Abstract
Despite their toxic potential, reactive oxygen species (ROS) play an integral role as signaling molecules in the regulation of a broad range of biological processes such as growth, development, and responses to biotic and/or abiotic stimuli in plants. To some extent, various functions of ROS signaling are attributed to differences in the regulatory mechanisms of respiratory burst oxidase homologs (RBOHs) that are involved in a multitude of different signal transduction pathways activated in assorted tissue and cell types under fluctuating environmental conditions. To acclimate or survive under abiotic stress conditions, plants possess powerful strategies involving systemic signaling, retrograde signaling, and programmed cell death (PCD), in which ROS signals are integrated with other pathways to generate highly coordinated signaling networks. In this chapter, beneficial roles of ROS as signaling molecules in the regulation of abiotic stress responses in plants will be addressed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Abiotic stress
- Hormones
- NADPH oxidase
- Programmed cell death
- Redox signaling
- ROS signal
- Systemic signaling
- Retrograde signaling
1 Introduction
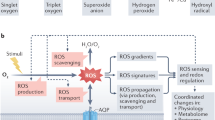
The reactive oxygen species (ROS) signaling network is highly conserved among aerobic organisms and controls a broad range of biological processes such as growth, development, and responses to biotic and/or abiotic stimuli (Mittler et al. 2011). Although early researches related to ROS metabolism focused on their potential toxicity and the different mechanisms to scavenge them, more recent studies have focused on the roles of ROS as signaling molecules. Why did plants acquire the ability to utilize ROS as signaling molecules during the evolutionary process? The existence of different types of ROS might be an advantage for the fine-tuning of complex signaling networks in cells, because coordinated production of ROS with different properties could at least partially contribute to generation of various signals. Indeed, specificity of ROS signaling was previously indicated by the finding that different sets of genes were upregulated in response to different types of ROS (Gadjev et al. 2006; Suzuki et al. 2011). In addition, almost any changes in cellular homeostasis could lead to alteration of redox state in particular organelles, followed by changes in cellular level of ROS. ROS can be therefore integrated with the activities of many other signaling components such as hormones, Ca2+, and kinases and involved in a number of pathways underlying different biological outcomes (Petrov and Van Breusegem 2012). Mobility of ROS, especially H2O2, is also an advantage to act as a signaling molecule. H2O2, a relatively long-lived and small ROS, readily penetrates cell membranes and is freely diffusible between cells (Bienert et al. 2006). Diffusion of H2O2 that is facilitated by plasma membrane aquaporins could also influence the efficiency and direction of H2O2 signaling between cells (Bienert et al. 2007; Dynowski et al. 2008).
To utilize ROS as signaling molecules, nontoxic levels of ROS must be maintained in a delicate balance between production involving enzymatic reactions and the unavoidable production during basic cellular processes and the metabolic counter-process involving ROS scavenging pathways (Mittler et al. 2004). Another possible advantage for using ROS as signaling molecules is the specific localization of their production/scavenging mechanisms in different organelles, cell types, and tissues (Mittler et al. 2004; Miller et al. 2009b; Suzuki et al. 2011). Local increase in ROS production can be limited to particular locations in the cell or plant. Therefore, level of ROS throughout the cell or plant as well as spatial coordination of ROS signals can be tightly regulated. In plants, many antioxidant mechanisms that function in different organelles modulate cellular redox state by detoxifying excess ROS produced during basic biological processes such as photosynthesis and respiration (Miller et al. 2009b). In addition, NADPH oxidases, respiratory burst oxidase homologs (RBOHs), deliberately produce ROS in different cell types, tissues, or stages (Suzuki et al. 2011). The tight regulation of RBOH protein activity makes these enzymes good candidates for the fine-tuning of ROS production in terms of amplitude, duration, and localization in plants (Marino et al. 2012; Gilroy et al. 2014) (Fig. 1).
Regulatory mechanisms of RBOH proteins in Arabidopsis. ROS can activate or suppress calcium channels to control the release of Ca2+ into cytosol. Ca2+ can directly or indirectly regulate the production of ROS by respiratory burst homolog (RBOH) proteins resulting in the generation of superoxide radicals that are dismutated to H2O2 spontaneously or via superoxide dismutase (SOD). H2O2 diffused into cytosol through aquaporin then functions as a signaling molecule. RBOHs have cytosolic FAD and NADPH-binding domains in the C-terminal region and six conserved transmembrane-spanning domains (pink cylinders). The N-terminal domain contains two EF-hand motifs and phosphorylation target sites that are important for activity of RBOHs. Binding of Ca2+ to EF-hand motifs is required for activation of RBOHs (Ogasawara et al. 2008; Drerup et al. 2013). Several kinases including OST1 (Sirichandra et al. 2009), CPK5 (Dubiella et al. 2013), and CBL1/9–CIPK26 complexes (Drerup et al. 2013) activate RBOH proteins by phosphorylation of the target sites (Ser or Arg residues). In addition, phosphatidic acid (PA) produced via function of phospholipase Dα1 (PLDα1) was also shown to activate RBOHD (Zhang et al. 2009b). Abbreviations: CBL1 calcineurin B-like protein 1, CBL9 calcineurin B-like protein 9, CIPK26 calcineurin B-like interacting protein 26, CPK5 calmodulin domain protein kinase 5, OST1 open stomata 1, FAD flavin adenine dinucleotide, NAD nicotinamide adenine dinucleotide, PA phosphatidic acid
Being sessile organisms, plants evolved sophisticated strategies to acclimate to or survive under fluctuating environmental conditions. These strategies involve systemic signaling, retrograde signaling, and programmed cell death (PCD), in which ROS play beneficial roles as signaling molecules (Woodson and Chory 2008; Baxter et al. 2014; Szechynska-Hebda and Karpinski 2013; Petrov et al. 2015). Systemic signaling mechanisms evolutionally improved the ability of plants to alert all remote and unstressed tissues of the plant to the existence of a biotic or abiotic threat and to trigger the activation of resistance or acclimation pathways in these tissues (Suzuki et al. 2013; Szechynska-Hebda et al. 2010). Recent studies have highlighted the importance of rapid systemic responses for acclimation of plants to abiotic stimuli, focusing on temporal–spatial coordination between several players of complex network of cell-to-cell communication, active propagation of ROS wave and calcium wave, hormones, and electric signals (Miller et al. 2009b; Mittler et al. 2011; Baxter et al. 2014; Gilroy et al. 2014). Signaling networks between the organelles employ ROS as second messenger. Alterations in redox state and ROS metabolism in the chloroplast and mitochondria are sources for retrograde signals to regulate nuclear gene expression, which play an important role in the acclimation of plants to environmental stimuli (Rhoads and Subbaiah 2007; Pogson et al. 2008; Woodson and Chory 2008; Shapiguzov et al. 2012; Szechynska-Hebda and Karpinski 2013). In addition, chloroplastic ROS production and photosynthetic functions were recently shown to be regulated by cues perceived by cell wall or apoplastic spaces (Padmanabhan and Dinesh-Kumar 2010), suggesting that chloroplastic retrograde signaling might be a part of a large signaling network involving various cues generated from different organelles, and ROS might be an important mediator that integrates these different signals. PCD is one of the essential strategies for survival of plants that are subjected to severe biotic or abiotic stresses, because only cells that are destined to die can be eliminated to prevent the spreading of damage to the neighboring cells and maintain appropriate metabolic status in the rest of cells and tissues (Petrov et al. 2015). It is an active and genetically controlled process in which cells are selectively eliminated in multistep fashion through the involvement of high concentrations of ROS and specific proteases and nuclease (Petrov et al. 2015).
In this chapter, beneficial roles of ROS as signaling molecules in the regulation of abiotic stress responses of plants will be addressed, especially in the context of ROS-producing mechanisms, integration of ROS signals with other pathways, and plant-specific mechanisms that underlie response of plants to abiotic stimuli.
2 ROS-Generating Pathways and Their Regulatory Mechanisms in Plants
In plants, NADPH oxidases, respiratory burst oxidase homologs (RBOHs), are responsible for the production of ROS that act as important signaling molecules (Torres and Dangl 2005; Suzuki et al. 2011). In Arabidopsis, RBOHs constitute a multigenic family comprised of ten genes (i.e., AtRBOHA–AtRBOHJ). Very specific function of each RBOH isoform was implicated by the finding that expression of genes encoding each RBOH protein, except for RBOHD, is restricted to one or two organs (Suzuki et al. 2011; Marino et al. 2012). Indeed, several studies have revealed that different plant RBOHs are involved in a multitude of different signaling pathways underlying growth, development, and response to biotic and abiotic stress (Torres et al. 2005; McInnis et al. 2006; Monshausen et al. 2007; Jammes et al. 2009; Nishimura and Dangl 2010; Miller et al. 2009b; Suzuki et al. 2011). In addition, a previous study revealed distinctive expression profiles of nine RBOHs in rice in response to various abiotic stimuli, which demonstrated their linked but diverse functions (Wang et al. 2013).
Plant RBOHs consist of C-terminal region containing cytosolic FAD and NADPH-binding domains, six conserved transmembrane-spanning domains, and N-terminal extension containing two Ca2+-binding EF-hand motifs and phosphorylation target sites that are important for their activity (Kobayashi et al. 2007; Oda et al. 2010; Kimura et al. 2012; Drerup et al. 2013). Previous studies in Arabidopsis have revealed several regulatory mechanisms of RBOH proteins, which involve protein phosphorylation, Ca2+, calcium-dependent protein kinases (CDPKs), and phospholipase Dα1 (PLDα1) (Lin et al. 2009; Monshausen et al. 2009; Zhang et al. 2009b; Jakubowicz et al. 2010; Dubiella et al. 2013; Drerup et al. 2013). Mechanical stimulation of plant tissue can induce an increase in cytosolic Ca2+ via an influx from the apoplast across the plasma membrane (Monshausen et al. 2009). The increased Ca2+ then enhances RBOHC-dependent ROS production followed by the activation of positive feedback loop between Ca2+ and RBOHC to regulate root hair development (Monshausen et al. 2007, 2009; Takeda et al. 2008). Ca2+ binding and phosphorylation synergistically activate RBOHD and RBOHF in Arabidopsis (Ogasawara et al. 2008; Kimura et al. 2012). A Ca2+ increase in the cytosol and conformational changes in EF-hand motifs by Ca2+ binding were found to be necessary for the activation of RBOHD (Ogasawara et al. 2008). PLDα1 and its lipid product phosphatidic acid (PA) play an essential role in abscisic acid (ABA)-induced production of ROS in guard cells via the function of RBOHD and RBOHF (Zhang et al. 2009b). ABA-dependent stomatal movement involves binding of PA to Arg residues 149, 150, 156, and 157 in RBOHD and phosphorylation of Ser13 and Ser174 in RBOHF by OPEN STOMATA 1 (OST1) kinase (Sirichandra et al. 2009). These findings indicate integration between RBOHD and RBOHF in the regulation of ABA-dependent stomatal closure. The coordination between PA and OST1 however, still needs to be addressed in future studies. In a recent study, systemic acquired resistance to pathogen was shown to involve phosphorylation of RBOHD by calcium-dependent protein kinase 5 (CPK5) and H2O2 production (Dubiella et al. 2013), supporting the hypothesis that Ca2+-dependent ROS production is required for the propagation of the ROS wave over long distances (Miller et al. 2009b). In addition, a recent finding demonstrated that the activity of RBOHF is regulated by direct Ca2+ binding to its EF-hands and Ca2+-dependent phosphorylation by CBL1/9–CIPK26 complexes (Drerup et al. 2013). Taken together, these findings indicate that the diverse functions of RBOH proteins in plants might be, at least partially, attributed to differences in regulatory mechanisms.
RBOH proteins are not the only source of ROS in plant cells. Numerous pathways for ROS production exist in plants and include photosynthesis, respiration, glycolate oxidase, oxalate oxidase, xanthine oxidase, amine oxidase, excited chlorophyll, fatty acid oxidation, and peroxidases (Mittler 2002). These pathways were also found to play important roles in the response of plants to abiotic stresses. For example, oxalate oxidase was shown to be involved in ROS production in root cells during drought stress (Voothuluru and Sharp 2013). In addition, recent studies uncovered a role for peroxidase-dependent ROS in the regulation of root growth and response to potassium deficiency (Kim et al. 2010; Jia 2011; Kwasniewski et al. 2013). Interestingly, ROS production by peroxidases might not be functionally equivalent to ROS generated by RBOH proteins (Daudi et al. 2012; Wrzaczek et al. 2013). This hypothesis can be supported by the finding that stomatal closure and ROS burst induced by a yeast elicitor were not inhibited in rbohD and rbohF mutants in Arabidopsis (Khokon et al. 2010). Functional differences between RBOH proteins and peroxidases may be at least partially attributed to differences in the types of ROS generated via functions of these enzymes. Superoxide (O2 •−), generated by RBOH proteins, can activate specific signaling pathways distinct from those activated by H2O2 (Suzuki et al. 2011). Another possibility is that diverse functions between these different types of enzymes might be due to differences in their respective reductants. RBOH proteins utilize NADPH as a reductant for the generation of O2 •−. In contrast, different chemicals or compounds including phenols, organic acids, and auxin have been suggested as candidate reductants employed in the peroxidase-dependent generation of H2O2 (O’Brien et al. 2012). Pathways involving these different reductants could be integrated with ROS signals activated via the different functions of these enzymes.
3 Involvement of ROS in the Regulation of Systemic Acquired Acclimation to Abiotic Stress
Recent findings highlight the significance of cell-to-cell communication mediating long-distance systemic signaling in plants. Plants evolved sophisticated acclimation and defense mechanisms that can be activated in the tissue(s) locally exposed to biotic or abiotic stimuli, as well as in distal portions not directly exposed to these stimuli. These mechanisms play an important role in preventing further damage to the entire plant when part of tissues is exposed to biotic or abiotic stimuli. The activation of defense or acclimation mechanisms in systemic or non-challenged tissues is termed systemic acquired resistance (SAR) or systemic acquired acclimation (SAA), respectively (Karpinski et al. 1999; Rossel et al. 2007; Carr et al. 2010; Szechynska-Hebda et al. 2010; Dempsey and Klessig 2012; Spoel and Dong 2012; Shah and Zeier 2013; Baxter et al. 2014). A recent study revealed the existence of an H2O2-dependent long-distance signal induced by various abiotic stimuli (Miller et al. 2009b). RBOHD was shown to be required for the initiation and self-propagation of a rapid cell-to-cell signal transduction that is dependent upon H2O2 accumulation in the apoplast to generate a “ROS wave” (yellow arrows in Fig. 2) (Mittler et al. 2011). In addition, more recent study demonstrated the significance of the ROS wave in the SAA of plants to heat or high-light stresses (Suzuki et al. 2013). The SAA of plants to abiotic stress is mediated by temporal–spatial interactions of the ROS wave, which function as a general priming signal, with stress-specific hormone or amino acid signals activated in systemic tissues. Calcium wave was recently shown to function as pivotal element of the systemic communication machinery (black arrows in Fig. 2) (Choi et al. 2014; Gilroy et al. 2014). In response to local stimulation with salt stress in the root tip, a wave of increased cytosolic Ca2+ level moves systemically through the plant paralleling with the ROS wave. The Ca2+ wave that travels at ~400 μm/s can spread through the root system and be transmitted to the aerial part of the plant. Application of the Ca2+ channel blocker lanthanum (La3+) inhibited systemic induction of marker gene expression associated with ROS as well as Ca2+ wave, implying the link between ROS and Ca2+ wave. In addition, plant deficient in two-pore channel 1 (TPC1), a vacuolar ion channel, exhibited disruption of the propagation of the systemic Ca2+ wave.
Cellular pathways that regulate ROS-dependent systemic signaling and retrograde signaling. H2O2 generated via function of RBOHD is required for rapid signal propagation from cell to cell in response to different environmental stimuli, such as high light, heat, or wounding (Miller et al. 2009). The ROS and Ca2+ waves in cells are integrated via the function of respiratory burst homolog (RBOH) proteins, Ca2+-dependent protein kinases, and calcium channels such as two-pore channel (TPC) 1. Changes in the redox state of PSII and the PQ pools in the chloroplast might activate electric signaling (red dotted arrow) at the plasma membrane involved in systemic responses (Szechynska-Hebda et al. 2010). The electric signal that is dependent upon the chloroplast redox state is also accompanied by ROS generation, implicating a cross talk between the RBOHD-dependent signal, electric signals on the plasma membrane, and ROS/redox signaling from chloroplast. Mitochondrial retrograde signaling (Rhoads and Subbaiah 2007; Pogson et al. 2008; Woodson and Chory 2008) could also play a key role in these responses as part of a cross talk network that senses different metabolic/environmental states and activates rapid systemic signaling. In addition, ROS produced via function of RBOH proteins or basic biological processes in different cellular components might induce different PCD pathways depending on abiotic stimuli. Abbreviations: PD plasmodesmata, CPK Ca2+-dependent protein kinase
4 Temporal Coordination Between ROS and Other Signals in the Regulation of Systemic Signaling in Plants
In response to changes in environmental conditions, early signaling events including increased Ca2+ levels in the cytosol, activation of MAPKs, accumulation of hormones, and production of ROS can all occur within seconds or minutes following application of abiotic stimuli (Benschop et al. 2007; Finka et al. 2012; Miller et al. 2009b). Early systemic responses of plants to abiotic stimuli have been previously described (Karpinski et al. 1999; Rossel et al. 2007; Muhlenbock et al. 2008; Szechynska-Hebda et al. 2010; Gordon et al. 2012). Studies employing transgenic plants expressing a luciferase reporter gene under the control of an APX1, APX2, or ZAT10 promoter demonstrated the activation of acclamatory responses within 5–20 min following application of high light both in leaves locally exposed to the stimuli and in distal tissues that were not directly exposed to the stimuli (Karpinski et al. 1999; Rossel et al. 2007; Szechynska-Hebda et al. 2010). Systemic responses to high light were shown to be associated with redox changes in the plastoquinone (PQ) pool, increased production of ROS and ethylene, reduction of maximal photochemical efficiency and non-photochemical quenching (NPQ), and changes in extracellular electric potential (Karpinski et al. 1999; Rossel et al. 2007; Szechynska-Hebda et al. 2010). In addition, amino acids involved in photorespiratory machinery, such as glycine, serine, and glycerate, rapidly accumulate in leaves directly exposed to high light within 60 s as well as in systemic tissues of plants at 15 and 45 min (Suzuki et al. 2013). Rapid local responses of these metabolites to high light were altered in the mutant lacking cytosolic APX1, demonstrating the involvement of H2O2 scavenging in this process.
In Arabidopsis, elevated levels of jasmonic acid (JA) accumulate in damaged tissues as well as undamaged systemic leaves within 30 s to 5 min in response to mechanical wounding (Glauser et al. 2009; Koo et al. 2009). The velocity of this long-distance signal leading to synthesis of JA in systemic tissues was 3.4–4.5 cm/min (Koo et al. 2009; Glauser et al. 2009). RBOHD-dependent long-distance signal is also a rapid auto-propagating systemic signal that travels at the rate of approximately 8.4 cm/min and is induced by various abiotic stimuli including mechanical wounding (Miller et al. 2009b). In addition, the potential involvement of electric signals that propagate with similar rates was also implicated in RBOHD-triggered rapid systemic signaling during wounding (Zimmermann et al. 2009; Mittler et al. 2011; Suzuki and Mittler 2012). These findings implicate the integration of JA and mobile signals such as ROS and electric signals. Peroxisomes that are responsible for the production of both H2O2 and JA might be a candidate of a key player to integrate JA and mobile ROS signals (Leon 2013). H2O2 is produced during the oxidation of glycolate to glyoxylic acid in photorespiratory processes (Mittler et al. 2004). JA is synthesized through the octadecanoid pathway involving the translocation of lipid intermediates from the chloroplast to the cytosol and later on into peroxisomes (Leon 2013). JA synthesized in the peroxisomes is transported to the cytosol, and JA-isoleucine conjugate, the bioactive form of the hormone, is then produced. Long-term responses to fluctuating environmental conditions regulate phenotypic changes such as growth, development, and survival of cells. Exposure of mature leaves to changes in light conditions and atmospheric CO2 induces alterations in photosynthetic rate and tolerance to high light in new developing leaves not directly exposed to these environmental changes (Coupe et al. 2006; Araya et al. 2008; Jiang et al. 2012). Although alterations in photosynthetic rate and response to high light implicate ROS and redox signaling in the systemic regulation of long-term responses in new developing leaves (Muhlenbock et al. 2008; Li et al. 2009; Mittler et al. 2011), links between ROS signaling and these responses are still not uncovered.
Previous studies demonstrated that the biphasic production of ROS consists of a primary phase that occurs within minutes and a secondary phase that occurs within hours/days (Nishimura and Dangl 2010; Soares et al. 2009; Kunihiro et al. 2011; Mittler et al. 2011). For example, mechanical wounding induced an initial burst of O2 •− within 3 min followed by later production of O2 •− and H2O2 after 6 h (Soares et al. 2009). Inhibition of early phase of ROS production by an NADPH oxidase inhibitor suppresses later production of O2 •− and accumulation of wound response proteins, indicating that an initial burst of ROS is required for the later phase of ROS production which regulates downstream acclamatory responses of plants to stress stimuli. In addition, a recent study suggests that these two phases of the ROS burst are linked via the ROS wave that communicates the initial ROS burst in the local tissue to the systemic tissue via a cell-to-cell relay mechanism (Miller et al. 2009b; Suzuki et al. 2013).
5 Spatial Coordination Between ROS and Other Signals in the Regulation of Systemic Signaling in Plants
To some extent, signals generated in plants during SAA are similar in local and systemic tissues. Rossel et al. (2007) compared the transcriptomes of local leaves, directly exposed to high light, and systemic leaves, not directly challenged by the stimulus. More than 70 % of the transcripts upregulated in local leaves in response to high light were also altered in their expression in systemic leaves, suggesting that similar signals exist between local and systemic tissues during SAA to high light. Similarities between local and systemic responses to high light is also supported by findings that alterations in ROS and redox signals and accumulation of amino acids associated with the photorespiratory pathway occurred both in local and systemic tissues (Muhlenbock et al. 2008; Szechynska-Hebda et al. 2010; Miller et al. 2009b). In addition, local application of heat or cold stimuli also can induce similar stress response proteins or transcripts in both local and systemic tissues (Gorsuch et al. 2010; Suzuki et al. 2013). In particular, induction of heat-responsive proteins in systemic tissue was shown to be RBOHD dependent (Suzuki et al. 2013).
Although signals generated in local and systemic tissues showed considerable overlap, previous studies have also demonstrated differences in alterations of transcripts or metabolites between these types of tissues. For example, ethylene accumulated both in local and systemic tissue in response to local application of high light; nevertheless, the signal regulated by EIN2 was shown to be required for induction of APX2 only in systemic tissues that are not directly subjected to high light (Muhlenbock et al. 2008). In addition, SID2 delays induction of APX2 only in leaves directly exposed to high light. These findings suggest that specific patterns of APX2 expression in local and systemic tissue might be regulated by the coordination between ethylene and salicylic acid (SA) signaling during SAA to high light. Moreover, spatial diversity in high-light responses between different leaves during SAA was also demonstrated by the findings that local high-light treatment resulted in the different expression levels of transcripts associated with regulation of ROS and redox signals depending on leaf position (Gordon et al. 2012).
How are signals generated in local and systemic tissues linked? The ROS wave may play a key role in propagating signals from local tissues to systemic tissues. The initial burst of ROS in a local group of plant cells triggers a cascade of cell-to-cell communication events that carries a systemic signal over long distances throughout different tissues of the plant (Miller et al. 2009b). Szechynska-Hebda et al. (2010) uncovered the pattern of systemic changes in NPQ, H2O2 concentration, and APX1 expression during SAA response of plants to high light. Wavelike patterns of APX1 expression in systemic tissue of plants correlate positively with H2O2 accumulation but negatively with NPQ (Szechynska-Hebda et al. 2010; Karpinski et al. 2013). The activation of systemic signals by local application of high light was recently shown to be accompanied by plasma membrane electrical signals in a light wavelength-specific manner (Szechynska-Hebda et al. 2010). In addition, the RBOHD-dependent ROS wave is associated with the generation and/or propagation of systemic potential variations (Suzuki et al. 2013). These finding suggest a link between electric signals in plants and ROS production.
6 Integration of ROS Signals with Other Signals
ROS signaling is integrated with various other signals including Ca2+ signaling, protein kinases, redox responses, and hormone signals. One good example of signaling networks associated with ROS signaling is MAPK cascade (Mittler et al. 2011; Petrov and Van Breusegem 2012). MPK3, MPK4, and MPK6 can all be activated by ROS and abiotic stresses, but different MKKs might transmit the signal depending on different stimuli (Jaspers and Kangasjarvi 2010). Arabidopsis overexpressing MKK2 exhibited constitutive MPK4 and MPK6 activity and resulted in increased tolerance of transgenic plans to salt and cold stress (Teige et al. 2004; Taj et al. 2010; Ismail et al. 2014). In contrast, overexpression of MKK9 that activates MPK3/MPK6 resulted in enhanced sensitivity of the transgenic plants to salt stress (Xu et al. 2008). In addition, activation of MPK6 by MKK3 was shown to be not required for salt stress response in plants (Takahashi et al. 2007). MEKK1 was suggested to be specifically required for the activation of MPK4 by H2O2 (Nakagami et al. 2006), and the signal between MEKK1 and MPK4 is mediated by MKK1 and MKK2 (Qiu et al. 2008). The MEKK1–MKK1/2–MPK4 pathway might play integral roles to regulate transcription factors that are highly responsive to ROS-generating conditions (Pitzschke et al. 2009). MPK8 could be a negative regulator of ROS wave (Marino et al. 2012). MPK8 which is activated by phosphorylation and direct binding of CaM in a Ca2+-dependent manner has been shown to negatively regulate ROS production via control of RBOHD (Takahashi et al. 2011). Various forms of abiotic stress result in increased production of ROS which can be liked to signals caused by changes in the regulation of plant hormones (Fujita et al. 2006). Ethylene biosynthesis was found to be modulated by positive regulation via RBOH proteins and negative regulation via CTR1 (constitutive triple response 1) (Jakubowicz et al. 2010). In Arabidopsis, CTR1 can be inhibited by phosphatidic acid (PA) that positively enhances activation of RBOHD and RBOHF (Jakubowicz et al. 2010). Previous studies revealed the involvement of ethylene in the regulation of SAA to high light induced by local high-light application (Muhlenbock et al. 2008; Karpinski et al. 2013). In response to high light, alterations in the redox state of the PQ pool can initiate a signal that induces production of 1-aminocyclopropane-1-carboxylate (ACC, the immediate precursor of ethylene), ROS, and the expression of ethylene-regulated genes (Muhlenbock et al. 2008). Increased ROS production results in bleaching of leaves and programmed cell death that relies on regulation of ethylene-insensitive 2 (EIN2) by lesion stimulating disease 1 (LSD1) (Muhlenbock et al. 2008; Karpinski et al. 2013).
Involvement of brassinosteroid (BR) signaling in ROS-dependent stress responses was also supported by previous findings. For example, exogenous BR treatments resulted in enhanced tolerance to oxidative stress accompanied by induction of H2O2 production in apoplast and expression of RBOH, MPK1, and MPK3 (Xia et al. 2009). More recent studies demonstrated the involvement of BR in SAA to high light in cucumber (Xia et al. 2009, 2011; Li et al. 2013a). Although BRs are not directly involved in long-distance signaling, they affect other signals such as auxins and polyamines (Li et al. 2013a). A recent study indicated the involvement of auxin in SAA response of plants to high light. Large portions of the transcripts that are altered in their expression in the distal leaves overlap with auxin-responsive transcripts (Gordon et al. 2012), indicating a connection between SAA and developmental processes mediated by auxin. Integration between ethylene and BRs during SAA response to HL needs to be elucidated in future studies.
ROS can affect auxin biosynthesis, transport, metabolism, and signaling underlying regulation of stress responses in plants (Krishnamurthy and Rathinasabapathi 2013b). The integration of RBOH functions with auxin has been evidenced by the analyses of the various RBOH expressions following the exogenous auxin (IAA: indole-3-acetic acid) treatment in Arabidopsis. It was observed that RBOHD was highly activated in response to auxin (Peer et al. 2013). Auxin transport mutant aux1 exhibited higher sensitivity to arsenite compared to WT plants, and H2O2 production was inhibited in aux1 under this stress condition (Krishnamurthy and Rathinasabapathi 2013a). These results indicate that auxin transport plays positive role in induction of ROS production and protection of plants against arsenite. In addition, the auxin signaling mutant tir afb2 showed reduced production of H2O2 and O2 •− and increased activity of catalase and ascorbate peroxidase accompanied by enhanced tolerance to salt stress (Iglesias et al. 2010).
ABA is involved in a broad range of biological functions, and its integration with ROS has been evidenced in many reports (Ma et al. 2012; Sagi et al. 2004; Drerup et al. 2013; Kwak et al. 2003). For example, RBOHD and RBOHF function together to regulate stomatal closure, seed germination, root elongation, and Na+/K+ homeostasis under salt stress (Ma et al. 2012; Kwak et al. 2003). Overexpression of 9-cis-epoxycarotenoid in tobacco, an enzyme of ABA synthesis, enhanced tolerance of transgenic plants to drought and salt stress accompanied by increased ABA-induced production of ROS via the function of RBOHs (Zhang et al. 2009a). In addition, mild salt stress triggers biphasic changes in ROS production in Arabidopsis and maize, and RBOHD in Arabidopsis is required for ROS production following mild salt stress (Lin et al. 2009; Xie et al. 2011). Moreover, SAA of plants to heat stress was shown to be correlated with activation of the ROS wave and transient accumulation of ABA in systemic tissues, and these responses were suppressed in a mutant lacking RBOHD (Suzuki et al. 2013). The SAA response to heat stress was also attenuated in mutants deficient in ABA signaling. These results indicate that temporal–spatial interactions between RBOHD-dependent ROS and ABA mediate SAA to heat stress (Suzuki et al. 2013). Moreover, ABA and SA treatment have been shown to result in transient increases in H2O2 production which induces tolerance to heat, salt, high-light, and oxidative stress (Xia et al. 2009).
Integration of SA or JA signaling with ABA underlying stomatal closure has been addressed in a recent review (Song et al. 2014). ABA-deficient mutant aba2-1 failed to close stomata in response to exogenous SA treatment, whereas guard cells of SA-deficient mutants sid2 and NahG responded to ABA (Zeng and He 2010; Montillet and Hirt 2013; Song et al. 2014), indicating that SA signaling functions upstream of ABA signaling. SA-induced stomatal closure was inhibited by DPI, an NADPH oxidase inhibitor, and plants deficient in RBOHD exhibited defect in stomatal response to exogenous SA treatment (Kalachova et al. 2013). In contrast, several lines of other evidences suggested that SA mediates ROS production, not via NADPH oxidases, but rather via a peroxidase-catalyzed reaction (Mori et al. 2001; Song et al. 2014). The reduced stomatal apertures in SA-accumulating siz1 mutant were rescued by the application of peroxidase inhibitors, but not by DPI (Miura et al. 2013). In addition, SA induces stomatal closure accompanied by extracellular ROS production mediated by peroxidase (Khokon et al. 2011). Mechanisms that regulate integration of SA signals to ROS-generating pathways need to be elucidated in future works. It was suggested that there is an overlap in the signals associated with stomatal closure between JA and ABA and that many common components including ROS production and Ca2+ oscillation are shared between these hormone signalings (Santino et al. 2013). Arabidopsis RBOHD and RBOHF were shown to be involved in the expression of methyl jasmonate (MeJA) response genes regulated by MYC2 transcription factor (Maruta et al. 2012).
7 Involvement of ROS in the Regulation of Retrograde Signaling
Under stress conditions, changes in the redox state of the chloroplast and mitochondria, ROS-producing organelles are the source of retrograde signaling that play crucial roles in stress acclimation of plants (Orange arrows in Fig. 2) (Suzuki et al. 2012; Choudhury et al. 2013). Three different processes in Arabidopsis were shown to induce chloroplast to nucleus signaling that alter the expression of nuclear genes, depending on the presence of GUN1 in the chloroplast and ABI4 in the nucleus: (1) accumulation of the chlorophyll biosynthesis intermediate Mg-protoporphyrin IX (Mg-Proto IX) and its methylester (Mg-Proto IX-ME), (2) inhibition of plastid gene expression (PGE) at the protein translation stage, and (3) changes in the redox state of the photosynthetic electron transfer (PET) chain (Koussevitzky et al. 2007; Woodson and Chory 2008).
Signals from the chloroplast to nuclei, modulated by changes in cellular redox state depending on the light intensity, might be an important process to respond to fluctuating light conditions (Szechynska-Hebda and Karpinski 2013). Under low light intensity, the transfer of signal from chloroplast to nucleus followed by changes in nuclear gene expression contributes to the adjustment of morphology of the leaves and cells, and number and structure of chloroplasts (Oelze et al. 2012). Light use efficiency is optimized at least partially by modulating stoichiometry of photosystems, light-harvesting antenna size, composition of the stromal enzymes, and activity of antioxidant systems (Muhlenbock et al. 2008; Pfannschmidt 2010; Foyer and Noctor 2011; Ruckle et al. 2012; Szechynska-Hebda and Karpinski 2013). Low light intensity can promote oxidation of PQ and reduction of thioredoxin, whereas high light intensity oppositely affects redox state in PQ pool and thioredoxin (Muhlenbock et al. 2008). Under high light intensity, PQ redox state and non-photochemical quenching (NPQ), the process to dissipate excess energy by heat, were shown to be potential regulators of retrograde signals that are required for at least regulation of APX1 and APX2 expression (Szechynska-Hebda et al. 2010). Imbalance between ATP and NADPH synthesis might be also an initiator of retrograde signaling (Szechynska-Hebda and Karpinski 2013). High proportion of NAD(P)H/NAD(P), ATP/ADP, and ATP/NADPH ratio could lead to inactivation of the photosynthetic electron transport chain components, resulting in production of 1O2 and O2 •− followed by deregulation of chloroplast metabolism and altered expression of stress response genes (Szechynska-Hebda and Karpinski 2013). In addition, excess excitation energy (EEE) also induces the changes in cellular ROS homeostasis and affects the expression of nuclear genes that control SAR and SAA (Rossel et al. 2007; Szechynska-Hebda et al. 2010). Progression of excess energy-induced PCD initiated by redox changes in PQ pool is regulated by the coordination between LSD1, EDS1, PAD4, and EIN2 that are associated with ethylene signaling and ROS production.
Upon illumination, chloroplast precursor, protochlorophyllide (PChlide), Mg-Proto IX, and Mg-Proto IX-ME produce 1O2 that can act as a signaling molecule from chloroplast to nuclei (Tripathy and Oelmuller 2012). The 1O2-dependent retrograde signaling has been extensively studied using the Arabidopsis flu mutant that massively accumulates PChlide and 1O2 and exhibits growth retardation and cell death under constant dark/light cycle (op den Camp et al. 2003; Apel and Hirt 2004; Wagner et al. 2004; Laloi et al. 2007; Lee et al. 2007). Transcriptome analysis revealed a set of genes that can be specifically activated by 1O2, not by H2O2 or O2 •− (op den Camp et al. 2003; Gadjev et al. 2006; Suzuki et al. 2011), suggesting that chloroplast to nucleus retrograde signaling is at least partially regulated by 1O2-specific signaling. Two plastid-localized proteins EXECUTER1 and EXECUTER2 are required for 1O2-dependent gene regulation and cell death (Lee et al. 2007). Moderate light exposure induces acclimation mechanisms that protect plants against more severe high-light stress, and 1O2 production and EXECUTER-dependent signal can be activated during this acclamatory process (Zhang et al. 2014), indicating the significance of 1O2-dependent retrograde signaling in the regulation of light acclimation of plants. In addition, nuclear topoisomerase VI and oxygenation derivatives of linoleic acid, the prominent polyunsaturated fatty acid of chloroplast membrane lipid, might also play an important role to integrate 1O2-dependent signal with regulation of nuclear gene expression (op den Camp et al. 2003; Tripathy and Oelmuller 2012). The oxidation of linoleic acid was shown to be not directly caused by 1O2, but enzymatic reactions regulated by 1O2 signals (op den Camp et al. 2003; Tripathy and Oelmuller 2012). Furthermore, 1O2-linked cell death activator (soldat8) that encodes SIGMA6 factor of the plastid RNA polymerase was identified as specific suppressor of 1O2-dependent stress responses in flu mutant (Coll et al. 2009). The other protein pleiotropic response locus 1 (PRL1) also affects the expression of 1O2 response genes in Arabidopsis (Baruah et al. 2009).
In a recent study, integration between ABI4 and redox metabolism was investigated using the ascorbic acid-deficient mutants, vtc1 and vtc2 (Kerchev et al. 2011). The transcriptome signatures of abi4, vtc1, and vtc2 mutants extensively overlap with large number of transcription factors and other regulatory genes (Kerchev et al. 2011). In addition, ABA and JA signaling synergistically function to regulate the growth of plants through ABI4 in ascorbic acid-dependent manner (Kerchev et al. 2011). Although it is still not clearly understood, H2O2 produced in chloroplasts was also implicated in retrograde signaling (Shapiguzov et al. 2012). H2O2-dependent retrograde signaling might be a combination of passive diffusion of H2O2 with indirect pathways involving ABA signaling (Mullineaux and Karpinski 2002; Galvez-Valdivieso and Mullineaux 2010). H2O2 might not be, however, the signaling molecule that directly affects nuclear gene expression; rather, redox-sensitive components such as oxidized proteins or peptides might mediate the signal transfer from the chloroplast to nucleus (Moller and Sweetlove 2010; Sierla et al. 2013).
Although mitochondrial retrograde signaling is poorly understood compared with the chloroplast to nucleus retrograde signaling, key regulators of mitochondrial retrograde signaling have been identified in recent studies. A membrane-bound NAC transcription factor, ANAC017, was found to be a regulator of AOX1a, a marker gene of mitochondrial retrograde signaling (Ng et al. 2013). ANAC017 mediates H2O2-induced alterations in transcript abundance. The abi4 mutants are insensitive to transcriptional derepression of AOX1a by the mitochondrial complex I inhibitor rotenone, indicating a role for ABI4 in redox regulation and mitochondria to nucleus retrograde signaling (Giraud et al. 2009). This work demonstrated the integral role of ABI4 to mediate mitochondrial and chloroplast retrograde signaling pathways, and perhaps it is the convergence point for mitochondria–plastid–nucleus coordination.
8 Programmed Cell Death Regulated by ROS Under Abiotic Stress
A low dose of ROS acts as signaling molecules that mediate at least part of stress responses, but they induce programmed cell death (PCD) at higher concentrations. PCD is a genetically controlled process in which only cells that are destined to die are selectively destroyed in a multistep fashion via the functions of specific proteases and nuclease (Petrov et al. 2015). Thus, no damage to the neighboring cells is inflicted. In addition, metabolism in the rest of cells and tissues can be appropriately adjusted to acclimate to or survive under stressed conditions by this process. Here, stress-specific pathways that positively regulate PCD will be mainly discussed. More detail of PCD under abiotic stress has been addressed in more extensive review (Petrov et al. 2015).
Severe drought enhances ROS production mainly due to decreased CO2 fixation accompanied by increased leakage of electron to O2, which may result in induction of PCD (Gechev et al. 2012). ROS production is inhibited by suppression of chlorophyll synthesis and photosynthetic activity, and modification of sucrose metabolisms under drought (Petrov et al. 2015; Liu et al. 2013). Although plants possess mechanisms to prevent unnecessary PCD, leaf senescence executed by ROS-triggered PCD acts as an important adaptation process of plants to drought (Petrov et al. 2015). ROS-triggered PCD under drought was shown to be regulated by coordination between ABA and cytokinin (Munn-Bosch and Alegre 2004). PCD characterized by degradation of organelles, increased size of the vacuole, and plasmalemma collapse can be induced in root tip meristems under drought (Duan et al. 2010). This adaptive mechanism in the root might be a strategy of plants to enhance lateral root growth. Reorientation of polyamine metabolism, as well as stomatal closure, is a drought response mechanism in which ABA plays pivotal roles. In grapevine, ABA induces accumulation of polyamine that is metabolized by amine oxidases, and H2O2 can be produced as a by-product of this reaction (Toumi et al. 2010). H2O2 produced in this reaction might be a regulator of further stress response or induction of PCD. PCD acts as an important process to protect plants against flooding stress. Under this stress condition, aerenchyma is formed by PCD in selected cells to produce air channels in roots (Gunawardena et al. 2001). Significance of ROS in the formation of aerenchyma has been indicated by the finding that exogenous application of H2O2 stimulated formation of aerenchyma in rice (Steffens et al. 2011). In addition, expression of RBOHD was highly enhanced during water logging conditions in maize, suggesting that RBOHD-dependent production of H2O2 play a key role in the formation of aerenchyma (Rajhi et al. 2011). Furthermore, mitochondrial dysfunction and accumulation of metal ion was also shown to increase ROS production under flooding stress (Shabala et al. 2014).
Although involvement of O2 •− produced via the function of NADPH oxidase is implicated in PCD pathway under salt and osmotic stress, signals activated by ionic salt and nonionic osmotic stress inducer (i.e., sorbitol) did not show extensive overlap (Monetti et al. 2014; Petrov et al. 2015), indicating the specificity of the mechanism regulating PCD under these stresses. Specific feature of salt-induced PCD is characterized by ion disequilibrium that may be due to invasion of Na+ into the cytosol accompanied by a decrease in K+ (Kim et al. 2014). Hydroxyl radicals in cells might affect K+/Na+ ratio and regulate enzymes involved in PCD (Demidchik et al. 2010). In addition, an anti-apoptotic protein BCL2 can regulate vacuolar processing enzymes (VPE) by modulating ion fluxes in rice (Kim et al. 2014). Overexpression of BCL2 exhibited enhanced PCD symptoms accompanied by significantly reduced K+ efflux and represses the expression of VPEs. These findings suggest that maintenance of proper Na+/K+ ratios could be a key process to enhance salt stress tolerance in plants (Huh et al. 2002; Teakle and Tyerman 2010).
Mitochondria are responsible for the regulation of PCD induced during heat stress (Vacca et al. 2004). Functionally active cytochrome c can be released from the mitochondria in a ROS-dependent manner, and caspase-like protease that induces PCD is then activated (Vacca et al. 2004). Proline that was implicated in mitochondrial ROS metabolism (Miller et al. 2009a) might be a specific component to cause cell death during heat stress. Although it acts as an osmolyte and ROS detoxifier under salt and osmotic stresses, it can negatively impact on cells under heat stress (Lv et al. 2011). In addition, exogenous application of ascorbate or glutathione (i.e., unspecific antioxidants) induced PCD in Arabidopsis cells under heat stress. On the other hand, catalase that specifically detoxifies H2O2 suppressed PCD. These finding suggest that H2O2 functions as PCD-inducing signal, but other types of ROS might act as negative regulator of PCD during heat stress (Doyle and McCabe 2010). Furthermore, MPK6 was found to be a component of PCD under heat stress. Enhanced accumulation of ROS and cytosolic Ca2+ can activate NPK6 that functions upstream to hydrolases and proteases associated with PCD (Li et al. 2012).
Low temperature is also able to induce PCD in plants when combined with elevated light intensity. LSD1, a negative regulator of PCD, interacts with catalase, and its deficiency can enhance low-temperature-induced PCD in plants (Huang et al. 2010; Li et al. 2013b). LSD1 and EDS1 proteins antagonistically regulate the acclimation of plants to UV-C stress. Plants deficient in LSD1 exhibited enhanced PCD following UV-C treatment. On the other hand, knocking out of EDS1 repressed UV-C-induced PCD (Wituszynska et al. 2015). The UV-C response associated with LSD1 and EDS1 is regulated by the modulation of ROS homeostasis. In response to high dose of UV-B, a defense program involving SA, JA, ethylene, and senescence-associated processes is activated to prevent oxidative damage in plants (Bandurska et al. 2013). These findings indicate the cross talks in the pathways associated with PCD between low-temperature, high-light, UV-B and UV-C, and pathogen responses.
9 Conclusions
ROS play an integral role in the regulation of numerous responses to abiotic stresses in plants. The complexity in ROS responses to various environmental stimuli might be, at least partially, attributed to different regulatory mechanisms of ROS production via basic biological processes and NADPH oxidases (RBOHs) that function in an array of organelles, tissue types, and developmental stages under various environmental conditions. A key mechanism in coordinating the complex spatial and temporal responses in plants is the cascade of cell-to-cell communication events that result in the formation of a wave of ROS production and increase in cytosolic Ca2+ that rapidly propagates throughout the different tissues of the plant. Networks of ROS/redox signaling in the chloroplast and mitochondria contribute to a delicate balance of homeostasis within each organelle, as well as to cross talk between different cellular components by regulating important biological pathways such as nuclear gene expression and energy metabolism under stress conditions. Functions of chloroplasts and mitochondria might be the key mediators for signal transductions in the cell because they are involved in the regulation of important pathways including systemic signaling, retrograde signaling, and PCD under stress conditions. In addition, there is a great overlap in the regulatory mechanisms between RBOHD-dependent systemic response, chloroplast/mitochondrial retrograde signaling and PCD, suggesting a cross talk between these pathways. Detailed mechanisms to coordinate or integrate these different ROS-dependent pathways need to be elucidated in future works. In addition, we also need to address how different pathways switched depending on the different environmental stimuli. This question could be answered by studying integration between ROS signaling and sensors of different stimuli.
Abbreviations
- ABA:
-
Abscisic acid
- ABI4:
-
ABA-insensitive 4
- ACC:
-
1-Aminocyclopropane-1-carboxylic acid
- ANAC017:
-
A membrane-bound NAC 017
- AOX:
-
Alternative oxidase
- APX:
-
Ascorbate peroxidase
- BCL2:
-
B-cell lymphoma 2
- BR:
-
Brassinosteroid
- CDPKs:
-
Calcium-dependent protein kinases
- CTR1:
-
Constitutive triple response 1
- DPI:
-
Diphenyleneiodonium
- EDS1:
-
Enhanced disease susceptibility 1
- EIN2:
-
Ethylene-insensitive 2
- EEE:
-
Excess excitation energy
- GUN1:
-
Genomes uncoupled 1
- IAA:
-
Indole-3-acetic acid
- JA:
-
Jasmonic acid
- LSD1:
-
Lesion simulating disease 1
- MAPK:
-
Mitogen-activated protein kinase
- NPQ:
-
Non-photochemical quenching
- MeJA:
-
Methyl jasmonate
- OST1:
-
Open stomata 1
- PA:
-
Phosphatidic acid
- PCD:
-
Programmed cell death
- PAD4:
-
Phytoalexin-deficient 4
- PRL1:
-
Pleiotropic response locus 1
- PQ:
-
Plastoquinone
- RBOH:
-
Respiratory burst oxidase homolog
- ROS:
-
Reactive oxygen species
- SA:
-
Salicylic acid
- SAA:
-
Systemic acquired acclimation
- SAR:
-
Systemic acquired resistance
- SID2:
-
Salicylic acid induction deficient 2
- TPC1:
-
Two-pore channel 1
- VPE:
-
Vacuolar processing enzymes
References
Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55:373–399
Araya T, Noguchi K, Terashima I (2008) Manipulation of light and CO2 environments of the primary leaves of bean (Phaseolus vulgaris L.) affects photosynthesis in both the primary and the first trifoliate leaves: involvement of systemic regulation. Plant Cell Environ 31:50–61
Bandurska H, Niedziela J, Chadzinikolau T (2013) Separate and combined responses to water deficit and UV-B radiation. Plant Sci Int J Exp Plant Biol 213:98–105
Baruah A, Simkova K, Hincha DK, Apel K, Laloi C (2009) Modulation of O-mediated retrograde signaling by the PLEIOTROPIC RESPONSE LOCUS 1 (PRL1) protein, a central integrator of stress and energy signaling. Plant J Cell Mol Biol 60:22–32
Baxter A, Mittler R, Suzuki N (2014) ROS as key players in plant stress signalling. J Exp Bot 65:1229–1240
Benschop JJ, Mohammed S, O’Flaherty M, Heck AJ, Slijper M, Menke FL (2007) Quantitative phosphoproteomics of early elicitor signaling in Arabidopsis. Mol Cell Proteomics 6:1198–1214
Bienert GP, Schjoerring JK, Jahn TP (2006) Membrane transport of hydrogen peroxide. Biochim Biophys Acta 1758:994–1003
Bienert GP, Moller AL, Kristiansen KA, Schulz A, Moller IM, Schjoerring JK, Jahn TP (2007) Specific aquaporins facilitate the diffusion of hydrogen peroxide across membranes. J Biol Chem 282:1183–1192
Carr JP, Lewsey MG, Palukaitis P (2010) Signaling in induced resistance. Adv Virus Res 76:57–121
Choi WG, Toyota M, Kim SH, Hilleary R, Gilroy S (2014) Salt stress-induced Ca2+ waves are associated with rapid, long-distance root-to-shoot signaling in plants. Proc Natl Acad Sci USA 111:6497–6502
Choudhury S, Panda P, Sahoo L, Panda SK (2013) Reactive oxygen species signaling in plants under abiotic stress. Plant Signal Behav 8:e23681
Coll NS, Danon A, Meurer J, Cho WK, Apel K (2009) Characterization of soldat8, a suppressor of singlet oxygen-induced cell death in Arabidopsis seedlings. Plant Cell Physiol 50:707–718
Coupe SA, Palmer BG, Lake JA, Overy SA, Oxborough K, Woodward FI, Gray JE, Quick WP (2006) Systemic signalling of environmental cues in Arabidopsis leaves. J Exp Bot 57:329–341
Daudi A, Cheng Z, O’Brien JA, Mammarella N, Khan S, Ausubel FM, Bolwell GP (2012) The apoplastic oxidative burst peroxidase in Arabidopsis is a major component of pattern-triggered immunity. Plant Cell 24:275–287
Demidchik V, Cuin TA, Svistunenko D, Smith SJ, Miller AJ, Shabala S, Sokolik A, Yurin V (2010) Arabidopsis root K+-efflux conductance activated by hydroxyl radicals: single-channel properties, genetic basis and involvement in stress-induced cell death. J Cell Sci 123:1468–1479
Dempsey DA, Klessig DF (2012) SOS - too many signals for systemic acquired resistance? Trends Plant Sci 17:538–545
Doyle SM, McCabe PF (2010) Type and cellular location of reactive oxygen species determine activation or suppression of programmed cell death in Arabidopsis suspension cultures. Plant Signal Behav 5:467–468
Drerup MM, Schlucking K, Hashimoto K, Manishankar P, Steinhorst L, Kuchitsu K, Kudla J (2013) The Calcineurin B-Like calcium sensors CBL1 and CBL9 together with their interacting protein kinase CIPK26 regulate the Arabidopsis NADPH oxidase RBOHF. Mol Plant 6:559–569
Duan Y, Zhang W, Li B, Wang Y, Li K, Sodmergen, Han C, Zhang Y, Li X (2010) An endoplasmic reticulum response pathway mediates programmed cell death of root tip induced by water stress in Arabidopsis. New Phytol 186:681–695
Dubiella U, Seybold H, Durian G, Komander E, Lassig R, Witte CP, Schulze WX, Romeis T (2013) Calcium-dependent protein kinase/NADPH oxidase activation circuit is required for rapid defense signal propagation. Proc Natl Acad Sci USA 110:8744–8749
Dynowski M, Schaaf G, Loque D, Moran O, Ludewig U (2008) Plant plasma membrane water channels conduct the signalling molecule H2O2. Biochem J 414:53–61
Finka A, Cuendet AF, Maathuis FJ, Saidi Y, Goloubinoff P (2012) Plasma membrane cyclic nucleotide gated calcium channels control land plant thermal sensing and acquired thermotolerance. Plant Cell 24:3333–3348
Foyer CH, Noctor G (2011) Ascorbate and glutathione: the heart of the redox hub. Plant Physiol 155:2–18
Fujita M, Fujita Y, Noutoshi Y, Takahashi F, Narusaka Y, Yamaguchi-Shinozaki K, Shinozaki K (2006) Crosstalk between abiotic and biotic stress responses: a current view from the points of convergence in the stress signaling networks. Curr Opin Plant Biol 9:436–442
Gadjev I, Vanderauwera S, Gechev TS, Laloi C, Minkov IN, Shulaev V, Apel K, Inze D, Mittler R, Van Breusegem F (2006) Transcriptomic footprints disclose specificity of reactive oxygen species signaling in Arabidopsis. Plant Physiol 141:436–445
Galvez-Valdivieso G, Mullineaux PM (2010) The role of reactive oxygen species in signalling from chloroplasts to the nucleus. Physiol Plant 138:430–439
Gechev TS, Dinakar C, Benina M, Toneva V, Bartels D (2012) Molecular mechanisms of desiccation tolerance in resurrection plants. Cell Mol Life Sci 69:3175–3186
Gilroy S, Suzuki N, Miller G, Choi WG, Toyota M, Devireddy AR, Mittler R (2014) A tidal wave of signals: calcium and ROS at the forefront of rapid systemic signaling. Trends Plant Sci 19:623–630
Giraud E, Van Aken O, Ho LH, Whelan J (2009) The transcription factor ABI4 is a regulator of mitochondrial retrograde expression of ALTERNATIVE OXIDASE1a. Plant Physiol 150:1286–1296
Glauser G, Dubugnon L, Mousavi SA, Rudaz S, Wolfender JL, Farmer EE (2009) Velocity estimates for signal propagation leading to systemic jasmonic acid accumulation in wounded Arabidopsis. J Biol Chem 284:34506–34513
Gordon MJ, Carmody M, Albrecht V, Pogson B (2012) Systemic and local responses to repeated HL stress-induced retrograde signaling in Arabidopsis. Front Plant Sci 3:303
Gorsuch PA, Sargeant AW, Penfield SD, Quick WP, Atkin OK (2010) Systemic low temperature signaling in Arabidopsis. Plant Cell Physiol 51:1488–1498
Gunawardena A, Pearce DM, Jackson MB, Hawes CR, Evans DE (2001) Characterisation of programmed cell death during aerenchyma formation induced by ethylene or hypoxia in roots of maize (Zea mays L.). Planta 212:205–214
Huang X, Li Y, Zhang X, Zuo J, Yang S (2010) The Arabidopsis LSD1 gene plays an important role in the regulation of low temperature-dependent cell death. New Phytol 187:301–312
Huh GH, Damsz B, Matsumoto TK, Reddy MP, Rus AM, Ibeas JI, Narasimhan ML, Bressan RA, Hasegawa PM (2002) Salt causes ion disequilibrium-induced programmed cell death in yeast and plants. Plant J Cell Mol Biol 29:649–659
Iglesias MJ, Terrile MC, Bartoli CG, D’Ippolito S, Casalongue CA (2010) Auxin signaling participates in the adaptative response against oxidative stress and salinity by interacting with redox metabolism in Arabidopsis. Plant Mol Biol 74:215–222
Ismail A, Takeda S, Nick P (2014) Life and death under salt stress: same players, different timing? J Exp Bot 65:2963–2979
Jakubowicz M, Galganska H, Nowak W, Sadowski J (2010) Exogenously induced expression of ethylene biosynthesis, ethylene perception, phospholipase D, and Rboh-oxidase genes in broccoli seedlings. J Exp Bot 61:3475–3491
Jammes F, Song C, Shin D, Munemasa S, Takeda K, Gu D, Cho D, Lee S, Giordo R, Sritubtim S, Leonhardt N, Ellis BE, Murata Y, Kwak JM (2009) MAP kinases MPK9 and MPK12 are preferentially expressed in guard cells and positively regulate ROS-mediated ABA signaling. Proc Natl Acad Sci USA 106:20520–20525
Jaspers P, Kangasjarvi J (2010) Reactive oxygen species in abiotic stress signaling. Physiol Plant 138:405–413
Jia L (2011) Is reactive oxygen species (ROS) the underlying factor for inhibited root growth in Osspr1? Plant Signal Behav 6:1024–1025
Jiang C, Belfield EJ, Mithani A, Visscher A, Ragoussis J, Mott R, Smith JA, Harberd NP (2012) ROS-mediated vascular homeostatic control of root-to-shoot soil Na delivery in Arabidopsis. EMBO J 31:4359–4370
Kalachova T, Iakovenko O, Kretinin S, Kravets V (2013) Involvement of phospholipase D and NADPH-oxidase in salicylic acid signaling cascade. Plant Physiol Biochem 66:127–133
Karpinski S, Reynolds H, Karpinska B, Wingsle G, Creissen G, Mullineaux P (1999) Systemic signaling and acclimation in response to excess excitation energy in Arabidopsis. Science 284:654–657
Karpinski S, Szechynska-Hebda M, Wituszynska W, Burdiak P (2013) Light acclimation, retrograde signalling, cell death and immune defences in plants. Plant Cell Environ 36:736–744
Kerchev PI, Pellny TK, Vivancos PD, Kiddle G, Hedden P, Driscoll S, Vanacker H, Verrier P, Hancock RD, Foyer CH (2011) The transcription factor ABI4 Is required for the ascorbic acid-dependent regulation of growth and regulation of jasmonate-dependent defense signaling pathways in Arabidopsis. Plant Cell 23:3319–3334
Khokon MA, Hossain MA, Munemasa S, Uraji M, Nakamura Y, Mori IC, Murata Y (2010) Yeast elicitor-induced stomatal closure and peroxidase-mediated ROS production in Arabidopsis. Plant Cell Physiol 51:1915–1921
Khokon AR, Okuma E, Hossain MA, Munemasa S, Uraji M, Nakamura Y, Mori IC, Murata Y (2011) Involvement of extracellular oxidative burst in salicylic acid-induced stomatal closure in Arabidopsis. Plant Cell Environ 34:434–443
Kim MJ, Ciani S, Schachtman DP (2010) A peroxidase contributes to ROS production during Arabidopsis root response to potassium deficiency. Mol Plant 3:420–427
Kim Y, Wang M, Bai Y, Zeng Z, Guo F, Han N, Bian H, Wang J, Pan J, Zhu M (2014) Bcl-2 suppresses activation of VPEs by inhibiting cytosolic Ca(2)(+) level with elevated K(+) efflux in NaCl-induced PCD in rice. Plant Physiol Biochem 80:168–175
Kimura S, Kaya H, Kawarazaki T, Hiraoka G, Senzaki E, Michikawa M, Kuchitsu K (2012) Protein phosphorylation is a prerequisite for the Ca2+-dependent activation of Arabidopsis NADPH oxidases and may function as a trigger for the positive feedback regulation of Ca2+ and reactive oxygen species. Biochim Biophys Acta Mol Cell Res 1823:398–405
Kobayashi M, Ohura I, Kawakita K, Yokota N, Fujiwara M, Shimamoto K, Doke N, Yoshioka H (2007) Calcium-dependent protein kinases regulate the production of reactive oxygen species by potato NADPH oxidase. Plant Cell 19:1065–1080
Koo AJ, Gao X, Jones AD, Howe GA (2009) A rapid wound signal activates the systemic synthesis of bioactive jasmonates in Arabidopsis. Plant J Cell Mol Biol 59:974–986
Koussevitzky S, Nott A, Mockler TC, Hong F, Sachetto-Martins G, Surpin M, Lim J, Mittler R, Chory J (2007) Signals from chloroplasts converge to regulate nuclear gene expression. Science 316:715–719
Krishnamurthy A, Rathinasabapathi B (2013a) Auxin and its transport play a role in plant tolerance to arsenite-induced oxidative stress in Arabidopsis thaliana. Plant Cell Environ 36:1838–1849
Krishnamurthy A, Rathinasabapathi B (2013b) Oxidative stress tolerance in plants: novel interplay between auxin and reactive oxygen species signaling. Plant Signal Behav. doi:10.4161/psb.25761
Kunihiro S, Hiramatsu T, Kawano T (2011) Involvement of salicylic acid signal transduction in aluminum-responsive oxidative burst in Arabidopsis thaliana cell suspension culture. Plant Signal Behav 6:611–616
Kwak JM, Mori IC, Pei ZM, Leonhardt N, Torres MA, Dangl JL, Bloom RE, Bodde S, Jones JD, Schroeder JI (2003) NADPH oxidase AtrbohD and AtrbohF genes function in ROS-dependent ABA signaling in Arabidopsis. EMBO J 22:2623–2633
Kwasniewski M, Chwialkowska K, Kwasniewska J, Kusak J, Siwinski K, Szarejko I (2013) Accumulation of peroxidase-related reactive oxygen species in trichoblasts correlates with root hair initiation in barley. J Plant Physiol 170:185–195
Laloi C, Stachowiak M, Pers-Kamczyc E, Warzych E, Murgia I, Apel K (2007) Cross-talk between singlet oxygen- and hydrogen peroxide-dependent signaling of stress responses in Arabidopsis thaliana. Proc Natl Acad Sci USA 104:672–677
Lee KP, Kim C, Landgraf F, Apel K (2007) EXECUTER1- and EXECUTER2-dependent transfer of stress-related signals from the plastid to the nucleus of Arabidopsis thaliana. Proc Natl Acad Sci USA 104:10270–10275
Leon J (2013) Role of plant peroxisomes in the production of jasmonic acid-based signals. Subcell Biochem 69:299–313
Li Z, Wakao S, Fischer BB, Niyogi KK (2009) Sensing and responding to excess light. Annu Rev Plant Biol 60:239–260
Li Z, Yue H, Xing D (2012) MAP Kinase 6-mediated activation of vacuolar processing enzyme modulates heat shock-induced programmed cell death in Arabidopsis. New Phytol 195:85–96
Li P, Chen L, Zhou Y, Xia X, Shi K, Chen Z, Yu J (2013a) Brassinosteroids-induced systemic stress tolerance was associated with increased transcripts of several defence-related genes in the phloem in. PLoS One 8:e66582
Li Y, Chen L, Mu J, Zuo J (2013b) LESION SIMULATING DISEASE1 interacts with catalases to regulate hypersensitive cell death in Arabidopsis. Plant Physiol 163:1059–1070
Lin F, Ding HD, Wang JX, Zhang H, Zhang AY, Zhang Y, Tan MP, Dong W, Jiang MY (2009) Positive feedback regulation of maize NADPH oxidase by mitogen-activated protein kinase cascade in abscisic acid signalling. J Exp Bot 60:3221–3238
Liu YH, Offler CE, Ruan YL (2013) Regulation of fruit and seed response to heat and drought by sugars as nutrients and signals. Front Plant Sci 4:282
Lv WT, Lin B, Zhang M, Hua XJ (2011) Proline accumulation is inhibitory to Arabidopsis seedlings during heat stress. Plant Physiol 156:1921–1933
Ma L, Zhang H, Sun L, Jiao Y, Zhang G, Miao C, Hao F (2012) NADPH oxidase AtrbohD and AtrbohF function in ROS-dependent regulation of Na(+)/K(+)homeostasis in Arabidopsis under salt stress. J Exp Bot 63:305–317
Marino D, Dunand C, Puppo A, Pauly N (2012) A burst of plant NADPH oxidases. Trends Plant Sci 17:9–15
Maruta T, Inoue T, Noshi M, Tamoi M, Yabuta Y, Yoshimura K, Ishikawa T, Shigeoka S (2012) Cytosolic ascorbate peroxidase 1 protects organelles against oxidative stress by wounding- and jasmonate-induced H(2)O(2) in Arabidopsis plants. Biochim Biophys Acta 1820:1901–1907
McInnis SM, Desikan R, Hancock JT, Hiscock SJ (2006) Production of reactive oxygen species and reactive nitrogen species by angiosperm stigmas and pollen: potential signalling crosstalk? New Phytol 172:221–228
Miller G, Honig A, Stein H, Suzuki N, Mittler R, Zilberstein A (2009a) Unraveling delta1-pyrroline-5-carboxylate-proline cycle in plants by uncoupled expression of proline oxidation enzymes. J Biol Chem 284:26482–26492
Miller G, Schlauch K, Tam R, Cortes D, Torres MA, Shulaev V, Dangl JL, Mittler R (2009b) The plant NADPH oxidase RBOHD mediates rapid systemic signaling in response to diverse stimuli. Sci Signal 2:ra45
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7:405–410
Mittler R, Vanderauwera S, Gollery M, Van Breusegem F (2004) Reactive oxygen gene network of plants. Trends Plant Sci 9:490–498
Mittler R, Vanderauwera S, Suzuki N, Miller G, Tognetti VB, Vandepoele K, Gollery M, Shulaev V, Van Breusegem F (2011) ROS signaling: the new wave? Trends Plant Sci 16:300–309
Miura K, Okamoto H, Okuma E, Shiba H, Kamada H, Hasegawa PM, Murata Y (2013) SIZ1 deficiency causes reduced stomatal aperture and enhanced drought tolerance via controlling salicylic acid-induced accumulation of reactive oxygen species in Arabidopsis. Plant J 73:91–104
Moller IM, Sweetlove LJ (2010) ROS signalling–specificity is required. Trends Plant Sci 15:370–374
Monetti E, Kadono T, Tran D, Azzarello E, Arbelet-Bonnin D, Biligui B, Briand J, Kawano T, Mancuso S, Bouteau F (2014) Deciphering early events involved in hyperosmotic stress-induced programmed cell death in tobacco BY-2 cells. J Exp Bot 65:1361–1375
Monshausen GB, Bibikova TN, Messerli MA, Shi C, Gilroy S (2007) Oscillations in extracellular pH and reactive oxygen species modulate tip growth of Arabidopsis root hairs. Proc Natl Acad Sci USA 104:20996–21001
Monshausen GB, Bibikova TN, Weisenseel MH, Gilroy S (2009) Ca2+ regulates reactive oxygen species production and pH during mechanosensing in Arabidopsis roots. Plant Cell 21:2341–2356
Montillet JL, Hirt H (2013) New checkpoints in stomatal defense. Trends Plant Sci 18:295–297
Mori IC, Pinontoan R, Kawano T, Muto S (2001) Involvement of superoxide generation in salicylic acid-induced stomatal closure in Vicia faba. Plant Cell Physiol 42:1383–1388
Muhlenbock P, Szechynska-Hebda M, Plaszczyca M, Baudo M, Mullineaux PM, Parker JE, Karpinska B, Karpinski S (2008) Chloroplast signaling and LESION SIMULATING DISEASE1 regulate crosstalk between light acclimation and immunity in Arabidopsis. Plant Cell 20:2339–2356
Mullineaux P, Karpinski S (2002) Signal transduction in response to excess light: getting out of the chloroplast. Curr Opin Plant Biol 5:43–48
Munn-Bosch S, Alegre L (2004) Die and let live: leaf senescence contributes to plant survival under drought stress. Funct Plant Biol 31:203–216
Nakagami H, Soukupova H, Schikora A, Zarsky V, Hirt H (2006) A mitogen-activated protein kinase kinase kinase mediates reactive oxygen species homeostasis in Arabidopsis. J Biol Chem 281:38697–38704
Ng S, Ivanova A, Duncan O, Law SR, Van Aken O, De Clercq I, Wang Y, Carrie C, Xu L, Kmiec B, Walker H, Van Breusegem F, Whelan J, Giraud E (2013) A membrane-bound NAC transcription factor, ANAC017, mediates mitochondrial retrograde signaling in Arabidopsis. Plant Cell 25:3450–3471
Nishimura MT, Dangl JL (2010) Arabidopsis and the plant immune system. Plant J Cell Mol Biol 61:1053–1066
O’Brien JA, Daudi A, Butt VS, Bolwell GP (2012) Reactive oxygen species and their role in plant defence and cell wall metabolism. Planta 236:765–779
Oda T, Hashimoto H, Kuwabara N, Akashi S, Hayashi K, Kojima C, Wong HL, Kawasaki T, Shimamoto K, Sato M, Shimizu T (2010) Structure of the N-terminal regulatory domain of a plant NADPH oxidase and its functional implications. J Biol Chem 285:1435–1445
Oelze ML, Vogel MO, Alsharafa K, Kahmann U, Viehhauser A, Maurino VG, Dietz KJ (2012) Efficient acclimation of the chloroplast antioxidant defence of Arabidopsis thaliana leaves in response to a 10- or 100-fold light increment and the possible involvement of retrograde signals. J Exp Bot 63:1297–1313
Ogasawara Y, Kaya H, Hiraoka G, Yumoto F, Kimura S, Kadota Y, Hishinuma H, Senzaki E, Yamagoe S, Nagata K, Nara M, Suzuki K, Tanokura M, Kuchitsu K (2008) Synergistic activation of the Arabidopsis NADPH oxidase AtrbohD by Ca2+ and phosphorylation. J Biol Chem 283:8885–8892
op den Camp RG, Przybyla D, Ochsenbein C, Laloi C, Kim C, Danon A, Wagner D, Hideg E, Gobel C, Feussner I, Nater M, Apel K (2003) Rapid induction of distinct stress responses after the release of singlet oxygen in Arabidopsis. Plant Cell 15:2320–2332
Padmanabhan MS, Dinesh-Kumar SP (2010) All hands on deck-the role of chloroplasts, endoplasmic reticulum, and the nucleus in driving plant innate immunity. Mol Plant Microbe Interact 23:1368–1380
Peer WA, Cheng Y, Murphy AS (2013) Evidence of oxidative attenuation of auxin signalling. J Exp Bot 64:2629–2639
Petrov VD, Van Breusegem F (2012) Hydrogen peroxide-a central hub for information flow in plant cells. AoB Plants 2012:pls014
Petrov V, Hille J, Mueller-Roeber B, Gechev TS (2015) ROS-mediated abiotic stress-induced programmed cell death in plants. Front Plant Sci 6:69
Pfannschmidt T (2010) Plastidial retrograde signalling–a true “plastid factor” or just metabolite signatures? Trends Plant Sci 15:427–435
Pitzschke A, Djamei A, Bitton F, Hirt H (2009) A major role of the MEKK1-MKK1/2-MPK4 pathway in ROS signalling. Mol Plant 2:120–137
Pogson BJ, Woo NS, Forster B, Small ID (2008) Plastid signalling to the nucleus and beyond. Trends Plant Sci 13:602–609
Qiu JL, Zhou L, Yun BW, Nielsen HB, Fiil BK, Petersen K, Mackinlay J, Loake GJ, Mundy J, Morris PC (2008) Arabidopsis mitogen-activated protein kinase kinases MKK1 and MKK2 have overlapping functions in defense signaling mediated by MEKK1, MPK4, and MKS1. Plant Physiol 148:212–222
Rajhi I, Yamauchi T, Takahashi H, Nishiuchi S, Shiono K, Watanabe R, Mliki A, Nagamura Y, Tsutsumi N, Nishizawa NK, Nakazono M (2011) Identification of genes expressed in maize root cortical cells during lysigenous aerenchyma formation using laser microdissection and microarray analyses. New Phytol 190:351–368
Rhoads DM, Subbaiah CC (2007) Mitochondrial retrograde regulation in plants. Mitochondrion 7:177–194
Rossel JB, Wilson PB, Hussain D, Woo NS, Gordon MJ, Mewett OP, Howell KA, Whelan J, Kazan K, Pogson BJ (2007) Systemic and intracellular responses to photooxidative stress in Arabidopsis. Plant Cell 19:4091–4110
Ruckle ME, Burgoon LD, Lawrence LA, Sinkler CA, Larkin RM (2012) Plastids are major regulators of light signaling in Arabidopsis. Plant Physiol 159:366–390
Sagi M, Davydov O, Orazova S, Yesbergenova Z, Ophir R, Stratmann JW, Fluhr R (2004) Plant respiratory burst oxidase homologs impinge on wound responsiveness and development in Lycopersicon esculentum. Plant Cell 16:616–628
Santino A, Taurino M, De Domenico S, Bonsegna S, Poltronieri P, Pastor V, Flors V (2013) Jasmonate signaling in plant development and defense response to multiple (a)biotic stresses. Plant Cell Rep 32:1085–1098
Shabala S, Shabala L, Barcelo J, Poschenrieder C (2014) Membrane transporters mediating root signalling and adaptive responses to oxygen deprivation and soil flooding. Plant Cell Environ 37:2216–2233
Shah J, Zeier J (2013) Long-distance communication and signal amplification in systemic acquired resistance. Front Plant Sci 4:30
Shapiguzov A, Vainonen JP, Wrzaczek M, Kangasjarvi J (2012) ROS-talk-how the apoplast, the chloroplast, and the nucleus get the message through. Front Plant Sci 3:292
Sierla M, Rahikainen M, Salojarvi J, Kangasjarvi J, Kangasjarvi S (2013) Apoplastic and chloroplastic redox signaling networks in plant stress responses. Antioxid Redox Signal 18:2220–2239
Sirichandra C, Gu D, Hu HC, Davanture M, Lee S, Djaoui M, Valot B, Zivy M, Leung J, Merlot S, Kwak JM (2009) Phosphorylation of the Arabidopsis AtrbohF NADPH oxidase by OST1 protein kinase. FEBS Lett 583:2982–2986
Soares NC, Francisco R, Vielba JM, Ricardo CP, Jackson PA (2009) Associating wound-related changes in the apoplast proteome of Medicago with early steps in the ROS signal-transduction pathway. J Proteome Res 8:2298–2309
Song Y, Miao Y, Song CP (2014) Behind the scenes: the roles of reactive oxygen species in guard cells. New Phytol 201:1121–1140
Spoel SH, Dong X (2012) How do plants achieve immunity? Defence without specialized immune cells. Nat Rev Immunol 12:89–100
Steffens B, Geske T, Sauter M (2011) Aerenchyma formation in the rice stem and its promotion by H2O2. New Phytol 190:369–378
Suzuki N, Mittler R (2012) Reactive oxygen species-dependent wound responses in animals and plants. Free Radic Biol Med 53:2269–2276
Suzuki N, Miller G, Morales J, Shulaev V, Torres MA, Mittler R (2011) Respiratory burst oxidases: the engines of ROS signaling. Curr Opin Plant Biol 14:691–699
Suzuki N, Koussevitzky S, Mittler R, Miller G (2012) ROS and redox signalling in the response of plants to abiotic stress. Plant Cell Environ 35:259–270
Suzuki N, Miller G, Salazar C, Mondal HA, Shulaev E, Cortes DF, Shuman JL, Luo X, Shah J, Schlauch K, Shulaev V, Mittler R (2013) Temporal-spatial interaction between reactive oxygen species and abscisic acid regulates rapid systemic acclimation in plants. Plant Cell 25:3553–3569
Szechynska-Hebda M, Karpinski S (2013) Light intensity-dependent retrograde signalling in higher plants. J Plant Physiol 170:1501–1516
Szechynska-Hebda M, Kruk J, Gorecka M, Karpinska B, Karpinski S (2010) Evidence for light wavelength-specific photoelectrophysiological signaling and memory of excess light episodes in Arabidopsis. Plant Cell 22:2201–2218
Taj G, Agarwal P, Grant M, Kumar A (2010) MAPK machinery in plants: recognition and response to different stresses through multiple signal transduction pathways. Plant Signal Behav 5:1370–1378
Takahashi F, Yoshida R, Ichimura K, Mizoguchi T, Seo S, Yonezawa M, Maruyama K, Yamaguchi-Shinozaki K, Shinozaki K (2007) The mitogen-activated protein kinase cascade MKK3-MPK6 is an important part of the jasmonate signal transduction pathway in Arabidopsis. Plant Cell 19:805–818
Takahashi F, Mizoguchi T, Yoshida R, Ichimura K, Shinozaki K (2011) Calmodulin-dependent activation of MAP kinase for ROS homeostasis in Arabidopsis. Mol Cell 41:649–660
Takeda S, Gapper C, Kaya H, Bell E, Kuchitsu K, Dolan L (2008) Local positive feedback regulation determines cell shape in root hair cells. Science 319:1241–1244
Teakle NL, Tyerman SD (2010) Mechanisms of Cl(-) transport contributing to salt tolerance. Plant Cell Environ 33:566–589
Teige M, Scheikl E, Eulgem T, Doczi R, Ichimura K, Shinozaki K, Dangl JL, Hirt H (2004) The MKK2 pathway mediates cold and salt stress signaling in Arabidopsis. Mol Cell 15:141–152
Torres MA, Dangl JL (2005) Functions of the respiratory burst oxidase in biotic interactions, abiotic stress and development. Curr Opin Plant Biol 8:397–403
Torres MA, Jones JD, Dangl JL (2005) Pathogen-induced, NADPH oxidase-derived reactive oxygen intermediates suppress spread of cell death in Arabidopsis thaliana. Nat Genet 37:1130–1134
Toumi I, Moschou PN, Paschalidis KA, Bouamama B, Ben Salem-Fnayou A, Ghorbel AW, Mliki A, Roubelakis-Angelakis KA (2010) Abscisic acid signals reorientation of polyamine metabolism to orchestrate stress responses via the polyamine exodus pathway in grapevine. J Plant Physiol 167:519–525
Tripathy BC, Oelmuller R (2012) Reactive oxygen species generation and signaling in plants. Plant Signal Behav 7:1621–1633
Vacca RA, de Pinto MC, Valenti D, Passarella S, Marra E, De Gara L (2004) Production of reactive oxygen species, alteration of cytosolic ascorbate peroxidase, and impairment of mitochondrial metabolism are early events in heat shock-induced programmed cell death in tobacco Bright-Yellow 2 cells. Plant Physiol 134:1100–1112
Voothuluru P, Sharp RE (2013) Apoplastic hydrogen peroxide in the growth zone of the maize primary root under water stress. I. Increased levels are specific to the apical region of growth maintenance. J Exp Bot 64:1223–1233
Wagner D, Przybyla D, Op den Camp R, Kim C, Landgraf F, Lee KP, Wursch M, Laloi C, Nater M, Hideg E, Apel K (2004) The genetic basis of singlet oxygen-induced stress responses of Arabidopsis thaliana. Science 306:1183–1185
Wang GF, Li WQ, Li WY, Wu GL, Zhou CY, Chen KM (2013) Characterization of rice NADPH oxidase genes and their expression under various environmental conditions. Int J Mol Sci 14:9440–9458
Wituszynska W, Szechynska-Hebda M, Sobczak M, Rusaczonek A, Kozlowska-Makulska A, Witon D, Karpinski S (2015) Lesion simulating disease 1 and enhanced disease susceptibility 1 differentially regulate UV-C-induced photooxidative stress signalling and programmed cell death in Arabidopsis thaliana. Plant Cell Environ 38:315–330
Woodson JD, Chory J (2008) Coordination of gene expression between organellar and nuclear genomes. Nat Rev Genet 9:383–395
Wrzaczek M, Brosche M, Kangasjarvi J (2013) ROS signaling loops-production, perception, regulation. Curr Opin Plant Biol 16:575–582
Xia XJ, Wang YJ, Zhou YH, Tao Y, Mao WH, Shi K, Asami T, Chen Z, Yu JQ (2009) Reactive oxygen species are involved in brassinosteroid-induced stress tolerance in cucumber. Plant Physiol 150:801–814
Xia XJ, Zhou YH, Ding J, Shi K, Asami T, Chen Z, Yu JQ (2011) Induction of systemic stress tolerance by brassinosteroid in Cucumis sativus. New Phytol 191:706–720
Xie YJ, Xu S, Han B, Wu MZ, Yuan XX, Han Y, Gu Q, Xu DK, Yang Q, Shen WB (2011) Evidence of Arabidopsis salt acclimation induced by up-regulation of HY1 and the regulatory role of RbohD-derived reactive oxygen species synthesis. Plant J Cell Mol Biol 66:280–292
Xu J, Li Y, Wang Y, Liu H, Lei L, Yang H, Liu G, Ren D (2008) Activation of MAPK kinase 9 induces ethylene and camalexin biosynthesis and enhances sensitivity to salt stress in Arabidopsis. J Biol Chem 283:26996–27006
Zeng W, He SY (2010) A prominent role of the flagellin receptor FLAGELLIN-SENSING2 in mediating stomatal response to Pseudomonas syringae pv tomato DC3000 in Arabidopsis. Plant Physiol 153:1188–1198
Zhang HJ, Fang Q, Zhang ZG, Wang YC, Zheng XB (2009a) The role of respiratory burst oxidase homologues in elicitor-induced stomatal closure and hypersensitive response in Nicotiana benthamiana. J Exp Bot 60:3109–3122
Zhang Y, Zhu H, Zhang Q, Li M, Yan M, Wang R, Wang L, Welti R, Zhang W, Wang X (2009b) Phospholipase dalpha1 and phosphatidic acid regulate NADPH oxidase activity and production of reactive oxygen species in ABA-mediated stomatal closure in Arabidopsis. Plant Cell 21:2357–2377
Zhang S, Apel K, Kim C (2014) Singlet oxygen-mediated and EXECUTER-dependent signalling and acclimation of Arabidopsis thaliana exposed to light stress. Philos Trans R Soc Lond B Biol Sci 369:20130227
Zimmermann MR, Maischak H, Mithofer A, Boland W, Felle HH (2009) System potentials, a novel electrical long-distance apoplastic signal in plants, induced by wounding. Plant Physiol 149:1593–1600
Acknowledgment
This work was supported by funding from Sophia University in Japan.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Suzuki, N. (2015). ROS as Key Players of Abiotic Stress Responses in Plants. In: Gupta, D., Palma, J., Corpas, F. (eds) Reactive Oxygen Species and Oxidative Damage in Plants Under Stress. Springer, Cham. https://doi.org/10.1007/978-3-319-20421-5_3
Download citation
DOI: https://doi.org/10.1007/978-3-319-20421-5_3
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-20420-8
Online ISBN: 978-3-319-20421-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)