Abstract
Aromatic amines are widely used industrial chemicals as their major sources in the environment include several chemical industry sectors such as oil refining, synthetic polymers, dyes, adhesives, rubbers, perfume, pharmaceuticals, pesticides and explosives. They result also from diesel exhaust, combustion of wood chips and rubber and tobacco smoke. Some types of aromatic amines are generated during cooking, special grilled meat and fish, as well. The intensive use and production of these compounds explains its occurrence in the environment such as in air, water and soil, thereby creating a potential for human exposure. Since aromatic amines are potential carcinogenic and toxic agents, they constitute an important class of environmental pollutants of enormous concern, which efficient removal is a crucial task for researchers, so several methods have been investigated and applied.
In this chapter the types and general properties of aromatic amine compounds are reviewed. As aromatic amines are continuously entering the environment from various sources and have been designated as high priority pollutants, their presence in the environment must be monitored at concentration levels lower than 30 mg L−1, compatible with the limits allowed by the regulations. Consequently, most relevant analytical methods to detect the aromatic amines composition in environmental matrices, and for monitoring their degradation, are essential and will be presented. Those include Spectroscopy, namely UV/visible and Fourier Transform Infrared Spectroscopy (FTIR); Chromatography, in particular Thin Layer (TLC), High Performance Liquid (HPLC) and Gas chromatography (GC); Capillary electrophoresis (CE); Mass spectrometry (MS) and combination of different methods including GC-MS, HPLC-MS and CE-MS. Choosing the best methods depend on their availability, costs, detection limit and sample concentration, which sometimes need to be concentrate or pretreated. However, combined methods may give more complete results based on the complementary information. The environmental impact, toxicity and carcinogenicity of many aromatic amines have been reported and are emphasized in this chapter too.
Lately, the conventional aromatic amines degradation and the alternative biodegradation processes are highlighted. Parameters affecting biodegradation, role of different electron acceptors in aerobic and anaerobic biodegradation and kinetics are discussed. Conventional processes including extraction, adsorption onto activated carbon, chemical oxidation, advanced oxidation, electrochemical techniques and irradiation suffer from drawbacks including high costs, formation of hazardous by-products and low efficiency. Biological processes, taking advantage of the naturally processes occurring in environment, have been developed and tested, proved as an economic, energy efficient and environmentally feasible alternative. Aerobic biodegradation is one of the most promising techniques for aromatic amines remediation, but has the drawback of aromatic amines autooxidation once they are exposed to oxygen, instead of their degradation. Higher costs, especially due to power consumption for aeration, can also limit its application. Anaerobic degradation technology is the novel path for treatment of a wide variety of aromatic amines, including industrial wastewater, and will be discussed. However, some are difficult to degrade under anaerobic conditions and, thus, other electron acceptors such as nitrate, iron, sulphate, manganese and carbonate have, alternatively, been tested.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
7.1 Introduction
Aromatic amines are widespread chemicals with considerable industrial and environmental importance (Fig. 7.1). Their major sources include several industrial sectors such as oil refining, dyes, cosmetics, medicines, rubber, textiles, agrochemicals, explosives and as reagent intermediates in many chemical syntheses synthetic polymers, dyes, adhesives and rubbers, pharmaceuticals, pesticides and explosives (Palmiotto et al. 2001). They are also found in environmental pollution such as diesel exhaust, combustion of wood chips and rubber, tobacco smoke, and substances in grilled meats and fish (DeBruin 1999). They are also used in the synthesis of organic colorants widely used in the textile, paper, leather, plastics, cosmetics, drugs, and food industries. Azo dye reduction produces aromatic amines that are generally higher toxic than the original dyes (Van der Zee and Villaverde 2005; Pinheiro et al. 2004). Consequently, aromatic amines appear in different environments, such as in air, water and soil thereby creating a potential for human exposure. Since these compounds are potential carcinogenic and toxic agents, they constitute an important class of environmental pollutants of enormous concern which efficient removal is a crucial task for researchers. Table 7.1 shows some examples of aromatic amines, and their major origins, known to be potential hazards to human health and to the environment.
Aromatic compounds constitute the second most abundant family of organic constituents present in the biosphere, after carbohydrates. Since the start of the industrial revolution, a wide variety of aromatic pollutants have also been introduced into the environment through anthropogenic activity (Bull et al. 2011; Carmona et al. 2009). The thermodynamic stability of the benzene ring, due to its resonance structure, has contributed to the widespread production and industrial use of natural and xenobiotic aromatic compounds, but has also contributed to the persistence of these compounds, many of which are toxic when released into the environment. Aromatic amines range from simplest aniline to highly complex molecules with conjugated aromatic or heterocyclic structures and multiple substituents. About 300 chemical products and intermediates are currently manufactured from aniline. According to the USA National Toxicology Program 2005, among the 415 chemicals recognized or suspected to be carcinogenic in humans, 12% are aromatic amines. Due to the wide use of aromatic amines together with the presence of relatively specific and industrial importance, very high exposures has determined the large toxicological experimentation and permitted the development of epidemiological knowledge unparalleled for other chemical classes. The US Environmental Protection Agency (EPA) has confirmed that, since 1970, several extremely toxic and potentially carcinogenic aromatic amines have been included in the list of priority pollutants (Sun et al. 2012a). Table 7.2 lists the aromatic amines banded in Europe (EU Directive 2002/61/EC).
Due to their high solubility in water, the aromatic amines can easily penetrate through the soil and enter into the water cycle in various forms, either in chemical effluents or as the breakdown products of herbicides. Their presence in ground waters or soil samples subject to industrial, agricultural or urban pollution is an increasing concern (Gan et al. 2004). Therefore, they constitute an important and diversified class of pollutants. Many of them are toxic to most living organisms due to their genotoxic or cytotoxic properties (Bull et al. 2011; Kim and Guengerich 2005).
In order to protect human health and environmental safety, it is important to monitor aromatic amines in water, with sensitive and reliable methods. In recent years an extensive research activity has been directed towards developing processes to efficiently remove highly complex structures of aromatic contaminants, including aromatic amines, from polluted water. Conventional processes for the removal of aromatic amines from industrial wastewaters include extraction, adsorption onto activated carbon, chemical oxidation, advanced oxidation, electrochemical techniques and irradiation. All of these methods suffer from drawbacks including high costs, formation of hazardous by-products and low efficiency (Franciscon et al. 2010; Mondal et al. 2010). Alternative biological methods appear to be a potentially economic, energy efficient and environmentally feasible option (Rieger et al. 2002).
Biological treatment of aromatic amines containing wastewaters, provides more specific conversions, is relatively inexpensive and usually results in complete mineralization. Microorganisms have evolved to degrade most naturally occurring organic compounds, including the persistent aromatics. Moreover, the promiscuity of the catabolic enzymes allows them to degrade, at least partially, xenobiotics that share similar structures with naturally occurring aromatic compounds (Díaz 2004). The bacterial catabolism of aromatic compounds involves several peripheral pathways that transform structurally diverse substrates into a limited number of intermediates that are further processed by a few central pathways to the central metabolism of the cell (McLeod and Eltis 2008). There are two major strategies to degrade aromatic compounds depending on the presence or absence of oxygen. In the aerobic catabolism of aromatics, oxygen is not only the final electron acceptor but also a co-substrate. In contrast, the anaerobic catabolism of aromatic compounds uses a completely different strategy, based on reductive reactions, to attack the aromatic ring. While the aerobic catabolism of aromatic compounds has been studied for several decades (Brown and Laboureur 1983; Pinheiro et al. 2004; Van der Zee and Villaverde 2005), this may not apply to all aromatic amines. Among the many different aromatic amines tested, only a few were degraded. Some of them, substituted with hydroxyl or carboxyl group, were degraded under methanogenic and sulphate reducing conditions (Kalyuzhnyi et al. 2000; Razo-Flores et al. 1999). It has been demonstrated that especially sulfonated aromatic amines are often difficult to degrade (Razo-Flores et al. 1996, 1997a; Tan et al. 2005). A drawback of using aerobic treatment, with the aim of degrading aromatic amines from azo dye cleavage, is that many of them are prone to autoxidation once they are exposed to oxygen. Since autoxidation often involves enlargement of the molecules, their biodegradability may consequently decrease. Alternatively, nitrate, instead of oxygen, can be used as electron acceptor (Pereira et al. 2011). Indeed, several ecosystems are characterized by lack of oxygen, such as aquatic sediments, stratified lakes, wetlands and some soil horizons. In those environments, microorganisms can utilize compounds like nitrate, iron, sulphate, manganese and carbonate as electron acceptors. It has been reported that at least some aromatic amines can be degraded coupled to nitrate reduction (Kahng et al. 2000; Pereira et al. 2011; Vázquez-Rodriguez et al. 2008; Wu et al. 2007).
The anaerobic degradation of aromatics is a more recently discovered microbial capacity that still awaits a deeper understanding despite the fact that microbial metabolism in the absence of oxygen is the most ancient of all life processes (Lovley 2001). In fact, many habitats containing large amounts of aromatic compounds are often anoxic, e.g., aquifers, aquatic sediments and submerged soils, sludge digesters, and intestinal contents, and at aerobic sites with high carbon concentrations, molecular oxygen is more rapidly consumed than replenished (Lovley 2003). Thus, anoxic conditions dominate in many natural habitats and contaminated sites, and the anaerobic catabolism of aromatic compounds by microorganisms becomes crucial for the biogeochemical cycles and for the sustainable development of the biosphere. The mineralization of aromatic compounds by facultative or obligate anaerobic bacteria (and some archaea) can be coupled to anaerobic respiration with a variety of electron acceptors, e.g., nitrate, sulfate, iron(III), manganese(II), and selenate, with each one conserving different yields of energy. The greatest energy conservation is reached when nitrate is the final electron acceptor, followed by ferric ion.
In this chapter, aromatic amines, sources and their environmental impact is outlined. The available methods for aromatic amines monitoring are described and degradation methods are reviewed, with special emphasis on biodegradation. Various parameters affecting the biodegradation, such as the type of electron acceptors, and some kinetic considerations are revised.
7.2 Types of Aromatic Amines and Structure
Aromatic amines are generally identified as those chemical compounds having in their molecular structure one or more aromatic rings, with one or more amino substituents. They range from the simplest aniline to highly complex molecules with conjugated aromatic or heterocyclic structures (Fig. 7.2). Therefore, they can be classified in three types: monocylic, polycyclic and heterocyclic. They contain single or multiple aromatic rings bonded to nitrogen aryl groups. Heterocyclic aromatic amines are also bearing one or more amino substituents with different functional group. The common denominator is an amino group bound to an aromatic system. The activity of aromatic amine depends upon the position and structure of amine group and aromatic ring, respectively. They are the second most abundant family of organic constituents present in the biosphere after carbohydrates. Since the start of the industrial revolution, a wide variety of aromatic pollutants have also been introduced into the environment through anthropogenic activity (Fekete et al 2010). Some examples or aromatic amines, their origin and impact are listed in Table 7.1.
Aniline, which is essentially phenylamine, is the simplest aromatic amine. Commercial aniline can be chemically synthesized from nitrobenzene which is prepared from benzene with nitric acid by electrophilic substitution reaction, as shown in Fig. 7.3, or from chlorobenzene by heating with ammonia in the presence of a copper catalyst. Some aromatic amines are natural, such as 2- and 4-aminobenzoic acids, others are xenobiotics, like 3-aminobenzoic acid, aminosalicylates, aniline and aminophenols. In addition, they can result from chemical or bio transformation of other organic compounds. Several substituted phenylenediamines, the benzenediamines, are intermediates in the synthesis of polyurethanes, and others are used in the dyestuff industry (Chung 2000). Certain substituted-benzenediamines are important commercial ingredients in semipermanent and permanent hair dyes (Garrigue et al. 2006; Nohynek et al. 2010). Non-industrial sources of aromatic amines are the combustion of tobacco, automobile exhaust fumes, the burning/pyrolysis of protein-rich vegetable matter, cooking and subsequent consumption of meats and they are also present in road tars (Lewtas 2007). Heterocyclic aromatic amines are formed, along with Polycyclic aromatic amines, when meats or fish are grilled or otherwise cooked at high temperatures (Combes and Haveland-Smith 1982). Heterocyclic aromatic amines are the major mutagenic compounds isolated from broiled and grilled meats and fish and have been shown to induce tumours in multiple organs, including the colon and mammary gland, in rodent bioassays (Melo et al. 2008). Depending on their chemical structure and their mechanism of formation, these xenobiotic genotoxic substances can be grouped into two main families. The first named 2-amino-3-methylimidazo[4,5-f]quinoline (IQ) type or aminoimidazoazaarenes, includes amines containing a 2-aminoimidazole group generated from the reaction of free amino acids (especially creatine and creatinine) and hexoses at ordinary cooking temperatures. The other amines, called non-IQ type or pyrolytic, are formed through the pyrolytic reaction of amino acids and proteins at temperatures between 200 and 300 °C (Szterk et al. 2012; Toribio et al. 2002).
Primary aromatic amines are used as a starting material for the manufacture of azo dyes. This class of dyes is often used in the colouring process of textiles and leather. Once these compounds hold the functional azo group -N = N-, they have the capacity to release certain aromatic amines by the reduction of this azo bond. Because many different types of sulfonated azo dyes are currently being utilized, a wide variety of sulfonated aromatic amines will be formed under anaerobic conditions that will not easily be biodegraded and will constitute an important part of untreated Chemical Oxygen Demand fraction in azo dyes containing wastewater treatment. A significant fraction of the alkylanilines is used in the synthesis of dyes and may be release after dye reduction. Examples of alkylanilines include 2-methylaniline, 3-methylaniline, 4-methylaniline, 2,3-dimethylaniline, 2,4-dimethylaniline, 2,5-dimethylaniline, 2,6-dimethylaniline, 3,4-dimethylaniline, 3,5-dimethylaniline, 2-ethylaniline, 3-ethylaniline and 4-ethylaniline. This group of aromatic amines are present in the environment as a result of other sources as well: a fraction of them is used as intermediate in the synthesis of pharmaceuticals, agrichemicals, that are example 2, 6-diethylamine and 2-methyl-6-ethylamine involved in the synthesis of chloroacetanilide herbicides, and photographic chemicals. A subclass alkylanilines, arylamines, has been documented in cigarette smokers (Skipper et al. 2010).
7.3 Analysis of Aromatic Amines
As aromatic amines are continuously entering the environment from various sources and have been designated as high priority pollutants, their presence in the environment must be monitored at concentration levels lower than 30 mg L−1, compatible with the limits allowed by the regulations. Efforts towards the development of accurate, reproducible and low detection limit methods for the quantification or aromatic amines in the environment have been made. Determination and monitoring of aromatic amines during their treatment and of the intermediates and final reaction products is also necessary. Methods based on voltammetry (Chey and Adams 1977), potentiometry with specific electrodes (Vytras et al. 1982) and spectrophotometric quantification after a specific colour-generating reaction or derivatisation to a chromophore (Verma et al. 1988; Zatar et al. 1998) were firstly proposed. However, despite the fact that the earliest simpler methods are not being discharged and are sometimes very useful, due to their poor sensitivity and selectivity they have been gradually replaced by modern advanced methods. Those include gas chromatography (GC) and high-performance liquid chromatography (HPLC) coupled with different detectors. Mass spectrometry (MS) and capillary electrophoresis (CE) are nowadays also common methods for determining aromatic amines (Table 7.3). Combined methods such as GC-MS, HPLC-MS and CE-MS have been also applied and will be discussed in this chapter. The most common disadvantages of the methods are the detection limits and need long pre-concentration processes for a good sensitivity. The costs involved, particularly in instrumentation and skilled staff requirements are also factors in consideration. Additionally, most of the times samples cannot be analyzed directly by instrumental methods and pretreatment is often considered as an indispensable step prior to determination and quantification methods (Moradi et al. 2010). Many methods have been reported for the extraction of aromatic amines from environmental water samples and will be described below.
Standard methods for aromatic amines arising from the reductive cleavage of azo dyes have been established in Europe, such as the French norm AFNOR XP G08-014 for dyed textiles or the German method DIN 53316 for dyed leather (Pinheiro et al. 2004).
7.3.1 Spectroscopy
7.3.1.1 UV/Visible
UV/visible spectroscopy is a very useful method for the routine monitoring of industrial effluent discharges. In addition, direct UV/visible spectrophotometry can be an ideal technique for the monitoring of treatment processes such as biodegradation, chemical oxidation and reduction, photo-oxidation, photolysis and adsorption, operated for the removal of residual amines and other aromatics.
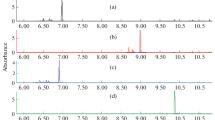
Spectrophotometry in the ultraviolet (UV) range has repeatedly proven to be a fast, inexpensive and reliable method for the monitoring of many compounds in urban and industrial wastewaters (Narayana and Sunil 2009; Pinheiro et al. 2004). Through the application of spectral analysis, quantitative and qualitative wastewater parameters can be estimated on direct samples in just a few minutes, using portable or online field instrumentation. Perez (2001) has successfully applied UV spectral deconvolution on wastewater monitoring in a chemical industry, for the estimation of aniline derivative concentrations. In the case of textile effluents, the use of the UV range of the spectra (200–350 nm) for aromatic amine determination is particularly useful to avoid interference by visible colour of dyes. The characteristic absorption of dyes in the visible region (400–700 nm), where most of the aromatic amines show little absorption, provides a way to monitor azo dye reduction with aromatic amines formation and independently assess their residual concentration level. Many researchers have followed the degradation of dyes by UV-visible and observed that the decrease of absorbance at the visible range, characteristic of the dyes, was followed by the increase and formation of new peaks at the UV range, indicating that a reaction occurred. For example, azo dyes reduction to the correspondent aromatic amines has been reported and the increase of absorbance on the UV region is a first sign of aromatic amines formation (Brás et al. 2001; Franciscon et al. 2009, 2012) (Fig. 7.4). However, dyes and other organic molecules also absorb in the UV region and, therefore, UV/visible spectroscopy may be very advantageous when complemented with other techniques. The UV spectrum of aniline in a basic solution presents two well defined maxima bands at 230 and 281 nm, while in acidic conditions, does bands are less defined and under streme acidity the spectrum shape does not show any specific absorption band and is not reliable (Fig. 7.5) (Gonzalez et al. 2007). The aromatic amine spectra show extensive peaks in the all UV range and identification only by using this technique may not be adequate. The UV/visible absorbance regions of some aromatic amines are listed in Table 7.4.
UV–vis spectra of the azo dyes before (straight line) and after decolourisation under microaerophilic (dashed line) and aerobic (dotted line) conditions, by the Brevibacterium sp. strain VN-15, isolated from an activated sludge process of a textile company. A: Reactive Yellow 107; B: Reactive Red 198; C: Reactive Black 5; D: Direct Blue 71
UV spectrum of aniline (15 mg L−1) under different pH aqueous solutions (Gonzalez et al. 2007)
7.3.1.2 Fourier Transform Infrared Spectroscopy
FTIR has also been applied to detect many compounds (Pielesz 1999). The infrared sensing is a powerful tool in the detection of organic species in aqueous solutions due to the detection speed and the abundant chemical information obtained. This spectroscopy technique deals with the infrared region of the electromagnetic spectrum, that is light with a longer wavelength and lower frequency than visible light. Spectral data is collected in a FTIR spectrometer in a wide spectral range (1000–4000 cm−1). This confers a significant advantage over a dispersive spectrometer which measures intensity over a narrow range of wavelengths at a time. Bond lengths and bond angles are continuously changing due to this vibration. A molecule absorbs infrared radiation when the vibration of the atoms in the molecule produces an oscillating electric field with the same frequency as the frequency of incident IR light. Infrared spectroscopy exploits the fact that molecules absorb specific frequencies that are characteristic of their structure; the frequency of the absorbed radiation matches the frequency of the bond or group that vibrates.
As example, the N–H stretches of amines are in the region 3300–3000 cm−1. These bands are weaker and sharper than those of the alcohol O–H stretches which appear in the same region. In primary amines (RNH2), there are two bands in this region, the asymmetrical N–H stretch and the symmetrical N–H stretch (Fig. 7.6). Secondary amines (R2NH) show only a single weak band in the 3300–3000 cm−1 region, since they have only one N–H bond. Tertiary amines (R3N) do not show any band in this region since they do not have an N–H bond. The N–H bending vibration of primary amines is observed in the region 1650–1580 cm−1. Usually, secondary amines do not show a band in this region and tertiary amines never show a band in this region. Another band attributed to amines is observed in the region 910–665 cm−1. This strong, broad band is due to N–H wag and observed only for primary and secondary amines. The C–N stretching vibration of aliphatic amines is observed as medium or weak bands in the region 1250–1020 cm−1. In aromatic amines, the band is usually strong and in the region 1335–1250 cm−1. The FTIR spectrum of aniline is shown in Fig. 7.7. This primary amine shows two N–H stretches (3442, 3360 cm−1). The shoulder band corresponds to an N–H bending vibration. The C–N stretch appears at 1281 cm−1 rather than at lower wavenumbers because aniline is an aromatic compound.
Some aromatic amines resulting from the reduction of azo dyes have been identified by FTIR (Franciscon et al. 2009; Pielesz 1999). However, FTIR by itself is not always sufficient, so Mass spectroscopy, Gas and/or Liquid chromatography are techniques currently being used as complementary ones for compounds identification.
7.3.1.3 Mass Spectroscopy
MS is an analytical technique that measures the mass-to-charge ratio (m/z) of charged particles. It is used for determining masses of particles, for determining the elemental composition of a sample or molecule, and for elucidating the chemical structures of molecules. Charged molecules or molecule fragments are generated by ionization of the chemical compounds. The technique has both qualitative and quantitative applications. In a complex mixture, where interference compounds and also in mixtures containing unknown molecules, fragmentation of the formed ion MS/MS is necessary to confirm the molecular structure for a certain mass. Indeed, the fragmentation pattern helps on determination of the structural information an unknown compound with a certain molar weight, once different compounds have the same molar weight.
An important enhancement to the mass resolving and mass determining capabilities of mass spectrometry is using it in sequence with chromatographic separation techniques. Indeed, most aromatic compounds identification when MS is applied result from the combination with chromatographic methods. This allows the sample separation prior to ionization in the mass spectrometer and we can even refer this as a requirement for complex mixtures. Combined methods will be described below in this chapter.
7.3.2 Chromatography
Chromatography can be used to monitor the progress of a reaction, identify and/or separate compounds present in a given mixture and determine the purity of a substance. The mixture is dissolved in a fluid called the mobile phase, which carries it through a structure holding another material called the stationary phase. The various constituents of the mixture travel at different speeds, causing them to separate. The separation is based on differential partitioning between the mobile and stationary phases. Subtle differences in a compound’s partition coefficient result in differential retention, namely retention time (Rt), on the stationary phase and thus changing the separation. The separation and identification of compounds by chromatography depends on their chromatographic behaviour and that is related with many factors that are related with the chemical structure. Therefore, chromatographic parameters need to be determined, independently of the method used. However, for the same compound, different conditions have been successfully applied. Chromatography may be preparative or analytical. The purpose of preparative chromatography is to separate the components of a mixture for more advanced use, being thus a form of purification. Analytical chromatography is done normally with smaller amounts of material and is used for measuring the relative proportions of analytes in a mixture.
7.3.2.1 Thin Layer Chromatography
TLC is performed on a sheet of glass, plastic, or aluminium foil, which is coated with a thin layer of adsorbent material, usually silica gel, aluminium oxide, or cellulose (blotter paper). This layer of adsorbent is known as the stationary phase. After the sample has been applied on the plate, a solvent or solvent mixture, known as the mobile phase, is drawn up the plate via capillary action. Because different analytes ascend the TLC plate at different rates, separation is achieved. TLC plates are usually commercially available, with standard particle size ranges to improve reproducibility.
Thin-layer and paper chromatography for aromatic amines identification were earlier reported in the 1970s-1980s (Franc and Koudelková 1979; Ghafoor and Bark 1982; Srivastava and Dua 1975). In their report, Ghafoor and Bark (1982) have tested halogen, alkyl and nitro aniline derivatives and showed how the substituents on the aromatic structure may affect the chromatographic behavior of aromatic amines. More recently, Janghel et al. (2005) have developed a new TLC technique based on the reaction of the aromatic amines o-nitroaniline, p-nitroaniline, m-nitroaniline, p-phenylenediamine and m-phenylenediamine, with orcinol on a TLC plate, leading to colour derivatives. The colour derivatives migrated by the action of temperature gradient and the intensity of the spots compared with those of the standards. Primary aromatic amines such as aniline, p-toluidine, o-toluidine, m-toluidine, p-anisidine, o-anisidine, m-anisidine, 2-aminophenol, 4-aminophenol, 3-aminophenol, p-phenylenediamine, o-phenylenediamine, m-phenylenediamine, p-(4-chlorobenzyl)aniline, 4,4-diaminobiphenyl, 2-aminonaphthalene have been identified on a TLC plate (Guo and Chen 2010). The colorimetric method is based on the reaction mechanism of cinnamaldehyde with the amines on a TLC plate. A yellow spot appears immediately from the mixing of a colorless solution of amines with a colorless solution of cinnamaldehyde on a TLC plate, meaning the presence of the aromatic amine (Fig. 7.8). The method is advantageous, as the reagent is inexpensive, commercially available and non-toxic, the detection is sensitive and selective, and the procedure is simple and fast.
Photo of TLC identification of aromatic amines by the colorimetric method based on the reaction mechanism with cinnamaldehyde (Guo and Chen 2010). When an aromatic amine is present, am yellow spot appears immediately from the mixing of a colorless solution of amines with a colorless solution of cinnamaldehyde on a TLC plate
7.3.2.2 High Performance Liquid Chromatography
Separation of aromatic amines by HPLC is presently a common practice for the analysis of these substances in water, avoiding the need for pre-derivatisation and the risk of thermal degradation in Gas Chromatography. HPLC is often used for direct analysis of aromatic amines. As example, Melo et al. (2008) have analysed by HPLC the presence of heterocyclic aromatic amines in Portuguese bovine meat dishes prepared by three different cooking methods. Other examples are given in Table 7.2.
Different columns such as C18, C8 and cyano, of various internal diameters, as example 2.1 and 4 mm, and different mobile phase compositions have been used. Both reversed-phase, using octyl or octadecyl silica derivatives, and ion chromatography, using cation exchange resins, methodologies have been developed, with fine tuning of separations being achieved by temperature, mobile phase composition and gradient adjustments. For reversed phase separations, mixtures of aqueous buffers with acetonitrile or methanol are widely used, though, for the separation of sulfonated amines, the addition of ion-pair reagents such as quaternary ammonium salts or tertiary amines has been reported (Ramalho et al. 2004). The most common used detection method is diode array detection (DAD) which allows on-line identification of the analytes in the whole spectra and has a low cost. However, the generally applicable UV detector sometimes lacks high sensitivity, especially when analysing real samples for which wavelengths below 230 nm often cannot be used for quantitation due to matrix interferences Fluorescence detection is sometimes used as a complement to DAD, because unavoidable interferences are frequently produced when using UV detection. Monitorization of aromatic amines degradation by HPLC has been done by many authors (Carvalho et al. 2010; Khalid et al. 2009). Aromatic amines resulted from azo dye reduction have also been commonly identified by HPLC, using standard compounds for comparison (Carvalho et al. 2008; Mendes et al. 2011; Ramalho et al. 2004) (Fig. 7.9).
HPLC chromatograms of the dyes (thin line) and 24 h of their enzymatic degradation (thick line): methyl red (a); Sudan orange G (b). Products of the reaction were identified, in comparison to the standards: 2-aminobenzoic acid (1), N,N′-dimethyl-p-phenylenediamine (2), 4-aminoresorcinol (3) and aniline (4) (Mendes et al. 2011)
Pre or post-column derivatisation are sometimes applied to improve peak shapes and to increase detection sensitivity. Many types of fluorescent derivatisation reagents have been developed, although there are still many reports describing various shortcomings in applications (Greaves et al. 2001; Kudlich et al. 1999). Zhao and Suo (2008), have synthesized two novel fluorescent labelling reagents, 2-(2- phenyl-1H-phenanthro-[9,10-d]imidazole-1-yl)-acetic acid (PPIA) and 2-(9-acridone)-acetic acid (AAA), which are easily accessible and very stable in solution or their crystal states. Effects of derivatisation conditions were investigated for the separation of six monocyclic aromatic amines, aniline, 2-methylaniline, 2-methoxyaniline, 4-methylaniline, 4-chloroaniline and 4-bromoaniline. The PPIA and AAA were compared through whole procedures and the results indicated that the PPIA was better than the AAA. The fluorescent detection sensitivity and peak shapes of PPIA-labelled aromatic amines were improved greatly, compared with direct UV detection without labelling. Furthermore, the mass spectra of PPIA labelled derivatives were more specific for their characterization than those of unlabelled ones. In addition, linearity, limits of detection, recovery, reproducibility and precision of the whole procedure were also determined.
7.3.2.3 Gas Chromatography
GC is a common type of chromatography used in analytical chemistry for separating and analyzing compounds that can be vaporized without decomposition. In gas chromatography, the mobile phase is a carrier gas, usually an inert gas such as helium or an unreactive gas such as nitrogen. The stationary phase is a microscopic layer of liquid or polymer on an inert solid support, inside a piece of glass or metal column. Devices reported for simple quantification or aromatic amine peaks in GC include flame ionisation, nitrogen-selective, flame photometric and electron capture detectors.
GC has been widely used for amine analysis because of its inherent advantages of simplicity, high resolving power, high sensitivity and short analysis time. In very complex mixtures, common in samples for aromatic amine analysis, GC offers better peak carrying capacity in chromatograms than liquid chromatography techniques. However, GC is sometimes unsatisfactory owing to the adsorption and decomposition of the solutes on the column. In addition, some of the amines cannot be separated at all by GC or exhibit problems of peak tailing. Due to the polar nature of aromatic amines, GC methodologies generally require derivatisation into apolar, volatile, thermally stable products prior to injection into the chromatographic column, though recent columns and equipments can allow separation of some aromatic amines without derivatisation. Another reason for derivatisation is to protect the compounds from chemical reactions prior to analysis. Sample concentration before injection is also often required. This can be achieved by liofilization of the aqueous solution and further dissolution in organic solvent or direct extraction with organic solvents. Derivatisation reactions for the determination of amines by GC with respect to reactivity, selectivity and sensitivity have been reviewed by Kataoka (1996). Applications to the determination of individual amines, ammonia and N-nitrosamines in various environmental samples are also described.
Skarping et al. (1983), have tested a method for the trace analysis of aromatic amines identification by capillary GC. It involved conversion of the amines into the corresponding amides by reaction with a perfluoro fatty acid anhydride prior to separation and quantification. Detection limits were in the low picogram range. Haas et al. (1997), have developed an analytical method for the determination of aromatic amines in water is introduced that uses iodination with a Sandmeyer-like reaction to replace the amino group by iodine in aqueous solution. The non-polar derivatives are extracted with pentane or toluene, separated with gas chromatography and sensitively detected with an ECD. Thirteen major metabolites of nitroaromatic explosives were investigated. Latter, a method of derivatisation based on the bromination of the aromatic ring in an acetic medium was experienced by the same group in order to improve determination of aromatic amines by GC and detection with an electron capture detector (Schmidt et al. 1998). From the 56 aromatic amines tested, only for 6 of them derivatives were not obtained. This derivatisation method can be easily and quickly performed and offers the possibility of separation of positional isomer derivatives. The authors have compared both derivatisation methods and concluded that the derivatisation of aromatic amines with iodination and bromination supplement each other in terms of sensitivity and selectivity. More recently, Dados et al. (2004), have tested a GC method whereby the aromatic amines originating from azo dyes in their derivatised form by bromination could be detected at ultra-low concentration levels by gas chromatography. Bromination of aromatic ring of the amines allowed their detection by GC with electron capture detection (ECD) exploiting the presence of the electron withdrawing groups in the analyte. Each derivative produced an easily interpretable mass spectrum with specific and prominent fragment ions. Out of the twenty aromatic amines tested only the bromination of 2,4-diaminoanisole was not feasible.
7.3.3 Capillary Electrophoresis
In traditional electrophoresis, electrically charged analytes move in a conductive liquid medium under the influence of an electric field. Introduced in the 1960s, the technique of CE was designed to separate species based on their size to charge ratio in the interior of a small capillary filled with an electrolyte. The technique uses fused silica capillaries under an electrical field, and separation occurs either solely on the basis of molecular weight and charge, electrophoretic mobility, or associated with differential solubilisation in surfactant micelles. Associated with pre-concentration, pre-derivatisation or incapillary derivatisation techniques in order to overcome the problem of inherent low sensitivity, this method has shown potential for excellent separation efficiency (Asthana et al. 2000; Cavallaro et al. 1995; Sun et al. 2009).
Aromatic amines are well known to show good electrochemical behaviour. Taking advantage of the polar, ionisable nature of aromatic amines, capillary electrophoresis methods, both in the capillary zone electrophoresis and the micellar electrokinetic chromatography modes, have also been proposed for their fractionation in environmental samples (Martínez et al. 2000; Oguri 2000; Ye and Huang 2007). A wide range of techniques have been applied for post-separation identification and quantification of CE peaks in aromatic amine analysis such as UV spectrophotometric, electrochemical (amperometric) and fluorescence detection (Asthana et al. 2000; Sun et al. 2009). Signal enhancement can be obtained through pre- or post-column derivatisation reactions with a chromophore or fluorophore (Asthana et al. 2000). In CE, indirect detection using a background electrolyte bearing also a chromophore or a fluorophore offers potentially higher sensitivity levels (Oguri 2000). Two novel pre-column fluorescent derivatisation reagents, 2-(2-Phenyl-1H-phenanthro-[9,10-d]imidazole-1-yl)-acetic acid (PPIA) and 2-(9-acridone)-acetic acid (AAA), have been developed and compared for analysis of primary aromatic amines by high performance liquid chromatographic fluorescence detection coupled with online mass spectrometric identification (Zhao and Suo 2008).
7.3.4 Combined Methods
The challenge of aromatic compounds identification and quantification has been to develop rapid analytical methods that unequivocally identify them in complex matrices and at low level. In combined methods, usually a purification step is carried out, followed by a separation technique such as HPLC, GC or CE, followed by MS. Peak identification is often a challenge in chromatographic aromatic amine analysis, due to the occurrence of multiple amine structures, interfering substances such as other aromatics and products of amine condensation. So, when chromatographic methods are used, the confirmation of the chromatographic peaks using selective techniques is common, since numerous co-elutions can occur leading to false peak identification. Commonly, for compounds identification by spectroscopy and chromatography, standard compounds are needed and this and this presupposes already having an idea of the products formed and their commercial availability.
The high basicity, reactivity and polar nature of aromatic amines are responsible for different problems involved on the extraction and detection by chromatographic analysis (Akyüz and Ata 2004). Though sample clean-up and specific derivatisation reactions at pre- or post-column level can minimise interferences, the coupling to spectral analysis methods, MS or diode array spectrophotometry, has emerged as a powerful aid. GC-MS has been widely used for the analysis of chemicals in environment because of the high selectivity and resolution of GC columns and mass spectrometry. This technique is suitable for the analysis of low polarity compounds; however, it is difficult to detect polar compounds directly by this technique. HPLC-MS, commonly referred as LC-MS, on the other hand, is an excellent and powerful tool for detection of polar compounds. There are several advantages in the use of LC-MS for the analysis of environmental samples, such as direct detection of highly polar compounds, no need of derivatisation, direct injection of polar solutions, and high selectivity (Zhao and Suo 2008). This combined technique has been frequently used in the last few years for environmental analysis of heterocyclic aromatic amines (Kataoka 1996).
Several reports on applications of combined methods such as GC–MS (DeBruin et al. 1999; Razo-Flores 1997), LC-MS (Fay et al. 1997; Moriwakia et al. 2003; Mortensen et al. 2005; Zhao and Suo 2008), HPLC coupled to an electrochemical detector, HPLC-ED, (Lizier and Zanoni 2012) for aromatic amine analysis have been published. As example, Fig. 7.10 shows the LC-MS analysis of the azo dye, Acid Red 37, reduction by an azoreductase isolated from Acinetobacter radioresistens bacteria, to the correspondent aromatic amine 7,8-diamino-3[(aminoxy)sulfonyl] naphthalene-1-ol (Ramya et al. 2010). Capillary electrophoresis, either with mass spectrometry (CE-MS) ultra-violet (CE-UV) or electrochemical (CE-ED) and florescent detection (CE-FD) has also been proposed (Asthana et al. 2000; Sentellas et al. 2003, 2004). The coupling of electrochemical detectors (ED) in HPLC systems has shown great potential in the quantification of trace organic compounds in various matrices (Lizier and Zanoni 2012). A new method for the determination of four aromatic amines in water samples was developed by Wang et al. (2008) using dispersive liquid–liquid microextraction (DLLME) technique combined with HPLC-variable wavelength detection (HPLC-VWD). An IR spectroscopy method combined with solid-phase-micro-extraction (SPME) was proposed by Yang and Tsai (2001) to detect chlorinated aromatic amines in aqueous solutions. The sensitivity of SPME-IR was enhanced as compared to common IR, because SPME films exclude water molecules interference. The method was greatly accurate and sensible, linear coefficients were around 0.995 and the detection could be lower than 100 ppb.
LC-MS analysis of Acid Red 37 reduction to the correspondent aromatic amines, 1-{3-amino-5-[(aminoxy)sulfonyl]phenyl}ethanol and 7,8-diamino-3[(aminoxy)sulfonyl] naphthalene-1-ol, catalyzed by an azoreductase isolated from Acinetobacter radioresistens bacteria (Ramya et al. 2010). (a) LC chromatogram and (b) MS spectra of Acid Red solution before bioreduction; (c) chromatogram and (d) MS spectra of Acid Red solution after 24 h of bioreduction
7.3.5 Sample Pre-treatment
Several sample pre-treatment methods have been proposed, aiming both at sample clean-up to reduce interferences and pre-concentration of which some have been duly validated and integrated in standardised analysis procedures and automated devices (Pinheiro et al. 2004). Those pre-treatments will increase the accuracy and sensitivity of the detection and lead to faster analyses and better reproducibility. Besides, these procedures may attain minimal sample volume requirements and avoid of analyte losses through evaporation. Liquid-phase extraction, LPE, (Alaejos et al. 2008; Sun et al. 2012b) solid-phase extraction, SPE, (Alaejos et al. 2008; Martínez et al. 2000), solid-phase microextraction, SPME, (Alaejos et al. 2008; DeBruin et al. 1999; Sharma et al. 2011), liquid–liquid–liquid microextraction, LLLME, (Hou and Lee 2003), liquid phase microextraction, LPME, (Tao et al. 2009), LLE–SPE tandem extraction (Alaejos et al. 2008) and headspace single-drop microextraction, HS-SDME, (Zhou and Ye 2008), have been reported for the extraction of aromatic amines from environmental water samples.
Due to the high solubility of aromatic amines in water, the extraction step is of great difficulty. Also, it is of large importance to achieve high performance in the following separation and detection. Liquid-liquid extraction and Solid-phase extraction are the two most frequently used methods for the extraction of these amines from aqueous samples. Solid-phase microextraction and liquid-phase microextraction can also be applied to reduce the requirements of sample and organic solvent, but are less common. For practical applications of these methods in routine inspection work, there are still some problems to resolve such as special interface, limited adsorption capacity, and flavoursome procedures. Zhang et al. (2009) have proposed an alternative approach based on sorbent trapping followed by thermal desorption (TD). Aromatic amines from azo dye reduction in liquid-phase could be directly entrapped on Tenax-TA, which then was subjected to TD for analyte recovery and the subsequent determination by GC-MS. Using this approach, the authors could detect 21 aromatic amines with high sensitivity and good reproducibility. The method was applied to determine aromatic amines in textile samples and the results showed good agreement with conventional solid extraction method. The main advantages of this method were the low organic solvents consumption and convenient procedure. On the other hand, this method was more durable than other miniaturized approaches such as solid-phase microextraction and liquid-phase microextraction, since the sorbent tube is durable enough to be reused many times. An additional device is needed to perform TD that would increase the cost of instrument. In addition, some aromatic amines with high boiling point have severe peak tailing since it is difficult to desorb them quickly.
Another important point to be considered is related with sample storage before analysis. Considering the analytical aspects, the knowledge of how stable a compound is in the particular environmental compartments, as well as of the degradation products, has great value for the validity and reliability of analytical results. It is important to ensure that the obtained measurements reflect the compound concentration in the investigated matrix at the moment of sample collection. Degradation during sample storage step, which frequently takes place, should be minimized as significantly as unlikely.
7.4 Toxicity of Aromatic Amines
Recent progress in cancer research has revealed the complexity of the interaction between exogenous exposures and the physiology of an organism. The existing knowledge favours the idea that most, if not all aromatic amines, have a carcinogenic potential. The epidemiological literature leaves little doubt that a specific few aromatic amines are the cause of bladder cancer in occupationally exposed persons and there is a convincing argument to be made that exposure to aromatic amines via tobacco smoke is a major, if not predominant, factor in causing bladder cancer in smokers (Yu and Ross 1998; Hecht 2003). The biochemical mechanisms by which aromatic amines might induce cancer have been investigated extensively and are now thought to be reasonably well understood (Skipper et al. 2010; Bull et al. 2011). Human population studies that have incorporated measures of metabolic genotype and phenotype tend to support the biochemical mechanisms inferred from experimental studies (Yu et al. 2002). It appears that, for this class of chemical carcinogens, the linkage between the experimental setting and the human condition is as strong as any. Non-occupational exposure to arylamines, a subclass of which are the alkylanilines, has been well documented in cigarette smokers (Skipper et al. 2010) and are believed to be the constituents of tobacco smoke that lead to the development of bladder cancer. Exposure to these alkylanilines potentially occurs through breathing ambient air containing combustion products or through the use of hair dyes. This class of aromatic amines is of particular interest because of demonstrated carcinogenicity in animals and humans and the broad exposure to many of these compounds (Kim and Guengerich 2005).
The investigators from the National Institute for Occupational Safety and Health have reported a correlation between o-toluidine and aniline exposure, noting the increased incidence of bladder cancer (Tannenbaum 1991; Robinson et al. 2001). Diphenylamine, which often contained p-aminobiphenyl, is reported as a human bladder carcinogenic agent as well (Tannenbaum 1991; Acquawell et al. 1991). The aromatic amine p-phenylenediamine, also listed as 1,4-benzenediamine; p-phenyldiamine and 4-phenylenediamine, is commonly found in hair dyes and is reported as a chemical that can damage the nervous system, cause lung irritation and cause severe allergic reactions (Chung et al. 1995).
The first concern with human exposure to carcinogenic aromatic amines arose in the dye manufacturing industry as early as the late nineteenth century (Weisburger 1997). It is worth noting that the many of the studies on the toxicity and carcinogenity of aromatic amines is due to the fact that they are the product of azo dye reduction. Only a few dyes have been found to be inhibitory to the microbial population in the aquatic environments (Pinheiro et al. 2004). However, there is ample evidence indicating that ingested azo dyes are reductively cleaved into aromatic amines. N,N-dimethylaminoazobenzene, commonly known as Methyl Yellow, had been used as a food colorant to enhance the colour of butter, but soon was discovered to be carcinogenic and toxic and was banned in the United States (Combes and Haveland-Smith 1982). Brown and De Vito (1993), in their extensive literature review, pointed out that there are three principal modes of activation for azo dyes: (i) azo dyes that are toxic only after reduction and cleavage of the azo linkage to give aromatic amines, (ii) azo dyes with a structure containing free aromatic amine groups that can be metabolically oxidized without azo reduction, and (iii) azo dyes that may be activated via direct oxidation of the azo linkage to highly reactive electrophilic diazonium salts. Azo reduction is, therefore, one of the most important metabolic activation steps in relation to the mutagenicity, carcinogenicity, and other possible biotoxicities of many azo dyes. Chung (2000) have reviewed the mutagenicity and carcinogenicity of aromatic amines metabolically produced from azo dyes reduction. They pointed out that azo reduction is an important step for the genotoxicity of many azo dyes and the mutagenic moieties of most of these compounds are phenylenediamine and benzidine and their derivatives. A minor difference in the type and position of substituents in the molecular structure of phenylenediamine and benzidine can cause major differences both in mutagenic and carcinogenic activities (Chung and Cerniglia 1992; Chung et al. 1995). For the phenylenediamine moiety, they have observed that methylation or substitution of a nitro group for an amino group did not decrease the mutagenicity. However, sulfonation, carboxylation, deamination, or substitution of an ethyl alcohol for the hydrogen in the amino groups leads to a decrease in mutagenic activity. Experiments conducted by Chung et al. (1995) using Ames Salmonella strains TA98 and TA100, proved that 2-methyl-p-phenylenediamine and 2-nitro-p-phenylenediamine were more mutagenic than p-phenylenediamine, whereas 2-sulfo-p-phenylenediamine was not mutagenic. For the benzidine moiety, Chung and Cerniglia (1992) also observed that methylation, methoxylation, halogenation or substitution of an acetyl group for hydrogen in the amino group did not affect or, in some cases, even increased the mutagenicity, but complexation with copper ions diminished the mutagenicity. The analysis of relationships between chemical structures and genotoxicity such as mutagenicity and carcinogenicity can therefore help the identification and synthesis of useful compounds that are neither mutagenic nor carcinogenic.
For a long time, it was believed that only the polycyclic aromatic amines, but not the monocyclic amines have carcinogenic potential. This conviction was abandoned when occupational exposure to 4-chloro-o-toluidine was shown to produce bladder tumours in workers. o-toluidine had also to be classified as a carcinogen and the experimental results with aniline eventually put an end to this hypothesis. With each of a vast variety of monocyclic aromatic amines, N-hydroxylamines are metabolically formed under suitable conditions, and reactions with DNA and mutagenic activity can be demonstrated (Marques et al. 1997). No criterion can be defined at present that would allow to separate genotoxic from non-genotoxic, or carcinogenic from non-carcinogenic monocyclic arylamines (Kim and Guengerich 2005). This is primarily due to results indicating that the role of genotoxicity was overestimated. This became particularly apparent with the recent developments concerning aniline and structurally related amines. The discussion focused for a long time on the question: is Tests for mutagenicity gave contradictory results and because of the low genotoxic potency these data were considered not to be sufficient to explain the spleen tumours observed in rats (Wilmer et al. 1984; Bomhard and Herbold 2005). The studies with the most basic aromatic amine, aniline, show how intimately genotoxic and non-genotoxic effects are connected and that genotoxicity alone will not answer the question (Zwirner-Baier et al. 2003).
7.5 Non-biological Removal of Aromatic Amines
Aromatic amines have been known to be carcinogenic in humans, therefore their is an huge task for the researchers.
Several degradation methods of aromatic amines have been investigated and proposed, namely biological, chemical, physical, photocatalytic, electrocatalytic and advanced oxidation processes (AOPs). All of those methods have their own advantages and limitations and their application will be dependent on the real scenery.
The chemical oxidation of aniline and substituted primary aromatic amines yields a variety of products depending on the particular oxidant, structure of the aromatic amine and reaction conditions and sometimes polymerization can occur. The most common oxidants for waste water treatment include chlorine, chromate and permanganate (Casero et al. 1997). Oxidation with permanganate has been commonly used and it results in ring-cleavage and subsequent complete breakdown of the molecule, but, unfortunately, it is unsuitable for wastewater containing oxidable solvents such as methanol or ethanol, or large amounts of other oxidable substances. Decontamination of large volumes of aqueous solution is also inconvenient, as it requires large amounts of permanganate. Since iron is ubiquitous in the environment and is largely tolerated in living systems, there is growing interest in the use of high-valent iron as an alternative to common oxidants (Huang et al. 2001).
Among physical processes, adsorption produces good-quality effluents and is one of the most effective processes for removing aromatic amines or other pollutants. Activated carbon, fly ash, serpentine, activated alumina, bauxite, clays, zeolites, bentonite and other ecofriendly adsorbent exhibits a good capacity for removing aromatic amine from wastewater (Hocine et al. 2004; Kostelníková et al. 2008; Oda and Yokokawa 1983; Yadav et al. 2011). Recently, other advanced absorbents such as the chemically synthesized polymeric adsorbents and modified with functional groups (Jianguo et al. 2005), modified activated carbon (Han et al. 2006) and Carbon Nanotubes (CNT) (Yang et al. 2008; Al-Johani and Salam 2011) have been proposed. Adsorption processes merely transfer aromatic amines from one phase to another and, therefore, invariably generate sludge that must be disposed off, or regenerated, by some other process. Moreover, an adsorption process removes the aromatic amines from the wastewater by concentrating them on the surface, retaining their structure practically unchanged. When the support needs to be regenerated, the fate of the resulting concentrated sludge of aromatic amines presents a problem of correct disposal.
AOPs are alternative methods for the complete degradation of pollutants. Such methods have been reported to be effective for the near ambient degradation of soluble organic contaminants from waters and soils, because they can provide an almost total degradation. These methods are based on the generation of a very powerful oxidizing agent such as hydroxyl radical (•OH) which serve as an oxidizing agent for pollutants (Oturan and Brillas 2007). AOPs include photocatalysis systems such as combination of a semiconductor such as TiO2, ZnO, WO3, SnO2, ZrO2, CeO2, CdS and ZnS, and UV light. TiO2 has been widely used because of its various merits such as the large surface area (7–50 m2 g−1), low cost, high photocatalytic activity, chemical activity and nontoxicity (Pereira et al. 2013). However, its applications have been limited for several reasons such as low photon utilization efficiency and need for a high power UV excitation source. One way to solve these problems is the modification of catalysts by doping them with various metals such as Ag, Pt, Fe, Au, etc. (Ganesh et al. 2007; Osterloh 2008). The metals deposited or doped on TiO2 act as electron traps, facilitating electron-hole separation and promoting the interfacial electron transfer process. In photocatalytic degradation of organic pollutants, the substrate molecules react with hole or, more probably, with hydroxyl radicals, to give a number of hydroxylated reaction intermediates. As example, Sánchez et al. (1998) have combined these TiO2-assisted photocatalysis and ozonation for the removal of aniline from water and found that the decomposition rate of aniline is larger than when individually treated by either of the two methods. The combination of ozonation and photocatalysis with TiO2 gives high yields of aniline degradation in aqueous solutions. Particularly, an ozonation pretreatment followed by photocatalysis significantly increases the yield of TOC removal in comparison to either ozonation or photocatalysis acting separately.
Fenton’s reagent has also been used for oxidation of aromatic amines This reagent is a mixture of hydrogen peroxide and ferrous iron that produces •OH radicals. Such radicals have proved to effectively react with a variety of compounds such as alcohols, ethers, dyes, chlorinated phenols, pesticides, polycyclic aromatics, etc., in aqueous solutions and waste waters degradation (Casero et al. 1997). The advantage of Fenton’s processes is the total mineralization of the organic compound treated to harmless compounds, for instance, carbon dioxide and water (Neyens and Baeyens 2003). Notwithstanding, it produces some unwanted compounds which are also not benign for the environment, such as ferric hydroxide sludge that requires additional separation and consequently Fenton’s processes appear combined with electrochemical others. As example, the kinetics of 2,6-dimethylaniline degradation by Fenton process, electro-Fenton process and photoelectro-Fenton process was investigated by Masomboon et al. (2011). 2,6-dimethylaniline degradation in the photoelectro-Fenton process was superior to the ordinary Fenton and electro-Fenton processes. Actually, electrochemical advanced oxidation processes (EAOPs), based on the in situ electrogeneration of the highly reactive hydroxyl radical (•OH) have being developed as a promising environmental friendly technique. Torres et al. (2003) have studied the electrochemical oxidation, on Pt anodes, of industrial wastewaters containing 5-amino-6-methyl-2-benzimidazolone. At the best conditions, the compound was 100 % degraded in 45 min. However, because the reaction intermediates exhibited high toxicity and non-biodegradability, the electrolysis had to continue for 3 more hours in order to obtain a biocompatible solution that could further be mineralized in a fixed bed biological reactor. In the anodic oxidation using boron doped diamond (BDD), as it is a high O2-overvoltage anode, degradation of the pollutants is mainly mediated by hydroxyl radicals formed at its surface from water oxidation (Panizza et al. 2008; Santos et al. 2010). These electrochemical processes using BDD are able to perform chemical conversion/combustion with high current efficiency, without the additional use of reactants and, consequently, in most cases without the formation of by-products. This electrode material presents also the advantages of chemical inertness and extended lifetime. BDD anodes have been used in the anodic oxidation of aromatic amines, such as aniline, metanilic and sulfanilic acids (Santos et al. 2010; Carvalho et al. 2006), p-aminophenol (Ratiu et al. 2010) and naphthalenesulfonates (Panizza et al. 2006). Electrodegradation of aniline and its three monosulfonated forms (ortanilic, metanilic and sulfanilic acids) was study by Santos et al. (2010) in order to understand the influence of introducing in the structure of the aniline a sulfonic group in different relative positions to the amino group. Efficiency of degradation followed the order aniline > metanilic acid > sulfanilic acid > ortanilic acid. More recently, the influence of the different groups in the structure of the aniline on electrodegradation using a BDD anode was assessed by Pacheco et al. (2011) by testing 4 aromatic amines with different substituent groups, 3-amino-4-hydroxy-5-nitrobenzenesulfonic acid (A1), 5-amino-2-methoxybenzenesulfonic acid (A2), 2,4-dihydroxyaniline hydrochloride (A3) and benzene-1,4-diamine (A3). Results have shown a good electrodegradation of all the amines tested and the efficiency was A4 > A3 > A2 > A1.
7.6 Biodegradation of Aromatic Amines
According to literature and industrial expertise, biodegradation have been seen as the most effective, economical and environmentally ecological processes to remove organic pollutants from water as well as soil. Microorganisms play an essential role in recycling carbon and maintaining the health of the biosphere (Aust et al. 1994). Biodegradation takes advantage of this capacity. Bacteria have evolved to degrade most naturally occurring organic compounds, including the persistent aromatics. Moreover, the promiscuity of the catabolic enzymes allows bacteria to degrade, at least partially, xenobiotics that share similar structures with naturally occurring aromatic compounds (Díaz 2004). The bacterial catabolism of aromatic compounds involves a wide variety of peripheral pathways that activate structurally diverse substrates into a limited number of common intermediates that are further cleaved and processed by a few central pathways to the central metabolism of the cell (McLeod and Eltis 2008). Electron acceptors play a vital role for aromatic amine degradation in aerobic/anaerobic environments. In this context, degradation of aromatic compounds can occur under aerobic or anaerobic conditions, depending on the presence or absence of oxygen. Under aerobic degradation of aromatics, oxygen is always the final electron acceptor, but is also a cosubstrate for the two key processes, hydroxylation and oxygenolytic ring cleavage of the aromatic ring, carried out by oxygenases (Parales and Resnick 2006; Vaillancourt et al. 2006). On the contrary, under anaerobic conditions, degradation of aromatic compounds uses a completely different strategy, based on reductive reactions, to attack the aromatic ring. Many habitats containing large amounts of aromatic compounds are often anoxic, e.g., aquifers, aquatic sediments and submerged soils, sludge digesters, and intestinal contents, and at aerobic sites with high carbon concentrations, molecular oxygen is more rapidly consumed than replenished. Local microbial communities are capable of using locally available electron donors and acceptors to perform biodegradation of those aromatics (Carmona et al. 2009). This anaerobic mineralization can be coupled to anaerobic respiration with a variety of electron acceptors, e.g., nitrate, sulfate, iron(III), manganese(II), and selenate, with each one conserving different yields of energy. The greatest energy conservation is reached when nitrate is the final electron acceptor, followed by ferric ion. The energy conservation when sulfate is the electron acceptor is much more limited (Carmona et al. 2009).
Since the redox potential of the electron-accepting system in the anaerobic breakdown of aromatic compounds dictates the biochemical strategy that is applied for the degradation of such compounds, there is wide biochemical diversity among anaerobic aromatic degraders. On the other hand, aromatic compounds can participate in anaerobic metabolism by serving as electron acceptors rather than electron donors, generally with accompanying modifications of ring substituents that do not perturb the benzene nucleus itself (Gibson and Harwood 2002).
7.6.1 Aerobic Biodegradation
Aniline is the simplest aromatic amine and extensive studies have been carried out on aerobic aniline degradation. Bacterial species of Pseudomonas (Hinteregger et al. 1992), Comamonas (Parales et al. 1997), Acinetobacter (Kim et al. 1997), Rhodococcus (Aoki et al. 1983), Frateuria (Murakumi et al. 1999), Moraxella (Zeyer et al. 1985) and Nocardia (Bachofer et al. 1975) have been shown to be able of degrading aniline and/or its derivatives. Highly aniline tolerant bacteria are desirable for environmental applications as well as for the biotransformation of aniline and its analogues into useful chemical products. Pseudomonas sp. are considered as the most aniline tolerant bacterial strains, utilizing concentrations of up to 32 mM (Konopka et al. 1989). Nevertheless, Liu et al. (2002) have isolated a novel bacterial strain, Delftia sp. AN3, that efficiently utilizes up to 53.8 mM (5000 mg L−1) of aniline. Delftia sp. AN3 uses a meta cleavage pathway for aniline degradation. The authors have proposed the pathway of aniline degradation (Fig. 7.11). Enzymes involved in aniline degradation were analyzed and their catalytic parameters were determined. Börnick et al. (2001) and Worch et al. (2002) both measured degradation rates of aromatic amines in river water fed to biofilm water treatment systems. The 20 tested substances included aniline and several of its chloro, bromo, methyl, nitro and mixed derivatives, N,N-dimethylaniline, N-ethylaniline and 1-naphthylamine. Half-degradation times from 0.5 h for 1-naphthylamine to 35 h for 2,4,5-trichloroaniline were reported. For the latter compound, a biofilm adaptation period of 6 weeks resulted in a 20-fold increase in the biodegradation rate. Six amines, including aniline and o-toluidine, showed half-degradation times under 1 h. Toräng et al. (2002) compared the degradation rates of aniline in laboratory shake flask simulation tests with field rates in the river Rhine. The estimated half-life of aniline in the Rhine at 15 and 22 °C was 9 h and was consistent with laboratory batch test performed with concentrations below 25 μg L−1. The results indicate that laboratory shake flask batch tests with low concentrations of test substance can be good predictors of degradation rates in natural water bodies. Wang et al. (2007) pointed out that the strain PN1001, isolated from the activated sludge of an oil refinery wastewater treatment plant, is a member of the Pseudomonas species capable of degrading aniline. A group of aromatic amines that are more difficult to degrade even under aerobic conditions are represented by aryl sulfonates. The presence of sulfonated group on aromatic ring not only confers a xenobiotic character, but also recalcitrant nature to these compounds (Barsing et al. 2011). Though, the aerobic degradation of sulfonic acid (SA) by isolated cultures and by microbial communities samples from sites previously contaminated with aromatic amines has been reported (Tan et al. 1999). Some authors found, however, that SA is not degraded under aerobic conditions with activated sludge from a plant treating domestic effluent (Tan et al. 2005; Yemashova and Kalyuzhnyi 2006). Of all the ten sulfonated aromatic amines tested by Tan et al. (2005) for their aerobic and anaerobic biodegradability, and toxicity potential in a variety of environmental inocula compounds, only two aminobenzenesulfonic acid (ABS) isomers (2- and 4-ABS) were degraded. The observed degradation occurred only under aerobic conditions with inocula sources that were historically polluted with sulfonated aromatic amines. These results indicate that cultures adapted to the compounds to be degraded, can easily undergo biodegradation and also that there is some specificity relatively to the type of species involved in biodegradation. Carvalho et al. (2008) investigated that sulfanilic acid and aniline are easily degraded by three different types of aerobic inocula: domestic wastewater and activated sludge from municipal and industrial treatment plants. The idea that an easier degradation of SA would occur with biomass from an industrial treatment plant was not confirmed in this results. On the contrary, a longer lag phase was required, corroborating the idea that degradation of SA has a higher specificity. Also, its biodegradation was not observed with activated sludge from a lab scale reactor fed with glucose.
Proposed pathway of aniline degradation by Delfia sp. AN3 (Liu et al. 2002)
Nitroanilines are another important type of aromatic amines which formed from reduction of azo dyes and anthropogenic activities. Khalid and coworkers (2009) have reported on the degradation of nitroanilines by isolated species and a mixed microbial culture. They found that most of the nitroanilines are biodegradable within 48 h of incubation and that the mixed microbial culture is more efficient than isolated species. 2-Aminobenzene sulfonic acid (2-ABS)/4-ABS was easily biodegradable if proper enrichment culture was available. Aminobenzene sulfonic acids are degraded through ring cleavage pathway to release ammonia and methane in stoichiometric amounts. More recently studies on aromatic amines biodegradation are those of Barsing et al. (2011), Jin et al. (2012) and Zhang et al. (2011).
A few bacterial cultures, utilizing napthyl amines as the sole organic carbon source, have been also reported. Sphingomonas sp. Starin ICX could decolorise AO7 and degrade 1-amino-2-napthol (Coughlin et al. 2002). Sulfonated naphthylamines are among the most common products of bacterial decolorisation of azo dyes. The most intensively studied bacterial strain with the ability to degrade naphthalenesulfonates is Sphingomonas xenophaga BN6, which degrades various amino and hydroxy naphthalenesulfonates to the corresponding amino or hydroxysalicylic acids (Stolz (1999). A complete mineralization requires a co-culture consisting of Sphingomonas xenophaga BN6 as well as Pseudomonas Sp. BN6 or other bacterial strains, which degrade substituted salicylic acids.
Recently, a Yeat strain, Candida methanosorbosa BP-6 was isolated from the wastewater pool of the old factory “Boruta” in Zgierz and tested, for the first time, on aniline biodegradation (Mucha et al. 2010). The strain growth well in the presence of aniline and could degrade it. Biodegradation intermediates and final products were identified by HPLC and the authors proposed the intradiolic pathway for aniline biodegradation.
Aerobic biodegradation of aromatic amines, however, can lead to the autooxidation of the aromatic amine with formation of larger and difficult to degradade molecules. Tan et al. (1999), have pointed out that 4-aminophenol rapidly suffered autoxidation to oligomeric or polymeric humic like substances, phenomena likely to happen with aromatic amines bearing hydroxyl substituents. The same phenomenon was mentioned for 1-amino-2-naphtol and 5-aminosalicylic acid, an effect which could effectively compete with biodegradation, increasing aromatic amine recalcitrance. Sulfonated o-amino hydroxybenzenes and o-amino-hydoxynaphthalenes were easily decomposed upon exposure to oxygen (Kudlich et al. 1999), Dimers and quinone derivatives were found among the oxidation products, some of which could be biodegraded by activated sludge.
7.6.2 Anaerobic Biodegradation
Aromatic amines are the reduced salt of nitro aromatics or azo compounds. The electron donating amino groups formed from the reduction of nitro and azo groups are estimated to carriage a serious problem to further reductive bio transformations by anaerobic granules. Aromatic amines with carboxy, hydroxyl and methoxy substituents are potentially mineralizable in methanogenic consortia (Razo-Flores et al. 1997a). Aromatic amine reduction is a tough task for biodegradation in methanogenic environment. In anaerobic condition, nitrate (NO3 −), sulfate (SO4 2−), metals such as iron (Fe3+) and manganese (Mn4+), or even CO2 can play the role of oxygen, accepting electrons from the degraded contaminant. In addition to new cell matter, the byproducts of anaerobic degradation may include nitrogen gas (N2), hydrogen sulfide (H2S), reduced forms of metals, and methane (CH4), depending on the electron acceptor.
Razo-Flores et al. (1997b) have published the first work on the completely biodegradation of an azo dye in the absence of oxygen. The electrons required for the reductive cleavage of azo dyes by anaerobic microorganisms are known to be derived from co-substrates. 5-aminosalicylic acid (5-ASA) was incubated with the methanogenic consortia in the presence of the specific methanogenic inhibitor 2-bromoethane-sulfonate. Acetate was identified as the major intermediate formed, indicating that the degradation of 5-ASA occurs via acetogenic fermentation. However, when the biodegradability of aniline, amino phenols, amino benzoates and 5-aminosalicylate by methanogenic sludge was tested, all the compounds, except aniline, were at least partially biodegraded, though with lag-phases between 25 and 110 days and in some cases requiring pre-adaptation of the culture to 2-nitrophenol. Batch methanogenic toxicity and biodegradability of 2-, 3- and 4-aminobenzoic acids (ABA) as well as 4- and 5-aminosalicylic acids (ASA) have been studied in the presence of two mesophilic (Shell and cattle) and one thermophilic sludges. 5-aminosalicylic acid (5-ASA) could be completely mineralized by all the sludges tested, but 4-aminosalicylic acid (4-ASA) could not be degraded at all by any of the sludges. All three aminobenzoic acid are principally biodegradable, but sludge source and adaptation are essential. Both mesophilic sludges were able to perform a complete mineralization of 2- aminobenzoic acid but this was not a case for the thermophilic sludge. 3-aminobenzoic acid was not biodegraded only in the presence of the Shell sludge. On the contrary, 4-aminobenzoic acid was quantitatively mineralized only by the Shell sludge. All the adapted sludges were able to mineralize the corresponding amino-aromatics in N-deprived media. Cross-acclimatization trials showed that 2-aminobenzoic acid, 5-aminosalicylic acid and salicylic acid adapted sludges were unable to degrade any other amino-aromatics tested that manifest about a different nature of key bacteria responsible for primary decomposition of these substrates. All the aromatic amines tested practically did not have any toxic effect on methanogenosis up to their concentration 3–7 g L−1, moreover some of them even exert a stimulating effect on acetoclastic activity, especially when not very active sludges are used.
Citrobacter freundii strain, WA1, was isolated from a 5-aminosalicylate degrading methanogenic consortium (Savelieva et al. 2004) The methanogenic enrichment culture degraded 5-aminosalicylate completely to CH4, CO2 and NH4 +, while C. freundii strain WA1 reduced 5-amino salicylate with simultaneous deamination to 2-hydroxy-benzyl alcohol during anaerobic growth with electron donors such as pyruvate, glucose or serine. When grown on pyruvate, C. freundii WA1 converted 3-aminobenzoate to benzyl alcohol and also reduced benzaldehyde to benzylalcohol. Pyruvate was fermented to acetate, CO2, H2 and small amounts of lactate, succinate and formate. Less lactate (30 %) was produced from pyruvate when C. freundii WA1 grew with 5-aminosalicylate as co-substrate. Chen et al. (2009) have demonstrated that an integrated anaerobic and aerobic biofilm reactor system, with a high level of aerobic effluent recirculation to the anaerobic reactor, can effectively treat highly concentrated and toxic nitrogenous aniline wastewater, with the simultaneous removal of carbon and nitrogen. In this system, ammonification, methanogenesis, and denitrification reactions occur simultaneously in a single anaerobic biofilm reactor, and the aerobic reactor is used for further COD reduction and autotrophic nitrification. Complete degradation of aniline by the facultative fraction of methanogenic granular sludge exposed to oxygen was described by Tan et al. (1999). Conversely, sulfanilic acid and 5-aminosalicylic acid mineralization required the addition of an aerobic enrichment culture obtained from river sediments exposed to sulfonated aromatic compounds. More recently, Pereira et al. (2011) investigated the fate of the aromatic amines aniline and sulfanilic acid, under denitrifying conditions in up flow anaerobic sludge blanket reactors (UASB). The results demonstrated that, in reactor 1 containing nitrate as electron acceptor, aniline could be degraded under denitrifying conditions while sulfanilic acid remained. In the reactor 2, containing nitrite as electron acceptor, a chemical reaction led to immediate disappearance of both aromatic amines and the formation of an intense yellow coloured solution. The reason to test nitrite was based on the fact that the first step of denitrification yields nitrite, a compound that has been found to react with aromatic amines, resulting in deamination, thereby yielding aromatics with a higher biodegradation potential (Seymour et al. 2002). Investigation on the degradation of aminoaromatic compounds by anaerobic microbial communities from lake sediments promotes apprehension of mechanisms of self-clarification of natural habitats (Lin’kova et al. 2011). Understanding of anaerobic biodegradation of (amino) aromatic substances allows predicting the destiny of these xenobiotics in nature and treatment facilities and is a fundamental basis for creation of specific biotechnologies for abatement with such pollutions.
7.6.3 Kinetics of Aromatic Amine Biodegradation
In order to determine the lifetimes of organic chemicals, under the assumption that degradation mechanism is being present, kinetics studies are carried out and can be evaluated by monitoring its disappearance, at different compound concentrations, during a certain time interval until steady state. The obtained results indicate the time required for the attainment of equilibrium during the biodegradation of the organic compound. The knowledge of kinetic parameters of aromatic amine biodegradation is also a very important issue both for the prediction of their fate and for the design of wastewater treatment plants. Batch assays are typically used to measure kinetic characteristics. Various analytical techniques are applied to monitor these studies, which were described before, however, for monitorisation over time of reaction, UV-vis in the case of coloured samples, and HPLC either for coloured and non-coloured samples, are the most common due to their simplicity, low maintenance and low labour and low cost.
Although biological degradation is very complex in nature, several investigations have shown that the overall kinetics are typically described by the pseudo-first order rate law (Mälzer et al. 1993; Worch et al. 2002). The first-order degradation kinetics may be expressed as follows (Eq. 7.1):
Where, C represents the concentration of a degraded compound at the time t and K 1 is the first-order rate constant. C 0 and C t , are the initial and the residual, at certain time, concentrations, respectively. . In practice, the first-order rate constant often is replaced by a half-life, H, and the degradation rate is expressed as follows where H is 0.693/K 1 (Eqs. 7.2 and 7.3):
If the initial rate of the reaction is measured over a range of substrate concentrations, denoted as [S], the reaction rate (v) increases as [S] increases. However, as [S] gets higher, the enzyme becomes saturated with substrate and the rate reaches the enzyme’s maximum rate, V max.
The kinetic constants, K M and V max, are critical to attempts to understand how enzymes work together to control metabolism and are calculated by the Michaelis–Menten equation (Eq. 7.4):
where V0 is the initial rate and Km is the concentration of substrate that leads to half-maximal velocity.
In many cases, at higher substrate concentration, inhibition occurs and the rate of reaction decreases.
The measured kinetics of biodegradation of a single organic compound as sole carbon source in a batch reactor depend on the history of the culture, the identifiability of the parameters, and the manner in which the experiment to measure them is run. The initial substrate to biomass ratio used in the experiment is particularly important because it influences both parameter identifiability and the expression of the culture history.
Carvalho et al. (2008) reported that sulfanilic acid biodegradation by aerobic microorganisms appears as a sigmoid curve and the kinetic parameters followed the pseudo first order kinetics. Worch et al. (2002) demonstrated that biodegradation kinetics of aromatic amines fitted by a pseudo-first order. Taking into account the possible analytical errors, from a first approximation it was assumed that the degradation curves follow a first order rate law. The rate constants estimated in that series of experiments range from about 2 h−1 for aniline t about 0.03 h−1 for 2,4,5-trichloroaniline. The half-lives ranged from 20 min for aniline to more than 20 h for 2,4,5-trichloroaniline.
The activities of aniline dioxygenase, catechol 2,3-dioxygenase and other enzymes involved in aniline degradation were determined by Liu et al. (2002). The Km and Vmax of aniline dioxygenase were 0.29 mM and 0.043 mmol mgprotein −1 min−1, respectively. The Km and Vmax of catechol 2,3-dioxygenase for catechol were 0.016 mM and 0.015 mmol mgprotein −1 min−1, respectively.
7.6.4 Factors Effecting Biodegradation
Different biological processes for aromatic amine degradation have been discussed previously. All of them present vantages and disadvantages and their appropriate adoption depends on the special application. In this chapter, one could not fail to mention that those processes and their rates depend in turn on the nature of the environment as well as the native properties of the aromatic amine. The aromatic amine biodegradation studies on aerobic/anaerobic technology have mainly been focused on general aspects on type of electron acceptors, effects of substrate type, organic loading rate, dissolved oxygen concentration, COD:N:P ratio, effect of pH, effect of temperature, types of reactor and characteristics of the sludge. Some other special factors may equally affect the biodegradation. Among these factors, the presence of heavy metals will also affect the reactions and is supposed as the reason behind microbial aggregation. Liu et al. (2002) have earlier described that the heavy metal ions Hg2+ and Ag+ are toxic to growing cells, and completely inhibition of growth occurred at 0.02 mM Hg2+ and 0.1 mM Ag+. In their study, a bacterial strain, AN3, was able to use aniline as sole carbon, nitrogen and energy sources. The optimal temperature and pH for growth and degradation of aniline were 30 °C and 7.0, respectively. The efficiency of aniline degradation increased gradually when the temperature was increased from 10 to 30 °C. Complete degradation occurred when aniline concentration was below 22 mM, but above this, the degree of degradation decrease and only 20 % was obtained at the maximal concentration tested. Cell growth simultaneous with aniline degradation and inhibition was observed at aniline concentrations above 32 mM, explaining the decrease on the process effectiveness.
Khalid and co-authors (2009) have studied the effect of many parameters on the biodegradation of 4-nitroaniline under aerobic conditions by pure and mixed bacterial cultures. Individual strains were unable to degrade 4-nitroaniline completely even with longer incubation period (168 h) but achieved rapid degradation when grown together: 92 % degradation in 48 h, followed by complete degradation at 72 h. Authors have assumed that the combined enzyme systems of the mixed bacterial culture were more effective than the enzymes from the individual isolate. Alternatively, cooperation within microbial communities can occur through exchange of growth cofactors and removal of toxic metabolites. Degradation of 4-nitroaniline by the bacterial consortium was inhibited when the substrate concentrations increased above 0.1 mmol L−1, and there was an inverse linear relationship between the rate of degradation reaction and 4-nitroaniline concentrations over the range between 0.1 and 1 mmol L−1. The authors have also observed that the addition of yeast extract up to 5 g L−1 to the medium accelerated the biodegradation of 4-nitroaniline by several orders of magnitude, possibly by acting as a bacterial growth-promoting factor. In contrast, addition of glucose, sucrose, mannitol or NH4NO3 had strong inhibitory effects on the degradation of 4-nitroaniline by the mixed bacterial culture, what was attributed to preferential use of these sources of carbon and nitrogen by the bacteria for their growth. The degradation of 4-nitroaniline by the bacterial consortium was optimal at slightly alkaline pH (7.2) with incubation at 35 °C under aerobic conditions. The degradation of 4-nitroaniline by the bacterial consortium was optimal at slightly alkaline pH, 7.2, with incubation at 35 °C under aerobic conditions. Similar optimal pH and temperature conditions were also found by Lin’kova et al. (2011) for the biodegradation of 2- and 4- aminobenzoic acid and 5-aminosalicylic acid by anaerobic microbial communities (30 °C and pH of 6.5–7.5).
Kalyuzhnyi et al. (2000) have studied the biodegradability of 2-, 3- and 4-aminobenzoic acids (ABA) as well as 4- and 5-aminosalicylic acids (ASA) in the presence of two mesophilic (Shell and cattle) and one thermophilic sludges. 5-ASA was completely mineralised by all the sludges used. On the other hand, 4-ASA was not degraded at all by any of the sludges used. Both mesophilic sludges were able to perform a complete mineralization of 2-ABA but this was not a case for the thermophilic sludge. 3-ABA was not biodegraded only in the presence of the Shell sludge. This finding emphasizes an extreme importance of substituent position in xenobiotic compounds to be biodegraded. At the same time, it is notice that the process is affected by the tip of biomass use. In addition, when previously adapted, sludges were able to mineralize, but only, the corresponding aminoaromatics.
The presence of salts, special in textile wastewaters, can also affect the process. O’Neill and co-authors (2000) have investigated the degradation of aniline by bacterial consortia under aerobic, fermentative, nitrate-reducing and sulphate-reducing conditions and a variety of salt concentrations, 0.2, 4 and 7 % NaCl w/v, and pH values, 5 and 7. Degradation of aniline was achieved under all combinations of salinity, however, the rate of bacterial growth decreased with increasing salinity. Cultures were able to adapt to changes in pH and to most changes in salinity, suggesting that in treatment plants the microflora may be able to cope with fluctuations in salinity and pH. Growth rates were unaffected by aniline concentration in the range of 0.25–1 g L−1 and an additional nitrogen source, two parameters of wastewater which are also likely to fluctuate.
7.7 Conclusions
Aromatic amines are important industrial compounds and their use is widespread. They range from the simple structure of aniline to most complex ones. Furthermore, aromatic amines are used in the synthesis of many compounds and are part of their integral structure, such as the azo dyes. Those compounds result also from common daily human activities such as cooking, smoking, diesel exhaust, combustion of wood chips and rubber, etc. The high impact of those compounds as pollutants and the increasing knowledge on their carcinogenic properties, leads to the rise interest on their detection, their fate, monitorization and degradation. Progress in the detection and treatment methods has, thus, being made. Combined methods such as HPLC-MS, GC-MS, CE-MS seem to be very effective, special for the monitorization of degrading reactions and when quantification rigor is need, once the results obtained from the complementary analyses give a more confident and complete information. Although, the complexity and costs of those methods make UV-visible spectroscopy and/or HPLC very common when only punctual monitorization is required.
Among the various methods on aromatic amines degradation, biodegradation seems to be promising. Researchers have been testing different biodegradation conditions and, with regard to electron acceptor, both aerobic and anaerobic conditions have been reported. However, scarce information has been stated to elucidate the mechanisms. Aromatic amines are commonly difficult to degrade under anaerobic conditions. Some of them, substituted with hydroxyl or carboxyl group, were degraded under methanogenic and sulphate reducing conditions. A drawback of using aerobic treatments is that many of them are prone to autoxidation once they are exposed to oxygen, resulting in bigger molecules with lower biodegradability. Other electron acceptors such as nitrate, iron, sulphate, manganese and carbonate have, alternatively, been tested and successful results achieved.
7.8 Future Perspectives
While the aerobic catabolism of aromatic compounds has been extensively studied, the anaerobic degradation of aromatics is a more recently discovered microbial capacity that still awaits a deeper understanding despite the fact that microbial metabolism in the absence of oxygen is the most ancient of all life processes. The natural attenuation of pollution carried by different microorganisms although may be improved by engineered techniques, either by bioaugmentation (addition of selected microorganisms) or by biostimulation (addition of nutrients) and by genetic engineer. However, the biochemical and genetic bases of the anaerobic degradation of aromatic compounds are not very well established yet due mainly to the difficulties in routinely growing and genetically manipulating the aromatic-degrading microorganisms. The reaction mechanisms are not yet clear, accordingly further studies are needed. On the other hand, the effect of reaction conditions still need to be better elucidated. The increased use of high-throughput techniques such as genomics, metagenomics, proteomics, metabolomics, as well as systems biology approaches for addressing biological complexity from a holistic perspective and the application of the network theory to biology will certainly contribute significantly to unraveling the intricate regulatory and metabolic networks that govern the anaerobic biodegradation of aromatic compounds as well as their distribution and ecophysiological relevance to carbon flux in the environment.
The knowledge of the environmental impact of aromatic amines is fundamental and, though the many toxicological studies, effect of this compounds at very little amount has to be continuously evaluated. In this way, the wide analytical techniques available are fundamental and studies may integrate combined methods to complement the information given by each. It is worth noting that also the analytical techniques are constantly evolving.
Researchers do not stop testing different methods of pollutants remediation. In view of biodegradation, different microorganisms and conditions will be tested. As pointed in this chapter, electron acceptors play a very important role and, therefore, other chemicals and/or novel materials need to be prepared and experienced. The advances in materials sciences, special on nanomaterials, may open a new world on the biotechnological processes and a big way has yet to be traveled.
A very important aspect of organic compound degradation in the environment is the possibility of applying these processes in natural and industrial environment, and at large scale. Accordingly, it is necessary to know the processes in full detail, so that their effectiveness can be controlled.
Concerning the chemical manufactures, the progress is to avoid the use of the most dangerous compounds and the substitution by similar innocuous ones. Also the technology has to be developed in order to release as less as possible chemicals to the environment.
References
Acquawell JF, Wilson JD, Conner P, Bannister R (1991) An alternative hypothesis for bladder cancer among workers exposed to o-toluidine and aniline. J Nat Cancer Inst 83:1686–1697. doi:10.1093/jnci/83.22.1686
Akyüz M, Ata S (2004) Ion-Par extraction and GC-MS determination of aliphatic and Aromatic amines in water and sediment samples. In: 4th AACD congress, 29 Sept–3 Oct 2004, Adnan Menderes University, Kuşadası-Aydin,Turkey, Proceedings Book 341, 623–628. http://www.srcosmos.gr/srcosmos/showpub.aspx?aa=5641
Alaejos MS, Ayala JH, Gonzalez V, Afonso AM (2008) Analytical methods applied to the determination of heterocyclic aromatic amines in foods. J Chromatogr B 862:15–42. doi:10.1016/j.jchromb.2007.11.040
Al-Johani H, Salam MA (2011) Kinetics and thermodynamic study of aniline adsorption by multi-walled carbon nanotubes from aqueous solution. J Colloid Interface Sci 360:760–767. doi:10.1016/j.jcis.2011.04.097
Aoki K, Ohtsuka K, Shinke R, Nishira H (1983) Isolation of aniline assimilation bacteria and physiological characterization of aniline biodegradation in Rhodococcus erythropolis AN13. Agric Biol Chem 47:2569–2575. http://www.doi.org/10.1271/bbb1961.47.2569
Asthana A, Bose D, Durgbanshi A, Sanghi SK, Kok WT (2000) Determination of aromatic amines in water samples by capillary electrophoresis with electrochemical and fluorescence detection. J Chromatogr A 895:197–203. doi:10.1016/S0021-9673(00)00522-7, PII: S0021-9673(00)00522-7
Aust SD, Bourquin A, Loper JC, Salanitro JR, Suk WA, Tiedjet J (1994) Biodegradation of hazardous wastes. Environ Health Perspect Suppl 102:245–252
Bachofer R, Lingens F, Schafer W (1975) Conversion of aniline into pyro-catechol by a Nocardia sp: incorporation of oxygen-18. FEBS Lett 50:288–290. http://www.doi.org/10.1016/0014-5793(75)80510-2
Barsing P, Tiwari A, Joshi T, Garg S (2011) Application of a novel bacterial consortium for mineralization of sulphonated aromatic amines. Bioresour Technol 102:765–771. http://www.doi.org/10.1016/j.biortech.2010.08.098
Bomhard EM, Herbold BA (2005) Genotoxic activities of aniline and its metabolites and their relationship to the carcinogenicity of aniline in the spleen of rats. Crit Rev Toxicol 35:783–835. doi:10.1080/10408440500442384
Börnick H, Eppinger P, Grischek T, Worch E (2001) Simulation of biological degradation of aromatic amines in river bed sediments. Water Res 35:619–624. http://www.doi.org/10.1016/S0043-1354(00)00314-6
Brás R, Ferra MIA, Pinheiro HM, Gonçalves IC (2001) Batch tests for assessing decolourisation of azo dyes by methanogenic and mixed cultures. J Biotechnol 89:155–162. http://www.doi.org/10.1016/S0168-1656(01)00312-1
Brown MA, De Vito SC (1993) Predicting azo dye toxicity. Crit Rev Environ Sci Technol 23(3):249–324. doi:10.1080/10643389309388453
Brown D, Laboureur P (1983) The aerobic biodegradability of primary aromatic amines. Chemosphere 12:405–414. http://www.doi.org/10.1016/S0045-6535(01)00074-1
Bull RJ, Reckhow DA, Li X, Humpaged AR, Joll C, Hrudey SE (2011) Potential carcinogenic hazards of non-regulated disinfection by-products: haloquinones, halo-cyclopentene and cyclohexene derivatives, N-halamines, halonitriles, and heterocyclic amines. Toxicology 286:1–19. doi:10.1016/j.tox.2011.05.004
Carmona M, Zamarro MT, Blázquez B, Durante-Rodríguez G, Juárez JF, Valderrama JA, Barragán MJL, García JL, Díaz E (2009) Anaerobic catabolism of aromatic compounds: a genetic and genomic view. Microbiol Mol Biol Rev 73(1):71–133. doi:10.1128/MMBR.00021-08
Carvalho C, Fernandes A, Lopes A, Pinheiro H, Gonçalves I (2006) Electrochemical degradation applied to the metabolites of Acid Orange 7 anaerobic biotreatment. Chemosphere 67:1316–1324
Carvalho MC, Pereira C, Goncalves IC, Pinheiro HM, Santos AR, Lopes A, Ferra MI (2008) Assessment of the biodegradability of a monosulfonated azo dye and aromatic amines. Int Biodeterior Biodegrad 62:96–103. doi:10.1016/j.ibiod.2007.12.008
Carvalho CJ, Lizier TM, Zanoni MVB (2010) Highly ordered TiO2 nanotube arrays and photoelectrocatalytic oxidation of aromatic amine. Appl Catal Environ 99:96–102. doi:10.1016/j.apcatb.2010.06.005
Casero I, Sicilia D, Rubio S, Bendito DP (1997) Chemical degradation of aromatic amines by Fenton’s reagent. Water Res 31(8):1985–1995. http://www.doi.org/10.1016/S0043-1354(96)00344-2
Cavallaro A, Piangerelli V, Nerini F, Cavalli S, Reshiotto C (1995) Selective determination of aromatic amines in water samples by capillary zone electrophoresis and solid-phase extraction. J Chromatogr A 709:361–366. doi:10.1016/0021-9673(95)00435-P
Chen S, Sun D, Chung JS (2009) Simultaneous methanogenesis and denitrification of aniline wastewater by using anaerobic–aerobic biofilm system with recirculation. J Hazard Mater 169:575–580. http://www.doi.org/10.1016/j.jhazmat.2009.03.132
Chey WM, Adams RN (1977) Anodic differential pulse voltammetry of aromatic amines and phenols at trace levels. J Electroanal Chem 75:731–738. http://www.doi.org/10.1016/S0022-0728(77)80212-X
Chung KT (2000) Mutagenicity and carcinogenicity of aromatic amines metabolically produced from Azo Dyes. Environ Carcinog Ecotoxicol Rev C 18(l):51–74. http://www.doi.org/10.1080/10590500009373515
Chung KT, Cerniglia CE (1992) Mutagenicity of azo dyes: structure activity relationships. Mutat Res 277:201–220. http://www.doi.org/10.1016/0165-1110(92)90044-A
Chung K-T, Murdock CA, Stevens SE, Li Y-S, Wei C-I, Huang T-S, Chou MW (1995) Mutagenicity and toxicity studies of p-phenylenediamine and its derivatives. Toxicol Lett 81(1):23–32. doi:10.1016/0378-4274(95)03404-8
Combes RD, Haveland-Smith RB (1982) A review of the genotoxicity of food, drug and cosmetic colours and other azo, triphenylmethane and xanthene dyes. Mutation Res/Rev Genetic Toxicol 98(2):101–243. doi:10/1016/016511108290015X
Coughlin MF, Kinkle BK, Bishop PL (2002) Degradation of acid orange 7 in an aerobic biofilm. Chemosphere 46:11–19. http://www.doi.org/10.1016/S0045-6535(01)00096-0
Dados AE, Stalikas CD, Pilidis GA (2004) Determination of aromatic amines in textile after bromination, by gas chromatography coupled with electron capture detection. Chromatographia 59(5/6):335–341. doi:10.1365/s10337-003-0156-x
DeBruin LS, Pawliszyn JB, Josephy PD (1999) Detection of monocyclic aromatic amines, possible mammary carcinogens, in human milk. Chem Res Toxicol 12:78–82. doi:10.1021/tx980168m CCC
Díaz E (2004) Bacterial degradation of aromatic pollutants: a paradigm of metabolic versatility. Int Microbiol 7:173–180. http://www.im.microbios.org/0703/0703173.pdf
Fay LB, Ali S, Gross GA (1997) Determination of heterocyclic aromatic amines in food products: automation of the sample preparation method prior to HPLC and HPLC–MS quantification. Mutat Res 376:29–35. http://www.doi.org/10.1016/S0027-5107(97)00022-5
Fekete A, Malik AK, Kumar A, Schmitt-Kopplin P (2010) Amines in the environment. Crit Rev Anal Chem 40:102–121. doi:10.1080/10408340903517495
Franc J, Koudelková V (1979) Thin-layer chromatography of aromatic amines and their derivatives after reactions with 1-fluoro-2,4-dinitrobenzene. J Chromatogr A 170:89–97. doi:10.1016/S0021-9673(00)84241-7
Franciscon E, Zille A, Dias GF, Ragagnin MC, Durrant LR, Cavaco-Paulo A (2009) Biodegradation of textile azo dyes by a facultative Staphylococcus arlettae strain VN-11 using a sequential microaerophilic/aerobic process. Int Biodeter Biodegr 63:280–288. doi:10.1016/j.ibiod.2008.10.003
Franciscon E, Piubeli F, Fantinatti-Garboggini F, Menezes CR, Silva IS, Cavaco-Paulo A, Grossman MJ, Durrant LR (2010) Polymerization study of the aromatic amines generated by the biodegradation of azo dyes using the laccase enzyme. Enzyme Microb Technol 46:360–365. doi:10.1016/j.enzmictec.2009.12.014
Franciscon E, Grossman MJ, Paschoal JAR, Reyes FGR, Durrant LR (2012) Decolorization and biodegradation of reactive sulfonated azo dyes by a newly isolated Brevibacterium sp. strain VN-15. Springerplus 1(37):1–10. doi:10.1186/2193-1801-1-37
Gan J, Skipper PL, Gago-Dominguez M, Arakawa K, Ross RK, Yu MC, Tannenbaum SR (2004) Alkylaniline-hemoglobin adducts and risk of non-smoking related bladder cancer. J Natl Cancer Inst 96(19):1425–1431. doi:10.1093/jnci/djh274
Ganesh R, Pala S, Metiu H (2007) Modification of the oxidative power of ZnO(1010) surface by substituting some surface Zn atoms with other metals. J Phys Chem C 111(24):8617–8622. doi:10.1021/jp071671n
Garrigue J-L, Ballantyne M, Kumaravel T, Lloyd M, Nohynek GJ, Kirkland D, Toutain H (2006) In vitro genotoxicity of para-phenylenediamine and its N-monoacetyl or N, N-diacetyl metabolites. Mutat Res 608:58–71. doi:10.1016/j.mrgentox.2006.05.001
Ghafoor A, Bark LS (1982) Thin layer chromatography of aromatic amines. J Chem Soc Pak 4(3):147–150
Gibson J, Harwood CS (2002) Metabolic diversity in aromatic compound utilization by anaerobic microbes. Annu Rev Microbiol 56:345–369. doi:10.1146/annurev.micro.56.012302.160749
Gonzalez C, Touraud E, Spinelli S, Thomas O (2007) Organic constituents. In: Thomas O, Burgess C (eds) UV-visible spectrophotometry of water and wastewater. Elsevier Science, Amsterdam, The Netherlands, p 64. ISBN 9780080489841
Greaves AJ, Churchley JH, Hutchings MG, Phillips DAS, Taylor JA (2001) A chemometric approach to understanding the bioelimination of anionic, water-soluble dyes by a biomass using empirical and semi-empirical molecular descriptors. Water Res 35:1225–1239. http://www.doi.org/10.1016/S0043-1354(00)00388-2
Guo K, Chen Y (2010) Simple and rapid detection of aromatic amines using a thin layer chromatography plate. Anal Methods 2:1156–1159. doi:10.1039/C0AY00316F
Haas R, Schmidt TC, Steinbach K, Von Löw E (1997) Derivatization of aromatic amines for analysis in ammunition wastewater II: derivatization of methyl anilines by iodination with a Sandmeyer-like reaction. Fresenius J Anal Chem 359:497–501
Han Y, Quan X, Chen S, Zhao H, Cui C, Zhao Y (2006) Electrochemically enhanced adsorption of aniline on activated carbon fibers. Sep Purif Technol 50:365–372. doi:10.1016/j.seppur.2005.12.011
Hecht SS (2003) Carcinogen derived biomarkers: applications in studies of human exposure to secondhand tobacco smoke. Tob Control 13:48–56. doi:10.1136/tc.2002.002816
Hinteregger C, Loidl M, Streichsbier F (1992) Characterization of isofunctional ring cleaving enzymes in aniline and 3-chloroaniline degradation by Pseudomonas acidovorans CA28. FEMS Microbiol Lett 97:261–266. http://www.doi.org/10.1016/0378-1097(92)90346-P
Hocine O, Boufatit M, Khouider A (2004) Use of montmorillonite clays as adsorbents of hazardous pollutants. Desalination 167:141–145
Hou L, Lee HK (2003) Dynamic three-phase microextraction as a sample preparation technique prior to capillary electrophoresis. Anal Chem 75:2784–2789. doi:10.1021/ac020753z
Huang H, Sommerfeld D, Dunn BC, Lloyd CR, Eyring EM (2001) Ferrate(VI) oxidation of aniline. J Chem Soc Dalton Trans 1301–1305. doi:10.1039/B008934F
Janghel EK, Rai JK, Rai MK, Gupta VK (2005) New analytical technique for the simultaneous determination of aromatic amines in environmental samples. J Sci Ind Res 64:594–597
Jianguo C, Aimin L, Hongyan S, Zhenghao F, Chao L, Quanxing Z (2005) Equilibrium and kinetic studies on the adsorption of aniline compounds from aqueous phase onto bifunctional polymeric adsorbent with sulfonic groups. Chemosphere 61(4):502–509. http://www.doi.org/10.1016/j.chemosphere.2005.03.001
Jin Q, Hu Z, Jin Z, Qiu L, Zhong W, Pan Z (2012) Biodegradation of aniline in an alkaline environment by a novel strain of the halophilic bacterium, Dietzia natronolimnaea JQ-AN. Bioresour Technol 117:148–154. http://www.doi.org/10.1016/j.biortech.2012.04.068
Kahng HY, Kukor Jerome J, Oh KH (2000) Characterization of strain HY99, a novel microorganism capable of aerobic and anaerobic degradation of aniline. FEMS Microbiol Lett 190:215–221 PII: S 0378–1097 (00) 00338–4
Kalyuzhnyi S, Sklyar V, Mosolova T, Kucherenko I, Russkova J, Degtyaryova N (2000) Methanogenic biodegradation of aromatic amines. Water Sci Technol 42(5–6):363–370
Kataoka H (1996) Derivatization reactions for the determination of amines by gas chromatography and their applications in environmental analysis. J Chromatogr A 733:19–34. SSDI 0021-9673(95)00726-1
Khalid A, Arshad M, Crowley DE (2009) Biodegradation potential of pure and mixed bacterial cultures for removal of 4-nitroaniline from textile dye wastewater. Water Res 43:1110–1116. doi:10.1016/j.watres.2008.11.045
Kim D, Guengerich FP (2005) Cytochrome P450 activation of arylamines and heterocyclic amines. Annu Rev Pharmacol Toxicol 45:27–49. doi:10.1146/annurev.pharmtox.45.120403.100010
Kim SI, Leem SH, Choi JS, Chung YH, Kim S, Park YM, Lee YN, Ha KS (1997) Cloning and characterization of two cat-A genes in Acinetobacter lwoffii K24. J Bacteriol 179:5226–5231. http://www.jb.asm.org/content/179/16/5226
Konopka A, Knight D, Turco RF (1989) Characterization of a Pseudomonas sp. capable of aniline degradation in the presence of secondary carbon sources. Appl Environ Microbiol 55:385–389. http://www.aem.asm.org/content/55/2/385
Kostelníková H, Praus P, Turicová M (2008) Adsorption of phenol and aniline by original and quaternary ammonium salts-modified montmorillonite. Acta Geodyn Geomater 5(149):83–88
Kudlich M, Hetheridge MJ, Knackmuss HJ, Stolz A (1999) Autoxidation reactions of different aromatic o-aminohydroxynaphthalenes that are formed during the anaerobic reduction of sulfonated azo dyes. Environ Sci Tech 33:896–901. doi:10.1021/es9808346
Lewtas J (2007) Air pollution combustion emissions: characterization of causative agents and mechanisms associated with cancer, reproductive, and cardiovascular effects. Rev Mutat Res 636(1–3):95–133. doi:10.1016/j.mrrev.2007.08.003
Lin’kova YV, Dyakonova AT, Gladchenko MA, Kalyuzhnyi SV, Kotova IB, Stams A, Netrusov AI (2011) Methanogenic degradation of (Amino) aromatic compounds by anaerobic microbial communities. Appl Biochem Microbiol 47(5):507–514. doi:10.1134/S0003683811050085
Liu Z, Yang H, Huang Z, Zhou P, Liu SJ (2002) Degradation of aniline by newly isolated, extremely aniline-tolerant Delftia sp. AN3. Appl Microbiol Biotechnol 58:679–682. doi:10.1007/s00253-002-0933-8
Lizier TM, Zanoni MVB (2012) Effect of ionic liquid on the determination of aromatic amines as contaminants in hair dyes by liquid chromatography coupled to electrochemical detection. Molecules 17:7961–7979. doi:10.3390/molecules17077961
Lovley DR (2001) Bioremediation anaerobes to the rescue. Science 293:1444–1446. doi:10.1126/science.1063294
Lovley DR (2003) Cleaning up with genomics: applying molecular biology to bioremediation. Nat Rev Microbiol 1:35–44. doi:10.1038/nrmicro731
Marques MM, Mourato LL, Amorim MT, Santos MA, Melchior WB Jr, Beland FA (1997) Effect of substitution site upon the oxidation potentials of alkylanilines, the mutagenicities of N-hydroxyalkylanilines, and the conformations of alkylaniline-DNA adducts. Chem Res Toxicol 10:1266–1274. doi:10.1021/tx970104w
Martínez D, Cugat MJ, Borrull F, Calull M (2000) Solid-phase extraction coupling to capillary electrophoresis with emphasis on environmental analysis. J Chromatogr A 902:65–89. PII: S0021-9673(00)00839-6
Masomboon N, Ratanatamskul C, Lu MC (2011) Kinetics of 2,6-dimethylaniline oxidation by various Fenton processes. J Hazard Mater 192:347–353. doi:10.1016/j.jhazmat.2011.05.034
McLeod MP, Eltis LD (2008) Genomic insights into the aerobic pathways for degradation of organic pollutants. In: Díaz E (ed) Microbial biodegradation: genomics and molecular biology. Caister Academic Press, Norfolk, pp 1–23
Melo A, Viegas O, Eça R, Petisca C, Pinho O, Ferreira IMPLVO (2008) Extraction, detection, and quantification of heterocyclic aromatic amines in Portuguese meat dishes by HPLC/Diode array. J Liq Chromatogr Relat Technol 31:772–787. doi:10.1080/10826070701855987
Mendes S, Pereira L, Batista C, Martins LO (2011) Molecular determinants of azo reduction activity in the strain Pseudomonas putida MET94. Appl Microbiol Biotechnol 92:393–405. doi:10.1007/s00253-011-3366-4
Mondal PK, Ahmad R, Usmani SQ (2010) Anaerobic biodegradation of triphenylmethane dyes in a hybrid UASFB reactor for wastewater remediation. Biodegradation 21:1041–1047. doi:10.1007/s10532-010-9364-x
Moradi M, Yamini Y, Esrafili A, Seidi S (2010) Application of surfactant assisted dispersive liquid-liquid microextraction for sample preparation of chlorophenols in water samples. Talanta 82:1864–1869. doi:10.1016/j.talanta.2010.08.002, ISSN: 0039–9140
Moriwakia H, Harino H, Hashimoto H, Arakawa R, Ohe T, Yoshikura T (2003) Determination of aromatic amine mutagens, PBTA-1 and PBTA-2, in river water by solid-phase extraction followed by liquid chromatography–tandem mass spectrometry. J Chromatogr A 995:239–243. doi:10.1016/S0021-9673(03)00514-4
Mortensen SK, Trier XT, Foverskov A, Petersen JH (2005) Specific determination of 20 primary aromatic amines in aqueous food simulants by liquid chromatography–electrospray ionization-tandem mass spectrometry. J Chromatogr A 1091:40–50. doi:10.1016/j.chroma.2005.07.026
Mucha K, Kwapisz E, Kucharska U, Okruszek A (2010) Mechanism of aniline degradation by yeast strain candida methanosorbosa BP-6. Pol J Microbiol 59(4):311–315
Murakumi S, Takashima A, Takemoto J, Takenaka S, Shinke R, Aoki K (1999) Cloning and sequence analysis of two catechol-degrading gene clusters from the aniline assimilating bacterium Frateuria species ANA-18. Gene 226:189–198. http://www.doi.org/10.1016/S0378-1119(98)00560-5
Mälzer HJ, Gerlach M, Gimbel R (1993) Effects of shock loads on bank filtration and their prediction by control filters. Water Supply 11:165–177
Narayana B, Sunil K (2009) A spectrophotometric method for the determination of nitrite and nitrate. Eurasian J Anal Chem 4(2):204–214. ISSN: 1306–3057
Neyens E, Baeyens J (2003) A review of classic Fenton’s peroxidation as an advanced oxidation technique. J Hazard Mater 98(1–3):33–50. PII: S0304-3894(02)00282-0
Nohynek GJ, Antignac E, Re T, Toutain H (2010) Safety assessment of personal care products/cosmetics and their ingredients. Toxicol Appl Pharmacol 243:239–259. doi:10.1016/j.taap.2009.12.001
O’Neill FJ, Brimley-Challenor KCA, Greenwood RJ, Knapp JS (2000) Bacterial growth of aniline: implications for the biotreatment of industrial wastewater. Water Res 34(18):4397–4409. doi:10.1016/S0043-1354(00)00215-3
Oda H, Yokokawa C (1983) Adsorption of aromatic amines and o-substituted derivatives of phenol from organic solutions by activated carbons-effect of surface acidity. Carbon 21(5):485–489. http://www.doi.org/10.1016/0008-6223(83)90141-0
Oguri S (2000) Electromigration methods for amino acids, biogenic amines and aromatic amines. J Chromatogr B 747:1–19. http://www.doi.org/10.1016/S0378-4347(00)00092-X
Osterloh FE (2008) Inorganic materials as catalysts for photochemical splitting of water. Chem Mater 20:35–54. doi:10.1021/cm7024203
Oturan MA, Brillas E (2007) Electrochemical Advanced Oxidation Processes (EAOPs) for environmental applications. Port Electrochim Acta 25:1–18
Pacheco MJ, Santos V, Ciríaco L, Lopes A (2011) Electrochemical degradation of aromatic amines on BDD electrodes. J Hazard Mater 186(2–3):1033–1041. doi:10.1016/j.jhazmat.2010.11.108
Palmiotto G, Pieraccini G, Moneti G, Dolara P (2001) Determination of the levels of aromatic amines in indoor and outdoor air in Italy. Chemosphere 433:355–361, PII:S0045-6535(00)00109-0
Panizza M, Zolezzi M, Nicolella C (2006) Biological and electrochemical oxidation of naphthalenesulfonates. J Chem Technol Biotechnol 81:225–232
Panizza M, Brillas E, Comninellis C (2008) Application of boron-doped diamond electrodes for wastewater treatment. J Environ Eng Manag 18:139–153
Parales RE, Resnick SM (2006) Aromatic ring hydroxylating dioxygenases. In: Ramos JL, Levesque RC (eds) Pseudomonas, vol 4, Molecular biology of emerging issues. Springer, New York, pp 287–340
Parales RE, Ontl TA, Gibson DT (1997) Cloning and sequence analysis of a catechol 2,3-dioxygenase gene from the nitrobenzene degrading strain Comamonas sp. JS765. J Ind Microbiol Biotechnol 19:385–391. doi:10.1038/sj.jim.2900420
Pereira R, Pereira L, Van der Zee FP, Alves MM (2011) Fate of aniline and sulfanilic acid in UASB bioreactors under denitrifying conditions. Water Res 45:191–200. doi:10.1016/j.watres.2010.08.027
Pereira L, Pereira R, Oliveira CS, Alves MM (2013) UV/TiO2 photocatalytic degradation of xanthene dyes. Photochem Photobiol, 2013, 89:33–50. doi:10.1111/j.1751-1097.2012.01208.x
Perez F (2001) Spectrophotometric study of industrial effluents – application in parameters estimation. PhD thesis, Universite’ d’Aix-Marseille; II
Pielesz A (1999) The process of the reduction of azo dyes used in dyeing textiles on the basis of infrared spectroscopy analysis. J Mol Struct 337–344. http://www.doi.org/10.1016/S0022-2860(99)00176-3
Pielesz A, Baranowska I, Rybak A, Włochowicz A (2002) Detection and determination of aromatic amines as products of reductive splitting from selected azo dyes. Ecotoxicol Environ Saf 53:42–47. doi:10.1006/eesa.2002.2191
Pinheiro HM, Touraud E, Thomas O (2004) Aromatic amines from azo dye reduction: status review with emphasis on direct UV spectrophotometric detection in textile industry wastewaters. Dyes Pigments 61:121–139. doi:10.1016/j.dyepig.2003.10.009
Ramalho PA, Helena CM, Cavaco-Paulo A, Teresa RM (2004) Characterization of azo reduction activity in a novel ascomycete yeast strain. Appl Environ Microbiol 70(4):2279–2288. doi:10.1128/AEM.70.4.2279-2288.2004
Ramya M, Iyappan S, Manju A, Jiffe JS (2010) Biodegradation and decolorization of acid red by acinetobacter radioresistens. J Bioremediat Biodegrad 1(105):2–6. doi:10.4172/2155-6199.1000105
Ratiu C, Manea F, Lazau C, Grozescu I, Radavon C, Schoonman J (2010) Electrochemical oxidation of p-aminophenol from water with boron-doped diamond anodes and assisted photocatalytically by TiO2-supported zeolite. Desalination 260(1–3):51–56. doi:10.1016/j.desal.2010.04.068
Razo-Flores E (1997) Biotransformation and biodegradation of N-substituted aromatics in methanogenic granular sludge. PhD thesis, Wageningen Agriculture University, The Netherlands. http://edepot.wur.nl/199864
Razo-Flores E, Donlon BA, Field JA, Lettinga G (1996) Biodegradability of N-substituted aromatics and alkylphenols under methanogenic conditions using granular sludge. Water Sci Technol 33(3):47–57. http://www.doi.org/10.1016/0273-1223(96)00320-4
Razo-Flores E, Donlon B, Lettinga G, Field JA (1997a) Biotransformation and biodegradation of N-substituted aromatics in methanogenic granular sludge. FEMS Microbiol Rev 20:525–538, PII S0168-6445(97)00031–4
Razo-Flores E, Luijten M, Donlon BA, Lettinga G, Field JA (1997b) Complete biodegradation of the azo dye azodisalicylate under anaerobic conditions. Environ Sci Tech 31:2098–2103
Razo-Flores E, Lettinga G, Field JA (1999) Biotransformation and biodegradation of selected nitroaromatics under anaerobic conditions. Biotechnol Prog 15:358–365. doi:10.1021/bp9900413
Rieger P-G, Meier H-M, Gerle M, Vogtb U, Groth T, Knackmuss H-J (2002) Xenobiotics in the environment: present and future strategies to obviate the problem of biological persistence. J Biotechnol 94(1):101–123. doi:10.1016/S0168-1656(01)00422-9
Robinson T, MacMullan G, Marchant R, Nigam P (2001) Remediation of dyes in textile effluents, current treatment technologies with a proposed alternative. Bioresour Technol 77:247–255. http://www.doi.org/10.1016/S0960-8524(00)00080-8
Sánchez L, Peral J, Domènech X (1998) Aniline degradation by combined photocatalysis and ozonation. Appl Catal Environ 19:59–65. PII: S0926- 3373(9 8)00058-7
Santos V, Diogo J, Pacheco MJ, Ciríaco L, Morão A, Lopes A (2010) Electrochemical degradation of sulfonated amines on SI/BDD electrodes. Chemosphere 79(6):637–645. doi:10.1016/j.chemosphere.2010.02.031
Savelieva O, Kotova I, Roelofsen W, Stams AJM, Netrusov A (2004) Utilization of aminoaromatic acids by a methanogenic enrichment culture and by a novel Citrobacter freundii strain. Arch Microbiol 181:163–170. doi:10.1007/s00203-003-0645-1
Schmidt TC, Haas R, Von Löw E, Steinbach K (1998) Derivatization of aromatic amines with bromine for improved gas chromatographic determination. Chromatographia 48(5–6):436–442. doi:10.1007/BF02467717
Sentellas S, Moyano E, Puignou L, Galceran MT (2003) Determination of heterocyclic aromatic amines by capillary electrophoresis coupled to mass spectrometry using in-line preconcentration. Electrophoresis 24:3075–3082. doi:10.1002/elps.200305523
Sentellas S, Moyano E, Puignou L, Galceran MT (2004) Optimization of a clean-up procedure for the determination of heterocyclic aromatic amines in urine by field-amplified sample injection–capillary electrophoresis–mass spectrometry. J Chromatogr A 1032:193–201. doi:10.1016/j.chroma.2003.11.011
Seymour EH, Lawrence NS, Pandurangappa M, Compton RG (2002) Indirect electrochemical detection of nitrite via diazotization of aromatic amines. Microchim Acta 140:211–217. doi:10.1007/s00604-002-0915-7
Sharma N, Jain A, Verma KK (2011) Headspace solid-phase microextraction and on-fibre derivatization of primary aromatic amines for their determination by pyrolysis to aryl isothiocyanates and gas chromatography-mass spectrometry. Anal Methods 3(4):970–976. doi:10.1039/C0AY00745E
Skarping G, Renman L, Dalene M (1983) Trace analysis of amines and isocyanates using glass capillary gas chromatography and selective detection. II Determination of aromatic amines as perfluoro fatty acid amides using nitrogen-selective detection. J Chromatogr A 270:207–218
Skipper PL, Kim MY, Sun H-LP, Wogan GN, Tannenbaum SR (2010) Monocyclic aromatic amines as potential human carcinogens: old is new again. Carcinogenesis 31(1):50–58. doi:10.1093/carcin/bgp267
Srivastava SP, Dua VK (1975) TLC separation of closely related amines. J Anal Chem 276:382
Stolz A (1999) Degradation of substituted naphthalene sulfonic acids by Sphingomonas xenophaga BN6. J Ind Microbiol Biotechnol 23:391–399. doi:10.1038/sj.jim.2900725
Sun Y, Liang L, Zhao X, Yu L, Zhana J, Shi G, Zhou T (2009) Determination of aromatic amines in water samples by capillary electrophoresis with amperometric detection. Water Res 43:41–46. doi:10.1016/j.watres.2008.10.004
Sun X-M, Sun Y, Wu L-W, Jiang C-Z, Yu X, Gao Y, Wang L-Y, Song D-Q (2012a) Determination of aromatic amines in environmental water samples. Anal Methods 4(7):2074–2080. doi:10.1039/C2AY25056J
Sun XM, Sun Y, Wu LW, Jiang CZ, Yu X, Gao Y, Wang LY, Song DQ (2012b) Development of a vortex-assisted ionic liquid microextraction method for the determination of aromatic amines in environmental water samples. Anal Methods 4:2074–2080. doi:10.1039/C2AY25056J
Szterk A, Roszko M, Małek K, Kurek M, Zbieæ M, Waszkiewicz-Robak B (2012) Profiles and concentrations of heterocyclic aromatic amines formed in beef during various heat treatments depend on the time of ripening and muscle type. Meat Sci 92:587–595. doi:10.1016/j.meatsci.2012.06.004
Tan NCG, Prenafeta-Boldu FX, Opsteeg J, Lettinga G, Field JA (1999) Biodegradation of azo dyes in cocultures of anaerobic granular sludge with aerobic aromatic amine degrading enrichment cultures. Appl Microbiol Biotechnol 51:865–871. doi:10.1007/s002530051475
Tan NCG, Van Leeuwen A, Van Voorthuizen EM, Slenders P, Prenafeta-Boldú FX, Temmink H, Lettinga G, Field JA (2005) Fate and biodegradability of sulfonated aromatic amines. Biodegradation 16:527–537
Tannenbaum SR (1991) Bladder cancer in workers exposed in aniline. J Nat Cancer Inst 83:1507–1508
Tao Y, Liu JF, Wang T, Jiang GB (2009) Simultaneous conduction of two- and three-phase hollow-fiber-based liquid-phase microextraction for the determination of aromatic amines in environmental water samples. J Chromatogr A 1216(5):756–762. doi:10.1016/j.chroma.2008.11.094
Toräng L, Reuschenbach P, Müller B, Nyholm N (2002) Laboratory shake flask batch tests can predict field biodegradation of aniline in the Rhine. Chemosphere 49:1257–1265. http://www.doi.org/10.1016/S0045-6535(02)00605-7
Toribio F, Moyano E, Puignou L, Galceran MT (2002) Ion-trap tandem mass-spectrometry for the determination of heterocyclic amines in food. J Chromatogr A 948:267–281. PII: S0021-9673(01)01476-5
Torres RA, Sarria V, Torres W, Peringer P, Pulgarin C (2003) Electrochemical treatment of industrial wastewater containing 5-amino-6-methyl-2-benzimidazolone: toward an electrochemical–biological coupling. Water Res 37:3118–3124. doi:10.1016/S0043-1354(03)00179-9
USA National Toxicology Program (2005) 11th report on carcinogens. U.S. Department of Health and Human Services
Vaillancourt FH, Bolin JT, Eltis LD (2006) The ins and outs of ring-cleaving dioxygenases. Crit Rev Biochem Mol Biol 41:241–267. doi:10.1080/10409230600817422
Van der Plas SE, De Clercq PJ, Madder A (2007) Fast and easy detection of aromatic amines on solid support. Tetrahedron Lett 48:2587–2589. doi:10.1016/j.tetlet.2007.02.023
Van der Zee FP, Villaverde S (2005) Combined anaerobic–aerobic treatment of azo dyes – A short review of bioreactor studies. Water Res 39:1425–1440. doi:10.1016/j.watres.2005.03.007
Vázquez-Rodríguez GA, Beltrán-Hernández RI, Lucho-Constantino CA, Blasco JL (2008) A method for measuring the anoxic biodegradability under denitrifying conditions. Chemosphere 71:1363–1368. doi:10.1016/j.chemosphere.2007.11.012
Verma KK, Sanghi SK, Jain A (1988) Spectrophotometric determination of primary aromatic amines with 4-N methylaminophenol and 2-iodylbenzoate. Talanta 35:409–411. http://www.doi.org/10.1016/0039-9140(88)80037-7
Vytras K, Kalous J, Kala´bova´ Z, Remes M (1982) Ion-selective electrodes in titrations involving azo-coupling reactions. Anal Chem Acta 141:163–171
Wang L, Barrington S, Kim JW (2007) Biodegradation of pentyl amine and aniline from petrochemical wastewater. J Environ Manage 83:191–197. http://www.doi.org/10.1016/j.jenvman.2006.02.009
Wang X, Fu L, Wei G, Hu J, Zhao X, Liu X, Li Y (2008) Determination of four aromatic amines in water samples using dispersive liquid–liquid microextraction combined with HPLC. J Sep Sci 31:2932–2938. doi:10.1002/jssc.200800273
Weisburger JH (1997) A perspective on the history and mutagenic, N-substituted aryle compounds in human health. Mutat Res 376:61–266
Wilmer JL, Rosenkranz HS, Pet-Edwards J, Chankong V, Haimes YY (1984) On the carcinogenicity of aniline. Environ Mutagen 6(5):629–632. doi:10.1002/em.2860060502
Worch E, Grischek T, Börnick H, Eppinger P (2002) Laboratory tests for simulating attenuation processes of aromatic amines in riverbank filtration. J Hydrol 266:259–268. http://www.doi.org/10.1016/S0022-1694(02)00169-5
Wu YG, Hui L, Li X, Zhang YZ, Zhang WC (2007) Degradation of aniline in weihe riverbed sediments under denitrification conditions. J Environ Sci Health, Part A: Tox Hazard Subst Environ Eng 42(4):413–419. doi;org/10.1080/10934520601187302
Yadav S, Tyagi DK, Yadav OP (2011) Equilibrium and kinetics studies on adsorption of aniline blue from aqueous solution onto rice husk carbon. Int J Chem Res 2(3):59–64
Yang J, Tsai FP (2001) Development of a solid-phase microextraction/reflection-absorption infrared spectroscopic method for the detection of chlorinated aromatic amines in aqueous solutions. Anal Sci 17:751–756
Yang K, Wu W, Jing Q, Zhu L (2008) Aqueous adsorption of aniline, phenol, and their substitutes by multi-walled carbon nanotubes. Environ Sci Tech 42:7931–7936. doi:10.1021/es801463v
Ye S, Huang F (2007) Separation of carcinogenic aromatic amines in the dyestuff plant wastewater treatment. Desalination 206:78–85. doi:10.1016/j.desal.2006.03.562
Yemashova N, Kalyuzhnyi S (2006) Microbial conversion of selected azo dyes and their breakdown products. Water Sci Technol 53(11):163–171. http://www.ukpmc.ac.uk/abstract/MED/16862786
Yu MC, Ross RK (1998) Epidemiology of bladder cancer. In: Petrovich Z, Baert L, Brady LW (eds) Carcinoma of the bladder. Innovations in management. Springer, Berlin, pp 1–13
Yu MC, Skipper PL, Tannenbaum SR, Chan KK, Ross RK (2002) Arylamine exposures and bladder cancer risk. Mutat Res/Fundam Mol Mech Mutagen 506–507:21–28. http://www.doi.org/10.1016/S0027-5107(02)00148-3
Zatar NA, Abu-Zuhri AZ, Abu-Shaweesh AA (1998) Spectrophotometric determination of some aromatic amines. Talanta 47:883–890. http://www.doi.org/10.1016/S0039-9140(98)00177-5
Zeng Z, Qiu W, Yang M, Wei X, Huang Z, Li F (2001) Solid-phase microextraction of monocyclic aromatic amines using novel fibers coated with crown ether. J Chromatogr A 934:51–57, PII: S0021-9673(01)01303-6
Zeyer J, Wasserfallen A, Timmis KN (1985) Microbial mineralization of ring-substituted anilines through an ortho-cleavage pathway. Appl Environ Microbiol 50(2):447–453. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC239144/pdf/aem00133-0350.pdf
Zhang Q, Wang C, Bai H, Wang X, Wu T, Ma Q (2009) Determination of aromatic amines from azo dyes reduction by liquid-phase sorbent trapping and thermal desorption-gas chromatography-mass spectrometry. J Sep Sci 32(14):2434–2441. doi:10.1002/jssc.200900089
Zhang S, Li A, Cui D, Yang JX, Ma F (2011) Performance of enhanced biological SBR process for aniline treatment by mycelial pellet as biomass carrier. Bioresour Technol 102(6):4360–4365. http://www.doi.org/10.1016/j.biortech.2010.12.079
Zhao X, Suo Y (2008) Analysis of primary aromatic amines using precolumn derivatization by HPLC fluorescence detection and online MS identification. J Sep Sci 31:646–658. doi:10.1002/jssc.200700400
Zhou QX, Ye CL (2008) Ionic liquid for improved single-drop microextraction of aromatic amines in water samples. Microchim Acta 162(1–2):153–159. doi:10.1007/s00604-007-0857-1
Zwirner-Baier I, Deckart K, Jäckh R, Neumann HG (2003) Biomonitoring of aromatic amines VI: determination of hemoglobin adducts after feeding aniline hydrochloride in the diet of rats for 4 weeks. Arch Toxicol 77:672–677. doi:10.1007/s00204-003-0473-8
Acknowledgement
The authors thank the FCT exploratory EXPL/AAG-TEC/0898/2013 and Strategic Project of UID/BIO/04469/2013 unit. Acknowledgements also to the project RECI/BBB-EBI/0179/2012 (FCOMP-01-0124-FEDER-027462) and the Project “BioEnv - Biotechnology and Bioengineering for a sustainable world”, REF. NORTE-07-0124-FEDER-000048, co-funded by the Programa Operacional Regional do Norte (ON.2 – O Novo Norte), QREN, FEDER.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Pereira, L., Mondal, P.K., Alves, M. (2015). Aromatic Amines Sources, Environmental Impact and Remediation. In: Lichtfouse, E., Schwarzbauer, J., Robert, D. (eds) Pollutants in Buildings, Water and Living Organisms. Environmental Chemistry for a Sustainable World, vol 7. Springer, Cham. https://doi.org/10.1007/978-3-319-19276-5_7
Download citation
DOI: https://doi.org/10.1007/978-3-319-19276-5_7
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-19275-8
Online ISBN: 978-3-319-19276-5
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)