Abstract
The intestine is one of the most rapidly proliferating tissues in the human body and serves as an important model to understand tissue stem cell function and homeostasis. The absorptive function of the intestine is served by the epithelium lining the villi, composed of differentiated cell types, which are replenished by the proliferative crypts that contain the intestinal stem cells. The concept of the intestinal stem cell niche was proposed almost 40 years ago and two different models of the intestinal stem cell location have since been developed. The characterization of genetic markers has allowed the identification of different stem cell populations in the intestinal epithelium and provided data supporting both these models. Recent studies of the behavior and interaction of intestinal stem cells have also revealed an important role of the intestinal stroma in maintaining the stem cells. More thorough characterization of the intestinal stem cell niche has allowed for the development of sophisticated methodologies to culture intestinal crypts ex vivo. Here we review various models of the intestinal stem cell populations and the interactions between them. The anatomical components and signaling pathways supporting the stem cell niche will also be described.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Intestinal Anatomy and Physiology
The mammalian small intestine and colon comprise the parts of the gastrointestinal system that perform the crucial function of nutrient absorption from food products digested in the stomach and also form an effective barrier against xenobiotics and microbes present in the gut lumen. In accord with the important function of the intestine for survival and the mechanical and chemical stressors that the intestinal epithelium is exposed to, the intestine is also one of the most rapidly proliferating tissues in the body. The entire small intestinal epithelium is capable of turning over in 3–5 days [1–3], compared to other tissues such as the skin with a turnover time of 40–56 days [4], or heart with a turnover time in years [5, 6].
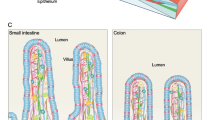
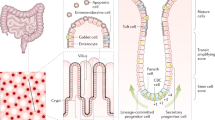
The luminal surface of the small intestine is made up of a layer of simple columnar epithelium organized into multiple finger-like projections called villi, which serve to increase its absorptive surface area. Between these villi lie the crypts of Lieberkühn, invaginations of the epithelial surface containing multipotent adult stem cells that maintain the proliferation and homeostasis of the small intestine. As these stem cells divide, they produce committed progenitors, known as transit amplifying cells, that continue to rapidly divide and move upward from the crypts to the villi where they differentiate into three major cell types: (i) enterocytes, the main absorptive cell type that comprise 80 % of the intestinal epithelium, (ii) enteroendocrine cells that produce hormones controlling intestinal function and metabolic homeostasis, and (iii) mucous-producing goblet cells that aid in the transport of material through the gut lumen [7]. The only cell type that does not undergo this upward migration is the Paneth cell, which remains at the base of the crypts within the intestinal stem cell niche , producing anti-microbial substances such as cryptdins, lysozyme and a multitude of signaling molecules (Fig. 1a). Unlike the other differentiated cell types, the Paneth cells do not cycle rapidly and can remain in the small intestinal crypts for 3–6 weeks. When the cells at the tip of the villus die, they are shed into the intestinal lumen and removed from the body [8]. The colon is similar to the small intestine except that it does not possess villi but has a flat epithelial surface and serves primarily to absorb water from the contents of the lumen. Furthermore, Paneth cells are only found in the ascending colon and it has thus been proposed that c-kit positive secretory cells may serve the same function in rest of the colon [9]. Immediately underlying the basement membrane of the epithelial layer is the lamina propria, which is a layer of connective tissue containing fibroblasts, myofibroblasts, nerves, blood vessels and lymphatic vessels. Besides providing structural support to the intestine, it also supplies blood to the epithelium and transports away absorbed nutrients from the intestinal lumen. We describe the self-renewal of the murine small intestine and the molecular pathways that regulate the differentiation of the stem cells into various epithelial cell types.
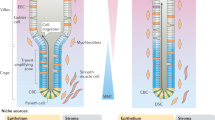
a Two different intestinal stem cell populations exist at the base of the crypts of Lieberkühn, the crypt base columnar cells and +4 position stem cells. These stem cells are surrounded by the intestinal stem cell niche, which is made up of Paneth cells interspersed between the crypt base columnar cells, as well as the surrounding stromal cells such as subepithelial myofibroblasts. This intestinal stem cell niche provides the correct signaling milieu in vivo for the controlled proliferation and differentiation of the stem cells. Wnt signaling is required for stem cell proliferation, while Notch signaling inhibits differentiation to the secretory lineage and are thus highest at the base of the crypt. Wnt signaling is also inhibited by BMP signaling which exists in an increasing gradient along the crypt-villus axis. Hedgehog signaling has diverse effects including increased BMP signaling, inhibited Wnt signaling and restricting niche-specific subepithelial myofibroblasts to the base of the crypt. b The stromal component of the stem cell niche plays a crucial role in most of the signaling pathways regulating the niche. Subepithelial myofibroblasts are sources of Wnt and R-spondins (Wnt agonists), as well as BMP antagonists such as gremlim1/2 and chordin-like 1. Their corresponding Wnt receptors, FZD and LGR5 can be found on the adjacent epithelial crypt base cells while BMP receptors are found at and above the +4 position of the epithelium. Other stromal cells also contribute by secreting Noggin (BMP antagonist) at the crypt base and BMP in an increasing gradient from the crypt to the villus. Similarly, Hedgehog is secreted by epithelial cells while its receptor, Patched, is found in the mesenchyme. This signaling is important to the localization of the subepithelial myofibroblasts and formation of the crypts. Notch-1 and Notch-2 receptors are found on both +4 stem cells as well as the crypt base columnar stem cells. Despite the fact that the two stem cell populations have different characteristics and expression profiles, significant plasticity exists within the small intestine, allowing for the inter-conversion of these populations
2 Intestinal Stem Cells
The renewal of each individual crypt is driven by a very small number of tissue stem cells . The first evidence for clonality of the human intestinal and colonic crypts and the stem cell derivation of all the epithelial lineages came from two studies. The first utilized in situ hybridization of a Y chromosome-specific probe on a XO/XY individual with familial adenomatous polyposis to demonstrate that each individual intestinal crypt possesses either XO or XY cells but not both. However, the villus epithelium comprises of a mixture of XO and XY cells [10], indicating that the villi derive from the stem cells of more than one crypt. The second study looked at female subjects heterozygous for a mutation on the X-linked gene, glucose-6-phosphate dehydrogenase (G6PD). Due to X-inactivation, these individuals are functionally mosaic and thus have a mixture of cells with low or high G6PD activity in the intestinal epithelium. Both the colonic and intestinal crypts however have only one phenotype, with no evidence of mixing [11]. More recently, a study utilizing R26R-Confetti;Lgr5-EGFP-CreERT2 mice shows that competition between stem cells at the crypt base for access to the stem cell niche maintains the stem cell population. Fate mapping studies using these mice demonstrate that crypts drift towards clonality within a period of 1–6 months. Stem cells further from the boundary of the niche experience a survival advantage and hence are more likely to colonize the crypt, consistent with the above observations [12, 13].
The key characteristics of stem cells are their capability to proliferate indefinitely to produce more stem cells, and their ability to differentiate into different cell types. Another characteristic of stem cells is quiescence that protects them from external chemical or physical stressors, as well as modifications of the genome from replicative errors or aging [14].
Two models have been proposed to explain the location and identity of small intestinal stem cells within the crypt, (i) the +4 position model and (ii) the stem cell zone model. The +4 model is based on the existence of a unique population of highly radiosensitive cells located 4 cells from the base of the crypt. This radiosensitivity has been theorized to be a safety mechanism to prevent stem cells from transmitting any DNA damage to their progeny [15]. These cells may also be highly sensitive to tamoxifen, which complicates interpretation of lineage tracing studies, as discussed later [16]. The stem cell zone model on the other hand argues that stem cells exist at the base of the crypt between positions 1–4 [17]. In vivo studies using lineage tracing and genetically engineered mice, as well as ex vivo studies using organoid assays (described below) have facilitated the identification of various markers for both of these populations.
For a rapidly proliferating tissue like the intestine, the adult stem cells have been theorized to be in two compartments, with one set remaining in a quiescent state as “back-up” while the other proliferates to maintain tissue homeostasis. Upon injury or degeneration of the active population, the quiescent population adapts its phenotype to replenish or support the proliferating cells [18, 19]. Evidence suggests that the +4 population of cells represents this quiescent stem cell population, while Lgr5, a cell marker described in greater detail below, marks the rapidly proliferating compartment.
2.1 In Vivo Assays for Identifying Intestinal Stem Cells
One of the most useful methods of identifying stem cell populations is to find a unique set of markers that can reliably distinguish them from other cell types. These markers facilitate the visualization, targeting and manipulation of stem cells and also allow sorting of these stem cells into pure populations for ex vivo analysis. One such analytical technique is lineage tracing whereby a label expressed in a single cell is transmitted to all of its progeny, allowing that single cell’s fate and behavior to be studied without affecting the normal function of the cell or its neighbors [20]. Alternatively, label-retaining experiments use a label that is incorporated into the nuclear material or cell membrane, and is lost with successive cell divisions, allowing the cycling rate of specific cell types to be quantified.
Multiple strains of genetically engineered mice have been generated to test the effects of specific genes on intestinal homeostasis and function in vivo. These studies have largely benefited from the use of Cre recombinase, which allows for precise gene regulation by site-specific recombination between loxP recognition sites [21]. Moreover, use of tissue specific promoters or inducible Cre also permits spatio-temporal regulation of gene expression [22, 23].
2.2 Ex Vivo Assays for Identifying Intestinal Stem Cells
Recent improvements in in vitro culturing techniques of the intestinal epithelium have also contributed to our understanding of the intestinal stem cells and its niche . Short-term primary culture of the intestine has been carried out for many years [24, 25]. However, while these in vitro cultures were useful for imaging and experimentation, they could not truly recapitulate the in vivo physiology, behavior or proliferative potential of the intestinal stem cells. Two techniques have been developed to overcome this limitation. In the first, intestinal epithelial cells are cultured without any mesenchymal support, while the second utilizes an air-liquid interface and includes both epithelial and stroma elements.
The first method utilizes matrigel, a mixture of extracellular proteins includings laminins and collagens to provide both 3-dimensional support and signaling factors for cell growth. Addition of R-Spondin1 (a Wnt agonist), epidermal growth factor and Noggin (a bone morphogenic protein (BMP) antagonist) into the culture medium mimics the normal intestinal crypt environment and signaling milieu. Using this method, isolated intestinal crypts without mesenchyme and even single cells can be successfully cultured ex vivo for more than 8 months and can be serially replated without loss of replating efficiency [26]. These “organoids” are made up of a single layer of villus-like epithelium, forming multiple crypt and villus-like structures around a central lumen into which apoptotic cells are shed, with their morphology closely resembling normal intestinal physiology. All terminally differentiated cell types of the intestine can be found throughout the organoid. Follow up studies on this technique show that increased organoid forming efficiency, with increased self-renewal and reduced differentiation of the single cells, can be achieved by adding a GSK3 inhibitor, CHIR99021, and high concentrations (1–2 mM) of valproic acid [27].
An air-liquid interface is introduced in the second technique to improve oxygenation of the cells, while a collagen gel and mesenchymal cells are included in the culture instead of individual signaling molecules to simulate the in vivo 3-dimensional intestinal stem cell niche . Cultures from murine neonatal intestine are able to grow up to 350 days in culture as cystic structures with representation of all the differentiated cell types of the intestine. Crypt-like structures and villus-like projections are also observed. There is however a decrease in proliferative capacity with time and with increasing age of the intestine, either due to developing mesenchymal defects with age or intrinsic defects in the intestinal stem cells. The induction of Wnt signaling by an exogenous R-spondin fusion protein improves proliferation and also increases the presence of putative stem cells [28].
Both these techniques capture the importance of the intestinal stem cell niche in the provision of structural support and an appropriate microenvironment in vivo for normal function of intestine stem cells. Numerous applications of this technology are currently being explored for therapeutic benefit such as creating patient-specific cystic fibrosis disease models from biopsy samples or growing replacement tissue for patients with diseased or dysfunctional intestines [29, 30].
2.3 Cell Specific Markers of the +4 Position
The initial discovery of the +4 position crypt cell notes its distinguishing characteristics, such as label-retention, slower cell cycling time, extreme radiosensitivity, involvement in post-injury regeneration and role as an origin for crypt cell migration [31, 32]. Further study of its label-retaining phenotype reveals the many safeguards these cells possess specifically to protect their genomic integrity, such as selective segregation of the DNA template and rigorous apoptotic pathways. By selectively segregating their DNA template, the newly synthesized strand (and any introduced mutation) is passed on to the proliferating and differentiating progeny and eventually lost while the original DNA strand remains in the stem line, known as the “immortal strand” hypothesis [33]. Meanwhile, the apoptotic pathways ensure that any errors in the template strand from environmental stressors are quickly eliminated [34]. Several markers of this intestinal stem cell population including Bmi 1, Dcamkl-1, mTert and Hopx have been reported.
Bmi1, a component of Polycomb group repressor complex, is required for the self-renewal of hematopoietic and neural stem cells. Given its broad tissue distribution, it was proposed to function in regulating self-renewal of other tissues. In situ hybridization and Bmi1-EGFP reporter mice confirm expression of Bmi1 in the small intestinal crypts, specifically at the +4 position. Lineage tracing using Bmi1-IRES-CreERT2;R26R-LacZ mice also indicates that Bmi1+ cells are both self-renewing, and capable of differentiation into all terminal cell types of the small intestine, fulfilling the criteria of stem cells [35, 36, 61]. The Bmi1+ cells can also generate epithelial organoids in culture. Follow-up studies using Bmi1 as a specific marker for these cells confirm that a few Bmi1-expressing cells retain label and are in fact slow cycling. Furthermore, stimulation of the Wnt pathway in these cells through β-catenin induction causes adenoma formation while ablation of the Bmi1+ cells through cell-specific expression of diphtheria toxin leads to a loss of intestinal crypts, capturing the importance of these stem cells for crypt proliferation [35, 36]. However, while Bmi1 expression is prominent in the duodenal crypts, it is poorly expressed in the ileal crypts [36], suggesting the existence of other stem cell populations that do not express Bmi1.
Doublecortin and Ca2+/calmodulin-dependent kinase-like 1 (Dcamkl-1), a microtubule-associated protein kinase, is also highly expressed around the +4 position (49 % of DCAMKL-1+ cells are in the +4 position and only 4 % of crypts contain DCAMKL-1+ crypt base columnar cells). Co-staining of DCAMKL-1 with proliferating cell nuclear antigen (PCNA) verifies its expression in quiescent PCNA negative, label-retaining cells [37]. While the expression of DCAMKL-1 is lost in the proliferative crypts 84 h after a dose of ionizing radiation greater than 8 Gy, its presence can be detected 7–10 days later, indicating that these cells and their appropriate niche can be regenerated within the intestine. Furthermore, pulse labeling with BrdU during this regeneration period shows that while BrdU labeled cells are present in the upper crypt and villi at day 7, only rare cells in the lower crypts are still labeled on day 10. On day 10, cells at the +4 position co-express BrdU and DCAMKL-1, but do not express PCNA, indicating a role of the DCAMKL-1+ population in regeneration after injury before their return to quiescence [37]. DCAMKL-1 positive cells isolated from a mouse small intestine are capable of generating spheroids that when dissociated and injected into nude mice form nodular structures that stain positive for glandular (cytokeratin 14), secretory (Math1) and epithelial progenitor/stem cell markers (Msi-1). DCAMKL-1 as a stem cell marker is still controversial, as lineage tracing studies have not been reported. Co-expression of DCAMKL-1 with tuft cell differentiation markers [38] and its expression in a subset of enteroendocrine cells indicates that its expression may not be limited to intestinal stem cells [39].
Other cell specific markers proposed to identify +4 stem cells include Hopx and Tert. Hopx is an atypical homeobox gene and analysis of Hopx-LacZ knock-in mice shows expression of Hopx in the crypts along the entire length of the intestine with the strongest expression at +4 position [40]. The location of Hopx+ cells in the intestinal crypt is distinct from the Lgr5+ cells. After irradiation, Hopx+ cells show label-retention following pulse labeling with BrdU and 14 days of regeneration. Moreover, lineage tracing studies using Hopx-IRES-ERCre;ROSA-LacZ mice show that Hopx+ cells repopulate the entire length of the crypt and villus and are capable of producing all the differentiated cell types of the intestine. Progeny of the Hopx+ cells persist for at least 13 months in the crypts despite the entire intestinal epithelial turnover rate being 5 days. Hopx-IRES-ERCre/ROSA-mTmG mice also confirm these results [40]. Most importantly, this study demonstrates a bidirectional relationship between the active and quiescent stem cells in their niches as discussed later.
The Tert gene encodes telomerase reverse transcriptase, a protein required to maintain telomere length and thus protect against cellular senescence in stem cells. Slow cycling cells expressing mTert-GFP localize to the +4 position and have strong overlap with the Bmi1+ cells but are distinct from the Lgr5+ cells [41]. Similar to Bmi1 and Hopx, lineage tracing studies using mTert-CreER;ROSA26 LacZ mice show that mTert expressing cells contribute to the regeneration of the intestinal epithelium and production of all four differentiated cell types after injury. However, in contrast to the original model, these cells are described to be radiation-resistant [41]. These studies suggest that mTert may mark an independent, quiescent and radiation resistant pool of intestinal stem cells .
An alternative method to label quiescent or very slowly dividing cells uses transient transgenic expression of a fluorescent histone (H2B-GFP). This marks a slow-cycling population of cells at the +4 position of the small intestinal crypt that do not express the proliferation markers Ki67 and phospho-histone H3. Interestingly and consistent with the findings in mTert-expressing cells, this population is also radiation resistant [42].
2.4 Cell Specific Markers of the Stem Cell Zone Model
Leucine-rich repeat-containing G-protein coupled receptor 5 (Lgr5) is the first specific genetic marker for the stem cell zone model [43]. Wnt signaling is known to be important in intestinal self-renewal, and Lgr5 has been identified as an intestinal Wnt/β-catenin target gene. Lgr5 is expressed only in proliferating, slender cells at the base of the intestinal crypt in between Paneth cells and below the +4 cells, termed crypt base columnar (CBC) cells. Lineage tracing experiments demonstrate that Lgr5 expressing cells are both actively self-renewing and pluripotent, suggesting they could be the intestinal stem cell [43]. However, more recently, Bulavin and co-workers have argued that all lineage tracing studies in the intestine are complicated by the finding that the dose of tamoxifen required to activate CreER also kills the +4 cells. In this model, the committed progenitor CBC/Lgr5+ cell may be recruited to become a +4 stem cell after radiation or tamoxifen damage to the +4 cells. This would lead to marking of +4 cells by Lgr5-driven Cre [16]. Thus, studies with Lgr5:CreER and tamoxifen may in fact also be studying the biology of the +4 cell.
The identification of Lgr5 as a marker for the crypt base columnar cells allowed for more in depth study on their properties and has revealed that these cells represent a rapidly proliferating population that does not possess the protective mechanisms for their genome as found in the +4 cell [37, 43]. The Lgr5+ crypt base columnar cells are actively cycling, as evidenced by their labeling kinetics and expression of proliferation markers Ki67 and phospho-histone H3. These cells are more radiation resistant compared to the label-retaining cells in the +4 position [43]. Further studies have shown that unlike the +4 position cells, Lgr5+ cells are not label-retaining and undergo symmetrical division with random segregation of chromosomes, implying that the “immortal strand” hypothesis is not a protective mechanism in these cells [44, 45]. This may be more consistent with Lgr5 cells being committed long-term progenitor cells, rather than immortal stem cells .
One theory of stem cell homeostasis is that the stem cells divide asymmetrically, producing one stem daughter cell, and one differentiating daughter cell (also known as the transit amplifying cell) to self-renew and produce differentiated progeny with each division. In the Lgr5 + putative stem cells however, homeostasis appears to be controlled by neutral drift dynamics instead. In the neutral drift theory, during symmetrical division the parent stem cell produces two identical daughter cells with potential to follow either fate resulting in two stem cells, two differentiated transit-amplifying cells or one of each. This being so, the regulation of the crypt follows a stochastic model in which the stem cells adopt fates depending on their environment such as the loss of neighboring stem cells or overcrowding within the niche [12, 13, 46]. This model implicates the stem cell niche as a key regulator of stem cell homeostasis instead of simply intrinsic properties of the stem cell itself.
Based on the gene expression signature of the Lgr5+ cells, other proposed stem cell markers have been identified, including various Wnt target genes such as Ascl2 (Achaete scute-like 2) [47], Tnfrsf19 (TNF receptor superfamily member 19), Ring finger nuclease 43 (Rnf43)/Zinc and ring finger 3 (Znrf3) [48] as well as Olfm4 [49]. Ascl2 is a basic helix loop helix transcription factor that together with β-catenin and Tcf4 regulates the expression of various genes including Lgr5 [50]. Ectopic expression of Ascl2 in the intestinal epithelium induces hyperproliferation. Rnf43 and Znrf3 are E3 ubiquitin ligases that negatively regulate the Wnt /β-catenin pathway by ubiquitinating Wnt receptors Frizzled and LRP6 on the cell surface. This ubiquitination targets the Wnt receptors for internalization and lysosomal degradation. Demonstrating their importance in regulation of crypt homeostasis, deletion of both Rnf43 and Znrf3 genes in mice results in greater numbers of proliferating cells, increased levels of β-catenin in these hyperproliferative cells, enlarged crypts and adenoma formation [48, 51]. Furthermore, loss of function mutations in RNF43 are found in several human cancers [52].
2.5 Other Stem Cell Specific Markers
Lrig1 (Leucine rich repeats and immunoglobulin like domains 1) is a transmembrane protein and a negative feedback inhibitor of ErbB signaling. Lrig1 positive cells are located primarily at positions 1–5, thus it was initially reported as a marker for the crypt base columnar stem cells [53]. However, a study using Lrig1 reporter (Lrig1-IRES-CreER;ROSA-LacZ) mice and Lrig1-specific antibody shows these cells to be distinct from the Lgr5+ population [54]. Lineage tracing studies also demonstrate that Lrig1+ cells can repopulate the crypt and the villus and generate all the differentiated progeny. Knock out of APC in the Lrig1-expressing cells also leads to adenoma formation, demonstrating their stem cell potential (keeping in mind the caveat regarding the effects of tamoxifen on stem cell dynamics). Moreover, BrdU labeling and Ki67 staining shows this cell population to be slowly cycling and less proliferative than the Lgr5+ cells, but more proliferative than the Bmi1 and mTert expressing cells. The authors propose that these cells serve as an intermediate population between the stem cell zone and the +4 position models [54].
Musashi-1, an RNA-binding protein, is expressed in both the +1 and +4 cells of the intestinal crypts and may therefore serve as a general marker of the intestinal stem cell [55–57].
Evidence has emerged of extensive plasticity of cell populations in the intestine and of interactions between these different putative stem cell populations [58]. As described above, long-term lineage tracing studies have demonstrated that mTert, Bmi1 and Hopx are bona-fide intestinal stem cell markers and that the Bmi1+ and mTert+ cells reside at +4 position whereas Lgr5 marks the mitotically active stem cells that are distinct from the mTert, Bmi1 and Hopx+ population. Lgr5+ cells are sensitive to Wnt perturbations, ablated by irradiation and contribute to homeostatic regeneration. The finding that Lgr5 marks a distinctive, highly proliferative population of the small intestinal and colonic stem cells has challenged the existence of quiescent stem cells. Specific elimination of Lgr5 expressing cells by knocking a human diphtheria toxin receptor gene into the Lgr5 locus does not change intestinal epithelial homeostasis, implying either that this cell population is not essential for intestinal function or that other cell populations are capable of compensating for its loss [59]. The only notable difference upon ablation of Lgr5+ cells is the increase of enteroendocrine cells in the crypts [35]. Following cessation of diphtheria treatment, the Lgr5+ cells rapidly regenerate in the intestinal crypts in vivo and in organoid cultures. However, depletion of Lgr5+ cells during radiation induced damage or Wnt pathway inhibition leads to the complete loss of intestinal architecture [59, 60]. This implies that these cells are not essential for normal intestinal homeostasis but are required for regeneration of intestinal epithelium following damage.
Tian et al. demonstrate that Bmi1+ cells can give rise to Lgr5+ cells in the small intestinal crypts after ablation of the Lgr5+ population under both normal conditions and during post-injury regeneration [35]. An independent study also demonstrates that Bmi1 and Lgr5 mark two functionally distinct intestinal stem cells in vivo [61]. Interconversion between Hopx expressing cells and Lgr5 expressing cells occurs as demonstrated by gene profiling and single cell organoid cultures [40, 61]. The Hopx positive +4 cells represent the quiescent population of reserve intestinal stem cells that are resistant to irradiation. Consistent with this model, a single Hopx expressing cell is able to generate rapidly proliferating Lgr5+ cells. Conversely, isolated single Lgr5 expressing cells from Lgr-EGFP-ERCre;Hopx:LacZ mice, cultured ex vivo to form organoids, are initially negative for Hopx, but express Hopx after 7 days. β-Gal expression (marking Hopx+ cells) also overlaps with GFP expression (derived from the Lgr5 locus) in the organoids derived from crypts of these mice [40]. Further in vivo fate mapping studies of the Lgr5 cells with Lgr-EGFP-ERCre;Hopx:LacZ;R26mTmG/+ mice provide evidence that the slow cycling intestinal stem cells at +4 position dynamically interconvert with the rapidly cycling Lgr5+ cells in the crypt base.
Following intestinal damage, certain non-stem cell populations are also able to regain stem-like properties. For example, Dll1+ secretory precursor cells normally produce short-lived secretory clones but are capable of reverting to organoid-producing Lgr5+ stem cells in vitro upon Wnt stimulation, as well as reverting to Lgr5+ stem cells in vivo upon irradiation [62]. Similarly, label-retaining Paneth or enteroendocrine precursors are also capable of replenishing the stem cell population and differentiating into multiple lineages under regenerative and post-injury conditions [63].
Taken together, these studies indicate that the intestinal epithelium possesses a highly complex signaling network to ensure maintenance of multiple cell populations with differing proliferative capabilities and differentiation states, and to allow the transition between these populations in response to insults or damage. This functional redundancy complicates the study of the normal physiology of the intestine because experimental techniques can be biased towards or against specific populations. For example, as mentioned above, +4 position stem cells are killed preferentially by tamoxifen in lineage tracing experiments causing them to be replaced by the Lgr5+ stem cells, which thus become over-represented. A question that is also raised by these discoveries is what signaling pathways regulate this plasticity and which components of the stem cell niche are responsible.
3 Lineage Specification of Intestinal Stem Cells
Intestinal homeostasis requires appropriate lineage specification of the intestinal stem cells . As mentioned earlier, intestinal stem cells differentiate into four major cell types that populate the intestinal epithelium: the absorptive enterocytes and the three secretory cell types—enteroendocrine cells, goblet cells and Paneth cells. The fate of these cell types is determined by various molecular signals.
Enterocytes, also termed columnar cells, constitute more than 80 % of the intestinal epithelium. Caudal-related homeobox transcription factor (Cdx1), thyroid hormone and Kruppel-like factor (Klf4) regulate the differentiation of enterocytes [64–68]. Notch signaling plays an important role in regulating the differentiation of secretory versus absorptive cell lineages. A basic helix-loop-helix transcription factor, Math1/ATOH1, which is regulated by the Delta-Notch signaling pathway regulates the development of a common secretory cell progenitor [69, 70]. Further differentiation of secretory precursors to enteroendocrine cells involves two other basic helix-loop-helix transcription factors Neurogenin 3 and NeuroD, as well as a pancreatic-duodenal homeobox 1 gene (Pdx1) [71, 72]. Spdef, an Ets-domain transcription factor and Sox9, an HMG-box transcription factor, are both Wnt target genes and promote terminal differentiation to goblet and Paneth cells [73–75].
4 The Intestinal Stem Cell Niche
The intestinal stem cell niche is essential for the maintenance of intestinal homeostasis by providing a suitable microenvironment and signaling milieu for the self-renewal and differentiation of stem cells [76–79]. The stem cell niche has multiple components, which may be divided into two separate parts: a specialized and a non-specialized niche. The specialized niche consists of the basement membrane and one or a few epithelial cell types that lie next to and locally regulate the stem cells. The non-specialized niche is comprised of the mesenchymal cells that lie in the lamina propria underneath the basement membrane and provide broader regulation of the stem cells. These include the mesenchymal stem cells, fibroblasts, myofibroblasts, vascular endothelium, lymphatic vessels, adipocytes, neurons and blood cells [80]. Two key signaling pathways controlling the intestinal stem cell niche are Wnt and Notch , while additional signaling pathways such as Bmp and Hedgehog are also involved.
4.1 Wnt Signaling
Wnt /β-catenin signaling is integral to normal intestinal homeostasis and is essential for the proliferation of the epithelial cells in the crypts [81]. Wnts are autocrine or paracrine signaling proteins essential for embryonic development, cell proliferation and differentiation. They are highly conserved across species and can stimulate multiple downstream pathways including the Wnt/β-catenin pathway, planar cell polarity pathway and Wnt /calcium pathway. Wnts act as ligands for the Frizzled family of receptors and also interact with co-receptors such as lipoprotein receptor-related proteins, (LRP5/6) and receptor tyrosine kinases (Ryk and Ror and Tyrosine-protein kinase-like 7 (PTK7)). Binding of Wnts to their receptors leads to the recruitment of Disheveled to the Frizzleds and this plays a crucial role in determining which downstream pathway is activated. In the Wnt/β-catenin pathway in the absence of Wnt ligand, a degradation complex composed of an Axin and APC scaffold facilitates the phosphorylation of β-catenin by GSK3 and CK1, which then targets β-catenin for ubiquitination by β-TrCP and proteosomal degradation [82]. Binding of Wnts to their receptors inhibits GSK3 and prevents the degradation of β-catenin leading to its cytoplasmic accumulation and eventual translocation to the nucleus. In the nucleus, β-catenin acts as a co-activator of transcription factors, TCF and LEF, triggering the transcription of numerous Wnt target genes. These target genes are then responsible for the migration and proliferation of the intestinal stem cell compartment [83, 84]. It should be noted that alternative pathways downstream of Wnt have been proposed [84].
Functional studies have demonstrated the importance of the Wnt/β-catenin pathway in regulating proliferation and differentiation of intestinal stem cells. Knockout of the downstream β-catenin effector Tcf-4 in mice prevents the proliferation of cells in the inter-villus region of the small intestine resulting in lethality within 24 h of birth [85]. Knockdown of β-catenin itself results in a similar loss of intestinal architecture and function [86, 87]. Inhibition of the Wnt pathway upstream using a Wnt inhibitor, Dkk1, confirms these results. Dkk1 interacts with LRP5/6 causing its internalization, thus inhibiting the interaction of Wnts with Frizzleds and LRP5/6 on the cell surface. Homozygous intestinal epithelium-specific expression of Dkk1 results in the development of grossly abnormal intestines with shorter and fewer villi as compared to non-transgenic controls [88]. A dramatic reduction in the size and number of crypts, goblet cells, enteroendocrine cells and Paneth cells, demonstrates the importance of Wnts for both proliferation and differentiation of the secretory lineage [88]. Transient adenoviral expression of Dkk1 in adult mice produces a similar phenotype of reduced proliferation in the small intestine and colon and progressive loss of crypts, villi and glands [89]. Finally, small molecule inhibitors of PORCN, which block Wnt secretion, also produce a lack of proliferation in the small intestine [60]. Thus, Wnt production and Wnt signaling is essential for the proliferation of the intestinal stem cell compartment.
Conversely, too much Wnt/β-catenin activity is detrimental. Activation of the Wnt signaling pathway by overexpressing Wnt agonist R-spondin 1 results in massive proliferation of intestinal crypts [28]. ApcMin/+ mice carrying a mutation in one allele of Apc spontaneously develop multiple adenomas in the intestinal epithelium, mimicking the human disease, familial adenomatous polyposis, caused by the truncation of APC. A key difference between the human disease and murine model is that the polyps are predominantly colonic in humans but present in the small intestine in mice [90]. Further study of these ApcMin/+ mice has also shown that different APC mutations result in different levels of activation of the Wnt pathway and hence different degrees of polyposis, indicating the fine control of Wnt signaling on phenotype [91, 92]. Activating the Wnt signaling pathway while simultaneously inhibiting the BMP pathway in normal human intestinal epithelial crypt cells increases their proliferation and induces a gene expression profile similar to that of crypt-base columnar cells [93]. Conversely, stimulation of Wnt signaling by expression of a Lef1/β-catenin fusion protein in progenitor cells in the small intestine of a chimeric mouse results in apoptotic cell death only of the cells expressing Lef-1/β-catenin. Cells without Lef1/β-catenin expression show normal apoptosis, and the intestine as a whole is morphologically and histologically normal [94].
Wnts are also essential for intestinal proliferation ex vivo. R-spondin1, a Wnt sensitizer, is an essential component for culturing intestinal organoids [26, 28]. R-spondins (RSPOs) are a family of four proteins containing thrombospondin repeats that enhance Wnt signaling by binding to LGR5 and its paralogs, LGR4 and LGR6, together with the E3 ubiquitin ligases RNF43/ZNRF3, thus inhibiting the activity of the latter [51, 95, 96]. This causes the accumulation of Frizzled receptors on the cell surface, and hence an increased sensitivity to Wnts. Chromosomal translocations resulting in increased expression of RSPO2 and RPSO3 have been identified in a number of human cancers including colorectal cancers. Consistent with their importance in the Wnt /β-catenin pathway, RSPO fusions are only found in colorectal cancers that do not have APC or β-catenin mutations [97]. While the role of Wnts in regulating intestinal homeostasis is well established, the cells making R-spondins and Wnts in the stem cell niche are still being defined [60].
One important conclusion from these studies is that the level of Wnt signaling in the small intestinal crypts is carefully modulated in normal homeostasis such that only cells which receive optimal amounts of Wnt will be able to survive and proliferate [98]. Excessive Wnt signaling results in unrestrained proliferation and neoplastic growth, indicating the existence of a complex regulatory network in vivo that maintains precise levels of Wnt signaling and therefore normal activity of the intestinal stem cells .
4.2 Notch Signaling
The Notch pathway plays a central role in cell fate decisions and differentiation of the intestinal epithelium. Notch is a single transmembrane receptor that undergoes proteolytic cleavage by γ-secretase upon ligand binding, freeing an intracellular domain (NICD) that translocates to the nucleus. The NICD then binds to the transcription factor CLS (or CBF1) to regulate transcription. There are four Notch receptors and several ligands, such as Delta-like and Jagged in mammals.
Notch signaling mostly works at very short distances, such as through contact of adjacent cells or by expression of ligand and receptor on the same cell [99, 100]. Studies using lineage tracing have confirmed the endogenous expression of Notch-1 and Notch-2 receptors specifically in the crypt stem cells , at both the +4 position and crypt base (Fig. 1b). Notch signaling is also active in the intestinal crypt stem cells and progenitors, but not in any of the three differentiated secretory cell types [101]. Similar to the Wnt pathway, Notch signaling is essential for maintaining the undifferentiated and proliferative state of the crypts. Labeling of all the cell types in lineage tracing experiments demonstrates activation of the Notch signaling in the adult intestinal stem cells [102].
The Notch pathway is also a key regulator of absorptive versus secretory cell fate decisions in the intestine. Notch signaling stimulates the expression of Hairy/Enhancer of Split (Hes1), which then inhibits the function of several basic helix-loop-helix transcription factors including Math1, which is critical for differentiation of the secretory lineage [103, 104]. Constitutive activation of the Notch1 receptor in the intestinal epithelium using the Villin promoter results in postnatal lethality, 3 days after birth. The mice have grossly abnormal intestinal architecture with impaired differentiation of the secretory lineage. Their intestines lack goblet cells and have reduced enteroendocrine and Paneth cells, but increased numbers of proliferating intestinal progenitors. This is accompanied by upregulation of Hes1 and downregulation of Math1 and neurogenin-3, while the components of the Wnt pathway such as β-catenin nuclear translocation and levels of Tcf4 or Lef1 are not affected [100].
Consistent with the importance of Notch signaling in promoting absorptive cell differentiation, inhibition of the Notch signaling pathway either through conditional knock out of the common downstream transcription factor CSL, or by using a γ-secretase inhibitor, causes a phenotype opposite to the constitutive Notch1 knock-in. In this case, proliferative crypt cells terminally differentiate to goblet cells, and proliferation of the intestinal epithelium ceases. The Paneth cell and enteroendocrine cell numbers and location remain normal and Wnt signaling remains active [105]. Likewise, conditional inactivation of both the Notch1 and Notch2 receptors also results in complete conversion of the intestinal epithelial cells to goblet cells [106]. The Hes 1 −/− mice also have more goblet cells and less enterocytes compared to wild-type controls, but show no difference in the proliferation of the intestinal precursors [103]. Inhibition of the Notch pathway by deletion of both Notch ligands Dll1 and Dll4 confirms these findings, with the complete differentiation of progenitors into goblet cells and loss of the proliferative crypt compartment [106]. Staining for Olfm4 using in situ hybridization also shows the absence of crypt base columnar stem cells in these mice. Taken together, these studies indicate that the Notch pathway is essential for the balance between proliferation and appropriate differentiation in the intestine crypt.
4.3 Bone Morphogenic Proteins (BMP)
BMPs, originally discovered to induce bone formation, belong to a family of growth factors that are integral to the normal development of various tissues. BMP4 is expressed in the intravillus mesenchyme of adult mice. The BMP receptor Bmpr1a is expressed highly in +4 position stem cells and in an increasing gradient along the crypt-villus axis but not in the proliferating cell zone [107] (Fig. 1a). This BMP signaling axis is believed to inhibit intestinal stem cell proliferation and self-renewal, thus maintaining the highly proliferative stem cell population only at the base of the crypts and promoting differentiation as cells move up the crypt. Consistent with this theory, expression of the BMP antagonist Noggin under the Villin promoter leads to increased proliferation causing the development of ectopic epithelial invaginations containing proliferating cells that later develop into crypt-villus units. These crypt-villus units are grossly normal, with all terminally differentiated cell types. After several months, these mice develop intestinal polyps characterized by branched villi with dilated cysts similar to the human disease, juvenile polyposis [108]. Studies inhibiting BMP signaling by conditionally inactivating the BMP receptor Bmpr1a confirm this phenotype and suggest cross-talk with the Wnt pathway by inhibition of β-catenin activity [107, 109]. Likewise Smad4 +/− mice also develop inflammatory polyposis lesions albeit at a later stage [110]. Studies in human colonic epithelium concur with the findings of BMP activity and interactions with the Wnt pathway [111, 112]. The requirement for Noggin to culture organoids ex vivo also reinforces the importance of inhibiting BMP signaling for self renewal and proliferation of intestinal stem cells [26].
4.4 Hedgehog
In the Hedgehog pathway, the binding of Hedgehog (Hh) ligands to Patched receptor relieves the inhibition of smoothened (SMO), leading to activation of Gli transcription factors, which then accumulate in the nucleus and control transcription of Hh target genes. The Hedgehog pathway, acting through Sonic (Shh) and Indian (Ihh) hedgehog proteins, is required for morphogenesis and embryonic development in a multitude of tissues. In the mouse, evidence of its role in the limbs, central nervous system [113] and foregut [114], among others, has been previously described. Ramalho-Santos and coworkers demonstrate that in the small intestine, both Shh (at very low levels) and Ihh are expressed at the base of the villi. Ihh is expressed throughout the epithelium in the colon and Shh is expressed mostly in the crypts (Fig. 1b). Importantly, they also demonstrate that hedgehog signaling is integral to anterior-posterior patterning, radial patterning, as well as proliferation and differentiation of the epithelial stem cells in the gastrointestinal tract. The intestines of Ihh −/− and Shh −/− mice show numerous intestinal abnormalities both gross and microscopic. Ihh −/− mice have smaller villi and less proliferation in the stem cell compartment, whereas Shh −/− mice show overgrown duodenal villi [115]. Inhibition of the Hedgehog pathway by expression of a pan-hedgehog inhibitor, Hhip, in the epithelial cells using the Villin promoter results in hyper-proliferation of the epithelium with formation of ectopic crypt-like structures and mislocalization of cells in the underlying stroma. This is accompanied by abnormally high and ectopic Wnt signaling [116]. This interaction of the Hedgehog pathway with the Wnt pathway is also demonstrated in rat colons. Expression of Wnt target genes is inhibited by ectopic expression of Ihh in vitro, and is restricted to the colon crypt base by Hedgehog signaling in vivo [117]. Besides inhibition of the Wnt pathway and a concomitant decrease in epithelial precursors, activation of Hedgehog signaling in the colon also increases epithelial Bmp signaling [118].
These signaling pathways are extremely complex and involve numerous interconnections and regulatory feedback loops. Numerous other signaling molecules and transcription factors have also been implicated including Forkhead [119], Yes-associated protein [120], Epithelial growth factor [26], Glucagon-like peptide-2 [121] and many more.
5 The Stem Cell Niche
5.1 Paneth Cells
In the small intestine, the Paneth cells are located at the base of the crypts, interspersed with the putative crypt base columnar stem cells just below the +4 position (Fig. 1a). The Paneth cell population is unique in that firstly, it is the only differentiated cell type which migrates down into the crypt instead of up into the villi, and secondly it has a much slower cycling time of around 60 days compared to the other differentiated cells with cycling times of 3–5 days [122]. The Paneth cells play a non-essential role in the physiology of the intestine. They secrete antimicrobial peptides that control gut flora as well as factors important for the development of the villus microvasculature [123]. Paneth cells also express components of the signaling pathways such as Wnt3, Wnt11, EGF, Tgf-α and Dll4, and therefore were originally hypothesized to be essential for intestinal proliferation and stem cell maintenance. The ability of the Paneth cells to support epithelial proliferation ex vivo is demonstrated by their enhancement of organoid forming efficiency when combined with Lgr5+ crypt base columnar stem cells compared to single Lgr5+ cells. Addition of exogenous Wnt3A in the absence of Paneth cells can also recapitulate this increase in organoid forming efficiency [124]. The adjacent location of the Notch ligand-expressing Paneth cells and the Notch receptor-expressing Lgr5+ crypt base columnar cells has been cited in support of a role for Paneth cells in Notch signaling [101].
Despite the role of Paneth cells ex vivo, the importance of their role in the intestinal stem cell niche in vivo has been called into question. Several different approaches to ablating the Paneth cells from the intestinal epithelium have not compromised intestinal proliferation. For example, even after more than 95 % of Paneth cells are killed by the expression of an attenuated diphtheria toxin gene under the Paneth cell-specific cryptdin-2 gene, no changes in tissue architecture, proliferation or differentiation are seen [125]. Growth factor independent 1 (Gfi1) deficient mice have no apparent Paneth cells, fewer goblet cells and more enteroendocrine cells and show normal crypt-villus structure and proliferation as assessed by both Ki67 staining and BrdU incorporation [126]. Deletion of Sox9 in the intestinal epithelium also results in the absence of differentiated Paneth cells in the intestinal crypts but these crypts are larger and full of proliferating cells. Sox9 −/− mice have normal body weight for up to 1 year [75]. The role of Sox9 in the development of Paneth cells and goblet cells was confirmed by an independent study [74].
Counter arguments to the redundancy of Paneth cells claim that the ablation of Paneth cells in many of the above techniques is incomplete with enough Paneth cells remaining to sustain the normal function of the intestine. For instance, Gfi1 −/− mice produced in two independent studies show Paneth cells are still present albeit in reduced numbers [124, 127]. Similarly, with cryptdin-controlled toxin expression and Sox9 deletion, depletion of Paneth cells is incomplete (95 % ablation) or temporary [124]. A more recent reassessment of this question utilizes an intestinal epithelial specific Atoh1 (Math1) knockout. Atoh1 is essential for differentiation of all secretory lineages upstream in their pathways, and also for Paneth cell survival. Therefore, this experiment maintains a complete and permanent absence of Paneth cells in the intestine. In crypts where Atoh1 is deleted, no Paneth cells are present, and Lgr5+ crypt base columnar cells occupy the whole crypt base with increased proliferation, normal differentiation and intact Wnt signaling [128]. These conclusions were replicated in a second study that additionally demonstrates that while loss of Paneth cells in vivo produces no phenotype, Math1-deficient crypts could not be cultured as organoids without exogenous Wnt supplementation [129].
These studies, taken as a whole, show that ex vivo Paneth cells supply important signaling factors such as Wnts, but that in vivo they are fully dispensable and not needed to sustain intestinal stem cells . This implies either functional redundancy, or that in vivo there is a different source of Wnts and other key factors that support the intestinal stem cell niche . This source has been proposed to be the underlying mesenchyme surrounding the niche [60].
5.2 Mesenchyme Provides Signals to the Intestinal Stem Cell Niche
The intestinal mesenchyme contains many different cell types that perform functions ranging from immune regulation to maintenance of proliferation and differentiation [130]. Recently, numerous studies have highlighted the role of the mesenchyme in regulating various signaling pathways essential for intestinal homeostasis.
Wnt signaling is required for the maintenance of intestinal homeostasis and various studies provide data to support the essential role of the stroma as the source of Wnts (Fig. 1b). Organoid cultures are one avenue of studying the role of the intestinal mesenchyme on stem cell function by allowing the isolation of components in an in vitro system. As described before, the Paneth cells produce Wnts and are therefore essential for the ex vivo culture of intestinal crypts, when supplemented with significant quantities of RSPO1. However, in the presence of mesenchymal cells, Wnt3 produced by Paneth cells is not required for ex vivo culture [131]. Furthermore, murine and human subepithelial myofibroblasts can support human intestinal organoid formation as well as increase the duration of ex vivo survival of organoids [132]. Epithelial and myofibroblast co-cultures implanted subcutaneously into mice form enteroids while crypt mono-cultures cannot, [132, 133] demonstrating that myofibroblasts can also provide support to isolated intestinal epithelium in vivo without supply of exogenous factors. This effect of myofibroblast co-culture holds true with whole crypts as well as single Lgr5+ stem cells.
In addition to Wnts , the stroma is also an important source of the Wnt agonists, R-spondins (Fig. 1b). The subepithelial myofibroblasts express high levels of R-spondins that are sufficient to sustain organoid growth even in the absence of exogenously supplied R-spondins [60]. Supplementing organoid or organoid/myofibroblast co-cultures with exogenous R-spondin does not enhance the enteroid forming efficiency, but leads to formation of larger and more complex enteroids in the co-cultures [133]. Similarly, co-culture of colonic crypts with immortalized colonic myofibroblasts also results in a significantly higher efficiency of colonoid formation than crypts alone or in co-culture with L cells or a cell line from a young adult mouse colon (YAMC) [134].
PORCN is a membrane bound O-acyl transferase that post-translationally palmitoleates all mammalian Wnts at a conserved serine residue, and is essential for the secretion and binding of Wnts to the Frizzled receptors [135–137]. Complete inhibition of Wnt secretion from the epithelium in the Porcn flox/flox /Villin Cre mice prevents the formation of organoids from the isolated crypts ex vivo unless supplied with exogenous Wnt3A, consistent with the critical role of Wnt signaling [60]. Importantly, intestinal proliferation, homeostasis and regenerative response to radiation damage are not affected in Porcn flox/flox /Villin Cre mice. This study provides evidence that the Wnts from the stroma are sufficient for maintaining the intestinal epithelium.
Mesenchymal cells are also an important source of BMPs. BMP4 is expressed in the intestinal intra-villus mesenchyme and phosphorylated SMAD1, 5 and 8 are observed in the nuclei of the villus epithelial cells indicative of paracrine BMP signaling to the adjacent villus epithelium [108]. This expression extends to the intercrypt mesenchymal cells, including those next to the +4 position putative stem cells (Fig. 1b). The stromal BMP signal appears to geographically restrict the crypt-forming region, since the crypts appear de novo at any place in the epithelium when BMP signaling is blocked. Noggin, a BMP antagonist, is expressed mostly in the submucosal layer at the crypt base and in lesser amounts near the +4 position [107] (Fig. 1b). Other BMP antagonists such as gremlin1/2 and chordin-like 1 are secreted by the myofibroblasts located near the crypts of the human colon [111]. Thus, the intestinal mesenchyme produces both activating and inhibitory signals that set up a gradient along the crypt-villus axis, limiting BMP activity at the crypt base and promoting proliferation of the epithelial stem cells (Fig. 1a).
Components of the Hedgehog signaling pathway are also expressed in both the epithelial and mesenchymal layers of the intestine. Specifically, Shh and Ihh are expressed in the epithelium while their receptors Ptch1 and Ptch2 as well as three downstream Gli transcription factors and target genes are expressed in the mesenchyme [116, 138] (Fig. 1b). This strongly suggests that Hedgehog signaling is paracrine from the epithelium to the mesenchyme. Furthermore, expression of a Hedgehog inhibitor in the intestinal epithelium results in mislocalization of the subepithelial myofibroblasts to the villus tips, near ectopic pre-crypt structures and proliferating epithelium [116]. Deletion of epithelial-specific Ihh also results in the absence of subepithelial myofibroblasts at the crypt base accompanied by abnormal proliferation and ectopic crypt-like structures of the epithelium. This phenotype is not observed when the Hedgehog pathway is inhibited in the epithelium itself by the deletion of Smoothened. This implies that the loss of myofibroblasts and their paracrine signaling is responsible for the abnormalities observed instead of autocrine Hedgehog signaling [139]. How this paracrine signal is transmitted from the producing mesenchymal cells to the receiving epithelial cells is still not fully understood but actin-based signaling filopodia called cytonemes have been proposed as the mechanism [140]. While more thoroughly studied in Drosophila systems, these cytonemes have been identified in vertebral development as well and may explain the precise spatial and temporal control of signaling in the intestinal stem cell niche [141].
5.3 An Integrated Model of the Intestinal Stem Cell Niche
The data in aggregate supports the conclusion that there are at least two different intestinal stem cell populations, quiescent and rapidly proliferating, which maintain normal intestinal homeostasis. Upon damage to the intestinal epithelium, these stem cells are capable of interconverting to replace the lost populations. The bidirectional signaling between the intestinal stroma and epithelium plays a key role in regulating the proliferation, differentiation and plasticity of the intestinal stem cells. As detailed above, subepithelial myofibroblasts are the main source of Wnt and R-spondins and are sufficient to sustain intestinal homeostasis even in the absence of epithelial Wnts. Receptors of the Wnt ligands (FZDs) and their agonists (LGRs), on the other hand, are abundant on the epithelial cells. Hedgehog signaling is also paracrine from the epithelium, which secretes Hedgehog , to the myofibroblasts bearing the receptors Patched. Hedgehog signaling regulates the localization of the subepithelial myofibroblasts at the base of the crypts and its inhibition leads to mislocalization of the subepithelial myofibroblasts to the tips of the villi. Additionally, BMP signaling, which allows for the differentiation of the epithelial cells by regulating Wnt signaling, is also bidirectional in an increasing gradient to the tip of the villi. Mesenchymal cells secrete BMPs, while their receptors are expressed by the +4 position cells. Taken together, subepithelial myofibroblasts and other stromal cells in the intestinal stem cell niche are source of the signals that regulate the proliferation, differentiation and plasticity of these intestinal stem cells (Fig. 1b).
5.4 Relevance of the Stem Cell Niche to Disease States and Therapeutics
Dysregulation of signaling in the stem cells or their niche can result in abnormal proliferation of the intestinal epithelium, which can then progress to cancer. Consistent with the role of Wnt signaling in intestinal homeostasis, one of the most common mutations found in human colon cancer is the inactivation of the APC gene, which results in stabilization of β-catenin and activation of additional pathways [142]. The loss of APC results in abnormal proliferation and neoplastic formation in the colon and is therefore integral to the development of familial adenomatous polyposis [143]. Dysregulation of the BMP signaling pathway caused by mutations of BMPR1A [144] and SMAD4 [145] results in juvenile polyposis, characterized by the formation of multiple polyps in the gastrointestinal tract and an increased risk of gastrointestinal adenocarcinomas. The cancer stem cell niche may also be integral for the maintenance of the cancer phenotype or the process of metastasis by cytokine and growth factor regulation, as well as by activation of inflammatory pathways [146, 147].
A profound understanding of the stem cell niche and its signaling pathways has permitted the long term ex vivo organoid culture of intestinal stem cells. Beyond their use in experimental analyses, these organoid cultures can be used to develop disease models from human patient biopsies for in depth study of disease pathophysiology as well as testing of novel therapeutics [29]. The ability to generate tissue-engineered small intestine for re-implantation into patients with short bowel syndrome or other intestinal diseases has also been explored. These tissue-engineered small intestines are created by attaching clusters of epithelial and mesenchymal cells isolated from the intestine onto scaffolds, and then implanting these loaded scaffolds into rats [148]. Human derived tissue-engineered small intestines have been produced by implantation into immunodeficient mice [149, 150]. In-depth knowledge of the intestinal stem cell niche can help improve the efficacy of these techniques [151].
6 Conclusion
The model of the intestinal stem cell and its niche is constantly evolving, as new pieces of the puzzle are discovered and fit into place. It appears that there are two different stem cell populations in the intestinal epithelium that control normal homeostasis—a rapidly proliferating population at the base of the crypt expressing markers like Lgr5, Ascl2, Olfm4, Rnf43 and Znrf3 and a quiescent population at the +4 position expressing Bmi1, Hopx and mTert. A great deal of plasticity exists within the intestine as these two stem cell populations are able to interconvert, and other intestinal epithelial cell types also have the potential to regain stemness post-injury. A complex array of signaling pathways involving both the epithelium and its underlying mesenchyme are involved in regulation of the stem cell niche. Crosstalk between different pathways, as well as spatial control and redundancy of signaling factors in the mesenchyme and epithelium allow for fine regulation of development and homeostasis. An improved understanding of the intestinal stem cell niche has also led to progress in the development of an in vitro model system of the intestine allowing for more physiologically relevant study of disease pathophysiology and testing of novel therapeutics. An integral part of understanding the behavior of these intestinal stem cells is characterizing the surrounding niche. Further studies to identify the source of the signaling molecules in the niche will provide an insight into how the sub-compartmentalization of the intestinal stem cells is maintained.
References
Lipkin M. Cell Replication in the gastrointestinal tract of man. Gastroenterology. 1965;48:616–24.
Heath JP. Epithelial cell migration in the intestine. Cell Biol Int. 1996;20(2):139–46.
Potten CS, Kovacs L, Hamilton E. Continuous labelling studies on mouse skin and intestine. Cell Tissue Kinet. 1974;7(3):271–83.
Halprin KM. Epidermal “turnover time”—a re-examination. Br J Dermatol. 1972;86(1):14–9.
Bergmann O, Bhardwaj RD, Bernard S, Zdunek S, Barnabé-Heider F, Walsh S, et al. Evidence for cardiomyocyte renewal in humans. Science. 2009;324(5923):98–102.
Kajstura J, Gurusamy N, Ogórek B, Goichberg P, Clavo-Rondon C, Hosoda T, et al. Myocyte turnover in the aging human heart. Circ Res. 2010;107(11):1374–86.
Cheng H, Leblond CP. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. V. Unitarian theory of the origin of the four epithelial cell types. Am J Anat. 1974;141(4):537–61.
Bjerknes M, Cheng H. The stem-cell zone of the small intestinal epithelium. I. Evidence from Paneth cells in the adult mouse. Am J Anat. 1981;160(1):51–63.
Rothenberg ME, Nusse Y, Kalisky T, Lee JJ, Dalerba P, Scheeren F, et al. Identification of a cKit(+) colonic crypt base secretory cell that supports Lgr5(+) stem cells in mice. Gastroenterology. 2012;142(5):1195–205.
Novelli MR, Williamson JA, Tomlinson IP, Elia G, Hodgson SV, Talbot IC, et al. Polyclonal origin of colonic adenomas in an XO/XY patient with FAP. Science. 1996;272(5265):1187–90.
Novelli M, Cossu A, Oukrif D, Quaglia A, Lakhani S, Poulsom R, et al. X-inactivation patch size in human female tissue confounds the assessment of tumor clonality. Proc Natl Acad Sci U S A. 2003;100(6):3311–4.
Ritsma L, Ellenbroek SIJ, Zomer A, Snippert HJ, de Sauvage FJ, Simons BD, et al. Intestinal crypt homeostasis revealed at single-stem-cell level by in vivo live imaging. Nature. 2014;507(7492):362–5.
Snippert HJ, van der Flier LG, Sato T, van Es JH, van den Born M, Kroon-Veenboer C, et al. Intestinal crypt homeostasis results from neutral competition between symmetrically dividing Lgr5 stem cells. Cell. 2010;143(1):134–44.
Gonzalez MA, Bernad A. Characteristics of adult stem cells. Adv Exp Med Biol. 2012;741:103–20.
Potten CS. Extreme sensitivity of some intestinal crypt cells to X and gamma irradiation. Nature. 1977;269(5628):518–21.
Zhu Y, Huang Y-F, Kek C, Bulavin DV. Apoptosis differently affects lineage tracing of Lgr5 and Bmi1 intestinal stem cell populations. Cell Stem Cell. 2013;12(3):298–303.
Bjerknes M, Cheng H. The stem-cell zone of the small intestinal epithelium. II. Evidence from Paneth cells in the newborn mouse. Am J Anat. 1981;160(1):65–75.
Fuchs E. The tortoise and the hair: slow-cycling cells in the stem cell race. Cell. 2009;137(5):811–9.
Li L, Clevers H. Coexistence of quiescent and active adult stem cells in mammals. Science. 2010;327(5965):542–5.
Kretzschmar K, Watt FM. Lineage tracing. Cell. 2012;148(1–2):33–45.
Sauer B, Henderson N. Targeted insertion of exogenous DNA into the eukaryotic genome by the Cre recombinase. New Biol. 1990;2(5):441–9.
Utomo AR, Nikitin AY, Lee WH. Temporal, spatial, and cell type-specific control of Cre-mediated DNA recombination in transgenic mice. Nat Biotechnol. 1999;17(11):1091–6.
Feil R, Brocard J, Mascrez B, LeMeur M, Metzger D, Chambon P. Ligand-activated site-specific recombination in mice. Proc Natl Acad Sci U S A. 1996;93(20):10887–90.
Yeh KY, Chopra DP. Epithelial cell cultures from the colon of the suckling rat. Vitro. 1980;16(11):976–86.
Kaeffer B. Mammalian intestinal epithelial cells in primary culture: a mini-review. Vitro Cell Dev Biol Anim. 2002;38(3):123–34.
Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459(7244):262–5.
Yin X, Farin HF, van Es JH, Clevers H, Langer R, Karp JM. Niche-independent high-purity cultures of Lgr5+ intestinal stem cells and their progeny. Nat Methods. 2014;11(1):106–12.
Ootani A, Li X, Sangiorgi E, Ho QT, Ueno H, Toda S, et al. Sustained in vitro intestinal epithelial culture within a Wnt-dependent stem cell niche. Nat Med. 2009;15(6):701–6.
Sato T, Clevers H. Growing self-organizing mini-guts from a single intestinal stem cell: mechanism and applications. Science. 2013;340(6137):1190–4.
Yui S, Nakamura T, Sato T, Nemoto Y, Mizutani T, Zheng X, et al. Functional engraftment of colon epithelium expanded in vitro from a single adult Lgr5+ stem cell. Nat Med. 2012;18(4):618–23.
Potten CS. Stem cells in gastrointestinal epithelium: numbers, characteristics and death. Philos Trans R Soc Lond B Biol Sci. 1998;353(1370):821–30.
Roth S, Fodde R. Quiescent stem cells in intestinal homeostasis and cancer. Cell Commun Adhes. 2011;18(3):33–44.
Rando TA. The immortal strand hypothesis: segregation and reconstruction. Cell. 2007;129(7):1239–43.
Potten CS, Owen G, Booth D. Intestinal stem cells protect their genome by selective segregation of template DNA strands. J Cell Sci. 2002;115(Pt 11):2381–8.
Tian H, Biehs B, Warming S, Leong KG, Rangell L, Klein OD, et al. A reserve stem cell population in small intestine renders Lgr5-positive cells dispensable. Nature. 2011;478(7368):255–9.
Sangiorgi E, Capecchi MR. Bmi1 is expressed in vivo in intestinal stem cells. Nat Genet. 2008;40(7):915–20.
May R, Sureban SM, Hoang N, Riehl TE, Lightfoot SA, Ramanujam R, et al. Doublecortin and CaM kinase-like-1 and leucine-rich-repeat-containing G-protein-coupled receptor mark quiescent and cycling intestinal stem cells, respectively. Stem Cells. 2009;27(10):2571–9.
Gerbe F, Brulin B, Makrini L, Legraverend C, Jay P. DCAMKL-1 expression identifies Tuft cells rather than stem cells in the adult mouse intestinal epithelium. Gastroenterology. 2009;137(6):2179–80.
Gagliardi G, Moroz K, Bellows CF. Immunolocalization of DCAMKL-1, a putative intestinal stem cell marker, in normal colonic tissue. Pathol Res Pract. 2012;208(8):475–9.
Takeda N, Jain R, LeBoeuf MR, Wang Q, Lu MM, Epstein JA. Interconversion between intestinal stem cell populations in distinct niches. Science. 2011;334(6061):1420–4.
Montgomery RK, Carlone DL, Richmond CA, Farilla L, Kranendonk ME, Henderson DE, et al. Mouse telomerase reverse transcriptase (mTert) expression marks slowly cycling intestinal stem cells. Proc Natl Acad Sci U S A. 2011;108(1):179–84.
Hughes KR, Gândara RMC, Javkar T, Sablitzky F, Hock H, Potten CS, et al. Heterogeneity in histone 2B-green fluorescent protein-retaining putative small intestinal stem cells at cell position 4 and their absence in the colon. Am J Physiol Gastrointest Liver Physiol. 2012;303(11):G1188–201.
Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449(7165):1003–7.
Escobar M, Nicolas P, Sangar F, Laurent-Chabalier S, Clair P, Joubert D, et al. Intestinal epithelial stem cells do not protect their genome by asymmetric chromosome segregation. Nat Commun. 2011;29(2):258.
Schepers AG, Vries R, van den Born M, van de Wetering M, Clevers H. Lgr5 intestinal stem cells have high telomerase activity and randomly segregate their chromosomes. EMBO J. 2011;30(6):1104–9.
Lopez-Garcia C, Klein AM, Simons BD, Winton DJ. Intestinal stem cell replacement follows a pattern of neutral drift. Science. 2010;330(6005):822–5.
van der Flier LG, van Gijn ME, Hatzis P, Kujala P, Haegebarth A, Stange DE, et al. Transcription factor achaete scute-like 2 controls intestinal stem cell fate. Cell. 2009;136(5):903–12.
Koo BK, Spit M, Jordens I, Low TY, Stange DE, van de Wetering M, et al. Tumour suppressor RNF43 is a stem-cell E3 ligase that induces endocytosis of Wnt receptors. Nature. 2012;488(7413):665–9.
van der Flier LG, Haegebarth A, Stange DE, van de Wetering M, Clevers H. OLFM4 is a robust marker for stem cells in human intestine and marks a subset of colorectal cancer cells. Gastroenterology. 2009;137(1):15–7.
Schuijers J, Junker JP, Mokry M, Hatzis P, Koo BK, Sasselli V, et al. Ascl2 acts as an R-spondin/Wnt-responsive switch to control stemness in intestinal crypts. Cell Stem Cell. 2015;16(2):158–70.
Hao HX, Xie Y, Zhang Y, Charlat O, Oster E, Avello M, et al. ZNRF3 promotes Wnt receptor turnover in an R-spondin-sensitive manner. Nature. 2012;485(7397):195–200.
Giannakis M, Hodis E, Mu XJ, Yamauchi M, Rosenbluh J, Cibulskis K, et al. RNF43 is frequently mutated in colorectal and endometrial cancers. Nat Genet. 2014;46(12):1264–6.
Wong VW, Stange DE, Page ME, Buczacki S, Wabik A, Itami S, et al. Lrig1 controls intestinal stem-cell homeostasis by negative regulation of ErbB signalling. Nat Cell Biol. 2012;14(4):401–8.
Powell AE, Wang Y, Li Y, Poulin EJ, Means AL, Washington MK, et al. The pan-ErbB negative regulator Lrig1 is an intestinal stem cell marker that functions as a tumor suppressor. Cell. 2012;149(1):146–58.
Kayahara T, Sawada M, Takaishi S, Fukui H, Seno H, Fukuzawa H, et al. Candidate markers for stem and early progenitor cells, Musashi-1 and Hes1, are expressed in crypt base columnar cells of mouse small intestine. FEBS Lett. 2003;535(1–3):131–5.
Potten CS, Booth C, Tudor GL, Booth D, Brady G, Hurley P, et al. Identification of a putative intestinal stem cell and early lineage marker; musashi-1. Differentiation. 2003;71(1):28–41.
Maria Cambuli F, Rezza A, Nadjar J, Plateroti M. Brief report: musashi1-eGFP mice, a new tool for differential isolation of the intestinal stem cell populations. Stem Cells. 2013;31(10):2273–8.
Philpott A, Winton DJ. Lineage selection and plasticity in the intestinal crypt. Curr Opin Cell Biol. 2014;31:39–45.
Metcalfe C, Kljavin NM, Ybarra R, de Sauvage FJ. Lgr5+ stem cells are indispensable for radiation-induced intestinal regeneration. Cell Stem Cell. 2014;14(2):149–59.
Kabiri Z, Greicius G, Madan B, Biechele S, Zhong Z, Zaribafzadeh H, et al. Stroma provides an intestinal stem cell niche in the absence of epithelial Wnts. Development. 2014;141(11):2206–15.
Yan KS, Chia LA, Li X, Ootani A, Su J, Lee JY, et al. The intestinal stem cell markers Bmi1 and Lgr5 identify two functionally distinct populations. Proc Natl Acad Sci U S A. 2012;109(2):466–71.
van Es JH, Sato T, van de Wetering M, Lyubimova A, Nee AN, Gregorieff A, et al. Dll1+secretory progenitor cells revert to stem cells upon crypt damage. Nat Cell Biol. 2012;14(10):1099–104.
Buczacki SJ, Zecchini HI, Nicholson AM, Russell R, Vermeulen L, Kemp R, et al. Intestinal label-retaining cells are secretory precursors expressing Lgr5. Nature. 2013;495(7439):65–9.
Alkhoury F, Malo MS, Mozumder M, Mostafa G, Hodin RA. Differential regulation of intestinal alkaline phosphatase gene expression by Cdx1 and Cdx2. Am J Physiol Gastrointest Liver Physiol. 2005;289(2):G285–90.
Soubeyran P, André F, Lissitzky JC, Mallo GV, Moucadel V, Roccabianca M, et al. Cdx1 promotes differentiation in a rat intestinal epithelial cell line. Gastroenterology. 1999;117(6):1326–38.
Li IC, Chan CT, Lu YF, Wu YT, Chen YC, Li GB, et al. Zebrafish krüppel-like factor 4a represses intestinal cell proliferation and promotes differentiation of intestinal cell lineages. PLoS ONE. 2011;6(6):e20974.
Hinnebusch BF, Siddique A, Henderson JW, Malo MS, Zhang W, Athaide CP, et al. Enterocyte differentiation marker intestinal alkaline phosphatase is a target gene of the gut-enriched Kruppel-like factor. Am J Physiol Gastrointest Liver Physiol. 2004;286(1):G23–30.
Malo MS, Zhang W, Alkhoury F, Pushpakaran P, Abedrapo MA, Mozumder M, et al. Thyroid hormone positively regulates the enterocyte differentiation marker intestinal alkaline phosphatase gene via an atypical response element. Mol Endocrinol. 2004;18(8):1941–62.
Yang Q, Bermingham NA, Finegold MJ, Zoghbi HY. Requirement of Math1 for secretory cell lineage commitment in the mouse intestine. Science. 2001;294(5549):2155–8.
VanDussen KL, Samuelson LC. Mouse atonal homolog 1 directs intestinal progenitors to secretory cell rather than absorptive cell fate. Dev Biol. 2010;346(2):215–23.
Li HJ, Ray SK, Singh NK, Johnston B, Leiter AB. Basic helix-loop-helix transcription factors and enteroendocrine cell differentiation. Diabetes Obes Metab. 2011;13(Suppl 1):5–12.
Yamada S, Kojima H, Fujimiya M, Nakamura T, Kashiwagi A, Kikkawa R. Differentiation of immature enterocytes into enteroendocrine cells by Pdx1 overexpression. Am J Physiol Gastrointest Liver Physiol. 2001;281(1):G229–36.
Gregorieff A, Stange DE, Kujala P, Begthel H, van den Born M, Korving J, et al. The ets-domain transcription factor Spdef promotes maturation of goblet and Paneth cells in the intestinal epithelium. Gastroenterology. 2009;137(4):1333–45.e1–3.
Bastide P, Darido C, Pannequin J, Kist R, Robine S, Marty-Double C, et al. Sox9 regulates cell proliferation and is required for Paneth cell differentiation in the intestinal epithelium. J Cell Biol. 2007;178(4):635–48.
Mori-Akiyama Y, van den Born M, van Es JH, Hamilton SR, Adams HP, Zhang J, et al. SOX9 is required for the differentiation of Paneth cells in the intestinal epithelium. Gastroenterology. 2007;133(2):539–46.
Fuchs E, Tumbar T, Guasch G. Socializing with the neighbors: stem cells and their niche. Cell. 2004;116(6):769–78.
Mikkers H, Frisén J. Deconstructing stemness. EMBO J. 2005;24(15):2715–9.
Schofield R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells. 1978;4(1–2):7–25.
Schofield R. The stem cell system. Biomed Pharmacother. 1983;37(8):375–80.
Ema H, Suda T. Two anatomically distinct niches regulate stem cell activity. Blood. 2012;120(11):2174–81.
Clarke AR. Wnt signalling in the mouse intestine. Oncogene. 2006;25(57):7512–21.
Holland JD, Klaus A, Garratt AN, Birchmeier W. Wnt signaling in stem and cancer stem cells. Curr Opin Cell Biol. 2013;25(2):254–64.
Holmberg J, Genander M, Halford MM, Annerén C, Sondell M, Chumley MJ, et al. EphB receptors coordinate migration and proliferation in the intestinal stem cell niche. Cell. 2006;125(6):1151–63.
Yu J, Virshup DM. Updating the Wnt pathways. Biosci Rep. 2014;34(5):e00142.
Korinek V, Barker N, Moerer P, van Donselaar E, Huls G, Peters PJ, et al. Depletion of epithelial stem-cell compartments in the small intestine of mice lacking Tcf-4. Nat Genet. 1998;19(4):379–83.
Ireland H, Kemp R, Houghton C, Howard L, Clarke AR, Sansom OJ, et al. Inducible Cre-mediated control of gene expression in the murine gastrointestinal tract: effect of loss of beta-catenin. Gastroenterology. 2004;126(5):1236–46.
Fevr T, Robine S, Louvard D, Huelsken J. Wnt/beta-catenin is essential for intestinal homeostasis and maintenance of intestinal stem cells. Mol Cell Biol. 2007;27(21):7551–9.
Pinto D, Gregorieff A, Begthel H, Clevers H. Canonical Wnt signals are essential for homeostasis of the intestinal epithelium. Genes Dev. 2003;17(14):1709–13.
Kuhnert F, Davis CR, Wang HT, Chu P, Lee M, Yuan J, et al. Essential requirement for Wnt signaling in proliferation of adult small intestine and colon revealed by adenoviral expression of Dickkopf-1. Proc Natl Acad Sci U S A. 2004;101(1):266–71.
Su LK, Kinzler KW, Vogelstein B, Preisinger AC, Moser AR, Luongo C, et al. Multiple intestinal neoplasia caused by a mutation in the murine homolog of the APC gene. Science. 1992;256(5057):668–70.
Oshima M, Oshima H, Kitagawa K, Kobayashi M, Itakura C, Taketo M. Loss of Apc heterozygosity and abnormal tissue building in nascent intestinal polyps in mice carrying a truncated Apc gene. Proc Natl Acad Sci U S A. 1995;92(10):4482–6.
Li Q, Ishikawa TO, Oshima M, Taketo MM. The threshold level of adenomatous polyposis coli protein for mouse intestinal tumorigenesis. Cancer Res. 2005;65(19):8622–7.
Guezguez A, Paré F, Benoit YD, Basora N, Beaulieu JF. Modulation of stemness in a human normal intestinal epithelial crypt cell line by activation of the WNT signaling pathway. Exp Cell Res. 2014;322(2):355–64.
Wong MH, Huelsken J, Birchmeier W, Gordon JI. Selection of multipotent stem cells during morphogenesis of small intestinal crypts of Lieberkuhn is perturbed by stimulation of Lef-1/beta-catenin signaling. J Biol Chem. 2002;277(18):15843–50.
de Lau W, Barker N, Low TY, Koo BK, Li VS, Teunissen H, et al. Lgr5 homologues associate with Wnt receptors and mediate R-spondin signalling. Nature. 2011;476(7360):293–7.
Carmon KS, Gong X, Lin Q, Thomas A, Liu Q. R-spondins function as ligands of the orphan receptors LGR4 and LGR5 to regulate Wnt/beta-catenin signaling. Proc Natl Acad Sci U S A. 2011;108(28):11452–7.
Seshagiri S, Stawiski EW, Durinck S, Modrusan Z, Storm EE, Conboy CB, et al. Recurrent R-spondin fusions in colon cancer. Nature. 2012;488(7413):660–4.
Wong MH. Regulation of intestinal stem cells. J Investig Dermatol Symp Proc. 2004;9(3):224–8.
Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: cell fate control and signal integration in development. Science. 1999;284(5415):770–6.
Fre S, Huyghe M, Mourikis P, Robine S, Louvard D, Artavanis-Tsakonas S. Notch signals control the fate of immature progenitor cells in the intestine. Nature. 2005;435(7044):964–8.
Fre S, Hannezo E, Sale S, Huyghe M, Lafkas D, Kissel H, et al. Notch lineages and activity in intestinal stem cells determined by a new set of knock-in mice. PLoS ONE. 2011;6(10):e25785.
Vooijs M, Ong CT, Hadland B, Huppert S, Liu Z, Korving J, et al. Mapping the consequence of Notch1 proteolysis in vivo with NIP-CRE. Development. 2007;134(3):535–44.
Jensen J, Pedersen EE, Galante P, Hald J, Heller RS, Ishibashi M, et al. Control of endodermal endocrine development by Hes-1. Nat Genet. 2000;24(1):36–44.
Ueo T, Imayoshi I, Kobayashi T, Ohtsuka T, Seno H, Nakase H, et al. The role of Hes genes in intestinal development, homeostasis and tumor formation. Development. 2012;139(6):1071–82.
van Es JH, van Gijn ME, Riccio O, van den Born M, Vooijs M, Begthel H, et al. Notch/gamma-secretase inhibition turns proliferative cells in intestinal crypts and adenomas into goblet cells. Nature. 2005;435(7044):959–63.
Pellegrinet L, Rodilla V, Liu Z, Chen S, Koch U, Espinosa L, et al. Dll1- and dll4-mediated notch signaling are required for homeostasis of intestinal stem cells. Gastroenterology. 2011;140(4):1230–1240.e1–7.
He XC, Zhang J, Tong WG, Tawfik O, Ross J, Scoville DH, et al. BMP signaling inhibits intestinal stem cell self-renewal through suppression of Wnt-beta-catenin signaling. Nat Genet. 2004;36(10):1117–21.
Haramis AP, Begthel H, van den Born M, van Es J, Jonkheer S, Offerhaus GJ, et al. De novo crypt formation and juvenile polyposis on BMP inhibition in mouse intestine. Science. 2004;303(5664):1684–6.
Tian Q, He XC, Hood L, Li L. Bridging the BMP and Wnt pathways by PI3 kinase/Akt and 14-3-3zeta. Cell Cycle. 2005;4(2):215–6.
Takaku K, Oshima M, Miyoshi H, Matsui M, Seldin MF, Taketo MM. Intestinal tumorigenesis in compound mutant mice of both Dpc4 (Smad4) and Apc genes. Cell. 1998;92(5):645–56.
Kosinski C, Li VS, Chan AS, Zhang J, Ho C, Tsui WY, et al. Gene expression patterns of human colon tops and basal crypts and BMP antagonists as intestinal stem cell niche factors. Proc Natl Acad Sci U S A. 2007;104(39):15418–23.
Reynolds A, Wharton N, Parris A, Mitchell E, Sobolewski A, Kam C, et al. Canonical Wnt signals combined with suppressed TGFβ/BMP pathways promote renewal of the native human colonic epithelium. Gut. 2014;63(4):610–21.
Chiang C, Litingtung Y, Lee E, Young KE, Corden JL, Westphal H, et al. Cyclopia and defective axial patterning in mice lacking Sonic hedgehog gene function. Nature. 1996;383(6599):407–13.
Litingtung Y, Lei L, Westphal H, Chiang C. Sonic hedgehog is essential to foregut development. Nat Genet. 1998;20(1):58–61.
Ramalho-Santos M, Melton DA, McMahon AP. Hedgehog signals regulate multiple aspects of gastrointestinal development. Development. 2000;127(12):2763–72.
Madison BB, Braunstein K, Kuizon E, Portman K, Qiao XT, Gumucio DL. Epithelial hedgehog signals pattern the intestinal crypt-villus axis. Development. 2005;132(2):279–89.
van den Brink GR, Bleuming SA, Hardwick JC, Schepman BL, Offerhaus GJ, Keller JJ, et al. Indian Hedgehog is an antagonist of Wnt signaling in colonic epithelial cell differentiation. Nat Genet. 2004;36(3):277–82.
van Dop WA, Uhmann A, Wijgerde M, Sleddens-Linkels E, Heijmans J, Offerhaus GJ, et al. Depletion of the colonic epithelial precursor cell compartment upon conditional activation of the hedgehog pathway. Gastroenterology. 2009;136(7):2195–2203.e1–7.
Kaestner KH, Silberg DG, Traber PG, Schütz G. The mesenchymal winged helix transcription factor Fkh6 is required for the control of gastrointestinal proliferation and differentiation. Genes Dev. 1997;11(12):1583–95.
Barry ER, Morikawa T, Butler BL, Shrestha K, de la Rosa R, Yan KS, et al. Restriction of intestinal stem cell expansion and the regenerative response by YAP. Nature. 2013;493(7430):106–10.
Rowland KJ, Brubaker PL. The “cryptic” mechanism of action of glucagon-like peptide-2. Am J Physiol Gastrointest Liver Physiol. 2011;301(1):G1–8.
Ireland H, Houghton C, Howard L, Winton DJ. Cellular inheritance of a Cre-activated reporter gene to determine Paneth cell longevity in the murine small intestine. Dev Dyn. 2005;233(4):1332–6.
Stappenbeck TS, Hooper LV, Gordon JI. Developmental regulation of intestinal angiogenesis by indigenous microbes via Paneth cells. Proc Natl Acad Sci U S A. 2002;99(24):15451–5.
Sato T, van Es JH, Snippert HJ, Stange DE, Vries RG, van den Born M, et al. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature. 2011;469(7330):415–8.
Garabedian EM, Roberts LJ, McNevin MS, Gordon JI. Examining the role of Paneth cells in the small intestine by lineage ablation in transgenic mice. J Biol Chem. 1997;272(38):23729–40.
Shroyer NF, Wallis D, Venken KJ, Bellen HJ, Zoghbi HY. Gfi1 functions downstream of Math1 to control intestinal secretory cell subtype allocation and differentiation. Genes Dev. 2005;19(20):2412–7.
Bjerknes M, Cheng H. Cell lineage metastability in Gfi1-deficient mouse intestinal epithelium. Dev Biol. 2010;345(1):49–63.
Kim TH, Escudero S, Shivdasani RA. Intact function of Lgr5 receptor-expressing intestinal stem cells in the absence of Paneth cells. Proc Natl Acad Sci U S A. 2012;109(10):3932–7.
Durand A, Donahue B, Peignon G, Letourneur F, Cagnard N, Slomianny C, et al. Functional intestinal stem cells after Paneth cell ablation induced by the loss of transcription factor Math1 (Atoh1). Proc Natl Acad Sci U S A. 2012;109(23):8965–70.
Powell DW, Pinchuk IV, Saada JI, Chen X, Mifflin RC. Mesenchymal cells of the intestinal lamina propria. Annu Rev Physiol. 2011;73:213–37.
Farin HF, van Es JH, Clevers H. Redundant sources of Wnt regulate intestinal stem cells and promote formation of Paneth cells. Gastroenterology. 2012;143(6):1518–1529.e7.
Lahar N, Lei NY, Wang J, Jabaji Z, Tung SC, Joshi V, et al. Intestinal subepithelial myofibroblasts support in vitro and in vivo growth of human small intestinal epithelium. PLoS ONE. 2011;6(11):e26898.
Lei NY, Jabaji Z, Wang J, Joshi VS, Brinkley GJ, Khalil H, et al. Intestinal subepithelial myofibroblasts support the growth of intestinal epithelial stem cells. PLoS ONE. 2014;9(1):e84651.
Hirokawa Y, Yip KH, Tan CW, Burgess AW. Colonic myofibroblast cell line stimulates colonoid formation. Am J Physiol Gastrointest Liver Physiol. 2014;306(7):G547–56.
Proffitt KD, Virshup DM. Precise regulation of porcupine activity is required for physiological Wnt signaling. J Biol Chem. 2012;287(41):34167–78.
Biechele S, Cox BJ, Rossant J. Porcupine homolog is required for canonical Wnt signaling and gastrulation in mouse embryos. Dev Biol. 2011;355(2):275–85.
Janda CY, Waghray D, Levin AM, Thomas C, Garcia KC. Structural basis of Wnt recognition by Frizzled. Science. 2012;337(6090):59–64.
Wang LC, Nassir F, Liu ZY, Ling L, Kuo F, Crowell T, et al. Disruption of hedgehog signaling reveals a novel role in intestinal morphogenesis and intestinal-specific lipid metabolism in mice. Gastroenterology. 2002;122(2):469–82.
Kosinski C, Stange DE, Xu C, Chan AS, Ho C, Yuen ST, et al. Indian hedgehog regulates intestinal stem cell fate through epithelial-mesenchymal interactions during development. Gastroenterology. 2010;139(3):893–903.
Gradilla AC, Guerrero I. Cytoneme-mediated cell-to-cell signaling during development. Cell Tissue Res. 2013;352(1):59–66.
Stanganello E, Hagemann AI, Mattes B, Sinner C, Meyen D, Weber S, et al. Filopodia-based Wnt transport during vertebrate tissue patterning. Nat Commun. 2015;5(6):5846.
Phelps RA, Chidester S, Dehghanizadeh S, Phelps J, Sandoval IT, Rai K, et al. A two-step model for colon adenoma initiation and progression caused by APC loss. Cell. 2009;137(4):623–34.
Fodde R. The APC gene in colorectal cancer. Eur J Cancer. 2002;38(7):867–71.
Howe JR, Bair JL, Sayed MG, Anderson ME, Mitros FA, Petersen GM, et al. Germline mutations of the gene encoding bone morphogenetic protein receptor 1A in juvenile polyposis. Nat Genet. 2001;28(2):184–7.
Houlston R, Bevan S, Williams A, Young J, Dunlop M, Rozen P, et al. Mutations in DPC4 (SMAD4) cause juvenile polyposis syndrome, but only account for a minority of cases. Hum Mol Genet. 1998;7(12):1907–12.
Ernst M, Ramsay RG. Colorectal cancer mouse models: integrating inflammation and the stroma. J Gastroenterol Hepatol. 2012;27(1):39–50.
Yeung TM, Buskens C, Wang LM, Mortensen NJ, Bodmer WF. Myofibroblast activation in colorectal cancer lymph node metastases. Br J Cancer. 2013;108(10):2106–15.
Vacanti JP, Morse MA, Saltzman WM, Domb AJ, Perez-Atayde A, Langer R. Selective cell transplantation using bioabsorbable artificial polymers as matrices. J Pediatr Surg. 1988;23(1 Pt 2):3–9.
Barthel ER, Levin DE, Speer AL, Sala FG, Torashima Y, Hou X, et al. Human tissue-engineered colon forms from postnatal progenitor cells: an in vivo murine model. Regen Med. 2012;7(6):807–18.
Levin DE, Barthel ER, Speer AL, Sala FG, Hou X, Torashima Y, et al. Human tissue-engineered small intestine forms from postnatal progenitor cells. J Pediatr Surg. 2013;48(1):129–37.
Spurrier RG, Grikscheit TC. Tissue engineering the small intestine. Clin Gastroenterol Hepatol. 2013;11(4):354–8.
Acknowledgments
Studies in the Virshup lab at the Duke-NUS Graduate Medical School are supported in part by the National Research Foundation Singapore and administered by the Singapore Ministry of Health’s National Medical Research Council under the STAR Award Program to D.M.V.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Chee, C.Y., Virshup, D.M., Madan, B. (2015). The Intestinal Stem Cell Niche. In: Turksen, K. (eds) Tissue-Specific Stem Cell Niche. Stem Cell Biology and Regenerative Medicine. Springer, Cham. https://doi.org/10.1007/978-3-319-21705-5_7
Download citation
DOI: https://doi.org/10.1007/978-3-319-21705-5_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-21704-8
Online ISBN: 978-3-319-21705-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)