Abstract
Regulatory T cells (Tregs) encompass an array of immunosuppressive cells responsible for the protection against exacerbated immune responses and the maintenance of tissue homeostasis. Various Treg subtypes, normally resident within distinct lymphoid and non-lymphoid tissues, can be recruited and expanded during inflammation, possibly undergoing functional and molecular re-programming. Generally, two processes have been reported in different settings of type-1 response: i) Treg subpopulations acquiring the ability to specifically suppress Th1 cells (called Th1-suppressing Tregs), and ii) Treg subsets rather polarizing into IFN-γ-producing (called Th1-like) Tregs.
Along the development of type-1 responses, Tregs are exposed to a variety of cytokines and other signals, exerting disparate activities. The combinatorial effects of typical Th1-driving cytokines, such as IL-12 (mostly produced by antigen-presenting cells during Th1 priming) and IFN-γ (mostly produced by pre-existing NK cells) lead to inhibition of Treg expansion and function, while promoting Th1-like Treg polarization. Conversely, cytokines produced at more advanced phases by Th1 effectors, such as IL-2, TNF-α and IFN-γ, promote Treg proliferation and/or Th1-suppressing Treg specialization. Some controversy exists around IL-27 and IFN-α, cytokines possibly released during bacterial or viral infections. Furthermore, cytokine signals can be finely tuned by the concomitant stimulation of costimulatory or coinhibitory receptors, such as OX40 and PD-1 respectively, within inflamed tissues.
A model may be envisaged of an alternate Treg response to type-1 cytokines, being hampered or boosted by early or late phase cytokines, respectively. Such regulation would unleash the development of protective type-1 immunity while constraining exacerbated Th1 responses, possibly causing immunopathology.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
- Th1 cells
- Regulatory T cells (Tregs)
- Th1-suppressing
- Th1-like
- Cytokine signals
- Type-1 cytokines
- CD4 T cells
Introduction
Most of the information about regulatory T cell (Treg) biology derives from the analysis of peripheral cells in the circulation and in lymphoid organs. While such data have provided important clues for the understanding of the crucial immune suppressive activity of Tregs in many settings, they may not recapitulate the complexity of Treg behavior within disparate non-lymphoid tissues. Many recent data, mostly obtained in experimental models, indicate a fine adaptation of Treg molecular program and suppressive functions in distinct tissues even under physiological conditions. For instance, the visceral adipose tissue and the skeletal muscle constitutively contain pools of resident Tregs characterized by PPARγ (Cipolletta et al. 2012) and amphiregulin (Burzyn et al. 2013b) overexpression respectively. Such molecules drive peculiar suppressive programs in the respective tissue-resident Tregs, shaped on the prevalent inflammatory mechanisms that need to be moldered to maintain or rescue homeostasis in each tissue (Burzyn et al. 2013a). Indeed, while visceral fat Tregs are particularly devoted to suppress metabolic inflammation, muscular Tregs are specialized in promoting muscle repair via the amphiregulin/epithelial growth factor receptor pathway.
In conditions of tissue injury, the development of immune and inflammatory responses is accompanied by, and possibly drives, the expansion of tissue-resident Tregs and the accrual of circulating Tregs, in an attempt to restore tissue homeostasis (Burzyn et al. 2013a). However, Treg suppression needs to be carefully regulated in the different phases of an inflammatory response, especially during chronic viral infections, such to achieve a compromise between pathogen containment and maintenance of tissue integrity and performance (Barnaba 2010).
Within tissues, Tregs are overwhelmed by a plethora of extracellular signals, such as cytokines, growth factors, chemokines and membrane-bound ligands, which finely and concurrently re-shape their functions, in line with the requirements of tissue preservation and immune response development (Smigiel et al. 2014). Cytokines represent crucial mediators in dictating and arranging the dominant type of immune response. At early phases of immune responses to virus-derived and also to other danger signals, both tissue and immune cells produce cytokines such as type I IFNs that orchestrate an innate defense program. Afterwards, appropriately stimulated antigen-presenting cells (APCs) release T helper 1 (Th1)-polarizing cytokines such as interleukin (IL)-12, IL-18 and IL-27, which drive Th1 differentiation and cytotoxic T lymphocyte (CTL) activation. At later phases, Th1- and CTL-derived cytokines, such as IL-2, interferon (IFN)-γ and tumor necrosis factor (TNF)-α, propagate inflammation and protective immunity. When recruited and/or expanded into inflamed tissues dominated by type-1 responses, Tregs may be exposed to combinations of APC- or T cell-derived type-1 cytokines , and differentially affected by them depending on cytokine receptor expression and sensitivity.
Treg Subsets Play Divergent Roles in Type-1 Inflammation
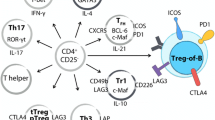
In the context of type-1 inflammation , two facets of Treg behavior have been described: some reports have underscored the existence of a Treg subset specialized in the suppression of Th1 responses (hereafter called “Th1-suppressing”) , while others have identified subpopulations of Tregs competent for IFN-γ production thus resembling Th1 cells (hence called “Th1-like”) . Different type-1 cytokines may shift the Treg balance between these two extremities, determining whether suppression or inflammation will prevail .
Specialized Th1-Suppressing Treg
A subpopulation of murine Tregs constitutively expresses CXCR3, the chemokine receptor typically associated to Th1 effector cells. Alike classical Th1 cells , CXCR3 expression in Tregs is strictly dependent on T-bet, the transcription factor orchestrating and stabilizing Th1 polarization (Koch et al. 2009). T-bet is markedly up-regulated in Tregs during type-1 inflammation and drives expansion and migration of Tregs specifically devoted to the regulation of type-1 responses (Koch et al. 2009). Contrary to classical Th1 cells, such Th1-suppressing Tregs are unable to secrete IFN-γ despite T-bet expression, a defect dependent on the low susceptibility of Tregs to IL-12, which is due to an epigenetic constrain in the expression of IL-12R-β2, the inducible subunit of IL-12 receptor (Koch et al. 2012). Expansion of Th1 suppressing Tregs has been observed not only in experimental models of type-1 inflammation (Koch et al. 2012; Koch et al. 2009) but also in human ovarian (Redjimi et al. 2012) and hepatic (Piconese et al. 2014) cancer, where the anti-tumor type-1 immune response cannot succeed in tumor eradication, being kept under strict control by populations of specialized Tregs .
Plastic Th1-Like Treg
In tissues characterized by acute, exacerbated and/or prolonged type-1 responses, it was possible to identify subsets of Tregs not only expressing T-bet but also producing detectable amounts of IFN-γ. Such Th1-like Tregs were observed in a variety of pathological conditions including graft-versus-host disease (Koenecke et al. 2012), viral infection (Zhao et al. 2011), parasite infection (Oldenhove et al. 2009), multiple sclerosis (Dominguez-Villar et al. 2011) and diabetes (Du et al. 2013; McClymont et al. 2011). Conceivably, the competence for IFN-γ production may render those Tregs more prone to contribute to, rather than suppress, inflammatory responses. In line with this possibility, IFN-γ-producing Tregs display reduced suppressive function in vitro (Dominguez-Villar et al. 2011), and conditions associated to Th1-like Treg polarization are also characterized by uncontrolled immunopathology (Lu et al. 2010; Oldenhove et al. 2009) .
Type-1 Cytokines Have Disparate Effects on Tregs
IL-12 Counteracts Treg Suppression
Tregs do not constitutively express IL-12R-β2, but IL-12-responsive Tregs can be found within tissues characterized by type-1 inflammation, and IL-12 susceptibility can be induced in vitro upon prolonged exposure to IFN-γ, in both murine (Koch et al. 2012) and human (Piconese et al. 2014) Tregs. In the resulting IFN-γ-sensitized, IL-12-responsive Tregs, IL-12 seems to exert a variety of functions aimed at destabilizing Treg suppression. At late phases of Mycobacterium tuberculosis infection, pathogen-specific highly activated Tregs undergo an IL-12-dependent contraction, thus unleashing protective immune responses (Shafiani et al. 2013). In vitro, IL-12 paralyzes Treg activity at different levels, inhibiting their proliferation, suppressive function, Foxp3 and CD25 expression (Dominguez-Villar et al. 2011; Zhao and Perlman 2012). Specifically, IL-12 produced by CD16-positive monocytes inhibits the proliferation of the Helioshigh Treg subpopulation, characterized by epigenetic stability and increased suppressive function (Zhong and Yazdanbakhsh 2013). Importantly, IL-12 appears as a pivotal signal in driving the polarization of Th1-like Tregs, both directly through IL-12R-β2/STAT4-mediated signaling pathway (Koch et al. 2012; Piconese et al. 2014), and indirectly by restraining IL-2 production in T cells and blocking CD25 expression on Tregs (Zhao and Perlman 2012). As discussed below, IL-2 is instead considered as one of the most relevant trophic factors for Treg maintenance and stability .
IL-2, IFN-γ and TNF-α Favor Treg Activation and Suppression
Under physiological conditions, IL-2 is released at the steady-state level mostly by T cells, and Tregs constitutively and highly express CD25, the high-affinity alpha-subunit of the IL-2 receptor (Boyman and Sprent 2012). Initially thought to be crucial for T cell clonal proliferation, IL-2 was soon recognized as the cytokine playing pivotal and non-redundant role in preserving tolerance, rather than immunity, through exerting a variety of functions on Treg homeostasis and activation (Malek and Bayer 2004). Indeed, IL-2 or IL-2 receptor deficiency or blockade has proven to severely impair Treg development and suppressive function, leading to lethal lymphoproliferation and autoimmunity, in a variety of experimental settings (Cheng et al. 2011). IL-2 determines not only the development of thymus-derived (previously called “natural”) Tregs, but also the homeostatic maintenance and survival of peripheral Tregs. IL-2 promotes the in vitro differentiation of induced Tregs (iTregs) and maintains their stability in opposition to other polarizing cytokines. Through STAT5 signaling, IL-2 directly induces and sustains Foxp3 expression, thus driving the molecular program of Treg suppressive function (Cheng et al. 2011) .
During immune responses, antigen-activated T cells (especially CD4) early release huge amounts of IL-2 and up-regulate high-affinity IL-2 receptor. IL-2 is captured by CD4 T cells , fostering their proliferation and directing their Th1 polarization, and by CD8 cells, optimizing their primary expansion and the development of long-lived memory cells (Boyman and Sprent 2012). A self-amplifying loop is established, with IL-2 expanding T cells that in turn further increase IL-2 amounts. To interrupt such circle turning from virtuous into vicious, Treg suppression intervenes: indeed Tregs, constitutively expressing CD25, promptly respond to IL-2 and proliferate concomitantly to activated T cells, until balancing and even outcompeting them.
At later phases of immune responses, CD4 T cells fully polarized into Th1 effectors, expressing high levels of T-bet, partially lose their competence for IL-2 production (Lazarevic et al. 2013). Therefore, it may be argued that control mechanisms other than IL-2-mediated Treg expansion may take place during an ongoing type-1 response. Self-limiting processes have been discovered that protect from collateral damage possibly triggered by excessive Th1 responses, such as IL-10 production (O’Garra and Vieira 2007) or, more recently, Twist expression (Niesner et al. 2008). However, also Th1-extrinsic mechanisms may contribute to the regulation of type-1 responses, again involving Tregs. Indeed, two cytokines abundantly released by Th1 effectors, namely TNF-α and IFN-γ, may replace IL-2 in activating Treg suppression at later phases of Th1 responses.
TNF-α may play crucial, possibly underestimated so far, roles in promoting Treg suppression not only in physiological conditions but also, and more importantly, in type-1 responses. At steady-state conditions, a subset of both human and mouse Tregs, constitutively expressing at high level the type-2 receptor for TNF-α (TNFR2), displays a more potent suppressive function (Chen et al. 2008; Chen et al. 2010). Tumor necrosis factor receptor (TNFR) 2, together with other members of the TNFR superfamily, plays non-redundant function in Treg thymic development (Mahmud et al. 2014). TNF-α stimulation promotes an activation program in Tregs, which is potently amplified by the induction of other members of the TNFR superfamily exerting similar functions (Chen et al. 2007; Hamano et al. 2011; Nagar et al. 2010). We have recently underscored the relevance of OX40, a receptor belonging to the same family, in fostering Treg activation and suppressive function (Piconese et al. 2014). OX40 was highly expressed in Tregs infiltrating tumor and pre-tumor liver tissues in chronic hepatitis C patients, in direct correlation with the high Treg frequency at those sites. OX40-expressing Tregs were mostly included among Helioshigh Tregs, characterized by signs of epigenetic and functional stability and by markers of operational immune suppression. In vitro, OX40 stimulation, by a soluble agonist or by monocytes expressing OX40 L, promoted Treg proliferation and stability (Piconese et al. 2014). Interestingly, TNF-α promptly induced OX40 up-regulation on Tregs and strongly enhanced Treg suppressive function in vitro (Piconese et al. 2014). In line with these data, others have shown TNF-α inhibiting preferentially Helioslow Tregs (Zhong and Yazdanbakhsh 2013) .
This effect may acquire utmost importance during type-1 inflammatory responses, in which not only Th1 and CTL but also other cells such as M1 macrophages produce vast amounts of TNF-α. In a mouse model of colitis, TNFR2- deficient Tregs failed to maintain Foxp3 expression within the inflamed tissue and could not suppress colitogenic T cells (Chen et al. 2013). In an experimental model of type 1 diabetes, diabetogenic effector cells boosted the expansion of islet-specific and polyclonal Tregs, in an IL-2-independent and rather TNF-α-dependent fashion (Grinberg-Bleyer et al. 2010). Of note, in that model, Tregs up-regulated CXCR3, a marker of the Th1-suppressing specialization program, when boosted by effector T cells-derived TNF-α (Grinberg-Bleyer et al. 2010). Therefore, the effector T cell response may exploit TNF-α to activate an immunoregulatory feedback loop when a potentially dangerous response needs to be moldered. In an experimental setting of human Th1-like Treg polarization in vitro, we have recently demonstrated that TNF-α completely counteracts the polarizing activity of IL-12 , and rather stabilizes the suppressive Treg phenotype (Piconese et al. 2014). It should be noted, however, that some studies have reported a negative effect of TNF-α on Treg stability and function. For instance, TNF-α in the synovium of rheumatoid arthritis patients was shown to reverse Treg suppressive function and destabilize FOXP3 expression by decreasing its phosphorylation (Nie et al. 2013), and therapies with TNF-α antagonists/inhibitors have shown to recover high proportions of Tregs in several autoimmune diseases (Di Sabatino et al. 2010; Nadkarni et al. 2007). Such controversy may arise from the intrinsic duality of TNF-α activities, pro-inflammatory (through the activation of innate cells) and anti-inflammatory (through the expansion of Tregs). Indeed, Treg rescue following TNF-α blockade approaches may be secondary to indirect effects on other inflammatory pathways depressing Treg expansion, rather than attributable to direct effects of TNF-α on Tregs.
IFN-γ is considered the prototypical cytokine released by NK cells during early innate responses and by Th1 cells and CTL at more advanced phases, thus conceivably abundant in microenvironments characterized by type-1 inflammation. IFN-γ has been recognized to directly induce (via STAT1 phosphorylation) T-bet expression that in turn promotes IL-12R-β2 transcription (Koch et al. 2012). Therefore, at early moments of type-1 responses, IFN-γ derived from innate cells can sensitize Tregs to IL-12, thus rendering them susceptible to Th1-like polarization. However, along the development the adaptive type-1 immunity, IFN-γ, mostly derived from T cells, paradoxically drives the expansion of Th1-suppressing Tregs (Koch et al. 2009). This likely occurs in advanced phases of Th1-responses, in which the depletion of IL-12 curtails the full differentiation into Th1-like Tregs and favors the establishment of Th1-suppressing Tregs producing low amount of IFN-γ. Such activity contributes to the regulation of immunopathology in a mouse model of toxoplasmosis (Hall et al. 2012). Similarly, we have shown that IFN-γ induces T-bet up-regulation in human Tregs (Piconese et al. 2014). Therefore, like IL-2 and TNF-α, also IFN-γ produced by Th1 effector cells can initiate a feedback regulatory loop through the expansion of Tregs specially addressed to controlling type-1 response. However, IFN-γ signaling should be finely regulated to prevent the conversion of Th1-suppressing into Th1-like Tregs. Indeed, a prolonged IFN-γ/STAT1 signaling, otherwise normally controlled by miR146, can paralyze Th1-suppressing Tregs and promote the polarization of IFN-γ producing Th1-like cells, disrupting Treg-mediated regulation of type-1 inflammation (Lu et al. 2010) .
Controversies About IL-27 and IFN-α
IL-27, belonging to the same family of IL-12, is released by APCs during type-1 responses and contributes to Th1 cell polarization by activating STAT1 and T-bet. However, contrary to IL-12, IL-27 also displays some immunoregulatory properties, promoting the induction of suppressive cytokines, such as IL-10, in a variety of immune cell types (Hunter and Kastelein 2012). With respect to Tregs, and again unlike IL-12, IL-27 did not down-regulate Foxp3 expression or molder their suppressive function (Hunter and Kastelein 2012). Rather, and similarly to IFN-γ, IL-27 induced Th1-suppressing Tregs, expressing T-bet and CXCR3, in experimental models of bacterial infection (Hall et al. 2012). Interestingly, the two cytokines showed a preferential anatomical competence, with IFN-γ being more relevant in lymphoid tissue and IL-27 particularly prominent at sites of inflammation (Hall et al. 2012) .
Controversy also exists about the role of type I IFNs, especially IFN-α, if predominantly pro-inflammatory or rather immunomodulatory cytokines in type-1 responses (Gonzalez-Navajas et al. 2012; Trinchieri 2010). Indeed, while on the one side IFN-α may induce IFN-γ in T and NK cells via STAT4 phosphorylation (which is anyway unstable), in some conditions it rather antagonizes IL-12 production and signaling (Trinchieri 2010). Controversy also exists about the effects of IFN-α in Treg expansion and function. Some reports assert that IFN-α promotes Treg-mediated suppression in mouse models of cancer (Stewart et al. 2013) and colitis (Lee et al. 2012). Other studies have instead underscored a negative effect of IFN-α on both murine (Pace et al. 2010) and human (Bacher et al. 2013; Le Buanec et al. 2011) Treg suppressive function. A recent report has clearly shown that, during an acute viral infection in mice, type I IFNs inhibited costimulation-dependent Treg proliferation thus unleashing the emergence of an optimal anti-viral adaptive immunity (Srivastava et al. 2014). In line with this data, we could observe that IFN-α strongly suppressed the proliferation of human Tregs in vitro, and significantly decreased Treg frequency in vivo in patients with chronic hepatitis C undergoing PEG-interferon/ribavirin therapy (unpublished data). It may be argued that different aspects, such as local dosage of IFN-α, timing of IFN-α release and concomitant presence of other cytokines may determine the prevalent effect of this cytokine on Treg functions.
Interplay Between Costimulatory/Coinhibitory Receptors and Cytokine Signals
Several examples can be quoted from the literature of the interplay between cytokine signaling pathways and costimulatory or coinhibitory receptors. Tregs may constitutively express some of these receptors in the peripheral lymphoid organs, and many of them have been shown to be strongly induced or up-regulated within inflamed tissues .
Tregs infiltrating human liver affected by chronic hepatitis C significantly up-regulate PD-1 (Franceschini et al. 2009; Piconese et al. 2014), a receptor belonging to the CD28/CTLA-4 family and recognized as a co-inhibitory molecule. Programmed cell death (PD)-1 was shown to temper the expansion of a peculiar Treg subtype known as T follicular regulatory cells, specialized in the suppression of T follicular helper cells that arrange humoral responses (Sage et al. 2013). Within the liver, PD-1 signal dampened Treg proliferation mainly through the inhibition of IL-2/STAT5 axis. Indeed, PD-1 blockade rescued Treg proliferation and STAT5 phosphorylation ex vivo (Franceschini et al. 2009). Such PD-1-mediated constraining of Treg expansion may contribute to mitigate immune suppression, allowing an immunological compromise between anti-viral immunity and immunopathology in chronic infections. Therefore, PD-1 seems to provide opposite signals to Tregs according to the phase of T cell activation: they contribute to Treg conversion from naïve Tconvs (induction signal), on the one hand, and constrain experienced Treg expansion and functions (inhibition signal), on the other hand (Barnaba and Schinzari 2013).
Contrary to PD-1, the costimulatory receptor OX40 seems to rather sustain immunoregulatory Treg activities in HCV-related cirrhosis and cancer (Piconese et al. 2014). OX40 may result in opposite effects depending on the cytokine contexts, whether non-inflammatory or pro-inflammatory. Indeed, in a mouse model of autoimmune disease, an OX40 agonist promoted the expansion of protective Tregs only when administered at priming, and not after disease onset (Ruby et al. 2009). This data suggests that the OX40 pathway may cooperate with steady-state homeostatic cytokines, mostly IL-2, and rather work in opposition to inflammatory cytokines. In line with this hypothesis, a defective Treg homeostasis and competitive fitness was observed in OX40-null mice, which was attributable to an impaired ability to optimally utilize IL-2 (Piconese et al. 2010). OX40-null Tregs showed reduced STAT5 phosphorylation in response to IL-2, an event possibly linked to an overexpression of the STAT5 inhibitor SOCS1, in turn sustained by low levels of miR155 (Piconese et al. 2010). Of note, such defect impaired Treg expansion in vivo not only in conditions of homeostatic proliferation but also during inflammatory responses in mouse models of colitis (Griseri et al. 2010; Piconese et al. 2010) .
OX40 engagement may promote Treg proliferation partly through the interaction with cytokine signaling. In mice, OX40 ligation synergized with IL-2 administration in promoting STAT5 phosphorylation and the expansion of fully suppressive Tregs (Xiao et al. 2012). Using human cells, others and we have demonstrated that OX40 stimulation promotes Treg proliferation (Hippen et al. 2008; Piconese et al. 2014). Of note, OX40 was up-regulated on human Treg surface by IL-2 and more massively by IL-2 and TNF-α co-exposure (Nagar et al. 2010; Piconese et al. 2010). The OX40/OX40 L axis may then represent an amplification loop of TNF-α signal towards Treg expansion and stabilization. In line with this idea, Tregs highly expressing OX40 in human hepatic cancer and cirrhosis were preferentially contained within the committed (Helioshigh) and specialized (Th1-suppressing) subpopulation, rather than in the unstable Th1-like counterpart. Supporting OX40 as a Treg-stabilizing signal, an OX40 agonist inhibited Th1-like Treg polarization in vitro (Piconese et al. 2014). It may be suggested that OX40- and TNF-α-initiated signaling pathways, mostly mediated by NF-kB activation (Nagar et al. 2010), may directly antagonize IL-12 axis by still unknown mechanism, possibly mediated by SOCS molecules. However, the antagonism between OX40/TNF-α and IL-12-mediated Th1-like polarization may also be explained taking into account the heterogeneity of Tregs: indeed, OX40 and TNF-α may preferentially promote the proliferation of those Treg subsets which are less susceptible to IL-12-mediated diversion. Supporting this view, we could observe a pattern of mutually exclusive expression between OX40 and IL-12R-β2 in human Tregs ex vivo (Piconese et al. 2014) .
Conclusions
Tregs reside in lymphoid and non-lymphoid tissues, hence being possibly exposed to distinct type-1 cytokines at different sites and phases of a type-1 response. Furthermore, the concomitant stimulation of some surface receptors may finely modulate Treg response to many cytokines, amplifying or antagonizing cytokine signals. From the above overview, a duality of Treg response to type-1 cytokines may be broadly delineated: on the one side, IL-12, mostly produced by APCs at the initial stages of Th1 priming in lymphoid organs, seems to antagonize Treg suppression and rather promote Th1-like Treg polarization; on the other side, cytokines released by proliferating T cells, such as IL-2, or by already differentiated Th1 cells , such as TNF-α and IFN-γ, at later phases of type-1 immunity mainly in inflamed tissues, seem to rather promote Treg proliferation, suppressive function and specialization into Th1-suppressing cells (Fig. 2.1). Of note, Tregs only inducibly express IL-12R-β2, while constitutively expressing at high levels the receptors for IL-2 and TNF-α. Similarly to IL-12, IFN-α is released by plasmacytoid dendritic cells and tissue cells relatively early during viral infections, and it may contribute to the initiation of innate and adaptive anti-viral responses also by antagonizing Tregs. We are tempted to speculate that such alternate processes may have evolved to ensure a transient Treg deactivation when type-1 responses need to be initiated, while promoting the expansion of specialized Tregs when type-1 inflammation should undergo resolution, to prevent collateral tissue damage.
Type-1 cytokines differentially affect Treg functions at distinct stages of type-1 responses. Cytokines produced during priming or effector phase of type-1 response may respectively antagonize or promote Treg-mediated suppression . At the initiation of type-1 responses (red area on the left), IFN-γ produced by innate lymphocytes (i.e., NK cells) induces (via STAT1/T-bet axis) IL-12R-β2 expression on Tregs making them susceptible to IL-12, which is mostly produced by myeloid dendritic cells (mDC). IL-12, together with IFN-α released by plasmacytoid dendritic cells (pDC) and epithelial cells, inhibits Treg proliferation and suppression; IL-12 even promotes the polarization of Th1-like Tregs, which possibly contribute to inflammation. At later phases of type-1 responses (green area on the right), Th0- and Th1-derived cytokines such as IL-2, TNF-α and IFN-γ foster Treg expansion and inhibitory function and promote their specialization into Th1-suppressing cells. Such alternate modulation would allow the development of type-1 responses while ensuring proper resolution of inflammation .
References
Bacher, N., Raker, V., Hofmann, C., Graulich, E., Schwenk, M., Baumgrass, R., Bopp, T., Zechner, U., Merten, L., Becker, C., & Steinbrink, K. (2013). Interferon-alpha suppresses cAMP to disarm human regulatory T cells. Cancer Research, 73(18), 5647–5656. doi:10.1158/0008–5472.CAN-12-3788.
Barnaba, V. (2010). Hepatitis C virus infection: A “liaison a trois” amongst the virus, the host, and chronic low-level inflammation for human survival. Journal of Hepatology, 53(4), 752–761. doi:10.1016/j.jhep.2010.06.003.
Barnaba, V., & Schinzari, V. (2013). Induction, control, and plasticity of Treg cells: The immune regulatory network revised? European Journal of Immunology, 43(2), 318–322. doi:10.1002/eji.201243265.
Boyman, O., & Sprent, J. (2012). The role of interleukin-2 during homeostasis and activation of the immune system. Nature Reviews Immunology, 12(3), 180–190. doi:10.1038/nri3156.
Burzyn, D., Benoist, C., & Mathis, D. (2013a). Regulatory T cells in nonlymphoid tissues. Nature Immunology, 14(10), 1007–1013. doi:10.1038/ni.2683.
Burzyn, D., Kuswanto, W., Kolodin, D., Shadrach, J. L., Cerletti, M., Jang, Y., Sefik, E., Tan, T. G., Wagers, A. J., Benoist, C., & Mathis, D. (2013b). A special population of regulatory T cells potentiates muscle repair. Cell, 155(6), 1282–1295. doi:10.1016/j.cell.2013.10.054.
Chen, X., Baumel, M., Mannel, D. N., Howard, O. M., & Oppenheim, J. J. (2007). Interaction of TNF with TNF receptor type 2 promotes expansion and function of mouse CD4+ CD25+ T regulatory cells. The Journal of Immunology, 179(1), 154–161.
Chen, X., Subleski, J. J., Kopf, H., Howard, O. M., Mannel, D. N., & Oppenheim, J. J. (2008). Cutting edge: Expression of TNFR2 defines a maximally suppressive subset of mouse CD4+CD25+FoxP3+ T regulatory cells: Applicability to tumor-infiltrating T regulatory cells. The Journal of Immunology, 180(10), 6467–6471.
Chen, X., Subleski, J. J., Hamano, R., Howard, O. M., Wiltrout, R. H., & Oppenheim, J. J. (2010). Co-expression of TNFR2 and CD25 identifies more of the functional CD4+FOXP3+ regulatory T cells in human peripheral blood. European Journal of Immunology, 40(4), 1099–1106. doi:10.1002/eji.200940022.
Chen, X., Wu, X., Zhou, Q., Howard, O. M., Netea, M. G., & Oppenheim, J. J. (2013). TNFR2 is critical for the stabilization of the CD4+Foxp3+ regulatory T. cell phenotype in the inflammatory environment. The Journal of Immunology, 190(3), 1076–1084. doi:10.4049/jimmunol.1202659.
Cheng, G., Yu, A., & Malek, T. R. (2011). T-cell tolerance and the multi-functional role of IL-2.R signaling in T-regulatory cells. Immunological Reviews, 241(1), 63–76. doi:10.1111/j.1600–065X.2011.01004.x.
Cipolletta, D., Feuerer, M., Li, A., Kamei, N., Lee, J., Shoelson, S. E., Benoist, C., & Mathis, D. (2012). PPAR-gamma is a major driver of the accumulation and phenotype of adipose tissue Treg cells. Nature, 486(7404), 549–553. doi:10.1038/nature11132.
Di Sabatino, A., Biancheri, P., Piconese, S., Rosado, M. M., Ardizzone, S., Rovedatti, L., Ubezio, C., Massari, A., Sampietro, G. M., Foschi, D., Porro, G. B., Colombo, M. P., Carsetti, R., MacDonald, T. T., & Corazza, G. R. (2010). Peripheral regulatory T cells and serum transforming growth factor-beta: Relationship with clinical response to infliximab in Crohn’s disease. Inflammatory Bowel Diseases, 16(11), 1891–1897. doi:10.1002/ibd.21271.
Dominguez-Villar, M., Baecher-Allan, C. M., & Hafler, D. A. (2011). Identification of T helper type 1-like, Foxp3+ regulatory T cells in human autoimmune disease. Nature Medicine, 17(6), 673–675. doi:10.1038/nm.2389.
Du, W., Shen, Y. W., Lee, W. H., Wang, D., Paz, S., Kandeel, F., & Liu, C. P. (2013). Foxp3+ Treg expanded from patients with established diabetes reduce Helios expression while retaining normal function compared to healthy individuals. PLos One, 8(2), e56209. doi:10.1371/journal.pone.0056209.
Franceschini, D., Paroli, M., Francavilla, V., Videtta, M., Morrone, S., Labbadia, G., Cerino, A., Mondelli, M. U., & Barnaba, V. (2009). PD-L1 negatively regulates CD4+CD25+Foxp3+ Tregs by limiting STAT-5 phosphorylation in patients chronically infected with HCV. Journal of Clinical Investigation, 119(3), 551–564. doi:10.1172/JCI36604.
Gonzalez-Navajas, J. M., Lee, J., David, M., & Raz, E. (2012). Immunomodulatory functions of type I interferons. Nature Reviews Immunology, 12(2), 125–135. doi:10.1038/nri3133.
Grinberg-Bleyer, Y., Saadoun, D., Baeyens, A., Billiard, F., Goldstein, J. D., Gregoire, S., Martin, G. H., Elhage, R., Derian, N., Carpentier, W., Marodon, G., Klatzmann, D., Piaggio, E., & Salomon, B. L. (2010). Pathogenic T cells have a paradoxical protective effect in murine autoimmune diabetes by boosting Tregs. Journal of Clinical Investigation, 120(12), 4558–4568. doi:10.1172/JCI42945.
Griseri, T., Asquith, M., Thompson, C., & Powrie, F. (2010). OX40 is required for regulatory T cell-mediated control of colitis. The Journal of Experimental Medicine, 207(4), 699–709. doi:10.1084/jem.20091618.
Hall, A. O., Beiting, D. P., Tato, C., John, B., Oldenhove, G., Lombana, C. G., Pritchard, G. H., Silver, J. S., Bouladoux, N., Stumhofer, J. S., Harris, T. H., Grainger, J., Wojno, E. D., Wagage, S., Roos, D. S., Scott, P., Turka, L. A., Cherry, S., Reiner, S. L., Cua, D., Belkaid, Y., Elloso, M. M., & Hunter, C. A. (2012). The cytokines interleukin 27 and interferon-gamma promote distinct Treg cell populations required to limit infection-induced pathology. Immunity, 37(3), 511–523. doi:10.1016/j.immuni.2012.06.014.
Hamano, R., Huang, J., Yoshimura, T., Oppenheim, J. J., Chen, X. (2011). TNF optimally activatives regulatory T cells by inducing TNF receptor superfamily members TNFR2, 4-1BB and OX40. European Journal of Immunology, 41(7), 2010–2020. doi:10.1002/eji.201041205.
Hippen, K. L., Harker-Murray, P., Porter, S. B., Merkel, S. C., Londer, A., Taylor, D. K., Bina, M., Panoskaltsis-Mortari, A., Rubinstein, P., Van Rooijen, N., Golovina, T. N., Suhoski, M. M., Miller, J. S., Wagner, J. E., June, C. H., Riley, J. L., & Blazar, B. R. (2008). Umbilical cord blood regulatory T-cell expansion and functional effects of tumor necrosis factor receptor family members OX40 and 4-1BB expressed on artificial antigen-presenting cells. Blood, 112(7), 2847–2857. doi:10.1182/blood-2008-01-132951.
Hunter, C. A., & Kastelein, R. (2012). Interleukin-27: Balancing protective and pathological immunity. Immunity, 37(6), 960–969. doi:10.1016/j.immuni.2012.11.003.
Koch, M. A., Tucker-Heard, G., Perdue, N. R., Killebrew, J. R., Urdahl, K. B, & Campbell, D. J. (2009). The transcription factor T-bet controls regulatory T cell homeostasis and function during type 1 inflammation. Nature Immunology, 10(6), 595–602. doi:10.1038/ni.1731.
Koch, M. A., Thomas, K. R., Perdue, N. R., Smigiel, K. S., Srivastava, S., & Campbell, D. J. (2012). T-bet(+) Treg cells undergo abortive Th1 cell differentiation due to impaired expression of IL-12 receptor beta2. Immunity, 37(3), 501–510. doi:10.1016/j.immuni.2012.05.031.
Koenecke, C., Lee, C. W., Thamm, K., Fohse, L., Schafferus, M., Mittrucker, H. W., Floess, S., Huehn, J., Ganser, A., Forster, R., & Prinz, I. (2012). IFN-gamma production by allogeneic Foxp3+ regulatory T cells is essential for preventing experimental graft-versus-host disease. The Journal of Immunology, 189(6), 2890–2896. doi:10.4049/jimmunol.1200413.
Lazarevic, V., Glimcher, L. H., & Lord, G. M. (2013). T-bet: A bridge between innate and adaptive immunity. Nature Reviews Immunology, 13(11), 777–789. doi:10.1038/nri3536.
Le Buanec, H., Gougeon, M. L., Mathian, A., Lebon, P., Dupont, J. M., Peltre, G., Hemon, P., Schmid, M., Bizzini, B., Kunding, T., Burny, A., Bensussan, A., Amoura, Z., Gallo, R. C., & Zagury, D. (2011). IFN-alpha and CD46 stimulation are associated with active lupus and skew natural T regulatory cell differentiation to type 1 regulatory T (Tr1) cells. Proceedings of the National Academy of Sciences of the United States of America, 108(47), 18995–19000. doi:10.1073/pnas.1113301108.
Lee, S. E., Li, X., Kim, J. C., Lee, J., Gonzalez-Navajas, J. M., Hong, S. H., Park, I. K., Rhee, J. H., & Raz, E. (2012). Type I interferons maintain Foxp3 expression and T-regulatory cell functions under inflammatory conditions in mice. Gastroenterology, 143(1), 145–154. doi:10.1053/j.gastro.2012.03.042.
Lu, L. F., Boldin, M. P., Chaudhry, A., Lin, L. L., Taganov, K. D., Hanada, T., Yoshimura, A., Baltimore, D., & Rudensky, A. Y. (2010). Function of miR-146a in controlling Treg cell-mediated regulation of Th1 responses. Cell, 142(6), 914–929. doi:10.1016/j.cell.2010.08.012.
Mahmud, S. A., Manlove, L. S., Schmitz, H. M., Xing, Y., Wang, Y., Owen, D. L., Schenkel, J. M., Boomer, J. S., Green, J. M., Yagita, H., Chi, H., Hogquist, K. A., & Farrar, M. A. (2014). Costimulation via the tumor-necrosis factor receptor superfamily couples TCR signal strength to the thymic differentiation of regulatory T cells. Nature Immunology, 15(5), 473–481. doi:10.1038/ni.2849.
Malek, T. R., & Bayer, A. L. (2004). Tolerance, not immunity, crucially depends on IL-2. Nature Reviews Immunology, 4(9), 665–674. doi:10.1038/nri1435.
McClymont, S. A., Putnam, A. L., Lee, M. R., Esensten, J. H., Liu, W., Hulme, M. A., Hoffmuller, U., Baron, U., Olek, S., Bluestone, J. A., Brusko, T. M. (2011). Plasticity of human regulatory T cells in healthy subjects and patients with type 1 diabetes. The Journal of Immunology, 186(7), 3918–3926. doi:10.4049/jimmunol.1003099.
Nadkarni, S., Mauri, C., & Ehrenstein, M. R. (2007). Anti-TNF-alpha therapy induces a distinct regulatory T cell population in patients with rheumatoid arthritis via TGF-beta. The Journal of Experimental Medicine, 204(1), 33–39. doi:10.1084/jem.20061531.
Nagar, M., Jacob-Hirsch, J., Vernitsky, H., Berkun, Y., Ben-Horin, S., Amariglio, N., Bank, I., Kloog, Y., Rechavi, G., & Goldstein, I. (2010). TNF activates a NF-kappaBregulated cellular program in human CD45RA-regulatory T cells that modulates their suppressive function. The Journal of Immunology, 184(7), 3570–3581. doi:10.4049/jimmunol.0902070.
Nie, H., Zheng, Y., Li, R., Guo, T. B., He, D., Fang, L., Liu, X., Xiao, L., Chen, X., Wan, B., Chin, Y. E., & Zhang, J. Z. (2013). Phosphorylation of FOXP3 controls regulatory T cell function and is inhibited by TNF-alpha in rheumatoid arthritis. Nature Medicine, 19(3), 322–328. doi:10.1038/nm.3085.
Niesner, U., Albrecht, I., Janke, M., Doebis, C., Loddenkemper, C., Lexberg, M. H., Eulenburg, K., Kreher, S., Koeck, J., Baumgrass, R., Bonhagen, K., Kamradt, T., Enghard, P., Humrich, J. Y., Rutz, S., Schulze-Topphoff, U., Aktas, O., Bartfeld, S., Radbruch, H., Hegazy, A. N., Lohning, M., Baumgart, D. C., Duchmann, R., Rudwaleit, M., Haupl, T., Gitelman, I., Krenn, V., Gruen, J., Sieper, J., Zeitz, M., Wiedenmann, B., Zipp, F., Hamann, A., Janitz, M., Scheffold, A., Burmester, G. R., Chang, H. D., & Radbruch, A. (2008). Autoregulation of Th1-mediated inflammation by twist1. The Journal of Experimental Medicine, 205(8), 1889–1901. doi:10.1084/jem.20072468.
O’Garra, A., & Vieira, P. (2007). T(H)1 cells control themselves by producing interleukin 10. Nature Reviews Immunology, 7(6), 425–428. doi:10.1038/nri2097.
Oldenhove, G., Bouladoux, N., Wohlfert, E. A., Hall, J. A., Chou, D., Dos Santos, L., O’Brien, S., Blank, R., Lamb, E., Natarajan, S., Kastenmayer, R., Hunter, C., Grigg, M. E., & Belkaid, Y. (2009). Decrease of Foxp3+ Treg cell number and acquisition of effector cell phenotype during lethal infection. Immunity, 31(5), 772–786. doi:10.1016/j.immuni.2009.10.001.
Pace, L., Vitale, S., Dettori, B., Palombi, C., La Sorsa, V., Belardelli, F., Proietti, E., & Doria, G. (2010). APC activation by IFN-alpha decreases regulatory T cell and enhances Th cell functions. The Journal of Immunology, 184(11), 5969–5979. doi:10.4049/jimmunol.0900526.
Piconese, S., Pittoni, P., Burocchi, A., Gorzanelli, A., Care, A., Tripodo, C., & Colombo, M. P. (2010). A non-redundant role for OX40 in the competitive fitness of Treg in response to IL-2. European Journal of Immunology, 40(10), 2902–2913. doi:10.1002/eji.201040505.
Piconese, S., Timperi, E., Pacella, I., Schinzari, V., Tripodo, C., Rossi, M., Guglielmo, N., Mennini, G., Grazi, G. L., Di Filippo, S., Brozzetti, S., Fazzi, K., Antonelli, G., Lozzi, M. A., Sanchez, M., & Barnaba, V. (2014). Human OX40 tunes the function of regulatory T cells in tumor and non-tumor areas of HCV-infected liver tissue. Hepatology. doi:10.1002/hep.27188.
Redjimi, N., Raffin, C., Raimbaud, I., Pignon, P., Matsuzaki, J., Odunsi, K., Valmori, D., & Ayyoub, M. (2012). CXCR3+ T regulatory cells selectively accumulate in human ovarian carcinomas to limit type I immunity. Cancer Research, 72(17), 43514360. doi:10.1158/0008-5472.CAN-12-0579.
Ruby, C. E., Yates, M. A., Hirschhorn-Cymerman, D., Chlebeck, P., Wolchok, J. D., Houghton, A. N., Offner, H., & Weinberg, A. D. (2009). Cutting Edge: OX40 agonists can drive regulatory T cell expansion if the cytokine milieu is right. The Journal of Immunology, 183(8), 4853–4857. doi:10.4049/jimmunol.0901112.
Sage, P. T., Francisco, L. M., Carman, C. V., & Sharpe, A. H. (2013). The receptor PD-1 controls follicular regulatory T cells in the lymph nodes and blood. Nature Immunology, 14(2), 152–161. doi:10.1038/ni.2496.
Shafiani, S., Dinh, C., Ertelt, J. M., Moguche, A. O., Siddiqui, I., Smigiel, K. S., Sharma, P., Campbell, D. J., Way, S. S., & Urdahl, K. B. (2013). Pathogen-specific Treg cells expand early during mycobacterium tuberculosis infection but are later eliminated in response to Interleukin-12. Immunity, 38(6), 1261–1270. doi:10.1016/j.immuni.2013.06.003.
Smigiel, K. S., Srivastava, S., Stolley, J. M., & Campbell, D. J. (2014). Regulatory T-cell homeostasis: Steady-state maintenance and modulation during inflammation. Immunological Reviews, 259(1), 40–59. doi:10.1111/imr.12170.
Srivastava, S., Koch, M. A., Pepper, M., & Campbell, D. J. (2014). Type I interferons directly inhibit regulatory T cells to allow optimal antiviral T cell responses during acute LCMV infection. The Journal of Experimental Medicine, 211(5), 961–974. doi:10.1084/jem.20131556.
Stewart, C. A., Metheny, H., Iida, N., Smith, L., Hanson, M., Steinhagen, F., Leighty, R. M., Roers, A., Karp, C. L., Muller, W., & Trinchieri, G. (2013). Interferon-dependent IL-10 production by Tregs limits tumor Th17 inflammation. Journal of Clinical Investigation, 123(11), 4859–4874. doi:10.1172/JCI65180.
Trinchieri, G. (2010). Type I interferon: Friend or foe? The Journal of Experimental Medicine, 207(10), 2053–2063. doi:10.1084/jem.20101664.
Xiao, X., Gong, W., Demirci, G., Liu, W., Spoerl, S., Chu, X., Bishop, D. K., Turka, L. A., & Li, X. C. (2012). New insights on OX40 in the control of T cell immunity and immune tolerance in vivo. The Journal of Immunology, 188(2), 892–901. doi:10.4049/jimmunol.1101373.
Zhao, J., & Perlman, S. (2012). Differential effects of IL-12 on Tregs and non-Treg T cells: Roles of IFN-gamma, IL-2 and IL-2R. PLos One, 7(9), e46241. doi:10.1371/journal.pone.0046241.
Zhao, J., Fett, C., Trandem, K., Fleming, E., & Perlman, S. (2011). IFN-gamma-and IL-10expressing virus epitope-specific Foxp3(+) T reg cells in the central nervous system during encephalomyelitis. The Journal of Experimental Medicine, 208(8), 1571–1577. doi:10.1084/jem.20110236.
Zhong, H., & Yazdanbakhsh, K. (2013). Differential control of Helios(+/-) Treg development by monocyte subsets through disparate inflammatory cytokines. Blood, 121(13), 2494–2502. doi:10.1182/blood-2012-11-469122.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this paper
Cite this paper
Piconese, S., Barnaba, V. (2015). Stability of Regulatory T Cells Undermined or Endorsed by Different Type-1 Cytokines. In: Schoenberger, S., Katsikis, P., Pulendran, B. (eds) Crossroads Between Innate and Adaptive Immunity V. Advances in Experimental Medicine and Biology, vol 850. Springer, Cham. https://doi.org/10.1007/978-3-319-15774-0_2
Download citation
DOI: https://doi.org/10.1007/978-3-319-15774-0_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-15773-3
Online ISBN: 978-3-319-15774-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)