Abstract
In addition to conditions specific to pregnancy such as eclampsia and peripartum cardiomyopathy, nearly any cardiac condition may occur during pregnancy. Common cardiovascular conditions may occur in 1 % of all pregnant women. Physiological increased volume loading during pregnancy may exacerbate cardiac conditions including valvular disease, shunts, or heart failure. Cardiac imaging examinations in the pregnant patient include radiography, echocardiology, and MRI.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Impact of Pregnancy on Cardiovascular Function
Building an understanding of the hemodynamic changes that occur during pregnancy and the peripartum period is critical to applying MRI as a tool to evaluate the cardiovascular system. During the first trimester, there is retention of sodium and water leading to an increase in total blood volume. Although there have been varying reports in the literature, it is estimated that overall there is an approximately 50 % increase in total blood volume [28]. This expansion of blood volume generally peaks in the second trimester and remains elevated through the peripartum period. Although the expansion in plasma volume is associated with a rise in red blood cell production, typically a mild decrease in the patient’s hematocrit is still observed resulting in a dilutional anemia [58]. The majority of the increased blood volume becomes physiologically distributed to the placenta, as well as the maternal kidneys and skin.
As a result of expansion in blood volume, there is a compensatory increase in cardiac output. Cardiac output, defined as stroke volume multiplied by heart rate, has been shown to rise to a maximum of approximately 50 % above baseline. Although these changes are associated with enlargement of the left atrium and left ventricle, progressive increase in valve orifice area, and left ventricular wall thickness [68], left ventricular function appears to be well preserved in the healthy pregnant patient [51]. The increase in cardiac output allows physiological compensatory response to increased preload from expansion in blood volume and decreased afterload from decreased systemic vascular resistance.
Additionally, there is a general increase in vascular compliance during pregnancy. This phenomenon is multifactorial, likely influenced by a variety of factors including change in hormonal balance, increased cardiovascular demands, and actual changes in the structure of the vascular wall [29]. Increased vascular compliance puts that patient at heightened risk for vascular complications such as dissection and aneurysm formation.
Pregnancy also results in a hypercoagulable state. Fundamentally, there is increased production of several coagulation factors, decreased production of protein S, and inhibition of the fibrinolytic system [74]. When paired with other influences such as increased venous distensibility, inhibition of venous return caused by compression of the inferior vena cava by the gravid uterus, and inactivity and prolonged bed rest, the risk for development of thromboembolism increases dramatically [74].
The process of labor and delivery can be associated with sudden and dramatic hemodynamic changes. Not only is there a physiological increase in cardiac output caused by return of blood to circulation, pain and anxiety as well as anesthesia used during labor all contribute to changes in cardiac output [29]. Of course, the prominent rise in cardiac output is not present in the case of a cesarean section. Only a mild increase in cardiac output during the postpartum period can be observed in the case of cesarean section [29].
During the immediate postpartum period, there is a rise in cardiac input, due to uterine involution, followed by a gradual return to baseline during the initial postpartum months [14].
Overall, the changes in cardiac function, intravascular volume, and vascular compliance create a unique set of clinical risk factors for the pregnant patient. Careful evaluation in light of knowledge of the dynamic cardiovascular system is critical for prompt and accurate diagnosis. This chapter discusses use of magnetic resonance imaging (MRI) as a tool for evaluation of the cardiovascular system in the pregnant patient.
2 Imaging Approach to Cardiovascular Diseases in the Pregnant Patient
The variety of cardiovascular diseases that can present during pregnancy and the peripartum period necessitates accurate and prompt diagnostic workup. Although cardiac computed tomography and traditional angiography are typically used in the nonpregnant population, their use is generally avoided in the pregnant patient due to the risks of fetal exposure to ionizing radiation (see [1] for a review of use of ionizing radiation in pregnancy).
Therefore, the primary imaging options include transthoracic and transesophageal echocardiography and MRI. Echocardiography, appropriately so, remains the initial diagnostic study of choice for workup of many different cardiovascular diseases during pregnancy. However, continued technological advances have allowed MRI to emerge as a technique that is often equivocal or even superior to echocardiography in some clinical circumstances.
As will be detailed in this chapter, MRI has a myriad of uses for the diagnostic evaluation of both congenital and acquired cardiovascular pathology. MRI is excellent at assessing left ventricular geometry and function, as well as valve pathology. MRI is thought to be superior to other imaging modalities, in particular for evaluation of pericardial disease [1]. Cine imaging allows for assessment of cardiac function and blood flow dynamics. Non-contrast MR angiographic techniques allow for detailed assessment of vascular pathology.
The decision to administer gadolinium-based contrast agents must be carefully considered. Gadolinium-based contrast agents are FDA class C medications, and teratogenic effects have been documented in animals exposed to doses of 3 to 33× clinical human daily doses for up to 12 days [18]. Further discussion of gadolinium and MRI safety with respect of the fetus is detailed elsewhere in this book.
When selecting an imaging technique, one must consider the notable amount of time needed to complete an MRI examination. In acute clinical situations, the length of the imaging exam might preclude its use. Furthermore, many MRI suites are not suited for critically ill patients requiring life support measurements and advanced ICU-level care. For this reason, faster exams or exams able to be completed at bedside may be preferred in critically ill patients.
Often echocardiography examinations can be limited by poor acoustic window or limited visualization of cardiac structures. In this case, MRI is often used as a troubleshooting tool to obtain a more complete evaluation of the patient [78]. MRI is particularly helpful for aortic pathology, complex congenital heart disease, and cardiac tumors (Fig. 17.1).
This 37-year-old woman G3P2 presented at 24 weeks’ gestation with increasing shortness of breath. Coronal gated spin echo (a) and (b) axial cine gated gradient echo images demonstrated a 5 cm right atrial pedunculated mass (*). She underwent operative delivery at 28 weeks followed by removal of a right atrial myxoma
In the CHIRP study (Cardiovascular magnetic resonance in pregnancy) by Ducas and colleagues [25], prospective comparison between MRI and transthoracic echocardiography demonstrated that there was good correlation between data on left ventricular mass, stroke volume, and cardiac output. Interestingly, transthoracic echocardiography was found to consistently underestimate the values. This study provides us with reference cardiac indexes for normal pregnancy for future comparison to various pathological conditions.
3 Basics of Cardiac MRI in the Pregnant Patient
Although a variety of both traditional and experimental MRI protocols can be used to assess the cardiovascular system, a few key protocols are essentially the workhorses of cardiovascular MRI. Basic anatomical assessment is traditionally carried out with black blood and bright blood imaging. In black blood imaging, a spin echo technique, the cardiac tissues demonstrate high signal intensity. The blood possesses low signal intensity, therefore appearing black. In bright blood imaging, a gradient echo technique, the cardiac tissues possess low signal intensity and the blood pool is bright, possessing high signal intensity. Not only is the bright blood technique good for anatomical assessment, it also allows evaluation of left ventricular and valve function as well as assessment of any intracardiac shunts. Similar to the bright blood technique, steady-state free precession is sometimes used to generate cine images with excellent temporal and spatial resolution. To complete functional assessment of the valves and any intracardiac shunts, flow velocity encoding technique or phase contrast is often used.
Cardiac and respiratory gating may be necessary to ensure good diagnostic quality of the images. Traditionally, cardiac gating is used throughout the imaging acquisition. Presence of atrial fibrillation or excessive irregular beats can interfere with cardiac gating and degrade image quality. Presence of respiratory motion can also degrade image quality. Either breath-hold technique or respiratory gating should be utilized depending on the length of time needed for image acquisition of each sequence. Alternatively, ultra-fast “real-time” cardiac MR may be performed without cardiac or respiratory triggering [60].
Particular attention should be paid to patient positioning. The gravid uterus may cause venous compression and therefore affect baseline hemodynamic function. A study performed by Rossi and colleagues [69] concluded that patient position affects venous return, stroke volume, and cardiac output. It is suggested that starting at the 20th week of gestation, patients should be positioned in the left lateral position, rather than the supine position. This is of utmost importance in patients that may receive serial exams throughout pregnancy. Positioning should remain in the left lateral position to assure changes in function are not confounded by the supine position.
The CHIRP study [25] detailed MRI findings in normal healthy pregnant patients. It is important to understand the expected physiological changes that occur during pregnancy and the associated finding on MRI. The authors demonstrated an increase in left ventricular end-diastolic volume and left ventricular end-diastolic diameter in the third trimester. There was also a significant increase in left ventricular mass. Increase in both heart rate and stroke volume resulted in calculated increase in cardiac output in the third trimester.
The CHIRP study also demonstrated significant increase in right ventricular end-diastolic diameter, right ventricular mass, and right ventricular volume. Additionally there was biatrial enlargement in the third trimester. Interestingly, there was no change in the size or geometry of the aortic root or ascending aorta during pregnancy.
4 Evaluation of Congenital Cardiovascular Diseases
4.1 ASD
Atrial septal defects (ASDs) are a fairly common congenital heart anomaly and have been found to make up approximately 13 % of congenital heart disease worldwide [76]. Abnormalities occurring during embryonic formation of the heart during organogenesis can result in a variety of different defects. The two most common types of ASD are primum ASD and secundum ASD. Primum ASD occurs with a defect in the most apical section of the intra-atrial septum. In this defect, there is an abnormality in the development of the endocardial cushions. This type of ASD is often associated with malformation of the atrioventricular valves as well as ventricular septal defects. The secundum type of ASD results from halted formation of the septum secundum. Alternatively, this defect also may result from over-absorption of the septum primum. Secundum ASDs are far more common than primum types and are seen twice as frequently in women [43]. ASDs can also involve the sinus venosus and the coronary sinus. Sinus venosus ASDs are quite rare, demonstrating connection between the superior or inferior vena cava and the right pulmonary veins. Ganigara and colleagues [34] point out that diagnosis can be particularly challenging, and MRI is an essential diagnostic tool. Involvement with the coronary sinus is considered the most rare type. In this form, an unroofed coronary sinus connects directly with the left atrium.
The clinical presentation of patients with ASDs can be widely varied depending on the size of the defect and the resultant potential left-to-right shunting of blood. Although some patients may be asymptomatic for quite some time, physiological situations, such as pregnancy, which increase demand on the cardiovascular system, may result in symptomatic presentation. Common symptoms include atrial arrhythmias or general fatigue, as well as dyspnea. On physical exam, patients may demonstrate parasternal right ventricular lift. Classically, on auscultation, a fixed split of the S2 heart sound can be heard. Although a systolic ejection murmur is often present, this can be easily overlooked as it may mimic the physiological murmur of pregnancy. During pregnancy, large ASDs may result in congestive heart failure, or the patient may also develop atrial arrhythmias, prompting a diagnostic workup. Given the hypercoagulable state of pregnancy, the development of peripheral deep vein thrombosis is of particular concern given the theoretical risk of paradoxical embolus.
Depending on the acuity of the clinical presentation, women may receive initial medical diagnostic workups that include EKGs and, in isolated circumstances, a chest radiograph. EKG may show a partial right bundle branch block. Depending on the size of the defect, initial radiograph may demonstrate enlargement of the right heart and signs of pulmonary vascular congestion. Echocardiography is the diagnostic test of choice for evaluation of the location and extent of the defect. However, in more complicated cases cardiac MRI is useful. In fact, more recently MRI is becoming an essential integral part of the complete diagnostic workup [67]. Of particular benefit, Beerbaum and colleagues [9] demonstrated that cardiac MRI could be used to quantify blood flow using the phase-contrast cine technique. Cardiac MRI can provide detailed assessment of defect size and location and also aid in detection of other associated conditions such as anomalous pulmonary venous return. Furthermore, MRI can be particularly useful in diagnosing the more rare types of ASD such as coronary sinus ASD [15]. In directly comparing cardiac MRI to transesophageal echocardiography, Piaw and colleagues [64] demonstrated cardiac MRI cine balanced fast field echo and phase-contrast technique to correlate well with measurements obtained via echocardiography and provide the added benefit of additional anatomical information.
Alpendurada and colleagues [3] recommend beginning the initial MRI evaluation with anatomical images in the transaxial, coronal, and sagittal planes using a sequence such as single-shot turbo spin echo and single-shot balanced steady-state free precession. Cine images are also recommended for adequate anatomical evaluation. For ASDs in particular, a short-axis stack in the atrioventricular plane may be particularly critical for accurate evaluation. Finally, flow measurements can be used to assess the dynamic impact of the ASD on cardiac function. Similarly, Gulati and colleagues [42], in describing how their institution evaluates ASDs, suggest axial black-blood-prepared fast spin echo images. This is followed by bright blood steady-state free precession cine sequences. Finally velocity-encoded cine sequences are used to assess flow.
In pregnant patients requiring acute invasive intervention, such as percutaneous transcatheter ASD closure, MRI has proven to be a valuable tool in assessing the impact of intervention. For example, Burgstahler and colleagues [13] demonstrated the use of MRI to accurately evaluate cardiac function post ASD closure. For more detailed and accurate assessment, Chen and colleagues [16] suggest a technique in which, following anatomical imaging acquisition, a cine gradient echo-planar sequence is used with a labeling pre-pulse to tag across the basal myocardium. Right ventricular tag displacement can then be measured and tracked to standardize measurement of right ventricular function.
4.2 VSD
Ventricular septal defects (VSDs) while considered the most common congenital heart defect are more rare in the adult population due to the common occurrence of spontaneous closure. As classified by Morello [47] there are four main types of VSD, divided according to anatomical location. Type 1 demonstrates infundibular septum defects. In this type there is a septal deficiency between the crista supraventricularis and the aortic and pulmonary valves. Aortic regurgitation is common. Type 2 defects are present in the membranous septum. This is considered the most common type of VSD. If the defect extends into the muscular septum, this may be classified as a perimembranous VSD. Type 3 VSD demonstrates a defect in the inlet septum. The defect is therefore located below the level of the mitral and tricuspid valves. Type 4 VSD demonstrates defects in the muscular septum. This type is not located in close proximity to the cardiac valves. An atrioventricular VSD, or Gerbode defect, is characterized by a defect in the septum separating the right atrium from the left ventricle. This type is exceptionally rare.
Severity of clinical symptoms depends on the size and location of the defect and the resultant severity of left-to-right shunting of blood. Although most women are asymptomatic throughout pregnancy, larger defects may potentially result in symptoms associated with left ventricular overload including fatigue and dyspnea. Development of pulmonary hypertension or aortic regurgitation is also a potential result of larger VSDs. In a study by Karamlou and colleagues [50], women with VSDs in particular, who are at increased risk of mortality and peripartum complications, often do not receive diagnostic work and care at academic centers. Proper diagnostic workup and treatment are critical given the known increased risk of preeclampsia, premature labor, and small for gestational age births in women with unrepaired VSDs [80].
Diagnostic workup often begins with an EKG demonstrating left ventricular hypertrophy. Chest radiography (if acquired) often shows nonspecific signs such as increased pulmonary vascular congestion and left heart enlargement. Echocardiography is often the diagnostic imaging test of choice to visualize and classify the defect. However, MRI may be necessary for more thorough evaluation and determining need for and timing of potential interventions. MRI allows for a more detailed anatomical assessment of the pulmonary vasculature and allows for quantification of left-to-right shunting using phase-contrast cine technique [24]. Specific techniques and recommended sequences are similar to that detailed in evaluation of the ASD.
4.3 PDA
A patent ductus arteriosus (PDA) can occur when the ductus arteriosus, or fetal connection between the aorta and the main pulmonary artery, fails to undergo natural postnatal closure. Although most PDAs are detected and treated early in childhood, a few may persist undetected into adulthood. Depending on the size of the PDA and the resultant left-to-right shunt, there is a potential risk for the development of elevated pulmonary vascular resistance. If the defect is significantly large, patients may present in preeclampsia and heart failure due to the increased cardiovascular demands of pregnancy [2].
Initial diagnostic workup often includes a chest radiograph demonstrating an enlarged left ventricle and pulmonary artery as well as increased pulmonary vascular congestion. If the PDA is small, the radiograph may appear within normal limits. Occasionally calcification of the ductus may be seen on lateral radiograph. Classically, the echocardiography workup shows continuous flow within the pulmonary artery [28]. MRI is useful for more complicated cases and for surgical planning. MRI allows accurate description of the size of the PDA and degree of calcifications [4].
Because many PDAs are identified and treated in childhood, the treatment has the potential to impact the patient throughout their adult life. Many of the treatment devices and coils are not MRI-compatible and therefore limit diagnostic evaluation of the patient during the pregnant and peripartum periods. Grifka and colleagues [40] demonstrate use of non-ferromagnetic embolization coils, used for transcatheter PDA closure, therefore enabling future successful evaluation with cardiac MRI.
4.4 Aortic Stenosis
Valvular aortic stenosis (AS) has a wide variety of potential underlying causes. Of note, stenosis can occur at the sub- and supravalvular levels in addition to the valvular level. Congenitally, AS may result from an abnormal unicuspid or bicuspid valve which develops calcifications. Alternatively, calcifications can develop on a trileaflet valve, resulting in stenosis. Rheumatic heart valve disease may also result in AS, discussed later in this chapter. AS is monitored and classified based on the antegrade velocity of blood across an abnormal valve, resulting in varying degrees of obstruction to left ventricular ejection.
AS can be asymptomatic until the outflow obstruction becomes hemodynamically significant. Symptoms are relatively nonspecific and include shortness of breath, presyncope/syncope, or angina. Ideally, AS is recognized and treated prior to pregnancy, as mortality and morbidity are increased in patients with AS during pregnancy and the peripartum period. Furthermore, women with severe AS who are symptomatic during pregnancy are at increased risk of requiring cardiac interventions postpartum [75]. The 2008 update of the 2006 ACC/AHA guidelines notes severe AS as a condition associated with high maternal risk during pregnancy. Furthermore, the guidelines suggest counseling to delay conception until after treatment.
Diagnostic workup often includes EKG and chest radiographs. Chest radiographs may be normal. Alternatively, one might see an enlarged left ventricle or post-stenotic aortic dilatation. Diagnosis and evaluation are routinely made by echocardiography, which allows for detailed analysis of the aortic valve. Although echocardiography is the gold standard for assessing the aortic valve, cardiac MRI has proven to be useful in assessing associated features. Cardiac MRI has been used for assessment of left ventricular volume and function. Furthermore MRI allows for more detailed assessment of the myocardium.
4.5 Mitral Valve Prolapse
Prolapse of the mitral valve can result in varying degrees of mitral regurgitation. Prolapse may be congenital or acquired in nature. Prolapse is technically defined as one or both mitral valve leaflets projecting into the left atrium during systole. This may be due to abnormalities in or disruption of the valve leaflets, the chordae, or the papillary muscles. A component of myxomatous degeneration of the valve leaflets may also be present. Mitral valve prolapse is present frequently, and the associated mortality and morbidity with the pregnant and peripartum periods are relatively low. However, one must consider the possibility of the cardiac demands of pregnancy precipitating an arrhythmia. Additionally, there is a risk of infective endocarditis, for which prophylactic antibiotics may be clinically indicated.
Although echocardiography is the most appropriate first step in assessment of the mitral valve and evaluation of degree of regurgitation associated with valve prolapse, MRI is emerging as a valuable tool to aid in diagnosis. Not only does MRI provide accurate assessment of the mitral valve function, it also often elucidates the underlying cause for functional abnormality. Cardiac MRI provides excellent insight into the geometry of the mitral annulus. As detailed by Morello and Gelfand [59], the degree of mitral valve prolapse is often best assessed by oblique coronal steady-state free precession imaging of the left ventricular outflow tract or alternatively in the four-chamber view. MRI can further be used to quantify severity of regurgitation. This can be assessed with volumetric methods or flow velocity mapping. The precise volume of mitral regurgitation can even be obtained through subtraction of the aortic flow from left ventricular inflow [59]. Cardiac MRI can also be used to assess the long-term effects of mitral regurgitation. Left ventricular hypertrophy and other changes in right ventricular geometry can be accurately detailed.
4.6 Coarctation of the Aorta
Coarctation of the aorta is generally defined as descending aorta narrowing, commonly at the insertion sites of the ductus arteriosus distal to the left subclavian artery. This anatomical variation, a congenital anomaly, results in left ventricular pressure overload. Of note, coarctation is usually present with other cardiac anomalies such as abnormal valves, ASD, or VSD.
If the anomaly is not diagnosed until adulthood, the most common clinical presentation is hypertension. In severe cases, sequel of hypertension such as stroke or aortic dissection can also be seen. On physical exam, there is a difference in systolic blood pressure between the upper and lower extremities. The upper extremities display hypertension, while the lower extremities are often hypotensive.
ECG is most often normal. Chest radiograph demonstrates typical findings such as rib notching and contour indentation of the aorta at the coarctation site, producing the classic “3” sign. Echocardiography is often the first advanced imaging modality of choice. However, the [12] ACC/AHA adult congenital heart disease guidelines specify that every adult with known coarctation, regardless of repair, is required to have at least one cardiac MRI (or CT) for a complete diagnostic workup. MRI allows for detailed anatomical analysis of the location and extent of the coarctation. Furthermore, MRI allows for mapping of collateral vessels. When the diagnosis is known prior to conception, MRI has been shown to allow for prediction of increased risk for hypertensive events. Jimenez-Juan and colleagues [48] demonstrated that smaller aortic dimensions correlated to increased risk of hypertensive events during pregnancy in women with coarctation of the aorta. MRI has also been proven as an accurate tool to predict catheterization gradient across a coarctation [61] and therefore an excellent alternative to angiography during pregnancy.
4.7 Pulmonary Stenosis
Pulmonary stenosis can occur at the valvular, subvalvular, or supravalvular level. Usually, a trileaflet valve with fibrous thickening is identified in a patient with congenital pulmonic stenosis. The leaflets are often described as having a characteristic “fish-mouth” appearance. PS is often associated with right ventricular hypertrophy. Rarely, stenosis can be seen in a bicuspid valve.
This anomaly is often missed in childhood and may present later in adulthood with increased cardiovascular demand such as pregnancy. Patients may present with fatigue, presyncope/syncope, or angina. On auscultation, one might detect splitting of the second heart sound or a right-sided fourth heart sound. Echocardiography is considered the gold standard for assessment of the pulmonic valve. In general, PS does not present with significantly increased mortality or morbidity in the peripartum period.
Although echocardiography is traditionally the gold standard for diagnostic workup, MRI can be of use for more complex cases or for planning intervention. Dedicated sequences through the pulmonary valve can be obtained using steady-state free precession for an accurate analysis of valve function. Flow across the valve is analyzed with velocity-encoded phase-contrast images. Furthermore, pulmonary vasculature can also be assessed with a 3D spoiled gradient echo angiographic sequence [66]. MRI cine images demonstrate thickened valve leaflets that take on a dome-shaped appearance due to restricted motion. Furthermore, cine images often show a dark jet across the pulmonic valve due to dephasing caused by high velocity [66].
4.8 Tetralogy of Fallot
Tetralogy of Fallot, first described in 1889 by Étienne-Louis Arthur Fallot, is one of the most common congenital cyanotic heart conditions. Although detection and subsequent surgical repair are becoming increasingly more common shortly after birth, rarely women do present during pregnancy or the peripartum period with an unrepaired defect. Tetralogy of Fallot consists of four key anatomical abnormalities. First, patients demonstrate pulmonary stenosis. The point of maximal stenosis can vary but is most commonly in the infundibular position. Second, there is a presence of a usually large ventricular septal defect (VSD). Of note, there may be multiple VSDs present. The VSD is typically present in the perimembranous region and may extend into the muscular septum. Third, there is deviation of the aorta rightward such that the aorta overrides the VSD, receiving blood flow from both ventricles. Fourth, pulmonary stenosis results in right ventricular hypertrophy. Postoperative evaluation will be discussed later in this chapter.
Given the altered hemodynamic flow, patients are acutely sensitive to changes in systemic vascular resistance. Changes that occur throughout pregnancy and particularly in labor and delivery create a potential situation of acute cyanosis that carries a high risk of mortality and morbidity for both the mother and the fetus.
Workup generally begins with a chest radiography demonstrating a “boot-shaped” cardiac silhouette. Echocardiography is typically the primary method for diagnosis and evaluation. Partington and Valente [62] describe in detail how to approach evaluation with cardiac MRI. It is critical to visualize and evaluate the entirety of the right ventricular outflow tract (RVOT). There is a spectrum of varying severities of obstruction that is seen in tetralogy of Fallot. The RVOT should be visualized in long-axis as well as two-chamber cine views, thus allowing accurate assessment of the hemodynamic flow through the tricuspid valve. One should look carefully for RVOT aneurysms as well as valve abnormalities. If needed in the case of distal pulmonary stenosis, additional sequences such as MRA can be added for further assessment of the pulmonary artery. Particularly in the peripartum period, detailed and accurate assessment of right ventricular function is needed. Potential dysfunction of the left ventricle should also be evaluated. Often found concurrently with RVOT, pulmonary regurgitation can be easily identified and assessed with cardiac MRI. A plethora of variations and anomalies can be found in tetralogy of Fallot, and cardiac MRI is excellent for providing detailed anatomical assessment. One can assess for variations in coronary anatomy, presence of a right-sided aortic arch, and anomalies in the pulmonary arteries. Cardiac MRI can provide critical diagnostic details, providing anatomical information about the entire heart, valves, and many associated vascular anomalies.
4.9 Ebstein Anomaly
Ebstein anomaly results from congenital malformation of the tricuspid valve and right ventricle. Normally, the valve consists of three leaflets: the anterior, posterior, and septal. In Ebstein anomaly, the leaflets extend below the anatomical annulus and form varying types of attachment to the ventricle. The leaflets themselves often display abnormal morphology with a wide spectrum of dysplasia and dysfunction. Several classification systems have been proposed to describe the wide range of pathology seen in this condition encompassing both anatomical and functional descriptions (see [35] for a review). Most recently, and perhaps most relevant to radiographic descriptions, a classification was proposed by Derani and Danielson [23] which incorporates both echocardiographic appearance and findings at the time of surgical evaluation, grading the defect I, II, III, or IV.
Although this congenital cyanotic heart anomaly is often recognized in infancy or childhood, it may have rare presentation in the adult population. In adults, Ebstein anomaly is most likely to present with arrhythmias. Certainly the increased cardiovascular demands of pregnancy can potentially precipitate symptoms such as arrhythmia.
If performed, initial chest radiography will often demonstrate features consistent with right atrial enlargement, and EKG will demonstrate congruent findings and frequently demonstrates right bundle branch block. More thorough evaluation is generally performed with echocardiography. However, MRI can be a useful tool for evaluation of more complex cases. MRI allows for further detailing of the tricuspid valve, detailing the severity of tricuspid regurgitation and providing functional data on the ventricles as well as ventricular volume. If present, as in some cases, the extent of right-to-left shunt can also be assessed. In Ebstein anomaly, the right ventricle is divided into two portions. The atrialized portion lies above the attachment of the abnormal leaflets, and the remaining functional right ventricle lies below the leaflet attachments. Cardiac MRI allows for full analysis of the atrialized portion, functional portion, and total right ventricle through the use of cine four-chamber views and axial and short-axis views [35].
4.10 Tricuspid Atresia
In tricuspid atresia there is either congenital absence or agenesis of the tricuspid valve. This results in lack of communication between the right atrium and right ventricle. There are varying types of atresia with classification based on the anatomical findings. There is, for example, muscular atresia, membranous atresia, and valvular atresia. Valvular anomalies are always associated with an atrial septal defect that allows communication of blood from the right to the left ventricle. There is also, due to alteration in hemodynamic flow, right ventricular hypertrophy. Quite often, there is a VSD as well. Other potential associations include obstruction of the pulmonary outflow tract and a hypoplastic pulmonary artery. The great arteries may be in normal anatomical position or transposed. Tricuspid atresia is generally classified into three types, based on anatomical findings.
Rarely, tricuspid atresia goes undetected into adulthood; however there have been several case reports of adults successfully delivering babies with uncorrected tricuspid atresia [10]. Cardiac MRI can be helpful in distinguishing tricuspid atresia from Ebstein anomaly. Initial imaging with chest radiography demonstrates nonspecific findings depending on pulmonary blood flow. Sometimes, findings consistent with right atrial enlargement are present. Patients are typically evaluated with echocardiography. However, MRI is becoming an increasingly more essential piece of the diagnostic workup, especially in pre- and postsurgical evaluation. MRI can provide essential information about ventricular volume and shunt dynamics, as well as provide detailed anatomical information about the valves. MRI is particularly useful in differentiating the different types (I-IV) of tricuspid atresia based on the signal intensities of the atrioventricular sulcus [32].
4.11 Transposition of the Great Vessels
Transposition of the great vessels is most commonly seen in the dextro variant, in which the pulmonary artery arises from the left ventricle and the aorta from the right ventricle.
Because transposition of the great vessels is nearly always detected and surgically corrected in infancy, the relevance in its native state during pregnancy and the peripartum period is small.
4.12 Truncus Arteriosus
In truncus arteriosus, a single large trunk arises from both ventricles and gives rise to both the aorta and the pulmonary arteries. In this cyanotic congenital lesion, there is a single semilunar valve, which can demonstrate a wide variety of morphologies. This anomaly is typically associated with a ventricular septal defect. Variant anomalies of the aorta, such as a right-sided aortic arch, are also commonly seen with truncus arteriosus. It is exceedingly rare for a patient to make it to childbearing age with this congenital lesion without some type of surgical correction.
If patients do survive into adulthood without surgery, they typically display clinical symptoms consistent with Eisenmenger syndrome (discussed later), which carries a high morbidity and mortality when associated with pregnancy. Cardiac MRI can be a critical tool for providing anatomical and functional information. As explained by Fogel and Crawford [33], detailed analysis using steady-state free precession, cine imaging, and velocity mapping allows for evaluation of the exact type of common trunk as well as branches and collateral vessels. Functional assessment of the semilunar valve is also achieved. Determining aortic arch hypoplasia or interruption is particularly important for determining where the lesion falls in the different classification schemes.
4.13 Single Ventricle
Patients with a single ventricle have a single large ventricular chamber that received blood from both the right and left atria. This rare condition is often associated with transposition of the great vessels and with pulmonary stenosis. Although there are scattered successful pregnancies reported, this lesion carries with it high peripartum morbidity and mortality for both the mother and the fetus. Patients are at greatest risk for thromboembolic disease and congestive heart failure. Use of MRI can profile critical information about the ventricular volume and function prior to and following any surgical intervention.
4.14 Eisenmenger Syndrome
Eisenmenger syndrome encompasses a spectrum of congenital lesions, all of which result in severe pulmonary hypertension and pulmonary vascular obstructive disease. Lesions producing this effect, including ASDs, VSDs, and PDAs, allow mixing of systemic and pulmonary circulation and therefore cyanosis. Pulmonary vascular occlusive disease results in shunt reversal or bidirectional shunting. In this rare condition there is a high risk of maternal mortality and morbidity during the peripartum period [53]. Women with this condition are recommended to avoid conception. As systemic vascular resistance decreases during pregnancy, there is an increase in the right-to-left shunting of blood, resulting in decreased arterial oxygen saturation. Ultimately, this creates heightened hypercoagulability and increases risk of thromboembolism. The sudden hemodynamic changes that occur during labor and delivery are poorly tolerated and particularly dangerous in this condition.
Detailed diagnostic evaluation of Eisenmenger syndrome is of utmost importance. Given the profound risk of both maternal and fetal mortality and morbidity with this condition, a diagnosis of Eisenmenger syndrome may lead some to consider termination of a pregnancy. Furthermore, accurate diagnosis during the prepartum period is important, as it will influence a patient’s decision to proceed with conception.
Initial chest radiography often demonstrates enlargement of the pulmonary arteries with peripheral pruning of the vasculature. On cardiac MRI, there is evidence of right ventricular hypertrophy and often paradoxical bulging of the septum toward the left ventricle. As the disease increases in severity, one may expect to see right ventricular enlargement and evidence of hypokinesis on cine imaging. Detailing the anatomical defect, allowing the shunt, and quantifying the degree of shunt can be done with MRI. Furthermore, the tricuspid and pulmonic valves can be evaluated for regurgitation. Full anatomical evaluation with MRI as well as other imaging modalities allows for accurate clinical decision-making and prognostication.
4.15 Marfan Syndrome
Marfan syndrome is an autosomal dominant connective tissue disorder. This disorder affects the entire body, with notable pathology often appreciated in the eyes, cardiovascular system, musculoskeletal system, pulmonary system, and skin. Specifically, within the cardiovascular system, there is often aneurysmal dilatation of the aortic root. Aortic valvular regurgitation may be present. Mitral valve prolapse may also be present with abnormal morphology of the mitral leaflets.
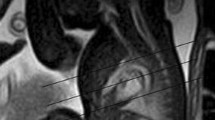
Due to the inherent abnormal dilatation of the aortic root in Marfan syndrome, peripartum patients are at particularly high risk of aortic dissection, aortic regurgitation, or even aortic rupture. Please see Fig. 17.2. Increased risk of dissection is multifactorial, likely due to changes in hemodynamics, shifts in hormonal balance, and the inherent deficit of aortic wall elastic fibers [39]. Chest radiography typically reveals dilatation of the thoracic aorta. In the pregnant patient, further anatomical evaluation with cardiac MRI is preferred over cardiac CT. Krishnam and colleagues [54] have found that use of unenhanced steady-state free precession magnetic resonance angiography can be used for accurate diagnosis and assessment of thoracic aortic disease without the need for gadolinium-based contrast agents. The 2010 [44] ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with thoracic aortic disease point out that MRI may in fact be superior to echocardiography given its ability to fully characterize anatomical variants and collateral branches while also assessing valve pathology and providing data on left ventricular function. Functional data and measurements of aortic root dilatation allow for accurate decision-making regarding need for medical management or surgical repair. Should a complication such as aortic dissection occur during pregnancy or the peripartum period, cardiac MRI is a valuable tool for assessment and follow-up after medical or surgical management.
A 27-year-old woman with Marfan syndrome with back pain and decreased right femoral pulse at 27 weeks’ gestation and history of mechanical aortic valve replacement. (a, b) Sagittal (a) and axial (b) gated steady-state free precession breath-hold MR images show sternal surgical artifact and mechanical aortic valve (oval, a), descending aortic dissection (asterisks indicate true lumen), fetus with placenta (p), and right iliac dissection extension (circle, b) (Reprinted with permission from the American Journal of Roentgenology Colletti et al. [19])
4.16 Absence of the Left Pericardium
Congenital absence of the pericardium is a rare but important diagnosis to accurately characterize. This congenital anomaly can either result in absence of the left pericardium or absence of the entire pericardium. While absence of the entire pericardium is typically asymptomatic and has a low mortality and morbidity rate in pregnancy, absence of the left pericardium can result in strangulation of the left atrial appendage. Patients can present with conspecific symptoms such as chest pain and presyncope/syncope. In the presence of other associated congenital abnormalities such as ASD, there is also a risk of emboli.
Chest radiography and echocardiography are not ideal for accurate evaluation of the pericardium. Therefore, in pregnancy MRI is the preferred diagnostic modality for evaluation of this congenital defect. In a case report by Yamano and colleagues [79], a patient presented with atypical chest pain. While radiography and echocardiography supported a diagnosis of complete absence of the pericardium, cardiac MRI allowed for more detailed evaluation and revealed absence of the left pericardium, prompting prophylactic surgical management.
5 Evaluation of Acquired Cardiovascular Diseases
5.1 Preeclampsia
Development of hypertension in pregnancy is a fairly common occurrence and prompts careful diagnostic workup and diligent clinical management to avoid potential maternal and fetal complications. Preeclampsia is diagnosed when a previously normotensive woman beyond 20 weeks’ gestation presents with new-onset hypertension and signs of end-organ dysfunction or proteinuria. Eclampsia is diagnosed with the development of seizures in a patient with preeclampsia. Although hypertension in pregnancy does not directly affect the myocardium in isolation, the change in hemodynamics creates potential functional changes that may become apparent on cardiac MRI evaluation. As detailed by Hamad and colleagues [65], the normal pregnant hemodynamic profile is characterized by increased cardiac output and decreased systemic vascular resistance. However, in preeclampsia there is decreased cardiac output and increased systemic vascular resistance [65]. This shift often leads to changes in left ventricular structure and function. Ultimately, the authors demonstrated impaired left ventricular function, increase in left ventricular wall thickness, and atrial enlargement in preeclamptic patients.
While cardiac MRI is not traditionally indicated in evaluation and follow-up of a patient with preeclampsia, many cardiac conditions may be complicated by preeclampsia, and patients may be imaged for a different diagnostic purpose. Therefore it is important to understand and recognize subtle changes in left ventricular appearance and function. Through anatomical and cine imaging, these changes can be appreciated.
5.2 Myocarditis
Myocarditis, an inflammatory process, can affect a focal portion of or diffusely involve the myocardium. There are a myriad of underlying causes of myocardial inflammation. Causes include infectious diseases such as bacterial and viral and other more rare etiologies such as mycotic or parasitic organisms. Noninfectious causes leading to myocardial inflammation should also be considered.
Clinical presentation is nonspecific and generally associated with typical viral symptoms such as fever and myalgia. Severity of cardiac-associated symptoms can vary widely with the degree of myocardial involvement. Cardiac symptoms are also nonspecific and often include fever, fatigue, shortness of breath, or palpitations. Tachycardia is often present. Severe cases with diffuse myocardial involvement may present with collapse of the circulatory system. Myocarditis is often associated with presence of a pericardial effusion and therefore may also present with cardiac tamponade. In the most severe form, myocarditis may result in sudden death.
Initial diagnostic workup with chest radiography often reveals enlargement of the cardiac silhouette, due to cardiac enlargement and/or pericardial effusion. Echocardiography is often performed and may reveal edema of the myocardium. Other typical findings include mural thrombi. Careful evaluation of the mitral and tricuspid valves must be performed to detect regurgitation.
Cardiac MRI is becoming an increasingly more prominent tool in the diagnostic workup of non-ischemic cardiac disease. Not only does cardiac MRI accurately characterize the left ventricular volume and function, post-contrast imaging demonstrates patchy areas of delayed contrast enhancement. A study by De Cobelli and colleagues [22] comparing biopsy to contrast-enhanced MRI revealed that MRI is a useful noninvasive tool for the diagnosis of myocarditis through demonstration of patchy areas of late enhancement. However, use of gadolinium-based contrast agents in pregnancy is generally avoided. Therefore, a new paper by Goenka and colleagues [38] details use of T2 mapping. The authors explain that prolongation of T2 relaxation, a result of increased mobility in edematous myocardium, results in increased T2 signal intensity. Quantitative T2 mapping can be employed for more sensitive and accurate interpretation of the data in the form of T2 mapping. The authors state a T2 steady-state free precession technique is preferred. Cardiac MRI can be used to monitor disease progression and/or response to treatment.
5.3 Peripartum Cardiomyopathy
Peripartum cardiomyopathy (or pregnancy-associated cardiomyopathy) is a rare but potentially very dangerous cause of heart failure during the third trimester or the immediate postpartum period. Peripartum cardiomyopathy is idiopathic. Although a variety of underlying pathophysiological processes have been proposed, none have been definitely proven to cause the condition. Peripartum cardiomyopathy is therefore a diagnosis of exclusion. Other causes of heart failure, such as congenital structural defects, and metabolic or toxic causes must be excluded.
Patients typically present with dyspnea and other classic symptoms of heart failure. Cough and hemoptysis are often present, as the association with pulmonary embolus is relatively high. Initial evaluation with chest radiography typically shows nonspecific findings such as cardiac silhouette enlargement. Findings consistent with pulmonary edema and pleural effusions may also be present. Echocardiography is typically performed and demonstrates marked decrease in left ventricular function, without obvious structural defect. The right ventricle may also be involved. Cardiac MRI is a useful tool for more specific and detailed evaluation and is generally thought to be required to make the diagnosis. In addition to assessment of left ventricular volume and function, cardiac MRI allows for detailed assessment of the myocardium.
Arora and colleagues [6] presented a case collection of peripartum cardiomyopathy evaluated with cardiac MRI. The authors suggest employing a standard cardiac MRI technique which includes two- and four-chamber views, balanced steady-state free precession, T2 breath-hold and double inversion recovery sequences, and T2 ratio data to evaluate edema. In particular, late gadolinium enhancement was also assessed with a breath-hold, inversion recovery gradient echo sequence following a 15 min delay from contrast administration. The authors concluded through retrospective review that late gadolinium enhancement is associated with poor prognosis in patients with peripartum cardiomyopathy. Similarly, in another case study [7], cardiac MRI demonstrated global hypokinesis, reduction in left ventricular function, evidence of myocardial inflammation/edema on T2 imaging, and, most notable, delayed myocardial enhancement. Please see Figs. 17.3, 17.4, 17.5, and 17.6. Cardiac MRI is also useful for identification of a potential high-yield myocardial biopsy site, should tissue sampling be needed for further management [11].
Patient is a 31-year-old female with history of postpartum cardiomyopathy. This study demonstrates cardiomyopathy with four-chamber dilatation and poor contractility as well as moderate mitral regurgitation and moderate to severe tricuspid regurgitation. A small pericardial effusion is seen. The left ventricle demonstrates hypocontractility and hypokinesis throughout the cardiac cycle. Ejection fraction: 11.5 % (normal range 50–70 %). End-diastolic volume index: 116.8 ml/m2 (normal range 50–84 ml/m2). End-systolic volume index: 102.8 ml/m2 (normal range 17–37 ml/m2). Stroke volume: 27.7 ml. Cardiac output: 0.12 lt min/m2 (normal range 2.6–4.2 lt/min/m2). Bilateral pleural effusions and bibasilar parenchymal consolidations also noted. (a) Steady-state free precession 3-chamber image demonstrating mitral regurgitation (arrow). (b) Steady-state free precession 4-chamber image demonstrating tricuspid regurgitation (arrow). (c) Steady-state free precession 4-chamber image from cine demonstrating end-diastole. (d) Steady-state free precession 4-chamber image from cine demonstrating end-systole
Patient is a 26-year-old female with acute chest pain, severe hypertension, and cardiomyopathy after a recent pregnancy. This study demonstrates marked left ventricular hypertrophy and left ventricular enlargement, consistent with biventricular cardiomyopathy. There is diffuse global systolic hypokinesis. Both mild mitral regurgitation and mild tricuspid regurgitation are present. There is a small pericardial effusion. Left ventricular end-diastolic volume is 246.2 craniocaudal (normal range for females is 52–141 cc). The end-systolic volume is 192.7 cc (normal range for females is 13–51 cc). The left ventricular stroke volume is 53.5 cc (normal range is 33–97 cc for females). Left ventricular mass is 279.4 g (normal range is 75–175 g for females). The ejection fraction is diminished at 21.7 % (normal range is 56–78 %). The right ventricular end-diastolic volume is 249.7 (normal range is 58–154 cc for females). The end-systolic volume is 153.5 cc (normal range is 12–68 cc for females). The stroke volume is 96.1 cc (normal range is 35–98 cc for females). The right ventricular ejection fraction is also diminished, to a lesser extent compared to the left ventricle, at 33.5 % (normal range is 47–80 % for females). (a) Steady-state free precession bright blood 2-chamber image from cine demonstrating end-diastole. (b) Steady-state free precession bright blood 2-chamber image from cine demonstrating end-systole
Patient is a 36-year-old female with peripartum cardiomyopathy. This study demonstrates global hypokinesis of left ventricle with mild left ventricular and left atrial enlargement. There is also mild mitral regurgitation and trace aortic regurgitation. Left ventricular end-diastolic volume is 162 craniocaudal (normal range for females is 52–141 cc). The end-systolic volume is 116 cc (normal range for females is 13–51 cc). The left ventricular stroke volume is 46 cc (normal range is 33–97 cc for females). The ejection fraction is diminished at 28 % (normal range is 56–78 %). (a) Steady-state free precession 4-chamber image from cine demonstrating end-diastole. (b) Steady-state free precession 4-chamber image from cine demonstrating end-systole
Patient is a 28-year-old female with peripartum cardiomyopathy. This study demonstrates dilated cardiomyopathy. There is suggestion of non-compaction of the left ventricle. Additionally, there is abnormal delayed gadolinium enhancement in a predominantly patchy mesocardial pattern. There is also moderate mitral and mild tricuspid valve regurgitation. There is a small-volume pericardial effusion and trace bilateral pleural effusions. Incidentally, it was noted that there was a normal variant bovine arch and a possible patent foramenovale. Left ventricular end-diastolic volume is 179.9 craniocaudal (normal range for females is 52–141 cc). The end-systolic volume is 119.4 cc (normal range for females is 13–51 cc). The left ventricular stroke volume is 60.5 cc (normal range is 33–97 cc for females). The ejection fraction is diminished at 33.6 % (normal range is 56–78 %). (a) Steady-state free precession 2-chamber image demonstrating mitral regurgitation (arrow). (b) Delayed enhancement 4-chamber image demonstrating delayed enhancement of nearly the entire left ventricle, predominantly of the mesocardium. (c) Delayed enhancement short-axis image demonstrating delayed enhancement of the left ventricle
5.4 Hypertrophic Cardiomyopathy
Hypertrophic cardiomyopathy occurs in genetically predisposed individuals, resulting in varying morphological manifestations of left ventricular hypertrophy. The structural changes lead to a hyperdynamic left ventricle and can, depending on the lesion location, result in left ventricular outflow tract obstruction or even diastolic dysfunction. Clinical presentation varies widely depending on the anatomical location and extent of the hypertrophy. Patients can range from a virtually asymptomatic state to severe heart failure or even death. Of note, even for patients that are asymptomatic prior to conception, there is a risk that the shift in hemodynamic function in pregnancy there could be precipitation of symptoms and worsening heart failure. There is also a theoretical increased risk of arrhythmias in pregnancy in patients with these underlying conditions. In an extensive case selection of patients with hypertrophic cardiomyopathy, Maron and colleagues [57] found with the use of cardiovascular MRI that areas of left ventricular hypertrophy are often segmental and non-diffuse. Furthermore, the case series demonstrated that the anterior left ventricular wall is more commonly and predominantly affected.
Although diagnostic evaluation is typically completed with echocardiography, many have recently noted that cardiovascular MRI may provide a more thorough and specific evaluation. In hypertrophic cardiomyopathy, there is typically asymmetric thickening of the intraventricular septum. MRI is thought to be superior to echocardiography in detailed evaluation of segmental areas of left ventricular hypertrophy that may be missed or misrepresented on echocardiography [56]. Furthermore, cardiac MRI potentially detects other associated pathologies such as scarred apical aneurysms, systolic dysfunction, and massive left ventricular hypertrophy [56]. In the postpartum patient, contrast-enhanced studies also allow assessment of myocardial fibrosis, which allows for better risk stratification and selection of appropriate management.
5.5 Acute Pericarditis
Acute pericarditis, or inflammation of the pericardium, can be due to a variety of etiologies. Although the most common form of pericarditis is idiopathic, other common causes include posttraumatic, iatrogenic, and infectious (viral and bacterial). Patients typically present with chest pain, which classically is exacerbated by leaning forward. Although the disease course of pericarditis is often mild and self-limited, increased cardiovascular demands of pregnancy can heighten symptoms. Chest radiography is nonspecific and may show an enlarged cardiac silhouette. Although echocardiography has traditionally been the imaging modality of choice in pregnancy, cardiac MRI is emerging as a useful tool for more detailed evaluation. Not only does MRI provide information on the extent of involved pericardium, it also potentially discovers other associated abnormalities. In the European Association of Cardiovascular Imaging position paper “Multimodality imaging in pericardial disease” [21], Cosyns and colleagues detail the strengths of cardiac MRI in evaluation of pericarditis. As detailed by the authors, the black blood T1 spin echo sequence is ideal for anatomical evaluation of the pericardium. Pericardial thickness can be measured and other thoracic abnormalities are detected. In contrast, black blood T2-weighted spin echo sequences are excellent to evaluate the pericardial fluid and presence of myocardial edema. Gradient echo cine images with steady-state free precession and cine imaging assess cardiac function. Tagging techniques can be used to further evaluate adhesions in constrictive pericarditis. In the postpartum patients, contrast-enhanced studies including dynamic contrast enhancement techniques and late gadolinium enhancement allow more in-depth evaluation of blood supply and potential myocardial involvement.
Naturally, similar MRI sequences are useful in evaluation of a pericardial effusion and pericardial cysts or for evaluation of pericardial restriction.
5.6 Coronary Artery Disease and Myocardial Infarction
The spectrum of coronary artery disease ranges from clinically insignificant lesions to significant pathology resulting in acute myocardial infarction. Typically the workup for anginal chest pain includes an EKG and chest radiography followed by echocardiography, stress testing, and angiographic evaluation with potential intervention if clinically appropriate. However, the pregnant patient presents a unique set of clinical challenges. Exposure to ionizing radiation should be minimized if at all possible. Within recent years, we have seen a gradual increase in the average maternal age, with patients conceiving in the fourth and even fifth decades. Therefore, there has been a concurrent rise in the amount and severity of cardiovascular disease in our pregnant population.
Not only does atherosclerotic vascular disease present in the pregnant population, other coronary pathologies such as dissection, thrombus, spasm, and embolism have also been reported in pregnant patients [27].
Fortunately, MRI has emerged as a tool to provide accurate evaluation of coronary artery pathology. Coronary MR angiography can be performed without the use of contrast and allows noninvasive assessment without use of ionizing radiation. Recent advances in technique have improved diagnostic quality of imaging, enabling increased clinical utility. Ishida and Sakuma [46] detail nuances of the angiographic technique. In short, the authors suggest a free-breathing, navigator echo respiratory gating technique with concurrent use of an abdominal belt to minimize patient movement. The authors additionally recommend a narrow data acquisition window. Further evaluation with other standard cardiac MRI sequences allows assessment of cardiac function in the case of resultant myocardial infarction. Specifically, T2-weighted images allow for detection of acute infarct-associated myocardial edema [27]. Of note, the region of myocardial edema on cardiac MRI is typically larger than the area of irreversible myocardial injury [81].
Interestingly, a large case review by Elkayam and colleagues [30] revealed that in contrast to nonpregnant patients, the majority of myocardial infarctions in pregnant patients were not actually due to atherosclerotic disease of the coronary arteries. Also of note, the authors found that specifically in pregnant patients, there was frequent involvement of the LAD and LM coronary arteries, and one of most common areas affected was the anterior wall. This can result in reduced function and potentially profound symptomatic presentation including cardiogenic shock.
5.7 Rheumatic Heart Disease
Although there is declining prevalence of rheumatic heart disease in the developed countries, the disease remains fairly pervasive in underdeveloped countries. Acute rheumatic fever results from infection with group A streptococcus. After the initial viral infection and a latent period of 2–3 weeks, patients may present with acute carditis. The presentation of acute disease typically involved the entirety of the heart and includes a component of valvulitis. Following the acute presentation of rheumatic fever, the sequel of chronic disease, rheumatic heart disease, can be evident.
Although pregnant patients are usually initially evaluated with echocardiography, MRI evaluation has the potential to provide a more detailed anatomical assessment of the degree of disease involvement. Acute rheumatic fever typically involves the pericardium. Pericardial effusions can often be complex and loculated [55]. Furthermore, MRI has the benefit of distinguishing constrictive pericarditis from restrictive cardiomyopathy [55]. As in other disease processes, MRI also enables assessments of cardiac volume and function.
Chronic rheumatic heart disease most frequently results in mitral stenosis. This ultimately results in a large diastolic gradient, elevation in left atrial pressure, and development of pulmonary edema [31]. Patients typically present with dyspnea and symptoms of fluid overload. With the sudden hemodynamic shifts during pregnancy, labor, and delivery, there is a high risk of mortality and morbidity in severe cases. Although mitral stenosis is the most common effect of chronic rheumatic heart disease, mitral regurgitation, aortic stenosis, and aortic regurgitation can also occur.
5.8 Primary Pulmonary Hypertension
Primary pulmonary hypertension is a rare but potentially very dangerous clinical condition. The diagnosis is one of exclusion, as pulmonary hypertension is present without a known underlying cause. This progressive clinical condition is associated with shortness of breath, fatigue, pain, and syncope. Symptoms progressively worsen, and patients often have a short life expectancy at the time of clinical presentation.
Given that primary pulmonary hypertension is a diagnosis of exclusion, diagnostic workup is often extensive. Echocardiography and pulmonary function tests often take a central role. Other diagnostic tests such as ventilation-perfusion scanning are also often used. Cardiac MRI has emerged as an additional tool, given its ability to accurately assess right ventricular size and function. Therefore, MRI aids in forming an accurate prognosis to help guide clinical decision-making [41]. Furthermore, Grothues and colleagues [41] demonstrated excellent reproducibility during cardiac MRI to evaluate right ventricular function. For this reason, cardiac MRI is an excellent tool for serial follow-up examination to monitor disease progression.
During pregnancy, efforts must be made to reduce ionizing radiation to the fetus. Therefore, other nontraditional diagnostic techniques should be considered for further evaluation of primary pulmonary hypertension. In addition to providing anatomical assessment and functional assessment of ventricular volume and function, phase-contrast velocity mapping can be used to assess blood vessel flow patterns [63], allowing for detailed assessment of blood flow dynamics within the pulmonary arteries. MRA can further be used for assessment of the pulmonary arteries.
5.9 Infective Endocarditis
Infective endocarditis can result from a variety of underlying conditions such as rheumatic heart disease and IV drug abuse. In the pregnant patient, timely diagnosis is critical to avoid serious maternal and fetal mortality and morbidity. Blood cultures may reveal organisms such as viridans streptococci, enterococci, or Staphylococcus aureus. Echocardiography is often the initial diagnostic modality of choice and is used to assess for valvular vegetation. In the case where echocardiography becomes limited, for example, if there is a difficult acoustic window, cardiac MRI may be used for further assessment of the valves.
Of note, in this systemic disease process, MRI becomes useful for evaluation of extracardiac complications. Brain MRI is particularly critical to assess for cerebrovascular complications. MR angiography is also a useful tool to assess for presence of or follow up mycotic aneurysms [73].
5.10 Vascular Dissections and Aneurysms
With the increasing prevalence of cardiovascular disease in the pregnant population, it is critical to be aware of diagnostic options for initial detection and subsequent follow-up of vascular pathology. Acute aortic syndrome encompasses a spectrum of diseases including aortic dissection, intramural hematoma, and penetrating aortic ulcers. Sudden changes in blood pressure and hemodynamic flow during pregnancy, labor, and delivery can lead to acute cardiovascular complications. Although MRI holds the potential to provide detailed anatomical assessment of vascular lesions, the long duration of time needed to complete an examination sometimes preclude its use in an acute clinical situation. However, after patient stability is achieved, MRI is the imaging modality of choice for follow-up examinations or assessment of postsurgical repair. As detailed by Clough and Nienaber [17], MR angiography can provide initial evaluation of the aorta, followed by T1 and T2 black blood images to assess the lumen caliber, wall thickness, and signal of the aortic wall. Furthermore, intramural hematoma age can be estimated based on T1 and T2 signal characteristics. Additionally, steady-state free precession imaging can be used for detailed evaluation of the aortic root and valve.
Splenic artery aneurysms are considered the most common visceral artery aneurysm [52]. In women with splenic artery aneurysms, there is a significant risk or rupture, particularly during the third trimester, labor, and the immediate postpartum period. In the case of acute rupture, pain and sudden cardiovascular collapse are the most common presenting symptoms [52]. Nausea and vomiting are also sometimes present. Ultrasound and immediate surgical intervention are often warranted. After patient stability is achieved, MR angiography can be used for further assessment and follow-up.
Similarly, there is also a risk of renal artery aneurysm rupture, ovarian artery aneurysm rupture, or rupture of the utero-ovarian vein, particularly during the third trimester, labor, and the postpartum period [8]. In these cases, patients are often not clinically stable enough to undergo extensive cross-sectional imaging prior to intervention. However, MR angiography is a powerful tool for assessment of intervention and serial follow-up [49].
6 Thromboembolic Disease
6.1 Deep Vein Thrombosis
Pregnant and postpartum patients are at elevated risk for development of a deep vein thrombosis. Prompt diagnosis is critical and however sometimes challenging given that normal pregnancy can mask classic symptoms. Interestingly, in pregnant females, the majority of deep vein thromboses have been reported in either the left lower extremity or pelvis [37]. Diagnosis and follow-up evaluation are typically conducted with ultrasonography. However, particularly for detection of pelvic vein thrombosis, MR venography is a reasonable alternative. Time-of-flight technique can be used to avoid need for gadolinium administration. Please see Fig. 17.7. Sampson and colleagues [70], through a large meta-analysis, concluded that MRI has equivalent sensitivity and is specific to ultrasound for diagnosis for DVT. However, it is important to understand that at this time, a relative paucity of robust data exists to validate MRI as a reasonable and trusted clinical alternative to ultrasound for the diagnosis of deep vein thrombosis.
This 32-year-old woman presented at 30 weeks with markedly swollen legs. Venous Doppler demonstrated bilateral patency without thrombosis. 2D time-of-flight MR venography with superior saturation (a) demonstrated bilateral compressive cutoff (open arrowheads) of the iliac veins with extensive collateralization via abdominal vessels (larger arrows) and paraspinal and epidural veins (small arrows). Incidentally noted are fetal vessels (f). An isolated reconstruction of the fetal vessels (b) demonstrates fetal subclavian vein (v), superior vena cava (s), aortic arch (a), and iliac artery (i). Selected vessels are detected as the fetus had cephalic presentation so that caudal flow was demonstrated due to maternal superior saturation. Repeat 2D time-of-flight MR venography with superior saturation after delivery (c) demonstrates normal pelvic veins. It is clear that pregnancy may cause significant external venous compression with collateral formation and increased risk for thrombosis (Colletti and Sylvestre [20 reprinted with permission)
6.2 Pulmonary Embolism
Development of a pulmonary embolism can result from the presence of deep vein thrombosis and, depending on the size, has potential for high mortality and morbidity during pregnancy and the peripartum period. Please see Fig. 17.8. The gold standard for diagnosis and evaluation of pulmonary embolism remains ventilation-perfusion scans and computed tomography pulmonary angiography. Although magnetic resonance pulmonary angiography has been proposed as a diagnostic tool for the diagnosis of pulmonary embolism in the pregnant patient, there is limited data supporting its efficacy.
A 36-year-old woman with shortness of breath in week 12 of pregnancy. (a, b) Coronal (a) and axial (b) steady-state free precession breath-hold localizer images show left main pulmonary artery filling defects (oval, a; circle, b) interpreted as acute pulmonary embolism. No further examination was required (Reprinted with permission from the American Journal of Roentgenology Colletti et al. 19])
6.3 Takayasu Arteritis
Takayasu arteritis is an idiopathic chronic vasculitis that primarily affects the aorta and its branches. Acutely, the disease may present with fever, fatigue, and weight loss. Diagnosis and appropriate management are critical as patients may present with hypertension due to the significant vascular disease.
The American College of Rheumatology 1990 [5] criteria for the classification of Takayasu arteritis describe the pathological imaging findings as “arteriographic narrowing or occlusion of the entire aorta, its primary branches, or large arteries in the proximal upper or lower extremities, not due to arteriosclerosis, fibromuscular dysplasia, or other causes”. These characteristic findings can be evident on non-contrast MR angiography using time-of-flight imaging technique [71]. Confirmation with conventional angiography or CT angiography can be obtained postpartum if needed.
6.4 Amniotic Fluid Embolism
Amniotic fluid embolism, also known as anaphylactoid syndrome of pregnancy, is a devastating clinical condition. If a communication forms between the uterine veins and the amniotic sac, then amniotic fluid can enter into venous circulation. This can precipitate anaphylaxis- and shocklike symptoms resulting in dramatic circulatory collapse and hypoxemia, carrying with it high maternal mortality and morbidity risks [36].
Should a patient survive the acute presentation, MRI can be a useful tool for evaluation of myocardial damage following the acute presentation. Evidence of late gadolinium may be present, indicating presence of focal myocardial damage [45]. Through analysis of a case report, Hosoya and colleagues [45] proposed that the finding of late gadolinium enhancement supports the theory that cardiac complications seen in amniotic fluid embolism may be due to direct left ventricular myocardial injury due to immune reactions and not due to pulmonary embolism.
7 Evaluation of Postsurgical Cardiovascular Diseases
7.1 Artificial Heart Valves
Although the topic of MRI safety with regard to the developing fetus is discussed extensively elsewhere in this book, there are specific safety considerations with regard to artificial valves. The majority of artificial heart valves are considered MRI-safe. However, the valves used in the TAVI procedure (transcatheter aortic valve implantation) are noted to be MRI-conditional. A careful patient history should be obtained prior to any imaging, and confirmation of MRI compatibility should be confirmed.
Given the potential for cardiovascular complications to arise or worsen during pregnancy and the peripartum period, the need for diagnostic evaluation of the postoperative heart may occur. MRI allows for detailed assessment of both native and prosthetic valves. Similar to assessment of native valves, MRI can be used to evaluate structure and function of the prosthesis. It is of particular importance to accurately diagnose prosthetic valve endocarditis.
7.2 Cardiac Surgery
Sternal wires are considered MRI-compatible and normally do not significantly degrade the diagnostic quality of the image [77]. Similarly, vascular clips, stents, annuloplasty rings, and many prostheses are also considered MRI-compatible [77].
Pacemakers and implantable cardioverter defibrillators may be MRI-conditional, and patients may be given a pocket card to carry with them listing device-specific safe and unsafe imaging conditions.
A variety of congenital heart conditions are often repaired at birth or shortly thereafter. MRI is an ideal imaging modality, allowing for detailed anatomical assessment of the postsurgical structure and function of the heart. Shunt dynamics can be assessed, and ventricular function can be measured. Because MRI is increasingly more common in the pediatric population for pre- and postoperative assessment of congenital defects, data acquired during pregnancy can theoretically be compared to earlier studies to gain accurate assessment of the effects of changing hemodynamics on the postsurgical heart. Please see Fig. 17.9. For example, in one study Assenza and colleagues [26] assessed pregnant patients with history of surgical repair of tetralogy of Fallot. In this small study, the authors demonstrated that pregnancy was associated with an accelerated rate of right ventricular remodeling with relative perseveration in right ventricular systolic function.
This 32-year-old woman, gravida 2, para 1, presents with shortness of breath at 28 weeks of pregnancy. Her first pregnancy was uneventful. Echocardiology demonstrated corrected transformation with prominent systemic AV valve insufficiency and dilated left ventricle and left atrium. Gated spin echo MRI (a–c) demonstrates corrected transposition of the great arteries. Image (d) demonstrates a dilated and hypertrophied systemic anatomical right ventricle. The moderator band “m” is hypertrophic, the left atrium “*” is dilated, and a small posterior pericardial effusion “e” is noted. Axial gradient echo cine images (e, f) demonstrate a prominent posterior low signal jet (arrows) of systemic AV valve insufficiency. With corrected transposition, the systemic ventricle is an anatomical right ventricle with a tricuspid AV valve. Patients with corrected transposition are prone to systemic ventricular failure and AV valve insufficiency. Both of these conditions are likely to appear during or immediately after pregnancy. Ao aorta, P main pulmonary artery, * dilated left atrium, m moderator band
7.3 Cardiac Transplantation
As transplant medicine continues to progress, increasing numbers of post-transplant pregnant patients create the need for complex peripartum monitoring and care. Cardiac MRI is ideal for post-transplant monitoring and assessment for potential acute and chronic transplant complications. Not only does MRI provide data on the structure of the left ventricle, but it also assesses function. In the pre- and postpartum period, contrast administration can allow for further assessment for infarction, viral myocarditis, cardiomyopathy, and signs of rejection [72].
8 Summary and Recommendations
Cardiac MRI holds tremendous potential for evaluation of cardiovascular disease during pregnancy and the peripartum period. The use of nonionizing radiation combined with the fact that most imaging techniques can be performed without the use of gadolinium-based contrast agents makes this technique ideal for evaluation of the pregnant patient. Furthermore, the ability to detail subtle anatomical abnormalities and elucidate precise differences in blood flow and function makes this technique ideal for evaluation of complex cardiovascular conditions.
Echocardiography is often the initial and preferred imaging modality for many of the described clinical conditions. However, this technique can be limited for a variety of reasons including poor acoustic window in the pregnant body habitus. MRI is then the preferred modality to complete diagnosis and fully characterize the disease.
As the science of MRI continues to progress at a rapid rate, image quality and the wide breadth of clinical applications will continue to expand.
References
Ain DL, Narula J, Sengupta PP (2012) Cardiovascular imaging and diagnostic procedures in pregnancy. Cardiol Clin 30(3):331–341
Akintunde AA, Opadijo OG (2011) Case report of a 26 year old primigravida with patent ductus arteriosus (PDA) in heart failure. Afr Health Sci 11(1):138–140
Alpendurada F, Wage R, Mohiaddin R (2008) Evaluation of a sinus venosus atrial septal defect by magnetic resonance: a case report. Rev Port Cardiol 27(10):1317–1321
Anilkumar M (2013) Patent ductus arteriosus. Cardiol Clin 31(3):417–430
Arend WP, Michel BA, Bloch DA, Hunder GG, Calabrese LH, Edworthy SM, Fauci AS, Leavitt RY, Lie JT, Lightfoot RW Jr et al (1990) The American College of Rheumatology 1990 criteria for the classification of Takayasu arteritis. Arthritis Rheum 33(8):1129–1134
Arora NP, Mohamad T, Mahajan N, Danrad R, Kottam A, Li T, Afonso LC (2014) Cardiac magnetic resonance imaging in peripartum cardiomyopathy. Am J Med Sci 347(2):112–117
Barone-Rochette G, Rodière M, Lantuejoul S (2011) Value of cardiac MRI in peripartum cardiomyopathy. Arch Cardiovasc Dis 104(4):263–264
Barrett JM, Van Hooydonk JE, Boehm FH (1982) Pregnancy-related rupture of arterial aneurysms. Obstet Gynecol Surv 37(9):557–566
Beerbaum P, Körperich H, Barth P, Esdorn H, Gieseke J, Meyer H (2001) Noninvasive quantification of left-to-right shunt in pediatric patients: phase-contrast cine magnetic resonance imaging compared with invasive oximetry. Circulation 103(20):2476–2482
Berg C, Lachmann R, Kaiser C, Kozlowski P, Stressig R, Schneider M, Asfour B, Herberg U, Breuer J, Gembruch U, Geipel A (2010) Prenatal diagnosis of tricuspid atresia: intrauterine course and outcome. Ultrasound Obstet Gynecol 35(2):183–190
Bhattacharyya A, Basra SS, Sen P, Kar B (2012) Peripartum cardiomyopathy: a review. Tex Heart Inst J 39(1):8–16
Bonow RO, Carabello BA, Chatterjee K, de Leon AC Jr, Faxon DP, Freed MD, Gaasch WH, Lytle BW, Nishimura RA, O’Gara PT, O’Rourke RA, Otto CM, Shah PM, Shanewise JS (2008) American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2008 focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to revise the 1998 guidelines for the management of patients with valvular heart disease). Endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol 52(13):e1–e142
Burgstahler C, Wöhrle J, Kochs M, Nusser T, Löffler C, Kunze M, Höher M, Gawaz MP, Hombach V, Merkle N (2007) Magnetic resonance imaging to assess acute changes in atrial and ventricular parameters after transcatheter closure of atrial septal defects. J Magn Reson Imaging 25(6):1136–1140
Capeless EL, Clapp JF (1991) When do cardiovascular parameters return to their preconception values? Am J Obstet Gynecol 165(4 Pt 1):883–886
Chaturvedi A, Dubinsky TJ, Maki JH (2012) MR findings of a rare defect, coronary sinus ASD. Int J Cardiovasc Imaging 28(2):429–430
Chen SS, Keegan J, Dowsey AW, Ismail T, Wage R, Li W, Yang GZ, Firmin DN, Kilner PJ (2011) Cardiovascular magnetic resonance tagging of the right ventricular free wall for the assessment of long axis myocardial function in congenital heart disease. J Cardiovasc Magn Reson 13:80
Clough RE, Nienaber CA (2015) Management of acute aortic syndrome. Nat Rev Cardiol 12(2):103–114
Colletti PM (2014) Magnetic resonance imaging in the pregnant patient. In: Shellock FG, Crues JV (eds) MRI bioeffects, safety, and patient management, 1st edn. Biomedical Research Publishing Group, Los Angeles, pp 217–241
Colletti PM, Lee KH, Elkayam U (2013) Cardiovascular imaging and the pregnant patient. Am J Roentgenol 200:1–7
Colletti PM, Sylvestre PB (1994) Magnetic resonance in pregnancy. MRI Clin North Am 2(2):291–307
Cosyns B, Plein S, Nihoyanopoulos P, Smiseth O, Achenbach S, Andrade MJ, Pepi M, Ristic A, Imazio M, Paelinck B, Lancellotti P, On behalf of the European Association of Cardiovascular Imaging (EACVI) and European Society of Cardiology Working Group (ESC WG) on Myocardial and Pericardial diseases (2015) European Association of Cardiovascular Imaging (EACVI) position paper: multimodality imaging in pericardial disease. Eur Heart J Cardiovasc Imaging 16(1):12–31
De Cobelli F, Pieroni M, Esposito A, Chimenti C, Belloni E, Mellone R, Canu T, Perseghin G, Gaudio C, Maseri A, Frustaci A, Del Maschio A (2006) Delayed gadolinium-enhanced cardiac magnetic resonance in patients with chronic myocarditis presenting with heart failure or recurrent arrhythmias. J Am Coll Cardiol 47(8):1649–1654
Dearani JA, Danielson GK (2000) Congenital Heart Surgery Nomenclature and Database Project: Ebstein’s anomaly and tricuspid valve disease. Ann Thorac Surg 69(4 Suppl):S106–S117
Debl K, Djavidani B, Buchner S, Heinicke N, Poschenrieder F, Feuerbach S, Riegger G, Luchner A (2009) Quantification of left-to-right shunting in adult congenital heart disease: phase-contrast cine MRI compared with invasive oximetry. Br J Radiol 82(977):386–391
Ducas RA, Elliott JE, Melnyk SF, Premecz S, daSilva M, Cleverley K, Wtorek P, Mackenzie GS, Helewa ME, Jassal DS (2014) Cardiovascular magnetic resonance in pregnancy: insights from the cardiac hemodynamic imaging and remodeling in pregnancy (CHIRP) study. J Cardiovasc Magn Reson 16:1
Egidy Assenza G, Cassater D, Landzberg M, Geva T, Schreier J, Graham D, Volpe M, Barker N, Economy K, Valente AM (2013) The effects of pregnancy on right ventricular remodeling in women with repaired tetralogy of Fallot. Int J Cardiol 168(3):1847–1852
El-Deeb M, El-Menyar A, Gehani A, Sulaiman K (2011) Acute coronary syndrome in pregnant women. Expert Rev Cardiovasc Ther 9(4):505–515
Elkayam U, Gleigher N (1998) Cardiac evaluation during pregnancy. In: Elkayam U, Gleigher N (eds) Cardiac problems in pregnancy, 3rd edn. Wiley-Liss, New York, pp p23–p32
Elkayam U, Hameed A (1998) Vascular dissections and aneurysms during pregnancy. In: Elkayam U, Gleigher N (eds) Cardiac problems in pregnancy, 3rd edn. Wiley-Liss, New York, pp p23–p32
Elkayam U, Jalnapurkar S, Barakkat MN, Khatri N, Kealey AJ, Mehra A, Roth A (2014) Pregnancy-associated acute myocardial infarction: a review of contemporary experience in 150 cases between 2006 and 2011. Circulation 129(16):1695–1702
Essop MR, Sareil P (1998) Theumatic valvular disease and pregnancy. In: Elkayam U, Gleigher N (eds) Cardiac problems in pregnancy, 3rd edn. Wiley-Liss, New York, pp p23–p32
Fletcher BD, Jacobstein MD, Abramowsky CR, Anderson RH (1987) Right atrioventricular valve atresia: anatomic evaluation with MR imaging. AJR Am J Roentgenol 148(4):671–674
Fogel MA, Crawford M (2012) Cardiac magnetic resonance of the common arterial trunk and transposition of the great arteries. Cardiol Young 22(6):677–686
Ganigara M, Tanous D, Celermajer D, Puranik R (2014) The role of cardiac MRI in the diagnosis and management of sinus venosus atrial septal defect. Ann Pediatr Cardiol 7(2):160–162
Geerdink LM, Kapusta L (2014) Dealing with Ebstein’s anomaly. Cardiol Young 24(2):191–200
Gilmore DA, Wakim J, Secrest J, Rawson R (2003) Anaphylactoid syndrome of pregnancy: a review of the literature with latest management and outcome data. AANA J 71(2):120–126
Ginsberg JS, Brill-Edwards P, Burrows RF, Bona R, Prandoni P, Büller HR, Lensing A (1992) Venous thrombosis during pregnancy: leg and trimester of presentation. Thromb Haemost 67(5):519–520
Goenka AH, Wang H, Flamm SD (2014) Cardiac magnetic resonance imaging for the investigation of cardiovascular disorders. Part 2: emerging applications. Tex Heart Inst J 41(2):135–143
Goland S, Elkayam U (2009) Cardiovascular problems in pregnant women with marfan syndrome. Circulation 119(4):619–623
Grifka RG, Fenrich AL, Tapio JB (2008) Transcatheter closure of patent ductus arteriosus and aorto-pulmonary vessels using non-ferromagnetic Inconel MReye embolization coils. Catheter Cardiovasc Interv 72(5):691–695
Grothues F, Moon JC, Bellenger NG, Smith GS, Klein HU, Pennell DJ (2004) Interstudy reproducibility of right ventricular volumes, function, and mass with cardiovascular magnetic resonance. Am Heart J 147(2):218–223
Gulati GS, Hoey ET, Gopalan D, Agrawal BS, Screaton NJ (2010) Sinus venosus atrial septal defect in adults: utility of cardiovascular MRI in resolving this diagnostic dilemma. Heart Lung Circ 19(10):615–619
Helgason H, Jonsdottir G (1999) Spontaneous closure of atrial septal defects. Pediatr Cardiol 20(3):195–199
Hiratzka LF, Bakris GL, Beckman JA, Bersin RM, Carr VF, Casey DE Jr, Eagle KA, Hermann LK, Isselbacher EM, Kazerooni EA, Kouchoukos NT, Lytle BW, Milewicz DM, Reich DL, Sen S, Shinn JA, Svensson LG, Williams DM, American College of Cardiology Foundation, American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, Society for Vascular Medicine (2010) 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with thoracic aortic disease: executive summary. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. Catheter Cardiovasc Interv 76(2):E43–E86
Hosoya Y, Watanabe M, Terashima M, Amiya E, Nakao T, Hasegawa A, Hyodo H, Ando J, Fujii T, Nagai R, Komuro I (2013) Cardiac magnetic resonance imaging in a patient with amniotic fluid embolism associated with severe cardiopulmonary complications. Int Heart J 54(2):119–122
Ishida M, Sakuma H (2015) Coronary MR angiography revealed: how to optimize image quality. Magn Reson Imaging Clin N Am 23(1):117–125
Jacobs JP, Burke RP, Quintessenza JA, Mavroudis C (2000) Congenital Heart Surgery Nomenclature and Database Project: ventricular septal defect. Ann Thorac Surg 69(4 Suppl):S25–S35
Jimenez-Juan L, Krieger EV, Valente AM, Geva T, Wintersperger BJ, Moshonov H, Siu SC, Colman JM, Silversides CK, Wald RM (2014) Cardiovascular magnetic resonance imaging predictors of pregnancy outcomes in women with coarctation of the aorta. Eur Heart J Cardiovasc Imaging 15(3):299–306
Kabul HK, Hagspiel KD (2006) Cross-sectional vascular imaging with CT and MR angiography. J Nucl Cardiol 13(3):385–401
Karamlou T, Diggs BS, McCrindle BW, Welke KF (2011) A growing problem: maternal death and peripartum complications are higher in women with grown-up congenital heart disease. Ann Thorac Surg 92(6):2193–2198; discussion 2198–2199
Katz R, Karliner JS, Resnik R (1978) Effects of a natural volume overload state (pregnancy) on left ventricular performance in normal human subjects. Circulation 58(3 Pt 1):434–441
Khurana J, Spinello IM (2013) Splenic artery aneurysm rupture: a rare but fatal cause for peripartum collapse. J Intensive Care Med 28(2):131–133
Krexi D, Sheppard MN (2015) Pulmonary hypertensive vascular changes in lungs of patients with sudden unexpected death. Emphasis on congenital heart disease, Eisenmenger syndrome, postoperative deaths and death during pregnancy and postpartum. J Clin Pathol 68(1):18–21
Krishnam MS, Tomasian A, Malik S, Desphande V, Laub G, Ruehm SG (2010) Image quality and diagnostic accuracy of unenhanced SSFP MR angiography compared with conventional contrast-enhanced MR angiography for the assessment of thoracic aortic diseases. Eur Radiol 20(6):1311–1320
Maksimović R, Seferović PM, Ristić AD, Vujisić-Tesić B, Simeunović DS, Radovanović G, Matucci-Cerinic M, Maisch B (2006) Cardiac imaging in rheumatic diseases. Rheumatology (Oxford) 45(Suppl 4):iv26–iv31
Maron BJ, Maron MS (2015) The 20 advances that have defined contemporary hypertrophic cardiomyopathy. Trends Cardiovasc Med 25(1):54–64
Maron MS, Maron BJ, Harrigan C, Buros J, Gibson CM, Olivotto I, Biller L, Lesser JR, Udelson JE, Manning WJ, Appelbaum E (2009) Hypertrophic cardiomyopathy phenotype revisited after 50 years with cardiovascular magnetic resonance. J Am Coll Cardiol 54(3):220–228
Metcalfe J, Ueland K (1974) Maternal cardiovascular adjustments to pregnancy. Prog Cardiovasc Dis 16(4):363–374
Morello A, Gelfand EV (2009) Cardiovascular magnetic resonance imaging for valvular heart disease. Curr Heart Fail Rep 6(3):160–166
Nayak KS, Cunningham CH, Santos JM, Pauly JM (2004) Real-time cardiac MRI at 3 tesla. Magn Reson Med 51(4):655–660
Nielsen JC, Powell AJ, Gauvreau K, Marcus EN, Prakash A, Geva T (2005) Magnetic resonance imaging predictors of coarctation severity. Circulation 111(5):622–628
Partington SL, Valente AM (2013) Cardiac magnetic resonance in adults with congenital heart disease. Methodist Debakey Cardiovasc J 9(3):156–162
Pawade T, Holloway B, Bradlow W, Steeds RP (2014) Noninvasive imaging for the diagnosis and prognosis of pulmonary hypertension. Expert Rev Cardiovasc Ther 12(1):71–86
Piaw CS, Kiam OT, Rapaee A, Khoon LC, Bang LH, Ling CW, Samion H, Hian SK (2006) Use of non-invasive phase contrast magnetic resonance imaging for estimation of atrial septal defect size and morphology: a comparison with transesophageal echo. Cardiovasc Intervent Radiol 29(2):230–234
Rafik Hamad R, Larsson A, Pernow J, Bremme K, Eriksson MJ (2009) Assessment of left ventricular structure and function in preeclampsia by echocardiography and cardiovascular biomarkers. J Hypertens 27(11):2257–2264
Rajiah P, Nazarian J, Vogelius E, Gilkeson RC (2014) CT and MRI of pulmonary valvular abnormalities. Clin Radiol 69(6):630–638
Rigatelli G, Cardaioli P, Hijazi ZM (2007) Contemporary clinical management of atrial septal defects in the adult. Expert Rev Cardiovasc Ther 5(6):1135–1146
Robson SC, Hunter S, Boys RJ, Dunlop W (1989) Serial study of factors influencing changes in cardiac output during human pregnancy. Am J Physiol 256(4 Pt 2):H1060–H1065
Rossi A, Cornette J, Johnson MR, Karamermer Y, Springeling T, Opic P, Moelker A, Krestin GP, Steegers E, Roos-Hesselink J, van Geuns RJ (2011) Quantitative cardiovascular magnetic resonance in pregnant women: cross-sectional analysis of physiological parameters throughout pregnancy and the impact of the supine position. J Cardiovasc Magn Reson 13:31
Sampson FC, Goodacre SW, Thomas SM, van Beek EJ (2007) The accuracy of MRI in diagnosis of suspected deep vein thrombosis: systematic review and meta-analysis. Eur Radiol 17(1):175–181
Shafi NA, Malik A, Silverman DI (2009) Management of Takayasu arteritis during pregnancy. J Clin Hypertens (Greenwich) 11(7):383–385
Steen H (2011) Cardiac magnetic resonance imaging in heart transplant patients. Transplantationsmedizin 23:91–95
Thuny F, Gaubert JY, Jacquier A, Tessonnier L, Cammilleri S, Raoult D, Habib G (2013) Imaging investigations in infective endocarditis: current approach and perspectives. Arch Cardiovasc Dis 106(1):52–62
Toglia MR, Weg JG (1996) Venous thromboembolism during pregnancy. N Engl J Med 335(2):108–114
Tzemos N, Silversides CK, Colman JM, Therrien J, Webb GD, Mason J, Cocoara E, Sermer M, Siu SC (2009) Late cardiac outcomes after pregnancy in women with congenital aortic stenosis. Am Heart J 157(3):474–480
van der Linde D, Konings EE, Slager MA, Witsenburg M, Helbing WA, Takkenberg JJ, Roos-Hesselink JW (2011) Birth prevalence of congenital heart disease worldwide: a systematic review and meta-analysis. J Am Coll Cardiol 58(21):2241–2247
von Knobelsdorff-Brenkenhoff F, Trauzeddel RF, Schulz-Menger J (2014) Cardiovascular magnetic resonance in adults with previous cardiovascular surgery. Eur Heart J Cardiovasc Imaging 15(3):235–248
Waksmonski CA (2014) Cardiac imaging and functional assessment in pregnancy. Semin Perinatol 38(5):240–244
Yamano T, Sawada T, Sakamoto K, Nakamura T, Azuma A, Nakagawa M (2004) Magnetic resonance imaging differentiated partial from complete absence of the left pericardium in a case of leftward displacement of the heart. Circ J 68(4):385–388
Yap SC, Drenthen W, Pieper PG, Moons P, Mulder BJ, Vliegen HW, van Dijk AP, Meijboom FJ, Jaddoe VW, Steegers EA, Boersma E, Roos-Hesselink JW, ZAHARA Investigators (2010) Pregnancy outcome in women with repaired versus unrepaired isolated ventricular septal defect. BJOG 117(6):683–689
Zaidi AN, Raman SV, Cook SC (2008) Acute myocardial infarction in early pregnancy: definition of myocardium at risk with noncontrast T2-weighted cardiac magnetic resonance. Am J Obstet Gynecol 198(3):e9–e12
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Hoque, K.E., Colletti, P.M. (2016). Magnetic Resonance Imaging of Cardiovascular Diseases in Pregnancy. In: Masselli, G. (eds) MRI of Fetal and Maternal Diseases in Pregnancy. Springer, Cham. https://doi.org/10.1007/978-3-319-21428-3_17
Download citation
DOI: https://doi.org/10.1007/978-3-319-21428-3_17
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-21427-6
Online ISBN: 978-3-319-21428-3
eBook Packages: MedicineMedicine (R0)