Abstract
Indications for liver transplantation after bile duct injury fall into two major categories: Chronic liver disease due to secondary biliary cirrhosis and acute liver failure due to an associated major vascular injury. The exact incidence of liver transplantation due to biliary injury is difficult to estimate because the etiology of liver failure for these patients is not always adequately captured in current transplant registries. There is a rare but important role and need for liver transplant in highly selected cases of bile duct injury. While the etiology of obstruction leading to secondary biliary cirrhosis is not consistently reported, most cases series describe the use of transplantation as a consequence of iatrogenic injury.
This chapter will review the existing literature, pathogenesis, and histology of liver disease associated with bile duct injury, evaluation of the potential transplant recipient, and technical factors in this patient group.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Secondary biliary cirrhosis
- Bile duct injury
- Liver transplantation
- Benign biliary stricture
- Hepatic failure
- Vasculobiliary injury
Liver Transplant for Bile Duct Injury
Indications for liver transplantation after bile duct injury fall into two major categories: Chronic liver disease due to secondary biliary cirrhosis and acute liver failure due to an associated major vascular injury. The exact incidence of liver transplantation due to biliary injury is difficult to estimate because the etiology of liver failure for these patients is not always adequately captured in current transplant registries. Furthermore, the current literature of iatrogenic injury resulting in liver transplantation is mostly limited to case reports and small case series (Table 34.1) [1–18]. There is a rare but important role and need for liver transplant in highly selected cases of bile duct injury. According to the U.S. United Network for Organ Sharing (UNOS) registry, between the years of 2000 and 2010, among 51,334 liver transplants in the United States, only 111 were performed for secondary biliary cirrhosis, and of these, less than one fourth specified an associated bile duct injury (UNOS data) [19]. Internationally, secondary biliary cirrhosis is the etiology of 1 % of transplants in the European Liver Transplantation Registry (ELTR) and approximately 2 % in the Argentinian population [1, 4, 20]. While the etiology of obstruction leading to secondary biliary cirrhosis is not consistently reported, most case series describe the use of transplantation as a consequence of iatrogenic injury [1, 3–5, 7–16, 18, 20–22].
The reasons for transplantation in the setting of bile duct injury can be grouped into the following categories:
-
Secondary biliary cirrhosis
-
Biliary stricture and portal hypertension
-
Hepatic failure and complex injury
-
Uncontrolled/recurrent sepsis of biliary tree
-
Bile duct injury in patient with underlying liver disease
-
Pruritus
-
Poor quality of life
This chapter will review the existing literature, pathogenesis, and histology of liver disease associated with bile duct injury, evaluation of the potential transplant recipient, and technical factors in this patient group.
Review of the Existing Literature
There are less than one hundred reported cases of liver transplantation secondary to bile duct injury in the existing literature (Table 34.1). The majority of reported cases are due to biliary injury at the time of cholecystectomy; however, there are several cases of injury for hydatid liver disease and for nonbiliary surgery [1, 3–5, 7–16, 18, 20–22]. While the majority of reported cases are referrals for transplant due to secondary biliary cirrhosis due to biliary injury, there are also reports of acute liver failure, usually secondary to an associated major vascular injury [3, 5, 9, 12, 15, 18]. Patients with secondary biliary cirrhosis were transplanted for the following reasons: cirrhosis, recurrent cholangitis, sequelae of portal hypertension, intractable ascites, pruritus, and/or poor quality of life [1, 3–5, 7–16, 18, 20–22].
This existing literature likely fails to completely capture the small number of patients seen at transplant centers with secondary biliary cirrhosis due to biliary injury. At our center, over the past 20 years, we have performed four liver transplants for iatrogenic injury to the bile duct (unpublished data). As noted previously, based on the UNOS data as currently reported, the number of transplants performed in the United States for this diagnosis is small.
In the largest existing series, Parilla et al. describe 27 patients over a 13-year period, all of whom sustained biliary injury after cholecystectomy (13 open and 14 laparoscopic) and subsequently underwent liver transplantation for either acute liver failure (14 patients) or secondary biliary cirrhosis (13 patients) [12]. They found a higher rate of vascular injuries associated with the laparoscopic procedure, consistent with other authors [14, 15, 21, 23–25]. Overall, the 5-year survival was 68 %, with the majority of deaths occurring in the early postoperative period in patients who were transplanted for acute liver failure [12].
Few conclusions regarding outcomes can be drawn from these small series. Liver transplantation is a treatment option for patients with acute liver failure or biliary cirrhosis after bile duct injury; however, patients who develop acute liver failure have poor survival and often die of infection-related complications. Patients with secondary biliary cirrhosis have acceptable long-term outcomes, with a 3-year survival of greater than 70 % [4, 7]. The major challenge of OLT, in patients with secondary biliary cirrhosis, is the extensive right upper quadrant surgery that so many have undergone with previous bile duct repairs.
Pathogenesis
Bile duct injuries leading to transplant have been described in open and laparoscopic cholecystectomy as well as nonbiliary surgery [1, 3–5, 7–16, 18, 20–22, 26]. The most common procedure associated with common bile duct injury is cholecystectomy, both open and laparoscopic [1, 3–5, 7–16, 18, 20–22, 26]. The incidence of bile duct injury during laparoscopic cholecystectomy in larger series is approximately 0.3–1 % compared to 0.1–0.2 % for historically reported rates in open cholecystectomy series [23, 27–31].
Case reports of patients requiring urgent liver transplant for acute liver failure are usually in the setting of a major vascular injury to either the common or proper hepatic artery and/or to the portal vein [3, 5, 9, 12, 15, 18, 21]. Fernandez et al. describe two cases, one in which portal vein injury during laparoscopic converted to open cholecystectomy resulted in portal and hepatic arterial injury and acute liver failure, and a second case in which hepatic arterial injury resulted in sufficient necrosis to cause acute liver failure. One of the patients received an urgent transplant, while the second died while on the waiting list [5]. Zaydfudim et al. reported on two cases of major vascular injury requiring emergent liver transplant. In this report, one such vascular injury occurred in a patient undergoing right adrenalectomy. The common bile duct, portal vein, and common hepatic artery were transected resulting in acute liver failure and referral for urgent transplant. Remarkably, the patient was alive at 6 years after transplant [18].
Injuries to the bile duct sustained during laparoscopic cholecystectomy are more often proximal injuries, rather than their open counterparts, and more likely to have an associated vascular injury [21, 23, 25]. Vascular injuries have been reported in up to 12–57 % of patients with bile duct injuries [14, 32, 33]. These patterns of injury are well described [15, 25] and frequently involve injury to the right branch of the proper hepatic artery or an aberrantly located replaced or accessory right hepatic artery. The contribution of vascular injury to formation of stricture might be overestimated. Alves et al., in a retrospective review of 55 patients with bile duct injury who were studied angiographically at the time of their repair, found associated vascular injury in 47 % of patients [32]. Forty three of the patients underwent Hepp-Couinaud repair (side to side anastomosis of the jejunal limb to the main left hepatic duct) and were followed to evaluate the influence of vascular injury on their outcome. With a mean follow-up of 56 and 61 months (without and with arterial injury respectively), there was no difference in the long-term stricture rate [32]. This can be explained by the blood supply to the left and right ductal systems, which consists in part by the hilar plate arterial plexus that connects the right and left hepatic arterial systems. This allows the confluence of the ducts and higher to maintain vascular supply in the setting of a contralateral arterial injury [34]. It additionally informs why a high bilioenteric repair, with dissection based anterior to the duct, is necessary to avoid ischemia in the repair when the blood supply from the ascending marginal vessels based of the pancreaticoduodenal and gastroduodenal arteries has been disrupted and the bile duct blood supply comes exclusively from the hepatic artery [35].
A high percentage of patients will go on to have biliary stricture, with reported rates as high as 50 % in some series [17, 27, 28, 33, 36–38]. The factors affecting the development of stricture and outcomes after repair including level of injury, timing and type of repair, surgeon experience, and presence of biliary peritonitis continue to be debated [6, 17, 21, 22, 27, 28, 33, 35–40]. The ability to accurately study these factors is limited by the variation in initial treatment and delay in presentation due to failed recognition of the injury or initial management at a low volume center. After repair, patients should be followed with imaging and lab studies for evaluation of liver function for years, as strictures may be a late development. In a series reported by Pitt et al., at 5 years, only 80 % of post repair strictures had been identified, some occurring as late at 19 years after repair [33, 37].
Early referral to a hepatobiliary specialist center is recommended, as a multimodality approach (with gastroenterologists and interventional and diagnostic radiologists) can be beneficial in establishing appropriate diagnosis, ensuring utilization of endoscopic treatment techniques, and involvement of a hepatobiliary surgery specialist. Surgeon experience has been found to correlate with increased patient survival [29].
Any sign of stricture needs to be timely and aggressively managed in order to avoid sequelae of portal hypertension and fibrosis from obstruction. If a stricture develops, a multimodality approach should be employed for management, with good cholangiographic success and symptom relief being reported using endoscopic balloon dilation and stenting [22, 41, 42]. Refractory strictures may require surgical revision.
Pathologic Consequences of Stricture: The Evolution of Portal Hypertension and Secondary Biliary Cirrhosis
Prolonged biliary obstruction can result in two major structural changes that require transplant: (1) portal hypertension and (2) progressive hepatic fibrosis with progression to secondary biliary cirrhosis.
The damage to the biliary system is the result of the chronic insult from high local concentration of hepatotoxic bile acids at the canalicular membrane leading to a process of ductular proliferation and portal inflammation along with fibrogenesis and matrix deposition, known as ductular reaction [3, 43, 44]. If not arrested, this process results in scarring. As this process progresses, mechanical interference with bile flow develops in the intrahepatic biliary radicles and perpetuates bile and bile salt accumulation in the parenchyma, i.e., cholestasis [45].
In patients with chronic cholestatic disease, histologic and vascular remodeling meeting the requirements for cirrhosis occur in the minority of patients, and the injury patterns are typically inhomogeneous; however, a higher proportion exhibit fibrosis and/or clinical sequelae of portal hypertension in the absence of cirrhosis [3, 43–47].
Portal hypertension, in the setting chronic large bile duct obstruction, is not completely understood. While intuitively portal hypertension would be a result of cirrhosis because of the deterioration of the normal vascular architecture and replacement of parenchyma by fibrous septa that contain only small shunt vessels, histologic studies confirmed that, in patients with primary sclerosing cholangitis and in secondary biliary cirrhosis, clinically evident portal hypertension exists in the absence of cirrhosis [3, 43–47]. In a histologic study of 28 patients with chronic biliary obstruction and portal hypertension and 76 patients with chronic biliary obstruction alone, Weinbren and colleagues found that most of these patients lacked the distorted vascular relations necessary to be considered cirrhotic. The clinical features were attributed to the combination of diffusely thickened hepatocyte plate and increased fibrous tissue in which the normal relation was maintained between the portal tracts and hepatic venous radicles [44]. Similarly, Abraham et al., in a review of 306 explants for cholestatic liver disease, found that the majority of patients with cholestatic liver disease have findings of cirrhosis on explant at the time of transplant, with only 26 of 306 (8.5 %) being precirrhotic [41].
In patients with chronic obstruction, there is no conclusive data regarding the timing of progression from fibrosis to cirrhosis or factors contributing to progression. It is well accepted that the longer the duration of the obstruction, the more likely it is that fibrosis will occur [22, 43, 47, 48]. Negi et al. reported a prospective series of 64 patients with postcholecystectomy bile duct strictures. Biopsies of the liver collected at the time of bile duct repair were reviewed and 35 (54 %) of the patients included had advanced fibrosis at the time of surgery, with a mean duration of biliary obstruction of 16.6 ± 3.4 months. Factors significantly associated with the presence of advanced fibrosis were duration of biliary obstruction, basal ALT level, and time to normalization of ALT after surgical repair. The grade of fibrosis correlated with the demonstrated positive correlation with the grade of portal inflammation, ductular proliferation, and cholestasis. Fibrotic changes occurred as early as 1 month after biliary obstruction with a mean duration of biliary obstruction associated with development of portal or periportal fibrosis at 3.9 months, severe fibrosis and numerous septa at 22.5 months, and development of cirrhosis at 62 months. There was no significant difference in the incidence of cirrhosis in patients with clinical cholangitis or infected bile [47].
In 71 patients, Sikora et al. found that all patients biopsied at the time of bile duct injury repair had some degree of fibrosis (mean time from injury to repair, 270 weeks in patients with cirrhosis and 90 weeks in patients with fibrosis only). Fibrous changes on liver biopsy were identified in patients as early as 11 weeks after bile duct injury at time of cholecystectomy [43]. Johnson et al. similarly studied hepatic injury looking at biopsies in patients after bile duct injury. Six of 16 patients had evidence of moderate to marked fibrosis and four of these had evidence of evolving cirrhosis, with the mean time from injury to repair of 480 days [39].
There is evidence in animal and human models that relief of obstruction can lead to recovery of fibrosis and portal hypertension [43, 44, 48, 49]. Therefore, if evaluation of biopsy shows mild fibrosis without evidence of cirrhosis, multidisciplinary review is mandatory to ensure that all interventional and surgical options are exhausted prior to being considered for transplant.
The exact time to regression of fibrosis is unknown but has been reported as quickly as weeks in a rat model and as early as 1 year in humans following surgical relief of obstruction. Depending on the degree of liver injury at time of relief of obstruction, the liver may or may not recover post-obstruction. Patients should be followed clinically and with repeat biopsy, after obstruction has been alleviated, to assess for regression.
Transplant Evaluation Preoperative Management
All patients being considered for transplant must undergo a thorough medical and psychosocial evaluation. General considerations and contraindications to transplant have been described previously and should be followed [50]. Specific considerations in the two categories of patients undergoing transplantation in the setting of bile duct injury are listed below.
Chronic Liver Disease
The evaluation of the patient with chronic liver disease will focus on the following: establishing the diagnosis, evaluation of alternative treatments, evaluation for complications of liver disease, and determining the appropriate timing for liver transplant.
A thorough history and physical examination should be obtained with attention to any underlying liver disease. Hepatitis serologies should be obtained. Hepatitis treatment should be pursued at the discretion of the treating medical team and requirements for alcohol abstinence are determined by transplant center. Prior surgical records and imaging should be obtained for review.
Liver biopsy can be used to establish the diagnosis and assess any other contributing factors to liver disease. Needle biopsy provides adequate tissue for diagnosis and can be performed percutaneously with ultrasound guidance or using a transjugular method. Transjugular biopsy may be preferred for patients with thrombocytopenia or ascites.
The presence and degree of portal hypertension is determined largely by clinical signs, including hepatosplenomegaly, ascites, dilated abdominal wall veins or caput medusae, and/or varices. It can also be confirmed by measurement of the hepatic venous pressure gradient if there is uncertainty, but we reserve this procedure only in equivocal cases.
Initial evaluation and management should ensure that the biliary injury has been appropriately treated, i.e., bilomas or abscesses have been drained, biliary drainage is adequate, and cholangiographic evaluation performed to characterize the injury and the current anatomy. ERCP, MRCP, or PTC can be utilized for this purpose and choice of modality may be directed by center preference and available expertise. While MRCP is noninvasive and sensitive for detection of fluid collection, biliary stricture, and biliary leak, its major limitation is its use for diagnostic purposes only. ERC may not evaluate proximal bile ducts in the setting of complete transection. PTC allows for evaluation of the proximal ducts, can be used in the setting of a Roux reconstruction, and can be used for treatment and diagnosis but is the most invasive of the other modalities.
Quality imaging should be obtained to evaluate the vascular anatomy and lesions suspicious for hepatocellular carcinoma. Portal vein patency is best evaluated with contrasted CT or MRI. If there is portal vein thrombosis, the extent of the thrombus needs to be determined as well as if the superior mesenteric vein (SMV) is patent and of adequate caliber for graft inflow. Hepatic artery occlusion, celiac axis occlusion, and portal vein thrombosis are not contraindications to transplant but should be considered in operative planning. Almost all patients can undergo adequate evaluation of their vasculature with contrast enhanced cross-sectional imaging (MR or CT), with the use of angiography reserved for highly selected cases where uncertainty exists.
Hepatocellular lesions should be evaluated and treated per transplant center protocol. In our center, patients undergoing evaluation for liver transplant with hepatocellular carcinoma are treated with chemoembolization to either downstage the tumor to within Milan criteria or treat while they are waitlisted.
Many patients referred will have percutaneous biliary drains (PTBD) in place at time of referral. If imaging suggests that they are not adequately decompressed, cholangiography should then be performed and drainage optimized. If drainage is optimized and recurrent cholangitis persists, antibiotic prophylaxis may be required in the interval to transplant.
The criteria for candidacy for liver transplant in the setting of secondary biliary cirrhosis are not well established but should include at least one of the following: cirrhosis by biopsy, MELD greater than 15, fibrosis and portal hypertension in setting of biliary stricture without percutaneous/endoscopic or surgical potential for revision, and/or poor quality of life: recurrent cholangitis requiring hospitalization with biliary tract stricture not amenable to surgical reconstruction.
Steps in evaluation of liver transplant candidate with secondary biliary cirrhosis:
-
1.
History and Physical
-
(a)
Evaluate for additional contributors to liver disease (alcohol use, hepatitis, NASH)
-
(a)
-
2.
Establish diagnosis of cirrhosis
-
(a)
Imaging
-
(b)
Biopsy
-
(a)
-
3.
Multidisciplinary review (hepatology, pathology, interventional and diagnostic radiology)
-
(a)
If biopsy does not show cirrhosis or portal hypertension, is there a chance for recovery of the liver with relief of obstruction via endoscopic/interventional or surgical methods
-
(b)
Review vascular and biliary anatomy
-
Is the biliary system adequately decompressed?
-
Will the patient require a vascular conduit for arterial and/or portal venous inflow at the time of transplant?
-
-
(a)
-
4.
Evaluate for surgical candidacy
-
(a)
Cardiopulmonary fitness
-
(b)
Comorbidities (HCC, HCV, Hepatopulmonary syndrome (HPS), Hepatorenal syndrome (HRS))
-
(a)
-
5.
Multidisciplinary evaluation (hepatology, anesthesia, surgery, social work, and psychology) and listing
Acute Liver Failure
For patients with acute liver failure, evaluation includes the likelihood of the patient to survive the procedure based on their overall clinical stability, the presence of any irreversible complications of liver failure (i.e., irreversible cerebral edema), and the presence of sepsis. Special consideration must be given to sepsis in these cases as there has been contamination at the original procedure, which is potentially ongoing, given the nature of the injury. Ongoing sepsis (except confined to the native liver) is a contraindication to transplant.
Coagulation parameters should be optimized in preparation for the operating room in anticipation of increased level of technical difficulty of the dissection due to prior surgery.
Imaging should be obtained to ensure vascular anatomy suitable for transplant.
Intraoperative Considerations
Donor Selection
Standard donor selection criteria apply, with the optimal donor being a young, otherwise healthy donor that sustained an injury that resulted in brain death. Special consideration should be given to the use of extended criteria donors.
Techniques for organ procurement from brain-dead, heart beating donors have been described previously and do not significantly differ in these circumstances [50–53].
Given the increased likelihood for arterial conduit and the need for available quality arterial vessels, donors at extremes of age, with known atherosclerotic disease, or imaging with atherosclerosis, should be used with caution. Donor iliac vessels should be procured as is standard for all cadaveric liver procedures. If there is a shortage of vessels or unexpected poor iliac quality, the carotid vessels may be procured as well. If a center has stored cadaveric vessels of appropriate blood type or cryopreserved vessels, these may also be utilized. Vein grafts from the iliac and, if needed, saphenous vein should be procured.
Due to the additional dissection time potentially required due to adhesions and disrupted anatomy, careful planning must occur between the procuring and recipient teams to minimize the cold ischemia time of the graft.
In some extreme cases, the anatomy encountered during the hepatectomy is such that the recipient is unsuitable for transplant. In patients where there have been multiple prior interventions or any concerns regarding the suitability of the patient’s vascular anatomy, we will backbench the graft in a separate sterile area so that, if needed, it can be used in an alternate recipient.
Given the risks associated with the use of deceased after cardiac death (DCD) grafts, including sensitivity to warm and cold ischemia and increased risk of hepatic arterial thrombosis, they should probably not be used in this group of recipients.
Recipient Operative Techniques
The technique of recipient hepatectomy has been described previously [50–54]. As with any reoperative surgery, increased difficultly in the dissection due to adhesions, altered anatomy due to prior surgical intervention, and/or ongoing inflammation and infection should be anticipated. This can be complicated in these patients by coagulopathy of cirrhosis and portal hypertension. Steib et al. found that prior surgery correlated with increased blood loss during liver transplant [55].
Several authors have attributed increased blood loss and increased perioperative morality to adhesions created from previous surgery in the setting of salvage transplant after resection for hepatocellular carcinoma and in patients with primary biliary cirrhosis [56–58]. Whether or not previous surgery has an adverse effect on outcomes remains controversial. However, prior right upper quadrant procedures, including prior biliary bypass, can present additional technical difficulties, sometimes making these very difficult liver transplant procedures [57, 58].
The importance of current imaging of the patient’s vascular and biliary anatomy to aid in operative planning cannot be overstated.
Regardless of the location of previous incisions, optimal exposure is critical and is achieved using a bilateral subcostal incision usually with the option for midline extension (Mercedes incision). The groin should be prepped in anticipation of the need for possible venovenous bypass. A generous amount of blood products should be readily available (at least 10 units of cross-matched blood, fresh frozen plasma, platelets, and cryoprecipitate). Perioperative antibiotics should be administered to cover suspected pathogens. If the patient has a percutaneous biliary drain in place, it should be prepped into the field as it can facilitate the portal dissection.
The hepatectomy should proceed as much as possible through the typical sequence. Early assessment of the hepatic arterial pulse should be performed to assess its integrity and the potential need for an arterial conduit. If there is obvious contamination from the injury or biloma, cultures should be obtained.
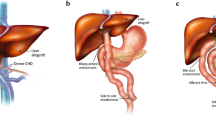
The portal vein is skeletonized to the level of the confluence of the splenic and superior mesenteric vein. In cases where a piggyback reconstruction is planned, the transection of the portal vein will often facilitate the dissection of the retrohepatic caval branches. The degree of adhesions may dictate whether or not the retrohepatic dissection can be achieved safely. Alternatively, a bicaval technique may be necessary. The decision to create a portocaval shunt or venovenous bypass will be dictated by the patient’s hemodynamics. If portal vein is thrombosed and cannot be removed with endovenothrombectomy or if the portal vein has been previously damaged and is unable to be used, an alternative inflow must be chosen. The superior mesenteric vein can be used for inflow with the donor iliac vein serving as the conduit between the native SMV and the donor portal vein. An adequate length of donor external iliac vein is prepared by ligating all side branches using fine synthetic monofilament suture, and the vein is marked to identify the inflow end (external iliac) to avoid twisting the graft at implantation. The SMV is exposed in the recipient. The colon is retracted cephalad and the SMV exposed at the root of its mesentery. A length of SMV adequate to allow a side-biting clamp is dissected. Ligation of 1–2 colonic branches may be necessary for mobilization. The external iliac end of the conduit is anastomosed in an end to side fashion to the recipient SMV using permanent fine monofilament suture (6-0 or 7-0 Prolene). The conduit is passed through tunnel over the neck of the pancreas, retrogastric into the former lesser sac, and the common iliac end of the conduit is anastomosed end to end to the donor portal vein [59]. The suture is tied with a growth factor of approximately one half of the diameter of the portal vein to avoid anastomotic stricture.
If the hepatic artery cannot be used for the arterial inflow, due to damage from prior surgery or inadequate flow, an arterial conduit may be used to establish inflow from the supraceliac or infrarenal aorta. Our preference is to use an infrarenal takeoff and donor iliac vessels as a conduit. The conduit is prepared by oversewing the internal iliac several millimeters from the bifurcation with the external. The infrarenal aorta is exposed. A side-biting vascular clamp is placed and an aortotomy is made and then enlarged using an aortic punch. The common iliac of the donor is anastomosed to the aorta using 5-0 Prolene. The conduit is then flushed with heparinized saline and passed through a window in the transverse mesocolon, behind the pylorus and into the subhepatic region [60]. The external iliac end of the donor artery conduit is then anastomosed to the donor hepatic artery using 6-0 Prolene.
There are several options to address the bile duct in these cases: choledochocholedochostomy (very rarely), revision of the existing biliary roux limb, or creation of a Roux-Y hepaticojejunostomy. While the recipient bile duct may be preserved in some cases, this should be done with special attention to the blood supply to the duct, which can be evaluated by assessing the backbleeding from the duct when the duct is transected. The duct should then be probed with an 8 French feeding tube to ensure that the ampulla is not stenosed.
When using the existing biliary roux limb, it is imperative that sufficient dissection be performed to verify that the limb was constructed correctly. The ligament of Treitz should be identified and followed to the level of the jejunojejunostomy to verify that this limb does not connect to the biliary system creating a backwards loop. Once this has been clarified, the blind end of the roux limb should be identified and disconnected from the native bile duct. This can be dissected several centimeters down the length of the intestine so that the previous enterostomy can be contained in the short segment enterectomy. Care is taken to avoid disrupting the mesentery of the roux. The limb must be adequately dissected to ensure sufficient length for creation of the new biliary anastomosis without tension (we prefer at least 50 cm). If a mesenteric trap/defect/hernia has been created, this is closed. The choledochojejunostomy is created using interrupted or running 6-0 PDS suture.
If a new Roux limb is required, it is created as previously described with an end of donor bile duct to side of jejunum anastomosis, with at least 50 cm of length to prevent reflux of intestinal contents into the biliary tree and avoid tension.
The postoperative care should be similar as in all patients undergoing liver transplant, with careful management of immunosuppression and prophylaxis for infectious complications. Patients should be placed on antiplatelet treatment with aspirin. If a vascular conduit was required, consideration should be given to anticoagulation.
Conclusions
There is a rare but important role and need for liver transplant in highly selected cases of bile duct injury, both in the acute and chronic setting. While its incidence is low, an understanding of special considerations is necessary to achieve a successful outcome in this challenging patient population. This includes multidisciplinary evaluation and delineation of complex anatomy, along with adequate surgical preparation and anticipation of the need for alternative reconstructive strategies.
Pearls and Pitfalls | |
|---|---|
Delineate recipient anatomy | • Define arterial and portal venous anatomy during the transplant evaluation |
• Update as the time to transplant approaches | |
Anticipate need for alternative vascular conduits | • Have suitable vessels for conduit use if needed (either from the donor or in the center vessel bank that are ABO compatible) |
Expect increased difficulty and increased blood loss | • Prepared anesthesia team – adequate blood availability – adequate vascular access |
• Prepared surgical team – adequate surgeon experience and availability | |
• Appropriate donor selection – anticipate increased operative time for hepatectomy | |
References
Ardiles V, McCormack L, Quinonez E, et al. Experience using liver transplantation for the treatment of severe bile duct injuries over 20 years in Argentina: results from a National Survey. HPB (Oxford). 2011;13(8):544–50.
Bacha EA, Stieber AC, Galloway JR, Hunter JG. Non-biliary complication of laparoscopic cholecystectomy. Lancet. 1994;344(8926):896–7.
Barbier L, Souche R, Slim K, Ah-Soune P. Long-term consequences of bile duct injury after cholecystectomy. J Visc Surg. 2014.
de Santibanes E, Ardiles V, Gadano A, Palavecino M, Pekolj J, Ciardullo M. Liver transplantation: the last measure in the treatment of bile duct injuries. World J Surg. 2008;32(8):1714–21.
Fernandez JA, Robles R, Marin C, Sanchez-Bueno F, Ramirez P, Parrilla P. Laparoscopic iatrogeny of the hepatic hilum as an indication for liver transplantation. Liver Transpl. 2004;10(1):147–52.
Frilling A, Li J, Weber F, et al. Major bile duct injuries after laparoscopic cholecystectomy: a tertiary center experience. J Gastrointest Surg. 2004;8(6):679–85.
Loinaz C, Gonzalez EM, Jimenez C, et al. Long-term biliary complications after liver surgery leading to liver transplantation. World J Surg. 2001;25(10):1260–3.
Lubikowski J, Chmurowicz T, Post M, et al. Liver transplantation as an ultimate step in the management of iatrogenic bile duct injury complicated by secondary biliary cirrhosis. Ann Transplant. 2012;17(2):38–44.
McCormack L, Quinonez EG, Capitanich P, et al. Acute liver failure due to concomitant arterial, portal and biliary injury during laparoscopic cholecystectomy: is transplantation a valid life-saving strategy? A case report. Patient Saf Surg. 2009;3(1):22.
Nordin A, Makisalo H, Isoniemi H, Halme L, Lindgren L, Hockerstedt K. Iatrogenic lesion at cholecystectomy resulting in liver transplantation. Transplant Proc. 2001;33(4):2499–500.
Oncel D, Ozden I, Bilge O, et al. Bile duct injury during cholecystectomy requiring delayed liver transplantation: a case report and literature review. Tohoku J Exp Med. 2006;209(4):355–9.
Parrilla P, Robles R, Varo E, et al. Liver transplantation for bile duct injury after open and laparoscopic cholecystectomy. Br J Surg. 2014;101(2):63–8.
Robertson AJ, Rela M, Karani J, Steger AC, Benjamin IS, Heaton ND. Laparoscopic cholecystectomy injury: an unusual indication for liver transplantation. Transpl Int. 1998;11(6):449–51.
Schmidt SC, Settmacher U, Langrehr JM, Neuhaus P. Management and outcome of patients with combined bile duct and hepatic arterial injuries after laparoscopic cholecystectomy. Surgery. 2004;135(6):613–8.
Strasberg SM, Gouma DJ. ‘Extreme’ vasculobiliary injuries: association with fundus-down cholecystectomy in severely inflamed gallbladders. HPB (Oxford). 2012;14(1):1–8.
Thomson BN, Parks RW, Madhavan KK, Garden OJ. Liver resection and transplantation in the management of iatrogenic biliary injury. World J Surg. 2007;31(12):2363–9.
Walsh RM, Vogt DP, Ponsky JL, Brown N, Mascha E, Henderson JM. Management of failed biliary repairs for major bile duct injuries after laparoscopic cholecystectomy. J Am Coll Surg. 2004;199(2):192–7.
Zaydfudim V, Wright JK, Pinson CW. Liver transplantation for iatrogenic porta hepatis transection. Am Surg. 2009;75(4):313–6.
US United Network for Organ Sharing (UNOS) registry. 2014; http://www.srtr.org/. Accessed 1 Oct 2014.
de Santibanes E, Ardiles V, Pekolj J. Complex bile duct injuries: management. HPB (Oxford). 2008;10(1):4–12.
Koffron A, Ferrario M, Parsons W, Nemcek A, Saker M, Abecassis M. Failed primary management of iatrogenic biliary injury: incidence and significance of concomitant hepatic arterial disruption. Surgery. 2001;130(4):722–8. discussion 728-731.
Mirza DF, Narsimhan KL, Ferraz Neto BH, Mayer AD, McMaster P, Buckels JA. Bile duct injury following laparoscopic cholecystectomy: referral pattern and management. Br J Surg. 1997;84(6):786–90.
Chaudhary A, Manisegran M, Chandra A, Agarwal AK, Sachdev AK. How do bile duct injuries sustained during laparoscopic cholecystectomy differ from those during open cholecystectomy. J Laparoendosc Adv Surg Tech A. 2001;11(4):187–91.
Nuzzo G, Giuliante F, Persiani R. The risk of biliary ductal injury during laparoscopic cholecystectomy. J Chir. 2004;141(6):343–53.
Strasberg SM. Avoidance of biliary injury during laparoscopic cholecystectomy. J Hepatobiliary Pancreat Surg. 2002;9(5):543–7.
Yan JQ, Peng CH, Ding JZ, et al. Surgical management in biliary restricture after Roux-en-Y hepaticojejunostomy for bile duct injury. World J Gastroenterol. 2007;13(48):6598–602.
Chapman WC, Abecassis M, Jarnagin W, Mulvihill S, Strasberg SM. Bile duct injuries 12 years after the introduction of laparoscopic cholecystectomy. J Gastrointest Surg. 2003;7(3):412–6.
Chapman WC, Halevy A, Blumgart LH, Benjamin IS. Postcholecystectomy bile duct strictures. Management and outcome in 130 patients. Arch Surg. 1995;130(6):597–602. discussion 602-594.
Flum DR, Cheadle A, Prela C, Dellinger EP, Chan L. Bile duct injury during cholecystectomy and survival in medicare beneficiaries. JAMA. 2003;290(16):2168–73.
Kern KA. Medicolegal analysis of bile duct injury during open cholecystectomy and abdominal surgery. Am J Surg. 1994;168(3):217–22.
Nilsson E. Incidence and nature of bile duct injuries following laparoscopic cholecystectomy: an audit of 5913 cases. Br J Surg. 1997;84(3):424.
Alves A, Farges O, Nicolet J, Watrin T, Sauvanet A, Belghiti J. Incidence and consequence of an hepatic artery injury in patients with postcholecystectomy bile duct strictures. Ann Surg. 2003;238(1):93–6.
Blumgart LH, Kelley CJ, Benjamin IS. Benign bile duct stricture following cholecystectomy: critical factors in management. Br J Surg. 1984;71(11):836–43.
Vellar ID. The blood supply of the biliary ductal system and its relevance to vasculobiliary injuries following cholecystectomy. Aust N Z J Surg. 1999;69(11):816–20.
Mercado MA, Chan C, Orozco H, Tielve M, Hinojosa CA. Acute bile duct injury. The need for a high repair. Surg Endosc. 2003;17(9):1351–5.
Kaman L, Behera A, Singh R, Katariya RN. Management of major bile duct injuries after laparoscopic cholecystectomy. Surg Endosc. 2004;18(8):1196–9.
Pitt HA, Miyamoto T, Parapatis SK, Tompkins RK, Longmire Jr WP. Factors influencing outcome in patients with postoperative biliary strictures. Am J Surg. 1982;144(1):14–21.
Walsh RM, Henderson JM, Vogt DP, Brown N. Long-term outcome of biliary reconstruction for bile duct injuries from laparoscopic cholecystectomies. Surgery. 2007;142(4):450–6. discussion 456-457.
Johnson SR, Koehler A, Pennington LK, Hanto DW. Long-term results of surgical repair of bile duct injuries following laparoscopic cholecystectomy. Surgery. 2000;128(4):668–77.
Thompson CM, Saad NE, Quazi RR, Darcy MD, Picus DD, Menias CO. Management of iatrogenic bile duct injuries: role of the interventional radiologist. Radiographics. 2013;33(1):117–34.
Abraham SC, Kamath PS, Eghtesad B, Demetris AJ, Krasinskas AM. Liver transplantation in precirrhotic biliary tract disease: Portal hypertension is frequently associated with nodular regenerative hyperplasia and obliterative portal venopathy. Am J Surg Pathol. 2006;30(11):1454–61.
Saad WE. Percutaneous management of postoperative anastomotic biliary strictures. Tech Vasc Interv Radiol. 2008;11(2):143–53.
Sikora SS, Srikanth G, Agrawal V, et al. Liver histology in benign biliary stricture: fibrosis to cirrhosis … and reversal? J Gastroenterol Hepatol. 2008;23(12):1879–84.
Weinbren K, Hadjis NS, Blumgart LH. Structural aspects of the liver in patients with biliary disease and portal hypertension. J Clin Pathol. 1985;38(9):1013–20.
Corvera CU, Alemi F, Jarnagin W. Benign Biliary Strictures. In: editor-in-chief WRJae, Jacques Belghiti … [and others]; editor emeritus, Leslie H. Blumgart, ed. Blumgart’s surgery of the liver, biliary tract, and pancreas. 5th Edition ed. Philadelphia: Elsevier Saunders; 2012.
Adson MA, Wychulis AR. Portal hypertension in secondary biliary cirrhosis. Arch Surg. 1968;96(4):604–12.
Negi SS, Sakhuja P, Malhotra V, Chaudhary A. Factors predicting advanced hepatic fibrosis in patients with postcholecystectomy bile duct strictures. Arch Surg. 2004;139(3):299–303.
Hammel P, Couvelard A, O’Toole D, et al. Regression of liver fibrosis after biliary drainage in patients with chronic pancreatitis and stenosis of the common bile duct. N Engl J Med. 2001;344(6):418–23.
Abdel-Aziz G, Lebeau G, Rescan PY, et al. Reversibility of hepatic fibrosis in experimentally induced cholestasis in rat. Am J Pathol. 1990;137(6):1333–42.
Conzen K, Doyle MBM, Chapman WC. Orthotopic Liver Transplantation In: editor-in-chief WRJae, Jacques Belghiti … [and others]; editor emeritus, Leslie H. Blumgart, ed. Blumgart’s surgery of the liver, biliary tract, and pancreas. Fifth edition ed. Philadelphia Elsevier Saunders; 2012.
Merkel FK, Jonasson O, Bergan JJ. Procurement of cadaver donor organs: evisceration technique. Transplant Proc. 1972;4(4):585–9.
Starzl TE, Hakala TR, Shaw Jr BW, et al. A flexible procedure for multiple cadaveric organ procurement. Surg Gynecol Obstet. 1984;158(3):223–30.
Starzl TE, Miller C, Broznick B, Makowka L. An improved technique for multiple organ harvesting. Surg Gynecol Obstet. 1987;165(4):343–8.
Porte RJ, Lerut J. Orthotopic liver transplant In: editors P-AC, Michael G. Sarr, and Yuman Fong; Panco Georgiev (associate editor), ed. Atlas of upper gastrointestinal and hepato-pancreato-biliary surgery. New York: Springer-Verlag; 2007
Steib A, Freys G, Lehmann C, Meyer C, Mahoudeau G. Intraoperative blood losses and transfusion requirements during adult liver transplantation remain difficult to predict. Can J Anaesth. 2001;48(11):1075–9.
Adam R, Azoulay D, Castaing D, et al. Liver resection as a bridge to transplantation for hepatocellular carcinoma on cirrhosis: a reasonable strategy? Ann Surg. 2003;238(4):508–18. discussion 518-509.
Cherqui D, Laurent A, Mocellin N, et al. Liver resection for transplantable hepatocellular carcinoma: long-term survival and role of secondary liver transplantation. Ann Surg. 2009;250(5):738–46.
Neuberger J, Gunson B, Komolmit P, Davies MH, Christensen E. Pretransplant prediction of prognosis after liver transplantation in primary sclerosing cholangitis using a Cox regression model. Hepatology. 1999;29(5):1375–9.
Burdick JF, Pitt HA, Colombani PM, et al. Superior mesenteric vein inflow for liver transplantation when the portal vein is occluded. Surgery. 1990;107(3):342–5.
Tzakis A, Todo S, Starzl TE. The anterior route for arterial graft conduits in liver transplantation. Transpl Int. 1989;2(2):121.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Collins, K.M., Chapman, W.C. (2015). Liver Transplantation for Common Bile Duct Injury. In: Dixon, E., Vollmer Jr., C., May, G. (eds) Management of Benign Biliary Stenosis and Injury. Springer, Cham. https://doi.org/10.1007/978-3-319-22273-8_34
Download citation
DOI: https://doi.org/10.1007/978-3-319-22273-8_34
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-22272-1
Online ISBN: 978-3-319-22273-8
eBook Packages: MedicineMedicine (R0)