Abstract
From the first moments of life, typically developing infants perceive social stimuli as relatively more salient than other competing stimuli. Through ongoing cycles of seeking and acting upon such stimuli, infants transform their understanding of the social world, leading to increasingly refined social abilities and specialized brain circuitry for social processing. By contrast, diminished interest in a wide variety of adaptive social stimuli is a pervasive, early emerging, and enduring feature of autism spectrum disorder (ASD). Reduced attention to such stimuli exerts a compounding influence on development as a child with ASD fails to accrue an increasingly longer list of social experiences that would otherwise play a foundational role in social development and functional brain specialization. In this chapter, we review the canalizing role of preferential attention to social stimuli on behavior and brain development in typical infancy. We also review what is known about the early development of infants who are later diagnosed with ASD, with a focus on early departures from normative developmental trajectories of social visual engagement. We focus primarily on visual attention to the eyes of others as a paradigmatic example of an early emerging foundational social ability, that, when disrupted, is both a marker of emerging social disability and a compounding influence on subsequent development. Finally, we examine the accumulative consequences of departures from normative developmental trajectories of social visual engagement, as children with ASD develop increasingly greater specialization in things other than the social world.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Autism spectrum disorder
- Social development
- Developmental trajectories
- Infancy
- Canalization
- Brain development
- Brain specialization
The ability to successfully navigate social interactions is deeply embedded in one’s history of social actions—in the accumulation of a vast number of experiences seeking and acting upon socially relevant information. The experiences themselves, as well as the abilities that result from those experiences, progress in a cyclical, iterative manner, both constrained by and then further constraining the way in which the environment is perceived (Jones & Klin, 2009; von Uexkull 1934). In human infants, these processes begin within the first hours after birth (at least): irrespective of the sensory “domain,” social stimuli in the environment are perceived by typically developing infants as relatively more salient than other competing stimuli (e.g., Batki, Baron-Cohen, Wheelwright, Connellan, & Ahluwalia, 2000; Bushneil, Sai, & Mullin, 1989; DeCasper & Fifer, 1980; Macfarlane, 1975; Simion, Regolin, & Bulf, 2008; Vouloumanos & Werker, 2007). Thereafter, through ongoing cycles of seeking and acting upon such stimuli, infants transform their understanding of the social world through active engagement (Smith & Gasser, 2005). This spontaneous seeking of and acting upon social information is an adaptive reaction displayed by typically developing children from infancy onward, if not before.
In contrast, the diminished interest in, and attention to, a wide variety of adaptive social stimuli has been identified as a pervasive, early emerging, and enduring feature of autism spectrum disorder (ASD). The diminished salience of social stimuli to individuals with ASD is a marker of social disability (e.g., Jones, Carr & Klin, 2008), but it also exerts a compounding influence on subsequent development as a child with ASD fails to accrue an increasingly longer list of social experiences that would otherwise lay the foundation for typical social development. Instead, individuals with ASD often engage with a range of nonsocial, physical stimuli (Kanner, 1943; Klin, Lin, Gorrindo, Ramsay, & Jones, 2009; Langdell, 1978; Shultz, Klin, & Jones, 2011), leading to an accumulation of experiences with “things” rather than people. This process, in turn, leads to atypical developmental profiles of functional brain specialization (Grelotti et al., 2005; Schultz, 2000), as that functional specialization is necessarily shaped through activity-dependent processes (LeDoux, 2003).
While reduced visual attention to social stimuli has been widely reported in children and adults with ASD, less is known about the early divergence of such behaviors in infancy, and even less is known about the impact thereof on subsequent development. In this chapter, we focus primarily on visual attention to the eyes of others as a paradigmatic example of an early emerging foundational social ability, that, when disrupted, is both a marker of emerging social disability and a compounding influence on subsequent development. While attention to eyes is only one of many critical mechanisms of social adaptive action, its phylogenetically conserved nature (Emery, 2000), early onset (Farroni, Csibra, Simion & Johnson, 2002), and foundational role in socialization (Brooks & Meltzoff, 2002; Emery, 2000; Kampe, Frith, Dolan, & Frith, 2001) are particularly well suited to investigations of when reduced social engagement is first observed in ASD and how such disruptions subsequently impact developmental outcomes.
Section 6.1 reviews the canalizing role of preferential attention to stimuli with social adaptive value, including the eyes of others, in typically developing infants’ first months of life. Section 6.2 discusses the relationship between changes in typical infants’ preferential attention to the eyes of others, changes in their interactions with conspecifics, and changes in related structural and functional brain maturation. Section 6.3 then reviews what is known about the early development of infants who are later diagnosed with ASD, with a focus on their early departures from normative developmental trajectories of social visual engagement with the eyes of others. Finally, Section 6.4 examines the accumulative consequences of such departures, as children with ASD develop increasingly greater specialization in things other than the social world.
Taken as a whole, this review of typical and atypical processes of social engagement highlights the canalizing role of early experience in child development: success in early social adaptive tasks leads to new social experiences and increasingly refined social abilities, but these iterative processes appear to work in much the same way for atypical social experiences (Jones & Klin, 2009). In the case of infants with ASD, early departures from normative processes of social engagement are likely to have profound and long-term effects on the social and cognitive development of these children (see Fig. 6.1). Whereas typically developing infants show an increase in preferential attention to the eyes of others from 2 until 6 months, infants later diagnosed with ASD show mean decline in eye fixation during this early period (with continuing declines thereafter) (Jones & Klin, 2013). While reduced attention to the eyes of others is not in itself a cause of autism, it does represent a marker of emergent social disability as well as a compounding influence on subsequent social and brain development . By mapping the unfolding of social disability in ASD, we hope to constrain future hypotheses about causal mechanisms underlying the disorder.
Summary of some early developmental milestones that serve to canalize and constrain typical social development in brain and behavior. In typical development (top panel), early predispositions and subcortical brain systems guide infants toward what is socially relevant. Seeking social interaction creates further opportunities for social learning and modulates normative brain development. In autism spectrum disorder (ASD) (bottom panel), disruption of active seeking of social information alters opportunities for learning; developing brain systems become co-opted in service of alternative goals
6.1 Adaptive Action in Response to Environmental Demands Constrains Typical Development
Given the fragility of human neonates at birth, engagement with a caregiver is the initial task upon which survival depends. Neonates’ remarkable attunement to their caregivers, even in the absence of extensive experience with others, presents a ready solution. From the first moments of life, human infants are drawn to the sight, sound, and smell of their caregivers (Bushneil et al., 1989; DeCasper & Fifer, 1980; Macfarlane, 1975). From rooting and sucking reflexes that help breastfed infants find their mother’s nipple to preferential attention to conspecifics, neonates display a host of adaptive skills that help them to successfully engage their caregivers (Nagy, 2011). When just 10-min old, infants already show tremendous sensitivity to faces, evinced by their proclivity to track a moving face-like pattern but not a scrambled or inverted face pattern (Goren, Sarty, & Wu, 1975; Johnson, Dziurawiec, Ellis, & Morton, 1991; Simion, Valenza, Umilta, & Barba, 1998; Valenza, Simion, Cassia, & Umiltà, 1996). By just 5 days, infants demonstrate special sensitivity not just to faces, but specifically to the eyes of another person, preferring to look at faces with eyes open rather than closed (Batki, 2000). Strikingly, and despite rather limited visual acuity (Kellman & Banks, 1998), they are also able, at approximately the same age, to distinguish faces whose gaze is directed toward them rather than away from them (Farroni et al., 2002). These preferences are widespread in other domains as well. Newborns distinguish and prefer their own mother’s voice to that of an unknown woman, but prefer the sound of an unknown woman’s voice to that of silence (DeCasper & Fifer, 1980). This evidence suggests that typically developing babies have a predisposition to engage with the social aspects of the world around them: the social dimension is most behaviorally salient and what consequently commands the greatest portion of the typically developing infant’s attention.
While preferential attention to caregivers has immediate survival value for newborn infants, an equally important effect is that it establishes new opportunities for social interaction and social learning . By directing attention toward the social world, the newborn embarks upon what will be its period of greatest postnatal change in brain and behavior, as success in early social adaptive tasks canalizes typical development toward increasingly refined forms of social and communicative competence. As infants gain increasing experience with their surroundings, their initially broad attentional preferences become increasingly attuned to specific signals that are most developmentally relevant. For instance, while newborns show a preference for both human speech and rhesus macaque vocalizations, 3-month olds prefer human speech over rhesus calls (Shultz & Vouloumanos, 2010; Vouloumanos, Hauser, Werker, & Martin, 2010). Similarly, infants’ discrimination skills become attuned to conspecific and own-race faces within the first 9 months (Kelly et al., 2007; Pascalis, de Haan & Nelson, 2002) and become similarly attuned to native language phonemic contrasts in the first 10 months (Werker & Tees, 1984). In addition to the refinement of preferential attention, infants display increasingly sophisticated social abilities as preferential orientation toward caregivers transitions into face-to-face communication: by 2 months, typical infants show improved ability to maintain eye contact (Blass & Camp, 2001), improved ability to maintain attention (Aslin, 1987), active exploration of internal facial features (Haith, Bergman, & Moore, 1977), and the ability to engage in coordinated affective and vocal exchanges with a communicative partner (Lavelli & Fogel, 2005).
While these feats of early infancy represent remarkable abilities in such otherwise fragile beings, neither the achievements themselves nor the patterns of developmental change are entirely unique to human infant development. In particular, the concept of canalization and the importance of experience in guiding development are well established in many other species. In bird species, for instance, early species-typical experiences, such as exposure to own-species vocalizations, are critical in guiding preferential attention toward socially relevant signals, such as the mother’s call (Gottlieb, 1981). When mallard ducklings are devocalized and thereby deprived of embryonic auditory self-stimulation, a preference for the mallard maternal call compared to the call of other species fails to emerge (Gottlieb, 1971, 1975). This demonstrates how the hatchling’s early experiences can canalize development, constraining the range of stimuli that elicits preferential attention and guiding the hatchling toward forms of stimulation that are most relevant for survival. In the absence of these early experiences, the auditory preferences of hatchlings remain broadly tuned, responsive to the maternal signals of many species (Gottlieb, 1991). Such a disruption could conceivably have widespread and cascading effects on subsequent development, as reduced preferential attention to conspecifics may diminish opportunities for species-typical learning and contact with conspecifics.
6.2 Adaptive Action and Developing Brain Systems Constrain and Shape One Another
In human infants, examination of neural systems that guide infants’ attention to caregivers reveals the ways in which developing brain systems and behavioral capacities both constrain and shape one another. Behavior exerts powerful influences on the development of neural systems by selecting inputs that dynamically modulate neural activity, thereby shaping brain structure and function (Byrge, Sporns, & Smith, 2014). Brain activity, in turn, modulates behavior, creating a circular process whereby “the brain’s outputs influence its inputs and these inputs in turn shape subsequent outputs—binding brain networks to the organism’s environment over short timescales and cumulatively over developmental time” (Byrge et al., 2014, p. 3).
This intrinsic dependency between brain and behavior is also found in the neural systems that subserve attention to the eyes of others. At birth, the relative maturity of subcortical visual structures, as compared to the relative immaturity of cortical visual structures, may actually function to facilitate the patterns of preferential visual attention described in the previous section. Newborns’ direct their rather limited visual attention, using the available subcortical neural resources, toward those stimuli that have the greatest survival value—the faces and eyes of others (Turkewitz & Kenny, 1982). The subcortical visual system (unlike the later-maturing cortical structures) is differentially responsive to visual properties of faces and eyes, such as low-spatial frequency components of the configuration of a face (Morton & Johnson, 1991), top-heavy vertically asymmetrical patterns (Simion, Cassia, Turati, & Valenza, 2001), and high contrast polarity (Farroni et al., 2005); this developmental affordance between available neural resources and physical regularities in the conspecific caregiving environment helps to ensure that newborns are more likely to fixate on faces, and especially the eyes, more than other competing stimuli in the natural environment (Johnson & de Haan, 2001; Johnson, 2005; Morton & Johnson, 1991).
Indirect evidence further supports this notion that neonates’ visual biases for face-like stimuli are primarily mediated by subcortical rather than cortical visual circuitry. By about 2 months prenatally, myelination of the subcortical visual pathway begins (Yakovlev & Lecours, 1967), and at birth, the subcortical visual pathway is fully functional. In contrast, the primary visual cortex is relatively immature at birth (Atkinson, 2000; Johnson, 1990; Martin et al., 1999; Morita et al., 2000) and has little influence over visually guided behavior (Csibra, Tucker, Volein, & Johnson, 2000). Myelination of cortical visual pathways begins at the time of birth and does not finish until approximately 4 months, a full month later than subcortical pathways (Yakovlev & Lecours, 1967). Similarly, while subcortical structures such as the lateral geniculate nucleus have completed the majority of their developmental change before birth (Garey & De Courten, 1983; Hitchcock & Hickey, 1980; Khan, Wadhwa, & Bijlani, 1994), primary visual cortical areas undergo a large increase in synaptogenesis in the first months of life (Huttenlocher, de Courten, Garey, & Van der Loos, 1982).
This evidence underscores the developmental affordance between the subcortical visual orienting system of newborns and a social adaptive action that is critical to their survival: attending to the faces and eyes of others. Together, these mechanisms facilitate survival, but they also conspire to encourage social interactions and social learning from the first months of life. As subcortical structures guide newborns’ visual attention toward socially relevant stimuli, the act of seeking and attending to such information in turn shapes the developing brain. The amygdala , a subcortical structure that plays a role in both directing and maintaining biases for attending to faces (Adolphs, Tranel, & Damasio, 1998; Bachevalier, 1994; Baron-Cohen et al., 2000; Brothers, 2002) and in reacting quickly to highly salient social stimuli (LeDoux, 1996; Schultz, 2000), has reciprocal connections with ventral visual areas (Amaral & Price, 1984) and projects information about salient stimuli to cortical areas involved in face processing in adults, such as the lateral occipital cortex, fusiform, and orbitofrontal cortex (Pasley, Mayes, & Schultz, 2004). During the first 2 months of life, connections between the amygdala and regions, such as the fusiform, lateral occipital cortex, and orbitofrontal cortex, may serve to increase the activity of cortical areas that would otherwise receive only weak input from functionally immature cortical visual areas (Johnson, 2005), leading to the subsequent specialization of these regions for processing faces and other social stimuli. As early experiences accrue, so too does the synaptogenesis to and from, and the myelination of, these cortical visual pathways.
Existing studies of brain function in infancy provide evidence of this type of neural specialization, specifically for stimuli that hold the greatest adaptive value to typically developing infants, even by the second month of life. Two-month olds show activation in cortical areas within the adult face-processing network, including the right fusiform gyrus and bilateral inferior occipital cortex, when viewing human faces (Tzourio-Mazoyer et al., 2002). Three-month olds show selective activation in left superior temporal and angular gyri in response to forward compared with reversed speech (Dehaene-Lambertz, Dehaene, & Hertz-Pannier, 2002) and a larger event-related potential (ERP) N290 component amplitude and shorter latency in response to human compared with monkey faces (Halit, De Haan, & Johnson, 2003). Finally, 3- to 7-month olds show activation of the right middle temporal gyrus in response to human vocalizations compared with nonspeech vocalizations and environmental sounds (Blasi et al., 2011).
Although studies examining the relationship between developmental change in brain function and behavior are scant, there is some evidence that cortical networks undergo a process of specialization that coincides with the refinement of social adaptive action in early infancy. A functional magnetic resonance imaging (fMRI) study of 1- to 4-month-old infants revealed increased neural selectivity for speech over biological nonspeech sounds during a developmental period that coincides with the attunement of infants’ listening preferences for human speech over rhesus monkey calls (Shultz, Vouloumanos, Bennett, & Pelphrey, 2014). Specifically, a negative correlation was observed between gestational age and response to biological nonspeech sounds within a speech-sensitive region of left temporal cortex. An increased selectivity for speech during this period in development represents a process that may be both a cause and consequence of the tuning of infants’ listening preference for speech: as infants’ active seeking of speech likely modulates neural activity, these neural changes, in turn, modulate behavior.
6.3 Early Departures from Normative Trajectories in ASD
While, as reviewed earlier, typically developing infants show a remarkable attunement to the social world, with evidence of maturational brain change that constrains and guides attunement, the available evidence suggests that this is not the case for individuals with autism. A striking feature of the disorder is that individuals with ASD, even intellectually capable adults, exhibit deficits in the very social adaptive actions that have immediate survival value and provide the platform for future social development (Kanner, 1943). For example, while typical newborns as young as 2-days old orient preferentially to socially relevant signals such as biological motion and the eyes of others (Farroni et al., 2002; Simion, Regolin, & Bulf, 2008), 2-year-old children with ASD fail to do so (Jones et al., 2008; Klin et al., 2009). By demonstrating that skills present from birth in typical newborns are disrupted in ASD, these findings point to early departures from normative processes of development. Following from a model of child development where success in social adaptive tasks guides typical development toward increasingly refined skills in an iterative process, early departures from such processes will likely lead to atypical outcomes as development becomes increasingly atypical (Jones & Klin, 2009).
The potentially devastating and accumulative consequences of early disruptions in basic mechanisms of social adaptive action highlight the period of early infancy as a target for research aimed at understanding the emergence of the syndrome. While reduced attention to social stimuli has been widely reported in children and adults with ASD, less is known about the course of such deviations from normative developmental trajectories in early infancy and their impact on subsequent outcomes. Because ASD is rarely diagnosed before 18 months (Klin et al., 2004), the method of choice for research of infancy in autism necessarily involves prospective study and longitudinal following of the “baby siblings” of children already diagnosed with an ASD. Within siblings followed prospectively, the recurrence rate of ASD is high, estimated at 18.7 % (Ozonoff et al., 2011).
To date, the main focus of longitudinal studies of baby siblings has been on the timing and diagnostic manifestation of autistic symptomatology. Within the first 18 months of life, infants with ASD already show signs characteristic of the disorder:
-
At 6 months: reduced attention to social scenes and faces (Chawarska, Macari, & Shic, 2013).
-
At 12 months: unusual patterns of object exploration and stereotyped, repetitive behaviors (Loh et al., 2007; Ozonoff et al., 2008), reduced social interest and atypicalities in eye contact, affect, orienting to one’s name, imitation, and social smiling (Hutman et al., 2010; Ozonoff et al., 2010; Wan et al., 2013; Zwaigenbaum et al., 2005), deficits in vocal production, language comprehension, and gesture production (Mitchell et al., 2006; Paul, Fuerst, Ramsay, Chawarska, & Klin, 2011), and lower rates of requesting behaviors (Rozga et al., 2011).
-
At 14 months: cognitive deficits, as measured by the Mullen Scales of Early Learning (Landa & Garrett-Mayer, 2006).
-
At 15 months: deficits in joint attention and triadic communication (Sullivan et al., 2007; Yoder, Stone, Walden, & Malesa, 2009).
-
At 18 months: atypicalities in play behavior (Christensen et al., 2010) .
While these studies provide important insights into the manifestation of ASD at developmental time points prior to the typical age at diagnosis, most of the symptomatology described is likely to have arisen as a consequence of atypical early social experiences. Few studies have examined disruptions to basic mechanisms of social adaptive action that are the putative building blocks of later social abilities, and no prospective longitudinal studies have examined departures from such processes in the first 6 months of life. By failing to measure the developmental antecedents of ASD symptomology, we run the risk of measuring the culmination, rather than the unfolding, of departures from typical social behavior and experience.
This state of affairs was the impetus for our most recent study of attention to the eyes of others in infants later diagnosed with ASD (Jones & Klin, 2013). Our motivation for measuring preferential attention to eyes was twofold. First, preferential attention to eyes is a phylogenetically well-conserved mechanism of social adaptive action (Emery, 2000) that plays a key role, as summarized earlier, in canalizing typical social development: eye-looking serves to entrain babies to the social signals of their caregivers and also establishes opportunities for learning through social interaction. Second, preferential attention to the eyes of others is developmentally early emerging, present from the first days of life in typical infants but significantly reduced in 2-year olds with ASD (Farroni et al., 2002; Jones et al., 2008).
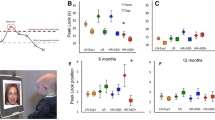
We collected data from infants at low-risk and at high-risk for ASD at 10 time points: at months 2, 3, 4, 5, 6, 9, 12, 15, 18, and 24. Diagnostic status was ascertained at 36 months. Infants viewed prerecorded video scenes of caregivers looking directly at the children, with the caregivers making entreating overtures and enacting typical infant routines (preparing for a meal, engaging in motherese vocal communication, singing a nursery rhyme, etc.). Infants’ visual scanning was measured with eye-tracking equipment while watching the videos. The percent of visual fixation time to eyes, mouth, body, and object regions was measured. Visual fixation time to eyes for the typical children created normative growth curves of social visual engagement against which to compare the data for infants later diagnosed with autism. Typically-developing (TD) children, from 2 to 24 months, looked more at the eyes than at any other region of the screen (mouth, body, objects); eye fixation increased steadily from 2 to 9 months and then remained relatively stable until the age of 24 months (Fig. 6.2a).
Growth charts of social visual engagement for typically developing children and children diagnosed with ASD, originally published in Nature (2013). a, b Fixation time to eyes, mouth, body, and object from 2 until 24 months in (a) typical development (TD) and (b) autism spectrum disorder (ASD). c, d Contrary to a congenital reduction in preferential attention to eyes in ASD, (d) children diagnosed with ASD exhibit mean decline in eye fixation. e–h Longitudinal change in fixation to (e) eyes; (f) mouth; (g) body; and (h) object regions. Dark lines indicate mean growth curves; light lines indicate 95 % confidence intervals (CI). Top panels in e-h plot percent fixation; middle panels plot change in fixation (the first derivative, in units of % change per month); and bottom panels plot F value functions for between-group pointwise comparisons as a function of age in months. Significant differences are shaded in medium gray for comparison of fixation data and light gray for comparison of change-in-fixation data
In infants later diagnosed with ASD, growth curves of social visual engagement follow a very different developmental course. Eye fixation began at a level similar to TD children, but then declined steadily from the 2-month starting point, arriving at a level that was approximately half that of TD children by the 24-month endpoint (Fig. 6.2e). This pattern holds two key implications for our understanding of the developmental pathogenesis of social disability in ASD. First, these results pinpoint the developmental onset of the widely reported reduction in preferential attention to eyes in ASD; rather than a congenital absence of attention to eyes in ASD, early levels of eye-looking at 2 months of age seem to begin at normative levels, with the decline in eye-looking beginning during the period from 2 to 6 months. Second, these results reveal the impact of deviations from normative trajectories of social visual engagement on subsequent outcomes. The decline in eye fixation from 2 to 6 months was significantly associated with diagnostic outcome at 36 months, providing a strong marker of later diagnosis 1½ years before children can be diagnosed conventionally and 2½ years before they can be diagnosed stably. In addition, the extent of decline in eye-looking among children with ASD was a strong predictor of their level of social disability at outcome (as measured with standardized clinical instruments): those whose levels of eye-looking declined most rapidly were also most socially disabled in later life.
To our surprise, however, these data contradicted prior hypotheses postulating a congenital absence of social adaptive orientation in ASD: early levels of eye-looking were not immediately diminished in infants with ASD; instead, infants with ASD exhibited a slight but significant increase in eye-looking at 2 months, which then declined (Fig. 6.2c and d). Several points are worth noting regarding this preliminary finding of “normative” eye-looking at 2 months of age in ASD. Although these data appear to show that orienting to eyes is present in early infancy in ASD, this does not necessarily mean that the behavior itself is “normative” or representative of eye-looking in typical social development. Put differently, although the superficial levels of eye-looking (i.e., high magnitude of eye-looking at 2 months of age) are present, the developmental processes underlying such eye-looking may be markedly different in infants with ASD. Indeed, the rate-of-change data indicate that the underlying rate of change in eye-looking already differs between typical infants and infants with ASD at 2 months of age. In addition, magnitude of eye-looking is slightly, but significantly, higher at 2 months of age in infants with ASD. While this finding should be replicated in a larger sample, the relatively high level of eye-looking at 2 months in ASD may already represent a departure from normative developmental processes. Thus, while eye fixation may look superficially similar in the early months, the underlying developmental processes may already be markedly different.
Despite the possibility that initial eye-looking in ASD may not reflect normative developmental processes, the mere presence of early eye-looking, as opposed to an outright absence, constrains hypotheses about what processes may be disrupted in ASD. Intriguingly, the finding of superficially normative levels of eye-looking, followed by a decline thereafter, maps onto preexisting literatures relating to early typical transitions in face expertise and early infant transitions in adaptive behavior.
In typical development, preferential orientation to faces (present at birth) declines between 4 and 6 weeks before reemerging at approximately 2 months (Johnson et al., 1991), a pattern that mirrors that of other neonatal reflexive actions, such as orienting to auditory sounds and imitating others (Dodwell, 1983; Field, Muir, Pilon, Sinclair, & Dodwell, 1980; Field, Goldstein, Vega-Lahr, & Porter, 1986; Maratos, 1982). A model for this transition has been proposed, moving from “experience-expectant” mechanisms (more “reflex-like,” and presumably more proximal to gene determination) to “experience-dependent” mechanisms (building on the iterative experiences and resultant learning that arise as a consequence of the initial, reflex-like behaviors; Bjorklund, 1987; Emde & Harmon, 1972; Johnson, 1990; Johnson et al., 1991).
The neural mechanism that underlies these changes is thought to be a shift from subcortical to cortical control, with initial predispositions (subserved by subcortical structures) declining as cortical control develops. This suggestion is strengthened by evidence of the early development of the visual system: as reviewed above, while the subcortical visual pathway is functional at birth, the primary visual cortex remains relatively immature and has little influence over visually guided behavior (Atkinson, 2000; Johnson, 1990; Martin et al., 1999; Morita et al., 2000). Retinocortical pathways become fully functional approximately 2 months after birth (Atkinson, 2000; Braddick, Wattam-Bell, & Atkinson, 1986)—when our data collection begins—and also at approximately the same age at which ERP and positron emission tomography (PET) studies show evidence of cortical specialization for attention to faces (Halit et al., 2003; Tzourio-Mazoyer et al., 2002).
This maturational timeline is well fitted by the changes in behavior that we observe in typically developing infants. At 2–3 months of age, typical infants appear to be in transition between reflex-like orientation and emerging, cortically controlled preferential attention: the 2-month time point would thus be situated between one downward developmental trend (the waning of subcortically controlled, reflex-like eye fixation) and one upward trend (the increasing, experience-dependent, cortically-mediated eye fixation). This maps onto both the relative low point in eye fixation by typical infants at 2 months of age and the increase in eye fixation from 2 until 9 months. In contrast, the data for infants later diagnosed with ASD suggest a reflex-like orientation that appears to persist beyond its developmentally appropriate time window (leading even to a slight increase in ASD relative to typical eye fixation at 2 months, Fig. 6.2e). In the absence of the emergence of cortically controlled, experience-dependent eye fixation, the reflex-like (subcortically mediated) orientation appears to persist before declining.
6.4 Departures from Normative Trajectories Yield Increasingly Atypical Behavior and Atypical Neural Specialization
Our recent eye-tracking results indicate that subcortically mediated reflex-like attention to eyes may fail to transition to cortically mediated experience-dependent eye fixation at around the second month of life in ASD. However, rather than suggesting an outright failure of cortical control of preferential visual attention in ASD, the available data suggest a co-opting of those mechanisms by attention to other features in the environment. For instance, instead of maintaining and reinforcing attention to eyes, infants with ASD showed high levels of mouth and object fixation, with fixation on others’ mouths increasing from month 2 until approximately month 18 and object fixation rising by 24 months to twice the level of typical controls (see Fig. 6.2f and h). Data from older infants and toddlers further support the notion that individuals with ASD use ostensibly intact attentional systems to actively seek out alternate experiences. For example, at 12 months of age, infants with ASD show reduced social interest, accompanied by a tendency to fixate on particular objects in the environment (Hutman, Chela, Gillespie-Lynch, & Sigman, 2012; Zwaigenbaum et al., 2005). In addition, unlike typical toddlers, toddlers with ASD show a preference for nonspeech compared with speech (Klin, 1991; Kuhl, Coffey-Corina, Padden, & Dawson, 2005) and for dynamic geometric images compared to dynamic social images (Pierce, Conant, Hazin, Stoner, & Desmond, 2011). Finally, studies from our laboratory demonstrate that toddlers with ASD are highly sensitive to the presence of nonsocial, physical contingencies (audiovisual synchronies between point lights) (Klin et al., 2009) and are more engaged by physical, rather than social events (Shultz et al., 2011).
Together these results suggest that a child with autism, from as early as 2 months of age, is learning from a world dominated by physical rather than social events. Given the canalizing role of preferential attention to social stimuli in typical development, how might attention toward physical, rather than social, stimuli impact developmental outcomes? Our longitudinal eye-tracking data provide an initial answer to this question by demonstrating that reduced attention to others’ eyes between 2 and 6 months of age is a strong predictor of a later diagnosis of ASD at 36 months. In addition, more pronounced atypical experiences may actually worsen developmental outcome, as steeper decline in eye fixation was associated with more severe social disability (Jones & Klin, 2013). Although not examined in our eye-tracking study, we further predict that disruptions to early mechanisms of social adaptive action may have widespread cascading effects on many areas of development, as children with ASD actively seek alternate experiences as they try to make sense of their surrounding world (Jones & Klin, 2009).
This hypothesis is nicely illustrated by the case of diverging developmental processes underlying attention to the mouth in typical toddlers and toddlers with ASD. While attention to the mouth is a social adaptive action in typical development, proposed to play a key role in the development of spoken communication in typical infancy (Lewkowicz & Hansen-Tift, 2012), research from our laboratory has revealed that attention to the mouth in toddlers with ASD may instead be driven by sensitivity to audiovisual synchrony (Klin et al., 2009). Put differently, the available evidence suggests that typical children and children with ASD seek and attend to the mouths of others for very different reasons, with typical children viewing mouths as a source of spoken social communication and toddler with ASD viewing the mouth as a source of audiovisual synchrony. These very different goals likely yield different learning experiences and expertise, and, at a later developmental time point, may result in different communication and language outcomes: typical children develop adaptive social communication skills (Locke, 1995), whereas children with ASD who do acquire language often do so in a way that is decoupled from social meaning, resulting in speech that is rote rather than contextualized (Tager-Flusberg, Paul, & Lord, 2005); facts that are memorized rather than episodic and personalized (Klin et al., 2007; O’Shea, Fein, Cillessen, Klin, & Schultz, 2005); and in extreme cases even results in instances of hyperlexia in ASD, when words are read without any concept of their meaning (Grigorenko, Klin, & Volkmar, 2003).
Given that infants contribute very actively to their own brain specialization by attending differentially to the surrounding environment (Byrge et al., 2014; Joseph E LeDoux, 2003), the accumulation of atypical social experiences in ASD is likely to have a profound impact on shaping brain structure and function. The brain, in turn, modulates behavior, creating an iterative process whereby atypical experiences and altered brain specialization become compounded over developmental time (Byrge et al., 2014). Although few studies have examined brain–behavior relationships prospectively and longitudinally in the first months of life, existing evidence supports the notion of early emerging alterations in brain structure and function in ASDs that are consistent with reduced social attention. Unlike typical controls, 6- to 10-month-olds later diagnosed with ASD fail to modulate the amplitude of a face-sensitive ERP component, the P400, in response to viewing eye-gaze shifts toward versus away from them (Elsabbagh et al., 2012). Similarly, 10-month-olds later diagnosed with ASD show a prolonged P400 latency along with late and less persistent gamma activity in response to direct eye gaze (Elsabbagh et al., 2009). Finally, in contrast to control infants, 9-month-olds at high risk for ASD do not show modulation of P400 latency when viewing their mother’s face compared with a stranger’s face (Key and Stone, 2012) and 10-month-olds at high risk for ASD show a faster N290 ERP component in response to objects compared with faces (McCleery, Akshoomoff, Dobkins, & Carver, 2009).
Consistent with altered brain specialization for social processing, older children and adults with ASD show reduced functional connectivity and hypoactivation in a network of brain regions implicated in social processing in typical adults (Gotts et al., 2012). For instance, hypoactivation has been reported in the superior temporal sulcus in response to biological motion (Freitag et al., 2008; Herrington et al., 2007; Kaiser et al., 2010); in the medial prefrontal cortex, superior temporal sulcus, and temporal poles during tasks that involve thinking about the mental states of others (Castelli, Frith, Happé, & Frith, 2002); in the mirror neuron system (including the inferior frontal gyrus and inferior parietal lobule) during imitation (Dapretto et al., 2006); and in the amygdala and fusiform gyrus in response to faces (Schultz, 2000). Although the relationship between reduced social attention and disruptions to social brain regions has not been systematically explored, some evidence suggests that brain structures typically subserving social processing become “co-opted” for processing alternate stimuli of greater interest to individuals with ASD. In one intriguing case (Grelotti et al., 2005), an adolescent with ASD honed his interests in Digimon cartoons over many years; he displayed activation of his amygdala and fusiform gyrus for perceptual discriminations involving Digimon, but not for those involving familiar or unfamiliar faces. This case suggests that abnormal functioning of the fusiform in ASD may arise as a consequence of years of reduced social interest and atypical experiences.
6.5 Conclusions
Adaptive action in response to environmental demands constrains development in an iterative process that builds on older structures to generate new ones. In this view, a child’s developmental outcome is shaped not only by genetic and neural predispositions, but also by the experiences that arise as a consequence of those predispositions (Jones & Klin, 2009). For typical infants, predispositions to attend to the social world from the first moments of life canalize development, resulting in successively more complex social cognitive abilities and neural specialization. For infants with autism, failure to attend to social stimuli, and looking at other parts of the world instead, suggests an altered path for learning, with cascading effects on further brain and behavioral development.
This framework highlights the pressing need to study deviations from the building blocks of typical development in order to understand developmental outcomes, echoing Karmiloff-Smith’s proposal that, “development itself is the key to understanding developmental disorders” (Karmiloff-Smith, 1998). By measuring departures from normative trajectories of brain and behavioral development, we can begin to identify disruptions in socialization (such as lack of attention to eyes) and map their timing and consequences for subsequent social cognitive growth, brain development , and syndrome expression.
This approach holds promise for informing understanding of brain-behavior pathogenesis in ASD. Given the late age of actual diagnosis of ASD (Centers for Disease Control and Prevention, 2014), the majority of current research findings are based on work with relatively older children. This leaves the possibility that much of our existing knowledge of autism reveals more about the consequences of having had autism (often for many years) than it does about the causes and underlying mechanisms from which a disability arose (Jones & Klin, 2009). Quantifying the earliest developmental antecedents of the condition—such as a decline in looking at the eyes of others—offers the opportunity to study the unfolding, rather than the culmination, of many years of often increasingly aberrant behavior and atypical experience (Grelotti, Gauthier, & Schultz, 2002). However, it is important to note that reduced orienting toward caregivers does not cause autism in and of itself. Clearly more work is needed to understand how these altered predispositions initially arise in infants with ASD. Nonetheless, early departures from normative processes of development represent both a sign of social disability and a compounding influence on subsequent social disability. Mapping these early departures in ASD holds promise for identifying important areas of future research and may constrain future hypotheses about the causal mechanisms underlying ASD. Indeed, our longitudinal eye-tracking findings refute hypotheses of a congenital absence of preferential social attention in ASD and instead highlight a narrow period of early infancy, spanning the transition from experience-expectant to experience-dependent mechanisms, as a critical focus for future investigation.
References
Adolphs, R., Tranel, D., & Damasio, A. R. (1998). The human amygdala in social judgment. Nature, 393(6684), 470–474.
Amaral, D. G., & Price, J. L. (1984). Amygdalo-cortical projections in the monkey (Macaca fascicularis). Journal of Comparative Neurology, 230(4), 465–496.
Aslin, R. N. (1987). Visual and auditory development in infancy. In J. Osofsky (Ed.), Handbook of infant development (2nd ed., pp. 5–97). Oxford: Wiley.
Atkinson, J. (2000). The developing visual brain. Oxford: Oxford University Press.
Bachevalier, J. (1994). Medial temporal lobe structures and autism: A review of clinical and experimental findings. Neuropsychologia, 32(6), 627–648.
Baron-Cohen, S., Ring, H. A., Bullmore, E. T., Wheelwright, S., Ashwin, C., & Williams, S. C. R. (2000). The amygdala theory of autism. Neuroscience & Biobehavioral Reviews, 24(3), 355–364.
Bates, E. (1976). Language and context. New York: Academic Press.
Batki, A., Baron-Cohen, S., Wheelwright, S., Connellan, J., & Ahluwalia, J. (2000). Is there an innate gaze module? Evidence from human neonates. Infant Behavior and Development, 23(2), 223–229.
Bjorklund, D. (1987). A note on neonatal imitation. Developmental Review, 7(1), 86–92.
Blasi, A., Mercure, E., Lloyd-Fox, S., Thomson, A., Brammer, M., Sauter, D., Deeley, Q., Barker, G. J., Renvall, V., Deoni, S., Gasston, D., Williams, S. C., Johnson, M. H., Simmons, A., & Murphy, D. G. (2011). Early specialization for voice and emotion processing in the infant brain. Current Biology, 21(14), 1220–1224.
Blass, E. M., & Camp, C. A. (2001). The ontogeny of face recognition: Eye contact and sweet taste induce face preference in 9- and 12-week-old human infants. Developmental Psychology, 37(6), 762–764.
Braddick, O. J., Wattam-Bell, J., & Atkinson, J. (1986). Orientation-specific cortical responses develop in early infancy. Nature, 320, 617–619.
Brooks, R., & Meltzoff, A. N. (2002). The importance of eyes: How infants interpret adult looking behavior. Developmental Psychology, 38(6), 958–966.
Brothers, L. (2002). The social brain: A project for integrating primate behavior and neurophysiology in a new domain. In J. T. Cacioppo (Ed.), Foundations in social neuroscience, (pp. 367–385). London: MIT Press.
Bushneil, I. W. R., Sai, F., & Mullin, J. T. (1989). Neonatal recognition of the mother’s face. British Journal of Developmental Psychology, 7(1), 3–15.
Byrge, L., Sporns, O., & Smith, L. B. (2014). Developmental process emerges from extended brain–body–behavior networks. Trends in Cognitive Sciences, 18(8), 395–403.
Castelli, F., Frith, C., Happé, F., & Frith, U. (2002). Autism, Asperger syndrome and brain mechanisms for the attribution of mental states to animated shapes. Brain, 125(8), 1839–1849.
Chawarska, K., Macari, S., & Shic, F. (2013). Decreased spontaneous attention to social scenes in 6-month-old infants later diagnosed with autism spectrum disorders. Biological Psychiatry, 74(3), 195–203.
Christensen, L., Hutman, T., Rozga, A., Young, G. S., Ozonoff, S., Rogers, S. J., Baker, B., & Sigman, M. (2010). Play and developmental outcomes in infant siblings of children with autism. Journal of Autism and Developmental Disorders, 40(8), 946–957.
Csibra, G., Tucker, L. A., Volein, Á., & Johnson, M. H. (2000). Cortical development and saccade planning: The ontogeny of the spike potential. Neuroreport, 11(5), 1069–1073.
Dapretto, M., Davies, M. S., Pfeifer, J. H., Scott, A., Sigman, M., Bookheimer, S. Y., & Iacoboni, M. (2006). Understanding emotions in others: Mirror neuron dysfunction in children with autism spectrum disorders. Nature Neuroscience, 9(1), 28–30.
DeCasper, A. J., & Fifer, W. P. (1980). Of human bonding: Newborns prefer their mothers’ voices. Science, 208(4448), 1174–1176.
Dehaene-Lambertz, G., Dehaene, S., & Hertz-Pannier, L. (2002). Functional neuroimaging of speech perception in infants. Science, 298(5600), 2013–2015.
Developmental Disabilities Monitoring Network Surveillance 2010 Year Principal Investigators; Centers for Disease Control and Prevention. (2014). Prevalence of autism spectrum disorder among children aged 8 years-autism and developmental disabilities monitoring network, 11 sites, United States, 2010. Morbidity and Mortality Weekly Report Surveillance Summaries, 63, 1–21.
Dodwell, P. C. (1983). Spatial sense of the human infant. In A. Hein & M. Jeannerod (Eds.), Spatially oriented behavior (pp. 197–213). New York: Springer.
Elsabbagh, M., Volein, A., Csibra, G., Holmboe, K., Garwood, H., Tucker, L., Krljes, S., Baron-Cohen, S., Bolton, P., Charman, T., Baird, G., & Johnson, M. H. (2009). Neural correlates of eye gaze processing in the infant broader autism phenotype. Biological Psychiatry, 65(1), 31–38.
Elsabbagh, M., Mercure, E., Hudry, K., Chandler, S., Pasco, G., Charman, T., Krljes, S., Baron-Cohen, S., Bolton, P., Charman, T., Baird, G., & Johnson, M. H. (2012). Infant neural sensitivity to dynamic eye gaze is associated with later emerging autism. Current Biology, 22(4), 338–342.
Emde, R. N., & Harmon, R. J. (1972). Endogenous and exogenous smiling systems in early infancy. Journal of the American Academy of Child Psychiatry, 11(2), 177–200.
Emery, N. J. (2000). The eyes have it: The neuroethology, function and evolution of social gaze. Neuroscience & Biobehavioral Reviews, 24(6), 581–604.
Farroni, T., Csibra, G., Simion, F., & Johnson, M. H. (2002). Eye contact detection in humans from birth. Proceedings of the National Academy of Sciences, 99(14), 9602–9605.
Farroni, T., Johnson, M., Menon, E., Zulian, L., Faraguna, D., & Csibra, G. (2005). Newborns’ preference for face-relevant stimuli: Effects of contrast polarity. Proceedings of the National Academy of Sciences of the United States of America, 102(47), 17245–17250.
Field, J., Muir, D., Pilon, R., Sinclair, M., & Dodwell, P. (1980). Infants’ orientation to lateral sounds from birth to three months. Child Development, 51, 295–298.
Field, T., Goldstein, S., Vega-Lahr, N., & Porter, K. (1986). Changes in imitative behavior during early infancy. Infant Behavior and Development, 9(4), 415–421.
Freitag, C. M., Konrad, C., Häberlen, M., Kleser, C., von Gontard, A., Reith, W., Troje, N. F., & Krick, C. (2008). Perception of biological motion in autism spectrum disorders. Neuropsychologia, 46(5), 1480–1494.
Garey, L. J., & De Courten, C. (1983). Structural development of the lateral geniculate nucleus and visual cortex in monkey and man. Behavioural Brain Research, 10(1), 3–13.
Goldfield, B. A., & Reznick, J. S. (1990). Early lexical acquisition: Rate, content and the vocabulary spurt. Journal of Child Language, 17(1), 171–183.
Goren, C. C., Sarty, M., & Wu, P. Y. K. (1975). Visual following and pattern discrimination of face-like stimuli by newborn infants. Pediatrics, 56(4), 544–549.
Gottlieb, G. (1971). Development of species identification in birds: An inquiry into the prenatal determinants of perception. Chicago: University of Chicago Press.
Gottlieb, G. (1975). Development of species identification in ducklings: I. Nature of perceptual deficit caused by embryonic auditory deprivation. Journal of Comparative and Physiological Psychology, 89(5), 387–399.
Gottlieb, G. (1981). Roles of early experience in species-specific perceptual development. In R. Aslin, J. R. Alberts, & M. R. Petersen (Eds.), Development of perception. Psychobiological perspectives, 1: Audition, somatic perception, and the chemical senses (pp. 5–44). New York: Academic Press.
Gottlieb, G. (1991). Experiential canalization of behavioral development: Theory. Developmental Psychology, 27(1), 4–13. (USA: American Psychological Association).
Gotts, S. J., Simmons, W. K., Milbury, L. A, Wallace, G. L., Cox, R. W., & Martin, A. (2012). Fractionation of social brain circuits in autism spectrum disorders. Brain, 135(9), 2711–2725.
Grelotti, D. J., Gauthier, I., & Schultz, R. T. (2002). Social interest and the development of cortical face specialization: What autism teaches us about face processing. Developmental Psychobiology, 40(3), 213–225.
Grelotti, D. J., Klin, A. J., Gauthier, I., Skudlarski, P., Cohen, D. J., Gore, J. C., Volkmar, F. R., & Schultz, R. T. (2005). fMRI activation of the fusiform gyrus and amygdala to cartoon characters but not to faces in a boy with autism. Neuropsychologia, 43(3), 373–385.
Grigorenko, E. L., Klin, A., & Volkmar, F. (2003). Annotation: Hyperlexia: Disability or superability? Journal of Child Psychology and Psychiatry, and Allied Disciplines, 44(8), 1079–1091.
Haith, M. M., Bergman, T., & Moore, M. J. (1977). Eye contact and face scanning in early infancy. Science, 198, 853–855.
Halit, H., De Haan, M., & Johnson, M. H. (2003). Cortical specialisation for face processing: Face-sensitive event-related potential components in 3-and 12-month-old infants. NeuroImage, 19(3), 1180–1193.
Herrington, J. D., Baron-Cohen, S., Wheelwright, S. J., Singh, K. D., Bullmore, E. T., Brammer, M., & Williams, S. (2007). The role of MT+/V5 during biological motion perception in Asperger syndrome: An fMRI study. Research in Autism Spectrum Disorders, 1(1), 14–27.
Hitchcock, P. F., & Hickey, T. L. (1980). Prenatal development of the human lateral geniculate nucleus. Journal of Comparative Neurology, 194(2), 395–411.
Howes, C. (1987). Social competence with peers in young children: Developmental sequences. Developmental Review, 7, 252–272.
Hutman, T., Rozga, A., DeLaurentis, A. D., Barnwell, J. M., Sugar, C. A., & Sigman, M. (2010). Response to distress in infants at risk for autism: A prospective longitudinal study. Journal of Child Psychology and Psychiatry, 51(9), 1010–1020.
Hutman, T., Chela, M. K., Gillespie-Lynch, K., & Sigman, M. (2012). Selective visual attention at twelve months: Signs of autism in early social interactions. Journal of Autism and Developmental Disorders, 42(4), 487–498.
Huttenlocher, P. R., de Courten, C., Garey, L. J., & Van der Loos, H. (1982). Synaptogenesis in human visual cortex—Evidence for synapse elimination during normal development. Neuroscience Letters, 33(3), 247–252.
Johnson, M. (1990). Cortical maturation and the development of visual attention in early infancy. Journal of Cognitive Neuroscience, 2(2), 81–95.
Johnson, M. H. (2005). Subcortical face processing. Nature Reviews Neuroscience, 6(10), 766–774.
Johnson, M. H., & de Haan, M. (2001). Developing cortical specialization for visual-cognitive function: The case of face recognition. In J. L. McLelland & R. S. Siegler (Eds.), Mechanisms of cognitive development: Behavioral and neural perspectives (pp. 253–270). Mahwah: Lawrence Erlbaum.
Johnson, M., Dziurawiec, S., Ellis, H., & Morton, J. (1991). Newborns’ preferential tracking of face-like stimuli and its subsequent decline. Cognition, 40(1–2), 1–19.
Jones, W., & Klin, A. (2009). Heterogeneity and homogeneity across the autism spectrum: The role of development. Journal of the American Academy of Child and Adolescent Psychiatry, 48(5), 471–473.
Jones, W., & Klin, A. (2013). Attention to eyes is present but in decline in 2-6-month-old infants later diagnosed with autism. Nature, 504(7480), 427–431.
Jones, W., Carr, K., & Klin, A. (2008). Absence of preferential looking to the eyes of approaching adults predicts level of social disability in 2-year-old toddlers with autism spectrum disorder. Archives of General Psychiatry, 65(8), 946–954.
Kaiser, M. D., Hudac, C. M., Shultz, S., Lee, S. M., Cheung, C., Berken, A. M., Deen, B., Pitskel, N. B., Sugrue, D. R., Voos, A. C., Saulnier, C. A., Ventola, P., Wolf, J. M., Klin, A., Vander Wyk, B. C., & Pelphrey, K. A. (2010). Neural signatures of autism. Proceedings of the National Academy of Sciences, 107(49), 21223–21228.
Kampe, K. K., Frith, C. D., Dolan, R. J., & Frith, U. (2001). Psychology: Reward value of attractiveness and gaze. Nature, 413(6856), 589.
Kanner, L. (1943). Autistic disturbances of affective contact. Nervous Child, 2(3), 217–250.
Karmiloff-Smith, A. (1998). Development itself is the key to understanding developmental disorders. Trends in Cognitive Sciences, 2(10), 389–398.
Kellman, P. J., & Banks, M. S. (1998). Infant visual perception. In R. S. Kuhn & S. Siegler (Eds.), Handbook of child psychology (1st ed., pp. 103–146). New York: Wiley.
Kelly, D. J., Quinn, P. C., Slater, A. M., Lee, K., Ge, L., & Pascalis, O. (2007). The other-race effect develops during infancy evidence of perceptual narrowing. Psychological Science, 18(12), 1084–1089.
Key, A. P. F., & Stone, W. L. (2012). Processing of novel and familiar faces in infants at average and high risk for autism. Developmental Cognitive Neuroscience, 2(2), 244–255.
Khan, A. A., Wadhwa, S., & Bijlani, V. (1994). Development of human lateral geniculate nucleus: An electron microscopic study. International Journal of Developmental Neuroscience, 12(7), 661–672.
Klin, A. (1991). Young autistic children’s listening preferences in regard to speech: A possible characterization of the symptom of social withdrawal. Journal of Autism and Developmental Disorders, 21(1), 29–42.
Klin, A., Chawarska, K., Paul, R., Rubin, E., Morgan, T., Wiesner, L., & Volkmar, F. (2004). Autism in a 15-month-old child. The American Journal of Psychiatry, 161(11), 1981–1988.
Klin, A., Saulnier, C. A., Sparrow, S. S., Cicchetti, D. V., Volkmar, F. R., & Lord, C. (2007). Social and communication abilities and disabilities in higher functioning individuals with autism spectrum disorders: The Vineland and the ADOS. Journal of Autism and Developmental Disorders, 37(4), 748–759.
Klin, A., Lin, D. J., Gorrindo, P., Ramsay, G., & Jones, W. (2009). Two-year-olds with autism orient to non-social contingencies rather than biological motion. Nature, 459(7244), 257–261.
Kuhl, P. K., Coffey-Corina, S., Padden, D., & Dawson, G. (2005). Links between social and linguistic processing of speech in preschool children with autism: Behavioral and electrophysiological measures. Developmental Science, 8(1), F1–F12.
Landa, R., & Garrett-Mayer, E. (2006). Development in infants with autism spectrum disorders: A prospective study. Journal of Child Psychology and Psychiatry, 47(6), 629–638.
Langdell, T. (1978). Recognition of faces: An approach to the study of autism. Journal of Child Psychology and Psychiatry, 19(3), 255–268.
Lavelli, M., & Fogel, A. (2005). Developmental changes in the relationship between the infant’s attention and emotion during early face-to-face communication: The 2-month transition. Developmental Psychology, 41(1), 265–280.
LeDoux, J. E. (1996). The emotional brain: The mysterious underpinnings of emotional life. New York: Simon & Schuster.
LeDoux, J. E. (2003). Synaptic self: How our brains become who we are. New York: Penguin.
Lewkowicz, D. J., & Hansen-Tift, A. M. (2012). Infants deploy selective attention to the mouth of a talking face when learning speech. Proceedings of the National Academy of Sciences, 109(5), 1431–1436.
Locke, J. L. (1995). The child’s path to spoken language. Cambridge: Harvard University Press.
Loh, A., Soman, T., Brian, J., Bryson, S. E., Roberts, W., Szatmari, P., Smith, I. M., & Zwaigenbaum, L. (2007). Stereotyped motor behaviors associated with autism in high-risk infants: A pilot videotape analysis of a sibling sample. Journal of Autism and Developmental Disorders, 37(1), 25–36.
Macfarlane, A. (1975). Olfaction in the development of social preferences in the human neonate. Parent–Infant interaction, Ciba Foundation Symposium 33 (pp. 103–113). Amsterdam: Ciba Foundation.
Maratos, O. (1982). Trends in the development of imitation in the first six months of life. In T. G. Bever (Ed.), Regressions in mental development: Basic phenomena and theories (pp. 81–101). Hillsdale: Erlbaum.
Martin, E., Joeri, P., Loenneker, T., Ekatodramis, D., Vitacco, D., Hennig, J., & Marcar, V. L. (1999). Visual processing in infants and children studied using functional MRI. Pediatric Research, 46(2), 135–140.
McCleery, J. P., Akshoomoff, N., Dobkins, K. R., & Carver, L. J. (2009). Atypical face versus object processing and hemispheric asymmetries in 10-month-old infants at risk for autism. Biological Psychiatry, 66(10), 950–957.
Mitchell, S., Brian, J., Zwaigenbaum, L., Roberts, W., Szatmari, P., Smith, I., & Bryson, S. (2006). Early language and communication development of infants later diagnosed with autism spectrum disorder. Journal of Developmental & Behavioral Pediatrics, 27(2), S69–S78.
Moore, D. R., & Jeffery, G. (1994). Development of auditory and visual systems in the fetus. In G. D. Thorburn & R. Harding (Eds.), Textbook of fetal physiology (pp. 278–286), Oxford: Oxford University Press.
Morita, T., Kochiyama, T., Yamada, H., Konishi, Y., Yonekura, Y., Matsumura, M., & Sadato, N. (2000). Difference in the metabolic response to photic stimulation of the lateral geniculate nucleus and the primary visual cortex of infants: A fMRI study. Neuroscience Research, 38(1), 63–70.
Morton, J., & Johnson, M. H. (1991). CONSPEC and CONLERN: A two-process theory of infant face recognition. Psychological Review, 98(2), 164–181.
Nagy, A. (2011). The newborn infant: A missing stage in developmental psychology. Infant and Child Development, 20(1), 3–19.
O’Shea, A. G., Fein, D. A., Cillessen, A. H. N., Klin, A., & Schultz, R. T. (2005). Source memory in children with autism spectrum disorders. Developmental Neuropsychology, 27(3), 337–360.
Oller, D. K. (1980). The emergence of the sounds of speech in infancy. In G. Yeni-Komshian, J. F. Kavanagh, & C. A. Ferguson (Eds.), Child phonology, 1: Production (pp. 93–112). New York: Academic Press.
Ozonoff, S., Macari, S., Young, G. S., Goldring, S., Thompson, M., & Rogers, S. J. (2008). Atypical object exploration at 12 months of age is associated with autism in a prospective sample. Autism, 12(5), 457–472.
Ozonoff, S., Iosif, A.-M., Baguio, F., Cook, I. C., Hill, M. M., Hutman, T., et al. (2010). A prospective study of the emergence of early behavioral signs of autism. Journal of the American Academy of Child & Adolescent Psychiatry, 49(3), 256–266.
Ozonoff, S., Young, G. S., Carter, A., Messinger, D., Yirmiya, N., Zwaigenbaum, L., Bryson, S., Carver, L. J., Constantino, J. N., Dobkins, K., Hutman, T., Iverson, J. M., Landa, R., Rogers, S. J., Sigman, M., & Stone, W. L. (2011). Recurrence risk for autism spectrum disorders: A Baby Siblings Research Consortium study. Pediatrics, 128(3), e488–e495.
Pascalis, O., de Haan, M., & Nelson, C. A. (2002). Is face processing species-specific during the first year of life? Science, 296(5571), 1321–1323.
Pasley, B. N., Mayes, L. C., & Schultz, R. T. (2004). Subcortical discrimination of unperceived objects during binocular rivalry. Neuron, 42(1), 163–172.
Paul, R., Fuerst, Y., Ramsay, G., Chawarska, K., & Klin, A. (2011). Out of the mouths of babes: Vocal production in infant siblings of children with ASD. Journal of Child Psychology and Psychiatry, 52(5), 588–598.
Pierce, K., Conant, D., Hazin, R., Stoner, R., & Desmond, J. (2011). Preference for geometric patterns early in life as a risk factor for autism. Archives of General Psychiatry, 68(1), 101–109.
Rozga, A., Hutman, T., Young, G. S., Rogers, S. J., Ozonoff, S., Dapretto, M., & Sigman, M. (2011). Behavioral profiles of affected and unaffected siblings of children with autism: Contribution of measures of mother–infant interaction and nonverbal communication. Journal of Autism and Developmental Disorders, 41(3), 287–301.
Sanefuji, W., Wada, K., Yamamoto, T., Mohri, I., & Taniike, M. (2014). Development of preference for conspecific faces in human infants. Developmental Psychology, 50(4), 979–985.
Schultz, R. T. (2000). Abnormal ventral temporal cortical activity during face discrimination among individuals with autism and Asperger syndrome. Archives of General Psychiatry, 57(4), 331–340.
Shultz, S., & Vouloumanos, A. (2010). Three-month-olds prefer speech to other naturally occurring signals. Language Learning and Development, 6(4), 241–257.
Shultz, S., Klin, A., & Jones, W. (2011). Inhibition of eye blinking reveals subjective perceptions of stimulus salience. Proceedings of the National Academy of Sciences, 108(52), 21270–21275.
Shultz, S., Vouloumanos, A., Bennett, R. H., & Pelphrey, K. (2014). Neural specialization for speech in the first months of life. Developmental Science, 17(5), 766–774.
Simion, F., Valenza, E., Umilta, C., & Barba, B. D. (1998). Preferential orienting to faces in newborns: A temporal–nasal asymmetry. Journal of Experimental Psychology: Human Perception and Performance, 24(5), 1399–1405.
Simion, F., Macchi Cassia, V., Turati, C., & Valenza, E. (2001). The origins of face perception: Specific versus non-specific mechanisms. Infant and Child Development, 10(1–2), 59–65.
Simion, F., Regolin, L., & Bulf, H. (2008). A predisposition for biological motion in the newborn baby. Proceedings of the National Academy of Sciences of the United States of America, 105(2), 809–813.
Smith, L., & Gasser, M. (2005). The development of embodied cognition: Six lessons from babies. Artificial Life, 11(1–2), 13–29.
Sullivan, M., Finelli, J., Marvin, A., Garrett-Mayer, E., Bauman, M., & Landa, R. (2007). Response to joint attention in toddlers at risk for autism spectrum disorder: A prospective study. Journal of Autism and Developmental Disorders, 37(1), 37–48.
Tager-Flusberg, H., Paul, R., & Lord, C. (2005). Language and communication in autism. In F. R. Volkmar, R. Paul, A. Klin, & D. Cohen. (Eds.), Handbook of autism and pervasive developmental disorders (3rd Ed.), 1: Diagnosis, development, neurobiology & behavior (pp. 335–364). Hoboken: Wiley.
Turkewitz, G., & Kenny, P. A. (1982). Limitations on input as a basis for neural organization and perceptual development: A preliminary theoretical statement. Developmental Psychobiology, 15(4), 357–368.
Twitchell, T. E. (1965). The automatic grasping responses of infants. Neuropsychologia, 3(3), 247–259.
Tzourio-Mazoyer, N., De Schonen, S., Crivello, F., Reutter, B., Aujard, Y., & Mazoyer, B. (2002). Neural correlates of woman face processing by 2-month-old infants. NeuroImage, 15(2), 454–461.
Von Uexkull, J. (1934). A stroll through the worlds of animals and men. In C. Schiller (Ed.), Instinctive behavior (pp. 5–80). New York: International Universities Press.
Valenza, E., Simion, F., Cassia, V. M., & Umiltà, C. (1996). Face preference at birth. Journal of Experimental Psychology: Human Perception and Performance, 22(4), 892–903.
Vouloumanos, A., & Werker, J. F. (2007). Listening to language at birth: Evidence for a bias for speech in neonates. Developmental Science, 10(2), 159–164.
Vouloumanos, A., Hauser, M. D., Werker, J. F., & Martin, A. (2010). The tuning of human neonates’ preference for speech. Child Development, 81(2), 517–527.
Wan, M. W., Green, J., Elsabbagh, M., Johnson, M., Charman, T., & Plummer, F. (2013). Quality of interaction between at-risk infants and caregiver at 12–15 months is associated with 3-year autism outcome. Journal of Child Psychology and Psychiatry, 54(7), 763–771.
Werker, J. F., & Tees, R. C. (1984). Cross-language speech perception: Evidence for perceptual reorganization during the first year of life. Infant Behavior and Development, 7(1), 49–63.
Yakovlev, P. I., & Lecours, A.-R. (1967). The myelogenetic cycles of regional maturation of the brain. In A. Minkowski (Ed.), Regional development of the brain in early life (pp. 3–70). Oxford: Blackwell.
Yoder, P., Stone, W. L., Walden, T., & Malesa, E. (2009). Predicting social impairment and ASD diagnosis in younger siblings of children with autism spectrum disorder. Journal of Autism and Developmental Disorders, 39(10), 1381–1391.
Zwaigenbaum, L., Bryson, S., Rogers, T., Roberts, W., Brian, J., & Szatmari, P. (2005). Behavioral manifestations of autism in the first year of life. International Journal of Developmental Neuroscience, 23(2), 143–152.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Shultz, S., Jones, W., Klin, A. (2015). Early Departures from Normative Processes of Social Engagement in Infants with Autism Spectrum Disorder. In: Puce, A., Bertenthal, B. (eds) The Many Faces of Social Attention. Springer, Cham. https://doi.org/10.1007/978-3-319-21368-2_6
Download citation
DOI: https://doi.org/10.1007/978-3-319-21368-2_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-21367-5
Online ISBN: 978-3-319-21368-2
eBook Packages: Behavioral ScienceBehavioral Science and Psychology (R0)