Abstract
Cerebral edema after stroke is associated with poor neurological outcomes. Current therapies are limited to osmotic agents, such as hypertonic saline (HS), which reduce intracranial pressure. Although studies have demonstrated edema reductions following HS, tissue survival has not been thoroughly examined. Additionally, the efficacy of promising pharmacological agents has not been evaluated for synergy with osmotic agents. Conivaptan is an FDA-approved vasopressin receptor antagonist that may exert both osmotic and anti-inflammatory effects. In this study, rats were subjected to middle cerebral artery occlusion prior to treatment with 5 % HS bolus +5 % HS maintenance (HS), conivaptan alone (Con), conivaptan +5 % HS maintenance (Con + HS), or conivaptan +5 % HS bolus +5 % maintenance (Con + HSb). Treatments were initiated at six (Early) or 24 h (Late) following stroke and rats were euthanized at 48 h to evaluate infarct volume, brain edema, and microglia/macrophage activation. Infarct volume and brain edema in the Early HS, Early Con, and Late HS groups were significantly reduced compared with controls. Interestingly, only the Early Con group demonstrated reduced microglia/macrophage activation. These data suggest an anti-inflammatory mechanism for conivaptan and provide support for a multipronged approach combining osmotic agents with compounds that inhibit the neuroinflammatory response to stroke.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Cerebral edema is characterized by pathologic increase in brain water content, one of the most important mechanisms of secondary injury and early mortality after ischemic stroke [29]. Despite the prevalence of this disorder, therapeutic options remain limited. In the case of ischemic stroke, cytotoxic edema is regarded by many to be the predominant injury mechanism in the early phase after stroke [13]. As injured brain tissue swells within the fixed volume of the skull and displaces healthy tissue, intracranial pressure (ICP) rises and cerebral perfusion is further decreased. These events promote additional ischemic events and herniation syndromes. In the clinical setting, the major goal of therapy is to preserve viable tissue by reducing ICP and maintaining cerebral blood flow.
Osmotherapy is the mainstay of treatment for cerebral edema [6]. Hypertonic saline (HS) has many of the properties of an ideal osmotherapeutic agent in that it is nontoxic [7], only slowly permeates or disrupts the blood-brain barrier (BBB) [8], and promotes the maintenance of intravascular volume [15]. Despite increasing use of HS, there is currently no optimized, validated clinical protocol for the treatment of cerebral edema. In current clinical practice, a target serum osmolality of 310–320 mOsm/L is commonly used. However, this target is largely arbitrary and insufficient for full therapeutic effect [6]. A number of recent animal studies examining different dosing strategies have determined that achieving higher serum osmolality levels (≥350 mOsm/l) with infusion of 7.5 % saline results in a robust attenuation of cerebral edema, and is well tolerated [7, 8, 31].
Although HS effectively reduces ICP and cerebral edema [27, 31], the mechanism of action is not fully understood and little is known regarding the net effect on neurodegenerative tissue damage. The benefits of HS are thought to arise from establishment of an osmotic gradient along which water travels from brain tissue into the intravascular compartment [8]. Astrocytic expression of the water channel aquaporin 4 (AQP4) has been shown to be involved in the rate-limiting effect on the bulk flow of water across membranes [36], suggesting that alterations in AQP4 expression and/or function may be linked to the therapeutic benefits of osmotherapy, including HS.
Interestingly, reduction of cerebral edema may not be the sole mechanism by which osmotherapy could promote tissue survival. Clinical data also suggest that HS may attenuate inflammation after brain injury [25]. Microgliosis is one of the most important components of post-stroke neuroinflammation [21], characterized by microglial activation that triggers intracellular signaling pathways. The net effect of these processes is production of cytotoxic and inflammatory mediators that facilitate tissue injury. Inflammation has also been linked to increased BBB permeability at both early and delayed time points after stroke [9], thereby promoting vasogenic edema [33], peripheral immune cell extravasation into brain parenchyma [18], and cytokine/chemokine production/release [14].
Targeting arginine vasopressin (AVP) signaling has been reported to be an effective osmotherapeutic therapy to reduce stroke-induced edema and infarct [7, 10, 17]. Evidence suggests that AVP has pro-inflammatory effects in traumatic brain injury and oxidative stress in the brain following dehydration [12, 30]. Conivaptan is a US Food and Drug Administration (FDA)-approved pan-AVP receptor antagonist and its osmotherapeutic and anti-inflammatory properties suggest a potential treatment for stroke. The present study was conducted to examine the effect of multiple HS and conivaptan administration strategies on brain edema, infarct volume, and neuroinflammation after ischemic stroke.
Methods
Animal Care
All animal procedures were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals with a protocol approved by the Institutional Animal Care and Use Committee at the University of South Florida. Male Sprague-Dawley rats (300–350 g), purchased from Harlan Labs (Indianapolis, IN, USA), were used for the experiments.
Laser Doppler Blood Flow Measurement, Permanent Focal Ischemia, and Jugular Catheter Placement
Blood perfusion in the brain was detected using the Moor Instruments Ltd. Laser Doppler (Devon, England) with MoorLAB proprietary Windows-based software on a standard laptop as previously described [1]. Rats that did not show ≥60 % reduction in perfusion during MCAO were excluded from the study [1].
MCAO surgery was performed using the intraluminal method originally described by Longa et al. [22] and previously reported [1]. An i-STAT handheld clinical analyzer was used to measure physiological blood parameters including hemoglobin, hematocrit, ionized calcium, glucose, sodium, potassium, pH, pCO2, HCO3, TCO2, base excess, pO2, and O2. No differences in these were found between groups of rats used in the treatment studies.
Following MCAO, the right jugular vein was exposed. Using forceps, two pieces of equal length of suture were passed under the jugular vein. The more distal suture was tied tightly to occlude blood flow from the head region. A small cut was made into the jugular vein between the two ligatures. A catheter filled with saline was inserted into the vein and threaded 0.5 cm toward the heart. The ligature closest to the heart was tightened around the vein and catheter to prevent dislodgement. The ends of the distal ligature were tied between the cuffs on the catheter for additional anchorage. After confirming patency, the catheter was tunneled subcutaneously and exteriorized at the nape of the neck. This incision was closed with a staple. The catheter was filled with lock solution (saline:glycerol 50:50 ratio). The neck was sutured closed and the rat was allowed to wake in a fresh cage. Following recovery, animals were randomly assigned into treatment groups.
Treatment Regimens
A series of injections were given intravenous (IV; jugular catheter) at 6 and 9 h post-MCAO, then every 4 h starting at 8:00 AM until 8:00 PM on the first day postoperatively, and a single 8:00 AM injection the second day postoperatively for a total of seven injections for every rat. Two treatment paradigms were used with treatment starting at either 6 h (Early) or 24 h (Late) post-stroke. The five treatment groups were normal saline (0.9 %, NS, control), 5 % saline infusion +5 % saline maintenance (HS), conivaptan only (Con), conivaptan +5 % saline maintenance (Con + HS), and conivaptan +5 % saline bolus +5 % saline maintenance (Con + HSb). The experimental dosages were chosen based on a weight-based extrapolation of typical human dosages. The initial dose of conivaptan was 0.35 mg/kg (0.5 ml of a 0.2 mg/ml stock) with follow-up doses of 0.7 mg/kg (0.1 ml). Treatment schedules for each group are summarized (Table 1).
Tissue Preparation and Fluoro-Jade Histochemistry
Animals were euthanized at 48 h post-MCAO and perfused with 0.9 % saline followed by 4 % paraformaldehyde in phosphate buffer (pH 7.4). The brains were harvested and processed as previously described [1]. Sections were collected from six bregma points, ranging from 1.7 to −2.3 mm. For determination of infarct volume, Fluoro-Jade (Histochem, Jefferson, AR, USA) staining was performed to label degenerating neurons. This method was adapted from that originally developed by Schmued et al. [26] and has been subsequently detailed [11].
Immunohistochemistry
Immunohistochemistry was performed to detect activated microglia [12]. Sections were incubated overnight at 4 °C with mouse anti-rat CD11b antibody (Ox-42; 1:3,000; Serotec, Raleigh, NC, USA) in PBS with 2 % goat serum, 0.3 % Triton X-100. The following day, slides were washed with PBS and incubated 1 h at room temperature with horse anti-mouse secondary antibody (1:300; Vector Laboratories Inc., Burlingame, CA, USA) in antibody solution (2 % serum, 0.3 % Triton X-100 in PBS). Slides were then washed with PBS and incubated in Avidin-Biotin Complex (ABC; Vector Laboratories Inc.) mixture for 1 h. Next the slides were washed and then visualized using a DAB/peroxide solution (Vector Laboratories Inc.).
Image Analyses
Images of the brain sections were taken with a 1× objective (for Fluoro-Jade and brain edema) on a Zeiss Axioskop2 microscope (Carl Zeiss Inc., Thornwood, NY, USA) using Open Lab software (Improvision Ltd., Lexington, MA, USA). Hemispheric areas were measured using Image J software (National Institutes of Health). Photomicrographs were processed using Jasc Paint Shop Pro to sharpen and enhance contrast to the same specifications for all slides.
Fluoro-Jade stained slides were used to calculate infarct volume. Total area of positive staining was measured in the ipsilateral and contralateral hemispheres for each section. The area values for six representative bregma points throughout the infarct were totaled for each animal, and infarct volumes were calculated as the percent of the contralateral hemisphere.
For edema, thaw-mounted slides were air-dried overnight before imaging. The area of each hemisphere was measured and the ventricular areas were subtracted from the hemispheric area. The total adjusted area for each hemisphere was determined across all six bregma points, and edema for each animal was calculated as a percentage of the contralateral hemisphere.
For CD11b immunohistochemistry, images were taken with a 10× objective. Three regions of the striatum were imaged (upper right, middle left, and lower center) for the first four bregma points collected (1.7 to −1.3). The area fraction of CD11b-positive staining was calculated for each image, and data are expressed as the average area fraction for each group.
Statistical Analyses
Data from all experiments were quantified and analyzed using GraphPad Prism 6.0 (GraphPad, La Jolla, CA, USA) software. For all statistical tests, the threshold for significant differences between groups was set at p < 0.05. For behavioral tests, significant effects of treatment were determined using unpaired, two-tailed t-tests with Welch’s correction. For all other data, main effects were determined using one-way or two-way analysis of variance (ANOVA) followed by Fisher’s least squares difference test for pairwise comparisons between treatment groups.
Results
Infarct Volume
Infarct volume was measured with Fluoro-Jade staining to determine the efficacy of HS and conivaptan, both alone and in combination, when administered either 6 h or 24 h following MCAO. Total area occupied by staining was calculated for each animal and was expressed as a percentage of Fluoro-Jade positive area in the ischemic hemisphere relative to the contralateral hemisphere.
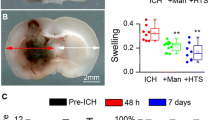
Both HS and conivaptan elicited protection when administered 6 h after MCAO (Fig. 1). NS controls showed large expanses of Fluoro-Jade throughout the striatum and adjacent cerebral cortex (Fig. 1a), whereas staining appeared markedly reduced in sections from animals treated with HS or Con (Fig. 1b, c). Quantification of total infarct volume (Fig. 1d) showed significant reductions in the 6 h HS (p = 0.0411, t = 1.825) and Con (p = 0.050, t = 1.678) relative to NS controls, whereas there were no significant differences in the combined Con + HS (p = 0.0922, t = 1.370) or Con + HSb groups (p = 0.3296, t = 0.4489) compared to NS controls.
(a–c) Representative micrographs of Fluoro-Jade stained sections from rats treated with NS, HS, or Con 6 h after MCAO. Fluoro-Jade staining was abundant throughout the striatum and cerebral cortex after treatment with NS (a), whereas staining was markedly reduced in sections from rats treated with HS (b) and Con (c). Quantification of infarct volume (d) showed significant reductions in rats treated with HS or Con (p < 0.05), whereas there was no significant difference between NS controls and rats that received Con + HS or Con + HSb. *p < 0.05, n = 4−6 per group. Scale bars = 2 mm. NS, normal (0.9 %) saline. HS, hypertonic (5 %) saline. Con, conivaptan. Con + HS, conivaptan + hypertonic (5 %) saline maintenance. Con + HSb, conivaptan/hypertonic (5 %) saline bolus + hypertonic (5 %) saline maintenance. Quantification of infarct volume in rats treated with NS, Con, Con + HS, or Con + HSb 24 h after MCAO (e). Data show a significant reduction in infarct volume following treatment with HS relative to NS controls (p < 0.05), whereas there were no significant differences after treatment with Con. Combined treatments produced no protective effect, as the Con + HS and Con + HSb groups showed no significant differences relative to NS controls. *p < 0.05, n = 4–6 per group. NS, normal (0.9 %) saline. HS, hypertonic (5 %) saline. Con, conivaptan. Con + HS, conivaptan + hypertonic (5 %) saline maintenance. Con + HSb, conivaptan/hypertonic (5 %) saline bolus + conivaptan/hypertonic (5 %) saline maintenance
Interestingly, the results from the 24 h post-treatment groups were not entirely consistent with those obtained from the 6 h groups (Fig. 2). Similar to the 6 h treatments, animals that received HS 24 h after MCAO showed significant reductions in infarct volume (p = 0.0207, t = 2.217) relative to NS controls, while there were no significant differences from controls in the combined Con + HS (p = 0.2722, t = 0.6204) or Con + HSb (p = 0.4399, t = 0.1532) groups. However, unlike the 6 h treatment, animals that received Con 24 h after MCAO showed no significant difference in infarct volume (p = 0.3753, t = 0.3235) compared with NS controls.
(a–d) Representative micrographs of CD11b immunohistochemistry in sections from rats treated with HS or Con 6 h after MCAO, or HS 24 h after MCAO. Immunohistochemical staining shows a profile of ubiquitous CD11b-positive cells within the striatum of an NS control (a) that resembles sections from rats treated with HS (b, d). Treatment with Con 6 h after MCAO resulted in reduced numbers of CD11b-positive cells (c). Quantification revealed a significant reduction in the area occupied by CD11b staining in the 6 h Con group (p < 0.05) relative to NS controls and HS groups. *p < 0.05, n = 4–6 per group (e). Quantification of brain swelling in rats treated with HS or Con 6 h after MCAO, or HS 24 h after MCAO (f). Brain swelling was calculated in groups that showed efficacy in reducing Fluoro-Jade staining to assess whether edema might account for the observed effects. Data show significantly reduced brain edema in 6 h HS, 6 h Con and 24 h HS rats relative to NS controls (p < 0.05), thus establishing a relationship between neurodegenerative injury and brain swelling. *p < 0.05, n = 4–6 per group. NS, normal (0.9 %) saline. HS 6 h, hypertonic (5 %) saline administered 6 h after MCAO. Con 6 h, conivaptan administered 6 h after MCAO. HS 24 h, hypertonic (5 %) saline administered 24 h after MCAO. Scale bars = 50 μm. NS, normal (0.9 %) saline. HS 6 h, hypertonic (5 %) saline administered 6 h after MCAO. Con 6 h, conivaptan administered 6 h after MCAO. HS 24 h, hypertonic (5 %) saline administered 24 h after MCAO
Microglia/Macrophage Activation
Adjacent sections were immunostained for the microglia/macrophage cell surface marker CD11b to gain insight into the anti-inflammatory effects of HS and Con (Fig. 2). Cells labeled with CD11b were abundant in sections from NS controls, particularly throughout the striatum (Fig. 2a) but also within the cortical tissue. Sections from animals treated with HS also displayed ubiquitous staining for CD11b (Fig. 2b, d) that resembled those of NS controls. However, sections from animals treated with Con 6 h after MCAO showed far fewer numbers of CD11b – positive cells, particularly within the striatum (Fig. 2c). Quantification (Fig. 2e) showed a significant reduction in the area occupied by CD11b staining for the 6 h Con group relative to NS (p = 0.0455, t = 1.786), 6 h HS (p = 0.0091, t = 2.651) and 24 h HS (p = 0.0031, t = 3.450). There were no significant differences between the NS controls and 6 h HS (p = 0.3231, t = 0.4659) or 24 h HS (p = 0.4872, t = 0.03247) groups.
Brain Swelling
Based on initial results, brain swelling was calculated for the treatment groups that showed reduced infarct volume to determine whether the protective doses of HS and Con also reduced brain edema (Fig. 2f). Brain edema in the ipsilateral hemisphere was measured by image analysis and expressed as a percentage of the contralateral hemisphere. Consistent with the Fluoro-Jade staining, animals that were protected from neurodegenerative injury also showed less brain edema. HS significantly attenuated brain swelling when administered either 6 h (p = 0.0015, t = 3.434) or 24 h (p = 0.0278, t = 2.102) after MCAO compared with NS controls, and Con significantly reduced brain swelling when administered 6 h following MCAO (p = 0.0221, t = 2.197).
Discussion
The present study evaluated the potential of two therapeutic strategies, both alone and in combination, to improve histological and functional outcomes when administered at clinically relevant time points after stroke onset. Although osmotherapy has been shown to be effective in reducing ICP and brain edema [6], studies that assess the relationship between these outcomes and neurodegenerative injury provide varied results [5, 24]. The 6 h HS and conivaptan groups showed significant reductions in both infarct volume and brain edema relative to controls, linking the effects of limiting edema to reduced cellular injury. Furthermore, HS significantly reduced edema even when administered 24 h after stroke onset. These data highlight the importance of controlling edema throughout the period of delayed infarct expansion [13] and establish a connection between brain swelling, neurodegenerative injury, and survival.
The mechanism of action of HS is not fully understood, but HS is believed to work primarily by establishment of an osmotic gradient along which water travels from brain tissue into the intravascular compartment through an intact BBB [8]. While cellular membranes are freely permeable to water, diffusion through membranes is relatively slow. For water in the capillary lumen to enter the astrocytic compartment in the brain, it must pass through three plasma membranes (luminal endothelial, abluminal endothelial, and luminal perivascular). Aquaporin channels allow cells to modulate the rapid bulk flow of water across cell membranes. AQP4 is the most abundant water channel in the brain. This channel has been implicated in the pathogenesis of cerebral edema, and also been shown to be a rate-limiter for water influx during edema formation [2, 34] and water efflux in the setting of an osmotic gradient created by hypertonic saline [37]. Expression levels of AQP4 also correlate with brain water content in untreated animals after ischemic stroke [35]. In ischemic stroke studies using transgenic mice, the deletion of AQP4 was shown to impair water uptake by ischemic cells and led to increased survival with improved neurologic outcomes [23]. This is consistent with other studies demonstrating the neuroprotective effects of limiting edema [8].
In addition to the effect of osmolar gradients on the development of cerebral edema, several lines of evidence suggest that AVP plays an important and complementary role. Elevated AVP levels have been demonstrated after ischemic stroke and other forms of brain injury [3, 7, 17]. Intracerebroventricular injections of AVP exacerbated acute ischemic brain edema, whereas injection of AVP antiserum significantly decreased cerebral edema [16]. Cerebral edema after ischemic stroke was also attenuated in vasopressin-deficient rats [10], while pharmacological AVP receptor inhibitors attenuated postischemic cerebral edema [4, 17, 19, 20, 28]. Indeed, a study by Chang et al. [4] demonstrated that treatment with HS led to a reduction in plasma AVP levels following experimental stroke in rats, which supports the idea that HS might have limited edema and infarction here through modulation of AVP.
It has been demonstrated that receptor antagonism of V1a, but not V2, reduced infarct volume and brain water content while increasing AQP4 expression following experimental stroke [28]. Conivaptan is a V1 and V2 receptor antagonist [32] that is currently FDA-approved for the treatment of hyponatremia. Based on this mechanism of action, conivaptan would be expected to increase serum sodium and osmolality, but may also reduce cerebral edema by accentuating AQP4 expression. This potential effect has not yet been investigated. However, the fact that conivaptan was ineffective in reducing infarct volume or edema when administered 24 h after MCAO suggests that its mechanism of action is likely distinct, at least in part, from that of HS. Notably, the 6 h conivaptan treatment also decreased microglial activation, which is implicated in delayed expansion of the infarct, while initiation of conivaptan treatment at 24 h did not alter microglial activation/recruitment. Vasopressin has been shown to exert pro-inflammatory effects in a model of traumatic brain injury [30], suggesting that conivaptan is blunting the stroke-induced neuroinflammatory response through vasopressin receptor antagonism. This finding suggests a potential additional mechanism to explain the beneficial effects of conivaptan on survival, tissue injury, and functional recovery that is independent of its role in fluid homeostasis.
Our results showed a positive result from HS or conivaptan alone but did not show a synergistic effect between HS and conivaptan. This suggests that conivaptan should be explored as a potentially useful alternative osmotherapeutic agent. The pharmacologic properties of conivaptan, that is, increasing serum sodium while reducing blood volume, may be particularly useful for treating patients who cannot tolerate the additional volume load associated with HS, such as those with congestive heart failure. In addition, the novel finding of reduced microglial activation offers a new potential mechanism to be exploited as means of improving outcomes after ischemic stroke.
References
Ajmo CT Jr, Vernon DO, Collier L, Hall AA, Garbuzova-Davis S, Willing A, Pennypacker KR (2008) The spleen contributes to stroke-induced neurodegeneration. J Neurosci Res 86:2227–2234
Amiry-Moghaddam M, Ottersen OP (2003) The molecular basis of water transport in the brain. Nat Rev Neurosci 4:991–1001
Barreca T, Gandolfo C, Corsini G, Del Sette M, Cataldi A, Rolandi E, Franceschini R (2001) Evaluation of the secretory pattern of plasma arginine vasopressin in stroke patients. Cerebrovasc Dis 11:113–118
Bemana I, Nagao S (1999) Treatment of brain edema with a nonpeptide arginine vasopressin V1 receptor antagonist OPC-21268 in rats. Neurosurgery 44:148–154, discussion 154–145
Bhardwaj A, Harukuni I, Murphy SJ, Alkayed NJ, Crain BJ, Koehler RC, Hurn PD, Traystman RJ (2000) Hypertonic saline worsens infarct volume after transient focal ischemia in rats. Stroke 31:1694–1701
Bhardwaj A, Ulatowski JA (2004) Hypertonic saline solutions in brain injury. Curr Opin Crit Care 10:126–131
Chang Y, Chen TY, Chen CH, Crain BJ, Toung TJ, Bhardwaj A (2006) Plasma arginine-vasopressin following experimental stroke: effect of osmotherapy. J Appl Physiol 100:1445–1451
Chen CH, Toung TJ, Sapirstein A, Bhardwaj A (2006) Effect of duration of osmotherapy on blood–brain barrier disruption and regional cerebral edema after experimental stroke. J Cereb Blood Flow Metab 26:951–958
Danton GH, Dietrich WD (2003) Inflammatory mechanisms after ischemia and stroke. J Neuropathol Exp Neurol 62:127–136
Dickinson LD, Betz AL (1992) Attenuated development of ischemic brain edema in vasopressin-deficient rats. J Cereb Blood Flow Metab 12:681–690
Duckworth EA, Butler TL, De Mesquita D, Collier SN, Collier L, Pennypacker KR (2005) Temporary focal ischemia in the mouse: technical aspects and patterns of Fluoro-Jade evident neurodegeneration. Brain Res 1042:29–36
Faraco G, Wijasa TS, Park L, Moore J, Anrather J, Iadecola C (2014) Water deprivation induces neurovascular and cognitive dysfunction through vasopressin-induced oxidative stress. J Cereb Blood Flow Metab 34(5):852–860
Forsyth LL, Liu-DeRyke X, Parker D Jr, Rhoney DH (2008) Role of hypertonic saline for the management of intracranial hypertension after stroke and traumatic brain injury. Pharmacotherapy 28:469–484
Hanisch UK, Kettenmann H (2007) Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci 10:1387–1394
Harukuni I, Kirsch JR, Bhardwaj A (2002) Cerebral resuscitation: role of osmotherapy. J Anesth 16:229–237
Hertz L, Chen Y, Spatz M (2000) Involvement of non-neuronal brain cells in AVP-mediated regulation of water space at the cellular, organ, and whole-body level. J Neurosci Res 62:480–490
Ikeda Y, Toda S, Kawamoto T, Teramoto A (1997) Arginine vasopressin release inhibitor RU51599 attenuates brain oedema following transient forebrain ischaemia in rats. Acta Neurochir (Wien) 139:1166–1171, discussion 1171–1162
Jander S, Kraemer M, Schroeter M, Witte OW, Stoll G (1995) Lymphocytic infiltration and expression of intercellular adhesion molecule-1 in photochemically induced ischemia of the rat cortex. J Cereb Blood Flow Metab 15:42–51
Kagawa M, Nagao S, Bemana I (1996) Arginine vasopressin receptor antagonists for treatment of vasogenic brain edema: an experimental study. J Neurotrauma 13:273–279
Laszlo FA, Varga C, Nakamura S (1999) Vasopressin receptor antagonist OPC-31260 prevents cerebral oedema after subarachnoid haemorrhage. Eur J Pharmacol 364:115–122
Leonardo CC, Hall AA, Collier LA, Ajmo CTJ, Willing AE, Pennypacker KR (2010) Human umbilical cord blood cell therapy blocks the morphological change and recruitment of CD-11b-expressing isolectin-binding proinflammatory cells after middle cerebral artery occlusion. J Neurosci Res 88:1213–1222
Longa E, Weinstein P, Carlson S, Cummins R (1989) Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 20:84–91
Manley GT, Fujimura M, Ma T, Noshita N, Filiz F, Bollen AW, Chan P, Verkman AS (2000) Aquaporin-4 deletion in mice reduces brain edema after acute water intoxication and ischemic stroke. Nat Med 6:159–163
Papangelou A, Toung TJ, Gottschalk A, Mirski MA, Koehler RC (2013) Infarct volume after hyperacute infusion of hypertonic saline in a rat model of acute embolic stroke. Neurocrit Care 18:106–114
Rhind SG, Crnko NT, Baker AJ, Morrison LJ, Shek PN, Scarpelini S, Rizoli SB (2010) Prehospital resuscitation with hypertonic saline-dextran modulates inflammatory, coagulation and endothelial activation marker profiles in severe traumatic brain injured patients. J Neuroinflammation 7:5
Schmued LC, Albertson C, Slikker W Jr (1997) Fluoro-Jade: a novel fluorochrome for the sensitive and reliable histochemical localization of neuronal degeneration. Brain Res 751:37–46
Schwarz S, Georgiadis D, Aschoff A, Schwab S (2002) Effects of hypertonic (10%) saline in patients with raised intracranial pressure after stroke. Stroke 33:136–140
Shuaib A, Xu Wang C, Yang T, Noor R (2002) Effects of nonpeptide V(1) vasopressin receptor antagonist SR-49059 on infarction volume and recovery of function in a focal embolic stroke model. Stroke 33:3033–3037
Silver FL, Norris JW, Lewis AJ, Hachinski VC (1984) Early mortality following stroke: a prospective review. Stroke 15:492–496
Szmydynger-Chodobska J, Fox LM, Lynch KM, Zink BJ, Chodobski A (2010) Vasopressin amplifies the production of proinflammatory mediators in traumatic brain injury. J Neurotrauma 27:1449–1461
Toung TJ, Chang Y, Lin J, Bhardwaj A (2005) Increases in lung and brain water following experimental stroke: effect of mannitol and hypertonic saline. Crit Care Med 33:203–208, discussion 259–260
Udelson JE, Smith WB, Hendrix GH, Painchaud CA, Ghazzi M, Thomas I, Ghali JK, Selaru P, Chanoine F, Pressler ML, Konstam MA (2001) Acute hemodynamic effects of conivaptan, a dual V(1A) and V(2) vasopressin receptor antagonist, in patients with advanced heart failure. Circulation 104:2417–2423
Uenohara H, Imaizumi S, Yoshimoto T, Suzuki J (1988) Correlation among lipid peroxidation, brain energy metabolism and brain oedema in cerebral ischaemia. Neurol Res 10:194–199
Vajda Z, Pedersen M, Fuchtbauer EM, Wertz K, Stodkilde-Jorgensen H, Sulyok E, Doczi T, Neely JD, Agre P, Frokiaer J, Nielsen S (2002) Delayed onset of brain edema and mislocalization of aquaporin-4 in dystrophin-null transgenic mice. Proc Natl Acad Sci U S A 99:13131–13136
Yang M, Gao F, Liu H, Yu WH, Sun SQ (2009) Temporal changes in expression of aquaporin-3, -4, -5 and -8 in rat brains after permanent focal cerebral ischemia. Brain Res 1290:121–132
Zeynalov E, Chen CH, Froehner SC, Adams ME, Ottersen OP, Amiry-Moghaddam M, Bhardwaj A (2008) The perivascular pool of aquaporin-4 mediates the effect of osmotherapy in postischemic cerebral edema. Crit Care Med 36:2634–2640
Ziai WC, Toung TJ, Bhardwaj A (2007) Hypertonic saline: first-line therapy for cerebral edema? J Neurol Sci 261:157–166
Acknowledgments
This work was supported by the University of South Florida Neuroscience Collaborative.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Decker, D. et al. (2016). The Effects of Clinically Relevant Hypertonic Saline and Conivaptan Administration on Ischemic Stroke. In: Applegate, R., Chen, G., Feng, H., Zhang, J. (eds) Brain Edema XVI. Acta Neurochirurgica Supplement, vol 121. Springer, Cham. https://doi.org/10.1007/978-3-319-18497-5_43
Download citation
DOI: https://doi.org/10.1007/978-3-319-18497-5_43
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-18496-8
Online ISBN: 978-3-319-18497-5
eBook Packages: MedicineMedicine (R0)