Abstract
Plants take up water via the root system and transpire water vapor through stomatal openings in the leaves. Surrounding guard and subsidiary cells control the magnitude of the openings, enabling transpiration but also CO2 transport for photosynthesis. Rhythmic transpiration reflects rhythmic cellular control by these cells and shows a range of short-term periods (typically from a few minutes to some hours). Hydraulic feedback models of water regulation and rhythmic transpiration via the stomatal cells have been developed, either for single or for coupled stomata oscillators. Coupling between stomata over a leaf is necessary to obtain overall transpiration rhythms. Modeling has been strongly extended in later years. The chapter concentrates on experimental findings of overt transpiration rhythms as well as on oscillatory events in the guard cells. Results on the occurrence of rhythms, their period, amplitude, and modulation are discussed. The impact of external environmental parameters on the rhythms is dealt with, e.g., humidity, light, osmotic changes, and ions. The relevance of hydraulic feedback models is discussed as well as the possibilities of calcium oscillations in the guard cells to participate in generating the transpiration rhythms. The overall transpiration pattern can be complicated in space and time: patchy transpiration can occur over a leaf surface, and period-doubling and period-n patterns have been recorded in the rhythms. There are indications that the control system can be chaotic and the advantages of such a system are shortly discussed. The behavior of transpiration rhythms reveals many dynamic features of the stomatal control system. Some comments and a short discussion on water-use efficiency (WUE) of plants and vegetation end the chapter.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction and Overview
Plants transpire water vapor through stomatal pores in the surfaces of their leaves or needles. The stomata have a typical dimension of about 10 μm. The width of the stomatal opening is regulated by an oppositely placed pair of guard cells , and the volumes of these cells are fundamental in controlling the size of the pore opening. Despite the small pore dimensions, the global transpiration through stomata is estimated to account for up to 80–90 % of the terrestrial evapotranspiration, thus responsible for the cycling of about 60,000 km3 water per year (or about 60 × 1015 kg per year; Jasechko et al. 2013, who also cite earlier estimates of the order of 14,000–41,000 km3 per year).
Thus, the stomatal pores in the terrestrial biomass play an outstanding role in the global water flux. But they also control CO2 transport from the atmosphere to the stomatal cavities (estimated to represent 125 × 1012 kg carbon per year; Beer et al. 2010) to be used in the photosynthetic processes of the plants. The stomata and the guard cells are thus the site of delicate and fundamental regulatory mechanisms that control the pore openings under varying external conditions.
The water transpiration of many plants (including bushes and trees) can be rhythmic, or oscillatory, under a wide variety of environmental and experimental conditions (Kaiser and Paoletti 2014; Barrs 1971; Hopmans 1971; Cowan 1972; Raschke 1979; Sack and Holbrook 2006; Yang et al. 2005; further references at end of chapter). The pore size and its characteristics then change rhythmically, and the guard cell volumes will change rhythmically and so will other variables in the water system of the plant.
Oscillations with a period of about 1–2 h or less have been studied both in monocots and in dicots, in whole plants/trees and in young plants as well as in old ones. Longer periods, e.g., circadian, also exist (see Sect. 7.4.2). Plants showing transpiration rhythms have quite different stomata anatomy. Furthermore, they occur in plants with varying numbers of stomata per surface area (see Kramer and Boyer 1995): about 175 per mm2 in Allium cepa and about 50 per mm2 in Avena sativa (in both species, on the upper and the lower leaf surface). Altogether, oscillatory transpiration is a general phenomenon, mirroring basic mechanisms in quite different species under quite different conditions.
The water transpired by a plant has to be replaced by water taken up from the soil/medium. It then enters the root system and moves via the xylem structures into storage elements and the leaves, underway experiencing different flow resistances. Storage tissue can be part of the trunk in trees. In the leaves, it reaches three important dynamic elements in the water regulation of the plant: the guard cells mentioned above with their neighboring subsidiary cells (or supporting tissue) and mesophyll cells. The water then evaporates through the stomatal pores via the stomatal cavities. A rhythmic transpiration will become accompanied by a rhythmic water transport as well as rhythmic changes in volume, in concentration of ions, in transport of ions , in membrane potentials, etc. of the cells involved. In Sect. 7.2, this description of water transport will be discussed.
Section 7.2 will also focus on the fact that an overall transpiration rhythm from an individual leaf can be ascertained only if enough individual stomata of the leaf are synchronized. On the other hand, a non-rhythmic transpiration could indicate either that the individual stomata are non-oscillating or that they are oscillating in a desynchronized fashion. Individual stomata and guard cells at a short distance from each other can be desynchronized in spite of an overall oscillatory transpiration pattern (e.g., Kaiser and Kappen 2001; Mott and Buckley 2000; Kamakura et al. 2012a, b). However, a sufficiently large number of synchronized stomata must be present when rhythmic transpiration is recorded.
During the last decades, much interest has been focussed on oscillations in the calcium concentration in guard cells (see reviews by Yang et al. 2004, 2005). [Ca2+] i oscillations form a signaling pathway and [Ca2+] i in the guard cells may act as a second messenger (McAinish et al. 1995; Blatt 2000b; Yang et al. 2003; Chap. 9 of the present volume). Water channels , aquaporins , might be downstream elements of [Ca2+] i oscillation signaling (Kaldenhoff and Fischer 2006; Yang et al. 2005; Lopez et al. 2004; Nielsen et al. 2002). In Sect. 7.3, we will discuss some aspects of these guard cell oscillations. In Sects. 7.4 and 7.5, we will focus on experimental findings in studies of oscillatory transpiration under different experimental and environmental conditions.
When the whole plant organism—for example, a bush or a tree—or big parts of it show a rhythmic overall transpiration, its different parts must also be synchronized. This means that coupling between the different parts (and their stomata) will exist and they constitute a network. Examples of such overall transpiration oscillations have been found in several species.
A complete description of the oscillations in a network should encompass features of water regulation at the molecular, ionic, cellular, and physiological levels. All the different levels of the network organization are involved in rhythmic transpiration. It can thus properly be described as a “complex system” (Goldenfeld and Kadanoff 1999). The network of cells can produce effects which are not found in studies of reactions and mechanisms in individual cells (or isolated, e.g. as protoplasts). One has, therefore, to describe the phenomena by means of appropriate models at the different levels. An effort to describe all properties of a complicated system on very basic mechanisms can be in vain. It can be too difficult a task to model a “bulldozer out from quarks” (Goldenfeld and Kadanoff 1999).
With a network concept in mind, results from measurements on oscillatory transpiration of plants are discussed in this chapter and in Sect. 7.5, some remarks and comments will be given.
2 Hydraulic Models for Rhythmic Water Transpiration
2.1 Overall Description—“Lumped,” Hydraulic Model of Leaf
Water will be transported between two points in the plant if they have different water potentials. The concept of water potential, ψw, is derived from the thermodynamic definition of electrochemical potential of water (see Nobel 1999). The water flux will be determined by the magnitude of water potential differences, Δψw, as well as by the conductivity, g, of the medium between the two points.
Representative values of ψw can be found in the literature (Nobel 1999), but some examples should be given here. Typical values for roots can be −5 × 105 N/m2, in xylem of roots about −6 × 105 N/m2, in the xylem of leaf at 10 m height −8 × 105 N/m2. All values are relative to pure water under standard conditions. The water (vapor) potential in the air outside the leaf, ψair, is dependent on the water content of the air and is lower the dryer is the air. ψair at 95 % relative humidity is about −70 × 105 N/m2 (= −70 bars), at 60 % about −700 × 105 N/m2, and at 50 % about −950 × 105 N/m2.
The transpiration through the guard cell pores depends on the water potential difference between the outside air and the air in the stomatal cavity, Δψw = (ψair − ψstoma), as well as on the conductance of the stomatal pore, gs. An open pore has a high gs and a totally closed pore a gs equal to zero. Since the pore opening in this way is crucial for the transpiration, the volumes of the guard cells and their supporting epidermal tissue (often denoted subsidiary cells) are important in regulating the transpiration. The volumes of these cells in turn depend on their water content. The Δψw between ambient air and the inside of the stomatal cavity is often high, which is a prerequisite for high transpiration rates through the pores. Δψw could easily be of the order of −900 × 105 N/m2, as exemplified by the data given above.
Transpiration through the cuticula of the leaves adds to the stomatal transpiration. However, the conductivity for this pathway is often much lower and is in many cases neglected. Typical values of the cuticular conductance are gcuticular = 0.004−0.02 mol m−2s−1 (Caird et al. 2007 list useful data) to be compared with about gs = 0.1−0.35 mol m−2s−1 for the stomatal conductance gs (Marenco et al. 2006; cotton daytime) and 0.01−0.25 mol m−2s−1 (Marenco et al. 2006; cotton nighttime). Stomatal conductance values for trees can be found in Wullschleger et al. 1998.
An overall, very simplified picture of the hydraulic water regulation is given in Fig. 7.1. When water is transpired from the stomatal cavity, it will be replaced from the leaf cells and from the xylem. If the water “supply” is limited, the cells will shrink. The decrease in guard cell volume leads to a closing of the stomatal pore, thus decreasing gs, and a counteracting decrease in water vapor transport through the transpiration. On the other hand, with abundant water present, the guard cell volumes will increase, and due to their structural features, the stomatal opening and gs will increase, thus increasing transpiration. The guard cell control of the stomatal opening thus functions as a control system and is an example of a feedback system.
Simplified feedback scheme for water regulation in plant. The water uptake of the plant occurs via the roots , lowermost two boxes. The water is transported via the xylem into the leaves (where mesophyll cells form the majority of cells). The stomatal openings in the leaf are the keys to the transpiration, being affected by the guard cells and subsidiary cells, to the right of figure. The stomatal opening is increased if guard cell volumes are increased, while it decreases when the subsidiary cells increase their volume (thus a positive and negative regulation). The stomatal openings are decisive for the plant transpiration, the most important regulation is via the guard cells—thick box. The transpiration in turn affects the water status of the plant (feedback connection, full line). Transpiration through the cuticle of the leaf is much less than through the stomata (feedback connection, broken line). The scheme emphasizes the feedback nature of the regulatory system
The guard cells are supported by neighboring subsidiary/epidermal cells. They exert a counteracting pressure on the guard cells when all the cells increase their volumes. In this way, they counteract the increase in pore opening initiated by the guard cell control. In a corresponding manner, the subsidiary cells counteract the guard cell’s closing of the pore when the water supply decreases. The main regulation of the stomatal pore occurs via the guard cells (therefore the more heavy line surrounding the symbol in Fig. 7.1), but the water transport into the subsidiary cells might be faster—thus causing a temporary regulation in the “wrong” direction.
The picture outlined is an overall description of the water transport system of, e.g., a typical leaf. It forms a frame for models of the overall transpiration and describes a feedback control system of the water regulation in a leaf. The model can be denoted as “lumped” model, in which all the guard cells of the leaf (or the plant) have been lumped into one box denoted guard cell, all stomatal cavities lumped into one stomatal cavity, etc. Since it is basic and descriptive, it is also a “frame” model to be taken into consideration when extensions and details will be added.
It is well known from control theory that oscillations often arise in feedback systems under certain conditions (amplification and phase change in the feedback loop should be large enough). A phase change in the closed loop will be particularly pronounced if there are time delays in any of the processes of the loop. Such time delays can be simple transport delays (of, e.g., xylem water) but also delays in biochemical reaction sequences and signaling in the guard cells. Hysteresis in reactions can likewise work as time delays (Ramos and Prado 2013) and increase oscillatory tendencies. Furthermore, the time constants of the volume changes are important for oscillations to arise. Since guard cells and the subsidiary cells counteract each other in their effects on the stomatal pores, there can be an increased tendency for regulatory “overshoots” and oscillations.
The hydraulic feedback model roughly outlined above has been studied in more elaborated forms with physiological concepts, parameter values, and stability criteria identified. Cowan (1972) showed that such a system can describe an oscillatory water regulation and thus lead to transpiration rhythms. The description forms the basis for several other approaches to rhythmic transpiration (e.g., Delwiche and Cooke (1977); review by Sack and Holbrook (2006) and Kaiser and Paoletti (2014) with references). It has been successful in describing an astonishingly large number of experimentally found features of transpiration oscillations (e.g., in series of studies on transpiration rhythms in excised Avena leaves, see the reference list). The model is also nonlinear, allowing complicated oscillatory patterns in transpiration. Of course, it does not go into descriptions of the important cellular mechanisms in the guard cells that respond to the water status. An overview of models for stomatal conductance is given by Damour et al. (2010).
However, there are several experimentally found features of the oscillatory transpiration which are not explicitly described in this hydraulic feedback model. Pro’s and con’s of the “Cowan model” were given fairly early (e.g., Johnsson 1976), but many new experimental results on the cellular level have to be taken into account in more complete models. Remembering the task of the stomata—to allow atmospheric CO2 to pass into the plant for optimal photosynthesis and, simultaneously, control the water vapor transpiration out from the plant—it is not astonishing that the modeling will be more and more complicated. Our information about how the water potentials in the guard cells are changed by cellular ion mechanisms like Ca2+ oscillations, and by the presence of ion pumps in guard cells, aquaporins at several levels in the plant, abscisic acid , etc. have increased substantially since the hydraulic models were first published.
This leads to a remark: Essential extensions of the essentially hydraulic model must take care of the questions of
-
how carbon dioxide, CO2, participates in the stomatal regulation and accompanies the rhythmic transpiration,
-
how the abscisic acid molecule, ABA, exerts its action in the cell regulation and affects the transpiration rhythms,
-
how water is transported across membranes (e.g., those of the guard cells) and thus how ψstoma, ψguard cell, and ψxylem, etc. are controlled and the role of aquaporins
-
how molecular ion pumps and transport channels of the guard cells affect the osmotic conditions
Detailed cellular approaches have thus to be included, and values of model parameters (e.g., for water conductance in membranes) have to be elaborated and new concepts introduced. Details of the guard cell regulation and mechanisms can be found in the present volume (see Chaps. 8 and 9 of the present volume).
2.2 Overall Description—“Composed” Hydraulic Models of Leaf
Composed models have been constructed to represent a higher level of complexity and to describe how individual stomata can be coupled to better represent the behavior of the overall, whole leaf (e.g., Rand et al. 1982). The nature of the coupling between stomata is a central issue (Mott et al. 1999; Gradmann and Buschmann 1996; Gradmann and Hoffstadt 1998; Sack and Holbrook 2006), but one mode is via the water potential of the mesophyll of the leaf. The volume change in guard cells of one stomatal unit can be visualized to affect the ψw in the neighboring stomatal units via this “hydraulic coupling.” Affecting transpiration in one part of the leaf will then affect the transpiration in neighboring parts. Coupling can of course also consist of transmissions of biochemical or electrical signals between cells and stomatal subunits (Gradmann and Buschmann 1996; Gradmann and Hoffstadt 1998).
The water system can now be modeled in a one-dimensional way (“stomata on a line”) so that waves of rhythmic transpiration could arise, waves moving from subunit to subunit along the line (could be illustrated, for example, by adding several stomatal units to the right in Fig. 7.1). Rand et al. (1982) found theoretically that a one-dimensional system of coupled stomatal oscillators could show spatially uniform behavior, i.e., the different surface sections were synchronized across the leaf surface. But under non-uniform light conditions the system could be desynchronized and would show more complicated patterns of transpiration. Haefner et al. (1997) coupled stomata hydraulically in a two-dimensional model (“stomata on a surface”) which then simulates a leaf with its stomata subunits. Simulations showed that all stomata oscillated synchronously but with phase differences across the leaf surface, if they were given the same parameters. However, a patchy organization (see Sect. 7.2.3) of subunits with different amplitudes and periods emerged under conditions where stomatal parameters were spatially randomized. Thus, partly uncoordinated stomatal behavior and uncoordinated transpiration occurred in this two-dimensional model.
The hydraulic coupling between different stomata (via water potential changes in the leaves) has been questioned (e.g., Kaiser and Kappen 2001), but alternative coupling modes can certainly be suggested as electric coupling (e.g., Gradmann and Buschmann 1996; Gradmann and Hoffstadt 1998), Ca2+ signaling (see below), or plant hormone signaling (Hetherington 2001). The hydraulic architecture of leaves was discussed by Cochard et al. (2004) concluding that in some species, at least the flow resistance has a low-resistance, vein component as well as a high-resistance, mesophyll component. Models for different types of oscillations in plants have also been presented, based on coupling between ion transporters (Gradmann 2001).
Also whole plants can show synchronized transpiration: In several cases, rhythmic transpiration has been recorded from trees in the field or in controlled laboratories, as well as from bushes and whole plants like orange trees (Dzikiti et al. 2007; Steppe et al. 2006a), rose bushes (Rose et al. 1994), and tomato trees (Wallach et al. 2010). The existence of oscillations requires coupling between leaves and/or parts of the plant or the tree. Figure 7.1 can be used to illustrate the overall situation—the hydraulic coupling may exist via water potential in root , xylem, trunk, etc. If one regards the water “pool” in the plant to represent a hydraulic coupling between leaves (or likewise between branches/twigs in trees and bushes), it is evident that oscillations in one part of the plant can affect other parts and possibly extend the oscillatory tendency.
Models similar to the one in Fig. 7.1 have also been used to discuss transpiration oscillations in trees, then including water flux resistances etc. in trunks and branches (Zweifel et al. 2007). Cavitation or embolism in xylem vessels and refilling of cavities are relevant phenomena in estimates of flux resistances. The wood water content is important to measure and model, and methods for non-destructive measurements are developed (Hao et al. 2013). Tuzet et al. (2003) designed a model for plant canopies in comprising coupled stomatal conductance, photosynthesis, leaf energy balance, and transport of water through the soil–plant–atmosphere continuum. Stomatal conductance in the model depends on light, temperature, leaf water potentials, and intercellular CO2 concentration via photosynthesis.
It can be noticed that the period of the transpiration oscillations in whole plants seems to be roughly the same as in single leaf oscillations, possibly indicating that stomata parameters are more important for the period of oscillations than other time constants in the overall hydraulic systems.
2.3 Models with “Patchy” Stomatal Areas. Distributed Stomata Parameters
In the models discussed up to now, one has by and large regarded the stomata as individual but identical units which can be coupled in different ways. A further description can take into consideration that the network of stomata of a leaf can be “patchy,” a property denoting that small “patches” of stomata respond differently from those in adjacent regions of the leaf, for example, due to “patchy stomatal conductance” (Ferraz et al. 2009; Mott and Buckley 2000; Haefner et al. 1997; Cardon et al. 1994). The patchy behavior can also be found in patchy photosynthesis as demonstrated in recording of leaves (e.g., in Quercus crispula, Kamakura et al. 2012b; Siebke and Weis 1995)
Basically, it is possible to introduce patchiness in the models by using statistical models for the stomatal parameters. For example, distributions of conductance parameters can lead to patchy transpiration behavior, as demonstrated by, e.g., Takanashi et al. (2006), who test both bell-shaped and bimodal stomatal distributions, and Haefner et al. (1997). A general discussion on stomatal patchiness and network operation is presented by Mott and Peak (2007).
3 Rhythmic Cellular Processes in Guard Cells
3.1 Self-Sustained Cell Oscillations
Oscillatory patterns are ubiquitous in organisms and cells and cover a broad frequency range. In most cases, they also exemplify so-called endogenous, self-sustained, or inherent oscillations. This might not be a very surprising finding, since in every cell the many biochemical processes and control chains are often of a feedback type —apt to be oscillating under certain conditions.
In the present context, it is pertinent to mention some examples of oscillatory, cellular phenomena which are relevant for water transport into and out from cells—as is the case for the guard cells. In Fig. 7.1, such oscillatory reactions will be situated in the box denoted “guard cell.” They might thus control the volume of the guard cells and can, therefore, subsequently contribute or even cause rhythmic transpiration.
Oscillatory water transport in plant cells is fairly common, and one example is provided from the pulvinus tissue of some leaf stalks, e.g., in the plant Desmodium (Codariocalyx), but several others (Mimosa, etc.) show a corresponding reaction pattern. In the cylindrical structure of the Desmodium (review Johnsson et al. 2012), water of leaflet pulvini is pumped into and out of the cells in a regular manner, giving rise to short-period movements of small leaflets. They have an up and down or elliptical movement with a period of about 4 min (at roughly 25 °C) and can be observed easily by eye inspection. The rhythmic water transport is concomitant with K+ transport out from or into the cells, of H+ transport, of membrane potential changes, etc. (Engelmann and Antkowiak 1998; Johnsson et al. 2012).
Guard cells show many similar mechanisms and some oscillatory processes will be mentioned below (concentrating on Ca2+ oscillations). However, we will not go into details (see Chap. 8 of this volume)—in the present chapter, the important point is to emphasize that self-sustained oscillations in the guard cells might be coupled to rhythmic and measurable transpiration from the leaves.
3.2 Oscillations in Membrane Transport , Membrane Potentials
Oscillations in several plant membrane transport processes have been recorded and studied, as well as concomitant voltage oscillations (Shabala et al. 1997a, b, 2006). The presence of such cellular oscillations has been documented since long, but the more detailed knowledge has increased substantially in the last decades. This is true also for cell processes where oscillatory water transport into and out of cells plays an important mechanistic role, as, for example, in guard cells.
In an important paper, Shabala et al. (2006) model in detail a range of oscillatory membrane transport processes connected with the H+ pump of the plant cells (and with the K+ transport and several other cell transport mechanisms across the cell membranes ). The period of the rhythms depends on many parameters but is found in a window ranging from 30 s to 15 min. The existence of slower oscillations (1–2 h) was also reported. The existence of these oscillations shows up in a wide variety of cells of which the presence in guard cells is of particular interest here.
The electric events accompanying ion transport causes voltage oscillations. Shabala (1997) recorded bioelectric potentials from plant leaves in response to white light pulses and discovered maximum voltages when the period of the pulses was 30–40 min. This resonance was interpreted to be associated with stomatal cell reactions in the leaves. Similar resonance experiments on primary Avena leaves (but with sine wave white light signals) have revealed maximum transpiration response in the same period region (Brogårdh and Johnsson 1974b).
3.3 Self-Sustained Guard Cell Calcium Oscillations
Intense work has been focused on the role of cytosolic calcium, (Ca2+)cyt in volume changes of guard cells: Relevant for plant transpiration rhythms , Ca2+ oscillations have been recorded by different techniques (e.g., fluorescence spectroscopy) and are often found to show up as regularly occurring concentration spikes. The period of these oscillations is about 5–10 min in plants (e.g., Allen et al. 2000; McAinsh et al. 1995; Trewavas and Malhó 1998; and others) but Berridge and Galione (1988) lists periods between ca 0.3 and ca 120 min in animal cells.
(Ca2+)cyt oscillations are shown to be an information carrier in plant signal chains and Ca2+ is a signaling ion also in guard cells (reviews by Blatt 2000b; Yang et al. 2003, 2004, 2005; Schroeder et al. 2001; see also Chap. 9 of this volume and other references at the end of the present chapter). The oscillations can be an endogenous basis for self-sustained volume oscillations in the guard cells (Blatt 2000a, b; Li et al. 2004) and can, as mentioned above, be coupled to several physiological chains in the water regulatory system, including the overt transpiration oscillations .
In wild-type Arabidopsis, guard cells, abscisic acid , oxidative stress, cold, and external calcium create (Ca2+)cyt oscillations of differing amplitudes and frequencies and induce stomatal closure. However, in guard cells of the V-ATPase mutant det3, external calcium and oxidative stress elicited non-oscillating prolonged calcium increased and stomatal closure was prevented. Conversely, cold and abscisic acid elicited calcium oscillations in det3, and stomatal closure occurred normally; furthermore, in det3 guard cells, external calcium-induced oscillations rescued stomatal closure. The coupling between calcium oscillations and guard cell movements, and implicit possibilities to rhythmic transpiration, is thus strongly indicated (Allen et al. 2000).
In the scheme of Fig. 7.1, the (Ca2+)cyt oscillations would represent water potential changes in the block denoted “guard cells.” It is understood that such self-sustained oscillations in the water potential of the guard cells could interfere with simultaneous, hydraulically initiated rhythmic tendencies and interact or even drive the overall transpiration oscillations in the regulatory feedback loop. This can give rise to interference between two oscillating systems, as was partly explored by Prytz (2001). Complicated entrainment patters could arise from such a coupled two-oscillator system—dependent on relative frequency and strength of the oscillators.
The properties and mechanisms of aquaporins are of great interest in the water regulation (Finkelstein 2013; Maurel et al. 2008; Tyerman et al. 1999, 2002; Moshelion et al. 2014; Lopez et al. 2013) and are probably downstream elements of [Ca2+]i in the oscillation signaling (Chaumont and Tyerman 2014; Kaldenhoff and Fischer 2006, Yang et al. 2005). This involves also ion transports, e.g., the guard cell K+ transport—which in turn affects the membrane voltages.
3.4 Coupling Between Water Stress and Calcium Regulation; Abscisic Acid
The phytohormone abscisic acid, ABA, plays a central role in numerous growth and developmental processes in plants (the name originates from its discovery in leaf abscission processes). It is important in the water regulation and control as well and in adaptation to water stress. An overview of ABA action—synthesis and response—is published by Finkelstein (2013), and the field expands rapidly.
In particular, under water stress, the root system of plants produces ABA and the guard cells respond to the ABA transported to the leaves (thus a phytohormone; review Finkelstein 2013; Pantin et al. 2013; Kuromori et al. 2014). It has been shown that ABA added to the transpiration stream accumulates in the guard cell apoplast and causes stomatal closure in Vicia faba (Zhang and Outlaw 2001). ABA induced a rapid increase in (Ca2+)cyt and this preceded stomatal closure (e.g., in Commelina communis McAinsh et al. 1990, 1995). ABA perception and transduction in the guard cells as well as the ABA signaling pathway have been studied and partly identified (Cutler et al. 2010; Joshi-Saha et al. 2011a, b; Pandey et al. 2007; Finkelstein 2013). Both S-type and R-type anion channels are activated and massive K+ outflux is a result—sucrose transport is also involved (Talbott and Zeiger 1998). Subsequent changes in ψguard and guard cell volume occur as well as in other membrane transport processes involved. External Ca2+ likewise affects the (Ca2+)cyt
Features to be discussed in our context are, inter alia, the following ones:
-
ABA induces (Ca2+)cyt oscillations in the guard cells (involving, e.g., phosphoinositide -specific phospholipase C) (http://www.pnas.org/content/96/4/1779.short )
-
ABA sensitizes many transporters in the guard cells (review Kim et al. 2010). H+-ATPase is one of them and is controlled both by ABA and by light (Assman and Shimazaki 1999), see also Sect. 7.4.3.3.)
-
ABA affects anion channels and Ca2+ channels and is essential in the stomatal closure (e.g., Zhang and Outlaw 2001)
The pattern of ABA controlled mechanisms in guard cells is complicated and the signaling network in guard cell is intensely studied (for further references, see Finkelstein 2013; Pantin et al. 2013; Hills et al. 2012; Kim et al. 2010; Sirichandra et al. 2009; Li et al. 2006; Pei and Kuchitsu 2005);Hetherington 2001).
4 Some Experimental Findings on Transpiration Oscillations
The transpiration rhythms are usually characterized by their period, frequency content, amplitude, curve form, and stability. Models for transpiration oscillations should be able to predict the changes in these characteristics as affected by variations in external or internal variables.
4.1 Basic Experimental Methods to Study Rhythmic Transpiration
Only a short summary of some methods will be given here (for further information, see textbooks on water relations of plants, e.g., Weyers and Meidner 1990).
Measurements of the water uptake of a plant mirror the overall transpiration from the plant. The method was adopted already by Stephen Hales (1727), probably the first one to publish scientifically on the water relations of plants and trees. The amount of water taken up can be weighed out automatically; it can also be replenished and recorded automatically (e.g., Brogårdh and Johnsson 1973a), and rhythmic water uptake thus documented.
Rhythmic transport of water to leaves, branches, etc. Rhythmic variation of water flux in stems and trunks has been measured by heat pulse techniques (a heat pulse given to the xylem fluid can be detected at a given distance, and the time difference between sent and picked up signals allows the velocity to be calculated), as well as with other flux techniques, e.g., frequency domain reflectometry (see Hao et al. 2013). Time lags between transpiration and sap flow were recorded in pine by using the heat probe (Phillips et al. 1997). Stem diameter can be useful in recording both rapid and more slow oscillations in the water regulation (Steppe et al. 2006a, b; Barlow et al. 2010).
Rhythmic transpiration has been recorded by enclosing the unit to be investigated (typically a leaf or parts of it, or a whole plant or bush) in a container or a cuvette which allows a controlled air flow to pass the unit. Modern integrated sensors or commercial instruments can conveniently be used to record changes in humidity, CO2, temperature, composition in general of the incoming, and outgoing air. The transpiration, etc. of the object can then be calculated.
Gas analysis has been used to record the patterns of CO2 assimilation (Lawson and Weyers 1999). Photosynthesis assimilation rate has often been regarded as an indirect measure of the distribution of the stomatal conductance and therefore studied (e.g., Genty and Meyer 1994), but a simple relation between photosynthesis and conductance has been questioned (Jones 1998).
Stomatal conductance has been widely studied (see review by Pospisilova and Santrucek 1994) with direct microscopic observations of stomatal aperture (e.g., scanning EM: van Gardingen et al. 1989), vacuum infiltration of leaves by water (Beyschlag and Pfanz 1992) and porometer techniques.
Measurements of transpiration oscillations and of simultaneous oscillations in other parameters of a water feedback loop provide information on the dynamical characteristics of water storage elements in the plant. Excision of root system or leaves has been used to study immediate dynamic effects on transpiration or pulse responses, thus mapping parts of the control system (Powles et al. 2006).
Applying a pressure across the xylem vessels in the stem/stalk can increase the water flow resistance, thus affecting the transpiration oscillations . A device to compress the very base of a leaf and then excise the root system below the water level was used to study the transpiration rhythms of the primary Avena leaf (Brogårdh and Johnsson 1973b) without roots. The physical compression can replace the water flux resistance of the root with the consequence that transpiration oscillations can occur while being absent in excised leaves. Since the roots and parts of the transport vessels were removed, ions and molecules in the root medium could now enter the xylem vessels directly and reach the stomatal regions (see Sect. 7.4.4). The method can, therefore, be used to study solute and ion effects on the ongoing transpiration rhythms without root influences.
Other indirect methods have also been used to study transpiration rhythms. The fact that water evaporation requires heat (about 540 cal/g water) means that transpiration through stomatal openings can cause a temperature decrease in neighboring parts of the stomata. Infrared thermography has been used to study water relations of plants (e.g., Cohen et al. 2005; Kümmerlen et al. 1999; Jones 1999; Fuchs 1990) and temperature rhythms of leaf surfaces simultaneously with transpiration rhythms (see, e.g., Prytz et al. 2003a).
Detection of fluorescence is a powerful technique once suitable probes have been found. Variations in Ca2+ concentration can be recorded using the fluorophore fura-2. Fluorescence techniques allow also investigations of patchy behavior in leaves (e.g., Kamakura et al. 2012b).
An interesting method to record non-invasively water stress and its effects on leaf parameters is based on a magnetic patch clamp pressure technique (review Zimmermann et al. 2013) and allows light effects on leaves and turgor oscillations to be recorded in real time with high precision (period about 12 min sugarcane; see also Ache et al. 2010 reporting period about 30 min for Arabidopsis).
The term water use efficiency, WUE , is used to characterize the plants’ uptake of water relative to the biomass generated. Methods based on imaging techniques are used to record WUE continuously (e.g., McAusland et al. 2013). Then, the water transpiration can be estimated from thermography, simultaneously with optical detection of chlorophyll fluorescence as a measure of biomass production. The data achieved allow calculating and mapping of the assimilation rate, the stomatal conductance, and the WUE.
4.2 Occurrence of Transpiration Rhythms. Period of Rhythms
As mentioned earlier, transpiration oscillations are widely found in outdoor and laboratory experiments on a variety of species. The period varies with the experimental conditions. Reports often present data for just a few transpiration cycles, but in order to properly specify the period of the rhythm, recordings of a sufficient amount of cycles should be analyzed. Examples can be found where the oscillations have continued for several days without appreciable variation in duration of individual cycles (see, e.g., recordings in Johnsson 1973, showing transpiration oscillations between typically 30 and 10 mm3 h−1 in the primary leaf of young Avena plants). With these reservations in mind, the period values published for oscillations in light cluster around a few minutes, around 30–50 min, and around 2–3 h (see Barrs and Klepper 1968; Barrs 1971, etc.). Also in older plants, bushes, and trees, etc., the period seems to cluster around 1–3 h (e.g., tomato plant Wallach et al. 2010). The periods mentioned above qualify the oscillations to be termed ultradian .
Circadian transpiration rhythms have been recorded (see Sect. 7.5.1), i.e., rhythmic endogenous transpiration with a period close to but not exactly 24 h. An example is the transpiration rhythm in the tree Tamarix aphylla (L.) Karst. with a mean period of around 22 h in light (Hagemeyer and Waisel 1987). The rhythmic transpiration can be entrained to—“locked to” or “in phase with”—external rhythmic signals, e.g., light signals. Entrainment can occur in a so-called entrainment region in which the period of the transpiration rhythm is equal to the period of the external signal.
4.3 Some Environmental Parameters Influencing Oscillations
4.3.1 Water Potential Conditions (Including Water Potential of the Root Medium)
The transpiration rate must be sufficiently high for oscillations to arise, i.e., the transpiration stream, indicatively given by gs multiplied by the difference in water potential (ψstomata − ψair), should not be too low (e.g., with gs close to zero). Obviously, if the stomata stay fully opened or fully closed, oscillations would cease.
Oscillations have often been recorded under low environmental relative humidity (e.g., Hopmans 1971; Cowan 1972; Johnsson 1973; Caldeira et al. 2014). The nonlinearities of the stomatal conductivity play an important role for the existence and the properties of the rhythms . Period is lengthened by increased xylem resistance—as shown in experiments where the xylem has been physically compressed (Brogårdh and Johnsson 1973b). This is in agreement with the feedback models (the loop gain can be said to be increased).
A recent model for the stomatal responses to humidity has been proposed by Mott and Peak (2013) based on the water potential equilibrium between the guard cells and the air at the bottom of the stomatal pore. The situation is complicated since temperature (and thus irradiation) affects the relative humidity of the air (see below, Sect. 7.4.3.2.)
A change of the water potential of the root medium around an intact root system also changes the ψxylem and thus the transpiration pattern. Oscillations have been studied both by varying the water potential of the root medium and keeping it constant or by short-term changes of the root medium, so-called pulse treatments (Brogårdh et al. 1974; Avena). Resulting phase shifts of the transpiration oscillations were studied—only slight adjustments of the nonlinearities in Cowan’s model were necessary to describe the results successfully. A non-oscillating state could be induced by a suitable pulse treatment (see Sect. 7.5).
The way in which water potential changes and water deficit of the root/xylem system is communicated to the shoot and the guard cells is discussed in the literature (e.g., Christmann et al. 2007). It has been demonstrated that abscisic acid , ABA, is produced in response to water stress and mediates stomatal closure (see Chaps. 8 and 9 in the present volume). Even under non-stress conditions, ABA is produced in the leaves and is necessary for the guard cell regulation.
4.3.2 Ambient Temperature
Temperature influences the transpiration rhythms : The period of the oscillations increases when temperature is lowered (period about 140 min at 16 °C lowers to about 90 min at 26 °C for wheat, Klockare and Falk 1981, see also Hopmans 1971). Sudden changes in root temperature can induce transpiration oscillations and induce phase shifts (Hopmans 1971; Prytz 2001) which might be interpreted as a response to increased root resistance.
The coupling between temperature effects in the leaves and the direct temperature effects on the water potential in the air makes it difficult to carry out decisive experiments. Mott and Peak (2010) chose to study stomatal response to temperature and humidity in darkness. They conclude that stomatal response to humidity is the same in darkness and in light and thus independent of the processes that cause stomatal opening in light.
Pieruschka et al. (2010) have suggested that plant transpiration is due to the total radiation (including infrared radiation) and not to reactions only in the visible light regions (see section below). This would then include the temperature effects. The hypothesis is discussed by Mott and Peak (2011, 2013), concluding that the published findings can be due to mismeasurements of leaf temperature using thermocouples and that blue and red light reactions are not consistent with the hypothesis (see section on light below).
4.3.3 Light
Most studies of transpiration rhythms have been carried out in continuous “white” light. Review articles on the light action on the guard cells have been published (e.g., Shimazaki et al. 2007, focusing on the differences between blue and red light action on the stomata and their interaction in stomatal regulation; Chen et al. 2012a).
White light. In continuous white light, the period of the transpiration oscillations is sensitive to the irradiance level, being in general somewhat shorter at higher light intensities and longer at lower intensities (Avena: Klockare et al. 1978; Phaseolus: Hopmans 1971). When the irradiance was stepwise lowered, a major change in period occurred in Avena transpiration rhythms at about 0.2 mW cm−2 where the period changed from roughly 30 min to about 110 min (Klockare et al. 1978). This longer period was also encountered in darkness. The authors indicate that two different approaches have to be used to describe the oscillations with period about 30–40 min (irradiances about 5–10 Wm−2) and oscillations with period around 100–120 min (irradiances below about 1 Wm−2). Complicated transpiration patterns occurred in the intermediate irradiance region. Possibly, the complicated transpiration pattern and the non-constant period could then be the result of two separate, but interacting, oscillatory mechanisms.
Single, short white light pulses can change the amplitude as well as the phase of the oscillations (Avena; Johnsson 1973). Both phase delays and phase advances were obtained. The transpiration oscillations could also be halted by a suitable, single, light pulse treatment (Sect. 7.5.2). The transpiration response to repeated light pulses demonstrated an underlying circadian control component, period being around 27 h (Brogårdh 1975). The general pulse responses to “broadband red” and “broadband blue” light pulses were different and complex (op.cit.)
Time varying light signals have been administered to leaves to study the resonance frequencies of the transpiration oscillations . For analysis purposes, sine signals are preferred and Brogårdh and Johnsson (1974b) found for Avena that resonance and maximum amplitude occurred for light periods that were equal to the period reported for the self-sustained transpiration rhythms . Responses to repeated pulse sequences of white light were used in corresponding transpiration studies Brogårdh and Johnsson (1975a). Shabla (1997) and Shabala et al. (1997a, b) recorded electrical activity from leaf surfaces also using white light pulses with different periods, an amplitude maximum in the electrical potentials occurred at a light period of 30–40 min. A wide variety of species all showed roughly the same resonance period. This correspondence in resonance curves of course points to simultaneous electrical (guard cell) events and transpiration oscillations .
Blue light response. Hopmans (1973) found that the transpiration rhythm period in Phaseolus was longer in broadband blue light than in red light and he suggested that blue light decreased the water conductivity of guard cells. The response of guard cells and the stomatal apparatus to blue light is ascribed to the photoreceptor carotenoid (see, e.g., Paolicchi et al. 2005; Kinoshita et al. 2001; Assmann and Shimazaki 1999; Karlsson 1986a, b; Shimazaki et al. 2007), zeaxanthin, and to phot1 and phot2 proteins (Kinoshita et al. 2001). The phot1 protein acts on (Ca2+)cyt. The blue light pathway as well as the ABA signaling pathway are known both to affect H + -ATPase (Assman and Shimazaki 1999). Green light reversal of blue light-induced stomatal opening has been interpreted as a cycling of isomeric zeaxanthin (Talbott et al. 2002). Rhodopsin-like retinal proteins might also be involved (Paolicchi et al. 2005).
Red light. The red light response in the transpiration rhythm is mediated via guard cell photosynthesis by chlorophyll and does thus also depend on the CO2 regulation of stomata (Shimazaki et al. 2007). Data of Olsen et al. (2002) suggest that the guard cell chloroplasts react to reduced CO2 and activate stomatal opening through ion uptake, depending on photosynthetic electron transport with downstream components of the blue light transduction cascade.
As emphasized in several publications, the stomata opening and thus transpiration increase is more rapid in response to blue light than after red light administration (Shimazaki et al. 2007; see also Karlsson 1986a; Hopmans 1973)
Darkness. It is interesting that also in darkness, rhythmic transpiration occurs, so-called nocturnal oscillations both in C3 and C4 plants (see, e.g., Caird et al. 2007 and Sect. 7.4.3.4 below). Photosynthesis is absent in darkness but respiration changes in stomatal CO2 could provide an explanation as to the change in gs. However, Klockare and Falk (1981) provide data for the rhythm in Avena leaves (in darkness) at different O2 and CO2 concentrations in air and conclude that lowering the oxygen concentration to 5 % did not affect the existing oscillations. When the CO2 concentration was raised to 0.3 or 3 % in darkness, the transpiration rate temporarily decreased, but the period of the oscillations was not changed.
4.3.4 CO2
The CO2 concentration in the ambient air influences the transpiration rhythms (see, e.g., experimental results by Hopmans 1971). Not all hydraulic models do include this regulation loop explicitly but allow the water potential of the guard cell to be influenced by CO2 much in the same way as by light (a review of guard cell signal transduction network, including CO2 mechanisms, is given by Kim et al. 2010). Upadhyaya et al. (1983) developed a mathematical model for the CO2 effects on the stomatal dynamics and water-use efficiency. Furthermore, CO2 increases the guard cell (Ca2+)cyt, providing an interesting pathway to calcium oscillations (Webb et al. 1996 )
Tuzet et al. (2003) discuss a coupled model where stomatal conductance is fitted empirically to the CO2 in the intercellular spaces and derive daily transpiration and photosynthesis variations.
In darkness, respiration causes higher CO2 in the stomata and one would expect them to stay closed. However, as mentioned in Sect. 7.4.3.3, nocturnal transpiration is reported in several species (e.g., Blom-Zandstra et al. 1995; Rawson and Clarke 1988; Kavanagh et al. 2007); and Klockare and Falk (1981) found oscillatory transpiration in darkness.
4.4 Ionic and Solution Interference with Transpiration Oscillations
Intact plant roots can represent an efficient barrier toward many ions and molecules and prevent studies of ongoing transpiration rhythms . In Avena, no effect on transpiration oscillations was detected when adding, e.g., Mg2+, Ca2+ or La3+ to the medium of intact plants (Brogårdh and Johnsson 1975b). But when using excised leaves and xylem compression (Sect. 7.3), solutes and ions could enter the stomatal regions and the period of the transpiration rhythm was clearly lengthened. In some cases, an effect on period, amplitude, or curve form of the transpiration rhythm could be detected already in the first oscillatory cycle after administration of the ions.
Ca2+ (20 mM chloride salt), Mg2+ (40 mM), and La3+ (2.5 mM) given to the xylem all prolonged the period of the transpiration oscillations with a concurrent increase in the amplitude of the rhythms (Brogårdh and Johnsson 1975b). Effects were reversible at the concentrations used. This period lengthening was ascribed to either ionic effects outside/inside the guard cells, e.g., affecting the K+ transports, or to changes of the water permeabilities of the guard cells. Simulations using the Cowan models with decreased water permeability of the guard cell membranes caused the same type of changes as found experimentally. K+ and Na+ did not seem to affect the period under the same experimental conditions.
Lithium chloride salt—given permanently to intact plants—caused no period changes (control plants had a period of about 40 min). Applying a pulse of 80 mM LiCl to the cut end of excised leaves caused, however, a rapid increase (about 10 %) in the period of the oscillation—within the first cycle after onset of the pulse (Brogårdh and Johnsson 1974a). Li+ caused a period increase in a dose-dependent manner. The action of the lithium ions was interpreted to affect the ion-pumping mechanisms and could also be due to interference with calcium oscillations and calcium signaling pathways in guard cells. The experiments should, therefore, be repeated in studies on guard cell oscillations to see whether corresponding period and amplitude changes are experimentally found. The ion has been shown to act on glycogen synthase kinase-3 as well as on processes involving inositol metabolism with several relevant downstream targets (see Quiroz et al. 2004).
10 mM LiCl given to intact Tamarix plants caused no lengthening in the circadian transpiration rhythm (Hagemeyer and Waisel 1987), although lithium slows down circadian rhythms in general, acts on aquaporins in renal functions (Nielsen et al. 2002), and has a broad spectrum of action mechanisms. It might be that the ion could not enter the leaves in the Tamarix case (cf. Avena experiment mentioned in the previous paragraph).
The short information given above concerns experiments of fairly short duration, mostly of some hours only. The same is true of experiments in which solutions of theophylline, kinetin, or valinomycin have been administered to oscillating, excised Avena leaves. In several cases, the substances cause drastic period lengthening (Johnsson 1976), as does ATP . In Arabidopsis, it is reported (Tanaka et al. 2010) that extracellular ATP causes an increased cytosolic Ca2+ level in plants cells; in root hair cells, one could localize temporal Ca2+ oscillations presumably from the nucleotide treatment. Several other ions, e.g., H+, Cl−, are important in guard cell reactions (see, e.g., Yang et al. 2005), but the direct action on rhythmic transpiration remains to be studied.
5 Further Experimental Findings on Transpiration Oscillations
5.1 Modulation of Oscillation. Entrainment
The parameters in the hydraulic control system change in a daily manner under the L/D conditions: root resistance, mean level of gs, etc. are all varying throughout the day and night (Barrs and Klepper 1968; Hopmans 1971; Cowan 1972, see also Caldeira et al. 2014). These changes might then modulate the ultradian , short-period transpiration oscillations . Their amplitudes are reported to increase toward the end of the day but usually die out in the night.
The daily responses could be direct results of the L/D variations. However, the transpiration of plants also shows a circadian rhythm , due to the endogenous biological clocks . To be truly circadian, the period should then be close to but not exactly 24 h under constant environmental conditions (see classic review by Bünning 1973). Willmer and Fricker (1996) give some examples showing that also Arabidopsis exhibits circadian transpiration (see also Fig. 2 in Webb 1998). The tree Tamarix aphylla (L.) showed a circadian transpiration rhythm in light with period about 22 h (Hagemeyer and Waisel 1987). Brinker et al. (2001) indicate that stomatal aperture was under circadian control in the gymnosperm Ginkgo biloba.
A circadian modulation of many transpiration variables, including the rhythmic ultradian ones, can be carried out by the internal biological clock (also under constant experimental conditions). The transpiration response to light steps has, as an example, been shown to be modulated in a circadian fashion (see Sect. 7.4.3.3) The range of entrainment can be studied either by administering light at fixed frequencies in a series of experiments or by using swept frequency techniques and sweeping the external frequency slowly over the range of interest. This possibility has partly been explored by using sinusoidal white light signals to entrain the transpiration oscillations in different parts of the primary Avena leaf (Brogårdh and Johnsson 1974b) and studying, e.g., the coupling across parts of the leaf.
5.2 Singularities of Transpiration Rhythms ; Test of Models
A short-term perturbation of an oscillating biological system often results in a temporary deviation from its regular amplitude and curve shape. When the perturbation ceases, it might return to its original shape but then often showing changes in phase and amplitude.
Originally discussed for circadian rhythms , the question has been asked whether biological oscillators in general could be perturbed to reach so-called singularities or fixed points. An oscillator in such a state is characterized by a halt of the oscillating variables, and it has stopped oscillating—temporarily or permanently. This state might be obtained by perturbations. Winfree (1970) explored the possibilities to stop a circadian eclosion rhythm in Drosophila by administering blue light pulses of suitable irradiance and duration at a certain phase of the rhythm, and the results could be interpreted to show that the rhythm had indeed been stopped due to a precisely administered light pulse of correct amplitude.
Along this line of thought, white light pulse experiments given to the Avena transpiration rhythm demonstrated, firstly, the phase resetting ability of light and dark pulses. Secondly, it was shown that a suitable pulse or combination of pulses could in fact halt the transpiration rhythm, Fig. 7.2. The oscillatory system was not irreversibly damaged since a subsequent strong light pulse could initiate the transpiration rhythm again (Johnsson et al. 1979).
Light/dark perturbations stopping transpiration rhythms in Avena plants. In the upper recording, a combination of two light pulses stopped the transpiration rhythm at about 4 h. Arrows I, II, and III indicate white light perturbations with duration of 30 s, 2 min, and 10 min, respectively. The two first perturbations caused small oscillatory responses but only a strong enough perturbation, III, could start the rhythm anew. In the lower recording, the damped transpiration rhythm spontaneously showed an oscillatory tendency after about 5 h but returned into a standstill. A stronger light pulse started the oscillations again (redrawn after Johnsson et al. 1979)
The nature of this particular singularity seemed to be stable in some cases; in others, it was evidently unstable since the halted rhythm could spontaneously start to oscillate again (see Fig. 7.2). The transpiration oscillations could also be stopped transiently by osmotic pulses (−32 bars, 3 or 6 min; Johnsson et al. 1979). This indicated that the action of the light perturbations in the singularity experiments may have been mediated via osmotic perturbations of the water control system. Singularity experiments could be critical tests of models for the transpiration rhythms . The results achieved were not possible to simulate with Cowan’s model as published, but nonlinear features introduced between the transpiration rate and the stomatal conductance allowed successful simulations.
5.3 Period Doubling and Bifurcations in Transpiration—a Way to Chaos ?
Several “lumped” models for the water regulatory system include three dynamic variables or elements (chosen as, e.g., water content of the guard cells , the subsidiary cells, and the mesophyll cells; cf. Gumowski 1981, 1983). Coupled models have a correspondingly higher number of dynamic variables. A nonlinear model with three variables can show period-doubling and chaotic behavior (Strogatz 1994). Complicated, non-sinusoidal waveforms have been encountered experimentally (Johnsson 1976, Johnsson and Prytz 2002) and some efforts to model their shape have been published (Gumowski 1981, 1983). If time delays are introduced in the reactions, the overall model becomes even more apt to show oscillations.
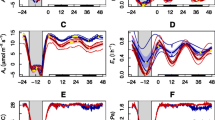
An example of period doubling in the water transpiration of the primary Avena leaf is given in Fig. 7.3, upper curve. One can conveniently introduce the concept of period-n oscillation (Strogatz 1994), characterized by a pattern repeating itself every n’th maximum of the transpiration. A period doubling is thus a period-2 oscillatory behavior.
Complex oscillatory transpiration patterns in Avena leaves (redrawn after Prytz et al. 2003a, b). Uppermost curve: Period-2 oscillations, triangles indicate maxima in rhythm (every second peak). A phase shift is induced by a pulse perturbation of 80 mM KCl to standard medium (middle of recording). Root excised, xylem compressed leaf. Middle curve: recording showing period-3 oscillations in intact plant. Filled circles indicate minima in rhythm (every third peak distinctly smaller than the rest of the peaks). Lowermost curves—to the left: period-3 oscillation. Triangles indicate maxima (every third peak) and pattern different from period-3 oscillation in the middle curve. 20 mM KCl added to medium. Root excised, xylem compressed leaf. Lowermost curves—to the right: recordings of a plant showing period-5 oscillations. Triangles indicate maxima. 20 mM KCl added to root medium. Root excised, xylem compressed leaf
The period doubling is but one of the many complicated oscillatory patterns that has been demonstrated in transpiration rhythms . The term bifurcation is used in the mathematical literature, and the phenomenon can be found in many nonlinear model systems at a critical value of the system’s control or bifurcation parameter. Period doubling was found in simulations of a mathematical model of the water regulatory system (for a description, see Johnsson and Prytz 2002) essentially based on the Cowan model. Period-doubling sequences have been reported in physical and chemical as well as in biological systems (see Lloyd 1997, Cvitanovic 1989 and references therein). However, studies of such oscillating systems are, to our knowledge, very few in plant physiology (a study by Shabala et al. 1997a, b is an exception, see below). Further increase in the bifurcation parameter may result in successive bifurcations giving period-4, period-8, period-16 oscillations, and so on. After an infinite number of bifurcations, the system becomes aperiodic or chaotic. The period-doubling phenomenon is often an indication of a system approaching such a chaotic state. In the chaotic region, period-3, period-5, period-6 oscillations, and others may exist.
Few studies have been performed to search for period-doubling sequences of the water control system. One attempt was made by Klockare et al. (1978) who varied light intensity (see Sect. 7.4.3.3). A rather abrupt transition in period from about 30 min to 110 min was encountered when irradiance was lower than about 0.2 mW/cm2. At the transition level, the transpiration rhythms showed irregular curve shapes and autocorrelation analysis indicated that both the periods mentioned could occur simultaneously. Shabala et al. (1997a, b) observed bifurcation and chaos in bioelectric leaf response to rhythmical light (experiments on Zea Mays)
Prytz et al. (2003b) demonstrated that the primary Avena leaf can show rhythms that are sometimes very complex (examples in Fig. 7.3). Thus, the overall transpiration system approaches under certain conditions chaotic behavior. These transpiration oscillations were recorded when the K+ concentration in the xylem medium was increased and used as a control parameter. It would be an elucidating step to look for possible simultaneous bifurcation patterns in transpiration rhythms and in calcium chain controlled rhythms in guard cells.
6 Remarks and Outlooks
This chapter has focussed on direct measurements of rhythmic transpiration of plants. The literature shows that oscillatory transpiration can exist in the regulatory feedback system in a wide variety of leaves of plants, including bushes and trees and plants with different guard cell anatomy. The hydraulic system comprises guard cells and their self-sustained volume changes and oscillatory reactions, e.g., the (Ca2+)cyt reactions, their ABA signaling, and the presence of ion pumps. These facets need to be extended and detailed in any models for the oscillating transpirations and so must the membrane water transport with relevant classes of aquaporins (see Sun et al. 2001; Tyerman et al. 2002). The aquaporins have up to now not been explicitly/quantitatively used in studies of rhythmic transpiration, but are frequent in plants and influence both root, stem, and leaf hydraulic conductivities (cf. Steudle 1997). Complex modeling is needed to approach a more complete picture of the water control system in plants, which also, of course, takes into account, for example, CO2 transport through stomata (reviews Kim et al. 2010 and Raschke 1979; Hubbard et al. 2012; Upadhyaya et al. 1983). Systems analysis and dynamic modeling already contribute constructively to the guard cell modeling (Chen et al. 2012b).
Details about the time constants involved in the molecular mechanisms to produce the (Ca2+)cyt oscillations are important (a simplified reaction scheme is shown by Yang et al. 2004). If ABA is controlling the (Ca2+)cyt oscillations and there is then a sequence of events leading to transpiration oscillations with certain characteristic period times—Which cellular constituents and reactions show the same period? And what phase relations exist between the constituents?
Perturbation experiments seem to be a fruitful way of studying the transpiration oscillations . If the transpiration rhythm is sent into a singularity as described in Sect. 7.5.2 and thus suddenly stopped, which other oscillations are stopped? Can, for example, the ABA concentration suddenly be halted by a suitable light pulse in such a way that the Ca2+ oscillations are stopped? How do models explain the pulse experiments where the oscillating transpiration is stopped?
Results in these areas will undoubtedly improve our models of the oscillatory transpiration of plants. So will also results from experiments focusing on the nature of the coupling between stomatal regions. And it would be interesting—but not an easy task—to establish whether the transpiration control system is really chaotic, as found, e.g., for the heart rhythm. Chaotic systems can use their sensitivity to stabilize a control by using small perturbations, thus increasing their flexibility and speed in response to varying environmental conditions (see, e.g., Shinbrot et al. 1993, Lloyd and Lloyd 1995 and others). This can be of much biological value in several contexts (Lloyd and Lloyd 1995; Strogatz 1994) and possibly also for the plants’ regulation of water transpiration (Souza et al. 2004).
We still have limited amount of data and limited number of oscillatory periods available for the transpiration oscillations —a drawback as compared with, e.g., the amount of data from the rhythmic human heart beat. The complex oscillatory patterns of the transpiration rhythms discussed in Sect. 7.5.3 might, however, be of interest and point in the direction of a possible chaotic underlying system.
The necessity of a rapid guard cell control system is also emphasized when looking at the structure of the irradiation onto leaves and stomata in the field—with rapid changes caused by shadow patterns from moving clouds, leaves, stems, and twigs. Optimizing the stomata control must certainly also involve a rapid control system.
The electric voltage changes which accompany the ion transports of the guard cells are concomitant variables of the oscillatory transpiration (see, e.g., Sects. 7.3.2 and 7.4.4). They could provide important further aspects on the simultaneous transpiration and guard cell rhythms (e.g., on patchiness). It might also be possible to stimulate the tissue by precise electric signals, precise both temporarily and spatially, to see whether voltage pulses influence the oscillations, for example, by phase shifting them. Corresponding experiments have been carried out on the rapid leaflet movement rhythm of Desmodium (review by Johnsson et al. 2012; early experiments by Bose 1913; Johnsson et al. 1993; Fostad et al. 1997; Sharma et al. 2001), where volume changes of pulvini cells are central elements in the movements . Electric direct current pulses phase shift these rhythmic leaflet movements, as do pulse exposures to magnetic (inhomogeneous) fields (Sharma et al. 2000).
We have touched upon some possible advantages that rapid control systems, probably chaotic ones, can show under several circumstances. Efforts to evaluate models on plant transpiration and transpiration rhythms can also imply another aspect, just mentioned in Sect. 7.4.1, namely the water-use efficiency, WUE. This relationship (or, simply, the ratio) between water transpiration and the CO2 absorption of the leaves (e.g., Cowan 1977) can reveal important practical aspects between necessary water loss to the atmosphere in relation to biomass production. A decrease in the stomatal pore size reduces the water transpiration as well as the CO2 absorption but the water transport reduction is proportionally the most affected one. The WUE (see review by Lawson and Blatt 2014) is therefore changing with the stomatal conductivity. Several approaches to calculate WUE for crops are used (e.g., Buckley et al. 1999), also starting from paradigm equations (see, e.g., Xu and Hsiao 2004), reaching useful predicted photosynthetic WUE (for cotton and sweet corn). The role of aquaporins is discussed in the literature in the frame of crop water-use efficiency (Moshelion et al. 2014).
It has been pointed out that the WUE can in fact increase during limited water supply if oscillatory behavior is present. This points at the beneficial nature of the oscillations (Upadhyaya et al. 1988; Lawson and Blatt 2014; Yang et al. 2004). If so, it is an extremely important work to penetrate the possibilities to increase the WUE of vegetation, thus reducing, for example, the crop need for water, but still with affordable, and hopefully increased, biomass production. The imaging technique by McAusland et al. (2013) to achieve intrinsic WUE has already been mentioned. Wang et al. (2014), using the OnGuard software (Hills et al. 2012), point at experimental modifications of dominant K+ channels as a favorable mean. However, other ways might also be relevant to follow and it seems reasonable to use a broad research design to achieve more efficient crops. Data must be available for crops and vegetation (e.g., water use of trees—some data in Wullschleger et al. 1998—and drought tolerance in Arabidopsis, see Nilson and Assmann 2007).
The oscillatory transpiration of plants reveals much of the dynamics of an important biological control system. As has been pointed out (Cowan 1977), the stomatal system provides more directly than any other aspect of the functions of plants a way to understand the dilemma of land plants, viz. to accumulate carbon without excessive loss of water. Detailed experimental studies of these oscillations provide a way to increased knowledge of some of the most basic and complex sensory and control systems in plants.
References
Ache P, Bauer H, Kollist H, Al-Rasheid KAS, Lautner S, Hartung W, Hedrich R (2010) Stomatal action directly feeds back on leaf turgor: new insights into the regulation of the plant water status from non-invasive pressure probe measurements. Plant J 62:1072–1082
Allen GJ, Chu SP, Schumacher K, Shimazaki CT, Vafeados D, Kemper A, Hawke SD, Tallman G, Tsien RY, Harper JF, Chory J, Schoeder JI (2000) Alteration of stimulus-specific guard cell calcium oscillations and stomatal closing in Arabidopsis det3 mutant. Science 289:2338–2342
Assmann SM, Shimazaki K (1999) The multisensory guard cell. Stomatal responses to blue light and abscisic acid. Plant Physiol 119(3):809–816
Barlow PW, Mikulecký M Sr, Strestík J (2010) Tree-stem diameter fluctuates with the lunar tides and perhaps with geomagnetic activity. Protoplasma 247:25–43
Barrs HD (1971) Cyclic variations in stomatal aperture, transpiration and leaf water potential under constant environmental conditions. Annu Rev Plant Physiol 22:223–236
Barrs HD, Klepper B (1968) Cyclic variations in plant properties under constant environmental conditions. Physiol Plant 21:711–730
Beer C et al (2010) Terrestrial gross carbon dioxide uptake: global distribution and covariation with climate. Science 329:834–838
Berridge MJ, Galione A (1988) Cytosolic calcium oscillators. FASEB J 2:3074–3082
Beyschlag W, Pfanz H (1992) A fast method to detect the occurrence of nonhomogeneous distribution of stomatal aperture in heterobaric plant leaves. Experiments with Arbutus unedo L. during diurnal course. Oecologia 82:52–55
Blatt MR (2000a) Ca2+ signalling and control of guard-cell volume in stomatal movements. Curr Opin Plant Biol 3:196–204
Blatt MR (2000b) Cellular signaling and volume control in stomatal movements in plants. Annu Rev Cell Dev Biol 16:221–241
Blom-Zandstra M, Pot CS, Maas FM, Schapendonk Ad HCM (1995) Effects of different light treatment on the nocturnal transpiration and dynamics of two Rose cultivars. Sci Horticul 61(3–4):251–262
Bose JC (1913) Researches on the irritability of plants. Longmans, Green & Co. London, NY, Bombay, Calcutta
Brinker M, Engelmann W, Kellmann JW, Piechulla B (2001) Circadian rhythms of leaf and stomatal movements in gymnosperm species. Biol Rhythm Res 32:471–478
Brogårdh T (1975) Regulation of transpiration in Avena. Responses to red and blue light steps. Physiol Plant 35:303–309
Brogårdh T, Johnsson A (1973a) A flexible apparatus for continuous recording of water uptake by plants. Med Biol Engn Comput 11(3):286–292
Brogårdh T, Johnsson A (1973b) Oscillatory transpiration and water uptake of Avena plants. II. Effects of deformation of xylem vessels. Physiol Plant 28:341–345
Brogårdh T, Johnsson A (1974a) Effects of lithium on stomatal regulation. Z Naturforsch 29c:298–300
Brogårdh T, Johnsson A (1974b) Oscillatory transpiration and water uptake of Avena plants. IV. Transpiratory response to sine shaped light cycles. Physiol Plant 31:311–322
Brogårdh T, Johnsson A (1975a) Regulation of transpiration in Avena. Responses to white light steps. Physiol Plant 35:115–125
Brogårdh T, Johnsson A (1975b) Effects of magnesium, calcium and lanthanum ions on stomatal oscillations in Avena sativa L. Planta 124:99–103
Brogårdh T, Johnsson A, Klockare R (1974) Oscillatory transpiration and water uptake of Avena plants. V. Influence of the water potential of the root medium. Physiol Plant 32:258–267
Buckley TN, Farquhar GD, Mott KA (1999) Carbon-water balance and patchy stomatal conductance. Oecologia 118:132–143
Bünning E (1973) The physiological clock, 3rd edn. Springer, New York
Caird MA, Richards JH, Donovan LA (2007) Nighttime stomatal conductance and transpiration in C3 and C4 plants. Plant Physiol 143:4–10
Caldeira CF, Jeanguenin L, Chaumont F, Tardieu F (2014) Circadian rhythms of hydraulic conductance and growth are enhanced by drought and improve plant performance. Nat Commun 5:5365. doi:10.1038/ncomms6365
Cardon ZG, Mott KA, Berry JA (1994) Dynamics of patch stomatal movements and their contribution to steady state and oscillating stomatal conductance calculated using gas-exchange techniques. Plant Cell Environ 17:995–1007
Chaumont F, Tyerman SD (2014) Aquaporins: highly regulated channels controlling plant water relations. Plant Physiol 164:1600–1618
Chen C, Xiao YG, Li X, Ni M (2012a) Light-regulated stomatal aperture in Arabidospsis. Mol Plant 5:566–572
Chen ZH, Hills A, Bätz U, Amtmann A, Lew VL, Blatt MR (2012b) Systems dynamics modeling of the stomatal guard cell predicts emergent behaviors in transport, signaling, and volume control. Plant Physiol 159:1235–1251
Christmann A, Weiler EW, Steudle E, Grill E (2007) A hydraulic signal in root-to-shoot signalling of water shortage. Plant J 52:167–174
Cochard H, Nardini A, Coll L (2004) Hydraulic architecture of leaf blades: where is the main resistance? Plant Cell Environ 27:1257–1267
Cohen Y, Alchanatis V, Meron M, Saranga Y, Tsipris J (2005) Estimation of leaf water potential by thermal imagery and spatial analysis. J Exp Bot 56:1843–1852
Cowan IR (1972) Oscillations in stomatal and plant functioning associated with stomatal conductance. Observations and a model. Planta 106:185–219
Cowan IR (1977) Stomatal behaviour and environment. Adv Bot Res 4:117–229
Cutler SR, Rodriquez PL, Finkelstein RR, Abrams SR (2010) Abscisic acid: emergence of a core signaling network. Annu Rev Plant Biol 61:651–679
Cvitanovic P (1989) Universality in chaos. IOP, Bristol
Damour G, Simonneau TH, Cochard H, Urban L (2010) An overview of models of stomatal conductance at the leaf level. Plant Cell Environ 33:1419–1438
Delwiche MJ, Cooke JR (1977) An analytical model of the hydraulic aspects of stomatal dynamics. J Theor Biol 69:113–141
Dzikiti S, Steppe K, Lemeur R, Milford JR (2007) Whole-tree level water balance and its implications on stomatal oscillations in orange trees (Citrus sinensis (L.) Osbeck) under natural climatic conditions. J Exp Bot 58(7):1893–1901
Engelmann W, Antkowiak B (1998) Ultradian rhythms in Desmodium (Minireview). Chronobiol Int 15:293–307
Ferraz MC, Souza GM, Prado CPC (2009) A model for patchy stomatal conductance in homobaric leaves. J Comput Interdisc Sci 1:83–88
Finkelstein R (2013) The Arabidopsis book. American Soc Plant Biologists (p 36). doi:10.1199/tab.0166
Fostad O, Johnsson A, Engelmann W (1997) Effects of electrical currents on Desmodium gyrans leaflet movements. Experiments using a currrent clamp technique. Biol Rhythm Res 28:244–259
Fuchs M (1990) Infrared measurement of canopy temperature and detection of plant water stress. Theor Appl Climatol 42:253–261
Genty B, Meyer S (1994) Quantitative mapping of leaf photosynthesis using chlorophyll fluorescence imaging. Austr J Plant Phys 22:277–284
Goldenfeld N, Kadanoff LP (1999) Simple lessons from complexity. Science 284:87–89
Gradmann D (2001) Models for oscillations in plants. Austr J Plant Physiol 28(7):577–590
Gradmann D, Buschmann P (1996) Electrocoupling causes oscillations of ion transport in plants. In: Greppin H, Degli Agosti R, Bonzon M (eds) Vistas on biorhythmicity. University of Geneva, pp 239–268
Gradmann D, Hoffstadt J (1998) Electrocoupling of ion transporters in plants; interaction with internal ion concentrations. J Membr Biol 166(1):51–59
Gumowski I (1981) Analysis of oscillatory plant transpiration. J Interdiscipl Cycle Res 12:273–291
Gumowski I (1983) Analysis of oscillatory plant transpiration II. J Interdiscipl Cycle Res 14:33–41
Haefner JW, Buckley TN, Mott KA (1997) A spatially explicit model of patchy stomatal responses to humidity. Plant Cell Environ 20:1087–1097
Hagemeyer J, Waisel Y (1987) An endogenous circadian rhythm of transpiration in Tamarix aphylla. Physiol Plant 70:133–138
Hales S (1727) Vegetable staticks. W and J Innys, London
Hao GY, Wheeler JK, Holbrook NM, Goldstein G (2013) Investigating xylem embolism formation, refilling and water storage in tree trunks using frequency domain reflectometry. J Exp Bot 64(8):2321–2332
Hetherington AM (2001) Guard cell signaling. Cell 107:711–714
Hills A, Chen ZH, Amtmann A, Blatt MR, Lew VI (2012) OnGuard, a computational platform for quantitative kinetic modeling of guard cell physiology. Plant Physiol 159:1026–1042
Hopmans PAM (1971) Rhythms in stomatal opening of bean leaves. Commun Agric Univ Wageningen 71:1–86
Hopmans PAM (1973) Effect of blue and red radiation on cycling stomata of bean. Neth J Agric Sci 21:117–123
Hubbard KE, Siegel RS, Valerio G, Brandt B, Schoeder JI (2012) Abscisic acid and CO2 ignalling via calcium sensitivity priming in guard cells, new CDPK mutant phenotypes and a method for improved resolution of stomatal stimulus-response analyses. Ann Bot 109:5–17
Jasechko S, Sharp ZD, Gibson JJ, Birks SJ, Yi Y, Fawcett PJ (2013) Terrestrial water fluxes dominated by transpiration. Nature 496:347–350
Johnsson A (1973) Oscillatory transpiration and water uptake of Avena plants. I. Preliminary observations. Physiol Plant 28:40–50
Johnsson A (1976) Oscillatory water regulation in plants. Bull Inst Math Appl 12:22–26
Johnsson A, Prytz G (2002) Period doubling of rhythmic water regulation in plants. In: Kumar V (ed) Biological rhythms. Narosa, Berlin, pp 38–45
Johnsson A, Brogårdh T, Holje Ø (1979) Oscillatory transpiration of Avena plants: perturbation experiments provide evidence for a stable point of singularity. Physiol Plant 45:393–398
Johnsson A, Bostrøm AC, Pedersen M (1993) Perturbation of the Desmodium leflet oscillation by electric current pulses. J Interdisc Cycle Res 24:17–32
Johnsson A, Sharma VK, Engelmann W (2012) The telegraph plant: codariocalyx motorius (formerly also Desmodium gyrans). In: Volkov AG (ed) Plant electrophysiology. Springer, Heidelberg, pp 85–123 (chap 4)
Jones HG (1998) Stomatal control of photosynthesis and transpiration. J Exp Bot 49:387–398
Jones HG (1999) Use of thermography for quantitative studies of spatial and temporal variation of stomatal conductance over leaf surfaces. Plant Cell Environ 22:1043–1055
Joshi-Saha A, Valon C, Leung J (2011a) A brand new START: Abscisic acid perception and transduction in the guard cell. Sci signal 4:re4
Joshi-Saha A, Valon C, Leung J (2011b) Abscisic acid signal off the STARTing block. Mol Plant 4(4):562–580
Kaiser H, Kappen L (2001) Stomatal oscillation at small apertures: indications for a fundamental insufficiency of stomatal feedback-control inherent in the stomatal turgor mechanism. J Exp Bot 52:1303–1313
Kaiser H, Paoletti E (2014) Dynamical stomatal changes. In: M Tausz, N Grulke (eds) Trees in a changing environment, Plant Ecophysiol, vol 9. Springer (chap 4), Dordrecht, pp 61–82
Kaldenhoff R, Fischer M (2006) Aquaporins in plants. Acta Physiol (Oxf) 187:169–176
Kamakura M, Kosugi Y, Muramatsu K, Muraoka H (2012a) Simulations and observations of patchy stomatal behavior in leaves of Quercus crispula, a cool-temperate deciduous broad-leaved tree species. J Plant Res 125:339–349
Kamakura M, Kosugi Y, Takanashi S, Tobita H, Uemura A, Utsugi H (2012b) Observation of the scale of patchy stomatal behavior in leaves of Quercus crispula using an imaging-PAM chlorophyll fluorometer. Tree Physiol 32:839–846
Karlsson PE (1986a) Blue light regulation of stomata in wheat seedlings. I. Influence of red background illumination and initial conductance level. Phys Plant 66(2):202–206
Karlsson PE (1986b) Blue light regulation of stomata in wheat seedlings. II. Action spectrum and search for action dichroism. Phys Plant 66(2):207–210
Kavanagh KL, Pangle R, Schotzko AD (2007) Nocturnal transpiration causing disequilibrium between soil and stem predawn water potential in mixed conifer forests of Idaho. Tree Physiol 27:621–629
Kim T-H, Böhmer M, Hu H, Nishimura N, Schroeder JI (2010) Guard cell signal transduction network: advances in understanding abscisic acid, CO2, and Ca2+ signaling. Annu Rev Plant Biol 61:561–591
Kinoshita T, Doi M, Suetsugu N, Kagawa T, Wada M, Shimazaki K (2001) Phot1 and phot2 mediate blue light regulation of stomatal opening. Nature 414:656–660
Klockare R, Falk SO (1981) Influence of O2− and CO2 concentrations on oscillations in the transpiration rate from oat plants in darkness. Physiol Plant 52:83–88
Klockare R, Johnsson A, Brogårdh T, Hellgren M (1978) Oscillatory transpiration and water uptake of Avena plants. VI. Influence of the irradiation level. Physiol Plant 42:379–386
Kramer PJ, Boyer JS (1995) Water relations of plants and soils. Academic Press, San Diego
Kümmerlen B, Dauwe S, Schmundt D, Schurr U (1999) Thermography to measure water relations of plant leaves. Handbook of computer vision and applications, vol 3. Academic Press, London, pp 763–781
Kuromori T, Sugimoto E, Shinozaki K (2014) Intertissue signal transfer of abscisic acid from vascular cells to guard cells. Plant Physiol 164:1587–1592
Lawson T, Blatt MR (2014) Stomatal size, speed, and responsiveness impact on photosynthesis and water use efficiency. Plant Physiol 164:1556–1570
Lawson T, Weyers J (1999) Spatial and temporal variation in gas exchange over the lower surface of Phaseolus vulgaris L. primary leaves. J Exp Bot 50:1381–1391
Li Y, Wang GX, Xin M, Yang HM, Wu XJ, Li T (2004) The parameters of guard cell calcium oscillation encode stomatal oscillation and closure in Vicia faba. Plant Sci 166:415–421
Li S, Assnmann S, Albert R (2006) Predicting essential components of signal transduction networks: a dynamic model of guard cell abscisic acid signaling. PlosBiol 4:e312
Lloyd D (1997) Chaos and ultradian rhythms. Biol Rhythm Res 28:134–143
Lloyd AL, Lloyd D (1995) Chaos: its significance and detection in biology. Biol Rhythm Res 26:233–252
Lopez F, Bousser A, Sissoëff I, Hoarau J, Mahé A (2004) Characterization in maize of ZmTIP2–3, a root-specific tonoplast intrinsic protein exhibiting aquaporin activity. J Exp Bot 55:539–541
Lopez D, Venisse J-S, Fumanal B, Chaumont F, Guillot E, Daniels MJ, Cochard H, Julien J-L, Gousset-Dupont A (2013) Aquaporins and leaf hydraulics: poplar sheds new light. Plant Cell Physiol 54(12):1963–1975
Marenco RA, Siebke K, Farquhar GD, Ball MC (2006) Hydraulically based stomatal oscillations and stomatal patchiness in Gossypium hirsutum. Funct Plant Biol 33:1103–1113
Maurel C, Verdoucq L, Luu D-T, Santoni V (2008) Plant aquaporins: membrane channels with multiple integrated functions. Annu Rev Plant Biol 59:595–624
McAinsh MR, Brownlee C, Hetherington AM (1990) Abscisic acid-induced elevation of guard cell cytosolic Ca2+ precedes stomatal closure. Nature 343:186–188
McAinsh MR, Webb AAR, Taylor JE, Hetherington AM (1995) Stimulus-induced oscillations in guard cell cytosolic free calcium. Plant Cell 7:1207–1219
McAusland L, Davey PA, Kanwal N, Baker NR, Lawson T (2013) A novel system for spatial and temporal imaging of intrinsic plant water use efficiency. J Exp Bot 64:4993–5007
Moshelion M, Halperin O, Wallach R, Oren R, Way DA (2014) Role of aquaporins in determining transpiration and photosynthesis in water-stressed plants: crop water-use efficiency, growth and yield. Plant Cell Environ. doi:10.1111/pce.12410
Mott KA, Buckley TN (2000) Patchy stomatal conductance: emergent collective behavior of stomata. Trends Plant Sci 5:258–262
Mott KA, Peak D (2007) Stomatal patchiness and task-performing networks. Ann Bot 99:219–226
Mott KA, Peak D (2010) Stomatal responses to humidity and temperature in darkness. Plant Cell Environ 33:1084–1090
Mott KA, Peak D (2011) Alternative perspective on the control of transpiration by radiation. Proc Natl Acad Sci USA 108:19820–19823
Mott KA, Peak D (2013) Testing a vapour-phase model of stomatal responses to humidity. Plant Cell Environ 36:936–944
Mott KA, Shope JC, Buckley TN (1999) Effects of humidity on light-induced stomatal opening: evidence for hydraulic coupling among stomata. J Exp Bot 50:1207–1213
Nielsen S, Frøkiaer J, Marples D, Kwan TH, Agre P, Knepper MA (2002) Aquaporins in the kidney: from molecules to medicine. Physiol Rev 82(1):205–244
Nilson SE, Assmann SM (2007) The control of transpiration. Insights from Arabidopsis. Plant Physiol 143:19–27
Nobel PS (1999) Physicochemical and environmental plant physiology. Academic Press, London. ISBN 0-12-520025-0
Olsen RL, Pratt RB, Gump P, Kemper A, Tallman G (2002) Red light activates a chloroplast-dependent ion uptake mechanism for stomatal opening under reduced CO2 concentrations in Vicia spp. New Phytol 153:497–508
Pandey S, Zhang W, Assman SM (2007) Roles of ion channels and transporters in guard cell signal transduction. FEBS Lett 581:2325–2336
Pantin F, Monnet F, Jannaud D, Costa JM, Renaud J, Muller B, Simonneau T, Genty B (2013) The dual effect of abscisic acid on stomata. New Phytol 197:65–72
Paolicchi F, Lombardi L, Ceccarelli N, Lorenzi R (2005) Are retinal and retinal-binding proteins involved in stomatal response to blue light? Funct Plant Biol 32:1135–1141
Pei Z-M, Kuchitsu K (2005) Early ABA signaling events in guard cells. J Plant Growth Regul 24:296–307
Phillips NP, Nagehaudhuri A, Oren R, Natul G (1997) Time constant for water transport in loblolly pine trees estimated from time series of evaporative demand and stem sapflow. Trees 11:412–419
Pieruschka R, Huber G, Berry JA (2010) Control of transpiration by radiation. Proc Natl Acad Sci USA 107(30):13372–13377
Pospisilova J, Santrucek J (1994) Stomatal patchiness. Biol Plantarum 36:481–510
Powles JE, Buckley TN, Nicotra AB, Farquhar GD (2006) Dynamics of stomatal water relations following leaf excision. Plant Cell Environ 29:981–992
Prytz G (2001) A biophysical study of oscillatory water regulation in plants. Measurements and models. PhD Thesis, Norwegian University of Science and Technology, Trondheim
Prytz G, Johnsson A, Futsaether CM (2003a) Thermography studies of the spatial and temporal variability in stomatal conductance of Avena leaves during stable and oscillatory transpiration. New Phytol 158:249–258
Prytz G, Futsæther C, Johnsson A (2003b) Self-sustained oscillations in plant water regulation: induction of bifurcations and anomalous rhythmicity. New Phytol 158:259–267
Quiroz JA, Gould TD, Manji H (2004) Molecular effects of lithium. Mol Interv 4:259–272
Ramos AMT, Prado CPC (2013) Role of hysteresis in stomatal aperture dynamics. Phys Rev E Stat Nonlin Soft Matter Phys 87(012):719
Rand RH, Storti DW, Upadhyaya SK, Cooke JR (1982) Dynamics of coupled stomatal oscillators. J Math Biol 15:131–149
Raschke K (1979) Movements of stomata. In: Haupt W, Feinlieb WE (eds) Encyclopedia of plant physiology, vol 7. Springer, Heidelberg/New York, pp 15–29
Rawson HM, Clarke JM (1988) Nocturnal transpiration in wheat. Austr J Plant Physiol 15(3):397–406
Rose MA, Beatti DJ, White JW (1994) Oscillations of whole-plant transpiration in ‘Moolight’ rose. J Amer Soc Hort Sci 119(3):439–445
Sack L, Holbrook NM (2006) Leaf hydraulics. Annu Rev Plant Biol 57:361–381
Schroeder JI, Allen GJ, Hugouvieux V, Kwak JM, Waner D (2001) Guard cell signal transduction. Annu Rev Plant Physiol Plant Mol Biol 52:627–658
Shabala SN (1997) Leaf bioelectric responses to rhythmical light: identification of the contributions from stomatal and mesophyll cells. Austr J Plant Phys 24:741–749
Shabala S, Delbourgo R, Newman I (1997a) Observations of bifurcation and chaos in plant physiological responses to light. Austr J Plant Physiol 24:91–96
Shabala S, Newman IA, Morris J (1997b) Oscillations in H+ and Ca2+ fluxes around the elongation region of corn roots and effects of external pH. Plant Physiol 113:111–118
Shabala S, Shabala L, Gradmann D, Chen Z, Newman I, Mancuso S (2006) Oscillations in plant membrane transport: model predictions, experimental validation, and physiological implications. J Exp Bot 57(1):171–184
Sharma VK, Engelmann W, Johnsson A (2000) Effect of static magnetic field on the ultradian lateral leaflet movement rhythm in Desmodium gyrans. Z Naturf 55c:638–642
Sharma VK, Jensen C, Johnsson A (2001) Phase response curve for the ultradian rhythm of the lateral leaflets of Desmodium gyrans using DC current Pulses. Z Naturforsch 56c:77–81
Shimazaki K-I, Doi M, Assman SM, Kinoshita T et al (2007) Light regulation of stomatal movements. Annu Rev Plant Biol 58:219–247
Shinbrot T, Grebogi C, Ott E, Yorke J (1993) Using small perturbations to control chaos. Nature 363:411–417
Siebke K, Weis E (1995) Assimilation images of leaves of Glechoma hederacea: analysis of nonsynchronous stomata related oscillations. Planta 196:155–165
Sirichandra C, Wasilewska A, Vlad F, Valon C, Leung J (2009) The guard cell as a single-cell model towards understanding drought tolerance and abscisic acid action. J Exp Bot 60(5):1439–1463
Souza GM, de Oloviera RF, Cardoso VJM (2004) Temporal dynamics of stomatal conductance of plants under water deficit: can homeostasis be improved by more complex dynamics? Brazilian Arch Biol Techn 47(3):423–431
Steppe K, Dzikiti S, Lemeur R, Milford JR (2006a) Stomatal oscillations in orange trees under natural climatic conditions. Ann Bot 97:831–835
Steppe K, de Pauw DJW, Lemeur R, Vanrolleghem PA (2006b) A mathematical model linking tree sap flow dynamics to daily stem diameter fluctuations and radial stem growth. Tree Physiol 26:257–273
Steudle E (1997) Water transport across plant tissue: role of water channels. Biol Cell 89:259–273
Strogatz SH (1994) Nonlinear dynamics and chaos. Addison-Wesley, München
Sun MH, Xu W, Zhu YF, Su W, Tang ZC (2001) A simple method for in situ hybridization to RNA in guard cells of Vicia faba L.: the expression of aquaporins in guard cells. Plant Mol Biol Rep 19:129–135
Takanashi S, Kosugi Y, Matsuo N, Tani M, Ohte N (2006) Patchy stomatal behavior in broad-leaved trees grown in different habitats. Tree Physiol 26:1565–1578
Talbott LD, Zeiger E (1998) The role of sucrose in guard cell osmoregulation. J Exp Bot 49:329–337
Talbott LD, Nikolova G, Ortiz A, Shmayevich I, Zeiger E (2002) Green light reversal of blue- light-stimulated stomatal opening is found in a diversity of plant species. Am J Bot 89:366–368
Tanaka K, Swanson SJ, Gilroy S, Stacey G (2010) Extracellular nucleotides elicit cytosolic free calcium oscillations in Arabidopsis. Plant Physiol 154:705–719
Trewavas AJ, Malhó R (1998) Ca2+ signalling in plant cells: the big network! Curr Opin Plant Biol 1:428–433
Tuzet A, Perrier A, Lkeuning R (2003) A coupled model of stomatal conductance, photosynthesis and transpiration. Plant Cell Environ 26:1097–1116
Tyerman SD, Bohnert HJ, Maurel C, Steudle E, Smith JAC (1999) Plant aquaporins: their molecular biology, biophysics and significance for plant water relations. J Exp Bot 50:1055–1071
Tyerman SD, Niermietz CM, Bramley H (2002) Plant aquaporins: multifunctional water and solute channels with expanding roles. Plant Cell Environ 25:173–194
Upadhyaya SK, Rand RH, Cooke JR (1983) A mathematical model of the effects of CO2 on stomatal dynamics. J Theor Biol 101:415–440
Upadhyaya SK, Rand RH, Cooke JR (1988) Role of stomatal oscillations on transpiration, assimilation and water use efficiency of plants. Ecol Model 41:27–40
van Gardingen PR, Jeffree CE, Grace J (1989) Variation in stomatal aperture in leaves of Avena fatua L. observed by low-temperature scanning electron microscopy. Plant Cell Environ 12:887–888
Wallach R, Da-Costa N, Raviv M, Moshelion M (2010) Development of synchronized, autonomous, and self-regulated oscillations in transpiration rate of whole tomato plant under water stress. J Exp Bot 61(12):3439–3449
Wang Y, Hills A, Blatt MR (2014) Systems analysis of guard cell membrane transport for enhanced stomatal dynamics and water use efficiency. Plant Physiol 164:1593–1599
Webb AAR (1998) Stomatal rhythms. In: Lumsden PJ, Millart AJ (eds) Biological rhythms and photoperiodism in plants. BIOS, Washington DC, pp 69–79
Webb AAR, McAinsh MR, Mansfield TA, Hetherington AM (1996) Carbon dioxide induces increases in guard cell cytosolic free calcium. Plant J 9:297–304
Weyers JDB, Meidner H (1990) Metods of stomatal research. Longman Scientific and Technical, Harlow
Willmer C, Fricker M (1996) Stomata. Chapman & Hall, London
Winfree AT (1970) Integrated view of resetting a circadian clock. J Theor Biol 28:327–374
Wullschleger SD, Meinzer FC, Vertessy RA (1998) A review of whole-plant water use studies in trees. Tree Physiol 18:499–512
Xu L-K, Hsiao TC (2004) Predicted versus measured photosynthetic water-use efficiency of crop stands under dynamically changing field environments. J Exp Bot 55(407):2395–2411
Yang HM, Zhang XY, Wang GX, Li Y, Wei XP (2003) Cytosolic calcium oscillation may induce stomatal oscillation in Vicia faba. Plant Sci 165:1117–1122
Yang HM, Zhang XY, Wang GX (2004) Cytosolic calcium oscillation signaling in guard cell. Plant Sci 166:549–556
Yang HM, Zhang JH, Zhang XY (2005) Regulation mechanisms of stomatal oscillations. J Integr Plant Biol 47:1159–1172
Zhang SQ, Outlaw SH (2001) Abscisic acid introduced into the transpiration stream accumulates in the guard-cell apoplast and causes stomatal closure. Plant Cell Environ 24(10):1045–1054
Zimmermann U, Bitter R, Marchiori PER, Rüger S, Ehrenberger W, Sukhorukov VL, Schüttler A, Ribeiro RV (2013) A non-invasive plant-based probe for continuous monitoring of water stress in real time: a new tool for irrigation scheduling and deeper insight into drought and salinity stress physiology. Theor Exp Plant Physiol 25 (1) Campo dos Goytacazes 2013. doi:10.1590/S2197-00252013000100002
Zweifel R, Steppe K, Sterck FJ (2007) Stomatal regulation by microclimate and tree water relations: interpreting ecophysiological field data with a hydraulic plant model. J Exp Bot 58(8):2113–2131
Acknowledgments
To colleagues who had performed hard and tedious experimental or theoretical investigations on oscillatory water regulation in plants: the author excuses himself for not having been able to cite all relevant results and papers in the field. Professor W Engelmann, Tübingen, is thanked for constructive contributions and support.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Johnsson, A. (2015). Oscillations in Plant Transpiration. In: Mancuso, S., Shabala, S. (eds) Rhythms in Plants. Springer, Cham. https://doi.org/10.1007/978-3-319-20517-5_7
Download citation
DOI: https://doi.org/10.1007/978-3-319-20517-5_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-20516-8
Online ISBN: 978-3-319-20517-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)