Abstract
The use of acellular dermal matrices (ADMs) in post mastectomy, two-stage, expander/implant breast reconstruction has been widely reported. Few studies have also described the utility of ADMs in the setting of post mastectomy radiation treatment. The main advantages of ADMs stem from coverage of the infero-lateral aspect of the implants during breast reconstruction. Seroma, infection, expander/implant loss are some of the commonly reported complications. Few well-designed studies have shown no significant increase in initial fill volume and postoperative tissue expansion in patients who had ADM assisted two-stage breast reconstruction. ADMs should not be used routinely in patients undergoing two-stage breast reconstruction. A case-by-case approach is recommended instead. More long-term and high-quality studies are required to truly evaluate the efficacy of ADMs.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
The techniques available for breast reconstruction following mastectomy have evolved over the past many years. One of the significant innovations in recent years has been the use of acellular dermal matrices (ADMs) in breast surgery. More specifically, ADMs have been used for breast reconstruction following mastectomy, for revision of secondary breast deformities following breast reconstruction, for breast augmentation and even nipple reconstruction.
2 Acellular Dermal Matrix (ADM)
An ADM is a soft tissue matrix that is derived by removing the cells and leaving the extracellular matrix intact. When placed against a well-vascularized surface, it incorporates into the host tissue, forming a new matrix for tissue regeneration [1]. Some of the allogenic as well as xenogenic ADMs currently available in the market for reconstruction are: AlloDerm® (LifeCell Corporation, USA), AlloMax™ (Bard Davol, USA), DermACELL™ (LifeNet, USA), DermaMatrix® (Synthes, USA), FlexHD® (Ethicon, USA), Permacol™ (Covidien, USA), Strattice™ (LifeCell, USA) and SurgiMend® (TEI Biosciences, USA) [2, 3]. The main differences in these products are in the source, the way they are processed and their costs.
3 Indications
ADMs are used for breast reconstruction as well as aesthetic surgeries. ADMs can be used generally in any woman undergoing tissue expander (TE) or implant-based breast reconstruction but are considered higher-risk in women with high BMI, active smokers and in women who have a history of prior radiation treatment [4–6]. ADMs allow for expansion of the sub-muscular space and are being used for one stage (direct-to-implant) as well as two-stage (expander/implant) immediate breast reconstructions. This chapter will primarily focus on the use of ADMs in two-stage TE/implant reconstruction.
4 Two-Stage Breast Reconstruction with ADM
ADMs are incorporated during the first stage of breast reconstruction at the time of expander placement. The purpose of ADM in this setting is to supplement partial muscle coverage, maintain and/or further define the inframammary fold, and maximize future breast ptosis and projection.
5 Operative Technique
Immediately following mastectomy, ADM is used to create the infero-lateral portion of the submuscular pocket. ADM is typically used as an inferior sling, which is attached superiorly to the dis-inserted, pectoralis major muscle, and inferiorly to the native inframammary fold. ADM placed along the lower breast pole can also recreate both the inframammary fold and lateral mammary fold. In using this technique, the need for elevation of the taut serratus anterior fascia and/or rectus muscle/fascia is thus eliminated. The result is a well-defined pocket in which an expander can be placed, thereby reducing the incidence of implant migration or visibility and ultimately improving breast aesthetics.
A fully deflated tissue expander is positioned within this pocket and direct contact is prevented between the skin and the expander prosthesis. Postoperatively, expansion of the submuscular pocket begins 10–14 days after surgery. Expansion occurs on a weekly basis. Typically, volumes up to 100 mL of normal saline are injected at each visit. The volume of fluid injected at each time point is limited by tissue tolerance and/or patient comfort. Weekly expansions are terminated once the expander is filled to a volume 20 % greater than the recommended volume of the expander. This overexpansion ultimately creates a looser skin/muscle envelope and a greater potential for breast ptosis. Exchange of the temporary expander for a permanent implant occurs at a subsequent operation.
6 Benefits
Many surgeons are of the opinion that ADMs improve aesthetic outcome by allowing for a more precise placement of tissue expanders, facilitating more rapid tissue expansion, improving inferior pole projection and reducing the development of capsular contracture [6–11]. Unfortunately, high-level evidence for these perceived clinical benefits is difficult to obtain. A randomized controlled trial was conducted by the senior author (CM) to evaluate the effects of AlloDerm® on patient reported pain and rate of tissue expansion following two-stage TE/implant breast reconstruction. Patients were randomly allocated into two groups: (1) ADM assisted, TE/Implant, and (2) submuscular, TE/Implant. Interestingly, no significant difference in immediate postoperative pain (p = 0.19), pain during expansion phase (p = 0.65), postoperative narcotic use (p = 0.38) and rate of postoperative expansion (p = 0.83) was noted between the two groups [12].
7 Complications
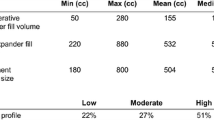
ADMs are thought to be associated with higher risk of infections and seroma formation. A systematic review by Kim et al. [13] compared 19 studies that used ADM for two-stage reconstruction versus 35 that did not. They noted higher complication rates in the former group i.e. seroma, 4.8 versus 3.5 %; infection, 5.3 versus 4.7 % and flap necrosis, 6.9 versus 4.9 %. Macadam and Lennox [14] reviewed complications post ADM use in two-stage breast reconstruction in 15 studies. They calculated a weighted average for each complication and compared it with Mentor and Allergan Core Study results that track complication rates following two-stage breast reconstruction without ADM. They reported higher rates of seroma (5.8 versus 4.9 %), infection (5.3 versus 3.2–5.7 %), flap necrosis (7.6 versus 2.3 %) and lower rates of capsular contracture (2.6 versus 8.3–17.1 %), late revision (10.7 versus 27–53 %) in the ADM group compared with Core studies. Sbitany and Serletti [11] reviewed six matched-cohort studies comparing ADM use with standard submuscular techniques. They reported significantly higher seroma rates in the former group (8.4 versus 4.3 %, p = 0.03) with higher hematoma (2 versus 1.2 %, p = 0.11), and infection (3.4 versus 3.2 %, p = 0.18) rates in the ADM group. Four studies in their analysis reported on expander/implant characteristics. Mean intraoperative fill volume was significantly higher in the ADM group compared to non-ADM group (68.5 versus 24.2 %, p = 0.01). Mean number of fills necessary to achieve final volume was significantly lower in the ADM group than non ADM group (2.4 versus 5.1, p = 0.03). A systematic review by Hoppe et al. [15] compared complications between ADM and non-ADM groups in seven studies. Four studies in their analysis reported significantly higher complications (seroma, hematoma, infection) in the ADM group and three reported no significant difference in complications between the two groups. Perminger et al. [16] performed a matched, retrospective cohort study comparing two groups of patients that underwent: (1) standard TE/implant reconstruction (n = 45), (2) ADM assisted TE/implant reconstruction (n = 45). No significant difference between the two groups was noted for mean initial fill volume (p = 0.18), median number of expansion (p = 0.11), postoperative tissue expansion (p = 0.90) and incidence of complications (p = 0.28).
8 Costs
Use of ADMs is associated with higher cost than traditional two-stage breast reconstruction not involving ADMs [17]. Krishnan et al. [18] performed a cost effectiveness analysis of use of ADMs in two-stage expander/implant breast reconstruction. Their baseline analysis revealed that use of ADM is cost-effective (Incremental cost utility ratio (ICUR) of $264.20/QALY).
9 ADM and Radiation Treatment
The use of two-stage expander/implant reconstruction using ADMs in the setting of radiation treatment has reported varied results in the literature. Proponents of ADM claim that ADM may in fact weather the long-term effects of radiation better than a traditional submuscular pocket. High-level evidence to support these claims are lacking at this time.
Blindingvale et al. [19] reported that 20 % patients who had radiation treatment following ADM use (n = 5) developed wound infection that necessitated explantation of TE and ADM. In contrast, in the non-irradiated patients (n = 36), wound infection, seroma formation and hematoma occurred in 2.7 %, 8.3 % women and 2.7 % women respectively. None of the patients had capsular contracture. Breuing and Colwell [20] reported the use of ADM in four patients that had expander/implant breast reconstruction. Over a 2–5 years period of follow up post radiation, none of the patients developed infection, capsular contracture or implant loss. Rawlani et al. [21] compared ADM use in patients who had radiation (n = 95) versus those who did not (n = 26). The radiation group had non-significantly higher overall complications (30.8 versus 13.7 %, p = 0.07), soft-tissue infection (11.5 versus 6.3 %, p = 0.40), flap necrosis (15.4 versus 4.2 %, p = 0.06) and exposure (15.4 versus 4.2 %, p = 0.06). Seroma formation was non-significantly higher in the non-radiation group (2.1 versus 0 %, p = 0.99). Spear et al. [22] reported significantly higher overall complication rates in women who had radiation after ADM assisted expander placement (n = 11 breasts) than in patients who did not have radiation treatment (n = 47 breasts), (45.45 versus 4.2 %, p = 0.002). Rate of infection was significantly higher in the former group (27.27 versus 2.12 %, p = 0.02). There was no significant difference in seroma formation between the two groups (p = 0.19). In a separate study, Spear et al. [23] reported no significant difference in infection, seroma and hematoma in between patients who had radiation treatment following first-stage reconstruction with ADM and the ones who did not have any radiation (p>0.05). Rate of capsular contracture was significantly higher in the radiation group (p<0.05). Following second stage of breast reconstruction, rates of infection, exposure and capsular contracture were higher in the radiation treatment groups (p<0.05). Clemens and Kronowitz [24] compared patients with ADM assisted breast reconstruction and those who had radiation treatment (n = 30) versus those who did not (n = 518). They reported higher overall complication rate (43.3 versus 15.6 %), loss of expander (13.3 versus 5.2 %), seroma (13.3 versus 5 %), infection (13.3 versus 6.2 %), mastectomy flap necrosis (26.7 versus 7.5 %), in irradiated patients. Hematoma rate was higher in the non-irradiated group (0.7 versus 0 %).
10 Sterile ADMs
Use of sterile ADMs in patients undergoing two-stage TE/implant breast reconstruction has conflicting results in the literature. Weichman et al. [25] compared complication rates between patients who had immediate implant based breast reconstruction (expander or permanent implant) with sterile and aseptic AlloDerm. The sterile ADM group had significantly lesser overall infectious complications than aseptic ADM group (8.5 versus 20 %, p = 0.008). Buseman et al. [26] reported complication rates following use of sterile and aseptic ADMs in patients undergoing breast reconstruction with implants or tissue expanders. Seroma occurrence was significantly higher in sterile ADM group versus aseptic ADM group (p = 0.003). Venturi et al. [27] used only sterile ADMs in 65 consecutive patients for tissue expander based breast reconstruction. They reported no seromas or explantations in any of the patients.
11 Discussion
ADMs may be associated with improved aesthetic outcomes at the cost of higher complication rates than traditional tissue expander/implant reconstruction techniques. A review of the literature reports mixed overall results from use of ADMs in two-stage breast reconstruction. Most of the evidence supports significantly increased complication rates whereas few studies report higher but not significantly increased complication rates than using standard techniques not involving ADMs. Most of the studies are case series from a single center and thus the results could have been influenced by the operative technique and experience of the surgeons involved. Results from two well designed studies, one a matched, retrospective cohort and the other a RCT, both suggest no greater benefit from the use of ADMs in two-stage breast reconstruction [12, 16]. There is also no consensus on use of ADMs in the setting of irradiation of breasts. Most of the studies that reported on results of ADM use in breast reconstruction, in the setting of radiation treatment, did that as a subgroup analysis and not as the primary outcome [19–24]. The American Society of Plastic Surgeons guidelines for use of ADM in breast reconstruction, have recommended a case-by-case approach to use of ADMs based on such varied and conflicting reports [28].
There is also a paucity of patient reported outcomes (PROs) data following use of ADMs in breast reconstruction. Most of the studies that have reported complications following ADMs have not used consistent and well-defined outcomes, making it difficult to compare results between different studies.
More, well-designed studies such as RCTs using PROs as part of measured outcomes are required to precisely assess the benefits and complications of ADMs in two-stage breast reconstructions.
Conclusions
ADMs are safe to use in two-stage expander/implant breast reconstructions. They may provide better aesthetic outcomes but are associated with higher incidence of infections and seromas. More long-term studies, involving PROs are required to accurately evaluate the effect of ADMs on immediate, two-stage breast reconstruction.
References
Novitsky YW, Rosen MJ (2012) The biology of biologics: basic science and clinical concepts. Plast Reconstr Surg 130(5 Suppl 2):9S–17S
Cheng A, Saint-Cyr M (2012) Comparison of different ADM materials in breast surgery. Clin Plast Surg 39(2):167–175
Ibrahim AM, Ayeni OA, Hughes KB, Lee BT, Slavin SA, Lin SJ (2013) Acellular dermal matrices in breast surgery: a comprehensive review. Ann Plast Surg 70(6):732–738
Ibrahim AM, Shuster M, Koolen PG, Kim K, Taghinia AH, Sinno HH, Lee BT, Lin SJ (2013) Analysis of the National Surgical Quality Improvement Program database in 19,100 patients undergoing implant-based breast reconstruction: complication rates with acellular dermal matrix. Plast Reconstr Surg 132(5):1057–1066
Davila AA, Seth AK, Wang E, Hanwright P, Bilimoria K, Fine N, Kim JY (2013) Human acellular dermis versus submuscular tissue expander breast reconstruction: a multivariate analysis of short-term complications. Arch Plast Surg 40(1):19–27
Liu AS, Kao HK, Reish RG, Hergrueter CA, May JW Jr, Guo L (2011) Postoperative complications in prosthesis-based breast reconstruction using acellular dermal matrix. Plast Reconstr Surg 127(5):1755–1762
Forsberg CG, Kelly DA, Wood BC, Mastrangelo SL, DeFranzo AJ, Thompson JT, David LR, Marks MW (2014) Aesthetic outcomes of acellular dermal matrix in tissue expander/implant-based breast reconstruction. Ann Plast Surg 72(6):S116–S120
Vardanian AJ, Clayton JL, Roostaeian J, Shirvanian V, Da Lio A, Lipa JE, Crisera C, Festekjian JH (2011) Comparison of implant-based immediate breast reconstruction with and without acellular dermal matrix. Plast Reconstr Surg 128(5):403e–410e
Sbitany H, Sandeen SN, Amalfi AN, Davenport MS, Langstein HN (2009) Acellular dermis-assisted prosthetic breast reconstruction versus complete submuscular coverage: a head-to-head comparison of outcomes. Plast Reconstr Surg 124(6):1735–1740
Lanier ST, Wang ED, Chen JJ, Arora BP, Katz SM, Gelfand MA, Khan SU, Dagum AB, Bui DT (2010) The effect of acellular dermal matrix use on complication rates in tissue expander/implant breast reconstruction. Ann Plast Surg 64(5):674–678
Sbitany H, Serletti JM (2011) Acellular dermis-assisted prosthetic breast reconstruction: a systematic and critical review of efficacy and associated morbidity. Plast Reconstr Surg 128(6):1162–1169
McCarthy CM, Lee CN, Halvorson EG, Riedel E, Pusic AL, Mehrara BJ, Disa JJ (2012) The use of acellular dermal matrices in two-stage expander/implant reconstruction: a multicenter, blinded, randomized controlled trial. Plast Reconstr Surg 130(5 Suppl 2):57S–66S
Kim JY, Davila AA, Persing S, Connor CM, Jovanovic B, Khan SA, Fine N, Rawlani V (2012) A meta-analysis of human acellular dermis and submuscular tissue expander breast reconstruction. Plast Reconstr Surg 129(1):28–41
Macadam SA, Lennox PA (2012) Acellular dermal matrices: use in reconstructive and aesthetic breast surgery. Can J Plast Surg 20(2):75–89
Hoppe IC, Yueh JH, Wei CH, Ahuja NK, Patel PP, Datiashvili RO (2011) Complications following expander/implant breast reconstruction utilizing acellular dermal matrix: a systematic review and meta-analysis. Eplasty 11, e40
Preminger BA, McCarthy CM, Hu QY, Mehrara BJ, Disa JJ (2008) The influence of AlloDerm on expander dynamics and complications in the setting of immediate tissue expander/implant reconstruction: a matched-cohort study. Ann Plast Surg 60(5):510–513
de Blacam C, Momoh AO, Colakoglu S, Slavin SA, Tobias AM, Lee BT (2012) Cost analysis of implant-based breast reconstruction with acellular dermal matrix. Ann Plast Surg 69(5):516–520
Krishnan NM, Chatterjee A, Rosenkranz KM, Powell SG, Nigriny JF, Vidal DC (2014) The cost effectiveness of acellular dermal matrix in expander-implant immediate breast reconstruction. J Plast Reconstr Aesthet Surg 67(4):468–476
Bindingnavele V, Gaon M, Ota KS, Kulber DA, Lee DJ (2007) Use of acellular cadaveric dermis and tissue expansion in postmastectomy breast reconstruction. J Plast Reconstr Aesthet Surg 60(11):1214–1218
Breuing KH, Colwell AS (2009) Immediate breast tissue expander-implant reconstruction with inferolateral AlloDerm hammock and postoperative radiation: a preliminary report. Eplasty 15(9), e16
Rawlani V, Buck DW 2nd, Johnson SA, Heyer KS, Kim JY (2011) Tissue expander breast reconstruction using prehydrated human acellular dermis. Ann Plast Surg 66(6):593–597
Spear SL, Parikh PM, Reisin E, Menon NG (2008) Acellular dermis-assisted breast reconstruction. Aesthetic Plast Surg 32(3):418–425
Spear SL, Seruya M, Rao SS, Rottman S, Stolle E, Cohen M, Rose KM, Parikh PM, Nahabedian MY (2012) Two-stage prosthetic breast reconstruction using AlloDerm including outcomes of different timings of radiotherapy. Plast Reconstr Surg 130(1):1–9
Clemens MW, Kronowitz SJ (2012) Acellular dermal matrix in irradiated tissue expander/implant-based breast reconstruction: evidence-based review. Plast Reconstr Surg 130(5 Suppl 2):27S–34S
Weichman KE, Wilson SC, Saadeh PB, Hazen A, Levine JP, Choi M, Karp NS (2013) Sterile “ready-to-use” AlloDerm decreases postoperative infectious complications in patients undergoing immediate implant-based breast reconstruction with acellular dermal matrix. Plast Reconstr Surg 132(4):725–736
Buseman J, Wong L, Kemper P, Hill JL, Nimtz J, Rinker B, Vasconez HC (2013) Comparison of sterile versus nonsterile acellular dermal matrices for breast reconstruction. Ann Plast Surg 70(5):497–499
Venturi ML, Mesbahi AN, Boehmler JH 4th, Marrogi AJ (2013) Evaluating sterile human acellular dermal matrix in immediate expander-based breast reconstruction: a multicenter, prospective, cohort study. Plast Reconstr Surg 131(1):9e–18e
Alderman A, Gutowski K, Ahuja A, Gray D, Postmastectomy Expander Implant Breast Reconstruction Guideline Work Group (2014) ASPS clinical practice guideline summary on breast reconstruction with expanders and implants. Plast Reconstr Surg 134(4):648e–655e
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Razdan, S.N., McCarthy, C.M. (2016). The Use of Acellular Dermal Matrices in Two-Stage Expander/Implant Reconstruction. In: Shiffman, M. (eds) Breast Reconstruction. Springer, Cham. https://doi.org/10.1007/978-3-319-18726-6_102
Download citation
DOI: https://doi.org/10.1007/978-3-319-18726-6_102
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-18725-9
Online ISBN: 978-3-319-18726-6
eBook Packages: MedicineMedicine (R0)