Abstract
This chapter provides some basic information regarding the origin of the MRI signal. The MRI signal is generated by the interaction of applied magnetic fields with the nuclei of hydrogen atoms in the body. Hydrogen nuclei (protons, or “spins”) tend to align themselves with the large static magnetic field generated by the MRI system, and rotate or precess about the direction of that field at a characteristic frequency called the Larmor frequency. The application of additional radiofrequency (RF) energy at the same frequency excites the magnetized protons causing them to tip into the plane perpendicular to the main field. The magnetized protons which have been perturbed in this fashion undergo a process of relaxation that returns them back into alignment with the main field. During the course of relaxation, a signal is emitted which is detected using receiver coils and digitally sampled. The relaxation of spins is governed by time constants known as T1 and T2, and these time constants play a major role in determining the contrast between tissues in an image. The encoding of spatial information is accomplished using magnetic field gradients that alter the precession frequency of spins based on their position in the scanner. The Fourier transform is then used to reconstruct an image from the encoded data. Many of the basic concepts introduced in this chapter are covered in greater detail in later chapters.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Larmor Frequency
- T1 relaxation
- T2 relaxation
- Spatial encoding
- Bloch Equations
- Slice selection gradient
- Phase encoding gradient
- Readout gradient
The Magnetic Resonance Imaging (MRI) signal is generated by the hydrogen nuclei (protons) in human tissue. Three fourths of the human body consists of water, and each water molecule includes two hydrogen atoms; thus, water is the primary source of MRI signal in medical imaging applications. This chapter covers the important steps required to generate the signal used to create MR images.
The MRI Signal
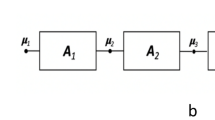
Any modern digital imaging system requires a probe to interact with the tissue to be imaged; this interaction results in a signal that is detected, digitized, and further processed to generate an image as shown in Fig. 1.1. In magnetic resonance imaging (MRI), radiofrequency (RF) pulses are used to excite the tissue, and the resulting signal is detected by receiver coils. MRI is thus based on the absorption and emission of energy in the RF range of the electromagnetic spectrum. Although several chemical elements can interact with magnetic fields to emit an MRI signal, the large amount of hydrogen found in the body, and the relatively large signal it produces, has made hydrogen-based the most widely used MRI technique for clinical diagnosis. The primary sources of MRI signal in the human body are the hydrogen nuclei found in tissues comprised mainly of water, and also fat (hydrocarbons). In the following discussion, the terms “hydrogen nuclei,” “protons,” and “spins” will be used interchangeably to describe the atomic particles that provide the primary source of the MRI signal.
Nuclear Spin in a Magnetic Field
Hydrogen has a nuclear property known as “spin” that results in a magnetic moment, μ. Because of this magnetic moment, when exposed to the static magnetic field (B0) generated by the main magnet of the MRI system, hydrogen nuclei will align parallel or anti-parallel to the field as shown in Fig. 1.2. The energy difference (E) between these two orientations is E = 2μB0. The small preference for the hydrogen nuclei to align toward the parallel, lower energy state, over the anti-parallel orientation, contributes to the development of the net longitudinal magnetization [1]. The net magnetization is the vector sum of magnetic moments from many individual protons.
Larmor Frequency
When a hydrogen nucleus is exposed to a magnetic field of strength B0, it precesses at a frequency, ω, due to the interaction of its angular momentum and the field, as illustrated in Fig. 1.3. The frequency, ω, depends on B0 and on the gyromagnetic ratio, γ, of the specific nucleus according to the Larmor Eq. 1.1.
For hydrogen nuclei, γ/2π = 42.58 MHz/T, and ωL is termed the Larmor frequency. Thus, the Larmor frequency of precession is dependent on the applied magnetic field strength; for example, protons will precess at approximately 64 MHz at 1.5T, and 128 MHz at 3.0T.
Excitation of Spins
In a three-dimensional (x, y, z) coordinate system, let us assume by convention that the static magnetic field is oriented in the z-direction. As a result, the net longitudinal magnetization vector at equilibrium (M0) will point in the z-direction as shown in Fig. 1.4a; this is due to the alignment of a majority of protons with the applied field.
Schematics showing (a) the direction of the main magnetic field and longitudinal magnetization, (b) nutation of a spin from the longitudinal axis to the transverse plane in response to an applied RF field (B1) (c) the final result of tipping all of the longitudinal magnetization by flip angle α = 90° into the transverse plane
Protons can be excited to the higher energy state by applying a radio frequency (RF) field oscillating at the Larmor frequency; this applied RF field is known as the B1 field. The magnitude of the net longitudinal magnetization in the z-direction is extremely small compared to the magnetic field strength, B0, and is undetectable. In order to measure a detectable signal from protons, the magnetization is tipped out of alignment with B0 and into the transverse plane. When an RF pulse is applied at the Larmor frequency, i.e., a B1 field is switched on briefly as shown in Fig. 1.4b, the net magnetization vector will respond and be tipped away from the z-axis and towards the x-y transverse plane (Fig. 1.4c). The angle that the magnetization vector rotates away from the z-axis is known as flip angle (α), and can be approximated as
where τ represents the length of time the RF pulse is applied with amplitude B1.
When the RF pulse is terminated, the net magnetization will begin to return back to the longitudinal axis as the protons return to equilibrium by releasing energy to the environment, a process known as relaxation as termed by Felix Bloch, one of the discoverers of the nuclear magnetic resonance phenomenon that forms the basis for MRI. Before the protons fully relax back to equilibrium, the signal generated by the transverse component of the precessing magnetization can be detected using an RF receiver coil.
MR Signal and Contrast Characteristics from Spin Relaxation
The MRI signal available from stationary tissue is determined by a combination of factors, including the density of protons and their relaxation rates; depending on the specific pulse sequence parameters, the image contrast will reflect these as well as many other factors such as flow and motion, diffusion, local differences in magnetic susceptibility and field homogeneity. Two types of relaxation take place: longitudinal, spin-lattice or T1 relaxation, and transverse, spin-spin, or T2 relaxation. The extent that T1 and T2-relaxations and proton density contribute to image intensity and contrast is controlled through manipulation of pulse sequence timing and RF pulse flip angles as explained in later chapters.
Longitudinal Relaxation (Spin-Lattice Relaxation)
The spin lattice relaxation time, or T1, is the characteristic tissue-specific exponential time constant that governs the regrowth of longitudinal magnetization (Mz) towards its equilibrium value, M0 (Fig. 1.5). T1 is the time required for Mz to regain 63 % of its equilibrium value when starting from zero, e.g., following the application of a 90° excitation pulse that tips the longitudinal magnetization completely into the transverse plane; this can be expressed as:
where t is the time following the excitation pulse.
Longitudinal, spin-lattice relaxation is caused by the transfer of thermal energy between excited nuclei and the surrounding atomic lattice [2]. Molecules that have an efficient means of energy transfer will exhibit a shorter T1 relaxation time, while those without effective transfer mechanisms demonstrate a longer T1 time. This is primarily dependent on the mobility of the lattice, and the related vibrational and rotational frequencies. The more closely these frequencies correspond to the energy gap, E, and the Larmor frequency, the more efficient is T1 relaxation. Thus, T1 relaxation is highly dependent on molecular motion, and hence, on the size of the molecules. The motion of very large molecules generally occurs at a frequency too low for efficient energy transfer, and likewise extremely mobile nuclei such as those in free water are moving at frequencies too high to facilitate relaxation; thus the T1 values of fluids are relatively long, while fat tissues demonstrate shorter T1 values [3].
T1 Differences Determine Image Contrast
As an example, consider tissues A and B shown in Fig. 1.6; tissue A has a shorter T1 relaxation time than tissue B, i.e., the longitudinal magnetization of tissue A will recover and realign with the main magnetic field more quickly than tissue B following RF excitation. After a 90° RF pulse, the magnetization vectors for both tissues A and B are tipped into the transverse plane and from there the longitudinal magnetization begins to recover. Magnetic resonance imaging typically requires multiple excitation pulses to collect all of the data needed to form an image; if the next excitation pulse is applied before full recovery occurs (which takes approximately five times T1), tissue A will have recovered more longitudinal magnetization than tissue B. Since tissue A has a larger longitudinal component prior to the next RF pulse, it will have a larger transverse component after the RF pulse, and therefore tissue A will have a higher signal than tissue B, and will appear brighter in the image as shown in Fig. 1.6. Thus, in a pulse sequence designed to generate image contrast sensitive to T1, i.e., a T1-weighted image [4], tissues with shorter T1 will have higher signal than tissues with longer T1. Table 1.1 shows the T1 values for various tissues at 1.5T [5].
Transverse Relaxation (Spin – Spin Relaxation)
The transverse magnetization created when an RF pulse is used to tip the longitudinal (Mz) magnetization into the transverse plane decays back to zero after the termination of the RF excitation pulse. The time constant, T2, describes the exponential rate of decay of the transverse magnetization (Fig. 1.7), which is given by:
where t is the time following the excitation pulse, and Mxy is the component of the magnetization in the transverse plane. By this equation, T2 is the time required for 63 % of the initial transverse magnetization to dissipate [6, p. 381].
Recall that the net magnetization vector is the sum of magnetic moments from many individual protons. To maintain a detectable net transverse magnetization, the protons must maintain phase coherence, that is, they must precess in phase with one another at exactly the same frequency. Over time, however, the individual precessing protons get out of sync with each other, or become “dephased” in the transverse plane; this means that the individual magnetic moments begin to point in different directions, decreasing the total net transverse magnetization [3]. Realizing from the Larmor Equation (Eq. 1.1) that the precession frequency of a proton is dependent on the magnetic field it experiences, it is easy to understand that the primary reasons for transverse dephasing are related to spatial and temporal variations in the magnetic field. One of the factors contributing to the dephasing of the protons is the interaction between the magnetic fields of individual protons, or so-called “spin-spin” dephasing [6]. Similar to spin-lattice relaxation, spin-spin relaxation is inefficient in highly mobile protons, and thus the T2 relaxation time tends to be longer in free water, and in tissue containing a high percentage of water. Besides these temporally varying spin-spin interactions, transverse phase coherence is also affected by static inhomogeneties in the local magnetic field; these can be caused by inhomogeneities in the applied field, or by local differences in the magnetization of tissues due to differences in their magnetic susceptibility. The effective transverse relaxation time (T2*) describes the exponential decay in signal that results from the combination of spin-spin relaxation (T2) and static field inhomogeneties (T2 ′) [3] as shown in Fig. 1.8 and in the equation:
T2 Differences Determine Image Contrast
Again, consider an example of two tissues, tissue A and tissue B; let tissue A have a shorter T2 time than tissue B, indicating that its transverse magnetization relaxes or decays more rapidly than tissue B. At any time following an excitation pulse, the amount of transverse magnetization in tissue A is less than tissue B, thus generating less signal in the image [4] as shown in Fig. 1.9. Thus, in a T2-weighted image, tissues with longer T2 will appear brighter than tissues with shorter T2. Table 1.1 shows the T2 relaxation times for different tissues.
Contrast Produced by Proton Density
Besides the tissue relaxation rates, contrast between tissues is also governed by differences in proton density. Proton density, as one would expect, is indicative of the number of protons per volume of tissue. The higher the number of protons in a given volume of tissue, the greater is the magnetization available to provide signal. Imaging pulse sequences can be designed to provide “proton density weighting,” i.e., to be sensitive to proton density and relatively insensitive to T1 or T2 relaxation [4], although this contrast mechanism is rarely used in cardiovascular MRI applications.
Signal Acquisition
Free Induction Decay
In the most basic example of a nuclear magnetic resonance signal, a 90° RF pulse is applied to rotate the net longitudinal magnetization vector completely into the x-y plane, where it can induce a signal in an RF receiver coil. This signal, which is a result of the free precession of the net magnetization in the transverse plane, is called the free induction decay, or FID, since it gradually decays due to the relaxation mechanisms previously described.
The FID in hydrogen magnetic resonance has the following characteristics:
-
1.
It oscillates at the Larmor frequency determined by the gyromagnetic ratio of hydrogen, and the applied magnetic field strength.
-
2.
It has an initial magnitude that is proportional to the density of protons (hydrogen nuclei) in the sample being measured.
-
3.
It decreases in amplitude exponentially with a time constant T2*, due to the combination of spin-spin relaxation and magnetic field inhomogeneties [2].
Imaging Procedure
To generate an image, a sequence of RF pulses in combination with magnetic field gradients is implemented as detailed in later chapters. The specific sequence and timing of RF pulses, magnetic field gradient pulses, and delay times is designed to manipulate the transverse and longitudinal magnetization to generate a specific type of image contrast. It is typically necessary to repeat a pulse sequence a number of times to acquire enough data to form an image. Therefore, the time for one cycle of a pulse sequence is called the repetition time, or TR.
Spatial Localization
It is necessary to encode the emitted signal so that the spatial position of the nuclei contributing to the signal can be located through the image reconstruction process. To spatially encode the signal, sets of magnetic field gradients are applied during the imaging procedure. Using coils that are embedded in the bore of the MRI scanner, it is possible to superimpose a magnetic field gradient (variation in the magnetic field with respect to position) onto the static main magnetic field. These gradients are weaker magnetic fields oriented in the same direction as the B0 field (i.e., the z-direction) and vary linearly with position. There are three independent gradient coils in the MRI system, each designed to create a linear variation in the static field along one of the Cartesian axes, x, y, and z; the gradients produced on each axis are respectively referred to as Gx, Gy, and Gz. Note that a linear gradient can be generated in any direction by appropriate combination of gradients along multiple axes simultaneously. This is what gives MRI the flexibility to generate images in any orientation.
As an example, Gx refers to a one-dimensional gradient superimposed on the B0 field that causes a linear variation in the field in the x direction. In Fig. 1.10, the length of the vectors represents the magnitude of the gradient field that changes positively and negatively. Recalling that the Larmor Equation (Eq. 1.1) states that the precessional frequency is proportional to magnetic field strength, the gradient causes the precessional frequency to vary as a function of position along the direction of the gradient. Thus, the position of a particular spin along the direction of an applied gradient can be determined from its precessional frequency.
Magnetic gradient field superimposes on the main magnetic field (B0) to create a linear variation in field strength in the direction of the gradient. By the Larmor equation, this creates a linear variation in precessional frequencies as a function of location. This control over the spatial distribution of precessional frequencies enables spatial localization of the MR signal, and image encoding
The imaging process can be divided into four fundamental operations:
-
1.
Slice selection
-
2.
Spatial encoding
-
3.
Signal read-out
-
4.
Image Reconstruction
Slice Selection
The slice selection gradient (Gz, by convention) and the RF pulse are applied simultaneously in order to excite (tip) the magnetization of protons only within a slice of discrete thickness (Fig. 1.11). The slice thickness is controlled by two factors: the amplitude of the magnetic field gradient, which affects the spatial distribution of the proton resonant frequencies, and the bandwidth of the RF pulse (i.e., the range of frequencies included in the pulse) [5] as shown in Fig. 1.12. We can see by Eq. 1.6,
Z-gradient coil, schematically represented by the red loops, generates a linear variation in the main magnetic field in the head to foot direction. Additional gradient coils create linear variations in the main field in X and Y directions. Using all three coils it is possible to select and encode an image in any orientation
RF excitation and slice select gradient are applied simultaneously to excite the spins only in the selected slice. The gradient creates a distribution of precessional frequencies along the direction of the gradient, and a narrow band RF pulse is applied to excite spins only within a location based on their frequency
that the slice thickness, Δz, is proportional to the RF pulse bandwidth, Δf, and inversely proportional to the slice select gradient amplitude, Gz. That is, for a given bandwidth RF pulse, a stronger slice select gradient will excite a thinner slice as compared to a weaker slice gradient, as shown in Fig. 1.13. The center frequency of the RF pulse is set to match the precessional frequency of protons at the location of the desired slice, as shown in Fig. 1.14. Therefore, the slice select gradient localizes the signals in one direction.
The gradient creates a variation in magnetic field, which creates a variation in precessional frequency by the relationship described by the Larmor equation. This schematic shows how slices can be selected in different locations (z1, z0, z2) by matching the frequency of the RF excitation pulse to the precessional frequency (ω1, ω0, ω2) of spins in a specific location
Spatial Encoding
The next step in the process of image formation is to localize the positions of the protons within the slice (that is, in the in-plane x- and y-directions). Two gradients are applied, one (Gy) in the phase-encoding direction (by convention, the y-direction) and one (Gx) in the frequency-encoding direction (by convention, the x-direction). The latter is also called the “read-out gradient”, because the signal is read or received during its application. When a phase-encoding gradient, Gy, is applied, the frequencies in the y direction are changed spatially. Similarly, when Gx is applied the frequencies in the x direction are changed spatially. This combination of gradients provides the basis for the application of the inverse two-dimensional Fourier transform (M(kx, ky)) which reconstructs the final image as shown in Eq. 1.7
where kx and ky are spatial frequencies in the x and y directions respectively. The time integrals of the applied gradients, Gx and Gy, control the sampling of spatial frequencies, kx and ky, as shown in Eqs. 1.8 and 1.9;
These relationships between gradient pulses and k-space sampling will be described in more detail in later chapters.
Signal Read-out
The MRI signal is sampled during the Gx gradient application. The time-varying signal is digitized and stored in a two-dimensional data matrix known as k-space. As will be described in detail in the following chapters, spatial frequencies kx and ky define the axes of k-space. The sampled signal typically fills k-space one row, or kx-line, at a time. Low spatial frequency data fill the center of k-space and provide information about general shapes and contrast in the image, while high spatial frequency data are stored in the periphery of k-space that represents image resolution and detail.
Image Reconstruction
In its most basic form, MR images are reconstructed by applying a two-dimensional Fourier transform to the raw k-space data row by row and column by column. This process effectively decodes the spatial position of the excited hydrogen nuclei based on variations in frequency and phase [2]. In the reconstruction process, a relative signal intensity value for each image voxel, or volume element, is calculated based on the strength of the signal from the hydrogen nuclei contained in the corresponding volume of tissue within the patient. The result is the final image, which illustrates spatial anatomical relationships and grayscale contrast between tissues based on the magnetization behavior of hydrogen within a slice of tissue [5]. Additional details on the process of image reconstruction are provided in the following chapters.
References
Slichter CP. Principles of magnetic resonance. 3rd ed. Berlin: Springer-Verlag; 1990.
Nishimura DG. Principles of magnetic resonance imaging. 11th ed. Raleigh: Lulu.com; 2010.
Elster AD, Burdette JH, editors. Questions and answers in magnetic resonance imaging. 2nd ed. Maryland Heights: Mosby; 1994.
Bushberg JT, et al. The essential physics of medical imaging. Philadelphia: Lippincott Williams & Wilkins; 1994.
Bernstein AM, et al. Hand book of MRI pulse sequences. Amsterdam: Elsevier; 2004.
Sprawls P. Physical principles of medical imaging. Philadelphia: Lippincott Williams & Wilkins; 1987.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Kolipaka, A. (2015). Signal Generation. In: Syed, M., Raman, S., Simonetti, O. (eds) Basic Principles of Cardiovascular MRI. Springer, Cham. https://doi.org/10.1007/978-3-319-22141-0_1
Download citation
DOI: https://doi.org/10.1007/978-3-319-22141-0_1
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-22140-3
Online ISBN: 978-3-319-22141-0
eBook Packages: MedicineMedicine (R0)