Abstract
Probiotics are defined as live microorganisms, which when administered in adequate amounts confer health benefits on the host. Lactic acid bacteria and Bifidobacterium strains are the most common groups of bacteria with claimed probiotic properties. These bacterial strains have been conventionally incorporated into food and beverage products as dietary adjuncts, aimed at promoting gastrointestinal health. Meanwhile, a growing number of studies have also revealed that probiotic strains could exert beneficial health effects beyond the gut, mainly attributed to their peculiar immunomodulatory properties. Probiotic strains are capable of modulating the innate and adaptive immune response through both immunostimulation and immunoregulation and can thereby exert prophylactic and therapeutic effects on the host. Indeed, experimental evidences have demonstrated that administration of live probiotics and/or probiotic-derived products can be potentially applied in the prevention and/or treatment of a wide range of non-gastrointestinal diseases, such as metabolic disorders, allergic and inflammatory skin disorders, respiratory diseases, osteoporosis, male hypogonadism, and rheumatoid arthritis. However, more clinical trials on the efficacy of different probiotic strains in the prevention and treatment of these health conditions are needed to generate more definitive results. The exact mechanisms by which specific probiotic strains can stimulate and/or regulate immune functions remain to be elucidated. Nonetheless, recent advances in biotechnology have provided rapid ways to explore possible immunomodulatory mechanisms of probiotics. Altogether, this chapter provides a succinct summary of the updated evidence on the immunomodulatory effects of probiotics and discusses their possible mechanisms of action. This chapter also presents the future directions to promote a better understanding of the underlying immunomodulatory actions of probiotics.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
In 1953, Werner Kollath introduced the term “probiotika” for “active substances that are essential for a healthy development of life” (Guarner et al. 2005). This term evolved to “probiotic,” which is derived from the Greek word “biotikos,” meaning “for life.” The term probiotic was first used by Lilly and Stillwell (1965) to describe “substances produced by one microorganism that stimulate the growth of another.” However, Parker (1974) applied the word probiotic to describe animal feed supplements that improved animal health and eventually introduced a new definition as “organisms and substances that contribute to intestinal microbial balance.” Since then, probiotics have received an increasing interest for the promotion of human and animal gastrointestinal health. Thereafter, Fuller (1989) revised Park’s definition by focusing on microorganisms and redefined probiotic as “a live microbial feed supplement which beneficially affects the host animal by improving its intestinal microbial balance.” The definition of probiotics has been re-modified throughout the years until 2001, when the joint Food and Agriculture Organization (FAO) and the World Health Organization (WHO) recommended the adoption of the definition of probiotics as “live microorganisms , which when administered in adequate amounts confer a health benefit on the host” (FAO/WHO 2001). This definition has been continuously retained and reinforced by an expert panel from the International Scientific Association for Probiotics and Prebiotics recently, as it is still relevant and capable of accommodating current and anticipated applications (Hill et al. 2014).
In order for a microorganism to be classified as a probiotic, it must fulfill the following criteria: (1) must be able to survive in the intestinal transit, (2) must be able to adhere to mucosal surfaces and colonize the intestine, (3) must be resistant to technological processes, (4) must be safe for consumption, (5) must possess documented beneficial health effects, and (6) must be taxonomically identified at the genus, species, and strain level (Borchers et al. 2009). The most common microorganisms with claimed probiotic properties are bacteria, Bifidobacterium, and lactic acid bacteria (LAB) strains, including the genera of Lactobacillus, Enterococcus, Lactococcus, Leuconostoc, Streptococcus, and Pediococcus. Additionally, Saccharomyces, a yeast genus, has also been identified as a microorganism associated with probiotic properties. Meanwhile, it is extremely important to note that the health-promoting effects of probiotics are strain-specific.
Lactobacillus and Bifidobacterium strains are among the primary candidates of probiotic bacteria inhabiting the gastrointestinal tract and are widely used as dietary adjuncts owing to their long history of safe use. There is unequivocal evidence that administration of specific strains of probiotic bacteria can help in the maintenance of a balanced intestinal microbiota and therefore potentially promote overall host health (Butel 2014). Dairy products have been considered as a desirable delivery vehicle for delivering probiotic bacteria to the human gastrointestinal tract (Xiao et al. 2014). Hence, probiotic strains are most often incorporated in dairy-based food and beverages globally, including fermented milk, yogurt, ice cream, frozen yogurt, cheeses, pasteurized milk, and kefir. Nevertheless, non-dairy probiotic products are also increasingly being developed due to the increase in vegetarianism as a dietary habit, lactose intolerance, and the cholesterol content of dairy products (Céspedes et al. 2013).
Among the many purported health benefits attributed to the administration of probiotics, the ability of probiotics to modulate the gut immune system has attracted more attention from the probiotic community. The intestinal microbiome plays an important role in the maintenance of the host immune homeostasis. It has been reported that probiotics may induce the production of intestinal defensin and immunoglobulin that inhibit the growth of pathogens, thereby maintaining a balanced intestinal microbiome (Thomas and Versalovic 2010). Probiotics have also been demonstrated to stimulate the production of cytokines, which can promote the activation of epithelial cells and both innate and adaptive immune cells, thus leading to the maintenance of a healthier intestinal microbiome and immune homeostasis (Hemarajata and Versalovic 2013). On the other hand, Furness et al. (1999) have mentioned that 70–80 % of the host’s immune cells are located in the gut. Therefore, in addition to the local gut immunomodulatory effects, probiotics may also extend its immunomodulatory activity to the systemic levels. Increasing interest has been focused on proving the previously proposed hypothesis that probiotics may stimulate and/or regulate the immune system beyond the gut, such as on the gut-liver axis and the gut-brain-skin axis (Arck et al. 2010; Smith et al. 2014). Indeed, compelling evidence has demonstrated that live probiotics and/or probiotic-derived bioactives extend its beneficial roles by modulating the immune system beyond the gut (Bowe and Logan 2011; Saulnier et al. 2013). This chapter documents up-to-date in vitro and in vivo evidences of immunomodulatory effects of probiotics in health and non-gastrointestinal diseases and discusses their possible molecular and cellular mechanisms of action.
2 Probiotics and Immune Modulation
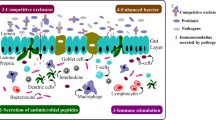
Innate and adaptive arms of the immune system work together to maintain the host immune homeostasis and to protect against external insults and undesirable inflammation (Yang and Polk 2011). However, imbalance of innate and adaptive immune responses can result in cell and tissue damage that further contribute to diseases. Experimental studies have proposed that probiotics can modulate humoral components and hematopoietic cells (natural killer cells, mast cells, neutrophils, macrophages, T cells, B cells, and dendritic cells) in both the innate (aspecific) and adaptive (specific) immune systems (Fig. 1), thereby promoting host defense.
Immunomodulatory actions of probiotics. Specific mechanisms: involvement of probiotics in cell-mediated and humoral immune responses. Aspecific mechanisms: enhancement of epithelial barrier function, competitive exclusion of bacteria along epithelium, modification of local microenvironment, and reduction of intestinal inflammation. Reprinted from Iacano et al. (2011) with permission from Elsevier (License number: 3537870510625)
Dong et al. (2012) have evaluated the immunomodulatory potential of live Lactobacillus and Bifidobacterium strains in human peripheral blood mononuclear cells in vitro. The authors found that L. casei Shirota, L. rhamnosus GG, L. plantarum NCIMB 8826, L. reuteri NCIMB 11951, B. longum SP 07/3, and B. bifidum MF 20/5 not only induced the expressions of CD69+ on T lymphocytes, T helper (Th) cells, cytotoxic T (Tc) cells, and natural killer cells but also CD25+ on T lymphocytes and NK cells, in a strain-dependent manner. These strains also increased the production of cytokines (IL-1ß, IL-6, IL-10, TNF-α), granulocyte-macrophage colony-stimulating factor, and macrophage inflammatory protein-1α (MIP-1α). It has also been reported that 16-month-old male Swiss mice orally administered with milk fermented with L. fermentum MTCC 5898 (3 × 109 CFU/day) for a period of 2 months showed significantly higher neutrophil phagocytosis and lower humoral antibodies (IgG1/IgG2a ratio and IgE levels) compared to the control, thereby demonstrating the potential of probiotics to improve immune functions in aging subjects (Sharma et al. 2014).
Macrophages serve as one of the immune sentinel cells with both protective and pathogenic roles in host defense (Galli et al. 2011). They express pattern recognition receptors (PRRs), such as Toll-like receptors (TLRs) and nucleotide-binding oligomerization domains (NODs) that recognize pathogen-associated molecular patterns (PAMPs) expressed by pathogens, which subsequently lead to the activation of a range of immune responses, including phagocytosis and cytokine production. It has been reported that peptidoglycans of L. johnsonii JCM 2012 and L. plantarum ATCC 14917 inhibited IL-12 production by macrophages that was induced by L. casei through PRRs such as TLR2 and NOD2, as well as suppressed IL-12p40 mRNA (Shida et al. 2009). The overproduction of IL-12, which subsequently results in the overstimulation of Th1 cells, is considered as one of the main factors that contribute to autoimmune and inflammatory diseases. In addition, IL-12p40 is a subunit of IL-23 that regulates the proliferation and maintenance of Th17 cells, which plays a major role in the development of inflammatory diseases. Hence, findings in this study suggest that the ability of peptidoglycans to downregulate the production of IL-12 and IL-23 may be useful for the treatment of inflammatory diseases. On the other hand, cell wall extracts of B. adolescentis BBMN23, B. longum BBMN68, and L. saliva Ren were shown to increase phagocytic activity and production of nitric oxide, IL-6, and TNF-α, thereby enhancing the overall activities of macrophage RAW264.7 cells (Zhu et al. 2011). Oral administration of dahi containing L. acidophilus LaVK2 (2–20 × 107 CFU) and B. bifidum BbVK3(2–20 × 107 CFU/g) for 4 months was also found to improve production of reactive oxygen intermediates (ROI), phagocytic activity, and adherence indices of peritoneal macrophages in aged mice, thus potentially improving immune functions in aging individuals (Kaushal and Kansal 2014).
Probiotics also modulate the function of mast cells , which specialize in stimulating or suppressing homeostatic or pathophysiological responses. Mast cells are well known for their role in allergic inflammation due to the ability of allergen-specific IgE to activate high-affinity IgE receptor (FcεR1) on the cell surface, which leads to the release of mediators and cytokines (Forsythe et al. 2012). Using global microarray analysis (Affymetrix GeneChip® Human Genome U133 Plus 2.0 Array), Oksaharju et al. (2011) have found that L. rhamnosus LGG and Lc705 downregulated genes encoding mast cell activation and mediator release [high-affinity IgE receptor subunits α (Fcer1a) and γ (Fcer1g) and histamine H4 receptor (Hrh4)] and immune responses (IL-8, TNF-α, CCL2, and IL-10) and thus could be applied in the cases of allergy mediated by mast cell activation. Oral administration of L. rhamnosus JB-1 (1 × 109 CFU/daily) for 9 days in healthy male Sprague-Dawley rats have also revealed that this probiotic strain systemically inhibited mast cell membrane potassium current and degranulation (enhanced mast cell stabilization) by inhibiting ß-hexosaminidase (mast cell mediator release) in response to the IgE-mediated activation, thus reducing passive cutaneous anaphylaxis and peritoneal mast cell responses to the KCa3.1 opener, a potent therapeutic target in a wide range of autoimmune diseases (Forsythe et al. 2012).
Dendritic cells are critical antigen-presenting cells for naïve T cells and generation of the T cell response. In vitro studies have demonstrated that L. rhamnosus Lcr35 can modulate the immunological functions of human dendritic cells. High dose of L. rhamnosus Lcr35 (MOI, 100) has been shown to upregulate nearly 1700 genes mainly involved in the immune response, with threefold changes (Evrard et al. 2011). Flow cytometry and ELISA analyses have also further revealed that L. rhamnosus Lcr35 upregulated the maturation of dendritic cell membrane phenotypes (CD86, CD83, HLA-DR, and TLR4) and increased the pro-Th1/Th17 cytokine levels in a dose-dependent manner. Despite pro-Th1/Th17 cytokines mediating inflammation and autoimmune diseases, in vivo studies have proposed that upregulation of Th1/Th17 responses could alleviate certain inflammation or allergic diseases (Lin et al. 2009). IgE-dependent allergies are diseases characterized by imbalance of Th1/Th2 responses. Yu et al. (2010) have demonstrated that L. rhamnosus Lcr35 is capable of regulating the impaired Th1/Th2 cytokine profile by modulating the Th1/Th2 responses toward the Th1 (/Th17) balance in dendritic cells. These findings suggest that L. rhamnosus Lcr35-treated dendritic cells have a higher potential of antigen presentation and co-stimulation and therefore may induce immune responses as required during inflammation and/or allergies. Till date, this is the first report of probiotics found to have a pro-Th1/Th17 effect on dendritic cell maturation that merits further investigation. On the other hand, You et al. (2014) have conducted a study to evaluate the effect of B. longum bv. infantis CCUG 52486, B. longum SP 07/3, L. rhamnosus GG, and L. casei Shirota on dendritic cell function in an allogeneic mixed leukocyte reaction model, using peripheral blood obtained from healthy young (20–30 years) and old (55–75 years) subjects. The authors found that these strains increased the expression of CD40, CD80, and CCR7 in both young and old dendritic cells and only enhanced cytokine production (TNF-α and TGF-ß) in old dendritic cells. Additionally, probiotic treatment was also found to stimulate the activation of young T cells activation by old dendritic cells, suggesting that probiotics may be able to modulate dendritic cell function in aging individuals.
Since probiotics are mainly used to maintain healthy intestines, a large proportion of research attention has focused on exploring the mechanisms of intestinal immunomodulation by probiotics. Probiotics have been found to exhibit their immunomodulatory effects in a strain-dependent and cell subset-specific manner. Suda et al. (2014) have evaluated the potential of L. jensenii TL2937 in modulating the immune response in cocultures of porcine intestinal epithelial cells and antigen-presenting cells from porcine Peyer’s patches. The authors found that L. jensenii TL2937 not only stimulated the expression of IL-10, MHC class II, Bcl-3, and CD80/CD86 in CD172a+CD11R1- and CD172a+CD11R1high− antigen-presenting cells but also suppressed TLR4 activation and subsequent inflammatory response by upregulating negative regulators (MKP1, Bcl3, and A20) of TLR4 in porcine intestinal epithelial cells. Smelt et al. (2013) have also demonstrated that specific probiotic strains exhibited specific immune responses in specific sites of the intestine in vivo. Although L. plantarum WCFS1, L. lactis MG1363, and L. salivarius UC118 increased the numbers of CD11c+MHC class II+ dendritic cells in the mice Peyer’s patches, L. salivarius UCC118 was the only strain which exhibited immunoregulatory effects by decreasing the number of effector T cells and increasing the number of regulatory T cells (Treg cells; CD4+CD25+Foxp3+ T cells) in the small intestine lamina propria. IgA is an important intestinal humoral component, which is secreted from B cells in gut-associated lymphoid tissues (Mora et al. 2006). Sakai et al. (2014) have revealed that oral administration of diet containing L. gasseri SBT 2055 (1.0 × 109 CFU/g/daily) for 5 weeks activated the TLR2 signaling pathway and increased the rate of IgA+ cells and IgA production in both the lamina propria and Peyer’s patch of the small intestine in healthy mice, as well as stimulated the expression of TGF-β in bone marrow-derived dendritic cells. These findings suggest that L. gasseri SBT 2055 could enhance host immune responses and protect against intestinal inflammation. In addition to animal studies, a number of clinical trials have also been conducted to elucidate the immunomodulatory actions and to evaluate the efficacy of probiotics in healthy populations (Table 1).
3 Non-gastrointestinal Diseases Modulated by Probiotics
The potential immunomodulatory roles of probiotics in non-gastrointestinal diseases are still emerging and might offer promising novel applications in the near future. Results from preliminary studies have proposed that probiotics may modulate immune responses in diseases such as metabolic syndrome, respiratory diseases, allergic and inflammatory skin disorders, osteoporosis, rheumatoid arthritis, and male hypogonadism, which will be further discussed in later sections (3.1–3.6). Although immunomodulatory effects of probiotics represent a new avenue for discovering the potential use of probiotics in non-gastrointestinal health, a number of clinical trials have already been performed. However, more clinical studies are needed in order to establish the immunomodulatory role of probiotics in the treatment of non-gastrointestinal diseases.
3.1 Metabolic Syndrome
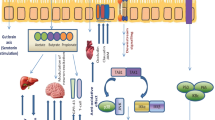
Metabolic syndrome is a constellation of multiplex factors, such as abdominal obesity, hyperlipidemia, hypertension, and nonalcoholic fatty liver disease that raises the risk of type 2 diabetes mellitus and cardiovascular diseases (Okubo et al. 2014; Fig. 2). It has been estimated that 25 % of the global population is suffering from metabolic syndrome (Shivakumar et al. 2014). Accumulating evidence has shown that altered gut microbiota mediated low-grade inflammation which contributed to the onset of metabolic syndrome and related diseases, via mechanisms responsible for intestinal barrier dysfunction (Cani et al. 2012; Zhang et al. 2014). Thus, the administration of probiotics appears to be a promising approach to treat metabolic syndrome owing to their ability to modulate gut microbiota and to restore impaired intestinal barrier function. Indeed, to date, probiotic approaches that target the gut microbiota have shown the most positive results in the prevention and treatment of abdominal obesity, hypertension, hyperlipidemia, and nonalcoholic fatty liver disease via immune-mediated mechanisms. Preliminary findings have shown that probiotics are capable of stimulating and regulating local and systemic immune responses in a diet-induced metabolic syndrome murine model. However, their potential warrants further investigation and clinical trials.
Probable immunological actions of probiotics to reduce the risk factors that contribute to type 2 diabetes mellitus and cardiovascular diseases. Reprinted from Panwar et al. (2013) with permission from John Wiley and Sons (License number: 3522340228488)
3.1.1 Abdominal Obesity
An increased high-fat diet has been linked to the development of abdominal obesity and increased risk of type 2 diabetes mellitus (Kaur 2014). Recently, Núñez et al. (2014) have conducted a study evaluating the effect of L. casei CRL 431 on intestinal and humoral responses using a high-fat-induced mouse model. The mice were orally administered a balanced diet or high-fat diet supplemented with milk, fermented milk containing L. casei CRL 431 (8 ± 2 × 108 CFU/mL/daily), water suspension containing L. casei CRL 431 (2 ± 1 × 108 CFU/mL/daily), or water for 60 days. The authors found that while both the administration of fermented milk or water suspension containing L. casei CRL 431 enhanced the phagocytic activity of macrophages, only fermented milk containing L. casei CRL 431 increased the number of macrophages and IgA+ cells in the small intestine. These results indicate that fermented milk containing L. casei CRL 431 can attenuate high-fat-diet-induced obesity by modulating mucosal immunity altered by obesity.
TNF-α is known as a cytokine that can stimulate the apoptosis of adipocytes and inhibit the synthesis of fatty acid synthase (Cawthorn and Sethi 2008). It has been reported that oral administration of L. rhamnosus PL 60 (1 × 107 or 1 × 109 CFU/mouse; daily) for 8 weeks significantly downregulated both TNF-α and fatty acid synthase mRNA expression levels in epididymal white adipose tissues, leading to an antiobesity effect in high-fat-diet-induced mice (Lee et al. 2006). Meanwhile, Park et al. (2013a, b) have also demonstrated that the mRNA expression of TNF-α, IL-1β, IL-6, and MCP1 (pro-inflammatory genes) in adipose tissues was downregulated in high-fat-diet-induced mice upon receiving L. curvatus HY 7601 (5 × 109 CFU/day) or L. plantarum KY 032 (5 × 109 CFU/day) treatment for 8 weeks. Recently, it has also been reported that oral administration of L. gasseri SBT 2055 (5 × 108 CFU/g; daily) for 24 weeks downregulated mRNA expression of CCL2 and CCR2 (pro-inflammatory genes) in epididymal adipose tissues of high-fat-induced mice (Miyoshi et al. 2014). These findings collectively suggest that probiotic strains may also attenuate inflammation of adipose tissues in high-fat-induced obesity by suppressing pro-inflammatory immune responses (TNF-α, IL-1β, IL-6, MCP1, CCL2, and CCR2). On the other hand, Yoo et al. (2013) have conducted an animal study involving 50 C57BL/6J mice that were fed with high-fat high-cholesterol diet (HFCD) supplemented with L. plantarum KY 1032 (1010 CFU/day), HFCD supplemented with L. curvatus HY 7601 (1010 CFU/day), or HFCD supplemented with L. plantarum KY 1032 and L. curvatus HY 7601 (1010 CFU/day) for 9 weeks. The authors found that only mice orally administered with L. plantarum KY 1032 were capable of suppressing plasma TNF-α and IL-1β (pro-inflammatory cytokine), indicating that probiotics may reduce the incidence of systemic low-grade inflammation in high-fat-diet-induced metabolic syndrome.
A reduction in the number of adipose tissue macrophage and macrophage infiltration has been shown to attenuate adipose inflammation and other obesity complications (Cani et al. 2008). Recently, Wang et al. (2015) have conducted an animal study involving 40 male high-fat diet -induced C57BL/6J mice fed with L. paracasei CNLM I-4270, L. rhamnosus I-3690, or B. animalis subsp. lactis I-2494 (108 CFU/day) for 12 weeks. The authors found that all three probiotic strains significantly reduced the number of adipose tissue macrophages and infiltration of pro-inflammatory macrophages (CD11c+ and MMP-12+) and may therefore attenuate inflammation in adipose tissues.
In addition to abdominal obesity, several studies have also determined the immune modulating potential of probiotics in the prevention of type 2 diabetes mellitus using an obesity-induced diabetes murine model . Inflammatory cytokines, such as TNF-α, IL-6, and IL-8, can cause insulin resistance and inflammation and consequently onset type 2 diabetes mellitus (King 2008). Hence, suppression of these inflammatory cytokines is a promising therapeutic approach to combat type 2 diabetes mellitus. Interestingly, several studies have demonstrated that probiotics exhibit antidiabetic effects by suppressing Th1 immune responses. Amar et al. (2011) have found that high-fat-diet-induced diabetic mice orally treated with B. lactis 420 (109 CFU/day) downregulated inflammatory cytokine (TNF-α, IL-1β, IL-6, and PAI-1) mRNA in mesenteric adipose tissues, the muscle, and the liver, thereby resulting in improved insulin sensitivity and overall inflammatory status. Another recent study also showed that oral administration of L. casei (4 × 109 CFU/day) inhibited the development of type 2 diabetes mellitus by suppressing plasma Th1-associated pro-inflammatory cytokines (IFN-γ and TNF-α) and downregulating Th1 responses related to Tbet gene mRNA levels in high-fat high-sucrose diet-induced pre-insulin-resistant rats (Zhang et al. 2014). Recently, a randomized, double-blind, controlled clinical trial was conducted on 44 patients (22 for placebo group and 22 for intervention group), BMI ≥ 25, over a period of 8 weeks to evaluate the effect of probiotic yogurt (3.7 × 106 CFU/g of L. acidophilus A5 and B. lactis BB12) consumption (300 g/day) on inflammatory biomarkers in patients with type 2 diabetes mellitus. The consumption of probiotic yogurt in the intervention group was found to reduce plasma TNF-α, thus ameliorating the onset of type 2 diabetes (Mohamadshahi et al. 2014).
On the other hand, probiotics can also improve type 2 diabetes by stimulating Th2 immune responses . Chen et al. (2014) have reported that oral administration of L. rhamnosus CCFM 0528 (109 CFU/day) for 12 weeks upregulated IL-4 and IL-10 (Th2 immune responses) expression and downregulated IL-6, IL-8, and TNF-α (Th1 immune responses) expression in the spleen, leading to improved glucose tolerance in high-fat-diet, streptozotocin-induced type 2 diabetic mice. Dendritic cells from nonobese diabetic mice have been shown to produce elevated levels of IL-12 that activate IFN-γ-producing T cells which can promote the development of diabetes (Trembleau et al. 1995). Meanwhile, nonobese diabetic dendritic cells stimulated with L. casei enhanced IL-10 over IL-12 in vitro, and their transfer reduced the incidence of diabetes in vivo, thus proposing another potential immunological role of probiotics for the treatment of type 2 diabetes mellitus (Manirarora et al. 2011).
3.1.2 Hypertension
Hypertension is one of the central metabolic syndromes that is increasing worldwide at an alarming rate. It has been estimated that the number of adult with hypertension will reach a total of 1.56 billion in the year 2025 (Kearney et al. 2005). Although the exact mechanisms underlying the immunomodulatory potentials of probiotics for hypertension still remain poorly understood, findings in animal studies have proposed that probiotics may attenuate pathogenesis in hypertension by targeting immune responses in the kidney and aorta.
Angiotensin type 1 receptors (AT1R) specifically activate immune and non-hematopoietic cells to promote pathogenesis in angiotensin II-dependent hypertension mice (Ryan 2013). A mutational analysis has revealed that CD3+ T cells and renal macrophages were increased in bone marrow-specific AT1R knockout mice associated with exaggerated hypertensive responses (Crowley et al. 2010). Mycophenolate mofetil is a drug that is used to improve hypertension (Herrera et al. 2006). It has been demonstrated that high-protein-diet-induced hypertension in Dahl salt-sensitive rats treated with mycophenolate mofetil reduced renal cortical T cell infiltration, thereby attenuating hypertension (De Miguel et al. 2011). Additionally, Barhoumi et al. (2011) have demonstrated that angiotensin II-induced hypertension is associated with the reduction of Treg cells in the renal cortex, and adoptive transfer to increase Treg cells suppressed TNF-α expression. On the other hand, inhibition of IL-6 has also been shown to downregulate IL-6 expression, T cell, and macrophage cell infiltration in cold-induced hypertension (Crosswhite and Sun 2010). Nonetheless, Chan et al. (2012) have also further revealed that deoxycorticosterone acetate-salt-induced hypertension was accompanied with an increase in the number of macrophage and expression levels of chemokine (CCR2, CC12, CCL7, and CC8) in the aorta. Altogether, these results propose that probiotics may attenuate hypertension by modulating immune cells (T cells, macrophages, Treg cells) and pro-inflammatory cytokines (TNF-α and IL-6) in the kidney, as well as chemokines (CCR2, CCL12, CCL7, and CC8) in the aorta.
3.1.3 Hyperlipidemia
Hyperlipidemia is the term used to describe the increased concentration of any or all lipids in the plasma (Stocks et al. 2005). Elevated very-low-density lipoproteins in the plasma can contribute to the risk of atherosclerosis. Apolipoprotein E-deficient mice (ApoE−/−) are characterized by elevated very-low-density lipoproteins and are widely used as murine models of atherosclerosis, steatosis, and hyperlipidemia (King et al. 2010). Interestingly, Mencarelli et al. (2012) have used this model to evaluate the potential of a probiotic cocktail (VSL#3) consisting of eight probiotic strains (L. acidophilus MB 443, L. delbrueckii subsp. bulgaricus MB 453, L. casei MB 451, L. plantarum MB 452, B. longum Y10, B. infantis Y1, B. breve Y8, and Streptococcus salivarius subsp. thermophilus MB 455) in protecting against low-grade intestinal inflammation in the development of arthrosclerosis. The ApoE−/− mice were orally administered with VSL#3 (20 × 109 CFU/kg/day) or 0.2 % dextran sulfate sodium (DSS) filtered drinking water containing VSL#3 (20 × 109 CFU/kg/day) six days a week for 12 weeks. The authors found that VSL#3 downregulated the expression of TNF-α and RANTES (inflammatory mediators) in both the colonic mucosa and mesenteric adipose tissues of ApoE−/− mice challenged with DSS. VSL#3 treatment was also shown to stimulate the expression of nuclear receptors (PPAR-γ and VDR) of positive regulators in the intestine and to increase IL-10 (anti-inflammatory cytokine) production from CD5+T cells in the spleen. Another set of results in this study also highlighted that the administration of VSL#3 downregulated the expression of inflammatory mediators (ICAM-1, VCAM, and RANTES) in the aorta, as well as decreased the percentage of CD35+ cells in circulating macrophages of ApoE−/− mice challenged with DSS, thereby preventing the generation of new plaques in the thoracoabdominal aortas and inhibiting the extension of atherosclerotic plaques (Fig. 3). Altogether, these results demonstrate that VSL#3 can potentially protect subjects with low-grade inflammation of hyperlipidemia against atherosclerosis by decreasing local and systemic inflammatory immune responses (aorta, intestine, adipose tissue, and plasma) while stimulating the expression of nuclear receptors of positive regulators and IL-10-producing T lymphocytes.
Effect of VSL# 3 administration on aortic plaques in ApoE−/− mice. The lipids in the vessel wall were stained with Sudan IV. ApoE−/−mice administered with VSL#3 for 12 weeks decreased the number of new plaques in thoracoabdominal aorta and inhibited the extension of atherosclerotic plaques. Reprinted from Mencarelli et al. (2012); free access from PLOS
3.1.4 Nonalcoholic Fatty Liver Disease
Nonalcoholic fatty liver disease is the common cause of chronic liver disease worldwide that increases with the prevalence of obesity, dyslipidemia, and insulin resistance (Kneeman 2012). Numerous studies have highlighted a causative relationship between liver (local) and systemic inflammation and nonalcoholic fatty liver disease. The invariant natural killer T (iNKT) cells are hepatic lymphocytes that play a role in the pathogenesis of nonalcoholic fatty liver disease (steatosis and nonalcoholic steatohepatitis). Although the exact mechanisms of NKT cells in liver diseases remain unclear, iNKT cells are capable of balancing the production of pro-inflammatory and anti-inflammatory cytokines (Seki et al. 2000). On the other hand, it has been reported that chronic liver inflammation is mediated by the Iκk-β/NF-κB signaling pathway, where increased NF-κB activity was associated with upregulated expression of inflammatory cytokines (TNF-α, IL-1β, and IL-6) in the liver of high-fat-diet-induced steatosis mouse model (Cai et al. 2005). Therefore, iNKT cells and Iκk-β/NF-κB signaling pathway could be promising targets in treating nonalcoholic fatty liver damage and inflammation.
Ma et al. (2008) have evaluated the potential of VSL#3 (a mixture of lyophilized Bifidobacterium, Lactobacillus, and Streptococcus thermophilus) to improve high-fat-diet-induced hepatic steatosis mice. The authors found that oral administration of VSL#3 (1.5 × 109 CFU/day) daily for 4 weeks significantly improved high-fat-diet-induced steatosis by stimulating IL-4 that contributes to an improvement in hepatic NKT cell depletion. Elevated TNF-α expression was found to activate Iκk-β activity (represented by ratio of phosphor-IKB-α/total IKB-α) in high-fat-induced hepatic steatosis mice. Nevertheless, VSL#3 treatment and adoptive transfer of NKT cells suppressed TNF-α expression a ssociated with reduced Iκκ-β activity and downstream signal for IKB-α (NF-KB p65 activity). Similarly, Liang et al. (2013) have also found that high-fat-diet mice orally administered with lipid extracts of VSL#3 for 4 weeks stimulated hepatic iNKT cells, suggesting that probiotic strains in VSL#3 may contain glycolipid antigens that directly confer effector functions to hepatic iNKT cells. Matrix metalloproteinases (MMPs) play an important role in the pathogenesis of liver diseases (Kurzepa et al. 2014). According to Esposito et al. (2009), VSL#3 treatment also suppressed the hepatic gelatinase activity of MMP-2 and MMP-9, thereby inhibiting liver inflammatory damage in rats receiving high-fat diet.
Several clinical studies have also been performed to evaluate the potential of probiotics for the treatment of nonalcoholic fatty liver disease, ranging from children to adult populations. Malaguarnera et al. (2012) have conducted a double-blind and randomized study to determine the effects of oral administration of B. longum with fructo-oligosaccharides in 66 patients (aged of 30–65 years) with abnormal hepatic steatosis. The authors found that patients who received B. longum with fructo-oligosaccharides and lifestyle modification (diet and exercise) had significantly decreased serum TNF-α and nonalcoholic steatohepatitis activity index compared to patients who received lifestyle modification alone. Furthermore, Eslamparast et al. (2014) have evaluated the effects of synbiotic capsules (Protexin) containing 2 × 108 CFU of seven probiotic strains (L. casei, L. rhamnosus, S. thermophilus, B. breve, L. acidophilus, B. longum, and L. bulgaricus) in a 28-week randomized, double-blind, placebo-controlled trial involving 52 patients with nonalcoholic fatty liver diseases. Synbiotic intervention significantly decreased TNF-α in plasma and NF-KB p65 in peripheral blood mononuclear cells. However, Vajro et al. (2011) reported that consumption of L. rhamnosus GG (12 × 109 CFU/day) for 8 weeks showed no significant effect on TNF-α production in twenty obese children (aged of 10.7 ± 2.1 years) with persisting hypertransaminasemia and ultrasonographic bright liver. Altogether, these findings warrant further investigation.
3.2 Respiratory Diseases
Respiratory diseases are a major leading cause of mortality and morbidity throughout the world, while respiratory infections have been reported to account for over four million deaths per year in low- or middle-income countries (World Health Organization 2008; Zar and Ferkol 2014). Thus, effective strategies are needed to properly tackle the burden of respiratory diseases. In brief, there are a growing number of in vitro and animal studies suggesting that probiotics may modulate pulmonary immune responses, thereby attenuating respiratory diseases, in particular asthma and influenza viral infections. However, clinical trials addressing the immunomodulatory potentials of probiotics in subjects with asthma and influenza are still very limited (Table 2).
3.2.1 Asthma
Asthma is a chronic inflammatory disease of the airways, with an estimated 300 million people of all ages and all ethnic backgrounds worldwide suffering from asthma (Masoli et al. 2004). Several studies have highlighted that Th2 lymphocytes, Th17 cells, as well as their cytokines (IL-4, IL-5, IL-12, IL-17A, IL-17F, and IL-23) are key inflammatory mediators that play critical roles in orchestrating the asthma pathogenesis (Li and Hua 2014). Thus, targeting these components may be a promising immunotherapeutic approach for the treatment of asthma. Interestingly, Jan et al. (2011) have demonstrated that oral administration of L. gasseri A5 (4 × 106 CFU/daily) starting from 2 weeks before sensitization for 4 weeks not only significantly decreased IL-17A production but also reduced TNF-α production and activation-regulated chemokines in bronchoalveolar lavage fluids of Dermatophagoides pteronyssinus-sensitized and Dermatophagoides pteronyssinus-challenged mice. Furthermore, the number of IL-17-producing alveolar macrophages was also decreased in L. gasseri A5-treated mice compared to untreated mice, suggesting that L. gasseri A5 treatment is capable of downregulating Th17 pro-inflammatory responses and improving asthma.
In addition to immunosuppressive effects on Th17 pro-inflammatory responses, probiotics (L. rhamnosus Lcr35, L. rhamnosus GG, B. lactis BB12, and L. reuteri strains) may attenuate asthmatic responses via a Treg cell-mediated mechanism (Jang et al. 2012; Karimi et al. 2009; Feleszko et al. 2007). The daily oral administration of L. rhamnosus Lcr35 at a dose of 1 × 109 CFU/mL for 7 days before the first sensitization was found to increase the percentage of Treg cells in the mouse spleen and also decrease Th2 (IL-4, IL-13, and IL-5) and Th1 (IFN-γ) cytokines in the mouse serum (Jang et al. 2012). Administration of anti-CD25 monoclonal antibody was also shown to inhibit the protective effects of L. rhamnosus Lcr35, confirming that Treg cells are essential targets in mediating the immune response of L. rhamnosus Lcr35 in asthma. Additionally, oral administration of L. rhamnosus Lcr35 (1 × 109 CFU/mL/daily; 1 week) prior to sensitization was also found to significantly decrease ovalbumin-specific IgE production in ovalbumin-induced murine model of asthma, to suppress Th2 responses (CCL11, Th2-related chemokines) and to increase Treg cells following the adoptive transfer of L. rhamnosus Lcr35-treated dendritic cells (Yu et al. 2010; Kim et al. 2013). These studies collectively suggest that L. rhamnosus Lcr35 can stimulate dendritic cell activation and suppress the Th2 responses by upregulating Treg cells in the murine model of asthma. However, the efficacy of L. rhamnosus Lcr35 in subjects with a sthma still remains unclear. Similar to L. rhamnosus Lcr35, L. reuteri strains have also been shown to increase the number of Treg cells in the spleen and to suppress Th2 responses (TNF, MCP-1/CCL2, IL-5) in an ovalbumin-induced murine model (Karimi et al. 2009).
3.2.2 Influenza
Influenza is a respiratory viral infectious disease caused by RNA viruses, belonging to the family of Orthomyxoviridae (influenza viruses). In vivo studies have proposed that probiotics may attenuate influenza virus infections by stimulating NK cells, which are major cellular components of the innate immunity that can recognize and control a broad spectrum of pathogens, including viruses. Intranasal administration of heat-killed L. pentosus S-PT84 (200 μg/day) for 3 days significantly increased IFN-γ and IL-12 (Th1 antiviral cytokines) production in mediastinal lymph node cells and bronchoalveolar lavage fluids, subsequently stimulating NK cell activity in the lung of mice infected with influenza virus PR8 (mouse-adapted H1N1 strain) (Izumo et al. 2010). A similar conclusion has also been drawn in another study by Harata et al. (2010), where daily intranasal administration of L. rhamnosus GG (200 μg/day) for three consecutive days upregulated mRNA expression of IL-1β, monocyte chemotactic protein (MCP), and TNF-α following activation of lung NK cells in mice infected with influenza virus A (H1N1). On the other hand, Park et al. (2013a, b) have also demonstrated that intranasal (109 CFU/mouse) or oral (109 CFU/daily for 10 days) administration of L. plantarum DK119 2055 can protect mice from influenza A/PR8 virus infection by increasing CD11c+ dendritic and macrophage cells and stimulating IFN-γ and IL-12 production. Altogether, these studies suggest that probiotics most probably exhibit their antiviral effects against influenza virus A by stimulating macrophage- and dendritic cell-mediated immune responses and NK cell activation.
3.3 Allergic and Inflammatory Skin Disorders
Contemporary studies have focused on the possible deployment of probiotics for alleviating allergic and inflammatory skin disorders due to their ability to balance intestinal microbiota, which ameliorates the immune system at both the local and systemic levels. Preliminary data suggest that probiotics could produce dermal bioactives such as bacteriocins and may thereby enhance the skin immune defense (Tan et al. 2014). A bacteriocin, CBT-SL5 produced from E. faecalis at a concentration of 100 ng/mL, was found to suppress P. acnes-induced NF-KB translocation, mRNA expression, and protein production of IL-8 in vitro, highlighting its potential as a topical agent for acne vulgaris (Jin et al. 2008). On the other hand, Pinto et al. (2011) have also demonstrated that plantaricin A, synthesized by L. plantarum, significantly induced proliferation and migration of human keratinocytes and increased expression of TGF-β1, VEGF-A, and IL-8, indicating a function in accelerating wound healing (Pinto et al. 2011). At a low concentration (10 μg/mL), plantaricin A was also found to increase antioxidant defenses of human keratinocytes and mRNA expression levels of filaggrin, involucrin, β-defensin 2, and TNF-α, which can promote antioxidant defenses, barrier functions, and antimicrobial activity of the skin.
The possible immunomodulatory actions of probiotics have also been investigated using animal models. Hacini-Rachinel et al. (2009) have revealed that oral administration of L. casei (DN-114 001) reduced the number of CD8+ effector T cells and increased the recruitment of CD4+ effector T cells and Treg cells in the skin of mice with antigen-specific-induced skin inflammation. L. casei (DN-114 001) treatment also enhanced the IL-10 production from Treg cells in skin-draining lymph nodes of hapten-sensitized mice, thus suggesting its potential to treat allergic skin diseases in human. It has also been further reported that oral administration of L. rhamnosus Lcr35 increased Treg cells, leading to the suppression of IL-4 production, and thymic stromal lymphopoietin (TSLP), which can promote Th1 cytokines that initiate the inflammation cascade in ovalbumin-induced atopic dermatitis SK-1 hairless mice via a mechanism that may be associated with Treg cells (Kim et al. 2012). Moreover, L. rhamnosus 1.3724 treatment was also found to prevent the development of atopic dermatitis in NC/NgaTnd mice by stimulating IFN-γ in skin, while L. sakei probio 65 treatment reduced plasma IgE level and IL-4 production in the spleen of sensitized mice (Tanaka et al. 2009; Park et al. 2008). On the other hand, several studies have also reported that probiotics can attenuate skin inflammation via the gut-skin axis. Inoue et al. (2007) have revealed that L. johnsonii NCC533 treatment can prevent the development of atopic dermatitis by stimulating intestinal IgA production, whereas Sawada et al. (2007) have suggested that oral administration of heat-treated L. rhamnosus LGG upregulated IL-10 mRNA expression levels in both Peyer’s patches and mesenteric lymph nodes in maternal and infant NC/Nga mice (a model of human atopic dermatitis) and may thus delay the onset and prevent the development of atopic dermatitis in human.
In addition to animal studies, Guéniche et al. (2010) have performed an ex vivo study to evaluate the immunomodulatory potential of L. paracasei CNCM I-2116 on substance P-induced skin inflammation. The authors found that L. paracasei CNCM I-2116 inhibited mast cell degranulation and TNF-α production induced by substance P ex vivo, thereby accelerating skin barrier recovery. Moreover, clinical trials have been performed in patients with other skin disorders, particularly on atopic dermatitis (Table 3). However, several clinical trials showed that probiotic strains were incapable of modulating immune responses in subjects with atopic dermatitis. Guéniche et al. (2009) have also conducted a randomized, double-blind, placebo-controlled trial to determine the immunomodulatory effects of probiotics in 57 volunteers upon exposure to ultraviolet (2 × 1.5 minimal erythema dose). The authors reported that volunteers who ingested L. johnsonii NCC 533 daily for 8 weeks had significantly increased production of regulating cytokines and growth factors such as TGF-β, which lead to the preservation of cutaneous immune homeostasis.
3.4 Osteoporosis
Estrogen deficiency is the primary risk factor of developing osteoporosis, a degenerative skeletal disease in postmenopausal women. In osteoporosis, osteoclastic bone resorption exceeds osteoblastic bone formation, thus leading to bone loss. Interestingly, probiotic treatment may attenuate osteoporosis in a postmenopausal woman by suppressing inflammatory cytokines (TNF-α and IL-1β) and T cell subsets (Treg and CD4+ T lymphocytes) involved in osteoclastogenesis. Recently, Ohlsson et al. (2014) have demonstrated that oral administration of either L. paracasei DSM 13434 or a cocktail of three strains (L. paracasei DSM 13434, L. plantarum DSM 15312, and DSM 15313) for 6 weeks, starting 2 weeks before ovariectomy, downregulated gene expression of TNF-α and IL-1β, maintained the number of Treg cells, and increased TGF-β1 production in ovariectomized mice. TGF-β1 has been shown to stimulate Treg cells, thereby inhibiting osteoclast differentiation and functions. Meanwhile, Britton et al. (2014) have also reported that ovariectomized mice fed with L. reuteri 6475 (1 × 109 CFU/mL; three times per week) for 4 weeks reduced the number of ovariectomized-induced CD4+ T lymphocytes, which prevented femur and vertebral trabecular bone loss (Fig. 4).
Micro-computed tomography isosurface images of femur and vertebral trabecular bone of mice. Ovariectomized (ovx) mice treated with L. reuteri 6475 for 4 weeks display femur and vertebral trabecular bone volumes that are similar to ovary-intact control mice. Reprinted from Britton et al. (2014) with permission from John Wiley and Sons (License number: 3534800317389)
3.5 Rheumatoid Arthritis
Rheumatoid arthritis is a chronic autoimmune inflammatory disease in which the Th1/Th2 balance shifts toward Th1 (pro-inflammatory responses), leading to joints deformities with destruction of cartilage and bone (Geusens et al. 2006). Owing to the immunosuppressive effect of probiotics, their potential use in reducing the pro-inflammatory responses were investigated in rats with type II collagen-induced rheumatoid arthritis. So et al. (2008a) have demonstrated that oral administration of L. casei (5 × 109 CFU/dose; 3 times/week) at day 7 after induction and continued for 11 weeks suppressed CD4+ T cells from releasing type II collagen reactive pro-inflammatory components, including IFN-γ, TNF-α, IL-1β, IL-2, IL-6, IL-12, IL-17, and Cox-2. Meanwhile, L. casei treatment also upregulated IL-10 expression and subsequently reduced the translocation of NF-KB, which is a key transcription factor that stimulates the production of certain pro-inflammatory cytokines (Driessler et al. 2004). Hence, L. casei may suppress pro-inflammatory cytokines by stimulating IL-10 production from CD4+ T cells. In another study, coadministration of L. casei (2 × 1010 CFU, 500 mg/kg) and collagen type II (250 mg/kg; glucosamine, 250 mg/kg) three times a week for 12 weeks has also been shown to suppress pro-inflammatory cytokines (IL-12, IL-17, IFN-γ, TNF-α, IL-1β, IL-2, and IL-6) and stimulate anti-inflammatory cytokines (TGF-β and IL-10) in mice with type II collagen-induced rheumatoid arthritis (So et al. 2008b). Meanwhile, this treatment also increased the numbers of Treg cells, upregulated Foxp3 expression, and suppressed collagen type II-reactive Th1-type IgG isotypes, IgG2a, and IgG2b. Although the ability of probiotics to suppress Th1-type cellular and humoral immune responses seems promising for the treatment of rheumatoid arthritis, more studies are needed to justify their potential.
3.6 Male Hypogonadism
High-energy and high-fat-diet-induced obesity has been shown to cause an increased risk for male hypogonadism (Cebler et al. 2010). Recently, Poutahidis et al. (2013) demonstrated that the pro-inflammatory cytokine IL-17 alone is capable of implicating a chronic inflammatory pathway in male hypogonadism, while probiotics can attenuate the incidence of male hypogonadism. Oral administration of L. reuteri ATCC PTA 6475 inhibited diet-induced obesity by restoring the Treg/Th17 balance and suppressing the systemic pro-inflammatory cytokine IL-17, thus demonstrating its potential in preventing male hypogonadism (Poutahidis et al. 2013). Poutahidis et al. (2014) then evaluated the anti-inflammatory effect of L. reuteri ATCC PTA 6475 in sustaining reproductive fitness in aging mice. Interestingly, the authors found that oral administration of L. reuteri ATCC PTA 6475 (3.5 × 105 CFU/mouse/day) at 2 months of age significantly increased the seminiferous tubule cross-sectional area, conspicuous Leydig cell area, and testicular weight of aging mice (aged of 5 months old).The beneficial effect of L. reuteri in the testicular health of 12-month-old mice was also recapitulated by blocking IL-17 signaling, indicating that systemic pro-inflammatory cytokine IL-17 could be a potential immunological target of probiotics in male hypogonadism. Recently, Al-Asmakh et al. (2014) have also reported that the gut microbiota plays a role in testicular health by modulating the permeability of the blood-testis barrier. Considering that probiotics can promote healthy gut microbiota to maintain immune homeostasis, probiotics may also be able to modulate the permeability of the blood-testis barrier and contribute to testicular health.
4 Future Trends
Despite the extensive use of probiotics as immune modulators in the treatment of gastrointestinal disorders , recent trends in probiotic research have clearly indicated a tendency toward discovering the potential applications of probiotics in non-gastrointestinal diseases. There are a growing number of studies suggesting that probiotics may stimulate and/or regulate gastrointestinal immune responses at both the local and systemic levels, thereby exerting beneficial effects beyond the gut (Vandenplas et al. 2014). Until today, the precise mechanisms of local and/or systemic immunomodulation by probiotics remain largely unknown. The turning point came in year 2009, when Ventura et al. (2009) suggested that probiogenomics could pave the way for the identification of genes and/or cell constituents in probiotics responsible for immune responses. Since then, omics high-throughput techniques, such as transcriptomics, proteomics, and metabolomics are continuously being introduced in the field of probiotics. Specifically, probiogenomics and these high-throughput analyses can be combined and used to bridge the mechanistic gap between genotype and phenotype, which could provide valuable insights into the way probiotics modulate the gastrointestinal and non-gastrointestinal immune responses in the human host (Sánchez et al. 2013). Meanwhile, we are approaching a new frontier, where computational (in silico) tools can be applied to screen and predict the immunological targets of probiotics through the discipline of immunoinformatics (Flower 2007). By using this approach, the immunogenicity of immunogenic substances of probiotics can be predicted based on the sum of predicted binding energies. On the other hand, Wendelsdorf et al. (2012) have also developed an in silico gut, known as ENteric Immunity Simulator (ENISI) for the studies of the inflammatory and regulatory immune responses in the gastrointestinal tract. With ENISI, we can now better understand immunological mechanisms of gastrointestinal pathogens in the gut and facilitate subsequent screening of probiotics that can be potentially deployed in the treatment of gastroenteric infections. Altogether, these prerequisites could not only facilitate and hasten the discovery of plausible and new immunity mechanisms of probiotic strains but also lead to a boost in the development of immunobiotic products in the near future. Nonetheless, a large number of clinical trials are still needed to determine the immunological efficacy and safety of beneficial probiotic strains for specific health claims.
5 Conclusions
In addition to regulating the gastrointestinal mucosal immunity, probiotics have the ability to modulate the activity of immune cells in both the innate and adaptive immune systems. Hence, probiotics represent a potential breakthrough therapeutic approach for other organs and systemic autoimmune disorders. It appears that immunostimulatory and/or immunoregulatory effects of probiotics on the human host are strain-specific and operate through a specific mechanism. Certainly, recent advances in experimental and computational tools, as well as animal models, can now be applied to unravel the mechanisms underpinning the immunomodulation effect of probiotic strains and to accelerate future development of probiotic-based products.
References
Al-Asmakh M, Stukenborg J, Reda A, Anuar A, Strand M, Hedin L, Petersson S, Soder O (2014) The gut microbiota and developmental programming of the testis in mice. PLoS One 9, e103809
Amar J, Chabo C, Waget A, Klopp P, Vachoux C, Bermudez-Humaran LG, Smirnova N, Berge M, Sulpice T, Lahtinen S, Ouwehand A, Langella P, Rautonen N, Sansonetti PJ, Burcelin R (2011) Intestinal mucosal adherence and translocation of commensal bacteria at the early onset of type 2 diabetes: molecular mechanisms and probiotic treatment. EMBO Mol Med 3:559–572
Arck P, Handjiski B, Hagen E, Pincus M, Bruenahl C, Bienenstock J, Paus R (2010) Is there a ‘gut-brain-skin axis’? Exp Dermatol 19:401–405
Barhoumi T, Kasal DA, Li MW, Shbat LS, Laurant P, Neves MF, Paradis P, Schiffrin EL (2011) T regulatory lymphocytes prevent angiotensin II-induced hypertension and vascular injury. Hypertension 57:469–476
Borchers AT, Selmi C, Meyers FJ, Keen CL, Gershwin ME (2009) Probiotics and immunity. J Gastroenterol 44:26–46
Bowe WP, Logan AC (2011) Acne vulgaris, probiotics and the gut-brain-skin axis- back to the future? Gut Pathog 3:1. doi:10.1186/1757-4749-3-1
Britton RA, Irwin R, Quach D, Schaefer L, Zhang J, Lee T, Parameswaran N, McCabe LR (2014) Probiotic L. reuteri treatment prevents bone loss in a menopausal ovariectomized mouse model. J Cell Physiol 229:1822–1830
Butel MJ (2014) Probiotics, gut microbiota and health. Med Mal Infect 44:1–8
Cai D, Yuan M, Frantz DF, Melendez PA, Hansen L, Lee J, Shoelson SE (2005) Local and systemic insulin resistance resulting from hepatic activation of IKK-beta and NF-kappaB. Nat Med 11:183–190
Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne NM, Burcelin R (2008) Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high fat diet-induced obesity and diabetes in mice. Diabetes 57:1470–1481
Cani PD, Osto M, Geurts L, Everard A (2012) Involvement of gut microbiota in the development of low-grade inflammation and type 2 diabetes associated with obesity. Gut Microbes 3:279–288
Cawthorn WP, Sethi JK (2008) TNF-α and adipocyte biology. FEBS Lett 9:117–131
Cebler S, Agarwal A, Flint M, Du Plessis SS (2010) Obesity: modern man’s fertility nemesis. Asian J Androl 12:480–489
Céspedes M, Cárdenas P, Staffolani M, Ciappini MC, Vinderola G (2013) Performance in nondairy drinks of probiotic L. casei strains usually employed in dairy products. J Food Sci 78:756–762
Chan CT, Moore JP, Budzyn K, Guida E, Diep H, Vinh A, Jones ES, Widdop RE, Armitage JA, Sakkal S, Ricardo SD, Sobey CG, Drummond GR (2012) Reversal of vascular macrophage accumulation and hypertension by a CCR2 antagonist in deoxycorticosterone/salt-treated mice. Hypertension 60:1207–1212
Chen P, Zhang Q, Dang H, Liu X, Tian F, Zhao J, Chen Y, Zhang H, Chen W (2014) Oral administration of Lactobacillus rhamnosus CCFM0528 improves glucose tolerance and cytokines secretion in high-fat-fed, streptozotocin-induced type 2 diabetic mice. J Funct Food 10:318–326
Cox AJ, Pyne DB, Saunders PU, Fricker PA (2010) Oral administration of the probiotic Lactobacillus fermentum VRI-003 and mucosal immunity in endurance athletes. Br J Sports Med 44:222–226
Crosswhite P, Sun Z (2010) Ribonucleic acid interference knockdown of interleukin 6 attenuates cold-induced hypertension. Hypertension 55:1484–1491
Crowley SD, Song YS, Sprung G, Griffiths R, Sparks N, Yan M, Burchette JL, Howell DN, Lin EE, Okeiyi B, Stegbauer J, Yang Y, Tharaux L, Ruiz P (2010) A role for angiotensin II type I receptors on bone marrow-derived cells in the pathogenesis of angiotensin II-dependent hypertension. Hypertension 55:99–108
De Miguel C, Lund H, Mattson DL (2011) High dietary protein exacerbates hypertension and renal damage in Dahl SS rats by increasing infiltrating immune cells in the kidney. Hypertension 57:269–274
Dong H, Rowland I, Yaqoob P (2012) Comparative effects of six probiotic strains on immune function in vitro. Br J Nutr 108:459–470
Dong H, Rowland I, Thomas LV, Yaqoob P (2013) Immunomodulatory effects of a probiotic drink containing Lactobacillus casei Shirota in healthy older volunteers. Eur J Nutr 52:1853–1863
Driessler F, Venstrom K, Sabat R, Asadullah K, Schottelius AJ (2004) Molecular mechanisms of interleukin-10-mediated inhibition of NF-kappaB activity: a role for p50. Clin Exp Immunol 135:64–73
Eslamparast T, Poustchi H, Zamani F, Sharafkhah M, Malekzadeh R, Hekmatdoost A (2014) Synbiotic supplementation in nonalcoholic fatty liver disease: a randomized, double-blind, placebo-controlled pilot study. Am J Clin Nutr 99:535–542
Esposito E, Iacono A, Bianco G, Autore G, Cuzzocrea S, Vajro P, Canani RB, Calignano A, Raso GM, Meli R (2009) Probiotics reduce the inflammatory response induced by a high-fat diet in the liver of young rats. J Nutr 139:905–911
Evrard B, Coudeyras S, Dosgilbert A, Charbonnel N, Alame J, Tridon A, Forestier C (2011) Dose-dependent immunomodulation of human dendritic cells by the probiotic Lactobacillus rhamnosus Lcr35. PLoS One 6, e18735
FAO/WHO (2001) Health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria. Report of a joint FAO/WHO expert consultation on evaluation of health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria. Córdoba, Argentina
Feleszko W, Jaworska J, Rha RD, Steinhausen S, Avagyan A, Jaudszus A, Ahrens B, Grobeberg DA, Wahn U, Hamelmann E (2007) Probiotic-induced suppression of allergic sensitization and airway inflammation is associated with an increase of T regulatory-dependent mechanism in a murine model of asthma. Clin Exp Allergy 37:498–505
Flower DR (2007) Immunoinformatics and the in silico prediction of immunogenicity: an introduction. Methods Mol Biol 409:1–15
Forsythe P, Wang B, Khambati I, Kunze WA (2012) Systemic effect of ingested Lactobacillus rhamnosus: inhibition of mast cell membrane potassium (IKCa) current and degranulation. PLoS One 7:41234. doi:10.137/journal.pone.0041234
Fuller R (1989) Probiotics in man and animal. J Appl Bacteriol 66:365–378
Furness JB, Kunze WA, Clerc N (1999) Nutrient tasting and signaling mechanisms in the gut. II. The intestine as a sensory organ: neural, endocrine, and immune responses. Am J Physiol 277:922–928
Galli SJ, Borregaard N, Wynn TA (2011) Phenotypic and functional plasticity of cells of innate immunity: macrophages, mast cells and neutrophils. Nat Immunol 12:1035–1044
Geusens PP, Landewe RB, Garnero P, Chen D, Dunstan CR, Lems WF, Stinissen P, van der Helide DM, van der Linden S, Boers M (2006) The ratio of circulating osteoprotegerin to RANKL in early rheumatoid arthritis predicts later joint destruction. Arthritis Rheum 54:1772–1777
Guarner F, Perdigon G, Cortheir G, Salminen S, Koletzko B, Morelli L (2005) Should yoghurt cultures be considered probiotic? Br J Nutr 93:783–786
Guéniche A, Philippe D, Bastien P, Blum S, Buyukpamukcu E, Castiel-Higounenc I (2009) Probiotics for photoprotection. Dermatoendocrinology 1:275–279
Guéniche A, Benyacoub J, Philippe D, Bastien P, Kusy N, Breton L, Blum S, Castiel-Higounenc I (2010) Lactobacillus paracasei CNCM-2116 (ST11) inhibits substance P-induced skin inflammation and accelerates skin barrier function recovery in vitro. Eur J Dermatol 20:731–737
Hacini-Rachinel F, Gheit H, Luduec JL, Dif F, Nancey S, Kaiserlian D (2009) Oral probiotic control skin inflammation by acting on both effector and regulatory T cells. PLoS One 4, e4903
Harata G, Hiruta N, Kawase M, Kubota A, Hiramatsu M, Yausi H (2010) Intranasal administration of Lactobacillus rhamnosus GG protects mice from H1N1 influenza virus infection by regulating respiratory immune responses. Lett Appl Microbiol 50:597–602
Hemarajata P, Versalovic J (2013) Effects of probiotics on gut microbiota: mechanism of intestinal immunomodulation and neuromodulation. Therap Adv Gastroenterol 6:39–51
Herrera J, Ferrebuz A, MacGregor EG, Rodriguez-Iturbe B (2006) Mycophenolate mofetil treatment improves hypertension in patients with psoriasis and rheumatoid arthritis. J Am Soc Nephrol 17:218–225
Hibberd PL, Kleimola L, Florino AM, Botelho C, Haverkamp M, Andreyeva I, Poutsiaka D, Fraser C, Solano-Aguilar G, Snydman DR (2014) No evidence of harms of probiotic Lactobacillus rhamnosus GG ATCC 53103 in healthy elderly- a phase I open label study to assess safety, tolerability and cytokine responses. PLoS One 9, e113456
Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, Morelli L, Canani RB, Flint HJ, Salminen S, Calder PC, Sanders ME (2014) The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol 11:506–514
Miyoshi M, Ogawa A, Higurashi S, Kadooka Y (2014) Anti-obesity effect of Lactobacillus gasseri SBT 2055 accompanied by inhibition of pro-inflammatory gene expression in the visceral adipose tissue in diet-induced obese mice. Eur J Nutr 53:599–606
Iacano A, Raso GM, Canani RB, Calignano A, Meli R (2011) Probiotics as an emerging therapeutic strategy to treat NAFLD: focus on molecular and biochemical mechanisms. J Nutr Biochem 22:699–711
Inoue R, Nishio A, Fukushima Y, Ushida K (2007) Oral treatment with probiotics Lactobacillus johnsonii NCC533 (La1) for specific part of the weaning period prevents the development of atopic dermatitis induced after maturation in model mice, NC/Nga. Br J Dermatol 156:499–509
Izumo T, Maekawa T, Noguchi A, Kitagawa Y, Shibata H, Yasui H, Kiso Y (2010) Effect of intranasal administration of Lactobacillus pentosus S-PT84 on influenza virus infection in mice. Int Immunopharmacol 10:1101–1106
Jan RL, Yeh KC, Hsieh MS, Lin YL, Kao HF, Li PH, Chang YS, Wang JY (2011) Lactobacillus gasseri suppresses Th17 pro-inflammatory response and attenuates allergen-induced airway inflammation in a mouse model of allergic asthma. Br J Nutr 108:130–139
Jang SO, Kim HJ, Kim YJ, Kang MJ, Kwon JW, Seo JH, Kim HY, Kim BJ, Yu J, Hong SJ (2012) Asthma prevention by Lactobacillus rhamnosus in a mouse model is associated with CD4+CD25+Fox3+Treg cells. Allergy Asthma Immunol Res 4:150–156
Jin LY, Choi HJ, Kang TW, Kim HO, Chung MJ, Park YM (2008) CBT-SL5, a bacteriocin from Enterococcus faecalis, suppresses the expression of interleukin-8 induced by Propionibacterium acnes in cultured human keratinocytes. J Microbiol Biotechnol 18:1308–1316
Karimi K, Inman MD, Bienenstock J, Forsythe P (2009) Lactobacillus reuteri-induced regulatory T cells protect against an allergic airway response in mice. Am J Respir Crit Care Med 179:186–193
Kaur J (2014) A comprehensive review on metabolic syndrome. Cardiol Res Pract 2014:943162. doi:10.1155/2014/943162
Kaushal D, Kansal VK (2014) Dahi containing Lactobacillus acidophilus and Bifidobacterium bifidum improves phagocytic potential of macrophages in aged mice. J Food Sci Technol 51:1147–1153
Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J (2005) Global burden of hypertension: analysis of worldwide data. Lancet 365:217–223
Kim HJ, Kim YJ, Kang MJ, Seo JH, Kim HY, Jeong SK, Lee SH, Kim JM, Hong SJ (2012) A novel mouse model of atopic dermatitis with epicutaneous allergen sensitization and the effect of Lactobacillus rhamnosus. Exp Dermatol 21:672–675
Kim HJ, Kim YJ, Lee SH, Kang MJ, Yu HS, Jung YH, Lee E, Seo JH, Kwon JW, Kim BJ, Yu J, Park HM, Hong SJ (2013) Effects of Lactobacillus rhamnosus on asthma with an adoptive transfer of dendritic cells in mice. J Appl Microbiol 115:872–879
King GL (2008) The role of inflammatory cytokines in diabetes and its complications. J Periodontol 79:1527–1534
King VL, Hatch NW, Chan HW, de Beer MC, de Beer FC, Tannock LR (2010) A murine model of obesity with accelerated atherosclerosis. Obesity 18:35–41
Kneeman JM (2012) Secondary causes of nonalcoholic fatty liver diseases. Therap Adv Gastroentrol 5:199–207
Kurzepa J, Madro A, Czechowska G, Kurzepa J, Celinski K, Kazmierak W, Slomka M (2014) Role of MMP-2 and MMP-9 and their natural inhibitors in liver fibrosis, chronic pancreatitis and non-specific inflammatory bowel diseases. Hepatobiliary Pancreat Dis Int 13:570–579
Lee HY, Park JH, Seok SH, Back MW, Kim DJ, Lee KE, Paek KS, Lee Y, Park JH (2006) Human originated bacteria, Lactobacillus rhamnosus PL60, produce conjugated linoleic acid and show anti-obesity effects in diet-induced obese mice. Biochim Biophys Acta 1761:736–744
Li Y, Hua S (2014) Mechanisms of pathogenesis in allergic asthma: role of interleukin 23. Respirology 19:663–669, Immunology 141: 203–210
Liang S, Webb T, Li Z (2013) Probiotic antigens stimulate hepatic natural killer T cells. Immunology 141:203–210
Lilly DM, Stillwell RH (1965) Probiotics: growth-promoting factors produced by microorganisms. Science 147:747–748
Lin L, Ibrahim AS, Xu X, Farber JM, Avanesian V, Baquir B, Fu Y, French SW, Edwards JE Jr, Spellberg B (2009) Th1-Th17 cells mediate protective adaptive immunity against Staphylococcus aureus and Candida albicans infection in mice. PLoS Pathog 5:1000703
Ma X, Hua J, Li Z (2008) Probiotics improve high fat diet-induced hepatic steatosis and insulin resistant by increasing hepatic NKT cells. J Hepatol 49:821–830
Malaguarnera M, Vacante M, Antic T, Giordano M, Chisari G, Acquaviva R, Mastrojeni S, Malaguarnera G, Mistretta A, Volti GL, Galvano F (2012) Bifidobacterium longum with fructo-oligosaccharides in patients with non alcoholic steatohepatitis. Dig Dis Sci 57:545–553
Manirarora JN, Parnell SA, Hu YH, Koslewicz MM, Alard P (2011) NOD dendritic cells stimulated with Lactobacillus preferentially produce IL-10 versus IL-12 and decrease diabetes incidence. Clin Dev Immunol 2011:630187. doi:10.1155/630187
Masoli M, Fabian D, Holt S, Beasley R, Global Initiative for Asthma (GINA program) (2004) The global burden of asthma: executive summary of the GINA Dissemination Committee Report. Allergy 59:469–478
Mencarelli A, Cipriani S, Renga B, Bruno A, D’Amore C, Distrutti E, Fiorucci S (2012) VSL#3 resets insulin signaling and protects against NASH and atherosclerosis in a model of genetic dyslipidemia and intestinal inflammation. PLoS One 7, e45425
Mohamadshahi M, Veissi M, Haidari F, Shahbazian H, Kaydani GA, Mohammadi F (2014) Effects of probiotic yogurt consumption on inflammatory biomarkers in patients with type 2 diabetes. Bioimpacts 4:83–88
Mora JR, Iwata M, Eksteen B, Song SY, Junt T, Senman B, Otipoby KL, Yokota A, Takeuchi H, Ricciardi-Castagnoli P, Rajewsky K, Adams DH, von Andrian UH (2006) Generation of gut-homing IgA-secreting B cells by intestinal dendritic cells. Science 17:1157–1160
Niers L, Martin R, Rijkers G, Sengers F, Timmerman H, van Uden N, Smidt H, Kimpen J, Hoekstra M (2009) The effects of selected probiotic strains on the development of eczema (the PandA study). Allergy 64:1349–1358
Núñez IN, Galdeano CM, de Moreno de LeBlanc A, Perdigon G (2014) Evaluation of immune response, microbiota, and blood markers after probiotic bacteria administration in obese mice induced by a high-fat diet. Nutrition 30:1423–1432
Ohlsson C, Engdahl C, Fak F, Andersson A, Windahl SH, Farman HH, Movérare-Skrtic S, Islander U, Sjögren K (2014) Probiotics protect mice from ovariectomy-induced cortical bone loss. PLoS One 9, e92368
Oksaharju A, Kankainen M, Kekkonen RA, Lindstedt KA, Kovanen PT, Korpela R, Miettinen M (2011) Probiotic Lactobacillus rhamnosus downregulates FCER1 and HRH4 expression in human mast cells. World J Gastroenterol 17:750–759
Okubo N, Matsuzaka M, Takahashi I, Sawada K, Sato S, Akimoto N, Umeda T, Nakaji S (2014) Relationship between self-reported sleep quality and metabolic syndrome in general population. BMC Public Health 14:562
Olivares M, Diaz-Ropero MP, Sierra S, Lara-Villoslada F, Fonolla J, Navas M, Rodriguez JM, Xaus J (2007) Oral intake of Lactobacillus fermentum CECT5716 enhances the effects of influenza vaccination. Nutrition 23:254–260
Panwar H, Rashmi HM, Batish VK, Grover S (2013) Probiotics as potential biotherapeutics in the management of type 2 diabetes- prospects and perspectives. Diabetes Metab Res Rev 29:103–112
Park CW, Youn M, Jung YM, Kim H, Jeong Y, Lee HK, Kim HO, Lee I, Lee SW, Kang KH, Park YH (2008) New functional probiotics Lactobacillus casei probio 65 alleviates atopic symptoms in the mouse. J Med Food 3:405–412
Park DY, Ahn Y, Park SH, Huh CS, Yoo SR, Yu R, Sung MK, McGregor RA, Choi MS (2013a) Supplementation of Lactobacillus curvatus HY 7601 and Lactobacillus plantarum KY 1032 in diet-induced obese mice is associated with gut microbial changes and reduction in obesity. PLoS One 8, e59470
Park MK, Ngo V, Kwon YM, Lee YT, Yoo S, Cho YH, Hong SM, Hwang HS, Ko EJ, Jung YJ, Moon DW, Jeong E, Kim MC, Lee YN, Jang JH, Oh JS, Kim CH, Kang SM (2013b) Lactobacillus plantarum DK119 as a probiotic confers protection against influenza virus by modulating innate immunity. PLoS One 8, e75368
Parker RB (1974) Probiotics, the other half of the antibiotic story. Anim Nutr Health 29:4–8
Peral MC, Rachid MM, Gobbato NM, Huaman Martinez MA, Valdez JC (2010) Interleukin-8 production by polymorphonuclear leukocytes from patients with chronic infected leg ulcers treated with Lactobacillus plantarum. Clin Microbiol Infect 16:281–286
Pinto D, Marzani B, Minervini F, Calasso M, Giuliani G, Gobbetti M, Angelis MD (2011) Plantaricin A synthesized by Lactobacillus plantarum induces in vitro proliferation and migration of human keratinocytes and increases the expression of TGF-β1, FGF7, VEGF-A and IL-8 genes. Peptides 32:1815–1824
Plaza-Diaz J, Gomez-Llorente C, Campana-Martin L, Matencio E, Ortuno I, Martinez-Silla R, Gomez-Gallego C, Periago MJ, Ros G, Chenoll E, Genoves S, Casinos B, Silva A, Corella D, Portoles O, Romero F, Ramon D, de la Cruz AP, Gill A, Fontana L (2013) Safety and immunomodulatory effect of three probiotic strains isolated from the feces of breast-fed infants in healthy adults: SETOPROB study. PLoS One 8, e78111
Poutahidis T, Kleinewietfeld M, Smillie C, Levkovich T, Perrota A, Bhela S, Varian BJ, Ibrahim YM, Lakritz JR, Kearney SM, Chatzigiagkos A, Hafler DA, Aim EJ, Erdman S (2013) Microbial reprogramming inhibits western-diet associated obesity. PLoS One 8, e68596
Poutahidis T, Springer A, Levkovich T, Qi P, Varian BJ, Lakritz JR, Ibrahim YM, Chatzigiagkos A, Alm EJ, Erdman SE (2014) Probiotic microbes sustain youthful serum testosterone levels and testicular size in aging mice. PLoS One 9, e84877
Prescott SL, Wicken K, Westcott L, Jung W, Currie H, Black PN, Stanley TV, Mitchell EA, Fitzharris P, Siebers R, Wu L, Crane, Probiotic Study Group (2008) Supplementation with Lactobacillus rhamnosus or Bifidobacterium lactis probiotics in pregnancy increases cord blood interferon-gamma and breast milk transforming growth factor-beta and immunoglobulin A detection. Clin Exp Allergy 38:1606–1614
Rose MA, Stieglitz F, Koksal A, Schubert R, Schulze J, Zielen S (2010) Efficacy of probiotic Lactobacillus GG on allergic sensitization and asthma in infants at risk. Clin Exp Allergy 40:1398–1405
Ryan MJ (2013) An update on immune system activation in the pathogenesis of hypertension. Hypertension 62:226–230
Sakai F, Hosoya T, Ono-Ohmachi A, Ukibe K, Ogawa A, Moriya T, Kadooka Y, Shiozaki T, Nakagawa H, Nakayama Y, Miyazaki T (2014) Lactobacillus gasseri SBT2055 induces TGF-β expression in dendritic cells and activates TLR2 signal to produce IgA in the small intestine. PLoS One 9, e105370
Sánchez B, Ruiz L, Gueimonde M, Margolles A (2013) Omics for the study of probiotic microorganisms. Food Res Int 54:1061–1071
Saulnier DM, Ringel Y, Heyman MB, Foster JA, Bercik P, Shulman RJ, Versalovic J, Verdu EF, Dinan TG, Hecht G, Guarner F (2013) The intestinal microbiome, probiotics and prebiotics in neurogastroenterology. Gut Microbes 4:17–27
Sawada J, Morita H, Tanaka A, Salminen S, He S, Matsuda H (2007) Ingestion of heat-treated Lactobacillus rhamnosus GG prevents development of atopic dermatitis in NC/Nga mice. Clin Exp Allergy 37:296–303
Seifert S, Bub A, Franz CMAP, Watzl B (2011) Probiotic Lactobacillus casei Shirota supplementation does not modulate immunity in healthy men with reduced natural killer cell activity. J Nutr 141:978–984
Seki S, Habu Y, Kawamura T, Takeda K, Dobashi H, Ohkawa T (2000) The liver as a crucial organ in the first line of host defense: the role of Kupffer cells, natural killer (NK) cells and NK1.1 Ag+ T cells in T helper 1 immune responses. Immunol Rev 174:35–46
Sharma R, Kapila R, Kapasiya M, Saliganti V, Dass G, Kapila S (2014) Dietary supplementation of milk fermented with probiotic Lactobacillus fermentum enhances systemic immune response and antioxidant capacity in aging mice. Nutr Res 34:968–981
Shida K, Kiyoshima-Shibata J, Kaji R, Nagaoka M, Nanno M (2009) Peptidoglycan from lactobacilli inhibits interleukin-12 production by macrophages induced by Lactobacillus casei through toll-like receptor 2-dependent and independent mechanisms. Immunology 128:858–869
Shivakumar V, Kandhare AD, Rajmane AR, Adil M, Ghosh P, Badgujar LB, Saraf MN, Bodhankar SL (2014) Estimation of the long-term cardiovascular events using UKPDS risk engine in metabolic syndrome patients. Indian J Pharm Sci 76:174–178
Smelt MJ, de Haan BJ, Bron PA, van Swam I, Meijerink M, Wells JM, Faas MM, de Vos P (2013) Probiotics can generate FoxP3 T-cell responses in the small intestine and simultaneously inducing CD4 and CD8 T cell activation in the large intestine. PLoS One 8, e68952
Smith CJ, Emge JR, Berzins K, Lung L, Khamishon R, Shah P, Rodrigues DM, Sousa AJ, Reardon C, Sherman PM, Barrett KE, Gareau MG (2014) Probiotics normalize the gut-brain-microbiota axis in immunodeficient mice. Am J Physiol Gastrointest Liver Physiol 307:793–802
So JS, Kwon HK, Lee CG, Yi HJ, Park JA, Lim SY, Hwang KC, Jeon YH, Im SH (2008a) Lactobacillus casei suppresses experimental arthritis by down-regulating helper 1 effector functions. Mol Immunol 45:2690–2699
So JS, Lee CG, Kwon HK, Yi HJ, Chae CS, Park JA, Hwang KC, Im SH (2008b) Lactobacillus casei potentiates induction of oral tolerance in experimental arthritis. Mol Immunol 46:172–180
Stocks N, Allan J, Mansfield PR (2005) Management of hyperlipidemia. Aust Fam Physician 34:447–453
Suda Y, Villena J, Takahashi Y, Hosoya S, Tomosada Y, Tsukida K, Shimazu T, Aso H, Tohno M, Ishida M, Makino S, Ikegami S, Kitazawa H (2014) Immunobiotic Lactobacillus jensenii as immune-health promoting factor to improve growth performance and productivity in post-weaning pigs. BMC Immunol 15:24
Surono IS, Martono PD, Kameo S, Suradji EW, Koyama H (2014) Effect of probiotic L. plantarum IS-10506 and zinc supplementation on humoral immune response and zinc status of Indonesian pre-school children. J Trace Elem Med Biol 28:465–469
Tan PL, Gan CY, Peh KK, Liong MT (2014) Bioactive dairy ingredients for food and non-food applications. Acta Aliment 43:113–123
Tanaka A, Jung K, Benyacoub J, Prioult G, Okamoto N, Ohmori K, Blum S, Mercenier A, Matsuda H (2009) Oral supplementation with Lactobacillus rhamnosus CGMCC 1.3724 prevents development of atopic dermatitis in NC/NgaTnd mice possibly by modulating local production of IFN-γ. Exp Dermatol 18:1022–1027
Thomas CM, Versalovic J (2010) Probiotics-host communication: modulation of signaling pathways in the intestine. Gut Microbes 1:148–163
Trembleau S, Penna G, Bosi E, Mortara A, Gately MK, Adorini L (1995) Interleukin 12 administration induces T helper type 1 cells and accelerates autoimmune diabetes in NOD mice. J Exp Med 181:817–821
Vajro P, Mandato C, Licenziati MR, Franzese A, Vitale DF, Lenta S, Caropreso M, Vallone G, Meli R (2011) Effects of Lactobacillus rhamnosus strain GG in pediatric obesity-related liver disease. J Pediatr Gastroenterol Nutr 52:740–743
van der Aa LB, Heymans HS, van Aalderen WM, Sillevis Smitt JH, Knol J, Ben Amor K, Goosens DA, Sprikkelman AB, Synbad Study Group (2010) Effect of a new synbiotic mixture on atopic dermatitis in infants: a randomized-controlled trial. Clin Exp Allergy 40:795–804
Vandenplas Y, Huys G, Daube G (2014) Probiotics: an update. J Pediatr 91(1):6–21. doi:10.1016/j.jped.2014.08.005
Ventura M, O’Flaherty S, Claesson MJ, Turroni F, Klaenhammer TR, van Sinderen D, O’Toole PW (2009) Genome-scale analyses of health-promoting bacteria: probiogenomics. Nat Rev Microbiol 7:61–71
Wang J, Tang H, Zhang C, Zhao Y, Derrein M, Rocher E, van-Hylckama Vlieg JET, Strissel K, Zhao L, Obin M, Shen J (2015) Modulation of gut microbiota during probiotic-mediated attenuation of metabolic syndrome in high fat diet-fed mice. ISME J 9:1–15
Wendelsdorf KV, Alam M, Bassaganya-Riera J, Bisset K, Eubank S, Hontecillas R, Hoops S, Marathe M (2012) ENteric Immunity SImulator: a tool for in silico study of gastroenteric infections. IEEE Trans Nanobiosci 11:273–288
Wickens K, Black PN, Stanley TV, Mitchell E, Fitzharris P, Tannock GW, Purdie G, Crane J, Probiotic Study Group (2008) A differential effect of 2 probiotics in the prevention of eczema and atopy: a double-blind, randomized, placebo-controlled trial. J Allergy Clin Immunol 122:788–794
World Health Organization (2008) The global burden of disease: 2004 update. WHO Press, Geneva, Switzerland, pp 39–52, http://www.who.int/healthinfo/global_burden_disease/GBD_report_2004update_full.pdf
Xiao J, Zhang Y, Yang Z (2014) Lactic acid bacteria in health and disease. In: Zhang H, Cai Y (eds) Lactic acid bacteria: fundamental and practice. Springer, London, pp 303–374
Yang F, Polk DB (2011) Probiotics and immune health. Curr Opin Gastroenterol 27:496–501
Yang JH, Min TK, Lee HW, Pyun BY (2014) Efficacy of probiotic therapy on atopic dermatitis in children: a randomized, double-blind, placebo-controlled trial. Allergy Asthma Immunol Res 6:208–215
Yoo SR, Kim YJ, Park DY, Jung UJ, Jeon SM, Ahn YT, Huh CS, McGregor R, Choi MS (2013) Probiotics L. plantarum and L. curvatus in combination alter hepatic lipid metabolism and suppress diet-induced obesity. Obesity 21:2571–2578
You J, Dong H, Mann ER, Knight SC, Yaqoob P (2014) Probiotic modulation of dendritic cell function is influenced by ageing. Immunology 219:138–148
Yu J, Jang SO, Kim BJ, Song YH, Kwon YH, Kwon JW, Kang MJ, Choi WA, Jung HD, Hong SJ (2010) The effects of Lactobacillus rhamnosus on the prevention of asthma in a murine model. Allergy Asthma Immunol Res 2:199–205
Zar HJ, Ferkol TW (2014) The global burden of respiratory disease-impact on child health. Pediatr Pulmonol 49:430–434
Zhang Y, Guo X, Guo J, He Q, Song Y, Zhang H (2014) Lactobacillus casei reduces susceptibility to type 2 diabetes via microbiota-mediated body chloride ion influx. Sci Rep 4:5854. doi:10.1038/srep05654
Zhu J, Zhao L, Guo H, Jiang L, Ren F (2011) Immunomodulatory effects of novel Bifidobacterium and Lactobacillus strains on murine macrophage cells. Afr J Microbiol Res 5:8–15
Conflict of Interest
No.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Tan, P., Eor, J., Chun, T., Kim, S. (2015). Immune Modulation by Probiotics. In: Liong, MT. (eds) Beneficial Microorganisms in Medical and Health Applications. Microbiology Monographs, vol 28. Springer, Cham. https://doi.org/10.1007/978-3-319-23213-3_5
Download citation
DOI: https://doi.org/10.1007/978-3-319-23213-3_5
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-23212-6
Online ISBN: 978-3-319-23213-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)