Abstract
Standard care of adjuvant treatment for potentially curable gastric cancer in Asia is to provide postoperative adjuvant chemotherapy after D2 surgery. To improve survival results of mainly for stage III patients, there are several clinical trials to test efficacy of new treatments, including new regimens as postoperative adjuvant, neoadjuvant chemotherapy, and postoperative or preoperative chemoradiotherapy.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Gastric cancer
- Adjuvant treatment
- Neoadjuvant chemotherapy
- D2 surgery
- Tegafur-gimeracil-oteracil
- Capecitabine
- Oxaliplatin
- Adjuvant chemoradiotherapy
Actual Standard of Adjuvant Therapy in the East
Principles
Due to results of several clinical trials [1–3], it is widely accepted that good local control by either radiation therapy or surgery is essential to cure gastric cancer. D2 dissection provides better local control of gastric cancer than D1 or D1 + radiation [4, 5]. High incidence of nodal disease in gastric cancer occurs in relatively early stage tumor as well, which justifies prophylactic application of D2 lymphadenectomy for stage IB or more advanced tumors [6].
Standard of Care in the Eastern Asian Countries
D2 dissection is widely accepted as common practice without serious increase of mortality with limited increase of morbidity in the East, due to high volume of patients with gastric cancer in each institution. Based on this surgical practice, adjuvant treatment does not include radiation therapy. Based on the results of two pivotal studies in this area [7, 8], postoperative adjuvant chemotherapy is the standard of care.
The advantage of postoperative setting of adjuvant treatment is that unnecessary toxic treatment can be avoided for patients who do not need any adjuvant treatment (stage I). This was a weak point of pre- or perioperative adjuvant treatment. In the MAGIC study of perioperative chemotherapy, 8.3 % of patients in surgery alone arm had T1 tumor, suggesting that similar proportion of patients in the peri-operative chemotherapy group were overtreated by unnecessary toxic agents [9]. Good prognosis of stage I patients after surgery alone means probability of occult residual cancer cells, which are target of adjuvant treatment, is very low (less than 10 %) in these patients [10]. Unlike Western countries, proportion of stage I patients in Korea and Japan is more than 50 %, thus this concept is quite important for patients and medical economy [11, 12].
Standard Regimen of Postoperative Chemotherapy
The adjuvant chemotherapy trial of TS-1 for gastric cancer (ACTS-GC) study showed significantly better overall survival (OS) and relapse-free survival (RFS) of the patients with stage II and III (Japanese classification [13]) gastric cancer using TS-1 monotherapy than surgery alone. Hazard ratio (HR) was 0.669 (95 % CI:0.540–0.828) for whole patients and that was 0.509, 0.708, 0.791 for stage II, IIIA, IIIB patients, respectively [8]. This monotherapy reduced mainly peritoneal (HR: 0.687) and nodal + local recurrence (0.505) but not remarkably distant metastasis (HR: 0.86). Based on these results, S-1 monotherapy is widely accepted in East Asian countries as one of the standard adjuvant treatment.
CLASSIC study showed significantly better disease-free survival (DFS) and OS for stage II and III (UICC TNM classification [14]) using XELODA + oxaliplatin (XELOX). HR of DFS was 0.56 (95 % CI: 0.44–0.72) for whole patients and that was 0.55, 0.57, 0.57 for stage II, IIIA, IIIB patients, respectively [7]. These results were confirmed after 5-year follow up [15]. The HR of OS was 0.66 (95CI: 0.51–0.85) for whole population and 0.54, 0.75, and 0.67 for stage II, IIIA, IIIB, respectively. The HR of DFS was 0.58 (95 % CI: 0.48–0.72). This doublet chemotherapy reduced mainly hematogenous (HR: 0.61) and nodal + local (HR: 0.51) but not peritoneal recurrence (HR: 0.87), which makes clear contrast with TS-1 monotherapy. Based on these results, XELOX is one of the standard treatments in Korea, China, and Taiwan. This study has two weak points: First, high proportion of patients did not receive allocated treatment (11% in surgery alone arm and 19% in chemotherapy arm) and secondly unusually large number of censored cases are seen in survival curves (both OS and DFS), suggesting lack of robustness of the statistical analyses.
Remaining Clinical Questions
-
1.
Is stronger or more intensive adjuvant treatment more efficient?
Since there was a tendency of worse HR with more advanced stage in the ACTS-GC study, more intensive treatment is searched for stage III patients. Theoretically, more intensive and therefore stronger chemotherapy might be better than single agent therapy, but expected worse feasibility (tolerance) of such treatment after D2 gastrectomy might ruin chemotherapeutic effect.
-
2.
Can OS be improved by adding preoperative chemotherapy to postoperative chemotherapy?
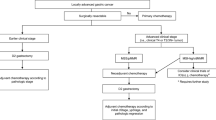
Advantage and disadvantage of preoperative chemotherapy (neoadjuvant chemotherapy (NAC)) is shown in Table 22.1. Unlike colon cancer, disturbance of oral intake is prominent after surgery in gastric cancer patients. Therefore, the most important benefit of NAC is high tolerability of rather intensive treatment, using multiple drugs. Another important benefit of NAC in gastric cancer is related with higher incidence of surgical complications which may hamper early start of adjuvant chemotherapy than in colorectal cancer.
-
3.
Is there any role of radiation therapy added to chemotherapy after D2 surgery?
The update analysis of INT0116 study showed that chemoradiotherapy in this trial reduced mainly local regional recurrence but not systemic recurrence. These data support the finding that this treatment is effective after D0/1 surgery but not after D2 surgery [16]. After the results of INT0116 study were published, a Korean group performed a phase III study to compare adjuvant chemoradiation with surgery with chemotherapy (XELOX) alone (ARTIST trial) [17]. Although there was a subgroup in which borderline benefit of this treatment was suggested, primary endpoints did not meet [17]. Chemoradiation after D2 surgery was not accepted as efficient adjuvant treatment for gastric cancer but there remains a question about role of radiation therapy after D2 surgery in advanced stage.
Ongoing Phase III Clinical Trials in Asia to Solve these Questions (Table 22.2)
-
1.
Comparing two adjuvant chemotherapy after D2 surgery
-
a.
S-1 + oxaliplatin (SOX) versus S-1 (POTENT study)
-
b.
Capecitabine + oxaliplatin (XELOX) versus XELOX + docetaxel
-
c.
S-1 versus S-1 + docetaxel
-
a.
-
2.
Evaluation of additional effect of NAC
-
a.
NAC by docetaxel + SOX followed by adjuvant S-1 after D2 surgery versus S-1 adjuvant after D2 surgery
-
b.
NAC by SOX followed by SOX after D2 versus SOX after D2
-
c.
NAC by SOX followed by SOX after D2 followed by S-1 versus SOX after D2 versus XELOX after D2
-
d.
NAC by XELOX followed by XELOX after D2 versus XELOX after D2
-
a.
-
3.
Role of radiation therapy added to adjuvant chemotherapy
-
a.
Surgery + S-1 versus surgery + SOX versus surgery + SOX + radiation (ARTIST II trial)
-
b.
NAC by XELOX + surgery followed by XELOX with or without concurrent preoperative radiotherapy
-
c.
Surgery + XELOX with radiation versus XELOX
-
a.
References
Wu CW, Hsiung CA, Lo SS, Hsieh MC, Chen JH, Li AFY, et al. Nodal dissection for patients with gastric cancer: a randomized controlled trial. Lancel Oncol. 2006;7:309–15.
Songun I, Putter H, Kranenbarg EM, Sasako M, van de Velde CJ. Surgical treatment of gastric cancer: 15-year follow-up results of the randomized nationwide Dutch D1D2 trial. Lancet Oncol. 2010;11:439–49.
Macdonald JS, Smalley SR, Benedetti J, Hundahl SA, Estes NC, Stemmermann GN, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med. 2001;345:725–30.
Sasako M. Gastric cancer—Eastern experience. Surg Oncol Clin N Am. 2012;21:71–7.
Sasako M, Saka M, Fukagawa T, Katai H, Sano T. Surgical treatment of advanced gastric cancer: Japanese perspective. Dig Surg. 2007;24:101–7.
Sasako M. Principles of surgical treatment for curable gastric cancer. J Clin Oncol. 2003;21(23s):274–5.
Bang YJ, Kim YW, Yang HK, Chung HC, Park YK, Lee KH, et al. for the CLASSIC trial investigators. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label randomized controlled trial. Lancet. 2012;379:315–21.
Sasako M, Sakuramoto S, Katai H, et al. Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J Clin Oncol. 2011;29:4387–93.
Cunningham D, Allum WH, Stenning SP, Thompson JN, van de Velde CJH, Nicolson M, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11–20.
Sano T, Sasako M, Kinoshita T, Maruyama K. Recurrence of early gastric cancer. Cancer. 1993;72:3174–8.
Nashimoto A, Akazawa K, Isobe Y, Miyashiro I, Katai H, Kodera Y, et al. Gastric cancer treated in 2002 in Japan: 2009 annual report of the JGCA nationwide registry. Gastric Cancer. 2013;16:1–27.
Jeong O, Park YK. Clinicopathological features and surgical treatment of gastric cancer in South Korea: the results of 2009 nationwide survey on surgically treated gastric cancer patients. J Gastric Cancer. 2011;11:69–77.
Japan Gastric Cancer Association. Japanese classification of gastric carcinoma. 2nd English ed. Gastric Cancer. 1998;1:10–24.
International Union Against Cancer (UICC). TNM classification of malignant tumours. 7th ed. Singapore: Wiley-Blackwell; 2009.
Noh SH, Park SR, Yang HK, Chung HC, Kim SW, Kim HH, Choi JH, Kim HK, Yu W, Lee JI, Shin DB, Ji J, Chen JS, Lim Y, Ha S, Bang YJ, on be half of the CLAASIC trial investigators. Adjuvant capecitabibe plus oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): 5-year follow-up of an open-label, randomised phase 3 trial. Lanect Oncol 2014;15:1389–96.
Smalley SR, Benditte JK, Haller DG, Hundahl SA, Estes NC, Ajani JA, et al. Update analysis of SWOG-directed intergroup study 0116: a phase III trial of adjuvant radiochemotherapy versus observation after curative gastric cancer resection. J Clin Oncol. 2012;30:2327–33.
Lee J, Lim DH, Kim S, Park SK, Park JO, Park YS, et al. Phase III trial comparing capecitabine plus cisplatin versus capecitabine plus cisplatin with concurrent Capecitabine radiotherapy in completely resected gastric cancer with D2 lymph node dissection: the ARTIST trial. J Clin Oncol. 2012;30:268–73.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Sasako, M. (2015). Adjuvant and Neoadjuvant Treatment: Standard Treatment and Clinical Trials in the East. In: Strong, V. (eds) Gastric Cancer. Springer, Cham. https://doi.org/10.1007/978-3-319-15826-6_22
Download citation
DOI: https://doi.org/10.1007/978-3-319-15826-6_22
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-15825-9
Online ISBN: 978-3-319-15826-6
eBook Packages: MedicineMedicine (R0)