Abstract
Perfluorooctanoic acid (PFOA) is an 8-carbon fully fluorinated chemical that has reported effects on endocrine-related systems in rodents, humans, and other species. Numerous endocrine organs may be targets for PFOA, including the brain, thyroid, pancreas, adipose tissue, ovary, uterus, testes, and breast. Developmental exposure effects have been reported on behavior, serum thyroid and gonadal steroid profiles, breast epithelial growth, and metabolic end points, such as serum insulin, leptin, and triglyceride levels and weight gain. Many of these PFOA-induced effects have been reported in two or more species. The mechanisms for these numerous effects are poorly understood and deserve further investigation to define the pathways that should be avoided as PFOA-replacement products enter the market.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
11.1 Introduction
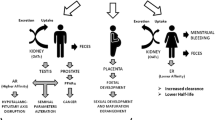
Perfluoroalkyl acids (PFAAs) are chemicals with carbon chains that are completely fluorinated. There are a wide variety of PFAAs in the environment that could have potential endocrine effects, but there has been minimal research on most PFAAs; this chapter will focus on the endocrine-related effects of perfluorooctanoic acid (PFOA). PFOA is persistent, lipophobic, can bind proteins, and is highly detectable in wildlife and human serum (White et al. 2011a). This chapter will focus on developmental exposures and their long-term effects. There has not been enough research on the other PFAAs to include for this focus. The exact mechanism(s) of action for endocrine-related effects of PFOA has not been discovered; however it has been shown that a variety of target tissues and health outcomes from PFOA exposure are endocrine-related such as mammary gland, thyroid, and adipose tissue (Lau et al. 2003; Macon et al. 2011; White et al. 2007; Hines et al. 2009) (see Fig. 11.1).
Perfluorooctanoic acid (PFOA) is known to have effects on numerous endocrine targets. Effects of the chemical have been associated with brain, pancreas, thyroid, breast, adipose, ovarian and uterine effects in female rodent research models and in women for some of the listed outcomes. Endocrine effects have also been reported in males, but there is less data available
There are key times in development where exposure to chemicals can have a long term effect. Developing infants from the fetal period to the prepubertal period are sensitive to environmental toxins because of the high growth rate (cell proliferation and differentiation) that takes place during this period (Birnbaum and Fenton 2003). Each time a cell proliferates or divides it offers an opportunity for mistakes in genomic repair, methylation, or programming to occur which may translate to growth of abnormal cells; thus the result may be cancer (Birnbaum and Fenton 2003). The placenta protects against the transmission of some compounds from the mother’s circulation into fetal circulation, however PFOA can bypass this protective mechanism (Fei et al. 2007; Gutzkow et al. 2012; Inoue et al. 2004). Early-life exposures may predispose individuals to more chronic adverse effects than later life exposures when rapid cell division no longer occurs (Landrigan et al. 2002). Therefore, exposure to PFOA that occurs during gestation and through the time of puberty is concerning in that it may reprogram endocrine-related signaling and result in long-term adverse health effects.
PFOA has been the target of much animal and human epidemiological research. Animal research primarily utilized mice because the elimination kinetics of PFOA in mice is more similar than rats to that of humans. Rats have a shorter PFOA elimination half-life than mice, and demonstrate a rapid elimination in reproductive-aged females, resulting in differences in excretion between males and females that do not lend well to developmental toxicity studies (Fenton et al. 2009; Lau et al. 2007). There are numerous studies that have evaluated human PFOA exposures because it is found in more than 99 % of the general U.S. population through contamination of food, water, house dust, and air (Winquist et al. 2013). One particular study, the C8 Health Project (C8HP), focuses on highly-exposed residents of the Mid-Ohio Valley (Winquist et al. 2013). The C8HP includes two cohorts: one made up of community members 20 years and older and a separate group comprised of occupationally-exposed individuals (Winquist et al. 2013). These cohorts, and others like them around the world, provide vital insight into the human effects correlated with varying levels of PFOA exposure.
11.2 Gestational Exposure and Pregnancy Outcome
Adult PFOA exposure primarily occurs through intake of contaminated food and water while transplacental transfer and breast milk are important routes of exposure for fetuses and infants (Mondal et al. 2012; Haug et al. 2011; Fromme et al. 2010). PFOA has the highest placental passing ratio of all the PFAAs (0.89:1), indicating that it can pass easily through the placental barrier and into the fetal environment, and may result in fetal exposures that are equivalent to years of exposure by the mother (Liu et al. 2011). The transfer ratio of PFOA was two times higher than that of perfluorooctane sulfonate (PFOS), another highly studied PFAA (PFOS ratio was 0.54:1) (Liu et al. 2011).
One way to measure human gestational PFOA exposure is to analyze its concentration in umbilical cord blood and to compare that to maternal serum levels. Declining serum levels of PFOA can be detected in the first trimester of pregnancy in women (Javins et al. 2013), indicating an early life transfer of serum PFOA to the developing fetus. Maternal sera and cord blood measurements for PFOA are reported to be strongly correlated (R > 0.5; p < 0.01) (Kim et al. 2011), and one study found higher PFOA concentrations in cord blood than in maternal serum with a cord blood:maternal PFOA ratio of 1.3 (Midasch et al. 2007). Most studies, however, report higher PFOA levels in maternal serum than in umbilical cord blood with ratios ranging from 0.67 to 0.87 for paired samples (umbilical serum:maternal serum) (Fei et al. 2007; Hanssen et al. 2010; Inoue et al. 2004; Kim et al. 2011; Monroy et al. 2008; Needham et al. 2011). World-wide, PFOA has been detected in cord blood from the general population in the U.S., Canada, Germany, Norway, Australia, South Africa, Korea, and Taiwan in concentrations ranging from 1.1 to 4.4 ng/ml (Post et al. 2012). These data provide strong evidence for transplacental transfer of PFOA between maternal and fetal circulation.
Developmental toxicology studies have found that PFOA has the potential to affect fetal growth and development. In studies designed to understand the transfer of PFOA from dam to fetus/neonate, CD-1 mice were given a single exposure to PFOA late in gestation (gestational day [GD] 17). This exposure did not affect the number of live fetuses, implantation sites, or weight of live-born pups on GD18 or post natal day (PND) 1 (Fenton et al. 2009). Interestingly, although dam serum concentrations were significantly greater than amniotic fluid levels collected the morning before birth, pups that were just a few hours old exhibited a significantly higher serum PFOA concentration than that of their dam due to the degree of placental transfer, and possibly from a short suckling period (Fenton et al. 2009). The levels of PFOA in mouse milk were 20–40 % that of maternal serum in early and late lactation when milk and blood volumes are low, relative to peak lactation, when milk PFOA levels were 10–20 % that in the maternal serum. Milk transfer of PFOA in rats (Hinderliter et al. 2005) and humans (Karrman et al. 2007) is reportedly less efficient than the mouse, with PFOA milk:maternal serum distribution ratios of 0.1 and 0.01, respectively.
There are a range of adverse developmental outcomes associated with PFOA in rodent models including decreased fetal weight and increased neonatal mortality (Lau et al. 2007; Olsen et al. 2009). PFOA effects in mice often occur in a dose-dependent manner. One study exposed pregnant mice to PFOA (doses from 1 to 40 mg/kg) from GD 1 through GD 17 (Lau et al. 2006). The exposures did not impact the number of implantations but there were significant increases in the number of full-litter resorptions starting at 5 mg/kg (Lau et al. 2006). There were no significant differences in live pup weights at doses at or equal to 10 mg/kg and significant prenatal losses were observed at the 20 mg/kg dose (Lau et al. 2006). Although most pups were born alive, there were increases in the incidences of stillbirth and neonatal mortality at doses of 5 mg/kg and higher (p < 0.05) (Lau et al. 2006). At lower doses (1 and 3 mg/kg), there were significant changes in postnatal growth and development, particularly in the form of growth retardation and delayed eye opening (Lau et al. 2006). These doses are much higher than ordinary human exposures.
As stated previously, PFOA transfer to the developing fetus can be detected in the first trimester of pregnancy for humans (Javins et al. 2013). The first trimester marks a period of time where there is abundant fetal development where the nervous, cardiovascular, digestive, respiratory, renal, and endocrine systems are forming, which makes perturbations during this time period potentially catastrophic (Javins et al. 2013). Although there has yet to be a thorough investigation of the endocrine-disrupting effects of perfluorinated compounds, there are numerous endocrine-related outcomes that are correlated with PFOA serum levels during pregnancy and birth. Pregnancy is a time of particular interest not only because of potential developmental insults to the fetus, but also because it can result in adverse pregnancy outcomes. Preterm birth (before 37 weeks gestation), pregnancy-induced hypertension (PIH), and low birth weight (less than 2,500 g) are often described as adverse pregnancy outcomes, all of which are linked to endocrine system dysregulation. There was little evidence of an association between PFOA and preterm birth or low birth weight among the C8HP cohort (Darrow et al. 2013), but two studies found modest inverse associations between birth weight and maternal PFOA concentration (Apelberg et al. 2007; Fei et al. 2007). These results have not been repeated in other studies (Savitz et al. 2012; Monroy et al. 2008; Washino et al. 2009; Nolan et al. 2009). However, a recent meta-analysis was performed on all of the available data from animal and human studies related to birth weight and PFOA exposures. The in-depth analyses indicated a significant −0.023 g reduction in birth weight of offspring of non-human mammalian species for each unit (mg/kg birth weight/day) increase in PFOA dose (Lam et al. 2014). The human data analysis, with numerous studies analyzed together, resulted in ‘sufficient’ evidence of PFOA negatively affecting fetal growth (Lam et al. 2014). Prior to this meta-analysis, there was no summary of human data to support an association between PFOA exposure and clinically significant endpoints of growth restriction.
To date there is no evidence to link PFOA exposures with preterm delivery in women (Savitz et al. 2012; Fei et al. 2007; Hamm et al. 2010; Darrow et al. 2013); however one of the endocrine-related health outcomes that may lead to preterm delivery, PIH, has been associated with PFOA exposures. An association between increased maternal PFOA levels and PIH in the C8HP cohort was reported based upon log-transformed and categorical analyses (27 % increased odds with 95 % CI) (Darrow et al. 2013). Modest associations between serum PFOA and self-reported preeclampsia have been reported (Stein et al. 2009), but this finding associating PFOA and PIH was not evident in a study involving birth records (Savitz et al. 2012) or in a Norwegian cohort with background PFAA levels (Starling et al. 2014a). Savitz and colleagues (2012) have suggested that the maternal physiology is a more important determinant of pregnancy outcomes than the degree of PFOA exposure. Therefore maternal age, parity, and BMI all impact the absorption and elimination and can determine the amount of exposure the fetus receives from any chemical which makes causal associations difficult to verify (Savitz et al. 2012).
11.3 Lactation
Lactation is a physiological state that is regulated and maintained by the endocrine system and endocrine disruptors or chemicals that interact with the endocrine system can alter mammals’ ability to lactate. Data indicate an association between the length of time a woman has lived near a source of PFAA contamination and the level of PFOA in her breast milk (von Ehrenstein et al. 2009). There is evidence that not only will PFOA be eliminated in milk to potentially affect the offspring, but it may also affect breast function. Animal models indicate that there are changes in maternal mammary gland structure after developmental PFOA exposure and that PFOA exposure inhibits production of normal milk proteins, leading to increased pup mortality (White et al. 2011a, b; Lau et al. 2006). These data are further described in Chap. 8 in this book.
Many chemicals can pass through the maternal system and be transferred to the infant through breast milk. As previously mentioned, human studies have found that there is not only placental transfer of PFOA but that lactational transfer occurs and may provide the majority of infant PFOA exposure (Inoue et al. 2004; Apelberg et al. 2007; Monroy et al. 2008; Tao et al. 2008; Mondal et al. 2012; Fei et al. 2007; Fromme et al. 2010; Kim et al. 2011; Needham et al. 2011). Epidemiological studies focusing on the C8HP cohort found a higher child:maternal serum PFOA ratio in children (12 months old) whose mothers breast fed exclusively versus those who were breast and/or bottle fed (1.83 for breast feeding only and 1.14 for breast/bottle fed) (Mondal et al. 2012). Another study estimated that breast milk contributes over 83 % of infant PFOA exposure even though the concentration of PFOA measured in breast milk was low (Haug et al. 2011). A small survey of Italian mothers found that the highest levels of PFOA in milk came from primiparous women (Barbarossa et al. 2013). None of the findings were statistically significant because of the low number of participants, however it appears to indicate that PFOA concentrations in breast milk decrease after the first lactation and, therefore, first born infants may have higher exposures of PFOA (Barbarossa et al. 2013; Tao et al. 2008).
Exposure to PFOA via transplacental transfer and milk leads to an elevated body burden in humans and rodents. One study estimated that infant PFOA exposure through milk is 2,173 ng, which is much higher than the 183 ng PFOA received through gestational exposure (Liu et al. 2011). Higher exposure results in elevated PFOA body burden for infants, from birth through 6 months of age, compared to adults (Fromme et al. 2010). Mouse studies found a similar increased body burden from GD18 through PND8 and decreased between PND8 and 18 when milk intake decreased (Fenton et al. 2009). One study in particular has generated a great deal of useful data concerning elimination of PFOA into breast milk and the burden in the infant over time. Breastfeeding mothers in the C8HP were found to have a lower geometric mean PFOA concentration than non-breastfeeding mothers in the same cohort (Mondal et al. 2014). Consequently, the breastfed infants in the cohort had a higher PFOA concentration (geometric mean of 49 ng/ml) than non-breast fed infants (geometric mean of 22 ng/ml) (Mondal et al. 2014). Overall the serum PFOA concentration of breastfeeding mothers decreased 3 % per month of breastfeeding to culminate in an estimated 34 % decrease in maternal serum PFOA concentration after breastfeeding for 12 months (Mondal et al. 2014). The infants who were breastfed for 12 months had PFOA concentrations that were 141 % higher than their formula-fed counterparts (Mondal et al. 2014). Concerns regarding the comparison of findings between labs because of differences in limits of detection and accidental PFOA contamination by lab equipment (Mondal et al. 2012) may be minimal as a second recent study evaluating PFOA burden in adolescent girls in the San Francisco Bay and Greater Cincinnati areas also reported highly significant increases in serum PFOA and other PFAAs correlated with the duration that the child was breastfed (Pinney et al. 2014).
11.4 Puberty
Puberty is another vulnerable life stage where environmental influences have been linked with health problems. Severe pubertal delays may be a risk factor for infertility while moderate delays may predispose females to endometriosis, osteoporosis, and psychosocial issues (Lopez-Espinosa et al. 2011). Human epidemiological studies are inconsistent in regards to associations between PFOA exposures and pubertal timing. One study found that PFOA was associated with earlier puberty (Pinney et al. 2009); other studies find PFOA concentration to be associated with later time to first menstruation (Lopez-Espinosa et al. 2011; Kristensen et al. 2013); and yet another study found no relationship between pubertal timing and PFOA concentration (Christensen et al. 2011). It should be noted that there are specific differences in how pubertal timing is measured. Some studies measure pubertal attainment by breast development (Pinney et al. 2009), sex steroid hormone levels (Lopez-Espinosa et al. 2011), and self-reported age at menarche (Lopez-Espinosa et al. 2011; Christensen et al. 2011). Kristensen et al. (2013) studied a group of women who had in utero PFOA exposure. These women reached menarche 5.9 months later than a reference group with a lower PFOA exposure and found a statistically significant delay in menarche in relation to prenatal PFOA exposure (p = 0.01). There was no association between PFOA exposure and menstrual cycle length, reproductive hormone levels, or number of ovarian follicles among this cohort (Kristensen et al. 2013).
Mouse studies have found associations between PFOA concentration and altered ovarian function (altered hormone/steroid receptor levels), delayed vaginal opening at high doses, delayed mammary gland development (at the lowest doses tested), and histopathological changes in the reproductive tract that would indicate delays in pubertal timing (Yang et al. 2009; White et al. 2011a, b; Dixon et al. 2012; Zhao et al. 2012; Macon et al. 2011; Tucker et al. in press). These effects appear to be dependent on PFOA exposure level, timing of exposure, and there may be some effects that are dependent on strain sensitivity (Macon et al. 2011; Tucker et al. in press).
11.5 Subfecundity/Subfertility
Subfecundity, or prolonged time to conceive, is often a measure used to determine the fertility of females. Similar to other endocrine-related endpoints discussed earlier in this chapter, the epidemiological data related to PFOA and subfecundity are mixed. Two studies found an association between increased time to pregnancy and PFOA serum concentration (Whitworth et al. 2012; Fei et al. 2009), while another found no association (Vestergaard et al. 2012). The study by Whitworth et al. (2012) specified that the odds ratio for subfecundity was elevated only in parous women (OR for the highest quartile = 2.1), while no effect was seen in the nulliparous women (OR for the highest quartile = 0.5). The proportion of women diagnosed with infertility (longer than 12 months without conceiving) in the Danish National Birth Cohort study was higher in the three higher quartiles of PFOA exposure versus the lowest quartile, indicating that PFOA may permanently alter an endocrine-related mechanism required for conception (Fei et al. 2009).
The mechanism of action by which PFOA alters female fertility is unknown, however it has been hypothesized that it may be an interaction with the hypothalamic-pituitary-ovarian axis, which in turn could trigger irregular menstruation, altered time of ovulation, or early spontaneous abortions (Fei et al. 2009). There is potential for the mode of action for increased time to pregnancy associated with high PFOA levels to include PFOA-induced irregular menstrual cycles (Fei et al. 2009). A report of longer menstrual cycles associated with the highest tertile of PFOA exposure was recently published using pooled estimates of over 1,600 women from Poland, Greenland, and Ukraine (Lyngso et al. 2014).
11.6 Thyroid
The thyroid gland is an integral part of the hormone regulatory system. It is needed for normal metabolic function. Thyroperoxidase catalyzes the transfer of iodine during thyroid hormone synthesis and PFOA decreases the activity of this enzyme in a cell-based system (Song et al. 2012). PFOA can also interfere with thyroid hormone levels and the sensitive feedback mechanisms they are associated with. Monkeys treated with PFOA were found to have decreased thyroid hormones thyroxine (T4) and triiodothyronine (T3), without the expected increase in thyroid stimulating hormone (TSH) (Calafat et al. 2007). This suggests that PFOA may block thyroid hormones from their binding proteins. Studies focused on individuals occupationally exposed to PFOA have had variable findings. PFOA exposure has ranged from no association with thyroid function (Olsen et al. 1998), to weakly positive changes in T3 (Olsen and Zobel 2007), to significant associations with elevated T4 and reduced T3 uptake (Huang et al. 2011). The C8HP recently published strong evidence for validated thyroid disease in relation to PFOA exposure estimates in combined cohorts of residents and workers in the Mid-Ohio Valley (n > 32,000 participants and n > 4,000 with reported disease) (Winquist and Steenland 2014). The trend for PFOA-related disease was more pronounced among women and absent in men, with hypothyroidism being the predominate disease. PFOA has also been linked to hypothyroidism in children (Lopez-Espinosa et al. 2012). A small and subclinical association between elevated serum free T4 and PFOA, without a concomitant decrease in TSH, was found in a cohort of Chinese adolescents and young adults (Lin et al. 2013). The group hypothesizes that PFAA exposure caused a syndrome of reduced thyroid responsiveness to thyroid hormone or that there may be TSH hypersecretion from the pituitary (Lin et al. 2013). The mechanism of interference between PFOA and the hypothalamic-pituitary-thyroid axis is still unclear.
There is a significant increase in serum T4 and reduction in T3 uptake in adults with 1 or more years of PFOA exposure (Knox et al. 2011). Using National Health and Nutrition Exposure Survey (NHANES data), TSH levels were found to increase with PFOA concentration, indicating that PFOA is associated with subclinical hypothyroidism in adults (Jain 2013). There was also a slight decrease in total T4 levels and no increase in total T3 in relation to PFOA concentration among this group (Jain 2013). In a separate analysis, NHANES data also indicated that there was an association between PFOA serum concentrations and self-reported thyroid disease (Melzer et al. 2010). Women in the highest PFOA exposure quartile (>5.7 ng/mL) were more than twice as likely to exhibit thyroid disease as women in the lower quartiles (<4 ng/mL) (Melzer et al. 2010). The significant effect in women was not recapitulated in men, although the trend was similar (Melzer et al. 2010). Because PFOA predominantly has thyroid effects on women, it has implications for pregnant mothers where thyroid hormone dysregulation can alter gene expression and development of the fetal brain (Javins et al. 2013). In utero thyroid levels are also involved in programming future body weight and therefore PFOA dysregulation of thyroid hormones may have implications for obesity later in life (Grun and Blumberg 2009).
11.7 Obesity/Fat Tissue/Lipid Metabolism
Obesity is a global problem that affects people of all ages and ethnicities. Over 10 years ago, researchers began suggesting that the rise in obesity is correlated with the marked increase in the number of industrial chemicals on the market (Baillie-Hamilton 2002) and that over-eating and less physical activity are not valid explanations for the epidemic. There are approximately 85,000 chemicals on the U.S. market. Of these, approximately 2 % have been tested and several have proved to be endocrine disrupting compounds. It is widely accepted that adipose tissue is an endocrine organ that produces hormones that act on other tissues in the body. The main hormones produced within adipose tissue are leptin, adiponectin, steroids, and resistin (Guerre-Millo 2002; Harwood 2012). Leptin is produced by white adipose tissue to regulate food intake, metabolism, and puberty progression (Gueorguiev et al. 2001). Low leptin levels or leptin resistance is related to adult overweight and obesity in a variety of animal models after developmental exposures to chemicals including PFOA (Newbold 2010).
A toxicology study in CD-1 mice investigated the metabolic effects of gestational and lactational PFOA exposure. They exposed mice to low doses of PFOA (0.01–5 mg/kg/day) during pregnancy and found that the doses of 0.01 mg/kg/day to 0.1 mg/kg/day induced elevated leptin, insulin, and body weight while the 1 mg/kg/day and 5 mg/kg/day doses caused decreased body weight after female offspring reached 10 weeks of age (Hines et al. 2009). Further, removing the ovaries prior to puberty prevented the body weight-related effects of the PFOA exposure indicating that the ovarian axis plays an important role in the PFOA-related metabolic effects (Hines et al. 2009). This study also dosed adult mice and found no effect on body weight related to PFOA exposure which indicates that gestation and early life is a vital window for these PFOA effects (Hines et al. 2009).
A human study by Halldorsson and coworkers (2012) found maternal PFOA concentration to be positively associated with body mass index (BMI) and waist circumference among 20-year old female offspring of women who had PFOA exposure during their pregnancy (p < 0.05; n = 345). The women whose mothers were in the highest quartile of PFOA exposure had a BMI that was 1.6 kg/m2 higher and a waist circumference that was 4.3 cm bigger than those females whose moms were in the lowest quartile (Halldorsson et al. 2012). There was no statistical difference between the males. Further, similar to the Hines study (2009), increased maternal PFOA levels were associated with increased insulin, leptin, and leptin-adiponectin ratio and inversely associated with adiponectin (Halldorsson et al. 2012).
One potential mechanism of action for these findings includes in utero PFOA exposure possibly interfering with ovary development or function which can lead to impaired estrogen synthesis (Hines et al. 2009). Another hypothesis is that PFOA interacts with peroxisome proliferator activated receptors (PPAR) alpha or gamma, signal transducers which are important in lipid metabolism in fat cells (Hines et al. 2009). Recent data utilizing prepubertal mammary tissue (mostly fat) suggests that PPAR gamma may indeed be an important modulator of effect following PFOA exposure (Macon et al. in press).
There are other important metabolic factors that are not directly weight-related, including cholesterol/triglyceride levels. Numerous studies have reported a positive association between PFOA levels and total cholesterol or LDL levels in humans (Nelson et al. 2010; Frisbee et al. 2010). A highly exposed occupational group not only had increased cholesterol in relation to PFOA exposure but they also exhibited increased triglycerides and lower HDL (the good cholesterol) (Olsen et al. 1998). The Norwegian Mother and Child Cohort Study, demonstrating low level exposures, found no evidence of an association between elevated triglycerides and PFAA concentrations in pregnant women (Starling et al. 2014b), suggesting that this may be an exposure-related effect.
In mice, however, serum cholesterol and PFOA are inversely correlated which indicates that there are different mechanisms of action for PFOA effects on cholesterol between mice and humans (White et al. 2011a; Quist et al. in press). Obesity and cholesterol-related heart disease are major contributors to adult morbidity so any modifying factors, such as preventing or limiting chemical exposures, could be potentially important in long term health and healthcare delivery systems.
11.8 Men
Much of the epidemiology and toxicology studies have focused primarily on female-related endpoints. Although fetal exposure to PFOA is inevitable there seems to be less focus on male outcomes. However, male mice who were developmentally exposed to >1 mg/kg/day PFOA may exhibit early onset puberty (Lau et al. 2006).
A longitudinal study of sons of women who were recruited during pregnancy was conducted by Vested et al. (2013) to determine if in utero PFOA exposure is related to semen quality and reproductive male hormone levels. The study found no relationship between the gestational PFOA exposure and abnormal spermatozoa morphology. This finding may be related to the fact that morphology and motility of spermatozoa are determined in adolescence and adulthood during sperm production. Two other studies focusing on PFOA and PFOS in combination found that the chemicals were negatively associated with the percentage of morphologically normal spermatozoa (Joensen et al. 2009; Toft et al. 2012). Statistically significant associations between PFOA and sperm count, sperm concentration, and LH were obtained after transforming data to obtain a normal distribution and correcting for confounders (Vested et al. 2013). There was a positive association between gestational PFOA exposure and LH and FSH in adulthood related to the idea that high gonadotropin concentrations are associated with low sperm concentration and sperm count (Vested et al. 2013; Appasamy et al. 2007; Gordetsky et al. 2012). Further studies need to be done to determine the male-specific effects of PFOA exposure, as the work by Vested et al. (2013) suggests that the fetal male reproductive system may be impacted by maternal PFOA exposures.
11.9 Conclusions
Although there has been little focus on PFOA as an endocrine disruptor, per se, there are numerous significant correlations between PFOA exposures and endocrine-related disease states (Fig. 11.1). Future studies should focus on modes of action in animal and human studies to identify the similar effect pathways. This will enable industry to design replacement chemicals that do not perturb those pathways and may in turn be a safer product.
Additional attention should be given to the timing of PFAA exposure and the disease end point, as the exposure that had the most impact on the end point may have been months, years or decades earlier, during a critical period of development for the endocrine-related tissue of interest. PFAA measurements made during meaningful life stages should be compared to latent disease end points. Further research is needed to determine the role of PFOA on many health outcomes, but the effects on the thyroid and adipose tissue are developing a weight of evidence to solidify a space for PFOA on the growing list of endocrine disrupting chemicals.
References
Apelberg BJ, Witter FR, Herbstman JB, Calafat AM, Halden RU, Needham LL, Goldman LR (2007) Cord serum concentrations of perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) in relation to weight and size at birth. Environ Health Perspect 115:1670–1676
Appasamy M, Muttukrishna S, Pizzey AR, Ozturk O, Groome NP, Serhal P, Jauniaux E (2007) Relationship between male reproductive hormones, sperm DNA damage and markers of oxidative stress in infertility. Reprod Biomed Online 14:159–165
Baillie-Hamilton PF (2002) Chemical toxins: a hypothesis to explain the global obesity epidemic. J Altern Complement Med 8:185–192
Barbarossa A, Masetti R, Gazzotti T, Zama D, Astolfi A, Veyrand B, Pession A, Pagliuca G (2013) Perfluoroalkyl substances in human milk: a first survey in Italy. Environ Int 51:27–30
Birnbaum LS, Fenton SE (2003) Cancer and developmental exposure to endocrine disruptors. Environ Health Perspect 111:389–394
Calafat AM, Wong LY, Kuklenyik Z, Reidy JA, Needham LL (2007) Polyfluoroalkyl chemicals in the U.S. population: data from the National Health and Nutrition Examination Survey (NHANES) 2003–2004 and comparisons with NHANES 1999–2000. Environ Health Perspect 115:1596–1602
Christensen KY, Maisonet M, Rubin C, Holmes A, Calafat AM, Kato K, Flanders WD, Heron J, Mcgeehin MA, Marcus M (2011) Exposure to polyfluoroalkyl chemicals during pregnancy is not associated with offspring age at menarche in a contemporary British cohort. Environ Int 37:129–135
Darrow LA, Stein CR, Steenland K (2013) Serum perfluorooctanoic acid and perfluorooctane sulfonate concentrations in relation to birth outcomes in the Mid-Ohio Valley, 2005–2010. Environ Health Perspect 121:1207–1213
Dixon D, Reed CE, Moore AB, Gibbs-Flournoy EA, Hines EP, Wallace EA, Stanko JP, Lu Y, Jefferson WN, Newbold RR, Fenton SE (2012) Histopathologic changes in the uterus, cervix and vagina of immature CD-1 mice exposed to low doses of perfluorooctanoic acid (PFOA) in a uterotrophic assay. Reprod Toxicol 33:506–512
Fei C, Mclaughlin JK, Tarone RE, Olsen J (2007) Perfluorinated chemicals and fetal growth: a study within the Danish National Birth Cohort. Environ Health Perspect 115:1677–1682
Fei C, Mclaughlin JK, Lipworth L, Olsen J (2009) Maternal levels of perfluorinated chemicals and subfecundity. Hum Reprod 24:1200–1205
Fenton SE, Reiner JL, Nakayama SF, Delinsky AD, Stanko JP, Hines EP, White SS, Lindstrom AB, Strynar MJ, Petropoulou SS (2009) Analysis of PFOA in dosed CD-1 mice. Part 2. Disposition of PFOA in tissues and fluids from pregnant and lactating mice and their pups. Reprod Toxicol 27:365–372
Frisbee SJ, Shankar A, Knox SS, Steenland K, Savitz DA, Fletcher T, Ducatman AM (2010) Perfluorooctanoic acid, perfluorooctanesulfonate, and serum lipids in children and adolescents: results from the C8 Health Project. Arch Pediatr Adolesc Med 164:860–869
Fromme H, Mosch C, Morovitz M, Alba-Alejandre I, Boehmer S, Kiranoglu M, Faber F, Hannibal I, Genzel-Boroviczeny O, Koletzko B, Volkel W (2010) Pre- and postnatal exposure to perfluorinated compounds (PFCs). Environ Sci Technol 44:7123–7129
Gordetsky J, Van Wijngaarden E, O’brien J (2012) Redefining abnormal follicle-stimulating hormone in the male infertility population. BJU Int 110:568–572
Grun F, Blumberg B (2009) Endocrine disrupters as obesogens. Mol Cell Endocrinol 304:19–29
Gueorguiev M, Goth ML, Korbonits M (2001) Leptin and puberty: a review. Pituitary 4:79–86
Guerre-Millo M (2002) Adipose tissue hormones. J Endocrinol Invest 25:855–861
Gutzkow KB, Haug LS, Thomsen C, Sabaredzovic A, Becher G, Brunborg G (2012) Placental transfer of perfluorinated compounds is selective – a Norwegian Mother and Child sub-cohort study. Int J Hyg Environ Health 215:216–219
Halldorsson TI, Rytter D, Haug LS, Bech BH, Danielsen I, Becher G, Henriksen TB, Olsen SF (2012) Prenatal exposure to perfluorooctanoate and risk of overweight at 20 years of age: a prospective cohort study. Environ Health Perspect 120:668–673
Hamm MP, Cherry NM, Chan E, Martin JW, Burstyn I (2010) Maternal exposure to perfluorinated acids and fetal growth. J Expo Sci Environ Epidemiol 20:589–597
Hanssen L, Rollin H, Odland JO, Moe MK, Sandanger TM (2010) Perfluorinated compounds in maternal serum and cord blood from selected areas of South Africa: results of a pilot study. J Environ Monit 12:1355–1361
Harwood HJ Jr (2012) The adipocyte as an endocrine organ in the regulation of metabolic homeostasis. Neuropharmacology 63:57–75
Haug LS, Huber S, Becher G, Thomsen C (2011) Characterisation of human exposure pathways to perfluorinated compounds–comparing exposure estimates with biomarkers of exposure. Environ Int 37:687–693
Hinderliter PM, Mylchreest E, Gannon SA, Butenhoff JL, Kennedy GL Jr (2005) Perfluorooctanoate: placental and lactational transport pharmacokinetics in rats. Toxicology 211:139–148
Hines EP, White SS, Stanko JP, Gibbs-Flournoy EA, Lau C, Fenton SE (2009) Phenotypic dichotomy following developmental exposure to perfluorooctanoic acid (PFOA) in female CD-1 mice: low doses induce elevated serum leptin and insulin, and overweight in mid-life. Mol Cell Endocrinol 304:97–105
Huang YF, Wu KY, Liou SH, Uang SN, Chen CC, Shih WC, Lee SC, Huang CC, Chen ML (2011) Biological monitoring for occupational acrylamide exposure from acrylamide production workers. Int Arch Occup Environ Health 84:303–313
Inoue K, Okada F, Ito R, Kato S, Sasaki S, Nakajima S, Uno A, Saijo Y, Sata F, Yoshimura Y, Kishi R, Nakazawa H (2004) Perfluorooctane sulfonate (PFOS) and related perfluorinated compounds in human maternal and cord blood samples: assessment of PFOS exposure in a susceptible population during pregnancy. Environ Health Perspect 112:1204–1207
Jain RB (2013) Association between thyroid profile and perfluoroalkyl acids: data from NHNAES 2007–2008. Environ Res 126:51–59
Javins B, Hobbs G, Ducatman AM, Pilkerton C, Tacker D, Knox SS (2013) Circulating maternal perfluoroalkyl substances during pregnancy in the C8 Health Study. Environ Sci Technol 47:1606–1613
Joensen UN, Bossi R, Leffers H, Jensen AA, Skakkebaek NE, Jorgensen N (2009) Do perfluoroalkyl compounds impair human semen quality? Environ Health Perspect 117:923–927
Karrman A, Ericson I, Van Bavel B, Darnerud PO, Aune M, Glynn A, Lignell S, Lindstrom G (2007) Exposure of perfluorinated chemicals through lactation: levels of matched human milk and serum and a temporal trend, 1996–2004, in Sweden. Environ Health Perspect 115:226–230
Kim SK, Lee KT, Kang CS, Tao L, Kannan K, Kim KR, Kim CK, Lee JS, Park PS, Yoo YW, Ha JY, Shin YS, Lee JH (2011) Distribution of perfluorochemicals between sera and milk from the same mothers and implications for prenatal and postnatal exposures. Environ Pollut 159:169–174
Knox SS, Jackson T, Frisbee SJ, Javins B, Ducatman AM (2011) Perfluorocarbon exposure, gender and thyroid function in the C8 Health Project. J Toxicol Sci 36:403–410
Kristensen SL, Ramlau-Hansen CH, Ernst E, Olsen SF, Bonde JP, Vested A, Halldorsson TI, Becher G, Haug LS, Toft G (2013) Long-term effects of prenatal exposure to perfluoroalkyl substances on female reproduction. Hum Reprod 28:3337–3348
Lam J, Koustas E, Sutton P, Johnson PI, Atchley DS, Sen S (2014) The navigation guide – evidence-based medicine meets environmental health: integration of animal and human evidence for PFOA effects on fetal growth. Environ Health Perspect 122:1040–1051
Landrigan PJ, Sonawane B, Mattison D, Mccally M, Garg A (2002) Chemical contaminants in breast milk and their impacts on children’s health: an overview. Environ Health Perspect 110:A313–A315
Lau C, Thibodeaux JR, Hanson RG, Rogers JM, Grey BE, Stanton ME, Butenhoff JL, Stevenson LA (2003) Exposure to perfluorooctane sulfonate during pregnancy in rat and mouse. II: postnatal evaluation. Toxicol Sci 74:382–392
Lau C, Thibodeaux JR, Hanson RG, Narotsky MG, Rogers JM, Lindstrom AB, Strynar MJ (2006) Effects of perfluorooctanoic acid exposure during pregnancy in the mouse. Toxicol Sci 90:510–518
Lau C, Anitole K, Hodes C, Lai D, Pfahles-Hutchens A, Seed J (2007) Perfluoroalkyl acids: a review of monitoring and toxicological findings. Toxicol Sci 99:366–394
Lin CY, Wen LL, Lin LY, Wen TW, Lien GW, Hsu SH, Chien KL, Liao CC, Sung FC, Chen PC, Su TC (2013) The associations between serum perfluorinated chemicals and thyroid function in adolescents and young adults. J Hazard Mater 244–245:637–644
Liu J, Li J, Liu Y, Chan HM, Zhao Y, Cai Z, Wu Y (2011) Comparison on gestation and lactation exposure of perfluorinated compounds for newborns. Environ Int 37:1206–1212
Lopez-Espinosa MJ, Fletcher T, Armstrong B, Genser B, Dhatariya K, Mondal D, Ducatman A, Leonardi G (2011) Association of Perfluorooctanoic Acid (PFOA) and Perfluorooctane Sulfonate (PFOS) with age of puberty among children living near a chemical plant. Environ Sci Technol 45:8160–8166
Lopez-Espinosa MJ, Mondal D, Armstrong B, Bloom MS, Fletcher T (2012) Thyroid function and perfluoroalkyl acids in children living near a chemical plant. Environ Health Perspect 120:1036–1041
Lyngso J, Ramlau-Hansen CH, Hoyer BB, Stovring H, Bonde JP, Jonsson BA, Lindh CH, Pedersen HS, Ludwicki JK, Zviezdai V, Toft G (2014) Menstrual cycle characteristics in fertile women from Greenland, Poland and Ukraine exposed to perfluorinated chemicals: a cross-sectional study. Hum Reprod 29:359–367
Macon MB, Villanueva LR, Tatum-Gibbs K, Zehr RD, Strynar MJ, Stanko JP, White SS, Helfant L, Fenton SE (2011) Prenatal perfluorooctanoic acid exposure in CD-1 mice: low-dose developmental effects and internal dosimetry. Toxicol Sci 122:134–145
Macon MB, Ren H, Fenton SE Prenatal PFOA exposure alters gene expression pathways in neonate murine mammary gland. Toxicol Sci (in press) http://dx.doi.org/10.1093/toxsci/kfu253 (Epub ahead of print)
Melzer D, Rice N, Depledge MH, Henley WE, Galloway TS (2010) Association between serum perfluorooctanoic acid (PFOA) and thyroid disease in the U.S. National Health and Nutrition Examination Survey. Environ Health Perspect 118:686–692
Midasch O, Drexler H, Hart N, Beckmann MW, Angerer J (2007) Transplacental exposure of neonates to perfluorooctanesulfonate and perfluorooctanoate: a pilot study. Int Arch Occup Environ Health 80:643–648
Mondal D, Lopez-Espinosa MJ, Armstrong B, Stein CR, Fletcher T (2012) Relationships of perfluorooctanoate and perfluorooctane sulfonate serum concentrations between mother-child pairs in a population with perfluorooctanoate exposure from drinking water. Environ Health Perspect 120:752–757
Mondal D, Weldon RH, Armstrong BG, Gibson LJ, Lopez-Espinosa MJ, Shin HM, Fletcher T (2014) Breastfeeding: a potential excretion route for mothers and implications for infant exposure to perfluoroalkyl acids. Environ Health Perspect 122:187–192
Monroy R, Morrison K, Teo K, Atkinson S, Kubwabo C, Stewart B, Foster WG (2008) Serum levels of perfluoroalkyl compounds in human maternal and umbilical cord blood samples. Environ Res 108:56–62
Needham LL, Grandjean P, Heinzow B, Jorgensen PJ, Nielsen F, Patterson DG Jr, Sjodin A, Turner WE, Weihe P (2011) Partition of environmental chemicals between maternal and fetal blood and tissues. Environ Sci Technol 45:1121–1126
Nelson JW, Hatch EE, Webster TF (2010) Exposure to polyfluoroalkyl chemicals and cholesterol, body weight, and insulin resistance in the general U.S. population. Environ Health Perspect 118:197–202
Newbold RR (2010) Impact of environmental endocrine disrupting chemicals on the development of obesity. Hormones (Athens) 9:206–217
Nolan LA, Nolan JM, Shofer FS, Rodway NV, Emmett EA (2009) The relationship between birth weight, gestational age and perfluorooctanoic acid (PFOA)-contaminated public drinking water. Reprod Toxicol 27:231–238
Olsen GW, Zobel LR (2007) Assessment of lipid, hepatic, and thyroid parameters with serum perfluorooctanoate (PFOA) concentrations in fluorochemical production workers. Int Arch Occup Environ Health 81:231–246
Olsen GW, Gilliland FD, Burlew MM, Burris JM, Mandel JS, Mandel JH (1998) An epidemiologic investigation of reproductive hormones in men with occupational exposure to perfluorooctanoic acid. J Occup Environ Med 40:614–622
Olsen GW, Butenhoff JL, Zobel LR (2009) Perfluoroalkyl chemicals and human fetal development: an epidemiologic review with clinical and toxicological perspectives. Reprod Toxicol 27:212–230
Pinney SM, Windham GC, Biro FM, Kushi LH, Yaghjyan L, Calafat AM, Kato K, Succop P, Brown MK, Hernick A, Bornschein R (2009) Perfluorooctanoic acid (PFOA) and pubertal maturation in young girls. Epidemiology 20:S80
Pinney SM, Biro FM, Windham GC, Herrick RL, Yaghjyan L, Calafat AM, Succop P, Sucharew H, Ball KM, Kato K, Kushi LH, Bornschein R (2014) Serum biomarkers of polyfluoroalkyl compound exposure in young girls in Greater Cincinnati and the San Francisco Bay Area, USA. Environ Pollut 184:327–334
Post GB, Cohn PD, Cooper KR (2012) Perfluorooctanoic acid (PFOA), an emerging drinking water contaminant: a critical review of recent literature. Environ Res 116:93–117
Quist EM, Filgo AJ, Cummings C, Kissling GE, Hoenerhoff MJ, Fenton SE (in press) Hepatic mitochondrial alteration in CD-1 mice associated with prenatal exposures to low doses of perfluorooctanoic acid (PFOA). Toxicol Pathol. http://dx.doi.org/10.1177/0192623314551841 (Epub ahead of print)
Tucker DK, Macon MB, Strynar MJ, Dagnino S, Andersen E, Fenton SE (in press) The mammary gland is a sensitive pubertal target in CD-1 and C57Bl/6 mice following perinatal perfluorooctanoic acid (PFOA) exposure. Reprod Toxicol. http://dx.doi.org/10.1016/j.reprotox.2014.12.002 (Epub ahead of print)
Savitz DA, Stein CR, Elston B, Wellenius GA, Bartell SM, Shin HM, Vieira VM, Fletcher T (2012) Relationship of perfluorooctanoic acid exposure to pregnancy outcome based on birth records in the mid-Ohio Valley. Environ Health Perspect 120:1201–1207
Song M, Kim YJ, Park YK, Ryu JC (2012) Changes in thyroid peroxidase activity in response to various chemicals. J Environ Monit 14:2121–2126
Starling AP, Engel SM, Richardson DB, Baird DD, Haug LS, Stuebe AM, Klungsoyr K, Harmon Q, Becher G, Thomsen C, Sabaredzovic A, Eggesbo M, Hoppin JA, Travlos GS, Wilson RE, Trogstad LI, Magnus P, Longnecker MP (2014a) Perfluoroalkyl substances during pregnancy and validated preeclampsia among nulliparous women in the Norwegian Mother and Child Cohort Study. Am J Epidemiol 179:824–833
Starling AP, Engel SM, Whitworth KW, Richardson DB, Stuebe AM, Daniels JL, Haug LS, Eggesbo M, Becher G, Sabaredzovic A, Thomsen C, Wilson RE, Travlos GS, Hoppin JA, Baird DD, Longnecker MP (2014b) Perfluoroalkyl substances and lipid concentrations in plasma during pregnancy among women in the Norwegian Mother and Child Cohort Study. Environ Int 62:104–112
Stein CR, Savitz DA, Dougan M (2009) Serum levels of perfluorooctanoic acid and perfluorooctane sulfonate and pregnancy outcome. Am J Epidemiol 170:837–846
Tao L, Kannan K, Wong CM, Arcaro KF, Butenhoff JL (2008) Perfluorinated compounds in human milk from Massachusetts, U.S.A. Environ Sci Technol 42:3096–3101
Toft G, Jonsson BA, Lindh CH, Giwercman A, Spano M, Heederik D, Lenters V, Vermeulen R, Rylander L, Pedersen HS, Ludwicki JK, Zviezdai V, Bonde JP (2012) Exposure to perfluorinated compounds and human semen quality in Arctic and European populations. Hum Reprod 27:2532–2540
Vested A, Ramlau-Hansen CH, Olsen SF, Bonde JP, Kristensen SL, Halldorsson TI, Becher G, Haug LS, Ernst EH, Toft G (2013) Associations of in utero exposure to perfluorinated alkyl acids with human semen quality and reproductive hormones in adult men. Environ Health Perspect 121:453–458, 458e1-5
Vestergaard S, Nielsen F, Andersson AM, Hjollund NH, Grandjean P, Andersen HR, Jensen TK (2012) Association between perfluorinated compounds and time to pregnancy in a prospective cohort of Danish couples attempting to conceive. Hum Reprod 27:873–880
Von Ehrenstein OS, Fenton SE, Kato K, Kuklenyik Z, Calafat AM, Hines EP (2009) Polyfluoroalkyl chemicals in the serum and milk of breastfeeding women. Reprod Toxicol 27:239–245
Washino N, Saijo Y, Sasaki S, Kato S, Ban S, Konishi K, Ito R, Nakata A, Iwasaki Y, Saito K, Nakazawa H, Kishi R (2009) Correlations between prenatal exposure to perfluorinated chemicals and reduced fetal growth. Environ Health Perspect 117:660–667
White SS, Calafat AM, Kuklenyik Z, Villanueva L, Zehr RD, Helfant L, Strynar MJ, Lindstrom AB, Thibodeaux JR, Wood C, Fenton SE (2007) Gestational PFOA exposure of mice is associated with altered mammary gland development in dams and female offspring. Toxicol Sci 96:133–144
White SS, Fenton SE, Hines EP (2011a) Endocrine disrupting properties of perfluorooctanoic acid. J Steroid Biochem Mol Biol 127:16–26
White SS, Stanko JP, Kato K, Calafat AM, Hines EP, Fenton SE (2011b) Gestational and chronic low-dose PFOA exposures and mammary gland growth and differentiation in three generations of CD-1 mice. Environ Health Perspect 119:1070–1076
Whitworth KW, Haug LS, Baird DD, Becher G, Hoppin JA, Skjaerven R, Thomsen C, Eggesbo M, Travlos G, Wilson R, Longnecker MP (2012) Perfluorinated compounds and subfecundity in pregnant women. Epidemiology 23:257–263
Winquist A, Steenland K (2014) Perfluorooctanoic acid exposure and thyroid disease in community and worker cohorts. Epidemiology 25:255–264
Winquist A, Lally C, Shin HM, Steenland K (2013) Design, methods, and population for a study of PFOA health effects among highly exposed mid-Ohio valley community residents and workers. Environ Health Perspect 121:893–899
Yang C, Tan YS, Harkema JR, Haslam SZ (2009) Differential effects of peripubertal exposure to perfluorooctanoic acid on mammary gland development in C57Bl/6 and Balb/c mouse strains. Reprod Toxicol 27:299–306
Zhao Y, Tan YS, Strynar MJ, Perez G, Haslam SZ, Yang C (2012) Perfluorooctanoic acid effects on ovaries mediate its inhibition of peripubertal mammary gland development in Balb/c and C57Bl/6 mice. Reprod Toxicol 33:563–576
Acknowledgements
This work was supported by the National Toxicology Program, National Institute of Environmental Health Sciences, NIH.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Reed, C.E., Fenton, S.E. (2015). Effects of PFOA on Endocrine-Related Systems. In: DeWitt, J. (eds) Toxicological Effects of Perfluoroalkyl and Polyfluoroalkyl Substances. Molecular and Integrative Toxicology. Humana Press, Cham. https://doi.org/10.1007/978-3-319-15518-0_11
Download citation
DOI: https://doi.org/10.1007/978-3-319-15518-0_11
Publisher Name: Humana Press, Cham
Print ISBN: 978-3-319-15517-3
Online ISBN: 978-3-319-15518-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)