Abstract
Although the diagnosis of primary headaches remains generally clinical, researchers have made great efforts in the investigation of neurotransmitter pathways, neuropeptides, hormones, and the vascular and trigeminal systems. The increasing evidence regarding the systems involved in migraines, in addition to tension-type and cluster headaches, has permitted better comprehension of the cerebral and extraneurological mechanisms underlying these types of headaches, leading, in some cases, to the identification of a treatment target. This chapter deals with the biochemical alterations that play a pivotal role in the pathophysiology of primary headaches.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Although the diagnosis of primary headaches remains generally clinical, researchers have made great efforts in the investigation of neurotransmitter pathways, neuropeptides, hormones, and the vascular and trigeminal systems. The increasing evidence regarding the systems involved in migraine, in addition to tension-type and cluster headaches, has permitted better comprehension of the cerebral and extraneurological mechanisms underlying these types of headaches, leading, in some cases, to the identification of a treatment target. This chapter deals with the biochemical alterations that play a pivotal role in the pathophysiology of primary headaches.
10.1 Markers of Trigeminovascular System Activation
10.1.1 Calcitonin Gene-Related Peptide
Calcitonin gene-related principle peptide (CGRP) is a 37-amino-acid neuropeptide that is widely distributed in the peripheral and central nervous systems. Following noxious stimulation, CGRP is released on the periphery, where it induces neurogenic inflammation and sensitization of sensory nociceptive fibers, including those distributing to meningeal vessels, and promotes excitatory neurotransmission at the central concentration (dorsal horn of the spinal cord and trigeminal nucleus caudalis), mediating the sensation of pain to the entire body. Head CGRP-containing nerves supply blood vessels to various parts of the body.
Approximately 50 % of trigeminal neurons express CGRP and CGRP receptor. The binding of CGRP to its functional receptor can lead to the activation of multiple pathways that modulate gene expression and ion channel activity with a positive feedback loop, which may in part explain why the peripheral injection of CGRP leads to a delayed migraine-like headache several hours after injection [1, 2].
CGRP may play a role in neuronal–glial interactions in the trigeminal ganglia. The mechanism proposed involves the release of CGRP during the neuronal activation of the trigeminal ganglia. This CGRP then stimulates the satellite glial cells, which release proinflammatory cytokines, thereby further modulating the neuronal response [3].
CGRP involvement in migraine pathophysiology is supported by the ability of this neuropeptide infused intravenously to induce attacks, and by the effectiveness of the CGRP antagonists, olcegepant and telcagepant, as acute antimigraine agents [4]. The latter positive findings are further sustained by other approaches aimed at stopping CGRP activity using antibodies against CGRP and the CGRP receptor.
It has been suggested that CGRP might be a marker of trigeminovascular activation [5]. In migraine patients CGRP concentrations were measured both in peripheral blood and in the extrajugular vein (EJV). While in the former studies an increase in CGRP in the EJV blood of migraine patients assessed during attacks was found compared with controls, this finding was not confirmed in most recent research [5, 6] (Table 10.1).
These discrepant results are difficult to reconcile. According to Tfelt-Hansen and Le, the bulk of EJV blood flow comes from the extracranial tissue of the head and face and only 22 % comes from the internal cerebral circulation in humans. Moreover, the middle meningeal veins contribute only to a small fraction of blood in the EJV. The CGRP in EJV blood, therefore, is most likely derived from part of the vasculature without the blood-brain barrier [7].
The effect of sumatriptan on CGRP is most likely caused by an antimigraine effect, which is not mediated by a decrease in CGRP. In fact, in human volunteers the subcutaneous administration of sumatriptan did not induce variations in EJV concentrations of CGRP [8].
Recent research focusing on the interictal plasma concentrations of chronic migraine (CM) patients showed higher values in these patients in the absence of a migraine attack and with no symptomatic medication. Variables such as age, analgesic overuse, depression, fibromyalgia, vascular risk factors, history of triptan consumption, or type of preventive treatment did not influence CGRP concentrations [9].
Further evidence of the involvement of CGRP in migraine is derived from studies using saliva as a specimen that is easy to obtain, providing information on the pathological states and representing a clinical model for studying neuronal mechanisms involved in migraine. Salivary CGRP concentrations have been reported to be elevated in migraineurs during a spontaneous migraine attack, with a return of concentrations to nearly interictal values in response to rizatriptan and onabotulinumtoxinA (onabotA) [10, 11].
CGRP resulted in increases in the EJV blood of cluster headache (CH) patients in the active periods, with normalization after either subcutaneous sumatriptan 6 mg or O2 inhalation [12–14].
Another study including patients with chronic tension-type headache (CTTH) showed no changes in CGRP concentrations measured in the cubital vein at 10, 20, and 60 min after nitroglycerin infusion, further supporting the specificity of CGRP for primary headaches involving trigeminovascular activation [15]. Similarly, no variations in CGRP concentrations were found in the EJV and antecubital vein samples from patients with cervicogenic headache assessed both in the presence and absence of headache [16].
10.1.2 Neurokinin A
Experimental evidence suggests that NKA, substance P (SP), and CGRP might act as neurotransmitters at the first central synapses of the trigeminal nociceptive pathway to transmit the sensory stimuli to the higher brain centers and could play the role of cotransmitters or comodulators [17]. Immunocytochemical studies have revealed that a contingent supplying cerebral blood is immunoreactive to neurokinin A (NKA) and is derived from the trigeminal ganglion [18].
A significant increase in NKA and in CGRP concentrations was demonstrated in the plasma of young migraine patients during attacks, suggesting, although indirectly, that CGRP and NKA might be involved in the pathogenesis of migraine attacks [19]. Furthermore, an increase in NKA and also CGRP, nitrite, and cyclic guanosine monophosphate (cGMP) concentrations were found in the internal jugular vein (IJV) blood of migraine patients during attacks. They reached their highest values during the first hour, then tended to decrease progressively and returned, after the end of the attacks, to values similar to or below those detected at the time of catheter insertion. Prostaglandin E2 (PGE2) and 6-keto prostaglandin F1α (6-k-PGF1α), in addition to cyclic adenosine monophosphate (cAMP) concentrations significantly increased during the first hour, but reached a peak during the second hour and remained within the same range until the fourth and sixth hours. These findings suggest early activation of the l-arginine/nitric oxide (NO) pathway, which accompanies the release of vasoactive peptides, including NKA, from trigeminal endings and a late rise in the synthesis of prostanoids with algogenic and vasoactive properties, which may intervene in maintaining the headache phase [20].
10.1.3 Pituitary Adenylate Cyclase-Activating Peptide
Pituitary adenylate cyclase-activating peptide (PACAP) is a multifunctional vasodilatory peptide that has recently been implicated in migraine pathogenesis. It belongs to the vasoactive intestinal polypeptide (VIP)-glucagon growth hormone-releasing factor secretin superfamily [21]. PACAP binds to three different G-protein-coupled receptors. The PACAP receptor can be associated with subunits of the CGRP receptor, including receptor activity modifying protein 1 (RAMP1); thus, a possible synergistic effect of CGRP and PACAP receptor cAMP signaling has been suggested, but this effect needs to be tested in future research [22].
In the trigeminovascular system, PACAP is expressed in the trigeminal nucleus caudalis (TNC) and trigeminal ganglia. PACAP and its receptors are also expressed in sphenopalatine ganglia (SPG) neurons, which control dural vessel tone and cranial blood flow. Like CGRP, PACAP binds to a variety of the central nervous system (CNS) sites, such as the dorsal horn of the spinal cord, brainstem, thalamus, and hypothalamus [23–26]. PACAP plays an excitatory role in pain transmission, suggesting that it might play a role in central sensitization [21]. Interestingly, PACAP and CGRP share the ability to induce light aversion in mice and just like CGRP, PACAP has been linked to anxiety-like behavior and chronic stress response [27]. This may be relevant for migraine, which recognizes both anxiety and stress as potential triggers of attacks.
Several lines of evidence relate PACAP to migraine pathophysiology. In human studies, PACAP-38-induced dilatation of human meningeal arteries, but was less potent than VIP. It seems that the ability of PACAP-38 to induce a migraine is not because of its weak vasodilatory effects, but rather because of its other central actions [28]. Moreover, PACAP-38 was able to induce dural mast cell degranulation, more potently than VIP and PACAP-27 [29].
This suggests that it might play role in triggering neurogenic inflammation in the dural vessels, which may be relevant to migraines [30].
In rats, at least, PACAP has also been linked to nitric oxide. This was suggested by the increase in PACAP concentrations within the TNC after nitroglycerin injection, which is more relevant in wild-type than in knockout mice and its increase in both plasma and TNC after electrical stimulation [31, 32]. These findings therefore support the coupling of PACAP and NO in the trigeminovascular system, which has also been observed for CGRP and NO [33].
The role of PACAP in migraine pathophysiology was confirmed by recent findings obtained from migraine patients. Like CGRP, administration of PACAP to migraineurs induced a delayed migraine-like headache after an initial nonspecific headache in contrast with controls, who experienced only the initial headache phase. Similarly, PACAP induced an early vasodilation of the middle cerebral artery and superficial temporal artery in both healthy controls and migraineurs, but only the latter experienced a delayed migraine-like headache [34]. Very recent clinical research in migraineurs demonstrated that PACAP plasma concentrations were elevated during attacks compared with interictal concentrations [35]. Interestingly, in this study interictal PACAP concentrations were lower than those of healthy subjects, whereas ictal PACAP concentrations of migraineurs were within the range of those of healthy subjects. Based on mechanisms partially shared with CGRP, which can be relevant for migraine attacks, PACAP is considered a potential target for antimigraine agents. PACAP and VIP have common receptors, VPAC1 and VPAC2, while the PAC1 receptor is specific to PACAP [36].
Only one PAC1 receptor agonist, maxadilan, is presently available, and has been isolated from the salivary glands of the sand fly Lutzomyia longipalpis. Maxadilan is a 61-amino acid peptide. The deletion of the amino acids between 25 and 41 generated a specific PAC1 receptor antagonist, termed M65 [37]. A recent study demonstrated that maxadilan had no effect on CGRP release and M65 did not block the PACAP-38-induced CGRP release in the trigeminal system [38].
10.1.4 Vasoactive Intestinal Peptide
Vasoactive intestinal peptide (VIP) is one of the parasympathetic signaling transmitters contributing to cranial parasympathetic outflow mediated through the SPG. The activation of parasympathetic cranial outflow during migraine and CH attacks due to the trigeminal–autonomic reflex may be mediated by the above neurotransmitter and receptors [12]. VIP is structurally and functionally related to PACAP-38, but differs from PACAP in migraine-inducing properties. The first experience in this regard did not show the ability of VIP to induce migraine-like attacks. It evoked only a very mild headache in healthy volunteers, despite eliciting intracranial vascular dilation.
In a recent study, only 18 % of patients reported migraine-like attacks after VIP infusion against 73 % of patients reporting migraine after PACAP-38 infusion. Both peptides induced marked dilatation of the extracranial but not intracranial arteries. VIP-induced dilatation was normalized after 2 h, whereas vasodilatation induced by PACAP-38 was longer lasting (>2 h) [39]. On this basis, VIP concentrations have been considered a marker of parasympathetic activation in migraine.
Recent evidence suggests that increased interictal VIP concentrations measured in peripheral blood might be a biomarker helping with CM diagnosis, but it was not able to clearly differentiate CM from episodic migraine (EM). Patients with EM showed, in fact, significantly higher concentrations of VIP compared with controls, but lower than those with CM [40].
Concentrations of CGRP and VIP were also indicated to be potential predictors of the efficacy of onabotA in CM. As in the previous study, VIP and CGRP plasma concentrations were significantly increased in CM patients compared with controls. Concentrations of CGRP and, to a lesser degree, VIP, were significantly increased in responders vs nonresponders. The probability of being a responder to onabotA was 28 times higher in patients with a CGRP concentration above the threshold of 72 pg/mL. Although the sensitivity in calculating the threshold for VIP was poor, the probability of CM patients with low CGRP concentrations responding to onabotA was significantly higher than in those patients with high VIP concentrations [41].
10.2 Monoaminergic Systems
10.2.1 Serotonin (5-hydroxytriptamine)
More than 50 years ago Sicuteri et al. [42] hypothesized the involvement of 5-hydroxytriptamine (5-HT) in migraine based on the demonstration of significantly higher concentrations in the urine of 5-hydroxyindoleacetic acid, the major stable metabolite of 5-HT, during migraine attacks. Since then, several studies have tried to verify whether or not biochemical anomalies of 5-HT occur in migraine, some of them using platelets as a peripheral model of serotonergic neurons. In agreement with Sicuteri’s results, during a migraine attack, the first studies showed a significantly increased 5-HT turnover in plasma [43] and saliva [44]. Furthermore, increased concentrations of 5-hydroxyindoleacetic acid have been reported in the cerebrospinal fluid (CSF) of migraineurs during attacks [45].
Conversely, interictal concentrations of plasma serotonin have been shown to be low in migraineurs [42]. Furthermore, concentrations of platelet 5-HT appear to fluctuate differently in female migraineurs than in healthy women during the different phases of the menses and, more importantly, the concentrations of indole decrease significantly in the luteal phase in menstrual migraine before the painful attacks [46]. An increase in serotonin transporter activity was found with medication-overuse headache (MOH), which was normalized after withdrawal in parallel to the improvement of headache frequency. However, the study was not able to differentiate whether the increase in serotonin uptake was caused by a regular intake of analgesics or triptans or was a consequence of frequent headache attacks [47]. In addition, a recent interictal neuroimaging study showed increased availability of 5-HT transporters in the brainstem of migraineurs, suggesting an imbalance in the serotonergic system [48].
All the above findings hypothesize that migraine might be a syndrome of a chronically low central serotonin system with consequent 5-HT receptor hypersensitivity, and that migraine attacks might be triggered by a sudden increase in 5-HT release.
10.2.2 Dopamine and Norepinephrine
An alteration in dopaminergic and noradrenergic systems has been suggested in migraine. Both neurotransmitters are derived from the metabolism of tyrosine. In particular, tyrosine hydroxylase generates 3,4-dihydroxyphenylalanine (DOPA). Dopamine (DA) and norepinephrine (NE) are synthesized by DOPA decarboxylase and dopamine b-hydroxylase enzymes (Db-h) respectively.
Increased concentrations of DA were found in platelets of migraine without aura (MwoA) patients, in comparison with healthy control subjects. Even higher concentrations were detected in patients with CH. The increased platelet DA concentrations have been interpreted as an abnormal biochemical phenotypic trait of primary neurovascular headaches [49]. The higher concentrations of DA can be the consequence of a reduction in Db-h activity. Reduced Db-H enzyme activity and reduced NE concentrations have been reported in MwoA patients [50].
More recently, particular polymorphisms in the gene encoding for Db-h protein have been associated with migraine [51]. All these findings support the notion that complex abnormalities in the metabolism of tyrosine could result in the possible derangement of dopaminergic and noradrenergic pathways in migraine and also in CH.
10.2.3 Elusive Amines
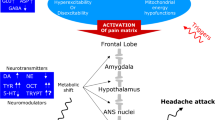
The involvement of the elusive amines, tyramine and octopamine, in migraine pathophysiology was proposed several years ago, but only in the last few years has there been a renewed interest in their role in migraine. These amines, together with DA and NE, are products of the alternative metabolic pathways of tyrosine. The renewed interest in elusive amines has also been derived from the discovery of a new class of G-protein coupled receptors, the trace amines receptors (TAARs), and there is a high affinity for these amines in rodents and humans [52]. TAARs have been identified in various tissues, including specific brain areas such as the rhinencephalon, limbic system, amygdala, hypothalamus, extrapyramidal system, and locus coeruleus. Some of these areas are relevant parts of the pain matrix modulating pain threshold. Their functions are mainly regulated by synapses using DA and NE as neurotransmitters.
Plasma concentrations of all trace amines have been determined in plasma and platelets of patients with primary neurovascular headaches. In particular, significantly higher concentrations of octopamine, synephrine, and tyramine were found in the plasma and platelets of CH patients, in both the remission and active phases, compared with control subjects or migraine patients. Additionally, intraplatelet concentrations were higher in CH patients than in control subjects.
In migraine patients assessed during headache-free periods, plasma concentrations of octopamine and synephrine were also higher than in controls, but in migraine with aura (MwA) patients, the difference did not reach the concentration of statistical significance [53]. Based on these results, an abnormality in the metabolism of tyrosine toward the increased synthesis of products of the decarboxylase pathway in migraine, resulting in increased synthesis of octopamine, synephrine, and tyramine in association with a decrease in NE, has been hypothesized.
10.3 Endocannabinoid System
Several lines of evidence support the central role of the endocannabinoid system in pain modulation [54]. It has been demonstrated that cannabinoid (CB) type-1 receptor (CB1R)-dependent retrograde mechanisms are involved in the release of neurotransmitters controlling nociceptive inputs and that the concentrations of these lipids are high in sensory terminals, skin, and dorsal root ganglia known to be involved in the transmission and modulation of pain signals. In the trigeminal system, activation CB1R has been shown to inhibit trigeminal neuron activity, which is pivotal in the pathogenic events of migraine [55].
Endocannabinoids are also involved in the descending modulation mediated by brainstem ventrolateral periaqueductal gray (PAG) of basal trigeminovascular neuronal tone and Aδ-fiber dural-nociceptive responses via CB1. Interaction between serotonergic and endocannabinoid systems in the processing of somatosensory nociceptive information suggests that at least part of the therapeutic action of triptans might occur via endocannabinoid-containing neurons in the ventrolateral PAG [56]. The endocannabinoid system is also involved in neurobiological mechanisms underlying drug addiction as it influences glutamatergic cortico-striatal transmission and the activity of the mesolimbic reward system [57].
Reduced concentrations of AEA were shown in the CSF of CM patients with and without medication overuse, reflecting impairment of the endocannabinoid system in these patients, which may contribute to chronic head pain and seems to be related to increased CGRP and NO production [58]. The activity of active endocannabinoids, anandamide membrane transporter (AMT) and fatty acid amide hydrolase (FAAH), and the concentration of cannabinoid receptors were also measured in peripheral platelets from patients with episodic MwoA, episodic tension-type headache (ETTH), and healthy controls. A significant increase in the activity of AMT and in FAAH activity was observed in female but not male patients with MwoA. No differences in the cannabinoid receptors emerged among the study groups. These findings suggest an increase in anandamide (N-arachidonoylethanolamine – AEA) degradation by platelets in women with migraine, which may be responsible for a reduction in AEA concentration in the blood, and which can lower the pain threshold and may explain the prevalence of migraine in women [59].
The activity of AMT and of FAAH was assessed in platelets of CM patients without symptomatic drug overuse, MOH patients, episodic migraine (EM) patients, and control subjects. AMT and FAAH appeared to be significantly reduced in both CM and MOH patients, compared with either controls or EM patients. This reduction was observed in both male and female patients in the CM and MOH groups. These changes observed in the endogenous cannabinoid degrading system have been hypothesized to reflect adaptive behavior to chronic headache and/or drug overuse [60].
In a more recent study a significant reduction in FAAH activity was demonstrated in MOH patients before withdrawal of treatment compared with controls. This was coupled with a significant improvement (reduction) in the facilitation of spinal cord pain processing (increased temporal summation threshold and related reduction in pain sensation) at both 10 and 60 days after treatment withdrawal. These findings could be a consequence of mechanisms aimed at reducing the endocannabinoid degradation and increased endocannabinoid system activity, resulting in an antinociceptive effect in patients undergoing withdrawal of treatment [61]. This supports the potential role of the CB1 receptor as a possible therapeutic target in CM [58].
A further confirmation of the dysfunction of the endocannabinoid system associated with a deficiency in the serotonergic system in CM and MOH is derived from a study investigating 2-acylglycerol (2-AG) and AEA concentrations in the platelets of these patients. These concentrations were significantly lower in MOH patients and CM patients than in the control subjects, with no significant differences between the two patient groups. Endocannabinoid and serotonergic systems appear to be both mutually related and defective in CM and MOH patients, and can contribute to headache chronification [62].
10.4 Glutamate
Glutamate, the major excitatory neurotransmitter in the CNS, is involved in several aspects of migraine pathophysiology. Preclinical data suggest its involvement in cortical spreading depression, trigeminovascular activation, and central sensitization. Based on these findings, glutamate concentrations have been determined in migraine patients both in peripheral blood and in CSF to assess possible dysfunction of the glutamatergic system in these patients [63]. An alteration in glutamate concentrations has been reported in migraine patients, with higher concentrations of CSF and plasma glutamate in episodic migraine patients compared with controls [64]. Furthermore, higher glutamate concentrations in CSF have been reported in chronic daily headache (CDH) patients related to the increase in the end-products of nitric oxide [65].
Platelet glutamate uptake and release have also been evaluated in patients with MwA and MwoA. Both glutamate release from stimulated platelets and plasma glutamate concentrations appeared to be increased in migraine patients, more markedly in MwA patients. Platelet glutamate uptake, assessed as 3H-glutamate intake, also appeared to be increased in MwA, whereas it was reduced in MwoA patients compared with controls. These findings suggest the involvement of different pathophysiological mechanisms in the two migraine types, with a more pronounced upregulation of glutamatergic metabolism in MwA [66].
Glutamate concentrations were also assessed using MR spectroscopy in vivo in women with migraine, and showed higher glutamate to glutamine ratios in occipital regions in women with migraine during the interictal state. These findings support the hypothesis that this increased ratio could arise from neuronal–glial coupling of glutamatergic metabolism differences compared with controls or an increased neuron/astrocyte ratio in the occipital cortex in migraine patients [67].
Glutamate and glutamine are strongly compartmentalized (in neurons for glutamate and in astrocytes for glutamine). The visual cortex is the brain region with a higher neuron/astrocyte ratio (the highest neuronal density and the relatively lowest density of astrocytes. Elevations in extracellular glutamate or potassium above certain thresholds are likely candidates to be the final common steps in the multiple distinct processes that can lead to cortically spreading depression. Astrocytes play a key role in this phenomenon, by acting as a sink for extracellular glutamate and potassium, in addition to generally acting as a buffer for the ionic and neurochemical changes that initiate and propagate cortical spreading depression.
In parallel to serotonin synthesis, the major route of tryptophan catabolism is the kynurenine pathway, which produces neuroactive metabolites. Among these substances, kynurenic acid has potential neuroprotective action blocking glutamate release and glutamatergic neurotransmission. Thus, kynurenines may affect several pathogenic events involving glutamate pathogenesis in migraine directly, by acting on glutamate receptors and exerting other neuromodulatory effects, and indirectly via an altered serotonin metabolism [68].
10.5 Nitric Oxide
Nitric oxide (NO) is a labile molecule with only a few seconds half-life, which is synthesized by endothelium, neuronal cells, and immune cells by specific NO synthases (NOS; endothelia, neuronal and inducible NOS). It is rapidly oxidized by tissue oxygen to the stable end products, nitrate and nitrite; the total concentration of both is the best index of overall NO production in the circulation. The most important effect of NO is the activation of the soluble guanylate cyclase. This enzyme induces the synthesis of cGMP and NO-cGMP pathways in smooth vascular muscles and is responsible for vascular dilatation and relaxation. Through these effects, NO is involved in the regulation of cerebral vessel tone in the cerebral blood flow.
Nitrite concentrations in plasma were investigated in patients with migraine and CH patients compared with a group of healthy nonheadache controls. Significantly higher nitrite concentrations were found in migraine patients, with and without aura, and in cluster headache patients, during the remission and cluster phases, than in controls, suggesting the involvement of a basal dysfunctioning in the l-arginine-NO pathway in the peripheral mechanisms, predisposing patients with neurovascular headaches to individual attacks [69].
In another study, basal plasma concentrations of NO metabolite nitrites (measured spectrophotometrically after the conversion of nitrates to NO2-), similar in patients and controls, were found to be significantly increased after glyceryl trinitrate (GTN) infusion at pain peak in patients and after 45 min in controls, but not after 120 min, with no differences between groups. These data do not support the presence of basal hyperactivity of the l-arginine-NO pathway in CH patients. The authors hypothesized that increased NO production might be of relevance in the mechanisms leading to CH attacks, but other factors are likely to render CH patients hyperresponsive to NO, ultimately causing the occurrence of pain and associated features [70].
It has been demonstrated that increased NO may interact with reactive oxygen substances (ROS). Enhanced endothelial NO and superoxide anion release may be involved in the induction of migraine through cerebral blood flow changes [71].
Dysfunction of the l-arginine/NO pathway activation was also investigated in migraine and tension type headache. It was demonstrated that the activation of the l-arginine/NO pathway in migraine patients, especially those with aura, increased basal and collagen-stimulated production of NO, and cGMP in the platelet cytosol of migraine patients, accompanied by a decrease in collagen-induced aggregation, especially in patients with aura, compared with controls and this production was further increased during attacks [72]. The increase in NO production in association with a decrease in platelet aggregation to collagen was also confirmed in female migraineurs compared with female controls with no headaches. This decrease was most evident at mid-cycle in nonmenstrual migraine patients and in the luteal phase in menstrual migraine patients, and was more accentuated during migraine attacks in both subgroups. The activation of the l-arginine/NO pathway was more accentuated in the luteal phase in menstrual migraine patients, and this could be responsible for the increased susceptibility to migraine attacks during perimenstrual and menstrual periods in these patients [73].
In patients with chronic daily headache (CDH) evolving from a previous episodic migraine and in patients with CTTH a significant reduction in platelet collagen aggregation was coupled with increased NO and cGMP production and a significant increase in cytosolic Ca(2+) concentration, which was more evident in patients with analgesic abuse compared with healthy control individuals. This was accompanied by a reduced platelet content and collagen-induced secretion of serotonin, and in patients with tension-type headache by increased glutamate content [74].
Based on the above findings, antagonizing NO production or the blockade of steps in the NO-cGMP pathway, or scavenging of NO have been proposed to be potential targets in the treatment of primary headaches for new drugs in treating migraine and other headaches. Nonselective NOS inhibitors are likely to have side effects, whereas the selective compounds, nNOS and i-NOS inhibitors, are now under investigation. Antagonizing the rate-limiting cofactor tetrahydrobiopterin could be another very likely new treatment. It is more unlikely that the antagonism of cGMP or its formation will also be feasible, but augmenting its breakdown via phosphodiesterase activation is a possibility, in addition to other ways of inhibiting the NO-cGMP pathway.
10.6 Neurotrophins
The involvement of nerve growth factor (NGF) in peripheral nociception clearly emerged from the evidence in experimental pain models of upregulation and increased delivery of this neurotrophin. Overexpression of the high affinity tyrosine kinase A (TrkA) receptors by nociceptive terminals was also observed and accompanied by enhanced SP and CGRP release [75]. The role of NGF as a peripheral pain mediator is further suggested by the effectiveness of NGF neutralizing molecules as analgesic agents in many models of persistent pain; these molecules have also been evaluated in exploratory clinical trials. A further mechanism mediated by NGF in experimental models of hyperalgesia is the enhancement of N-methyl-d-aspartate (NMDA)-evoked responses through the induction of brain-derived neurotrophic factor (BDNF) synthesis by TrkA-positive sensory neurons and its interaction with Trk-B receptors.
Experimental data also suggest that NGF might activate mast cells through the collaborative interaction with lysophosphatidyl serine expressed on membranes of activated platelets. This could be of relevance for the neurogenic inflammation mechanisms, assumed to constitute an experimental model of acute migraine attack, where the occurrence of activated platelets has been demonstrated.
All these findings strongly suggest the potential implication of both NGF and BDNF maintaining chronic pain states in humans, including headache.
Higher concentrations of BDNF were also demonstrated on the periphery in patients affected by neurovascular primary headaches. In one report, patients with all primary headaches showed significantly decreased platelet concentrations of BDNF, but a selective reduction of platelet NGF was found only in migraineurs and not in CH patients. No changes were observed in the plasma concentrations of either of the neurotrophins in the two patient groups compared with controls. These findings further suggested the potential involvement of BDNF and NGF in the pathophysiology of both headache disorders, and raised the possibility that it might be helpful in differentiating migraine biologically from CH [76].
Tumor necrosis factor (TNF)-alpha, sTNF-R1, sTNF-R2 receptors were also determined in a further study aimed at verifying changes in BDNF serum concentrations in migraine patients assessed during migraine attacks. BDNF was increased during a migraine attack, whereas there was no significant difference in the serum concentrations of TNF-alpha, sTNF-R1, and sTNF-R2 between attacks and headache-free periods. This report therefore reinforces the hypothesis of the involvement of BDNF in migraine pathophysiology [77].
Further studies have been performed to assess NGF and BDNF in the CSF of patients with chronic headache. Significantly higher concentrations of NGF were demonstrated in the CSF of patients with CDH, with no differences between patients with and those without analgesic abuse [78]. Similar findings were found in a subsequent study revealing higher concentrations of both neurotrophins in the CSF of both CM and fibromyalgia patients. Values of BDNF and those of NGF correlated positively with those of glutamate [79].
In the spinal cord after the stimulation of primary sensory neurons various substrates are released from the central terminals, such as ATP or BDNF. ATP is able to influence the activity of the microglia, which causes the release of BDNF from the microglia through the activation of the P2X4 receptors. BDNF activates the TrkB receptors, which results in the downregulation of the K+-Cl--contransporter of the second-order neurons, which convey information to the thalamus. This process is associated with the development of allodynia.
Experimental findings demonstrated that somatostatin (SOM) is a regulatory peptide in both the central and peripheral nervous systems. In humans, SOM is used to treat opioid-resistant pain. In animal models, SOM and its stable analog octreotide (OCT) have analgesic effects and SOM released from sensory neurone peripheral endings exerts anti-inflammatory actions. Activity-induced release of endogenous SOM can be modulated by the trophic factor glial-cell-line-derived neurotrophic factor (GDNF).
Glial-cell-line-derived neurotrophic factor and SOM were measured in the CSF of CM and fibromyalgia patients both with and without analgesic abuse and control subjects. Significantly lower concentrations of GDNF and SOM were found in the CSF of both CM and fibromyalgia patients compared with controls, with no significant differences between those with analgesic overuse and those without. The abuse of simple or combination analgesics does not seem to influence the biochemical changes investigated, which appear to be more strictly related to the chronic pain state [80] (Table 10.2).
10.7 Neurometabolic Systems
10.7.1 Hypothalamic Orexinergic System
The hypothalamus has been involved in several headache disorders, including migraine. Its role in migraine has initially been suggested owing to the observations of premonitory symptoms in migraineurs, such as changes in thirst, food cravings, and mood and sleep disturbances. More recently, functional imaging showed hypothalamic activation during acute migraine attacks [81].
It has also been demonstrated that several hypothalamic peptides, proteins, and neurotransmitters involved in feeding are also involved in migraine pathophysiology. Among them orexin, adiponectin, and leptin should be mentioned.
Specifically, orexin (OX) A- and OXB-containing neurons are primarily located in the lateral hypothalamus. They project to the cortex, thalamus, hypothalamus, brainstem (including the locus coeruleus and the raphe nucleus), in addition to the gastrointestinal tract. Orexins interact with 2G-protein coupled receptors, OXR1 and OXR2, which have been shown to contribute to regulate food intake. Further evidence suggests that orexin might also modulate adipose tissue metabolism by inhibiting lipolysis.
The hypothalamic orexinergic system may act as a key regulator of involvement in the modulation of trigeminovascular activation and processing and may therefore be involved in the pathophysiology of a variety of primary headaches, including cluster headaches and chronic migraine [82].
Based on the above evidence, OXA and corticotropin-releasing factor (CRF) were determined in the CSF of CM patients without analgesic abuse and in that of MOH patients. Significantly higher concentrations of both OXA and CRF were found in the CSF of MOH and to a lesser extent in patients with CM without analgesic abuse compared with controls. A significant positive correlation was also found between CSF OXA and CRF values, monthly drug intake group, and scores of a self-completion ten-item instrument to measure dependence upon a variety of substances, the Leeds Dependence Questionnaire (LDQ) in the MOH patient group. The significantly higher OXA concentrations found in CM, mainly in MOH, can be interpreted as a compensatory response to chronic head pain or, alternatively, as an expression of hypothalamic response to stress due to chronic pain. A potential role for orexin-A in driving drug-seeking in MOH patients through the activation of stress pathways in the brain can also be hypothesized [83].
Changes in the concentrations of hypothalamic neuropeptides in migraineurs under preventive treatment with amitriptyline and flunarizine were assessed in another study. Thirty-nine migraine patients with a body mass index <25 kg/m2 and with no endocrinological or metabolic diseases were assigned to receive amitriptyline, or flunarizine, for 3 months. Orexin-A and orexin-B concentrations were significantly reduced in both groups. Conversely, plasma neuropeptide-Y concentrations were markedly increased, with the highest concentrations at the second and third months, in both patient groups. Correlation of orexin-A concentrations with weight gain suggest their involvement in body weight increase occurring in migraineurs during amitriptyline or flunarizine prophylactic treatment [84].
10.7.2 Adipokines
Adiponectin (ADP) is a protein primarily secreted from adipocytes, and its receptors are expressed in the brain (particularly in the proopiomelanocortin [POMC] and neuropeptide Y [NPY] neurons of the hypothalamus), the blood vessel endothelium, in addition to liver and muscle.
As far as migraine is concerned, a pattern similar to that observed with serotonin has been hypothesized in migraineurs, with low concentrations of ADP interictally and increased concentrations during acute attacks. ADP concentrations appeared to be raised in migraine and this increase was independent of psychiatric comorbidities, migraine severity and allodynia [85].
Conversely, in a pilot study of female episodic migraineurs, the high molecular weight (HMW]):low molecular weight (LMW) ADP ratio concentration was associated with migraine severity and was predictive of acute treatment response to sumatriptan. In responder patients, the HMW:LMW ratio concentration was in fact greater at pain onset compared with nonresponders. Responders also showed a decrease in the HMW:LMW ratio at 60 and 120 min after treatment compared with onset. These changes in responders remained significant after adjusting for covariates, including measured body mass index (m-BMI). Although nonresponders showed no significant changes in unadjusted T-ADP or ADP oligomer or ratio concentrations, the HMW:LMW ratio appeared to be increased in nonresponders after adjustments [86]. ADP and the HMW:LMW ratio of ADP were therefore suggested to be potential novel biomarkers and drug targets for episodic migraine.
In the recent Atherosclerosis Risk in Communities Study, the prevalence of migraine was significantly associated with total adiponectin only in older men, but not in older women [87].
10.7.3 Leptin
Leptin is an adipocytokine involved in appetite suppression and the modulation of inflammatory processes. It is primarily produced by adipocytes such as adiponectin and leptin, but can also be produced by several other tissues, including the brain. Interestingly, leptin receptors are abundantly expressed in the arcuate nucleus (ARC) and dorsomedial nucleus (DM) hypothalamus. Leptin has also been shown to modulate inflammation.
In the first study leptin concentrations in migraineurs were determined in serum pre- and post-treatment in two small groups of patients treated with amitriptyline or flunarizine. Body mass index (BMI) and serum leptin concentrations were found to be increased at 4 and 12 weeks post-treatment with both preventive drugs compared with baseline concentrations. It would not be possible to discriminate from the above results if the changes in leptin concentrations were entirely due to weight gain or a therapeutic response [88].
In more recent research, Guldiken et al. [89] assessed interictal serum leptin concentrations in migraineurs compared with age- and gender-matched controls. Lower leptin concentrations and lower fat mass were found in episodic migraineurs. However, there was no significant difference in leptin concentrations between the groups after adjusting for fat mass.
No conclusive results can be drawn from the above research on leptin. Further research is therefore needed evaluating serum leptin concentrations in migraineurs that should take into account disease duration, sex hormones, and the phase of the menstrual cycle in women.
10.7.4 Neuropeptide Y
Neuropeptide Y is widely distributed throughout sympathetic nerve endings where it is costored and cosecreted with noradrenaline. It is considered a marker of noradrenergic function. NPY participates in the autonomic control of cerebral circulation and can be involved in disorders characterized by neurogenically mediated changes in the cerebral blood flow, such as migraine, cluster headache, and stroke.
Studies on this marker are dated. Significantly lower plasma concentrations of NPY in young MwA patients and, to a lesser extent, those with MwoA were found in the interictal period, compared with a control group. Plasma NPY concentrations tended to increase significantly during migraine attacks, particularly in patients with MwA. No significant variations were observed between headache-free periods and attacks in tension-type headache patients.
Reduced NPY concentrations in the interictal period have been interpreted as evidence of the derangement of the sympathetic function in the course of migraine (especially MwA), whereas the increase in NPY concentrations during migraine attacks was suggested to be an expression of sympathetic activation, even though this system is less functionally efficient [90].
These results denied an increased sympathetic tone in migraine patients with either migraine or subarachnoid hemorrhage (SAH), and suggested that the higher CSF NPY might originate centrally. Findings in subarachnoid hemorrhage patients argue in favor of a decreased sympathetic tone, which could represent a homeostatic response counterbalancing vasoconstriction mediated by other mechanisms [91].
10.7.5 Gamma-Amino Butyric Acid
Gamma-amino butyric acid (GABA) is the major inhibitory neurotransmitter in the CNS where it plays an important role in pain modulation. The most effective drugs for migraine prevention, valproate and topiramate, exert a potent GABAergic agonism. Direct evidence of the role of GABA in migraine is scarce. A dated research reported that this neurotransmitter could be detected during attacks only in migraineurs, but not in TTH patients, suggesting an increase in this inhibitory neurotransmitter in the brain of migraine patients ictally aimed at antagonizing pain [92].
The GABA concentrations were also measured in the CSF of CM patients, and only those with comorbid depression showed significantly lower concentrations of this inhibitory neurotransmitter with no difference when comparing patients with controls supporting the possibility that a GABA deficiency might underlie mechanisms of depression in CM and that preventive therapies modulating GABA neurotransmission might be useful in this condition [93].
More recently, GABA concentrations were measured using magnetic resonance spectroscopy (MRS), in migraine patients. They did not differ significantly in migraineurs and controls during attacks and interictally. GABA concentrations, however, did vary significantly as a function of severe headache attacks and of attack-related disability, further suggesting the role of GABA in suppressing headache attacks [94].
Despite the potential role of the GABAergic system in migraine, no association was found between common variants of the GABA A receptor and migraine, denying their major contribution to migraine susceptibility [95].
10.8 Substance P
The neuropeptide substance P (SP) is widely distributed in both the central and the peripheral nervous system. In the trigeminal system, SP, present in sensory afferent neurons, mediates vasodilation and nociceptive information.
Experimental findings demonstrated that stimulation of the trigeminal ganglion in cats and humans elicits release of SP and CGRP. Immunoreactivity of SP was found to be increased in the saliva of migraine and cluster headache patients during attacks. This increase was accompanied by a rise in CGRP in both migraine and cluster headache patients, and also VIP, but only in cluster headache patients [96]. A further study reported significantly higher concentrations of SP, together with those of CGRP and NGF in the plasma and saliva of patients with CM compared with control subjects. Plasma concentrations of SP and CGRP correlated significantly with their concentrations in saliva. There was a significant positive correlation between NGF and both neuropeptide concentrations in plasma, and between the neuropeptide concentrations in both plasma and saliva. NGF and both neuropeptides were highly associated with pain intensity [97].
A central sensitization has been advocated to explain chronic daily headache due to sustained trigeminovascular system activation. Glutamate-NO-cGMP-mediated mechanisms and increased release of sensory neuropeptides have been shown to play an important role in the maintenance of chronic head pain. In particular, among sensory neuropeptides from the trigeminal system, SP, CGRP, and, to a lesser extent, neurokinin A, appeared to be significantly increased in the CSF of CDH patients.
Substance P and somatostatin-like immunoreactivity in addition to enkephalinase activity have also been evaluated in a dated study in the CSF during spontaneous and histamine-induced attacks in cluster headache patients in the active phase. During the histamine-provoked attacks, CSF and plasma somatostatin and enkephalinase activity were unchanged, while plasma SP decreased significantly. During spontaneous attacks, a significant lowering of SP without changes in somatostatin was found in plasma compared with controls. Notably, both during and between attacks in the cluster phase, plasma enkephalinase activity was increased in comparison with the values in controls and this could explain the reduced concentrations of SP detected [98].
In a further study, SP and 5-HT concentrations were determined in platelets of migraine and TTH patients. SP concentration was significantly higher, whereas 5-HT concentration was significantly lower in both patient groups compared with controls. Furthermore, there was significant negative correlation between the concentrations of platelet SP and those of platelet 5-HT. These findings may reflect similar changes in monoaminergic pathways involved in trigeminal pain processing in both types of primary headache [99].
10.9 Endothelins
Endothelin-1 (EDN-1), encoded by the EDN1 gene on chromosome 6p24, is one of the most potent vasoconstrictors in humans, but also exerts neuronal effects. EDN-1 is in fact able to induce cortical spreading depression (CSD) in animal models and its involvement in migraine is demonstrated by increased concentrations during attacks [100]. In preclinical studies expression of ET(A) and ET(B) receptor mRNA was detected in human cerebral arteries with different effects: endothelin A receptor agonism mediates strong vasoconstriction, whereas agonism to ET (B) receptor determines concentration-dependent vasodilation. In one study, EDN-1 ictal values were markedly elevated at the beginning of the migraine crisis (<2 h) and declined to interictal or even lower concentrations later (4–6 h) in the course of an attack. The local vasoconstriction at the beginning of a migraine attack was interpreted to be EDN-1-mediated secondary to serotonin activation [101]. Vasopressin, which is known to induce EDN-1 synthesis in endothelial cells, seems to play a role as a mediator of elevated plasma EDN-1 in migraine, as suggested by the finding of increased vasopressin concentrations 3 h after the attack onset, preceding an EDN-1 increase at 6 h [102]. In contrast to the above results, only one study reported decreased concentrations of plasma EDN-1 during migraine attacks compared with interictal conditions, which returned to basal values after pain relief [103]. In a more recent study, EDN-1 appeared to correlate with the duration of migraine, systolic and diastolic blood pressure, carotid artery intima media thickness, and impaired endothelial vasoreactivity. An increase in EDN-1 was indicated to be an expression of endothelial injury in migraineurs, independent of attacks and associated with vascular risk factors [104].
10.10 Hormonal Alterations in Primary Headaches
In primary headache disorders, contributions are made to the premorbid state by genetic and epigenetic factors. Hormonal fluctuations alter the triggering of the migraine attack. Estrogen has mainly an excitatory effect, while progesterone has an inhibitory effect in the CNS. The progesterone concentration is decreased in the premenstrual phase and its withdrawal could cause cortical hyperexcitability. CSD is a propagating transient negative direct potential shift, which occurs in the aura phase of MwA. CSD is influenced by estrogen [105]. Estrogen receptors, which are expressed by the microglia, become activated during CSD, with the consequence of the release of cytokines [106]. In the migraine-related structures (e.g., hypothalamus, periaqueductal gray), estrogen has been revealed. Neuronal hyperexcitability during the menstrual cycle is modulated by the brain-derived neurotrophic factor, which is induced by estrogen and is decreased by progesterone. It is supposed that degranulation of the dural mast cells could activate the perivascular nerve fibers. The mast cells synthesize gonadotropin-releasing hormone, which can modulate the neuronal activity [105].
10.10.1 Migraine
The prevalence of migraine changes with age. Migraine occurs in 3–10 % of prepubertal children (puberty begins at 8–14 years in girls and 9–15 years in boys), and the rates are similar among boys and girls. In the postpubertal period, the hypothalamus resets its neurohormonal systems [107]. Migraine affects close to three times as many adult women (15–17 %) as adult men (6 %), and the prevalence of migraine decreases in postmenopausal women. Alterations in the hormonal milieu of the reproductive cycles of women are associated with the frequency of migraine headache. The patterns of migraine correlate with menarche, pregnancy, lactation, and the menopause.
10.10.2 Tension-Type Headache
A literature review has indicated that TTH is triggered by the menses. Population studies have revealed that 12.7 % of women have menstrual TTH, but pregnancy, the clinical features of TTH may improve. TTH remains unchanged or worsens in 70 % of postmenopausal women. A connection between the hormonal fluctuation and the pathomechanism of TTH is currently lacking [108].
10.10.3 Cluster Headache
No strong link has been detected between CH and hormones, nor is there any association between CH and menarche, menstruation, hormonal contraceptive use or menopause. In spite of this, data exist that point to the remission of CH during pregnancy, with a relapse several days after delivery and a worsening in the perimenopausal period. The results of clinical studies revealed no relationship between menstruation and CH or between the use of hormonal contraceptives and CH [108].
10.11 Immunological Findings in Primary Headaches
10.11.1 Migraine
Among the mechanisms thought to intervene in migraine pathophysiology the involvement of allergic factors has been hypothesized for many years [109]. High comorbidity between migraine and atopic diseases such as eczema and asthma has been shown in dated studies, whereas the role of dietary factors as triggers of migraine via immunological mechanisms has been criticized over the years [110].
Evidence for the involvement of the immune system in migraine comes from findings of changes of serum concentrations in complement and immunoglobulins, histamine, and immune cells in migraine patients, without clarification of the mechanisms involved [111].
Research into cytokines in patients with migraine has demonstrated fluctuations of cytokine concentrations in migraine. The involvement of proinflammatory cytokines in migraine attacks is emphasized in a study showing a trend toward an increase in plasma concentrations of TNF-α in children with migraine compared with controls. In the same study, TNF-α and IL-1 α, in addition to sTNF-RI concentrations, were significantly higher in MwA than in MwoA [107]. Prophylactic drugs seemed to reduce all proinflammatory cytokine concentrations with no significant differences from each other [112].
More recent research concerning the distribution of undetectable and detectable anti-inflammatory cytokines in children and adolescents with migraine or TTH did not demonstrate differences among the two groups and age-matched control subjects [113].
Chronic migraine with MOH is also characterized by alterations of some immune parameters compared with episodic migraine patients indicating an inflammatory state, as in other chronic pain conditions, especially with comorbid depression [114]. They include changes in white blood cell and total lymphocyte count and number and percentages of some lymphocyte subsets, such as CD4, CD19, CD8, and CD3 [115, 116]. These changes are accompanied by a reduction in beta-endorphin lymphocyte concentrations, according to previous findings in migraine and tension-type headache.
The assessment of cytokine concentrations in the cerebrospinal fluid of headache patients assessed during attacks showed significant group differences in IL-1ra, transforming growth factor-beta (TGF)-β1, and monocyte chemoattractant protein (MCP)-1 in episodic TTH and migraine compared with controls, and a significant difference in MCP-1 between cervicogenic headache and MwA. Intrathecal MCP-1 appeared to correlate with IL-1ra, IL-10, and TGF- β 1 in episodic TTH, and MCP-1 with IL-10 in MwA patients. Cytokine changes are modest compared with those found in other serious neurological conditions, and have been interpreted as being a mild response to pain [117].
More information on the involvement of immune cells in migraine is derived from research into meningeal sterile inflammation. Sterile inflammation is believed to play a role in the persistent activation of meningeal trigeminal nociceptors and related blood vessels underlying migraine attack precipitation and maintenance.
Mast cells surrounding dural nociceptors are activated in the course of sterile meningeal inflammation. Activated mast cells degranulate and release several proinflammatory mediators such as histamine and proinflammatory cytokines, which contribute to sustained trigeminal nociceptor activation. Among proinflammatory mediators, tumor necrosis factor (TNF)-α, in particular, is thought to intervene in the sensitization of meningeal nociceptors and the induction of intracranial throbbing pain through a complex meningeal immunovascular mechanism implicating activation of the TNF receptor (TNFR) 1 and 2 on both vascular and immune cells, in addition to downstream activation of meningeal NOS, COX-1, and COX-2, and phosphorylation of MAP kinase p38 in vascular cells. IL-1β, but not IL-6, also promotes the activation and increased mechanosensitivity of intracranial meningeal nociceptors.
Other mediators potentially implicated in promoting the persistent activation of meningeal nociceptors after CSD might be vasoconstricting mediators released during the prolonged reduction in CBF that follows CSD [118]. One potential factor in this regard is the arachidonic acid-derived 20-hydroxyeicosatetraenoic acid (20-HETE), because of its ability to activate the ion-channel TRPV1 on nociceptors.
The involvement of TNF-α in trigeminovascular activation is supported by its transient increase together with that of soluble intercellular adhesion molecule (ICAM)-1 in the internal jugular blood of MwoA patients assessed ictally. The increase in TNF-α can be induced by sensory neuropeptides, mainly CGRP released from activated trigeminal endings. sICAM-1 concentrations then progressively decrease and the very late activation antigen (VLA)-1 expression by lymphocytes is downregulated during migraine attacks. This could antagonize their transvascular migration in the brain, to further support the hypothesis of “sterile” inflammation in the dura mater [119].
A further finding in the jugular venous blood of MwoA patients is the transient increase in the concentrations of the neutrophil chemotactic chemokine IL-8, but not the monocyte chemotactic chemokine MCP-1 or the lymphocyte chemotactic chemokine RANTES during attacks, consistent with the increase in CGRP. Experimental evidence demonstrated a CGRP-induced activation of IL-8 gene expression, but not RANTES and MCP-1, via transcriptional factor AP-2 transduction in response to cyclic adenosine monophosphate in sensory neurons involved in inflammatory pain models. Although IL-8 is transiently increased during migraine attacks, it is unlikely to be due to an accumulation of leukocytes secondary to neurogenic inflammation, as it is for other neuroinflammatory conditions affecting CNS [120].
Several preclinical and clinical data suggest the putative role of matrix metalloproteinases in migraine. These proteolytic enzymes involved in the remodeling of the majority of protein components of the extracellular matrix may alter the composition and function of the blood–brain barrier and promote a local inflammatory process with accumulation of inflammatory mediators capable of discharging and sensitizing meningeal nociceptors. In experimental models, they are upregulated by CSD [121].
10.11.2 Tension-Type Headache
Few studies have been performed in TTH patients, suggesting dysfunction of the immune system in patients, mainly in the chronic form (CTTH).
Decreased serum concentrations of IL-2 were found in patients with CTTH and in those with CM compared with controls. Decreased serum IL-2 concentrations have been interpreted to be a reflection of the reduction in 5-HT or catecholamine concentrations in CNS [122]. Conversely, concentrations of interleukin (IL)-1β were significantly elevated in participants diagnosed with CTTH compared with healthy controls, while IL-18 concentrations were found to be significantly elevated in men with CTTH [123].
In a recent study, children with CTTH showed lower IgA concentrations, but not lower cortisol concentrations. A significant negative association between the number of years with headache and IgA concentration was found [124].
10.11.3 Cluster Headache
Some evidence suggests the involvement of an inflammatory process in CH. Reduced activity of the NK cells and an increase in lymphokine-activated killer (LAK) generation has been shown in patients with CH compared with controls [125, 126]. Impairment of the cytolytic and proliferative responsiveness of peripheral blood mononuclear cells to IL-2 was also found [127].
Further findings in cluster headache patients concern the increase in the number of monocyte and NK cell populations (despite the reduced activity of these cells), alterations in NK+, CD3+, and CD4+ concentrations found in the cluster period, probably pain- or stress-related, reduced activity of Gi proteins, and a marked downregulation of Gi α mRNA [128, 129]. An increase in IL-1 β concentrations in CH patients between attacks has been shown compared with controls. IL-1 β was further increased during the ictal phase of CH compared with patients between attacks and normal individuals [130]. These data were contradicted by a more recent study on systemic changes in the IL-1 or IL-6 systems in CH patients [130]. In the same study, elevated soluble IL-2 receptors indicative of T cell activation were also found in cluster headache patients assessed during attacks. It has been hypothesized that IL-2 might be involved in the activation of the hypothalamus by stimulating CRF release.
10.11.4 Other Primary Headaches
Elevated TNF alpha concentrations were found in the CSF, but not in the plasma, of almost all new daily persistent headache (NDPH) patients assessed; similar concentrations were found in CM patients. An inflammatory status in the CNS has been hypothesized in both conditions and is considered one of the causes of refractoriness to treatment [131].
10.12 Magnesium in Primary Headaches
Magnesium (Mg) is an essential element that controls several cellular functions. Its physiological roles include control of neuronal activity, cardiac excitability, muscular contraction, neuromuscular transmission, vasomotor tone, blood pressure, and peripheral and cerebral blood flow. A deficiency of Mg in migraineurs was proposed for the first time by Durlach [132], who found that migraineurs may excrete excessive amounts of Mg owing to stress. More recently, a Mg load test study [133] revealed that migraine patients had greater retention of Mg than healthy controls, suggesting its systemic deficiency. Lower concentrations of Mg were found in CSF and were detected by 31P-magnetic resonance spectroscopy in the brain of migraine patients assessed interictally [134]. Low free Mg in the brain was also associated with deficient energy metabolism in patients with migraine and cluster headaches [135].
Low Mg concentrations have been associated with vasoconstriction, platelet aggregation, neurotransmitter release, including SP. Based on the preclinical and clinical evidence, Mg was assessed in the serum, but contrasting results were obtained in this regard. In particular, they did not appear to be significantly different among episodic (both with and without aura) and chronic migraine patients [136], and in hemiplegic migraine [137], or conversely to be reduced interictally and more consistently ictally [138]. Interestingly, an inverse correlation between increased P100 amplitude and lowered serum Mg concentrations was found in a study involving children suffering from migraine with and without aura assessed in a headache-free period. Both groups expressed neuronal hyperexcitability of the visual pathways related to a lowered threshold for migraine attacks, which can be normalized in most patients by treatment with oral magnesium pidolate [139].
Studies on Mg in erythrocytes and lymphocytes are more consistent, all showing on average a significant reduction in migraine patients compared with controls or TTH patients with no significant variations between ictal and interictal concentrations [140, 141]. It has been hypothesized that this reduction might be due to abnormal regulation of intracellular Mg that can reflect at the peripheral concentration changes observed in the brain of a migraineur. Red blood Mg deficiency may also explain premenstrual syndrome including migraine.
Serum ionized magnesium (IMg2+) was also measured by an ion-selective electrode for Mg2+ in patients with various headache syndromes. Low serum IMg2+ and a high ICa2+/IMg2+ ratio were found in 42 % of migraine patients assessed with attacks, but in only 23 % of patients with other severe, continuous headaches. Conversely, total serum Mg was normal in all patients [142]. In another study, 30.8 % of chronic migraine patients had low serum ionized but not total Mg concentrations, and 61.5 % had high ionized Ca/Mg ratios. Proportions for chronic tension-type headache patients were 4.5 and 36.4 % respectively. A high incidence of IMg2+ deficiency and elevated ICa2+/IMg2+ ratio has also been demonstrated during menstrual attacks (45 %), confirming previous suggestions of the involvement of Mg deficiency in its development [143].
Taking into account the above results, some Mg formulations have been used for prophylactic treatment (magnesium pidolate, trimagnesium dicitrate, magnesium oxide) and for the treatment of acute headaches (magnesium sulfate).
Two double-blind, randomized, placebo-controlled trials have shown the therapeutic efficacy of Mg supplementation in migraine patients, one involving women with menstrual migraine treated with two cycles of Mg or placebo taken daily from ovulation to the first day of flow [144, 145]. A third trial using a different Mg salt showed no effect of oral Mg on migraine [146].
In a randomized, double-blind, placebo-controlled study involving children and adolescents (aged 3–17 years), the administration of magnesium oxide induced a statistically significant trend toward reduction in headache frequency [147].
Further trials have also shown that intravenous magnesium sulfate is effective in the treatment of acute migraine [148]. Intravenous magnesium sulfate with prochlorperazine was also able to abort a prolonged migrainous aura [149].
In addition, Mg was found to be effective for the treatment of CH. After i.v. administration of magnesium sulfate, 41 % of patients reported meaningful relief defined as a complete cessation of attacks or relief for more than 3 days [150].
Based on the promising findings described above, Mg in the sulfate salt formulation can be recommended for migraine and possibly CH attacks. This may be particularly relevant for migraine patients) considering that up to 50 % of patients during attacks have low ionized Mg concentrations. Furthermore, Mg in chelated formulations may be recommended for the prophylactic treatment of patients with MwA and MwoA. Mg may also be indicated for patients with other headaches, such as TTH, with pericranial muscle tenderness, if peripheral Mg deficiency is demonstrated [151].
10.13 Conclusion
In this chapter we reviewed the most important aspects of the biochemistry of primary headaches. The most consistent results have been obtained for migraine (Tables 10.1 and 10.2). Further investigations are needed to broaden the horizons of this topic. The integration of the different fields of the pathophysiology of headaches could, in future, allow for a significant improvement in the knowledge and the management of such a relevant and common ailment.
References
Eftekhari S, Salvatore CA, Calamari A et al (2010) Differential distribution of calcitonin gene-related peptide and its receptor components in the human trigeminal ganglion. Neuroscience 169:683–696
Walker CS, Conner AC, Poyner DR et al (2010) Regulation of signal transduction by calcitonin gene-related peptide receptors. Trends Pharmacol Sci 31:476–483
Thalakoti S, Patil VV, Damodaram S et al (2007) Neuron-glia signaling in trigeminal ganglion: implications for migraine pathology. Headache 47:1008–1023
Durham PL, Vause CV (2010) Calcitonin gene-related peptide (CGRP) receptor antagonists in the treatment of migraine. CNS Drugs 24:539–548
Goadsby PJ, Edvinsson L (1993) The trigeminovascular system and migraine: studies characterizing cerebrovascular and neuropeptide changes seen in humans and cats. Ann Neurol 33:48–56
Sarchielli P, Alberti A, Codini M et al (2000) Nitric oxide, prostaglandin and trigeminal vasoactive peptides in internal jugular vein blood during spontaneous migraine attacks. Cephalalgia 20:903–918
Tvedskov JF, Lipka K, Ashina M et al (2005) No increase of calcitonin gene-related peptide in jugular blood during migraine. Ann Neurol 58:561–563
Tfelt-Hansen P, Le H (2009) Calcitonin gene-related peptide in blood: is it increased in the external jugular vein during migraine and cluster headache? A review. J Headache Pain 10:137–143
Hansen JM, Petersen J, Wienecke T et al (2009) Sumatriptan does not change calcitonin gene-related peptide in the cephalic and extracephalic circulation in healthy volunteers. J Headache Pain 10:85–91
Cernuda-Morollón E, Larrosa D, Ramón C et al (2013) Interictal increase of CGRP levels in peripheral blood as a biomarker for chronic migraine. Neurology 81:1191–1196
Cady RK, Vause CV, Ho TW et al (2009) Elevated saliva calcitonin gene-related peptide levels during acute migraine predict therapeutic response to rizatriptan. Headache 49:1258–1266
Cady R, Turner I, Dexter K et al (2014) An exploratory study of salivary calcitonin gene-related peptide levels relative to acute interventions and preventative treatment with onabotulinumtoxinA in chronic migraine. Headache 54:269–277
Goadsby PJ, Edvinsson L (1994) Human in vivo evidence for trigeminovascular activation in cluster headache. Neuropeptide changes and effects of acute attack therapies. Brain 117:427–434
Fanciullacci M, Alessandri M, Figini M et al (1995) Increase in plasma calcitonin gene-related peptide from the extracerebral circulation during nitro-glycerin-induced cluster headache attack. Pain 60:119–123
Fanciullacci M, Alessandri M, Sicuteri R et al (1997) Responsiveness of the trigeminovascular system to nitroglycerin in cluster headache patients. Brain 20:283–288
Ashina M, Bendtsen L, Jensen R et al (2001) Calcitonin gene-related peptide during nitric-oxide headache in patients with chronic tension-type headache. Eur J Neurol 8:173–178
Uddman R, Edvinsson L (1989) Neuropeptides in the cerebral circulation. Cerebrovasc Brain Metab Rev 1:230–252
Frese A, Schilgen M, Edvinsson L et al (2005) Calcitonin gene-related peptide in cervicogenic headache. Cephalalgia 25:700–703
Samsam M, Coveñas R, Ahangari R et al (2000) Simultaneous depletion of neurokinin A, substance P and calcitonin gene-related peptide from the caudal trigeminal nucleus of the rat during electrical stimulation of the trigeminal ganglion. Pain 84:389–395
Gallai V, Sarchielli P, Floridi A et al (1995) Vasoactive peptide levels in the plasma of young migraine patients with and without aura assessed both interictally and ictally. Cephalalgia 15:384–390
Vaudry D, Gonzalez BJ, Basille M et al (2000) Pituitary adenylate cyclase-activating polypeptide and its receptors: from structure to functions. Pharmacol Rev 52:269–324
Schytz HW, Olesen J, Ashina M (2010) The PACAP receptor: a novel target for migraine treatment. Neurotherapeutics 7:191–196
Csati A, Tajti J, Kuris A et al (2012) Distribution of vasoactive intestinal peptide, pituitary adenylate cyclase-activating peptide, nitric oxide synthase, and their receptors in human and rat sphenopalatine ganglion. Neuroscience 202:158–168
Masuo Y, Ohtaki T, Masuda Y et al (1992) Binding sites for pituitary adenylate cyclase activating polypeptide (PACAP): comparison with vasoactive intestinal polypeptide (VIP) binding site localization in rat brain sections. Brain Res 575:113–123
Narita M, Dun SL, Dun NJ et al (1996) Hyperalgesia induced by pituitary adenylate cyclase-activating polypeptide in the mouse spinal cord. Eur J Pharmacol 311:121–126
Tajti J, Uddman R, Edvinsson L (2001) Neuropeptide localization in the “migraine generator” region of the human brainstem. Cephalalgia 21:96–101
Sandor K, Kormos V, Botz B et al (2010) Impaired nocifensive behaviours and mechanical hyperalgesia, but enhanced thermal allodynia in pituitary adenylate cyclase-activating polypeptide deficient mice. Neuropeptides 44:363–371
Markovics A, Kormos V, Gaszner B et al (2012) Pituitary adenylate cyclase-activating polypeptide plays a key role in nitroglycerol-induced trigeminovascular activation in mice. Neurobiol Dis 45:633–644
Robert C, Bourgeais L, Arreto CD et al (2013) Paraventricular hypothalamic regulation of trigeminovascular mechanisms involved in headaches. J Neurosci 33:8827–8840
Vécsei L, Tuka B, Tajti J (2014) Role of PACAP in migraine headaches. Brain 137:650–651
Chan KY, Baun M, de Vries R et al (2011) Pharmacological characterization of VIP and PACAP receptors in the human meningeal and coronary artery. Cephalalgia 31:181–189
Hashimoto H, Shintani N, Baba A (2006) New insights into the central PACAPergic system from the phenotypes in PACAP- and PACAP receptor-knockout mice. Ann N Y Acad Sci 1070:75–89
Baun M, Pedersen MH, Olesen J et al (2012) Dural mast cell degranulation is a putative mechanism for headache induced by PACAP-38. Cephalalgia 32:337–345
Schytz HW, Birk S, Wienecke T et al (2009) PACAP38 induces migraine-like attacks in patients with migraine without aura. Brain 132:16–25
Tuka B, Helyes Z, Markovics A et al (2013) Alterations in PACAP-38-like immunoreactivity in the plasma during ictal and interictal periods of migraine patients. Cephalalgia 33:1085–1095
Dickson L, Finlayson K (2013) VPAC and PAC receptors: from ligands to function. Pharmacol Ther 121:294–316
Lerner EA, Iuga AO, Reddy VB (2007) Maxadilan, a PAC1 receptor agonist from sand flies. Peptides 28:1651–1654
Jansen-Olesen I, Baun M, Amrutkar DV et al (2014) PACAP-38 but not VIP induces release of CGRP from trigeminal nucleus caudalis via a receptor distinct from the PAC1 receptor. Neuropeptides 48:53–64
Amin FM, Hougaard A, Schytz HW et al (2014) Investigation of the pathophysiological mechanisms of migraine attacks induced by pituitary adenylate cyclase-activating polypeptide-38. Brain 137:779–794
Morollón E, Martínez-Camblor P, Alvarez R et al (2014) Increased VIP levels in peripheral blood outside migraine attacks as a potential biomarker of cranial parasympathetic activation in chronic migraine. Cephalalgia
Cernuda-Morollón E, Martínez-Camblor P, Ramón C et al (2014) CGRP and VIP levels as predictors of efficacy of Onabotulinumtoxin type A in chronic migraine. Headache 54:987–995
Sicuteri F, Testi A, Anselmi B (1961) Biochemical investigations in headache: Increase of hydroyindoleacetic acid excretion during migraine attack. Int Arch Allergy 19:265–271
Ferrari MD, Odink J, Tapparelli C et al (1989) Serotonin metabolism in migraine. Neurology 39:1239–1242
Marukawa H, Shimomura T, Takahashi K (1996) Salivary substance P, 5-hydroxytryptamine, and gamma-aminobutyric acid levels in migraine and tension-type headache. Headache 36:100–104
Kovacs K, Bors L, Tothfalusi L et al (1989) Cerebrospinal fluid (CSF) investigations in migraine. Cephalalgia 9:53–57
Fioroni L, D’Andrea G, Alecci M et al (1996) Platelet serotonin pathway in menstrual migraine. Cephalalgia 16:427–430
Ayzenberg I, Obermann M, Leineweber K et al (2008) Increased activity of serotonin uptake in platelets in medication overuse headache following regular intake of analgesics and triptans. J Headache Pain 9:109–112
Schuh-Hofer S, Richter M, Geworski L et al (2007) Increased serotonin transporter availability in the brainstem of migraineurs. J Neurol 254:789–796
D’Andrea G, Granella F, Perini F et al (2006) Platelet levels of dopamine are increased in migraine and cluster headache. Headache 46:585–591
Gallai V, Gaiti A, Sarchielli P et al (1992) Evidence for an altered dopamine b-hydroxylase activity in migraine and tension type headache. Acta Neurol Scand 86:403–446
Fernandez F, Lea RA, Colson NJ et al (2006) Association between a 19 bp deletion polymorphism at dopamine betahydroxylase (DBH) locus and migraine with aura. J Neurol Sci 251:118–123
Borowsky B, Adham N, Jones KA et al (2001) Trace amines: identification of a family of mammalian G protein-coupled receptors. Proc Natl Acad Sci U S A 98:933–941
D’Andrea G, Terrazzino S, Leon A et al (2004) Elevated levels of circulating trace amines in primary headaches. Neurology 62:1701–1705
Wilson RI, Nicoll RA (2002) Endocannabinoid signaling in the brain. Science 296:678–682
Greco R, Gasperi V, Maccarrone M, Tassorelli C (2010) The endocannabinoid system and migraine. Exp Neurol 224:85–91
Akerman S, Holland PR, Lasalandra MP et al (2013) Endocannabinoids in the brainstem modulate dural trigeminovascular nociceptive traffic via CB1 and “triptan” receptors: implications in migraine. J Neurosci 33:14869–14877
Maldonado R, Valverde O, Berrendero F (2006) Involvement of the endocannabinoid system in drug addiction. Trends Neurosci 29:225–232
Cupini LM, Bari M, Battista N et al (2006) Biochemical changes in endocannabinoid system are expressed in platelets of female but not male migraineurs. Cephalalgia 26:277–281
Cupini LM, Costa C, Sarchielli P et al (2008) Degradation of endocannabinoids in chronic migraine and medication overuse headache. Neurobiol Dis 30:186–189
Perrotta A, Arce-Leal N, Tassorelli C et al (2012) Acute reduction of anandamide-hydrolase (FAAH) activity is coupled with a reduction of nociceptive pathways facilitation in medication-overuse headache subjects after withdrawal treatment. Headache 52:1350–1361
Sarchielli P, Pini LA, Coppola F et al (2007) Endocannabinoids in chronic migraine: CSF findings suggest a system failure. Neuropsychopharmacology 32:1384–1390
Rossi C, Pini LA, Cupini ML et al (2008) Endocannabinoids in platelets of chronic migraine patients and medication-overuse headache patients: relation with serotonin levels. Eur J Clin Pharmacol 64:1–8
Ramadan NM (2003) The link between glutamate and migraine. CNS Spectr 8:446–449
Zukerman E, Minatti-Hannuch SN, Mazzacoratti MGN et al (1993) Cerebrospinal fluid neurotransmitter amino acids in migraine. Cephalalgia 13(Suppl 13):92
Gallai V, Alberti A, Gallai B et al (2003) Glutamate and nitric oxide pathway in chronic daily headache: evidence from cerebrospinal fluid. Cephalalgia 23:166–174
Vaccaro M, Riva C, Tremolizzo L et al (2007) Platelet glutamate uptake and release in migraine with and without aura. Cephalalgia 27:35–40
González de la Aleja J, Ramos A, Mato-Abad V et al (2013) Higher glutamate to glutamine ratios in occipital regions in women with migraine during the interictal state. Headache 53:365–375
Párdutz A, Fejes A, Bohár Z et al (2012) Kynurenines and headache. J Neural Transm 119:285–296
D’Amico D, Ferraris A, Leone M et al (2002) Increased plasma nitrites in migraine and cluster headache patients in interictal period: basal hyperactivity of L-arginine-NO pathway? Cephalalgia 22:33–36
Costa A, Ravaglia S, Sances G et al (2003) Nitric oxide pathway and response to nitroglycerin in cluster headache patients: plasma nitrite and citrulline levels. Cephalalgia 23:407–413
Silva FA, Rueda-Clausen CF, Silva SY et al (2007) Endothelial function in patients with migraine during the interictal period. Headache 47:45–51
Gallai V, Floridi A, Mazzotta G et al (1996) L-arginine/nitric oxide pathway activation in platelets of migraine patients with and without aura. Acta Neurol Scand 94:151–160
Sarchielli P, Tognoloni M, Russo S et al (1996) Variations in the platelet arginine/nitric oxide pathway during the ovarian cycle in females affected by menstrual migraine. Cephalalgia 16:468–475
Sarchielli P, Alberti A, Russo S et al (1999) Nitric oxide pathway, Ca2+, and serotonin content in platelets from patients suffering from chronic daily headache. Cephalalgia 19:810–816
Mendell LM, Albers KM, Davis BM (1999) Neurotrophins, nociceptors, and pain. Microsc Res Tech 45:252–261
Blandini F, Rinaldi L, Tassorelli C et al (2006) Peripheral levels of BDNF and NGF in primary headaches. Cephalalgia 26:136–142
Tanure MT, Gomez RS, Hurtado RC et al (2010) Increased serum levels of brain-derived neurotropic factor during migraine attacks: a pilot study. J Headache Pain 11:427–430
Sarchielli P, Alberti A, Floridi A et al (2001) Levels of nerve growth factor in cerebrospinal fluid of chronic daily headache patients. Neurology 57:132–134
Sarchielli P, Mancini ML, Floridi A et al (2007) Increased levels of neurotrophins are not specific for chronic migraine: evidence from primary fibromyalgia syndrome. J Pain 8:737–745
Sarchielli P, Alberti A, Candeliere A et al (2006) Glial cell line-derived neurotrophic factor and somatostatin levels in cerebrospinal fluid of patients affected by chronic migraine and fibromyalgia. Cephalalgia 26:409–415
Denuelle M, Fabre N, Payoux P et al (2007) Hypothalamic activation in spontaneous migraine attacks. Headache 47:1418–1426
Holland P, Goadsby PJ (2007) The hypothalamic orexinergic system: pain and primary headaches. Headache 47:951–962
Sarchielli P, Rainero I, Coppola F et al (2008) Involvement of corticotrophin-releasing factor and orexin-A in chronic migraine and medication-overuse headache: findings from cerebrospinal fluid. Cephalalgia 28:714–722
Caproni S, Corbelli I, Pini LA et al (2011) Migraine preventive drug-induced weight gain may be mediated by effects on hypothalamic peptides: the results of a pilot study. Cephalalgia 31:543–549
Duarte H, Teixeira AL, Rocha NP, Domingues RB (2014) Increased serum levels of adiponectin in migraine. J Neurol Sci 342:186–188
Peterlin BL, Tietjen GE, Gower BA et al (2013) Ictal adiponectin levels in episodic migraineurs: a randomized pilot trial. Headache 53:474–490
Dearborn JL, Schneider AL, Gottesman RF, Kurth T et al (2014) Adiponectin and leptin levels in migraineurs in the Atherosclerosis Risk in Communities Study. Neurology 83:2211–2218
Berilgen MS, Bulut S, Gonen M et al (2005) Comparison of the effects of amitriptyline and flunarizine on weight gain and serum leptin, C peptide and insulin levels when used as migraine preventive treatment. Cephalalgia 25:1048–1053
Guldiken B, Guldiken S, Demir M et al (2008) Low leptin levels in migraine: a case control study. Headache 40:1103–1107
Gallai V, Sarchielli P, Trequattrini A et al (1994) Neuropeptide Y in juvenile migraine and tension-type headache. Headache 34:35–40
Valenzuela RF, Donoso MV, Mellado PA et al (2000) Migraine, but not subarachnoid hemorrhage, is associated with differentially increased NPY-like immunoreactivity in the CSF. J Neurol Sci 173:140–146
Welch KMA, Chabi E, Nell JH et al (1975) Cerebrospinal fluid gamma aminobutyric acid levels and migraine. Br Med J 3:516–517
Vieira DS, Naffah-Mazacoratti MG, Zukerman E et al (2006) Cerebrospinal fluid GABA levels in chronic migraine with and without depression. Brain Res 1090:197–201
Bigal ME, Hetherington H, Pan J et al (2008) Occipital levels of GABA are related to severe headaches in migraine. Neurology 70:2078–2080
Chen T, Murrell M, Fowdar J et al (2012) Investigation of the role of the GABRG2 gene variant in migraine. J Neurol Sci 318:112–114
Nicolodi M, Del Bianco E (1990) Sensory neuropeptides (substance P, calcitonin gene-related peptide) and vasoactive intestinal polypeptide in human saliva: their pattern in migraine and cluster headache. Cephalalgia 10:39–50
Jang MU, Park JW, Kho HS et al (2011) Plasma and saliva levels of nerve growth factor and neuropeptides in chronic migraine patients. Oral Dis 17:187–193
Sicuteri F, Fanciullacci M, Geppetti P et al (1985) Substance P mechanism in cluster headache: evaluation in plasma and cerebrospinal fluid. Cephalalgia 5:143–149
Nakano T, Shimomura T, Takahashi K et al (1993) Platelet substance P and 5-hydroxytryptamine in migraine and tension-type headache. Headache 33:528–532
Gallai V, Sarchielli P, Firenze C et al (1994) Endothelin 1 in migraine and tension-type headache. Acta Neurol Scand 89:47–55
Kallela M, Färkkilä M, Saijonmaa O et al (1998) Endothelin in migraine patients. Cephalalgia 18:329–332
Hasselblatt M, Köhler J, Volles E et al (1999) Simultaneous monitoring of endothelin-1 and vasopressin plasma levels in migraine. Neuroreport 10:423–425
Nattero G, Mengozzi G, Inconis T et al (1996) Nitric oxide, endothelin-1, and transcranial Doppler in migraine. Findings in interictal conditions and during migraine attack. Headache 36:307–311
Hamed SA, Hamed EA, Ezz Eldin AM et al (2010) Vascular risk factors, endothelial function, and carotid thickness in patients with migraine: relationship to atherosclerosis. J Stroke Cerebrovasc Dis 19:92–103
Borsook D, Erpelding N, Lebel A et al (2014) Sex and the migraine brain. Neurobiol Dis 68:200–214
Mor G, Nilsen J, Horvath T et al (1999) Estrogen and microglia: a regulatory system that affects the brain. J Neurobiol 40:484–496
Alstadhaug KB (2009) Migraine and the hypothalamus. Cephalalgia 29:809–817
Lieba-Samal D, Wöber C (2011) Sex hormones and primary headaches other than migraine. Curr Pain Headache Rep 15:407–414
Kemper RH, Meijler WJ, Korf J et al (2001) Migraine and function of the immune system: a meta-analysis of clinical literature published between 1966 and 1999. Cephalalgia 21:549–557
Mortimer MJ, Kay J, Gawkrodger DJ et al (1993) The prevalence of headache and migraine in atopic children: an epidemiological study in general practice. Headache 33:427–431
Gazerani P, Pourpak Z, Ahmadiani A et al (2003) A correlation between migraine, histamine and immunoglobulin e. Scand J Immunol 57:286–290
Boćkowski L, Sobaniec W, Zelazowska-Rutkowska B (2009) Proinflammatory plasma cytokines in children with migraine. Pediatr Neurol 41:17–21
Boćkowski L, Smigielska-Kuzia J, Sobaniec W et al (2010) Anti-inflammatory plasma cytokines in children and adolescents with migraine headaches. Pharmacol Rep 62:287–291
Forcelini CM, Dantas DCM, Luz C et al (2011) Analysis of leukocytes in medication-overuse headache, chronic migraine and episodic migraine. Headache 51:1228–1238
Grazzi L, Corsini E, Ciusani E et al (2014) Evaluation of immune parameters in chronic migraine with medication overuse. Neurol Sci 35(Suppl 1):171–173
Bø SH, Davidsen EM, Gulbrandsen P et al (2009) Cerebrospinal fluid cytokine levels in migraine, tension-type headache and cervicogenic headache. Cephalalgia 29:365–372
Levy D (2012) Endogenous mechanisms underlying the activation and sensitization of meningeal nociceptors: the role of immuno-vascular interactions and cortical spreading depression. Curr Pain Headache Rep 16(3):270–277
Sarchielli P, Alberti A, Baldi A et al (2006) Proinflammatory cytokines, adhesion molecules, and lymphocyte integrin expression in the internal jugular blood of migraine patients without aura assessed ictally. Headache 46:200–207
Sarchielli P, Alberti A, Vaianella L et al (2004) Chemokine levels in the jugular venous blood of migraine without aura patients during attacks. Headache 44:961–968
Gursoy-Ozdemir Y, Qiu J, Matsuoka N et al (2004) Cortical spreading depression activates and upregulates MMP-9. J Clin Invest 113:1447–1455
Shimomura T, Araga S, Esumi E et al (1991) Decreased serum interleukin-2 level in patients with chronic headache. Headache 31:310–313
Della Vedova C, Cathcart S, Dohnalek A et al (2013) Peripheral interleukin-1ß levels are elevated in chronic tension-type headache patients. Pain Res Manag 18:301–306
Fernández-de-Las-Peñas C, Fernández-Mayoralas DM, Arroyo-Morales M et al (2011) Lower immunglobulin A levels but not lower cortisol or α-amylase activity in children with chronic tension-type headache. Cephalalgia 31:481–487
Martelletti P, Stirparo G, De Stefano L et al (1987) Reduced activity of the NK cells from patients with cluster headache and the “in vitro” response to beta-interferon. Headache 27:548–551
Giacovazzo M, Stirparo G, DeStefano L et al (1989) Lymphokine-activated killer (LAK) cell phenomenon in cluster headache. “In vitro” activation by recombinant interleukin-2. Headache 29:177–179
Stirparo G, Martelletti P, Giacovazzo M et al (1992) Impairment of cytolytic and proliferative responsiveness of peripheral blood mononuclear cells from cluster headache patients to IL-2. Pharmacol Res 26(Suppl 2):194–195
Bussone G, Salmaggi A, Leone M et al (1992) Immunological alterations in cluster headache during remission and cluster period. Comparison with low back pain patients. Cephalalgia 12:250–253
Galeotti N, Ghelardini C, Zoppi M et al (2001) Hypofunctionality of Gi proteins as aetiopathogenic mechanism for migraine and cluster headache. Cephalalgia 21:38–45
Martelletti P, Granata M, Giacovazzo M (1993) Serum interleukin-1 beta is increased in cluster headache. Cephalalgia 13:343–345
Empl M, Förderreuther S, Schwarz M, Müller N, Straube A (2003) Soluble interleukin-2 receptors increase during the active periods in cluster headache. Headache 43(1):63–68
Rozen T, Swidan SZ (2007) Elevation of CSF tumor necrosis factor alpha levels in new daily persistent headache and treatment refractory chronic migraine. Headache 47:1050–1055
Durlach J (1976) Neurological manifestations of magnesium imbalance. In: Vinken PJ, Bruyn GW (eds) Handbook of clinical neurology. North-Holland Publishing Co, Amsterdam, pp 545–579
Trauninger A, Pfund Z, Koszegi T et al (2002) Oral magnesium load test in patients with migraine. Headache 42:114–119
Ramadan NM, Halvorson H, Vande-Linde A et al (1989) Low brain magnesium in migraine. Headache 29:590–593
Lodi R, Iotti S, Cortelli P et al (2001) Deficient energy metabolism is associated with low free magnesium in the brains of patients with migraine and cluster headache. Brain Res Bull 54:437–441
Schoenen J, Sianard-Gainko J, Lenaerts M (1991) Blood magnesium levels in migraine. Cephalalgia 11:97–99
Smeets MC, Vernooy CB, Souverijn JH et al (1994) Intracellular and plasma magnesium in familial hemiplegic migraine and migraine with and without aura. Cephalalgia 14:29–32
Sarchielli P, Coata G, Firenze C et al (1992) Serum and salivary magnesium levels in migraine and tension-type headache. Results in a group of adult patients. Cephalalgia 12:21–27
Aloisi P, Marrelli A, Porto C et al (1997) Visual evoked potentials and serum magnesium levels in juvenile migraine patients. Headache 37:383–385
Gallai V, Sarchielli P, Morucci P et al (1993) Red blood cell magnesium levels in migraine patients. Cephalalgia 13:94–98
Gallai V, Sarchielli P, Morucci P et al (1994) Magnesium content of mononuclear blood cells in migraine patients. Headache 34:160–165
Mauskop A, Altura BT, Cracco RQ et al (1993) Deficiency in serum ionized magnesium but not total magnesium in patients with migraines. Possible role of ICa2+/IMg2+ ratio. Headache 33:135–138
Mauskop A, Altura BT, Altura BM (2002) Serum ionized magnesium levels and serum ionized calcium/ionized magnesium ratios in women with menstrual migraine. Headache 42:242–248
Facchinetti F, Sances G, Borella P et al (1991) Magnesium prophylaxis of menstrual migraine: effects on intracellular magnesium. Headache 31:298–301
Peikert A, Wilimzig C, Kohne-Volland R (1996) Prophylaxis of migraine with oral magnesium: results from a prospective, multicenter, placebo-controlled and double-blind randomized study. Cephalalgia 16:257–263
Pfaffenrath V, Wessely P, Meyer C et al (1996) Magnesium in the prophylaxis of migraine-A double-blind, placebo-controlled study. Cephalalgia 16:436–440
Wang F, Van Den Eeden SK, Ackerson LM (2003) Oral magnesium oxide prophylaxis of frequent migraine headache in children: a randomized, double-blind, placebo-controlled trial. Headache 43:601–610
Bigal ME, Bordini CA, Tepper SJ et al (2002) Intravenous magnesium sulphate in the acute treatment of migraine without aura and migraine with aura. A randomized, double-blind, placebo-controlled study. Cephalalgia 22:345–353
Rozen TD (2003) Aborting a prolonged migrainous aura with intravenous prochlorperazine and magnesium sulfate. Headache 43:901–903
Mauskop A, Altura BT, Cracco RQ, Altura BM (1995) Intravenous magnesium sulfate relieves cluster headaches in patients with low serum ionized magnesium levels. Headache 35:597–600
Mauskop A, Varughese J (2012) Why all migraine patients should be treated with magnesium. J Neural Transm 119:575–579
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Sarchielli, P., Caproni, S., Costa, C., Szok, D., Tajti, J. (2015). Biochemistry of Primary Headaches. In: Ashina, M., Geppetti, P. (eds) Pathophysiology of Headaches. Headache. Springer, Cham. https://doi.org/10.1007/978-3-319-15621-7_10
Download citation
DOI: https://doi.org/10.1007/978-3-319-15621-7_10
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-15620-0
Online ISBN: 978-3-319-15621-7
eBook Packages: MedicineMedicine (R0)