Abstract
Mucosal melanoma is an exceedingly rare variant of cutaneous melanoma that, due to its rarity, is poorly described and infrequently studied. Primary sites of origin include the head and neck, anorectum and vulvovaginal regions. It is uniquely different from cutaneous melanoma with respect to epidemiology, etiology, pathogenesis and prognosis. The etiology and pathogenesis remain unclear. Unlike cutaneous melanoma, exposure to UV light is not an apparent risk factor. Furthermore, distinct molecular features including a lower incidence of BRAF oncogene mutations but a higher incidence of KIT oncogene mutations suggest divergent genetic etiologies. Mucosal melanomas generally present at a later stage, are more aggressive and carry a worse prognosis regardless of the stage at diagnosis. Establishing standardized treatment guidelines has been challenging due to the rarity of the disease. Early detection provides the best chance at survival but is often difficult due to anatomic location. Surgery remains the primary therapeutic intervention if complete resection is technically feasible given the anatomic location. Radiotherapy may be used to achieve local control when resection is not feasible, or adjuvantly to enhance locoregional control, but most studies have failed to demonstrate an improvement in overall survival. There are no consensus guidelines on the optimal systemic therapy, and regimens are often extrapolated from data based on therapies used to treat advanced cutaneous melanoma. Clinical trials, particularly utilizing newer targeted therapies and immunotherapies, are investigating novel treatment approaches.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Mucosal melanoma is an exceedingly rare variant of cutaneous melanoma [7, 14, 24, 29, 41, 45–49, 54, 57, 63], representing approximately 0.03 % of all cancer diagnoses [38] and 1.3 % of all melanomas [3, 7, 9, 10, 14, 29, 31, 38, 41, 46, 48, 54–57]. It was first described in 1856 by Weber et al., but not classified as its own distinct disease process until 1869 by Lucke [28, 38, 57]. Unfortunately, because of its rarity, it is a poorly described and infrequently studied disease process, and as a result there is a paucity of consistent data regarding its epidemiology, etiology and pathogenesis, as well as limited data to support general recommendations regarding its proper diagnosis and treatment [14, 28, 29, 46, 57].
Mucosal melanomas can arise from any mucosal surface [9, 14, 29, 31, 41, 45, 48, 55, 57], most typically the mucosal epithelium of the respiratory, alimentary and genitourinary tracts, where melanocytes are present [3, 10, 28, 31, 41, 45, 47, 54, 57]. Primary sites of origin include the head and neck (55 %), the anorectum (24 %) and the vulvovaginal region (18 %) [3, 7, 9, 10, 14, 29, 38, 41, 45, 47, 50, 54, 56, 57]. Less frequently, mucosal melanoma has been found in the urinary tract (3 %) [3, 7, 10, 29, 46, 56, 57], as well as the tracheobronchial tree, esophagus, stomach, small and large intestine, gall bladder and cervix [9, 10, 28].
2 Etiology
Mucosal melanoma is a distinct entity from cutaneous melanoma with respect to epidemiology, etiology, pathogenesis and prognosis [24, 26, 28, 29, 41, 46, 48, 49, 54, 55, 57]. While the incidence of cutaneous melanoma is rapidly increasing in the United States, the incidence of mucosal melanoma appears stable [9, 10, 21, 25, 29, 41, 46, 48, 56, 57]. In general, patients with mucosal melanoma present at a much later age, about one to two decades later than cutaneous melanoma [14, 29, 46, 54], with a majority of cases reported between the ages of 50–80 [10, 14, 28, 29, 31, 38, 41, 45, 54, 56, 63], and a median age at diagnosis of 70 [9, 21, 46, 48]. Females are diagnosed more often than males [7, 9, 10, 14, 21, 28, 29, 31, 41, 46, 48, 56], in many reports up to twice as often [9, 14, 29, 41, 48], due to cases of vulvovaginal disease, with estimated prevalence in the United States at 2.8 cases per million women versus 1.5 cases per million men [29, 41]. Notably, a slight male predominance in cases of the head and neck mucosal melanoma subtypes has been suggested [14, 45, 46]. Overall mucosal melanoma does not seem to have a racial predilection, although it makes up a higher percentage of overall melanoma cases diagnosed in Black, Asian and Hispanic populations, likely reflecting the lower incidence of cutaneous melanoma in these populations. It has also been described that considered separately, mucosal melanoma of the oral cavity may be more common in Black and Japanese populations [9, 10, 38].
The etiology and pathogenesis of mucosal melanoma remain unclear [14, 38, 41, 46]. To date there are no clearly established risk factors for its development [7, 9, 14, 48, 57]. Unlike cutaneous melanoma, the common anatomic locations for mucosal melanoma preclude exposure to UV light as a risk factor [7, 9, 14, 21, 24, 26, 28, 29, 38, 41, 46, 48, 54, 56, 57]. There is no definitive evidence that common carcinogens such as tobacco and formaldehyde, or exposure to carcinogenic viruses such as the human papilloma viruses, human herpes viruses or polyomavirus have a role in its pathogenesis [7, 29, 38, 41, 48, 54]. Nevertheless, some studies have suggested various correlations between the mucosal melanoma subtypes and various predisposing risk factors, though strong evidence for any of these correlations is lacking [9]. Melanocytes have an established role in the sinonasal region in the metabolization of polycyclic aromatic hydrocarbons, suggesting a link between inhaled environmental and immune factors and the development of sinonasal mucosal melanoma [9, 38, 46, 57]. Because of the higher incidence of oral cavity mucosal melanoma in the Japanese population, some researchers have suggested a correlation between this particular subtype and unidentified common hereditary or environmental factors [38, 41, 46, 54, 57, 63]. In addition, up to one third of oral cavity mucosal melanomas appear to be preceded by melanosis, which has been suggested to represent the radial growth phase prior to the vertical growth phase [9, 38, 41, 43, 45, 46, 48, 54, 57, 63], a phenomenon reported in head and neck mucosal melanoma as well [43, 57]. Although no direct correlation has been established, 66 % of patients with laryngeal or pharyngeal mucosal melanoma have a history of smoking [38]. Anorectal mucosal melanoma appears to be more prevalent in patients with HIV [9, 28, 54, 63]. Finally, vulvovaginal mucosal melanoma has been suggested to be associated with chronic inflammatory conditions, viral infections and chemical irritants. Genetic factors may also play in the development of vulvovaginal disease, as 15 % of patients with vulvar mucosal melanoma provide a family history of cutaneous melanoma, and one study reported a patient with vulvar mucosal melanoma with a germline mutation in the melanocortin type I receptor [9, 46, 54].
3 Molecular Biology
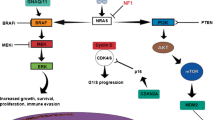
Mucosal melanomas have distinct molecular features that suggest divergent genetic etiologies (see Fig. 1) [8, 29, 31, 41, 46, 49, 54]. Studies of comparative genomic hybridization have shown distinct chromosomal aberrations such as gains of 1q, 6p and 8q [29, 54], gain of function mutations such as K642E, L576P, D816H and V559A [38, 54] and amplifications of the 4q12 locus [54]. While the incidence of activating mutations in the BRAF oncogene is quite common in cutaneous melanoma, it appears to be rare in mucosal melanoma, with an incidence estimated at 10 % [3, 7–9, 19, 29, 38, 41, 46, 48–50, 54, 56, 61]. More salient from a treatment perspective is that mucosal melanomas appear to have a high incidence of activating mutations and/or amplifications in the KIT oncogene. Curtin et al. first demonstrated the presence of KIT mutations in mucosal melanomas and estimated the rate of these mutations to be 39 %, while similar mutations were found in cases of non-chronic, sun damaged cutaneous melanoma cases [3, 12, 38, 41, 50, 54]. Beadling et al. found KIT mutations in 15.6 % of mucosal melanoma cases studied [4, 41, 62]. Finally, Carvajal et al. reported that of 295 tumor samples screened for the presence of KIT mutations or amplifications, abnormalities were found in 25 % [9, 48], a rate of KIT mutations and/or amplifications that has been similar across multiple reports [7–9, 19, 24, 26, 29, 31, 38, 39, 48–50, 54, 61, 62].
4 Prognosis
Mucosal melanomas are often aggressive and carry a worse prognosis, regardless of the stage at the time of diagnosis [3, 9, 14, 22, 26, 28, 29, 31, 32, 38, 41, 45, 46, 48, 54–57, 62, 63]. In contrast to cutaneous melanomas, mucosal melanomas more frequently are amelanotic and present in a multifocal fashion [2]. While different staging systems are in place for mucosal melanomas of different primary sites, a generalized staging system can be utilized as follows: Stage I, clinically localized disease; Stage II, regional nodal involvement; and Stage III, distant metastatic involvement [2]. The often concealed locations of mucosal melanoma present a challenge for routine screening and result in frequent presentations of advanced disease [3, 9, 14, 22, 26, 28, 29, 31, 32, 38, 41, 45, 46, 48, 54–57, 62, 63]. In addition, unique to these anatomic locations are vast vascular and lymphatic networks in close proximity to the primary tumor, allowing for diffuse spread [9, 14, 28, 29, 46, 54, 57], with approximately one third of patients having nodal involvement at diagnosis [10, 14, 29, 38, 45, 46]. Local treatment failure is common [48, 54, 55], with recurrences rates suggested to be as high as 50–90 % even with complete surgical resection [9]. Local recurrences are considered a harbinger for simultaneous or subsequent metastatic spread [43, 48, 54]. In all likelihood, most patients have micrometastatic disease at the time of presentation, resulting in a disease course characterized by local recurrences followed by metastatic disease, even despite aggressive surgical resection and adjuvant therapy [3, 7, 14, 29, 31, 32, 38, 46, 48, 54, 55, 57]. Thus, even for patients with presumed early-stage disease, the prognosis is poor, with 5 year survival rates of only 25 % [3, 9, 10, 22, 28, 29, 31, 32, 38, 43, 45, 46, 54, 55].
5 Treatment
Establishing guidelines for the clinical course of mucosal melanoma has been challenging due to the rarity of the disease. This renders conducting large, randomized, controlled trials to investigate various treatment modalities in this particular melanoma subtype difficult [3, 7, 14, 29, 46, 54], thus standards of care have not been formulated. As well, assumptions regarding the natural history and appropriate management of mucosal melanoma are based on retrospective case series, which are often limited by small numbers of cases and inconsistent treatment regimens [29, 43, 48]. Like most malignancies, early detection still provides the best chance at survival, but is difficult as previously discussed [14, 45, 46, 54]. Surgical considerations are often dictated by the anatomic location of the tumor, with adjuvant radiotherapy a consideration for local control. Limited data exist concerning adjuvant systemic therapy for the disease, and systemic treatments for distant disease often follow established paradigms for cutaneous melanoma [7, 14, 28, 29, 31, 41, 57], unless targets unique to mucosal melanoma are discovered within the primary or metastatic tumor.
5.1 Surgery
Surgery remains the primary therapeutic intervention for mucosal melanoma [9]. Regardless of subtype, when technically feasible, complete resection for local control provides the best chance at prolonged disease-free survival and cure, especially in light of the lack of effective systemic treatment options [9, 10, 14, 28, 46, 54, 57]. Unfortunately, complete resection is often challenging due to the anatomy of the commonly involved regions and the tendency for these tumors to have a lentiginous growth pattern [9, 14, 31, 48, 54, 55, 57]. Historically, the surgical management of mucosal melanoma involved radical procedures such as abdominoperineal resections (APR) for anorectal disease and pelvic exenteration for vulvovaginal disease [7, 9, 32, 48]. These operations resulted in significant morbidity and functional impairments [7, 9, 32]. While studies have shown improved local control with aggressive surgical resection, retrospective reports suggest they confer no overall survival benefit over more conservative techniques [7, 9, 32, 48]. Furthermore, because most patients will develop local recurrences and ultimately metastatic disease regardless of the intervention chosen, multiple additional considerations such as patient preference and quality of life often become more relevant considerations [9, 14, 32, 48, 57]. Thus, in general, conservative procedures by way of wide local excisions have replaced aggressive procedures as primary management [9, 48]. In addition, recent evidence has suggested that less-invasive endoscopic resections may be a feasible alternative for achieving aggressive local control with less morbidity for patients. This may become particularly relevant for patients with multiple local recurrences requiring repeat resections and patients with multifocal disease [9].
Lymph node status remains a controversial topic when discussed in relationship to mucosal melanoma. Unlike cutaneous melanoma, where lymph node status is a vital prognostic factor, the implications of lymph node status in cases of mucosal melanoma are less apparent. Toward that end, there is no widely accepted standard of care for the management of lymph nodes in mucosal melanoma [9]. As with other types of malignancies, evaluation of the sentinel lymph node has become routine in cases of cutaneous melanoma [9]. While this is often feasible in cases of mucosal melanoma, because of the uncertainty surrounding the prognostic importance of a positive finding, the role of sentinel lymph node biopsies remains undefined. Moreover, in light of the known course of this disease to involve frequent recurrences and distant metastases despite aggressive therapy, the question of if and how a positive sentinel lymph node should change subsequent therapy remains [9, 48]. Similarly, while most are in agreement that therapeutic lymph node dissection is reasonable to address clinically apparent, bulky or symptomatic disease, the performance of prophylactic lymphadenectomy is falling out of favor [14]. Anatomic location of a mucosal melanoma also influences surgical treatment options, as summarized below.
5.1.1 Mucosal Melanomas of the Head and Neck
Mucosal melanoma of the head and neck region constitutes 55 % of all mucosal melanomas, but <10 % of all melanomas of the head and neck region [3, 7, 9, 10, 14, 28, 29, 38, 41, 45, 47, 50, 54, 56, 57]. A majority of these tumors are found in the sinonasal regions (~55 %), while the rest are located in the oral cavity (25–40 %) [9, 28, 38, 41, 46–48, 54, 56, 57]. Among sinonasal cases, approximately 80 % are located in the nasal cavity itself, most commonly the turbinates and lateral nasal wall [9, 28, 38, 41, 43, 46, 54], while 20 % occur in the paranasal sinuses, most commonly the maxillary and ethmoid sinuses, followed by the frontal and sphenoid sinuses [9, 28, 38, 41, 46, 54]. Lesions in the oral cavity are most commonly found in the large palate and upper alveolus [9, 28, 38, 41, 46, 47, 54, 57]. Lesions within the larynx or pharynx are extremely rare, with only sixty cases reported in the literature. These tumors are most commonly located in the supraglottic region (62.2 %) followed by the vocal cords (37.8 %) [28, 38, 41, 46].
The American Joint Committee on Cancer (AJCC) staging system for head and neck mucosal melanoma is often utilized, beginning at stage III (see Table 1). The primary therapeutic modality is complete surgical resection for mostly localized stage III and IVA disease [3, 14, 41, 43, 46–48, 54, 57, 63]. The type of surgical approach used is dependent upon the location and extension of the tumor, but the goal is negative margins with minimal cosmetic or functional derangements [43, 47]. Surgery is not advised for very advanced stage IVB or metastatic stage IVC disease aside from attempting to achieve local control for symptomatic purposes [47, 48]. Unfortunately, achieving melanoma-free margins is often procedurally challenging due to the anatomical complexity of the region and the close proximity of critical anatomic structures [41, 43, 44, 48, 54, 57, 63]. The primary approach should be accompanied by appropriate surgical reconstruction for the area [14, 48, 54, 57]. However, rates of recurrence and widespread disease remain high [43, 48, 57]; it is thus difficult to justify the use of radical surgical procedures in a majority of patients, and morbidity should be a prime consideration [43]. In the setting of local recurrence, repeat surgical resections may be considered, but only after performing an extensive re-staging work-up [48]. For the multitude of reasons above, endoscopic resections are being performed more frequently to avoid the morbidity associated with open procedures [43, 48].
As discussed previously, although biopsy of the sentinel lymph node is technically feasible in head and neck mucosal melanoma, because of the uncertain prognostic implications provided by the sentinel node and the ambiguity of how it guides further management, its role in diagnosis is still under investigation [9, 27, 48, 54]. Unlike many other malignancies, the clinical significance of detecting a positive sentinel lymph node remains uncertain, as there are no clear data to suggest that outcomes are improved if management is altered to address discovered nodal disease [48]. The role of prophylactic lymph node dissection is contested as well. It is often recommended in cases of oral cavity mucosal melanoma because of the high incidence of lymph node involvement, but in cases of sinonasal disease, where the lymph nodes are less commonly involved, elective dissection is not routinely recommended [47, 54]. There is also evidence to suggest that, even in cases of oral cavity melanoma, prophylactic neck dissection does not change ultimate outcomes [48, 57]. Therapeutic neck dissection is considered in patients with clinically evident nodal disease for the purposes of local control and to address symptomatic concerns [47, 48, 57]. Much like elective lymph node dissection, there is no compelling evidence to suggest therapeutic dissection results in an overall survival benefit, even in patients without an eventual lymph node recurrence, suggesting that this procedure should be reserved for patients with clinically apparent nodal disease requiring local control, and not done empirically [48, 57].
5.1.2 Mucosal Melanomas of the Anus and Rectum
Anorectal mucosal melanoma represents 24 % of all mucosal melanomas, but <1 % of all malignant tumors of the anorectal region [3, 7, 9, 10, 14, 28, 29, 32, 38, 41, 45–47, 50, 54, 56, 57, 63]. The area known as the transitional zone of the anal canal harbors a variety of epithelial cells, including squamous-type, and the presence of melanocytes is known here, particularly beneath the dentate line [7, 28, 46, 54, 57]. The concentration of melanocytes increases distally from the dentate line toward the anoderm. For this reason, it is presumed anorectal mucosal melanoma arises within melanocytes distal to the dentate line, then has a tendency to extend proximally into the rectum [7, 28, 46, 54, 57]. There are rare cases of melanocytes present in the intestinal mucosal epithelium above the dentate line in normal patients, thus, melanoma at times can arise in the proximal anus or distal rectum [7, 54]. It is important to note, however, that only 1/3 of anorectal mucosal melanomas are pigmented, and that the presence of amelanotic melanoma is an adverse prognostic sign. Mucosal anorectal melanomas must be differentiated from anal melanomas of cutaneous origin, as the latter are more likely to behave like typical cutaneous melanomas. Approximately one third of anorectal mucosal melanoma cases are thought to originate within the anal canal, while 42 % arise from the rectum and 25 % have an indeterminate origin [9, 48]. Perineural invasion, tumor size, and thickness have also been found to be adverse prognostic factors.
Similarly to head and neck mucosal melanoma, the mainstay of treatment for anorectal melanoma is surgical resection, though there is no standard of care or agreed upon optimal approach as in colorectal carcinoma [7, 46, 50, 54, 57, 63]. Historically more aggressive surgical approaches were considered to be superior, and abdominoperineal resection (APR) was the standard of care [7, 9, 32, 48]. Once retrospective reviews of clinical outcomes data were available, and it was suggested that the extent of the surgical intervention did not significantly improve overall survival [7, 9, 14, 32, 46, 48, 54, 57], more conservative, sphincter-sparing, wide local excisions became the procedure of choice [7, 14, 32, 41, 48, 54, 57]. There has been some suggestion that performing an APR improves local control and recurrence rates over wide local excisions, but the benefit of that control appears limited. Local recurrence rates have been estimated at 8 % with APR versus 20 % with wide local excision, but as stated, this has not resulted in an improvement in overall survival [7, 14, 32, 41, 46, 48, 54, 57]. Furthermore, on multivariate analysis, type of resection was not shown to be significantly associated with prognosis [57].
The goal, of course, remains to achieve negative surgical margins if feasible, as studies have suggested a survival benefit in patients where negative margins are obtained. One study showed a 5-year survival rate of 19 % in patients with negative surgical resection margins, versus 6 % in those with positive margins [7]. APR remains an option for patients with bulky locally confined disease, and in some patients with local recurrences after conservative excision, though it still confers high morbidity and functional limitations [14, 32, 54]. As in the other subtypes of mucosal melanoma, because most patients develop recurrences and distant disease regardless of primary surgical intervention, and because of the aforementioned controversy over the impact of the extent of surgery on clinical outcomes, quality of life considerations should be a top priority [7, 32, 48].
The diagnostic and therapeutic considerations surrounding lymph nodes in cases of head and neck mucosal melanoma translate to cases of anorectal melanoma. Nodal involvement is also of uncertain prognostic significance in this mucosal melanoma subtype, as studies have suggested that regional lymph node metastases have not affected disease recurrence or survival rates. Additionally, data suggest prophylactic lymph node dissection is not associated with improved long-term prognosis. Thus, there is no established role for sentinel lymph node biopsy or indication for elective lymph node dissection [7, 9, 48, 57]. Therapeutic lymphadenectomy should be offered in cases of clinically apparent disease. It should be noted, however, that because the lymphatic drainage of the anorectum differs by tumor location, either superficial inguinal lymph nodes or hypogastric and obturator lymph nodes (and subsequently the sigmoid and peri-aortic rectal groups) may be involved [48, 57]. Thus, there may be significant morbidity associated with such lymph node dissections.
5.1.3 Mucosal Melanomas of the Vulva and Vagina
Vulvovaginal mucosal melanoma represents 18 % of all mucosal melanomas, while vulvar melanoma accounts for 10 % of all vulvar malignancies and vaginal <3 % of all vaginal malignancies [3, 7, 9, 10, 14, 21, 22, 28, 29, 38, 41, 45–47, 50, 54, 56, 57, 63]. Melanoma of the cervix and uterus are quite rare [28, 41, 46]. Vulvar melanomas vastly outnumber vaginal melanomas, with <5 % of vulvovaginal mucosal melanomas arising from the vagina [9, 21, 22, 41, 46, 48, 54]. Some reports suggest up to 20 % of cases are multi-focal, and the precise site is unidentifiable [9, 41, 54]. This is also complicated by the fact that vulvar melanomas may extend to the mucocutaneous vaginal border, obscuring the primary site of origin [46]. Of vulvar melanomas, most cases arise from the labia minora, clitoris, or inner labia majora [9, 22, 41, 46, 57, 59, 63]. The periurethral area and vaginal introitus are less commonly involved [26, 41, 57, 63]. Of vaginal melanomas, most are confined to the lower third of the vagina and the anterior wall [5, 9, 17, 28, 41, 46, 57, 63]. Bleeding, discharge, and palpation of a discernible mass are common presenting signs [5, 17, 59]. Vulvar melanomas are actually staged according to the 2002 TNM staging system for melanoma, whereas vaginal melanomas are staged by the generalized staging system discussed earlier in this chapter [5, 46, 59]. Five year survival rates for vulvar melanoma range from 24 to 77 %, and are poorer for vaginal melanoma, with rates of 5–25 % [5, 59].
Similar to anorectal melanoma, aggressive surgical approaches were the standard of care in the past, and among the options were vulvectomy, vaginectomy, urethrocystectomy, radical hysterectomy and pelvic exenteration [9, 14, 21, 22, 41, 46, 48, 54, 57]. As with anorectal melanoma, though studies have shown improved local control with these more aggressive approaches, retrospective data suggests they result in no significant improvement in overall survival as compared to more conservative, wide local excisions, thus they have fallen out of favor [9, 14, 21, 22, 41, 46, 48, 54, 57, 59]. In light of the high rates of local recurrence and metastatic spread, the benefit of improved local control is called into question, making patient preference and functional deformity important considerations [9, 14, 48, 57]. Unfortunately, obtaining negative margins without aggressive surgical procedures may be technically difficult due to the multifocality of these tumors and the anatomic constraints of the region [57]. In general, 1 cm margins should be obtained for small melanomas <1 mm; 2 cm margins can be entertained for patients with larger tumors, if possible. When wide local excision is not technically feasible, more aggressive approaches may be judiciously considered, or excision may be combined with adjuvant radiation in the setting of close or positive margins [41]. Cervical and uterine mucosal melanomas are generally still treated with radical procedures such as radical hysterectomies and vaginectomies with lymphadenectomies [28, 41].
Again, the issue of lymph node management in vulvovaginal mucosal melanoma is unclear [48]. However, sentinel lymph node mapping is feasible in patients with vulvar melanoma and recommended by some experts [35], although studies have yielded conflicting results regarding the prognostic relevance and effects on overall survival [21, 48]. While prophylactic lymph node dissection may reduce the chance of recurrence in the lymph node bed, it does not appear to impact outcomes [21, 54, 57]. Lymphadenectomy may be considered on a therapeutic basis in the setting of clinically apparent or symptomatic disease [48]. In contrast, sentinel lymph node mapping for vaginal melanomas is likely to be difficult given the complexity of lymph node drainage to the pelvic and/or inguinal basins, and cannot routinely be recommended [35].
5.1.4 Mucosal Melanomas of Other Rare Sites
Mucosal melanoma has been reported in a number of exceedingly rare sites, including the tracheobronchial tree, esophagus, stomach, small and large intestine, biliary tract, and urinary tract. Primary melanoma of the lung, specifically the tracheobronchial tree is extremely rare, with only approximately thirty cases reported in the literature [28, 41]. The treatment of choice is lobectomy and pneumonectomy in conjunction with lymph node resection. The role of adjuvant therapies, including both radiotherapy and chemotherapy, is undefined [41]. Mucosal melanoma of the esophagus is also rare, representing 0.1–0.2 % of all esophageal malignancies [28, 41, 46, 63] with just over 300 cases reported as of 2011 [63]. It is predominantly confined to the middle and lower parts of the esophagus, with only 10 % of cases located in the upper third [28, 41, 57, 63]. Radical surgical resections with nodal dissections are often the chosen primary treatment, though they have not been demonstrated to improve survival [28, 41, 46, 57]. Adjuvant therapy has been used in these cases in a palliative role [41]. Mucosal melanoma of the stomach constitutes 2.7 % of mucosal melanomas of the GI tract, with <20 cases reported in the literature [41]. Melanoma of the small intestine comprises 2.3 %, and is most commonly located in the ileum [41]. Melanoma of the biliary tract represents 1.4 % of mucosal melanomas of the GI tract, with only nine cases of bile duct and thirty cases of gallbladder melanoma reported [28, 41]. Finally, mucosal melanoma of the large intestine makes up 0.9 %, with only twelve cases reported to date [41]. Surgery is still the mainstay of therapy, and as is the trend in mucosal melanoma, has not improved overall survival [41].
Mucosal melanoma of the urinary tract includes melanomas of the urethra and bladder. Urethral melanoma represents 3 % of all mucosal melanomas, and only 4 % of all urethral malignancies, with only about 25 cases in males and 40 cases in females reported [28, 41, 46, 63]. There have only been around 20 cases of bladder melanoma reported [28, 41]. Urethral melanoma is most commonly located in the distal urethra, followed next in frequency by the meatus [41, 46, 57, 63]. Thus, treatment typically involves partial penectomy or urethrectomy with or without inguinal lymph node dissection. In the cases of more proximal urethral lesions, radical cystoprosto urethectomy or anterior exenteration may be required [46]. The optimal extent of surgery still remains undefined, and additional options have been used in female patients including radiotherapy and cryosurgery [41, 46]. Bladder melanoma is predominantly treated with surgical resection [41]. Regardless of approach used, the survival benefit is limited [46].
5.1.5 Radiotherapy
Radiotherapy may be used to achieve local control in patients with mucosal melanoma for whom surgical resection is not possible, or to enhance control after surgery particularly when resection is suboptimal, a not infrequent occurrence given the anatomic locations of these tumors. Most studies have failed to demonstrate an improvement in overall survival with adjuvant radiotherapy, although these findings are complicated by the fact that there is a tendency for radiotherapy to be favored in more advanced cases [9, 43]. Radiation is also clearly useful for most of these tumors in the palliative setting to control symptomatic local disease [41, 46, 57]. Multiple ongoing studies to investigate newer technologies that are capable of more precise delivery of radiotherapy such as protons and heavy ions that take advantage of higher linear energy transfer [9] may better define the role for radiation in the upfront management of these tumors.
With regard to mucosal melanomas of the head and neck, most clinicians agree on the use of adjuvant radiotherapy when surgery is not appropriate or feasible, and in the setting of extracapsular disease, two or more nodes involved, large nodes (3 cm or greater), positive or close margins and in the setting of residual disease or recurrence after primary surgical resection [43, 46, 47, 54, 57], and on omission of RT in clinically sensitive locations such as the eye. While several studies have accounted improved local control with the addition of radiotherapy, overall survival is not significantly affected [41, 43, 47, 48, 54, 57]. The use of prophylactic radiotherapy without clinically apparent disease post-operatively or in the above indications is contested, with some recommending post-operative radiotherapy in almost all cases because of the high risk of potentially devastating local recurrence, while others argue against its use as it has not been demonstrated to improve recurrence rates even in spite of that risk [54]. Although not an exclusive test of radiotherapy in mucosal melanoma, the Trans-Tasman randomized trial of radiotherapy or observation after lymphadenectomy for patients with high-risk melanoma can safely be extrapolated to mucosal presentations. This trial demonstrated a significant improvement in LR recurrence (HR 0.56), but without improvements in RFS or OS. The fractionation used in the RT arm was 48 Gray (Gy) in 20 fractions [6]. With respect to additional reports of optimal dosing and fractionation, a series of 28 patients reported a 49 % local control rate at 3 years using a treatment schedule of 50–55 Gy in 15–16 fractions; a similar 44 % local control rate was achieved in 25 patients treated with 8 Gy on days 7 and 21 [18, 23]. Of note, Moreno et al. reported standard fractionation at a dose greater than 54 Gy resulted in superior results as compared to a hypofractionated schedule, with locoregional failure rates of 54.6 % versus 100 % respectively [43]. Some retrospective studies noted better local control with modification of dose schedule, whereas others note both improved local control and overall survival [9, 43].
Importantly, radiotherapy has also been useful as an adjunct to sphincter-sparing local excision in anorectal melanomas, as an alternative to APR in order to preserve quality of life [32, 41, 48], although even in reports of high local control rates, 5 year survival remains low [30]. Similarly in patients with vaginal melanomas, where pelvic exenteration would be required for complete excision with unclear impact on survival, radiation may be an important tool to enhance local control when used adjuvantly and may reduce morbidity if used in the neoadjuvant setting. The fractionation schedule for radiation used for anal or vulvovaginal melanoma is based on patient and tumor anatomy with careful consideration of expected acute and chronic toxicities; with standard fractionation schedules used in the adjuvant or neoadjuvant setting and hypofractionation used primarily in the palliative or limited postoperative setting. However, no improvement in overall survival in vaginal or vulvovaginal melanoma has been reported in two recent studies, respectively [17, 33]; as such the role of radiation is largely to palliate local or metastatic disease.
5.2 Systemic Therapy
There are no consensus guidelines on the optimal systemic therapy for mucosal melanoma. Most conclusions regarding systemic therapies to date are based on case reports on a limited number of patients [3, 22, 32]. As a result, systemic therapy regimens vary widely [3, 22, 32] and are extrapolated from data based on therapies used to treat advanced cutaneous melanoma [3, 9, 50]. Contributing to the ambiguity is that many past and ongoing studies have excluded patients with mucosal melanoma [3]. Even still, with limited data available, no systemic therapy has been shown to significantly improve outcomes [9, 14, 19, 31, 57]. As it stands, the medial overall survival reported with most treatment regimens is 4.9–9.7 months [25]. Chemotherapy, targeted therapies and immunotherapies have each been examined in small studies (Table 2) and form the basis of ongoing clinical trials examining novel approaches.
5.2.1 Chemotherapy
The benefit of adjuvant chemotherapy in mucosal melanoma is unclear. There has been some experience among these patients with cisplatin and interferon, but outcomes in general show limited benefit. Some studies even suggest a possible decrease in survival rates in cases where these agents have been tried [46, 57]. However, as mentioned above, systematic trials of chemotherapy regimens specifically involving mucosal melanoma patients are lacking [54]. In a single study of 189 Chinese patients with resected mucosal melanoma, chemotherapy prolonged relapse free survival versus interferon or observation (20.8 vs. 9.4 and 5.4 months) and significantly increased overall survival (49 vs. 40 and 21 months) [36].
There are limited data regarding the efficacy of additional chemotherapeutic agents in mucosal melanoma patients, and while some retrospective series suggest these therapies produce responses in these patients equivalent to those seen in cases of cutaneous melanoma, others have demonstrated mucosal melanoma patients have worse outcomes in similar dacarbazine-based regimens [9, 48]. A trial examining the combination of chemotherapy with bevacizumab in advanced patients is under way (Table 3). Small retrospective series exist reporting antitumor activity of biochemotherapy based regimens similar to those in cutaneous melanoma [3, 32]. Kim et al. reported the retrospective evaluation of cisplatin, vinblastine, dacarbazine, IFN-a 2b and/or IL-2 in patients with anorectal mucosal melanoma [3, 22, 32, 48]. Of 18 treated patients, 8 patients (44 %) had major responses, including two (11 %) with complete responses. Median time to progression of evaluated patients was 6.2 months and the median overall survival was 12.2 months. Prolonged survival was seen in a subset of treated patients ranging from 14.0 to 43.7 months, and response rates were highest in those who received treatment first line [32, 48]. Harting et al. looked at eleven patients with advanced vulvovaginal mucosal melanoma treated with biochemotherapy in the first line, with roughly a third of the patients achieving a partial response, and median follow-up at 10 months demonstrating median overall survival of 10 months [22, 48]. Finally, a third study by Bartell et al. evaluated variations of the above regimen in fifteen patients with advanced head and neck mucosal melanoma, demonstrating four patients (27 %) with complete responses and three patients (20 %) with partial responses. The median time to progression was 10 months, though again a longer time to progression (50 months) was observed among responding patients; median overall survival was 22 months [3, 48].
These studies discussed suggest response rates in advanced mucosal melanoma patients that parallel rates in cutaneous melanoma patients treated with comparable regimens [48]. Unfortunately, a phase three trial of patients with cutaneous melanoma treated with identical biochemotherapy regimens failed to confirm a survival benefit, and significant toxicity is sustained; the authors of these studies concluding that this approach could be considered in judiciously selected patients [3, 22, 48]. While this introduces doubt into the strength of evidence in favor of biochemotherapy, it also hints at the possibility that, like other alternative therapies, there is a subset of melanoma patients that may derive benefit from these regimens [3, 48].
5.2.1.1 Targeted Therapies
A better comprehension of the pathogenesis of mucosal melanoma, in particular its molecular aberrations, has provided important insights into targets for future therapies [9, 29, 54, 62]. As mentioned previously, a large portion of mucosal melanomas, regardless of subset, have mutations and/or amplifications in the KIT oncogene which encodes for a receptor tyrosine kinase protein involved in multiple processes of cell division and survival [3, 7–9, 19, 24, 26, 29, 31, 38, 39, 41, 48–50, 54, 61, 62]. Imatinib is an inhibitor of multiple tyrosine kinases, including KIT [8, 9, 19, 24, 26]. The initial clinical trials of imatinib showed the agent to be ineffective in a general population of patients with advanced cutaneous melanoma [8, 19, 24, 26, 61, 62]. While the early studies of the drug were coming to a close, Curtin et al. published their identification of KIT gene aberrations (discussed above), prompting researchers to begin examining tumors for KIT abnormalities and treat identified cases with tyrosine kinase inhibitors with activity against KIT [8, 12, 26, 62]. Since that time there has been anecdotal evidence that imatinib results in rapid and durable tumor responses to KIT inhibitors specifically in patients with KIT mutations [29, 31, 41, 48, 54, 61]. Lutzky et al. were among the first investigators to report a complete response to imatinib therapy in the case of a 69 year old woman with advanced loco-regional mucosal melanoma of the anus harboring both a mutation and an amplification of KIT [39, 62]. Hodi et al. then reported a patient with primary anal melanoma with a mutation of KIT, most specifically a seven-codon duplication in exon 11. This patient demonstrated a near-complete response as measured by PET/CT as well as a greater than 50 % reduction in tumor volume after only 2 weeks of therapy with imatinib [19, 24, 62].
After these observational reports, several clinical trials of imatinib in patients with melanoma specifically bearing KIT alterations have subsequently taken place [8, 19, 26, 29, 41, 48, 54, 62]. Carvajal et al. conducted a single-arm, open-label, phase two clinical trial and reported the effects of imatinib on 25 evaluable patients with melanoma harboring KIT mutations. Their study showed 2 complete responses and 4 partial responses, two of which were transient, and five had stable disease for 12 weeks or more. The overall durable response rate was 16 %, with the four patients with durable responses maintaining disease stability for more than a year. The median overall survival was 46.3 weeks. All six patients with responses were noted to have mutations in L576P on exon 11 or K642E on exon 13; both with complete responses had both an L576P exon 11 mutation and a concomitant KIT amplification [8, 26, 48, 62].
In a second single-arm, open-label phase II clinical trial of imatinib in patients with KIT mutations or amplifications, Guo et al. studied 43 patients, with partial responses observed in 10 (23 %) while 13 patients (30 %) had stable disease. A majority of the patients who responded had KIT mutations in exons 11 and 13, though one patient with a KIT amplification alone responded. The median progression free survival time was 3.5 months, and the median overall survival was 14 months [19, 26].
Finally, Hodi et al. conducted a multicenter, phase II clinical trial of imatinib in 24 patients with advanced acral, mucosal or chronically sun-damaged melanoma with KIT mutations and/or amplifications, including 17 patients with mucosal melanoma. There was an overall disease control rate of 50 %, again favoring patients with KIT aberrations. Partial responses were seen in 7 of the 13 patients with KIT mutations (54 %), but no responses in patients with KIT amplifications or without KIT deviations [26].
Patients with KIT anomalies have also been reported to positively respond to other KIT inhibitors such as sorafenib, dasatinib and sunitinib [31, 50, 54, 61, 62]. Quintas-Cardama et al. described a case of a 79 year old male with KIT positive metastatic anal mucosal melanoma who was treated with temozolomide and sorafenib and achieved a complete response for 5 months before eventually expiring from progressive disease [50, 61, 62]. Woodman, et al. described two cases of metastatic mucosal melanoma in patients with L576P KIT mutations treated with dasatinib. Both of these patients had a significant reduction in tumor burden (>50 %) and elimination of tumor by PET imaging, one of which who had previously failed therapy with imatinib. This also suggests melanoma with L576P KIT mutations that are resistant to imatinib may be sensitive to additional KIT inhibitors. Unfortunately, both patients developed tumor re-growth by PET imaging after 4 months of treatment [61, 62]. Finally, Zhu et al. detailed a patient with KIT mutated metastatic nasal melanoma who received sunitinib and had a partial response with tumors shrinking by 70 % which was maintained 5 months after the initiation of therapy at the time of publication [64].
Taken collectively, these reports demonstrate that, while genetically selected tumors with KIT anomalies have better response rates to KIT inhibitors than the general population, these responses are variable. However, there is a pattern that suggests tumors with specific KIT alterations may be more likely to respond to these agents than others. Multiple reports suggested aberrations in exon 11 (most commonly L576P) and exon 13 (most commonly K642E) have better and longer sustained responses over tumors with KIT amplifications or KIT alterations in other regions [8, 9, 19, 26, 48, 50, 61, 62, 64]. These findings suggest perhaps only a few KIT variations are truly oncogenic and are appropriate targets for therapy [8, 26]. Furthermore, they suggest not all KIT alterations equivocally forecast benefit from KIT inhibition, but that further molecular discrimination may be required to better identify patients for which these agents are appropriate [8, 9, 19, 26, 48, 62].
Unfortunately, most patients who demonstrate an initial response to KIT inhibiting agents will only achieve brief periods of disease response and resistance ultimately leads to progressive disease. This is in contrast to other malignancies where KIT inhibition is commonly used, such as chronic myelogenous leukemia (CML) and gastrointestinal stromal tumors (GIST), where durable responses commonly occur [48]. While the mechanism of resistance in GIST tumors is understood to involve the acquisition of additional, unique KIT mutations, the mechanisms behind resistance to KIT inhibition in mucosal melanoma are unclear [19, 26, 48]. Some have suggested it may be due to pre-existing concomitant mutations in a variety of other oncogenes, while others have implicated acquired resistance mechanisms, particularly in the case of KIT amplifications. There is limited data available for both hypotheses [26], with more investigation in this area warranted. Indeed, multiple clinical trials are ongoing to further define the activity of KIT inhibition in populations of KIT mutant tumors, and to examine the use of additional KIT inhibiting agents (Table 3).
Finally, some mention of the role of BRAF inhibition in the treatment of mucosal melanomas is warranted given the albeit small presence of BRAF V600E mutations in about 10 % of mucosal melanomas [3, 7–9, 19, 29, 38, 41, 46, 48–50, 54, 56, 61]. As covered more thoroughly in Chap. 10, vemurafenib and dabrafenib are inhibitors of BRAF that specifically harbor an activating mutation wherein valine is substituted for glutamic acid at position 600. These agents have been approved for the treatment of metastatic melanoma based on trials that have shown superior response rates, progression free and overall survival in melanoma compared with chemotherapy [1, 11]. As almost all tumors become resistant to single agent BRAF inhibition, combinatorial therapy with MEK inhibition was studied in two randomized phase III trials, both of which have shown superior progression free survival [34, 37] and most recently overall survival with trametnib/dabrafenib [52]. The question remains whether patients with mucosal melanoma with BRAF mutations will see similar response rates to BRAF or BRAF/MEK inhibition as has been seen in patients with cutaneous melanoma. This has yet to investigated [9], although BRAF and MEK inhibitor therapy in mucosal melanomas with BRAF V600 mutations is a reasonable scientific approach for patients not able to participate in protocols.
5.2.2 Immunotherapies
In 2011, the FDA issued approval for ipilimumab, which has been shown to improve overall survival in advanced cutaneous melanoma [8, 9, 25, 48, 49, 51]. The approval of immune checkpoint inhibitor therapies for the treatment of advanced melanoma, which have the ability to produce long term durable remissions, has revolutionized the treatment of this disease and are detailed further in Chap. 10. Ipilimumab is a fully human, IgG antibody that targets cytotoxic T lymphocyte-associated antigen 4 (CTLA-4), an inhibitor receptor on T cells. By blocking this receptor, ipilimumab enhances T cell activation and proliferation, thus enhancing anti-tumor immunity [9, 11, 25, 48, 49, 51]. Ipilimumab is the first therapy to demonstrate a survival advantage in a randomized phase III trial [9, 25]. Mucosal melanoma patients were not specifically excluded from this study, but there were few in the study population [25, 48].
To date there have been no randomized trials of ipilimumab in mucosal melanoma patients, but anecdotal cases of benefit with use of this agent have been reported [9, 48, 49]. A multicenter, retrospective analysis of 33 patients with either unresectable or advanced mucosal melanoma treated with ipilimumab described one complete response, one partial response, six cases of stable disease and twenty two with progressive disease after 12 weeks of therapy. The overall durable response rate was 6.7 %, consistent with the rates of 4.2–10.9 % reported in patients with cutaneous melanoma who underwent ipilimumab monotherapy. The median overall survival was 6.4 months with a range of 1.8–26.7 months. Although these response rates were comparatively low, this study demonstrated ipilimumab could result in antitumor effects in patients specifically with mucosal melanoma [49]. An additional analysis of 71 patients with mucosal melanoma treated as part of an expanded access program in Italy showed a 12 % response rate and immune related disease control rate of 36 %; progression free survival and overall survival were 4.3 and 6.4 months [13].
The landscape of treatment for cutaneous melanoma has been further transformed by the development of inhibitors of programmed death 1 (PD-1) receptor and programmed death receptor ligand (PDL 1). Anti PD-1 inhibitors have yielded impressive durable responses in phase I trials [20, 58], as well as improved progression free survival in melanoma compared with chemotherapy [15], and improved overall survival was observed in patients wild type for BRAF mutation compared with chemotherapy [53]. Response rates are yet higher in patients treated with anti-CTLA-4 and anti-PD-1 combinations accompanied by higher toxicity [60]. To date, one case report documents a durable response of a mucosal melanoma patient treated with the anti-PD 1 antibody pembrolizumab following treatment with ipilimumab; this patient also experienced hypothyroidism and rhabdomyolysis as a consequence of therapy [42]. Clinical trials to assess the efficacy of checkpoint inhibition in the subset of patients with mucosal melanoma will further evaluate potential therapeutic benefits of anti-PD-1 inhibitors, alone or in combination with other checkpoint inhibitors or immunomodulatory agents.
Given the ever-evolving intricacies of tumor pathogenesis as well as the complex mechanics of our own immune response, combination therapy is the next logical step in cancer research in order to broaden clinical responses and prevent the development of resistance to single agents, which has been shown to develop rapidly [40]. In addition, novel agents that have been shown to be efficacious in one setting should continue to be investigated in additional settings that make scientific sense. The aforementioned therapies are only the beginning in a promising future for mucosal melanoma patients.
References
Ascierto PA, Minor D, Ribas A, Lebbe C, O’Hagan A, Arya N, Guckert M, Schadendorf D, Kefford RF, Grob JJ, Hamid O, Amaravadi R, Simeone E, Wilhelm T, Kim KB, Long GV, Martin AM, Mazumdar J, Goodman VL, Trefzer U (2013) Phase II trial (BREAK-2) of the BRAF inhibitor dabrafenib (GSK2118436) in patients with metastatic melanoma. J Clin Oncol 31(26):3205–3211. doi:10.1200/JCO.2013.49.8691
Ballantyne AJ (1970) Malignant melanoma of the skin of the head and neck. An analysis of 405 cases. Am J Surg 120(4):425–431
Bartell HL, Bedikian AY, Papadopoulos NE, Dett TK, Ballo MT, Myers JN, Hwu P, Kim KB (2008) Biochemotherapy in patients with advanced head and neck mucosal melanoma. Head Neck 30(12):1592–1598. doi:10.1002/hed.20910
Beadling C, Jacobson-Dunlop E, Hodi FS, Le C, Warrick A, Patterson J, Town A, Harlow A, Cruz F 3rd, Azar S, Rubin BP, Muller S, West R, Heinrich MC, Corless CL (2008) KIT gene mutations and copy number in melanoma subtypes. Clin Cancer Res 14(21):6821–6828. doi:10.1158/1078-0432.CCR-08-0575
Borazjani G, Prem KA, Okagaki T, Twiggs LB, Adcock LL (1990) Primary malignant melanoma of the vagina: a clinicopathological analysis of 10 cases. Gynecol Oncol 37(2):264–267
Burmeister BH, Henderson MA, Ainslie J, Fisher R, Di Iulio J, Smithers BM, Hong A, Shannon K, Scolyer RA, Carruthers S, Coventry BJ, Babington S, Duprat J, Hoekstra HJ, Thompson JF (2012) Adjuvant radiotherapy versus observation alone for patients at risk of lymph-node field relapse after therapeutic lymphadenectomy for melanoma: a randomised trial. Lancet Oncol 13(6):589–597. doi:10.1016/S1470-2045(12)70138-9
Carcoforo P, Raiji MT, Palini GM, Pedriali M, Maestroni U, Soliani G, Detroia A, Zanzi MV, Manna AL, Crompton JG, Langan RC, Stojadinovic A, Avital I (2012) Primary anorectal melanoma: an update. J Cancer 3:449–453. doi:10.7150/jca.5187
Carvajal RD, Antonescu CR, Wolchok JD, Chapman PB, Roman RA, Teitcher J, Panageas KS, Busam KJ, Chmielowski B, Lutzky J, Pavlick AC, Fusco A, Cane L, Takebe N, Vemula S, Bouvier N, Bastian BC, Schwartz GK (2011) KIT as a therapeutic target in metastatic melanoma. JAMA 305(22):2327–2334. doi:10.1001/jama.2011.746
Carvajal RD, Spencer SA, Lydiatt W (2012) Mucosal melanoma: a clinically and biologically unique disease entity. J Natl Compr Cancer Netw JNCCN 10(3):345–356
Chang AE, Karnell LH, Menck HR (1998) The National Cancer Data Base report on cutaneous and noncutaneous melanoma: a summary of 84,836 cases from the past decade. The American College of Surgeons Commission on Cancer and the American Cancer Society. Cancer 83(8):1664–1678
Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, Dummer R, Garbe C, Testori A, Maio M, Hogg D, Lorigan P, Lebbe C, Jouary T, Schadendorf D, Ribas A, O’Day SJ, Sosman JA, Kirkwood JM, Eggermont AM, Dreno B, Nolop K, Li J, Nelson B, Hou J, Lee RJ, Flaherty KT, McArthur GA, Group B-S (2011) Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med 364(26):2507–2516. doi:10.1056/NEJMoa1103782
Curtin JA, Busam K, Pinkel D, Bastian BC (2006) Somatic activation of KIT in distinct subtypes of melanoma. J Clin Oncol 24(26):4340–4346. doi:10.1200/JCO.2006.06.2984
Del Vecchio M, Di Guardo L, Ascierto PA, Grimaldi AM, Sileni VC, Pigozzo J, Ferraresi V, Nuzzo C, Rinaldi G, Testori A, Ferrucci PF, Marchetti P, De Galitiis F, Queirolo P, Tornari E, Marconcini R, Calabro L, Maio M (2014) Efficacy and safety of ipilimumab 3 mg/kg in patients with pretreated, metastatic, mucosal melanoma. Eur J Cancer 50(1):121–127. doi:10.1016/j.ejca.2013.09.007
DeMatos P, Tyler DS, Seigler HF (1998) Malignant melanoma of the mucous membranes: a review of 119 cases. Ann Surg Oncol 5(8):733–742
Dummer R, Daud A, Puzanov I, Hamid O, Schadendorf D, Robert C, Schachter J, Pavlick A, Gonzalez R, Hodi F, Cranmer L, Blank C, O’Day S, Ascierto P, Salama A, Li NX, Zhou W, Lis J, Ebbinghaus S, Kang P, Ribas A (2015) A randomized controlled comparison of pembrolizumab and chemotherapy in patients with ipilimumab-refractory melanoma. Journal of Translational Medicine 13(Suppl 1):O5
Edge SB, Compton CC (2010) The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 17(6):1471–1474. doi:10.1245/s10434-010-0985-4
Frumovitz M, Etchepareborda M, Sun CC, Soliman PT, Eifel PJ, Levenback CF, Ramirez PT (2010) Primary malignant melanoma of the vagina. Obstet Gynecol 116(6):1358–1365. doi:10.1097/AOG.0b013e3181fb8045
Gilligan D, Slevin NJ (1991) Radical radiotherapy for 28 cases of mucosal melanoma in the nasal cavity and sinuses. Br J Radiol 64(768):1147–1150
Guo J, Si L, Kong Y, Flaherty KT, Xu X, Zhu Y, Corless CL, Li L, Li H, Sheng X, Cui C, Chi Z, Li S, Han M, Mao L, Lin X, Du N, Zhang X, Li J, Wang B, Qin S (2011) Phase II, open-label, single-arm trial of imatinib mesylate in patients with metastatic melanoma harboring c-Kit mutation or amplification. J Clin Oncol 29(21):2904–2909. doi:10.1200/JCO.2010.33.9275
Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, Wolchok JD, Hersey P, Joseph RW, Weber JS, Dronca R, Gangadhar TC, Patnaik A, Zarour H, Joshua AM, Gergich K, Elassaiss-Schaap J, Algazi A, Mateus C, Boasberg P, Tumeh PC, Chmielowski B, Ebbinghaus SW, Li XN, Kang SP, Ribas A (2013) Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med 369(2):134–144. doi:10.1056/NEJMoa1305133
Hardie C, Siddiqui N (2011) Primary Malignant Melanoma of the Vulva and Vagina. In: Reed N, Green JA, Gershenson DM, Siddiqui N, Connor R (eds) Rare and uncommon gynecologic cancers. Springer, New York
Harting MS, Kim KB (2004) Biochemotherapy in patients with advanced vulvovaginal mucosal melanoma. Melanoma Res 14(6):517–520
Harwood AR, Cummings BJ (1982) Radiotherapy for mucosal melanomas. Int J Radiat Oncol Biol Phys 8(7):1121–1126
Hodi FS, Friedlander P, Corless CL, Heinrich MC, Mac Rae S, Kruse A, Jagannathan J, Van den Abbeele AD, Velazquez EF, Demetri GD, Fisher DE (2008) Major response to imatinib mesylate in KIT-mutated melanoma. J Clin Oncol 26(12):2046–2051. doi:10.1200/JCO.2007.14.0707
Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJ, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbe C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ (2010) Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 363(8):711–723. doi:10.1056/NEJMoa1003466
Hodi FS, Corless CL, Giobbie-Hurder A, Fletcher JA, Zhu M, Marino-Enriquez A, Friedlander P, Gonzalez R, Weber JS, Gajewski TF, O’Day SJ, Kim KB, Lawrence D, Flaherty KT, Luke JJ, Collichio FA, Ernstoff MS, Heinrich MC, Beadling C, Zukotynski KA, Yap JT, Van den Abbeele AD, Demetri GD, Fisher DE (2013) Imatinib for melanomas harboring mutationally activated or amplified KIT arising on mucosal, acral, and chronically sun-damaged skin. J Clin Oncol 31(26):3182–3190. doi:10.1200/JCO.2012.47.7836
Hurria A, Dale W, Mooney M, Rowland JH, Ballman KV, Cohen HJ, Muss HB, Schilsky RL, Ferrell B, Extermann M, Schmader KE, Mohile SG, Cancer, Aging Research G (2014) Designing therapeutic clinical trials for older and frail adults with cancer: U13 conference recommendations. Journal Clin Oncol 32 (24):2587–2594. doi:10.1200/JCO.2013.55.0418
Hussein MR (2008) Extracutaneous malignant melanomas. Cancer Invest 26(5):516–534. doi:10.1080/07357900701781762
Keller DS, Thomay AA, Gaughan J, Olszanski A, Wu H, Berger AC, Farma JM (2013) Outcomes in patients with mucosal melanomas. J Surg Oncol 108(8):516–520. doi:10.1002/jso.23445
Kelly P, Zagars GK, Cormier JN, Ross MI, Guadagnolo BA (2011) Sphincter-sparing local excision and hypofractionated radiation therapy for anorectal melanoma: a 20-year experience. Cancer 117(20):4747–4755. doi:10.1002/cncr.26088
Kim HS, Kim EK, Jun HJ, Oh SY, Park KW, Lim do H, Lee SI, Kim JH, Kim KM, Lee DH, Lee J (2010) Noncutaneous malignant melanoma: a prognostic model from a retrospective multicenter study. BMC Cancer 10:167. doi:10.1186/1471-2407-10-167
Kim KB, Sanguino AM, Hodges C, Papadopoulos NE, Eton O, Camacho LH, Broemeling LD, Johnson MM, Ballo MT, Ross MI, Gershenwald JE, Lee JE, Mansfield PF, Prieto VG, Bedikian AY (2004) Biochemotherapy in patients with metastatic anorectal mucosal melanoma. Cancer 100(7):1478–1483. doi:10.1002/cncr.20113
Kirschner AN, Kidd EA, Dewees T, Perkins SM (2013) Treatment approach and outcomes of vaginal melanoma. International J Gynecol Cancer 23(8):1484–1489. doi:10.1097/IGC.0b013e3182a1ced8
Larkin J, Ascierto PA, Dreno B, Atkinson V, Liszkay G, Maio M, Mandala M, Demidov L, Stroyakovskiy D, Thomas L, de la Cruz-Merino L, Dutriaux C, Garbe C, Sovak MA, Chang I, Choong N, Hack SP, McArthur GA, Ribas A (2014) Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N Engl J Med 371(20):1867–1876. doi:10.1056/NEJMoa1408868
Leitao MM Jr (2014) Management of vulvar and vaginal melanomas: current and future strategies. American Society of Clinical Oncology educational book/ASCO American Society of Clinical Oncology Meeting e277–281. doi:10.14694/EdBook_AM.2014.34.e277
Lian B, Si L, Cui C, Chi Z, Sheng X, Mao L, Li S, Kong Y, Tang B, Guo J (2013) Phase II randomized trial comparing high-dose IFN-alpha2b with temozolomide plus cisplatin as systemic adjuvant therapy for resected mucosal melanoma. Clin Cancer Res 19(16):4488–4498. doi:10.1158/1078-0432.CCR-13-0739
Long GV, Stroyakovskiy D, Gogas H, Levchenko E, de Braud F, Larkin J, Garbe C, Jouary T, Hauschild A, Grob JJ, Chiarion Sileni V, Lebbe C, Mandala M, Millward M, Arance A, Bondarenko I, Haanen JB, Hansson J, Utikal J, Ferraresi V, Kovalenko N, Mohr P, Probachai V, Schadendorf D, Nathan P, Robert C, Ribas A, DeMarini DJ, Irani JG, Casey M, Ouellet D, Martin AM, Le N, Patel K, Flaherty K (2014) Combined BRAF and MEK inhibition versus BRAF inhibition alone in melanoma. N Engl J Med 371(20):1877–1888. doi:10.1056/NEJMoa1406037
Lourenco SV, Fernandes JD, Hsieh R, Coutinho-Camillo CM, Bologna S, Sangueza M, Nico MM (2014) Head and neck mucosal melanoma: a review. Am J Dermatopathol 36(7):578–587. doi:10.1097/DAD.0000000000000035
Lutzky J, Bauer J, Bastian BC (2008) Dose-dependent, complete response to imatinib of a metastatic mucosal melanoma with a K642E KIT mutation. Pigm Cell Melanoma Res 21(4):492–493. doi:10.1111/j.1755-148X.2008.00475.x
Mellman I, Coukos G, Dranoff G (2011) Cancer immunotherapy comes of age. Nature 480(7378):480–489. doi:10.1038/nature10673
Mihajlovic M, Vlajkovic S, Jovanovic P, Stefanovic V (2012) Primary mucosal melanomas: a comprehensive review. Int J Clin Exp Pathol 5(8):739–753
Min L, Hodi FS (2014) Anti-PD1 following ipilimumab for mucosal melanoma: durable tumor response associated with severe hypothyroidism and rhabdomyolysis. Cancer Immunol Res 2(1):15–18. doi:10.1158/2326-6066.CIR-13-0146
Moreno MA, Roberts DB, Kupferman ME, DeMonte F, El-Naggar AK, Williams M, Rosenthal DS, Hanna EY (2010) Mucosal melanoma of the nose and paranasal sinuses, a contemporary experience from the M.D. Anderson Cancer Center. Cancer 116(9):2215–2223. doi:10.1002/cncr.24976
Nieder C (2012) Ipilimumab in patients with melanoma and brain metastases. Lancet Oncol 13(7):e277; author reply e277–278. doi:10.1016/S1470-2045(12)70303-0
Pandey M, Mathew A, Abraham EK, Ahamed IM, Nair KM (1998) Primary malignant melanoma of the mucous membranes. European J Surg Oncol 24(4):303–307
Patrick RJ, Fenske NA, Messina JL (2007) Primary mucosal melanoma. J Am Acad Dermatol 56(5):828–834. doi:10.1016/j.jaad.2006.06.017
Pfister DG, Ang KK, Brizel DM, Burtness B, Cmelak AJ, Colevas AD, Dunphy F, Eisele DW, Gilbert J, Gillison ML, Haddad RI, Haughey BH, Hicks WL Jr, Hitchcock YJ, Kies MS, Lydiatt WM, Maghami E, Martins R, McCaffrey T, Mittal BB, Pinto HA, Ridge JA, Samant S, Sanguineti G, Schuller DE, Shah JP, Spencer S, Trotti A 3rd, Weber RS, Wolf G, Worden F, National Comprehensive Cancer N (2012) Mucosal melanoma of the head and neck. J Natl Compr Cancer Network JNCCN 10(3):320–338
Postow MA, Hamid O, Carvajal RD (2012) Mucosal melanoma: pathogenesis, clinical behavior, and management. Curr Oncol Rep 14(5):441–448. doi:10.1007/s11912-012-0244-x
Postow MA, Luke JJ, Bluth MJ, Ramaiya N, Panageas KS, Lawrence DP, Ibrahim N, Flaherty KT, Sullivan RJ, Ott PA, Callahan MK, Harding JJ, D’Angelo SP, Dickson MA, Schwartz GK, Chapman PB, Gnjatic S, Wolchok JD, Hodi FS, Carvajal RD (2013) Ipilimumab for patients with advanced mucosal melanoma. Oncologist 18(6):726–732. doi:10.1634/theoncologist.2012-0464
Quintas-Cardama A, Lazar AJ, Woodman SE, Kim K, Ross M, Hwu P (2008) Complete response of stage IV anal mucosal melanoma expressing KIT Val560Asp to the multikinase inhibitor sorafenib. Nat Clin Pract Oncol 5(12):737–740. doi:10.1038/ncponc1251
Robert C, Thomas L, Bondarenko I, O’Day S, Weber J, Garbe C, Lebbe C, Baurain JF, Testori A, Grob JJ, Davidson N, Richards J, Maio M, Hauschild A, Miller WH Jr, Gascon P, Lotem M, Harmankaya K, Ibrahim R, Francis S, Chen TT, Humphrey R, Hoos A, Wolchok JD (2011) Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med 364(26):2517–2526. doi:10.1056/NEJMoa1104621
Robert C, Karaszewska B, Schachter J, Rutkowski P, Mackiewicz A, Stroiakovski D, Lichinitser M, Dummer R, Grange F, Mortier L, Chiarion-Sileni V, Drucis K, Krajsova I, Hauschild A, Lorigan P, Wolter P, Long GV, Flaherty K, Nathan P, Ribas A, Martin AM, Sun P, Crist W, Legos J, Rubin SD, Little SM, Schadendorf D (2015) Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med 372(1):30–39. doi:10.1056/NEJMoa1412690
Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, Hassel JC, Rutkowski P, McNeil C, Kalinka-Warzocha E, Savage KJ, Hernberg MM, Lebbe C, Charles J, Mihalcioiu C, Chiarion-Sileni V, Mauch C, Cognetti F, Arance A, Schmidt H, Schadendorf D, Gogas H, Lundgren-Eriksson L, Horak C, Sharkey B, Waxman IM, Atkinson V, Ascierto PA (2015) Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 372(4):320–330. doi:10.1056/NEJMoa1412082
Seetharamu N, Ott PA, Pavlick AC (2010) Mucosal melanomas: a case-based review of the literature. Oncologist 15(7):772–781. doi:10.1634/theoncologist.2010-0067
Smyth EC, Flavin M, Pulitzer MP, Gardner GJ, Costantino PD, Chi DS, Bogatch K, Chapman PB, Wolchok JD, Schwartz GK, Carvajal RD (2011) Treatment of locally recurrent mucosal melanoma with topical imiquimod. J Clin Oncol 29(33):e809–811. doi:10.1200/JCO.2011.36.8829
Tas F, Keskin S, Karadeniz A, Dagoglu N, Sen F, Kilic L, Yildiz I (2011) Noncutaneous melanoma have distinct features from each other and cutaneous melanoma. Oncology 81(5–6):353–358. doi:10.1159/000334863
Tomicic J, Wanebo HJ (2003) Mucosal melanomas. Surg Clin North Am 83(2):237–252. doi:10.1016/S0039-6109(02)00100-7
Topalian SL, Sznol M, McDermott DF, Kluger HM, Carvajal RD, Sharfman WH, Brahmer JR, Lawrence DP, Atkins MB, Powderly JD, Leming PD, Lipson EJ, Puzanov I, Smith DC, Taube JM, Wigginton JM, Kollia GD, Gupta A, Pardoll DM, Sosman JA, Hodi FS (2014) Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol 32(10):1020–1030. doi:10.1200/JCO.2013.53.0105
Verschraegen CF, Benjapibal M, Supakarapongkul W, Levy LB, Ross M, Atkinson EN, Bodurka-Bevers D, Kavanagh JJ, Kudelka AP, Legha SS (2001) Vulvar melanoma at the M. D. Anderson Cancer Center: 25 years later. Int J Gynecol Cancer 11(5):359–364
Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, Segal NH, Ariyan CE, Gordon RA, Reed K, Burke MM, Caldwell A, Kronenberg SA, Agunwamba BU, Zhang X, Lowy I, Inzunza HD, Feely W, Horak CE, Hong Q, Korman AJ, Wigginton JM, Gupta A, Sznol M (2013) Nivolumab plus ipilimumab in advanced melanoma. New Engl J Med 369(2):122–133. doi:10.1056/NEJMoa1302369
Woodman SE, Trent JC, Stemke-Hale K, Lazar AJ, Pricl S, Pavan GM, Fermeglia M, Gopal YN, Yang D, Podoloff DA, Ivan D, Kim KB, Papadopoulos N, Hwu P, Mills GB, Davies MA (2009) Activity of dasatinib against L576P KIT mutant melanoma: molecular, cellular, and clinical correlates. Mol Cancer Ther 8(8):2079–2085. doi:10.1158/1535-7163.MCT-09-0459
Woodman SE, Davies MA (2010) Targeting KIT in melanoma: a paradigm of molecular medicine and targeted therapeutics. Biochem Pharmacol 80(5):568–574. doi:10.1016/j.bcp.2010.04.032
Wu E, Golitz LE (2000) Primary noncutaneous melanoma. Clinics Lab Med 20(4):731–744
Zhu YSL, Kong Y, Chi Z, Yuan X, Cui C, Sheng X, Guo J, Shen L (2009) Response to sunitinib in Chinese KIT-mutated metastatic mucosal melanoma. J Clin Oncol 27(15s):e20017
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Spencer, K.R., Mehnert, J.M. (2016). Mucosal Melanoma: Epidemiology, Biology and Treatment. In: Kaufman, H., Mehnert, J. (eds) Melanoma. Cancer Treatment and Research, vol 167. Springer, Cham. https://doi.org/10.1007/978-3-319-22539-5_13
Download citation
DOI: https://doi.org/10.1007/978-3-319-22539-5_13
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-22538-8
Online ISBN: 978-3-319-22539-5
eBook Packages: MedicineMedicine (R0)