Abstract
Increasing soil salinity is a major threat to crop production in many agricultural areas throughout the world. Although sodium chloride (NaCl) is one of the most abundant salts in soils, others viz. sulfate salts may also be present in high concentrations in some soil types. Sulfate salts, e.g. Na2SO4, are still widely under-represented amongst salt stress studies and the mechanism of its toxicity is poorly understood. Exposure of Chinese cabbage to Na2SO4 already reduced growth at levels ≥20 mM, accompanied by an increase in the total sulfur content of both roots and shoots, which in the shoot for a greater part could be ascribed to an accumulation of sulfate. Moreover, there was an increase in the total water-soluble non-protein thiol content (glutathione) in roots and shoots. Enhanced sulfur metabolite levels (sulfate, glutathione) would down-regulate the expression and activity of the sulfate transporters and APS reductase (glutathione). Indeed, Na2SO4 exposure resulted in a down-regulation of the sulfate uptake capacity of the roots at ≥5 mM, whereas the transcript level of the sulfate transporters Sultr1;2 and Sultr4;1 and APS reductase in the roots was reduced at ≥20 mM. Apparently in the shoot this regulatory signal transduction pathway was overruled by the toxic effects of Na2SO4, since in contrast to the roots, the transcript levels of Sultr4;1 and APS reductase were enhanced in the shoot at ≥30 mM and ≥5 mM Na2SO4, respectively.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Salt tolerance of plants and its improvement is one of the most prominent topics in crop research due to both the acuteness of the threats of salinity for agriculture and the complex physiology that underlies salt tolerance in plants (Flowers 2004; Parida and Das 2005; Peleg et al. 2011). Sulfur metabolism may have significance in the tolerance of plants to salinity. For instance, salt stress may result in an enhanced glutathione level, which presumably has adaptive significance in the protection of plants against reactive oxygen species (Noctor et al. 1998; Mittler 2002; Tausz et al. 2004; Szalai et al. 2009). The production of reactive oxygen species may be increased if Na+ accumulates in other cell compartments than the vacuole (Zhu et al. 2007). The enhanced glutathione levels appeared to be coupled to increased levels of Na+ in the cytosol, since an enhanced level of glutathione (and cysteine) was absent in transgenic Brassica napus that over-expressed a vacuolar Na+/H+ antiporter upon NaCl exposure (Ruiz and Blumwald 2002). However, the most important mechanisms in plants to avoid Na+ toxicity are the so-called includer/excluder strategies where Na+ is actively transported either back to the outside of the cell/plant and/or into the vacuole in order to prevent cytosolic Na+ accumulation (Blumwald 2000; Munns and Tester 2008). These strategies also evolved in halophytes, accompanied by anatomical adaptations such as succulence (increased cell size by salt accumulation in vacuoles) or specialized organs for salt exclusion via the leaves (salt glands). Another crucial factor is the cellular K+/Na+ ratio, which needs to be kept high in order to prevent an inhibition of enzymes regulated by K+ (Maathuis and Amtmann 1999; Tester and Davenport 2003; Chen et al. 2005; Zhu 2007; Cuin et al. 2008). In addition to NaCl plants also may have to deal with Na2SO4 salinity (Garcia and Hernandez 1996) and many areas are even dominated by sulfate salts (Chang et al. 1983; Keller et al. 1986). Although salt stress is usually mainly attributed to Na+ toxicity many studies showed that the accompanying anion might change the severity of the toxicity (e.g. Renault et al. 2001). In many species it had been shown that sulfate salinity might be more toxic than chloride salinity (Eaton 1942; Paek et al. 1988; Bilski et al. 1988; Datta et al. 1995; Renault et al. 2001). The physiological basis of the toxic effects of sulfate salinity has still to be resolved. In this study the impact of Na2SO4 salinity on the uptake, distribution and assimilation of sulfate was studied in Chinese cabbage.
Chinese cabbage ( Brassica pekinensis (Lour.) Rupr. cv. Kasumi F1 (Nickerson-Zwaan, Made, The Netherlands)) was germinated in vermiculite. Ten day-old seedlings were pre-grown on a 10 % Hoagland nutrient solution for 2 days and subsequently grown on a 25 % Hoagland nutrient solution (pH 5.9; for composition see Koralewska et al. 2007) at Na2SO4 concentrations of 0.5, 5, 10, 20, 30 and 40 mM in 30 l containers (ten sets per container, three plants per set) which were placed in a climate controlled room for 11 days. Day and night temperatures were 21 and 17 °C (±1 °C), respectively, relative humidity was 60–70 % and the photoperiod was 14 h at a photon flux rate of 230 ± 20 μmol m−2 s−1 (within the 400–700 nm range) at plant height, supplied by Philips HPI-T (400 W) lamps. After 11 days of exposure plants were harvested and shoot and root fresh weight were determined. For determination of the dry matter content, fresh plant tissue was dried at 80 °C for 24 h. For anion analysis, roots were rinsed in ice-cold de-mineralized water (for 3 × 20 s) to remove sulfate from the free space. Shoots and roots were separated, weighed, frozen in liquid N2 and stored at −20 °C until further analysis. Anions were extracted from frozen plant material and determined refractometrically after separation by HPLC (Shahbaz et al. 2010). Water -soluble non-protein thiols were extracted from freshly harvested plant tissue (Shahbaz et al. 2010) and the total water-soluble non-protein content was determined colorimetrically according to De Kok et al. (1988). For determination of the total sulfur and nitrogen contents, oven-dried plant material was pulverized by a Retsch Mixer-Mill (type MM2; Haan, Germany). Total sulfur content was determined with the barium sulfate precipitation method (Koralewska et al. 2008) and total nitrogen content was determined according to a modified Kjeldahl method (Barneix et al. 1988). Sulfate uptake capacity was determined as described by Koralewska et al. (2007). Three sets of plants (three plants per set) per treatment were transferred to 25 % Hoagland solution labeled with 35S-sulfate (2 MBq l−1) and incubated for 30 min at 30 °C, at 0.5 mM Na2SO4. Subsequently, plants were removed and roots rinsed in ice-cold non-labeled nutrient solution for 3 × 20 s. Roots and shoots were separated and digested in 1 N HCl at room temperature for 7 days. The extracts were filtered through one layer of Miracloth and 100 μl of the filtrate was mixed with 1 ml Emulsifier Scintillator Plus (Perkin Elmer, Boston, MA, USA). Radioactivity was measured with a liquid scintillation counter (TRI-CARB 2000 CA Liquid Scintillation Analyzer, Perkin Elmer, Waltham, MA, USA). Total RNA from roots and shoots was isolated by a method based on Verwoerd et al. (1989), which involved an additional phenol-chloroform-isoamyl alcohol extraction of the aqueous phase after the first centrifugation, or by using TRI REAGENT™ (SIGMA), a mixture of guanidine thiocyanate and phenol in a mono-phase solution. The final air-dried pellet was dissolved in an appropriate volume of diethyl pyrocarbonate-treated water. The quality of the RNA preparations was checked by electrophoresis of a 2 μg aliquot on a 1 % (w/v) Tris-acetate/agarose gel. The concentration was calculated from the absorbance at 260 nm in water. Determination of the expression of sulfate transporter was carried out according to Church and Gilbert (1984), with pre-hybridization and hybridization at 65 and 60 °C, respectively. Ten μg of total RNA per slot was separated on a 1.2 % (w/v) agarose/formaldehyde gel and blotted onto a positively charged nylon membrane (Hybond-N+). Sequence diversity, especially in the 3′ non-coding region, allowed the use of partial cDNA fragments for gene-specific hybridization to the respective Brassica sulfate transporter mRNA . The cDNA fragments were labeled with 32P-dCTP and used as hybridization probes. After hybridization with probes for sulfate transporters, membranes were washed at 65 °C twice with 2xSSC, 0.1 % SDS for 5 and 30 min, once with 1xSSC, 0.1 % SDS and twice with 0.1xSSC, 0.1 % SDS for at least 30 min each, and exposed to Kodak BioMax MS film or to Cyclone MultiPurpose Phosphor Screen (Perkin Elmer, UK). Statistical analysis was performed with an unpaired Student’s t-test.
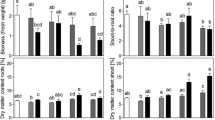
Although in natural ecosystems Brassicaceae species occur in dry and saline habitats and even in extreme sulfur-enriched gypsum-bearing soils (Ernst 1990; Dixon 2007), some of the modern cultivated hybrids and cultivars appear to be very sensitive to sulfate salinity. The results of the present study showed that the shoot growth of Chinese cabbage was already inhibited at 20 mM Na2SO4 (Fig. 1). The shoot growth was slightly more susceptible to salt stress than root growth and the latter was only significantly reduced at 40 mM Na2SO4, resulting in a slight decrease in the shoot to root ratio at ≥30 mM Na2SO4. Dry matter content of shoots was enhanced at Na2SO4 concentrations ≥30 mM, up to twofold at 40 mM, whereas that of roots was only slightly enhanced at 40 mM Na2SO4 (Fig. 2).
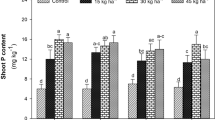
Exposure of plants to Na2SO4 salinity resulted in an increase in the total sulfur content of both roots and shoots (Fig. 3), which in the shoot for a greater part could be ascribed to an accumulation of sulfate (Fig. 4). The sulfate content increased gradually with the Na2SO4 concentration, but it was strongly enhanced at 40 mM Na2SO4 and its content in roots and shoots was increased 1.5-fold and fourfold, respectively. Apparently, the regulatory control of the uptake of sulfate by the roots was overruled at Na2SO4 concentrations exceeding 30 mM. In both shoots and roots, total nitrogen and nitrate decreased with the Na2SO4 concentration, indicating that the uptake and assimilation of nitrate was negatively affected by sulfate salinity (Figs. 3 and 4). Similar to previous observations there was apparently no direct linkage between the uptake and assimilation of sulfate and nitrate (Stulen and De Kok 2012).
Similar to observations with NaCl (Ruiz and Blumwald 2002), Na2SO4 salinity resulted in an increase in the total water-soluble non-protein thiol content (presumably GSH ) in roots and shoots at concentrations ≥20 mM (Fig. 5). Evidently this increase remained relatively low and only at toxic Na2SO4 concentrations, and at sulfate levels in shoots fourfold higher than that of the control, a substantial increase of thiol content (twofold) occurred. From the present data, the increased thiol/glutathione level appears to be a consequence of the excessive sulfate accumulation and not an adaptive protective response against salinity.
Sulfate uptake plays a major role in the control of plant sulfur homeostasis (Vauclare et al. 2002). The uptake and distribution of sulfate is mediated by distinct sulfate transporters, which activity may be controlled at a transcriptional, translational and/or post-translational level, and is regulated by the plant sulfur requirement for growth (Hawkesford and De Kok 2006; De Kok et al. 2011). Sulfate salinity had a substantial effect on the expression and activity of the sulfate transporters and the expression of APS reductase of Chinese cabbage ( APR; Fig. 6). However, there were considerable differences in response of the different sulfate transporters and APS reductase between roots and shoots of Chinese cabbage. The Group 1 transporters are responsible for the primary uptake of sulfate by the root and in sulfate-sufficient Brassica species only Sultr1;2 is expressed (Hawkesford and De Kok 2006; Koralewska et al. 2007, 2008, 2009; De Kok et al. 2011). Also upon Na2SO4 salinity Sultr1;2 was the sole Group 1 sulfate transporter expressed in roots and the transcript levels of Sultr1;1 were negligible (Fig. 6). Na2SO4 salinity resulted in a decreased expression of Sultr1;1 in the roots at ≥30 mM, whereas the sulfate uptake capacity was already decreased ≥5 mM. The latter further decreased with the Na2SO4 concentration and was reduced more than 2.5-fold at 40 mM (Fig. 6). Apparently, Na2SO4 salinity affected the regulation of the sulfate transporters in the roots already at lower concentrations at translational and/or post translational than at transcriptional level. The Group 4 transporters are involved in the vacuolar efflux of sulfate (Hawkesford 2003; Kataoka et al. 2004; Hawkesford and De Kok 2006; De Kok et al. 2011) and there was also a decrease in the transcript level of Sultr4;1 in roots at 40 mM Na2SO4, whereas Sultr4;2 was hardly expressed at all. The latter was in agreement with previous observations that Sultr1;1 and Sultr4;2 were only expressed in sulfate-deprived Brassica tissue (Koralewska et al. 2009; De Kok et al. 2012; Shahbaz et al. 2014). The transcript level of APR, the key regulating enzyme in the sulfate reduction pathway (Hawkesford and De Kok 2006; De Kok et al. 2011) was also reduced in the roots at ≥30 mM (Fig. 6). Sultr1;1 was hardly and Sultr4;2 was only slightly expressed in both roots and shoots. Sulfate salinity only resulted in an increased transcript level of Sultr4;1 and Sultr4;2 at 40 mM Na2SO4, whereas that of APR was increased at ≥5 mM Na2SO4 (Fig. 6). Of the Group 1 and 4 transporters, only Sulftr4;2 was substantially expressed in the shoot of Chinese cabbage (Fig. 5). Contrary to the observations in the root, its transcript levels increased at ≥30 mM Na2SO4, together with that of APS reductase (Fig. 6). The latter needs to be further investigated. It has been suggested that sulfate itself, or reduced sulfur compounds, may have a role in the regulation of expression and activity of the sulfate transporters and APS reductase (Hawkesford and De Kok 2006; De Kok et al. 2011). For instance, high tissue levels of these compounds would down-regulate the expression and activity of the sulfate transporters (sulfate, glutathione) and APS reductase (glutathione). Indeed, in the roots this relationship between the content of these sulfur compounds and the expression and activity of the sulfate transporters and expression of APS reductase does exist although in the shoot the toxic effects of Na2SO4 salinity apparently overruled this regulatory signal transduction pathway.
The impact of Na2SO4 salinity on sulfate uptake capacity and gene expression of sulfate transporters (Sultr) and APS reductase (APR; Northern blot analysis) of shoot and roots of Chinese cabbage. Equal RNA loading was determined by ethidium bromide staining of gels (shown in the bottom panels). Data on sulfate uptake capacity represent the mean of three measurements with three plants in each ±SD (* = p < 0.01; unpaired Student’s t-test)
Sulfate salinity was described as having a greater inhibitory effect on growth than chloride salinity in wheat (Datta et al. 1995), sugar beet and tomato (Eaton 1942), wild potato (Bilski et al. 1988), pepper (Navarro et al. 2003) and on germination in barley (Huang and Redmann 1995), alfalfa (Redmann 1974) and wheat (Hampson and Simpson 1990). Comparative studies within Brassica species are still very scarce. Additionally, the results of a study by Paek et al. (1988) on calli of B. campestris revealed that Na2SO4 had a stronger negative impact on biomass. The authors of the study also noted that sulfate accumulated much less under Na2SO4 than chloride under NaCl salinity. This unequal uptake of Na+ and its anion under sulfate salinity could explain the increased inhibitory effect on growth (also concluded by Meiri et al. 1971; Navarro et al. 2003). Another observation from older studies is that excess sulfate inhibits calcium uptake (Hayward and Wadleigh 1949) but this also holds true for NaCl and the application of additional calcium usually leads to an amelioration of salt stress (Cramer 2002; Kaya et al. 2002; Shabala et al. 2006). However, Johansen and Loneragan (1975) observed that Na2SO4 reduced 75 % of K+ uptake compared to the absence of Na+ while the same concentration of NaCl reduced it only by 50 %. A link to cation homeostasis therefore seems likely. Interestingly, Na+ accumulated mainly in shoots when jack pines were exposed to NaCl whereas it mainly accumulated in the roots under Na2SO4 salinity (Apostol et al. 2002). This is another hint that the translocation to the shoot and its control might be a crucial process under salt stress. Besides promoting the toxicity of Na+, sulfate could also have direct toxic effects (Visscher et al. 2010).
Salt tolerance is known to be a physiological complex and trait, which challenges attempts at improvement. As Brassica is a diverse genus with high agricultural importance, many efforts have been made to identify and develop salt tolerant cultivars. Salt tolerance in B. napus , for example, could be increased tremendously in transgenic plants with an enhanced Na+ accumulation in the vacuole (Zhang et al. 2001). In another transgenic approach, plants of B. juncea with an introduced bacterial pathway for the synthesis of glycine-betaine showed increased germination rates and seedling growth (Prasad et al. 2000) but the occurrence of sulfate salinity and its increased toxicity compared with chloride salinity also suggests correlations of salt tolerance with sulfate uptake, assimilation and whole plant distribution. More research needs to be carried out on the toxicity of excessive sulfate and its possible role in exacerbating Na+ toxicity.
References
Apostol KG, Zwiazek JJ, MacKinnon MD (2002) NaCl and Na2SO4 alter responses of jack pine (Pinus banksiana) seedlings to boron. Plant Soil 240:321–329
Barneix AJ, Cooper HD, Stulen I, Lambers H (1988) Metabolism and translocation of nitrogen in two Lolium perenne populations with contrasting rates of mature leaf respiration and yield. Physiol Plant 72:631–636
Bilski JJ, Nelson DC, Conlon RL (1988) Response of six wild potato species to chloride and sulfate salinity. Am Potato J 65:605–612
Blumwald E (2000) Sodium transport and salt tolerance in plants. Curr Opin Cell Biol 12:431–434
Chang C, Sommerfeldt TG, Carefoot JM, Schaalje GB (1983) Relationships of electrical conductivity with total dissolved salts and cation concentration of sulfate-dominant soil extracts. Can J Soil Sci 63:79–86
Chen Z, Newman I, Zhou M, Mendham N, Zhang G, Shabala S (2005) Screening plants for salt tolerance by measuring K+ flux: a case study for barley. Plant Cell Environ 28:1230–1246
Church GM, Gilbert W (1984) Genomic sequencing. Proc Natl Acad Sci U S A 81:1991–1995
Cramer GR (2002) Sodium-calcium interactions under salinity stress. In: Läuchli A, Lüttge U (eds) Salinity: environment-plants-molecules. Springer, Dordrecht, pp 205–227
Cuin TA, Betts SA, Chalmandrier R, Shabala S (2008) A root’s ability to retain K+ correlates with salt tolerance in wheat. J Exp Bot 59:2697–2706
Datta KS, Kumar A, Varma SK, Angrish R (1995) Differentiation of chloride and sulphate salinity on the basis of ionic distribution in genetically diverse cultivars of wheat. J Plant Nutr 18:2199–2212
De Kok LJ, Buwalda F, Bosma W (1988) Determination of cysteine and its accumulation in spinach leaf tissue upon exposure to excess sulfur. J Plant Physiol 133:502–505
De Kok LJ, Stulen I, Hawkesford MJ (2011) Sulfur nutrition in crop plants. In: Hawkesford MJ, Barraclough P (eds) The molecular basis of nutrient use efficiency in crops. Wiley-Blackwell, Oxford, pp 295–309
De Kok LJ, Stuiver CEE, Shahbaz M, Koralewska A (2012) Regulation of expression of sulfate transporters and APS reductase in leaf tissue of Chinese cabbage (Brassica pekinensis). In: De Kok LJ, Tausz M, Hawkesford MJ, Hoefgen R, McManus MT, Norton RM, Rennenberg H, Saito K, Schnug E, Tabe L (eds) Sulfur metabolism in plants: mechanisms and application to food security, and responses to climate change. Springer, Dordrecht, pp 47–52
Dixon GR (2007) Vegetable brassicas and related crucifers (No. 14). CABI, Cambridge, MA
Eaton FM (1942) Toxicity and accumulation of chloride and sulfate salts in plants. J Agr Res 64:357–399
Ernst WHO (1990) Ecological aspects of sulfur metabolism. In: Rennenberg H, Brunold C, De Kok LJ, Stulen I (eds) Sulfur nutrition and sulfur assimilation in higher plants – fundamental environmental and agricultural aspects. SPB Academic Publishing, The Hague, pp 131–144
Flowers TJ (2004) Improving crop salt tolerance. J Exp Bot 55:307–319
Garcia C, Hernandez T (1996) Influence of salinity on the biological and biochemical activity of a calciorthird soil. Plant Soil 178:255–263
Hampson CR, Simpson GM (1990) Effects of temperature, salt, and osmotic potential on early growth of wheat (Triticum aestivum). I. Germination. Can J Bot 68:524–528
Hawkesford MJ (2003) Transporter gene families in plants: the sulphate transporter gene family – redundancy or specialization? Physiol Plant 117:155–163
Hawkesford MJ, De Kok LJ (2006) Managing sulfur metabolism in plants. Plant Cell Environ 29:382–395
Hayward HE, Wadleigh CH (1949) Plant growth on saline and alkali soils. Adv Agron 1:1–38
Huang J, Redmann RE (1995) Salt tolerance of Hordeum and Brassica species during germination and early seedling growth. Can J Plant Sci 75:815–819
Johansen C, Loneragan JF (1975) Effects of anions and cations on potassium absorption by plants of high potassium chloride content. Funct Plant Biol 2:75–78
Kataoka T, Watanabe-Takahashi A, Hayashi N, Onishi M, Mimura T, Buchner P, Hawkesford MJ, Yamaya T, Takahashi H (2004) Vacuolar sulfate transporters are essential determinants controlling internal distribution of sulfate in Arabidopsis. Plant Cell 16:2693–2704
Kaya C, Kirnak H, Higgs D, Saltali K (2002) Supplementary calcium enhances plant growth and fruit yield in strawberry cultivars grown at high (NaCl) salinity. Sci Hortic 93:65–74
Keller LP, McCarthy GJ, Richardson JL (1986) Mineralogy and stability of soil evaporites in North Dakota. Soil Sci Soc Am J 50:1069–1071
Koralewska A, Posthumus FS, Stuiver CEE, Buchner P, Hawkesford MJ, De Kok LJ (2007) The characteristic high sulfate content in Brassica oleracea is controlled by the expression and activity of sulfate transporters. Plant Biol 9:654–661
Koralewska A, Stuiver CEE, Posthumus FS, Kopriva S, Hawkesford MJ, De Kok LJ (2008) Regulation of sulfate uptake, expression of the sulfate transporters Sultr1;1 and Sultr1;2, and APS reductase in Chinese cabbage (Brassica pekinensis) as affected by atmospheric H2S nutrition and sulfate deprivation. Funct Plant Biol 35:318–327
Koralewska A, Buchner P, Stuiver CEE, Posthumus FS, Kopriva S, Hawkesford MJ, De Kok LJ (2009) Expression and activity of sulfate transporters and APS reductase in curly kale in response to sulfate deprivation and re-supply. J Plant Physiol 166:168–179
Maathuis FJ, Amtmann A (1999) K+ nutrition and Na+ toxicity: the basis of cellular K+/Na+ ratios. Ann Bot 84:123–133
Meiri A, Kamburoff J, Poljakoff-Mayber A (1971) Response of bean plants to sodium chloride and sodium sulphate salinization. Ann Bot 35:837–847
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7:405–410
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Annu Rev Plant Biol 59:651–681
Navarro JM, Garrido C, Martínez V, Carvajal M (2003) Water relations and xylem transport of nutrients in pepper plants grown under two different salts stress regimes. Plant Growth Regul 41:237–245
Noctor G, Arisi ACM, Jouanin L, Kunert KJ, Rennenberg H, Foyer CH (1998) Glutathione: biosynthesis, metabolism and relationship to stress tolerance explored in transformed plants. J Exp Bot 49:623–647
Paek KY, Chandler SF, Thorpe TA (1988) Physiological effects of Na2SO4 and NaCl on callus cultures of Brassica campestris (Chinese cabbage). Physiol Plant 72:160–166
Parida AK, Das AB (2005) Salt tolerance and salinity effects on plants: a review. Ecotoxicol Environ Saf 60:324–349
Peleg Z, Apse MP, Blumwald E (2011) Engineering salinity and water-stress tolerance in crop plants: getting closer to the field. Adv Bot Res 57:405–443
Prasad KVSK, Sharmila P, Kumar PA, Saradhi PP (2000) Transformation of Brassica juncea (L.) Czern with bacterial codA gene enhances its tolerance to salt stress. Mol Breeding 6:489–499
Redmann RE (1974) Osmotic and specific ion effects on the germination of alfalfa. Can J Bot 52:803–808
Renault S, Croser C, Franklin JA, Zwiazek JJ (2001) Effects of NaCl and Na2SO4 on red-osier dogwood (Cornus stolonifera Michx) seedlings. Plant Soil 233:261–268
Ruiz J, Blumwald E (2002) Salinity-induced glutathione synthesis in Brassica napus. Planta 214:965–969
Shabala S, Demidchik V, Shabala L, Cuin TA, Smith SJ, Miller AJ, Davies JM, Newman IA (2006) Extracellular Ca2+ ameliorates NaCl-induced K+ loss from Arabidopsis root and leaf cells by controlling plasma membrane K+-permeable channels. Plant Physiol 141:1653–1665
Shahbaz M, Hwei Tseng M, Stuiver CEE, Koralewska A, Posthumus FS, Venema JH, Parmar S, Schat H, Hawkesford MJ, De Kok LJ (2010) Copper exposure interferes with the regulation of the uptake, distribution and metabolism of sulfate in Chinese cabbage. J Plant Physiol 167:438–446
Shahbaz M, Stuiver CEE, Posthumus FS, Parmar S, Hawkesford MJ, De Kok LJ (2014) Copper toxicity in Chinese cabbage is not influenced by plant sulfur status, but interferes with correlations between sulfur metabolism-related gene expression and the suggested regulatory metabolites. Plant Biol 16:68–78
Stulen I, De Kok LJ (2012) Exploring interactions between sulfate and nitrate uptake at a whole plant level. In: De Kok LJ, Tausz M, Hawkesford MJ, Hoefgen R, McManus MT, Norton RM, Rennenberg H, Saito K, Schnug E, Tabe L (eds) Sulfur metabolism in plants: mechanisms and application to food security, and responses to climate change. Springer, Dordrecht, pp 1–8
Szalai G, Kellős T, Galiba G, Kocsy G (2009) Glutathione as an antioxidant and regulatory molecule in plants under abiotic stress conditions. J Plant Growth Regul 28:66–80
Tausz M, Šircelj H, Grill D (2004) The glutathione system as a stress marker in plant ecophysiology: is a stress-response concept valid? J Exp Bot 55:1955–1962
Tester M, Davenport R (2003) Na+ tolerance and Na+ transport in higher plants. Ann Bot 91:503–527
Vauclare P, Kopriva S, Fell D, Suter M, Sticher L, Von Ballmoos P, Krähenbühl U, Den Camp RO, Brunold C (2002) Flux control of sulphate assimilation in Arabidopsis thaliana: adenosine 5′-phosphosulphate reductase is more susceptible than ATP sulphurylase to negative control by thiols. Plant J 32:729–740
Verwoerd TC, Dekker BM, Hoekema A (1989) A small-scale procedure for the rapid isolation of plant RNAs. Nucleic Acids Res 17:2362
Visscher AM, Paul AL, Kirst M, Guy CL, Schuerger AC, Ferl RJ (2010) Growth performance and root transcriptome remodeling of Arabidopsis in response to Mars-like levels of magnesium sulfate. PLoS One 5:e12348
Zhang HX, Hodson JN, Williams JP, Blumwald E (2001) Engineering salt-tolerant Brassica plants: characterization of yield and seed oil quality in transgenic plants with increased vacuolar sodium accumulation. Proc Natl Acad Sci U S A 98:12832–12836
Zhu J, Fu X, Koo YD, Zhu JK, Jenney FE, Adams MW, Zhu Y, Shi H, Yun DY, Hasegawa PM, Bressan RA (2007) An enhancer mutant of Arabidopsis salt overly sensitive 3 mediates both ion homeostasis and the oxidative stress response. Mol Cell Biol 27:5214–5224
Zhu JK (2007) Plant salt stress. In: Encyclopedia of life sciences. Wiley, Chichester
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Reich, M., Aghajanzadeh, T., Stuiver, C.E.E., Koralewska, A., De Kok, L.J. (2015). Impact of Sulfate Salinity on the Uptake and Metabolism of Sulfur in Chinese Cabbage. In: De Kok, L., Hawkesford, M., Rennenberg, H., Saito, K., Schnug, E. (eds) Molecular Physiology and Ecophysiology of Sulfur. Proceedings of the International Plant Sulfur Workshop. Springer, Cham. https://doi.org/10.1007/978-3-319-20137-5_25
Download citation
DOI: https://doi.org/10.1007/978-3-319-20137-5_25
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-20136-8
Online ISBN: 978-3-319-20137-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)