Abstract
The concept of natural orifice translumenal endoscopic surgery (NOTES) was seamlessly adapted to colorectal resections, enabled by the availability of a wide array of transanal endoscopic platforms. Since the demonstration of the feasibility of this approach in experimental models and human cadavers, transanal endoscopic proctectomy has rapidly emerged as one of the most exciting new trends in rectal cancer surgery. Indications for this approach range from benign inflammatory conditions to malignant disease of the rectum, particularly of the mid- and low rectum, where transanal endoscopic access can be applied to completion proctectomy, total proctocolectomy, abdominoperineal resection (APR), restorative proctectomy or proctocolectomy with ileoanal J pouch reconstruction, and low anterior resection with total mesorectal excision (TME). Whether it is performed using a pure or hybrid approach, transanal endoscopic proctectomy has the potential to reduce the technical complexity, morbidity, and operative time associated with open and laparoscopic proctectomy. Preliminary outcomes from 16 published series on this approach for benign and malignant pathology suggest that this approach is safe in carefully selected patients. Specific indications, preoperative work-up, surgical techniques, and expected complications associated with transanal endoscopic proctectomy, as well as tips and tricks for success, will be reviewed, based on the rapidly expanding literature on this approach.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Transanal endoscopic proctectomy
- Total mesorectal excision
- NOTES
- Rectal cancer
- Inflammatory bowel disease
- Completion proctectomy
Introduction
Total mesorectal excision (TME) has become the standard of care for resection of rectal cancer, with en-bloc removal of the rectum and the surrounding lymphatic tissue by sharp dissection within the plane between the pelvic visceral and parietal fascia, with or without preservation of the sphincter mechanism [1]. Widespread adoption of TME technique in combination with systematic use of neoadjuvant chemoradiation has substantially improved oncologic outcomes of resectable locally advanced rectal cancer. Laparoscopic approaches for TME have slowly gained wider acceptance over traditional open approaches in low anterior resection (LAR) or abdominoperineal resection (APR) when possible, due to well-documented advantages from multiple randomized trials including decreased blood loss and length of hospital stay, faster return of bowel function, with equivalent oncologic outcomes [2–4]. However, laparoscopic TME is associated with longer operative time and similar surgical morbidity as the open approach, including wound-related complications, as it does not obviate creation of a sizeable abdominal incision for specimen extraction. For tumors located within the distal 5 cm of the rectum, laparoscopic TME is associated with a long learning curve due to the technical complexity of achieving negative resection margins both circumferentially and distally, in addition to sphincter preservation, particularly in obese patients with a narrow pelvis. Rates of positive circumferential resection margins (CRM) with laparoscopic TME remain 10–18 %, and conversion rates are as high as 30 %, even in experienced hands [2–4]. Not surprisingly, adoption rates of laparoscopic techniques for rectal cancer resections remain approximately 30 % of less, with recent interest in robotic techniques as a potential solution to bridge the technical gap required for these complex procedures [5].
An even greater technical challenge is posed by ultralow rectal tumors , located in close proximity to the dentate line, and that require either partial or complete intersphincteric resection (ISR) in combination with TME in order to achieve oncologically adequate resection while preserving the sphincter complex. Combined abdominal and perineal dissection is required, where inferior mesenteric vessel ligation, splenic flexure mobilization, and TME down to the level of the levator ani are performed using an open or laparoscopic transabdominal approach, followed by the perineal ISR phase. During the perineal dissection, a variable length of distal rectum and mesorectum are dissected beyond incision of the anorectal mucosa and internal anal sphincter [6] and is typically limited by poor exposure with conventional anorectal instruments. Abdominal and perineal dissection planes are ultimately connected and the specimen is extracted transanally when feasible, followed by coloanal anastomosis . While ISR has been associated with good short- and long-term oncologic outcomes [7, 8], its widespread adoption has been limited by the technical challenges of achieving an R0 resection, as well as concerns over poor functional outcomes, which have largely favored APR over ISR, especially in the United States.
The concept of Natural Orifice Translumenal Endoscopic Surgery (NOTES) , whereby complex abdominal procedures can be performed endoscopically without the need for abdominal incisions, is ideally suited for colorectal applications. By virtue of the availability of multitasking and multiport transanal endoscopic platforms (TEM, TEO, TAMIS), transanal NOTES rectosigmoid resection has rapidly transitioned from experimental animal models and human cadavers into clinical practice [9]. The adoption of NOTES techniques in colorectal surgery led to the evolution of transanal specimen extraction, and transanal NOTES TME (taTME), performed either using a hybrid approach with laparoscopic, robotic, or open transabdominal assistance, or using a pure transanal NOTES approach [10]. Since the report of the first case of a laparoscopic-assisted transanal NOTES TME for a mid-rectal cancer in 2010 [11], taTME has been gaining momentum with over 250 cases published to date. Cumulatively, the published data from case series on transanal endoscopic TME demonstrates the technical feasibility and preliminary oncologic safety of this approach in carefully selected patients with resectable upper, mid, and low rectal cancers, with overall good quality TME, adequate lymph node harvest, adequate distal margins and CRM’s, as well as morbidity comparable to that following laparoscopic TME [10, 12–22].
Although taTME is used for tumors throughout the rectum, the majority of reports describe the use of taTME for mid and lower rectal tumors. The quoted benefits of a transanal endoscopic approach for very low rectal cancers in particular include the ability to expand the upper limit of intersphincteric resection (ISR) and facilitate completion of a complete rectal and mesorectal dissection using a primarily transanal approach, particularly in patients with substantial visceral obesity and narrow pelvises with anticipated difficulties completing the TME from an abdominal approach. Additional benefits include improved visualization provided by transanal endoscopic platforms (rigid and disposable platforms including TEM, TEO, and TAMIS platforms, combined with HD or 3D imaging), early identification of the distal resection margins which may reduce the incidence of margin positivity, and avoidance of an abdominal extraction site when transanal specimen extraction is feasible.
Indications and Contraindications for Transanal Endoscopic Proctectomy
Although the data from published series has not yet matured with respect to oncologic and functional outcomes, transanal endoscopic proctectomy, with or without TME, has been shown to be feasible and effective in the treatment of benign and malignant diseases of the rectum. Based on the data published on this approach to date, there is a growing consensus regarding specific indications and contraindications for this approach based on specific pathology, tumor stage, and favorable vs. unfavorable anatomic factors.
Benign Indications
Transanal endoscopic completion proctectomy is a particularly attractive approach when seeking to avoid abdominal entry during removal of retained rectal stumps. Indications for a transanal endoscopic approach are the same as for any other approach to completion proctectomy and include refractory proctitis from diversion, ulcerative colitis (UC), or Crohn’s disease (CD). Depending on the length of residual rectal stump to be removed, a pure transanal endoscopic approach or hybrid transanal/abdominal approach can be performed. The transanal approach also lends itself well to intersphincteric proctectomy in cases of refractory radiation proctitis or fecal incontinence, strictures, rectovaginal fistulas, or other complex pelvic fistula, as well as anastomotic complications from prior proctectomy. Depending on the specific pathology warranting proctectomy, rectal dissection can be carried out along the rectal wall with preservation of the mesorectum, or in combination with total mesorectal dissection.
To date, there have been four series published on transanal endoscopic proctectomy for benign indications, describing outcomes in a total of 36 patients. Procedures performed included transanal endoscopic completion proctectomy, transanal endoscopic-assisted proctectomy, transanal endoscopic-assisted restorative proctectomy [13, 23–25] and proctocolectomy with IPAA [25] for refractory diversion and radiation proctitis, IBD, large carpeting villous adenomas of the rectum, fecal incontinence, rectal strictures, and complex fistulas (Table 11.1). The length of the resected retained rectal stumps ranged from 8 to 30 cm. There were no mortality or major procedural complications except for conversion to open proctectomy due to intraabdominal adhesion [13]. The cumulative morbidity across the series was 39 % (14/36 cases) and included urinary tract infections, a hematoma, several cases of delayed perineal wound healing, a perineal dehiscence requiring reoperation, an incarcerated parastomal hernia, and a colocutaneous fistula to the perineum requiring reoperation [13, 24, 25] (Table 11.1).
Rectal Cancer
Unlike benign disease, proctectomy for rectal adenocarcinoma strictly requires total mesorectal excision. Oncologically, adequate resection with a complete mesorectum and negative margins is critical to minimize the chance of local recurrence, with the CRM being a major determinant of overall survival following curative rectal cancer resection. Of critical importance in the early stages of adoption of transanal endoscopic TME for rectal cancer (taTME) was the demonstration of the feasibility of achieving adequate mesorectal dissection and satisfactory short-term oncologic outcomes. The major drive behind increased adoption on this approach has been the suggested improvement in access to the low rectum and mesorectum relative to open and laparoscopic approaches and an enhanced view of dissection planes achieved through the transanal platforms. This bottom-up approach may provide a less obstructed view and manipulation of the perirectal and mesorectal planes, facilitating the mesorectal dissection, especially for low rectal tumors in narrow pelvises.
Since the first published case report of laparoscopic-assisted taTME in 2010 for a mid-rectal T2N1 cancer treated with neoadjuvant therapy [11], at least 12 case series including 247 patients have since published their preliminary perioperative and oncologic results with taTME performed with either open, laparoscopic, robotic, or with no abdominal assistance. Across the 12 published series, 8 % (21/247) of taTME cases were performed as part of an APR, and 92 % as part of sphincter-preserving restorative proctectomy (Tables 11.2 and 11.3).
Tumor Stage
With respect to tumor selection for taTME , the large majority of studies performed taTME for non-obstructing, resectable tumors including preoperatively staged T1, T2, and T3, N0 or N1 tumors. When studies were performed under protocol [15, 21, 22], and early in their operative experience, most authors specifically excluded T4 and metastatic tumors, local recurrences, and tumors with threatened CRM margins based on staging MRI. Cumulatively, across the 12 published series with sample size ranging from 4 to 56 patients, the mesorectum was complete in 90 % and near complete in 8.7 % of patients, with negative resection margins achieved in 95 %, and an average of 12–33 lymph nodes harvested (Tables 11.2 and 11.3). Although five publications included one to two unsuspected T4 rectal tumors [10, 16, 18, 20, 21], only one study specifically selected unfavorable tumors in male patients for this approach, including large T3 and T4 tumors, located anteriorly, in the distal 5 cm of rectum, and with threatened positive CRM’s [14], all radiated preoperatively. The rationale for this patient selection was to facilitate completion of sphincter-preserving good-quality TME in cases that were otherwise predicted to be technically challenging and associated with a high risk of incomplete mesorectal specimens. The authors were able to achieve a complete mesorectum in every case, but reported a 13 % incidence of positive margins, and 80.5 % overall survival at 24 months, reflecting the advanced stage of the tumors [14].
In the largest and only multicenter taTME series published to date, 56 patients with locally advanced tumors ≤ 5 cm from the anal verge, most of which treated with neoadjuvant treatment (84 %), underwent taTME with laparoscopic assistance with complete or near complete mesorectum achieved in all cases, and a 95 % R0 resection rate [21]. There were three conversions and six cases of delayed anastomosis, no mortality, and a 26 % morbidity rate including anastomotic leakage, pelvic sepsis urinary dysfunction, bleeding, and a cerebrovascular accident. Local recurrence rate at a median follow-up of 29 months was 1.7 %, with 96.4 % overall survival [21].
Although the international experience with taTME is still preliminary with no randomized trial yet comparing taTME with open or laparoscopic TME, two retrospective studies compared outcomes of matched cohorts of patients who underwent taTME vs. laparoscopic TME [19, 20]. Fernandez-Hevia et al. retrospectively case-matched 37 cases of laparoscopic-assisted taTME with 37 cases of laparoscopic TME for rectal cancer and demonstrated no significant differences with respect to quality of the mesorectal specimen, lymph node harvest, resection margins, or intraoperative complications [19]. They also demonstrated comparable 30-day postoperative complications, but a statistically significant lower readmission rate in the taTME group (2 % vs. 6 %) [19]. Velthuis et al. retrospectively matched 25 cases of laparoscopic-assisted taTME with 25 cases of laparoscopic TME, and interestingly, they found that taTME was associated with a significantly higher rate of complete mesorectum than laparoscopic TME (92 % vs. 72 %) [19].
Tumor Location
With respect to location, there are no absolute contraindications to performance of the TME, either in part or completely, using a transanal endoscopic approach. As demonstrated in the published series on transanal proctectomy for benign indications, a pure transanal endoscopic approach can be used in completion proctectomy for short rectal stumps. For longer stumps, or when there is concern of possible pelvic adhesions to the proximal stump, transanal procedures are performed with abdominal assistance [13, 23, 24].
With respect to rectal cancer, based on the 12 series of taTME cases published to date, the majority of tumors were located in the mid- and low rectum (<10 cm from the anal verge), with only five studies including high rectal tumors (≥10 cm from the anal verge) [10, 14, 15, 20, 22] (Tables 11.2 and 11.3). Most authors determined that the benefit of using a transanal approach was less evident for upper rectal tumors, where standard laparoscopic techniques can usually achieve adequate TME and sphincter preservation. TME for mid- and low rectal tumors can be performed using a pure transanal endoscopic approach when feasible, or with a hybrid approach with abdominal assistance. Depending on the level of distal rectal or anorectal transection, stapled or handsewn coloanal anastomosis is performed, with or without creation of a colonic J pouch, based on the surgeons’ preference.
Across the series reporting on outcomes of taTME for tumors of the distal third of the rectum, advantages of the transanal approach cited by authors include early and accurate assessment of the distal resection margins which is an essential prerequisite for achieving sphincter preservation and R0 resection. For tumors located ≤1 cm from the anorectal ring, taTME is performed with intersphincteric resection with handsewn coloanal anastomosis , in order to achieve negative margins. For tumors invading the external sphincter muscle or for other contraindications to sphincter preservation such as baseline fecal incontinence, taTME was performed in conjunction with APR [13, 16, 18, 20, 21].
Anatomic Factors
Across published series, and reflecting the centers’ learning curve, most patients were carefully selected with respect to BMI, prior abdominal and pelvic operations, prior pelvic radiation, and any other anatomic features that might significantly complicate transanal dissection and increase intraoperative complications such as rectal perforation, organ injury, bleeding, and conversion. The average BMI across 12 series including 247 patients ranged from 23.4 to 27.9 (Tables 11.2 and 11.3) with a cumulative incidence of intraoperative complications of 8 %, which included mostly conversion to open proctectomy and rectal perforation (Tables 11.2 and 11.3). In Rouanet’s series of 30 males with high-risk low rectal tumors, two urethral injuries occurred early in the authors’ experience, highlighting the importance of careful patient selection early in the surgeon’s operative experience [14]. This is particularly true in males with very low rectal tumors, when ISR is required and is extended cephalad using a transanal endoscopic approach. Anterior rectal dissection within a radiated field can be particularly arduous, and as with APR, can result in rectal perforation and urethral injury.
Taken altogether, taTME has thus far been demonstrated to be safe and effective in the oncologic resection of carefully selected resectable rectal cancer and is particularly well-suited for tumors of the mid- and low rectum. Relative contraindications, particularly early in the operator’s learning curve, include bulky tumors, T4 tumors, prior pelvic surgery and radiation with anticipated dense pelvic adhesions, visceral obesity, and any other unfavorable anatomic factors, as they have been associated with a higher rate of intraoperative complications, as well as higher risk of positive margins. However, preliminary oncologic data from taTME series, including the analysis of the quality of mesorectal excision, have shown that taTME is associated with a high rate of complete mesorectal specimens, which may surpass that achieved using a laparoscopic approach. Further comparative studies, including randomized trials, will be needed to evaluate these differences further.
Preoperative Workup
Evaluation of surgical candidates for transanal endoscopic proctectomy follows the same principles as for any other approaches to the rectum. Preoperative workup includes a complete medical and surgical history, colonoscopy with biopsies, and a comprehensive physical exam, including a digital rectal exam (DRE) . Preoperative assessment should take into account patients’ baseline activity level, defecatory function, as well as urinary and sexual function. For newly diagnosed rectal cancer, laboratory studies include complete blood count, serum chemistries, liver function tests, and baseline serum carcinoembryonic antigen level. Staging CT scans of the chest, abdomen, and pelvis should be completed in addition to a pelvic MRI for tumor staging and to assess the status of the CRM. Endorectal ultrasound can be performed in conjunction with pelvic MRI. Patients with locally advanced disease should undergo standard long-course neoadjuvant treatment, although in some cases, short-course radiation may be elected, and neoadjuvant treatment avoided altogether in carefully selected T3a rectal tumors [19].
Preoperative DRE should assess anal sphincter function and localize the tumor along the rectum, determine fixity and distance from the anorectal ring, and dentate line and anal verge. Preoperative evaluation should also include proctoscopy or flexible sigmoidoscopy to visualize the rectal tumor or affected region of the rectum for surgical planning. DRE, office endoscopy, and/or pelvic MRI may be repeated following completion of neoadjuvant treatment, to assess tumor response, as that may impact the operative plan with respect to sphincter preservation. Transanal proctectomy for rectal cancer is typically performed 6–10 weeks following completion of neoadjuvant treatment, which is standard of care in the management of locally advanced rectal cancer.
Candidates for sphincter-preserving proctectomy using transanal assistance should be extensively counseled regarding temporary fecal diversion, as well as anticipated functional disturbances and quality of life issues following ileostomy closure, especially if radiation was administered preoperatively. This is particularly important for very low rectal tumors, when partial or complete ISR might be required in order to achieve negative resection margins.
Operative Details
With the exception of completion proctectomy, patients undergoing transanal endoscopic restorative proctectomy (LAR or IPAA) usually undergo mechanical bowel preparation, either orally and enemas, or with enemas alone, the night prior to surgery [13, 23–25]. Standard perioperative antibiotic prophylaxis is administered parentally, and while most surgeons perform these procedures with patient in lithotomy position, completion proctectomy can be performed in prone position, which can be helpful in cases where hip flexion is severely limited [25]. Most authors perform on-table rectal irrigation with dilute betadine. A Foley catheter is routinely placed, and the abdomen and perineum are both prepped and draped to allow simultaneous or sequential access during hybrid transanal procedures.
Hybrid Procedures
If abdominal assistance is planned, it can be provided using standard laparoscopic access (single-incision, hand-assisted, multiport, or robotic) or using an open approach. Laparoscopic access is usually obtained as the first step, which allows thorough evaluation of the abdominal cavity. Laparoscopic assistance can then be performed simultaneously with, prior to, or following transanal proctectomy. There may be some advantages with a dual-team approach where the abdominal and perineal teams work simultaneously with respect to operating time (Fig. 11.1). While the transanal team performs the proctectomy, the abdominal team proceeds with splenic flexure takedown, inferior mesenteric vessel dissection and division, followed by assistance during completion of the transanal TME. In a cohort of 20 patients undergoing hybrid taTME, Chen et al. found that using a two-team approach in 8 patients significantly reduced the operative time relative to a single team approach in 12 patients (226 ± 32 vs. 157.5 ± 31.7 min) [17]. Furthermore, in their comparative study of 37 hybrid taTME cases performed using two surgical teams to a matched cohort who underwent laparoscopic TME for rectal cancer, the authors reported significantly shorter mean operative time in the taTME group (215 ± vs. 252 ± 50 min) [19].
Dual team set-up for laparoscopic-assisted transanal endoscopic proctectomy with TME (taTME). The abdominal and transanal teams are working simultaneously. While the abdominal team performs laparoscopic splenic flexure and mesenteric vessel division, the transanal team performs endoscopic rectal and mesorectal dissection through the transanal platform
Transanal Endoscopic Completion Proctectomy, Proctocolectomy, and Apr
In cases of proctocolectomy, laparoscopic, robotic, or open abdominal total colectomy is usually performed first followed by ligation of the inferior mesenteric vessels, creation of an end-ileostomy, or end-colostomy. For APR, the rectosigmoid colon is mobilized and transected, with or without splenic flexure takedown, followed by mesenteric dissection and creation of the end-colostomy. Transanal endoscopic perineal proctectomy is performed simultaneously or sequentially. Whether performed first, during or following abdominal procedures, the transanal team prepares the rectum for proctectomy by suturing the anus closed, initiating intersphincteric or extrasphincteric proctectomy using a standard open transanal approach, and continuing the dissection until the puborectalis is reached [25]. At that level, the transanal endoscopic platform (rigid metal or disposable) is inserted, CO2 insufflated, and rectal dissection is extended proximally through the endoscopic platform. Depending on the pathology, the posterior dissection is carried out either within the mesorectum, or along the presacral plane, according to the principles of TME. Alternatively, in cases of completion proctectomy for benign indications, the endoscopic platform is first inserted, the rectum is divided full-thickness circumferentially starting above the dentate line, and the rectal and mesorectal dissection carried until the rectal stump is entirely mobilized and exteriorized transanally. Then, intersphincteric dissection of the anal canal and distal rectal wall is carried out [23]. Transanal endoscopic dissection of the rectum can be safely performed without abdominal assistance, as long as there are no pelvic adhesions precluding safe dissection of intraperitoneal portion of the rectal stump. If extensive pelvic adhesions prevent adequate visualization and safe dissection of the rectal stump, abdominal assistance should be used. The rectum is subsequently removed transanally, the cavity irrigated, and the perineal wound closed in layers with or without the use of drains.
Transanal Endoscopic-Assisted Restorative Proctectomy
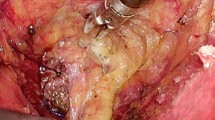
Transanal endoscopic proctectomy with TME is most commonly performed for resectable rectal cancer of the mid- and low rectum. Transanal procedures typically start with confirmation of the location of the tumor in relationship to the anorectal ring and dentate line (Fig. 11.2a). For tumors that are well above the dentate line and ≥1 cm above the anorectal ring, the rectum is occluded with a pursestring suture below the tumor (Fig. 11.2b), and a transanal endoscopic platform is inserted with insufflation of CO2. Through the transanal platform, the rectal mucosa is scored circumferentially with cautery (Fig. 11.3a) and full thickness rectal and mesorectal dissection is initiated (Fig. 11.3b) and extended proximally along the anterior, lateral, and posterior planes (Fig. 11.4).
For rectal tumors that are less than 1 cm from the top of the anorectal ring and abutting the dentate line, partial ISR is first performed using standard technique. For tumors that are at the level of or just below the dentate line, total ISR is performed [21], with preservation of the uninvolved external sphincter muscle for later coloanal anastomosis . ISR is extended cephalad using an open approach until the levator ani is identified posteriorly, and the rectoprostatic or rectovaginal plane is identified anteriorly. At that point, depending on the preference of the operator, additional intersphincteric dissection is completed using an open approach, or through the transanal endoscopic platform, after airtight closure of the anal stump. Further superior mobilization includes division of the anococcygeal raphe posteriorly, leading to the presacral space and bottom of the mesorectum. Anteriorly, further mobilization includes extending sharp dissection along the rectoprostatic or rectovaginal plane. At that point, if not done earlier, the anorectal stump is tightly occluded with a pursestring suture to avoid fecal spillage, leak of CO2 into the colon, and potential spillage of tumor cells in the operative field. The endoscopic platform is inserted transanally, and rectal and mesorectal dissection is completed circumferentially using monopolar cautery, with or without bipolar energy (Fig. 11.4). Typically, the anterior dissection is extended proximally until the peritoneal reflection is reached, divided, and the peritoneal cavity is entered. Posteriorly, the dissection is extended towards S1–S2 levels and is usually limited by the sharp angle of the sacral promontory, preventing further cephalad exposure. If technically feasible, the taTME can be extended into the abdominal cavity with division of the inferior mesenteric vessels using a bipolar device or surgical stapler inserted through the transanal platform. In some cases, the left colon is mobile enough not to require splenic flexure takedown, and alternatively, the splenic flexure can be mobilized transanally until it is mobile enough to allow a tension-free coloanal anastomosis [16]. In most cases, however, abdominal assistance is required for splenic flexure takedown, mesenteric vessel ligation, and completion of the mesorectal division (Fig. 11.5).
Following completion of the TME, the specimen is exteriorized either transanally or through a small abdominal incision, if the specimen is too bulky [14, 22], followed by handsewn coloanal anastomosis or stapled colorectal anastomosis , depending on the height of distal anorectal cuff and the surgeon’s preference (Fig. 11.6). Either end-end or side-end anastomosis is constructed with or without creation of a colonic J pouch. In the large majority of published cases, a diverting loop ileostomy is performed to protect the anastomosis, with liberal use of pelvic drains.
Of note, when restorative proctectomy or proctocolectomy is used in combination with ileoanal J pouch reconstruction in IBD, the colectomy and pouch creation are completed using an abdominal approach followed by transanal proctectomy. Transanal procedures are typically initiated by placement of a self-retaining retractor and circumferential sleeve mucosectomy starting at the dentate line is then followed by full-thickness rectal transection as described above [25]. Alternatively, following pursestring occlusion of the low rectum just above the anorectal ring, full-thickness rectal transection is initiated transanally followed by completion of the proctectomy, with or without TME [23].
Robotic Transanal Dissection
Most recently, several groups have described laparoscopic-assisted taTME, with the transanal dissection performed using the robotic arms inserted through a TAMIS platform (Table 11.4) [26–29]. The robot is docked over the left or right hip, and transanal dissection is performed using 2 robotic arms and the camera, with or without the use of an assistant port. Although the data is relatively preliminary, with only four case series with sample size ranging from 1 to 7, outcomes from the 16 patients who have undergone this procedure suggest the feasibility and preliminary safety of this approach in carefully selected patients with rectal cancer [26–29] by highly skilled robotic surgeons. There were wide variations in the average operative time across the series, ranging from 165.7 to 398 min, likely reflecting the learning curve. With the majority of tumors located in the low rectum (≤5 cm from the anal verge), R0 resection was achieved in all cases, and the mesorectum was complete in 81 % of cases, or nearly complete in 19 % of all cases. There were no conversions or mortality, and the morbidity rate was 25 % (4/16 cases).
Postoperative Care
Patients are admitted to the surgical service postoperatively. A urinary catheter is typically kept in place for at least 48 h postprocedure given the relatively high incidence of postoperative urinary retention following perineal dissection, especially in males [14, 15, 17, 21]. A total of one to two doses of parenteral antibiotics are administered postoperatively as is standard of care. Patients are usually managed using enhanced recovery protocols including immediate initiation of oral intake as tolerated. Pain control is provided as per enhanced recovery pathways including aggressive non-narcotic regimens. Patients are extensively counseled regarding management of ostomies prior to discharge, especially with respect to hydration. Average length of hospital stay ranges from 2 to 5 days for benign disease [13, 23–25] and 4.5–12 days following taTME based on published reports (Tables 11.2 and 11.3). In the retrospective case-matched study by Fernandez-Hevia comparing 37 patients who underwent hybrid taTME to 37 patients who underwent laparoscopic TME, although there were no differences in the length of hospital stay, there were statistically more readmissions in the laparoscopic group than in the taTME group (22 % vs. 6 %) [19].
Possible Complications
Based on the published reports on transanal completion proctectomy for benign disease, the cumulative rate of postoperative complications was 39 % (Table 11.1) with no mortality. The majority of complications were minor with the most serious and frequent complication consisting in non-healing perineal wounds [24, 25].
Based on the 12 published series of pure and hybrid taTME for rectal cancer , the cumulative intraoperative complication rate was 8 % (20/247 cases) and mostly consisted in conversions to open proctectomy due to technical difficulties during transanal dissection (Tables 11.2 and 11.3). Other intraoperative complications included urethral injuries, air embolism, rectal perforation, and the need for delayed anastomosis due to technical difficulties. Forty percent of all reported intraoperative complications (5/20 cases) occurred in the Rouanet study, which was not entirely surprising given selection of high-risk patients, including males’ very low, bulky, and mostly anterior tumors [14]. The authors pointed out that the two urethral injuries occurred early in their learning curve and during dissection of bulky anterior tumors, one of which with concomitant prostatic carcinoma [14].
The incidence of postoperative complications based on the 12 published case series is within the range of that anticipated from laparoscopic TME, and cumulatively, that rate was 30 % (70/247 cases). There was no 30-day mortality. Major complications included anastomotic leak, intraabdominal abscess, sepsis, SBO, bleeding, ileus, and transient urinary retention (Tables 11.2 and 11.3). In the only comparative matched series of taTME to laparoscopic TME that evaluated early oncologic as well as perioperative outcomes, there were no statistically significant differences in complication rates between the groups (32 % vs. 51 %) [19].
Follow-Up
Postoperative visits and evaluation following taTME are routine and per standard following rectal cancer resection. In patients with locally advanced rectal cancer treated with neoadjuvant treatment, ileostomy closure is usually deferred until completion of adjuvant treatment. Endoscopic and radiographic evaluation of the coloanal anastomosis is performed prior to reversal, and anastomotic complications such as strictures, leaks, and fistulas are managed using standard protocols. Oncologic surveillance following rectal cancer resections also follows standard NCCN guidelines. Regarding functional outcomes, patients who have undergone partial or complete intersphincteric resection are at increased risk for poor functional outcomes and require long-term monitoring of their defecatory function and aggressive management of their fecal incontinence.
Tips and Tricks
Procedural Training
Despite the lack of published data on the effect of the learning curve or the impact of inanimate training model on surgeon’s performance during transanal proctectomy, data from prior experimental studies on this technique have highlighted the importance of fresh human cadavers as the best suited training model for this technique [30]. Total mesorectal dissection is accurately reproducible in human cadavers, as most of the dissection in patients is bloodless, as long as rectal and mesorectal dissection proceeds along the anatomically correct planes. In their series of consecutive transanal endoscopic rectosigmoid resection in 32 human cadavers, based on the significant decrease in operative time in completing the procedures after five cases, the authors concluded that the learning curve for taTME was likely around five cadavers with regard to procedural training [30].
Operating Teams
Although not absolutely necessary, a dual team approach may have the potential to reduce operative time as well as intraoperative complications. Simultaneous visualization of the pelvis from the transabdominal and transanal sides may increase the accuracy of the dissection, particularly with regard to the pelvic side walls (to avoid nerve and ureteral injury), and during anterior peritoneal entry (to avoid inadvertent organ injury).
Smoke Evacuation
With the exception of one of the rigid metal platforms that provides continuous CO2 insufflation and suction, all other commercially available transanal endoscopic platforms lack a built-in mechanism for balanced smoke evacuation. Cyclical insufflation through standard laparoscopic insufflators result in intermittent and bothersome rectal flapping as a result of the fluctuations in pressures as occurs with smoke suctioning. It was recently suggested that the use of commercially available high-flow CO2 insufflators might solve this technical issue by maintaining a set working pressure via high-flow CO2 insufflation in response to smoke evacuation [31].
Anterior Dissection for a Very Low Rectal Tumor in a Male
In cases of a rectal tumor located ≤1.5 cm from the dentate line, it is safest to avoid initiating intersphincteric dissection directly through the transanal endoscopic platform. It is much safest to initiate ISR using standard open transanal techniques and to only insert the transanal platform once the anatomic landmarks have been identified, including the puborectalis and inferior aspect of the mesorectum posteriorly, and the rectovaginal or rectoprostatic plane anteriorly. As is the case in a difficult APR , there is a risk of dissecting above the anal sphincters during anterior perineal dissection, and erroneously dissect too anteriorly which could result in dissection of a plane above the prostate rather than in the rectoprostatic plane. Prostatic urethral injury is then likely to result and has been reported during taTME, which might be more likely to occur when intersphincteric resection is attempted endoscopically.
Abbreviations
- APR:
-
Abdominoperineal resection
- BMI:
-
Body mass index
- CD:
-
Crohn’s disease
- CRM:
-
Circumferential resection margin
- DRE:
-
Digital rectal examination
- IBD:
-
Inflammatory bowel disease
- IPAA:
-
Ileoanal pouch anastomosis
- ISR:
-
Intersphincteric resection
- LAR:
-
Low anterior resection
- NOTES:
-
Natural Orifice Endoscopic Surgery
- TAMIS:
-
Transanal minimally invasive surgery
- taTME:
-
Transanal endoscopic total mesorectal excision
- TME:
-
Total mesenteric excision
- UC:
-
Ulcerative colitis
References
Heald RJ, Ryall RD. Recurrence and survival after total mesorectal excision for rectal cancer. Lancet. 1986;1:1479–82.
Green BL, Marshall HC, Collinson F, Quirke P, Guillou P, Jayne DG, et al. Long-term follow-up of the Medical Research Council CLASICC trial of conventional versus laparoscopically assisted resection in colorectal cancer. Br J Surg. 2013;100:75–82.
Guillou PJ, Quirke P, Thorpe H, Walker J, Jayne DG, Smith AM, et al. Short-term endpoints of conventional versus laparoscopic-assisted surgery in patients with colorectal cancer (MRC CLASICC trial): multicentre, randomised controlled trial. Lancet. 2005;365:1718–26.
van der Pas M, Haglind E, Cuesta M, et al. COlorectal cancer Laparoscopic or Open Resection II (COLOR II) Study Group. Laparoscopic versus open surgery for rectal cancer (COLOR II): short-term outcomes of a randomised, phase 3 trial. Lancet Oncol. 2013;14:210–8.
Trastulli S, Farinella E, Cirocchi R, Cavaliere D, Avenia N, Sciannameo F, et al. Robotic resection compared with laparoscopic rectal resection for cancer: systematic review and meta-analysis of short-term outcome. Colorectal Dis. 2012;14:e134–56.
Rullier E, Sa Cunha A, Couderc P, Rullier A, Gontier R, Saric J. Laparoscopic intersphincteric resection with coloplasty and coloanal anastomosis for mid and low rectal cancer. Br J Surg. 2003;90:445–51.
Bretagnol F, Rullier E, Laurent C, Zerbib F, Gontier R, Saric J. Comparison of functional results and quality of life between intersphincteric resection and conventional coloanal anastomosis for low rectal cancer. Dis Colon Rectum. 2004;47:832–8.
Dumont F, Ayadi M, Goéré D, Honoré C, Elias D. Comparison of fecal continence and quality of life between intersphincteric resection and abdominoperineal resection plus perineal colostomy for ultra-low rectal cancer. J Surg Oncol. 2013;108:225–9.
Emhoff IA, Lee GC, Sylla P. Transanal colorectal resection using natural orifice translumenal endoscopic surgery (NOTES). Dig Endosc. 2014;26 Suppl 1:29–42.
Zorron R, Phillips HN, Wynn G, Neto MP, Coelho D, Vassallo RC. “Down-to-Up” transanal NOTES Total mesorectal excision for rectal cancer: preliminary series of 9 patients. J Minim Access Surg. 2014;10(3):144–50. doi:10.4103/0972-9941.134878.
Sylla P, Rattner DW, Delgado S, Lacy AM. NOTES transanal rectal cancer resection using transanal endoscopic microsurgery and laparoscopic assistance. Surg Endosc. 2010;24(5):1205–10. doi:10.1007/s00464-010-0965-6. Epub 2010 Feb 26.
Dumont F, Goéré D, Honoré C, Elias D. Transanal endoscopic total mesorectal excision combined with single-port laparoscopy. Dis Colon Rectum. 2012;55(9):996–1001. doi:10.1097/DCR.0b013e318260d3a0.
Wolthuis AM, de Buck van Overstraeten A, D’Hoore A. Dynamic article: transanal rectal excision: a pilot study. Dis Colon Rectum. 2014;57:105–9.
Rouanet P, Mourregot A, Azar CC, Carrere S, Gutowski M, Quenet F, Saint-Aubert B, Colombo PE. Transanal endoscopic proctectomy: an innovative procedure for difficult resection of rectal tumors in men with narrow pelvis. Dis Colon Rectum. 2013;56(4):408–15. doi:10.1097/DCR.0b013e3182756fa0.
Sylla P, Bordeianou LG, Berger D, Han KS, Lauwers GY, Sahani DV, Sbeih MA, Lacy AM, Rattner DW. A pilot study of natural orifice transanal endoscopic total mesorectal excision with laparoscopic assistance for rectal cancer. Surg Endosc. 2013;27(9):3396–405. doi:10.1007/s00464-013-2922-7. Epub 2013 Apr 10.
Chouillard E, Chahine E, Khoury G, Vinson-Bonnet B, Gumbs A, Azoulay D, Abdalla E. NOTES total mesorectal excision (TME) for patients with rectal neoplasia: a preliminary experience. Surg Endosc. 2014;28(11):3150–7. doi:10.1007/s00464-014-3573-z. Epub 2014 May 31.
Chen CC, Lai YL, Jiang JK, Chu CH, Huang IP, Chen WS, et al. The evolving practice of hybrid natural orifice transluminal endoscopic surgery (NOTES) for rectal cancer. Surg Endosc. 2014.
Atallah S, Martin-Perez B, Albert M, deBeche-Adams T, Nassif G, Hunter L, Larach S. Transanal minimally invasive surgery for total mesorectal excision (TAMIS-TME): results and experience with the first 20 patients undergoing curative-intent rectal cancer surgery at a single institution. Tech Coloproctol. 2014;18(5):473–80. doi:10.1007/s10151-013-1095-7. Epub 2013 Nov 23.
Fernández-Hevia M, Delgado S, Castells A, Tasende M, Momblan D, Díaz del Gobbo G, DeLacy B, Balust J, Lacy AM. Transanal total mesorectal excision in rectal cancer: short-term outcomes in comparison with laparoscopic surgery. Ann Surg. 2015;261(2):221–7. doi:10.1097/SLA.0000000000000865.
Velthuis S, Nieuwenhuis DH, Ruijter TE, Cuesta MA, Bonjer HJ, Sietses C. Transanal versus traditional laparoscopic total mesorectal excision for rectal carcinoma. Surg Endosc. 2014;28(12):3494–9.
Tuech JJ, Karoui M, Lelong B, De Chaisemartin C, Bridoux V, Manceau G, Delpero JR, Hanoun L, Michot F. A step toward NOTES total mesorectal excision for rectal cancer: endoscopic transanal proctectomy. Ann Surg. 2015;261(2):228–33. doi:10.1097/SLA.0000000000000994.
de Lacy AM, Rattner DW, Adelsdorfer C, Tasende MM, Fernández M, Delgado S, Sylla P, Martínez-Palli G. Transanal natural orifice transluminal endoscopic surgery (NOTES) rectal resection: “down-to-up” total mesorectal excision (TME)—short-term outcomes in the first 20 cases. Surg Endosc. 2013;27(9):3165–72. doi:10.1007/s00464-013-2872-0. Epub 2013 Mar 22.
Bremers AJ, van Laarhoven KJ, van der Kolk BM, de Wilt JH, van Goor H. Transanal endoscopic microsurgery approach for rectal stump resection as an alternative to transperitoneal stump resection. Br J Surg. 2013;100:568–71.
Liyanage C, Ramwell A, Harris GJ, Levy BF, Simson JN. Transanal endoscopic microsurgery: a new technique for completion proctectomy. Colorectal Dis. 2013;15:e542–7.
McLemore ECL, Devaraj B, Pola S, Docherty MJ, Patel DR, Levesque BG, et al. Transanal endoscopic surgical proctectomy for proctitis case series report: diversion, radiation, ulcerative colitis, and Crohn’s disease. Glob J Gastroenterol Hepatol. 2013;1:51–7.
Atallah S, Martin-Perez B, Pinan J, Quinteros F, Schoonyoung H, Albert M, et al. Robotic transanal total mesorectal excision: a pilot study. Tech Coloproctol. 2014;18(11):1047–53.
Verheijen PM, Consten EC, Broeders IA. Robotic transanal total mesorectal excision for rectal cancer: experience with a first case. Int J Med Robot. 2014;10(4):423–6.
Huscher CGS, Bretagnol F, Ponzano C. Robotic-assisted transanal total mesorectal excision: the key against the Achilles’ heel of rectal cancer ? Ann Surg. 2015. doi:10.1097/SLA.0000000000001089.
Gómez Ruiz M, Parra IM, Palazuelos CM, Martín JA, Fernández CC, Diego JC, Fleitas MG. Robotic-assisted laparoscopic transanal total mesorectal excision for rectal cancer: a prospective pilot study. Dis Colon Rectum. 2015;58(1):145–53.
Telem DA, Han KS, Kim MC, Ajari I, Sohn DK, Woods K, et al. Transanal rectosigmoid resection via natural orifice translumenal endoscopic surgery (NOTES) with total mesorectal excision in a large human cadaver series. Surg Endosc. 2013;27(1):74–80.
Bislenghi G, Wolthuis AM, de Buck van Overstraeten A, D’Hoore A. AirSeal system insufflator to maintain a stable pneumorectum during TAMIS. Tech Coloproctol. 2015;19:43–5.
Chen WH, Kang L, Luo SL, Zhang XW, Huang Y, Liu ZH, Wang JP. Transanal total mesorectal excision assisted by single-port laparoscopic surgery for low rectal cancer. Tech Coloproctol. 2015;19(9):527–34. doi:10.1007/s10151-015-1342-1. Epub 2015 Jul 29.
Velthuis S, van den Boezem PB, van der Peet DL, Cuesta MA, Sietses C. Feasibility study of transanal total mesorectal excision. Br J Surg. 2013;100(6):828–31. doi:10.1002/bjs.9069; discussion 831. Epub 2013 Feb 25.
Leroy J, Barry BD, Melani A, Mutter D, Marescaux J. No-scar transanal total mesorectal excision: the last step to pure NOTES for colorectal surgery. JAMA Surg. 2013;148(3):226–30; discussion 231.
Zhang H, Zhang YS, Jin XW, Li MZ, Fan JS, Yang ZH. Transanal single-port laparoscopic total mesorectal excision in the treatment of rectal cancer. Tech Coloproctol. 2013;17(1):117–23. doi:10.1007/s10151-012-0882-x. Epub 2012 Aug 31.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Sachdeva, U.M., Sylla, P. (2018). Natural Orifice Approaches in Rectal Surgery: Transanal Endoscopic Proctectomy. In: Pigazzi, A. (eds) Techniques in Minimally Invasive Rectal Surgery. Springer, Cham. https://doi.org/10.1007/978-3-319-16381-9_11
Download citation
DOI: https://doi.org/10.1007/978-3-319-16381-9_11
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-16380-2
Online ISBN: 978-3-319-16381-9
eBook Packages: MedicineMedicine (R0)