Abstract
There are several kinds of craniopharyngioma that can be managed via two variations of the endoscopic endonasal transsphenoidal procedure: the “standard” approach to the sellar region and the “extended” approach to the suprasellar area. These two variations have different indications.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Indications
There are several kinds of craniopharyngioma that can be managed via two variations of the endoscopic endonasal transsphenoidal procedure: the “standard” approach to the sellar region and the “extended” approach to the suprasellar area. These two variations have different indications.
The indications for the standard approach were postulated more than 50 years ago, in the early 1960s, when Guiot and Derome [1] identified the possibilities of transsphenoidal surgery for craniopharyngiomas retaining suitable for this approach infradiaphragmatic lesions, with enlarged sella that preferably already caused panhypopituitarism. Still today these guidelines are absolutely valid for both the microsurgical and endoscopic transsphenoidal approaches. The standard transsphenoidal route for infradiaphragmatic craniopharyngiomas provides the advantage of accessing the tumor immediately after dural opening, without entering the subarachnoid space.
Later on, during the 1980s, some authors expanded the classic indications of transsphenoidal microscopic approach, adopting this route for the management of craniopharyngiomas with extension above the diaphragma sellae, i.e., with an involvement of the subarachnoid space. Accordingly, in order to allow the proper handling of surgical instruments and the adequate exposure of the tumor, the refinement of bone and dural opening beyond the limits of the sella, i.e., over the tuberculum sellae and the posterior portion of the planum sphenoidale, was described. A new variation of the transsphenoidal pathway, i.e., the so-called “extended” transsphenoidal approach, was though defined [2].
During more recent years, there have been a worldwide diffusion and acceptance of the endoscope in transsphenoidal approaches. The panoramic and wider view offered by the endoscope increased the versatility of the transsphenoidal pathway, thus permitting the removal even of supradiaphragmatic lesions, including craniopharyngiomas [3–6]. The use of the endoscope through this route provides an access to the suprasellar supradiaphragmatic area, regardless of the sellar size (even a not enlarged sella). It was the group of Pittsburgh, initially Jho [7] and later Kassam and Carrau [8], that defined and popularized the use of the endoscope in the so-called “extended” endoscopic transsphenoidal approaches for the removal of suprasellar lesions.
The proposed indications for an extended endoscopic transsphenoidal approach are different from the standard one: the relationship of lesions with the diaphragma sellae is a key aspect for choosing either a standard or an extended transsphenoidal approach. However, craniopharyngiomas that can be considered amenable for an extended endoscopic endonasal approach should have particular features, such as midline position, without a solid parasellar component or encasement of the main neurovascular structures, small-to-medium size, with main pattern of growth along the stalk-infundibulum axis, as these can be identified running along the same path of the endoscopic endonasal route [9–13]. Nevertheless, other characteristics, above all the degree of tumor and capsule adhesion to critical neurovascular structures, could make this surgery difficult and risky. On the contrary, it is possible to assume that patients who have undergone earlier operations, radiation therapy, or both could harbor lesions presenting such troublesome features. However, if a previous open craniotomy has been used, the endoscopic endonasal approach may offer the benefit of a naive surgical route, thus providing a safer option for recurrence surgery.
Finally, it should be reminded that when endoscopic endonasal route is chosen, many different aspects in regard to the approach itself should be taken into account.
First of all, the sphenoid sinus pneumatization has to be considered. Differently from tuberculum sellae meningiomas, when dealing with a craniopharyngioma, the degree of pneumatization of the sphenoid sinus is not a limiting factor. Indeed, if the craniopharyngioma develops in the sub- and/or retrochiasmatic areas, it is not necessary to have a large sphenoid sinus to obtain a proper exposure of these regions.
Secondarily, the location of the pituitary stalk in relation to the craniopharyngioma should be highlighted: the correlation between the craniopharyngioma and the pituitary stalk is of utmost importance for properly planning the optimal surgical strategy. As a matter of fact, the surgical classification proposed by Kassam et al. [14] relates the craniopharyngioma to the infundibulum and therefore identifies type I, preinfundibular; type II, transinfundibular; type III, post- or retroinfundibular, further subdivided based on rostral or caudal extension; and type IV, isolated third ventricular (generally, not removable via endonasal route).
The size and location of the craniopharyngioma should be assessed in relation to the width of osteo-dural defect that is necessary to realize an adequate exposure of the lesion. In case of a very large predominantly cystic craniopharyngioma with components of the lesion getting into the retro and/or parasellar space, extending beyond the limit of the surgical maneuverability of the instruments, the endoscopic endonasal route should not be adopted. Moreover, a very narrow surgical corridor due to minimal distance between the supraclinoid tracts of the internal carotid arteries could represent a relative contraindication to the use of this route.
The endoscopic endonasal approach, with both its standard and extended variation, provides a shorter intradural corridor, a median closeup and symmetric view of suprasellar neurovascular structures, and, above all, a greater exposure of the subchiasmatic and retrochiasmatic areas, as well as of the stalk-infundibulum axis and the anterior part of the third ventricle, which definitely represents a crucial area in the growth path of craniopharyngiomas [15, 16].
Anyway it is important to underline that the endonasal approach is not the only route used to treat craniopharyngiomas. Many transcranial approaches have been and are successfully used to manage such kind of lesions [17–21].
2 Neuroradiology
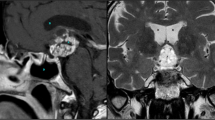
Magnetic resonance imaging (MRi) is more commonly used to define the features of a craniopharyngioma. High-resolution sequences of the sellar region, pre- and post-contrast enhancement should be obtained in all cases, thus providing more details regarding the relationship of the tumor to the nearby neurovascular structures (Figs. 6.1, 6.2, and 6.3).
(a) Sagittal, (b) coronal, and (c) axial preoperative MRi scans showing an intra- and suprasellar infradiaphragmatic craniopharyngioma, partially cystic, displacing upward the optic chiasm and the floor of the third ventricle. (d) Sagittal, (e) coronal, and (f) axial postoperative MRi scans showing complete tumor removal
Postoperative sagittal MRi scans of the cases shown in Fig. 6.2. The tumors have been removed via an extended endoscopic endonasal approach
Adamantinous craniopharyngioma, the most common type, appears as a lobulated mass located within the suprasellar area or, less frequently, in the sellar, parasellar, and retrosellar regions; it could present cystic, calcic, and solid components.
Cysts can be found in 90 % of cases, commonly hypointense on the T1- and hyperintense on the T2-weighted (w) images; the typical hyperintense signal on the T1-w images and hypointense signal on the T2-w images, eliciting a “motor-oil” content, are mainly influenced by high protein concentrations and by the presence of blood degradation products; cholesterol and triglycerides concentrations have little effect on the signal. A fluid-fluid level can be present.
The cystic part usually shows a post-gad rim enhancement.
A calcified ring, not always complete, or calcified clusters can be found in 90 % of cases, better showed on CT images; x-rays of the skull is nowadays rarely performed, sometimes demonstrating the presence of calcifications and providing some information about the size and shape of the sella.
The identification of a cystic, partially calcified suprasellar mass, is suggestive for a craniopharyngioma.
The solid portion of the tumor appears isointense and/or hypointense on the T1-w images, almost always hyperintense on the T2-w images; it is characterized by a strong post-gad enhancement.
Hyperintense signal in the parenchyma adjacent to tumor may indicate gliosis, tumor invasion, or indirect injury due to cyst fluid leaking and/or edema from compression of optic chiasm/tracts. Obstructive hydrocephalus is a possible concomitant characteristic of large tumors.
Magnetic resonance angiography (MRA) images may better characterize vascular displacement and/or encasement, in particular when the anterior cerebral artery complex is involved.
Squamopapillary craniopharyngioma appears as a rounded, solid, or both solid and cystic mass located in the third ventricle; it shows a hypointense signal on the T1-w images and hyperintense signal on the T2-w images and enhances strongly post-gad injection, although not homogeneously, because of the presence of necrotic areas.
However, it has to be reminded that there are no radiologic features that can absolutely discriminate among the subtypes of craniopharyngioma; lobulated shape, vessel encasement, and calcification have all been postulated to be indicative of the adamantinomatous subtype.
3 Anatomy of the Approach
The main structures related to the standard endoscopic endonasal approach have been already described in details in the homonymous section of the pituitary adenomas chapter (see Chap. 1). Although the anatomy of the nasal and paranasal cavities is the same, during surgical treatment of craniopharyngiomas, there are some relevant structures, which it is useful to focus on, especially in case of extended approaches.
First of all, whether a pedicled nasoseptal flap should be used, its anatomy has to be understood. The flap consists of the mucoperiosteum and mucoperichondrium of the nasal septum, and it is pedicled on the posterior septal artery, a branch of the sphenopalatine artery. The posterior septal artery arises from the sphenopalatine artery, branch of the internal maxillary artery, in the pterygopalatine fossa and bifurcates into a superior and inferior branch with the latter being the larger one. The branches of the posterior septal artery then form a dense network along the septum to supply the inferior two thirds of the septum and a large portion of the nasal floor.
As well, the configuration of the sphenoid sinus has to be addressed properly: the sphenoid sinus could present an extreme variability in terms of size and shape and, above all, degree of pneumatization. Accordingly, in the adulthood, the sphenoid sinus could be identified as follows: sellar (≅75 %), presellar (≅24 %), and conchal (≅1 %). The sellar type of sphenoid sinus is the most common; in this case the air cavity extends into the body of sphenoid below the sella and as far posteriorly as the clivus. On the other hand, in the presellar type of sphenoid sinus, the air cavity does not penetrate beyond a vertical plane parallel to the sellar floor, whereas in the conchal type, the area below the sella is a solid block of bone without an air cavity [22].
During the approach, after all the sphenoidal septa have been flattened down, the posterior and lateral walls of the sphenoid sinus are visible. The sellar floor is positioned at the center of the surgical field with the cavernous sinuses laterally to it and the planum sphenoidale above with the bony protuberances of the optic nerves laterally to it and the clival indentation below. The bony prominences of C4 and C5 segments of the internal carotid artery (ICA) [23] can be seen laterally to the sellar floor and, above them, the optic nerves prominences can be observed; between them the optocarotid recesses lie. The ICA prominences are more evident as more the sphenoid sinus is pneumatized; the molding can be different depending on the shape and course of the carotid arteries. It is useful to remind that the bone covering the artery at the level of the sphenoid sinus may be very thin, especially at bony lateral aspects of the tuberculum sellae. The lateral optocarotid recess (LOCR) lies in between the inferior aspect of the optic nerve and the lateral aspect of the carotid artery bone protuberances and corresponds to the optic strut; it varies in depth accordingly to its degree of pneumatization. On the other hand, the medial optocarotid recess (MOCR) can be identified at the medial confluence of the optic and carotid bone protuberances.
Immediately above the sellar floor, the angle formed by the convergence of the sphenoid planum with the sellar floor can be observed; this latter structure, recently named “suprasellar notch” [24], from an endoscopic standpoint resembles a “mirror image” of the tuberculum sellae. It is limited superiorly by a line joining the 2 lateral optocarotid recesses, inferiorly by a line crossing just above the superior margin of the sella, and laterally by the medial aspect of the parasellar tract of the carotid arteries.
Once the bone of the sella and of the planum sphenoidale has been removed, venous sinuses that interconnect the cavernous sinuses appear: the intercavernous connections are named on the basis of their relationship to the pituitary gland; the anterior, or superior, intercavernous sinus passes anterior to the hypophysis, while the posterior, or inferior, intercavernous sinus passes behind the gland. However, these intercavernous connections can run at any site along the anterior, inferior, or posterior surface of the gland, or eventually they may be absent. There is also a large intercavernous venous connection, i.e., the basilar sinus that passes posteriorly to the dorsum sellae and upper clivus and connects the inferior and posterior aspects of both cavernous sinuses.
The dura is opened and it is possible to explore the entire suprasellar region. It should be said that a brief intercarotid distance – measured at the level of the supraclinoid tracts – could narrow the width of the endonasal corridor. Though, when adopting an extended endoscopic endonasal approach, this feature should be carefully evaluated preoperatively.
As well, the anatomical variability of the optic chiasm as related to the anterior skull base and surrounding structures should be highlighted: several anatomical studies showed that about 80 % of the optic chiasms overlie the diaphragm sellae, so defined as normal positioning. The remaining 20 % was equally distributed between the prefixed variant – when sitting above the tuberculum sellae – and the postfixed variant – when lying over to the dorsum sellae.
From the endoscopic endonasal perspective, the suprasellar area can be divided into four areas by two ideal planes, one passing through the inferior surface of the chiasm and the mammillary bodies and another passing through the posterior margin of chiasm and the dorsum sellae; these two lines define four regions, i.e., the suprachiasmatic, the subchiasmatic, the retrosellar, and the ventricular (Fig. 6.4) [3].
In the suprachiasmatic region, the chiasmatic and lamina terminalis cisterns with relative contents are accessible. The anterior margin of the chiasm and the medial portion of the optic nerves, the anterior cerebral arteries, the anterior communicating artery, and the recurrent Heubner arteries, together with the gyri recti of the frontal lobes, are identified.
In the subchiasmatic space, the pituitary stalk is at the center of the view, below the chiasm, with the superior hypophyseal arteries and its perforating branches, supplying the inferior surface of the chiasm and the optic nerves. The superior aspect of the pituitary gland and the dorsum sellae are also visible. The superior hypophyseal arteries supply the optic chiasm, the floor of the hypothalamus, and the median eminence. On the other side, the inferior hypophyseal artery divides into a medial and a lateral branch, which anastomose with the corresponding vessels of the opposite side, forming an arterial ring around the hypophysis.
The retrosellar area can be explored passing with the endoscope between the pituitary stalk and the internal carotid artery, sliding above the dorsum sellae; it encloses the upper third of the basilar artery, the pons, the superior cerebellar arteries, the oculomotor nerves, the posterior cerebral arteries, and lastly the mammillary bodies and the floor of the third ventricle at the level of the tuber cinereum (Fig. 6.5).
Endoscopic endonasal anatomical view of the (a) infrachiasmatic, (b) suprachiasmatic, (c) retrosellar, and (d) intraventricular areas. Pg pituitary gland, Ps pituitary stalk, PcoA posterior communicating artery, BA basilar artery, ICA internal carotid artery, ONL left optic nerve, ONR right optic nerve, Ch chiasm, A2L post-communicating tract of the left anterior cerebral artery, A2R post-communicating tract of the right anterior cerebral artery, IIIR right oculomotor nerve, IIIL left oculomotor nerve, P1R pre-communicating tract of the right posterior cerebral artery, P1L pre-communicating tract of the left posterior cerebral artery, sca superior cerebellar artery, + choroid plexus, * fronto-polar artery, ITC interthalamic commissure, MB mammillary bodies, ** tuber cinereum, white arrows access to the foramina of Monro, T thalamus
Concerning the third ventricle area, it has to be highlighted that, as seen from the endonasal perspective, this cavity can be divided into four areas by means of two ideal planes, one passing through the optic chiasm and the interthalamic commissure and one passing through the posterior edge of the foramen of Monro and the interthalamic commissure. Accordingly, two anterior (infundibular and foraminal) and two posterior (mesencephalic and tectal) areas can be defined (Fig. 6.6). Furthermore, two separate endoscopic endonasal corridors can be identified, namely, the suprachiasmatic and the subchiasmatic [25].
Artistic drawing showing a sagittal view of the third ventricle. The third ventricle chamber has been divided into four areas by means of two ideal lines passing the first one between the optic chiasm and the interthalamic commissure and the second one between the posterior edge of the foramen of Monro and the interthalamic commissure. 1 anterior-inferior (infundibular) area, 2 anterior-superior (foraminal) area, 3 posterior-inferior (mesencephalic) area, 4 posterior-superior (tectal) area
Through the suprachiasmatic pathway, the lamina terminalis cistern is entered above the optic chiasm. Once the lamina terminalis is opened, the infundibular area of the third ventricle can be accessed. As soon as the third ventricle chamber is entered, the endoscopic inspection with 0° endoscope permits to visualize the thalami laterally and the interthalamic commissure. The use of angled endoscopes permits a better view, especially of the foraminal area.
On the other hand, the subchiasmatic route allows the entry into the third ventricle cavity through its floor. In this case the third ventricle is accessed through the tuber cinereum, which is located on the floor of the third ventricle between the pituitary stalk and the mammillary bodies. It has to be stressed that, entering the third ventricle through the tuber cinereum, the extradural removal of the dorsum sellae and eventually of the posterior clinoids along with the anterior transposition of the pituitary gland are useful.
As the endoscope is advanced in an inferior-superior trajectory through the tuber cinereum inside the ventricular cavity, a wide panoramic view is obtained (not only its infundibular area): the thalami and the interthalamic commissure and the foramen of Monro are visualized anteriorly, while the bulging of mammillary bodies can be seen posteriorly.
Advancing further inside the ventricular chamber, a panoramic view of the foraminal area is obtained: the endoscopic endonasal exploration of the foraminal area permits to show the inner surface of the foramina of Monro, i.e., the aspect appearing at the third ventricle. As seen from this perspective, the body of the fornix is located on the middle of the field and it continues upward and laterally with its columns; on the other hand, the inferior-lateral surface of each foramen of Monro, as seen from below, is formed by the ipsilateral thalamus. The choroid plexus extends within each foramen of Monro, surrounding the body of the fornix like a collar before entering the lateral ventricle through the choroidal fissure. The anterior commissure is identified anteriorly to the foramen of Monro.
Finally, passing under the interthalamic commissure, the posterior portion of the third ventricle, i.e., the mesencephalic area, can be reached up to the pineal and suprapineal recesses, the posterior commissure, the habenular commissure, the habenular trigone, the stria medullaris, the tela choroidea, and the beginning of the cerebral aqueduct. The pineal gland and the internal cerebral veins lateral to the pineal gland can be seen as well (Fig. 6.7). The tectal area is generally not accessible through an endoscopic endonasal approach, neither via a suprachiasmatic or a subchiasmatic route.
Endoscopic endonasal anatomical view of the (a) infundibular, (b) foraminal, and (c) mesencephalic regions of the third ventricle. T thalamus, ITC interthalamic commissure, FM foramina of Monro, f fornix, AC anterior commissure, TC tela coroidea, sm striae medullaris, HC habenular commissure, PC posterior commissure, * mesencephalic region, ** aqueduct of Sylvius
4 Technique
As a general rule, it should be said that as position and orientation of the microscope could turn around the patient’s head, the same concept should be adopted during the endoscopic endonasal procedure. The position of the endoscope and instruments inside the nasal cavities should be variable and versatile, according to the target surgical area. Whether the surgeon is right-handed or left-handed, the endoscope is inserted, respectively, into the right or left nostril, at the high or low portion of the nasal cavity, with the aim of reaching the area of the surgical field that must be displayed. The first surgeon uses the nostril not occupied by the scope for his first instrument and the other for a second tool, while the second surgeon holds the scope in one nostril and another instrument in the contralateral nostril. This strategy attains to the strictly proper neurosurgical part of the operation, let’s say from the sphenoid sinus on, where a microsurgical attitude with using an instrument for each surgeon’s hand is mandatory. The same principle has to be applied to the surgical instruments; as a matter of fact, their position inside the nasal cavities should allow the most comfortable trajectory in order to reach each visible area of the surgical field, avoiding any conflict with the endoscope.
In case of infradiaphragmatic craniopharyngiomas, the surgical procedure is the same as described for pituitary adenomas (see Chap. 1). The endoscopic endonasal approach begins in the same way; in case of a craniopharyngioma, after the dura is opened, any cystic component of the lesion is aspirated, while the solid component has to be sharply dissected from the sellar walls and/or from the suprasellar cistern trying to not damage this structure to avoid intraoperative CSF leak (Figs. 6.8, 6.9, and 6.10). It has to be stressed that infradiaphragmatic craniopharyngiomas may be purely intrasellar or, more frequently, present a considerable suprasellar extension, pushing upward the diaphragma sellae. Those tumors can be approached via a standard endoscopic endonasal pathway as well. During the removal of infradiaphragmatic craniopharyngiomas, it is often possible to preserve the integrity of the suprasellar cistern: thus far, the sellar floor can remain open allowing the drainage of any eventual fluid part of the lesion inside the sphenoid sinus. The introduction of an X-shaped silastic catheter inside the sella grants the communication of the sella with the sphenoid sinus and/or the nasal cavities also after tissue healing (see Fig. 6.21) [26].
In case of supradiaphragmatic craniopharyngioma, an “extended” endoscopic endonasal approach has to be performed.
Lumbar drainage is placed at the beginning of the procedure. The patient is placed supine and the head is positioned in a slightly extended position in order to optimize the access to the anterior cranial base. The face is turned 5–10° toward the surgeons. The procedure starts, dealing with nasal steps as previously described for pituitary adenomas, with some modifications according to the principle of the extended endoscopic endonasal surgery [27, 28, 14]. Besides, the periumbilical area has to be prepped and draped in order to allow to harvest autologous fat for the reconstruction phase.
Cottonoids, soaked with diluted adrenaline and lidocaine, are inserted in both nostrils between the middle turbinate and the nasal septum and left in place for about 5 min; then, unilateral middle turbinectomy with removal of the posterior ethmoidal cells is performed in the same cavity where the nasoseptal flap will be raised. Moreover, the posterior aspect of the nasal septum is removed minding to not extend anteriorly to the head of the contralateral middle turbinate to reduce scars to a single nasal cavity and therefore limit the patient’s postoperative breathing discomfort.
At the beginning of the surgical procedure, the nasoseptal flap is usually drawn – tailored according to size and shape of the defect – on the septum while raised and rotated on the osteo-dural defect at the end of the surgery. Two parallel incisions are performed following the sagittal plane of the septum, one over the maxillary crest and the other 1–2 cm below the most superior aspect of the septum (this preserves the olfactory epithelium) and joined anteriorly by a vertical cut. Elevation of the flap is realized at the end of the procedure, in order to reduce the nasal bleeding during surgery and eventually avoid the ischemia of the flap due to the twisting of its pedicle and, at the same time, increase the adhesive property of the same flap that is lifted from the septum and immediately placed on the osteo-dural defect. The nasoseptal flap is elevated with a Cottle dissector or similar instrument taking care of preserving the posterolateral neurovascular pedicle [29].
At this point, the nasal septum is detached from the anterior wall of the sphenoid sinus with the aid of a dissector or with a high-speed microdrill and the posterior edge of the nasal septum (1–2 cm) is resected with backbiting forceps. It is crucial to widely open the anterior wall of the sphenoid sinus in order to gain a proper working angle for the instruments. Subsequently, the contralateral middle turbinate is laterally displaced. All intrasinus septae are flattened down. The main anatomical landmark of the posterior wall of the sphenoid sinus must be recognized (Fig. 6.1). These include the optic nerves and intracavernous carotid artery (ICA) canals as well as lateral and medial opticocarotid recesses (MOCR and LOCR, respectively) and the clival recess.
A complete removal of the tuberculum sellae, i.e., the suprasellar notch [24] as seen from the endonasal perspective up to both medial optocarotid recesses, is mandatory to enter the suprasellar area and reach the third ventricle: the bone is thinned with the drill and then removed with a Kerrison rongeur. The extent of bone removal, in anteroposterior direction, is determined by the size and location of the tumor and can be measured intraoperatively using neuronavigation.
Before opening the dura, it can be useful to dissect the epidural space alongside the edge of the bone opening in order to allow a comfortable extradural reconstruction at the final stage of the surgery.
Prior than dural opening, micro-Doppler ultrasonography can be used to avoid injury to the ICA, especially when its proximal supraclinoid portion courses toward midline and the superior intercavernous sinus should be coagulated and closed (Fig. 6.11). The dura is initially incised horizontally few millimeters above and below the superior intercavernous sinus, with the aim of dissecting the two dural layers in which the sinus lies; thereafter, it is totally closed with bipolar coagulation and cut in its median portion. This technique avoids the use of hemoclips that conversely can narrow the dural opening, hindering the access to the suprasellar space.
Management of the superior intercavernous sinus during extended endoscopic endonasal approach to the planum sphenoidale. (a) The sinus is first coagulated and then the dura is cutted (b) below and (c) above it. (d) A dissector is inserted underneath the superior intercavernous sinus in order to detach it from the arachnoid and the pituitary gland. Then, (e) both layers of the dura mater containing the venous connection are coagulated and the sinus is (f) divided to allow safe entry inside the subarachnoid space. sis superior intercavernous sinus, s sella
The dural incision is though performed in a median position passing over the superior intercavernous sinus diverging superiorly in a “Y shape” toward both the optic prominences and downward as well in “Y shape” to the infero-lateral corners of the pituitary gland. It is crucial to avoid coagulation and/or removal of the dural leaflets, as they can help supporting the reconstruction.
The removal of a craniopharyngioma via an endoscopic transsphenoidal approach suits the same goals and principles of the microsurgical transcranial routes: internal debulking of the solid part and/or cystic evacuation, followed by fine tumor dissection from the main surrounding neurovascular structures.
Hence, upon dural opening the craniopharyngioma will came into view covered by the arachnoidal layers of the suprasellar cistern (Figs. 6.12 and 6.13): the surgical removal proceeds in an anteroposterior direction taking extreme care while managing the lower part of the tumor, in close contact with to the pituitary gland and stalk, and, as well, dealing with the upper part, in a strict relationship with the optic chiasm and optic nerves. During the early intracapsular debulking of the craniopharyngioma, it is mandatory to identify and preserve the superior hypophyseal arteries as they greatly contribute to the blood supply of the optic chiasm (Fig. 6.14).
Proceeding with the removal of the posterior portion of the lesion, the relationships of the craniopharyngioma with the infundibulum and the floor of the third ventricle have to be ruled out (Figs. 6.15, 6.16, and 6.17): in these terms the endoscopic endonasal route for the management of craniopharyngiomas provides the advantages of a peculiar surgical trajectory that follows the origin and development of craniopharyngioma (Fig. 6.18). The endoscopic closeup view gives the opportunity to understand the amount of involvement of the main neurovascular structures along the axis stalk-infundibulum-third ventricle and their supplying vessels (Fig. 6.19).
Intraoperative endoscopic endonasal picture showing a preinfundibular craniopharyngioma (type I). (a) Identification of the tumor in front of the pituitary stalk. (b) Tumor dissection and removal from the main neuronal structure. (c) Exploration of the surgical field after total tumor removal. T tumor, Pg pituitary gland, P2L post-communicating tract of the left posterior cerebral artery, PcoA posterior communicating artery, ICA internal carotid artery, A1L pre-communicating tract of the left anterior cerebral artery, OTL left optic tract, Ps pituitary stalk, ds dorsum sellae
Intraoperative endoscopic endonasal picture showing an infundibular craniopharyngioma (type II). (a–c) The infundibulum has been enlarged by the craniopharyngioma and through it the tumor is gradually removed. (d) At the end of the procedure, the infundibular part of the third ventricle cavity comes into view. T tumor, OT R right optic tract, OT L left optic tract, ThV third ventricle
Intraoperative endoscopic endonasal picture (a) showing retroinfundibular craniopharyngioma (type III). View of the retrosellar area after the removal of a retroinfundibular craniopharyngioma (b–d). Pg pituitary gland, Ps pituitary stalk, ThV third ventricle, PcoA posterior communicating artery, BA basilar artery, III R right oculomotor nerve, IIIL left oculomotor nerve, P 1R pre-communicating tract of the right posterior cerebral artery, P1 L pre-communicating tract of the left posterior cerebral artery, sca superior cerebellar artery, + post-communicating tract of the right posterior cerebral artery, * hypoplastic left posterior communicating artery
Endoscopic views showing third ventricle cavity exploration during removal of craniopharyngiomas. (a) Infundibular area of the third ventricle cavity; (b) closeup view of the foraminal area of the third ventricle after the removal of a large intra and suprasellar craniopharyngioma in a patient with hydrocephalus. (c) Surgical view of the third ventricle mesencephalic area. At this level the third ventricle floor can present different degrees of pial invasion: (d) no involvement; (e) partial involvement; (f) diffuse involvement. f fornix, FM foramina of Monro, PcoA posterior communicating artery, + choroid plexus, MB mammillary bodies, * striae medullaris, T thalamus, ITC interthalamic commissure, fThV floor of the third ventricle, ** tuber cinereum
Debulking of the craniopharyngioma can be effectively performed according to its consistency using mostly the ultrasonic aspirator; delicate “countertraction” can be very useful to identify the arachnoid cleavage plane that could allow sharp dissection from other neurovascular structures [30, 31].
The endoscopic endonasal approach provides some advantages, i.e., a shorter intradural corridor, a median closeup view of suprasellar neurovascular structures, and, above all, a great exposure of sub- and retrochiasmatic areas, as well as of stalk-infundibulum axis, which definitely represents a crucial area in the craniopharyngiomas growth path. Indeed, a minimal displacement of the surrounding neurovascular structures is performed as compared to transcranial microsurgical approaches [9, 32].
4.1 Reconstruction Techniques
It has to be minded that the reconstruction technique is different among infradiaphragmatic craniopharyngiomas and those with supradiaphragmatic extension.
For infradiaphragmatic craniopharyngiomas, which require in most cases a standard endoscopic endonasal approach, the reconstruction phase of the surgical procedure, when no intraop CSF leak has occurred, aims to protect the suprasellar cistern, placing over it collagen sponge or dural substitute foils (Fig. 6.20), and to fill then the sellar dead space. In case of cystic craniopharyngiomas, after the emptying of the lesion, the insertion of an X-shaped silastic catheter into the residual cavity may be performed, in order to avoid cyst reformation and continuously drain fluid contents into the sphenoid sinus and/or nasal cavities during complementary postop radiotherapy and radiosurgery (Fig. 6.21).
Reconstruction technique used in case of infradiaphragmatic craniopharyngioma: (a) the suprasellar cistern has descent into the sellar cavity. Though, (b) it is protected with collagen sponge and (c) the floor is closed with dural substitute wedged in the extradural space. SC suprasellar cistern, + collagen sponge, * dura mater substitute
On the other hand, for supradiaphragmatic craniopharyngioma, which requires an “extended” endoscopic endonasal approach, meticulous closure is mandatory for minimizing the risk of postoperative CSF leakage that may lead to potential fatal complications. A variety of reconstruction methods and materials are currently available and used (Fig. 6.22). First of all, intradural closure with obliteration of the dead space, using autologous fat and fibrin glue, is performed. Then, we rather prefer to use the so-called sandwich technique: the surgical cavity is filled with fat graft sutured to three layers of fascia lata or dural substitute. The first two layers are positioned intradurally and the third one extradurally, wedged in between the dura and the bone. A pedicled nasoseptal flap is used to cover the skull base defect and the posterior wall of the sphenoid sinus (Fig. 6.23). An inflated Foley balloon catheter is placed, inside the sphenoid sinus to support the reconstruction [33].
Reconstruction technique used after endoscopic endonasal removal of a supradiaphragmatic craniopharyngioma. (a) Obliteration of the suprasellar dead space, using autologous fat. (b, c) Closure of the osteo-dural defect using the so-called sandwich technique. Finally, (d) the nasoseptal flap is reflected posteriorly over the skull base defect. F fat, dm dura mater, S “sandwich”, NSF nasoseptal flap, Co choana
Finally, bipolar hemostasis is performed over the lateral wall of the sphenoidotomy and irrigation of the nasal cavities is obtained. Lumbar drainage is usually left in place for 3–5 days.
4.2 Postoperative Management
Craniopharyngiomas treated via an endoscopic endonasal approach require an intensive and watchful postoperative care [34]. New-onset diabetes insipidus (DI) should be diagnosed promptly and treated with desmopressin (DDAVP).
The patient should rest in bed with the head raised at 30° in order to facilitate venous return and CSF flow toward the spine; besides, patients are asked to adopt postoperative habits in order to prevent any ICP increase and the eventual displacement of skull base reconstruction. It is preferable to avoid the use of straws, to cough and/or sneeze with the mouth open, to assume as early as possible a stand-up position and start walking, to avoid bending over nor squatting, and to assume stool softeners.
Computed tomography (CT) scan is performed routinely at POD#1 in order to evaluate any neurosurgical complication and/or the amount of pneumocephalus. According to a recent contribution [35], frontal and intraventricular pneumocephalus is not necessarily associated with a postoperative CSF leak; on the other hand, a “suspicious” pattern of air, namely, pneumocephalus in the convexity, interhemispheric fissure, and sella, parasellar, or perimesencephalic locations, may be significantly associated with a postoperative CSF leak occurrence and for such reason these patients require closer observation. An initial sign of not yet recognizable CSF leak may be a sudden fever onset in the early post-op period (fever sign).
It has to be highlighted that patients with minimal postoperative CSF leak can be managed without reoperation: repeated endoscope-guided fibrin glue injections – while they were awake in the outpatient operating room – can be performed if the CSF leak is minimal or moderate [36]. However, in case of severe CSF leak, displacement of the reconstruction materials, and/or evident communication of the sphenoid sinus with the intradural compartment, immediate transsphenoidal reoperation is needed.
References
Guiot G, Derome P (1972) Indications for trans-sphenoid approach in neurosurgery. 521 cases. Ann Med Interne (Paris) 123(8):703–712
Weiss MH (1987) The transnasal transsphenoidal approach. In: Apuzzo MLJ (ed) Surgery of the third ventricle. Williams & Wilkins, Baltimore, pp 476–494
Cavallo LM, de Divitiis O, Aydin S, Messina A, Esposito F, Iaconetta G, Talat K, Cappabianca P, Tschabitscher M (2008) Extended endoscopic endonasal transsphenoidal approach to the suprasellar area: anatomic considerations – part 1. Neurosurgery 62(6 Suppl 3):1202–1212. doi:10.1227/01.neu.0000333786.98596.33
Couldwell WT, Weiss MH, Rabb C, Liu JK, Apfelbaum RI, Fukushima T (2004) Variations on the standard transsphenoidal approach to the sellar region, with emphasis on the extended approaches and parasellar approaches: surgical experience in 105 cases. Neurosurgery 55(3):539–550
Dusick JR, Esposito F, Kelly DF, Cohan P, DeSalles A, Becker DP, Martin NA (2005) The extended direct endonasal transsphenoidal approach for nonadenomatous suprasellar tumors. J Neurosurg 102(5):832–841
de Divitiis E, Cappabianca P, Cavallo LM (2002) Endoscopic transsphenoidal approach: adaptability of the procedure to different sellar lesions. Neurosurgery 51(3):699–705; discussion 705–707
Jho HD (2001) The expanding role of endoscopy in skull-base surgery. Indications and instruments. Clin Neurosurg 48:287–305
Kassam A, Snyderman CH, Mintz A, Gardner P, Carrau RL (2005) Expanded endonasal approach: the rostrocaudal axis. Part I. Crista galli to the sella turcica. Neurosurg Focus 19(1):E3
Cavallo LM, Frank G, Cappabianca P, Solari D, Mazzatenta D, Villa A, Zoli M, D’Enza AI, Esposito F, Pasquini E (2014) The endoscopic endonasal approach for the management of craniopharyngiomas: a series of 103 patients. J Neurosurg 121(1):100–113. doi:10.3171/2014.3.JNS131521
Koutourousiou M, Gardner PA, Fernandez-Miranda JC, Tyler-Kabara EC, Wang EW, Snyderman CH (2013) Endoscopic endonasal surgery for craniopharyngiomas: surgical outcome in 64 patients. J Neurosurg 119(5):1194–1207. doi:10.3171/2013.6.JNS122259
Cappabianca P, Frank G, Pasquini E, de Divitiis O, Calbucci F (2003) Extended endoscopic endonasal transsphenoidal approaches to the suprasellar region, planum sphenoidale & clivus. In: de Divitiis E, Cappabianca P (eds) Endoscopic endonasal transsphenoidal surgery. Springer, Wien
Cappabianca P, Cavallo LM, Esposito F, de Divitiis O, Messina A, de Divitiis E (2008) Extended endoscopic endonasal approach to the midline skull base: the evolving role of transsphenoidal surgery. In: Pickard JD, Akalan N, Di Rocco C et al (eds) Advances and technical standards in neurosurgery. Springer, Wien/New York, pp 152–199
Jane JA Jr, Kiehna E, Payne SC, Early SV, Laws ER Jr (2010) Early outcomes of endoscopic transsphenoidal surgery for adult craniopharyngiomas. Neurosurg Focus 28(4):E9. doi:10.3171/2010.1.FOCUS09319
Kassam AB, Gardner PA, Snyderman CH, Carrau RL, Mintz AH, Prevedello DM (2008) Expanded endonasal approach, a fully endoscopic transnasal approach for the resection of midline suprasellar craniopharyngiomas: a new classification based on the infundibulum. J Neurosurg 108(4):715–728. doi:10.3171/JNS/2008/108/4/0715
Cavallo LM, Prevedello DM, Solari D, Gardner PA, Esposito F, Snyderman CH, Carrau RL, Kassam AB, Cappabianca P (2009) Extended endoscopic endonasal transsphenoidal approach for residual or recurrent craniopharyngiomas. J Neurosurg 111(3):578–589. doi:10.3171/2009.2.JNS081026
Cappabianca P, Cavallo LM (2012) The evolving role of the transsphenoidal route in the management of craniopharyngiomas. World Neurosurg 77(2):273–274
Samii M, Samii A (2000) Surgical management of craniopharyngiomas. In: Schmidek HH (ed) Schmidek & Sweet operative neurosurgical techniques, vol 1, Indications, methods and results. W. B. Saunders, Philadelphia, pp 489–502
Yasargil MG, Abdulrauf SI (2008) Surgery of intraventricular tumors. Neurosurgery 62(6 Suppl 3):1029–1040. doi:10.1227/01.neu.0000333768.12951.9a, 00006123-200806001-00010 [pii]; discussion 1040–1021
Yasargil MG, Curcic M, Kis M, Siegenthaler G, Teddy PJ, Roth P (1990) Total removal of craniopharyngiomas. Approaches and long-term results in 144 patients. J Neurosurg 73(1):3–11
Fatemi N, Dusick JR, de Paiva Neto MA, Malkasian D, Kelly DF (2009) Endonasal versus supraorbital keyhole removal of craniopharyngiomas and tuberculum sellae meningiomas. Neurosurgery 64(5 Suppl 2):269–284. doi:10.1227/01.NEU.0000327857.22221.53; discussion 284–266
Gerganov V, Metwali H, Samii A, Fahlbusch R, Samii M (2014) Microsurgical resection of extensive craniopharyngiomas using a frontolateral approach: operative technique and outcome. J Neurosurg 120(2):559–570. doi:10.3171/2013.9.JNS122133
Rhoton AL Jr (2002) The sellar region. Neurosurgery 51(4 Suppl):S335–S374
Bouthillier A, van Loveren HR, Keller JT (1996) Segments of the internal carotid artery: a new classification. Neurosurgery 38(3):425–432; discussion 432–423
de Notaris M, Solari D, Cavallo LM, D’Enza AI, Ensenat J, Berenguer J, Ferrer E, Prats-Galino A, Cappabianca P (2012) The “suprasellar notch,” or the tuberculum sellae as seen from below: definition, features, and clinical implications from an endoscopic endonasal perspective. J Neurosurg 116(3):622–629. doi:10.3171/2011.11.JNS111162
Cavallo LM, Di Somma A, de Notaris M, Prats-Galino A, Aydin S, Catapano G, Solari D, de Divitiis O, Somma T, Cappabianca P (2015) Extended endoscopic endonasal approach to the third ventricle. Multimodal anatomical study with surgical implications. World Neurosurg. doi:10.1016/j.wneu.2015.03.007
Spaziante R, De Divitiis E, Irace C, Cappabianca P, Caputi F (1989) Management of primary or recurring grossly cystic craniopharyngiomas by means of draining systems. Topic review and 6 case reports. Acta Neurochir (Wien) 97(3–4):95–106
de Divitiis E, Cappabianca P, Cavallo LM, Esposito F, de Divitiis O, Messina A (2007) Extended endoscopic transsphenoidal approach for extrasellar craniopharyngiomas. Neurosurgery 61(5 Suppl 2):219–227. doi:10.1227/01.neu.0000303220.55393.73, 00006123-200711001-00006 [pii]; discussion 228
Frank G, Pasquini E, Doglietto F, Mazzatenta D, Sciaretta V, Farneti G, Calbucci F (2006) The endoscopic extended transsphenoidal approach for craniopharyngiomas. Neurosurgery 59(Suppl 1):ONS75–ONS83
Hadad G, Bassagasteguy L, Carrau RL, Mataza JC, Kassam A, Snyderman CH, Mintz A (2006) A novel reconstructive technique after endoscopic expanded endonasal approaches: vascular pedicle nasoseptal flap. Laryngoscope 116(10):1882–1886
Conger AR, Lucas J, Zada G, Schwartz TH, Cohen-Gadol AA (2014) Endoscopic extended transsphenoidal resection of craniopharyngiomas: nuances of neurosurgical technique. Neurosurg Focus 37(4):E10. doi:10.3171/2014.7.FOCUS14364
Kassam A, Snyderman CH, Mintz A, Gardner P, Carrau RL (2005) Expanded endonasal approach: the rostrocaudal axis. Part I. Crista galli to the sella turcica. Neurosurg Focus 19(1):E3:1–12
Cavallo LM, Solari D, Esposito F, Cappabianca P (2013) The endoscopic endonasal approach for the management of craniopharyngiomas involving the third ventricle. Neurosurg Rev 36(1):27–37. doi:10.1007/s10143-012-0403-4; discussion 38
Snyderman CH, Kassam AB, Carrau R, Mintz A (2007) Endoscopic reconstruction of cranial base defects following endonasal skull base surgery. Skull Base 17(1):73–78. doi:10.1055/s-2006-959337
Ditzel Filho LFS, Prevedello DM, Kerr EE, Jamshidi AO, Ottoy BA, Carrau RL (2015) Endonasal resection of craniopharyngiomas: post-operative management. In: Evans JJ, Kenning TJ (eds) Craniopharyngiomas: comprehensive diagnosis, treatment and outcome. Elsevier, Inc. USA, pp 271–280
Banu MA, Szentirmai O, Mascarenhas L, Salek AA, Anand VK, Schwartz TH (2014) Pneumocephalus patterns following endonasal endoscopic skull base surgery as predictors of postoperative CSF leaks. J Neurosurg 121(4):961–975. doi:10.3171/2014.5.JNS132028
Cavallo LM, Solari D, Somma T, Savic D, Cappabianca P (2014) The awake endoscope-guided sealant technique with fibrin glue in the treatment of postoperative cerebrospinal fluid leak after extended transsphenoidal surgery: technical note. World Neurosurg 82(3–4):e479–e485. doi:10.1016/j.wneu.2013.01.017
Acknowledgment
The editors wish to thank Doctor Carmela Chiaramonte for the original drawings prepared for this chapter.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
1 Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Cavallo, L.M. et al. (2016). Endoscopic Endonasal Transsphenoidal Approach. In: Cappabianca, P., Cavallo, L., de Divitiis, O., Esposito, F. (eds) Midline Skull Base Surgery. Springer, Cham. https://doi.org/10.1007/978-3-319-21533-4_6
Download citation
DOI: https://doi.org/10.1007/978-3-319-21533-4_6
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-21532-7
Online ISBN: 978-3-319-21533-4
eBook Packages: MedicineMedicine (R0)