Abstract
The lack of well-controlled studies on treatment efficacy, along with the immense costs of education and care for patients with autism spectrum disorder (ASD) makes ASD a public health problem. Likewise, the enormous heterogeneity of the clinical and behavioral symptoms has made it rather difficult to delineate the neural circuitry affiliated with this condition. The objective of this review is to provide a summary of recent data on the clinical neurophysiology in patients with ASD. Electroencephalographic (EEG) findings with emphasis on quantitative EEG (power EEG, coherence EEG) are summarized since EEG studies provide evidence of brain functional aspects in this disorder. Besides addressing current knowledge of the relationship among EEG abnormalities, epilepsy, and regression in ASD, this chapter further highlights the most recent literature on sleep disorders and polysomnography (PSG) findings in this pathology. Lastly, we discuss limitations in available research that may contribute to understand the inconsistencies in the literature, and offer suggestions for future research in this area for advancing the understanding of ASD.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Autism spectrum disorder
- Electroencephalography
- Quantitative electroencephalography
- Epileptiform abnormalities
- Epilepsy
- Autistic regression
- Polysomnography
4.1 Introduction
Autism spectrum disorder (ASD) is a new Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) disorder encompassing the previous Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSMIV) autistic disorder (autism), Asperger’s disorder, childhood disintegrative disorder, Rett’s disorder, and pervasive developmental disorder not otherwise specified. It is characterized by deficits in two core domains: deficits in social communication and social interaction and restricted repetitive patterns of behavior, interests, and activities (Volkmar and McPartland 2014; Kelly 2014) . This pathology is a behaviorally defined syndrome of early life, with varied symptoms reflecting many biological and environmental influences that are unique to each individual’s brain and that shape its unique developmental trajectory (Jeste 2011; Toth and Stobbe 2011) .
ASD has been associated with disturbance in cerebral organization and function (Palau-Baduell et al. 2013) . The goal of neurologic, genetic, electrophysiologic, imaging, and other biologic tests performed in this disease is not only to diagnose autism but to attempt to define some of its many potential etiologies and understand their pathogenic effects on the brain (Silver and Rapin 2012) .
The objective of this review is to present research data on the electroencephalography (EEG) findings, with emphasis on quantitative EEG (power EEG, coherence EEG), epileptiform EEG abnormalities and sleep disorders in patients with ASD. Besides addressing current knowledge of the relationship among EEG abnormalities, epilepsy, and regression in ASD this chapter further highlights the most recent literature on sleep disorders and PSG findings in this pathology.
4.2 Electroencephalography (EEG) and Autism Spectrum Disorder (ASD)
ASD involving multiple neural system dysfunctions and EEG studies provide evidence of brain functional aspects in this disorder (Palau-Baduell et al. 2011; Palau-Baduell et al. 2013) .
The first EEG study in autism reported EEG abnormalities in 58 % of the autistic children studied (White et al. 1964) ; later research focused on epileptiform activity. However, other EEG findings have been reported such as: suppression of mu waves, reduced alpha power, photic driving on the right, and low levels of coherence and synchronization. Some of these EEG results are discussed below.
Mu rhythm at 7–12 Hz can be recorded from the central scalp areas and is reactive in the normal population (mu suppression) by movements or thoughts of movement and also when the subject is observing others’ movements. The latter refers to the mirror neurons system (MNS) and is normally active when observing others, proposed as deficient in autism (Hughes 2010) . Mirror neurons active during both observation and execution of actions, are thought to play a crucial role in imitation and other social-communicative skills that are often impaired in ASD (Oberman et al. 2008; Martineau et al. 2008; Hamilton 2013) . Subjects with ASD show significant mu suppression to self-movements but they fail to react to the movements performed by others. These findings support the hypothesis of a dysfunctional MNS in individuals with ASD (Palau-Baduell et al. 2011; Southgate and Hamilton 2008; Leighton et al. 2008)
Oberman et al. (2008) reported that the MNS responded in individuals with autism, but only when observing a familiar face (Oberman et al. 2008). The same year, another author indicated that females showed stronger mu suppression than males, supporting the theory that autism represents an “extreme male brain” (Cheng et al. 2008) .
A significant correlation between age and mu suppression in response only to the observation of actions (not during execution), for individuals with ASD and typical individuals was reported by Oberman et al. (2013) . This result provides evidence against the argument that mirror neuron dysfunction improves with age in individuals with ASD, and suggests that a diagnosis-independent developmental change may be at the root of the correlation of age and mu suppression (Oberman et al. 2013) .
To examine the integrity of the mirror system in autism Hamilton et al. (2013) systematically reviewed 25 suitable papers using neuroscience methods. They showed that the only well localized measure of mirror system function was functional magnetic resonance imaging (fMRI; Hamilton 2013) .
The study of Ruysschaert et al. (2014) challenged the “broken mirror” hypothesis of ASD, suggesting that impaired neural mirroring is not a distinctive feature of ASD (Ruysschaert et al. 2014) . Mu suppression was investigated in children with ASD during the observation of goal-directed actions and nongoal-directed mimicked hand movements, as well as during action execution. No significant correlations between mu suppression, quality of imitation, age, and social communication questionnaire scores were found by these authors. Overall, there is little evidence for a global dysfunction of the mirror system in autism.
Other available theories of the pathophysiology of ASD have focused on abnormal temporal coordination of neural activity in cortical circuits as a core impairment of the disorder. Synchronous neural oscillatory activity in the gamma range (30–80 Hz) has been shown to be abnormal in individuals with ASD and their first-degree relatives in response to simple auditory and language stimuli (McFadden et al. 2012; Milne et al. 2009) . Magnetoencephalography (MEG) data also provides evidence that gamma-band activity may be involved in abnormal brain functioning in ASD. Limin Sun’s findings highlighted the contribution of impaired gamma-band activity toward complex visual processing, suggesting atypical modulation of high-frequency power in fronto-posterior networks (Sun et al. 2012) .
Lastly, an emerging focus of research on autism targets the identification of early-developing ASD endophenotypes using infant siblings of the affected children. Gabard-Durnam et al. (2013) demonstrated that low- and high-risk infants show different patterns of alpha asymmetry at 6 months of age and opposite growth trajectories in asymmetry over the following 12 months. These results support the candidacy of alpha asymmetry as an early neural ASD endophenotype (Gabard-Durnam et al. 2013; Carson et al. 2014) .
4.2.1 Quantitative EEG
Analysis of resting state brain activity, using electrophysiological measures like complexity as well as functional connectivity, is of growing interest in the study of ASD, in this respect quantitative electroencephalography (QEEG) may help in detecting the regions of altered brain function and connectivity abnormalities (Billeci et al. 2013) .
Children with ASD exhibit regionally specific elevations in delta, theta, alpha, and high-frequency (20–120 Hz) power, supporting an imbalance of neural excitation/inhibition as a neurobiological feature of this disorder. Moreover, increased temporal and parietal alpha power has been associated with greater symptom severity (Cornew et al. 2012) .
Resting-state EEG studies of ASD suggest a U-shaped profile of electrophysiological power alterations, with excessive power in low-frequency and high-frequency bands, abnormal functional connectivity , and enhanced power in the left hemisphere of the brain. These studies have documented differences associated with ASD, particularly in frontal areas functionally linked to cognitive functions which are disrupted in individuals with ASD (Billeci et al. 2013; Wang et al. 2013; Shimizu et al. 1982) .
Neuroimaging technologies and EEG studies have shown that autism is largely a disorder of neuronal connectivity (Coben et al. 2014; Palau-Baduell et al. 2012) . Functional findings revealed that patients with ASD had deficit in long-distance connections (under-connectivity), with a most prominent deficit in fronto-posterior connections, as well as an excess of local connections (over-connectivity) and of long-distance under-connectivity, with some nonuniformities, along with disruptions were also described, more severe in later-developing cortical regions (Wass 2011; Palau-Baduell et al. 2012) . Furthermore, lower synchronization in non-rapid eye movement (NREM) sleep stages, and low coherence for most frequency bands confirm the validity of the underconnectivity model in autism (Coben et al. 2008; Kulisek et al. 2008)
Recently, Barttfeld P. showed a decay in functional connectivity mainly within the delta and theta bands (the lower part of the EEG spectrum) associated with an increasing number of autistic traits. According to this author, EEG functional connectivity at low frequencies and its associated network properties may be related to some autistic features in the general population (Barttfeld et al. 2013) . Resting state MEG has also revealed band-specific group differences in connectivity measure that agreed with other functional studies in fMRI and EEG (Ghanbari et al. 2013) .
Cantor et al. (1986), were the first to examine the utility of pairwise coherence measures for representing connectivity impairments in autism (Cantor et al. 1986) . Using phase coherence in multiple frequency EEG bands as a measure of functional connectivity , Pineda et al. (2012) have shown evidence for both global hypoconnectivity and local hyperconnectivity in individuals with ASD (Pineda et al. 2012) . Carson et al. (2014) also found decreased interhemispheric connectivity in frontal and temporal-parietal regions in these children compared to controls using EEG coherence (Carson et al. 2014) .
A large case control study conducted by Duffy et al. (2012) found a stable pattern of EEG spectral coherence distinguishing children with autism from neurotypical controls. They proposed that the predominantly reduced short-distance coherences may indicate poor local network function, whereas, the increased long-distance coherences might represent compensatory processes or reduced neural pruning (Duffy and Als 2012) . It has been suggested that preferential attention to detail (perceptual domain) is connected with both, lower levels of alpha activity and reduced coherence in posterior regions in the ASD group (Mathewson et al. 2012) .
EEG coherence has also been evaluated during the intermittent photic stimulation at fixed frequencies of 3–24 Hz in 14 boys with autism, aged 6–14 years, with relatively intact verbal and intellectual functions and without differences in the spontaneous EEG. However, the number of interhemispheric coherent connections pertaining to the 20 highest connections of each individual was significantly lower in autistic patients than in the controls at all the EEG beta frequencies corresponding to those of stimulation (Lazarev et al. 2013) .
In this respect, there are a number of extant questions. First, whether aberrant connectivity observed in ASD should be seen as part of their primary pathogenesis, or whether it disrupted connectivity emerging over time. Second, how the patterns of disrupted connectivity found in ASD might relate to those found in a range of other disorders.
4.2.2 EEG Biofeedback as Treatment in ASD
Neurofeedback (NF) training is an intervention based on operant conditioning that results in self-regulation of brain electrical oscillations (Kubik 2010) . NF exploits the brain’s plasticity to normalize aberrant connectivity patterns apparent in the autistic brain, by grounding this training in known anatomical (e.g., mirror neuron system) and functional markers (e.g., mu rhythms) of autism. So, NF training holds promise to support current treatments for this complex disorder. Pineda et al. proposed a hypothesis which states that neurofeedback-induced alpha mu (8–12 Hz) rhythm suppression or desynchronization, a marker of cortical activation, should induce neuroplastic changes and lead to the normalization in relevant mirroring networks that have been associated with higher-order social cognition(Pineda et al. 2012) .
Holtmann et al. (2011) reviewed available studies on the effectiveness of NF as a method of treatment for ASD core symptoms. The existing evidence at that time did not support the use of this tool in the treatment of ASD (Holtmann et al. 2011) . Studies with outcomes in favor of NF might show an improvement in comorbid attention-deficit-hyperactivity disorder symptoms, rather than a true improvement in core ASD symptoms (Coben and Myers 2010; Holtmann et al. 2011; Kubik 2010) .
Recently, a randomized pretest–posttest control group design showed that EEG biofeedback seems to be an applicable tool to regulate EEG activity, with specific effects on cognitive flexibility, but it did not result in significant reductions in symptoms of ASD (Kouijzer et al. 2013) .
Despite conflicting results, literature analysis suggests that QEEG features are sensitive to modification in neuronal regulation dysfunction which characterizes autistic brain. The use of advanced techniques for the increase of the specificity and of spatial localization could allow finding distinctive patterns of QEEG abnormalities in ASD subjects, paving the way for the development of tailored intervention strategies .
4.3 Relationship Between EEG Epileptiform Abnormalities, Epilepsy, and Regression in ASD
4.3.1 EEG Epileptiform Abnormalities
Confounding interpretations of findings related to the co-occurrence of autism and epilepsy is that the standards used to determine epileptiform abnormalities (EA) in children with ASD have varied among studies (Tuchman et al. 2010) . Both nonspecific changes, such as slowing or asymmetry, and epileptiform discharges, consisting of spikes or sharp wave discharges, sharp slow waves, generalized spike-wave, and generalized polyspikes are seen in this disorder (Spence and Schneider 2009) . On the other hand, the term subclinical or nonconvulsive seizure is used to refer to electrographic patterns, without clinically recognizable cognitive, behavioral, or motor functions or any apparent impairment of consciousness (Chez et al. 2006) .
Many authors have reported a prevalence of epileptiform abnormalities in 20–70 % of the children with autism and of epilepsy in 10–40 % of them (Rutter 1970; Tuchman 1997; Tuchman 2004; Rossi et al. 1995; Hashimoto et al. 2001; Hrdlicka et al. 2004; Hughes and Melyn 2005; Kawasaki et al. 1997; Ballaban-Gil and Tuchman 2000; Lewine et al. 1999) . Moreover, high rates of interictal epileptiform EEG abnormalities in children with ASD have also been observed in patients with or without a history of seizures (Palau-Baduell et al. 2013; Chez et al. 2006; Kim et al. 2006; Parmeggiani et al. 2002; Amiet et al. 2008; Bolton et al. 2011; Francis et al. 2013) . EA rates as high as 60 % have been seen even in the absence of epilepsy, so their presence should not be considered evidence of epilepsy. This raises questions about whether these discharges could be considered a biomarker of cortical dysfunction in this population, and whether these discharges have a causal association with any of the autism phenotypes . Some investigators propose that these abnormalities may play a causal role in the autism phenotype (Munoz-Yunta et al. 2003; Bolton et al. 2011; Dawson et al. 2005; Francis et al. 2013) .
Kagan-Kushnir et al. (2005) summarized 13 EEG studies from 1966 to 2003. None of the studies received a “good” quality rating, three were rated “fair,” and the other ten were rated “poor.” In examining the prevalence of epileptiform abnormalities in all patients, irrespective of clinical seizure history, wide variants in prevalence rates were obtained, ranging from 10.3 to 72.4 %. When only the “fair” studies were considered, the rates ranged from 38.3 to 60.8 %. Out of 13, 8 studies examined the prevalence rate of epileptiform abnormalities in patients with no clinical history of seizures and it was found to range from 6.1 to 31 % (Kagan-Kushnir et al. 2005) .
In the general population the occurrence of EA observed ranges from 2 to 8.7 %, and decreases during puberty (Danielsson et al. 2005; Hara 2007; Trevathan 2004) , while EA occurrence in ASD ranges from 6.7 to 83.0 %, tends to disappear during puberty (Parmeggiani et al. 2007) , and is present predominantly in female subjects (Chez et al. 2006) . On the other hand, the prevalence of epilepsy declines with age in individuals with static processes like cerebral palsy and intellectual disability, nevertheless increases of autism and epilepsy raise the possibility of an ongoing degenerative process in these individuals, like the occurrences in Down syndrome (Silver and Rapin 2012; Tuchman et al. 2010) . Epilepsy generally persists into adult life, however a remission of only 16 % has been reported in adults with autism (Levisohn 2007) . The heterogeneous data reported in the literature are probably due to (a) different samples with different ages and clinical features and (b) the methodological variability in collecting and interpreting EEGs.
It has also been demonstrated that the occurrence of EA is higher when using 24- to 48-h EEG recordings than in routine studies; in fact, this may be present only during sleep (Parmeggiani et al. 2010; Hrdlicka et al. 2004) . According to one report EEG sleep study, could be best achieved in patients with autism by using dexmedetomidine, (Ray and Tobias 2008) .
MEG and sleep EEG are more sensitive for correctly detecting epileptiform EEG abnormalities in autism than wake EEGs (Lewine et al. 1999; Hrdlicka 2008) . Mulligan et al. (2014) completed a retrospective chart review of 101 patients with ASD who had overnight EEGs they suggested that increasing severity of autistic symptoms may be associated with a higher likelihood of epileptiform abnormalities (Mulligan and Trauner 2014) . MEG epileptiform activity is frequently documented in children with early-onset ASD and subclinical epileptiform activity is present especially in the perisylvian regions for many patients with this pathology (Munoz-Yunta et al. 2008) . The focus of spike discharges has been reported to be mainly in the centro-temporal or temporal (Olsson et al. 1988; Tuchman and Rapin 1997; Parmeggiani et al. 2010) , frontal (Kawasaki et al. 1997; Hashimoto et al. 2001) , or rarely in the occipital regions (Nass et al. 1998) . Kanemura et al. (2013) observed that the presence of frontal paroxysms was significantly associated with the later development of epilepsy compared with centrotemporal paroxysmus (Kanemura et al. 2013) .
In a recent study, our team in the International Center for Neurological Restoration in Havana Cuba evaluated 70 ASD patients between the age of 2 and 14 years (5.8 ± 2.86) with male predominance (63.3 %), 55 % of the patients were classified as primary ASD. Sleep EEGs, wake EEGs, or both were performed in this study. Sleep EEGs recording was performed in 41.6 % of the patients. The overall rate of epileptiform EEG abnormalities in the whole sample was 71.6 %. These EEG findings were not found to be associated with clinical severity of autism. No significant relationship between EA occurrence, and ASD subtypes was observed (Fisher exact test p = 0.46). Epilepsy was diagnosed in 11, 6 %, of the study participants. Temporal lobe localization of the EA was associated with epilepsy diagnosis , (unpublished data).
In general, the significance of EA is controversial. Moreover, a clinical diagnosis of ASD cannot be confirmed by pathognomic EEG or PSG features but these data may help to determine the role of abnormal electrical activity in the development of ASD .
4.3.2 EEG Epileptiform Abnormalities and Epilepsy
Epilepsy is the most common neurological comorbidity in autism. Approximately one third of the children with autism develop epilepsy (Giovanardi et al. 2000; Olsson et al. 1988) , with prevalence estimates between 8 and 42 % (Volkmar and Nelson 1990; Rossi et al. 1995; Spence and Schneider 2009; Tuchman and Rapin 2002; Hughes and Melyn 2005; Kagan-Kushnir et al. 2005; Danielsson et al. 2005; Francis et al. 2013) .
The onset of epilepsy in autism has two peaks: one before 5 years of age and the other after 10–12 years of age, with most cases presenting after 10 years of age (Volkmar et al. 2005) . In these cases all types of epilepsy have been observed (Volkmar et al. 2005; Gillberg 1991) .
The high frequency of autism in some of the early-onset developmental encephalopathic epilepsies, and the high prevalence of interictal EEG discharges in children with autism is frequently cited as evidence of the relationship between autism and epilepsy (Berg and Plioplys 2012) .
Some studies have ascertained that mental retardation, cerebral lesions, and rare diseases associated with ASD increase the risk of epilepsy (Spence and Schneider 2009; Hughes and Melyn 2005) . The prevalence of epilepsy reported in autism has varied across studies depending on the age distribution of the sample, the degree of mental retardation, and the type of language disorder (Volkmar et al. 2005; Kagan-Kushnir et al. 2005) . However, in “idiopathic” ASD seizure the occurrence remains higher than in the general population, suggesting that autism itself is associated with an enhanced risk of epilepsy (Spence and Schneider 2009) . A paper published by Ekinci et al. (2010) examined the possible associations of epilepsy/interictal epileptiform abnormalities with asthma/allergy, hyperactivity, and familial factors in ASD. They observed that epilepsy was associated with a family history of epilepsy and psychiatric problems in the mother during pregnancy (Ekinci et al. 2010) .
It has been reported that some ASD patients with late-onset epilepsy showed severe EEG abnormalities, which included continuous spike-waves during slow-wave sleep (CSWS), generally demonstrate an improvement in EEG and clinical symptoms in the long-term follow up (Lee et al. 2011) .
The complexity of the relationship of ASD, epilepsy, and epileptiform EEG activity is highlighted in tuberous sclerosis complex (TSC). This is a key clinical model for at least four reasons. First, 1–5 % of the children with autism have TSC (de et al. 2005). Second, a more careful analysis reveals that TSC is present in 8–14 % of those with the autism–epilepsy phenotype (Smalley 1998) .Third, autism or ASD have been reported in up to 50 % of the individuals with TSC (de et al. 2005). Fourth, approximately 60 % of the children with TSC have epilepsy and 50 % have infantile spasms (Guerrini and Aicardi 2003) . All of these reasons make TSC a unique clinical model to study the complex interplay between genetics, seizures onset, and location of epileptiform activity to the development of ASD.
The consensus emerging from studies on ASD and epilepsy is that the same brain pathology accounts for the majority of children with co-occurring ASD and epilepsy or with an epileptiform EEG. This brain pathology may represent a set of uniform underlying genetics as well. The current understanding of the association between epilepsy and ASD is still limited, but from a clinical point of view, this association should not be overlooked. Controversy also seems to exist regarding the rate of seizures and EEG abnormalities.
4.3.3 EEG Epileptiform Abnormalities and Autistic Regression
Another interesting point emerging from studying the association of epilepsy, EA, and autism is the fact of autistic regression (usually occurring between 18 and 24 months of age) in which the developmental trajectory of approximately 30 % of the children with ASD is characterized by a regression of verbal and nonverbal communication skills. This phenomenon is currently well accepted, but poorly understood at a biological level (Werner et al. 2005; Luyster et al. 2005; Lord et al. 2004; Goldberg et al. 2003; Meilleur and Fombonne 2009; Baird et al. 2008) .
Many studies have addressed the question of the influence of epilepsy and/or EA on autistic regression (Tuchman 1997; Kobayashi and Murata 1998; Hrdlicka et al. 2004; Baird et al. 2006; Deonna and Roulet 2006) . However, the relationship between them continues to be an area of active research interest and controversy with studies showing mixed results. Some studies during the past decade have found no differences in history of autistic regression in ASD children with epileptiform EEGs and epilepsy versus ASD children with a normal EEG and no epilepsy (Hara 2007; Canitano et al. 2005; Hrdlicka 2008; Parmeggiani et al. 2010) . Contrary to results showing no relationship of regression to epilepsy in autism there are other case reports linking epilepsy or an epileptiform EEG to autistic regression (Deonna and Roulet 2006; Chilosi et al. 2013; Hrdlicka et al. 2004; Giannotti et al. 2008) . This latter study also found that children with autistic regression had more disrupted sleep than those with ASD without regression (Giannotti et al. 2008) .
For many years, a number of studies have pointed out the role of EEG abnormalities in language and cognitive processes suggesting that interictal epileptiform activity could impair brain function (Patry and Naquet 1971; Deonna 1996; Rapin 1996; Aarts et al. 1984; Binnie et al. 1987; Kasteleijn-Nolst Trenite et al. 1987) while, uncontrolled and few randomized controlled trials of antiepileptic treatment of interictal epileptiform discharges have suggested that the suppression of discharges is associated with significant improvement in psychosocial function (Garcia-Penas 2011) .
Furthermore, despite the high prevalence of interictal EEG epileptiform activity in children with ASD and the overlap of Landau–Kleffner syndrome (LKS) and ASD, little evidence to date has shown that spikes contribute to the pathogenesis or to the worsening of language, social, or behavioral dysfunction in children with ASD (Tharp 2004) . However, there are clear differences between LKS and autistic regression (Szatmari et al. 2008) .
An attempt to clarify the relationship between regressions or any type of problematic clinical findings and severity of EEG abnormalities in ASD patients with late-onset epilepsy was made by Lee et al. (2011) . They found that severe EEG abnormalities tended to be related to the neuropsychological function. Nevertheless they were not able to conclude that the treatment of EEG abnormality was related to the improved neuropsychological symptoms (Lee et al. 2011) .
In summary, although there is no clear understand on the biology of regression in autism, we have much information of their phenomenology. The decision to treat EA remains controversial, and the treatment of seizures should be pursued utilizing drugs generally indicated in international epilepsy guidelines. It is quite evident that the results of different studies are argumentative and the relationship of epilepsy and EEG abnormalities to developmental regression in autism remains unknown and should be clarified and investigated.
4.4 Sleep Disorders and Polysomnography in ASD
Just as with epileptiform discharges and epilepsy, the prevalence of sleep problems in children with ASD is very high, ranging between 40 and 80 % (Thirumalai et al. 2002; Silver and Rapin 2012; Richdale 1999; Schreck and Mulick 2000; Wiggs and Stores 2004; Polimeni et al. 2005; Liu et al. 2006; Malow 2004; Malow et al. 2009; Bruni et al. 2007; Goldman et al. 2009) . A growing body of literature contains reports of sleep disorders as a third indicator of abnormal neural functioning in autism and, therefore, a characteristic of the ASD phenotype (Anders et al. 2011; Wiggs and Stores 2004; Richdale 1999; Cortesi et al. 2010; Baker et al. 2013) . Parental surveys indicate a 50–80 % prevalence of sleep problems in children with ASD, compared with a 9–50 % prevalence rate in age-matched, typically developing subjects (Polimeni et al. 2005; Kotagal and Broomall 2012; Allik et al. 2006; Richdale and Schreck 2009) .
The degree of cognitive impairment likely does not influence the prevalence of sleep problems in ASD because they are observed in those who are severely mentally handicapped as well as in those who are high functioning, with intelligence quotients greater than 70 (Richdale and Schreck 2009; Krakowiak et al. 2008) . Sleep problems also do not seem to be influenced by ASD subtype (Polimeni et al. 2005) . A positive correlation has also been observed between the number of seizures and total scores on the Child Sleep Habits Questionnaire, where higher scores denote more sleep problems (Giannotti et al. 2008) . Parents of children with ASD and epilepsy are more likely to report difficulties with sleep than parents of children without epilepsy (Cotton and Richdale 2006; Goldman et al. 2012; Wiggs and Stores 2004) .
The main problems described in ASD include insomnia, difficulty with sleep initiation and sleep maintenance, parasomnias such as rapid eye movement (REM) and non-REM arousal disorders, rhythmical movement disorders, and periodic limb movements during sleep (Malow et al. 2006; Liu et al. 2006; Krakowiak et al. 2008; Hoshino et al. 1984) . The multiplicity of sleep problems in children with ASDs was confirmed by Wiggs and Stores using sleep diaries from 69 parents, together with actigraphy (a device to detect and record muscle activity), to monitor sleep pattern (Wiggs and Stores 2004) . Although some sleep disorders might be of behavioral origin (as in the typically developing children with poor sleep hygiene), circadian rhythm disturbances have also been reported in children with ASD (Patzold et al. 1998; Wiggs and Stores 2004) .
The consequences of the difficulties with sleep initiation and maintenance in children with ASD may include alterations in daytime behavior, memory, learning, and also significant stress in caretakers (Kotagal and Broomall 2012) . In 2004 Schreck et al. demonstrated that in children with autism, fewer hours of sleep per night were highly correlated with a more severe behavioral phenotype (Schreck et al. 2004). Previous studies had also suggested that some symptoms specific to autism may be directly associated with disturbed sleep, and that the improved sleep in children with autism is associated with enhancement in daytime behavior (Johnson et al. 1996; Patzold et al. 1998; Richdale 1999; Segawa and Nomura 1992) .
In an interesting study Giannotti et al. (2006) observed persistent sleep problems in children with autism, in over 50 % of the sample (Giannotti et al. 2006) , with a peak age of onset during the second year of life, similar to what is known for regression. Later, this author also reported significantly more severe and persistent abnormalities of sleep wake patterns in regressed children (Giannotti et al. 2008; Giannotti et al. 2011) .
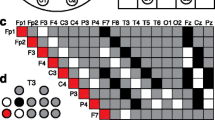
Polysomnography (PSG) consists of monitoring multiple simultaneous, physiologic parameters during sleep Fig. 4.1 and it is indicated when sleep-disordered breathing, restless legs syndrome, parasomnias, or nocturnal seizures are suspected. PSG studies have confirmed the presence of disrupted sleep architecture in children with ASD. PSG abnormalities include reduction of REM sleep; longer sleep latency, increased arousals, lower sleep efficiency, increased stage 1 sleep, and decreased slow wave sleep as well as decreased density of spindle activity (Palau-Baduell et al. 2013) .
Polysomnography study in a 4-year-old patient with ASD without epileptic seizures. Recordings involve complete electroencephalogram (EEG), chin electromyography, eye movements and electrocardiogram. Note EEG epileptiform abnormality in channels containing the right centroparietotemporal leads, C4, P4, T4, and T6
Relatively few reports of PSG-based sleep studies in children with autism have been published. Overall, these have focused on abnormalities in REM sleep, including immaturity in the organization of eye movements into discrete bursts (Tanguay et al. 1976) , increased muscle twitches during REM sleep (Elia et al. 2000) , and undifferentiated sleep in which features of non-REM and REM sleep are intermixed (Diomedi et al. 1999; Limoges et al. 2005) . The prolonged sleep times, early wake times, and frequent interruptions in sleep noted in the survey literature have not been commented on in these PSG studies. Although subjective sleep parameters appear to be roughly similar in adults and children with ASD, there are inconsistencies in the objective sleep profiles obtained from actual sleep recordings (Elia et al. 2000) .
The characterization of sleep manifestations by specific ASD subtype (preferably by using the International Classification of Sleep Disorders) will help gather better longitudinal data about diagnosis and responses to specific treatments.
4.5 Conclusions
EEG and MEG provide evidence of disrupted brain connectivity in ASD and reveal that gamma-band activity may be crucially involved in aberrant brain functioning in ASD. However, researchers have only recently begun to link patterns of brain activity and connectivity to behavior.
Although there have been relatively few reports of PSG-based sleep studies in ASD, both non-REM and REM sleep abnormalities have been observed, which support the multiplicity of sleep problems in this pathology.
One of the best-known associations with central nervous system dysfunction in ASD is the high risk of epilepsy; though the relationship among epileptiform abnormalities , epilepsy, and regression is not yet well understood. Thus, a greater number of controlled studies are required in order to confirm this relationship. That is why, identification of the early processes that leads to ASD, and to epileptogenicity, or both is a challenge that is worth pursuing.
Abbreviations
- ASD:
-
Autism spectrum disorder
- EA:
-
Epileptiform abnormalities
- EEG:
-
Electroencephalography
- MEG:
-
Magnetoencephalography
- MNS:
-
Mirror neurons system
- MRI:
-
Magnetic resonance imaging
- NF:
-
Neurofeedback
- PSG:
-
Polysomnography
- QEEG:
-
Quantitative electroencephalography
References
Aarts JH, Binnie CD, Smit AM, Wilkins AJ (1984) Selective cognitive impairment during focal and generalized epileptiform EEG activity. Brain 107:293–308
Allik H, Larsson JO, Smedje H (2006) Sleep patterns of school-age children with Asperger syndrome or high-functioning autism. J Autism Dev Disord 36:585–595
Amiet C, Gourfinkel-An I, Bouzamondo A, Tordjman S, Baulac M, Lechat P, Mottron L, Cohen D (2008) Epilepsy in autism is associated with intellectual disability and gender: evidence from a meta-analysis. Biol Psychiatry 64:577–582
Anders TF, Iosif AM, Schwichtenberg AJ, Tang K, Goodlin-Jones BL (2011) Six-month sleep-wake organization and stability in preschool-age children with autism, developmental delay, and typical development. Behav Sleep Med 9:92–106
Baird G, Robinson RO, Boyd S, Charman T (2006) Sleep electroencephalograms in young children with autism with and without regression. Dev Med Child Neurol 48:604–608
Baird G, Charman T, Pickles A, Chandler S, Loucas T, Meldrum D, Carcani-Rathwell I, Serkana D, Simonoff E (2008) Regression, developmental trajectory and associated problems in disorders in the autism spectrum: the SNAP study. J Autism Dev Disord 38:1827–1836
Baker E, Richdale A, Short M, Gradisar M (2013) An investigation of sleep patterns in adolescents with high-functioning autism spectrum disorder compared with typically developing adolescents. Dev Neurorehabil 16:155–165
Ballaban-Gil K, Tuchman R (2000) Epilepsy and epileptiform EEG: association with autism and language disorders. Ment Retard Dev Disabil Res Rev 6:300–308
Barttfeld P, Amoruso L, Ais J, Cukier S, Bavassi L, Tomio A, Manes F, Ibanez A, Sigman M (2013) Organization of brain networks governed by long-range connections index autistic traits in the general population. J Neurodev Disord 5:16–5
Berg AT, Plioplys S (2012) Epilepsy and autism: is there a special relationship? Epilepsy Behav 23:193–198
Billeci L, Sicca F, Maharatna K, Apicella F, Narzisi A, Campatelli G, Calderoni S, Pioggia G, Muratori F (2013) On the application of quantitative EEG for characterizing autistic brain: a systematic review. Front Hum Neurosci 7:442. doi:10.3389/fnhum.2013.00442. eCollection;%2013.:442
Binnie CD, Kasteleijn-Nolst Trenite DG, Smit AM, Wilkins AJ (1987) Interactions of epileptiform EEG discharges and cognition. Epilepsy Res 1:239–245
Bolton PF, Carcani-Rathwell I, Hutton J, Goode S, Howlin P, Rutter M (2011) Epilepsy in autism: features and correlates. Br J Psychiatry 198:289–294
Bruni O, Ferri R, Vittori E, Novelli L, Vignati M, Porfirio MC, Arico D, Bernabei P, Curatolo P (2007) Sleep architecture and NREM alterations in children and adolescents with Asperger syndrome. Sleep 30:1577–1585
Canitano R, Luchetti A, Zappella M (2005) Epilepsy, electroencephalographic abnormalities, and regression in children with autism. J Child Neurol 20:27–31
Cantor DS, Thatcher RW, Hrybyk M, Kaye H (1986) Computerized EEG analyses of autistic children. J Autism Dev Disord 16:169–187
Carson AM, Salowitz NM, Scheidt RA, Dolan BK, Van Hecke AV (2014) Electroencephalogram coherence in children with and without autism spectrum disorders: decreased interhemispheric connectivity in autism. Autism Res10
Cheng Y, Lee PL, Yang CY, Lin CP, Hung D, Decety J (2008) Gender differences in the mu rhythm of the human mirror-neuron system. PLoS ONE 3:e2113
Chez MG, Chang M, Krasne V, Coughlan C, Kominsky M, Schwartz A (2006) Frequency of epileptiform EEG abnormalities in a sequential screening of autistic patients with no known clinical epilepsy from 1996 to 2005. Epilepsy Behav 8:267–271
Chilosi AM, Brovedani P, Ferrari AR, Ziegler AL, Guerrini R, Deonna T (2013) Language regression associated with autistic regression and electroencephalographic (EEG) abnormalities: a prospective study. J Child Neurol
Coben R, Myers TE (2010) The relative efficacy of connectivity guided and symptom based EEG biofeedback for autistic disorders. Appl Psychophysiol Biofeedback 35:13–23
Coben R, Clarke AR, Hudspeth W, Barry RJ (2008) EEG power and coherence in autistic spectrum disorder. Clin Neurophysiol 119:1002–1009
Coben R, Mohammad-Rezazadeh I, Cannon RL (2014) Using quantitative and analytic EEG methods in the understanding of connectivity in autism spectrum disorders: a theory of mixed over- and under-connectivity. Front Hum Neurosci 8:45. doi:10.3389/fnhum.2014.00045. eCollection;%2014
Cornew L, Roberts TP, Blaskey L, Edgar JC (2012) Resting-state oscillatory activity in autism spectrum disorders. J Autism Dev Disord 42:1884–1894
Cortesi F, Giannotti F, Ivanenko A, Johnson K (2010) Sleep in children with autistic spectrum disorder. Sleep Med 11:659–664
Cotton S, Richdale A (2006) Brief report: parental descriptions of sleep problems in children with autism, Down syndrome, and Prader-Willi syndrome. Res Dev Disabil 27:151–161
Danielsson S, Gillberg IC, Billstedt E, Gillberg C, Olsson I (2005) Epilepsy in young adults with autism: a prospective population-based follow-up study of 120 individuals diagnosed in childhood. Epilepsia 46:918–923
Dawson G, Webb SJ, Wijsman E, Schellenberg G, Estes A, Munson J, Faja S (2005) Neurocognitive and electrophysiological evidence of altered face processing in parents of children with autism: implications for a model of abnormal development of social brain circuitry in autism. Dev Psychopathol 17:679–697
de VP, Humphrey A, McCartney D, Prather P, Bolton P, Hunt A (2005) Consensus clinical guidelines for the assessment of cognitive and behavioural problems in Tuberous Sclerosis. Eur Child Adolesc Psychiatry 14:183–190
Deonna T (1996) Autism and related conditions. J Am Acad Child Adolesc Psychiatry 35:403–405
Deonna T, Roulet E (2006) Autistic spectrum disorder: evaluating a possible contributing or causal role of epilepsy. Epilepsia 47(Suppl 2):79–82
Diomedi M, Curatolo P, Scalise A, Placidi F, Caretto F, Gigli GL (1999) Sleep abnormalities in mentally retarded autistic subjects: Down’s syndrome with mental retardation and normal subjects. Brain Dev 21:548–553
Duffy FH, Als H (2012) A stable pattern of EEG spectral coherence distinguishes children with autism from neuro-typical controls—a large case control study. BMC Med 10:64. doi:10.1186/1741-7015-10-64.:64-10
Ekinci O, Arman AR, Isik U, Bez Y, Berkem M (2010) EEG abnormalities and epilepsy in autistic spectrum disorders: clinical and familial correlates. Epilepsy Behav 17:178–182
Elia M, Ferri R, Musumeci SA, Del GS, Bottitta M, Scuderi C, Miano G, Panerai S, Bertrand T, Grubar JC (2000) Sleep in subjects with autistic disorder: a neurophysiological and psychological study. Brain Dev 22:88–92
Francis A, Msall M, Obringer E, Kelley K (2013) Children with autism spectrum disorder and epilepsy. Pediatr Ann 42:255–260
Gabard-Durnam L, Tierney AL, Vogel-Farley V, Tager-Flusberg H, Nelson CA (2013) Alpha asymmetry in infants at risk for autism spectrum disorders. J Autism Dev Disord 45(2): 473–480. doi:10.1007/s10803-013-1926-4
Garcia-Penas JJ (2011) Interictal epileptiform discharges and cognitive impairment in children. Rev Neurol 52(Suppl 1):S43–S52
Ghanbari Y, Bloy L, Christopher EJ, Blaskey L, Verma R, Roberts TP (2013) Joint analysis of band-specific functional connectivity and signal complexity in autism. J Autism Dev Disord 45(2):444–460. doi:10.1007/s10803-013-1915-7
Giannotti F, Cortesi F, Cerquiglini A, Bernabei P (2006) An open-label study of controlled-release melatonin in treatment of sleep disorders in children with autism. J Autism Dev Disord 36:741–752
Giannotti F, Cortesi F, Cerquiglini A, Miraglia D, Vagnoni C, Sebastiani T, Bernabei P (2008) An investigation of sleep characteristics, EEG abnormalities and epilepsy in developmentally regressed and non-regressed children with autism. J Autism Dev Disord 38:1888–1897
Giannotti F, Cortesi F, Cerquiglini A, Vagnoni C, Valente D (2011) Sleep in children with autism with and without autistic regression. J Sleep Res 20:338–347
Gillberg C (1991) The treatment of epilepsy in autism. J Autism Dev Disord 21:61–77
Giovanardi RP, Posar A, Parmeggiani A (2000) Epilepsy in adolescents and young adults with autistic disorder. Brain Dev 22:102–106
Goldberg WA, Osann K, Filipek PA, Laulhere T, Jarvis K, Modahl C, Flodman P, Spence MA (2003) Language and other regression: assessment and timing. J Autism Dev Disord 33:607–616
Goldman SE, Surdyka K, Cuevas R, Adkins K, Wang L, Malow BA (2009) Defining the sleep phenotype in children with autism. Dev Neuropsychol 34:560–573
Goldman SE, Richdale AL, Clemons T, Malow BA (2012) Parental sleep concerns in autism spectrum disorders: variations from childhood to adolescence. J Autism Dev Disord 42:531–538
Guerrini R, Aicardi J (2003) Epileptic encephalopathies with myoclonic seizures in infants and children (severe myoclonic epilepsy and myoclonic-astatic epilepsy). J Clin Neurophysiol 20:449–461
Hamilton AF (2013) Reflecting on the mirror neuron system in autism: a systematic review of current theories. Dev Cogn Neurosci 3:91–105. doi:10.1016/j.dcn.2012.09.008. Epub 2012 Oct 13.
Hara H (2007) Autism and epilepsy: a retrospective follow-up study. Brain Dev 29:486–490
Hashimoto T, Sasaki M, Sugai K, Hanaoka S, Fukumizu M, Kato T (2001) Paroxysmal discharges on EEG in young autistic patients are frequent in frontal regions. J Med Invest 48:175–180
Holtmann M, Steiner S, Hohmann S, Poustka L, Banaschewski T, Bolte S (2011) Neurofeedback in autism spectrum disorders. Dev Med Child Neurol 53:986–993
Hoshino Y, Watanabe H, Yashima Y, Kaneko M, Kumashiro H (1984) An investigation on sleep disturbance of autistic children. Folia Psychiatr Neurol Jpn 38:45–51
Hrdlicka M (2008) EEG abnormalities, epilepsy and regression in autism: a review. Neuro Endocrinol Lett 29:405–409
Hrdlicka M, Komarek V, Propper L, Kulisek R, Zumrova A, Faladova L, Havlovicova M, Sedlacek Z, Blatny M, Urbanek T (2004) Not EEG abnormalities but epilepsy is associated with autistic regression and mental functioning in childhood autism. Eur Child Adolesc Psychiatry 13:209–213
Hughes JR (2010) A review of Savant syndrome and its possible relationship to epilepsy. Epilepsy Behav 17:147–152
Hughes JR, Melyn M (2005) EEG and seizures in autistic children and adolescents: further findings with therapeutic implications. Clin EEG Neurosci 36:15–20
Jeste SS (2011) The neurology of autism spectrum disorders. Curr Opin Neurol 24:132–139
Johnson CD, Matt MK, Dennison D, Brown RS, Koh S (1996) Preventing factitious gingival injury in an autistic patient. J Am Dent Assoc 127:244–247
Kagan-Kushnir T, Roberts SW, Snead OC III (2005) Screening electroencephalograms in autism spectrum disorders: evidence-based guideline. J Child Neurol 20:197–206
Kanemura H, Sano F, Tando T, Sugita K, Aihara M (2013) Can EEG characteristics predict development of epilepsy in autistic children? Eur J Paediatr Neurol 17:232–237
Kasteleijn-Nolst Trenite DG, Riemersma JB, Binnie CD, Smit AM, Meinardi H (1987) The influence of subclinical epileptiform EEG discharges on driving behaviour. Electroencephalogr Clin Neurophysiol 67:167–170
Kawasaki Y, Yokota K, Shinomiya M, Shimizu Y, Niwa S (1997) Brief report: electroencephalographic paroxysmal activities in the frontal area emerged in middle childhood and during adolescence in a follow-up study of autism. J Autism Dev Disord 27:605–620
Kelly PA (2014) Textual Standardization and the DSM-5 “Common Language”. J Med Humanit 35(2):171–189. doi:10.1007/s10912-014-9281-9
Kim HL, Donnelly JH, Tournay AE, Book TM, Filipek P (2006) Absence of seizures despite high prevalence of epileptiform EEG abnormalities in children with autism monitored in a tertiary care center. Epilepsia 47:394–398
Kobayashi R, Murata T (1998) Setback phenomenon in autism and long-term prognosis. Acta Psychiatr Scand 98:296–303
Kotagal S, Broomall E (2012) Sleep in children with autism spectrum disorder. Pediatr Neurol 47:242–251
Kouijzer ME, van Schie HT, Gerrits BJ, Buitelaar JK, de Moor JM (2013) Is EEG-biofeedback an effective treatment in autism spectrum disorders? A randomized controlled trial. Appl Psychophysiol Biofeedback 38:17–28
Krakowiak P, Goodlin-Jones B, Hertz-Picciotto I, Croen LA, Hansen RL (2008) Sleep problems in children with autism spectrum disorders, developmental delays, and typical development: a population-based study. J Sleep Res 17:197–206
Kubik A (2010) Neurofeedback and personal development. Przegl Lek 67:716–720
Kulisek R, Hrncir Z, Hrdlicka M, Faladova L, Sterbova K, Krsek P, Vymlatilova E, Palus M, Zumrova A, Komarek V (2008) Nonlinear analysis of the sleep EEG in children with pervasive developmental disorder. Neuro Endocrinol Lett 29:512–517
Lazarev VV, Pontes A, Mitrofanov AA, Deazevedo LC (2013) Reduced interhemispheric connectivity in childhood autism detected by electroencephalographic photic driving coherence. J Autism Dev Disord 45(2):537–547. doi:10.1007/s10803-013-1959-8
Lee H, Kang HC, Kim SW, Kim YK, Chung HJ (2011) Characteristics of late-onset epilepsy and EEG findings in children with autism spectrum disorders. Korean J Pediatr 54:22–28
Leighton J, Bird G, Charman T, Heyes C (2008) Weak imitative performance is not due to a functional ‘mirroring’ deficit in adults with autism spectrum disorders. Neuropsychologia 46:1041–1049
Levisohn PM (2007) The autism-epilepsy connection. Epilepsia 48(Suppl 9):33–35
Lewine JD, Andrews R, Chez M, Patil AA, Devinsky O, Smith M, Kanner A, Davis JT, Funke M, Jones G, Chong B, Provencal S, Weisend M, Lee RR, Orrison WW Jr (1999) Magnetoencephalographic patterns of epileptiform activity in children with regressive autism spectrum disorders. Pediatrics 104:405–418
Limoges E, Mottron L, Bolduc C, Berthiaume C, Godbout R (2005) Atypical sleep architecture and the autism phenotype. Brain 128:1049–1061
Liu X, Hubbard JA, Fabes RA, Adam JB (2006) Sleep disturbances and correlates of children with autism spectrum disorders. Child Psychiatry Hum Dev 37:179–191
Lord C, Shulman C, DiLavore P (2004) Regression and word loss in autistic spectrum disorders. J Child Psychol Psychiatry 45:936–955
Luyster R, Richler J, Risi S, Hsu WL, Dawson G, Bernier R, Dunn M, Hepburn S, Hyman SL, McMahon WM, Goudie-Nice J, Minshew N, Rogers S, Sigman M, Spence MA, Goldberg WA, Tager-Flusberg H, Volkmar FR, Lord C (2005) Early regression in social communication in autism spectrum disorders: a CPEA Study. Dev Neuropsychol 27:311–336
Malow BA (2004) Sleep disorders, epilepsy, and autism. Ment Retard Dev Disabil Res Rev 10:122–125
Malow BA, Marzec ML, McGrew SG, Wang L, Henderson LM, Stone WL (2006) Characterizing sleep in children with autism spectrum disorders: a multidimensional approach. Sleep 29:1563–1571
Malow BA, Crowe C, Henderson L, McGrew SG, Wang L, Song Y, Stone WL (2009) A sleep habits questionnaire for children with autism spectrum disorders. J Child Neurol 24:19–24
Martineau J, Cochin S, Magne R, Barthelemy C (2008) Impaired cortical activation in autistic children: is the mirror neuron system involved? Int J Psychophysiol 68:35–40
Mathewson KJ, Jetha MK, Drmic IE, Bryson SE, Goldberg JO, Schmidt LA (2012) Regional EEG alpha power, coherence, and behavioral symptomatology in autism spectrum disorder. Clin Neurophysiol 123:1798–1809
McFadden KL, Hepburn S, Winterrowd E, Schmidt GL, Rojas DC (2012) Abnormalities in gamma-band responses to language stimuli in first-degree relatives of children with autism spectrum disorder: an MEG study. BMC Psychiatry 12:213. doi:10.1186/1471-244X-12-213
Meilleur AA, Fombonne E (2009) Regression of language and non-language skills in pervasive developmental disorders. J Intellect Disabil Res 53:115–124
Milne E, Scope A, Pascalis O, Buckley D, Makeig S (2009) Independent component analysis reveals atypical electroencephalographic activity during visual perception in individuals with autism. Biol Psychiatry 65:22–30
Mulligan CK, Trauner DA (2014) Incidence and behavioral correlates of epileptiform abnormalities in autism spectrum disorders. J Autism Dev Disord 44:452–458
Munoz-Yunta JA, Salvado B, Ortiz-Alonso T, Amo C, Fernandez-Lucas A, Maestu F, Palau-Baduell M (2003) Clinical features of epilepsy in autism spectrum disorders. Rev Neurol 36(Suppl 1):S61–S67
Munoz-Yunta JA, Ortiz T, Palau-Baduell M, Martin-Munoz L, Salvado-Salvado B, Valls-Santasusana A, Perich-Alsina J, Cristobal I, Fernandez A, Maestu F, Dursteler C (2008) Magnetoencephalographic pattern of epileptiform activity in children with early-onset autism spectrum disorders. Clin Neurophysiol 119:626–634
Nass R, Gross A, Devinsky O (1998) Autism and autistic epileptiform regression with occipital spikes. Dev Med Child Neurol 40:453–458
Oberman LM, Ramachandran VS, Pineda JA (2008) Modulation of mu suppression in children with autism spectrum disorders in response to familiar or unfamiliar stimuli: the mirror neuron hypothesis. Neuropsychologia 46:1558–1565
Oberman LM, McCleery JP, Hubbard EM, Bernier R, Wiersema JR, Raymaekers R, Pineda JA (2013) Developmental changes in mu suppression to observed and executed actions in autism spectrum disorders. Soc Cogn Affect Neurosci 8:300–304
Olsson I, Steffenburg S, Gillberg C (1988) Epilepsy in autism and autisticlike conditions. A population-based study. Arch Neurol 45:666–668
Palau-Baduell M, Valls-Santasusana A, Salvado-Salvado B (2011) Autism spectrum disorders and mu rhythm. A new neurophysiological view. Rev Neurol 52(Suppl 1):S141–S146
Palau-Baduell M, Salvado-Salvado B, Clofent-Torrento M, Valls-Santasusana A (2012) Autism and neural connectivity. Rev Neurol 54(Suppl 1):S31–S39
Palau-Baduell M, Valls-Santasusana A, Salvado-Salvado B, Clofent-Torrento M (2013) Interest of electroencephalogram in autism. Rev Neurol 56(Suppl 1):S35–S43
Parmeggiani A, Posar A, Giovanardi-Rossi P, Andermann F, Zifkin B (2002) Autism, macrocrania and epilepsy: how are they linked? Brain Dev 24:296–299
Parmeggiani A, Posar A, Antolini C, Scaduto MC, Santucci M, Giovanardi-Rossi P (2007) Epilepsy in patients with pervasive developmental disorder not otherwise specified. J Child Neurol 22:1198–1203
Parmeggiani A, Barcia G, Posar A, Raimondi E, Santucci M, Scaduto MC (2010) Epilepsy and EEG paroxysmal abnormalities in autism spectrum disorders. Brain Dev 32:783–789
Patry G, Naquet R (1971) Action of dipropylacetic acid upon photosensitive Papio papio. Can J Physiol Pharmacol 49:568–572
Patzold LM, Richdale AL, Tonge BJ (1998) An investigation into sleep characteristics of children with autism and Asperger’s Disorder. J Paediatr Child Health 34:528–533
Pineda JA, Juavinett A, Datko M (2012) Self-regulation of brain oscillations as a treatment for aberrant brain connections in children with autism. Med Hypotheses 79:790–798
Polimeni MA, Richdale AL, Francis AJ (2005) A survey of sleep problems in autism, Asperger’s disorder and typically developing children. J Intellect Disabil Res 49:260–268
Rapin I (1996) Practitioner review: developmental language disorders: a clinical update. J Child Psychol Psychiatry 37:643–655
Ray T, Tobias JD (2008) Dexmedetomidine for sedation during electroencephalographic analysis in children with autism, pervasive developmental disorders, and seizure disorders. J Clin Anesth 20:364–368
Richdale AL (1999) Sleep problems in autism: prevalence, cause, and intervention. Dev Med Child Neurol 41:60–66
Richdale AL, Schreck KA (2009) Sleep problems in autism spectrum disorders: prevalence, nature, & possible biopsychosocial aetiologies. Sleep Med Rev 13:403–411
Rossi PG, Parmeggiani A, Bach V, Santucci M, Visconti P (1995) EEG features and epilepsy in patients with autism. Brain Dev 17:169–174
Rutter ML (1970) Psycho-social disorders in childhood, and their outcome in adult life. J R Coll Physicians Lond 4:211–218
Ruysschaert L, Warreyn P, Wiersema JR, Oostra A, Roeyers H (2014) Exploring the role of neural mirroring in children with autism spectrum disorder. Autism Res 7(2):197–206. doi:10.1002/aur.1339. Epub 2014 Feb 10
Schreck KA, Mulick JA (2000) Parental report of sleep problems in children with autism. J Autism Dev Disord 30:127–135
Schreck KA, Mulick JA, Smith AF (2004) Sleep problems as possible predictors of intensified symptoms of autism. Res Dev Disabil 25:57–66
Segawa M, Nomura Y (1992) Polysomnography in the Rett syndrome. Brain Dev 14(Suppl):S46–S54
Shimizu Y, Niwa S, Ohta M, Kurita H, Saito Y (1982) Quantitative analysis of awake EEG in infantile autism. Seishin Shinkeigaku Zasshi 84:545–558
Silver WG, Rapin I (2012) Neurobiological basis of autism. Pediatr Clin North Am 59:45–61, x
Smalley SL (1998) Autism and tuberous sclerosis. J Autism Dev Disord 28:407–414
Southgate V, Hamilton AF (2008) Unbroken mirrors: challenging a theory of Autism. Trends Cogn Sci 12:225–229
Spence SJ, Schneider MT (2009) The role of epilepsy and epileptiform EEGs in autism spectrum disorders. Pediatr Res 65:599–606
Sun L, Grutzner C, Bolte S, Wibral M, Tozman T, Schlitt S, Poustka F, Singer W, Freitag CM, Uhlhaas PJ (2012) Impaired gamma-band activity during perceptual organization in adults with autism spectrum disorders: evidence for dysfunctional network activity in frontal-posterior cortices. J Neurosci 32:9563–9573
Szatmari P, Merette C, Emond C, Zwaigenbaum L, Jones MB, Maziade M, Roy MA, Palmour R (2008) Decomposing the autism phenotype into familial dimensions. Am J Med Genet B Neuropsychiatr Genet 147B:3–9
Tanguay PE, Ornitz EM, Forsythe AB, Ritvo ER (1976) Rapid eye movement (REM) activity in normal and autistic children during REM sleep. J Autism Child Schizophr 6:275–288
Tharp BR (2004) Epileptic encephalopathies and their relationship to developmental disorders: do spikes cause autism? Ment Retard Dev Disabil Res Rev 10:132–134
Thirumalai SS, Shubin RA, Robinson R (2002) Rapid eye movement sleep behavior disorder in children with autism. J Child Neurol 17:173–178
Toth K, Stobbe G (2011) Diagnosis of autism spectrum disorders. Pediatr Ann 40:488–492
Trevathan E (2004) Seizures and epilepsy among children with language regression and autistic spectrum disorders. J Child Neurol 19(Suppl 1):S49–S57
Tuchman RF (1997) Language disorders: is EEG clinically useful?. Rev Neurol 25:744–749
Tuchman R (2004) AEDs and psychotropic drugs in children with autism and epilepsy. Ment Retard Dev Disabil Res Rev 10:135–138
Tuchman RF, Rapin I (1997) Regression in pervasive developmental disorders: seizures and epileptiform electroencephalogram correlates. Pediatrics 99:560–566
Tuchman R, Rapin I (2002) Epilepsy in autism. Lancet Neurol 1:352–358
Tuchman R, Cuccaro M, Alessandri M (2010) Autism and epilepsy: historical perspective. Brain Dev 32:709–718
Volkmar FR, McPartland JC (2014) From kanner to DSM-5: autism as an evolving diagnostic concept. Annu Rev Clin Psychol 10:193–212. doi:10.1146/annurev-clinpsy-032813-153710. Epub 2013 Dec 9
Volkmar FR, Nelson DS (1990) Seizure disorders in autism. J Am Acad Child Adolesc Psychiatry 29:127–129
Volkmar F, Chawarska K, Klin A (2005) Autism in infancy and early childhood. Annu Rev Psychol 56:315–336
Wang J, Barstein J, Ethridge LE, Mosconi MW, Takarae Y, Sweeney JA (2013) Resting state EEG abnormalities in autism spectrum disorders. J Neurodev Disord 5:24–25
Wass S (2011) Distortions and disconnections: disrupted brain connectivity in autism. Brain Cogn 75:18–28
Werner E, Dawson G, Munson J, Osterling J (2005) Variation in early developmental course in autism and its relation with behavioral outcome at 3–4 years of age. J Autism Dev Disord 35:337–350
White Pt, Demyer W, Demyer M (1964) Eeg abnormalities in early childhood schizophrenia: a double-blind study of psychiatrically disturbed and normal children during promazine sedation. Am J Psychiatry 120:950–958
Wiggs L, Stores G (2004) Sleep patterns and sleep disorders in children with autistic spectrum disorders: insights using parent report and actigraphy. Dev Med Child Neurol 46:372–380
Acknowledgments
We thank Odalys Morales Chacón for her English assistance. We would also like to thank Abel Sanchez, Daymet Grass, Maydelin Alfonso, and Maria Luisa Rodriguez for their useful cooperation.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Morales Chacón, L., Báez Martin, M. (2015). Autism Spectrum Disorder. A Clinical Neurophysiology Approach I. In: Robinson-Agramonte, M. (eds) Translational Approaches to Autism Spectrum Disorder. Springer, Cham. https://doi.org/10.1007/978-3-319-16321-5_4
Download citation
DOI: https://doi.org/10.1007/978-3-319-16321-5_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-16320-8
Online ISBN: 978-3-319-16321-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)