Abstract
Background: The recent increase in popularity of acellular dermal matrix (ADM) assistance in immediate expander/implant breast reconstruction has led to variety of viewpoints. Many studies are published indicating an increase in complications with the use of ADM, while others indicate there is no increase in complications.
Methods: This meta-analysis utilizes information from all available studies that directly compare ADM with traditional methods of immediate expander/implant breast reconstruction. There was over a twofold increase in the number of infections and explanations in the ADM group compared to the control. There was a threefold increase in seroma formation in the ADM group compared to the control. There was a significant difference of intraoperative fill volumes between the ADM group compared to the control. This study illustrates that after pooling all available date regarding the use of ADM in immediate expander/implant breast reconstruction there appears to be an increased rate of complications. However, the increased intraoperative fill volume may lead to ultimately greater patient satisfaction.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
The use of AlloDerm (LifeCell, Branchburg, NJ), an acellular dermal matrix (ADM), in breast reconstruction to facilitate complete coverage of the implant/expander gained popularity in 2005 [1]. The introduction of this biomaterial has made it possible to provide complete tissue expander coverage without dissection of the serratus anterior or rectus abdominis muscle/fascia. In addition, it has been shown that when using ADM, the intraoperative tissue expander fill volume was increased and the total number of expansions needed was decreased [2]. However, ADM use does not come without complications; increased rates of infection and seroma formation have been linked with ADM use [3–5].
Almost a decade following the adoption of ADM in expander/implant breast reconstruction it is still unclear whether the benefits of its use presents an increased risk of infection and/or seroma formation. The results of most individual studies are oftentimes difficult to interpret. This chapter attempts to clarify complication rates associated with the use of ADM by performing an examination of existing literature and pooling selected outcomes in the form of a meta-analysis. Selected studies were examined for rates of complications in patients undergoing ADM-assisted and conventional expander/implant breast reconstruction. A literature search was performed to select high-quality observational studies that examine the relevant data. Stroup et al. [6] consensus article regarding meta-analyses of observational studies was used in the reporting of methods and results of this study. This method has been validated previously for the reporting of observational studies in the field of plastic and reconstructive surgery [7]. Guidelines compiled specifically for meta-analyses in plastic surgery literature were also followed [8]. The contents of this chapter are an expansion of a meta-analysis previously published by the authors incorporating since published data [9].
2 Search Strategy
One author (I.C.H) conducted all initial searches. PubMed was searched with the keywords, “alloderm”, “biocompatible materials”, “acellular dermal matrix”, “breast”, “expander implant”, and “infection” through February 2011. Daily updates of new papers that matched the search criteria were provided by email. In addition, reference lists were scrutinized to find any studies that may have been inadvertently excluded in the initial search. Abstracts were initially used to select relevant articles utilizing inclusion criteria (Table 148.1). Full-text articles were retrieved and submitted to the exclusion criteria (Table 148.2). A diagram of the initial search process is presented in Fig. 148.1. Since the publication of the original article, daily email updates with articles matching the search criteria were scrutinized for inclusion in a follow-up meta-analysis. This resulted in seven additional articles that met inclusion/exclusion criteria.
3 Data Extraction
All 15 studies included in the meta-analysis evaluated the rates of complications in ADM-assisted compared to traditional implant/expander breast reconstruction following mastectomy. All data was extracted directly from each study. Two researchers evaluated and independently extracted data from each study using a standardized form. The researchers were not blinded to the study being examined as this has been shown to be unnecessary [10].
4 Statistical Analysis
Statistical analysis was performed utilizing Review Manager ((RevMan) [Computer program]. Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration 2012). A fixed effect model and the Mantel–Haenszel test were utilized to provide pooled odds ratios for the variables under examination.
5 Results
Thirteen observational studies and one prospective randomized trial were found to fit the inclusion and exclusion criteria [2–4, 11–21]. One study [5] reported only explantation rates and was only included in that analysis. Table 148.3 provides a summary of the characteristics of each study.
6 Included Studies
Antony et al. [13] retrospective comparative study examines immediate 2-stage tissue expander breast reconstruction over a 4 year period at a single institution. In this period, there were 153 breasts included in the ADM group and 2910 breasts included in the control group. The control group was defined as a traditional musculofascial method. Descriptive characteristics are provided in Table 148.4. Notably, the mean BMI for the non-ADM group was lower than the ADM group and the rate of preoperative radiation therapy was higher in the ADM group. No formal comparison between ADM and control groups was performed. Outcomes examined included seroma, cellulitis, hematoma, and premature explantation of the expander. Overall, there was an increased incidence of complications noted in the ADM group. Age, BMI, and axillary dissection were determined to be independent risk factors for development of one or more complications.
Chun et al. [3] retrospective comparative study examines immediate breast reconstruction utilizing tissue expanders or implants over a 6 year period at a single institution. During this period, there were 269 breasts included in the ADM group and 146 breasts in the control group. The control group was defined as expanders/implants with total submuscular coverage or partial submuscular coverage with corresponding partial subcutaneous coverage. Within this group, 68 latissimus dorsi and 1 pedicled transverse rectus abdominis muscle flaps were included because they utilized a tissue expander or an implant. Descriptive characteristics are provided in Table 148.5. Notably, there was a significant (p = 0.002) difference between the BMI of the two groups and the mastectomy specimen (p < 0.001) weight of each group. Outcomes examined included hematoma, seroma, necrosis, intraoperative fill volume, and infection. Infection was broken down into minor (successfully treated with outpatient antibiotics) and major infections (required admission), both of which were combined to determine the rate of infection. Overall there was an increased rate of complications in the ADM group. BMI and the use of ADM were determined to be significant risk factors for the development of infection and seroma.
Collis et al. [15] retrospective review examined immediate 2-stage expander/implant breast reconstruction performed over a 4.5 year period at a single institution. During this period there were 106 breasts included in the ADM group and 68 breasts included in the control group. The ADM utilized in this study was of human origin, and not strictly Alloderm. The control group was only defined as those undergoing 2-stage reconstructions without the use of ADM. No mention is made of complete submuscular coverage. The only descriptive characteristics provided were mean age (53 in both groups) and that 100 % of each group was an immediate reconstruction. Outcomes examined included tissue expander dynamics, skin necrosis, seroma, expander malposition, and infection requiring explantation. It was found that a significantly higher intraoperative fill volume was achieved in the ADM group, but that this group was also associated with a higher rate of overall complications. There was no significant difference noted between the groups with regard to explantation.
Hanna et al. [16] retrospective review examined 2-stage expander/implant breast reconstructions performed by 2 senior authors over a 4 year period at a single institution. During this period, there were 38 breasts included in the ADM group and 62 breasts included in the control group. The control group was defined as complete submuscular coverage beneath the pectoralis superiorly and the serratus anterior inferiorly. Eleven delayed reconstructions were included in this study. Descriptive characteristics are provided in Table 148.6. Outcomes examined included seroma, hematoma, cellulitis (defined as a minor infection), skin necrosis, wound separation, major infection, infection requiring intravenous antibiotics, and explantation. For the purposes of the meta-analysis infections requiring IV antibiotics were used for the infection variable. No statistical differences were noted between the ADM and control groups with regards to complications. Tissue expander dynamics were also reported, however were not included in the meta-analysis due to the authors’ protocol of not inflating the expander intraoperatively in the control group. It was thought that including this would skew the results of the meta-analysis of that variable. An evaluation of patient satisfaction was performed between the 2 groups with no significant difference found.
Lanier et al. [4] retrospective comparative study examines immediate 2-stage tissue expander breast reconstruction over a 3 year period at a single institution. During this period, 52 breasts were included in the ADM group and 75 breasts in the control group. The control group was defined as the creation of a sub-pectoral pocket and a lateral pocket utilizing serratus anterior muscle. Descriptive characteristics are provided in Table 148.7. All characteristics were compared to find significant differences among groups. Significant differences were noted in BMI (p < 0.001) and mean breast tissue removed (p = 0.005) between groups. Outcomes examined included seroma, hematoma, necrosis requiring revision, capsular contracture, intraoperative fill volume, and cellulitis or infection. Overall there was an increased rate of complications in the ADM group.
Liu et al. [14] retrospective comparative study examines immediate 2-stage tissue expander breast reconstruction over a 5 year period at a single institution. During this period, 266 breasts were included in the ADM group and 204 breasts in the control group. The control group was defined as either total or partial submuscular placement of the expander. Descriptive characteristics are provided in Table 148.8. Significant differences were noted in mean breast tissue removed (p = 0.0184). Outcomes included infection, implant removal, skin flap necrosis, seromas, intraoperative fill volume, and hematomas. Overall, complications were higher in the ADM group.
McCarthy et al. [17] multicenter, blinded, randomized controlled trial examined immediate expander/implant reconstruction over a 4 year period at 2 institutions. During this time period 56 breasts were randomized to the ADM group and 50 breasts were randomized to the control group. The control group was defined as submuscular coverage utilizing serratus muscle/fascia and rectus abdominis fascia. Descriptive characteristics are provided in Table 148.9. Outcomes examined included visual analog scale rating throughout expansion, BREAST-Q Physical Wellbeing: Chest and Upper Body Scale scores throughout expansion, narcotic use, tissue expander dynamics, and perioperative complications (hematoma, seroma, infection, premature removal of tissue expander). No difference was found between groups with regards to perioperative and expansion-phase pain, physical well-being scores, narcotic use, intraoperative expander fill volume, or perioperative complications. There were significantly fewer percutaneous expansions in the ADM group. This was an incredibly well-designed study that was terminated early due to an interim review that revealed a very small likelihood of discovering a difference between the groups with continued patient enrollment.
Nahabedian [11] retrospective comparative study examines breast reconstruction utilizing prosthetic devices over a period of 11 years. Specifically, this study examined the rates of complications with and without chemotherapy and/or radiation. During this period, 100 breasts were included in the ADM group and 376 breasts were included in the control group. The control group was defined as device placement beneath the pectoralis major and lower mastectomy skin flap. Few descriptive characteristics were included regarding the patients. Outcomes examined included infection, implant removal, and ADM removal. Overall, there was no difference in the rate of complications between groups.
Parks et al. [18] retrospective review examined immediate 2-stage expander/implant breast reconstruction performed over a 10 year period at a single institution. During this period there were 346 breasts in the ADM group and 165 breasts in the control group. There is no description of the control group surgical procedure, but mention is made regarding non-uniformity of surgical technique amongst surgeons. Also of note, all non-ADM reconstructions were performed in the first 4 years followed by all ADM reconstructions in the following 6 years. Descriptive characteristics are provided in Table 148.10. Outcomes examined included tissue expander dynamics, seroma, mastectomy skin necrosis, and loss of tissue expander. A significantly increased rate of seromas was found in the ADM group. Prior radiation, increased intraoperative percentage of tissue expander fill volume, mastectomy flap necrosis, and seroma were all identified as risk factors for tissue expander loss.
Peled et al. [19] prospective cohort study examined immediate 2-stage expander-implant breast reconstruction performed by a single surgeon over a 5 year period at a single institution. During this time period there were 100 breasts in the ADM group and 90 breasts in the control group. The control group was defined as a dual-plane position of the expander without muscular coverage of the inferior pole. The non-ADM group was performed over the course of the first year, and the ADM group was performed over the course of years 2–3 of the study. A 3rd group was included in this study in which ADM was used selectively based on mastectomy skin flap thickness. This group was excluded from the meta-analysis because it failed to separate reconstructions utilizing ADM. Descriptive characteristics are provided in Table 148.11. Outcomes examined included infection, unplanned return to operating room, skin flap necrosis, expander-implant exposure or loss, seroma, hematoma, and nipple necrosis. It is difficult to interpret the results of this study due to the inclusion of a 3rd cohort of patients, but it appears that infections, return to operating room, and expander loss were decreased in the ADM group. The analysis of the 3rd cohort is important however, in that it implies an individualized approach to selection of ADM use in breast reconstruction.
Preminger et al. [12] matched, retrospective cohort study examines immediate tissue expander implant breast reconstruction over a 2 year period. Matching criteria included median expander size, history of radiation, and indication for mastectomy. Matched cohorts of 45 breasts each were prepared. The control group was defined as creation of a subpectoral pocket with elevation of the serratus anterior and superior rectus abdominis muscle/fascia. Few descriptive characteristics were included regarding the patients, but due to the matched cohort nature of the study it can be assumed that there are likely few differences between groups. Outcomes examined included seroma, hematoma, intraoperative fill volume and cellulitis. For the purposes of the meta-analysis, the rate of cellulitis was used as the rate of infection. Overall, there was no difference in the rate of complications between groups.
Sbitany et al. [2] retrospective comparative study examines tissue expander implant breast reconstruction over a 4 year period. During this period 92 breasts were included in the ADM group and 84 breasts were included in the control group. The control was defined as placement in a subpectoral pocket with elevation of the serratus anterior laterally. Descriptive characteristics are provided in Table 148.12. No significant differences were found between these characteristics. Outcomes examined included seroma, cellulitis, intraoperative fill volume, and infection requiring expander removal. For the purpose of this meta-analysis the rate of cellulitis was used as the rate of infection. Overall, there was no difference in the rate of complications between groups. During the reporting of complications, the authors used the number of patients instead of the number of reconstructions. The decision was made to utilize these values in terms of the number of reconstructions for the purpose of this meta-analysis to avoid discrepancies in reporting.
Vardanian et al. [20] retrospective review examined 2-stage expander/implant breast reconstruction performed over a 9 year period at a single institution. During this period there were 208 breasts in the ADM group and 129 breasts in the control group. The control group was defined as either total or partial submuscular coverage, with a majority of the reconstruction being performed with partial submuscular coverage. Descriptive characteristics are provided in Table 148.13. Of note, 21 reconstructions were performed for indications other than breast cancer, including BRCA mutation, silicone mastitis, and congenital breast asymmetry. Outcomes examined included capsular contracture, inframammary fold problems, bottoming out, rippling, mechanical shift, wound infection, seroma/hematoma, dehiscence, and skin problems. Because seroma and hematoma were tabulated in the same category they were unable to be included within each respective meta-analysis. It was found that a significantly decreased number of capsular contractures, inframammary fold problems, episodes of bottoming out, rippling, and mechanical shift were associated with the use of ADM. No differences were noted with regard to wound infection, seroma/hematoma, dehiscence, or skin problems between groups.
Weichman et al. [21] retrospective review examined immediate 2-stage expander/implant breast reconstruction performed over a 3 year period at a single institution. During this period there were 442 patients in the ADM group and 186 breasts in the control group. The control group was defined as total submuscular coverage with use of a serratus flap and rectus fascia as needed. Descriptive characteristics are provided in Table 148.14. Outcomes examined included tissue expander dynamics, mastectomy flap necrosis, infection, seroma, hematoma, and explantation. It was found that a significantly higher intraoperative fill volume was achieved in the ADM group. The ADM group had significantly higher rates of mastectomy flap necrosis, infection and explantation.
7 Meta-analysis of Studies
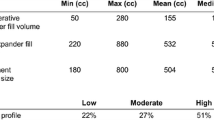
A forest plot of the odds ratio of infection across all studies is provided in Fig. 148.2, a funnel plot is provided in Fig. 148.3. The meta-analysis reports an increased in rate of infections for the ADM-assisted group (odds ratio of 1.71; 95 % confidence interval, 1.29–2.28). A forest plot of the odds ratio of seroma formation across the 11 studies reporting this statistic is provided in Fig. 148.4, a funnel plot is provided in Fig. 148.5. There was over a twofold increase in the incidence of seromas in the ADM-assisted group (odds ratio of 2.14; 95 % confidence interval, 1.61–2.84). A forest plot of the odds ratio of hematoma formation across the nine studies reporting this outcome is provided in Fig. 148.6, a funnel plot is provided in Fig. 148.7. There was no significant difference in the rate of hematoma formation between groups. A forest plot of the odds ratio of tissue expander explantation across the 13 studies reporting this outcome is provided in Fig. 148.8, a funnel plot is provided in Fig. 148.9. The meta-analysis reports an increased rate of explantations for the ADM-assisted group (odds ratio of 1.77; 95% confidence interval, 1.32–2.36). A forest plot of the mean difference of intraoperative fill volumes of the tissue expanders is provided in Fig. 148.10, a funnel plot is provided in Fig. 148.11. The meta-analysis reports a mean difference of 94.45 mL (95 % confidence interval, 84.73–104.17)
8 Discussion
As breast reconstruction following mastectomy utilizing ADM becomes an acceptable reconstructive option it is important to understand the risks and benefits of its use. In the case of expander/implant breast reconstruction, several variables are important to the end result and ultimately patient satisfaction. These include postoperative complications such as infections, seromas, hematomas, and the rate of expander explantation following one of these complications.
While there is much in the current literature regarding ADM use in expander/implant breast reconstruction, the majority focuses on the rates of complications and the dynamics of expansion [1, 22–27]. Unfortunately, most of these publications are case series with no control group.
It is oftentimes difficult to evaluate the results of each individual study due to the presence of confounding factors such as body mass index (BMI), surgeon expertise (both plastic surgeon and breast surgeon), and unclear methods of study design. The 15 studies included in this analysis were dissenting regarding whether complication rates were higher in the ADM group. This presents a problem for the surgeon attempting to determine the true risks and benefits of the use of ADM. It is the goal of this meta-analysis to pool all available data to obtain a clearer picture of the risks inherent with the use of ADM in expander/implant breast reconstruction.
This meta-analysis demonstrates that ADM use in expander/implant reconstruction results in increased rates of infection, seroma, and explantation compared to a control. This is not conceptually difficult to understand given that ADM is a foreign body, despite its biologic properties. Foreign bodies incite an inflammatory reaction that may result in increased rates of infection. This does not preclude the use of ADM in breast reconstruction as the benefits may outweigh the risks reported by this study. These benefits may include decreased postoperative pain and morbidity, decreased operative time, increased initial expander fill volume, and an increased rate of expansion. This meta-analysis showed a significant difference between the ADM group and the control regarding intraoperative fill volume. This may lead to a fewer number of expansions and ultimately greater patient satisfaction.
Weaknesses of this meta-analysis include the introduction of publication bias and a slightly different definition of outcome measurements across studies. Through the meticulous literature search the authors attempted to include all published data on the topic. Unfortunately, researchers are less likely to publish unfavorable results, introducing a degree of bias. An attempt was made to generalize outcome measurements throughout each study as described in each synopsis.
Specific mention is warranted regarding the study performed by McCarthy et al. [17]. This study prospectively evaluated pain and patient satisfaction between groups and found no significant difference. In the ADM-assisted group there were significantly fewer percutaneous expansions, which may ultimately lead to increased patient satisfaction.
Several other studies that were not included deserve mention. Ibrahim et al. [28] examination of the National Surgical Quality Improvement Program database provides a large number of patients undergoing expander/implant breast reconstruction. It is difficult to include a study such as this in a meta-analysis due to the method of recording complications and the lack of information regarding treatment. No difference was found in perioperative complications between those undergoing ADM-assisted reconstruction and those [presumably] undergoing traditional reconstruction. Similarly, the study performed by Pannucci et al. [29] examined a large cohort of patients from the Tracking Outcomes and Operations in Plastic Surgery database to determine rate of expander/implant loss in patients undergoing traditional and ADM-assisted breast reconstruction. This data was not included due to several limitations of the study, including the voluntary reporting nature of the database, and difficulties in determining timing of complications. Interestingly this study showed that from 2008 to 2011 there was a significant decrease in expander/implant losses, possibly explained by the learning curve associated with the use of these devices.
As in any reconstructive procedure, the surgeon must use their judgment when deciding on an operative plan. This chapter simply attempts to clear up an intensely debated issue in breast reconstruction. The answer likely lies in individual patient evaluation to determine suitability for placement of ADM. The surgeon should be aware of the possible risks associated with its use and convey this to the patient prior to surgery.
References
Breuing KH, Warren SM (2005) Immediate bilateral breast reconstruction with implants and inferolateral AlloDerm slings. Ann Plast Surg 55(3):232–239
Sbitany H, Sandeen SN, Amalfi AN, Davenport MS, Langstein HN (2009) Acellular dermis-assisted prosthetic breast reconstruction versus complete submuscular coverage: a head-to-head comparison of outcomes. Plast Reconstr Surg 124(6):1735–1740
Chun YS, Verma K, Rosen H, Lipsitz S, Morris D, Kenney P, Eriksson E (2010) Implant-based breast reconstruction using acellular dermal matrix and the risk of postoperative complications. Plast Reconstr Surg 125(2):429–436
Lanier ST, Wang ED, Chen JJ, Arora BP, Katz SM, Gelfand MA, Khan SU, Dagum AB, Bui DT (2010) The effect of acellular dermal matrix use on complication rates in tissue expander/implant breast reconstruction. Ann Plast Surg 64(5):674–678
Nguyen MD, Chen C, Colakoglu S, Morris DJ, Tobias AM, Lee BT (2010) Infectious complications leading to explantation in implant-based breast reconstruction With AlloDerm. Eplasty 10, e48
Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB (2000) Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 283(15):2008–2012
Man LX, Selber JC, Serletti JM (2009) Abdominal wall following free TRAM or DIEP flap reconstruction: a meta-analysis and critical review. Plast Reconstr Surg 124(3):752–764
Haase SC (2011) Systematic reviews and meta-analysis. Plast Reconstr Surg 127(2):955–966
Hoppe IC, Yueh JH, Wei CH, Ahuja NK, Patel PP, Datiashvili RO (2011) Complications following expander/implant breast reconstruction utilizing acellular dermal matrix: a systematic review and meta-analysis. Eplasty 11, e40
Berlin JA (1997) Does blinding of readers affect the results of meta-analyses? University of Pennsylvania Meta-analysis Blinding Study Group. Lancet 350(9072):185–186
Nahabedian MY (2009) AlloDerm performance in the setting of prosthetic breast surgery, infection, and irradiation. Plast Reconstr Surg 124(6):1743–1753
Preminger BA, McCarthy CM, Hu QY, Mehrara BJ, Disa JJ (2008) The influence of AlloDerm on expander dynamics and complications in the setting of immediate tissue expander/implant reconstruction: a matched-cohort study. Ann Plast Surg 60(5):510–513
Antony AK, McCarthy CM, Cordeiro PG, Mehrara BJ, Pusic AL, Teo EH, Arriaga AF, Disa JJ (2010) Acellular human dermis implantation in 153 immediate two-stage tissue expander breast reconstructions: determining the incidence and significant predictors of complications. Plast Reconstr Surg 125(6):1606–1614
Liu AS, Kao HK, Reish RG, Hergrueter CA, May JW, Guo L (2011) Postoperative complications in prosthesis-based breast reconstruction using acellular dermal matrix. Plast Reconstr Surg 127(5):1755–1762
Collis GN, TerKonda SP, Waldorf JC, Perdikis G (2012) Acellular dermal matrix slings in tissue expander breast reconstruction: are there substantial benefits? Ann Plast Surg 68(5):425–428
Hanna KR, DeGeorge BR Jr, Mericli AF, Lin KY, Drake DB (2013) Comparison study of two types of expander-based breast reconstruction: acellular dermal matrix-assisted versus total submuscular placement. Ann Plast Surg 70(1):10–15
McCarthy CM, Lee CN, Halvorson EG, Riedel E, Pusic AL, Mehrara BJ, Disa JJ (2012) The use of acellular dermal matrices in two-stage expander/implant reconstruction: a multicenter, blinded, randomized controlled trial. Plast Reconstr Surg 130(5 Suppl 2):57S–66S
Parks JW, Hammond SE, Walsh WA, Adams RL, Chandler RG, Luce EA (2012) Human acellular dermis versus no acellular dermis in tissue expansion breast reconstruction. Plast Reconstr Surg 130(4):739–746
Peled AW, Foster RD, Garwood ER, Moore DH, Ewing CA, Alvarado M, Hwang ES, Esserman LJ (2012) The effects of acellular dermal matrix in expander-implant breast reconstruction after total skin-sparing mastectomy: results of a prospective practice improvement study. Plast Reconstr Surg 129(6):901e–908e
Vardanian AJ, Clayton JL, Roostaeian J, Shirvanian V, Da Lio A, Lipa JE, Crisera C, Festekjian JH (2011) Comparison of implant-based immediate breast reconstruction with and without acellular dermal matrix. Plast Reconstr Surg 128(5):403e–410e
Weichman KE, Wilson SC, Weinstein AL, Hazen A, Levine JP, Choi M, Karp NS (2012) The use of acellular dermal matrix in immediate two-stage tissue expander breast reconstruction. Plast Reconstr Surg 129(5):1049–1058
Nahabedian MY, Tsangaris T, Momen B, Manson PN (2003) Infectious complications following breast reconstruction with expanders and implants. Plast Reconstr Surg 112(2):467–476
Spear SL, Parikh PM, Reisin E, Menon NG (2008) Acellular dermis-assisted breast reconstruction. Aesthetic Plast Surg 32(3):418–425
Breuing KH, Colwell AS (2007) Inferolateral AlloDerm hammock for implant coverage in breast reconstruction. Ann Plast Surg 59(3):250–255
Gamboa-Bobadilla GM (2006) Implant breast reconstruction using acellular dermal matrix. Ann Plast Surg 56(1):22–25
Namnoum JD (2009) Expander/implant reconstruction with AlloDerm: recent experience. Plast Reconstr Surg 124(2):387–394
Zienowicz RJ, Karacaoglu E (2007) Implant-based breast reconstruction with allograft. Plast Reconstr Surg 120(2):373–381
Ibrahim AM, Shuster M, Koolen PG, Kim K, Taghinia AH, Sinno HH, Lee BT, Lin SJ (2013) Analysis of the National Surgical Quality Improvement Program database in 19,100 patients undergoing implant-based breast reconstruction: complication rates with acellular dermal matrix. Plast Reconstr Surg 132(5):1057–1066
Pannucci CJ, Antony AK, Wilkins EG (2013) The impact of acellular dermal matrix on tissue expander/implant loss in breast reconstruction: an analysis of the tracking outcomes and operations in plastic surgery database. Plast Reconstr Surg 132(1):1–10
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Hoppe, I.C., Ahuja, N.K., Datiashvili, R.O. (2016). Complications Following Expander/Implant-Based Breast Reconstruction Utilizing Acellular Dermal Matrix. In: Shiffman, M. (eds) Breast Reconstruction. Springer, Cham. https://doi.org/10.1007/978-3-319-18726-6_148
Download citation
DOI: https://doi.org/10.1007/978-3-319-18726-6_148
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-18725-9
Online ISBN: 978-3-319-18726-6
eBook Packages: MedicineMedicine (R0)