Abstract
The term sphingolipid was coined by J.L.W. Thudichum before the turn of the nineteenth century, referring to the enigmatic (related to the Sphinx myth) nature of this class of molecules. One hundred thirty years later, the enigma is not yet completely solved. Nevertheless, much progress has been made, shedding light on the numerous roles these lipids play in eukaryotes. How sphingolipids are synthesized, transformed and degraded in mammalian cells, and how some of them transduce signals and regulate biological functions is reviewed in this chapter. Special attention is given to those sphingolipid species which regulate key aspects of the development of malignancies in humans, and therefore represent potential targets for therapy.
An erratum to this chapter can be found at http://dx.doi.org/10.1007/978-3-319-20750-6_21
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction: Sphingolipids Are Bioactive Metabolites
Sphingolipids (SLs) are found in all eukaryotes and represent a class of lipids with considerable structural diversity. In mammals, there are likely tens of thousands of SL molecular species [1]. Because of their amphiphilic nature, most of them are membrane components; some are present in biological fluids, being constituents of circulating lipoproteins or transported by other proteins.
The smallest molecule common to all SLs is an aliphatic amino-alcohol, also known as long chain base, the most frequent one being 4-sphingenine (also termed sphingosine). Its condensation to a fatty acid, through an amide bond rather unusual in the lipid world, forms the core of most SLs, ceramide (Cer). This structural analogue of diacylglycerol (DAG) constitutes the hydrophobic anchor of all complex SLs, determining the membrane location of both, glycosphingolipids (GSLs) and sphingomyelin (SM), the two major classes of complex SLs. The analogy between DAG and Cer extends to their common ability to convey signals; these signals, however, often are of diverging nature (see Sect. 3). With regard to the signalling properties of SLs, the best documented roles are undoubtedly attributed to sphingosine 1-phosphate (S1P), the phosphorylated derivative of sphingosine, which is abundant in plasma while one of the least represented SLs within the cells. Nevertheless, Cer and S1P are not the only SLs that behave as SL signalling intermediates or second messengers. Glycosylated or N-methylated forms of sphingoid bases, sphingosylphosphocholine (SPC), phosphorylated Cer, as well as some GSLs can transduce signals and/or modulate biological functions.
The last 2–3 decades have witnessed an enormous improvement in our knowledge of the structural diversity, metabolism, cell biology and pathology of SLs. Such a progress has been facilitated thanks to (1) the development of new technologies for analyzing the sphingolipidome (see Part II of this book), (2) the cloning of most enzymes of SL metabolism, (3) the synthesis and use of SL analogues for studying their metabolism, location, targets (or interacting partners) and mechanism of action, (4) the generation of animal models harboring alterations of SL metabolism [2, 3], and (5) the identification of new human genetic disorders characterized by disturbed homeostasis of SLs [4]. Moreover, the involvement of SLs in the pathophysiology of numerous conditions, including multiple types of solid cancers and hematological malignancies, has been explored. The intent of this overview is not to cover in a comprehensive manner the many effects and modes of actions of SLs, but to highlight some important aspects of SL metabolism and signalling in mammalian organisms. The reader is also referred to recent excellent reviews [5–9]. In the field of cancer biology and treatment, SLs are currently viewed as multifaceted mediators and, as a consequence, potentially new therapeutic targets. The biological and physiological effects modulated by SLs include cancer (and cancer stem) cell death, survival, differentiation, cell cycle arrest, cell motility, autophagy, epithelial–mesenchymal transition, but also effects on the tumor microenvironment through angiogenesis, recruitment of inflammatory and immune cells, or phenotypic changes of adjacent fibroblasts. This astounding variety is SL-dependent, sometimes SL species-dependent (underscoring the importance of the metabolic source of SLs) [10], and cellular context-dependent. Figure 1 illustrates some of these diverse effects.
Sphingolipid-mediated biological effects in cancer. The figure summarizes how SLs affect hallmarks of cancer (adapted from [95]). Note that not all bioactive SLs are indicated in this scheme. SLs written in red promote tumor development whereas those written in green have antitumor effects. Numbers in brackets indicate some key references. Cer ceramide, Cer1P ceramide 1-phosphate, 2′-OHCer, 2′-hydroxyceramide, DMS N,N-dimethyl-sphingosine, GalCer galactosylceramide, GlcCer glucosylceramide, S1P sphingosine 1-phosphate, So sphingosine, SPC sphingosylphosphocholine, TMS N,N,N-trimethyl-sphingosine. References cited: (1) [96], (2) [97], (3) [8], (4) [98], (5) [99], (6) [54], (7) [100], (8) [101], (9) [102], (10) [103], (11) [104], (12) [56], (13) [105], (14) [106], (15) [107], (16) [108], (17) [109], (18) [110], (19) [111], (20) [112], (21) [113], (22) [114], (23) [115], (24) [116], (25) [117], (26) [118], (27) [119], (28) [120], (29) [121], (30) [122], (31) [52], (32) [123]
2 How Are Sphingolipids Formed, Transformed and Degraded?
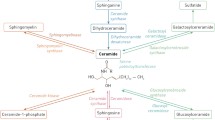
The astonishing variety of biological effects of SLs is not only related to their structural diversity but also their tissue and subcellular distribution [thanks to the newly developed imaging mass spectrometry methods, subtle differences in tissue distribution of particular molecular species of SLs begin to be appreciated; see Part II of this book]. Understanding which, where (in the cell or in the body) and how a given SL can exert a biological effect requires prior knowledge of SL metabolism and transport. Although there are still a few gaps, the picture of SL biosynthesis and catabolism is almost complete. Figures 2 and 3 depict the pathways of SL synthesis and turnover in humans (for details, the reader is referred to www.sphingomap.org).
Subcellular compartmentalization of sphingolipid biosynthesis. For each enzymatic reaction or transport event, the names of the corresponding protein (in boxes) and gene are indicated. Cer ceramide, Cer1P ceramide 1-phosphate, CERK ceramide kinase, CERPase ceramide 1-phosphate phosphatase, CerpE ceramide phosphoethanolamine, CERS ceramide synthase, CERT ceramide transport protein, CGT UDP-galactose: ceramide galactosyltransferase, CPTP ceramide 1-phosphate transfer protein, DES dihydroceramide desaturase, DHCer dihydroceramide, FAPP four-phosphate-adaptor protein, GalCer galactosylceramide, GBA beta-glucosidase, GCS glucosylceramide synthase, GlcCer glucosylceramide, GSLs glycosphingolipids, KSa 3-ketosphinganine, KDSR ketosphinganine reductase, LacCer lactosylceramide, LCS lactosylceramide synthase, Palm-CoA palmitoyl-CoA, Sa sphinganine, SM sphingomyelin, SMS sphingomyelin synthase, SMSr sphingomyelin synthase-related protein, SPT serine palmitoyltransferase
Subcellular compartmentalization of sphingolipid catabolism and interconversion. For each enzymatic reaction, the names of the corresponding protein (in boxes) and gene are indicated. ACDase acid ceramidase, AlkCDase alkaline ceramidase, ASMase acid sphingomyelinase, Cer ceramide, CerpE ceramide phosphoethanolamine, CERS ceramide synthase, EP ethanolamine 1-phosphate, FA fatty acid, GALC galactosylceramidase, GalCer galactosylceramide, GBA beta-glucosidase, GCase glucosylceramidase, GlcCer glucosylceramide, GSLs glycosphingolipids, LacCer lactosylceramide, NCDase neutral ceramidase, NSMase neutral sphingomyelinase, SM sphingomyelin, So sphingosine, S1P sphingosine 1-phosphate, SK sphingosine kinase, SPL sphingosine 1-phosphate lyase
SL biosynthesis starts in the endoplasmic reticulum (ER) with the condensation of l-serine and a fatty acyl-CoA, usually palmitoyl-CoA, by serine-palmitoyltransferase (note that instead of l-serine, l-alanine or l-glycine can be used under some pathological conditions, leading to the formation of 1-deoxysphingolipids [11, 12]). The resultant 3-keto-sphinganine is then reduced to sphinganine (also named dihydrosphingosine) before N-acylation by Cer synthases. In the de novo pathway, these enzymes form dihydroceramides. They are encoded by six different genes that exhibit distinct tissue expression and produce enzymes with different acyl-CoA (or 2-hydroxyacyl-CoA) chain length specificities, leading to numerous non-hydroxy and 2-hydroxy-dihydroceramide species with possibly distinct properties [5]. The de novo synthesis of Cer is completed by a desaturase that introduces a trans-Δ4 double bond in the sphingoid backbone.
Newly synthesized Cer can be transformed to five different lipids: (1) ceramide 1-phosphate (Cer1P), by Cer kinase; (2) sphingomyelin, one of the most abundant SL, by sphingomyelin synthases; (3) ceramide phosphoethanolamine, by sphingomyelin synthase 2 or sphingomyelin synthase-related protein; (4) galactosylceramide, a myelin lipid, by galactosylceramide synthase; and (5) glucosylceramide (GlcCer), the precursor of most GSLs, by glucosylceramide synthase. As shown in Fig. 2, these reactions occur in the ER or in discrete compartments of the Golgi apparatus, either on the cytosolic or luminal side. Selection of one of these pathways may be dictated by the expression of the corresponding enzymes. Nevertheless, it implies some sophisticated regulation of Cer channelling to these distinct locations in order to serve specific metabolic needs. Although this issue is imperfectly solved, recent progress has been made regarding SL transport. Cer can be transported from the ER to the Golgi either by a vesicular process [13] or by a CERT-mediated non-vesicular mechanism [14]. Once synthesized, GlcCer might be retrogradely transported to the ER by FAPP2 [15], and Cer1P carried from the trans-Golgi to other membrane destinations by CPTP [16]. Besides Cer-metabolizing enzymes, these transporters represent additional targets to manipulate SL metabolism and biological effects, as illustrated by the role of CERT in modulating the sensitivity of cancer cells to chemotherapeutic drugs [17].
Most of the GSLs are synthesized from GlcCer at the luminal face of Golgi vesicles (as this part of SL metabolism is beyond the topic of the present chapter, the reader is referred to recent reviews, e.g., [18, 19]). Because of their luminal orientation and vesicular transport to the cell surface, GSLs and SM become constituents of the extracellular leaflet of the plasma membrane, and concentrate, along with cholesterol, within the so-called microdomains. A fraction of these complex SLs is incorporated in the circulating lipoproteins.
Constitutive turnover of plasma membrane elements, endocytosis of extracellular lipoproteins as well as (macro)autophagy result in the entry of SLs into the endosomal/lysosomal compartment where the bulk of SL catabolism takes place (Fig. 3). Stepwise degradation of GSLs occurs through a unique sequence of reactions catalyzed by exoglycosidases that ends by the release of Cer [20]. Formation of Cer in endosomes/lysosomes also results from the action of acid sphingomyelinase. Eventually, Cer is cleaved by acid ceramidase, which releases a fatty acid and sphingosine. How these end-products exit the lysosome is still unclear.
Whether protein-mediated or not, the efflux out of the acidic compartments of the sphingoid base initiates the SL ‘salvage’ pathway that allows re-use of the sphingoid moiety for SL synthesis. Unless sphingosine gets access to the ER because of close vicinity with lysosomal membranes, a prerequisite for this recycling pathway might be the phosphorylation of sphingosine by sphingosine kinase(s). The transient formation of S1P, a much less hydrophobic molecule than sphingosine, would represent a way to transport the sphingoid base to the ER, where S1P is dephosphorylated prior to N-acylation by Cer synthases. An alternative destination for S1P is its irreversible degradation by a single enzyme, S1P lyase, which produces phosphoethanolamine and a fatty aldehyde, two molecules that connect SL and glycerophospholipid metabolisms [21]. What directs S1P either to be recycled (after dephosphorylation) or broken down is still unknown; clarifying this issue is, however, critical for understanding the bioactive properties of S1P and manipulating its levels for therapeutic purposes.
Of note, turnover of GSLs and SM is not restricted to the acidic compartments, and it can occur at the plasma membrane level, both—at least for SM—on the extracellular side and the cytosolic side [22]. This location has long been envisioned as the starting point for the generation, or modulation, of bioactive signals. As a matter of fact, cleavage of SM and Cer, followed by phosphorylation of sphingosine can occur at the level of the plasma membrane. These reactions can be catalyzed by plasma membrane-located enzymes but also by secreted or translocated enzymes such as acid sphingomyelinase [23]. In addition, secreted hydrolases or ectoenzymes ensure SL degradation in the intestinal lumen to digest dietary lipids [24], providing not only nutrients but also potential bioactive lipids to prevent colon carcinogenesis. Moreover, although information remains scarce, GSL, SM and Cer turnover likely exists at the level of mitochondria, the nucleus, Golgi and ER membranes that are closely associated with mitochondria [25–27]. For instance, neutral and alkaline ceramidases localize to mitochondria, ER and Golgi [28].
Finally, the pathway that leads to the generation of some minor SLs, including sphingosylphosphocholine (SPC), psychosine or glucosylsphingosine, remains to be unambiguously identified. These lysosphingolipids are normally present in trace amounts but they mediate biological effects and underlie the molecular pathogenesis of some inherited disorders [29].
In summary, SL metabolism starts from structurally simple molecules, i.e., an amino acid and an acyl-CoA, produces thousands of distinct lipids, and ends by the release of small metabolites that are recycled into glycerophospholipids. Complex SLs are abundant membrane constituents that act as reservoirs for the production of simple, bioactive lipids. Transformations occur in multiple subcellular compartments, serving different functions and possibly also spatially restricting the biological effects of these lipids. Such a sophisticated metabolism is undoubtedly regulated to comply with the cell’s demand and to control the levels of highly bioactive metabolites. However, how this metabolism is regulated and how the many pathways aforementioned are coordinated is still poorly understood [30]. These are key issues to appreciate the importance of the dysregulated expression of SL-metabolizing enzymes seen in tumor cells [8].
3 How Do Sphingolipids Signal and Mediate Biological Effects?
SLs are no longer considered just as inert membrane components. Some of them behave as intracellular second messengers to transduce extracellular signals; some elicit cellular outcomes through binding to cell surface receptors; some others alter the cell’s responses by changing the physicochemical properties of membranes. Here, we will not describe the numerous physiological effects that can be modulated by SLs (see Fig. 1) but will emphasize their mode of action at the cellular level through some selected examples that are particularly relevant to cancer biology or therapy.
3.1 History of Signalling Sphingolipids: Where Are We One Generation Later?
Perhaps, the first SL recognized to mediate pathological changes is psychosine (i.e., galactosylsphingosine). While almost undetectable in normal brain, this lysolipid was found to accumulate in the cerebral white matter of patients affected with Krabbe disease (globoid cell leukodystrophy) [31, 32], a lysosomal disorder due to deficient activity of beta-galactosylceramidase. Psychosine was already known to be a cytotoxic substance; later studies suggested that this lysolipid, like sphingosine, inhibits protein kinase C (PKC) [29]. It also disturbs cytokinesis (thus explaining the formation of globoid, multinucleated cells) through binding to the G-protein coupled receptor TDAG8 [33]. However, whether psychosine regulates cytokinesis via TDAG8 [34] or antagonizes the proton-sensing properties of TDAG8 [35] is still debated.
Further indication that SLs could act as signal transducers was provided 25 years ago by the demonstration that the effects of vitamin D3 on leukemic cell differentiation were preceded by an increased activity of a neutral sphingomyelinase, transient hydrolysis of SM and concomitant production of Cer, and recapitulated by treatment with an exogenous sphingomyelinase [36]. This report immediately followed the observation that the phenothiazine trifluoroperazine stimulated SM breakdown and Cer generation in pituitary cells [37]. These data were the first of a series documenting the “sphingomyelin cycle”, that is the transient formation of Cer from SM via activation of a sphingomyelinase by a wide variety of physiological or pathological agents [38, 39]. This pathway was viewed as a means to serve the production of bioactive SLs mediating the biological outcome triggered by the applied stimulus. However, it soon appeared that SM turnover is not the only source of signalling Cer. Activation of the Cer biosynthetic (de novo) pathway was reported as an alternative mechanism, especially in mediating cell death [40, 41]. Other mechanisms, such as the inhibition of SM synthesis [42, 43] or of ceramidase activity [44], likely contribute to stress-induced Cer generation. In addition, more recently production of bioactive Cer from glycolipids has been reported [45, 46].
Of note, whatever its metabolic source, Cer may not be necessarily the “signalling” SL that directly transduces the observed effect. Other SLs display biological roles, including in cancer (see Fig. 1). For instance, bioactive S1P, some effects of which were already described in 1990–1991 [47, 48], can originate from the metabolic cascade starting with SM breakdown [49]. Even products of S1P catabolism appear to be biologically active [50, 51].
Studies reported in the beginning of the 90s disclosed opposite effects of some SLs. Such a dichotomy is typically illustrated by the antagonistic actions of Cer and S1P on cell growth/death and survival, which led to the “rheostat” concept [52]. This concept initially proposed that the balance between Cer and S1P levels controls the cell fate via distinct effects on members of the MAPK family. However, such an equilibrium is presumably not maintained by the overall intracellular concentrations of these two SLs, which differ by about two orders of magnitude. One can imagine that the subcellular location (and thus, the accessibility to the protein target) and/or the nature of the molecular species are key determinants in the action of these lipid mediators. Accordingly, even a minute amount of a given Cer species may counteract the action of S1P if appropriately located.
To substantiate such hypotheses, considerable technological progress has been made during the last two decades that allowed a “higher-resolution view” of SL metabolism and signalling (see Part II of this book). In particular, mass spectrometry-based techniques for analysis of individual SL molecular species have permitted to reveal the specific role of particular SL molecules. Examples of the diversity/divergence of biological outcomes include differences due to the acyl chain-length, unsaturation or hydroxylation of Cer [10, 53]. Thus, current questions in the SL signalling field include which precise SL molecule is bioactive, in which subcellular compartment or even in which cell membrane domain it is formed or transported to, and which protein it targets. The answer to these questions is linked to the identification of the pathway that generates the candidate SL molecule. However, one should not forget that SL metabolism is a very dynamic process involving multiple reactions occurring in different compartments (and likely regulated by mechanisms that we have yet to discover). This implies that changes in the local concentration of one SL bioactive molecule may be accompanied by changes in the content of another bioactive SL, in the same or another subcellular location, indicating the need for a full spatiotemporal picture of SLs in order to accurately appreciate any variation of potential biological significance.
3.2 Sphingosine 1-Phosphate: A Paradigmatic Signalling Molecule
S1P meets the criteria that define a lipid mediator, coupling cell stimulation and functional activation, with regulation of cell proliferation being one of the first examples of its role as second messenger [54]. Not only is this small lipid, characterized by very low intracellular levels, able to activate specific cell surface receptors but also some intracellular targets (see Figs. 4 and 5). For detailed information regarding S1P signalling, the reader is referred to some recent reviews [6, 7, 9, 55, 56].
Signalling cascades regulated by sphingosine 1-phosphate. Upon stimulation by growth factors and cytokines, sphingosine kinase 1 (SK1) gets activated by ERK1/2 and phosphorylates sphingosine (So) to sphingosine 1 phosphate (S1P). S1P controls cell fate by regulation of Bcl-2 members [124–127]. In the nucleus S1P, produced by sphingosine kinase 2 (SK2), binds and inhibits histone deacetylases (HDACs) that regulates gene transcription [60]. S1P can function as an intracellular messenger or is secreted out of the cell by SPNS2 or ATP-binding cassette (ABC) transporters to signal through G-protein-coupled receptors (S1PR) in an autocrine and/or paracrine manner to regulate proliferation, migration, angiogenesis or autophagy in cancer cells and tumor microenvironment. Activation of S1P1 receptor leads to the activation of signal transducer and activator of transcription 3 (STAT3) [128]. STAT3 is a transcription factor for S1P1, which then reciprocally activates STAT3, resulting in its persistent activation and interleukin-6 production, a pro-inflammatory cytokine. S1P binds to and activates TNF receptor-associated factor 2 (TRAF2) that is implicated in activation of NF-κB in response to TNF. Activation of NF-κB and STAT3 induce chronic inflammation that promotes cancer cell progression
Upon activation of sphingosine kinase(s), for instance in response to cytokines or growth factors, S1P can be transported out of the cell (possibly by SPNS2) to activate its cognate G-protein coupled receptors. These receptors belong to a family of proteins, initially described as products of endothelial differentiation genes, some of which can bind the S1P-related lipid lysophosphatidic acid [57]. Engagement of S1P receptors modulates cell growth, migration, angiogenesis, lymphangiogenesis and the immune response [58]. Distinct outcomes arise from differential coupling of these five receptors to heterotrimeric G proteins and their downstream effectors (Fig. 5). Whereas no bona fide intracellular receptor of S1P has been reported, S1P can physically interact with and regulate some intracellular proteins. Indeed, S1P can function as a cofactor for the E3 ubiquitin ligase activity of the adaptor protein tumor necrosis factor receptor-associated factor 2 (TRAF2), regulating NF-κB activation [59] and thus explaining, among other processes, the cytoprotective function of S1P. In the nucleus, S1P binds to and inhibits HDAC1 and HDAC2, leading to increased levels of histone acetylation and gene transcription [60]. At the level of mitochondria, S1P is able to bind prohibitin-2 [61] and BAK [51], providing links with mitochondrial respiration and apoptosis, respectively. Finally, interaction of S1P with Cer synthase 2 provides a potential regulatory mechanism of the functional crosstalk between Cer and S1P [62].
3.3 How Are Other Sphingolipids Bioactive?
Early reports demonstrated that free sphingoid bases can inhibit PKC by preventing its interaction with DAG/phorbol esters [63–65]. Subsequent studies indicated that sphingosine regulates the function of many additional proteins, including members of the MAPK pathway, the sphingosine-dependent protein kinase 1, Akt and caspases (for a review see [66]). Modulation of these effectors by long-chain bases or sphingoid base analogues (i.e., N,N-dimethylsphingosine or N,N,N-trimethylsphingosine) has been classically associated with induction of cell death. Interestingly, natural unusual sphingoid bases called sphingadienes (because of the presence of an additional double bond at the C6 or C8 position) were found to inhibit colon tumorigenesis in animal models [67]. These sphingadienes, which derive from the catabolism of SLs present in food sources, inhibit the phosphoinositide 3-kinase/Akt and Wnt signalling pathways and promote apoptosis [68, 69]. They may also suppress intestinal tumorigenesis by inducing S1P lyase expression and reducing S1P colonic levels [70].
The way Cer mediates biological effects has attracted much attention. However, unlike DAG—a structurally similar lipid—the answer is not univocal and remains unclear. The search for potential Cer-binding proteins has faced technical difficulties [71] and has not yet provided unambiguous targets that could transduce all Cer actions. Nevertheless, diverse cellular proteins have been identified that relay the anti-mitogenic effects of Cer (Fig. 6; see also reviews by [8, 72–74]). Indeed, through modulation of cell cycle effectors, caspase-dependent and independent pathways and macroautophagy, Cer-activated cascades converge to antiproliferative signals. Some of them appear to be initiated by direct interaction of Cer with kinases such as PKC isoforms [75, 76], KSR1 [77], or the inhibitor 2 of protein phosphatase 2A [78]. Because of its rather hydrophobic nature, this membrane-localized SL may interact with proteins in distinct subcellular compartments, including the plasma membrane, ER, mitochondria or mitochondria-associated membranes. Of note, however, Cer may also modulate signalling pathways by changing the properties of membranes (i.e., membrane fluidity and curvature) or microdomains. For instance, ceramide synthase 6 expression is down-regulated during the epithelial-to-mesenchymal transition process, increasing plasma membrane fluidity as a consequence of reduced C16-Cer levels, and enhancing breast cancer cell motility [79]. Even modest variations (a few percent) in overall Cer levels largely exceed total S1P levels, suggesting that some actions of Cer might be related to membrane alterations that in turn influence the recruitment of key protein effectors or the activation of membrane receptors or channels [10, 80–82].
Signalling cascades regulated by ceramides. Various stress stimuli, such as chemotherapy, radiotherapy and death receptor (DR) ligands, trigger the accumulation of Cer as a consequence of (1) increased de novo Cer synthesis, (2) inhibition of Cer conversion to complex SLs, (3) hydrolysis of SM or GlcCer and/or (4) inhibition of ceramidase. Cer behaves as an anti-oncometabolite, inhibiting cancer cell motility and proliferation or inducing cancer cell death. For the sake of clarity, not all signalling pathways modulated by Cer are depicted. The anti-proliferative signalling pathway triggered by Cer involves the activation of both Kinase Suppressor of Ras/Cer-Activated Protein Kinase (KSR/CAPK) and p53, inhibiting the cdk-dependent phosphorylation of Rb, which sequesters E2F. Cer can activate the extrinsic apoptotic signalling pathway by facilitating DR oligomerization and subsequent caspase cascade activation. Alternatively, Cer stimulates the mitochondrial (i.e., intrinsic) apoptotic signalling pathway as a consequence of (1) PKCζ and PP2a-dependent Akt inhibition and subsequent Bad activation, (2) PP2a-dependent Bcl-2 inhibition, (3) cathepsins and/or caspase-8-dependent Bid cleavage. All these events lead to the mitochondrial outer membrane permeabilization followed by cytochrome c (Cyto c) release and caspase-9 activation. Both initiator caspases-8 and -9 cleave and activate effector caspases, such as caspase-3. AIF release from the mitochondria and ROS production are involved in Cer-induced caspase-independent cell death. Cer-triggered cell death can be amplified by ER stress and macroautophagy. Whereas p8 is involved in Cer-induced ER stress, the inhibition of mTOR and nutrient transporters, as well as the JNK-dependent Beclin-1 up-regulation and activation, facilitate the macroautophagy process
What about glycolipids? Although this topic is not discussed in this book, it is important to note that, because they localize in the extracellular leaflet of the plasma membrane, these SLs not only can affect membrane processes but also actively interact with the environment [83–85]. Hence, gangliosides, whose expression is altered in many tumors, can modulate the proliferation of malignant cells as well as cell–cell and cell–matrix adhesion. The biological effects of GSLs are likely mediated by interactions of their carbohydrate moieties, either on the same cell surface or between two different cells [86]. Perturbations of the membrane properties, the subsequent binding of ligands to membrane receptors, as well as the activity of multidrug resistance transporters could also be implicated [87]. In addition, shedding of tumor gangliosides provides both immunosuppressive and angiogenic signals [88, 89].
4 Current and Future Challenges
A renewed interest in SLs manifested 25 years ago with the discovery of specific biological functions mediated by members of this class. Since then, not only has our knowledge of metabolic pathways, enzymes and transport proteins greatly improved but also the way some SL molecules exert their regulatory roles has been molecularly characterized. Their abilities to modulate critical events in cancer development and progression (Fig. 1) suggest that certain SLs (e.g., S1P) behave as “oncometabolites” while some others (e.g., Cer) as “anti-oncometabolites”. Even though mutations in the genes encoding SL-metabolizing enzymes that would confer neomorphic activity (as mutations in the IDH1 gene do) have not—yet—been identified in tumors or leukemic cells, some SL molecules share properties with known oncometabolites such as 2-hydroxyglutarate [90]. For instance, levels of S1P-forming enzymes (mRNA and/or protein) are higher in tumor vs. normal tissues, and S1P acts as a pro-oncogenic signal, influencing the epigenome [60], transcriptional programs (e.g., [91]), hypoxia-inducible factor biology [92], tumor development [93] (see also numerous examples of reduced tumor growth upon treatment with inhibitors of the sphingosine kinase/S1P/S1P receptors axis) and sensitivity to anticancer regimens (for a review see [6]).
That said, despite considerable advances in our understanding, the world of SLs-and-cancer still faces a number of challenges. First, the metabolism of some SLs needs to be unequivocally elucidated. This includes the way: (1) some minor sphingoid bases (i.e., sphingadiene), omega-esterified Cer species and other unusual SLs are produced; (2) some lysosphingolipids, e.g., SPC, glucosylsphingosine and psychosine, are synthesized; (3) free sphingoid bases are transported into the cell, (4) S1P gets out of the cell; (5) Cer is oriented to spatially distinct compartments for the biosynthesis of SM, GlcCer, GalCer or Cer1P. Enzymes and transport proteins/transporters for such pathways are to be identified. Equally important is the need to fully understand how SL metabolism is regulated (little is known about transcriptional regulation of the genes encoding SL-metabolizing enzymes, regulation by microRNAs, or possible sensors of SL levels).
Second, the mode of action of bioactive SLs has to be further deciphered, implying the identification of direct protein targets and receptors [71]. In this regard, one should consider the possibility that changes in SL metabolism and composition in a given cell (e.g., the cancer cell) impact surrounding cells (i.e., the tumor microenvironment) through exosomes [94]. Third, efforts to characterize alterations in the sphingolipidome of tumor cells (or even plasma from patients with cancer) as well as (epi)genomic and transcriptomic changes in the genes of SL metabolism will hopefully define novel markers. They could serve as diagnostic, prognostic and/or predictive biomarkers, useful for the stratification of cancers or the tumor response to therapy. It is anticipated that advances in the above directions will help understand the roles of SLs in cancer and develop targeted therapies based on the manipulation of SL metabolism.
Abbreviations
- Cer:
-
Ceramide
- Cer1P:
-
Ceramide 1-phosphate
- DAG:
-
Diacylglycerol
- ER:
-
Endoplasmic reticulum
- GalCer:
-
Galactosylceramide
- GlcCer:
-
Glucosylceramide
- GSL:
-
Glycosphingolipid
- S1P:
-
Sphingosine 1-phosphate
- SL:
-
Sphingolipid
- SM:
-
Sphingomyelin
- SPC:
-
Sphingosylphosphocholine
References
Merrill AH Jr, Stokes TH, Momin A et al (2009) Sphingolipidomics: a valuable tool for understanding the roles of sphingolipids in biology and disease. J Lipid Res 50(Suppl):S97–102
Sabourdy F, Kedjouar B, Sorli SC et al (2008) Functions of sphingolipid metabolism in mammals—lessons from genetic defects. Biochim Biophys Acta 1781:145–183
Jennemann R, Grone HJ (2013) Cell-specific in vivo functions of glycosphingolipids: lessons from genetic deletions of enzymes involved in glycosphingolipid synthesis. Prog Lipid Res 52:231–248
Albinet V, Bats ML, Bedia C et al (2013) Genetic disorders of simple sphingolipid metabolism. Handb Exp Pharmacol 7:127–152. doi:10.1007/978-3-7091-1368-4
Hannun YA, Obeid LM (2008) Principles of bioactive lipid signalling: lessons from sphingolipids. Nat Rev Mol Cell Biol 9:139–150
Pyne NJ, Pyne S (2010) Sphingosine 1-phosphate and cancer. Nat Rev Cancer 10:489–503
Maceyka M, Harikumar KB, Milstien S, Spiegel S (2012) Sphingosine-1-phosphate signaling and its role in disease. Trends Cell Biol 22:50–60
Morad SA, Cabot MC (2013) Ceramide-orchestrated signalling in cancer cells. Nat Rev Cancer 13:51–65
Kunkel GT, Maceyka M, Milstien S, Spiegel S (2013) Targeting the sphingosine-1-phosphate axis in cancer, inflammation and beyond. Nat Rev Drug Discov 12:688–702
Grosch S, Schiffmann S, Geisslinger G (2012) Chain length-specific properties of ceramides. Prog Lipid Res 51:50–62
Penno A, Reilly MM, Houlden H et al (2010) Hereditary sensory neuropathy type 1 is caused by the accumulation of two neurotoxic sphingolipids. J Biol Chem 285:11178–11187
Bertea M, Rutti MF, Othman A et al (2010) Deoxysphingoid bases as plasma markers in diabetes mellitus. Lipids Health Dis 9:84
Funato K, Riezman H (2001) Vesicular and nonvesicular transport of ceramide from ER to the Golgi apparatus in yeast. J Cell Biol 155:949–959
Hanada K, Kumagai K, Yasuda S et al (2003) Molecular machinery for non-vesicular trafficking of ceramide. Nature 426:803–809
Halter D, Neumann S, van Dijk SM et al (2007) Pre- and post-Golgi translocation of glucosylceramide in glycosphingolipid synthesis. J Cell Biol 179:101–115
Simanshu DK, Kamlekar RK, Wijesinghe DS et al (2013) Non-vesicular trafficking by a ceramide-1-phosphate transfer protein regulates eicosanoids. Nature 500:463–467
Swanton C, Marani M, Pardo O et al (2007) Regulators of mitotic arrest and ceramide metabolism are determinants of sensitivity to paclitaxel and other chemotherapeutic drugs. Cancer Cell 11:498–512
Wennekes T, van den Berg RJ, Boot RG, van der Marel GA, Overkleeft HS, Aerts JM (2009) Glycosphingolipids—nature, function, and pharmacological modulation. Angew Chem Int Ed Engl 48:8848–8869
D’Angelo G, Capasso S, Sticco L, Russo D (2013) Glycosphingolipids: synthesis and functions. FEBS J 280:6338–6353
Kolter T, Sandhoff K (2010) Lysosomal degradation of membrane lipids. FEBS Lett 584:1700–1712
Kihara A (2014) Sphingosine 1-phosphate is a key metabolite linking sphingolipids to glycerophospholipids. Biochim Biophys Acta 1841:766–772
Tani M, Ito M, Igarashi Y (2007) Ceramide/sphingosine/sphingosine 1-phosphate metabolism on the cell surface and in the extracellular space. Cell Signal 19:229–237
Jenkins RW, Canals D, Hannun YA (2009) Roles and regulation of secretory and lysosomal acid sphingomyelinase. Cell Signal 21:836–846
Duan RD (2011) Physiological functions and clinical implications of sphingolipids in the gut. J Dig Dis 12:60–70
Birbes H, El Bawab S, Obeid LM, Hannun YA (2002) Mitochondria and ceramide: intertwined roles in regulation of apoptosis. Adv Enzyme Regul 42:113–129
Morales A, Colell A, Mari M, Garcia-Ruiz C, Fernandez-Checa JC (2004) Glycosphingolipids and mitochondria: role in apoptosis and disease. Glycoconj J 20:579–588
Lucki NC, Sewer MB (2012) Nuclear sphingolipid metabolism. Annu Rev Physiol 74:131–151
Mao C, Obeid LM (2008) Ceramidases: regulators of cellular responses mediated by ceramide, sphingosine, and sphingosine-1-phosphate. Biochim Biophys Acta 1781:424–434
Hannun YA, Bell RM (1987) Lysosphingolipids inhibit protein kinase C: implications for the sphingolipidoses. Science 235:670–674
Breslow DK, Weissman JS (2010) Membranes in balance: mechanisms of sphingolipid homeostasis. Mol Cell 40:267–279
Miyatake T, Suzuki K (1972) Globoid cell leukodystrophy: additional deficiency of psychosine galactosidase. Biochem Biophys Res Commun 48:539–543
Vanier MT, Svennerholm L (1975) Chemical pathology of Krabbe’s disease. III. Ceramide-hexosides and gangliosides of brain. Acta Paediatr Scand 64:641–648
Im DS, Heise CE, Nguyen T, O’Dowd BF, Lynch KR (2001) Identification of a molecular target of psychosine and its role in globoid cell formation. J Cell Biol 153:429–434
Radu CG, Cheng D, Nijagal A et al (2006) Normal immune development and glucocorticoid-induced thymocyte apoptosis in mice deficient for the T-cell death-associated gene 8 receptor. Mol Cell Biol 26:668–677
Wang JQ, Kon J, Mogi C et al (2004) TDAG8 is a proton-sensing and psychosine-sensitive G-protein-coupled receptor. J Biol Chem 279:45626–45633
Okazaki T, Bell RM, Hannun YA (1989) Sphingomyelin turnover induced by vitamin D3 in HL-60 cells. Role in cell differentiation. J Biol Chem 264:19076–19080
Kolesnick RN (1989) Sphingomyelinase action inhibits phorbol ester-induced differentiation of human promyelocytic leukemic (HL-60) cells. J Biol Chem 264:7617–7623
Hannun YA (1994) The sphingomyelin cycle and the second messenger function of ceramide. J Biol Chem 269:3125–3128
Levade T, Jaffrezou JP (1999) Signalling sphingomyelinases: which, where, how and why? Biochim Biophys Acta 1438:1–17
Bose R, Verheij M, Haimovitz-Friedman A, Scotto K, Fuks Z, Kolesnick R (1995) Ceramide synthase mediates daunorubicin-induced apoptosis: an alternative mechanism for generating death signals. Cell 82:405–414
Park JW, Park WJ, Futerman AH (2014) Ceramide synthases as potential targets for therapeutic intervention in human diseases. Biochim Biophys Acta 1841:671–681
Watanabe M, Kitano T, Kondo T et al (2004) Increase of nuclear ceramide through caspase-3-dependent regulation of the “sphingomyelin cycle” in Fas-induced apoptosis. Cancer Res 64:1000–1007
Lafont E, Milhas D, Carpentier S et al (2010) Caspase-mediated inhibition of sphingomyelin synthesis is involved in FasL-triggered cell death. Cell Death Differ 17:642–654
Bedia C, Casas J, Andrieu-Abadie N, Fabrias G, Levade T (2011) Acid ceramidase expression modulates the sensitivity of A375 melanoma cells to dacarbazine. J Biol Chem 286:28200–28209
Valaperta R, Chigorno V, Basso L et al (2006) Plasma membrane production of ceramide from ganglioside GM3 in human fibroblasts. FASEB J 20:1227–1229
Sorli SC, Colie S, Albinet V et al (2013) The nonlysosomal beta-glucosidase GBA2 promotes endoplasmic reticulum stress and impairs tumorigenicity of human melanoma cells. FASEB J 27:489–498
Ghosh TK, Bian J, Gill DL (1990) Intracellular calcium release mediated by sphingosine derivatives generated in cells. Science 248:1653–1656
Zhang H, Desai NN, Olivera A, Seki T, Brooker G, Spiegel S (1991) Sphingosine-1-phosphate, a novel lipid, involved in cellular proliferation. J Cell Biol 114:155–167
Auge N, Nikolova-Karakashian M, Carpentier S et al (1999) Role of sphingosine 1-phosphate in the mitogenesis induced by oxidized low density lipoprotein in smooth muscle cells via activation of sphingomyelinase, ceramidase, and sphingosine kinase. J Biol Chem 274:21533–21538
Kumar A, Byun HS, Bittman R, Saba JD (2011) The sphingolipid degradation product trans-2-hexadecenal induces cytoskeletal reorganization and apoptosis in a JNK-dependent manner. Cell Signal 23:1144–1152
Chipuk JE, McStay GP, Bharti A et al (2012) Sphingolipid metabolism cooperates with BAK and BAX to promote the mitochondrial pathway of apoptosis. Cell 148:988–1000
Cuvillier O, Pirianov G, Kleuser B et al (1996) Suppression of ceramide-mediated programmed cell death by sphingosine-1-phosphate. Nature 381:800–803
Mesicek J, Lee H, Feldman T et al (2010) Ceramide synthases 2, 5, and 6 confer distinct roles in radiation-induced apoptosis in HeLa cells. Cell Signal 22:1300–1307
Olivera A, Spiegel S (1993) Sphingosine-1-phosphate as second messenger in cell proliferation induced by PDGF and FCS mitogens. Nature 365:557–560
Strub GM, Maceyka M, Hait NC, Milstien S, Spiegel S (2010) Extracellular and intracellular actions of sphingosine-1-phosphate. Adv Exp Med Biol 688:141–155
Spiegel S, Milstien S (2011) The outs and the ins of sphingosine-1-phosphate in immunity. Nat Rev Immunol 11:403–415
Lee MJ, Van Brocklyn JR, Thangada S et al (1998) Sphingosine-1-phosphate as a ligand for the G protein-coupled receptor EDG-1. Science 279:1552–1555
Blaho VA, Hla T (2014) An update on the biology of sphingosine 1-phosphate receptors. J Lipid Res 55:1596–1608
Alvarez SE, Harikumar KB, Hait NC et al (2010) Sphingosine-1-phosphate is a missing cofactor for the E3 ubiquitin ligase TRAF2. Nature 465:1084–1088
Hait NC, Allegood J, Maceyka M et al (2009) Regulation of histone acetylation in the nucleus by sphingosine-1-phosphate. Science 325:1254–1257
Strub GM, Paillard M, Liang J et al (2011) Sphingosine-1-phosphate produced by sphingosine kinase 2 in mitochondria interacts with prohibitin 2 to regulate complex IV assembly and respiration. FASEB J 25:600–612
Laviad EL, Albee L, Pankova-Kholmyansky I et al (2008) Characterization of ceramide synthase 2: tissue distribution, substrate specificity, and inhibition by sphingosine 1-phosphate. J Biol Chem 283:5677–5684
Hannun YA, Loomis CR, Merrill AH Jr, Bell RM (1986) Sphingosine inhibition of protein kinase C activity and of phorbol dibutyrate binding in vitro and in human platelets. J Biol Chem 261:12604–12609
Merrill AH Jr, Sereni AM, Stevens VL, Hannun YA, Bell RM, Kinkade JM Jr (1986) Inhibition of phorbol ester-dependent differentiation of human promyelocytic leukemic (HL-60) cells by sphinganine and other long-chain bases. J Biol Chem 261:12610–12615
Wilson E, Olcott MC, Bell RM, Merrill AH Jr, Lambeth JD (1986) Inhibition of the oxidative burst in human neutrophils by sphingoid long-chain bases. Role of protein kinase C in activation of the burst. J Biol Chem 261:12616–12623
Cuvillier O (2002) Sphingosine in apoptosis signaling. Biochim Biophys Acta 1585:153–162
Symolon H, Schmelz EM, Dillehay DL, Merrill AH Jr (2004) Dietary soy sphingolipids suppress tumorigenesis and gene expression in 1,2-dimethylhydrazine-treated CF1 mice and ApcMin/+ mice. J Nutr 134:1157–1161
Fyrst H, Oskouian B, Bandhuvula P et al (2009) Natural sphingadienes inhibit Akt-dependent signaling and prevent intestinal tumorigenesis. Cancer Res 69:9457–9464
Kumar A, Pandurangan AK, Lu F et al (2012) Chemopreventive sphingadienes downregulate Wnt signaling via a PP2A/Akt/GSK3beta pathway in colon cancer. Carcinogenesis 33:1726–1735
Degagne E, Pandurangan A, Bandhuvula P et al (2014) Sphingosine-1-phosphate lyase downregulation promotes colon carcinogenesis through STAT3-activated microRNAs. J Clin Invest 124:5368–5384
Snook CF, Jones JA, Hannun YA (2006) Sphingolipid-binding proteins. Biochim Biophys Acta 1761:927–946
Saddoughi SA, Ogretmen B (2013) Diverse functions of ceramide in cancer cell death and proliferation. Adv Cancer Res 117:37–58
Young MM, Kester M, Wang HG (2013) Sphingolipids: regulators of crosstalk between apoptosis and autophagy. J Lipid Res 54:5–19
Garcia-Barros M, Coant N, Truman JP, Snider AJ, Hannun YA (2014) Sphingolipids in colon cancer. Biochim Biophys Acta 1841:773–782
Huwiler A, Fabbro D, Pfeilschifter J (1998) Selective ceramide binding to protein kinase C-alpha and -delta isoenzymes in renal mesangial cells. Biochemistry 37:14556–14562
Wang G, Silva J, Krishnamurthy K, Tran E, Condie BG, Bieberich E (2005) Direct binding to ceramide activates protein kinase Czeta before the formation of a pro-apoptotic complex with PAR-4 in differentiating stem cells. J Biol Chem 280:26415–26424
Yin X, Zafrullah M, Lee H, Haimovitz-Friedman A, Fuks Z, Kolesnick R (2009) A ceramide-binding C1 domain mediates kinase suppressor of ras membrane translocation. Cell Physiol Biochem 24:219–230
Mukhopadhyay A, Saddoughi SA, Song P et al (2009) Direct interaction between the inhibitor 2 and ceramide via sphingolipid-protein binding is involved in the regulation of protein phosphatase 2A activity and signaling. FASEB J 23:751–763
Edmond V, Dufour F, Poiroux G et al (2015) Downregulation of ceramide synthase-6 during epithelial-to-mesenchymal transition reduces plasma membrane fluidity and cancer cell motility. Oncogene 34:996–1005. doi:10.1038/onc.2014.55
van Blitterswijk WJ, van der Luit AH, Veldman RJ, Verheij M, Borst J (2003) Ceramide: second messenger or modulator of membrane structure and dynamics? Biochem J 369:199–211
Goni FM, Alonso A (2006) Biophysics of sphingolipids I. Membrane properties of sphingosine, ceramides and other simple sphingolipids. Biochim Biophys Acta 1758:1902–1921
Zhang Y, Li X, Becker KA, Gulbins E (2009) Ceramide-enriched membrane domains—structure and function. Biochim Biophys Acta 1788:178–183
Hakomori S (2002) Glycosylation defining cancer malignancy: new wine in an old bottle. Proc Natl Acad Sci U S A 99:10231–10233
Fredman P, Hedberg K, Brezicka T (2003) Gangliosides as therapeutic targets for cancer. BioDrugs 17:155–167
Furukawa K, Hamamura K, Aixinjueluo W, Furukawa K (2006) Biosignals modulated by tumor-associated carbohydrate antigens: novel targets for cancer therapy. Ann N Y Acad Sci 1086:185–198
Handa K, Hakomori SI (2012) Carbohydrate to carbohydrate interaction in development process and cancer progression. Glycoconj J 29:627–637
Giussani P, Tringali C, Riboni L, Viani P, Venerando B (2014) Sphingolipids: key regulators of apoptosis and pivotal players in cancer drug resistance. Int J Mol Sci 15:4356–4392
Hossain DM, Mohanty S, Ray P, Das T, Sa G (2012) Tumor gangliosides and T cells: a deadly encounter. Front Biosci (Schol Ed) 4:502–519
Liu Y, Wondimu A, Yan S, Bobb D, Ladisch S (2013) Tumor gangliosides accelerate murine tumor angiogenesis. Angiogenesis 17:563–571. doi:10.1007/s10456-013-9403-4
Ward PS, Patel J, Wise DR et al (2010) The common feature of leukemia-associated IDH1 and IDH2 mutations is a neomorphic enzyme activity converting alpha-ketoglutarate to 2-hydroxyglutarate. Cancer Cell 17:225–234
Avery K, Avery S, Shepherd J, Heath PR, Moore H (2008) Sphingosine-1-phosphate mediates transcriptional regulation of key targets associated with survival, proliferation, and pluripotency in human embryonic stem cells. Stem Cells Dev 17:1195–1205
Ader I, Malavaud B, Cuvillier O (2009) When the sphingosine kinase 1/sphingosine 1-phosphate pathway meets hypoxia signaling: new targets for cancer therapy. Cancer Res 69:3723–3726
Xia P, Gamble JR, Wang L et al (2000) An oncogenic role of sphingosine kinase. Curr Biol 10:1527–1530
Trajkovic K, Hsu C, Chiantia S et al (2008) Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science 319:1244–1247
Hanahan D, Weinberg RA (2000) The hallmarks of cancer. Cell 100:57–70
Moon SK, Kim HM, Lee YC, Kim CH (2004) Disialoganglioside (GD3) synthase gene expression suppresses vascular smooth muscle cell responses via the inhibition of ERK1/2 phosphorylation, cell cycle progression, and matrix metalloproteinase-9 expression. J Biol Chem 279:33063–33070
Wang H, Isaji T, Satoh M, Li D, Arai Y, Gu J (2013) Antitumor effects of exogenous ganglioside GM3 on bladder cancer in an orthotopic cancer model. Urology 81(210):e211–215
Shirahama T, Sweeney EA, Sakakura C et al (1997) In vitro and in vivo induction of apoptosis by sphingosine and N, N-dimethylsphingosine in human epidermoid carcinoma KB-3-1 and its multidrug-resistant cells. Clin Cancer Res 3:257–264
Desai NN, Carlson RO, Mattie ME et al (1993) Signaling pathways for sphingosylphosphorylcholine-mediated mitogenesis in Swiss 3T3 fibroblasts. J Cell Biol 121:1385–1395
Endo K, Igarashi Y, Nisar M, Zhou QH, Hakomori S (1991) Cell membrane signaling as target in cancer therapy: inhibitory effect of N,N-dimethyl and N,N,N-trimethyl sphingosine derivatives on in vitro and in vivo growth of human tumor cells in nude mice. Cancer Res 51:1613–1618
Webb TJ, Li X, Giuntoli RL 2nd et al (2012) Molecular identification of GD3 as a suppressor of the innate immune response in ovarian cancer. Cancer Res 72:3744–3752
Zhou D (2006) The immunological function of iGb3. Curr Protein Pept Sci 7:325–333
Shimizu K, Kurosawa Y, Taniguchi M, Steinman RM, Fujii S (2007) Cross-presentation of glycolipid from tumor cells loaded with alpha-galactosylceramide leads to potent and long-lived T cell mediated immunity via dendritic cells. J Exp Med 204:2641–2653
Maru M, Haraguchi M, Higashi H et al (1993) Anti-tumor activity of ceramides and glycosphingolipids in a murine tumor system. Int J Cancer 53:645–650
van Vlerken LE, Duan Z, Little SR, Seiden MV, Amiji MM (2010) Augmentation of therapeutic efficacy in drug-resistant tumor models using ceramide coadministration in temporal-controlled polymer-blend nanoparticle delivery systems. AAPS J 12:171–180
Parameswaran R, Lim M, Arutyunyan A et al (2013) O-acetylated N-acetylneuraminic acid as a novel target for therapy in human pre-B acute lymphoblastic leukemia. J Exp Med 210:805–819
Lavie Y, Cao H, Bursten SL, Giuliano AE, Cabot MC (1996) Accumulation of glucosylceramides in multidrug-resistant cancer cells. J Biol Chem 271:19530–19536
Ponnusamy S, Meyers-Needham M, Senkal CE et al (2010) Sphingolipids and cancer: ceramide and sphingosine-1-phosphate in the regulation of cell death and drug resistance. Future Oncol 6:1603–1624
Bocci G, Fioravanti A, Orlandi P et al (2012) Metronomic ceramide analogs inhibit angiogenesis in pancreatic cancer through up-regulation of caveolin-1 and thrombospondin-1 and down-regulation of cyclin D1. Neoplasia 14:833–845
Seyfried TN, Mukherjee P (2010) Ganglioside GM3 is antiangiogenic in malignant brain cancer. J Oncol 2010:961243
Visentin B, Vekich JA, Sibbald BJ et al (2006) Validation of an anti-sphingosine-1-phosphate antibody as a potential therapeutic in reducing growth, invasion, and angiogenesis in multiple tumor lineages. Cancer Cell 9:225–238
Cazet A, Groux-Degroote S, Teylaert B et al (2009) GD3 synthase overexpression enhances proliferation and migration of MDA-MB-231 breast cancer cells. Biol Chem 390:601–609
Yan Q, Bach DQ, Gatla N et al (2013) Deacetylated GM3 promotes uPAR-associated membrane molecular complex to activate p38 MAPK in metastatic melanoma. Mol Cancer Res 11:665–675
Ratajczak MZ, Suszynska M, Borkowska S, Ratajczak J, Schneider G (2014) The role of sphingosine-1 phosphate and ceramide-1 phosphate in trafficking of normal stem cells and cancer cells. Expert Opin Ther Targets 18:95–107
Takenaga M, Igarashi R, Matsumoto K et al (1999) Lipid microsphere preparation of a lipophilic ceramide derivative suppresses colony formation in a murine experimental pulmonary metastasis model. J Drug Target 7:187–195
Beil M, Micoulet A, von Wichert G et al (2003) Sphingosylphosphorylcholine regulates keratin network architecture and visco-elastic properties of human cancer cells. Nat Cell Biol 5:803–811
Sadahira Y, Ruan F, Hakomori S, Igarashi Y (1992) Sphingosine 1-phosphate, a specific endogenous signaling molecule controlling cell motility and tumor cell invasiveness. Proc Natl Acad Sci U S A 89:9686–9690
Birks SM, Danquah JO, King L, Vlasak R, Gorecki DC, Pilkington GJ (2011) Targeting the GD3 acetylation pathway selectively induces apoptosis in glioblastoma. Neuro Oncol 13:950–960
Bennaceur K, Popa I, Chapman JA et al (2009) Different mechanisms are involved in apoptosis induced by melanoma gangliosides on human monocyte-derived dendritic cells. Glycobiology 19:576–582
Obeid LM, Linardic CM, Karolak LA, Hannun YA (1993) Programmed cell death induced by ceramide. Science 259:1769–1771
Kota V, Dhople VM, Fullbright G et al (2013) 2′-Hydroxy C16-ceramide induces apoptosis-associated proteomic changes in C6 glioma cells. J Proteome Res 12:4366–4375
Sakakura C, Sweeney EA, Shirahama T, Hakomori S, Igarashi Y (1996) Suppression of bcl-2 gene expression by sphingosine in the apoptosis of human leukemic HL-60 cells during phorbol ester-induced terminal differentiation. FEBS Lett 379:177–180
Bleicher RJ, Cabot MC (2002) Glucosylceramide synthase and apoptosis. Biochim Biophys Acta 1585:172–178
Bektas M, Jolly PS, Muller C, Eberle J, Spiegel S, Geilen CC (2005) Sphingosine kinase activity counteracts ceramide-mediated cell death in human melanoma cells: role of Bcl-2 expression. Oncogene 24:178–187
Sauer B, Gonska H, Manggau M et al (2005) Sphingosine 1-phosphate is involved in cytoprotective actions of calcitriol in human fibroblasts and enhances the intracellular Bcl-2/Bax rheostat. Pharmazie 60:298–304
Li QF, Wu CT, Guo Q, Wang H, Wang LS (2008) Sphingosine 1-phosphate induces Mcl-1 upregulation and protects multiple myeloma cells against apoptosis. Biochem Biophys Res Commun 371:159–162
Colie S, Van Veldhoven PP, Kedjouar B et al (2009) Disruption of sphingosine 1-phosphate lyase confers resistance to chemotherapy and promotes oncogenesis through Bcl-2/Bcl-xL upregulation. Cancer Res 69:9346–9353
Lee H, Deng J, Kujawski M et al (2010) STAT3-induced S1PR1 expression is crucial for persistent STAT3 activation in tumors. Nat Med 16:1421–1428
Acknowledgement
Support to TL’s team by INSERM, Université Paul Sabatier, ANR (SphingoDR program), RITC, Ligue Nationale Contre le Cancer (Equipe Labellisée 2013), and VML is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Colacios, C., Sabourdy, F., Andrieu-Abadie, N., Ségui, B., Levade, T. (2015). Basics of Sphingolipid Metabolism and Signalling. In: Hannun, Y., Luberto, C., Mao, C., Obeid, L. (eds) Bioactive Sphingolipids in Cancer Biology and Therapy. Springer, Cham. https://doi.org/10.1007/978-3-319-20750-6_1
Download citation
DOI: https://doi.org/10.1007/978-3-319-20750-6_1
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-20749-0
Online ISBN: 978-3-319-20750-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)