemisphere. In nfvFTD, VBM studies found atrophy in the region of the including the pars opercularis of inferior frontal lobe (Broca’s area), upper part of the temporal pole, the lentiform nucleus, middle frontal gyrus (see Fig. 7.3c). Atrophy of anterior insula and basal ganglia has also been described in this variant.
In lvFTD, the variant most commonly associated with AD rather than FTD pathology, VBM findings have shown more posterior abnormalities with the angular and supramarginal gyrus and other posterior perisylvian regions involved.
Although most MRI studies focused on the differences between FTD clinical variants, Whitewell et al. examined the pattern of atrophy in different genetic sub-groups suggesting specific radiological signatures in these subgroups: symmetrical anteromedial temporal atrophy in MAPT-related FTD; symmetric frontal atrophy with variable involvement of other brain regions including the cerebellum in C9orf2-related FTD; and asymmetrical inferior frontal and tempero-parietal atrophy in GRN-related disease.
7.10.1.1.2 Diffusion Tensor Imaging (DTI)
While the above mentioned studies examined pattern of grey matter loss, DTI is a MRI-based technique that examines white matter integrity. Several DTI studies have reported widespread white matter injury in FTD patients most prominent in the white matter tracts underlying the frontotemporal cortex. Recent evidence suggests that DTI may be superior to volumetric grey matter analyses in differentiating FTD-tau and FTD-TDP-43.
Agosta and colleagues descried bilateral and widespread white matter damage in bvFTD including frontal and temporal areas such as the anterior superior and inferior longitudinal fasciculus, the inferior fronto-occipital fasciculus, the anterior cingulate, and parts of the corpus callosum. DTI changes in FTD language variants tended to be more focal and asymmetrical (more on the left). The best predictors svPPA were involvement of the left uncinate and inferior longitudinal fasciculus, while nfvPPA was associated with changes in the left arcuate fasciculus and superior longitudinal fasciculus.
Notably, a large study of FTD gene carriers (MAPT or GRN) with no clinical evidence of the disease showed changes in the right uncinate fasciculus compared to controls suggesting that this technique may play a role in detection of early and pre-symptomatic FTD patients.
7.10.1.1.3 Functional MRI (fMRI)
Functional MRI (fMRI) is a recent technique employed to investigate regional activation during specific tasks (activation fMRI) and functional connectivity between brain regions as measure neural network integrity (resting state or functional connectivity fMRI). The latter endeavour is of particular interest to FTD researchers in the context of the emerging hypotheses of preferential targeting of specific brain networks in different in neurodegenerative disorders.
The two main networks examined in FTD studies are the “Default Mode Network” (DMN) and the “Salience Network” (SN). The DMN is believed to be associated introspective cognitive processes e.g. strategy making, daydreaming, memory retrieval, contemplating the motives of other individuals etc. Thus the DMN is activated during wakeful rest and deactivated during cognitive tasks requiring redirection of attention to the external goal-directed behaviour. Brain regions associated with the DMN include the memory centres in the medial temporal lobe, medial prefrontal cortex (one of the core regions involved in theory of mind), the posterior cingulate cortex, the ventral precuneus and the medial, lateral and inferior parietal cortex. Decreased resting-state functional connectivity among DMN regions has been consistently shown in AD patients including patients with early or presymptomatic disease.
The SN, on the other hand, is linked to processing and adapting to salient emotional and external stimuli and goal-oriented behaviour. Components of the SN include the anterior cingulate, anterior insula, amygdala, and dorsal striatum.
Using fMRI findings, Zhou and colleagues proposed preferential damage to the SN in FTD (akin to the preferential damage of DMN in AD). This postulate was supported by decreased connectivity of the SN and increased connectivity of the DMN. As the SN may play a role in modulating the activity of other networks to ensure optimum performance during goal-directed behaviour, the damage to the SN may be connected to the observed lack of deactivation of the DMN. A combined index of DMN and SN activity differentiated FTD and AD cases with high levels of accuracy approaching 100 % in genetically and pathologically confirmed cases. Decreased SN activity would be expected to lead to poor emotional processing and decline in executive control of goal oriented tasks. An overactive DMN would facilitate excessive reliance on self-generated narratives and over-learnt behaviour and a reduced ability to adapt to external social and environmental stimuli.
Subsequent work by Farb et al. demonstrated correlations between changes in connectivity and both FTD subtypes and specific behavioural manifestations. Disrupted fronto-limbic connectivity and increased local connectivity within the prefrontal cortex was demonstrated in both bvFTD and svPPA. Prefrontal hyperconnectivity was more diffuse in bvFTD and was associated in this subgroup with higher apathy and disinhibition scores. Increased DMN connectivity in the right angular gyrus was associated with stereotypic and apathetic behaviours, a finding that was also exclusive to bvFTD.
More recent work showed within the bvFTD variant, different genetic subgroups (such as patients with and without the pathogenic c9orf72 repeat) display similar patterns of network connectivity (diminished SN activity and heightened DMN activity) despite differences in patterns of cortical atrophy on structural MRI. Moreover, the connectivity changes correlated with the severity of reported behavioural abnormalities.
Finally, there is evidence suggesting that alterations in DMN activity in FTD gene carriers may predate the emergence of clinical symptoms and cortical atrophy, which supports a potential role in pre-symptomatic screening.
7.10.1.2 Non-MRI Based Functional Imaging
[99mTc]-hexamethylpropyleneamine oxime single-photon emission computed tomography (SPECT) and [18 F]-fluorodeoxyglucose (FDG)-PET are increasingly being used to help with the diagnosis of FTD, mainly by detecting regional hypometabolism in cases where there is no evidence of focal atrophy of structural imaging.
Changes in frontal regions extending to the anterior temporal regions on SPECT and FDG-PET have been shown to be sensitive but not specific for bvFTD. However, specificity increases in individual cases where asymmetrical frontal abnormalities are demonstrated in the context of little or no atrophy MR imaging. PET changes can be widespread but are most significant in the mesial frontal regions, consistent with the focal onset of many bvFTD patients.
The value of functional imaging in svFTD patients in the setting of typical clinical and radiological findings is limited. There is usually dramatic bilateral hypometabolism in the anterior temporal lobes, cortical regions that are universally affected by regional atrophy in this condition. In nfvPPA, functional imaging may show widespread abnormalities but the focus is usually left posterior fronto-insular regions. The imaging of lvPPA shows changes in the left posterior perisylvian, lateral temporal and parietal cortex.
Functional imaging, particularly FDG-PET can be regarded as an established imaging tool in FTD and changes in FDG-PET and SPECT are now incorporated in recent diagnostic criteria for FTD syndromes (see Sect. 7.11 below).
7.10.1.3 Other Techniques
Amyloid-PET neuroimaging, using amyloid-β-detecting 11C-Pittsburgh compound B (PiB), is a sensitive and specific marker for underlying Aβ amyloid deposition. Amyloid deposition is an early event in AD while it is rare in FTD. As such there is increasing evidence that Amyloid-PET has a promising role in diagnosing AD even in early or pre-manifest stages and in differentiating it from FTD. However, the use of amyloid PET is restricted by its prohibitive costs and logistics which largely relate to the short half-life of PiB, the most established ligand in the market. Newly developed 18 F-labelled tracers with longer half-lives are increasingly gaining FDA approval and may result in wider use of this technology in a clinical context. Clinicians using this technology to exclude FTD must be aware of the rare patients reported in the literature with mixed FTD and AD pathology.
Other advances in neuroimaging include techniques designed to identify protein aggregates in vivo including ligands that bind to tau and to TDP-43. As these techniques are still in progress with only a handful in-human studies, their potential utility in a clinical context is yet to be established.
7.10.2 Neurophysiology
7.10.2.1 Electroencephalography (EEG)
The use of EEG in dementia was more widespread before the advent of brain imaging though even then its clinical and diagnostic use was limited. There is a tendency for the background organization features of the EEG to be preserved in FTD whereas in AD the emergence of background slowing is common as the disease progresses. The reasons for such preservation in FTD are unclear but the observation may be related to the relatively rare association between FTD and seizures compared to AD.
In the research lab, quantitative EEG (qEEG), which is a digital algorithm of the different wave frequencies, has tended to confirm the preservation of resting alpha rhythm but the loss of some faster frequencies in the Beta range. Further work in this area is required before qEEG is to be considered a useful biomarker.
7.10.2.2 Nerve Conduction Studies and Electromyography (NCS/ EMG)
Because of the co-existence of ALS and FTD, NCS and EMG studies have become an important diagnostic tool in FTD patients who have associated motor or swallowing difficulties (See Chap. 8). Limited data suggests the presence of motor neuron dysfunction in FTD patients on the neurophysiological evaluations in the context of minimal or absent clinical signs or symptoms. As yet, the status of EMG as a biomarker is unclear, since the use of EMG in unselected FTD cohort is not likely to be either cost-effective or clinically valuable.
7.10.2.3 Transcranial Magnetic Stimulation (TMS)
TMS is a promising non-invasive neurophysiological tool that examines cortical networks by testing excitatory and inhibitory properties of the cortex, conduction in the cortico-spinal tracts, and functional integrity of cortical structures including the corpus callosum. Advances in TMS have enabled in vivo investigations of the cortical cholinergic, glutaminergic and GABAergic circuits.
Although TMS investigation in FTD is still in its early stages, the available data provide fascinating insights into the disease such as the presence of motor circuit abnormalities in the absence of clinical evidence of pyramidal involvement. Limited data also suggest that TMS may have a potential role in disease therapeutics as evidenced by improved language function in PPA patients following high frequency TMS over the dorsolateral prefrontal cortical region. Further research is needed to confirm or refute the applicability of TMS to FTD clinical care.
7.10.3 Proteomics
The identification of a reliable protein biomarker in the cerebrospinal fluid (CSF) or serum facilitates in depth investigation of disease proteomics during life (as opposed to neuropathological examinations on autopsy). In addition, such a biomarker would potentially be useful in diagnoses of atypical, early stage, and or even pre-symptomatic patients as well as monitoring of disease progression and/or response to therapeutic agent. In general, CSF biomarkers have attracted more interest in neurodegenerative conditions as they are more likely to mirror the pathological processes taking place in the CNS.
The progress in CSF biomarkers (tau and abeta-42) in AD has been a major milestone in AD research, and these biomarkers have already been incorporated in the updated AD diagnostic criteria (see Chap. 4). The identification of reliable CSF (or serum) biomarkers in FTD remains elusive.
The detection of low or high levels of tau, progranulin, or TDP-43 are considered to be the “holy grail” of biomarker research in FTD since they would be conforming with key elements of what is already known about its molecular pathogenesis. The most obvious reason our failure to find such a biomarker lies in the pathological heterogeneity of FTD compared to AD. For example, measuring tau is less likely to be of value in tau negative FTD. This is complicated by the overlap between clinical and pathological phenotypes which means that a group of patients belonging the same FTD variant could eventually prove to have either tau-positive, or ubiquitin positive, or even occasionally AD pathology. Moreover, there is the problem of FTD phenocopy syndrome, which may include normal brains.
7.10.3.1 Tau and aBeta1-42
By far, the most investigated CSF biomarkers in FTD are tau and aBeta1-42, mainly in the context of studies evaluating the use of these biomarkers in differentiating FTD from AD.
Studies of CSF tau in FTD have yielded contradictory results (normal or high). It is important to note that CSF tau does not necessarily reflect brain pathology, as normal tau levels have been documented in Tau positive FTD patients (e.g. MAPT-related FTD).
Levels of CSF aBeta1-42 in FTD have been shown to be more consistently, though not universally, low in FTD. However, this observation is not useful in differentiating FTD from AD, which is also associated with low level of the same biomarker. Interestingly, other amyloid Beta1 sub-species have also been reported to be low in FTD, with data suggesting that a reduction in aBeta1-38 may be more specific to FTD and that levels of some species (e.g. aBeta1-37) vary in different FTD variants. Further research is needed to elucidate the role of abeta1 protein as a disease biomarker in FTD.
7.10.3.2 TDP-43
Studies of CSF TDP-43 in FTD have also been inconclusive. Some studies showed similar levels to controls. Other studies reported TDP-43 levels that were significantly higher than those of controls, but not significantly higher than the respective serum levels, raising the possibility that the identified protein was blood-derived.
More promising is the attempt to focus on more CNS specific isoforms of the protein, such as abnormally phosphorylated TDP-43 which is the main constituent of protein aggregates in TDP-43 proteinopathies. A recent study documented that FTD patients with likely TDP-43 pathology (positive gene mutations in C9orf72 or GRN) had higher serum and CSF levels of phosphorylated TDP-43 compared to other FTD patients and controls, though in case of controls only the differences in serum levels reached statistical significance.
7.10.3.3 Progranulin
Serum prograulin has been shown to low in FTD patents with null pathogenic mutations of the GRN gene including those in the pre-symptomatic stage. However, proganulin levels are normal in FTD patients without these mutations, limiting the usefulness of this biomarker to clinical trials targeting GRN-related FTD.
7.10.3.4 Ubiquitin
Ubiquitin is another major constituent of abnormal protein aggregates in FTLD-U. CSF ubiquitin levels in FTD patients have been reported to be significantly higher than those in AD patients, but not significantly different from that of healthy controls. This suggests a potential role for CSF ubiquitin in differentiating AD and FTD, but further research is needed to replicate these findings.
7.10.3.5 Other Biomarkers
Neurofilaments (NFH), often in phosphorylated isoforms, constitute an integral part of the axonal cytoskeleton. The high levels of NFH in neurons have led to an interest in investigating their CSF levels in several neurodegenerative disorders as a surrogate marker of neuronal degeneration and loss. Several studies have shown remarkably high CSF levels of both light chain and hyperphosphorylated heavy chain neurofilaments in FTD. The degree of NFH phosphorylation is increased in FTD compared to both AD and controls. Of note, levels were normal in gene carriers with pre-manifest disease. The pathological significance of these neurofilaments remains to be determined.
Less established is the role of pro-inflammatory cytokines (e.g. TNF-alpha and IL6) as biomarkers in FTD as the few studies conducted in this area arrived at contradictory conclusions.
Finally, several recent studies employed advanced mass-spectrometric techniques to simultaneously examine multiple analytes simultaneously (15 to more than 2000) in an attempt to identify a reliable biomarker in FTD. Candidates proteins proposed to date (alone or in combination) include the neurosecretory protein VGF, transthyretin, S-cysteinylated transthyretin, truncated cystatin C and a fragment of chromogranin B. However, there is still a considerable journey ahead prior to the translation of these efforts into a biomarker of practical value in research or clinical setting.
7.11 Diagnostic Criteria
The diagnostic criteria in FTD have been subject to a number of changes over the years. The first set of criteria was devised at a consensus meeting in 1996 (Neary et al. 1998) where it was decided to separate the three clinical syndromes in FTD (bvFTD, progressive non-fluent aphasia and semantic dementia) and criteria were devised for each. Core diagnostic features thought to be integral to each syndrome had to be present to make the diagnoses. Supportive diagnostic features were not considered necessary for a diagnosis but were considered characteristic of the syndrome and “added more weight” to the diagnosis. Exclusion criteria were listed to prevent the inclusion of other forms of cortical dementia, specifically AD and psychiatric disorders. All must be absent in order to make a diagnosis.
Some researchers (McKhann et al. 2001) have suggested simplifying the clinical criteria into either behaviour or language presentation of FTD and then qualifying this classification further with a neuropathological diagnosis when and if a patient comes to autopsy. However, most researchers of the language variants of FTD would view svPPA and nfvPPA as distinct and separate entities. The risk of combining these syndromes is that important clinical findings, including potential biomarkers are missed because the population studied is heterogeneous.
Indeed, the tripartite division of FTD (bvFTD, svFTD, nfvFTD) have been preserved in the new diagnostic criteria, published by Rascovsky in 2011 (Table 7.5). However, the inclusion criteria have been substantially modified. In the same year, a separate revision of the diagnostic criteria and of classification the language syndromes has been agreed upon and published by Gorno-Tempini et al. (2011). The proposed criteria create a four step diagnostic framework. The first step is the diagnoses of PPA as condition where the earliest, most salient and most disabling feature of the condition, at least in the initial stages, is decline in language function. The symptoms must also not be better accounted for by a non-neurodegenerative or a medical condition, The subsequent steps of the diagnostic framework aim to identify the different variants of PPA with increasing levels of certainty (see table 7.6).
7.12 Therapy
Before the treatment, comes the diagnosis. Indeed, appropriate care of FTD patients starts with communicating the diagnoses. The diagnoses and its implications should be explained to patients, families and caregivers in lay terms and in a sensitive manner. This should be carried out in a specialist environment with an understanding of the unique features of FTD such as the personality changes, loss of empathy and socially embarrassing behaviours. Multidisciplinary support should be offered.
7.12.1 Disease Modifying Therapy
Currently there are no FDA approved disease modifying therapies for FTD.
However, preclinical and early clinical phase trials of true disease modifying therapies are underway. The main targets are protein pathways known to be integral to the pathological process in FTD including tau, progranulin, and TDP-43 (described in the Sect. 7.8). This approach has produced several promising candidates. However, the logistic difficulties intrinsic to a disease such as FTD are significant. The first clinical trial for a disease modifying therapy in bvFTD was initiated in 2013. This involved TRx0237 (also called LMTX™) which acts by reducing levels of aggregated or misfolded tau protein. Many patients had to be excluded because of lack of supportive MRI changes and/or diagnostic uncertainty raising the possibility of “FTD phenocopies”. Others displayed advanced cognitive impairment, lack of interest and/or inability to give informed consent. Other challenges included the reduced ability of bvFTD patients to wait for prolonged periods or to tolerate MRI scanning. The patients’ behavioural changes posed significant challenges to untrained staff. Of the first 275 potential subjects who were pre-screened, 55 progressed to formal screening, and only 20 patients proceeded to the randomisation. The results of the trial are still pending.
Granulin, the product of the GRN gene, displays low levels in FTD patients with this mutation. Granulin is a growth factor believed to play a role in multiple essential biological processes like regulating inflammatory reactions, energy and protein homeostasis, neurite outgrowth, and neuronal survival. Several new therapies are being developed to increase granulin including PTC124-a new chemical entity that selectively induces ribosomal read through premature but not normal termination codons. Early trials have demonstrated safety in healthy volunteers is in preclinical trials for GRN related FTD.
Davunetide is an intranasal neuropeptide therapy derived from a growth factor called activity-dependent neurotrophic protein and is believed to have neuroprotective effects. Despite early promising Phase II trials in MCI and AD patients, a more recent trial in FTD with predicted tau pathology (which included CBS and PSP) was halted following a large multicentre trial involving PSP patients reporting negative results in all outcome measures.
Preclinical studies are also investigating the therapeutic value of immune therapy or efforts to block cleavage in removing abnormal TDP-43.
7.13 Symptomatic Treatment
There are no FDA approved therapies for symptomatic treatment of the cognitive or behavioural difficulties in FTD. A few small randomised controlled clinical trials have been undertaken in FTD including those for memantine, paroxetine, trazadone, oxytocin, methylphenidate, and galantamine. These largely yielded negative results, though a few reported modest improvements in behavioural symptoms (e.g. nasally administered oxytocin, methylphenidate, trazodone, paroxetine in 1 of 2 trials).
Current therapeutic management of FTD involves the off-label use of medications based on efficacy in treating other neurodegenerative or psychiatric disorders with similar behavioural problems. Selective serotonin reuptake inhibitors or serotonin-norepinephrine reuptake inhibitors are now considered first line treatments to help control the behavioural symptoms in FTD particularly disinhibition and poor impulse control. This is based on open label studies as case controlled randomised trials have produced mixed results. A meta-analyses of trazadone and SSRIs studies suggest that the use of these medications may indeed be helpful in ameliorating behavioural changes in FTD. Case reports suggest that atypical antipsychotic agents such as quetiapine and anti-epileptics may be considered for treatment of agitation, delusions and aggression.
No drug to date has been shown to have any significant benefit on cognitive function in FTD. In some cases (e.g. paroxetine) the drugs had a negative effect on cognitive performance. There is some evidence suggesting that intra-nasal oxytocin may have a beneficial effect of recognition of facial emotions, but it has no effect on other measures of cognitive function including executive functions.
Limitations of most FTD trials published to date include small sample size and in many cases poor selection of cognitive measures.
Supportive therapy remains the cornerstone of treatment in FTD. A multidisciplinary approach is essential to tackle the range of behaviour and neuropsychiatric manifestations bvFTD. Neuropsychological strategies can help patients and caregivers cope with the worst effects of the cognitive and behavioural impairment.
Sleep disturbances should be managed by maintaining a regular sleep regime and may be aided by the use of the sedating anti-depressant e.g. Trazadone.
Speech and language therapy including the utilization of communication aids by carers .may offer some benefit to PPA patients early in the course of the illness,
Parkinsonism and amyotrophy are the most common motor manifestations of advanced FTD. Multidisciplinary care including physiotherapy, occupational therapy and speech language therapy are the cornerstone of treating motor symptoms. Riluzole in the only FDA approved treatment for motor system degeneration associated ALS. Although the parkinsonian symptoms in FTD rarely respond to dopamine replacement therapies, it is worth noting that a small proportion of these patients do report some benefit from these therapies. More detailed discussion of these disorders can be found in the relevant chapters.
Prominence of early bladder difficulty should give rise to suspicion of disorders of autonomic control such as PSP rather than FTD. However early involvement of the medial frontal lobe in both bvFTD and CBD can lead to incontinence which should be managed with intermittent catheterization, indwelling catheters, and the use of a leg bag or in some cased urinary diversion. The use of anti-cholinergic must be tempered by the possibility of increasing confusion.
The inexorable decline in function and quality of life that currently follows a diagnosis of FTD should always be met with a plan for palliation and appropriate end of life care. There has been significant improvement in the knowledge and understanding of this process amongst palliative care specialists. For further discussion see Chap. 17 .
Supportive care should always include the patient’s carer. It has been recognized that carers of patients with FTD report significant distress and depression even when compared to carers of patients with other forms of dementia. This is most likely due to the FTD patient’s behavioural dysfunction and poor social cognition. The multi-disciplinary team should be aware of these stressors on the carer. Support to the carer can be provided through advice and instruction on how to manage disruptive behaviour and the availability of respite care.
7. Conclusion
FTD researchers world-wide are working on translating the progress made over the last decade in furthering our understanding of FTD molecular pathology and proteomic into clinically relevant disease biomarkers and potentially targeted disease modifying therapy. However, until then the corner stone of caring for FTD patients continues to be the use of symptomatic pharmacological therapy and comprehensive multidisciplinary care that includes the expertise of neurologists with a special interest in cognitive neurology, clinical nurse specialists, clinical psychologists, speech therapist, physiotherapists, and palliative care specialists along with social workers.
Further Reading
Amador-Ortiz C, Lin WL, Ahmed Z, Personett D, Davies P, Duara R, Graff-Radford NR, Hutton ML, Dickson DW. TDP-43 immunoreactivity in hippocampal sclerosis and Alzheimer’s disease. Ann Neurol. 2007;61(5):435–45.
Cairns NJ, Bigio EH, Mackenzie IR, Neumann M, Lee VM, Hatanpaa KJ, White 3rd CL, Schneider JA, Grinberg LT, Halliday G, Duyckaerts C, Lowe JS, Holm IE, Tolnay M, Okamoto K, Yokoo H, Murayama S, Woulfe J, Munoz DG, Dickson DW, Ince PG, Trojanowski JQ, Mann DM, Consortium for Frontotemporal Lobar Degeneration. Neuropathologic diagnostic and nosologic criteria for frontotemporal lobar degeneration: consensus of the Consortium for Frontotemporal Lobar Degeneration. Acta Neuropathol. 2007;114(1):5–22.
Chare L, Hodges JR, Leyton CE, McGinley C, Tan RH, Kril JJ, Halliday GM. New criteria for frontotemporal dementia syndromes: clinical and pathological diagnostic implications. J Neurol Neurosurg Psychiatry. 2014;85(8):865–70.
Cummings JL. Frontal-subcortical circuits and human behaviour. Arch Neurol. 1993;50:873–80.
Diehl-Schmid J, Onur OA, Kuhn J, Gruppe T, Drzezga A. Imaging frontotemporal lobar degeneration. Curr Neurol Neurosci Rep. 2014;14:489.
Hodges JR, editor. Frontotemporal dementia syndromes. Cambridge: Cambridge University Press; 2007.
Mackenzie IR, Neumann M, Baborie A, Sampathu DM, Du Plessis D, Jaros E, Perry RH, Trojanowski JQ, Mann DM, Lee VM. A harmonized classification system for FTLD-TDP pathology. Acta Neuropathol. 2011;122(1):111–3.
McKhann GM, et al. Clinical and pathological diagnosis of frontotemporal dementia. Arch Neurol. 2001;58:1803–9.
Neary D, et al. Frontotemporal lobar degeneration. A consensus on clinical diagnostic criteria. Neurology. 1998;51:1546–54.
Neumann M, Sampathu DM, Kwong LK, Truax AC, Micsenyi MC, Chou TT, Bruce J, Schuck T, Grossman M, Clark CM, McCluskey LF, Miller BL, Masliah E, Mackenzie IR, Feldman H, Feiden W, Kretzschmar HA, Trojanowski JQ, Lee VM. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314(5796):130–3.
Nilsson C, Landqvist Waldö M, Nilsson K, Santillo A, Vestberg S. Age-related incidence and family history in frontotemporal dementia: data from the Swedish Dementia Registry. PLoS One. 2014;9(4), e94901.
Onyike CU, Diehl-Schmid J. The epidemiology of frontotemporal dementia. Int Rev Psychiatry. 2013;25(2):130–7.
Piguet O, et al. Sensitivity of current criteria for the diagnosis of behavioural variant frontotemporal dementia. Neurology. 2009;72:732–7.
Rascovsky K, Hodges JR, Knopman D, Mendez MF, Kramer JH, Neuhaus J, van Swieten JC, Seelaar H, Dopper EG, Onyike CU, Hillis AE, Josephs KA, Boeve BF, Kertesz A, Seeley WW, Rankin KP, Johnson JK, Gorno-Tempini ML, Rosen H, Prioleau-Latham CE, Lee A, Kipps CM, Lillo P, Piguet O, Rohrer JD, Rossor MN, Warren JD, Fox NC, Galasko D, Salmon DP, Black SE, Mesulam M, Weintraub S, Dickerson BC, Diehl-Schmid J, Pasquier F, Deramecourt V, Lebert F, Pijnenburg Y, Chow TW, Manes F, Grafman J, Cappa SF, Freedman M, Grossman M, Miller BL. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134(Pt 9):2456–77.
Snowden JS, Bathgate D, Varma A, Blackshaw A, Gibbons ZC, Neary D. Distinct behavioural profiles in frontotemporal dementia and semantic dementia. J Neurol Neurosurg Psychiatry. 2001;70(3):323–32.
Vossel KA, Miller BL. New approaches to the treatment of frontotemporal lobar degeneration. Cur Opin Neurol. 2008;21(6):708–16.
Weintraub S, Mesulam M. With or without FUS, it is the anatomy that dictates the dementia phenotype. Brain. 2009;132:2906–8.
Whitwell JL, Weigand S, Boeve BF, Senjem ML, Gunter JL, DeJesus-Hernandez M, Rutherford NJ, Baker M, Knopman DS, Wszolek ZK, Parisi JE, Dickson DW, Petersen RC, Rademakers R, Jack Jr CR, Josephs KA. Neuroimaging signatures of frontotemporal dementia genetics: C9orf72, tau, progranulin and sporadics. Brain. 2012;135:794–806.
Whitwell JL, Przybelski SA, Weigand SD, Ivnik RJ, Vemuri P, Gunter JL, Senjem ML, Shiung MM, Boeve BF, Knopman DS, Parisi JE, Dickson DW, Petersen RC, Jack Jr CR, Josephs KA. Distinct anatomical subtypes of the behavioural variant of frontotemporal dementia: a cluster analysis study. Brain. 2009;132(Pt 11):2932–46.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Elamin, M., Omer, T., Hutchinson, S., Doherty, C.P., Bak, T.H. (2016). Fronto-Temporal Dementia (FTD). In: Hardiman, O., Doherty, C., Elamin, M., Bede, P. (eds) Neurodegenerative Disorders. Springer, Cham. https://doi.org/10.1007/978-3-319-23309-3_7
Download citation
DOI: https://doi.org/10.1007/978-3-319-23309-3_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-23308-6
Online ISBN: 978-3-319-23309-3
eBook Packages: MedicineMedicine (R0)