Abstract
There has been a continuous increase in the incidence and prevalence of diabetes mellitus over the past 20 years, both globally and in the United States of America. A 20 to 69% increase was projected from 2010 to 2030 in developing and developed countries, respectively. The majority of this increase is attributed to type 2 diabetes (T2DM) the most common type of diabetes (87 to 95% of cases). In 2015, it was estimated that 1 in 11 persons in the world had diabetes. T2DM prevalence in the U.S. has quadrupled since 1980s. In 2012, the prevalence was highest amongst American Indians/Alaska Natives (15.9%), lowest amongst Non-Hispanic Whites (7.6%) and intermediate amongst Non-Hispanic Blacks, Hispanics and Asian Americans (13.2, 12.8 and 9.0%, respectively). Recent trends in the U.S. reveal an overall plateau in prevalence, increased incidence amongst youth and an almost equal distribution of T2DM in men and women. This increase in diabetes prevalence in the U.S. and throughout the world has been attributed to an increase in the ability to diagnose diabetes, an increase in lifespan, and the worsening obesity and physical inactivity epidemics seen globally. Differences between groups exposed to similar environments implicates a genetic contribution to the development of diabetes. Data suggests that the modern lifestyle with consequent obesity and sedentarism may interact with preexisting diabetes genes and lead to epigenetic modifications.
The incidence of type 1 diabetes (T1DM) is also on the rise both globally and in the U.S., particularly amongst children under the age of 15. It is estimated that by the year 2050, there will be a 20 to 70% increase in the prevalence of T1DM, depending on age and geographic location. It is unclear whether this is due to improved ability of diagnosis versus a true increase in genetically stable populations under the inducing influence of non-genetic factors changing over time and place.
Once diabetes is diagnosed, efforts must be made to prevent secondary complications through strict glycemic control and control of other metabolic risk factors such as hypertension and hyperlipidemia. Recently in the U.S. there has been a decrease in complications such as stroke, myocardial infarction, amputations, and death due to hyperglycemia. Since many complications are present before T2DM is diagnosed, early diagnosis and prevention of T2DM is key to further decreasing the incidence of complications.
Access provided by CONRICYT-eBooks. Download reference work entry PDF
Similar content being viewed by others
Keywords
- Diabetes mellitus prevalence/incidence
- Diabetes mellitus type 2 epidemiology
- Diabetes mellitus type 1 epidemiology
- Diabetes mellitus complications epidemiology
Epidemiology of Type 2 Diabetes Mellitus
Type 2 diabetes mellitus (T2DM) accounts for ~87–95% [1, 2] of all cases of diabetes in the world, one of the greatest global health challenges of the twenty-first century. Type 1 diabetes accounts for 5–12%, and other types of diabetes account for less than 5% of all cases of diabetes according to available reports. T2DM (also known as non-insulin-dependent or adult-onset diabetes) is a complex syndrome with hyperglycemia as its defining manifestation resulting from defects in insulin secretion, insulin action, or both [1]. The chronic hyperglycemia is accompanied in most cases by other metabolic features conferring increased risk for vascular and heart disease. Patients with T2DM have an increased incidence of atherosclerotic cardiovascular, peripheral arterial, and cerebrovascular disease [1]. Both genetic and epigenetic predispositions along with behavioral and environmental factors have been implicated as contributing to its pathophysiology [3].
Geographical Variation
Global Prevalence of Diabetes Mellitus: Regional Trends
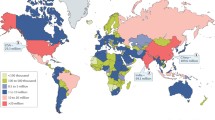
The prevalence of diabetes varies in different regions of the world. In low- and middle-income countries, reports on prevalence distinguishing between the various types of diabetes are not available. Therefore the best estimates of the regional variation in T2DM prevalence follow the variation of the overall diabetes prevalence, as T2DM by far accounts for most cases of diabetes in the world [2]. According to the International Diabetes Federation (IDF) statistics (Fig. 1), in 2015, globally 415 million people were estimated to have diabetes, which is roughly one in every 11 adults about 8.8% of the population, (4.72 billion people) [2]. More people with diabetes live in urban (269.7 million) than in rural (145.1 million) areas. This pattern is similar even in low- and middle-income countries, with prevalence of diabetes more in urban areas (186.2 million) than in rural areas (126.7 million). By 2040, globally the difference is expected to widen, with 477.9 million diabetics living in urban areas and 163.9 million in rural areas [2].
Estimated number of people with diabetes worldwide and per region in 2015 and 2040 (20–79 years) (Adapted from Ref. [2] with permission)
Due to factors such as improved diagnosis of diabetes, aging of the population, urbanization with subsequent lifestyle changes, and an increase in obesity - the global diabetes prevalence has doubled from 4.7% in 1980 to 8.5% of the adult population in 2014. According to the IDF statistics, currently 75% of the population with diabetes live in low- and middle-income countries, while 81.1% of the population with diabetes is undiagnosed globally [2]. Asia has emerged as the “diabetes epicenter” of the world due to the rapid increase in prevalence over a relatively short period of time; this has been attributed to a disproportionately high diabetes burden in young to middle-aged adults and to the presence of the “metabolically obese” phenotype [6]. In addition to Asia, the Gulf region in the Middle East [4] and Africa [7, 8] are other hot spots for higher diabetes prevalence, with a higher proportion of undiagnosed diabetes of 40.6% and 66.7%, respectively [2].
It is interesting to note that North America and the Caribbean region have the highest prevalence of diabetes per capita with one out of eight adults with diabetes (12.9%). However the heavily populated Western Pacific Region has 153 million adults with diabetes (9.3%) substantially more than any other region; 90.2% live in low- and middle-income countries and account for 36.9% of the population with diabetes worldwide. In the South and Central American region, the number of people with diabetes is currently 29.6 million (9.4%); the population in this region is relatively young, and thus the prevalence is expected to increase by 65% by 2040 as the population ages. In the Middle East and North African region, diabetes is largely underdiagnosed with at least two out of five people not diagnosed. It is projected to become a hot spot for diabetes due to urbanization and population aging. It is particularly challenging to estimate the total number of people with diabetes in the African region, as more than three-quarters of countries lack nationwide data [2].
Prevalence of Type 2 Diabetes Mellitus in the United States of America
The estimates and prevalence of people with diagnosed and undiagnosed diabetes in the United States (USA) were derived from the 2009 to 2012 National Health and Nutrition Examination Survey (NHANES), 2010–2012 National Health Interview Survey (NHIS), 2012 IHS data, and 2012 US resident population estimates. The United States ranks third in the world with 29.1 million people with diabetes which account for 9.3% of the population. Among them 21 million people are diagnosed, while 8.1 million (27.8%) remain undiagnosed [9]. Diagnosed diabetes was determined by self-report among survey respondents and by diagnostic codes for American Indians and Alaska Natives. Both fasting glucose and hemoglobin A1c (HbA1c) levels collected in a subset of the survey responders were used to derive estimates for undiagnosed diabetes. Although this data does not discriminate between T2DM and other types of diabetes, the facts apply mostly to T2DM, by far the most common type. In addition the surveys and blood glucose data were used to estimate prediabetes in the United States (impaired fasting glucose or glucose intolerance and elevated HbA1c). In prediabetes, blood glucose is elevated but not in the diabetes range; prediabetes confers higher risk for progression to T2DM. The Centers for Disease Control and Prevention (CDC) estimates that 86 million adults (more than 1 in 3 adults) had prediabetes in 2012. Among the 37% of adults estimated to have prediabetes in the United States, only 11% are aware of having this condition. The prevalence of prediabetes has increased from 29.2% in 1999–2002 to 36.2% in 2007–2010 among adults aged ≥ 18 years of age [10].
Within the United States, the rate of diabetes varies from 6.2% in Montana up to 11.7% in Mississippi. The CDC has now defined a geographical area called the “diabetes belt” [11]. It is based on the county data; the prevalence of diabetes in this area is on average 11.7%, whereas outside this region it is 8.5%. This area includes 644 counties in 15 states in the southeastern part of the United States. Some of the factors contributing to the higher prevalence of diabetes in this region are higher rates of obesity and physical inactivity, lower levels of education, and the higher prevalence of certain racial/ethnic groups who are at higher risk for diabetes.
Temporal Trends
Globally, diabetes was a relatively rare health problem in developing countries some decades ago, but a projected increase of 69% from 2010 to 2030 is expected in developing countries compared to 20% in developed countries [4]. For example, the prevalence of the disease was <1% in China in 1980 [6]; this has increased significantly to 10.6% in 2015 with 109 million people diagnosed with diabetes. Diabetes is on the rise in most regions, but the greatest increase is expected in the low- to middle-income countries [2, 12]. If the current trends continue, the population living with diabetes in the Western Pacific Region is estimated to increase from 153 million to 214.8 million adults by 2040 (Fig. 1). These trends are likely due to a combination of increased incidence as well as improved detection due to increased awareness.
The increase in diabetes incidence is postulated to be related to the increase in the prevalence of obesity all over the world, most rapidly occurring in Asian countries [13]. Add change in lifestyle with increased energy dense food and decreased physical activity is the major contributor to these trends. Higher prevalence of T2DM in immigrants from the Middle East living in Sweden than that of native Swedes has been reported [14], identifying the importance of the interplay between genetic predisposition and environmental factors for the expression of the T2DM phenotype. The variation in the ethnicity prevalence and heritability of T2DM (see below) clearly indicates that a genetic etiology needs to be explored. However, despite multiple genetic loci found to be associated with the risk of T2DM, the discriminative ability of genetic scores based on a number of risk alleles to predict diabetes incidence has been so far unsatisfactory [15]. Epigenetic modifications [16–18] such as those related to poor fetal and infant nutrition [19] have also been postulated to confer risk for the development of T2DM later in life. Regardless, the rise in T2DM prevalence has taken place too quickly to be explained by altered gene frequencies or by sustained epigenetic modifications alone. Although the wide difference in prevalence between ethnic groups exposed to similar environments implicates a significant genetic contribution, it can be safely postulated that the Western lifestyle is what has unmasked the effects of the preexisting genes [20] and of the epigenetic modifications.
The prevalence of diabetes has been increasing in the United States similar to the increase in the global diabetes prevalence. The statistics from CDC show that the prevalence of diagnosed T2DM was 5.5 million in 1980, which has quadrupled to 22 million in 2014 [21]. A recent study using National Health Interview Survey data suggests that diagnosed diabetes prevalence increased between 1990 and 2008, but remained steady between 2008 and 2012 [22] (Fig. 2). This plateauing can be attributed to the obesity trends in the United States, which showed a leveling off around the same period [23]; less likely to be contributing is the use of HbA1c rather than fasting glucose for the diagnosis of diabetes in a subset of the surveilled population as HbA1c may diagnose fewer cases [1]. Regardless, as the incidence of diagnosed diabetes has almost doubled from 6.9 to 12.1 per 1000 from 1980 to 2014, these trends cannot be attributed to difference in the methodology of data collection.
Number (in millions) of civilian, noninstitutionalized persons with diagnosed diabetes, the United States, 1980–2014 (Adapted from Ref. [21] with permission)
Age
Of the 415 million people reported to have diabetes in the world in 2015, 320.5 million are of working age (20–64 years), and 94.2 million people are aged 65–79 years. T2DM is usually late in onset and is more common in the older population. Global epidemiology of T2DM is changing, however; from an almost exclusive chronic adult disorder occurring commonly in middle-aged and elderly populations to also being increasingly prevalent in young adults, adolescents, and children. These trends are likely due to the increased rates of obesity in the younger age groups. Although still lower than in the older individuals, the incidence of diabetes in children and adolescents has been increasing [20], both globally and in the United States. The latest data show that the prevalence of diabetes in the United States including diagnosed and undiagnosed individuals in the age group of 20–44 is 4.3 million (4.1%), between 45 and 64 is 13.4 million (16.2%), and peaks in the age group 65 and above at 11.2 million (25.9%) [9] (Fig. 3). The SEARCH study [24] showed that diabetes affects 191,986 youth aged <20 years in 2009 (~1 in 433 of the ~3.3 million youth aged <20 years), a leading chronic disease in youth. Among these, T2DM accounted for 20,262 as opposed to type 1 diabetes, which accounted for 166,984. Approximately 4,740 had secondary, other, and unknown diabetes in the United States in 2009. An increase in the prevalence of both T1DM and T2DM in youth occurred between 2001 and 2009 [25].
Diagnosed and undiagnosed diabetes among people aged 20 years or older, the United States, 2012 (Adapted from Ref. [9])
Gender
Diabetes showed a pronounced female excess in the first half of the last century (1935–1936) but then became equally prevalent among men and women in most populations (by the end of the last century), with slight male preponderance in early middle age [26]. It is possible that men may have been more susceptible than women to the consequences of the decrease in physical activity and the rise in obesity, possibly due to the differences in insulin sensitivity and regional fat deposition [27]. According to IDF statistics in 2015 globally, there were about 15.6 million more men than women with diabetes (215.2 million men vs. 199.5 million women). This difference is expected to marginally decrease to about 15.1 million more men than women (328.4 million men vs. 313.3 million women) by 2040, since the difference in incidence rate between men and women has decreased at present.
Currently, in the United States the difference in diabetes incidence rates among men and women is minimal. The age-adjusted rates of diagnosed diabetes per 100 US civilian, noninstitutionalized persons was almost similar in both men and women from 1980 to 2000, then there was a slight increase in male compared to female from 2000 to 2008 with a peak rate of 7.2 in male in 2010 (Fig. 4) [28]. As per the 2014 statistics, the age-adjusted rate of diagnosed diabetes in males was slightly higher at 6.6 compared to 5.9 in females [28] (Fig. 4).
Age-adjusted rates of diagnosed diabetes per 100 civilian, noninstitutionalized population, by sex, the United States, 1980–2014 (Adapted from Ref. [28] with permission)
Race and Ethnicity
In the United States, racial and ethnic groups at increased risk for T2DM, (which includes most groups other than non-Hispanic whites) represent a proportionally increasing percentage of the population [29]. In 1980, the non-Hispanic black and Hispanic population represented 11.7% and 6.4% of the United States population diagnosed with diabetes, respectively, and their numbers grew to 12.6% and 16.3%, respectively, in 2010 [30, 31].
Among adults aged 20 years or older in the United States – Alaska Natives or American Indians report the highest percentage of having diagnosed diabetes (15.9%), while only 7.6% of non-Hispanic whites report diabetes diagnosis, according to the National Health Interview Survey 2010–2012 and the Indian Health Services’ National Patient Information Reporting System in 2012 [32] (Fig. 5). Non-Hispanic blacks, Hispanic, and Asian-Americans have intermediate prevalence at 13.2%, 12.8%, and 9.0% [32] (Fig. 5). The higher prevalence of diabetes in Native Americans, in whom this disease was virtually unknown 50 years ago, is likely a result of “collision” of the old hunter-gatherer genes with the new twentieth-century way of life [33]. The increased prevalence in the Asian-American population is again likely due to lifestyle/environmental factors in a community that tends to have more insulin resistance at a lower body mass index (BMI) compared to the non-Hispanic whites [34]. The “metabolically obese” phenotype, with abdominal obesity as the central feature, might explain the mismatch between the rates of obesity in general and rates of diabetes in Asia [35]. Data from the Obesity in Asia Collaboration, which includes information on >263,000 individuals from 21 studies in the Asia-Pacific region, have shown that measures of central adiposity, such as waist circumference, have a stronger association with diabetes prevalence than BMI [36 ].
Percentage of US adults aged 20 or older with diagnosed diabetes, by racial and ethnic group, 2010–2012. Note: percentages are age adjusted to the 2000 US standard population (Adapted from Ref. [32] with permission)
Epidemiology of Type 1 Diabetes Mellitus
Type 1 diabetes (T1DM) is the most common form of diabetes in childhood and in youth throughout the world [25]. T1DM is caused by destruction of the insulin-producing pancreatic beta cells, which leads to an absolute insulin deficiency. This is usually due to an autoimmune destruction of the beta cells, specifically seen in type 1A diabetes [37]. T1DM was reported to account for roughly 5–10% of all cases of diabetes worldwide [38]. As of 2012, there are 1.25 million adults and children living with T1DM in the United States [9]. T2DM is the most common form of diabetes throughout the world, while other types of diabetes account for <5% of cases and include gestational diabetes, mature-onset diabetes of the young (MODY), secondary diabetes, etc.
Geographical Variation
Worldwide Variation in T1DM
Majority of the epidemiologic data on T1DM throughout the world come from large registries in Europe and North America. Data on the incidence and/or prevalence of T1DM is limited from countries such as Africa and Asia [2] (Fig. 6). Two major collaborative projects, the Diabetes Mondiale study (DIAMOND) [39] and the Europe and Diabetes study (EURODIAB) [40], have been instrumental in monitoring trends in the incidence of T1DM throughout the world. These studies suggest that the incidence of T1DM among children is increasing in many countries, particularly in children under the age of 15. The estimated number of children under the age of 15 living with T1DM worldwide is 542,000 [2].
Countries and territories with data available on the incidence or prevalence of type 1 diabetes in children (0–14 years of age) (Adapted from Ref. [2] with permission)
According to the IDF, roughly 79,100 children under 15 years of age are estimated to develop T1DM annually worldwide [2]. It is estimated that of all the children living with T1DM, 26% live in Europe and 22% live in North America and the Caribbean [2]. Data from the IDF also suggest that the incidence rates of T1DM vary throughout the world as seen in Fig. 7 [2]. The prevalence of T1DM is highest in the United States, India, and Brazil (Table 1) [2]. The highest incidence rates of T1DM were found in Finland, Sweden, and Kuwait (Table 2) [2].
Estimated new cases of type 1 diabetes in children (0–14 years of age) per 100,000 children per year, 2015 (Adapted from Ref. [2] with permission
The DIAMOND project focused on children less than or equal to 14 years of age from 100 centers in 50 different countries [39]. The DIAMOND project demonstrated a >350-fold variation in the incidence among 100 different populations worldwide. The highest incidence of T1DM was found in Sardinia, Finland, Sweden, Norway, Portugal, the United Kingdom, Canada, and New Zealand. The largest age-adjusted incidence of T1DM was in Sardinia and Finland. The lowest incidence was found in populations from China and Venezuela [39].
In addition to the variation in incidence between countries, there was also variation in the incidence of T1DM within countries as well. For example, the incidence of T1DM in Sardinia was three to five times higher than the rates in continental Italy [39]. DIAMOND [39] and EURODIAB [40] revealed variations in the incidence of T1DM throughout the globe, but did not answer the important question as to why these variations exist. Many studies suggest that these variations in incidence of T1DM are likely due to environmental factors which either initiate or accelerate ongoing beta-cell destruction.
There are many prenatal, perinatal, and postnatal factors implicated in the development of autoimmune T1DM [41] (Fig. 8). Some researchers have also suggested other environmental risk factors including distance from the equator (the farther the distance from the equator, the higher the incidence of T1DM) [42], seasonality (specifically being born during certain months) [43], temperature [44], differences in environmental exposure and diet leading to changes in gut microbiota [45], etc. Nutritional factors tied to increased T1DM incidence include early introduction of infants to cow’s milk, short duration of time breastfeeding, wheat gluten in the diet, and vitamins D and E deficiencies; however, majority of this data has been inconsistent [46].
Prenatal, perinatal, and postnatal factors implicated in the development of autoimmune type 1 diabetes mellitus (Adapted from Ref. [45] with permission)
Given the inconsistent findings pertaining to environmental factors reported from observational studies and clinical trials, the Environmental Determinants of Diabetes in the Young (TEDDY) was established by the National Institute for Health [47]. TEDDY assessed newborns with high-risk HLA-DR, DQ genotypes beginning at age 4.5 months until 15 years of age from six clinical centers in the United States and Europe. It is a prospective study identifying environmental factors predisposing to, or protective against, islet autoimmunity and T1DM. The wealth of data from this study will provide a foundation for future randomized clinical trials [47].
T1DM in the United States
The CDC reports 1.25 million adults and children living with T1DM in the United States in 2012 [9]. This accounts for <1% of the US population. According to the IDF, the United States is home to the world’s largest number of children living with T1DM who are less than 15 years of age, roughly 84,100 children (Table 1) [2].
The SEARCH for Diabetes in Youth Study in the United States has been designed to examine diabetes mellitus in individuals <20 years of age, by sex, age, race/ethnicity, and diabetes type [24, 25]. The study was performed at ten study locations in the United States, including Ohio, Washington, South Carolina, Colorado, Hawaii, and California. They also gathered data from American Indian reservations in Arizona and New Mexico. The SEARCH study revealed an increase in the prevalence of T1DM in all sex, age, and race/ethnic subgroups except for those with the lowest prevalence (i.e., those age 0–4 years and American Indians) between 2001 and 2009. The SEARCH study was limited in that it did not cover all populations throughout the United States; therefore, we cannot interpret geographical trends and/or variations in T1DM incidence/prevalence throughout the United States.
Age and Ethnicity
T1DM typically affects youth and accounts for >80% of diabetes diagnoses in those less than 20 years of age [48]. It is estimated that 86,000 children under age 15 will develop T1DM annually worldwide [2].
The DIAMOND project (which evaluated children less than or equal to 14 years of age) demonstrated that the incidence of T1DM increased with age and was highest among children 10–14 years of age [39]. Most studies show two peaks in the distribution of the age of onset of T1DM in children – the first in early childhood and the second at the time of puberty [49]. The reason for this bimodal distribution may be related to certain genotypes and their interaction with different environmental risk factors (as described in the first section of this chapter). The SEARCH study estimated that more than 18,000 new cases of T1DM will be diagnosed among US youth younger than age 20 each year [24, 25].
The incidence of T1DM is higher in certain ethnic groups, especially in Europeans versus non-Europeans, as demonstrated in the DIAMOND [39] and EURODIAB [40] studies. There are ethnic differences among those with T1DM living in the United States. In 2009, the SEARCH study revealed that 18,436 US youth were newly diagnosed with T1DM – 12,945 non-Hispanic white; 3,098 Hispanic; 2,070 non-Hispanic black; 276 Asian-Pacific Islander; and 47 American Indians [25]. Between 2000 and 2009, the SEARCH study revealed an increased prevalence of T1DM in white, black, Hispanic, and Asian-Pacific Islander youth and in those aged 5 years or older [24, 25].
There are not many studies evaluating the incidence of T1DM in adulthood. One study in Kronoberg, Sweden found that the incidence of T1DM in those <19 years of age was 37.8 (per 100,000 patient years) and 27.1 (per 100 000 patient years) in those between ages 20 and 100. There appeared to be a bimodal distribution with equal peaks in the 0–9-year-old age group and in the 50–80-year-old age group [48] (Fig. 9). These trends were similar to research findings in earlier studies in other countries like Denmark and Finland [50, 51]. The reason for the peak in adulthood is unclear but may be due to the interaction between genotype and environmental factors and/or loss of beta-cell function over time [52]. Some adults might also develop antibodies associated with T1DM later in life. One study suggests that roughly 10% of adults initially diagnosed with T2DM are found to have pancreatic autoantibodies associated with T1DM [53].
Gender
While most autoimmune conditions affect women more than males, T1DM impacts both genders equally. Some studies have revealed that there is a higher prevalence in males, especially when looking at European people age 15–40 years old. One study reports an approximate 3:2 male to female ratio. This ratio has remained constant in young adults over two or three generations in some populations [26]. Gender differences also appear to vary by age. A large study in Sweden revealed no gender differences in children 0–14 years of age with T1DM, but did find a twofold male preponderance in subjects 15–39 years of age [54]. Although T1DM is mainly due to loss of insulin secretion via destruction of the beta cells, some authors hypothesize that the male preponderance in T1DM from age 15 to 40–50 could be due to hormonal influences associated with higher peripheral insulin resistance among men in young adulthood and middle age.
Temporal Trends
Just as the incidence of T2DM is increasing throughout the world, so is the incidence of T1DM. Diabetes was not common in the eighteenth century. The 1892 edition of Osler’s Principles and Practice of Medicine had 10 pages of text dedicated to diabetes (compared with >50 pages for other illnesses) and mentions that only 10 of 35,000 patients treated at Johns Hopkins had diabetes during that time [55]. In the 1930s, there was an improvement with data collection and statistics. The NHIS in the late twentieth century reported an incidence of diabetes mellitus (all types) of 1.30 (per 1,000 person years) in 1973 and 1.60 (per 1,000 person years) in 1976 in those under age 16 [56].
Data collected from 37 different studies in 27 countries from 1960 to 1996 showed a significant increase in the incidence of T1DM over time at roughly 3% each year, on average [57]. Data collected from the twenty-first century suggests an annual increase in the incidence of T1DM worldwide to be similar at 3% [2, 39, 40].
The multinational trial in Europe, EURODIAB, attempted to predict 15-year incidence trends in children less than 5 years of age diagnosed with T1DM. The trial estimated a doubling of new cases of T1DM in European children (less than 5 years of age) between 2005 and 2020. Researchers suspect the prevalence of T1DM in those less than 15 years of age will also rise by 70% between 2005 and 2020 [40] (Fig. 10).
Estimated (2005) and predicted cases of newly diagnosed type 1 diabetes. Predicted new cases for future years in Europe (excluding Belarus, the Russian Federation, Ukraine, Moldova, and Albania) on the basis of the best-fitting Poisson regression model (Adapted from Ref. [40] with permission)
The data seem to suggest that the incidence of T1DM has been increasing in the United States similarly to the rest of the world [38]. The SEARCH study conducted in the United States confirmed that there was a 21.1% increase in T1DM between 2001 and 2009 [25]. Another study group used the 2001 prevalence and the 2002 incidence data of T1DM from the SEARCH for Diabetes in Youth Study and US Census Bureau population demographic projections to predict future incidence rates in T1DM in the United States in people less than 20 years of age – The study projected that the number of youth with T1DM would rise from 166,018 in 2010 to 203,382 in 2050 [58].
The Epidemiology of Complications Due to Diabetes Mellitus
The accurate global prevalence of most complications associated with diabetes is difficult to obtain due to the lack of internationally agreed standards for diagnosis and assessment of diabetes complications [2]. Complications associated with diabetes mellitus, both T2DM and T1DM, are still common despite some recent decreasing trends reported in the United States. These trends are likely a result of increased detection of diabetes associated with an increase in the prevalence of diabetes and a decrease in the overall mortality of the population [59]. A large proportion of people with T2DM (50% or more in some studies) have at least one complication at the time of diagnosis [60].
Complications are thought to be largely a consequence of long-term exposure to elevated blood glucose [61–63] and associated risk factors such as elevated blood pressure [64–66] and other components of the metabolic syndrome [67, 68]. In general, diabetes-related complications can be prevented or delayed by maintaining blood glucose as close to normal as possible as evidenced by the Diabetes Control and Complications Trial (DCCT) [69] and follow-up Epidemiology of Diabetes Interventions and Complications (EDIC) study [61] for T1DM and the United Kingdom Prospective Diabetes Study (UKPDS) [62, 63] and Kumamoto study [70] for T2DM. The UKPDS conducted in patients newly diagnosed with T2DM showed that a 1% reduction in HgbA1c can reduce the risk of complications by 35% [62]. Further, in the DCCT conducted in patients with T1DM, intensive glycemic control (maintaining a HgbA1c of 7% vs. 9% in the control group for 6.5 years) was associated with 76% risk reduction for retinopathy, 50% for nephropathy and 60% for neuropathy, and a 35–76% reduction in overall microvascular diabetes complication [69]. EDIC, a follow-up study for the DCCT, showed that the reduction in the microvascular complications in the intensive glucose control group persisted over time despite the HgbA1c being no different in the two study groups during the follow-up period, suggesting that the earlier intensive glucose control over 6.5 years had a lasting, “memory” effect in reducing complications [61]. The “metabolic memory” postulates that both early and long-term reduction in blood glucose may further decrease microvascular complications. In addition in EDIC, there was a 42% risk reduction in cardiovascular disease (CVD) events and a 57% reduction in CVD mortality related to the intensive control of blood glucose [71].
Retinopathy
Diabetic retinopathy (DR) occurs due to microvascular changes in the blood vessels of the retina. It is staged based on the International Clinical Diabetic Retinopathy Scale into mild, moderate, and severe nonproliferative DR (NPDR) based on the severity of changes observed upon dilated ophthalmoscopy [72]. Later, new blood vessel formation occurs due to growth factors leading to proliferative diabetic retinopathy (PDR) [72]. The retinal thickening that occurs due to the leaking blood vessels leads to diabetic macular edema (DME) which can develop at all stages of retinopathy. Tractional retinal detachment, preretinal or vitreous hemorrhage, and neovascular glaucoma are further complications following neovascularization in PDR and can result in loss of vision. DR may be present at the time of diagnosis and can progress fast to an advanced stage before vision is affected. Retinopathy can develop as early as 7 years before diabetes is diagnosed in patients with T2DM [73]. Thus, regular screening, early diagnosis, and interventions with treatments like panretinal laser photocoagulation and the use of anti-vascular endothelial growth factors are of significance to prevent or reverse loss of vision [74].
The global estimate of DR prevalence, obtained by pooled analysis of individual data from population-based studies around the world, was about 93 million (age standardized to the 2010 world population age 20–79 years). Approximately 28 million had vision-threatening DR, 17 million people had PDR, and 21 million people had DME [75]. The prevalence of any DR was 34.6%, PDR was 7.0%, DME was 6.8%, and vision-threatening DR (VTDR) was 10.2% in the diabetic population. Higher prevalence rates were observed in T1DM, in those with increased duration of diabetes, and also in cases of poor glycemic control, inadequate blood pressure control and poor lipid control.
In the United States, DR is the leading cause of new cases of blindness in American adults aged 20–74 years [76]. The National Eye Institute statistics have reported an 89% increase in the prevalence of DR, from 4.06 million in 2000 to 7.69 million in 2010. According to the 2012 data, the prevalence of DR varied based on ethnicity with 68% non-Hispanic whites affected compared to 16% Hispanics and 11% blacks. The prevalence was almost equal in both men and women. In parallel to the projected massive increase in diabetes prevalence, the prevalence of DR is expected to nearly double, from 7.7 million in 2010 to 14.6 million in 2050.
CVD
CVD is the most common cause of death and disability among people with diabetes. The CVD that accompanies diabetes includes angina, myocardial infarction, cerebrovascular disease and stroke, peripheral arterial disease, and congestive heart failure. There is a twofold to fourfold increased risk for CVD in T2DM compared to the population without diabetes. Both established diabetes and prediabetes (impaired fasting glucose and impaired glucose tolerance) have been proven to be independent risk factors for the development of CVD, as well as independent predictors of CV mortality after adjusting for other CVD risk factors [77].
In most of the regions in the world, higher-than-optimum blood glucose is a leading cause of cardiovascular mortality. One in five deaths from ischemic heart disease (21%) and one in eight (13%) deaths from stroke are attributable to high blood glucose as per the comparative risk assessment done to estimate global and regional mortality attributable to high blood glucose [78]. More than three-quarters of cardiovascular deaths due to high blood glucose have occurred in the low- and middle-income countries.
In 2010, in the United States, the age-adjusted population statistics, according to the CDC, show that CVD death was 1.7 times higher in people with diabetes aged 18 years or older compared to people without diabetes. Similarly the hospitalization rates for CVD and stroke were 1.8 times and 1.5 times higher in those with diabetes than without [9, 79]. Among people with diabetes in the United States, aged 35 years and above, 5 million self-reported having coronary heart disease, 3.7 million reported having other heart disease, and 2.1 million reported having stroke [80].
Hypertension and dyslipidemia, that commonly coexist with diabetes, are clear risk factors for CVD, but diabetes alone confers an independent risk. During the period of 1999–2010, there has been significant improvement in the control of risk factors for microvascular and macrovascular complications among US adults with diabetes [81]. The rate of CVD mortality for the same period has decreased as evidenced by the decline in crude and age-adjusted hospital discharge rates for major cardiovascular disease based on the first-listed diagnosis per 1,000 diabetic population [82].
Diabetic Foot Complications
Microvascular and macrovascular changes in diabetes may lead to nerve damage of various degrees as well as impaired circulation in the lower limbs leading to increased risk and development of ulcerations, pathological fractures, and bone damage. Infections and amputations ensue. With strict glycemic control, the nerve damage can be prevented, as shown in the DCCT, EDIC, and UKPDS [61, 63]. Regular foot examinations and intensive and early detection and care of ulcerations, infections, and pathological fractures can prevent amputations of any part of the lower limbs [74].
In 2010, the number of nontraumatic amputations among the US adult population with diagnosed diabetes aged 20 years or older was 73,000, and this accounted for 60% of the total number of nontraumatic lower limb amputations [9]. As per the 1999–2000 NHANES data, the prevalence of peripheral arterial disease (PAD) was 9.5%, peripheral neuropathy (PN) was 28.5%, and any lower-extremity disease (LED) was 30.2% among the population diagnosed with diabetes aged ≥40 years; this was approximately twice as high compared to the overall prevalence of PAD (5%), PN (15%), and LED (19%) in the general population. The prevalence of foot ulcers in those with diabetes was 7.7%, which was almost three times higher than the general population [83].
Kidney Disease
Diabetes is one of the leading causes of chronic kidney disease. The disease is caused by hyperglycemia-related microvascular changes in the kidney leading to progressive decrease in renal function and culminating in renal failure over decades. Hypertension, dyslipidemia, and smoking are the other main risk factors for chronic kidney disease [84]. Diabetic nephropathy is clinically characterized by the presence of macroalbuminuria (>300 mg/24 h). This is often preceded by microalbuminuria, which is defined as albumin excretion of 30–299 mg/24 h. Without intervention, microalbuminuria typically progresses to macroalbuminuria and overt diabetic nephropathy. Microalbuminuria may be present in about 7% of T2DM patients at the time of diagnosis.
The international comparison of pooled data from 54 countries reports that the percentage of incident end-stage renal disease (ESRD) patients due to diabetes varies from 12% in Ukraine to 66% in Singapore [85]. In 2011 diabetes was the leading cause of kidney failure in the United States accounting for 44% of all new cases. The number of patients with diabetes in all age groups starting treatment for ESRD was 49,677, and the number of patients with diabetes of all ages on chronic dialysis or with a kidney transplant was 228,924 [9].
Prevention of Complications
Complications of diabetes mellitus can be reduced, delayed, and prevented by maintaining tight glycemic control along with control of comorbidities like elevated blood pressure and abnormal blood lipids. Early detection of the complications of diabetes by regular screening is essential as early treatment can reduce the morbidity and mortality due to complications. Recently published data in the United States has shown that between 1990 and 2010, there has been a decline in the rates of diabetes complications such as myocardial infarction, stroke, lower-extremity amputations, end-stage renal disease, and deaths due to hyperglycemic crisis likely as a result of significantly improved preventive care in the United States [79]. Figure 11 demonstrates that the rates of myocardial infarction and deaths due to hyperglycemic crisis among adults diagnosed with diabetes decreased by more than 60% between 1990 and 2010. Rates of stroke and amputations of the legs and feet fell by about 50%, and rates of kidney failure fell by about 30% [79]. This decline in diabetes complications can be attributed to advances in clinical care, increased availability of preventive measures, control of risk factors, and increased awareness of the potential complications of diabetes.
Trends in rates of diabetes complications among US adults with diagnosed diabetes, 1990–2010 (Adapted from Ref. [79] with permission)
Summary
There has been a continuous increase in diabetes incidence and prevalence over the past 20 years, both globally and in the United States. Although data collection methodology did not allow precise identification of cases as T2DM or other cases of diabetes, the very large increases and the fact that T2DM is by far the most common type of diabetes (87–95% of cases) point to the majority of the increase in prevalence and incidence being attributed to T2DM. With an increase in the ability to diagnose T2DM, the aging of the population and increased life span, and no reprieve from the relentless obesity and physical inactivity epidemics, (especially in the developing countries), these global trends are expected to continue. This will be true in all regions of the world with variations which will follow the aforementioned factors. Improved early detection of risk, including detection of prediabetes, followed by aggressive and widely applied public health preventive measures in those at highest risk for developing T2DM, will hopefully slow these increasing trends.
Currently it is unclear whether the increase in incidence of T1DM is due to improved ability of diagnosis and screening versus a true increase in genetically stable populations under the inducing influence of non-genetic factors changing over time and place. Given that the prevalence of T1DM is also predicted to increase over time throughout the world, it is essential for all healthcare providers to familiarize themselves with the clinical signs and symptoms of diabetes in youth and the possibility of T1DM in late adulthood. Early recognition and diagnosis can help prevent hospitalizations for life-threatening diabetic ketoacidosis (DKA) and complications of diabetes. Education early on can help improve lifelong compliance with insulin treatment and allow for a better quality of life.
Once diabetes is established, efforts to secondarily prevent complications include tight glucose control, routine screening for diabetic complications (i.e., foot examinations, monofilament testing, urine microalbumin testing, dilated eye examinations, podiatric evaluation, etc.), and control of other metabolic risk factors. Secondary prevention efforts have recently been fruitful with encouraging results. Since many complications are present before T2DM is diagnosed, early diagnosis of risk and prevention of T2DM is key in further decreasing the incidence of complications. Prevention of T2DM is possible as shown by the The Diabetes Prevention Program (DPP) and other trials [86]; T2DM prevention is a team effort and requires the support of physicians, diabetes educators, dietitians, and the patient. Furthermore, both primary and secondary prevention programs need to be more individualized and centered on the patient in order to have the greatest impact on prevention and control of diabetes and its complications.
References
American Diabetes Association. Classification and diagnosis of diabetes. Sec. 2 in standards of medical care in diabetes. Diabetes Care. 2016;39 Suppl 1:S13–22.
International Diabetes Federation. IDF diabetes atlas. 7th ed. Brussels: International diabetes federation; 2015.
Zimmet P, Alberti KG, Shaw J. Global and societal implications of the diabetes epidemic. Nature. 2001;414(6865):782–7.
Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87(1):4–14.
World Health Organization. Global report on diabetes. Geneva: WHO; 2016. Available at: http://apps.who.int/iris/bitstream/10665/204871/1/9789241565257_eng.pdf. Accessed 24 June 2016.
Chan JC, Malik V, Jia W, et al. Diabetes in Asia: epidemiology, risk factors, and pathophysiology. JAMA. 2009;301(20):2129–40.
Mbanya JC, Motala AA, Sobngwi E, et al. Diabetes in sub-Saharan Africa. Lancet. 2010;375(9733):2254–66.
Abubakari AR, Lauder W, Jones MC, et al. Prevalence and time trends in diabetes and physical inactivity among adult West African populations: the epidemic has arrived. Public Health. 2009;123(9):602–14.
Centers for Disease Control and Prevention (CDC). National diabetes statistics report: estimates of diabetes and its burden in the United States, 2014. Atlanta: U.S. Department of Health and Human Services. 2014. Available at: https://www.cdc.gov/diabetes/pubs/statsreport14/national-diabetes-report-web.pdf. Accessed 24 June 2016.
Bullard KM, Saydah SH, Imperatore G, et al. Secular changes in U.S. Prediabetes prevalence defined by hemoglobin A1c and fasting plasma glucose: National Health and Nutrition Examination Surveys, 1999–2010. Diabetes Care. 2013;36(8):2286–93.
Barker LE, Kirtland KA, Gregg EW, et al. Geographic distribution of diagnosed diabetes in the U.S.: a diabetes belt. Am J Prev Med. 2011;40(4):434–9.
Whiting DR, Guariguata L, Weil C, et al. IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract. 2011;94(3):311–21.
Seidell JC. Obesity, insulin resistance and diabetes – a worldwide epidemic. Br J Nutr. 2000;83 Suppl 1:S5–8.
Wandell PE, Johansson SE, Gafvels C, et al. Estimation of diabetes prevalence among immigrants from the Middle East in Sweden by using three different data sources. Diabetes Metab. 2008;34:328–33.
McCarthy MI. Genomics, type 2 diabetes, and obesity. N Engl J Med. 2010;363(24):2339–50.
Bird A. Perceptions of epigenetics. Nature. 2007;447:396–8.
Gluckman PD, Hanson MA, Cooper C, et al. Effect of in utero and early-life conditions on adult health and disease. N Engl J Med. 2008;359(1):61–73.
Pinney SE, Simmons RA. Epigenetic mechanisms in the development of type 2 diabetes. Trends Endocrinol Metab. 2010;21(4):223–9.
Hales CN, Barker DJ. The thrifty phenotype hypothesis. Br Med Bull. 2001;60:5–20.
Zimmet P. Globalization, coca-colonization and the chronic disease epidemic: can the Doomsday scenario be averted? J Int Med. 2000;247(3):301–10.
Centers for Disease Control and Prevention (CDC), National Center for Health Statistics, Division of Health Interview Statistics, data from the National Health Interview Survey. Number (in Millions) of civilian, non-institutionalized persons with diagnosed diabetes, United States, 1980–2014. Available at: http://www.cdc.gov/diabetes/statistics/incidence/fig1.htm. Accessed 24 June 2016.
Geiss LS, Wang J, Cheng YJ, et al. Prevalence and incidence trends for diagnosed diabetes among adults aged 20 to 79 years, United States, 1980–2012. JAMA. 2014;312(12):1218–26.
Ogden CL, Carroll MD, Kit BK, et al. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA. 2014;311(8):806–14.
Pettitt DJ, Talton J, Dabelea D, et al. Prevalence of diabetes in U.S. youth in 2009: the SEARCH for diabetes in youth study. Diabetes Care. 2014;37(2):402–8.
Dabelea D, Mayer-Davis EJ, Saydah S, et al. SEARCH for Diabetes in Youth Study. Prevalence of type 1 and type 2 diabetes among children and adolescents from 2001 to 2009. JAMA. 2014;311(17):1778–86.
Gale EA, Gillespie KM. Diabetes and gender. Diabetologia. 2001;44(1):3–15.
Logue J, Walker JJ, Colhoun HM, et al. Do men develop type 2 diabetes at lower body mass indices than women? Diabetologia. 2011;54(12):3003–6.
Centers for Disease Control and Prevention (CDC), National Center for Health Statistics, Division of Health Interview Statistics, data from the National Health Interview Survey. Age-adjusted rates of diagnosed diabetes per 100 civilian, non-institutionalized population, by Sex, United States, 1980–2014. Available at: http://www.cdc.gov/diabetes/statistics/incidence/fig4.htm. Accessed 24 June 2016.
Zhang Q, Wang Y, Huang ES. Changes in racial/ethnic disparities in the prevalence of Type 2 diabetes by obesity level among U.S. adults. Ethn Health. 2009;14(5):439–57.
Humes KR, Jones NA, Ramirez RR. Overview of race and Hispanic origin: 2010. Washington, DC: U.S.Department of Commerce, Economics and Statistics Administration, US Census Bureau; 2011.
Bureau of the Census. 1980 census of population: general population characteristics. Washingon, DC: U.S. Department of Commerce, Bureau of the Census; 1983.
Centers for Disease Control and Prevention (CDC). Diabetes report card 2014. Atlanta: Centers for Disease Control and Prevention, US Dept of Health and Human Services. 2015. Available at: https://www.cdc.gov/diabetes/pubs/statsreport14/national-diabetes-report-web.pdf. Accessed 18 July 2016.
Diamond JM. Human evolution. Diabetes running wild. Nature. 1992;357:362–3.
Deurenberg P, Deurenberg-Yap M, Guricci S. Asians are different from Caucasians and from each other in their body mass index/body fat per cent relationship. Obes Rev. 2002;3:141–6.
Yoon KH, Lee JH, Kim JW, et al. Epidemic obesity and type 2 diabetes in Asia. Lancet. 2006;368:1681–8.
Huxley R, James WP, Barzi F, et al. Ethnic comparisons of the cross-sectional relationships between measures of body size with diabetes and hypertension. Obes Rev. 2008;9 Suppl 1:53–61.
Chiang JL, Kirkman MS, Laffel LM, et al. Type 1 diabetes through the life span: a position statement of the American Diabetes Association. Diabetes Care. 2014;37(7):2034–54.
Patterson CC, Gyurus E, Rosenbauer J, et al. Trends in childhood type 1 diabetes incidence in Europe during 1989–2008: evidence of non-uniformity over time in rates of increase. Diabetologia. 2012;55(8):2142–7.
The DIAMOND Project Group. Incidence and trends of childhood Type 1 diabetes worldwide 1990–1999. Diabet Med. 2006;23(8):857–66.
Patterson CC, Dahlquist GG, Gyurus E, et al. EURODIAB Study Group. Incidence trends for childhood type 1 diabetes in Europe during 1989–2003 and predicted new cases 2005–20: a multicentre prospective registration study. Lancet. 2009;373(9680):2027–33.
Ma RC, Chan JC. Incidence of childhood type 1 diabetes: a worrying trend. Nat Rev Endocrinol. 2009;5(10):529–30.
Rosenbauer J, Herzig P, von Kries R, et al. Temporal, seasonal, and geographical incidence patterns of type I diabetes mellitus in children under 5 years of age in Germany. Diabetologia. 1999;42(9):1055–9.
Moltchanova EV, Schreier N, Lammi N, et al. Seasonal variation of diagnosis of Type 1 diabetes mellitus in children worldwide. Diabet Med. 2009;26(7):673–8.
Waernbaum I, Dahlquist G. Low mean temperature rather than few sunshine hours are associated with an increased incidence of type 1 diabetes in children. Eur J Epidemiol. 2016;31(1):61–5.
Knip M, Siljander H. The role of the intestinal microbiota in type 1 diabetes mellitus. Nat Rev Endocrinol. 2016;12(3):154–67.
Virtanen SM, Knip M. Nutritional risk predictors of beta cell autoimmunity and type 1 diabetes at a young age. Am J Clin Nutr. 2003;78(6):1053–67.
TEDDY Study Group. The environmental determinants of diabetes in the young (TEDDY) Study. Ann N Y Acad Sci. 2008;1150:1–13.
Thunander M, Petersson C, Jonzon K, et al. Incidence of type 1 and type 2 diabetes in adults and children in Kronoberg, Sweden. Diabetes Res Clin Pract. 2008;82(2):247–55.
Felner EI, Klitz W, Ham M, et al. Genetic interaction among three genomic regions creates distinct contributions to early- and late-onset type 1 diabetes mellitus. Pediatr Diabetes. 2005;6(4):213–20.
Molbak AG, Christau B, Marner B, et al. Incidence of insulin-dependent diabetes mellitus in age groups over 30 years in Denmark. Diabet Med. 1994;11(7):650–5.
Lammi N, Taskinen O, Moltchanova E, et al. A high incidence of type 1 diabetes and an alarming increase in the incidence of type 2 diabetes among young adults in Finland between 1992 and 1996. Diabetologia. 2007;50(7):1393–400.
Leslie RD, Williams R, Pozzilli P. Clinical review: type 1 diabetes and latent autoimmune diabetes in adults: one end of the rainbow. J Clin Endocrinol Metab. 2006;91(5):1654–9.
Turner R, Stratton I, Horton V, et al. UKPDS 25: autoantibodies to islet-cell cytoplasm and glutamic acid decarboxylase for prediction of insulin requirement in type 2 diabetes. UK Prospective Diabetes Study Group. Lancet. 1997;350:1288–93.
Wandell PE, Carlsson AC. Time trends and gender differences in incidence and prevalence of type 1 diabetes in Sweden. Curr Diabetes Rev. 2013;9(4):342–9.
Osler W. The principles and practice of medicine. New York: D. Appleton and Company; 1892.
National Diabetes Data Group. Diabetes in America. 2nd ed. Bethesda: National Institutes of Health; 1995.
Onkamo P, Vaananen S, Karvonen M, et al. Worldwide increase in incidence of Type I diabetes – the analysis of the data on published incidence trends. Diabetologia. 1999;42(12):1395–403.
Imperatore G, Boyle JP, Thompson TJ, et al. Projections of type 1 and type 2 diabetes burden in the U.S. population aged <20 years through 2050: dynamic modeling of incidence, mortality, and population growth. Diabetes Care. 2012;35(12):2515–20.
Boyle JP, Thompson TJ, Gregg EW. Projection of the year 2050 burden of diabetes in the U.S. adult population: dynamic modeling of incidence, mortality, and prediabetes prevalence. Popul Health Metr. 2010;8:29.
Spijkerman AM, Dekker JM, Nijpels G, et al. Microvascular complications at time of diagnosis of type 2 diabetes are similar among diabetic patients detected by targeted screening and patients newly diagnosed in general practice: the Hoorn Screening Study. Diabetes Care. 2003;26(9):2604–8.
Nathan DM. The diabetes control and complications trial/epidemiology of diabetes interventions and complications study at 30 years: overview. Diabetes Care. 2014;37(1):9–16.
UK Prospective Diabetes Study (UKPDS) Group. (UKPDS 33). Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes. Lancet. 1998;352:837–53.
Holman RR, Paul SK, Bethel MA, et al. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359(15):1577–89.
UK Prospective Diabetes Study (UKPDS) Group. (UKPDS 38). Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes. BMJ. 1998;317:703–13.
Holman RR, Paul SK, Bethel MA, et al. Long-term follow-up after tight control of blood pressure in type 2 diabetes. N Engl J Med. 2008;359(15):1565–76.
Volpe M, Cosentino F, Tocci G, et al. Antihypertensive therapy in diabetes: the legacy effect and RAAS blockade. Curr Hypertens Rep. 2011;13(4):318–24.
Isomaa B, Henricsson M, Almgren P, et al. The metabolic syndrome influences the risk of chronic complications in patients with type II diabetes. Diabetologia. 2001;44(9):1148–54.
Tandon N, Ali MK, Narayan KM. Pharmacologic prevention of microvascular and macrovascular complications in diabetes mellitus: implications of the results of recent clinical trials in type 2 diabetes. Am J Cardiovasc Drugs. 2012;12(1):7–22.
The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977–86.
Ohkubo Y, Kishikawa H, Araki E, et al. Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non-insulin-dependent diabetes mellitus: a randomized prospective 6-year study. Diabetes Res Clin Pract. 1995;28(2):103–17.
Lachin JM, Orchard TJ, Nathan DM. Update on cardiovascular outcomes at 30 years of the diabetes control and complications trial/epidemiology of diabetes interventions and complications study. Diabetes Care. 2014;37(1):39–43.
Fong DS, Aiello L, Gardner TW, et al. Retinopathy in diabetes. Diabetes Care. 2004;27 Suppl 1:S84–7.
Harris MI. Undiagnosed NIDDM: clinical and public health issues. Diabetes Care. 1993;16(4):642–52.
American Diabetes Association. Microvascular complications and foot care. Sec 9. in standards of medical care in diabetes. Diabetes Care. 2016;39 Suppl 1:S72–80.
Yau JW, Rogers SL, Kawasaki R, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012;35(3):556–64.
The National Eye Institute Statistics. Diabetic Retinopathy. NEI. Available at: https://nei.nih.gov/eyedata/diabetic. Accessed 18 July 2016.
Barr EL, Zimmet PZ, Welborn TA, et al. Risk of cardiovascular and all-cause mortality in individuals with diabetes mellitus, impaired fasting glucose, and impaired glucose tolerance: the Australian Diabetes, Obesity, and Lifestyle Study (AusDiab). Circulation. 2007;116(2):151–7.
Danaei G, Lawes CM, Vander Hoorn S, et al. Global and regional mortality from ischaemic heart disease and stroke attributable to higher-than-optimum blood glucose concentration: comparative risk assessment. Lancet. 2006;368:1651–9.
Gregg EW, Li Y, Wang J, et al. Changes in diabetes-related complications in the United States, 1990–2010. N Engl J Med. 2014;370(16):1514–23.
Centers for Disease Control and Prevention (CDC), National Center for Health Statistics, Division of Health Interview Statistics, data from the National Health Interview Survey. Number (in Millions) of people with diabetes aged 35 years or older with self-reported heart disease or stroke, United States, 1997–2011. Available at http://www.cdc.gov/diabetes/statistics/cvd/fig1.htm. Accessed 24 June 2016.
Ali MK, Bullard KM, Saaddine JB, et al. Achievement of goals in U.S. diabetes care, 1999–2010. N Engl J Med. 2013;368(17):1613–24.
Centers for Disease Control and Prevention, National Center for Health Statistics, Division of Health Care Statistics. Crude and age-adjusted hospital discharge rates for major cardiovascular disease as first-listed diagnosis per 1,000 diabetic population, United States, 1988–2006. Available at: http://www.cdc.gov/diabetes/statistics/complications_national.htm. Accessed 24 June 2016.
Gregg EW, Sorlie P, Paulose-Ram R, et al. Prevalence of lower-extremity disease in the US adult population > = 40 years of age with and without diabetes: 1999–2000 national health and nutrition examination survey. Diabetes Care. 2004;27(7):1591–7.
Gross JL, de Azevedo MJ, Silveiro SP, et al. Diabetic nephropathy: diagnosis, prevention, and treatment. Diabetes Care. 2005;28(1):164–76.
United States Renal Data System. 2014 USRDS annual data report: Epidemiology of kidney disease in the United States: international comparisons. Bethesda: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases. 2014. Available at: https://www.usrds.org/2014/view/v2_10.aspx. Accessed 24 June 2016.
Diabetes Prevention Program (DPP) Research Group. The Diabetes Prevention Program (DPP): description of lifestyle intervention. Diabetes Care. 2002;25(12):2165–71.
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this entry
Cite this entry
Adimoolam, D., Muthukrishnan, V., Albu, J.B. (2017). Epidemiology. In: Poretsky, L. (eds) Principles of Diabetes Mellitus. Springer, Cham. https://doi.org/10.1007/978-3-319-18741-9_8
Download citation
DOI: https://doi.org/10.1007/978-3-319-18741-9_8
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-18740-2
Online ISBN: 978-3-319-18741-9
eBook Packages: MedicineReference Module Medicine