Abstract
Clival chordomas are lesions located at the anterolateral aspect of the brainstem, involving the extradural and seldom the intradural spaces. These tumors have been historically accessed through posterolateral approaches, which offer a comfortable surgical corridor for this kind of lesions: indeed, the retrosigmoid approach is considered adequate for chordomas that involve mainly the cerebellomesencephalic and cerebellopontine cisterns (Samii A, Gerganov VM, Herold C, Hayashi N, Naka T, Mirzayan MJ, Ostertag H, Samii M (2007) Chordomas of the skull base: surgical management and outcome. J Neurosurg 107(2):319–324), whereas in the far lateral approach, it is more suitable for those mainly interested in the cerebellomedullary cistern (Liu JK, Couldwell WT (2005) Far-lateral transcondylar approach: surgical technique and its application in neurenteric cysts of the cervicomedullary junction. Report of two cases. Neurosurg Focus 19(2), E9; Wen HT, Rhoton AL Jr, Katsuta T, de Oliveira E (1997) Microsurgical anatomy of the transcondylar, supracondylar, and paracondylar extensions of the far-lateral approach. J Neurosurg 87(4):555–585).
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Indications
Clival chordomas are lesions located at the anterolateral aspect of the brainstem, involving the extradural and seldom the intradural spaces. These tumors have been historically accessed through posterolateral approaches, which offer a comfortable surgical corridor for this kind of lesions: indeed, the retrosigmoid approach is considered adequate for chordomas that involve mainly the cerebellomesencephalic and cerebellopontine cisterns [55], whereas in the far lateral approach, it is more suitable for those mainly interested in the cerebellomedullary cistern [45, 61].
Besides, it has been possible to expose chordomas via complex and often morbid procedures such as the presigmoid approach, anterior and posterior petrosectomies with or without labyrinthectomy [9], and the extreme lateral approach [4]. These approaches, however, provide narrow dissection corridors with limited working area and mandatory angle of attacks, above all, in between the cranial nerves. Hence, such surgical procedures have been burdened often by significant brain tissue and cranial nerve morbidity.
Because chordomas displace dorsally the neurovascular structures, ventral corridors have been preferred: indeed, in transfacial [2, 12, 18, 34, 46, 58], transsphenoidal [1, 13, 22, 30, 39, 42, 43, 47, 51, 56], and transoral [11, 17, 33] techniques, lesions have been adopted and refined along years.
In recent times, the endoscope brought in transsphenoidal surgery a wider and panoramic view, pushing the refinement of several routes targeted mainly to entire midline of the skull base from the anterior skull base to the cranio-vertebral junction and adjacent areas. These corridors represent a direct and minimally invasive approach to the suprasellar, retrosellar, and retroclival space, obviating brain retraction, visualizing safely and effectively the surgical field, and granting the lowest rates of morbidity and mortality in a safe and effective way. Hence, the extended endoscopic approaches (EEAs) provided ideal surgical alternative options [5, 16, 29, 60] also for clival chordomas and, in particular, those with a major component ventral to the brainstem and/or to the lower cranial nerves [10, 26, 35, 57]. By using a direct anterior approach, the EEAs allow the surgeon to work in a large window and to work between and not cross the cranial nerves, achieving early devascularization of the tumor and microsurgical dissection under closeup view.
Chordoma surgery, as any other skull base procedure, requires careful and specific postoperative management and long-term patient follow-up, which can make the difference between a satisfactory result and a poor result.
Detailed anatomy, neuroimaging, pathophysiology, and natural history of the disease are knowledge prerequisite pillars to build up a multidisciplinary team, which becomes familiar with all the different therapeutic options and fully informed about current therapeutic possibilities in the interest of the patient and of the institution where the operation is done.
Indications for surgery have changed through time and refinements of the surgical techniques, as well as according to the evaluation of results and experience, to the development of knowledge about disease’s molecular biology, and to the use of effective new pharmacological agents and radiation techniques [3, 6, 14, 24, 32, 42, 43, 48, 59, 62].
Whether an EEA represents an appropriate surgical management strategy for a clival chordoma, the epicenter and extent of the tumor’s bony and/or dural relationships, as well as neurovascular structures involvement, should be carefully evaluated preoperatively.
Though, it is worth reminding that tumor to be resected should be ventral to the brainstem and medial to the cranial nerves, i.e., when extending into the interpeduncular cistern, the mass should not extend beyond the third cranial nerve; when involving the prepontine cistern, it should be medial to the sixth cranial nerve; or when in the premedullary cistern, it should be preferably enclosed within the hypoglossal nerves [10, 23, 25, 26, 39, 40, 52, 57].
Frequently, the nerves and perforating arteries are pushed away by the tumor, which allows direct visualization and safe tumor debulking. Hence, the possibility of running tumor removal with the vital structures located lateral and/or posterior to it and without any neural tissue retraction or dissection in between cranial nerves should be considered as a main advantage of the endonasal endoscopic approach.
Nevertheless, there are conditions that could limit and somehow hinder the choice of the endonasal route, either related to the anatomy of the surgical route or to the inner features of the lesion itself: the size and the pneumatization of the sphenoid sinus and/or carotid arteries position and shape, the caudal extension of the lesion, as well as the encasement of the main neurovascular structures and above all the lateral extension beyond the ascending tract of ICAs.
2 Radiology
Clivus chordoma originates at the spheno-occipital synchondrosis (35 % of all chordomas) and usually spreads as an expansile multilobulated mass from the midline toward the lateral neighboring regions. In fact, after eroding of the occipital bone, the neoplasm tends to invade anteriorly the sphenoid sinus and the rhinopharynx and, posterosuperiorly, the subarachnoid spaces of the brainstem. The brain parenchyma is seldom infiltrated, while the brainstem is compressed on the midline [50].
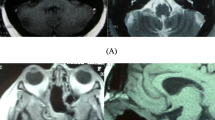
The first-choice imaging modality is contrast-enhanced magnetic resonance imaging, which demonstrates the different histological components of the mass [27]: typical chordomas show large areas with increased water content and prolonged relaxation times compared with the forms where chondroid tissue predominates. The lesion therefore usually appears isointense (75 %) or hypointense in T1-weighted images and markedly hyperintense in T2-weighted images, reflecting the high fluid content of vacuolated cellular components [20]. Often, within the mass it is possible to spot areas of signal void representing sequestered fragments of destroyed cortical bone (rather than calcific foci), fibrous connective septa, vessels or hemorrhage, or, conversely, areas with increased signal intensity in T1-weighted images that may reveal cyst-like mucoid material or hemorrhage (see Figs. 25.1 and 25.2).
(a) Sagittal and (b) axial preoperative MRI scans showing an invasive extradural sellar chordoma extending to the clivus. This latter T2-weighted image shows the typical inhomogeneous hyperintensity known as “thumb sign.” (c) Axial and (d) sagittal postoperative scans showing complete tumor removal via an extended endoscopic endonasal approach
Besides providing an optimal view on the sagittal plane, MRI permits the accurate definition of the intracranial extension of the tumor and its relationships with the brainstem – the impinging on the pons is typical and defined as “thumb sign” (see Fig. 25.1b) – the third ventricle, the sellar and hypothalamic structures, and the rhinopharynx [50].
Moreover, a better and earlier detection of the pathological substitution of the normal yellow marrow of the clivus can be obtained with MRI. Instead, the definition of intratumoral calcifications and osteolytic areas is more problematic compared to CT.
The CT appearance of chordoma (see Fig. 25.3) is that of centrally located, well-circumscribed, inhomogeneous soft-tissue mass (hyperdense compared to the brain), with irregular intratumoral calcific spots and destructive osteolysis, sometimes with marginal sclerosis [27].
After contrast media administration, both on CT and MRI, the tumor shows variable areas of moderate to marked enhancement, creating a “honeycomb” appearance of the enhancement pattern [19].
As the usual clinical presentation is lined out by visual disturbances – i.e., oculomotor nerve palsies – and orbitofrontal headache, the diagnostic workup should start with contrast-enhanced MRI, with angiographic sequences to evaluate the encasement/displacement of carotid or vertebral arteries (80 %); then, unenhanced spiral CT may be used for the evaluation of the osseous skull base.
As for the differential diagnosis, chondroma and chondrosarcoma, clival meningioma, giant invasive pituitary macroadenoma, plasmacytoma, and nasopharyngeal carcinoma must be considered:
-
Chondromas and chondrosarcomas, while similar in T1 and T2 signal intensity, tend to have a more lateral origin (the petro-occipital fissure) and show larger (archlike) calcifications compared to chordoma.
-
In meningioma, the signs of bone destruction are usually less evident and a dural attachment may be seen.
-
Macroadenomas of course cannot be separated by the pituitary gland, unlike chordomas, which usually displace but do not invade the gland.
-
Plasmacytoma typically shows intermediate to low signal intensity in T2-weighted images.
-
Nasopharyngeal malignancies usually extend more anteriorly and are associated with head and neck lymphadenopathy.
Chordomas very rarely metastatize, but they frequently recur, given also the difficulty in obtaining a radical resection in advanced cases. Therefore, radiation therapy is frequently employed and neuroradiological follow-up with MRI is warranted, to assess whether the residual disease is under control (i.e., if there is lack of progression) and also to evaluate the possible complications of radiation therapy, such as optic neuritis, edema, gliosis, or even necrosis in the temporal lobes [15].
3 Anatomy of the Approach
According to the anatomical classification introduced by Rhoton [53], the clivus can be divided into three segments in a cranio-caudal direction: the upper third extends from the level of dorsum sellae and posterior clinoids down to the sellar floor; the middle third limits are represented by the lower aspect of the sellar floor, superiorly, and the level of the sphenoid sinus floor, inferiorly; and finally, the lower segment goes from the sphenoid sinus floor to the foramen magnum. As described also in a recent publication by Prevedello et al. [52], this anatomical scheme could be adopted when exploring this area from endoscopic endonasal route (see Figs. 25.4 and 25.5)
Endoscopic endonasal view of the clival region. The main anatomical landmarks can be observed: (a) in this case the venous system has been injected with blue latex. The bone of the upper clivus has been removed, and (b) the transposition of the pituitary gland has been made. OP optic protuberance, PS planum sphenoidale, ICA internal carotid artery, ocr opto-carotid recess, sis superior intercavernous sinus, Pg pituitary gland, FL foramen lacerum, ds dorsum sellae, VI abducens nerve, V2 maxillary branch of the trigeminal nerve, dm dura mater of the clivus, * inferior hypophyseal artery, ** pericarotid sympathetic plexus
Intradural exploration of the upper and middle clival area. The dura mater has been opened in order to show the main neurovascular structures. ON optic nerve, Ch optic chiasm, Pg pituitary gland, ICA internal carotid artery, BA basilar artery, VI abducens nerve, V2 maxillary branch of the trigeminal nerve, aica anteroinferior cerebellar artery, VA vertebral artery, XII hypoglossal nerve, pica, postero-inferior cerebellar artery, * inferior hypophyseal artery, ** ophthalmic artery, dotted lines proximal and distal dural rings of the internal carotid artery
3.1 Upper Third of the Clivus
The utmost superior aspect of the clivus is represented by the dorsum sellae and the posterior clinoids on both sides.
The anterior aspect of this area encloses posteriorly the sella: two layers of dura cover its bony surface. the periosteal and meningeal layers, the same two layers covering the sellar floor, between which run the superior, inferior, and posterior intercavernous sinuses (PIS) [54]. This latter venous channel is located behind the pituitary gland and could be seen upon elevation of the gland; the dorsum sellae could be identified posterior to the PIS. Upon bone removal, the clival dura harboring the basilar venous plexus is exposed (see Fig. 25.4): dural opening at this level gives access to the interpeduncular cistern encompassed laterally by Liliequist membrane and the posterior communicating arteries, the respective perforating arteries, and the third cranial nerves (see Figs. 25.5 and 25.6a, b). The mesencephalon, the basilar bifurcation, the posterior cerebral arteries, and the superior cerebellar arteries are noble neurovascular structure encountered in a deep level, being the epicenter of this area. The horizontal lamina of Liliequist membrane represents the inferior limit of this area [52, 53].
Endoscopic endonasal panoramic view of the clival area with (a) the basilar trunk is clearly seen on the ventral side of the pons. (b) Closeup view of the upper clivus and (c) of the inferior third of the clivus. Sca superior cerebellar artery, III oculomotor nerve, ICA internal carotid artery, BA basilar artery, VI abducens nerve, Pg pituitary gland, MB mammillary bodies, * inferior hypophyseal artery, PCA posterior cerebral artery, sca superior cerebellar artery, III oculomotor nerve, aica anteroinferior cerebellar artery, VI abducens nerve, VA vertebral artery, ASA anterior spinal artery, ** postero-inferior cerebellar artery
3.2 Middle Third of the Clivus
The bony aspect of this clival area is enclosed between the sellar floor superiorly and the sphenoid floor inferiorly; the protuberances of the ascending segments of ICAs, the so-called paraclival tract, represent the lateral limits of the area [41]. The identification of this region from a ventral endonasal route depends on the degree of pneumatization of the sphenoid sinus: it is well represented in sellar and presellar types, while it could be troublesome to recognize its boundaries in case of conchal sinuses (see Fig. 25.4).
Once the bone has been removed, the dura is exposed with the basilar venous plexus within: a segment of the sixth cranial nerve’s root runs in between the two layers of clival dura just before piercing Dorello’s canal [31]. The dural opening reveals the prepontine cistern with the sixth cranial nerves laterally; the pons with the basilar artery and its branches and the anteroinferior cerebellar arteries lie deeply (see Fig 25.6b). The pontomedullary junction and the vertebrobasilar junction (VBJ) are considered as the inferior edge of this region [7, 35].
3.3 Inferior Third of Clivus
The inferior third has its superior border at the level of the floor of the sphenoid sinus and reaches down the foramen magnum; on both sides, the inferior third of the clivus is not limited directly by the ICA as in the middle third, so that dissection can be safely extended further laterally. Upon the lateral aspects of the clival bone, the petroclival synchondrosis can be identified and followed all the way to the jugular foramen (see Fig. 25.6c). The occipital condyles are found in the anterior portion of the foramen magnum: the lateral exposure can be increased by removing the condyles up to the hypoglossal canal, wherein the twelfth cranial nerve runs, this latter being fixed as the limit of maximum lateral extent [7, 35]. Once the dura has been opened, the premedullary cistern and the medulla oblongata are identified as the epicenter of this area. Whether a supracondylar approach is extended through the jugular tubercle, the ninth, tenth, and eleventh cranial nerves are exposed, in the lateral aspect of the cistern [49].
4 Surgical Technique
Surgical approach for a clival chordoma should be planned according to the location, dimension, and spread of the tumor. Clival lesions located predominantly in the midline are more fit to the endoscopic endonasal approach, which could offer a safer and more direct anatomical route. When tumor involves lateral aspects of the area, the endoscopic transclival approach can be implemented, gaining more exposure by the opening of bony surfaces around the different segments of the ICA. The concept behind this kind of surgery is to minimize the opening at the most superficial compartment while expanding the exposure in close proximity of the targeted area [25, 26, 39, 40, 52, 57].
The initial segments of the procedure are run according to the paradigm of Pittsburgh school for the expanded endoscopic endonasal approaches: middle turbinectomy in one nostril, accompanied by posterior ethmoidectomy, and a wider anterior sphenoidotomy are accomplished. At this time, whether reconstruction should rely on naso-septal flap [28, 37], it should be harvested and stored in the maxillary sinus or down into the choana. Upon sphenoid sinus opening, different steps are required to achieve a complete exposure of the clivus in each of the three portions as considered in the anatomical classification.
4.1 Superior Third
Once the main landmarks, i.e., clival indent, carotid protuberances, and sellar floor, have been identified, the bone removal can start at the level of the sellar floor: dura is exposed from the superior intercavernous sinus (SIS), down to inferior intercavernous sinus (IIS) and, posteriorly, at the sella-clival junction. Once circular sinuses have been managed, the dura is opened up to the supradiaphragmatic space to allow freeing the pituitary stalk. In order to gain a more comfortable corridor, Kassam et al. [36] introduced the so-called pituitary transposition/trans-dorsum sellae technique: the gland is mobilized superiorly after ligaments connecting the pituitary capsule to the medial cavernous sinus have been dissected. The posterior sellar dura is coagulated and transected so that the dorsum sellae and posterior clinoids are exposed; these are then drilled and carefully removed, minding attention to avoid injuries to the ICA and third and sixth cranial nerves. The retroclival dura harboring the basilar plexus is visualized: basilar plexus can determine intense venous bleeding, which can be controlled with hemostatic agents such as oxidized cellulose or thrombin/gelatin matrix.
Once the dura has been opened tumor, debulking is first performed and then the dissection from surrounding neurovascular structures completes the removal maneuvers. Chordomas in this location can be tightly attached to the branches of the superior hypophyseal artery, which should be preserved in order to avoid vascular damages to the pituitary stalk and the optic chiasm; as well, the basilar apex and its perforators are pushed posteriorly and attached to tumor capsule, while, laterally, the lesion can impinge the third cranial nerve and the posterior communicating artery from which it should be dissected carefully. Owing that, these maneuvers should be performed sharply under direct closeup view. Finally, it is useful to remark that when performing removal of the inferior aspects of tumor involving this area, the preservation of the inferior horizontal lamina of Liliequist membrane helps to decrease subarachnoid blood dissemination to other cisterns.
4.2 Middle Third (Clival Recess of Sphenoid Sinus)
In well-pneumatized sphenoid sinus, the middle third of the clivus is that thin bone, i.e., the clival recess, lying over the posterior wall of the sphenoid sinus, enclosed between the ascending tracts (paraclival) of the ICAs.
The bone is drilled away and the dura and the basilar plexus are exposed; laterally, the exposure is limited by the paraclival ICAs; particularly in cases of chordomas located immediately behind this vessel, removal maneuvers are hindered via this route. In such situations, to increase exposure and instruments maneuverability, the ICA bony canals should be opened and the periosteum exposed in order to allow ICA mobilization. It is important to identify the vidian nerve as referring landmark for this approach: indeed it points toward the anterior genu of the ICA, at the level of the foramen lacerum, thus helping the identification of the petrous ICA in non-pneumatized sphenoid sinuses and/or cases in which the anatomy is altered by the disease [38].
In case of extradural chordomas, dissection maneuvers are run in a deep median plane according to bimanual microsurgical concept: this route offers a direct, safe corridor (see Fig. 25.7).
Whether chordomas have breached the dura, this is opened at the midline, under neurophysiology and nerve stimulation monitoring: it is of utmost importance to recognize at this level the sixth cranial nerve that could have been displaced medially by the tumor. Furthermore, image guidance system should also be used to determine the positioning of the vertebrobasilar junction (VBJ): dural opening should be performed below the VBJ, in order to ensure that the sixth cranial nerve origin remains above, at the brainstem.
When dissecting posterior aspects of the tumor, care should be taken to avoid injuries to the basilar artery and its perforator branches as well as their relationship with the pons.
4.3 Inferior Third
To properly expose this area, the nasal septum has to be detached from the anterior surface of the sphenoid bone, and the sphenoid sinus floor is completely drilled down to create a unique working room constituted of the sphenoid sinus and the nasopharynx; the basopharyngeal fascia is then stripped from the sphenoid rostrum and the clival bone.
Careful drilling of the anterior surface of the clivus down to the foramen magnum is carried out under image guidance; Kerrison rongeurs are used to bite off the bony speckles under endoscopic direct visual control. Dural exposure and whether or not the dura itself is opened depend on chordoma’s features, each approach being tailored to the single patient. In case of intradural tumor, the vertebral arteries, VBJ superiorly, and medulla represent the posterior limit of the dissection.
According to Prevedello et al., the dissection can be refined in a coronal plane identifying three modules [52]:
-
1.
Infrapetrous extension: in this module, the petrous bone below the ICA is removed and the area of the foramen lacerum exposed; the dense fibrous tissue attached to eustachian tube is transected.
-
2.
Supracondylar or transjugular tubercle approach: the occipital bone medial to the petroclival synchondrosis and above the occipital condyle is removed; the dissection follows the petroclival synchondrosis inferiorly in order to expose ninth, tenth, and twelfth cranial nerves. In such module investigation of these inferior nerves with neurophysiology monitoring is mandatory.
-
3.
Transcondylar route: a medial condylectomy is performed. The hypoglossal nerve represents the lateral limit of occipital condyles removal: this allows identification of the proximal aspect of the vertebral artery.
4.4 Reconstruction
This step of the procedure could tremendously affect the final surgical outcome. We usually adopt a multilayer technique that addresses all the single compartments of the osteodural defect [8, 21, 44]. Initially, intradural closure, consisting of the obliteration of dead space, with fibrin glue and/or autologous fat is achieved. Thereafter, the extradural closure with positioning of one or multiple dural substitute layers, free mucosal flaps, and/or fascia lata in the extradural space and/or over the bony aperture is performed. Recently, we perform what we call “sandwich technique”: the surgical cavity is filled with fat sutured the inner layer of a three-layer foil of fascia lata or dural substitute; the first layer is positioned intradurally, the second between the dura and the bone, and the third outside to cover the bone. A vascular flap of septal mucosa harvested according to the Hadad-Bassagasteguy [28, 37] method is used to cover the skull base defect and a moderate inflated Foley balloon catheter is then placed in the sphenoid sinus to support the reconstruction.
References
Al-Mefty O, Kadri PA, Hasan DM, Isolan GR, Pravdenkova S (2008) Anterior clivectomy: surgical technique and clinical applications. J Neurosurg 109(5):783–793
Anand VK, Harkey HL, Al-Mefty O (1991) Open-door maxillotomy approach for lesions of the clivus. Skull Base Surg 1(4):217–225
Ares C, Hug EB, Lomax AJ, Bolsi A, Timmermann B, Rutz HP, Schuller JC, Pedroni E, Goitein G (2009) Effectiveness and safety of spot scanning proton radiation therapy for chordomas and chondrosarcomas of the skull base: first long-term report. Int J Radiat Oncol Biol Phys 75(4):1111–1118
Arnold H, Sepehrnia A (1995) Extreme lateral transcondylar approach. J Neurosurg 82(2):313–314
Cappabianca P, Cavallo LM, Esposito F, de Divitiis O, Messina A, de Divitiis E (2008) Extended endoscopic endonasal approach to the midline skull base: the evolving role of transsphenoidal surgery. In: Pickard JD, Akalan N, Di Rocco C, Dolenc VV, Lobo Antunes J, Mooij JJA, Schramm J, Sindou M (eds) Advances and technical standards in neurosurgery. Springer, Wien/New York, pp 152–199
Catton C, O'Sullivan B, Bell R, Laperriere N, Cummings B, Fornasier V, Wunder J (1996) Chordoma: long-term follow-up after radical photon irradiation. Radiother Oncol 41(1):67–72
Cavallo LM, Messina A, Cappabianca P, Esposito F, de Divitiis E, Gardner P, Tschabitscher M (2005) Endoscopic endonasal surgery of the midline skull base: anatomical study and clinical considerations. Neurosurg Focus 19(1), E2
Cavallo LM, Messina A, Esposito F, de Divitiis O, Dal Fabbro M, de Divitiis E, Cappabianca P (2007) Skull base reconstruction in the extended endoscopic transsphenoidal approach for suprasellar lesions. J Neurosurg 107(4):713–720
Chanda A, Nanda A (2002) Partial labyrinthectomy petrous apicectomy approach to the petroclival region: an anatomic and technical study. Neurosurgery 51(1):147–159; discussion 159–160
Chibbaro S, Cornelius JF, Froelich S, Tigan L, Kehrli P, Debry C, Romano A, Herman P, George B, Bresson D (2014) Endoscopic endonasal approach in the management of skull base chordomas--clinical experience on a large series, technique, outcome, and pitfalls. Neurosurg Rev 37(2):217–224; discussion 224–215
Choi D, Crockard HA (2013) Evolution of transoral surgery: three decades of change in patients, pathologies, and indications. Neurosurgery 73(2):296–303; discussion 303–304
Cocke EW Jr, Robertson JH, Robertson JT, Crook JP Jr (1990) The extended maxillotomy and subtotal maxillectomy for excision of skull base tumors. Arch Otolaryngol Head Neck Surg 116(1):92–104
Couldwell WT, Weiss MH, Rabb C, Liu JK, Apfelbaum RI, Fukushima T (2004) Variations on the standard transsphenoidal approach to the sellar region, with emphasis on the extended approaches and parasellar approaches: surgical experience in 105 cases. Neurosurgery 55(3):539–550
Crockard HA, Steel T, Plowman N, Singh A, Crossman J, Revesz T, Holton JL, Cheeseman A (2001) A multidisciplinary team approach to skull base chordomas. J Neurosurg 95(2):175–183
Curtin HD, Rabinov JD, Som PM (2003) Central skull base. In: Head and neck imaging. Mosby, Saint Louis
de Divitiis E, Cappabianca P, Cavallo LM (2002) Endoscopic transsphenoidal approach: adaptability of the procedure to different sellar lesions. Neurosurgery 51(3):699–705; discussion 705–707
Delgado TE, Garrido E, Harwick RD (1981) Labiomandibular, transoral approach to chordomas in the clivus and upper cervical spine. Neurosurgery 8(6):675–679
DeMonte F, Diaz E Jr, Callender D, Suk I (2001) Transmandibular, circumglossal, retropharyngeal approach for chordomas of the clivus and upper cervical spine. Technical note. Neurosurg Focus 10(3), E10
Doucet V, Peretti-Viton P, Figarella-Branger D, Manera L, Salamon G (1997) MRI of intracranial chordomas. Extent of tumour and contrast enhancement: criteria for differential diagnosis. Neuroradiology 39(8):571–576
Erdem E, Angtuaco EC, Van Hemert R, Park JS, Al-Mefty O (2003) Comprehensive review of intracranial chordoma. Radiographics 23(4):995–1009
Esposito F, Dusick JR, Fatemi N, Kelly DF (2007) Graded repair of cranial base defects and cerebrospinal fluid leaks in transsphenoidal surgery. Neurosurgery 60(4 Suppl 2):295–303; discussion 303–304
Fatemi N, Dusick JR, Gorgulho AA, Mattozo CA, Moftakhar P, De Salles AA, Kelly DF (2008) Endonasal microscopic removal of clival chordomas. Surg Neurol 69(4):331–338
Fernandez-Miranda JC, Gardner PA, Snyderman CH, Devaney KO, Mendenhall WM, Suarez C, Rinaldo A, Ferlito A (2014) Clival chordomas: a pathological, surgical, and radiotherapeutic review. Head Neck 36(6):892–906
Feuvret L, Noel G, Weber DC, Pommier P, Ferrand R, De Marzi L, Dhermain F, Alapetite C, Mammar H, Boisserie G, Habrand JL, Mazeron JJ (2007) A treatment planning comparison of combined photon-proton beams versus proton beams-only for the treatment of skull base tumors. Int J Radiat Oncol Biol Phys 69(3):944–954
Frank G, Sciarretta V, Calbucci F, Farneti G, Mazzatenta D, Pasquini E (2006) The endoscopic transnasal transsphenoidal approach for the treatment of cranial base chordomas and chondrosarcomas. Neurosurgery 59(1 Suppl 1):ONS50–ONS57; discussion ONS50–ONS57
Fraser JF, Nyquist GG, Moore N, Anand VK, Schwartz TH (2010) Endoscopic endonasal transclival resection of chordomas: operative technique, clinical outcome, and review of the literature. J Neurosurg 112(5):1061–1069
Gupta A, Harnsberger HR (2004) Chordoma, clivus. In: Diagnostic imaging. Head and neck. Amyrsis Inc, Salt Lake City
Hadad G, Bassagasteguy L, Carrau RL, Mataza JC, Kassam A, Snyderman CH, Mintz A (2006) A novel reconstructive technique after endoscopic expanded endonasal approaches: vascular pedicle nasoseptal flap. Laryngoscope 116(10):1882–1886
Hardy J (2007) Transsphenoidal hypophysectomy. 1971. J Neurosurg 107(2):458–471
Hardy J, Vezina JL (1976) Transsphenoidal neurosurgery of intracranial neoplasm. Adv Neurol 15:261–273
Iaconetta G, Fusco M, Cavallo LM, Cappabianca P, Samii M, Tschabitscher M (2007) The abducens nerve: microanatomic and endoscopic study. Neurosurgery 61(3 Suppl):7–14; discussion 14
Igaki H, Tokuuye K, Okumura T, Sugahara S, Kagei K, Hata M, Ohara K, Hashimoto T, Tsuboi K, Takano S, Matsumura A, Akine Y (2004) Clinical results of proton beam therapy for skull base chordoma. Int J Radiat Oncol Biol Phys 60(4):1120–1126
James D, Crockard HA (1991) Surgical access to the base of skull and upper cervical spine by extended maxillotomy. Neurosurgery 29(3):411–416
Janecka IP, Nuss DW, Sen CN (1991) Facial translocation approach to the cranial base. Acta Neurochir Suppl 53:193–198
Kassam A, Snyderman CH, Mintz A, Gardner P, Carrau RL (2005) Expanded endonasal approach: the rostrocaudal axis. Part II. Posterior clinoids to the foramen magnum. Neurosurg Focus 19(1), E4
Kassam AB, Prevedello DM, Thomas A, Gardner P, Mintz A, Snyderman C, Carrau R (2008) Endoscopic endonasal pituitary transposition for a transdorsum sellae approach to the interpeduncular cistern. Neurosurgery 62(3 Suppl 1):57–72; discussion 72–74
Kassam AB, Thomas A, Carrau RL, Snyderman CH, Vescan A, Prevedello D, Mintz A, Gardner P (2008) Endoscopic reconstruction of the cranial base using a pedicled nasoseptal flap. Neurosurgery 63(1 Suppl 1):ONS44–ONS52; discussion ONS52–ONS53
Kassam AB, Vescan AD, Carrau RL, Prevedello DM, Gardner P, Mintz AH, Snyderman CH, Rhoton AL (2008) Expanded endonasal approach: vidian canal as a landmark to the petrous internal carotid artery. J Neurosurg 108(1):177–183
Komotar RJ, Starke RM, Raper DM, Anand VK, Schwartz TH (2011) The endoscope-assisted ventral approach compared with open microscope-assisted surgery for clival chordomas. World Neurosurg 76(3–4):318–327; discussion 259–362
Koutourousiou M, Gardner PA, Tormenti MJ, Henry SL, Stefko ST, Kassam AB, Fernandez-Miranda JC, Snyderman CH (2012) Endoscopic endonasal approach for resection of cranial base chordomas: outcomes and learning curve. Neurosurgery 71(3):614–624; discussion 624–625
Labib MA, Prevedello DM, Carrau R, Kerr EE, Naudy C, Abou Al-Shaar H, Corsten M, Kassam A (2014) A road map to the internal carotid artery in expanded endoscopic endonasal approaches to the ventral cranial base. Neurosurgery 10(Suppl 3):448–471; discussion 471
Lanzino G, Dumont AS, Lopes MB, Laws ER Jr (2001) Skull base chordomas: overview of disease, management options, and outcome. Neurosurg Focus 10(3), E12
Laws E (1993) Clivus chordomas. In: Sekhar LN, Janecka IP (eds) Surgery of cranial base tumors. Raven, New York, pp 679–685
Leng LZ, Brown S, Anand VK, Schwartz TH (2008) “Gasket-seal” watertight closure in minimal-access endoscopic cranial base surgery. Neurosurgery 62(5 Suppl 2):ONSE342–ONSE343; discussion ONSE343
Liu JK, Couldwell WT (2005) Far-lateral transcondylar approach: surgical technique and its application in neurenteric cysts of the cervicomedullary junction. Report of two cases. Neurosurg Focus 19(2), E9
Liu JK, Decker D, Schaefer SD, Moscatello AL, Orlandi RR, Weiss MH, Couldwell WT (2003) Zones of approach for craniofacial resection: minimizing facial incisions for resection of anterior cranial base and paranasal sinus tumors. Neurosurgery 53(5):1126–1135; discussion 1135–1137
Maira G, Pallini R, Anile C, Fernandez E, Salvinelli F, La Rocca LM, Rossi GF (1996) Surgical treatment of clival chordomas: the transsphenoidal approach revisited. J Neurosurg 85(5):784–792
Mendenhall NP, Malyapa RS, Su Z, Yeung D, Mendenhall WM, Li Z (2011) Proton therapy for head and neck cancer: rationale, potential indications, practical considerations, and current clinical evidence. Acta Oncol 50(6):763–771
Morera VA, Fernandez-Miranda JC, Prevedello DM, Madhok R, Barges-Coll J, Gardner P, Carrau R, Snyderman CH, Rhoton AL Jr, Kassam AB (2010) “Far-medial” expanded endonasal approach to the inferior third of the clivus: the transcondylar and transjugular tubercle approaches. Neurosurgery 66(6):211–219; discussion 219–220
Osborn AG (2012) Clival chordomas. In: Osborn's brain: imaging, pathology and anatomy. Lippincott Williams & Wilkins, Philadelphia
Pallini R, Maira G, Pierconti F, Falchetti ML, Alvino E, Cimino-Reale G, Fernandez E, D'Ambrosio E, Larocca LM (2003) Chordoma of the skull base: predictors of tumor recurrence. J Neurosurg 98(4):812–822
Prevedello DM, Ditzel Filho LF, Solari D, Carrau RL, Kassam AB (2010) Expanded endonasal approaches to middle cranial fossa and posterior fossa tumors. Neurosurg Clin N Am 21(4):621–635, vi
Rhoton AL Jr (2000) The cerebellar arteries. Neurosurgery 47(3 Suppl):S29–S68
Rhoton AL Jr (2002) The sellar region. Neurosurgery 51(4 Suppl):S335–S374
Samii A, Gerganov VM, Herold C, Hayashi N, Naka T, Mirzayan MJ, Ostertag H, Samii M (2007) Chordomas of the skull base: surgical management and outcome. J Neurosurg 107(2):319–324
Sen C, Triana AI, Berglind N, Godbold J, Shrivastava RK (2010) Clival chordomas: clinical management, results, and complications in 71 patients. J Neurosurg 113(5):1059–1071
Stippler M, Gardner PA, Snyderman CH, Carrau RL, Prevedello DM, Kassam AB (2009) Endoscopic endonasal approach for clival chordomas. Neurosurgery 64(2):268–277; discussion 277–288
Swearingen B, Joseph M, Cheney M, Ojemann RG (1995) A modified transfacial approach to the clivus. Neurosurgery 36(1):101–104; discussion 104–105
Tamaki N, Nagashima T, Ehara K, Motooka Y, Barua KK (2001) Surgical approaches and strategies for skull base chordomas. Neurosurg Focus 10(3), E9
Weiss MH (1987) The transnasal transsphenoidal approach. In: Apuzzo MLJ (ed) Surgery of the third ventricle. Williams & Wilkins, Baltimore, pp 476–494
Wen HT, Rhoton AL Jr, Katsuta T, de Oliveira E (1997) Microsurgical anatomy of the transcondylar, supracondylar, and paracondylar extensions of the far-lateral approach. J Neurosurg 87(4):555–585
Yasuda M, Bresson D, Chibbaro S, Cornelius JF, Polivka M, Feuvret L, Takayasu M, George B (2012) Chordomas of the skull base and cervical spine: clinical outcomes associated with a multimodal surgical resection combined with proton-beam radiation in 40 patients. Neurosurg Rev 35(2):171–182; discussion 182–183
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
1 Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Video 25.1
(MP4 63577 kb)
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Solari, D. et al. (2016). Endoscopic Endonasal Transsphenoidal Approach. In: Cappabianca, P., Cavallo, L., de Divitiis, O., Esposito, F. (eds) Midline Skull Base Surgery. Springer, Cham. https://doi.org/10.1007/978-3-319-21533-4_25
Download citation
DOI: https://doi.org/10.1007/978-3-319-21533-4_25
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-21532-7
Online ISBN: 978-3-319-21533-4
eBook Packages: MedicineMedicine (R0)