Abstract
MD registries—collections of secondary data related to patients with one of the nine types of muscular dystrophy—can vary in sophistication from simple spreadsheets accessible only by a small group of physicians, to complex databases accessed online across multiple institutions. Registries can help identify MD patients for scientific research, clinical trials, and later, as products/drugs are approved for the treatment of MD, in the post-marketing surveillance of pharmaceuticals. Registries can also provide healthcare providers or patients with reminders of the need to undergo certain tests in order to reach quality goals. At present, many registries cover only one geographic area or one or two types of MD. The Muscular Dystrophy Association (MDA) is attempting to remedy this disparity with two initiatives. First it is seeking participation from patients with certain myopathies in a patient registry and world map. This database is designed to allow researchers to better understand certain diseases and locate participants for clinical trials and other research studies. Second, to address the lack of a fully operational central registry database for patients with MD, the MDA and Quintiles, a biopharmaceutical services company, formed a partnership in October 2013 to develop and implement the US, Neuromuscular Disease Registry.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Amyotrophic Lateral Sclerosis
- Muscular Dystrophy
- Spinal Muscular Atrophy
- Patient Registry
- Neuromuscular Disease
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
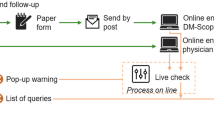
An muscular dystrophy (MD) patient registry is a collection of secondary data related to patients (and therefore may include family members) with one of the nine types of MD. Registries can vary in sophistication from simple MS Excel spreadsheets that can be accessed only by a small group of physicians, to very complex databases that are accessed online across multiple institutions.
Due to the small numbers of patients with MD, it is important to identify patients quickly in order to share rapidly evolving scientific advances with them, advocate for them, and provide opportunities to advance our scientific knowledge about each type of MD so that, ultimately, a cure can be found.
Registries can play multiple roles including identifying MD patients for scientific research, clinical trials, and later, as products/drugs are approved for the treatment of MD, in the post-marketing surveillance of pharmaceuticals. Registries can also provide healthcare providers or patients with reminders of the need to undergo certain tests in order to reach quality goals.
Registries are less complex and simpler to set up than an electronic medical record, which keeps track of all the patients a doctor follows, while a registry only keeps track of a small subpopulation of patients with a specific condition.
Currently, many registries are only offered in one geographic area or for just one or two types of MD. The Muscular Dystrophy Association (MDA), the largest MD patient advocacy group in the world, recognizing how difficult it is to identify and find patients to study treatments for MD, is attempting to remedy this disparity and has posted two important news items applicable to patients afflicted with MD [1]:
-
1.
The first, and most recent news item, is the solicitation for a patient registry and world map of people with certain types of myopathies (e.g., centronuclear [CNM]/myotubular myopathy [MTM]), which are being developed and are seeking participation from people with these disorders or their family members. This database is designed to allow researchers to better understand certain diseases and locate participants for clinical trials and other research studies.
-
The registry site will initially make possible the study of the natural history of each disease, which is the first step towards understanding the progression or course of the disease. This is especially important when determining if a new treatment or therapy has the potential to alter or stop disease progression (as compared with using a placebo or sugar pill) in a clinical study. Natural history is defined by the National Cancer Institute [2] as “a study that follows a group of people over time who have, or are at risk of developing, a specific medical condition or disease. A study that collects health information to understand how the medical condition or disease develops and how to treat it.” An alternative is the definition provided by Posada and Groft [3]: “The natural course of a disease from the time immediately prior to its inception, progressing through its presymptomatic phase and different clinical stages to the point where it has ended and the patient is either cured, chronically disabled or dead without external intervention.”
-
Given the potential for this information to be misused (e.g., individuals being targeted by insurance companies), patient privacy is protected and de-identified information will be shared only with “selected members of the research community” and a Scientific Advisory Board.
-
Registrants will receive email updates on research progress and be notified of trial participation opportunities. A de-identified “pin” will be added to the global map after a participant has given his or her approval for the posting.
-
“This information is crucial for helping us to understand the demographics of our community,” says the foundation’s website. “If you know of anyone affected with CNM/MTM, please direct them to this website and ask them to register.”
-
-
2.
Currently, the lack of a fully operational central registry database for patients with MD is problematic. With so many smaller registries scattered across the U.S., it is difficult for researchers to find enough patients to fulfill enrollment requirements for a proposed MD clinical study. Therefore, to address this issue, Quintiles, a biopharmaceutical services company, and the MDA announced a new partnership in October 2013 [4], to develop and implement the U.S. Neuromuscular Disease Registry, a patient registry that will play an important role in determining effective treatments for people with MD and related muscle diseases.
-
According to MDA’s Website, “We are making remarkable progress in researching new lifesaving treatments and cures for neuromuscular diseases as we move from bench to bedside in clinical trials,” said MDA Executive Vice President & Chief Medical and Scientific Officer Valerie Cwik, M.D. “We are committed to changing and saving the lives of the individuals and families we serve, and the U.S. Neuromuscular Disease Registry brings us one step closer to answering critical clinical and research questions that will improve quality of care.”
-
Quintiles was awarded the project based on its depth of experience in post-marketing research, multistakeholder strategy, and systems-oriented approach to registry design and development [3]. “Patient registries are an increasingly important component of real-world evidence development for understanding the cause of disease and identifying effective treatments,” said Richard Gliklich, M.D., then president of Quintiles Real World & Late Phase Research. “In designing the U.S. Neuromuscular Disease Registry, our goal is to create a research and collaboration platform that will enable physicians, patients, caregivers and others involved in MDA’s mission to collaborate to advance new treatments for patients.”
-
MDA will use the registry to study the natural history of MD and related muscle diseases, collect information on practice patterns, inform care guidelines, and improve quality of care for patients. The registry is currently available at 25 medical clinics within the organization’s national network, with plans to expand to their full network of 200 clinics. It will gather data in a common format across neuromuscular diseases, starting with amyotrophic lateral sclerosis (ALS), Becker muscular dystrophy/Duchene muscular dystrophy (BMD/DMD), and spinal muscular atrophy (SMA), with plans to collect data on three other neuromuscular diseases within three years.
-
Other registries are available throughout the world and may be specific for certain types of MD [5–7]. These registries can help patients with MD network with others who have the same disease or have a special interest in developing therapies for patients afflicted with a certain type of MD (e.g., caregivers, physicians, and research scientists).
Take, for example, The Myotubular and Centronuclear Myopathy Patient Registry (also referred to as “MTM and CNM Registry”), which is an international database managed from the UK and operated by the Myotubular Trust. According to their Website, the registry was developed in partnership with TREAT-NMD (Neuro Muscular Network) and with a number of leading neuromuscular researchers and plans to:
-
Help identify patients for relevant clinical trials as they become available
-
Encourage further research into MTM and CNM
-
Provide researchers with specific patient information to support their research
-
Assist doctors and other health professionals by providing them with up-to-date information on managing MTM and CNM, to help them deliver better standards of care for their patients.
-
The requirements for registration include:
-
All patients, with a MTM or CNM diagnosis, which has been confirmed via genetic testing or muscle biopsy.
-
Any carrier females of x-linked MTM, especially if they have manifested MTM type symptoms.
-
Any patient who is deceased, but who had a confirmed diagnosis.
-
Any patient who wishes to receive information only.
-
-
Their aim is, in part, to “get a good insight into the numbers of people affected.”
Another example is a patient registry for myotonic dystrophy (DM) and FSHD which was started in the U.S. as part of a National Institutes of Health (NIH) grant (e.g., NIH contract # N01-AR-02250). Starting in 2001, the project established a national research registry for people with the diseases and their families. The registry—established by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) and the National Institute of Neurological Disorders and Stroke (NINDS), both parts of the NIH—is based at the University of Rochester (Rochester, NY).
Registry scientists sought out and classified patients with clinically diagnosed forms of myotonic dystrophy (DM) and FSHD and stored their medical and family history data. The registry is a central information source where researchers can obtain data for analysis associated with these diseases.
The registry’s scientific advisory committee made recommendations about enrollment criteria, monitored and improved ways to recruit patients and investigators, and assessed progress. It also revised and extended methods for collecting and handling data and determined possible clinical studies.
NIAMS Director Stephen I. Katz, M.D., Ph.D., said, “This national registry will be an important resource to provide hope to families and encourage scientists in finding a cure for these two disabling diseases. It will also hasten the course of research for more in-depth answers to what happens in muscular dystrophy.”
Richard Moxley III, M.D., was the lead investigator for the registry. “Research has uncovered recent clues to genetic, chromosomal and DNA errors in those with DM and FSHD,” he said. “I am pleased to lead scientists in collecting and analyzing new research data for better treatments for these two diseases.”
DM and FSHD are two of the nine types of MD. They can be detected through testing at birth and may be passed from one generation to the next. Both cause progressive, disabling weakness. In addition, DM sometimes results in sudden death.
Similar to the MDA in the U.S., the EU has important groups dedicated to helping those afflicted with MD, such as Treat-NMD. This group’s Web site posts its mission statement as follows:
-
TREAT-NMD is a network for the neuromuscular field that provides an infrastructure to ensure that the most promising new therapies reach patients as quickly as possible. Since its launch in January 2007 the network’s focus has been on the development of tools that industry, clinicians and scientists need to bring novel therapeutic approaches through preclinical development and into the clinic, and on establishing best-practice care for neuromuscular patients worldwide.
The Web site also includes a useful section on patient registries that demonstrates the fundamental thinking—and potential scientific utility—of any registry:
-
A patient registry collects information about patients who are affected by a particular condition.
-
When planning a clinical trial, it is very important that eligible patients can be found and contacted quickly. The best way for this to happen is through a database or “registry” that contains all the information that researchers will need. Patients’ clinical and genetic details are collected and made easily available for the researchers.
-
Scientific advances over recent years have led to substantial changes in the treatment of many neuromuscular diseases. Several new therapeutic strategies which target specific genetic defects are being developed. For some of these treatments, plans are already in place for large studies involving patients from more than one country.
-
Any potential new treatment needs to be tested under strictly controlled circumstances to make sure that not only are participants safe but, that any notable changes can be attributed to the treatment and not to external factors. This allows researchers to ensure that all potential new therapies are both safe and effective.
-
Due to the nature of rare diseases, scientific approaches differ from those used for common diseases. Finding enough patients who might be eligible to participate in trials for rare neuromuscular conditions can take years without a patient registry, delaying the testing of potential therapies.
Because there are multiple registries, it can be confusing for both the newly diagnosed patient and the caregiver. In general, for those who are unfamiliar with a certain type of MD, most patient advocacy groups provide links and post information for patients and their caregivers [8]. For example, the FSH Society Website has a “For Patients” link that lists how FSHD researchers and clinical trials are connected through their own list as well as their connection to the NIH. According to their Website:
-
A disease registry or patient registry is a database of information on patients with a particular disease, such as FSHD, that can be accessed and used by researchers, clinicians, and physicians interested in working on the disease. Registries are especially valuable in diseases like FSHD where access to patients and materials is limited. The collected information contained within the registry is used to increase the understanding of FSHD by allowing doctors, clinicians, and researchers to access patients and biomaterials. Many more research projects and avenues of investigation will result from FSHD patients and their families signing up and becoming involved!
-
There are several disease research or patient registries available for FSHD in the U.S.:
-
The FSH Society maintains a FSHD registry of patients and families wishing to become involved in research.
-
The NIH funds the National Registry of Myotonic Dystrophy and Facioscapulohumeral Muscular Dystrophy Patients and Family Members. The National Registry helps individuals and families with FSHD participate in research on their disease. It helps investigators accomplish their research by connecting them with people who have FSHD and it acts as a resource to facilitate more research on FSHD.
-
Summary
Due to the small numbers of patients with MD, it is important for patients with MD (and their caregivers) to be aware of patient and disease registries in order to access the latest scientific information and be provided with the opportunity to become part of scientific investigations. Registries can be a good way to gain access to clinical studies which, after a positive risk/benefit assessment is made by the patient and the patient’s caregivers, provide opportunities to obtain disease modifying, or possibly even curative, therapies.
For patients living in the U.S., contacting the MDA is the logical first step to find a registry for a particular type of MD. Other smaller patient advocacy groups, such as the FSH Society, may offer more patient-specific details, as they are sometimes staffed or founded by individuals afflicted by the disease.
References
MDA, Quest. http://quest.mda.org/news/cnm-mtm-registry-world-map-seek-participants. Accessed 9 Nov 2014.
National Cancer Institute, August 2014.
Posada de la Paz M, Groft SC, editors. Rare diseases epidemiology, Vol. 6862010, XXII, 542; 2010.
Quintiles selected by Muscular Dystrophy Association to develop US Disease Registry. [news release]. Quintiles Media Relations. 8 Oct 2013. http://www.quintiles.com/library/press-releases/quintiles-selected-by-muscular-dystrophy-association-to-develop-u-s-disease-registry/. Accessed 25 Oct 2013.
The Myotubular and Centronuclear Myopathy Registry. http://www.mtmcnmregistry.org/. Accessed 22 Nov 2013.
National Registry Established for Two Muscular Dystrophy Types [news release]. National Institute of Arthritis and Musculoskeletal and Skin Diseases Office of Communications. 11 Dec 2000. http://www.niams.nih.gov/News_and_Events/Press_Releases/2000/12_11.asp. Accessed 22 Nov 2013.
Muscular Dystrophy Campaign Registry (UK). http://www.treat-nmd.eu/. Accessed 22 Nov 2013.
FSH Society. For patients. http://www.fshsociety.org/pages/patPatReg.html. Accessed 10 Nov 2014.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Huml, R.A. (2015). Global and National Patient Registries. In: Huml, R. (eds) Muscular Dystrophy. Springer, Cham. https://doi.org/10.1007/978-3-319-17362-7_14
Download citation
DOI: https://doi.org/10.1007/978-3-319-17362-7_14
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-17361-0
Online ISBN: 978-3-319-17362-7
eBook Packages: MedicineMedicine (R0)