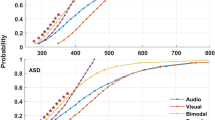
Abstract
One of the most controversial issues in the study of autism spectrum disorder (ASD) is the involvement and dysfunction of sensory systems that underlie atypical behaviors, which could be evaluated through neuroimaging and electrophysiological techniques. Auditory disturbances are the most frequently reported sensory deficits that could explain language and communication deficits shown in ASD patients. Event-related potentials such as auditory brainstem response, mismatch negativity, P50, and P300 have shown differences between patients and normal subjects, supporting the existence of an abnormal auditory processing. Somesthetic perception is also distorted in this heterogeneous group of patients, probably associated with deficits in communication, motor ability, and social skills. It has been confirmed by abnormalities in short- and long-latency somatosensory evoked potentials. Visual processing impairment has also been described in children and young adults with autism. Dipole source analysis revealed that the visual cortex, fusiform gyrus, and medial prefrontal lobe are less active in autism compared with control subjects during the execution of emotion processing tasks. ASD patients also have problems to integrate information from multiple sensory sources necessary to a successful social behavior, and consequently, they show deficits with social and cognitive processes. Electrophysiological and imaging techniques may constitute useful tools in the diagnosis, classification, and therapeutic strategies of ASD patients, considering the diversity of this spectrum.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Auditory brainstem response
- Autism spectrum disorder
- Middle latency response
- Sensory integration
- Somatosensory evoked response
- Somesthetic perception
- Visual perception
5.1 Introduction
Autism spectrum disorder (ASD) refers to a group of disorders including autistic disorder, Asperger’s disorder, and pervasive developmental disorder (American Psychiatric Association 2013).
One of the most controversial issues in the study of ASD is the involvement and dysfunction of sensory systems that underlie atypical behaviors.
In an extensive review of the literature published in 2008 (1300 reports), Hughes summarizes this contradiction finding equally a low and high frequency of sensory symptoms in patients with autism, and hyper- or hypofunctioning of the sensory systems (Hughes 2009).
Techniques like electroencephalography (EEG), magnetoencephalography (MEG), and functional Magnetic Resonance Imaging (fMRI) have demonstrated their utility to evaluate unimodal sensory processing and multisensory integration (Marco et al. 2011). Neurophysiological responses to auditory, tactile, and visual stimuli in autistic patients have been studied to explain the neural bases of the atypical behaviors previously mentioned (Marco et al. 2011). Other sensory processing deficits, including olfactory and gustatory, have been also described in children with ASD (Tomchek and Dunn 2007; Bennetto et al. 2007).
This chapter aims to summarize the electrophysiological and imaging evidences published in the past years that support the existence of sensory deficits in ASD patients.
5.2 Auditory Perception
Disturbances in auditory processing are the most frequently reported sensory deficits in patients with ASD. In fact, children with autism show a major impairment during the processing of auditory and verbal information compared with visual information (Duncan et al. 2009).
The main trends point out the disturbances of auditory perception of linguistic and social auditory stimulus, and enhanced perception of pitch and music. These discordant findings in auditory abnormality reported in ASD might be the consequence of differences between global and local processing of auditory information occurring in these patients (Kellerman et al. 2005).
Diverse studies in ASD have been designed to test the integrity of the auditory pathway using electrophysiological techniques, such as auditory brainstem response (ABR), but the results are not coincident. In consequence, some authors state that these studies do not support the involvement of brainstem in autism, despite the presence of peripheral hearing impairment in a nonnegligible number of autistic subjects (Klin 1993). For example, Novick et al. show that abnormalities in the brainstem are scarce in patients with ASD using evoked potentials to clicks, pitch changes, and tones. These authors suggest that the registration and storage of stimulus information in the higher levels of processing are more engaged, and they could explain the severe language disorders in ASD (Novick et al. 1980).
Most reports support the hypothesis of impairment in the brainstem auditory pathway to explain the disturbances in auditory processing demonstrated in ASD subjects (Kwon et al. 2007; Magliaro et al. 2010; Maziade et al. 2000; Tas et al. 2007).
The objective evaluation of hearing in a group of children with ASD was carried out by Taset et al. (2007) using transient evoked otoacoustic emissions (TEOAEs) and ABR. Both these techniques were obtained in the majority of ASD children bilaterally (83.3 %), while the remaining cases showed an increase in duration of III–V interpeak latency compared with a control group (Tas et al. 2007). Similar results were reported by Kwon et al. (2007) studying 121 autistic children compared with a control group. They reported a prolonged latency of wave V, and an increase in duration of III–V interpeak interval in the ASD group, suggesting a dysfunction or immaturity of the central auditory pathway, particularly at the level of mesencephalon (Kwon et al. 2007).
Autistic patients and unaffected first degree relatives showed the prolongation of I–III interpeak interval of ABR in relation to control subjects. So, this finding could be proposed as an endophenotype of the disease (Maziade et al. 2000).
The auditory abnormalities mentioned could contribute to sensory deprivation and consequently to communication deficits. Furthermore, aberrations in the perception and processing of the audiological stimuli, like maturational defects and atypical lateralization, has been proposed (Hitoglou et al. 2010).
Other techniques have been used to assess hearing in autistic subjects: pure tone and speech audiometry, acoustic immitance measures, ABR, auditory middle latency response (MLR), and cognitive potential (P300). All auditory evoked potentials showed differences with respect to a group of normal subjects. Particularly, statistically significant differences between groups were observed in the latency of waves III and V, and in the duration of interpeak intervals I–III and I–V of ABR, confirming the impairment of the brainstem auditory pathway. The absence of P300 component suggests the dysfunction of cortical areas in the same patients (Magliaro et al. 2010).
Abnormal auditory symptoms include peripheral and sensorineural hearing deficit, both hypo- and hypersensitivity to auditory stimulation with bizarre reactions to sounds, and limitations to filter relevant auditory information in the presence of environmental background noise (Källstrand et al. 2010). Khalfa et al. explained that difficulty in filtering information presupposes the participation of active cochlear mechanisms regulated by the efferent olivocochlear system and evaluated with TEOAEs. The abnormalities in lateralization at the auditory periphery observed in ASD patients are probably related to dysfunction at higher levels of the auditory processing mediated by the medial olivocochlear system (Khalfa et al. 2001).
Limitations to filter auditory information in a noisy background also include speech. Evidence of this was provided by Russo et al., evaluating speech-evoked responses in quiet and noise background in ASD patients and typically developing children. Differences between quiet and noise responses were minimal in ASD children, probably related to a severe depression of the responses in a noise background (Russo et al. 2009).
On the contrary, other authors reported that nonmentally retarded children with autism had a sensory gating to auditory stimulus similar to a control group using the P50 gating paradigm (Kemner et al. 2002). All these apparent differences in published papers are probably related to the nature of the auditory stimuli used.
Another aspect that has to be taken into account is the automatic and active processing of auditory stimuli evaluated with the mismatch negativity. The amplitude of this event-related potential is smaller in autistic than in normal children in unattended conditions. On the other hand, when the stimuli are attended the differences between groups disappear. All these results confirmed the existence of an abnormal automatic auditory processing in autism (Dunn et al. 2008).
Larger P300 component to target auditory stimuli has been also found in autistic children during active conditions with respect to healthy, dyslexic, and attention deficit/hyperactivity disorder (AD/HD) groups, while other authors found larger P300a in younger children with respect to older (Hughes 2009).
An interesting finding reported by Lai et al. in low-functional autistic patients showed that functional systems which process speech and song are more related to the last one. Some structures, like the left inferior frontal gyrus, are more activated during song stimulation and less with speech stimulation in autistic patients compared with control normal subjects. Measures like functional connectivity between this gyrus and superior temporal gyrus was increased for songs compared to speech in patients. At the same time, there were increased frontal-posterior connections. These and other findings of this study support the assumption that functional systems related to speech and song are more active for song in this subgroup of autistic patients (Lai et al. 2012).
Deficits in communication in ASD patients include a variety of disturbances in language, ranging from semantic-pragmatic deficits to the absence of speech. But these problems are probably dependent, at least in part, on auditory aberrant perception (Kujala et al. 2013).
Deficient prosody, for example, is a distinguishing characteristic of the language impairment in ASD patients related to acoustic cues such as pitch contour. The sensory encoding of pitch was evaluated by evoked brainstem responses to pitch syllables in a group of ASD patients with normal intelligence and hearing, some of them showed deficient pitch tracking compared with normal children. Obviously, there is a subcortical origin of prosody encoding deficits demonstrated by electrophysiological techniques. This should be taken into account in the diagnosis and treatment of this subgroup of ASD patients (Russo et al. 2008).
Of course, ASD include a wide spectrum of entities with a diversity of responses. Matas et al. studied the audiological and electrophysiological profile of patients with autism and Asperger syndrome, and compared their results with a group of normal subjects. They did not find abnormalities in the audiological evaluation in all subjects, but 50 % of patients with autism and 30 % with Asperger syndrome showed ABR alterations, with statistically significant differences between groups. Auditory MLR presented abnormalities in all groups without differences between them, while P300 component showed differences between Asperger patients and control subjects. These results suggest that auditory information processing at different levels of the pathway has specific profiles in the diverse forms of the disease (Matas et al. 2009).
Other authors have found normal hearing levels in high-functioning autistic children with a pervasive development disorder (PDD) and speech delay (Psillas et al. 2006).
Bruneau et al. demonstrated in a group of mentally retarded children with ASD the existence of electroclinical correlations between the amplitude of the right temporal N1 cortical response and the verbal and nonverbal communication abilities, suggesting a reorganization of the hemisphere functions, with a greater activation of the right hemisphere for functions that correspond to the left one. This especially was true for the lateral aspect of the superior temporal gyrus which belongs to secondary auditory areas (Bruneau et al. 2003).
5.3 Somesthetic Perception
In comparison with auditory disturbances in ASD patients, fewer studies have been conducted to evaluate the dysfunction of somesthetic perception. Some of the symptoms include hyper- or hypoesthesia to touch, pain, and temperature (Miyazaki et al. 2007). Somatosensory input is fundamental for motor development, while touch is also critical for healthy social and communication skills in early childhood and beyond.
Short-latency somatosensory evoked potentials (S-SEPs) elicited by the stimulation of median nerve were evaluated and correlated with clinical symptoms in a group of 24 children with autism. The abnormalities include prolonged peak latency of N20, increase in central conduction time, and a prevalence of the amplitude of N20/P25 in the right hemisphere (more than twofold) that indicates hyperactivity in the right primary somatosensory area of these patients (Miyazaki et al. 2007).
Similar results have been published by Cabrera et al., who obtained S-SEPs with stimulation of posterior tibial nerve. In this study, 92 % of ASD children showed abnormalities related to an increase of the central conduction time (65 %) and morphological distortion or absence of the evoked cortical response (Cabrera et al. 2011).
It has also been reported a strong positive valence to tactile stimuli, which provokes the excitation of patients, and enhanced responsiveness mediated by hypersensitivity to vibration and thermal pain (Hughes 2008).
In 2010, Cascio published a review about somatosensory processing in neurodevelopmental disorders that include autism. This author found that somatosensory perception is aberrant in ASD, associated with deficits in communication, motor ability, and social skills, which should be considered in the etiology and treatment of these disorders (Cascio 2010).
More surprising were the results reported by Wong et al., who found slower and larger amplitude of the event-related potential P300 in the parietal somatosensory cortices of children with autism during the processing of facial gender and emotion, besides the activation of visual cortex and fusiform gyrus (Wong et al. 2008). These results suggest an abnormal specialization of the cortex engaged to social brain networks.
5.4 Visual Perception
Visual processing abnormalities have also been described in children and young adults with autism. For example, Milne et al. stated that perception of simple visual stimuli is atypical in ASD patients (Milne et al. 2009). These authors found differences between patients and control subjects in the electroencephalographic activity during visual perception with a cortical topographic differentiation (striate-extrastriate cortex and cingulate gyrus) (Milne et al. 2009).
There are specific abnormalities in early processing of visual stimuli that are probably related to perception. It has been demonstrated in patients with PDD, verifying a reduction in amplitude of event-related potentials at the occipital cortex in response to visual stimuli with high and low spatial frequencies (Kemner and van Engeland 2006).
More specific results were described by Boeschoten et al. using short-latency visual-evoked potentials and dipole sources of their components. They found abnormalities for high spatial frequency stimuli during the early processing, and in late phases for both high and low spatial frequency stimuli (Boeschoten et al. 2007). This atypical response might suggest the existence of difficulties in visual processing from the primary levels of analysis in the visual pathway of PDD subjects.
The same authors explained that the previously described anomalies had an effect in the abnormal face processing in PDD subjects, provoking disturbances of social behavior (Boeschoten et al. 2007b).
Nevertheless, most papers support the idea of a higher level of dysfunction that involves extrastriate cortices (Deeley et al. 2007; Gunji et al. 2009; Koshino et al. 2008; Pierce and Redcay 2008; Wong et al. 2008; O’Connor et al. 2007).
Event-related functional MRI demonstrated a lesser activation of fusiform and extrastriate cortices to detect facial expressions of primary emotions in Asperger syndrome compared with control subjects (Deeley et al. 2007).
The distinction between self, familiar, and unfamiliar faces is disturbed in PDD patients as shown in the study of Gunji et al., whose results suggest a deficit of semantic encoding of faces in this group of patients (Gunji et al. 2009).
When recognition of faces has an emotional connotation the results differ. Event-related potentials that evaluate recognition of emotional facial expressions (N1,N170, and P2 components) are in general not affected in ASD, but dipole source analysis revealed that the visual cortex, fusiform gyrus, and medial prefrontal lobe are less active in autism compared with control subjects during the execution of emotion processing tasks (Wong et al. 2008).
Autistic patients need to make an excessive processing of the visual information to successfully differentiate target and a novel stimuli during the execution of a three-stimulus oddball paradigm task (Sokhadze et al. 2009).
5.5 Integration of Sensory Information
The impairment in social cognition and the abnormalities in sensory and perceptual processing are characteristics of patients with ASD. These subjects especially have problems to integrate information from multiple sensory sources necessary to a successful social behavior, and consequently, they show deficits with social and cognitive processes.
ASD patients can also have problems with earlier stages of multisensory information processing, evidenced by the recording of the cerebral electrical activity and the reaction time during a simple audiovisual task (Brandwein et al. 2013).
It has been explained that communicative impairment of PDD subjects is based on a disturbed complex audiovisual integration of speech stimuli, while low-level abilities integration is relatively spared (Magnee et al. 2008).
Auditory-somatosensory integration is also damaged in ASD patients compared to matched typically developing children, different to auditory or somatosensory stimuli alone (Russo et al. 2010).
Visual-somatosensory integration was evaluated by Kemner et al. using a three-stimulus oddball task (standard, deviant, and novel stimuli) in autistic and control children. They reported that autistic children differed from controls with respect to visual (P2, N2, and P3) and somatosensory (P3) responses, showing abnormal processing of proximal and distal stimuli, but they could not find evidence of abnormal lateralization of the event-related potential components (Kemner et al. 1994).
5.6 Conclusions
The main sensory modalities and multisensory processing are impaired in autistic patients, conducting to a global sensory dysfunction that could be in the background of other disturbances such as language and social disabilities.
Electrophysiological and imaging techniques may constitute useful tools in the diagnosis, classification, and therapeutic strategies of ASD patients, considering the diversity of this spectrum.
Abbreviations
- ABR:
-
Auditory brainstem response
- AD/HD:
-
Attention deficit/hyperactivity disorder
- ASD:
-
Autism spectrum disorder
- MLR:
-
Middle latency response
- MRI:
-
Magnetic resonance imaging
- PDD:
-
Pervasive development disorder
- S-SEPs:
-
Short-latency somatosensory evoked potentials.
- TEOAEs:
-
Transient evoked otoacoustic emissions
References
American Psychiatric Association (2013) American psychiatric association: diagnostic and statistical manual of mental disorders, DSM-5. American Psychiatric Publishing, Washington, DC
Bennetto L, Kuschner ES, Hyman SL (2007) Olfaction and taste processing in autism. Biol Psychiatry 62:1015–1021
Boeschoten MA, Kenemans JL, van Engeland H, Kemner C (2007a) Abnormal spatial frequency processing in high-functioning children with pervasive developmental disorder (PDD). Clin Neurophysiol 118:2076–2088
Boeschoten MA, Kenemans JL, van Engeland H, Kemner C (2007b) Face processing in pervasive developmental disorder (PDD): the roles of expertise and spatial frequency. J Neural Transm 114:1619–1629
Brandwein AB, Foxe JJ, Butler JS, Russo NN, Altschuler TS, Gomes H, Molholm S (2013) The development of multisensory integration in high-functioning autism: high-density electrical mapping and psychophysical measures reveal impairments in the processing of audiovisual inputs. Cereb Cortex 23:1329–1341
Bruneau N, Bonnet-Brilhault F, Gomot M, Adrien JL, Barthelemy C (2003) Cortical auditory processing and communication in children with autism: electrophysiological/behavioral relations. Int J Psychophysiol 51:17–25
Cabrera I, Báez M, Maragoto C, Galvizu R, Vera H, Ortega M (2011) Evaluación funcional de sistemas sensoriales mediante potenciales evocados en niños con trastornos del espectro autista. Enfermería Global 24:39–44
Cascio CJ (2010) Somatosensory processing in neurodevelopmental disorders. J Neurodevelop Disord 2:62–69
Deeley Q, Daly EM, Surguladze S, Page L, Toal F, Robertson D, Curran S, Giampietro V, Seal M, Brammer MJ, Andrew C, Murphy K, Phillips ML, Murphy DG (2007) An event related functional magnetic resonance imaging study of facial emotion processing in Asperger syndrome. Biol Psychiatry 62:207–217
Duncan CC, Barry RJ, Connolly JF, Fischer C, Michie PT, Naatanen R, Polich J, Reinvang I, Van Petten C (2009) Event-related potentials in clinical research: guidelines for eliciting, recording, and quantifying mismatch negativity, P300, and N400. Clin Neurophysiol 120:1908
Dunn MA, Gomes H, Gravel J (2008) Mismatch negativity in children with autism and typical development. J Autism Dev Disord 38:52–71
Gunji A, Inagaki M, Inoue Y, Takeshima Y, Kaga M (2009) Event-related potentials of self-face recognition in children with pervasive developmental disorders. Brain Dev 31:139–147
Hitoglou M, Ververi A, Antoniadis A, Zafeiriou DI (2010) Childhood autism and auditory system abnormalities. Pediatr Neurol 42:309–314
Hughes JR (2008) A review of recent reports on autism: 1000 studies published in 2007. Epilepsy Behav 13:425–437
Hughes JR (2009) Update on autism: a review of 1300 reports published in 2008. Epilepsy Behav 16:569–589
Källstrand J, Olsson O, Fristedt Nehlstedt S, Ling Sköld M, Nielzén S (2010) Abnormal auditory forward masking pattern in the brainstem response of individuals with Asperger syndrome. Neuropsychiatr Dis Treat 6:289–296
Kellerman GR, Fan J, Gorman JM (2005) Auditory abnormalities in autism: toward functional distinctions among findings. CNS Spectr 10:748–756
Kemner C, van Engeland H (2006) ERPs and eye movements reflect atypical visual perception in pervasive developmental disorder. J Autism Dev Disord 36:45–54
Kemner C, Verbaten MN, Cuperus JM, Camfferman G, van Engeland H (1994) Visual and somatosensory event-related brain potentials in autistic children and three different control groups. Electroencephalogr Clin Neurophysiol 92:225–237
Kemner C, Oranje B, Verbaten MN, van Engeland H (2002) Normal P50 gating in children with autism. J Clin Psychiatry 63:214–217
Khalfa S, Bruneau N, Roge B, Georgieff N, Veuillet E, Adrien JL, Barthelemy C, Collet L (2001) Peripheral auditory asymmetry in infantile autism. Eur J Neurosci 13:628–632
Klin A (1993) Auditory brainstem responses in autism: brainstem dysfunction or peripheral hearing loss? J Autism Dev Disord 23:15–35
Koshino H, Kana RK, Keller TA, Cherkassky VL, Minshew NJ, Just MA (2008) fMRI investigation of working memory for faces in autism: visual coding and underconnectivity with frontal areas. Cereb Cortex 18:289–300
Kujala T, Lepisto T, Naatanen R (2013) The neural basis of aberrant speech and audition in autism spectrum disorders. Neurosci Biobehav Rev 37:697–704
Kwon S, Kim J, Choe BH, Ko C, Park S (2007) Electrophysiologic assessment of central auditory processing by auditory brainstem responses in children with autism spectrum disorders. J Korean Med Sci 22:656–659
Lai G, Pantazatos SP, Schneider H, Hirsch J (2012) Neural systems for speech and song in autism. Brain 135:961–975
Magliaro FCL, Scheuer CI, Assumpção Júnior FB, Matas CG (2010) Study of auditory evoked potentials in autism (original title: Estudo dos potenciais evocados auditivosem autismo). Pró-Fono Revista de Atualização Científica 22:31–36
Magnee MJ, de Gelder B, van Engeland H, Kemner C (2008) Audiovisual speech integration in pervasive developmental disorder: evidence from event-related potentials. J Child Psychol Psychiatry 49:995–1000
Marco EJ, Hinkley LB, Hill SS, Nagarajan SS 2011 Sensory processing in autism: a review of neurophysiologic findings. Pediatr Res 69:48R–54R
Matas CG, Goncalves IC, Magliaro FC (2009) Audiologic and electrophysiologic evaluation in children with psychiatric disorders. Braz J Otorhinolaryngol 75:130–138
Maziade M, Merette C, Cayer M, Roy MA, Szatmari P, Cote R, Thivierge J (2000) Prolongation of brainstem auditory-evoked responses in autistic probands and their unaffected relatives. Arch Gen Psychiatry 57:1077–1083
Milne E, Scope A, Pascalis O, Buckley D, Makeig S (2009) Independent component analysis reveals atypical electroencephalographic activity during visual perception in individuals with autism. Biol Psychiatry 65:22–30
Miyazaki M, Fujii E, Saijo T, Mori K, Hashimoto T, Kagami S, Kuroda Y (2007) Short-latency somatosensory evoked potentials in infantile autism: evidence of hyperactivity in the right primary somatosensory area. Dev Med Child Neurol 49:13–17
Novick B, Vaughan HG Jr, Kurtzberg D, Simson R (1980) An electrophysiologic indication of auditory processing defects in autism. Psychiatry Res 3:107–114
O’Connor K, Hamm JP, Kirk IJ (2007) Neurophysiological responses to face, facial regions and objects in adults with Asperger’s syndrome: an ERP investigation. Int J Psychophysiol 63:283–293
Pierce K, Redcay E (2008) Fusiform function in children with an autism spectrum disorder is a matter of “who”. Biol Psychiatry 64:552–560
Psillas G, Psifidis A, Antoniadou-Hitoglou M, Kouloulas A (2006) Hearing assessment in pre-school children with speech delay. Auris Nasus Larynx 33:259–263
Russo NM, Skoe E, Trommer B, Nicol T, Zecker S, Bradlow A, Kraus N (2008) Deficient brainstem encoding of pitch in children with autism spectrum disorders. Clin Neurophysiol 119:1720–1731
Russo N, Zecker S, Trommer B, Chen J, Kraus N (2009) Effects of background noise on cortical encoding of speech in autism spectrum disorders. J Autism Dev Disord 39:1185–1196
Russo N, Foxe JJ, Brandwein AB, Altschuler T, Gomes H, Molholm S (2010) Multisensory processing in children with autism: high-density electrical mapping of auditory-somatosensory integration. Autism Res 3:253–267
Sokhadze E, Baruth J, Tasman A, Sears L, Mathai G, El-Baz A, Casanova MF (2009) Event-related potential study of novelty processing abnormalities in autism. Appl Psychophysiol Biofeedback 34:37–51
Tas A, Yagiz R, Tas M, Esme M, Uzun C, Karasalihoglu AR (2007) Evaluation of hearing in children with autism by using TEOAE and ABR. Autism 11:73–79
Tomchek S, Dunn W (2007) Sensory processing in children with and without autism: a comparative study using the short sensory profile. Am J Occup Ther 61:190–200
Wong TK, Fung PC, Chua SE, McAlonan GM (2008) Abnormal spatiotemporal processing of emotional facial expressions in childhood autism: dipole source analysis of event-related potentials. Eur J Neurosci 28:407–416
Acknowledgments
We thank Odalys Morales-Chacon for her English assistance and Jorge Bergado-Rosado for his contributions.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Baez Martin, M., Morales Chacón, L., Cabrera Abreu, I. (2015). Autism Spectrum Disorder. A Clinical Neurophysiology Approach II. In: Robinson-Agramonte, M. (eds) Translational Approaches to Autism Spectrum Disorder. Springer, Cham. https://doi.org/10.1007/978-3-319-16321-5_5
Download citation
DOI: https://doi.org/10.1007/978-3-319-16321-5_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-16320-8
Online ISBN: 978-3-319-16321-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)