Abstract
Intussusception represents the most common cause of gastrointestinal obstruction in children aged between 3 months and 3 years. It is the second most frequent acute abdominal surgical emergency in pediatrics after acute appendicitis. Left untreated, intussusception can have serious, potentially life-threatening sequelae. All clinicians involved in the assessment and care of pediatric patients should therefore have a sound understanding of this condition and its management. This chapter provides a comprehensive clinical overview of intussusception, including its pathogenesis, etiology, classification, presentation, epidemiology, diagnosis, management, and prognosis.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
History and Introduction
First described by Dutch physician Paul Barbette in 1674, intussusception is the full-thickness telescoping or invagination of a proximal portion of the intestine into an adjacent, more distal portion. The term derives from the Latin words “intus” (“within”) and “suscipere” (“to receive”). Ladd published the first radiograph with contrast enema demonstrating intussusception in 1913. While he considered contrast enema a useful diagnostic tool, Ladd did not appreciate its therapeutic potential in reducing intussusception. Diagnosis of intussusception was later expedited with the use of ultrasound. Burke and Clarke first described the distinctive ultrasonographic pattern of intussusception, including the pathognomonic “target sign” and “pseudo kidney,” in 1977 [1].
Before reduction techniques were developed and refined, infant intussusception was almost universally fatal in the early nineteenth century. Samuel Mitchell reported the first successful reduction of childhood intussusception by air enema using an enema tube and “common pair of bellows” in 1836. Hirschsprung first described controlled hydrostatic reduction in 1876. Successful reduction by hydrostatic pressure using saline or contrast solutions was reported by Hipsley in Australia in 1926 and by Retan and Stephens in America, Pouliquien in France, and Olsson in Scandinavia in 1927. Despite these reported successes, many surgeons remained skeptical of the potential benefits of hydrostatic reduction in the mid-twentieth century. Reduction by barium enema under fluoroscopy was popularized in the 1950s by Ravitch at Johns Hopkins, but has since fallen out of favor in the UK due to the risk of leakage and subsequent barium peritonitis. In 1959, Fiorito and colleagues reintroduced pneumatic reduction with pressure control, and this remains the method of choice in the UK today [1].
Although Barbette had suggested the possibility of surgical reduction in his early description of intussusception , the first successful operation for intussusception in an infant after failed hydrostatic reduction did not take place until 1873 under British surgeon Jonathan Hutchinson. As has been the way with many other surgical procedures, it is now possible to reduce intussusceptions laparoscopically. This approach is of particular use in recurrent cases to avoid repeat laparotomy and as a prelude to possible laparotomy in cases of failed conservative treatment [1].
Intussusception represents the most common cause of gastrointestinal obstruction in children aged between 3 months and 3 years [2]. It is the second most frequent acute abdominal surgical emergency in pediatrics after acute appendicitis [3]. Left untreated, intussusception can have serious, potentially life-threatening sequelae.
Pathogenesis and Natural History
The drawing up of the proximal portion of the intestine (the “intussusceptum”) into the lumen of the distal portion of the intestine (the “intussuscepiens”) is driven by peristalsis. As the mesentery becomes progressively incorporated into the intussusception, it is compressed, resulting first in impaired lymphatic return and, second, poor venous drainage, culminating in congestion and edema. This builds pressure on the mesenteric vasculature, eventually causing arterial compromise, infarction, ischemia and necrosis. The mucous membrane lining the lumen is highly sensitive to ischemia and therefore begins to slough off and bleed. Mucous, shed blood, and sloughed mucosa combine and are expelled as “red currant jelly stools.” If the built-up pressure is not relieved, it will result in the complete obstruction of the bowel and transmural gangrene of the intussusceptum. This can produce fluid sequestration, perforation of the bowel, leakage of intestinal contents into the peritoneal cavity, and peritonitis [2]. If the associated mesentery is lax, the intussusceptum can be drawn up as far as the distal colon or sigmoid (Fig. 48.1) and eventually prolapse through the anus. The later intussusception presents, the more manifest the natural history of the disease will become. Timely diagnosis and management is therefore extremely important.
Spontaneous reduction is another possible outcome and reportedly occurs in almost 20 % of intussusceptions [4].
Etiology
The pathogenesis of intussusception is possibly caused by an imbalance in the longitudinal forces acting along the intestinal wall.
The majority of intussusceptions (~ 95 %) are idiopathic as no obvious etiology can be identified. In these so-called idiopathic cases, it is thought that Peyer’s patches, hypertrophied in response to a respiratory or gastrointestinal infection, function as a lead point . Peyer’s patches are oval masses of aggregated lymphoid follicles on the mucous membrane lining the small intestine. Peyer’s patches are distributed irregularly along the anti-mesenteric wall, becoming more numerous and forming a lymphoid ring in the distal ileum. These structures have been labeled the “immune sensors of the intestine’ owing to their role in “sampling” the contents of the gut lumen, taking up antigens and microorganisms and, if appropriate, stimulating a protective mucosal immune response [5]. This probably explains why an antecedent viral infection is present in as many as 20 % of intussusceptions. Specifically, adenovirus [6], cytomegalovirus, and live rotavirus vaccines [7] have been variably associated with intussusception. Bacterial enteritis involving, for example, Salmonella, E. coli, Shigella, and Campylobacter, also increases the risk of intussusception in children [8].
This imbalance may be caused by a mass protruding into the intestinal lumen, which represents a “lead point” upon which peristalsis acts in an attempt to clear it as if it were a bolus of food. A pathological lead point has been defined as “a recognizable intraperitoneal anomaly or abnormality that tethers or obstructs the bowel, initiating the process of intussusception” [4]. Pathological lead points are identified in 2–12 % of intussusceptions. While a lead point is rarely identified in patients < 2 years, 20 % of patients > 2 years are found to have a lead point [2]. Pathological lead points are more commonly identified in ileoileal or colocolic intussusceptions.
Meckel’s diverticulum [9], benign and malignant intestinal or mesenteric tumors including lipomas [10], lymphomas [11], and polyps associated with Peutz–Jeghers syndrome [12], duplication cysts [13, 14], intestinal abnormalities associated with cystic fibrosis (e.g., hypertrophied mucosal glands or thickened feces) [15], hematomas secondary to abdominal trauma [16] or—Henoch–Schönlein purpura and other vascular/coagulation disorders [17], foreign bodies [18], intestinal hemangiomas [19], Kaposi sarcoma [20], abnormalities associated with posttransplantation lymphoproliferative disorder [21], and anastomotic sutures and staples and indwelling tubes [22] have all been reported as providing pathological lead points.
A small percentage of intussusceptions (typically ileoileal) arise postoperatively, usually after laparotomy with extensive bowel manipulation, although it has been reported following other abdominal and non-abdominal procedures. The precise mechanism underlying postoperative intussusception is unknown, but disorganized peristalsis, early postoperative adhesions, electrolyte disturbances, anesthetic drugs, and/or neurogenic factors may be implicated [23].
Epidemiology
In the UK, the reported incidence of intussusception stands at around 1.6–4 cases per 1000 live births [2]. Males are affected more frequently than females, with an incidence ratio of 3:2. The male preponderance becomes more evident after 9 months of age.
Ninety percent of intussusceptions will occur within the first 3 years of life, 65 % of cases arising within the first year of life and 50 % between 3 and 10 months [2]. Incidence peaks between 5 and 7 months [24]. Intussusception in utero is rarely reported [25, 26], and perinatal intussusception in newborns accounts for only 0.3 % of all cases. Several reasons for the increase in incidence from around 3 months of age have been advanced, including changes in feeding practices that affect the gut, maturation of lymphoid tissue, fattening of the mesentery which increases the likelihood of it becoming trapped, or a decline in the protection afforded by maternal antibodies against microorganisms that might precipitate intussusception [2, 27] .
Adult intussusception is rare, representing around 5 % of all cases. Unlike in the pediatric population, where intussusception is the most common cause of acute intestinal obstruction and is usually idiopathic, intussusception only accounts for 1–5 % of cases of intestinal obstruction in adults, often presents atypically and subacutely [28], and it is attributable to a pathological process in 90 % of cases. A more definitive, surgical approach (often resection) is warranted when managing adult intussusception compared to pediatric intussusception, due to the significant risk of associated malignancy (~ 65 % cases) [29] and high risk of perforation and leakage of microorganisms [30].
Evidence suggests that the relative risk of intussusception may vary according to race or ethnicity. For example, in their review of pediatric hospitalization data in the USA between 1993 and 2004, Tate et al. [31] found that in infants over 16 weeks of age, non-Hispanic black and Hispanic infants had higher rates of hospitalization for intussusception compared with non-Hispanic white infants. In line with the USA findings, research from the UK and Republic of Ireland suggests that Black Caribbean and African infants have higher incidence rates of intussusception than in White British and Asian groups [27]. Justice et al. [32] and Webby et al. [33] have identified a lower risk of intussusception among indigenous Australian children compared to nonindigenous children. However, as these studies rely on data gathered from hospitals, the differences they identify may reflect differences in admission and access rather than any actual ethnic variation in intussusception incidence [34].
Incidence of intussusception is also thought to vary by geographic region. Compared to other regions, it has been observed that incidence is higher than average in populations in Australia, Hong Kong, Vietnam, South Korea, and Japan and lower than average in populations in Finland, India, Malaysia, and Bangladesh [24, 35]. However, data upon which the assertion of geographic variability is based is problematic [27].
Finally, it is suggested that incidence of intussusception varies by season. Incidence has been found to peak during winter (Dec–Feb) and spring (March–May) in the UK and Republic of Ireland [27], although no such trend has been demonstrated in studies in equatorial regions [36] or indeed in those performed on a global scale [24]. Absence of any significant seasonal variation in incidence of intussusception goes against there being any strong association between intussusception and natural rotavirus, which has a highly seasonal pattern [37] .
Classification
Intussusception is classified anatomically, with the proximal portion of the intestine (the “intussusceptum”) first, followed by the more distal portion (the “intussuscepiens”). The majority of intussusceptions (~80 %) involve the terminal ileum telescoping into the cecum or ascending colon and are thus termed ileocecal or ileocolic intussusceptions. Less frequently, segments of ileum invaginate into ileum (ileoileal) or segments of colon invaginate into colon (colocolic) or a combination arises (ileo-ileo-colic).
Clinical Presentation
Sudden onset of severe, colicky abdominal pain is the most common presenting feature of intussusception [38], present in around 85 % of cases [39]. Infants will typically present with episodes of inconsolable crying while drawing up their legs in conjunction with spasms of peristalsis. These episodes occur every 10–15 min and last around 2–3 min. The pain becomes more constant after around 12 h. Between episodes, the infant may appear normal or increasingly pale, clammy, quiet, and lethargic. It is hypothesized that lethargy may be induced by the release of endogenous opioids or endotoxins from the ischemic bowel [40].
Vomiting (non-bilious, undigested gastric contents becoming bilious) can be an early indication of intestinal obstruction. Evacuation of small, loose stools from the colon distal to the obstruction will occur early in the course of the disease in some patients. Around 50 % of patients pass “red currant jelly” stool [39]. Overall, 1/3 patients will have the classic triad of abdominal pain, vomiting, and bloody stool [2].
The combination of reduced fluid intake, increased fluid loss through vomiting, anticipated losses into the obstructed bowel, and perhaps some reactive vasodilation can culminate in dehydration and hypovolemic shock. If the bowel perforates resulting in bacteremia, the child will become febrile, tachycardic, and hypotensive.
Examination can be unremarkable in between “attacks.” A “sausage-shaped” mass is palpable in around 65 % of cases, usually in the right upper quadrant extending to the left along the line of the transverse colon (Fig. 48.2). The mass can be tender and is sometimes seen on clinical inspection. It can become harder to detect this mass as the disease progresses and the abdomen distends [39]. The right lower quadrant can become flat or empty due to the absence of bowel (“Dance” sign). In around 5 % of cases, the apex of the intussusceptum can be palpated on rectal examination. It rarely prolapses out of the anus.
Investigation
Diagnostic work up of a patient with clinically suspected intussusception might include a plain abdominal radiograph, abdominal ultrasound, contrast enema, and CT scan. Laboratory tests are not specifically diagnostic but may show leukocytosis, acidosis, and electrolyte disturbance associated with bowel ischemia.
A plain radiograph is usually only performed if the diagnosis on presentation is unclear [3]. Suggestive signs of intussusception on a plain radiograph include an elongated soft tissue mass typically in the right upper quadrant, abnormal distribution of gas and fecal contents, dilated bowel loops, no gas in the transverse or descending colon, and air–fluid levels in the presence of bowel obstruction. Although plain radiographs can aid diagnosis of intussusception, they may appear normal in the early stages and they lack sensitivity (i.e., high incidence of false positives) [41].
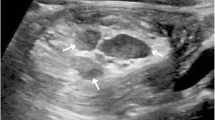
Ultrasonography is an extremely effective imaging modality for diagnosing intussusception. It has a sensitivity over 98 % and a specificity of 100 % when diagnosing ileocolic or colocolic intussusceptions. These parameters are slightly reduced for ileoileal intussusceptions [2]. The “doughnut” or “target” sign on transverse section (concentric rings created by the telescoping bowel) and a “pseudo-kidney” (bowel wall and mesentery mimic renal structures) are characteristic signs of intussusception on ultrasound [2].
If the diagnosis is still in doubt, a contrast enema is the gold standard for diagnosing intussusception. Barium or (more commonly) air is introduced via a catheter inserted into the rectum. When the contrast substance enters the lumen of the intussusceptum and the intraluminal space, this creates the “coiled-spring” sign. This test is contraindicated if there is evidence of perforation on plain radiograph. Enemas can be therapeutic as well as diagnostic [41].
Finally, CT scans are more commonly used in adults with suspected intussusception. Transversely, concentric rings of telescoping bowel will form the equivalent of an ultrasonographic “target” sign. Longitudinally, a soft tissue sausage-shaped mass may be observed. Hyper-dense rings at the proximal end of the intussusception formed by the intussusceptum and the folded edge of the intussuscipiens might also be visualized. Occasionally, a lead point may be picked up on CT [41].
Management
Initial management focuses on stabilizing the child. This includes fluid resuscitation using normal saline—20 mL/kg intravenous (IV) bolus to start. Nasogastric (NG) tube should be placed. It should be regularly aspirated and free draining. Prophylactic broad-spectrum antibiotics (e.g., cefuroxime and metronidazole) should be administered.
Focus then shifts to reducing the intussusception nonsurgically or surgically. The majority of cases of intussusception are reduced nonsurgically with sustained intra-colonic pressure delivered by enema using various different substances [42]. Some centers have reported successful reduction of intussusceptions with saline [43].
A catheter, often with an inflatable balloon and attached pressure gauge, is inserted into the rectum, and the child’s buttocks are held together to create a tight seal. Air is then passed through the catheter at carefully monitored pressures up to 120 mmHg. Up to three attempts at reduction can be made, each lasting up to 3 min [2]. If the first three attempts fail but the child is clinically well, the air enema can be repeated after 4–6 h (Fig. 48.3a, b). A radiologist usually performs the procedure, but a surgeon must be present in case complications arise, the most serious of which is an acute tension pneumoperitoneum. This compromises venous return from the lower body and causes cardiovascular collapse. Decompression of the abdominal cavity is achieved by inserting a large-bore cannula into the peritoneum. A total of 75–90 % of intussusceptions are successfully reduced by air enema [2].
In the remaining cases, laparotomy can be performed. A transverse incision is made in the right lower quadrant, although occasionally can be transumbilical or in the right upper quadrant. Once the intussusception is located and delivered through the incision, the intussuscipiens is gently squeezed or milked so that the intussusceptum is pushed distally and the telescoping is reduced (Fig. 48.4). The reduced bowel should be carefully examined for signs of ischemic damage and signs of a pathological lead point . Resection with anastomosis may be necessitated by presence of nonviable ischemic bowel or an obvious lead point after manual reduction. Indications for laparotomy include perforation, peritonism, and unsuccessful nonsurgical reduction. Many centers will bypass attempts at nonsurgical reduction in atypical cases or if intussusception is recurrent or related to a pathological lead point [2].
Minimally invasive laparoscopic surgery is becoming increasingly popular for reduction of intussusception [44]. Gentle pressure is applied distally using atraumatic graspers inserted through the ports. A recent systematic review reported a 71 % success rate for laparoscopic reduction of intussusception [44]. Compared to open reduction, laparoscopic reductions are associated with shorter operating times, shorter time to first postoperative feed, reduced use of IV narcotics, and earlier discharge [45]. It has therefore been suggested that tertiary centers with adequate facilities should use laparoscopy as the primary surgical approach to reducing intussusceptions. The major disadvantage to using laparoscopy is that it reduces a surgeon’s tactile acuity, meaning that extra care should be taken to search for pathological lead points .
Complications and Prognosis
Complications of pneumatic reduction include perforation causing tension pneumothorax and failure to reduce the intussusception.
Complications associated with surgical reduction of intussusception include perforation, systemic infection (e.g., sepsis and meningitis), bleeding, wound infection, leak or breakdown of an anastomosis, incisional hernia, adhesions, and bowel obstruction [4]. Resection rarely has any long-term consequences, although removal of the ileocecal valve may cause increased stool frequency [2].
Recurrence of intussusception following reduction is not uncommon. Recurrence usually arises within 2–3 days of the first reduction (~ 60 % within 6 months), presents early, and is treated in the same way as the initial episode. Recurrence is more likely in the presence of a pathological lead point.
Death is a rare outcome, usually associated with late presentation. Prognosis varies globally as diagnosis and treatment usually occur earlier in developed countries compared to the developing world [36].
Conclusion
Intussusception is a common emergency in pediatric surgery, which must be diagnosed and managed swiftly to avoid life-threatening complications. It should be suspected in children presenting between 3 months and 3 years with colicky abdominal pain, vomiting, and bloody stools and reduced as soon as possible to avoid irreversible ischemic damage to the bowel. Therapeutic pneumatic air enema forms the main form of treatment modality. Surgical reduction of intussusceptions, when required, is becoming less invasive.
References
Davis CF, McCabe AJ, Raine PAM. The ins and outs of intussusception: history and management over the past fifty years. J Paediatr Surg. 2003;38 (Suppl 7):60–4.
R obb A, Lander A. Intussusception in infants and young children. Surgery 2008;26(7):291–3.
Saliakellis E, Borrelli O, Thapar N. Paediatric GI emergencies. Best Pract Res Clin Gastroenterol. 2013;27(5):799–817.
Morrison SC, Stork E. Documentation of spontaneous reduction of childhood intussusception by ultrasound. Paediatr Radiol. 1990;20(5):358–9.
Jung C, Hugot J, Barreau F. Payer’s patches: the immune sensors of the intestine. Int J Inflam. 2010;2010:823710. (published online 19/09/2010).
Bines JE, Liem NT, Justice FA, Son TN, Kirkwood CD, de Campo M, et al. Risk factors for intussusception in infants in Vietnam and Australia: adenovirus implicated, but not rotavirus. J Paediatr. 2006;149(4):452.
Patel MM, Haber P, Baggs J, Zuber P, Bines JE, Parashar UD. Intussusception and rotavirus vaccination: a review of the available evidence. Expert Rev Vaccines. 2009;8(11):1555–64.
Nyland CM, Denson LA, Noel JM. Bacterial enteritis as a risk factor for childhood intussusception: a retrospective cohort study. J Paediatr. 156(5):761.
Milbrandt K, Sigalet D. Intussusception associated with a Meckel’s diverticulum and a duplication cyst. J Paediatr Surg. 2008;43(12):e21–3.
Howard N, Pranesh N, Carter P. Colo-colonic intussusception secondary to a lipoma, Int. J Surg Case Rep. 2012;3(2):52–4.
Brichon P, Bertrand Y, Plantaz D. Burkitt’s lymphoma revealed by acute intussusception in children. Ann Chir. 2001;126(7):649–53.
Sasaki T, Fukumori D, Sato M, Sakai K, Ohmori H, Yamamato F. Peutz–Jeghers syndrome associated with intestinal intussusception: a case report. Int Surg. 2002;87(4):256–9.
Verma S, Bawa M, Rao K et al. BMJ Case Rep. 2013. doi:10.1136/ bcr-2012-008056.
Deigaard SB, Trap R. Intestinal duplication—an important differential diagnosis to intussusception. Ugeskr Laeger. 2008;170(35):2708.
Nash EF, Stephenson A, Helm EJ, Ho T, Thippanna CM, Ali A, et al. Intussusception in adults with cystic fibrosis: a case series with review of the literature. Dig Dis Sci. 2011;56(12):3695–700.
Lu S, Goh P. Traumatic intussusception with intramural haematoma. Paediatr Radiol. 2009;39(4):403–5.
Chang WL, Yang YH, Lin YT, Chiang BL. Gastrointestinal manifestations in Henoch–Schonlein purpura: a review of 261 patients. Acta Paediatr. 2004;93(11):1427–31.
Dalshaug GB, Wainer S, Hollaar GL. The Rapunzel syndrome (Trichobezoar) causing atypical intussusception in a child: a case report. J Paediatr Surg. 1999;34(3):479–80.
Guthrie SO, Rhodes M, Janco R, Stein, SM, Jabs K, Engelhardt B. An infant with Kasabach–Merritt syndrome with associated renal haematoma and intussusception. J Perinatol. 2005;25:143–5.
Ramdial PK, Sing Y, Hadley GP, Chotey NA, Mahlakwane MS, Singh B. Paediatric intussusception caused by acquired immunodeficiency syndrome-associated Kaposi sarcoma. Paediatr Surg Int. 2010;26(8):783–87.
Earl TM, Wellen JR, Anderson CD, Nadler M, Doyle MM, Shenoy SS, et al. Small bowel obstruction after paediatric liver transplantation: the unusual is the usual. J Am Coll Surg. 2011;212(1):62–7.
Furuya Y, Wakahara T, Akimoto H, Long CM, Yanagie H, Yasuhara H. A case of postoperative recurrent intussusception associated with indwelling bowel tube. World J Gastrointest Surg. 2010;2(3):85–8.
Yang G, Wang X, Jiang W, Ma J, Zhao J, Liu W. Postoperative intussusception in children and infants: a systematic review. Paediatr Surg Int. 29:1273–9.
Jiang J, Jiang B, Parashar U, Nguyen T, Bines J, Patel MM. Childhood intussusception: a literature review. PLoS One. 2013;8(7):e68482.
Nguyen DT, Lai E, Cunningham T, and Moore TC. In utero intussusception producing ileal atresia and meconium peritonitis with and without free air. Paediatr Surg Int. 1995;10(5–6):406–8.
Huebner BR, Azarow KS, Cusick RA. Intestinal atresia due to intrauterine intussusception of a Meckel’s diverticulum. J Paediatr Surg Case Rep. 2013;1(8):232–4.
Samad L, Cortina-Borja M, El Bashir H, Sutcliffe AG, Marven S, Cameron JC, et al. Intussusception incidence among infants in the UK and Republic of Ireland: a pre- rotavirus vaccine prospective surveillance study. Vaccine 2013;31(38):4098–102.
Wong KB, Lui CT, Fung HT. How do adult and paediatric intussusceptions differ? A 10-year retrospective study. Hong Kong J Emerg Med. 2012;19(4).
Marinis A, Yiallourou A, Samanides L, Dafnios N, Anastasopoulos G, Vassiliou J, et al. Intussusception of the bowel in adults: a review. World J Gastroenterol. 2009;15(4):407–11.
Erkan N, Haciyanli M, Yildirim M, Sayhan H, Vardar E, Polat AF. Intussusception in adults: an unusual and challenging condition for surgeons. Int J Colorectal Dis. 2005;20(5):452.
Tate JE, Simonsen L, Viboud C, Steiner C, Patel MM, Curns AT, et al. Trends in intussusception hospitalizations among US infants, 1993–2004: implications for monitoring the safety of the new rotavirus vaccination program. Pediatrics 2008;121(5):e1125–32.
Justice F, Carlin J, Bines J. Changing epidemiology of intussusception in Australia. J Paediatr Child Health. 2005;41(9–10):475–8.
Webby RJ, Bines J, Barnes GL, Tindall H, Krause V, Patel M. Intussusception in the Northern Territory: the incidence is low in Aboriginal and Torres Strait Islander children. J Paediatr Child Health. 2006;42(5):235–9.
http://whqlibdoc.who.int/hq/2002/WHO_V&B_02.19.pdf. Accessed 12 Dec 2013.
Takeuchi M, Osamura T, Yasunaga H, Horiguchi H, Hashimoto H, Matsuda S. Intussusception among Japanese children: an epidemiologic study using an administrative database. BMC Pediatr. 2012;12:36.
Boudville IC, Phua KB, Quak SH, Lee BW, Han HH, Verstraeten T, et al. The epidemiology of paediatric intussusception in Singapore: 1997–2004. Ann Acad Med Singapore. 2006;35(10):674–9.
Rennels MB, Parashar UD, Homlam RC, Le CT, Chang HG, Glass RI. Lack of an apparent association between intussusception and wild or vaccine rotavirus infection. Pediatr Infect Dis J. 1998;17:924–5.
Mandeville K, Chien M, Willyerd FA, Mandell G, Hostetler MA, Bulloch B. Intussusception: clinical presentations and imaging characteristics. Pediatr Emerg Care. 2012;28(9):842–4.
Hutson JM, O’Brien M, Woodward AA, Beasley SW, editors. Jones’ clinical paediatric surgery. 6th ed. Oxford: Blackwell; 2008.
http://www.hawaii.edu/medicine/pediatrics/pedtext/s10c04.html. Accessed 12 Dec 2013.
Byrne AT, Geoghegan T, Govender P, Lyburn ID, Colhoun E, Torreggiani WC. The imaging of intussusception. Clin Radiol. 2005;60(1):39–46.
Shehata S, El Kholi N, Sultan A, El Sahwi E. Hydrostatic reduction of intussusception: barium, air, or saline. Pediatr Surg Int. 2000;16(5–6):380–2.
Nayak D, Jagdish S. Ultrasound guided hydrostatic reduction of intussusception in children by saline enema: our experience. Indian J Surg. 2008;70(1):8–13.
Apelt N, Featherstone N, Giuliani S. Laparoscopic treatment of intussusception in children: a systematic review. J Paediatr Surg. 2013;48(8):1789–93.
Hill SJ, Koontz CS, Langness SM, Wulkan ML. Laparoscopic versus open reduction of intussusception in children: experience over a decade. J Laproendosc Adv Surg Tech. 2013;23(2):166–9.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
O’Sullivan, L., Desai, A. (2016). Intussusception. In: Guandalini, S., Dhawan, A., Branski, D. (eds) Textbook of Pediatric Gastroenterology, Hepatology and Nutrition. Springer, Cham. https://doi.org/10.1007/978-3-319-17169-2_48
Download citation
DOI: https://doi.org/10.1007/978-3-319-17169-2_48
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-17168-5
Online ISBN: 978-3-319-17169-2
eBook Packages: MedicineMedicine (R0)