Abstract
Gliomas are primary cancers of the brain and the most lethal cancers known to man. In recent years the discovery of germinal regions in the postnatal brain containing neuronal stem and progenitor cell populations has led to the hypothesis that these cells may themselves serve as an origin of brain tumors. Stem cells that reside within the glioma tumor have been shown to display nonneoplastic stem-like characteristics, including expression of various stem cell markers, as well as capacity for self-renewal and multipotency. Furthermore, glioma tumors display marked similarities to the germinal regions of the brain. Investigations of human neural stem cells and their potential for malignancy may finally identify a cell-of-origin for human gliomas. This, in turn, may facilitate better therapeutic targeting leading to improved prognosis for glioma patients.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Subventricular zone (SVZ)
- Neuro-oncogenesis
- Glioma cell of origin
- Neural stem cells and glioma
- Germinal regions and oncogenesis
- Neurogenesis and oncogenesis of glioma
Introduction
Despite progress in research on the molecular aspects of malignant gliomas, the prognosis of these primary brain tumors continues to be dismal. In grade IV glioma, or glioblastoma, the most common glioma in adults, the median survival has changed only slightly in the last decade, increasing from 9 to 12 months in 2005 to the current median survival of 13–14 months. One reason for the lack of clinical advances is ignorance of the cellular origin of this disease, which delays the application of molecular analyses to treatment and impairs the ability to anticipate tumor behavior reliably.
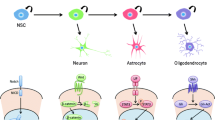
Historically, the neoplastic transformation of fully differentiated glia was widely assumed to be the only mechanism for gliomagenesis. Astrocytes and oligodendrocytes, once thought to be the sole dividing cells in the postnatal brain, were assumed to represent the cellular component most susceptible to transformation. More recently, however, this hypothesis has been challenged by the discovery of stem cell and progenitor populations residing in the postnatal brain, which may themselves serve as an origin of brain tumors. Phenotypic and behavioral similarities between gliomas and adult neural stem cells raise the possibility that stem or progenitor cells can give rise to gliomas. Resident tumor glioma stem cells display adult neural stem cell characteristics, including expression of stem cell markers (e.g., nestin), the ability to self-renew, and conserved multipotent potential. Candidate cells-of-origin include astrocytic neural stem cells (B cells) or transient amplifying precursors (C cells) of the adult subventricular zone (SVZ) and glial progenitor cells of the subcortical white matter. While a direct link remains to be established between any one of these cellular compartments and the formation of gliomas, recent advances have provided ample evidence to support the hypothesis.
Shared Features of Adult Germinal Regions and Gliomas
Adult germinal regions, such as the SVZ, are restricted to specialized microenvironments which allow for the survival and regulation of neural stem cells. Such specialized microenvironments consist of structural and molecular elements resulting in the appropriate conditions to support stem cell self-renewal and capacity for differentiation. For instance, capillaries can be found in close proximity to cells of the SVZ and hippocampus. Secreted factors and proteins, such as instructive growth factors, regulate neural stem cell behavior and may be absorbed from SVZ capillaries. Such growth factors include epidermal growth factor (EGF), brain-derived neurotrophic factor (BDNF), and vascular endothelial growth factor C (VEGFC), among others. Gliomas are highly vascularized tumors and many tumors overexpress receptors to these growth factors. Furthermore, within the tumor, CD133+;nestin+ glioma stem cells reside in close proximity to the vasculature [1]. In 3D culture systems, glioma stem cells preferentially home to areas of vasculature, while other cells of the glioma tumor do not display such preference. The factors provided by the vascular endothelial cells were shown to contribute to the maintenance of glioma stem cell proliferation and self-renewal thus explaining their homing preference. Therefore, a strong association exists between vascular and neurogenic niches in adult germinal regions [2, 3]. Vascular endothelial growth factor (VEGF) and its receptors are expressed by neurospheres derived from rodent SVZ [4]. VEGF has also been implicated in glioma growth and is secreted by glioma cells that act on tumor endothelial cells expressing VEGF receptors [5]. Similarly, cancer stem cells isolated from gliomas generate markedly elevated levels of VEGF [6]. Taken together, these data suggest that targeting proangiogenic factors is a potential therapeutic strategy against gliomas and their putative cancer stem cell fraction.
The extracellular matrix (ECM) of germinal regions must contain specialized molecules in order to regulate neuronal differentiation and proper development. The ECM of the SVZ germinal niche is enriched in ECM proteins such as tenascin, collagen, and chondroitin sulfate proteoglycans. Tenascin C is an extracellular matrix molecule that modulates cellular adhesion [7]. The SVZ has increased expression of tenascin C, and it acts to regulate neural stem cell development through the modulation of cell–matrix interactions [8]. Tenascin expression, while concentrated in the human SVZ, is upregulated in GBM and able to stimulate the proliferation of glioma tumor cells. Furthermore, tenascin expression leads to changes in glioma cell gene expression resulting in a more aggressive phenotype [7].
CD44 is a glycoprotein transmembrane receptor and has been used to select for glioma stem cells [9, 10]. It functions as an ECM adhesion protein and its expression is important for non-neoplastic stem cell niche homing and maintenance [11]. CD44 expression in glioma is correlated with more aggressive tumor growth [12] and its expression increases glioma cell migration and invasion [13].
Nestin is a member of a class of intermediate filaments (class VI) that is expressed by neural progenitors during development [14]. It is widely expressed in the brain at birth, but its expression is downregulated in the adult brain and becomes restricted to the SVZ. Nestin+ cells also exist in human gliomas [15], lending further support to the hypothesis that neural stem cells may be implicated in glioma formation. Furthermore, nestin expression appears to be significantly correlated to high-grade gliomas in addition to its expression being a predictor for reduced overall survival [16].
Transcription factors play an important role in the regulation of cell fate and are capable of inducing transcriptional programs leading to oncogenesis. The hedgehog family of regulatory pathways is a key regulator of nonneoplastic progenitor proliferation in the SVZ, where the Shh-Gli pathways maintain the stem cell population and facilitate the survival and proliferation of stem cell progeny. It is important to note that Gli is expressed in both low-grade and high-grade gliomas, and that the Shh-Gli pathway may mediate the initiation and maintenance of these tumors as it does for neural stem cells [17]. As might be expected, treatment with cyclopamine (a specific inhibitor of hedgehog signaling) can inhibit the growth of some glioma cell lines in vitro. Thus, the hedgehog family of signaling pathways is implicated in both gliomagenesis and regulation of adult neural stem cell proliferation.
Beyond ECM proteins and transcription factors, growth factor signaling pathways also play an important role in both gliomagenesis and germinal zone regulation. Nearly half of high-grade astrocytomas demonstrate EGF receptor amplification. Not surprisingly, EGFR amplification is a potential transformation mechanism in the development of glioblastoma multiforme. EGF-responsive C cells within the SVZ constitute a large population of migratory, rapidly dividing progenitor. EGF-mediated stimulation prevents C cell differentiation of these cell types and releases their infiltrative potential, similar to the infiltration seen in high-grade gliomas [18].
A population of platelet-derived growth factor (PDGF)+ B cells in the adult SVZ have been identified and shown to give rise to both neurons and oligodendrocytes in vivo [19]. Excessive PDGF activation in the rodent SVZ arrests neuroblast production, induces SVZ cellular proliferation, and creates areas of hyperplasia with features of early glioma formation. There also appears to be a link between these PDGFR+ B cells and the early changes associated with tumor initiation, suggesting that they may be targets of neoplastic transformation.
Models of SVZ Stem Cell Transformation
The genetic manipulation of various known oncogenes, specifically in mouse neural stem cells, has led to the development of numerous animal models of glioma. These models demonstrate the potential for neural stem cell transformation, which often results in neuro-oncogenesis within SVZ cells. Such studies have led to the conclusion that neural stem cell populations are more sensitive to chemical or viral oncogenesis than are areas with a low proportion of proliferating cells. Early models transformation in cells of the SVZ investigated the effects of prenatal exposure to the classic mutagen n-ethyl-n-nitrosourea (ENU), a well characterized neurocarcinogen [20]. These studies found that cells of the SVZ, in particular, undergo genetic transformations resulting in increased proliferation and immortalization [21]. Interestingly, tumor formation in this model is limited to regions of the SVZ. Genetic mutations in SVZ cells that resulted from ENU exposure included the deletion of INK4a/ARF, cell-cycle genes which are have also been shown to be significantly mutated in GBM samples. The Cancer Genome Atlas 2008 examination of over 200 GBM patient samples found a homozygous deletion or mutation in 49 and 52 % of patients for ARF and INK4a, respectively [22]. Neoplastic cells of mice treated with ENU were also found to upregulate nestin, whose expression is limited to neuronal precursors [23], thus further implicating the role of neural stem cell transformation in the development of glioma.
In addition, an investigation of the loss of tumor suppressor p53 results in increased proliferation of relatively quiescent astrocyte-like SVZ type B cells both in vitro and in vivo [24–26]. Tp53 is a tumor suppressor protein whose signaling is altered in 87 % of GBM patients [22]. When p53−/− mice are prenatally treated with the mutagen ENU, the result is the development glioblastoma-like tumors in 60 % of mice. These tumors form periventricularly and display glioblastoma characteristics, including infiltration into surrounding areas, areas of necrosis, and heterogeneous cell populations. Furthermore, early inactivation of p53 has also been shown to cooperate with the neurofibromatosis-1 (NF1) tumor suppressor gene mutation, resulting in malignant astrocytoma formation in a mouse tumor model [27, 28]. The NF1 tumor suppressor neurofibromin is a functional Ras GTPase-activating protein and its loss results in abnormal activation of Ras, a central mediator of receptor tyrosine kinase (RTK) signaling. Furthermore, mutation or homozygous deletion in NF1 has been noted in 18 % of GBM samples [22]. Mice that carry germline mutations in both p53 and NF1 develop both low and intermediate-grade astrocytomas. These astrocytomas express Nestin, the progenitor-associated intermediate filament, and were consistently associated with the SVZ. Based on these results, it appears that SVZ cells are most susceptible to p53/NF1-mediated astrocytoma formation and that the cell-of-origin for malignant astrocytomas in p53/NF1 mutant mice may reside within the SVZ.
Various other mouse models of neural stem cell transformation have provided clues as to the potential molecular events that lead to neuro-oncogenesis [27, 29–33]. The genetic manipulation of Harvey-Ras (H-Ras) and AKT in as little as 60 GFAP+ precursor cells of the SVZ or hippocampus results in the development of high-grade gliomas in these regions [33]. Both the H-Ras pathway and the AKT pathway are highly associated with gliomas. Recent studies have reported 2 % of gliomas experience gain-of-function mutations in Ras and 36 % of gliomas experience a loss of the tumor suppressor phosphatase and tensin homolog (PTEN), a negative regulator of the Ras signaling pathway. Both a gain-of-function mutation in Ras and loss-of-function mutation in PTEN result in AKT pathway activation. These pathways participate in RTK signaling, which has been shown to be altered in 88 % of GBM patients [22]. Importantly, the injection of active AKT and H-RasV12 into the cortex fails to result in any significant tumor formation. However, mice with injection in the hippocampus or SVZ results in neuro-oncogenesis of glioma-like tumors, indicating GFAP+ NSCs of the SVZ and hippocampus to be putative cells of origin [33]. These tumors display pathological characteristics of glioblastomas, including microvascular proliferation, pseudo-palisading necrosis, and increased cell density. In addition to displaying these hallmark characteristics of glioblastoma, the tumors also exhibit cellular heterogeneity, evidenced by the expression of various cellular markers, including the astrocytic marker GFAP, the oligodendrocyte marker, myelin basic protein, and a neuronal specific marker, tuj1. Furthermore, the tumors in the hippocampus and/or SVZ were mostly GFP+, indicating that a majority of the cells in the tumor were derived from a smaller infected cell population. After isolating GFP+ cells from the tumors of GFAP-Cre;TP53+/− mice injected with H-RasV12/AKT and culturing them in neural stem cell media, the cells formed neurosphere structures in vitro. Proliferation and differentiation assays confirmed that these cells were both self-renewing and multipotent, as they were proliferative in neural stem cell media and differentiated upon serum stimulation. Forty to fifty percent of these cells express CD133, a speculative marker for glioma initiating cells. This study provides strong evidence towards the SVZ neural stem cell as the putative cell-of-origin for GBM as tumors arise in the SVZ and hippocampus and fail to develop in the cortex. Furthermore, these glioma initiating cells of the SVZ maintained their self-renewal and differentiation capacities, indicating that stemness is a contributing factor to astrocytoma development.
PTEN (phosphatase and tensin homologue) is a recognized tumor suppressor mutated or deleted in 36 % of GBM samples [22]. PTEN protein is a phosphatidylinositol phosphate (PIP) phosphatase that lowers PIP3 levels and enhances the rate of apoptosis. PTEN also decreases cell motility via G protein-coupled mechanisms. PTEN is expressed in SVZ precursor cells during neuronal differentiation [34]. A loss of PTEN and p53 in neural stem cells isolated from the SVZ results in significantly increased proliferation, self-renewal capacity, and impaired differentiation potential [35]. In vivo, 42 out of 57 hGFAP-Cre+;p53fl/fl;Ptenfl/+ mice develop malignant gliomas which display classic features of human GBM including cellular pleomorphism, microvascular proliferation, and areas of necrosis. In another mouse model, heterozygous loss of FN1 and PTEN coupled with homozygous loss of p53 in nestin-cre+ cells results in 100 % astrocytoma tumor development [32]. Again, it is important to note that viral injection of cre into the SVZ of these mice results in astrocytoma formation in 100 % of mice, while injection of cre into other regions, such as the cortex and striatum, does not. Studies such as these, which uncover the innate tumor competence of SVZ neural stem cells, provide strong evidence for the neural stem cell as the cell-of-origin for glioma.
Clinical and Therapeutic Implications
Clinical correlations have been drawn between GBMs and their anatomical relationship to the SVZ [36–38]. An examination of 53 patients with newly diagnosed GBM and the classification of their tumors based on spatial relationship to the SVZ showed that patients whose tumors made contact with the SVZ had a significantly higher incidence of multifocal disease at diagnosis [37]. Interestingly, upon follow-up MRI, all of the patients whose tumors had direct contact to the SVZ had recurred in a noncontiguous manner with the original lesion. However, of the patients whose tumors did not have contact with the SVZ, none had any evidence of tumor noncontiguous with the primary lesion [37]. A similar analysis of 91 GBM patients showed that progression free survival is significantly reduced in patients whose tumor has contact with both the SVZ and the cortex [36]. Furthermore, those patients with SVZ involvement had an overall reduced survival as well as a decreased time until recurrence compared to those tumors not involving the SVZ. In addition, an analysis of 39 newly diagnosed GBM patients revealed that tumor SVZ involvement was a significant predictor in reduced overall survival [38].
Based on the cancer stem cell theory, any brain tumor therapy that fails to eradicate cancer stem cells will result in recurrence or regrowth of the residual tumor stem cells, resulting in eventual disease progression [39]. Recently, studies have attempted to ask whether irradiation of the ipsilateral SVZ can improve GBM prognosis [40–45]. The first of such studies analyzed 55 patients with glioma who received surgery, radiotherapy and chemotherapy [42]. SVZ radiation dose was found to be significantly predictive of progression free survival, and bilateral radiation to the SVZ yielded a significant hazard ratio for death, leading to the author’s conclusion that SVZ radiation may provide a significant benefit for GBM survival. In a pooled analysis of 173 GBM patients from two academic centers, these results were confirmed as high radiation therapy doses to the ipsilateral SVZ led to significantly longer progression free survival. An analysis of 40 GBM patients found statically significant improved overall survival in patients who received high dose of ipsilateral SVZ radiation [43]. In a recent investigation of 100 GBM patients—50 long term survivors (>3 years) and 50 short term survivors (<1 year), SVZ tumor contact was found to be a significant predictor of prolonged survival, in addition to age and total resection status [40].
While these data are suggestive, the topic of the neural stem cell as the cell-of-origin for glioma remains controversial. Other hypotheses of glioma initiating cells exist, including the notion of cellular dedifferentiation resulting in neuro-oncogenesis [46]. In this model, terminally differentiated cells within the tumor are capable of dedifferentiating and reverting back to multipotency. Others have shown differentiated terminal astrocytes, in addition to NSCs, are capable of dedifferentiation under the influence of Ink4a/Arf inactivation and EGFR activation [47]. Furthermore, the oligodendrocyte precursor cell (OPC) is also postulated as a candidate cell of origin for glioma [48–50]. Using mosaic analysis with double markers (MADM) in mice, Liu et al. [49] show that only OPCs are capable of gliomagenesis upon genetic manipulation. Yet another OPC cell of origin model shows NG2+ oligodendrocytes may undergo a loss of asymmetric divisions resulting in proliferative, self-renewing cells with tumor-initiating potential [50]. Moving forward, increased focus on the development of these brain tumors, including cellular and molecular transformations in different cell types, may lead to the discovery of new therapeutic targets able to arrest neuro-oncogenesis early in its track.
Conclusions
The discovery of neural stem cells in the adult human brain has led to the emergence of a new area of scientific inquiry connecting neuro-oncology with developmental neurobiology. This field has gained prominence in recent years with the identification and characterization of stem-like cells within glioma tumors which retain the capacity to both self-renew and differentiate into neuronal subtypes. Glioma tumors have been shown to resemble neuronal germinal niches in structural, functional, and molecular characteristics. Findings such as these may pave the way for the identification of a cell-of-origin for human glioma allowing for the development of novel therapeutic agents and strategies to improve glioma prognosis.
References
Calabrese C, Poppleton H, Kocak M, Hogg TL, Fuller C, Hamner B, Allen M. A perivascular niche for brain tumor stem cells. Cancer Cell. 2007;11(1):69–82.
Gilbertson RJ, Rich JN. Making a tumour’s bed: glioblastoma stem cells and the vascular niche. Nat Rev Cancer. 2007;7(10):733–6.
Palmer TD, Willhoite AR, Gage FH. Vascular niche for adult hippocampal neurogenesis. J Comp Neurol. 2000;425(4):479–94.
Knizetova P, Darling JL, Bartek J. Vascular endothelial growth factor in astroglioma stem cell biology and response to therapy. J Cell Mol Med. 2008;12(1):111–25.
Jain RK, Di Tomaso E, Duda DG, Loeffler JS, Sorensen AG, Batchelor TT. Angiogenesis in brain tumours. Nat Rev Neurosci. 2007;8(8):610–22.
Bao S, Wu Q, Sathornsumetee S, Hao Y, Li Z, Hjelmeland AB, Rich JN. Stem cell-like glioma cells promote tumor angiogenesis through vascular endothelial growth factor. Cancer Res. 2006;66(16):7843–8.
Ruiz C, Huang W, Hegi ME, Lange K, Hamou M-F, Fluri E, Orend G. Differential gene expression analysis reveals activation of growth promoting signaling pathways by tenascin-C. Cancer Res. 2004;64(20):7377–85.
Garcion E, Halilagic A, Faissner A. Generation of an environmental niche for neural stem cell development by the extracellular matrix molecule tenascin C. Development. 2004;131(14):3423–32.
Anido J, Sáez-Borderías A, Gonzàlez-Juncà A, Rodón L, Folch G, Carmona MA, Prudkin L. TGF-β Receptor Inhibitors Target the CD44 high/Id1 high Glioma-Initiating Cell Population in Human Glioblastoma. Cancer Cell. 2010;18(6):655–68.
Fu J, Yang Q-Y, Sai K, Chen F-R, Pang JC, Ng H-K, Chen Z-P. TGM2 inhibition attenuates ID1 expression in CD44-high glioma-initiating cells. Neuro Oncol. 2013;15(10):1353–65.
Haylock DN, Nilsson SK. Perspective stem cell regulation by the hematopoietic stem cell niche. Cell Cycle. 2005;4(10):1353–5.
Pietras A, Katz AM, Ekström EJ, Wee B, Halliday JJ, Pitter KL, Holland EC. Osteopontin-CD44 signaling in the glioma perivascular niche enhances cancer stem cell phenotypes and promotes aggressive tumor growth. Cell Stem Cell. 2014;14(3):357–69.
Chetty C, Vanamala SK, Gondi CS, Dinh DH, Gujrati M, Rao JS. MMP-9 induces CD44 cleavage and CD44 mediated cell migration in glioblastoma xenograft cells. Cell Signal. 2012;24(2):549–59.
Wiese C, Rolletschek A, Kania G, Blyszczuk P, Tarasov K, Tarasova Y, Wobus A. Nestin expression: a property of multi-lineage progenitor cells? Cell Mol Life Sci CMLS. 2004;61(19–20):2510–22.
Yang XH et al. Nestin expression in different tumours and its relevance to malignant grade. J Clin Pathol. 2008;61(4):467–473.
Arai H, Ikota H, Sugawara K-I, Nobusawa S, Hirato J, Nakazato Y. Nestin expression in brain tumors: its utility for pathological diagnosis and correlation with the prognosis of high-grade gliomas. Brain Tumor Pathol. 2012;29(3):160–7.
Dahmane N, Sánchez P, Gitton Y, Palma V, Sun T, Beyna M, Ruiz i Altaba A. The Sonic Hedgehog-Gli pathway regulates dorsal brain growth and tumorigenesis. Development. 2001;128(24):5201–12.
Doetsch F, Petreanu L, Caille I, Garcia-Verdugo J-M, Alvarez-Buylla A. EGF converts transit-amplifying neurogenic precursors in the adult brain into multipotent stem cells. Neuron. 2002;36(6):1021–34.
Jackson EL, Garcia-Verdugo JM, Gil-Perotin S, Roy M, Quinones-Hinojosa A, VandenBerg S, Alvarez-Buylla A. PDGFRα-positive B cells are neural stem cells in the adult SVZ that form glioma-like growths in response to increased PDGF signaling. Neuron. 2006;51(2):187–99.
Sanai N. Adult neural stem cells and gliomagenesis. In: Glioblastoma. Springer; 2010. p. 153–65.
Savarese TM, Jang T, Pang Low H, Salmonsen R, Litofsky NS, Matijasevic Z, Recht LD. Isolation of immortalized, INK4a/ARF-deficient cells from the subventricular zone after in utero N-ethyl-N-nitrosourea exposure. J Neurosurg. 2005;102(1):98–108.
McLendon R, Friedman A, Bigner D, Van Meir EG, Brat DJ, Mastrogianakis GM, Aldape K. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455(7216):1061–8.
Jang T, Litofsky NS, Smith TW, Ross AH, Recht LD. Aberrant nestin expression during ethylnitrosourea-(ENU)-induced neurocarcinogenesis. Neurobiol Dis. 2004;15(3):544–52.
Gil-Perotin S, Marin-Husstege M, Li J, Soriano-Navarro M, Zindy F, Roussel MF, Casaccia-Bonnefil P. Loss of p53 induces changes in the behavior of subventricular zone cells: implication for the genesis of glial tumors. J Neurosci. 2006;26(4):1107–16.
Katayama K-I, Ueno M, Yamauchi H, Nagata T, Nakayama H, Doi K. Ethylnitrosourea induces neural progenitor cell apoptosis after S-phase accumulation in a p53-dependent manner. Neurobiol Dis. 2005;18(1):218–25.
Leonard JR, D’Sa C, Klocke BJ, Roth KA. Neural precursor cell apoptosis and glial tumorigenesis following transplacental ethyl-nitrosourea exposure. Oncogene. 2001;20(57):8281–6.
Wang Y, Yang J, Zheng H, Tomasek GJ, Zhang P, McKeever PE, Zhu Y. Expression of mutant p53 proteins implicates a lineage relationship between neural stem cells and malignant astrocytic glioma in a murine model. Cancer Cell. 2009;15(6):514–26.
Zhu Y, Guignard F, Zhao D, Liu L, Burns DK, Mason RP, Parada LF. Early inactivation of p53 tumor suppressor gene cooperating with NF1 loss induces malignant astrocytoma. Cancer Cell. 2005;8(2):119–30.
Chen J, McKay RM, Parada LF. Malignant glioma: lessons from genomics, mouse models, and stem cells. Cell. 2012;149(1):36–47.
Chow LM, Endersby R, Zhu X, Rankin S, Qu C, Zhang J, Baker SJ. Cooperativity within and among Pten, p53, and Rb pathways induces high-grade astrocytoma in adult brain. Cancer Cell. 2011;19(3):305–16.
Lei L, Sonabend AM, Guarnieri P, Soderquist C, Ludwig T, Rosenfeld S, Canoll P. Glioblastoma models reveal the connection between adult glial progenitors and the proneural phenotype. PLoS One. 2011;6(5):e20041.
Llaguno SRA, Chen J, Parada LF. Signaling in malignant astrocytomas: role of neural stem cells and its therapeutic implications. Clin Cancer Res. 2009;15(23):7124–9.
Marumoto T, Tashiro A, Friedmann-Morvinski D, Scadeng M, Soda Y, Gage FH, Verma IM. Development of a novel mouse glioma model using lentiviral vectors. Nat Med. 2009;15(1):110–6.
Li L, Liu F, Salmonsen RA, Turner TK, Litofsky NS, Di Cristofano A, Ross AH. PTEN in neural precursor cells: regulation of migration, apoptosis, and proliferation. Mol Cell Neurosci. 2002;20(1):21–9.
Zheng H, Ying H, Yan H, Kimmelman AC, Hiller DJ, Chen A-J, Ding Z. p53 and Pten control neural and glioma stem/progenitor cell renewal and differentiation. Nature. 2008;455(7216):1129–33.
Jafri NF, Clarke JL, Weinberg V, Barani IJ, Cha S. Relationship of glioblastoma multiforme to the subventricular zone is associated with survival. Neuro Oncol. 2013;15(1):91–6.
Lim DA, Cha S, Mayo MC, Chen M-H, Keles E, VandenBerg S, Berger MS. Relationship of glioblastoma multiforme to neural stem cell regions predicts invasive and multifocal tumor phenotype. Neuro Oncol. 2007;9(4):424–9.
Young GS, Macklin EA, Setayesh K, Lawson JD, Wen PY, Norden AD, Kesari S. Longitudinal MRI evidence for decreased survival among periventricular glioblastoma. J Neurooncol. 2011;104(1):261–9.
Schulenburg A, Ulrich-Pur H, Thurnher D, Erovic B, Florian S, Sperr WR, Zielinski CC. Neoplastic stem cells: a novel therapeutic target in clinical oncology. Cancer. 2006; 107(10):2512–20.
Adeberg S, Bostel T, König L, Welzel T, Debus J, Combs SE. A comparison of long-term survivors and short-term survivors with glioblastoma, subventricular zone involvement: a predictive factor for survival? Age (years). 2014;64:8.
Corn BW, Raizer J, Kanner AA. Should the subventricular zone be part of the “rad” zone? J Neurooncol. 2014;118:423–4.
Evers P, Lee PP, DeMarco J, Agazaryan N, Sayre JW, Selch M, Pajonk F. Irradiation of the potential cancer stem cell niches in the adult brain improves progression-free survival of patients with malignant glioma. BMC Cancer. 2010;10(1):384.
Gupta T, Nair V, Paul SN, Kannan S, Moiyadi A, Epari S, Jalali R. Can irradiation of potential cancer stem-cell niche in the subventricular zone influence survival in patients with newly diagnosed glioblastoma? J Neurooncol. 2012;109(1):195–203.
Lee P, Eppinga W, Lagerwaard F, Cloughesy T, Slotman B, Nghiemphu PL, Demarco J. Evaluation of high ipsilateral subventricular zone radiation therapy dose in glioblastoma: a pooled analysis. Int J Radiat Oncol Biol Phys. 2013;86(4):609–15.
Sonoda Y, Saito R, Kanamori M, Kumabe T, Uenohara H, Tominaga T. The association of subventricular zone involvement at recurrence with survival after repeat surgery in patients with recurrent glioblastoma. Neurol Med Chir. 2014;54(4):302–9.
Friedmann-Morvinski D, Bushong EA, Ke E, Soda Y, Marumoto T, Singer O, Verma IM. Dedifferentiation of neurons and astrocytes by oncogenes can induce gliomas in mice. Science. 2012;338(6110):1080–4.
Bachoo RM, Maher EA, Ligon KL, Sharpless NE, Chan SS, You MJ, Weissleder R. Epidermal growth factor receptor and Ink4a/Arf: Convergent mechanisms governing terminal differentiation and transformation along the neural stem cell to astrocyte axis. Cancer Cell. 2002;1(3):269–77.
Lindberg N, Kastemar M, Olofsson T, Smits A, Uhrbom L. Oligodendrocyte progenitor cells can act as cell of origin for experimental glioma. Oncogene. 2009;28(23):2266–75.
Liu C, Sage JC, Miller MR, Verhaak RG, Hippenmeyer S, Vogel H, Luo L. Mosaic analysis with double markers reveals tumor cell of origin in glioma. Cell. 2011;146(2):209–21.
Sugiarto S, Persson AI, Munoz EG, Waldhuber M, Lamagna C, Andor N, Siu J. Asymmetry-defective oligodendrocyte progenitors are glioma precursors. Cancer Cell. 2011;20(3):328–40.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Kusne, Y., Sanai, N. (2015). The SVZ and Its Relationship to Stem Cell Based Neuro-oncogenesis. In: Ehtesham, M. (eds) Stem Cell Biology in Neoplasms of the Central Nervous System. Advances in Experimental Medicine and Biology, vol 853. Springer, Cham. https://doi.org/10.1007/978-3-319-16537-0_2
Download citation
DOI: https://doi.org/10.1007/978-3-319-16537-0_2
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-16536-3
Online ISBN: 978-3-319-16537-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)