Abstract
Hydrometallurgical processes are used to recover metals from solutions obtained after leaching steps. This chapter will present liquid-liquid extraction and cementation processes used to recover metals from WEEE.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Hydrometallurgical processes are used to recover metals from solutions obtained after leaching steps. This chapter will present liquid-liquid extraction and cementation processes used to recover metals from WEEE.
6.1 Liquid-Liquid Extraction
Liquid-liquid extraction, sometimes called solvent extraction, is the separation of the constituents of a liquid solution by contact with another insoluble liquid [1]. The process enables the separation of the constituents of an aqueous solution by contact with another insoluble liquid, an organic solution. Through intensive contact, the solute transfers from the aqueous solution (raffinate) into the organic solvent (extract). After mixing, the two phases are separated, which happens either by gravity or by centrifugal forces. To recover the solvent and to obtain the solute in pure form, a further separation process is necessary (rectification or re-extraction).
In the ideal case, the component to be extracted is soluble in the solvent and the other components are insoluble. This means the solute is the only component of the initial mixture transferred to the solvent phase. The initial mixture becomes a raffinate as the solute is extracted. The solvent phase becomes the extract as it receives the solute. In practice, all components are potentially soluble to some degree in each other, and the separation is only feasible when the solubilities are sufficiently different. In any case, the component that is not extracted (inert) must be sufficiently insoluble so as to form two phases that can be separated [2].
The separation of a component from a homogeneous solution is obtained by the addition of another insoluble constituent, the solvent, in which the desired component, the solute, is preferably soluble. The solute diffuses with a characteristic velocity until equilibrium concentrations are reached in each stage. For example, zinc and cadmium are extracted from the selectively mixed electrolyte solutions containing zinc, cadmium and cobalt sulphates, using di-2-ethylhexyl phosphoric acid (DEHPA) as extractant [3].
Liquid-liquid extraction is used to remove undesirable components from lubricating oils and other fractions from crude oil to produce concentrated phosphoric acid, to separate/concentrate metals and rare earth elements, and in many other applications. Many metal separations, particularly those that are expensive through chemical routes, such as uranium-vanadium, niobium-tantalum, and hafnium-zirconium, can be done economically by extraction [1, 2].
Solvent extraction is an equilibrium process:
where HR is the organic extractant, MRn is the metal-organic extracted species, H+ is the proton released by the organic extractant in exchange for the cationic metal species M +n . The distribution of the metal ions between the organic and aqueous phases depends on a number of variables, such as solution composition, extraction/equilibrium pH, extractant concentration and type, aqueous to organic volume ratio and, sometimes, on the type of diluent used to dilute the extractant [3].
Liquid-liquid extraction operations may be carried out either as a batch or as a continuous process. In the single-stage batch process illustrated in Fig. 6.1, the solvent and solution are mixed together and then allowed to separate into the two phases—the extract E containing the required solute in the added solvent and the raffinate R, the weaker solution with some associated solvent. With this simple arrangement, mixing and separation can occur in the same vessel [4].
An example of a countercurrent extraction in a series of mixing and separating vessels is shown in Fig. 6.2. Each box corresponds to a mixer and a separator. The initial solution of the solute B in solvent A, F0, is fed into the first unit and leaves as raffinate F1. This stream passes through the units and leaves from the Nth unit as stream F N . The fresh solvent ON+1 enters the Nth unit and passes in the reverse direction through the units, leaving as extract O1 [4].
According to Geankoplis [5], if the components B and C are immiscible in each other and the solvent current ON+1 contains the components A and C, and if A and B are contained in the feed stream F0, then the number of stages can be determined. In this configuration, therefore, the solute A is transferred from F0 to ON+1. According to these considerations, a mass balance for the system (Fig. 6.2) can be done to the first n stages:
F′ and O′ represent the mass of the inert components B and C per unit of time, respectively, y is the mass fraction of A in stream O and x is the mass fraction of A in stream F. The obtained equation is the operating line. The operating line can be a straight line if y and x are in low concentrations. Therefore, the number of equilibrium stages can be obtained as shown in Fig. 6.3.
Graphical method for determining the number of stages using immiscible solvents [4]
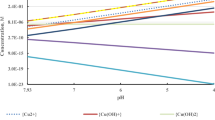
The pH has an important effect on the extractions. Figure 6.4 shows the effect of pH on the extractions of zinc, cobalt and cadmium from a synthetic solution. One can see that negligible cobalt and cadmium co-extractions take place at pH < 3. Thus, at an equilibrium pH < 3, zinc could be extracted from the mixed electrolyte solution with little or no contamination from the other metals. At 3 < pH < 4.5, cadmium extraction is significant but co-extraction of cobalt is negligible. The implication of this observation is that by carefully controlling the extraction/equilibrium pH, a solution containing these electrolytes can be separated into their respective fractions [3].
Extent of metal extraction (qualitative behavior): [DEHPA] = 30 v/o; [TBP] = 4 v/o; diluent—SX-1; A: O = 1; temperature = 25 °C; feed solution—21 g/1 Zn, 12.6 g/1 Cd and 2 g/1 Co. (adapted from Owusu [3])
Obtaining metals from aqueous solutions is a technological challenge. It is very difficult to separate the metals among themselves and from other industrial metals present in the leached solutions. Among the various techniques of separation or purification in hydrometallurgy, solvent extraction has great versatility for the separation of different types of elements, and can occur in a wide range of concentrations of solutions. As such, this technique has been widely used for the treatment of leachate concentrates [6].
Extractants are divided into three classes: acidic, basic and neutral or anionic. The difference between them is the chemistry of their reactions, but they must always present specific features for effective extraction. Some of the most important features are: selectivity, high extraction capacity, stability, reversibility and low cost. In general, extractants do not have all these characteristics, so they may be diluted with other components in order to provide the most suitable physical properties (viscosity, density, etc.) and increase their extraction potential and selectivity. The mixture of extractants is a very interesting way to improve extraction efficiency and selectivity, this procedure is called synergistic extraction [7–9].
The application of solvent extraction is important for the purification of solutions in the battery recycling process, and for the recovery of metals of economic importance. The studies presented next have evaluated the liquid-liquid extraction of metals using different extractants.
Devi et al. [10] studied the liquid-liquid extraction of Zn and Mn from sulphate solutions using Cyanex 272. They observed that the extraction becomes more efficient with increasing pH and by increasing the concentration of the extractant to 0.05 M. Kongolo et al. [11] studied the recovery of Co and Zn from a copper sulfate solution by solvent extraction. The use of LIX 984 allowed the recovery of Cu. After CaCO3 precipitation. Fe, Zn and Co were extracted with DEHPA. They obtained high recovery percentages of 95 % for Cu and 90 % for Co and Zn.
Safarzadeh et al. [12] evaluated the solvent extraction of Cd with the following extractors: D2EHPA, Cyanex 272, TBP, TOPS99, PC88A, DIPSA, TIBPS, HRJ-4277 and BTMHA. They found that the extractor D2EHPA was more widespread inapplications in industrial solutions for the recovery of Zn and Cd with pH 1–2. They also found from the equilibrium curves with carboxylic acids (Versatic and naphthenic acids) for Zn and Cd, that the extraction was possible only with pH above 6.5 [12].
Parus et al. [13] studied solvent extraction of Cd (II) from chloride solutions using [1-(2-pyridyl)-tridecane-1-one (2PC12)], [1-(2-pyridyl)-pentadecane-1-one (2PC14)], [1-(4-pyridyl)-tridecane-1-one (4PC12)] and [1-(4-pyridyl)-pentadecane-1-one (4PC14)] oximes. The influence of the extractant concentration, metal concentration, concentrations of chloride ions and various polar and non-polar solvents was evaluated. They found that only the 2-pyridyl ketoximes extracted Cd (II) and that the extraction depends on the solvent, on the reagent concentration and on the concentration of chloride ions. Cd (II) was extracted efficiently by using a mixture of chloroform and hydrocarbons as diluents with decan-1-ol, and removed from the loaded organic phase with aqueous ammonia and water.
Nan et al. [14] studied a new process for recovering metals from a mixture of worn lithium-ion and nickel-metal hydride (NiMH) batteries. In the developed process, liquid-liquid extraction was used to remove copper, cobalt and nickel from the obtained leachate. Using the developed process, it was possible to recover more than 94 % of all metals.
Innocenzi and Veglio [15] studied the use of solvent extraction for purifying leach solutions of NiMH batteries. Dissolved nickel was separated from manganese and zinc by liquid-liquid extraction using DEHPA. Two extractants were investigated: DEHPA and Cyanex 272 in n-dodecane. DEHPA was more efficient in separating manganese and zinc from the leach solutions. The results suggest that a two-step liquid-liquid cross flow system is sufficient to extract 100 % of zinc and about 95 % of manganese, while residual nickel is about 80 % of the initial content. These researchers have proposed a process flow that includes two stages of extraction, 20 % v/v DEHPA in n-dodecane (1/1, at room temperature, 30 min of contact, pH ≤ 2.5) and 4 M H2SO4 (1/0.5, at room temperature, 15 min of contact).
Larsson et al. [16] studied the properties of extraction with the leaching liquors of nickel metal hydride dissolved in hydrochloric acid 8 M using Cyanex 923.
The obtainment and recovery of rare earth elements (REE) has been studied extensively in recent years. These elements are very relevant from an industrial point of view [17]. REE took on essential importance because of the rapidly growing demand for them in several areas, such as electronics, metallurgy, magnetism, catalysts, ceramics and laser technology. Although there are many different uses for REE, they are not recycled in large quantities. This could change, however, if recycling became mandatory or if very high prices of rare earth metals (REM) made recycling feasible. In-plant recycling activities have been reported by the NEOMAX group (Hitachi Metals Ltda), including its plants in Japan. This process includes a solvent extraction step to recover REE from permanent magnet scraps. Recently, an hydrometallurgical process was developed by Rhodia (France) for the recycling of rare earth oxides (REO) from slag generated after the pyrometallurgical treatment of NiMH batteries. This recovery of REO is a business secret, but the process includes different solvent extraction units [18].
The reported separation processes of REE are based on the use of the following classes of extractants: tri-n-butilfosfate (TBP), quaternary amines, acid versatic and acid di-2-etilhexilfosforic (DEHPA).
Banda et al. [19] developed a process for the separation of Pr from La and Nd in a chloride solution. Among the extractants (Cyanex 272, DEHPA, PC88A, and Cyanex 301), Cyanex 272 and saponified Cyanex 272 showed a better potential for extracting La than Pr and Nd.
Alstad et al. [20] studied the solvent extraction of rare earth metal ions (La, Pr, Nd, Sm, Eu, Tb, Dy, Ho, Er, Tm, Yb and Lu) from an ionic aqueous solution of sodium perchlorate, using carbon tetrachloride as the organic phase. Tian et al. [21] also conducted a study of the synergistic extraction of Sm (III) in a chloride medium. Nasab et al. [22] evaluated the optimal process conditions for the separation of lanthanum, cerium and yttrium using three acids, Cyanex 272, Cyanex 302 and TBP (HA), at concentrations ranging from 0.01 to 0.5 M. The use of Cyanex 272 was more effective for the separation of thorium and rare earths when compared with TBP.
Torkoman et al. [23] conducted studies on the extraction of Sm (III) from nitrate in aqueous solutions, using Cyanex 301 alone and in combination with DEHPA. They evaluated the influence of various operating parameters, such as extraction time, acidity of the aqueous solution, concentration of the extractor and temperature. The synergistic effect was also evaluated, and the addition of DEHPA to Cyanex 301 in low concentrations (0.01–0.05 M) increased the efficiency of the separation.
Tian et al. [21] also studied the synergistic solvent extraction of rare earth elements (La, Nd, Sm, Tb, Ho, Tm) from nitrate mixtures with 8-hydroxyquinoline (HQ) and acidic organophosphorus extractants, Cyanex 301 and Cyanex 302.
Catalysts are widely used in various operations in the petroleum refining and petrochemical industry. These catalysts undergo a process of deactivation due to coke deposits. They are therefore periodically regenerated and replaced by fresh catalysts. However, when the regeneration process is no longer possible, catalysts are treated for the recovery of the noble metals present in their composition [24, 25]. According to Li et al. [26] spent catalysts are dangerous waste, but they are valuable secondary materials. This has motivated companies and research groups to develop efficient methods for regenerating and reusing saturated or deactivated catalysts.
Chen et al. [27] studied the extraction of molybdenum and vanadium from the ammonia leaching residue of spent catalysts by roasting the residue with soda carbonate, followed by a hydrometallurgical treatment of the roasted products. The researchers found that the proposed roasting, leaching and separation steps provide a feasible alternative for the processing of ammonia leaching residue of spent catalysts, and that it can be applied in the comprehensive utilization of low grade molybdenum ore.
Marinho et al. [25] evaluated a hydrometallurgical route for processing spent commercial catalysts (Pt and PtSnIn/A2O3) used in Brazilian refineries. They studied the recovery of noble metals with less generation of final waste. They found that Aliquat 336 (15 vol.% in kerosene) extracted more than 99 wt% of platinum in one stage at 25 °C with an A/O phase ratio equal to 1 (v/v). No other metals were extracted. Barakat and Mahmoud [28] studied the recovery of platinum from spent catalysts. Platinum was separated from the leach liquor by direct precipitation and by solvent extraction using trioctylamine in kerosine.
6.2 Supercritical Extraction
The increasing environmental problems caused by the pollution of organic compounds and the restrictions imposed by environmental legislation have limited the use of conventional solvents. In this context, the use of supercritical fluids (SCF) is a promising alternative for the replacement of conventional toxic organic solvents [29].
According to Fox et al. [30], supercritical fluids (SCFs) have been successfully used in industrial-scale extractions since the 1930s–1940s. It is most commonly used in the extraction and separation of organic compounds, and the traditionally employed solvents are Carbon dioxide and light hydrocarbon gases. These authors studied the complex formation reactions of praseodymium nitrate hexahydrate, and neodymium nitrate hexahydrate salts with tri-n-butyl phosphate (TBP) and several other neutral organophosphorus reagents in supercritical carbon dioxide.
Tai et al. [29] studied the kinetics of metal ion (Zinc II as model species) extraction using an in situ chelation-SFE method with Cyanex 302 as the chelating agent and supercritical carbon dioxide as the solvent, which extracts the metal-chelate complex from aqueous solutions. Their main conclusion was that the extraction rate of zinc (II) ions increases with an increase in stirring rate, but decreases with pressure.
Goreishi et al. [31] evaluated the extraction of toxic heavy metals, uranium (U), hafnium (Hf) and zirconium (Zr), using supercritical carbon dioxide (SC-CO2) and Cyanex 301 as chelating agent from a synthetic wastewater sample. The researchers found that the extraction yield of U, Hf and Zr at optimal conditions was 98.1, 28.9 and 65.2 %, respectively.
Liang et al. [32] studied the extraction of cobalt ions from a fireproof board using a mixture of D2EPHA (Di-2-Ethyl Hexyl Phosphoric Acid) and n-hexane in supercritical carbon dioxide. The authors found that the cobalt on the spiked sample can be totally removed in the evaluated pilot unit.
6.3 Cementation
Cementation is a unit process in hydrometallurgical engineering and it is used to precipitate a metal from its solution onto another metal that is more electropositive. The thermodynamic conditions necessary for cementation can be determined by considering the reduction potentials of the species involved in the reaction. In general, the cementation reaction can be expressed as:
The precipitation of copper on iron from a natural solution is a classical example of a relatively ancient art that has been applied successfully for centuries to produce copper on a commercial scale. According to Lung [33], this method has been applied in China since 1086 AD for the extraction of copper from mine water.
In the case of copper, for example, dilute solutions of copper are put into contact with metallic iron, leading to the general reaction,
Cementation reactions involve the transfer of electrons and are thus by definition electrochemical reactions. The deposition of copper ions and the transfer of iron ions into the solution can be treated as a corrosion reaction.
Cementation is used extensively as a primary metal recovery method for cadmium, copper, gold and silver, and as an electrolyte purification technique in electrolytic processes.
The most common industrial cementation operations include the use of zinc dust to precipitate gold and silver from cyanide solutions, and the use of iron to recover copper from copper-bearing solutions [33].
Furthermore, several researchers have used the cementation process to concentrate/recover unusual metals. Cao [34] used cementation to obtain cobalt using two different ferromanganese alloys. The results show that the reaction is quick and reached levels close to 100 % recovery of cobalt.
In another study, Safarzadeha et al. [35] evaluated cadmium cementation using zinc dust. The results showed a recovery rate of 95.83 % for cadmium. Anacleto [36] studied Mercury cementation using zinc, iron and metallic aluminium as reducing agents. The reaction efficiency is strongly dependent on pH. For each metal under study, an ideal pH was established. It was shown that mercury cementation with metallic zinc is a first-order process referring to mercury concentration.
Aktas [37] investigated the cementation of rhodium from waste chloride solutions using a metallic copper powder as reducing agent. The method used in this study resulted in a fine rhodium powder with a purity of 98.65 %. The copper content of the powder was found to be 1,220 ppm. The impure powder can be directly sold to any refinery, but it requires further purification prior to being used for the preparation of a rhodium sulfate plating solution.
The recovery of metals, especially gold, silver and copper, from electronic waste with the cementation process has also been extensively investigated by several authors [38–42].
References
Treybal RE (1968) Mass transfer operations, 2nd edn. McGraw-Hill, New York
Foust AS, Wenzel LA, Clump CW, Maus L, Andersen LB (1980) Principles of unit operations. 2nd edn. Wiley, New York
Owusu G (1998) Selective extractions of Zn and Cd from Zn-Cd-Co-Ni sulphate solution using di-2-ethylhexyl phosphoric acid extractant. Hydrometallurgy 47:205–215
Harker JH, Backhurst JR, Richardson JF (2002) Chemical engineering, vol 2: particle technology and separation process, vol 5. Elsevier, London
Geankoplis CJ (2003) Transport process and separation process principles, 4th edn. Prentice Hall, upper Saddle River
Thornton JD (1992) Science and practice of liquid-liquid extraction. Clarendon Press, Oxford
Habashi F (1999) Textbook of hydrometallurgy. 2nd edn. Laval University Canada
Erkey C (2000) Supercritical carbon dioxide extraction of metals from aqueous solutions: a review. J Supercrit Fluids 17:259–287
Tong S, Zhao X, Song N, Jia Q, Zhou W, Liao W (2009) Solvent extraction study of rare earth elements from chloride medium by mixtures of sec-nonylphenoxy acetic with Cyanex301 and Cyanex302. Hydrometallurgy 100:15–19
Devi NB, Nathsarma KC, Chakravortty V (1997) Extraction and separation of Mn(II) and Zn(II) from sulphate solutions by sodium salt of Cyanex 272. Hydrometallurgy 45(1–2):169–179
Kongolo K, Mwema MD, Banza AN, Gock E (2003) Cobalt and zinc recovery from copper sulphate solution by solvent extraction. Miner Eng 16(12):1371–1374
Safarzadeh MS, Bafghi MS, Moradkhani D, Ojaghi IM (2007) A review on hydrometallurgical extraction and recovery of cadmium from various resources. Miner Eng 20:211–220
Parus A, Wieszczycka K, Olszanowski A (2011) Solvent extraction of cadmium(II) from chloride solutions by pyridyl ketoximes. Hydrometallurgy 105(3–4):284–289
Nan J, Han D, Yang M, Cui M, Hou X (2006) Recovery of metal values from a mixture of spent lithium-ion batteries and nickel-metal hydride batteries. Hydrometallurgy 84(1–2):75–80
Innocenzi V, Veglio F (2012) Separation of manganese, zinc and nickel from leaching solution of nickel-metal hydride spent batteries by solvent extraction. Hydrometallurgy 129–130:50–58
Larsson K, Ekberg C, Odegaard-Jensen A (2012) Using Cyanex 923 for selective extraction in a high concentration chloride medium on nickel metal hydride battery waste. Hydrometallurgy 129–130:35–42
Humphries M (2010) Rare Earth elements: the global supply chain. Congressional Research Service, United States Congress
Worrell E, Reuter M (eds) (2014) Handbook of recycling. Elsevier, London
Banda R, Nguyen TH, Sohn SH, Lee MS (2013) Recovery of valuable metals and regeneration of acid from the leaching solution of spent HDS catalysts by solvent extraction. Hydrometallurgy 133:161–167
Alstad J, Augustson JH, Farbu L (1974) Solvent extraction of rare-earth metal ions with thenoyltrifluoroacetone in carbon tetrachloride. J Inorg Nucl Chem 36(4):899–903
Miaomiao T, Qiong J, Wuping L (2013) Studies on synergistic solvent extraction of rare earth elements from nitrate medium by mixtures of 8-hydroxyquinoline with Cyanex 301 or Cyanex 302. J Rare Earths 31(6):604
Nasab ME, Sam A, Milani SA (2011) Determination of optimum process conditions for the separation of thorium and rare earth elements by solvent extraction. Hydrometallurgy 106:141–147
Torkaman R, Moosavian MA, Torab-Mostaedi M, Safdari J (2013) Solvent extraction of samarium from aqueous nitrate solution by Cyanex301 and D2EHPA. Hydrometallurgy 137:101–107
Kar BB, Murthy BVR, Misra VN (2005) Extraction of molybdenum from spent catalyst by salt-roasting. Int J Miner Process 76:143–147
Marinho RS, Afonso JC, Cunha JWSD (2010) Recovery of platinum from spent catalysts by liquid-liquid extraction in chloride médium. J Hazard Mater 179:488–494
Li W, Peng J, Zhang L, Zhang Z, Li L, Zhang S, Guo S (2008) Pilot-scale extraction of zinc from the spent catalyst of vinyl acetate synthesis by microwave irradiation. Hydrometallurgy 92:79–85
Chen Y, Feng Q, Shao Y, Zhang G, Ou L, Lu Y (2006) Investigations on the extraction of molybdenum and vanadium from ammonia leaching residue of spent catalyst. Int J Miner Process 79:42–48
Barakat MA, Mahmoud MHH (2004) Recovery of platinum from spent catalyst. Hydrometallurgy 72:179–184
Tai CY, You GS, Chen SL (2000) Kinetics study on supercritical fluid extraction of zinc(II) ion from aqueous solutions. J Supercrit Fluids 18:201–212
Fox RV, Duane Ball R, Harrington PB, Rollins HW, Jolley JJ, Wai CM (2004) Praseodymium nitrate and neodymium nitrate complexation with organophosphorus reagents in supercritical carbon dioxide solvente. J Supercrit Fluids 31:273–286
Ghoreishia SM, Ansaria K, Ghaziaskarb HS (2012) Supercritical extraction oftoxic heavy metals from aqueous waste via Cyanex 301 as chelating agente. J Supercrit Fluids 72:288–297
Liang MT, Liang RC, Lin CH, Hsu PJ, Wu LY, Chen HF, Wu YW, Lee WC (2013) Metal extraction of a spiked solid with supercritical carbon dioxide. J Supercrit Fluids 79:324–329
Lung TN (1986) The history of copper cementation on iron—the world’s first hydrometallurgical process from medieval China. Hydrometallurgy 17(1):113–129
Cao Y, Duby P (2001) Cobalt cementation with ferromanganese. Hydrometallurgy 61:195–205
Safarzadeha MS, Moradkhanib D, Ilkhchid MO (2007) Determination of the optimum conditions for the cementation of cadmium with zinc powder in sulfate medium. Chem Eng Process 46:1332–1340
Anacleto AL, Carvalho JR (1996) Mercury cementation from chloride solutions using iron, zinc and aluminium. Miner Eng 9(4):385–397
Aktas S (2012) Cementation of rhodium from waste chloride solutions using copper powder. Int J Miner Process 114–117:100–105
Yap CY, Mohamed N (2007) An electrogenerative process for the recovery of gold from cyanide solutions. Chemosphere 67:1502–1510
Tuncuka A, Stazi V, Akcil A, Yazici EY, Deveci H (2012) Aqueous metal recovery techniques from e-scrap: hydrometallurgy in recycling. Miner Eng 25:28–37
Naseri Joda N, Rashchi F (2012) Recovery of ultra fine grained silver and copper from PC board scraps. Sep Purif Technol 92:36–42
Syed S (2012) Recovery of gold from secondary sources—a review. Hydrometallurgy 115–116:30–51
Gros F, Baupb S, Aurousseau M (2011) Copper cementation on zinc and iron mixtures: part 1: results on rotating disc electrode. Hydrometallurgy 106:127–133
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Bertuol, D.A., Tanabe, E.H., Meili, L., Veit, H.M. (2015). Hydrometallurgical Processing. In: Veit, H., Moura Bernardes, A. (eds) Electronic Waste. Topics in Mining, Metallurgy and Materials Engineering. Springer, Cham. https://doi.org/10.1007/978-3-319-15714-6_6
Download citation
DOI: https://doi.org/10.1007/978-3-319-15714-6_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-15713-9
Online ISBN: 978-3-319-15714-6
eBook Packages: EngineeringEngineering (R0)