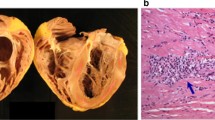
Abstract
The initial presentation of cardiac sarcoidosis (CS) may be in the acute care setting with heart block, ventricular tachycardia (VT) or acute heart failure (HF). Cardiovascular clinicians should consider sarcoidosis in the differential diagnosis when confronting these relatively common problems, especially where the patient is relatively young and once coronary heart disease has been excluded. Corticosteroids are the principal immunosuppressant used in the acute setting, owing to its relatively rapid effect. Although minimal controlled data are available to guide the use of corticosteroids, they have been most effective in resolving AV block. Accordingly, conventional management of VT and HF should be also be utilized.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Ventricular Tachycardia
- Cardiac Sarcoidosis
- Acute Care Setting
- Cardiac Manifestation
- Noncaseating Granuloma
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
Clinicians encountering cardiac sarcoidosis (CS) in an acute care setting are charged with managing a potentially life threatening disease without the benefit of a robust database of clinical trials on which to base their management. Accordingly, this chapter reflects one group’s approach to this disease based upon the limited information available in the medical literature and enhanced by clinical experience. Hopefully this is a salvo in a sustained effort to improve our collective abilities to diagnose, risk stratify and manage patients with this vexing illness.
One key factor in managing acute presentations of CS is recognizing the disease. Indeed there are several practical reasons to include CS in the differential diagnosis of atrioventricular (AV) block, ventricular tachycardia (VT) and acute heart failure with systolic dysfunction. For one, anti-inflammatory therapies are generally considered more effective if initiated prior to end-stage disease. Second, diagnosis of CS may influence device utilization in the setting of conduction disease. As reviewed in Chap. 12 and elsewhere, the general practice is to implant a cardioverter defibrillator (ICD) in lieu of a pacemaker (PPM) [1]. Moreover, a CS diagnosis may be unnecessarily delayed by conventional management of heart rhythm disorders. For example, PPM or ICD implantation typically precludes future cardiac magnetic resonance imaging (CMR) and delays endomyocardial biopsy as newly placed leads could by displaced by the bioptome. Moreover, radiofrequency ablation may confound the use of 18F-labeled fluorodeoxyglucose (FDG) positron emission tomography (PET) to identify cardiac inflammation.
When to Suspect Cardiac Sarcoidosis
Infrahissian AV block (either Mobitz II or 3rd degree), monomorphic VT (often multifocal) and heart failure with reduced left ventricular systolic function are the principle cardiac manifestations of sarcoidosis which can culminate in presentation to an acute care setting. Associated symptoms include syncope, cardiac arrest, dyspnea, and reduced exercise capacity. Much less commonly an acute presentation of CS is secondary to mitral regurgitation or pericardial effusion.
Heart failure and arrhythmia are amongst the most common general reasons for cardiac hospitalization. How then to recognize a rare cause (sarcoidosis) hidden amongst the many presentations of ischemic and hypertensive heart disease? The approach below is to consider different scenarios, where the pre-test probability of CS ranges from relatively high (cardiac presentation in patients with known systemic sarcoidosis) to low (index presentation of isolated CS). In each of these different scenarios, alternate diagnoses should be considered, especially ischemic heart disease, which by virtue of its high prevalence, is a likely cause of cardiac hospitalization regardless of the pretest probability for CS.
Cardiac Manifestations in Patients with Known Systemic Sarcoidosis
Patients presenting with acute cardiac manifestations of sarcoidosis in the context of an established diagnosis of systemic sarcoidosis should be readily recognized by providers. Different diagnostic criteria can be used in this setting (Table 10.1). These tools are expert consensus documents that have not been empirically derived nor well validated. The Japanese Ministry of Health and Welfare (JMHW) criteria were first established in 1993 [2] and refined in 2007 [1]. Definite diagnosis of CS according to the JMHW criteria are present if a noncaseating granuloma is seen in the myocardium – or – probable diagnosis of CS is made if a patient with proven extra cardiac sarcoidosis has a combination of electrocardiographic findings plus either abnormal cardiac imaging or hemodynamics. The Heart Rhythm Society (HRS) and the World Association of Sarcoidosis and Other Granulomatous (WASOG) disorders have recently provided similar consensus recommendations for the diagnosis of cardiac sarcoidosis. Like the JHMW criteria, definite CS diagnosis can be made if microscopic examination of the heart reveals noncaseating granulomas – or – probable diagnosis can be made in the context of pathologically confirmed systemic disease and non-invasive evidence of cardiac abnormalities [1, 3].
Cardiac Manifestations in Patents with Hitherto Unknown Systemic Sarcoidosis
Although there is a broad differential diagnosis for the presenting symptoms of CS, in young patients without ischemic heart disease, the likelihood of underlying CS increases. Accordingly, patients without previously known systemic sarcoidosis should be queried for history suggestive of multisystem involvement (e.g. cough, iritis, dermatologic abnormalities). Here, the identification of non-cardiac involvement and establishment of diagnosis through histologic evaluation (e.g. lymph node biopsy) can be pivotal.
Cardiac Manifestations in Patients with IsolatedCardiac Sarcoidosis
The diagnosis of sarcoidosis limited to the heart without extra-cardiac features is challenging. The classic teaching is that isolated CS is rare, however the epidemiologic data are suspect. Anecdotal experience includes patients only recognized to have CS at the time of cardiac transplantation, when the explant is carefully examined by a pathologist, or at autopsy. Our general approach is to extensively evaluate for CS in young patients presenting with infrahissian block, dilated cardiomyopathy with conduction disease and/or arrhythmia, or repetitive multifocal monomorphic VT.
A relatively high prevalence of CS has been reported in adult patients younger than 60 presenting with AV block [4]. In a single center prospective study utilizing FDG-PET in 32 young and middle aged adults presenting with idiopathic AV block, CS was identified in 34 % of subjects. All were subsequently found to have systemic sarcoidosis. In addition to ischemic heart disease, Lyme carditis and inherited neuromuscular disease should be considered alongside CS in these patients.
In patients without ischemic heart disease who present with sustained VT, cardiac sarcoidosis may be present in up to 10 % of cases [5, 6]. These patients are usually middle aged with multifocal monomorphic VT and electrophysiological evidence of scar reentry. Left ventricular systolic dysfunction and AV block often, but not universally, coexist with VT in these patients.
Dilated cardiomyopathy with conduction disease and/or arrhythmia (DCM+E) has been variably described in the literature as conduction cardiomyopathy [7], DCM+E [8] or arrhythmogenic cardiomyopathy [9]. By recognizing a heavy burden of conduction disease and/or arrhythmia in DCM, the differential diagnosis can be narrowed to a number of etiologies with specific therapeutic and prognostic implications [8]. Broadly categorized, DCM+E can be caused by ischemic, genetic, infections, and inflammatory etiologies. Once ischemic heart disease has been excluded, key clinical features can be used to distinguish amongst the other pathologies and definitive testing is often available.
A family history of unexplained sudden death, heart failure or cardiac transplantation should be obtained. Genetic testing, inclusive of genes commonly mutated in DCM+E (LMNA, DES, SCN5A, EMD, DSP, PKP2, DSC2), is now widely available. Although results will not be available for up to 14 weeks, the identification of a disease causing DNA variant in the appropriate clinical context can provide definitive diagnostic information allowing the reasonable exclusion of alternate etiologies including CS along with the identification of at risk family members [10, 11].
Residence or extended travel to areas where infection with Trypanosoma cruzi is endemic is key to identifying patients with Chagas heart disease [12]. Like CS, Chagas heart disease can present with conduction disease, regional wall motion abnormalities and ventricular tachyarrhythmia. Serologic testing in the appropriate setting can identify patients with Chagas heart disease and can enable therapy with anti-parasitic therapies such as benznidazole which may alter the natural history of this otherwise unrelenting disease [13]. Moreover, erroneous use of immunosuppression for presumed CS in a patient with Chagas heart disease could lead to accelerated pathogenesis [14].
Giant cell myocarditis (GCM) is a rare but devastating inflammatory cardiomyopathy with many of the same clinical features of CS, albeit with a rapidly progressive course. Amongst patients presenting with rapidly progressive cardiomyopathy, often without dilated remodeling, GCM should be considered. Although therapy for giant cell myocarditis is unrefined, its identification should prompt consideration for cardiac transplantation owing to its generally poor prognosis. Unlike CS where the yield of endomyocardial biopsy is generally low (see Chap. 9) the diffuse myocardial involvement in GCM is usually readily apparent on biopsy.
Acute Evaluations for Cardiac Sarcoidosis
Our approach to the evaluation of sarcoidosis is context dependent, as enumerated above. In patients with proven systemic disease, the exclusion of coronary heart disease and either FDG-PET or CMR findings suggestive of CS are usually sufficient to make a diagnosis. However, we prioritize this testing to precede ICD implantation or radiofrequency ablation due to the limiting/confounding effects of these therapies on non-invasive testing. Amongst patients with suspected isolated CS, we perform a thorough evaluation for extra-cardiac sarcoidosis, enhanced with FDG-PET. If not present, we have a low threshold to perform endomyocardial biopsy. In this setting, the performance of voltage guided biopsy may increase the diagnostic yield and is generally favored [15, 16]. As noted earlier, the implantation of a PPM or ICD usually limits the performance of biopsy for at least a month due to concerns of lead dislodgement.
Acute Medical Management of Cardiac Sarcoidosis
Medical therapy initiated in the acute care setting should be undertaken based on the severity of cardiac involvement and with a broader perspective of the patient’s multisystem involvement, prior therapies, and clinical trajectory. The response to prior immunosuppressive therapy, including corticosteroids and the use of steroid sparing agents is an important consideration. The presence of severe extra-cardiac disease may justify immunosuppressive therapy ipso facto. To the contrary, patients with advanced or end-stage cardiac involvement may have little to gain from immunosuppression and may only suffer its adverse consequences.
The content below focuses on the use of corticosteroid immunosuppression and presupposes that conventional therapies for heart failure (diuretics, neuro-hormonal antagonists) and arrhythmia (antiarrhythmic drugs, radiofrequency ablation) are used. In general, a diagnosis of CS should lead to the addition of immunosuppressive medications on top of conventional therapies.
As described in detail in Chap. 11, immunosuppressive therapies are frequently utilized in CS, albeit without prospective or well-designed clinical studies to inform dose, duration or extent of efficacy. The published studies likely reflect some degree of publication and ascertainment bias and usually only describe an individual center’s approach to management. Indeed the limitations of the published literature were highlighted in a recently published systemic review of the literature by Nery and colleagues [17]. They concluded that the best data exist for the efficacy of steroids for the management of AV block related to sarcoidosis and that the existing literature pertaining to VT and heart failure do not enable a statement of efficacy.
Of the different immunosuppressive agents used in sarcoidosis, the experience is greatest with corticosteroids. Because corticosteroids have a rapid onset of action, they are generally the agent used in the acute setting where rapid control of inflammation is desired. Steroid sparing agents such as methotrexate typically require weeks to take effect and accordingly are not reviewed here.
Our general approach to corticosteroid therapy is to initiate prednisone at high dose (0.5 mg/kg), which is then gradually tapered off over the ensuing 6–12 months. The use of non-invasive imaging and clinical cues to guide the weaning of steroids is detailed elsewhere in this text. However, prior to the initiation of high dose corticosteroids for CS, providers are advised to assess for risk of complications and prepare the patient accordingly (Table 10.2). This includes a test for latent tuberculosis infection and subsequent management, as well as an assessment for osteoporosis and glucose intolerance.
Scenarios Where Corticosteroids May Be Useful in the Acute Management of Cardiac Sarcoidosis
Atrioventricular Block
The best data in support of corticosteroid therapy for CS are for patients presenting with AV block. As summarized by Sadek and colleagues [17], 6 studies, including a total of 73 patients, have reported the outcomes associated with steroid therapy in patients presenting AV block. Amongst 57 patients treated with steroids, nearly half had resolution of AV block, whereas recovery of conduction occurred in none of the 16 patients not receiving steroids. As noted previously, ICD implantation in lieu of a standard dual chamber pacemaker should be strongly considered in patients requiring pacing for symptomatic conduction disease [1].
Ventricular Tachycardia
There are extremely limited data to inform the use of corticosteroids to manage symptomatic VT in CS. As the underlying etiology appears to be related to scar reentry [5], and reduction in inflammation is not expected to reduce scar burden, steroids may have limited effect of VT burden. Accordingly, it is advised that ICD implantation for VT in CS not be deferred for course of steroid therapy [1]. Amiodarone has been used effectively for VT in CS, although controlled studies are lacking. Catheter radiofrequency ablation has been used to reduce the burden of VT in small series of patients [18], but may be less efficacious than when used for VT in other forms of non-ischemic cardiomyopathy [19].
Worsening Systolic Function
Patients presenting with a new decline in LV systolic function, especially without significant dilated remodeling are generally treated aggressively with corticosteroids. As with other forms of cardiomyopathy, once significant dilated remodeling has occurred, the prospects of recovery with therapy (anti-inflammatories in the case of CS) is generally limited. In an uncontrolled retrospective study of CS treated with corticosteroids, including 24 with systolic dysfunction (LVEF <55 %), Chiu et al. reported an improvement in LVEF (40 ± 10–51 ± 12 %, p = 0.008) in patients with moderate systolic dysfunction at baseline. However there was no improvement in the subset with severe systolic dysfunction (LVEF <30 %) prior to starting steroids [20]. The time course of recovery of systolic function has not been well described.
Our approach is to consider corticosteroids in patients with severe systolic dysfunction where ventricular dilation is not present and/or where FDG-PET suggests active inflammation. Conversely the absence of inflammation by FDG PET may identify a subset of patients with mild-moderate systolic dysfunction who may not benefit from steroid therapy. Of the different patterns of CS activity on FDG-PET, the presence of a perfusion defect with FDG avidity (“mismatch pattern”) and/or right ventricular FDG uptake have been associated with worse prognosis and hence may have the most to benefit from corticosteroid therapy [21]. Sarcoidosis recurrence has been reported in patients who have undergone cardiac transplantation for CS, representing a challenging subset with recurrent disease despite active immunosuppression [22].
Pearls of Wisdom
-
1.
The diagnosis of CS should be considered early in an acute presentation of symptomatic heart disease, as certain therapies (i.e., pacemaker or ICD implantation) can preclude/confound/delay tests used to diagnose CS.
-
2.
Corticosteroid therapy is likely most effective for patients with symptomatic AV block, and may benefit patients with mild systolic dysfunction. Steroid therapy may not benefit patients with symptomatic VT or advanced cardiomyopathy.
-
3.
Prior to initiating corticosteroid therapy for CS, patients should be assessed for side effects and toxicities.
References
Birnie DH, Sauer WH, Bogun F, et al. HRS expert consensus statement on the diagnosis and management of arrhythmias associated with cardiac sarcoidosis. Heart Rhythm. 2014;11:1305–23.
Diagnostic standard and guidelines for sarcoidosis. Jpn J Sarcoidosis Granulomatous Disord. 2007;27:89–102.
Judson MA, Costabel U, Drent M, et al. The WASOG sarcoidosis organ assessment instrument: an update of a previous clinical tool. Sarcoidosis Vasc Diffuse Lung Dis. 2014;31:19–27.
Nery PB, Beanlands RS, Nair GM, et al. Atrioventricular block as the initial manifestation of cardiac sarcoidosis in middle-aged adults. J Cardiovasc Electrophysiol. 2014;25:875–81.
Koplan BA, Soejima K, Baughman K, Epstein LM, Stevenson WG. Refractory ventricular tachycardia secondary to cardiac sarcoidosis: electrophysiologic characteristics, mapping, and ablation. Heart Rhythm. 2006;3:924–9.
Nery PB, Mc Ardle BA, Redpath CJ, Leung E, Lemery R, Dekemp R, Yang J, Keren A, Beanlands RS, Birnie DH. Prevalence of cardiac sarcoidosis in patients presenting with monomorphic ventricular tachycardia. Pacing Clin Electrophysiol. 2014;37:364–74.
Hershberger RE, Siegfried JD. Update 2011: clinical and genetic issues in familial dilated cardiomyopathy. J Am Coll Cardiol. 2011;57:1641–9.
Lakdawala NK, Givertz MM. Dilated cardiomyopathy with conduction disease and arrhythmia. Circulation. 2010;122:527–34.
Saffitz JE. Arrhythmogenic cardiomyopathy: advances in diagnosis and disease pathogenesis. Circulation. 2011;124:e390–2.
Lakdawala NK, Funke BH, Baxter S, et al. Genetic testing for dilated cardiomyopathy in clinical practice. J Card Fail. 2012;18:296–303.
Lakdawala NK. Using genetic testing to guide therapeutic decisions in cardiomyopathy. Curr Treat Options Cardiovasc Med. 2013;15:387–96.
Fox MC, Lakdawala N, Miller AL, Loscalzo J. Clinical problem-solving. A patient with syncope. N Engl J Med. 2013;369:966–72.
Viotti R, Vigliano C, Lococo B, Bertocchi G, Petti M, Alvarez MG, Postan M, Armenti A. Long-term cardiac outcomes of treating chronic Chagas disease with benznidazole versus no treatment: a nonrandomized trial. Ann Intern Med. 2006;144:724–34.
Lattes R, Lasala MB. Chagas disease in the immunosuppressed patient. Clin Microbiol Infect. 2014;20:300–9.
Lee JC, Seiler J, Blankstein R, Padera RF, Baughman KL, Tedrow UB. Images in cardiovascular medicine. Cardiac sarcoidosis presenting as heart block. Circulation. 2009;120:1550–1.
Nery PB, Keren A, Healey J, Leug E, Beanlands RS, Birnie DH. Isolated cardiac sarcoidosis: establishing the diagnosis with electroanatomic mapping-guided endomyocardial biopsy. Can J Cardiol. 2013;29:1015.e1–3.
Sadek MM, Yung D, Birnie DH, Beanlands RS, Nery PB. Corticosteroid therapy for cardiac sarcoidosis: a systematic review. Can J Cardiol. 2013;29:1034–41.
Jefic D, Joel B, Good E, Morady F, Rosman H, Knight B, Bogun F. Role of radiofrequency catheter ablation of ventricular tachycardia in cardiac sarcoidosis: report from a multicenter registry. Heart Rhythm. 2009;6:189–95.
Tokuda M, Tedrow UB, Kojodjojo P, Inada K, Koplan BA, Michaud GF, John RM, Epstein LM, Stevenson WG. Catheter ablation of ventricular tachycardia in nonischemic heart disease. Circ Arrhythm Electrophysiol. 2012;5:992–1000.
Chiu C-Z, Nakatani S, Zhang G, Tachibana T, Ohmori F, Yamagishi M, Kitakaze M, Tomoike H, Miyatake K. Prevention of left ventricular remodeling by long-term corticosteroid therapy in patients with cardiac sarcoidosis. Am J Cardiol. 2005;95:143–6.
Blankstein R, Osborne M, Naya M, et al. Cardiac positron emission tomography enhances prognostic assessments of patients with suspected cardiac sarcoidosis. J Am Coll Cardiol. 2014;63:329–36.
Osborne M, Kolli S, Padera RF, Naya M, Lewis E, Dorbala S, Di Carli MF, Blankstein R. Use of multimodality imaging to diagnose cardiac sarcoidosis as well as identify recurrence following heart transplantation. J Nucl Cardiol. 2013;20:310–2.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Lakdawala, N.K., Stewart, G.C. (2015). Acute Management of Cardiac Sarcoidosis. In: Freeman, A., Weinberger, H. (eds) Cardiac Sarcoidosis. Springer, Cham. https://doi.org/10.1007/978-3-319-14624-9_10
Download citation
DOI: https://doi.org/10.1007/978-3-319-14624-9_10
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-14623-2
Online ISBN: 978-3-319-14624-9
eBook Packages: MedicineMedicine (R0)