Abstract
Oxidative stress results from an imbalance between the production of free radicals and the ability of an organism to ameliorate their effects through the actions of enzymes and small molecules that function as antioxidants. Many of the pollutants found in freshwater and marine systems are known to promote a pro-oxidant cellular environment that leads to the production of reactive oxygen species (ROS). Considerable research efforts are beginning to provide a mechanistic understanding of oxidative stress in fishes as related to the basic biochemical and molecular mechanisms of oxidative stress in fishes, its relevance to basic biomedical sciences, and its application toward an understanding of the impact of environmental stressors on fish population health. This chapter will discuss the chemicals known to cause oxidative stress in fishes, and the variety of small molecule and enzymatic defenses employed by fish to counteract the effects of ROS. We will also consider how the molecular-level understanding is informing the development of Adverse Outcome Pathways to link environmentally-induced oxidative stress to ecological risk assessments.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Oxidative Stress
- Reactive Oxygen Species
- Oxidative Stress Response
- Adverse Outcome Pathway
- Protective Target
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
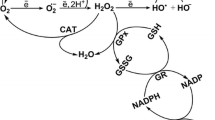
Oxidative stress can be defined as the deleterious cellular effects arising from the production of reactive oxygen species (ROS) beyond the capacity of antioxidant defense systems to detoxify them. ROS are reactive O2-based molecules including the superoxide anion radical (O2 −.), the hydroxyl radical (.OH), hydrogen peroxide (H2O2), ozone (O3) and singlet oxygen (1O2). The importance of oxidative stress and antioxidant defenses in human health and disease has been a major topic of research and clinical application for decades (see review by [1]). More recently, there has been an increasing appreciation for these phenomena in fishes, particularly in the context of pollution of freshwater and marine systems. This has spurred substantial research into a mechanistic understanding of oxidative stress in fishes. The mechanistic study of oxidative stress in fishes serves many purposes: (1) increase our understanding of the basic biochemical and molecular mechanisms related to oxidative stress in fish; (2) explore evolutionary adaptations to oxidative stress to inform our understanding in other vertebrate species, including humans; and (3) understand the impact of prooxidant environmental stressors on fish population health. Many xenobiotics induce the production of ROS by several biochemical mechanisms (Fig. 26.1) such as the impairment of membrane-bound electron transport (e.g., mitochondrial, microsomal electron transport), redox cycling, inactivation of antioxidant enzymes, depletion of free radical scavengers, photosensitization, and facilitation of Fenton reactions [2]. A number of reviews have been published that discuss the nature of ROS, mechanisms by which they are produced naturally and via xenobiotics, antioxidant defense systems, cellular targets and organismal consequences [2, 3].
Many environmental chemicals of varying chemical classes have been shown to cause oxidative stress in marine and freshwater fishes. The severity of the oxidative stress can be influenced by temperature [4–6], salinity [7–9], and hypoxia [10–12]. It is unclear in many cases whether these stressors are acting synergistically or additively with xenobiotic chemicals since antioxidants and oxidative stress responses can be influenced by many environmental factors, including temperature [13–15], dissolved oxygen [16–19], salinity [20], and acidification [21]. A very interesting case is the notothenoid ice fish that have adapted to the cold, oxygen-rich waters of Antarctica through genomic loss or gene amplification [22, 23] and have a pronounced loss of heat shock response and a non-traditional battery of oxidative stress-responsive genes [24]. This suggests that they might be highly sensitive to the effects of oxidative stress or any other stressor that might promote oxidative stress [25]; therefore, questions arise regarding their ability to adapt to warming in the Antarctic and the increased environmental pollution via global transport mechanisms and expanding ecotourism.
The association of pesticide exposure and oxidative stress is well established for many environmentally-relevant agents, and their structural diversity leads to a spectrum of effects. For example, a number of pesticides have been shown to impact catalase activity [26–28], lipid peroxidation [29–31], and mitochondrial function [32–34]. A relatively recent and thorough review of pesticide-induced oxidative stress in fish was produced by Slaninova and coworkers [35].
Metals are known inducers of oxidative stress in many species of fish, promoting the formation of ROS through either redox cycling [36–38] or interaction with antioxidant defenses, especially with thiol-containing antioxidants and enzymes [39–41]. Sevcikova et al. have published a thorough review of metals and oxidative stress in fish [42].
Aromatic hydrocarbons are found in nearly all aquatic environments around the globe. They are structurally diverse, but all are composed of one or more benzene rings. In general, aromatic hydrocarbons are metabolized in fish by Phase I enzymes (e.g., cytochrome P450s or epoxide hydrolase) to reactive intermediates that are substrates for conjugation by Phase II enzymes to create hydrophilic metabolites for excretion. As part of this metabolic process, reactive oxygen species are produced that lead to the induction of antioxidant enzymes and protective molecules [43–45]. Also, the metabolic products of PAHs include quinones that can generate ROS via redox cycling [46]. The toxicity of aromatic hydrocarbons can also be increased by exposure to UV light in the environment, which has been demonstrated in a number of fish species and life stages [47–49].
Microcystins are potent cyanobacterial toxins produced by Microcystis spp. during bloom events, and their effects on fish and other aquatic animals have been reviewed recently [50, 51]. In cultured fish cells, purified microcystin causes increases in lipid peroxidation and the expression of several antioxidant enzymes [52]. In many whole organism studies using fish, the exposures occur via the addition of cultured Microcystis spp. to the water in which the fish are being held. Under these conditions, there is also an increase in lipid peroxidation and the expression of several antioxidant enzymes with the liver being the most affected organ followed by kidney and gills [53]. A few studies have observed that Microcystis spp. exposure also inhibits protein serine/threonine phosphatase in liver that may initiate a metabolic response to the toxin (Olivares [54]). Interestingly, Amado et al. suggest that hyperphosphorylation linked to ROS is responsible for inducing and maintaining the antioxidant response to Microcystis spp. exposure [50]. The exact mechanistic linkages between oxidative stress, phosphorylation state and oxidative stress responses induced by Microcystis spp. remain to be determined.
1 Small Molecule Antioxidant Defenses
A number of small molecules have been shown to have a protective effect in fish under environmentally-induced oxidative stress. We will briefly discuss what are often considered the most important small molecules in the prevention of ROS-induced damage—glutathione, ascorbic acid (vitamin C), and tocopherol (vitamin E). Glutathione is considered the most important small molecule for cellular defense against ROS-induced damage. As observed in mammals, tissue glutathione levels are often depleted after short-term oxidant exposures but elevated after long-term exposures [3, 55, 56]. Glutathione-associated antioxidant enzymes—glutathione peroxidase, glutathione reductase, and glutathione S-transferase—(discussed below) are also critical for maintenance of normal cellular redox status and protection against ROS [2]. Non-glutathione small molecules are increasingly under scrutiny and evidence suggests they also play a critical role in protection against ROS. Ascorbic acid (vitamin C) levels are modulated by environmental chemicals [57, 58] and other environmental stressors including osmotic stress [20] and redox stress associated with air breathing [59]. Tissue ascorbic acid levels have been shown to protect against lipid peroxidation [60]. The majority of studies using fish to investigate the protective effect of tocopherol (vitamin E) are based on dietary supplementation, which can reduce oxidative stress-related biomarkers [61–63]. Environmental toxicants can modulate the levels of tocopheral which may influence ROS-induced damage [64, 65].
2 Antioxidant Transcriptional Response
Cellular homeostatic mechanisms have evolved to deal with low levels of oxidative stress through modulation of the basal cellular concentrations of glutathione and the transcription factor commonly known as NRF2 (HGNC approved name: nuclear factor, erythroid 2-like 2, symbol: NFE2L2). With elevated levels of oxidative stress, cells can adapt by up-regulation of networks of responsive proteins regulated by NRF2 or NFkB (Fig. 26.2). Most proteins whose expression is regulated by NRF2 activity function as cryoptotectants [66]. The cellular pool of NRF2 is regulated through binding to KEAP1 (kelch-like ECH-associated protein 1), which promotes NRF2 ubiquitination and limits protein half-life. Under oxidative stress conditions, ubiquitination of NRF2 is dramatically reduced and KEAP1 binding sites are rapidly saturated. This leads to an increase in free cytosolic NRF2, which acts as a redox probe and translocates to the nucleus under oxidative conditions [66]. In the nucleus, NRF2 dimerizes with small MAF proteins to up-regulate the transcription of numerous target genes via binding to antioxidant response elements (also known as electrophile response elements) [67].
Transcriptional activation in response to oxidative stress via NRF2 and NFkB pathways. Both pathways play a key role in the transcriptional responses to oxidative stress. Interestingly, the two pathways have been described as mutually stimulatory and inhibitory in an apparently cell-types specific manner
NFkB is known to regulate proteins that are cryoprotectants and some that are pro-oxidant. While these two functions seem contradictory, the expression of NFkB target genes typically promotes cellular survival in response to numerous cellular stressors [68]. The canonical NFkB pathway is activated mostly under proinflammatory conditions. The NFKB1-RELA dimer is held inactive via interaction with IkB inhibitory proteins. Under oxidative stress conditions, IkB is phosphorylated and subsequently ubiquinated, which allows the NFKB1-RELA dimer to translocate to the nucleus and bind to NFkB binding sites [68].
3 Antioxidant Enzymes and Protective Targets
These two pathways are largely responsible for transcriptional activation of gene products that protect the cell from oxidative stress-associated damage (Table 26.1). These proteins can be broadly described as antioxidant enzymes or protective targets. Antioxidant enzymes catalyze the elimination of reactive metabolic intermediates and ROS, or are important modulators of these processes. Enzymes such as superoxide dismutase and catalase act directly to detoxify the inorganic free radicals superoxide and hydrogen peroxide, respectively [69]. Enzymes such as glutathione S-transferase and UDP glucuronosyltransferases generally catalyze the conjugation of small molecules (glutathione and glucuronic acid, respectively) to reactive intermediates which makes them readily excretable [70, 71]. In contrast, the enzyme glutamate-cysteine ligase, which has a catalytic subunit (GCLC) and a modifier subunit (GCLM), is the rate-limiting enzyme in glutathione synthesis [72].
Proteins that function as protective targets can generally be considered ROS scavengers. Many members of the globin gene family (including myoglobin, neuroglobin, cytoglobin) are thought to play an important role in ROS scavenging and are prominent stress-responsive proteins in fish [73–75]. The globin X member of the gene family is found only in fishes and may either protect the lipids in cell membrane from oxidation or may act as a redox-sensing or signaling protein [76]. As in other vertebrates, metallothioneins and thioredoxins are also important free radical scavengers [16, 77].
4 Adverse Outcome Pathways, Oxidative Stress, and the Health of Wild Fish Populations
The effects of oxidative stress can be measured and interpreted at the level of individual fish as correlations between biomarker activation and measured changes in physiology. However, when considering impacts on wildlife, it is the impacts at the population level that are most relevant. This relationship between molecular responses, individual health, and population effects are rarely straightforward or readily apparent. The Adverse Outcome Pathway (AOP) framework as defined by Ankley et al. [78] is an approach toward understanding the linkages from a molecular initiating event, through a series of biological processes, to an ultimate adverse outcome of relevance to human or ecological risk assessors (Fig. 26.3). This approach is based on the 2007 report by U.S. National Research Council (NRC) Committee on Toxicity Testing and Assessment of Environmental Agents that sought to transform toxicity testing and embrace newly-developed high-throughput and computational approaches focused on pathways to inform risks to humans (NRC 2007). One primary difference between the NRC approach and the AOP framework approach for ecotoxicology or public health is the focus on population-level effects in the AOP.
Schematic of a generalized Adverse Outcome Pathway. Points where oxidative stress and subsequent and transcriptional responses play a role are indicated. The gray arrows indicate the multiplicity of cellular- and organ-level responses to oxidative stress. Biomarkers are commonly used to indicate (1) oxidative damage that initiates stress responses (e.g. lipid peroxidation, oxidative DNA damage, depleted glutathione levels) to indicate (2) processes that are initiated when homeostatic mechanisms are overwhelmed (e.g. apoptosis, necrosis)
Oxidative stress and oxidative stress responses are a very important component of many AOPs; however, the nature of oxidative stress responses as homeostatic pathways precludes consideration as a defined AOP [79]. For example, oxidative stress is a key component of proposed AOPs for drug-induced cholestasis and chemical-induced liver fibrosis [80]. At this time, the role of oxidative stress in the initiation of specific disease or dysfunction processes (or AOPs) is rarely well-defined. There are numerous biomarkers of oxidative stress that include macromolecular damage (e.g., lipid peroxidation, oxidative DNA damage) or changes in expression levels of oxidative stress-responsive genes (Table 26.1). There is a substantial body of scientific literature that clearly connects toxicant exposure with the generation of oxidative stress and the expression or certain biomarkers. Enhancing our understanding of these linkages to disease and dysfunction will increase the certainty with which one can apply oxidative stress biomarkers to predict and assess the potential for adverse effects at the organism and population level.
References
Halliwell, Barry, John Gutteridge (2007) Free radicals in biology and medicine—Barry Halliwell; John Gutteridge—Oxford University Press. Oxford University Press, Oxford. http://global.oup.com/academic/product/free-radicals-in-biology-and-medicine-9780198568698?cc=us&lang=en&
Di Giulio RT, Meyer JN (2008) Reactive oxygen species and oxidative stress. In: Di Giulio RT, Hinton DE (eds) The toxicology of fishes. CRC Press/Taylor and Francis Group, Boca Raton, pp 273–324
Valavanidis A, Vlahogianni T, Dassenakis M, Scoullos M (2006) Molecular biomarkers of oxidative stress in aquatic organisms in relation to toxic environmental pollutants. Ecotoxicol Environ Saf 64(2):178–189. doi:10.1016/j.ecoenv.2005.03.013
Dorts J, Bauwin A, Kestemont P, Jolly S, Sanchez W, Silvestre F (2012) Proteasome and antioxidant responses in cottus gobio during a combined exposure to heat stress and cadmium. Comp Biochem Physiol Toxicol Pharmacol 155(2):318–324. doi:10.1016/j.cbpc.2011.10.003
Olsvik PA, Ornsrud R, Lunestad BT, Steine N, Fredriksen BN (2014) Transcriptional responses in Atlantic salmon (Salmo Salar) exposed to deltamethrin, alone or in combination with azamethiphos. Comp Biochem Physiol Toxicol Pharmacol 162(May):23–33. doi:10.1016/j.cbpc.2014.03.005
Vergauwen L, Hagenaars A, Blust R, Knapen D (2013) Temperature dependence of long-term cadmium toxicity in the zebrafish is not explained by liver oxidative stress: evidence from transcript expression to physiology. Aquat Toxicol (Amsterdam, Netherlands) 126(January):52–62. doi:10.1016/j.aquatox.2012.10.004
Baysoy E, Atli G, Gürler CÖ, Dogan Z, Eroglu A, Kocalar K, Canli M (2012) The effects of increased freshwater salinity in the biodisponibility of metals (Cr, Pb) and effects on antioxidant systems of Oreochromis niloticus. Ecotoxicol Environ Saf 84(October):249–253. doi:10.1016/j.ecoenv.2012.07.017
Kupsco A, Schlenk D (2014) Mechanisms of selenomethionine developmental toxicity and the impacts of combined hypersaline conditions on Japanese medaka (Oryzias Latipes). Environ Sci Technol 48(12):7062–7068. doi:10.1021/es5019948
Villarreal, Fernando D, Gautom Kumar Das, Aamir Abid, Ian M Kennedy, Dietmar Kültz (2014) Sublethal effects of CuO nanoparticles on Mozambique Tilapia (Oreochromis mossambicus) are modulated by environmental salinity. PloS One 9(2):e88723. doi:10.1371/journal.pone.0088723
Dolci GS, Dias VT, Roversi K, Kr R, Pase CS, Segat HJ, Teixeira AM et al (2013) Moderate hypoxia is able to minimize the manganese-induced toxicity in tissues of silver catfish (Rhamdia Quelen). Ecotoxicol Environ Saf 91(May):103–109. doi:10.1016/j.ecoenv.2013.01.013
Thomaz JM, Martins ND, Monteiro DA, Rantin FT, Kalinin AL (2009) Cardio-respiratory function and oxidative stress biomarkers in Nile tilapia exposed to the organophosphate insecticide trichlorfon (NEGUVON). Ecotoxicol Environ Saf 72(5):1413–1424. doi:10.1016/j.ecoenv.2008.11.003
Wu Y, Zhou Q (2012) Dose- and time-related changes in aerobic metabolism, chorionic disruption, and oxidative stress in embryonic medaka (Oryzias Latipes): underlying mechanisms for silver nanoparticle developmental toxicity. Aquat Toxicol (Amsterdam, Netherlands) 124–125:238–246. doi:10.1016/j.aquatox.2012.08.009
Jeffries, Ken M, Scott G Hinch, Thomas Sierocinski, Paul Pavlidis, Kristi M Miller (2014) Transcriptomic responses to high water temperature in two species of pacific salmon. Evol Appl 7(2):286–300. doi:10.1111/eva.12119
Machado, Cintia, Tania Zaleski, Edson Rodrigues, Cleoni Dos Santos Carvalho, Silvia Maria Suter Correia Cadena, Gustavo Jabor Gozzi, Priscila Krebsbach, Flávia Sant’Anna Rios, Lucélia Donatti (2014) Effect of Temperature Acclimation on the Liver Antioxidant Defence System of the Antarctic Nototheniids Notothenia Coriiceps and Notothenia Rossii. Comp Biochem Physiol B Biochem Mol Biol 172–173:21–28. Accessed 30 June. doi:10.1016/j.cbpb.2014.02.003
Madeira D, Narciso L, Cabral HN, Vinagre C, Diniz MS (2013) Influence of temperature in thermal and oxidative stress responses in estuarine fish. Comp Biochem Physiol A Mol Integr Physiol 166(2):237–243. doi:10.1016/j.cbpa.2013.06.008
Hauser-Davis, Rachel Ann, Frederico Freire Bastos, Rafael Ferreira Dantas, Santiago Alonso Leitão Tobar, Jayme da Cunha Bastos Neto, Vera Lucia Freire da Cunha Bastos, Roberta Lourenço Ziolli, Marco Aurélio Zezzi Arruda (2014) Behaviour of the oxidant scavenger metallothionein in hypoxia-induced neotropical fish. Ecotoxicol Environ Saf 103:24–28. doi:10.1016/j.ecoenv.2014.01.015
Joyner-Matos, Joanna, Lauren J Chapman (2013) Persisting in papyrus: size, oxidative stress, and fitness in freshwater organisms adapted to sustained hypoxia. Comp Biochem Physiol A Mol Integr Physiol 165(4):405–416. doi:10.1016/j.cbpa.2013.03.032
Pérez-Jiménez A, Peres H, Rubio VC, Oliva-Teles A (2012) The effect of hypoxia on intermediary metabolism and oxidative status in gilthead sea bream (Sparus Aurata) fed on diets supplemented with methionine and white tea. Comp Biochem Physiol Toxicol Pharmacol 155(3):506–516. doi:10.1016/j.cbpc.2011.12.005
Riffel APK, Garcia LO, Finamor IA, Saccol EMH, Meira M, Kolberg C, Horst A et al (2012) Redox profile in liver of leporinus macrocephalus exposed to different dissolved oxygen levels. Fish Physiol Biochem 38(3):797–805. doi:10.1007/s10695-011-9563-3
Wong, Samuel Z H, Biyun Ching, You R Chng, Wai P Wong, Shit F Chew, Yuen K Ip (2013) Ascorbic acid biosynthesis and brackish water acclimation in the euryhaline freshwater white-rimmed stingray, himantura signifer. PloS One 8(6):e66691. doi:10.1371/journal.pone.0066691
Tiedke J, Cubuk C, Burmester T (2013) Environmental acidification triggers oxidative stress and enhances globin expression in zebrafish gills. Biochem Biophys Res Commun 441(3):624–629. doi:10.1016/j.bbrc.2013.10.104
Buckley BA, Hedrick MS, Hillman SS (2014) Cardiovascular oxygen transport limitations to thermal niche expansion and the role of environmental P O 2 in antarctic notothenioid fishes. Physiol Biochem Zool 87(4):000–000. doi:10.1086/676664
Chen Z, Christina Cheng C-H, Zhang J, Cao L, Chen L, Zhou L, Jin Y et al (2008) Transcriptomic and genomic evolution under constant cold in antarctic notothenioid fish. Proc Natl Acad Sci U S A 105(35):12944–12949. doi:10.1073/pnas.0802432105
Patarnello T, Verde C, di Prisco G, Bargelloni L, Zane L (2011) How will fish that evolved at constant sub-zero temperatures cope with global warming? Notothenioids as a case study. Bioessays 33(4):260–268. doi:10.1002/bies.201000124
Abele D, Puntarulo S (2004) Formation of reactive species and induction of antioxidant defence systems in polar and temperate marine invertebrates and fish. Comp Biochem Physiol A Mol Integr Physiol 138(4):405–415. doi:10.1016/j.cbpb.2004.05.013
Atamaniuk, Tetiana M, Olga I Kubrak, Kenneth B Storey, Volodymyr I Lushchak (2013) Oxidative stress as a mechanism for toxicity of 2,4-dichlorophenoxyacetic acid (2,4-D): studies with Goldfish gills. Ecotoxicology (London, England) 22(10):1498–1508. doi:10.1007/s10646-013-1136-z
Yang, Ye, Huihui Ma, Jinghua Zhou, Jing Liu, Weiping Liu (2014) Joint toxicity of permethrin and cypermethrin at sublethal concentrations to the embryo-larval zebrafish. Chemosphere 96:146–154. doi:10.1016/j.chemosphere.2013.10.014
Yonar, Serpil Mişe, Mevlüt Şener Ural, Sibel Silici, M Enis Yonar (2014) Malathion-induced changes in the haematological profile, the immune response, and the oxidative/antioxidant status of cyprinus carpio carpio: protective role of propolis. Ecotoxicol Environ Saf 102:202–209. doi:10.1016/j.ecoenv.2014.01.007
Ibrahim, Ahmed Th A, Ahmed S A Harabawy (2014) Sublethal toxicity of carbofuran on the African Catfish clarias gariepinus: hormonal, enzymatic and antioxidant responses. Ecotoxicol Environ Saf 106:33–39. doi:10.1016/j.ecoenv.2014.04.032
Ma, Junguo, Yang Liu, Daichun Niu, Xiaoyu Li (2013) Effects of chlorpyrifos on the transcription of CYP3A cDNA, activity of acetylcholinesterase, and oxidative stress response of goldfish (Carassius Auratus). Environ Toxicol doi:10.1002/tox.21918
Nwani CD, Nagpure NS, Kumar R, Kushwaha B, Lakra WS (2013) DNA damage and oxidative stress modulatory effects of glyphosate-based herbicide in freshwater fish, Channa punctatus. Environ Toxicol Pharmacol 36(2):539–547. doi:10.1016/j.etap.2013.06.001
Birceanu, Oana, Grant B McClelland, Yuxiang S Wang, Jason C L Brown, Michael P Wilkie (2011) The lampricide 3-trifluoromethyl-4-nitrophenol (TFM) uncouples mitochondrial oxidative phosphorylation in both sea lamprey (Petromyzon marinus) and TFM-tolerant rainbow trout (Oncorhynchus Mykiss). Comp Biochem Physiol Toxicol Pharmacol 153(3):342–349. doi:10.1016/j.cbpc.2010.12.005
Bravo, MI Segnini de, Medina J, Marcano S, Finol HJ, Boada-Sucre A (2005) Effects of herbicide on the kidneys of two Venezuelan cultured fish: Caquetaia Kraussii and Colossoma Macropomum (Pisces: Ciclidae and Characeae). Revista de Biología Tropical 53(Suppl 1):55–60. http://www.ncbi.nlm.nih.gov/pubmed/17465144
Liu X-M, Shao J-Z, Xiang L-X, Chen X-Y (2006) Cytotoxic effects and apoptosis induction of atrazine in a grass carp (Ctenopharyngodon Idellus) cell line. Environ Toxicol 21(1):80–89. doi:10.1002/tox.20159
Slaninova, Andrea, Miriam Smutna, Helena Modra, Zdenka Svobodova (2009) A review: oxidative stress in fish induced by pesticides. Neuro Endocrinol Lett 30(Suppl 1):2–12. doi:NEL300709R01 [pii]
Bagnyukova, Tetyana V, Oxana I Chahrak, Volodymyr I Lushchak (2006) Coordinated response of goldfish antioxidant defenses to environmental stress. Aquat Toxicol (Amsterdam, Netherlands) 78(4):325–331. doi:10.1016/j.aquatox.2006.04.005
Gravato C, Teles M, Oliveira M, Santos MA (2006) Oxidative stress, liver biotransformation and genotoxic effects induced by copper in Anguilla anguilla L.–the influence of pre-exposure to beta-naphthoflavone. Chemosphere 65(10):1821–1830. doi:10.1016/j.chemosphere.2006.04.005
Kubrak, Olha I, Oleh V Lushchak, Julia V Lushchak, Ihor M Torous, Janet M Storey, Kenneth B Storey, Volodymyr I Lushchak (2010) Chromium effects on free radical processes in goldfish tissues: comparison of Cr(III) and Cr(VI) exposures on oxidative stress markers, glutathione status and antioxidant enzymes. Comp Biochem Physiol Toxicology Pharmacol 152(3):360–370. doi:10.1016/j.cbpc.2010.06.003
Bhattacharya A, Bhattacharya S (2007) Induction of oxidative stress by arsenic in Clarias batrachus: involvement of peroxisomes. Ecotoxicol Environ Saf 66(2):178–187. doi:10.1016/j.ecoenv.2005.11.002
Miller LL, Wang F, Palace VP, Hontela A (2007) Effects of acute and subchronic exposures to waterborne selenite on the physiological stress response and oxidative stress indicators in juvenile rainbow trout. Aquat Toxicol (Amsterdam, Netherlands) 83(4):263–271. doi:10.1016/j.aquatox.2007.05.001
Monteiro DA, Rantin FT, Kalinin AL (2010) Inorganic mercury exposure: toxicological effects, oxidative stress biomarkers and bioaccumulation in the tropical freshwater fish matrinxã, Brycon amazonicus (Spix and Agassiz, 1829). Ecotoxicology (London, England) 19(1):105–123. doi:10.1007/s10646-009-0395-1
Sevcikova M, Modra H, Slaninova A, Svobodova Z (2011) Metals as a cause of oxidative stress in fish: a review. Vet Med 2011:537–546
Crowe KM, Joseph C, Newton BK, Johnson C (2014) Oxidative stress responses of gulf killifish exposed to hydrocarbons from the deepwater horizon oil spill: potential implications for aquatic food resources. Environ Toxicol Chem 33(2):370–374. doi:10.1002/etc.2427
Meland S, Heier LS, Salbu B, Tollefsen KE, Farmen E, Rosseland BO (2010) Exposure of brown trout (Salmo trutta L.) to tunnel wash water runoff–chemical characterisation and biological impact. Sci Total Environ 408(13):2646–2656. doi:10.1016/j.scitotenv.2010.03.025
Milinkovitch T, Ndiaye A, Sanchez W, Le Floch S, Thomas-Guyon H (2011) Liver antioxidant and plasma immune responses in juvenile golden grey mullet (Liza aurata) exposed to dispersed crude oil. Aquat Toxicol (Amsterdam, Netherlands) 101(1):155–164. doi:10.1016/j.aquatox.2010.09.013
Penning TM, Burczynski ME, Hung CF, McCoull KD, Palackal NT, Tsuruda LS (1999) Dihydrodiol dehydrogenases and polycyclic aromatic hydrocarbon activation: generation of reactive and redox active O-quinones. Chem Res Toxicol 12(1). American Chemical Society: 1–18. doi:10.1021/tx980143n
Barron MG, Mark G, Carls JW, Short SD, Rice RA, Heintz MR, Di Giulio R (2005) Assessment of the phototoxicity of weathered Alaska North slope crude oil to juvenile pink salmon. Chemosphere 60(1):105–110. doi:10.1016/j.chemosphere.2004.12.006
Incardona, John P, Tanya L Swarts, Richard C Edmunds, Tiffany L Linbo, Allisan Aquilina-Beck, Catherine A Sloan, Luke D Gardner, Barbara A Block, Nathaniel L Scholz (2013) Exxon valdez to deepwater horizon: comparable toxicity of both crude oils to fish early life stages. Aquat Toxicol (Amsterdam, Netherlands) 142–143:303–316. doi:10.1016/j.aquatox.2013.08.011
Pelletier E, Sargian P, Payet J, Demers S (2014) Ecotoxicological effects of combined UVB and organic contaminants in coastal waters: a review. Photochem Photobiol 82(4):981–993, Accessed July 5. doi:10.1562/2005-09-18-RA-688
Amado LL, Monserrat JM (2010) Oxidative stress generation by microcystins in aquatic animals: why and how. Environ Int 36(2):226–235. doi:10.1016/j.envint.2009.10.010
Paskerova H, Hilscherova K, Blaha L (2012) Oxidative stress and detoxification biomarker responses in aquatic freshwater vertebrates exposed to microcystins and cyanobacterial biomass. Environ Sci Pollut Res 19:2024–2037. doi:10.1007/s11356-012-0960-7
Puerto, María, Silvia Pichardo, Angeles Jos, Ana M Cameán (2009) Oxidative stress induced by microcystin-LR on PLHC-1 fish cell line. Toxicol In Vitro 23(8):1445–1449. doi:10.1016/j.tiv.2009.08.011
Jos, Angeles, Silvia Pichardo, Ana I. Prieto, Guillermo Repetto, Carmen M Vázquez, Isabel Moreno, Ana M Cameán (2005) Toxic cyanobacterial cells containing microcystins induce oxidative stress in exposed tilapia fish (Oreochromis Sp.) under laboratory conditions. Aquat Toxicol (Amsterdam, Netherlands) 72(3):261–271. doi:10.1016/j.aquatox.2005.01.003
Olivares Rubio, Hugo F, M Lysset Martínez-Torres, Minerva Nájera-Martínez, Ricardo Dzul-Caamal, María Lilia Domínguez-López, Ethel García-Latorre, Armando Vega-López (2014) Biomarkers involved in energy metabolism and oxidative stress response in the liver of Goodea Gracilis hubbs and turner, 1939 exposed to the microcystin-producing microcystis aeruginosa LB85 strain. Environ Toxicol, doi:10.1002/tox.21984
Lesser MP (2006) Oxidative stress in marine environments: biochemistry and physiological ecology. Annu Rev Physiol 68(3):253–278. doi:10.1146/annurev.physiol.68.040104.110001
Lushchak VI (2011) Environmentally induced oxidative stress in aquatic animals. Aquat Toxicol (Amsterdam, Netherlands) 101(1):13–30. doi:10.1016/j.aquatox.2010.10.006
Eyckmans M, Celis N, Horemans N, Blust R, De Boeck G (2011) Exposure to waterborne copper reveals differences in oxidative stress response in three freshwater fish species. Aquat Toxicol (Amsterdam, Netherlands) 103(1–2):112–120. doi:10.1016/j.aquatox.2011.02.010
Sinhorin, Valéria Dornelles Gindri, Adilson Paulo Sinhorin, Jhonnes Marcos Dos Santos Teixeira, Kelly Márcia Lazarotto Miléski, Paula Carine Hansen, Paula Sueli Andrade Moreira, Nair Honda Kawashita, Amanda Martins Baviera, Vania Lúcia Loro (2014) Effects of the acute exposition to glyphosate-based herbicide on oxidative stress parameters and antioxidant responses in a hybrid amazon fish surubim (Pseudoplatystoma Sp). Ecotoxicol Environ Saf 106:181–87. doi:10.1016/j.ecoenv.2014.04.040
Paital B (2014) Modulation of redox regulatory molecules and electron transport chain activity in muscle of air breathing fish heteropneustes fossilis under air exposure stress. J Comp Physiol B 184(1):65–76. doi:10.1007/s00360-013-0778-8
González PM, Aguiar MB, Malanga G, Puntarulo S (2013) Electronic Paramagnetic Resonance (EPR) for the study of ascorbyl radical and lipid radicals in marine organisms. Comp Biochem Physiol A Mol Integr Physiol 165(4):439–447. doi:10.1016/j.cbpa.2013.02.021
Amin, Kamal A, Khalid S Hashem (2012) Deltamethrin-induced oxidative stress and biochemical changes in tissues and blood of catfish (Clarias Gariepinus): antioxidant defense and role of alpha-tocopherol. BMC Vet Res 8:45. doi:10.1186/1746-6148-8-45
Izquierdo MS, Scolamacchia M, Betancor M, Roo J, Caballero MJ, Terova G, Witten PE (2013) Effects of dietary DHA and Α-tocopherol on bone development, early mineralisation and oxidative stress in Sparus Aurata (Linnaeus, 1758) larvae. Br J Nutr 109(10):1796–1805. doi:10.1017/S0007114512003935
Prieto, Ana I, Angeles Jos, Silvia Pichardo, Isabel Moreno, Ana M Cameán (2008) Protective role of vitamin E on the microcystin-induced oxidative stress in Tilapia fish (Oreochromis niloticus). Environ Toxicol Chem 27(5):1152–1159. doi:10.1897/07-496.1
Miller LL, Rasmussen JB, Palace VP, Hontela A (2009) The physiological stress response and oxidative stress biomarkers in rainbow trout and brook trout from selenium-impacted streams in a coal mining region. J Appl Toxicol 29(8):681–688. doi:10.1002/jat.1458
Xu, WeiNa, WenBin Liu, KangLe Lu, YangYang Jiang, GuiFeng Li (2012) Effect of trichlorfon on oxidative stress and hepatocyte apoptosis of Carassius auratus Gibelio in vivo. Fish Physiol Biochem 38(3):769–775. doi:10.1007/s10695-011-9559-z
Li W, Kong A-N (2009) Molecular mechanisms of Nrf2-mediated antioxidant response. Mol Carcinog 48(2):91–104. doi:10.1002/mc.20465
Ma Q (2013) Role of nrf2 in oxidative stress and toxicity. Annu Rev Pharmacol Toxicol 53(January):401–426. doi:10.1146/annurev-pharmtox-011112-140320
Morgan MJ, Liu Z-g (2011) Crosstalk of reactive oxygen species and NF-κB signaling. Cell Res 21(1):103–115. doi:10.1038/cr.2010.178
Bauer G (2014) Targeting extracellular ROS signaling of tumor cells. Anticancer Res 34(4):1467–1482, http://www.ncbi.nlm.nih.gov/pubmed/24692674
Board PG, Menon D (2013) Glutathione transferases, regulators of cellular metabolism and physiology. Biochim Biophys Acta 1830(5):3267–3288. doi:10.1016/j.bbagen.2012.11.019
Rowland, Andrew, John O Miners, Peter I Mackenzie (2013) The UDP-glucuronosyltransferases: their role in drug metabolism and detoxification. Int J Biochem Cell Biol 45(6):1121–1132. doi:10.1016/j.biocel.2013.02.019
Lu SC (2013) Glutathione synthesis. Biochim Biophys Acta 1830(5):3143–3153. doi:10.1016/j.bbagen.2012.09.008
Burmester T, Hankeln T (2009) What is the function of neuroglobin? J Exp Biol 212(Pt 10):1423–1428. doi:10.1242/jeb.000729
Hankeln T, Ebner B, Fuchs C, Gerlach F, Haberkamp M, Tilmann L, Laufs AR et al (2005) Neuroglobin and cytoglobin in search of their role in the vertebrate globin family. J Inorg Biochem 99(1):110–119. doi:10.1016/j.jinorgbio.2004.11.009
Wakasugi K, Takahashi N, Uchida H, Watanabe S (2011) Species-specific functional evolution of neuroglobin. Mar Genomics 4(3):137–142. doi:10.1016/j.margen.2011.03.001
Blank, Miriam, Jessica Wollberg, Frank Gerlach, Katja Reimann, Anja Roesner, Thomas Hankeln, Angela Fago, Roy E Weber, Thorsten Burmester (2011) A membrane-bound vertebrate globin. Edited by Vladimir N. Uversky. PloS One 6(9). Public Library of Science: e25292. doi:10.1371/journal.pone.0025292
Pacitti D, Wang T, Martin SAM, Sweetman J, Secombes CJ (2014) Insights into the fish thioredoxin system: expression profile of thioredoxin and thioredoxin reductase in rainbow trout (Oncorhynchus mykiss) during infection and in vitro stimulation. Dev Comp Immunol 42(2):261–277. doi:10.1016/j.dci.2013.09.013
Ankley, Gerald T, Richard S Bennett, Russell J Erickson, Dale J Hoff, Michael W Hornung, Rodney D Johnson, David R Mount et al (2010) Adverse outcome pathways: a conceptual framework to support ecotoxicology research and risk assessment. Environ Toxicol Chem 29(3):730–741. doi:10.1002/etc.34
Jennings P (2013) Stress response pathways, toxicity pathways and adverse outcome pathways. Arch Toxicol 87(1):13–14. doi:10.1007/s00204-012-0974-4
Vinken M (2013) The adverse outcome pathway concept: a pragmatic tool in toxicology. Toxicology 312(October):158–165. doi:10.1016/j.tox.2013.08.011
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Carvan, M.J., Di Giulio, R.T. (2015). Oxidative Stress Responses in Aquatic and Marine Fishes. In: Roberts, S., Kehrer, J., Klotz, LO. (eds) Studies on Experimental Toxicology and Pharmacology. Oxidative Stress in Applied Basic Research and Clinical Practice. Humana Press, Cham. https://doi.org/10.1007/978-3-319-19096-9_26
Download citation
DOI: https://doi.org/10.1007/978-3-319-19096-9_26
Publisher Name: Humana Press, Cham
Print ISBN: 978-3-319-19095-2
Online ISBN: 978-3-319-19096-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)