Abstract
The morbidity and mortality of lung diseases such as chronic obstructive pulmonary disease (COPD), idiopathic pulmonary fibrosis, and silicosis have been increasing in the past decades. Moreover, the currently available treatments are not able to reverse the pathological damage or to restore normal organ function. Thus, there is an urgent search for new regenerative strategies. In parallel, while advances in supportive care have improved survival in other lung diseases and critical illnesses, such as the acute respiratory distress syndrome (ARDS) and septic shock, mortality for each of these still remains high. In this context, current advances in comprehending the potential roles of stem cells and cell therapy offer new promise for lung diseases and critical illness. In this chapter, the potential role of one type of adult stem cell, mesenchymal stromal (stem) cells (MSCs), in pre-clinical experimental models of pulmonary disorders and critical illnesses as well as in ongoing clinical trials will be reviewed. Relevant mechanisms of MSC actions and next steps in the translational pathway for MSC therapy for lung diseases will be discussed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Pulmonary diseases, including acute respiratory distress syndrome (ARDS), asthma, chronic obstructive pulmonary disease (COPD), idiopathic pulmonary fibrosis (IPF), bronchopulmonary dysplasia, and occupational diseases such as silicosis, remain important causes of morbidity and mortality worldwide. Some of these, such as COPD and asthma, in contrast to many other major diseases, are increasing in prevalence. COPD, the fourth leading cause of disease mortality worldwide, is expected to be the third by 2020, and thus remains a major public health concern. Current available treatments for lung diseases may lessen the severity of symptoms, but there is still a pressing need for new therapeutic approaches, since no existing treatment has been shown to reduce disease progression, reverse the pathological changes, and restore the organ functionality. Lung transplantation is considered the only curative approach for end-stage chronic diseases; however, there is a significant shortage of suitable donor lungs and many on waiting lists die before a lung becomes available. Further, lung transplantation requires lifelong immunosuppression and five-year mortality after transplantation is approximately 50 %. Lung transplantation is also not a realistic option for patients in many parts of the world. New therapeutic approaches are thus desperately needed (Weiss 2014).
Approaches utilizing cell-based therapies for lung diseases have progressed rapidly in recent years. Systemic or local (intratracheal) administration of different stem and progenitor cell types has been demonstrated to have efficacy in different pre-clinical models of lung diseases (Weiss et al. 2013a, b; Kotton 2012; Lau et al. 2012). The different cell types have included endothelial progenitor cells (EPCs), bone marrow-derived mononuclear cells, amniotic fluid cells, and mesenchymal stromal (stem) cells (MSCs) (Weiss 2014; Weiss et al. 2013a, b). However, the majority of available pre-clinical data have focused on investigation of MSCs.
2 Mesenchymal Stromal (Stem) Cells
MSCs were first described in 1968, and since then, have been widely investigated for their applications in stem cell-based regeneration studies. The nomenclature has evolved over time as MSCs were initially named fibroblastic colony-forming units, subsequently as marrow stromal cells, mesenchymal stem cells, mesenchymal stromal cells, or as multipotent mesenchymal stromal cells. Nowadays, the application of the more commonly currently utilized terms, mesenchymal stem cell or mesenchymal stromal cell, is inconsistent in the literature. This nomenclature loosely depends on whether MSCs are being used for their ability to differentiate into lineages potentially useful in regenerative medicine efforts and structural repair, or utilizing their immunomodulatory properties in the absence of structural engraftment (Lotfinegad et al. 2014).
MSCs are described as self-renewal, fibroblastoid, non-phagocytic, adherent cells which are able to differentiate in vitro into some cell lineages, in particular, culture systems (Mafi et al. 2011; Paunescu et al. 2007; Oswald et al. 2004; Tran et al. 2011). In addition to the bone marrow (Li and Ikehara 2013; Kern et al. 2006), MSCs have been found in other sources, including liver (Najimi et al. 2007), lung (Sabatini et al. 2005; Lama et al. 2007), brain (Kang et al. 2010), adipose tissue (Kern et al. 2006; Pawitan 2009; Zuk et al. 2002; Zannettino et al. 2008), peripheral blood (Chong et al. 2012), cornea (Choong et al. 2007), synovium (Jones et al. 2010), thymus (Krampera et al. 2007), dental pulp (Gronthos 2011; Gronthos et al. 2000), periosteum (Nakahara et al. 1990), tendon (Bi et al. 2007), fallopian tube (Jazedje et al. 2009), placenta (Sabapathy et al. 2012; Igura et al. 2004), amniotic fluid (You et al. 2008), Wharton’s jelly (Wang et al. 2004), umbilical cord (Capelli et al. 2011; Romanov et al. 2003), and umbilical cord blood (Kern et al. 2006).
However, definition and investigation of MSCs continue to be confounded by several issues. For instance, there can be important differences in MSC properties, such as cell surface epitopes, secretome, immunomodulatory properties, lineage tendencies, and genomic stability, according to the tissue, strain, and species that MSCs are derived from (Keating 2012; Prockop and Oh 2012a, b; Romieu-Mourez et al. 2012; Baer and Geiger 2012). Further, there is growing evidence that MSCs are heterogeneous and that different MSC subtypes exist, even in cells isolated from the same source. Thus, delineating functional differences between MSCs isolated from different sources is an area of current intense investigation (Viswanathan et al. 2014).
To foster a more uniform characterization of MSCs and facilitate the exchange of data among investigators, the Mesenchymal and Tissue Stem Cell Committee of the International Society for Cellular Therapy (ISCT) have proposed minimal criteria to define human MSCs, which are listed as: (1) MSCs must be plastic-adherent when maintained in standard culture conditions; (2) MSCs must express CD105, CD73, and CD90 in at least 95 % of cell population, and lack expression of CD45, CD34, CD14 or CD11b, CD79 alpha or CD19, and HLA-DR surface molecules as measured by flow cytometry; (3) MSCs must differentiate into osteoblasts, adipocytes, and chondroblasts in vitro (Dominici et al. 2006; Horwitz et al. 2005). These criteria are currently being updated given the continued advances in understanding MSC biology with particular focus on developing potency assays applicable to clinical applications (Viswanathan et al. 2014). To address some of the variations in properties of cultured MSCs, an NCRR/NIH-sponsored Center for Preparation and Distribution of Adult Stem Cells (MSCs) serves as a pre-clinical resource for standardized preparations of mouse, rat, and human MSCs (http://medicine.tamhsc.edu/irm/msc-distribution.html). The NHBLI also sponsors the Production Assistance in Cellular Therapies (PACT) program, a training and GMP manufacturing resource that supports pre-clinical, IND preparation, and clinical investigations with MSCs and other cell therapy (https://secure.emmes.com/pactweb/Facilities).
3 Mechanisms of Action
While therapeutic interest in MSCs initially focused on exploring their capacity for multilineage differentiation to directly regenerate tissues and organs (Pittenger et al. 1999; Caplan and Bruder 2001), they are now also viewed as potent immunomodulators of disease-associated tissue microenvironments (Caplan 2009). Thus, the current translational landscape for MSCs includes therapeutic models involving direct tissue regeneration as well as indirect, through their anti-inflammatory and immunomodulatory effects on damaged and diseased tissues (Bianco et al. 2013; Griffin et al. 2013; Le Blanc and Mougiakakos 2012; Prockop and Oh 2012a, b) (Fig. 6.1). The capacity of MSCs to broadly modify the activity of most major components of the innate and adaptive immune system is now seen, along with their pro-angiogenic and cytoprotective effects, as an essential component of their therapeutic potential for many disease targets (Caplan and Bruder 2001; Bianco et al. 2013; Griffin et al. 2013; Le Blanc and Mougiakakos 2012).
The mechanisms by which MSCs might alleviate inflammation and injury are not completely understood and, as in other organ systems, likely involve multiple pathways including release of soluble mediators and/or microsomal particles as well as cell–cell contact. Importantly, the mechanisms of MSCs actions are different in different lung diseases and reflect the ability of the MSCs to sense and respond differently to different inflammatory environments (Weiss 2014; Weiss et al. 2013a, b). Much current interest in MSCs has focused on soluble factors due to their ability to secrete multiple paracrine factors such as growth factors, factors regulating endothelial and epithelial permeability, factors regulating innate and adaptive immunity, anti-inflammatory cytokines, and more recently, antimicrobial peptides. Some of the soluble mediators implicated in the different model systems include IL-6, IL-10, indoleamine 2,3-dioxygenase (IDO), Nitrous Oxide (NO), hepatocyte growth factor (HGF), and transforming growth factor (TGF)-β (Lotfinegad et al. 2014). Transduction or transfection of the MSCs to over-express secreted mediators including angiopoietin-1 or keratinocyte growth factor (KGF) further decreases endotoxin-mediated lung injury presumably through abrogation of endotoxin-mediated endothelial injury (Xu et al. 2008; Chen et al. 2013). While native MSCs are effective, MSCs transduced to over-express eNOS, IL-10, KGF, or a CCL2 inhibitor were found to be more effective in preventing monocrotaline-induced pulmonary hypertension, ischemia-reperfusion-induced lung injury, or bleomycin-induced pulmonary inflammation and subsequent fibrosis, respectively (Prockop and Oh 2012a, b; Keating 2012; Weiss 2013; Antunes et al. 2014; Kanki-Horimoto et al. 2006). MSCs appear also to act in part by decreasing the increased endothelial permeability found in acute lung injury, by secreting antibacterial peptides, by promoting an anti-inflammatory M2 phenotype in alveolar macrophages, by increasing monocyte phagocytic activity, and by reducing collagen fiber content associated with increased metalloproteinase-8 expression and decreased expression of tissue inhibitor of metalloproteinase-1 (Weiss et al. 2013a, b). However, MSCs may not always ameliorate lung injury with some pre-clinical data suggesting that MSCs may contribute to established lung fibrosis (Epperly et al. 2003; Yan et al. 2007; Weiss and Ortiz 2013).
In addition, the ability to secrete microparticles that contain not only proteins but RNA or miRNA species which can modulate the expression of multiple genes make these packaging vesicles an attractive and quite plausible means for MSCs to regulate multiple pathways and produce a robust therapeutic effect in different lung injury models (Lee et al. 2012; Aliotta et al. 2012; Zhang et al. 2012a, b; Thebaud and Stewart 2012; Islam et al. 2012). Besides this, direct mitochondrial transfer from MSCs to ATII cells through connexin 43-mediated cell–cell bridges has been demonstrated to replenish endotoxin-depleted ATP stores and restore surfactant secretion (Islam et al. 2012).
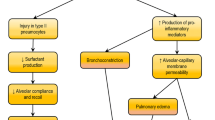
Importantly, MSCs can also exert effects on lung inflammation and injury through primary interactions with the immune system rather than through direct actions in lung. For example, available information demonstrates that MSCs alleviate endotoxin-induced acute lung injury in mouse models inhibiting Th1 response through release of soluble anti-inflammatory, anti-bacterial, and angiogenic substances, including IL-10, angiopoietin 1, KGF, and others (Mei et al. 2007; Lee et al. 2009a, b; Danchuk et al. 2011; Gupta et al. 2012; Ionescu et al. 2012a, b). In contrast, MSC administration in mouse models of asthma (allergic airways inflammation) ameliorates airways hyper-responsiveness by reducing Th2/Th17-mediated inflammation through effects on antigen-specific T lymphocytes and by up-regulating T-regulatory cells (Cho et al. 2009; Park et al. 2010; Nemeth et al. 2010; Firinci et al. 2011; Goodwin et al. 2011). As such, as MSC-based therapies are developed for lung diseases, the specific disease pathogenesis in the context of the known actions of the MSCs must be carefully considered (Fig. 6.2).
4 Localization of MSCs in Lung After Systemic MSC Administration
Following systemic administration of MSCs isolated from bone marrow, adipose, placenta, or cord blood, a number of studies demonstrate that the cells initially localize in the lung vascular bed and that lung injury results in increased localization and/or retention of marrow-derived cells in lung (reviewed by Weiss 2013; Antunes et al. 2014). Whether this represents formation of cell emboli in the lung vasculature or specific adherence to pulmonary vascular adhesion or other molecules remains unclear. Further, the source of the MSCs may influence retention in the lung. For example, MSCs derived from human umbilical cord blood are cleared more rapidly from the lungs than are human bone marrow-derived MSCs (Nystedt et al. 2013). This reflects both differences in size of the MSCs from different sources as well as differential expression of specific integrin and proteoglycan patterns. Retention in the lung may also trigger the MSCs to have functional effects. For example, embolization of systemically administered MSCs in lung was felt to result in secretion of an anti-inflammatory protein, TSG-6 (Lee et al. 2009a, b). However, although bone marrow- or adipose-derived MSCs can be induced in vitro to express phenotypic markers of alveolar or airway epithelial cells, retention of MSCs in the lung is generally transient. Structural engraftment of MSCs as lung epithelium is a rare event of uncertain physiologic significance in lung (Loi et al. 2006; Sueblinvong et al. 2008; Ma et al. 2011; Maria and Tran 2011; Li et al. 2012; Yan et al. 2012; Baer 2011). However, some available data suggests that systemically administered MSCs can engraft as fibroblasts or myofibroblasts under certain fibrosing injury conditions, further discussed below (Antunes et al. 2014; Kanki-Horimoto et al. 2006). This is a potential undesirable effect of the MSCs.
5 Use of MSCs in Lung Diseases
A steadily increasing number of articles demonstrate efficacy of either systemic or intratracheal administration of MSCs obtained from bone marrow, adipose, cord blood, or placenta in a growing spectrum of lung injury models in mice and in a slowly growing number of clinical investigations in lung diseases (reviewed by Weiss 2013; Antunes et al. 2014). This includes mouse models of acute lung injury and bacterial lung infection (Gupta et al. 2012; Ionescu et al. 2012a, b; Danchuk et al. 2011; Kim et al. 2011; Sun et al. 2011a, b; Xu et al. 2012; Zhang et al. 2013), asthma (Firinci et al. 2011; Goodwin et al. 2011; Kapoor et al. 2011; Kavanagh and Mahon 2011; Lee et al. 2011; Ou-Yang et al. 2011; Ionescu et al. 2012a, b; Lathrop et al. 2014), bronchopulmonary dysplasia (Chang et al. 2011; Pierro et al. 2013; Zhang et al. 2010, 2012a, b; Tropea et al. 2012; Sutsko et al. 2013), COPD (Hoffman et al. 2011; Katsha et al. 2011; Schweitzer et al. 2011; Ingenito et al. 2012; Kim et al. 2012), ischemia re-perfusion injury (Yang et al. 2009; Manning et al. 2010; Sun et al. 2011a, b), post-inflammatory lung fibrosis (Ortiz et al. 2003, 2007; Rojas et al. 2005; Zhao et al. 2008; Aguilar et al. 2009; Kumamoto et al. 2009; Moodley et al. 2009; Cargnoni et al. 2010; Cabral et al. 2011; Lee et al. 2010; Saito et al. 2011), pulmonary hypertension (Lee et al. 2012; Baber et al. 2007; Umar et al. 2009; Kanki-Horimoto et al. 2006; Hansmann et al. 2012; Liang et al. 2011), sepsis and burns (Gonzalez-Rey et al. 2009; Nemeth et al. 2009; Iyer et al. 2010; Mei et al. 2010; Yagi et al. 2010a, b; Krasnodembskaya et al. 2012), and other critical illness or autoimmune-related lung injuries including hemorrhagic shock, lupus, pancreatitis, silicosis, and ventilator-induced lung injury (Shi et al. 2012; Pati et al. 2011; Wang et al. 2012; Lassance et al. 2009; Chimenti et al. 2012; Curley et al. 2012). Systemically administered MSCs can also home to tumors, through as yet unclear chemotactic mechanisms, and have been utilized for delivery of chemotherapeutic and other anti-tumor agents in mouse lung tumor models. This may provide a viable therapy for lung cancers, particularly with MSCs engineered to express anti-tumor compounds such as tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) or Interferon beta (IFN-β) (Kanehira et al. 2007; Rachakatla et al. 2007; Stoff-Khalili et al. 2007; Xin et al. 2007; Zhang et al. 2008; Matsuzuka et al. 2010; Loebinger et al. 2009a, b; Heo et al. 2011; Hu et al. 2012). MSC administration has also been demonstrated to alleviate inflammation and injury produced by intratracheal instillation of either endotoxin or bacterial in human lung explants (Lee et al. 2009a, b, 2013).
In parallel with robust pre-clinical data, a slowly growing number of clinical investigations of MSC-based therapy in different lung diseases including ARDS, COPD, IPF, and silicosis are occurring (Table 6.1). In the following sections, the rationale for potential MSC effects, available pre-clinical data, and considerations of clinical trials of MSCs in ARDS, COPD, IPF, and silicosis will be considered.
6 Acute Respiratory Distress Syndrome
The immunomodulatory and reparative potential of MSCs makes them potential therapeutic tools for the acute inflammatory response to infection and pulmonary injury seen in ARDS. Several pre-clinical studies on ARDS have demonstrated that MSCs may improve the pulmonary and systemic inflammation characteristic of the disease (Rojas et al. 2005; Nemeth et al. 2009; Mei et al. 2010; Gupta et al. 2007). In models of endotoxin- or bacterial-induced ARDS mice and in explanted human lungs, MSC administration not only attenuates inflammation by decreasing several inflammatory mediators, including tumor necrosis factor-alpha (TNF-α), macrophage inflammatory protein 2-alpha (MIP-2), IFN-γ, IL-1β, MIP-1α, IL-6, IL-8, and keratinocyte-derived cytokine in plasma and bronchoalveolar lavage fluid, but it is also able to rescue epithelial cells with mitochondrial dysfunction by mitochondria transfer (Islam et al. 2012; Spees et al. 2006). In addition, MSCs favorably influence the host response to bacterial infections, the commonest and most severe cause of ARDS. MSC therapy can reduce bacterial counts via a number of mechanisms, including increased antimicrobial peptide secretion, such as lipocalin-2 (Gupta et al. 2012), and enhanced macrophage phagocytosis (Krasnodembskaya et al. 2012; Nemeth et al. 2009). MSCs also enhance repair following lung injury, as evidenced by the findings that both intravenous (Curley et al. 2012) and intratracheal (Curley et al. 2013) MSC therapy restore lung function following ventilator-induced lung injury via a KGF-dependent mechanism. Based on these promising preclinical findings, a number of early-phase clinical trials have begun to investigate the potential of MSC therapy for severe ARDS.
Currently, five studies of MSC therapy safety in patients with ARDS are listed in ClinicalTrials.gov. At the University of California, San Francisco, a phase I, multicenter, open-label dose escalation clinical trial is in progress to assess the safety of intravenous infusion of allogeneic bone marrow-derived human MSCs in ARDS (NCT01775774) and a phase II, multicenter study was initiated in March, 2014, to assess the safety and efficacy of a single dose of allogeneic bone marrow-derived human MSCs infusion in patients with ARDS. In Sweden, a phase I, multi-center, open-label, non-randomized controlled trial is also testing the safety of bone-marrow-derived MSCs in ARDS (NCT02215811). Two phase I, randomized, double-blind, placebo-controlled trials are also taking place in China to test the safety of systemic infusion of allogeneic human adipose MSCs (NCT01902082) and of MSCs derived from menstrual blood (NCT02095444) in ARDS patients.
7 Chronic Obstructive Pulmonary Disease
In several preclinical studies, MSC administration has been demonstrated to attenuate inflammation by decreasing levels of inflammatory mediators, such as IL-1β, TNF-α, IL-8, as well as decrease apoptosis (Huh et al. 2011; Zhen et al. 2010), improve parenchymal repair (increased levels of KGF, HGF, and epidermal growth factor), and increase lung perfusion (Huh et al. 2011; Shigemura et al. 2006; Guan et al. 2013). Based on these preclinical findings, several groups are investigating the therapeutic potential of MSC therapy in COPD patients.
The first safety trial registered in ClinicalTrials.gov (NCT01110252) assessed systemic administration of autologous bone marrow mononuclear cells in four Brazilian patients/volunteers with advanced COPD (stage IV dyspnea) and found no obvious adverse effects after 1 year (Ribeiro-paes et al. 2011). In a recent trial carried out in the United States (NCT00683722), using non-HLA matched allogeneic bone marrow-derived MSCs obtained from healthy volunteers (Prochymal®; Osiris Therapeutics Inc), sixty-two patients were randomized to double-blinded intravenous infusions of either allogeneic MSCs or vehicle control. Patients received four monthly infusions (100 × 106 cells/infusion) and were subsequently followed for 2 years after the first infusion (Weiss et al. 2013a, b). This trial demonstrated that use of MSCs in COPD patients may be considered safe, as there were no infusion reactions and no deaths or serious adverse events deemed related to MSC administration. However, no significant differences were observed in the overall number of adverse events, frequency of COPD exacerbations, or severity of disease in patients treated with MSCs. A significant decrease was observed in circulating C-reactive protein in MSC-treated patients giving a potential mechanistic clue of MSC actions.
A phase I, non-randomized, open-label study in Brazil is currently recruiting patients diagnosed with severe heterogeneous emphysema to evaluate the safety of one-way endobronchial valves combined with bone-marrow MSCs (NCT01872624). Another phase I, non-randomized, non-blinded, prospective study to test the safety and feasibility of administration of bone-marrow MSCs before and after lung volume reduction surgery for severe pulmonary COPD has been concluded in the Netherlands (NCT01306513). Results for this study are pending. An open-label, non-randomized, multicenter study is currently underway in Mexico to evaluate the safety and efficacy of autologous adipose-derived stem cell transplantation in GOLD moderate-severe patients (NCT01559051).
8 Idiopathic Pulmonary Fibrosis
When administered early after injury is instituted, MSCs attenuate inflammation and prevent development of bleomycin-induced lung fibrosis in mice, the most commonly utilized experimental model. However, administration of MSCs at time intervals longer than 7 days after bleomycin administration had no effect on established fibrotic changes in either mouse or pig lungs (Ortiz et al. 2003). Further, using a different model of lung fibrosis induced by radiation exposure in rodents, MSCs administered at time points at which established fibrotic changes were present, MSCs were detected in the interstitium as myofibroblasts suggesting that fibroblastic differentiation of MSC occurred in response to mediators produced in the injured tissue (Epperly et al. 2003; Yan et al. 2007). These data suggest that MSC administration in the setting of an established or ongoing fibrotic response may worsen the disease process and augment scarring in injured tissue rather than reversing it. As such, available data only supports a potential ameliorating effect of MSC administration in fibrotic lung diseases if administered early in the disease course during active inflammation (Weiss et al. 2013a, b). At present, there is no data to support an ameliorating effect of MSCs on established lung fibrosis. Thus, careful consideration must be given to clinical investigations of MSCs in fibrotic lung diseases.
Despite these concerns, there are three trials listed in ClinicalTrials.gov that are taking place to evaluate the safety and feasibility of MSC therapy in IPF patients. In the United States, a phase I/ll, randomized, blinded, and placebo-controlled trial is recruiting 25 IPF patients to investigate the safety, tolerability, and potential efficacy of intravenous infusion of allogeneic human MSCs (NCT02013700). Another phase I, open-label, multicenter, non-randomized study will evaluate the safety and feasibility of the endobronchial infusion of autologous bone-marrow MSCs at escalating doses in patients with mild-to-moderate IPF at Navarra University in Spain (NCT01919827). A third phase I, open-label, single-center, non-randomized dose-escalation study in Australia evaluated the safety and feasibility of placental-derived MSC infusion in IPF patients (NCT01385644). Initial results from this trial demonstrate no adverse effects over the 6-month follow-up period. A fourth trial, not listed in clinicaltrials.gov, reported no adverse effects of endobronchial administration of autologous adipose-derived MSCs over a 1-year follow-up period (Tzouvelekis et al. 2013).
9 Silicosis
Preclinical studies using an experimental model of silicosis demonstrated that both systemic and intratracheal administration of autologous BMMCs reduce inflammation and fibrosis (Lassance et al. 2009; Lopes-Pacheco et al. 2013). These positive effects encouraged a non-randomized, phase I trial of endobronchial administration of autologous BMMCs in patients with chronic and accelerated silicosis in Brazil (NCT01239862). In this study, three patients each received 2 × 107 bone marrow-derived cells labeled with 99mTc. The cellular infusion procedure was well tolerated by the patients, and no respiratory, cardiovascular, or hematological complications were observed. Scintigraphy showed an increase in lung perfusion in the basal region up to day 180 after the infusion, while the apex and midzone areas presented reduced perfusion at day 180 (Loivos et al. 2010; Souza et al. 2012). However, no subsequent clinical study of MSCs in silicosis has occurred.
10 Conclusions and Future Directions
Cell therapy approaches for lung diseases and critical illnesses including ARDS, COPD, IPF, and silicosis continue to evolve at a rapid pace. Pre-clinical studies with MSCs have generated a great amount of enthusiasm as a beneficial therapy for lung diseases and critical illnesses. Initial clinical trials have demonstrated that MSC administration is safe, with few adverse effects, but substantial challenges still have to be overcome before MSCs can be used for clinical practice. As such, further studies focusing on understanding the mechanisms of action of MSCs must be more investigated in order to continue to develop rational approaches for clinical trials. Nonetheless, cell-based therapies with MSCs and other cell types offer potential hope for these devastating and incurable pulmonary diseases.
Abbreviations
- ARDS:
-
Acute respiratory distress syndrome
- CCL2:
-
Chemokine ligand 2
- COPD:
-
Chronic obstructive pulmonary disease
- eNOS:
-
Endothelial nitric oxide synthase
- EPC:
-
Endothelial progenitor cell
- GMP:
-
Good manufacturing process
- HGF:
-
Hepatocyte growth factor
- IDO:
-
Indoleamine 2,3-dioxygenase
- IFN-β:
-
Interferon beta
- IL-10:
-
Interleukin 10
- IL-6:
-
Interleukin 6
- IND:
-
Investigational new drug
- IPF:
-
Idiopathic pulmonary fibrosis
- ISCT:
-
International Society for Cellular Therapy
- KGF:
-
Keratinocyte growth factor
- MIP-2:
-
Macrophage inflammatory protein 2-alpha
- miRNA:
-
Microribonucleic acid
- MSC:
-
Mesenchymal stromal (stem) cells
- NHBLI:
-
National Heart Blood and Lung Institute
- NO:
-
Nitric oxide
- PACT:
-
Production Assistance in Cellular Therapies
- TGF-β:
-
Transforming growth factor beta
- TNF-α:
-
Tumour necrosis factor alpha
- TRAIL:
-
Tumour necrosis factor-related apoptosis-inducing ligand
References
Aguilar S, Scotton CJ, McNulty K et al (2009) Bone marrow stem cells expressing keratinocyte growth factor via an inducible lentivirus protects against bleomycin-induced pulmonary fibrosis. PLoS One 4:e8013
Aliotta JM, Lee D, Puente N, Faradyan S, Sears E, Amaral A, Goldberg L, Dooner MS, Pereira M, Quesenberry PJ (2012) Progenitor/stem cell fate determination: interactive dynamics of cell cycle and microvesicles. Stem Cells Dev 21(10):1627–1638
Antunes MA, Laffey JG, Pelosi P, Rocco PR (2014) Mesenchymal stem cell trials for pulmonary diseases. J Cell Biochem. doi:10.1002/jcb.24783
Baber SR, Deng W, Master RG, Bunnell BA, Taylor BK, Murthy SN, Hyman AL, Kadowitz PJ (2007) Intratracheal mesenchymal stem cell administration attenuates monocrotaline-induced pulmonary hypertension and endothelial dysfunction. Am J Physiol Heart Circ Physiol 292:H1120–H1128
Baer PC (2011) Adipose-derived stem cells and their potential to differentiate into the epithelial lineage. Stem Cells Dev 20(10):1805–1816
Baer PC, Geiger H (2012) Adipose-derived mesenchymal stromal/stem cells: tissue localization, characterization, and heterogeneity. Stem Cells Int 2012:812693
Bi Y, Ehirchiou D, Kilts TM, Inkson CA, Embree MC, Sonoyama W et al (2007) Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nat Med 13(10):1219–1227
Bianco P, Cao X, Frenette PS et al (2013) The meaning, the sense and the significance: translating the science of mesenchymal stem cells into medicine. Nat Med 19:35–42
Cabral RM, Branco E, Rizzo Mdos S, Ferreira GJ, Gregores GB, Samoto VY, Stopiglia AJ, Maiorka PC, Fioretto ET, Capelozzi VL, Borges JB, Gomes S, Beraldo MA, Carvalho CR, Miglino MA (2011) Cell therapy for fibrotic interstitial pulmonary disease: experimental study. Microsc Res Tech 74:957–962
Capelli C, Gotti E, Morigi M, Rota C, Weng L, Dazzi F et al (2011) Minimally manipulated whole human umbilical cord is a rich source of clinical-grade human mesenchymal stromal cells expanded in human platelet lysate. Cytotherapy 13(7):786–801
Caplan AI (2009) Why are MSCs therapeutic? New data: new insight. J Pathol 217:318–324
Caplan AI, Bruder SP (2001) Mesenchymal stem cells: building blocks for molecular medicine in the 21st century. Trends Mol Med 7:259–264
Cargnoni A, Gibelli L, Tosini A et al (2010) Transplantation of allogeneic and xenogeneic placenta-derived cells reduces bleomycin-induced lung fibrosis. Cell Transplant 18:405–422
Chang YS, Choi SJ, Sung DK, Kim SY, Oh W, Yang YS, Park WS (2011) Intratracheal transplantation of human umbilical cord blood-derived mesenchymal stem cells dose-dependently attenuates hyperoxia-induced lung injury in neonatal rats. Cell Transplant 20(11):1843–1854
Chen J, Li C, Gao X, Li C, Liang Z, Yu L, Li Y, Xiao X, Chen L (2013) Keratinocyte growth factor gene delivery via mesenchymal stem cells protects against lipopolysaccharide-induced acute lung injury in mice. PLoS One 8(12)
Chimenti L, Luque T, Bonsignore MR, Ramirez J, Navajas D, Farre R (2012) Pre-treatment with mesenchymal stem cells reduces ventilator-induced lung injury. Eur Respir J 40:939–948
Cho KS, Park HK, Park HY et al (2009) IFATS collection: immunomodulatory effects of adipose tissue-derived stem cells in an allergic rhinitis mouse model. Stem Cells 27:259–265
Chong PP, Selvaratnam L, Abbas AA, Kamarul T (2012) Human peripheral blood derived mesenchymal stem cells demonstrate similar characteristics and chondrogenic differentiation potential to bone marrow derived mesenchymal stem cells. J Orthop Res 30(4):634–642
Choong PF, Mok PL, Cheong SK, Then KY (2007) Mesenchymal stromal cell-like characteristics of corneal keratocytes. Cytotherapy 9(3):252–258
Curley GF, Hayes M, Ansari B, Shaw G, Ryan A, Barry F, O’Brien T, O’Toole D, Laffey JG (2012) Mesenchymal stem cells enhance recovery and repair following ventilator-induced lung injury in the rat. Thorax 67:496–501
Curley GF, Ansari B, Hayes M, Devaney J, Masterson C, Ryan A, Barry F, O’Brien T, Toole DO, Laffey JG (2013) Effects of intratracheal mesenchymal stromal cell therapy during recovery and resolution after ventilator-induced lung injury. Anesthesiology 118:924–932
Danchuk S, Ylostalo JH, Hossain F, Sorge R, Ramsey A, Bonvillain RW, Lasky JA, Bunnell BA, Welsh DA, Prockop DJ, Sullivan DE (2011) Human multipotent stromal cells attenuate lipopolysaccharide-induced acute lung injury in mice via secretion of tumor necrosis factor-alpha-induced protein 6. Stem Cell Res Ther 2(3):27
Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D et al (2006) Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8(4):315–317
Epperly MW, Guo H, Gretton JE, Greenberger JS (2003) Bone marrow origin of myofibroblasts in irradiation pulmonary fibrosis. Am J Respir Cell Mol Biol 29(2):213–224
Firinci F, Karaman M, Baran Y, Bagriyanik A, Ayyildiz ZA, Kiray M, Kozanoglu I, Yilmaz O, Uzuner N, Karaman O (2011) Mesenchymal stem cells ameliorate the histopathological changes in a murine model of chronic asthma. Int Immunopharmacol 11:1120–1126
Gonzalez-Rey E, Anderson P, Gonzalez MA, Rico L, Buscher D, Delgado M (2009) Human adult stem cells derived from adipose tissue protect against experimental colitis and sepsis. Gut 58:929–939
Goodwin M, Sueblinvong V, Eisenhauer P, Ziats NP, Leclair L, Poynter ME, Steele C, Rincon M, Weiss DJ (2011) Bone marrow derived mesenchymal stromal cells inhibit Th2-mediated allergic airways inflammation in mice. Stem Cells 29:1137–1148
Griffin MD, Ryan AE, Alagesan S et al (2013) Anti-donor immune responses elicited by allogeneic mesenchymal stem cells: what have we learned so far? Immunol Cell Biol 91:40–51
Gronthos S (2011) The therapeutic potential of dental pulp cells: more than pulp fiction? Cytotherapy 13(10):1162–1163
Gronthos S, Mankani M, Brahim J, Robey PG, Shi S (2000) Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci U S A 97(25):13625–13630
Guan XJ, Song L, Han FF, Cui ZL, Chen X, Guo XJ, Xu WG (2013) Mesenchymal stem cells protect cigarette smoke-damaged lung and pulmonary function partly via VEGF-VEGF receptors. J Cell Biochem 114:323–335
Gupta N, Su X, Popov B, Lee JW, Serikov V, Matthay MA (2007) Intrapulmonary delivery of bone marrow-derived mesenchymal stem cells improves survival and attenuates endotoxin-induced acute lung injury in mice. J Immunol 179:1855–1863
Gupta N, Krasnodembskaya A, Kapetanaki M, Mouded M, Tan X, Serikov V, Matthay MA (2012) Mesenchymal stem cells enhance survival and bacterial clearance in murine Escherichia coli pneumonia. Thorax 67:533–539
Hansmann G, Fernandez-Gonzalez A, Aslam M, Vitali SH, Martin T, Mitsialis SA, Kourembanas S (2012) Mesenchymal stem cell-mediated reversal of bronchopulmonary dysplasia and associated pulmonary hypertension. Pulm Circ 2:170–181
Heo SC, Lee KO, Shin SH, Kwon YW, Kim YM, Lee CH, Kim YD, Lee MK, Yoon MS, Kim JH (2011) Periostin mediates human adipose tissue-derived mesenchymal stem cell-stimulated tumor growth in a xenograft lung adenocarcinoma model. Biochim Biophys Acta 1813(12):2061–2070
Hoffman AM, Paxson JA, Mazan MR, Davis AM, Tyagi S, Murthy S, Ingenito EP (2011) Lung-derived mesenchymal stromal cell post-transplantation survival, persistence, paracrine expression, and repair of elastase-injured lung. Stem Cells Dev 20(10):1779–1792
Horwitz EM, Le Blanc K, Dominici M, Mueller I, Slaper-Cortenbach I, Marini FC et al (2005) Clarification of the nomenclature for MSC: The International Society for Cellular Therapy position statement. Cytotherapy 7(5):393–395
Hu YL, Huang B, Zhang TY, Miao PH, Tang GP, Tabata Y, Gao J (2012) Mesenchymal stem cells as a novel carrier for targeted delivery of gene in cancer therapy based on nonviral transfection. Mol Pharm 9:2698–2709
Huh JW, Kim SY, Lee JH, Lee JS, Van Ta Q, Kim M, Oh YM, Lee YS, Lee SD (2011) Bone marrow cells repair cigarette smoke-induced emphysema in rats. Am J Physiol Lung Cell Mol Physiol 301(3):L255–L266
Igura K, Zhang X, Takahashi K, Mitsuru A, Yamaguchi S, Takashi TA (2004) Isolation and characterization of mesenchymal progenitor cells from chorionic villi of human placenta. Cytotherapy 6(6):543–553
Ingenito EP, Tsai L, Murthy S, Tyagi S, Mazan M, Hoffman A (2012) Autologous lung-derived mesenchymal stem cell transplantation in experimental emphysema. Cell Transplant 21(1):175–189
Ionescu L, Byrne RN, van Haaftern T, Vadivel A, Alphonse RS, Rey-Parra GJ, Weissmann G, Hall A, Eaton F, Thebaud B (2012a) Stem cell conditioned medium improves acute lung injury in mice: in vivo evidence for stem cell paracrine action. Am J Physiol Lung Cell Mol Physiol 303:L967–L977
Ionescu LI, Alphonse RS, Arizmendi N, Morgan B, Abel M, Eaton F, Duszyk M, Vliagoftis H, Aprahamian TR, Walsh K, Thebaud B (2012b) Airway delivery of soluble factors from plastic-adherent bone marrow cells prevents murine asthma. Am J Respir Cell Mol Biol 46:207–216
Islam MN, Das SR, Emin MT, Wei M, Sun L, Westphalen K, Rowlands DJ, Quadri SK, Bhattacharya S, Bhattacharya J (2012) Mitochondrial transfer from bone-marrow-derived stromal cells to pulmonary alveoli protects against acute lung injury. Nat Med 8(5):759–765
Iyer SS, Torres-Gonzalez E, Neujahr DC, Kwon M, Brigham KL, Jones DP, Mora AL, Rojas M (2010) Effect of bone marrow-derived mesenchymal stem cells on endotoxin-induced oxidation of plasma cysteine and glutathione in mice. Stem Cells Int 2010:868076
Jazedje T, Perin PM, Czeresnia CE, Maluf M, Halpern S, Secco M et al (2009) Human fallopian tube: a new source of multipotent adult mesenchymal stem cells discarded in surgical procedures. J Transl Med 7:46
Jones E, Churchman SM, English A, Buch MH, Horner EA, Burgoyne CH et al (2010) Mesenchymal stem cells in rheumatoid synovium: enumeration and functional assessment in relation to synovial inflammation level. Ann Rheum Dis 69(2):450–457
Kanehira M, Xin H, Hoshino K, Maemondo M, Mizuguchi H, Hayakawa T, Matsumoto K, Nakamura T, Nukiwa T, Saijo Y (2007) Targeted delivery of NK4 to multiple lung tumors by bone marrow-derived mesenchymal stem cells. Cancer Gene Ther 14:894–903
Kang SG, Shinojima N, Hossain A, Gumin J, Yong RL, Colman H et al (2010) Isolation and perivascular localization of mesenchymal stem cells from mouse brain. Neurosurgery 67(3):711–720
Kanki-Horimoto S, Horimoto H, Mieno S et al (2006) Implantation of mesenchymal stem cells overexpressing endothelial nitric oxide synthase improves right ventricular impairments caused by pulmonary hypertension. Circulation 114(1 Suppl):I181–I185
Kapoor S, Patel SA, Kartan S, Axelrod D, Capitle E, Rameshwar P (2011) Tolerance-like mediated suppression by mesenchymal stem cells in patients with dust mite allergy-induced asthma. J Allergy Clin Immunol 129(4):1094–1101
Katsha AM, Ohkouchi S, Xin H, Kanehira M, Sun R, Nukiwa T, Saijo Y (2011) Paracrine factors of multipotent stromal cells ameliorate lung injury in an elastase-induced emphysema model. Mol Ther 19:196–203
Kavanagh H, Mahon BP (2011) Allogeneic mesenchymal stem cells prevent allergic airway inflammation by inducing murine regulatory T cells. Allergy 66(4):523–531
Keating A (2012) Mesenchymal stromal cells: new directions. Cell Stem Cell 10(6):709–716
Kern S, Eichler H, Stoeve J, Kluter H, Bieback K (2006) Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells 24(5):1294–1301
Kim ES, Chang YS, Choi SJ, Kim JK, Yoo HS, Ahn SY, Sung DK, Kim SY, Park YR, Park WS (2011) Intratracheal transplantation of human umbilical cord blood-derived mesenchymal stem cells attenuates Escherichia coli-induced acute lung injury in mice. Respir Res 12:108
Kim SY, Lee JH, Kim HJ, Park MK, Huh JW, Ro JY, Oh YM, Lee SD, Lee YS (2012) Mesenchymal stem cell-conditioned media recovers lung fibroblasts from cigarette smoke-induced damage. Am J Physiol Lung Cell Mol Physiol 302:L891–L908
Kotton DN (2012) Next generation regeneration: the hope and hype of lung stem cell research. Am J Respir Crit Care Med 12:1255–1260
Krampera M, Sartoris S, Liotta F, Pasini A, Angeli R, Cosmi L et al (2007) Immune regulation by mesenchymal stem cells derived from adult spleen and thymus. Stem Cells Dev 16(5):797–810
Krasnodembskaya A, Samarani G, Song Y, Zhuo H, Su X, Lee JW, Gupta N, Petrini M, Matthay MA (2012) Human mesenchymal stem cells reduce mortality and bacteremia in gram-negative sepsis in mice in part by enhancing the phagocytic activity of blood monocytes. Am J Physiol Lung Cell Mol Physiol 302:L1003–L1013
Kumamoto M, Nishiwaki T, Matsuo N et al (2009) Minimally cultured bone marrow mesenchymal stem cells ameliorate fibrotic lung injury. Eur Respir J 34:740–748
Lama VN, Smith L, Badri L, Flint A, Andrei AC, Murray S et al (2007) Evidence for tissue-resident mesenchymal stem cells in human adult lung from studies of transplanted allografts. J Clin Invest 117(4):989–996
Lassance RM, Prota LF, Maron-Gutierrez T, Garcia CS, Abreu SC, Passaro CP, Xisto DG, Castiglione RC, Carreira H Jr, Ornellas DS, Santana MC, Souza SA, Gutfilen B, Fonseca LM, Rocco PR, Morales MM (2009) Intratracheal instillation of bone marrow-derived cell in an experimental model of silicosis. Respir Physiol Neurobiol 169:227–233
Lathrop MJ, Brooks EM, Bonenfant NR, Sokocevic D, Borg ZD, Goodwin M, Loi R, Cruz FF, Dunaway CW, Steele C, Weiss DJ (2014) Mesenchymal stromal cells mediate aspergillus hyphal extract-induced allergic airways inflammation by inhibition of the Th17 signaling pathway. Stem Cells Transl Med 3(2):194–205
Lau AN, Goodwin M, Kim CF, Weiss DJ (2012) Stem cells and regenerative medicine in lung biology and diseases. Mol Ther 20:1116–1130
Le Blanc K, Mougiakakos D (2012) Multipotent mesenchymal stromal cells and the innate immune system. Nat Rev Immunol 12:383–396
Lee RH, Pulin AA, Seo MJ et al (2009a) Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell 5(1):54–63
Lee JW, Fang X, Gupta N et al (2009b) Allogeneic human mesenchymal stem cells for treatment of E. coli endotoxin-induced acute lung injury in the ex vivo perfused human lung. Proc Natl Acad Sci U S A 106:16357–16362
Lee S, Jang A, Kim Y, Cha J, Kim T, Jung S, Park S, Lee Y, Won J, Kim Y, Park C (2010) Modulation of cytokine and nitric oxide by mesenchymal stem cell transfer in lung injury/fibrosis. Respir Res 11:16
Lee SH, Jang AS, Kwon JH, Park SK, Won JH, Park CS (2011) Mesenchymal stem cell transfer suppresses airway remodeling in a toluene diisocyanate-induced murine asthma model. Allergy Asthma Immunol Res 3(3):205–211
Lee C, Mitsialis SA, Aslam M, Vitali SH, Vergadi E, Konstantinou G, Sdrimas K, Fernandez-Gonzalez A, Kourembanas S (2012) Exosomes mediate the cytoprotective action of mesenchymal stromal cells on hypoxia-induced pulmonary hypertension. Circulation 126:2601–2611
Lee JW, Krasnodembskaya A, McKenna DH, Song Y, Abbott J, Matthay MA (2013) Therapeutic effects of human mesenchymal stem cells in ex vivo human lungs injured with live bacteria. Am J Respir Crit Care Med 187(7):751–760
Li M, Ikehara S (2013) Bone-marrow-derived mesenchymal stem cells for organ repair. Stem Cells Int 2013:132642
Li H, Xu Y, Fu Q, Li C (2012) Effects of multiple agents on epithelial differentiation of rabbit adipose-derived stem cells in 3D culture. Tissue Eng Part A 18(17–18):1760–1770
Liang OD, Mitsialis SA, Chang MS, Vergadi E, Lee C, Aslam M, Fernandez-Gonzalez A, Liu X, Baveja R, Kourembanas S (2011) Mesenchymal stromal cells expressing heme oxygenase-1 reverse pulmonary hypertension. Stem Cells 29:99–107
Loebinger MR, Eddaoudi A, Davies D, Janes SM (2009a) Mesenchymal stem cell delivery of TRAIL can eliminate metastatic cancer. Cancer Res 69:4134–4142
Loebinger MR, Kyrtatos PG, Turmaine M, Price AN, Pankhurst Q, Lythgoe MF, Janes SM (2009b) Magnetic resonance imaging of mesenchymal stem cells homing to pulmonary metastases using biocompatible magnetic nanoparticles. Cancer Res 69:8862–8867
Loi R, Beckett T, Goncz KK, Suratt BT, Weiss DJ (2006) Limited restoration of cystic fibrosis lung epithelium in vivo with adult bone marrow-derived cells. Am J Respir Crit Care Med 173(2):171–179
Loivos LP, Fonseca LMB, Lima MA, Rocco PRM, Silva JRL, Morales MM (2010) Phase-1 study of autologous bone marrow cells intrabronchial instillation for patients silicosis. ClinicalTrials.gov Identifier: NCT01239862
Lopes-Pacheco M, Xisto DG, Ornellas FM, Antunes MA, Abreu SC, Rocco PR, Takiya CM, Morales MM (2013) Repeated administration of bone marrow-derived cells prevents disease progression in experimental silicosis. Cell Physiol Biochem 32(6):1681–1694
Lotfinegad P, Shamsasenjan K, Movassaghpour A, Majidi J, Baradaran B (2014) Immunomodulatory nature and site specific affinity of mesenchymal stem cells: a hope in cell therapy. Adv Pharm Bull 4(1):5–13
Ma N, Gai H, Mei J, Ding FB, Bao CR, Nguyen DM, Zhong H (2011) Bone marrow mesenchymal stem cells can differentiate into type II alveolar epithelial cells in vitro. Cell Biol Int 35(12):1261–1266
Mafi R, Hindocha S, Mafi P, Griffin M, Khan WS (2011) Sources of adult mesenchymal stem cells applicable for musculoskeletal applications—a systematic review of the literature. Open Orthop J 5(Suppl 2):242–248
Manning E, Pham S, Li S, Vazquez-Padron RI, Mathew J, Ruiz P, Salgar SK (2010) Interleukin-10 delivery via mesenchymal stem cells: a novel gene therapy approach to prevent lung ischemia-reperfusion injury. Hum Gene Ther 21:713–727
Maria OM, Tran S (2011) Human mesenchymal stem cells cultured with salivary gland biopsies adopt an epithelial phenotype. Stem Cells Dev 20(6):959–967
Matsuzuka T, Rachakatla RS, Doi C, Maurya DK, Ohta N, Kawabata A, Pyle MM, Pickel L, Reischman J, Marini F, Troyer D, Tamura M (2010) Human umbilical cord matrix-derived stem cells expressing interferon-beta gene significantly attenuate bronchioloalveolar carcinoma xenografts in SCID mice. Lung Cancer 70:28–36
Mei SH, McCarter SD, Deng Y, Parker CH, Liles WC, Stewart DJ (2007) Prevention of LPS-induced acute lung injury in mice by mesenchymal stem cells overexpressing angiopoietin 1. PLoS One Med 4:e269
Mei SHJ, Haitsma JJ, Dos Santos CC, Deng Y, Lai PFH, Slutsky AS, Liles WC, Stewart DJ (2010) Mesenchymal stem cells reduce inflammation while enhancing bacterial clearance and improving survival in sepsis. Am J Respir Crit Care Med 182:1047–1057
Moodley Y, Atienza D, Manuelpillai U, Samuel CS, Tchongue J, Ilancheran S, Boyd R, Trounson A (2009) Human umbilical cord mesenchymal stem cells reduce fibrosis of bleomycin-induced lung injury. Am J Pathol 175:303–313
Najimi M, Khuu DN, Lysy PA, Jazouli N, Abarca J, Sempoux C et al (2007) Adult-derived human liver mesenchymal-like cells as a potential progenitor reservoir of hepatocytes? Cell Transplant 16(7):717–728
Nakahara H, Bruder SP, Haynesworth SE, Holecek JJ, Baber MA, Goldberg VM et al (1990) Bone and cartilage formation in diffusion chambers by subcultured cells derived from the periosteum. Bone 11(3):181–188
Nemeth K, Leelahavanichkul A, Yuen PS, Mayer B, Parmelee A, Doi K, Robey PG, Leelahavanichkul K, Koller BH, Brown JM, Hu X, Jelinek I, Star RA, Mezey E (2009) Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med 15:42–49
Nemeth K, Keane-Myers A, Brown JM et al (2010) Bone marrow stromal cells use TGF-beta to suppress allergic responses in a mouse model of ragweed-induced asthma. Proc Natl Acad Sci U S A 107:5652–5657
Nystedt J, Anderson H, Tikkanen J et al (2013) Cell surface structures influence lung clearance rate of systemically infused mesenchymal stromal cells. Stem Cells 31(2):317–326. doi:10.1002/stem.1271
Ortiz LA, Gambelli F, McBride C, Gaupp D, Baddoo M, Kaminski N, Phinney DG (2003) Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proc Natl Acad Sci U S A 100:8407–8411
Ortiz LA, Dutreil M, Fattman C, Pandey AC, Torres G, Go K, Phinney DG (2007) Interleukin 1 receptor antagonist mediates the antiinflammatory and antifibrotic effect of mesenchymal stem cells during lung injury. Proc Natl Acad Sci U S A 104:11002–11007
Oswald J, Boxberger S, Jørgensen B, Feldmann S, Ehninger G, Bornhäuser M et al (2004) Mesenchymal stem cells can be differentiated into endothelial cells in vitro. Stem Cells 22(3):377–384
Ou-Yang H-F, Huang Y, Hu X-B, Wu C-G (2011) Suppression of allergic airway inflammation in a mouse model of asthma by exogenous mesenchymal stem cells. Exp Biol Med 236(12):1461–1467
Park HK, Cho KS, Park HY et al (2010) Adipose-derived stromal cells inhibit allergic airway inflammation in mice. Stem Cells Dev 19:1811–1818
Pati S, Gerber M, Menge TD et al (2011) Bone marrow derived mesenchymal stem cells inhibit inflammation and preserve vascular endothelial integrity in the lungs after hemorrhagic shock. PLoS One 6:e25171
Paunescu V, Deak E, Herman D, Siska IR, Tanasie G, Bunu C et al (2007) In vitro differentiation of human mesenchymal stem cells to epithelial lineage. J Cell Mol Med 11(3):502–508
Pawitan JA (2009) Prospect of adipose tissue derived mesenchymal stem cells in regenerative medicine. Cell Tissue Transplant Ther 2:7–9
Pierro M, Ionescu L, Montemurro T, Vadivel A, Weissmann G, Oudit G, Emery D, Bodiga S, Eaton F, Peault B, Mosca F, Lazzari L, Thebaud B (2013) Short-term, long-term and paracrine effect of human umbilical cord-derived stem cells in lung injury prevention and repair in experimental bronchopulmonary dysplasia. Thorax 68(5):475–484
Pittenger MF, Mackay AM, Beck SC et al (1999) Multilineage potential of adult human mesenchymal stem cells. Science 284:143–147
Prockop DJ, Oh JY (2012a) Medical therapies with adult stem/progenitor cells (MSCs): a backward journey from dramatic results in vivo to the cellular and molecular explanations. J Cell Biochem 113(5):1460–1469
Prockop DJ, Oh JY (2012b) Mesenchymal stem/stromal cells (MSCs): role as guardians of inflammation. Mol Ther 20:14–20
Rachakatla RS, Marini F, Weiss ML, Tamura M, Troyer D (2007) Development of human umbilical cord matrix stem cell-based gene therapy for experimental lung tumors. Cancer Gene Ther 14:828–835
Ribeiro-Paes JT, Bilaqui A, Greco OT, Ruiz MA, Marcelino MY, Stessuk T, de Faria CA, Lago MR (2011) Unicentric study of cell therapy in chronic obstructive pulmonary disease/pulmonary emphysema. Int J Chron Obstruct Pulmon Dis 6:63–71
Rojas M, Xu J, Woods CR, Mora AL, Spears W, Roman J, Brigham KL (2005) Bone marrow-derived mesenchymal stem cells in repair of the injured lung. Am J Respir Cell Mol Biol 33:145–152
Romanov YA, Svintsitskaya VA, Smirnov VN (2003) Searching for alternative sources of postnatal human mesenchymal stem cells: candidate MSC-like cells from umbilical cord. Stem Cells 21(1):105–110
Romieu-Mourez R, Coutu DL, Galipeau J (2012) The immune plasticity of mesenchymal stromal cells from mice and men: concordances and discrepancies. Front Biosci 4:824–837
Sabapathy V, Ravi S, Srivastava V, Srivastava A, Kumar S (2012) Long-term cultured human term placenta-derived mesenchymal stem cells of maternal origin displays plasticity. Stem Cells Int 2012:174328
Sabatini F, Petecchia L, Tavian M, Jodon De Villeroche V, Rossi GA, Brouty-Boye D (2005) Human bronchial fibroblasts exhibit a mesenchymal stem cell phenotype and multilineage differentiating potentialities. Lab Invest 85(8):962–971
Saito S, Nakayama T, Hashimoto N, Miyata Y, Egashira K, Nakao N, Nishiwaki S, Hasegawa M, Hasegawa Y, Naoe T (2011) Mesenchymal stem cells stably transduced with a dominant-negative inhibitor of CCL2 greatly attenuate bleomycin-induced lung damage. Am J Pathol 179:1088–1094
Schweitzer K, Johnstone BH, Garrison J, Rush N, Cooper S, Traktuev DO, Feng D, Adamowicz JJ, Van Demark M, Fisher AJ, Kamocki K, Brown MB, Presson RG Jr, Broxmeyer HE, March KL, Petrache I (2011) Adipose stem cell treatment in mice attenuates lung and systemic injury induced by cigarette smoking. Am J Respir Crit Care Med 183:215–225
Shi D, Wang D, Li X, Zhang H, Che N, Lu Z, Sun L (2012) Allogeneic transplantation of umbilical cord-derived mesenchymal stem cells for diffuse alveolar hemorrhage in systemic lupus erythematosus. Clin Rheumatol 31:841–846
Shigemura N, Okumura M, Mizuno S, Imanishi Y, Nakamura T, Sawa Y (2006) Autologous transplantation of adipose tissue-derived stromal cells ameliorates pulmonary emphysema. Am J Transplant 6:2592–2600
Souza S, Loivos L, Lima M, Szklo A, Goldenberg R, Rocco PRM, Silva JRL, Morales MM, Fonseca L, Gutffilen B (2012) Intrabronchial instillation of bone marrow derived mononuclear cells in silicotic patients: nuclear medicine analysis and follow-up. J Nucl Med 53(Suppl 1):606
Spees JL, Olson SD, Whitney MJ, Prockop DJ (2006) Mitochondrial transfer between cells can rescue aerobic respiration. Proc Natl Acad Sci U S A 103:1283–1288
Stoff-Khalili MA, Rivera AA, Mathis JM, Banerjee NS, Moon AS, Hess A, Rocconi RP, Numnum TM, Everts M, Chow LT, Douglas JT, Siegal GP, Zhu ZB, Bender HG, Dall P, Stoff A, Pereboeva L, Curiel DT (2007) Mesenchymal stem cells as a vehicle for targeted delivery of CRAds to lung metastases of breast carcinoma. Breast Cancer Res Treat 105:157–167
Sueblinvong V, Loi R, Eisenhauer PL, Bernstein IM, Suratt BT, Spees JL, Weiss DJ (2008) Derivation of lung epithelium from human cord blood-derived mesenchymal stem cells. Am J Respir Crit Care Med 177(7):701–711
Sun CK, Yen CH, Lin YC, Tsai TH, Chang LT, Kao YH, Chua S, Fu M, Ko SF, Leu S, Yip HK (2011a) Autologous transplantation of adipose-derived mesenchymal stem cells markedly reduced acute ischemia-reperfusion lung injury in a rodent model. J Transl Med 9:118
Sun J, Han ZB, Liao W, Yang SG, Yang Z, Yu J, Meng L, Wu R, Han ZC (2011b) Intrapulmonary delivery of human umbilical cord mesenchymal stem cells attenuates acute lung injury by expanding CD4+CD25+ Forkhead Boxp3 (FOXP3)+ regulatory T cells and balancing anti- and pro-inflammatory factors. Cell Physiol Biochem 27(5):587–596
Sutsko RP, Young KC, Ribeiro A, Torres E, Rodriguez M, Hehre D, Devia C, McNeice I, Suguihira C (2013) Long-term reparative effects of mesenchymal stem cell therapy following neonatal hyperoxia-induced lung injury. Pediatr Res 73(1):46–53
Thebaud B, Stewart DJ (2012) Exosomes: cell garbage can, therapeutic carrier, or trojan horse? Circulation 126(22):2553–2555
Tran TC, Kimura K, Nagano M, Yamashita T, Ohneda K, Sugimori H et al (2011) Identification of human placenta-derived mesenchymal stem cells involved in re-endothelialization. J Cell Physiol 226(1):224–235
Tropea KA, Leder E, Aslam M, Lau AN, Raiser DM, Lee JH, Balasubramaniam V, Fredenburgh LE, Mitsialis A, Kourembanas S, Kim CF (2012) Bronchioalveolar stem cells increase after mesenchymal stromal cell treatment in a mouse model of bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol 302:L829–L837
Tzouvelekis A, Paspaliaris V, Koliakos G, Ntolios P, Bouros E, Oikonomou A, Zissimopoulos A, Boussios N, Dardzinski B, Gritzalis D, Antoniadis A, Froudarakis M, Kolios G, Bouros D (2013) A prospective, non-randomized, no placebo-controlled, phase Ib clinical trial to study the safety of the adipose derived stromal cells-stromal vascular fraction in idiopathic pulmonary fibrosis. J Transl Med 11:171
Umar S, de Visser YP, Steendijk P et al (2009) Allogenic stem cell therapy improves right ventricular function by improving lung pathology in rats with pulmonary hypertension. Am J Phys Heart Circ Phys 297:H1606–H1616
Viswanathan S, Keating A, Deans R, Hematti P, Prockop DJ, Stroncek D, Stacey G, Weiss DJ, Mason C, Rao M (2014) Soliciting strategies for developing cell-based reference materials to advance MSC research and clinical translation. Stem Cells Dev 23(11):1157–1167
Wang HS, Hung SC, Peng ST, Huang CC, Wei HM, Guo YJ et al (2004) Mesenchymal stem cells in the Wharton’s jelly of the human umbilical cord. Stem Cells 22(7):1330–1337
Wang L, Tu XH, Zhao P, Song JX, Zou ZD (2012) Protective effect of transplanted bone marrow-derived mesenchymal stem cells on pancreatitis-associated lung injury in rats. Mol Med Rep 6:287–292
Weiss DJ (2013) Stem cells, cell therapies and bioengineering in lung biology and diseases: comprehensive review of the literature. Ann Am Thorac Soc 10(5):S45–S97
Weiss DJ (2014) Concise review: current status of stem cells and regenerative medicine in lung biology and diseases. Stem Cells 32(1):16–25. doi:10.1002/stem.1506
Weiss DJ, Ortiz LA (2013) Invited editorial: cell therapy trials for lung diseases: progress and cautions. Am J Respir Crit Care Med 188(2):123–125
Weiss DJ, Bates JHT, Gilbert T, Liles WC, Lutzko C, Rajagopal J, Prockop DJ (2013a) Vermont stem cell conference report: stem cells and cell therapies in lung biology and diseases. Ann Am Thorac Soc 10(5):S25–S44
Weiss DJ, Casaburi R, Flannery R, LeRoux-Williams M, Tashkin DP (2013b) A placebo-controlled, randomized trial of mesenchymal stem cells in COPD. Chest 143(6):1590–1598
Xin H, Kanehira M, Mizuguchi H, Hayakawa T, Kikuchi T, Nukiwa T, Saijo Y (2007) Targeted delivery of CX3CL1 to multiple lung tumors by mesenchymal stem cells. Stem Cells 25:1618–1626
Xu J, Qu J, Cao L, Sai Y, Chen C, He L, Yu L (2008) Mesenchymal stem cell-based angiopoietin-1 gene therapy for acute lung injury induced by lipopolysaccharide in mice. J Pathol 214(4):472–481
Xu YL, Liu YL, Wang Q, Li G, Lu XD, Kong B (2012) Intravenous transplantation of mesenchymal stem cells attenuates oleic acid induced acute lung injury in rats. Chin Med J 125(11):2012–2018
Yagi H, Soto-Gutierrez A, Kitagawa Y, Tilles AW, Tompkins RG, Yarmush ML (2010a) Bone marrow mesenchymal stromal cells attenuate organ injury induced by LPS and burn. Cell Transplant 19:823–830
Yagi H, Soto-Gutierrez A, Navarro-Alvarez N, Nahmias Y, Goldwasser Y, Kitagawa Y, Tilles AW, Tompkins RG, Parekkadan B, Yarmush ML (2010b) Reactive bone marrow stromal cells attenuate systemic inflammation via sTNFR1. Mol Ther 18:1857–1864
Yan X, Liu Y, Han Q, Jia M, Liao L, Qi M, Zhao RC (2007) Injured microenvironment directly guides the differentiation of engrafted Flk-1(+) mesenchymal stem cell in lung. Exp Hematol 35(9):1466–1475
Yan C, Qu P, Du H (2012) Myeloid-specific expression of Stat3C results in conversion of bone marrow mesenchymal stem cells into alveolar type II epithelial cells in the lung. Sci China Life Sci 55(7):576–590
Yang Z, Sharma AK, Marshall M, Kron IL, Laubach V (2009) NADPH oxidase in bone marrow-derived cells mediates pulmonary ischemia-reperfusion injury. Am J Respir Cell Mol Biol 40:375–381
You Q, Cai L, Zheng J, Tong X, Zhang D, Zhang Y (2008) Isolation of human mesenchymal stem cells from third-trimester amniotic fluid. Int J Gyn Obstet 103(2):149–152
Zannettino AC, Paton S, Arthur A, Khor F, Itescu S, Gimble JM et al (2008) Multipotential human adipose-derived stromal stem cells exhibit a perivascular phenotype in vitro and in vivo. J Cell Physiol 214(2):413–421
Zhang X, Zhao P, Kennedy C, Chen K, Wiegand J, Washington G, Marrero L, Cui Y (2008) Treatment of pulmonary metastatic tumors in mice using lentiviral vector-engineered stem cells. Cancer Gene Ther 15:73–84
Zhang H, Fang J, Su H, Yang M, Lai W, Mai Y, Wu Y (2010) Bone marrow mesenchymal stem cells attenuate lung inflammation of hyperoxic newborn rats. Pediatr Transplant 16(6):589–598
Zhang H, Liu X, Huang S, Bi X, Wang H, Xie L, Wang Y, Cao X, Xiao F, Yang Y, Guo Z (2012a) Microvesicles derived from human umbilical cord mesenchymal stem cells stimulated by hypoxia promote angiogenesis both in vitro and in vivo. Stem Cells Dev 21:3289–3297
Zhang X, Wang H, Shi Y, Peng W, Zhang S, Zhang W, Xu J, Mei Y, Feng Z (2012b) Role of bone marrow-derived mesenchymal stem cells in the prevention of hyperoxia-induced lung injury in newborn mice. Cell Biol Int 36(6):589–594
Zhang S et al (2013) Comparison of the therapeutic effects of human and mouse adipose-derived stem cells in a murine model of lipopolysaccharide-induced acute lung injury. Stem Cell Res Ther 4(1):13. doi:10.1186/scrt161
Zhao F, Zhang YF, Liu YG, Zhou JJ, Li ZK, Wu CG, Qi H (2008) Therapeutic effects of bone marrow-derived mesenchymal stem cells engraftment on bleomycin-induced lung injury in rats. Transplant Proc 40:1700–1705
Zhen G, Xue Z, Zhao J, Gu N, Tang Z, Xu Y, Zhang Z (2010) Mesenchymal stem cell transplantation increases expression of vascular endothelial growth factor in papain-induced emphysematous lungs and inhibits apoptosis of lung cells. Cytotherapy 12:605–614
Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H et al (2002) Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell 13(12):4279–4295
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Cruz, F.F., Rocco, P.R.M., Weiss, D.J. (2015). Challenges of Cell Therapy for Lung Diseases and Critical Illnesses. In: Firth, A., Yuan, JJ. (eds) Lung Stem Cells in the Epithelium and Vasculature. Stem Cell Biology and Regenerative Medicine. Springer, Cham. https://doi.org/10.1007/978-3-319-16232-4_6
Download citation
DOI: https://doi.org/10.1007/978-3-319-16232-4_6
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-16231-7
Online ISBN: 978-3-319-16232-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)