Abstract
Increasing pressure to develop green and eco-friendly methods for synthesizing nanoparticles has provoked researchers to shift to microorganisms and biological systems. Plants can be used in large-scale production of nanoparticles, and in order to improve their potential in nanoparticle synthesis, it is necessary to investigate the biochemical and molecular mechanisms of nanoparticle formation. This chapter reviews phytosynthesis of nanoparticles, the influences of reaction conditions, and mechanistic aspects.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
11.1 Introduction
Development of reliable and environmentally friendly process for synthesis of metallic nanoparticles (NPs) is an important step in the field of application of nanobiotechnology and nanoscience. There are various methods for the synthesis of NPs, but most of them are environmentally unfriendly and expensive (Senapati 2005; Klaus-Joerger et al. 2001). Consequently, there is an ever-growing need to develop nontoxic and eco-friendly procedures for synthesis and assembly of NPs with desired morphologies and sizes, fast, and clean. One of the options to achieve this objective is to use natural processes such as use of microorganisms and biological systems. One approach that shows immense potential is based on the phytosynthesis of NPs using plants (Table 11.1) (Iravani 2011; Iravani et al. 2014a, b; Korbekandi et al. 2009; Iravani and Zolfaghari 2013). The use of plants in this area is rapidly developing due to their growing success and ease of formation of NPs. The objectives of recent studies tend to provide a controlled and upscalable process for synthesis of monodispersed and highly stable NPs (Iravani 2011; Korbekandi et al. 2009; Iravani et al. 2014a). In this chapter, most of the plants used in nanoparticle synthesis are mentioned, and detailed examination of numerous syntheses examples and case studies is presented.
11.2 Phytosynthesis of Metal NPs
11.2.1 Silver and Gold NPs
Gardea-Torresdey et al. (2002a) reported phytosynthesis of gold (Au) NPs within live Medicago sativa (alfalfa) plants by gold ion uptake from solid media. The alfalfa plants were grown in an AuCl4 – rich environment (Gardea-Torresdy et al. 2002b, 2003). Moreover, colloidal silver (Ag) NPs were synthesized by reacting aqueous silver nitrate with M. sativa seed exudates under nonphotomediated conditions (Lukman et al. 2011). Upon contact, rapid reduction of Ag+ ions was observed in <1 min with silver nanoparticle formation reaching 90 % completion in <50 min. It was observed that largely spherical NPs (~5–51 nm) were produced at [Ag+] = 0.01 M and 30 °C, while flower-like particle clusters (~104 nm) were observed on treatment at higher silver concentrations. Predilution of the exudate induced the formation of single-crystalline silver nanoplates, forming hexagonal particles and nanotriangles with edge lengths of about 86–108 nm, while pH adjustment to 11 resulted in monodisperse silver NPs (~12 nm). It was mentioned that repeated centrifugation and redispersion enhanced the percentage of nanoplates from 10 to 75 % in solution (Lukman et al. 2011).
Armendariz et al. (2004b) reported the formation of rod-shaped gold NPs by biomaterials. They characterized the gold NPs formed by wheat biomass exposed to a 0.3 mM potassium tetrachloroaurate solution at pH values of 2–6 at room temperature. It was concluded that wheat biomass was able to reduce Au(III)–Au(0) forming face-centered cubic (FCC) tetrahedral, hexagonal, decahedral, icosahedral multitwinned, irregular shape, and rod shape NPs. In another study, pH-dependent synthesis of rod-shaped gold NPs using Avena sativa has shown that biomass might carry more positive functional groups such as positive amino groups, sulfhydryl groups, and carboxylic groups which allowed the gold ions to get more closure to binding sites and approved the reduction of Au(III)–Au(0) (Gardea-Torresdey et al. 2002a; Armendariz et al. 2004a). A 0.1 mM solution of Au(III) was reacted with powdered oat biomass at pH values of 2–6 for 1 h. As in the case of wheat, oat biomass produced FCC tetrahedral, hexagonal, decahedral, icosahedral multitwinned, irregular , and rod shaped NPs. It was reported that most of the NPs synthesized by using alfalfa, wheat, and oat at pH 2 had an irregular shape. However, it seems that pH has a major impact on the size of the synthesized NPs rather than on the shape of them.
Sastry and coworkers have explored the formation mechanism of triangular gold nanoprisms by Cymbopogon flexuosus extracts, the nanotriangles seemed to grow by a process involving rapid bioreduction, assembly, and room temperature sintering of spherical gold NPs (Shankar et al. 2004b). Also rapid synthesis of stable gold nanotriangles using Tamarindus indica leaf extract as a reducing agent could be achieved (Ankamwar et al. 2005a). The shape of metal NPs considerably changed their optical and electronic properties (Kelly et al. 2003). They have demonstrated synthesis of gold NPs with variety of shapes (spherical and triangular) and sizes using Aloe vera plant extracts, as well (Chandran et al. 2006). It was explained that only biomolecules of molecular weights less than 3 kDa caused reduction of chloroaurate ions, leading to the formation of gold nanotriangles.
The aqueous solution of gold ions when exposed to Coriandrum sativum leaf extract was reduced and resulted in the extracellular biosynthesis of gold NPs with spherical, triangle, truncated triangles, and decahedral morphologies ranging from 6.75 to 57.91 nm. These NPs were stable in solution over a period of 1 month at room temperature (Badri Narayanan and Sakthivel 2008). Gold NPs synthesized using Azadirachta indica leaf broth appeared to have a propensity to form thin, planar structures rather than just spherical particles. The planar particles formed were predominantly triangular with a very small percentage of hexagonal-shaped particles (Shankar et al. 2004a). Ghosh et al. (2012a) reported the synthesis of gold NPs using Gnidia glauca flower extract. The concentration of chloroauric acid and temperature was optimized to be 0.7 mM and 50 °C, respectively. The NPs varied in morphology from nanotriangles to nanohexagons majority being spherical. Spherical particles (~10 nm) were found in majority. However, particles of larger dimensions were in range between 50 and 150 nm. The gold NPs exhibited remarkable catalytic properties in a reduction reaction of 4-nitrophenol to 4-aminophenol by NaBH4 in aqueous phase (Ghosh et al. 2012a).
Magnolia kobus and Diospyros kaki were capable of eco-friendly extracellular synthesis of gold NPs (~5–300 nm) with different triangular, pentagonal, hexagonal, and spherical shapes within a few minutes (for up to 90 % conversion at a reaction temperature of 95 °C) (Song et al. 2009). It was suggested that the rate of synthesis of the NPs was related to the reaction and incubation temperature, and increased temperature levels allowed nanoparticle growth at a faster rate. Moreover, by increasing the temperatures and leaf broth concentrations, size of NPs became smaller. Fourier Transformed Infrared Spectroscopy (FTIR) analysis has shown that gold NPs produced by M. kobus extract were surrounded by proteins and metabolites (such as terpenoids having functional groups of amines, aldehydes, carboxylic acid, and alcohols). It was also reported that the use of low concentration of phyllanthin extract reacting with HAuCl4 led to synthesis of hexagonal or triangular gold NPs, but spherical NPs could be formed by addition of higher concentration of the extract (Song et al. 2009).
Triangular and hexagonal gold NPs (~200–500 nm) were synthesized using pear fruit extract (Ghodake et al. 2010). Pear extract contains essential phytochemicals consisting of organic acids, peptides, proteins, and amino acids. In addition, it contains saccharides which provide synergetic reduction power for the bioreduction of chloroaurate ions into gold NPs. The pear fruit extract when exposed to chloroaurate ions in an alkaline condition resulted in gold NPs with plate-like morphologies in a highly productive state. The gold NPs formed under normal conditions also exhibited plate-like morphologies with a low productivity. In addition, the synthesis of gold NPs (~11 nm) with spherical and triangular shapes by fruit extract of Tanacetum vulgare was reported (Dubey et al. 2010b). Carbonyl group was involved in synthesis of these NPs. In another study, Singh et al. (2013) reported the green synthesis of silver NPs (~40–100 nm) using Dillenia indica fruit extract. It was reported that the stability of the colloidal silver NPs for more than 6 days (166 h) might be attributed to the citrate component of the D. indica fruit juice (Singh et al. 2013).
Green synthesis of biocompatible gold NPs from chloroauric acid using water extract of Eclipta alba leaves at room temperature was reported. The gold NPs were produced in very short time, even in less than 10 min. The in vitro stability of as-synthesized gold NPs was studied in different buffer solutions. Mukherjee et al. (2012) designed and developed a gold NPs-based drug delivery system containing doxorubicin, an FDA-approved anticancer drug. Consequently, administration of this to breast cancer cells (MCF-7 and MDA-MB-231) showed significant inhibition of breast cancer cell proliferation compared to pristine doxorubicin. They suggested this method for large-scale synthesis of biocompatible gold NPs which can be used as a delivery vehicle for the treatment of cancer diseases (Mukherjee et al. 2012).
Silver and gold NPs were synthesized using an aqueous extract of the seaweed Turbinaria conoides. Spherical and triangular nanostructures of the silver and gold NPs were observed between the size ranges of 2–17 and 2–19 nm, respectively. The synthesized silver NPs were efficient in controlling the bacterial biofilm formation, but, gold NPs did not show any remarkable anti-biofilm activity. The maximum zone of inhibition was recorded against Escherichia coli (17.6 ± 0.42 mm), followed by Salmonella sp., Serratia liquefaciens, and Aeromonas hydrophila (Vijayan et al. 2014). Moreover, silver NPs were synthesized from aqueous silver nitrate through a simple green route using the leaf extract of Coccinia grandis (as the reducing and capping agents) (Arunachalam et al. 2012). The synthesized NPs were in range of 20–30 nm and were crystallized in face-centered cubic symmetry. The thermal stability of NPs was studied using Thermo Gravimetric Analyzer (TGA) which showed that the NPs synthesized by plant extracts began to degrade at around 300 °C. Moreover, there was a steady weight loss until 800 °C. The total weight loss up to 800 °C for the synthesized silver NPs was about 36.26 %. The observed behavior was most likely as a consequence of the surface desorption of bio-organic compounds present in nanoparticle powder. Therefore, plant leaf extract-stabilized silver NPs were expected to be made up of molecules responsible for the reduction of metal ion and stabilizing particles in the solution. Further, the thermal stability of NPs was studied using differential scanning calorimeter (DSC). As a result, the synthesized silver NPs showed an endothermic peak at 65 °C. Photocatalytic property of the silver NPs were investigated by degradation of Coomassie Brilliant Blue G-250 under UV light. The results showed that silver NPs have suitable activity for the degradation of Coomassie Brilliant Blue G-250. FTIR spectrum was examined to identify the possible biomolecules responsible for capping and efficient stabilization of the silver NPs synthesized by plant leaf extract. The peaks observed for silver NPs formed through reduction by C. grandis, at 1,228 cm−1 (ether linkages), 1,376 cm−1 (‒O‒H bending), 1,050 cm−1 (ether linkages), 1,484 cm−1 (=NH), and 1,632 cm−1 (amide I) suggested the presence of alkaloids and terpenoids adsorbed on the surface of silver NPs (Arunachalam et al. 2012).
Silver NPs were synthesized by reaction of the biomass of Cinnamomum camphora leaf with aqueous silver precursors at ambient temperature (Huang et al. 2007). Size dispersity of quasi-spherical silver NPs could be facilely controlled by simple variation of the amount of biomass reacting with aqueous solution of silver nitrate. The polyol components and the water-soluble heterocyclic components were mainly found to be responsible for the reduction of silver ions and the stabilization of the NPs, respectively. Furthermore, Huang et al. (2008) investigated biological formation of silver NPs by lixivium of sun-dried C. camphora leaf in continuous-flow tubular microreactors. They introduced polyols in the lixivium as possible reducing agents. In another study, silver and gold NPs with a particle size of 10–20 nm, using Zingiber officinale root extract as a reducing and capping agent were synthesized (Velmurugan et al. 2014). Chloroauric acid and silver nitrate were mixed with Z. officinale root extract for the synthesis of silver and gold NPs. As a result, optimum nanoparticle formation was achieved at pH 8 and 9, 1 mM metal ion, a reaction temperature 50 °C, and reaction time of 150–180 min for silver NPs (~10–20 nm) and gold NPs (~5–20 nm), respectively. FTIR spectroscopy analysis showed the respective peaks for the potential biomolecules in the ginger rhizome extract, which were responsible for the bioreduction in metal ions and synthesized silver and gold NPs. Further, it was observed that the synthesized silver NPs showed a moderate antibacterial activity against bacterial food pathogens (Velmurugan et al. 2014).
Spherical and ovoid crystalline silver NPs (~7–15 nm) were synthesized by phytoreduction of silver nitrate using aqueous leaf extract of Ocimum tenuiflorum (Vignesh et al. 2013). FTIR spectrum was analyzed to identify the feasible biological functional groups responsible for the reduction of silver ions and stabilizing the synthesized NPs. A glycosidic linkage present in the FTIR spectrum of silver NPs suggested that the plant-based polysaccharides might contribute for the reduction of silver ion into silver NPs. The colloidal solution of silver NPs showed significant antimicrobial activity against variety of human and fish pathogens in solid medium. The implications of colloidal silver NPs on hematological and biochemical functions of fresh water fish Labeo rohita were tested. The experiments revealed that the amount of hemoglobin and total count of blood cells were considerably increased. The activity of functional enzymes such as glutamic oxaloacetic transaminase (GOT), glutamate pyruvate transaminase (GPT), anti-protease, and myeloperoxidase were radically improved due to the treatment of fish with colloidal silver NPs. The authors of this study mentioned that the colloidal silver NPs having prominent immunomodulatory effects in fresh water fish, thus these NPs can be used as an immunomodulator in aquaculture sectors instead of synthetic growth factors (Vignesh et al. 2013).
Brassica juncea and M. sativa can be used for phytosynthesis of silver NPs. Harris and Bali (2008) have investigated the limits (substrate metal concentration and time exposure) of uptake of metallic silver by two common metallophytes, B. juncea and M. sativa. B. juncea, when exposed to an aqueous substrate containing 1,000 ppm silver nitrate for 72 h, accumulated up to 12.4 wt% silver. M. sativa accumulated up to 13.6 wt% silver when exposed to an aqueous substrate containing 10,000 ppm silver nitrate for 24 h. In the case of M. sativa, an increase in metal uptake was observed with a corresponding increase in the exposure time and substrate concentration. In both cases, Transmission Electron Microscopy (TEM) analysis showed the presence of roughly spherical silver NPs, with a mean size of 50 nm. In addition, the in vivo formation of silver NPs was observed in B. juncea, Festuca rubra, and M. sativa (Marchiol et al. 2014). Marchiol et al. (2014) reported that the mentioned plants were grown in Hoagland’s solution for 30 days and then exposed for 24 h to a solution of 1,000 ppm AgNO3. Despite the short exposure time, the silver uptake and translocation to plant leaves was very high, reaching 6,156 and 2,459 mg kg−1 in B. juncea and F. rubra, respectively. TEM images of plant fractions showed the in vivo formation of silver NPs in the roots, stems, and leaves of the plants. In the roots, silver NPs were present in the cortical parenchymal cells, on the cell wall of the xylem vessels and in regions corresponding to the pits. In leaf tissues, silver NPs of different sizes and shapes were located close to the cell wall, as well as in the cytoplasm and within chloroplasts. The NPs were not observed in the phloem of the three plant species. The contents of reducing sugars and antioxidant compounds, proposed as being involved in the green synthesis of silver NPs. Ascorbic acid has been proposed as the reducing agent responsible for this process. In contrast, F. rubra had a level of reducing sugars much higher than B. juncea and M. sativa. The authors of this study mentioned that nanoparticle synthesis started in healthy cells, which then rapidly undergo a progressive alteration until they were completely disrupted due to silver toxicity. Thus, nanoparticle synthesis was initiated within the chloroplasts in a healthy cell and ends in the cytoplasm of the same cell, which has been damaged (Marchiol et al. 2014). Further, in another study, after a 9-week growth in gold-, silver-, and copper-enriched soil, seeds of B. juncea grow into a plant containing Au–Ag–Cu alloy NPs (Haverkamp et al. 2007). Starnes et al. (2010) detected the formation of gold NPs in M. sativa and other species as early as 6 h after the start of exposure to KAuCl4. It was also verified that plant growth conditions (e.g., variations in temperature, pH, and photosynthetically active radiation) influenced the size and shape of growing gold NPs (Starnes et al. 2010). Beattie and Haverkamp (2011) demonstrated that in B. juncea, the sites of the most abundant reduction of metal salts to NPs were the chloroplasts, in which high reducing sugars (i.e., glucose and fructose) may be responsible for the metal reduction (Beattie and Haverkamp 2011).
Rodríguez-León et al. (2013) reported the synthesis of silver NPs (~2–40 nm) from silver nitrate solutions using extracts of Rumex hymenosepalus. High-resolution transmission electron microscopy and fast Fourier transform analysis show that two kinds of crystal structures are obtained: FCC and hexagonal. They observed that the FCC NPs displayed two size populations: one with a small average diameter (~10 nm) and a second one with a larger diameter (~28 nm). On the other hand, the hexagonal NPs have only one size population and larger diameters (~38 nm). The polyphenols contained in the R. hymenosepalus extracts acted effectively as the reducing agents for the Ag+ ions due to their antioxidant activity.
Shankar et al. (2004a) reported the synthesis of silver NPs by the reduction of aqueous Ag+ ions and also the synthesis of bimetallic core-shell NPs of silver by simultaneous reduction of aqueous Ag+ ions with the broth of A. indica leaves. They observed that the metal particles were stable in solution even 4 weeks after their synthesis. Moreover, stabilization of NPs was possibly facilitated by reducing sugars and/or terpenoids present in A. indica leaf broth. The synthesized silver NPs were predominantly spherical and polydisperse with diameters in the range 5–35 nm. Furthermore, Pelargonium graveolens (geranium) leaf broth, when exposed to aqueous silver nitrate solution, resulted in enzymatic synthesis of stable crystalline silver NPs, extracellularly (Shankar et al. 2003a). The bioreduction of the metal ions was fairly rapid, occurred readily in solution, and resulted in a high density of stable silver NPs in the size range 16–40 nm. The synthesized NPs appeared to be assembled into open, quasilinear superstructures and were predominantly spherical in shape. It was believed that proteins, terpenoids, and other bio-organic compounds in the geranium leaf broth participated in the bioreduction of silver ions and in the stabilization of the NPs thus formed by surface capping. Shankar et al. (2003b) reported the possibility of terpenoids from geranium leaf in the silver nanoparticle synthesis. Polyols such as terpenoids, polysaccharides, and flavones in the C. camphora leaf were believed to be the main cause of the reduction of silver and chloroaurate ions (Huang et al. 2007). Moreover, green synthesis of silver NPs using methanol extract of Eucalyptus hybrida leaf was reported. Flavonoid and terpenoid constituents present in E. hybrid leaf extract are responsible for the stabilization of produced silver NPs (~50–150 nm) (Dubey et al. 2009). Cinnamon zeylanicum bark extract could be used in biosynthesis of cubic and hexagonal silver nanocrystals (~31–40 nm) (Sathishkumar et al. 2009b). The particle size distribution varied with variation in the dosage of C. zeylanicum bark extract. The number of particles increased with increasing dosage due to the variation in the amount of reductive biomolecules. Small NPs were formed at high pH. The shape of silver NPs at high pH was more spherical in nature rather than ellipsoidal. Moreover, bactericidal effect of produced nanocrystalline silver particles was tested against E. coli strain. As a result, the various tested concentrations of 2, 5, 10, 25, and 50 mg/L produced inhibition of 10.9, 32.4, 55.8, 82, and 98.8 %, respectively. The minimum inhibitory concentration was found to be 50 mg/L. C. zeylanicum bark is rich in terpenoids (linalool, methyl chavicol, and eugenol) and in chemicals (such as cinnamaldehyde, ethyl cinnamate, and β-caryophyllene) which contribute to its special aroma. Furthermore, proteins are also present in the bark. It was believed that water-soluble organics present in C. zeylanicum bark were the reasons of the bioreduction of silver ions to silver NPs. Moreover, proteins from C. zeylanicum bark capped the synthesized NPs either through free amine groups or cysteine residues, and thus stabilized them (Sathishkumar et al. 2009b).
The reaction of aqueous silver ions with Desmodium trifolium extract resulted in synthesis of silver NPs at room temperature (Ahmad et al. 2011). The authors of this article believed that H+ ions produced along with NAD during glycolysis were responsible for the formation of nanosilver particles as well as water-soluble antioxidative agents (e.g., ascorbic acids). These antioxidative agents were especially participating in reduction of silver ions. Jha and Prasad (2010) demonstrated that cycas leaf extract could be used in order to produce stable silver NPs. X-ray data indicated that silver NPs had FCC unit cell structure. Phytochemicals such as polyphenols, glutathiones, metallothioneins, and ascorbates probably were responsible for formation of the NPs.
High density of extremely stable silver NPs (~16–40 nm) were rapidly synthesized by challenging silver ions with Datura metel leaf extract (Kesharwani et al. 2009). Leaf extracts of this plant contains biomolecules such as alkaloids, proteins/enzymes, amino acid, alcoholic compound, and polysaccharides which could be used as reluctant to react with silver ions and scaffolds to direct the formation of silver NPs in solution. Quinol and chlorophyll pigment were responsible for the reduction of silver ions and stabilization of produced NPs. In another study, green synthesis of silver NPs (~20–30 nm) from silver nitrate (1 mM) solution through the extract of D. metel flower (as the reducing and capping agents) was reported. In addition, the synthesized NPs showed antimicrobial activity (Nethradevi et al. 2012).
Geraniol as a volatile compound obtained from P. graveolens was used for biosynthesis of silver NPs (~1–10 nm). The cytotoxicity of the synthesized silver NPs was evaluated in vitro against Fibrosarcoma Wehi 164 cell line at different concentrations (1–5 µg/mL) (Safaepour et al. 2009). The presence of silver NPs (5 µg/mL) significantly inhibited the cell line’s growth (up to 60 %). Therefore, it seems that silver NPs have inhibitory effects against the proliferation of cancer cells.
Song and Kim (2008) have elucidated that Pinus desiflora, D. kaki, Ginko biloba, M. kobus, and Platanus orientalis leaves broth synthesized stable silver NPs with average particle size ranging from 15 to 500 nm, extracellularly. In the case of M. kobus and D. kaki leaves broth, synthesis rate and final conversion to silver NPs became faster, when reaction temperature increased. But the average particle sizes produced by D. kaki leaf broth decreased from 50 to 16 nm, when temperature increased from 25 to 95 °C. They also illustrated that only 11 min was required for more than 90 % conversion at the reaction temperature of 95 °C using M. kobus leaf broth (Song and Kim 2008). Moreover, phytosynthesis of silver NPs (~44–64 nm) using Iresine herbstii and evaluation of their antibacterial, antioxidant, and cytotoxic activities were reported (Dipankar and Murugan 2012). The synthesized NPs were cubic and face-centered cubic in shape. The synthesized silver NPs showed potent antibacterial activity against human pathogenic bacteria. These NPs exhibited strong antioxidant activity as well as cytotoxicity against HeLa cervical cell lines. Furthermore, Suman et al. (2013) reported the phytosynthesis of spherical silver NPs (~30–55 nm) from the root of Morinda citrifolia. FTIR showed that the NPs were capped with plant compounds. The X-ray diffraction spectrum XRD pattern clearly indicated that the silver NPs formed in the present synthesis were crystalline in nature. The synthesized NPs were also proved to exhibit excellent cytotoxic effect on HeLa cell (Suman et al. 2013). Logeswari et al. (2013) reported the green synthesis of silver NPs from silver nitrate solution by commercially available plant powders, such as Solanum tricobatum, Syzygium cumini, Centella asiatica, and Citrus sinensis. Atomic Force Microscopy (AFM) showed the irregular shapes of silver NPs and the formation of them were found to be 53, 41, 52, and 42 nm, corresponding to S. cumini, C. sinensis, S. tricobatum, and C. asiatica, respectively. FTIR spectroscopy confirmed the presence of protein as the stabilizing agent surrounding the silver NPs. Antimicrobial activity of the silver bionanoparticles was performed by a well diffusion method. The highest antimicrobial activity of silver NPs synthesized by C. sinensis and C. asiatica was found against Pseudomonas aeruginosa (Logeswari et al. 2013).
Vilchis-Nestor et al. (2008) used Camellia sinensis (green tea) extract to produce gold NPs and silver nanostructures in aqueous solution at ambient conditions. They also investigated the control of size, morphology, and optical properties of the nanostructures and reported initial concentrations of metal ions and tea extract as the controlling factors. It was investigated that when the amount of C. sinensis extract was increased, the resulted NPs were slightly bigger and more spherical. Authors of this study believed that phenolic acid-type biomolecules present in C. sinensis extract were responsible for synthesis and stabilization of silver and gold NPs. Caffeine and theophylline present in tea extracts might be responsible for catalysis and synthesis of NPs (Vilchis-Nestor et al. 2008). In another study, green synthesis of spherical gold NPs (~2.94–45.58 nm) was done by using fresh young leaves and leaf buds of C. sinensis (Boruah et al. 2012). Phytoreduction of HAuCl4 by polyphenols present in young leaves and leaf buds of tea extract at room temperature produced gold NPs. The kinetics of the reaction suggested that the reaction was fast and completed in 28 min. In addition, black tea leaf extracts were used in synthesis of gold and silver NPs (Begum et al. 2009). The NPs are stable and have different shapes, such as spheres, trapezoids, prisms, and rods. Findings of this study demonstrated that polyphenols and flavonoids were responsible for synthesis of silver and gold NPs. Sun et al. (2014) reported a simple and cost-effective method for synthesis of silver NPs using tea leaf extract. The synthesized NPs were nearly spherical, with the sizes ranging from 20 to 90 nm. In addition, the antibacterial activity of silver NPs was determined by monitoring the growth curve and also by the Kirby-Bauer disk diffusion method. But, the silver NPs produced by tea extract showed low antibacterial activity against E. coli (Sun et al. 2014).
Mude et al. (2009) reported synthesis of spherical silver NPs (~60–80 nm) using callus extract of Carica papaya. Proteins and other ligands seemed to be responsible for the synthesis and stabilization of silver NPs. Furthermore, FCC silver NPs (~10–20 nm) were synthesized by using the latex of Jatropha curcas as reducing and capping agent (Bar et al. 2009c). It was demonstrated that leaf extracts from the aquatic medicinal plant, Nelumbo nucifera (Nymphaeaceae), could be able to reduce silver ions and produce silver NPs (~45 nm) in different shapes (Santhoshkumar et al. 2010). Biosynthesized silver NPs showed larvicidal activity against malaria (Anopheles subpictus) and filariasis (Culex quinquefasciatus) vectors. Silver NPs were synthesized biologically by using Sorbus aucuparia leaf extract within 15 min. The synthesized NPs were found to be stable for more than 3 months. The authors of this study believed that sorbate ion in the leave extract of S. aucuparia encapsulated the NPs and this matter was responsible for maintenance of the stability (Dubey et al. 2010a). The effect of leaf extract quantity, substrate concentration, temperature, and contact time were also evaluated to optimize the process of producing NPs. With increase in the concentration of metal ions from 10−4 to 10−2 M, increase in particle size was also found (Dubey et al. 2010a).
The aqueous extract of Alternanthera sessilis L. (Amaranthaceae) was used for synthesizing silver NPs from silver nitrate aqueous (Niraimathi et al. 2013). Phytochemical analysis of the extract revealed the presence of alkaloid, tannins, ascorbic acid, carbohydrates, and proteins and they served as effective reducing and capping agents for converting silver nitrate into NPs. FTIR spectrum showed the silver NPs having a coating of proteins indicating a dual role of biomolecules responsible for capping and efficient stabilization of the silver NPs (Niraimathi et al. 2013). Moreover, polyethylene glycol-stabilized colloidal silver NPs (~7–14 nm) were synthesized using the reductive potency of the aqueous extract of Thuja occidentalis leaves under ambient conditions (Barua et al. 2013). MTT assay revealed the dose-dependent cytocompatibility and toxicity of the NPs with the L929 normal cell line. On the other hand, the antiproliferative action of the NPs was evaluated on HeLa cell line. Fluorescence and phase contrast microscopic imaging indicated the appearance of multinucleate stages with aggregation and nuclear membrane disruption of the HeLa cells post treatment with the NPs. The interaction at the prokaryotic level was also assessed via differential antibacterial efficacy against Staphylococcus aureus and E. coli (Barua et al. 2013).
Silver NPs were prepared by using Bryophyllum sp., Cyperus sp., and Hydrilla sp. plant extracts (Jha et al. 2009b). X-ray analysis demonstrated that silver NPs (~2–5 nm) have FCC unit cell structure. The reduction of silver ions was due to water-soluble phytochemicals such as flavones, quinones, and organic acids (e.g., oxalic, malic, tartaric, and protocatechuic acid) present in plant tissues. It was demonstrated that silver NPs might have resulted due to different aforementioned metabolites or fluxes and other oxidoreductively labile metabolites such as ascorbates or catechol/protocatechuic acid (Jha et al. 2009b). In the case of Chenopodium album, organic acids were also responsible for nanoparticle biosynthesis. The plant leaf has high level of oxalic acid as well as lignin which can act as reducing agents. C. album leaf extract was used for the single-pot bio-inspired synthesis of spherical silver and gold NPs (Dwivedi and Gopal 2010). Quasi-spherical shapes were observed for produced NPs within range of about 10–30 nm.
Silver NPs (~20–40 nm) were successfully synthesized using switchgrass (Panicum virgatum) extract used at room temperature. Phytosynthesis of silver NPs through this process was fairly rapid, with 90 % of silver ion reduction completed within 90 min. XRD analysis indicated the formation of phase pure silver NPs with FCC symmetry (Mason et al. 2012). In another study, spherical silver NPs (~16–28 nm) were synthesized using plant fruit bodies (Tribulus terrestris L.) (Gopinath et al. 2012). The active phytochemicals present in the plant were responsible for the quick reduction of silver ions to metallic silver NPs. The antibacterial property of synthesized NPs was observed by Kirby-Bauer method with clinically isolated multidrug resistant bacteria such as Streptococcus pyogens, P. aeruginosa, E. coli, Bacillus subtilis and S. aureus (Gopinath et al. 2012). Sathiya and Akilandeswari (2014) reported the phytoreduction of silver nitrate into silver NPs using the leaf extract of Delonix elata. SEM analysis revealed the spherical shape of the NPs with sizes in the range of 35–45 nm and Energy Dispersive Spectroscopy (EDS) spectrum confirmed the presence of silver along with other elements in the plant metabolite. The X-ray diffraction analysis showed that the synthesized NPs were crystalline in nature and had FCC structure. FTIR analysis revealed the presence of phenolic compounds along with the silver NPs, and flavonoids present in the leaf broth of D. elata were responsible for the bioreduction and stabilization of silver NPs (Sathiya and Akilandeswari 2014).
Santhosh kumar et al. (2012) reported the efficacies of antiparasitic activities of biosynthesized silver NPs using stem aqueous extract of Cissus quadrangularis against the adult of hematophagous fly, Hippobosca maculata, and the larvae of cattle tick, Rhipicephalus (Boophilus) microplus. Contact toxicity method was followed to determine the potential of parasitic activity. Twelve milliliters of stem aqueous extract of C. quadrangularis was treated with 88 mL of 1 mM silver nitrate (as the substrate) solution at room temperature for 30 min. FTIR analysis confirmed that the bioreduction of silver ions to silver NPs were due to the reduction by capping material of plant extract. The synthesized silver NPs (~42.46 nm) were spherical and oval in shape. The mortality obtained by the synthesized silver NPs from the C. quadrangularis was more effective than the aqueous extract of C. quadrangularis and silver nitrate solution (1 mM). The adulticidal activity was observed in the aqueous extract, silver nitrate solution, and produced silver NPs against the adult of H. maculata with LC50 values of 37.08, 40.35, and 6.30 mg/L; LC90 values of 175.46, 192.17, and 18.14 mg/L and r2 values of 0.970, 0.992, and 0.969, respectively. The maximum efficacy showed in the aqueous extract, silver nitrate solution, and produced silver NPs against the larvae of R. (B.) microplus with LC50 values of 50.00, 21.72, and 7.61 mg/L; LC90 values of 205.12, 82.99, and 22.68 mg/L and r2 values of 0.968, 0.945, and 0.994, respectively (Santhoshkumar et al. 2012). In another study, the antiparasitic activities of hexane, chloroform, ethyl acetate, acetone, methanol, and the aqueous leaf extracts of Euphorbia prostrata Ait. (Euphorbiaceae) and synthesized silver NPs using aqueous leaf extract against the adult cattle tick Haemaphysalis bispinosa Neumann (Acarina: Ixodidae) and the haematophagous fly H. maculata Leach (Diptera: Hippoboscidae) were determined (Zahir and Rahuman 2012). Parasites were exposed to varying concentrations of plant extracts and produced silver NPs for 24 h. All extracts showed the maximum toxic effect on parasites; however, the highest mortality was found in the hexane, chloroform, ethyl acetate, acetone, methanol, and aqueous leaf extracts of E. prostrate and biosynthesized silver NPs against the adult of H. bispinosa (LC50 = 45.24, 40.07, 21.91, 25.32, 19.30, 10.16, and 2.30 ppm; LC90 = 86.95, 88.66, 70.92, 83.22, 48.28, 70.27, and 8.28 ppm) and against H. maculata (LC50 = 39.37, 41.98, 19.92, 27. 93, 21.97, 9.79, and 2.55 ppm; LC90 = 89.44, 98.52, 76.59, 90.18, 55.07, 54.35, and 9.03 ppm), respectively. Mortality of 100 % was found in biosynthesized silver NPs at a concentration of 10 mgL−1. It was observed that the synthesized silver NPs (~25–80 nm) were rod shaped. The chemical composition of aqueous leaf extract was analyzed by gas chromatography–mass spectrometry (GC–MS). The major chemical constituent was identified as 2-phenylethanol. In addition, toxicity tests were conducted to analyze the toxicological effects of particle size on Daphnia magna and Ceriodaphnia dubia, and the animal model test was evaluated against Bos indicus for 24-h treatment. No toxicity on daphnids and no adverse effects were noted on animals after exposure to solvent extracts and produced silver NPs (Zahir and Rahuman 2012). In addition, silver NPs were synthesized by employing an aqueous peel extract of Annona squamosa in silver nitrate (Kumar et al. 2012). The synthesized NPs were irregular spherical in shape and the average particle size was about 35 ± 5 nm. The authors of this study reported that reducing sugars (aldoses) and terpenoids are proposed to play a key role for the phytoreduction of silver ions and the formation of corresponding NPs, while ketones/aldehydes bind to emerging spherical NPs to form large nanotriangles and hexagons. Moreover, to identify the capping reagents and the molecules responsible for the reduction of silver ions in A. squamosa aqueous peel extract GC–MS has been evaluated. GC–MS chromatogram of aqueous extract of A. squamosa revealed that a major constituent contains ‒OH/‒CHO as functional group in the moiety. Further, 1H NMR spectrum of A. squamosa L. aqueous peel extract revealed that strong signal observed at δ = 4.76–4.81 ppm is due to =C–H. Signal at δ = 5.35 ppm could be due to aliphatic ‒OH group, whereas the signals appearing between δ = 1.26–1.32 ppm are related to aliphatic C‒CH2‒C groups. Further, water-soluble hydroxy functional group containing compounds were reported to be responsible for the reduction of Ag+ ions. Consequently, the hydroxyl compounds bind to the NPs and enhance the stability as well as it will forms a layer (capping material) over silver NPs which provide stability to NPs (Kumar et al. 2012).
Jagtap and Bapat (2013) reported the phytosynthesis of silver NPs (with an average size 10.78 nm) from aqueous solution of silver nitrate using Artocarpus heterophyllus Lam. seed powder extract, as a reducing agent. The reaction of A. heterophyllus Lam. seed powder extract and silver nitrate was carried out in an autoclave at 15 psi, 121 °C for 5 min. The synthesized NPs were generally found to be irregular in shapes. The FTIR spectra indicated the role of amino acids, amides group I in the synthetic process. The seed contains Jacalin, a lectin which is a single major protein representing more than 50 % of the proteins from the jackfruit crude seed extract having several biological activities. The synthesized NPs showed highly potent antibacterial activity toward B. cereus, B. subtilis, S. aureus, and P. aeruginosa (Jagtap and Bapat 2013). In another study, the extract of Benincasa hispida seeds (as the reducing and capping agents) was used for the biosynthesis of stable and nearly spherical gold NPs (~10–30 nm). The particle size could be easily tuned by the reaction conditions including quantity of extract, temperature, and pH (Aswathy Aromal and Philip 2012a). It was found that the NPs were more stable at pH 6. Moreover, from FTIR spectrum, it was suggested that the possible reducing agent was polyols and the capping material responsible for stabilization was proteins present in the extract. Carboxylic group (COOH) present in the extract becomes COO–, and this carboxylate group present in proteins can act as a surfactant to attach on the surface of gold NPs and it stabilizes gold NPs through electrostatic stabilization (Aswathy Aromal and Philip 2012a).
11.2.2 Palladium NPs
Sathishkumar et al. (2009a) have investigated phytocrystallization of palladium (Pd) through reduction process using Cinnamomum zeylanicum bark extract. As a result, nanocrystalline palladium particles (~15–20 nm) were successfully synthesized. It was demonstrated that reaction conditions such as pH, temperature, and biomaterial dosage had no major effects on the shape and size of produced NPs. In another study, nanocrystalline palladium particles (10–15 nm) have been synthesized using Curcuma longa tuber extract as the biomaterial. Temperature and pH had no major effect on size and shape of the NPs. It was found that the zeta potential of formed palladium NPs was negative and it increased with increase in pH. It has been also reported that palladium NPs could be synthesized by using coffee and tea extract. The synthesized NPs were in the size range of about 20–60 nm and crystallized in face-centered cubic symmetry (Nadagouda and Varma 2008). It was reported that Gardenia jasminoides E.′ water crude extract could be used for bioreduction of palladium chloride. Identified antioxidants, including geniposide, chlorogenic acid, crocins, and crocetin were reducing and stabilizing agents for synthesizing palladium NPs (~3–5 nm) in water crude extract. The particle size and disparity of NPs were temperature dependent, and the best dispersity was revealed at 70 °C (Jia et al. 2009). Roopan et al. (2012a) reported the green synthesis of spherical shape palladium NPs (~80 ± 5 nm) using A. squamosa L. (Annonaceae), commonly known as custard apple. The report revealed the presence of secondary metabolites contained −OH group which was responsible for the reduction of Pd(II)–Pd(0) (Roopan et al. 2012a). In another study, a facile and environmentally friendly method was reported for the synthesis of palladium NPs using an aqueous solution of Pulicaria glutinosa, a plant widely found in a large region of Saudi Arabia, as a bioreductant (Khan et al. 2014). The hydroxyl groups of the plant extract molecules were found mainly responsible for the reduction and growth of palladium NPs. Results from FTIR analysis confirmed the dual role of the P. glutinosa extract, both as a bioreductant as well as a capping ligand, which stabilized the surface of the NPs. It was demonstrated that the synthesized NPs were crystalline NPs, and the results of XRD analysis confirmed the formation of a face-centered cubic structure. These palladium NPs demonstrated excellent catalytic activity toward the Suzuki coupling reaction under aqueous and aerobic conditions. Moreover, kinetic studies of the catalytic reaction monitored using GC confirmed that the reaction completes in less than 5 min (Khan et al. 2014).
Palladium NPs were synthesized by using Piper betle leave extract. The FTIR spectrum indicated the influence of flavonoids, terpenoids, and proteins in palladium NPs synthesis and stabilization in the aqueous medium. The peaks at 1,600 and 1,406 cm−1 showed the presence of flavonoids and terpenoids. It was demonstrated that the water-soluble flavonoids present in the P. betle extract could have been adsorbed on the surface of the NPs and same might have induced the reduction of Pd+2 to stable Pd0 NPs with interaction of carbonyl groups. Further, it was reported that the protein molecules in the P. betle extract might also participate in the process of capping and stabilization of NPs (Mallikarjuna et al. 2013).
11.2.3 Platinum NPs
Platinum (Pt) NPs (~2–12 nm) could be synthesized using the leaf extract of D. kaki. The leaf extract of D. kaki was reacted with an aqueous H2PtCl6 · 6H2O solution at various temperatures between 25 and 95 °C. It was reported that more than 90 % of the platinum ions were converted into NPs with a 10 % leaf biomass concentration at 95 °C (Song et al. 2010). The authors proved that the size platinum NPs could be controlled by the temperature or the composition of the reaction mixture. The average size of the NPs decreased from 12 nm at 25 °C to 5 nm at 95 °C, and decreased from 11 nm at 5 % to 3 nm at 50 % the lead broth concentration. The size of platinum NPs increased from 3 to 9 nm as PtCl6 ion concentration increased from 0.1 to 2 mM (Song et al. 2010). The leaf extract of Ocimum sanctum was used as a reducing agent for the synthesis of platinum NPs (~23 nm). Fourier transform infrared spectroscopy revealed that the compounds such as ascorbic acid, gallic acid, terpenoids, certain proteins and amino acids act as reducing agents for platinum ions reduction. It was reported that the reduced platinum showed similar hydrogen evolution potential and catalytic activity like pure platinum using linear scan voltammetry (Soundarrajan et al. 2012). In another study, one-pot synthesis of platinum and palladium NPs using lignin isolated from red pine (Pinus resinosa) was reported. In the TEM images, spherical NPs of diameters in the range of 16–20 nm were observed, in the case of palladium, and smaller ones of not so well-defined shapes for platinum. These NPs showed good catalytic activity in the reduction reaction (Coccia et al. 2012).
11.2.4 Copper NPs
Copper (Cu) NPs were biosynthesized using Magnolia leaf extract. When aqueous solutions of CuSO4 · 5H2O treated with the leaf extract, stable copper NPs (~40–100 nm) were formed. Foams coated with biologically synthesized copper NPs showed higher antibacterial activity against E. coli cells (Lee et al. 2011). In another study, extracellular synthesis of copper NPs was carried out using stem latex of a medicinally important plant, Euphorbia nivulia. The synthesized NPs were stabilized and subsequently capped by peptides and terpenoids present within the latex. The copper NPs are toxic to adenocarcinomic human alveolar basal epithelial cells (A549 cells) in a dose-dependent manner. It was concluded that the nontoxic aqueous formulation of latex capped copper NPs could be directly used for administration/in vivo delivery of NPs for cancer therapy (Valodkar et al. 2011). Moreover, an eco-friendly method for synthesis of copper NPs (~15 ± 1.7 nm) was reported (Harne et al. 2012). Cysteine proteases present in the latex of Calotropis procera L. were used to fabricate copper NPs from copper acetate. FTIR was performed to confirm capping behavior of the latex proteins that contributed to long term stability of copper NPs (6 months) in aqueous medium. The FTIR spectrum results suggested that antioxidant enzymes (AOEs), cysteine protease, and tryptophan with functional groups of amines, alcohols, ketones, aldehydes, and carboxylic acids might be adsorbed on the surface of the synthesized copper NPs using C. procera L. latex. Further, cytotoxicity studies of latex-stabilized copper NPs were carried out on HeLa, A549, and BHK21 cell lines by MTT dye conversion assay. HeLa, A549, and BHK21 cells showed excellent viability even at 120 μM concentration of copper NPs (Harne et al. 2012).
Terminalia arjuna bark extract was used to produce copper NPs (~23 nm) under microwave irradiation (Yallappa et al. 2013). FTIR result indicated the presence of flavonones and terpenoids which might be responsible for the reduction and stabilization process. A solid-state 13C NMR spectrum also indicated the presence of biomolecules on copper NPs. As a result, the prominent peaks at 211 and 200 ppm were attributed to ketones and aldehydes (flavonones, terpenoid aldehydes) from the plant extract on copper NPs. The peak at 187 ppm indicated the presence of amides while the peaks at 155, 145, 132, and 77 ppm indicated unsaturated compounds, reducing sugar, aromatic benzenes, and carbonyl groups, respectively (Yallappa et al. 2013). In another study, extract of leaves of henna (Lawsonia inermis) was used for synthesizing spherical copper NPs (~27–45 nm). Results from FTIR spectrum showed that the synthesized copper NPs were coated with lawsone and oxide layers. The authors fabricated a conducting nanobiocomposite films using the prepared copper NPs and collagen fibers discarded from leather industry. They demonstrated that the synthesized copper NPs and nanobiocomposite films had potential for various electronic device applications (Cheirmadurai et al. 2014).
11.2.5 Lead NPs
Rapid synthesis of lead (Pb) NPs (~10–12.5 nm) by using 0.5 % aqueous extract of J. curcas L. latex was reported. Lead NPs were characterized initially by UV-Vis spectroscopy and shown distinct peak at 218 nm. This peak was highly specific for lead NPs. FTIR was performed to find the role of cyclic peptides namely curcacycline A (an octapeptide) and curcacycline B (a nonapeptide) as possible reducing and capping agents present in the latex of J. curcas L. (Joglekar et al. 2011).
11.2.6 Gold–Palladium Bimetallic NPs
Gold–palladium (Au–Pd) bimetallic NPs (~7.4 ± 0.8 nm) were produced based on simultaneous bioreduction of Au(III) and Pd(II) precursors (aqueous HAuCl4/PdCl2 mixture) with Cacumen platycladi leaf extract in aqueous environment. TEM micrograph demonstrated that the gold–palladium bimetallic NPs were well-defined spherical in shape. FTIR showed that the C=O and C‒O groups in the plant extract played a critical role in capping the NPs. It was reported that some water-soluble polyhydroxy biomolecules in the C. platycladi leaf extract such as flavonoid and reducing sugar were responsible for the phytoreduction of the NPs (Zhan et al. 2011).
11.2.7 Zero-Valent Iron NPs
Generally, several methods can be used for the synthesis of zero-valent iron NPs including vacuum sputtering, decomposition of iron pentacarbonyl (Fe(CO)5) in organic solvents, and the reaction of iron(II) or iron(III) salts with sodium borohydride (Kuhn et al. 2002; Karlsson et al. 2005; Wang and Zhang 1997). Further, green methods for synthesis of zero-valent iron NPs have been developed. Nadagouda et al. (2010) compared the zero-valent iron NPs produced using the green and borohydride methods and observed that some of the green zero-valent iron NPs were found to be nontoxic when compared with control samples prepared using the conventional borohydride reduction protocols. In this study, tea polyphenols were used for the rapid generation of nanoscale zero-valent iron particles. The zero-valent iron NPs were subsequently examined for in vitro biocompatibility using the human keratinocyte cell (HaCaT) line as a representative skin exposure model. The cells were exposed to zero-valent iron NPs for time periods of 24 and 48 h. Biocompatibility was assessed using the methyl tetrazolium, or MTS, (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium)) and lactate dehydrogenase (LDH) assays to determine in vitro cytotoxicity. The evaluation of mitochondrial function (MTS) and membrane integrity (LDH) in human keratinocytes showed that these green synthesized zero-valent iron NPs were nontoxic in the human keratinocytes exposed when compared with control samples synthesized using a borohydride protocol (Nadagouda et al. 2010). It was demonstrated that these NPs could have an adverse effect on pure cultures of bacteria, such as E. coli, Pseudomonas fluorescens, and B. subtilis var. niger (Auffan et al. 2008; Lee et al. 2008; Diao and Yao 2009).
Machado et al. (2013) evaluated the viability of the utilization of several plant leaves to produce extracts which were capable of reducing iron(III) in aqueous solution to form zero-valent iron NPs (~10–20 nm). The extracts could be classified into three categories according to their antioxidant capacity (expressed as Fe(II) concentration): >40 mmol L−1; 20–40 mmol L−1; and 2–10 mmol L−1; with oak, pomegranate, and green tea leaves producing the richest extracts. It was concluded that the best results were obtained when the extraction is performed at 80 °C, while the best contact time and leaf mass: solvent volume ratio depended on the type of leaf. The authors of this chapter believed that polyphenols were responsible for synthesis of zero-valent iron NPs (Machado et al. 2013).
11.3 Phytosynthesis of Metal Oxide NPs
11.3.1 Iron Oxide NPs
It was reported that iron oxide NPs could be synthesized by using alfalfa biomass (Herrera-Becerra et al. 2008). Results showed that pH 10 yielded smaller particles with greater proportion of the Fe2O3, and the size could be controlled in the range of 1–4 nm. When pH decreased (pH 5), larger NPs were produced. In another study, Au–Fe3O4 composite NPs were prepared with a combined chemical and biological reducing process (“semi-biosynthesis method”). Magnetic cores were primarily produced using a fabrication method consisting of co-precipitation of Fe2+ and Fe3+. An ethanol extract of Eucalyptus camaldulensis was used for the reduction of Au+3 on the surface of the magnetite NPs and for the functionalization of the Au–Fe3O4 nanocomposite particles (Haratifar et al. 2009). Iron oxide NPs (Fe3O4) were rapidly synthesized by reduction of ferric chloride solution with Tridax procumbens leaf extract containing carbohydrates as a major component which act as reducing agent (Senthil and Ramesh 2012). The results indicated that water-soluble carbohydrates which have aldehyde groups may cause the formation of iron oxide NPs. The purification process of the Fe3O4 product did not require any expensive method, since solid product was obtained from the reaction in liquid phase. The antibacterial effect of iron oxide NPs against P. aeruginosa was also investigated. Bacteriological tests were performed in Potato Dextrose Agar (PDA) medium on solid agar plates and in liquid systems supplemented with different concentrations of iron oxide NPs. These particles were shown to be an effective bactericide (Senthil and Ramesh 2012).
11.3.2 Indium Oxide NPs
It was reported that indium oxide (In2O3) NPs were biologically synthesized using Aloe barbadensis Miller (A. vera) plant extracted solution. In2O3 NPs (~5–50 nm) were produced by using indium acetylacetonate and A. vera plant extracted solution (Maensiri et al. 2008). The NPs were formed after calcinations the dried precursor of indium oxide in air at 400–600 °C. The morphologies and sizes of indium oxide materials were affected by the temperature of calcinations.
11.3.3 Copper Oxide NPs
Copper oxide NPs were synthesized using aqueous extract of Acalypha indica leaf. The synthesized NPs were highly stable, spherical, and particle size was in the range of 26–30 nm. The antimicrobial activity of A. indica-mediated copper oxide NPs was tested against selected pathogens. These NPs showed efficient antibacterial and antifungal effect against E. coli, P. fluorescens, and Candida albicans. The cytotoxic activity of A. indica-mediated copper oxide NPs was evaluated by MTT assay against MCF-7 breast cancer cell lines and confirmed that these NPs have cytotoxicity activity (Sivaraj et al. 2014). Moreover, Gunalan et al. (2012a) reported that A. barbadensis-mediated copper oxide NPs were spherical in nature and sizes ranging from 15 to 30 nm. Results from FTIR indicated the existence of some phenolic compounds, terpenoids, or proteins which were bound to the surface of copper oxide NPs that remained despite repeated washing. The stability of copper oxide NPs might be due to the free amino and carboxylic groups which had interacted with the copper surface. Moreover, the proteins in the medium prevented agglomeration and stabilized the NPs (Gunalan et al. 2012a).
11.3.4 Chromium Oxide NPs
Chromium oxide (Cr2O3) NPs were rapidly synthesized by reduction of potassium dichromate solution with Arachis hypogaea leaf extract containing reducing sugars which act as reducing agent (Ramesh et al. 2012). The results indicated that the aldehyde groups present in the plant extract played an important role in the formation of chromium oxide NPs. The antibacterial effect of chromium oxide NPs against E. coli was investigated. Bacteriological tests were performed in PDA medium on solid agar plates and in liquid systems supplemented with different concentrations of chromium oxide NPs. These particles were shown to be an effective bactericide. The shapes of the chromium oxide NPs appeared like hexagonal and cubic with rough surfaces (Ramesh et al. 2012). In another study, chromium oxide NPs (~65 nm) were phytosynthesized by the reduction of potassium dichromate solution with Mukia maderaspatana plant extract. For this, 20 mL of potassium dichromate solution was mixed with 20 mL of plant extract in a beaker and stirred for 10–15 min. The color of the solution changed from orange to green indicating the formation of chromium(III) oxide NPs. The solution was kept at room temperature for evaporation of aqueous phase. The green solid product was dried in hot air oven at 65–70 °C for an hour. The resulting solid was calcined at 650–700 °C for 3 h. The addition of potassium dichromate solution to the plant extract containing mild reducing agents caused the reduction of orange dichromate(VI) ions to green chromium(III) ions. The enhancing influence of chromium oxide NPs as a catalyst for the decomposition of KMnO4 was studied. The antibacterial effect of chromium oxide NPs against E. coli was investigated. These particles were shown to have an effective bactericide (Rakesh et al. 2013).
11.3.5 Zinc Oxide NPs
It was reported that crystalline zinc oxide (ZnO) NPs (~72.5 nm) were synthesized using Physalis alkekengi L. The TEM micrographs demonstrated that the synthesized NPs were polydispersed and not uniformly distributed (Qu et al. 2011b). Furthermore, the branches (stems) of Euphorbia tirucalli was used for synthesizing spherical zinc oxide NPs (~20 nm) (Hiremath et al. 2013). The synthesis of hexagonal wurtzite zinc oxide NPs from Zn-hyperaccumulator (Sedum alfredii Hance) plants was reported, as well. The formed NPs were agglomerated, and single zinc oxide NPs were pseudospherical in shape with a mean size of 53.7 nm (Qu et al. 2011a). In another study, the exploit of aqueous leaf extract of Corriandrum sativum as an eco-friendly agent for the pattern of zinc oxide nanoparticle using zinc acetate and sodium hydroxide as a surrogate for chemical method was reported. FTIR studies of aqueous Corriandrum leaf extract revealed the presence of phytoconstituents such as alcohol, aldehyde, and amine which were the surface active molecules stabilized the NPs and this phytochemicals have interacted with the zinc surface and stabilized zinc oxide NPs (Gnanasangeetha and SaralaThambavani 2013b). Moreover, the antimicrobial efficacy of green and chemical synthesized zinc oxide NPs against various bacterial and fungal pathogens was determined. Zinc oxide NPs are known to be one of the multifunctional inorganic NPs with effective antimicrobial activities. Actually, metal oxide powders (e.g., ZnO, MgO, and CaO) showed antimicrobial activities against S. aureus, E. coli, and fungi. It is considered that the detected active oxygen species generated by these metal oxide particles could be the main mechanism of their antibacterial activity. Various microbiological tests were performed using varying concentrations of green and chemical zinc oxide NPs with sizes 40 and 25 nm, respectively. It was reported that green zinc oxide NPs showed more enhanced biocidal activity against various pathogens when compared to chemical synthesized zinc oxide NPs. Also effectiveness of NPs were increased with increasing particle dose, treatment time, and synthesis method. This study demonstrated that the particle size variation and surface area to volume ratio of green zinc oxide nanoparticle were responsible for significant higher antimicrobial activity (Gunalan et al. 2012b). Aqueous leaf extract of A. indica were used to green synthesis of zinc oxide NPs (~100–200 nm). This green method showed that the environmentally benign and renewable aqueous leaf extract of A. indica could be used as a stabilizing agent for the synthesis of zinc oxide NPs. XRD pattern illustrated that the synthesized NPs were in wurtzite nature. The flavonoid and terpenoid constituents present in A. indica leaf extract were the surface dynamic molecules stabilized the NPs (Gnanasangeetha and SaralaThambavani 2013a). Moreover, green synthesis of spherical zinc oxide NPs (~30–35 nm) by zinc nitrate and utilizing the biocomponents of leaves extract of Calotropis gigantea was reported. The X-ray patterns showed hexagonal crystal type for zinc oxide (Vidya et al. 2013).
Nagajyothi et al. (2013) reported the green synthesis of zinc oxide NPs (~8.48–32.51 nm) using trifoliate orange (Poncirus trifoliata). Scanning electron microscope (SEM) image showed that the morphology of zinc oxide NPs was nearly spherical shape. The utility of phytosynthesized zinc oxide NPs as catalyst in Claisen Schmidt Condensation reaction was described. Accordingly, this eco-friendly, inexpensive and chemically stable catalyst was used for the condensation of 3,4-dimethyl benzaldehyde and acetophenone (Nagajyothi et al. 2013). Furthermore, zinc oxide nanocrystals were synthesized by employing Nephelium lappaceum L., peel extract as a natural ligation agent. Green synthesis of zinc oxide nanocrystals was carried out via zinc–ellagate complex formation using rambutan peel wastes. It was reported that zinc oxide nanocrystals-coated cotton showed good antibacterial activity toward E. coli and S. aureus (Yuvakkumar et al. 2014).
Zinc oxide NPs were synthesized by using Borassus flabellifer fruit extract (~50–60 nm) (Vimala et al. 2014). The UV/Vis spectrum showed an absorption peak at 368 nm that reflects surface plasmon resonance zinc oxide NPs. The synthesized NPs were porous in nature and rod-like structure. The possible mechanism for the green synthesis of zinc oxide NPs involved reduction of zinc nitrate ions that can form intermediate complexes with phenolic OH groups present in hydrolyzable tannins, which subsequently undergo oxidation to quinine forms with consequent reduction of zinc to zinc oxide NPs. Results from analysis proved the existence of some phenolic compounds, terpenoids or proteins which were bound to the surface of zinc oxide NPs. Moreover, it was suggested that the proteins prevented agglomeration and stabilized the NPs by forming a coat, covering the NPs. The authors examined the synthesized doxorubicin-zinc oxide NPs exhibited a dose-dependent cytotoxicity against MCF-7 and HT-29. The inhibitory concentration (IC50) was found to be 0.125 μg mL−1 for MCF-7 and HT-29 cells. An induction of apoptosis was evidenced by nuclear stain Hoechst 33,258. In vivo toxicity assessment showed that doxorubicin-zinc oxide NPs had low systemic toxicity in murine model system. The results proved that the synthesized zinc oxide NPs can be considered as a promising candidate for a drug delivery system (Vimala et al. 2014).
11.3.6 Titanium Dioxide NPs
An eco-friendly approach for synthesis of titanium dioxide (TiO2) NPs from titanium isopropoxide solution using Nyctanthes arbor-tristis (night-flowering jasmine) leaves extract has been reported. SEM analysis demonstrated that the average size was from 100 to 150 nm, and the shapes were uniformed spherical (Sundrarajan and Gowri 2011). In another study, titanium dioxide NPs were synthesized using A. squamosa peel extract and their catalytic applications were studied on the 2,3-disubstituted dihydroquinazolin-4(1H)-one synthesis. Synthesized compounds were confirmed using FTIR, 1H NMR, 13C NMR and GC–MS analyses (Bharathi et al. 2014). Moreover, Roopan et al. (2012b) reported the phytosynthesis of rutile titanium dioxide NPs using fruit peel A. squamosa aqueous extract. The UV-Vis spectrophotometer results were promising and showed a rapid synthesis of titanium dioxide NPs with a surface plasmon resonance occurring at 284 nm. The TEM images showed polydisperse NPs with spherical shapes and size 23 ± 2 nm ranges. The authors of this study used GC–MS to find the possible mechanisms for the formation of titanium dioxide NPs. Consequently, results from GC–MS analysis showed that the aqueous extract of A. squamosa fruit peel contained compounds having hydroxyl group as a functional group in the structure. Titanyl hydroxide could be dehydrated to give titanium dioxide NPs by heating it with an A. squamosa aqueous peel extract at about 60 °C. Here, the plant extract served as catalysts due to the present of compounds having hydroxyl group as a functional group in the structures. Further, water-soluble compounds containing hydroxyl functional group were reported to be responsible for the stabilization of titanium dioxide NPs (Roopan et al. 2012b).
Eclipta prostrata leaf extract was used for synthesizing of titanium dioxide NPs (Rajakumar et al. 2012). FTIR peak implicated the role of carboxyl group O‒H stretching amine N‒H stretch in the formation of titanium dioxide NPs. The results indicated that alcohols, phenols, alkanes, primary amines, and aliphatic amines in E. prostrata might be participating in the process of nanoparticle synthesis. Authors mentioned that the E. prostrata leaves contained beta-amyrin, wedelolactone, triterpenoids, flavonoids, luteolin-7-o-glucoside, L-terthienyl methanol and stigmasterol. Water-soluble heterocyclic compounds such as flavones were the reducing and capping ligands of the NPs. Functional groups associated with these the cause for the bioreduction of TiO(OH)2 to TiO2 NPs. Field emission scanning electron microscopy analysis showed that the synthesized NPs were spherical clusters, quite polydisperse and they ranged in size from 36 to 68 nm with calculated average size of 49.5 nm (Rajakumar et al. 2012).
11.4 Reaction Conditions
The important challenges frequently encountered in the green synthesis of NPs are to control the shape and size of the nanosized particles as well as to achieve the monodispersity in solution phase. Moreover, the rate of nanoparticle synthesis and stability of the NPs are very important especially in industrial production. Therefore, effects of reaction conditions should be checked. In this section, we mention the effects of some important parameters including pH, temperature, amount of biomass or plant extract, substrate concentration, and reaction time:
11.4.1 pH
The pH of the solution is an important factor in controlling the size and morphology of NPs, and in the location of nanoparticle deposition (Diao and Yao 2009; Machado et al. 2013; Herrera-Becerra et al. 2008). For instance, it was demonstrated that in the case of Cocos nucifera, high monodispersity was obtained when the reaction was conducted at pH 11, with an average size of 23 nm, while at pH 2 no reaction occurred. It was observed that upon adjusting the pH of the reactants with NaOH, the light brown extract and colorless silver nitrate solution developed murky brown, respectively. It was revealed that when silver nitrate solution was mixed with NaOH solution first it would favor the formation a pure silver oxide (Ag2O) precipitate and if a reducing reagent (e.g., C. nucifera coir extract) was also added, then the product became pure silver and that the reduction of Ag+–Ag(0) occurred on the surface of the existing colloids in the system (Roopan et al. 2013). Sathishkumar et al. (2009b) reported the effect of pH on the formation of silver NPs using C. zeylanicum powder and bark extract over a wider pH range (1–11) and concluded that the pH of the solution dropped in most of the cases after the synthesis of silver NPs. As a result, small NPs were formed at high pH. Moreover, the formation of large-sized ellipsoidal silver NPs was observed at lower or acidic pH, while at higher or alkaline pH highly dispersed, small-sized, and spherical NPs tended to form. Andreescu et al. (2007) reported the acceleration in the rate of silver nanoparticle synthesis (rapid and complete reduction of silver ions) by using elevated pH. They reported that increase in pH results in increase the absolute value of the negative zeta potential which led to the formation of highly dispersed NPs. This phenomenon could be related to the electrostatic repulsion at high pH or attributed to the high absolute value of the negative zeta potential (Sadowski et al. 2008). Dubey et al. (2010b) have shown the effect of pH on zeta potential of produced NPs. Zeta potential values reveal details about the surface charge and stability of the synthesized metal NPs (Dubey et al. 2010b). Actually, stability of produced metal NPs are evaluated with zeta potentiometer and corresponding surface Plasmon spectra (Dwivedi and Gopal 2010; Parashar et al. 2009a, b). Ag NPs demonstrated lower zeta potential value at strongly acidic pH, but when the pH increased higher zeta potential values were obtained. Furthermore, larger particle size could be achieved by decreasing the pH. Gardea-Torresdey et al. (1999) found that pH is an important factor in the biosynthesis of colloidal gold using alfalfa biomass and concluded that the size of NPs varied with the change in pH. Mock et al. (2002) also have reached to similar conclusions and reported that pH is responsible for the formation of NPs of various shapes and size. As different plant extracts and even the extracts coming from different parts of the same plant may have different pH values which further need optimization for the efficient synthesis of NPs, it has been reported by several researchers that larger NPs formed at lower pH (2–4) as compared to higher pH. Moreover, Armendariz et al. (2004a) reported that the size of gold nanoparticle produced by A. sativa was highly dependent on the pH value. At pH 2, large size NPs (~25–85 nm) were formed albeit in a small quantity but at pH 3 and 4, smaller sized NPs were formed in a large quantity. They speculated that at low pH (pH 2), the gold NPs prefer to aggregate to form larger NPs rather than to nucleate and form new NPs. In contrast, at pH 3 and 4, more functional groups (carbonyl and hydroxyl) are available for gold binding; thus a higher number of Au(III) complexes would bind to the biomass at the same time. Dwivedi and Gopal (2010) revealed that silver and gold NPs are stable in a wider range of pH as they observed very small variation in the zeta potential values between pH 2–10 in their study using Chenopodium album. Veerasamy et al. (2011) while working on mangosteen leaf extract reported that at low pH, aggregation of silver NPs is favored over the nucleation. However, higher pH facilitates the nucleation and subsequent formation of large number of NPs with smaller diameter. Nalawade et al. (2014) reported the synthesis of spherical silver NPs (~20–150 nm) using an aqueous extract of Acacia auriculiformis (as the reducing and capping agents). FTIR indicated the role played by hydroxyl groups of polyphenols and ester groups of terpenoids in the reduction of Ag+ ions. Increase in the pH of the extract caused the formation of the NPs immediately after addition of Ag+ ions. At pH 11, instant formations of silver NPs was observed. The color of the silver solution was also found to be changed from brown to yellow when pH of the extract was increased up to pH 11. It was observed that as pH of the solution increased above 2.3, the wavelength of absorption maximum due to silver NPs also decreased linearly from 432 to 403 nm. It was also observed that the silver NPs obtained at pH higher than 9.5 have shown enhanced colloidal stability compared to those obtained at lower pH. Moreover, the silver NPs obtained at pH 11 were stable for more than 2 months at room temperature and above 7 months at 4 °C. Further, it was observed that the NPs formed at higher pH are nearly monodispersed with polygonal morphology (Nalawade et al. 2014).
In case of Pinus eldarica, by increasing the pH of the reaction mixture, an increase in absorbance was observed, which could be due to the increase in production of colloidal silver NPs and reduction rate. It seems that pH affected the amount of nanoparticle formation and stability of them. Furthermore, pH influenced the rate of the reduction reaction. The reaction mixture turned brown when silver was reduced, and the reaction mixture coloring accelerated with increasing pH. Furthermore, the formation of large-sized silver NPs was observed at lower or acidic pH; while higher or alkaline pH highly dispersed, small-sized NPs tended to form (Iravani and Zolfaghari 2013).
11.4.2 Temperature
Temperature might be one of the crucial factors dominating the size and shape of NPs. It was reported that when the reaction temperature was increased from 25 to 150 °C, the size of silver and gold NPs became smaller which resulted into sharpness of plasmon resonance band of them (Dubey et al. 2010b). This is so because with the increase in temperature the rate of reaction will be increased which thus enhances the synthesis of NPs (Dwivedi and Gopal 2010; Philip 2009). Since the sharpness in absorbance peak depends on the size of the synthesized NPs, as with higher temperature, the particle size may be smaller, which results into the sharpening of the plasmon resonance band of silver and gold NPs (Shaligram et al. 2009). Andreescu et al. (2007) reported a rapid synthesis rate of silver NPs at higher temperatures. The increase in the reaction rate due to increasing the reaction temperature have been also reported by Rai et al. (2006) in case of gold nanotriangles biosynthesis using lemongrass extract as well as Song et al. (2009) in case of silver nanoparticle synthesis using persimmon and magnolia leaf extract. Song et al. (2009) have shown that silver NPs could be rapidly synthesized by using Magnolia leaf extract requiring 11 min for up to 90 % conversion at a reaction temperature of 95 °C. It can be concluded that the rate of synthesis of the NPs was related to the reaction and incubation temperature, and increased temperature levels allowed nanoparticle growth at a faster rate. Gericke and Pinches (2006) observed that nanorod and platelet-shaped gold NPs were synthesized at higher temperatures while the spherical-shaped NPs formed at lower temperatures. Moreover, Sathiskumar et al. (2010) realized the increase in the surface plasmon resonance with the increase in temperature, confirming the positive correlation between the yield of the NPs and the temperature.
11.4.3 Amount of Biomass/Plant Extract
Song et al. (2009) reported that the use of a low concentration of phyllanthin extract reacting with HAuCl4 led to synthesis of hexagonal or triangular gold NPs, but spherical NPs could be formed by addition of higher concentrations of the extract. Dubey et al. (2010b) have used different quantities of fruit extracts. They found that larger quantities of fruit extract leads to an increase in the peak absorbance in UV/Vis spectrum. Moreover, a decrease in particle size of Ag and Au NPs has been reported due to an increase in the extract amount. Dwivedi and Gopal (2010) demonstrated that with increasing ratio of leaf extract, the color of reaction mixture changed from reddish-yellow to deep red and pink to reddish pink for silver and gold NPs, respectively. In addition, the influences of the initial concentrations of the tea extract on the silver NPs productivity were studied at 25 °C. The stock solution of tea extract was diluted to 1, 5, 10, 25, 50, and 100 % (v/v) and was used as working solution, with the total organic carbon (TOC) of 1.0, 2.0, 5.0, 10.1, and 20.2 g/L, respectively. Consequently, the synthesis efficiencies of silver NPs were 99.1, 99.7, 99.9, 99.8, 94.6, and 95.3 % (w/w) with 1, 5, 10, 25, 50, and 100 % (v/v) tea extract, respectively. The production efficiencies of silver NPs were all above 94 % (w/w), while the highest nanosilver synthesis efficiency was achieved with 5 % (v/v) tea extract (Sun et al. 2014).
11.4.4 Substrate Concentration
One of the important measures to make the reaction more economical and efficient is finding the maximum concentration of the substrate which could be converted to the final product. We demonstrated green synthesis of spherical silver NPs using P. eldarica bark extract. The results obtained from time course of reaction indicated that by gradual increase in concentration of silver nitrate, the nanoparticle synthesis was also increased (Iravani and Zolfaghari 2013). In another study, Kora et al. (2012) reported the synthesis of spherical silver NPs (~7.5 ± 3.8 nm) using the aqueous extract of gum olibanum (Boswellia serrata), a renewable natural plant biopolymer. The water-soluble compounds in the gum serve as dual functional reducing and stabilizing agents. It was demonstrated that upon increasing the concentration of metal precursor, the intensity of the absorption band was increased, due to an enhancement in the nuclei formation, resulted in the synthesis of larger number of NPs.
11.4.5 Reaction Time
It was observed that the contact time may play a crucial role in the synthesis of NPs. It seems that due to the instability of the NPs formed, an optimum duration is required for complete nucleation and subsequent stability of the synthesized NPs. For instance, in one study, it was reported that the optimum time required for the completion of the reaction for silver nanoparticle synthesis was 60 min (Veerasamy et al. 2011). The requirement of optimum reaction time for the stability of synthesized silver and gold NPs using Rosa damascene has been reported, as well (Ghoreishi et al. 2011). Dwivedi and Gopal (2010) reported an increase in the sharpness of UV absorption spectra peaks with the increase in the contact time, while working with Chenopodium leaf extract. They reported that NPs appeared within 15 min of the reaction and were increased up to 2 h, but after that only slight variation was occurred. Furthermore, Dubey et al. (2010b) observed that in T. vulgare mediated synthesis, the formation of silver and gold NPs started with in 10 min of the reaction. In addition, they found that the increase in the contact time is responsible for the sharpening of the peaks in both silver and gold NPs. Roopan et al. (2012b) studied the effect of duration for the formation of titanium dioxide NPs using aqueous extract of A. squamosa fruit peel. Consequently, at each and every 2 h interval the reaction mixture was subjected to UV-Vis analysis. It was found that the sample which was heated for 6 h gave the higher absorbance value and that the absorbance value decreased with further increase in the time of heating. Therefore, in order to form titanium dioxide NPs, 6 h was found to be most effective (Roopan et al. 2012b).
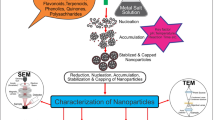
11.5 Mechanistic Aspects
In general, several mechanisms have been proposed (summarized in Table 11.2). Shankar et al. (2004a) reported that gold nanoparticle synthesis takes place due to the reduction of aqueous chloroaurate by reducing aldoses and ketones. Richardson et al. (2006) also reported that leaf extracts containing carbohydrates and proteins serve as reducing agents for silver ions. Jacob et al. (2012) reported a cost-effective and eco-friendly technique for green synthesis of spherical silver NPs (~17.6–41 nm) from silver nitrate solution (1 mM) using the extract of Piper longum leaf as reducing as well as capping agent. The synthesized NPs were also exhibiting excellent cytotoxic effect on HEp-2 cell lines. The FTIR spectrum of silver NPs showed distinct peaks 1,660 cm−1, which represent the involvement of C=N in the plane vibrations of amino acids, 1,031–1,225 cm−1 represent the involvement of C–N in the plane vibrations of aliphatic amines. The above bonds commonly occur in proteins indicating the presence of proteins as ligands for silver NPs, which increase the stability of synthesized NPs. A peak at 1,225 cm−1 was assigned to the C–O group of polyols, indicating that polyols are playing a major role in the reduction of silver NPs. Two new alkaloids, piperlongumine and piperlonguminine, were isolated from Piper longum, and might be responsible for the reduction of silver nitrate into silver NPs. Rest of the bands showed resemblance to alkenes (617–702 cm−1) and aromatic (900 cm−1) groups, which is present in the plant extract and might have an effect in the synthesis of NPs (Jacob et al. 2012). Huang et al. (2007) reported green synthesis of silver NPs in the presence of reductive biomolecules present in C. camphora leaf extract. In the FTIR spectra, the presence of functional groups like –C–O–C, –C–O–, –C=C, and –C=O–, derived from several heterocyclics, was observed. These bioactive compounds are presumed to act as reducing and capping agents for the silver NPs. Li et al. (2007) proposed recognition reduction-limited nucleation besides a growth model to explain the silver NPs production. In the recognition phase, the silver ions were trapped on the surface of proteins present in the Capsicum annum extract through electrostatic interaction, then, probably the proteins reduce the silver ions, resulting in nucleation of silver. Afterward, the proteins and biomolecules present in the reaction mixture lead to isotropic growth of silver nuclei with stabilization of silver NPs.
Sathishkumar et al. (2012) reported the green synthesis of FCC crystalline silver NPs (~10–60 nm) using leaf extract of Morinda citrifolia L. under different temperatures and reaction periods. It was observed that these NPs had moderate inhibitory effects against human pathogens than the crude plant extract, demonstrating its antimicrobial value against pathogenic diseases. The FTIR spectrum analysis of the synthesized silver NPs and the plant extract shows variation in transmittance and modification stretches at 3394, 2078, 1408, 1025, 1638, and 672 cm−1 in M. citrifolia plant extract, which might be due to the interaction of biomolecules with silver NPs. The results depicted the presence of phenolic compounds such as flavonoids, triterphenoids present in the leaf extract which might possibly influence the reduction and stabilization of the synthesized silver NPs (Sathishkumar et al. 2012). Shankar et al. (2003a) reported the presence of proteins and secondary metabolites in the water-soluble fractions of geranium leaves and postulated that terpenoids contributes in the reduction of silver ions and are oxidized to carbonyl groups. They suggested that hydroxyls in the terpenoids present in geranium leaf extract, like citronellol and geraniol, are oxidized to carbonyl groups and hence act as a reducing agent for silver ions. They have assigned a band of 1,748 cm−1 to ester C=O group of chlorophyll in FTIR analysis supporting their hypothesis. In another study, the proteins were found to be involved in the surface capping of gold NPs synthesized using geranium leaf extract (Shankar et al. 2003b). Vanaja and Annadurai (2012) suggested that the presence of aromatic amine, amide(I) group, phenolic groups, and secondary alcohols in Coleus aromaticus leaf extract may act as reducing agents for the synthesis of silver NPs. Moreover, Safaepour et al. (2009) used geraniol extract for the reduction of silver ions and found that geraniol possesses the ability to synthesize silver NPs by reducing silver ions. The study reported synthesis of uniformly dispersed silver NPs in the size range of 1–100 nm. Kasthuri et al. (2009a) isolated phyllanthin from the plant Phyllanthus amarus and used different concentrations of phyllanthin extract for the synthesis of gold and silver NPs. The FTIR studies demonstrated a shift in the methoxy (–OCH3) band (1,088 cm−1) of the phyllanthin extract after silver or gold nanoparticle formation. This change can be attributed to the binding of the –OCH3 group with the NPs. This shift, in gold NPs, was slightly higher than with the silver NPs, indicating that the adsorption of phyllanthin on the surface of gold NPs was more efficient than on silver NPs. Depending on the phyllanthin concentration, different shapes of the gold and silver NPs were obtained.
Haverkamp and Marshall (2009) reported that plants have limited capacities for reducing the metal ions and this capacity relies on the reduction potential of the metal species. During their work on the uptake of various silver salts solution by hydroponically grown Barssica juncea, they proposed that metal nanoparticle formation by plants is restricted to precious and semiprecious metals (Haverkamp and Marshall 2009). Jatropha latex obtained from J. curcas was used as reducing and capping agent to form silver NPs (~20–30 nm). The data based on this plant revealed that the major peptide constituents of the latex are curcacycline A (cyclic octapeptide) and curcacycline B (cyclic nonapeptide) (Bar et al. 2009a). The FTIR analysis before and after silver nanoparticle formation showed decreased intensity of the secondary amine band and a shift of the stretching vibration of the (NH) C=O group. These changes can be attributed to the reduction of silver ions and to the binding of the (NH) C=O group to the NPs, respectively. These results indicated that the cyclic peptides curcacycline A and B were involved in the reduction of silver ions to silver NPs and in their stabilization (Bar et al. 2009a). The authors of this article suggest a possible mechanism to form silver NPs. In this mechanism, first of all the silver ions are entrapped in the core structure of the cyclic peptide. Then, the ions are reduced and stabilized in situ by the amide group of the host peptide under the experimental conditions. The sizes of these silver NPs were similar to the radius of the cavity of the cyclic peptides, confirming this mechanism (Bar et al. 2009a).
Ganeshkumar et al. (2013) developed a method to synthesize monodispersed gold NPs by mixing gold solution with fruit peel extract of Punica granutum without using any surfactant or external energy. Casein, being a biocompatible polymer, is used to couple the prepared gold NPs for functionalization of folic acid, which is highly expressed in cancer cells. These functionalized gold NPs could be used for targeted drug delivery for cancer therapy with enhanced therapeutic efficacy and minimal side effects. Hemocompatibility of the synthesized gold NPs was evaluated in human blood samples and found that the particles were hemocompatible. The gold NPs were facilely obtained via the addition of plant extract into Au3+ solution within 30 s. The preparation of gold NPs was so efficient that the synthetic process could be completed within 60 s, which indicated a great practical value for the large-scale preparation of gold NPs. Epicalocatechine present in the plant extract could be a reason for the reduction of Au3+ to form Au0. The authors of this study mentioned that the gold NPs prepared using tea extract did not show much stability, but the gold NPs prepared by this method were found to be very stable, which might be due to the presence of ellagitannins like punicalagin. The punicalagin molecule first chelated with Au3+ through its adjacent phenolic hydroxyls to form a five-membered chelate ring. Due to the high oxidation-reduction potential of Au3+, the reacted adjacent phenolic hydroxyls of punicalagin were inductively oxidated to the corresponding quinones, and thus the Au3+ were reduced to Au0 atoms. These neighbor Au0 atoms further collided with each other to form gold NPs with high stability. The FTIR spectrum of pomegranate extract showed a peak at 3,380 cm−1 which could be attributed to the stretching vibration of phenolic hydroxyls (O‒H bond) in extracts, the absorption peaks at 1445, and 1623 cm−1 represent aromatic ring present in the extract. However, in the FTIR spectrum of the synthesized gold NPs, the peak at 3,400 cm−1 becomes relatively narrow which could be attributed to the stretching vibration of phenolic hydroxyls (O‒H bond), confirm the interactions of phenolic hydroxyls to gold NPs, resulting in the partial destruction of hydrogen bonds among pomegranate extract molecules. A shift in the peak at 1,346–1,384 cm−1 also indicated that pomegranate extract interacted with gold NPs through its adjacent phenolic hydroxyls and/or formed quinones (Ganeshkumar et al. 2013).
Silver NPs were prepared by using plant extracts from xerophytes (Bryophyllum sp.), mesophytes (Cyperus sp.), and hydrophytes (Hydrilla sp.). For xerophytes (like Bryophyllum sp.), a mechanistic scheme was suggested. In this mechanism, malic acid undergoes oxidative decarboxylation to produce pyruvate in the presence of NAD+/NADP+-dependent malic enzyme. Furthermore, pyruvate in the presence of phosphoenolpyruvate carboxykinase produces phosphoenolpyruvate that is used in the glycolytic pathway. Also, emodin, which is an anthraquinone, undergoes redial tautomerization that leads to reduction of silver ions (Jha et al. 2009b). A different mechanism was proposed for mesophytes (Cyperus sp.) which have well-defined metabolic pathways. In this mechanism, silver nanoparticle synthesis might result due to tautomerization of quinones. Benzoquinones, for example, cyperquinone (type I), dietchequinone (type II), and remirin (type III), are reported to undergo redial tautomerization for reduction of silver ions (Jha et al. 2009b). In hydrophytes (Potamogeton sp. or Hydrilla sp.), the antioxidant ascorbate is oxidized in antioxidative reactions and the enzyme dehydroascorbate reductase catalyzes the re-reduction of dehydroascorbate to ascorbate. Apart from the antioxidants, the hydrophytes are also known to possess catechol and protocatechuic acid. In alkaline conditions, catechol oxidizes to protocatechuic acid through protocatechaldehyde, presumably with hydrogen participation in the reduction of silver ions and synthesis of silver NPs. Jha et al. (2009b) suggested possible mechanisms of silver nanoparticle synthesis using plant extracts based on different metabolites or metabolic pathways. In the same direction, using leaf extract from Psidium guajava on an aqueous gold chloride solution gave stable poly-shaped gold NPs. Zeta potential experiments showed that the biogenic colloidal functionalized NPs kept their stabilities up to 30 weeks of storage. It was found that flavonoids in the extracellular solution from the leaves were responsible for the biosynthesis of the gold NPs (Raghunandan et al. 2009). A biosynthesis of silver and gold NPs from aqueous leaf extract of Chenopodium album, an obnoxious weed, was carried out given quasi-spherical-shaped NPs (~10–30 nm), probably through some chemical constituent in the extract acting as reductant (e.g., oxalic acid and aldehyde groups) (Dwivedi and Gopal 2010). During the biomimetic synthesis of silver NPs by Citrus lemon, Prathna et al. (2011) demonstrated the ability of citric acid as both the principal reducing agent for nanoparticle synthesis process and the probable stabilizing agent. In another study with tamarind leaf broth, Ankamwar et al. (2005a) investigated the possibility of the acid group (tartaric acid) to act as a capping agent and to become responsible for the stability of bio-reduced gold NPs.
We reported the formation of spherical silver NPs (~10–40 nm) using Pinus eldarica bark extract (Iravani and Zolfaghari 2013). P. eldarica bark contains phenolic compounds such as catechin, taxifolin, caffeic acid, and ferulic acid (Iravani and Zolfaghari 2011). We determined the chemical composition of P.eldarica bark extract. A reversed-phase high pressure liquid chromatography (RP-HPLC) method for the determination of catechin, caffeic acid, ferulic acid, and taxifolin in P. eldarica bark extract was developed. A mixture of 0.1 % formic acid in deionized water and 0.1 % formic acid in acetonitrile was used as the mobile phase, and chromatographic separation was achieved on a Nova pack C18 at 280 nm. Among four marker compounds, the main substances identified in P. eldarica was catechin (Iravani and Zolfaghari 2014). These phenolic compounds were possibly responsible for the synthesis of NPs (Iravani and Zolfaghari 2013).
FTIR analysis was carried out to identify possible functional groups responsible for the synthesis of silver NPs by using G. jasminoides E. extract (Fenfen et al. 2014). Consequently, through the peak identification, it was reported that the compounds responsible for the synthesis of silver NPs might include saccharides, polyphenols, aldehydes, alcohol ketones, and carbonyl compounds. The authors of this study speculated that the synthesis of silver NPs was due to the reduction of aqueous silver nitrate by the saccharides, carbonyl compounds or phenolic hydroxyl group, and the aldehydes ketones binding to silver nanoparticles act as protective group. Results from the component analysis showed that proteins, flavonoid, reducing sugar, polyphenol, geniposide and chlorogenic acid were important for the formation of silver NPs. Especially, the content of flavonoid, polyphenols and chlorogenic acid had a significant drop after reaction. To further ascertain the role of the components in G. jasminoides E. extract, pure chemicals of these active components were utilized and corresponding identifying experiments showed that rutin, gallic acid and chlorogenic acid had reducing and protecting capacities, and bovine albumin was a potential capping agent. Moreover, geniposide exhibited good shape-directing capacity for the formation of silver nanowires (Fenfen et al. 2014).
In the case of tea extract, results from FTIR spectroscopy showed a shift in peaks: 3,420–3,371 (due to N–H stretching, amides), 2,931–2,925 (due to C–H stretching, alkanes), 1,383–1,371 (characteristic of hydroxyl groups, phenolic hydroxyl), and 1,051–1,044 cm−1 (due to C-stretching, ether groups). Further, the synthesized silver NPs showed peaks at 1695, 1452, 1241, and 926 cm−1 related to alkene groups (C=C stretching), tertiary ammonium ions, poly phenols, aliphatic amines (C–N stretching vibrations), and alkene groups (C–H stretching), respectively. The FTIR analysis indicated the involvement of amides, carboxyl, amino groups and poly phenols in the synthesized silver NPs (Sun et al. 2014). Moreover, Begum et al. (2009) suggested that polyphenols or flavonoids present in black tea leaf extracts were responsible for silver and gold nanoparticle synthesis. They reached to this conclusion silver or gold nanoparticle was not observed in the leaf extract lacking polyphenols/flavonoids. Sathishkumar et al. (2009c) published similar results during palladium nanoparticle synthesis using C. longa tuber extract. Aswathy Armol and Philip (2012b) reported that fenugreek (Trigonella foenum-graecum) seed extract has the ability to perform dual function of reduction and stabilization of gold NPs (~15–25 nm). They elaborated that flavonoids present in seed extract are powerful reducing agents which may be responsible for the reduction of chloroauric acid, whereas the carboxylate group present in proteins can act as surfactant to attach on the surface of gold NPs and stabilize them through electrostatic stabilization. Kasthuri et al. (2009c) used apiin, which is a flavonoid glycoside isolated from L. inermis leaves for the synthesis of gold and silver NPs. Results obtained from FTIR spectroscopy confirmed that the carbonyl group of apiin contributes to the interaction between the NPs and apiin. Moreover, Raghunandan et al. (2009) found that flavonoids in the guava (Psidium guajava) leaf extract were responsible for the synthesis of gold NPs. Song et al. (2009) also reported that various plant extracts can be used to synthesize the metallic NPs owing to the existence of terpenoids and reducing sugars in them. During their study on the synthesis of gold nanoparticle with M. kobus, they reported that terpenoids having the functional groups of amines, alcohols, ketones, aldehydes, and carboxylic acids were responsible for the stabilization of gold NPs. Furthermore, Song et al. reported that platinum nanoparticle synthesis using Diopyros kaki leaf extract is not an enzyme mediated process because the rate of nanoparticle synthesis was found to be greatest at the temperature as high as 95 °C and there were no peaks associated with protein/enzymes on the FTIR analysis (Song et al. 2010). Moreover, Narayanan and Sakthivel (2011) reported that plant-mediated synthesis could not be happened by enzymes as generally the plant extract is heated up to 90 °C during the synthesis process. According to them, phytochemicals such as phenolics, terpenoids, sesquiterpenes, and flavonoids and the functional groups present in these phytochemicals are involved in the reduction and capping of NPs. Similarly, saponins present in the aqueous leaf extract of Memecylon edule also contributed to the reduction of silver and gold ions to nanosized silver and gold particles respectively (Elavazhagan and Arunachalam 2011). Mesocarp layer extract of C. nucifera was used and assessed for the synthesis of silver NPs (~23 ± 2 nm) (Roopan et al. 2013). The reduction of silver ions occurred when silver nitrate solution was treated with aqueous extract of C. nucifera coir at 60 °C. In this study, GC–MS of coir extract confirmed the presence of hydrocarbon such as nonacosane and heptacosane which may possibly influence the reduction process and stabilization of silver NPs. Synthesized silver NPs were effective antilarvicidal agents against Anopheles stephensi and Culex quinquefasciatus (Roopan et al. 2013). Nabikhan et al. (2010) reported the effect of extracts from tissue culture-derived callus and leaf of the saltmarsh plant, Sesuvium portulacastrum L. on synthesis of antimicrobial silver NPs using silver nitrate as a substrate. It was demonstrated that the callus extract could be able to produce silver NPs, better than leaf extract. There were prominent peaks in the extracts corresponding to amide I, II, and III indicating the presence of the protein, as revealed by FTIR spectroscopy measurement. There were also peaks that were corresponding to aromatic rings, geminal methyls, and ether linkages, indicating the presence of flavones and terpenoids responsible for the stabilization of the silver NPs (Nabikhan et al. 2010).
11.6 Conclusion
In general, different methods have been developed to obtain NPs of various shapes and sizes, but most of them can cause contamination, from precursor chemicals, organic solvents, and hazardous by-products, which often raise environmental concerns. Therefore, there is a significant benefit in developing nontoxic, cheap, and eco-friendly processes for nanoparticle synthesis. Plants are one of the best candidates for synthesizing NPs. Green synthesis of NPs using plants is regarded as a safe, cost-effective, sustainable, and clean process. Plants have successfully been used to produce NPs such as magnetite, silica, silver, gold, titania, selenium, platinum, palladium, quantum dots, zirconia, indium oxide, zinc oxide, titanium dioxide, silver–gold alloy and etc. The use of plants in green nanotechnology and nanobiotechnology is developing rapidly because of the ease of handling and formation of NPs. By using the plants (biomass or extracts) in the reaction mixture, the formation of NPs with controlled morphologies and sizes can be achieved. The synthesized NPs have wide applications in catalysis, biomedicine, electronics, sensing, photonics, environmental clean-up, imaging, bio-labeling, drug and gene delivery, and pharmaceutical sciences. Concluding remarks and research challenges for phytosynthesis of NPs using plants are mentioned below:
-
1.
Actually, most of the researches focused their attention on silver and gold nanoparticle synthesis, and it seems that more efforts are needed for green synthesis of other metallic NPs.
-
2.
Major drawbacks associated with phytosynthesis of NPs are tedious purification steps and poor understanding of the mechanisms. Some investigations have put forward hypothetical mechanisms proposing that the reduction of the metal ions to metal NPs may be due to the distinct compounds present in the extracts including reducing sugars, phenolic compounds, proteins/enzymes, amino acids, flavonoids, polysaccharides, alkaloids, tartaric acid, tannic acid, alcoholic compounds, vitamins, citric acids, phyllanthin, pralines, tartaric acid, tannic acid, and etc. In addition to use of whole plant extracts, the pure natural products (small molecules or macromolecules) can be used for reducing and stabilizing the metallic NPs. It seems that by using these natural products, there is better control over the size, shape, and agglomeration. Then the synthesized NPs are stable and destined for many potential applications. In this context, there have been significant advances.
-
3.
Qualitative routine-based spectrophotometric analysis such as FTIR, colorimetric assays, and UV/Vis spectroscopy can give information about reducing agents responsible for metallic ions reduction. However, these methods cannot unambiguously differentiate the presence or absence of the different families of compounds in the extracts and the resulting NPs. Thus, the role of the various components of plant extracts in the metal ions reduction or stabilization has not been clearly understood or addressed. Therefore, more efforts such as using advanced chromatographic techniques (e.g., HPLC-MS and NMR) are needed to find the exact mechanisms of nanoparticle formation which may lead to fine tuning of the process ultimately leading to the synthesis of NPs with a strict control over the size and shape parameters. In some studies, advanced chromatographic techniques have been used.
-
4.
Phytosynthesis of NPs is still in a developing stage, and the stability and aggregation of NPs, control of crystal growth, morphology, size, and size distribution are the most experienced problems. The important challenges frequently encountered in the synthesis of NPs are to control the shapes and sizes of the particles as well as to achieve the monodispersity in solution phase. Several important parameters including substrate concentration, electron donor (e.g., glucose or fructose), reaction or incubation time, pH, temperature, buffer strength, mixing speed, and light should be optimized. It seems that by optimization of these critical parameters, highly stable NPs with desired sizes and morphologies can be achieved. In addition, purification, isolation, and stabilization of the synthesized NPs are very important, and challenges in this regard must be overcome.
-
5.
It seems that several important challenges, and technical problems and otherwise, should be overcome before this green method will be a successful and competitive alternative for industrial synthesis of NPs. An important challenge is scaling up for production level processing. Furthermore, little is known about the mechanistic aspects, and information in this regard is necessary for economic and rational development of phytosynthesis of NPs. The large-scale phytosynthesis of NPs is interesting because it does not need any hazardous, toxic, and expensive chemicals for synthesis and stabilization of the synthesized NPs.
References
Ahmad N, Sharma S, Alam Md K et al (2010) Rapid synthesis of silver nanoparticles using dried medicinal plant of basil. Colloids Surf B 81:81–86
Ahmad N, Sharma S, Singh VN et al (2011) Biosynthesis of silver nanoparticles from Desmodium triflorum: a novel approach towards weed utilization. Biotechnol Res Int. doi:10.4061/2011/454090
Ahmed KBA, Subramanian S, Sivasubramanian A et al (2014) Preparation of gold nanoparticles using Salicornia brachiata plant extract and evaluation of catalytic and antibacterial activity. Spectrochim Acta Part A Mol Biomol Spectrosc 130:54–58
Ajitha B, Ashok Kumar Reddy Y, Reddy PS (2014) Biosynthesis of silver nanoparticles using Plectranthus amboinicus leaf extract and its antimicrobial activity. Spectrochim Acta Part A Mol Biomol Spectrosc 128:257–262
Ali DM, Thajuddin N, Jeganathan K et al (2011) Plant extract mediated synthesis of silver and gold nanoparticles and its antibacterial activity against clinically isolated pathogens. Colloids Surf B 85:360–365
Amin M, Anwar F, Janjua MRSA et al (2012) Green synthesis of silver nanoparticles through reduction with Solanum xanthocarpum L. berry extract: characterization, antimicrobial and urease inhibitory activities against Helicobacter pylori. Int J Mol Sci 13:9923–9941
Andreescu D, Eastman C, Balantrapu K et al (2007) A simple route for manufacturing highly dispersed silver nanoparticles. J Mater Res 22:2488–2496
Ankamwar B (2010) Biosynthesis of gold nanoparticles (green-gold) using leaf extract of Terminalia catappa. E-J Chem 7:1334–1339
Ankamwar B, Chaudhary M, Mural S (2005a) Gold nanotriangles biologically synthesized using tamarind leaf extract and potential application in vapor sensing. Synth React Inorg Metal-Org Nanometal Chem 35:19–26
Ankamwar B, Damle C, Ahmad A et al (2005b) Biosynthesis of gold and silver nanoparticles using Emblica officinalis fruit extract, their phase transfer and transmetallation in an organic solution. J Nanosci Nanotechnol 5(10):1665–1671
Antony JJ, Sivalingam P, Siva D et al (2011) Comparative evaluation of antibacterial activity of silver nanoparticles synthesized using Rhizophora apiculata and glucose. Colloids Surf B 88:134–140
Armendariz V, Herrera I, Peralta-Videa JR et al (2004a) Size controlled gold nanoparticle formation by Avena sativa biomass: use of plants in nanobiotechnology. J Nanopart Res 6:377–382
Armendariz V, Jose-Yacaman M, Duarte Moller A et al (2004b) HRTEM characterization of gold nanoparticles produced by wheat biomass. Rev Mex Fis Suppl 50:7–11
Arunachalam R, Dhanasingh S, Kalimuthu B et al (2012) Phytosynthesis of silver nanoparticles using Coccinia grandis leaf extract and its application in the photocatalytic degradation. Colloids Surf B 94:226–230
Aswathy Aromal S, Philip D (2012a) Benincasa hispida seed mediated green synthesis of gold nanoparticles and its optical nonlinearity. Physica E 44:1329–1334
Aswathy Aromal S, Philip D (2012b) Green synthesis of gold nanoparticles using Trigonella foenum-graecum and its size-dependent catalytic activity. Spect Acta A 97:1–5
Auffan M, Achouak W, Rose J et al (2008) Relation between the redox state of iron-based nanoparticles and their cytotoxicity toward Escherichia coli. Environ Sci Technol 42:6730–6735
Badri Narayanan K, Sakthivel N (2008) Coriander leaf mediated biosynthesis of gold nanoparticles. Mater Lett 62:4588–4590
Bankar A, Joshi B, Kumara AR et al (2010) Banana peel extract mediated novel route for the synthesis of silver nanoparticles. Colloids Surf A 368:58–63
Bar H, Bhui DK, Sahoo GP et al (2009a) Green synthesis of silver nanoparticles using latex of Jatropha curcas. Colloids Surf A 339:134–139
Bar H, Bhui DK, Sahoo GP et al (2009b) Green synthesis of silver nanoparticles using seed extract of Jatropha curcas. Colloids Surf A 348:212–216
Bar H, Bhui DKr, Sahoo GP et al (2009c) Green synthesis of silver nanoparticles using latex of Jatropha curcas. Colloids Surf A 339(1–3):134–139
Barua S, Konwarh R, Bhattacharya SS et al (2013) Non-hazardous anticancerous and antibacterial colloidal ‘green’ silver nanoparticles. Colloids Surf B 105:37–42
Beattie IR, Haverkamp RG (2011) Silver and gold nanoparticles in plants: sites for the reduction to metal. Metallomics 3:628–632
Begum NA, Mondal S, Basu S et al (2009) Biogenic synthesis of Au and Ag nanoparticles using aqueous solutions of black tea leaf extracts. Colloids Surf B 71:113–118
Bharathi A, Roopan SM, Kajbafvala A et al (2014) Catalytic activity of TiO2 nanoparticles in the synthesis of some 2,3-disubstituted dihydroquinazolin-4(1H)-ones. Chin Chem Lett 25:324–326
Bindhu MR, Umadevi M (2012) Synthesis of monodispersed silver nanoparticles using Hibiscus cannabinus leaf extract and its antimicrobial activity. Spectrochim Acta A Mol Biomol Spectrosc 101:184–190
Bindhu MR, Umadevi M (2014) Silver and gold nanoparticles for sensor and antibacterial applications. Spectrochim Acta Part A Mol Biomol Spectrosc 128:37–45
Boruah SK, Boruah PK, Sarma P et al (2012) Green synthesis of gold nanoparticles using Camellia sinensis and kinetics of the reaction. Adv Mater Lett 3:481–486
Chandran SP, Chaudhary M, Pasricha R et al (2006) Synthesis of gold nanotriangles and silver nanoparticles using Aloe vera plant extract. Biotechnol Prog 22:577
Chauhan S, Upadhyay MK, Rishi N et al (2011) Phytofabrication of silver nanoparticles using pomegranate fruit seeds. Int J Nanomater Biostruct 1:17–21
Cheirmadurai K, Biswas S, Murali R et al (2014) Green synthesis of copper nanoparticles and conducting nanobiocomposites using plant and animal sources. RSC Adv 4:19507–19511
Coccia F, Tonucci L, Bosco D et al (2012) One-pot synthesis of lignin-stabilised platinum and palladium nanoparticles and their catalytic behaviour in oxidation and reduction reactions. Green Chem 14:1073–1078
Coman C, Leopold LF, Rugină OD et al (2013) Green synthesis of gold nanoparticles by Allium sativum extract and their assessment as SERS substrate. J Nanopart Res 16:2158
Daniel SC, Ayyappan S, Philiphan NJP et al (2011) Green synthesis and transfer of silver nanoparticles in a food chain through Chiranamous Larva to zebra fish—a new approach for therapeutics. Int J Nanosci Nanotech 2:159–169
Das J, Das MP, Velusamy P (2013) Sesbania grandiflora leaf extract mediated green synthesis of antibacterial silver nanoparticles against selected human pathogens. Spectrochim Acta Part A Mol Biomol Spectrosc 104:265–270
Dash SS, Majumdar R, Sikder AK et al (2014) Saraca indica bark extract mediated green synthesis of polyshaped gold nanoparticles and its application in catalytic reduction. Appl Nanosci 4:485–490
Deshpande R, Bedre MD, Basavaraja S et al (2010) Rapid biosynthesis of irregular shaped gold nanoparticles from macerated aqueous extracellular dried clove buds (Syzygium aromaticum) solution. Colloids Surf B 79:235–240
Diao M, Yao M (2009) Use of zero-valent iron nanoparticles in inactivating microbes. Water Resour 43:5243–5251
Dinesh S, Karthikeyan S, Arumugam P (2012) Biosynthesis of silver nanoparticles from Glycyrrhiza glabra root extract. Arch Appl Sci Res 4:178–187
Dineshkumar B, Krishnakumar K, Paul D et al (2012) Musa sapientum L. leaves: synthesis of silver nanoparticles. Int J Nanomater Biostruct 2:22–24
Dipankar C, Murugan S (2012) The green synthesis, characterization and evaluation of the biological activities of silver nanoparticles synthesized from Iresine herbstii leaf aqueous extracts. Colloids Surf B 98:112–119
Donda MR, Kudle KR, Alwala J et al (2013) Synthesis of silver nanoparticles using extracts of Securinega leucopyrus and evaluation of its antibacterial activity. Int J Curr Sci 7:1–8
Dubey M, Bhadauria S, Kushwah B (2009) Green synthesis of nanosilver particles from extract of Eucalyptus hybrida (Safeda) leaf. Dig J Nanomater Biostruct 4:537–543
Dubey SP, Lahtinen M, Särkkä H et al (2010a) Bioprospective of Sorbus aucuparia leaf extract in development of silver and gold nanocolloids. Colloids Surf B 80:26–33
Dubey SP, Lahtinen M, Sillanpää M (2010b) Tansy fruit mediated greener synthesis of silver and gold nanoparticles. Process Biochem 45:1065–1071
Dwivedi AD, Gopal K (2010) Biosynthesis of silver and gold nanoparticles using Chenopodium album leaf extract. Colloids Surf A 369:27–33
Dwivedi AD, Gopal K (2011) Plant- mediated biosynthesis of silver and gold nanoparticles. J Biomed Nanotechnol 7:163–164
Elavazhagan T, Arunachalam KD (2011) Memecylon edule leaf extract mediated green synthesis of silver and gold nanoparticles. Int J Nanomed 6:1265–1278
Elumalai EK, Prasad TNVKV, Hemachandran J et al (2010) Extracellular synthesis of silver nanoparticles using leaves of Euphorbia hirta and their antibacterial activities. J Pharm Sci Res 2:549–554
Fazaludeena MF, Manickamb C, Ashankytyc MAI et al (2012) Synthesis and characterizations of gold nanoparticles by Justicia gendarussa Burm F leaf extract. J Microbiol Biotech Res 2:23–34
Fenfen L, Yixian G, Jiale H et al (2014) Roles of biomolecules in the biosynthesis of silver nanoparticles: case of Gardenia jasminoides extract. Chin J Chem Eng 22:706–712
Gade A, Gaikwad S, Duran N et al (2014) Green synthesis of silver nanoparticles by Phoma glomerata. Micron 59:52–59
Ganeshkumar M, Sathishkumar M, Ponrasu T et al (2013) Spontaneous ultra fast synthesis of gold nanoparticles using Punica granatum for cancer targeted drug delivery. Colloids Surf B 106:208–216
Gardea-Torresdey JL, Tiemann KJ, Gamez G et al (1999) Gold nanoparticles obtained by bio-precipitation from gold (III) solutions. J Nanopart Res 1:397–404
Gardea-Torresdey JL, Tiemann KJ, Parsons JG et al (2002a) Characterization of trace level Au (III) binding to alfalfa biomass (Medicago sativa) by GFAA. Adv Environ Res 6:313–323
Gardea-Torresdey JL, Parsons JG, Gomez E et al (2002b) Formation and growth of Au nanoparticles inside live alfalfa plants. Nano Lett 2:397–401
Gardea-Torresdey JL, Gomez E, Videa JRP et al (2003) Alfalfa Sprouts: a natural source for the synthesis of silver nanoparticles. Langmuir 19:1357
Garg S (2012) Rapid biogenic synthesis of silver nanoparticles using black pepper (Piper nigrum) corn extract. Int J Innov Biol Chem Sci 3:5–10
Geethalakshmi R, Sarada DVL (2010) Synthesis of plant mediated silver nanoparticles using Trianthema decandra extract and evaluation of their anti microbial activities. Int J Eng Sci Technol 2:970–975
Gericke M, Pinches A (2006) Biological synthesis of metal nanoparticles. Hydrometallurgy 83:132–140
Ghodake GS, Deshpande NG, Lee YP et al (2010) Pear fruit extract-assisted room-temperature biosynthesis of gold nanoplates. Colloids Surf B 75:584–589
Ghoreishi SM, Behpour M, Khayatakashani M (2011) Green synthesis of silver and gold nanoparticles using Rosa damascena and its primary applications in electrochemistry. Phys A 44:97–104
Ghosh S, Patil S, Ahire M et al (2012a) Gnidia glauca flower extract mediated synthesis of gold nanoparticles and evaluation of its chemocatalytic potential. J Nanobiotechnol 10:17
Ghosh S, Patil S, Ahire M et al (2012b) Synthesis of silver nanoparticles using Dioscorea bulbifera tuber extract and evaluation of its synergistic potential in combination with antimicrobial agents. Int J Nanomed 7:483–496
Gnanajobitha G, Annadurai G, Kannan C (2012) Green synthesis of silver nanoparticles using Elettaria cardamomom and assessment of its antimicrobial activity. Int J Pharm Sci Res 3:323–330
Gnanajobitha G, Vanaja M, Paulkumar K et al (2013) Green synthesis of silver nanoparticles using Millingtonia hortensis and evaluation of their antimicrobial efficacy. Int J Nanomater Biostruct 3:21–25
Gnanasangeetha D, SaralaThambavani D (2013a) Biogenic production of zinc oxide nanoparticles using Acalypha Indica. J Chem Biol Phys Sci 4:238–246
Gnanasangeetha D, SaralaThambavani D (2013b) One pot synthesis of zinc oxide nanoparticles via chemical and green method. Res J Mater Sci 1(7):1–8
Gopalakrishnan K, Ramesh C, Ragunathan V et al (2012) Antibacterial activity of copper oxide nanoparticles on E. coli Synthesized from Tridax procumbens leaf extract and surface coating with polyaniline. Dig J Nanomater Bios 7:833–839
Gopinath V, MubarakAli D, Priyadarshini S et al (2012) Biosynthesis of silver nanoparticles from Tribulus terrestris and its antimicrobial activity: a novel biological approach. Colloids Surf B 96:69–74
Gunalan S, Sivaraj R, Venckatesh R (2012a) Aloe barbadensis Miller mediated green synthesis of mono-disperse copper oxide nanoparticles: optical properties. Spectrochim Acta Part A Mol Biomol Spectrosc 97:1140–1144
Gunalan S, Sivaraj R, Rajendran V (2012b) Green synthesized ZnO nanoparticles against bacterial and fungal pathogens. Progr Nat Sci: Mater Int 22:693–700
Haratifar Emad al din, Shahverdi HR, Shakibaie M et al (2009) Semi-biosynthesis of magnetite-gold composite nanoparticles using an ethanol extract of Eucalyptus camaldulensis and study of the surface chemistry. J Nanomater 200963:1–5. doi:10.1155/2009/962021
Harne S, Sharma A, Dhaygude M et al (2012) Novel route for rapid biosynthesis of copper nanoparticles using aqueous extract of Calotropis procera L. latex and their cytotoxicity on tumor cells. Colloids Surf B 95:284–288
Harris AT, Bali R (2008) On the formation and extent of uptake of silver nanoparticles by live plants. J Nanopart Res 10:691–695
Haverkamp RG, Marshall AT (2009) The mechanism of metal nanoparticle formation in plants: limits on accumulation. J Nanopart Res 11:1453–1463
Haverkamp RG, Marshall AT, Agterveld DV (2007) Pick your carats: nanoparticles of gold-silver-copper alloy produced in vivo. J Nanopart Res 9:697–700
Herrera-Becerra R, Zorrilla C, Rius JL et al (2008) Electron microscopy characterization of biosynthesized iron oxide nanoparticles. Appl Phys A 91:241–246
Hiremath S, Vidya C, Antonyraj MAL et al (2013) Biosynthesis of ZnO nano particles assisted by Euphorbia tirucalli (Pencil Cactus). Int J Current Eng Technol (Special Issue1):176–179
Huang J, Li Q, Sun D et al (2007) Biosynthesis of silver and gold nanoparticles by novel sundried Cinnamomum camphora leaf. Nanotechnology 18(105):104
Huang J, Lin L, Li Q et al (2008) Continuous-flow biosynthesis of silver nanoparticles by lixivium of sundried Cinnamomum camphora leaf in tubular microreactors. Ind Eng Chem Res 47:6081–6090
Hudlikar M, Joglekar S, Dhaygude M et al (2012) Latex-mediated synthesis of ZnS nanoparticles: green synthesis approach. J Nano Res 14:1–6
Im A-R, Han L, Kim ER et al (2012) Enhanced antibacterial activities of Leonuri herba extracts containing silver nanoparticles. Phytother Res 26:1249–1255
Iravani S (2011) Green synthesis of metal nanoparticles using plants. Green Chem 13(10):2638–2650
Iravani S, Zolfaghari B (2011) Pharmaceutical and nutraceutical effects of Pinus pinaster bark extract. Res Pharm Sci 6(1):1–11
Iravani S, Zolfaghari B (2013) Green synthesis of silver nanoparticles using Pinus eldarica bark extract. Biomed Res Int. doi:10.1155/2013/639725:5
Iravani S, Zolfaghari B (2011) Phytochemical analysis of Pinus eldarica bark. Res Pharm Sci 9(4):243–250
Iravani S, Korbekandi H, Mirmohammadi SV et al (2014a) Plants in nanoparticle synthesis. Rev Adv Sci Eng 3(3):261–274
Iravani S, Korbekandi H, Mirmohammadi SV et al (2014b) Synthesis of silver nanoparticles: chemical, physical, and biological methods. Res Pharm Sci 9:385–406
Jacob SJP, Finub JS, Narayanan A (2012) Synthesis of silver nanoparticles using Piper longum leaf extracts and its cytotoxic activity against Hep-2 cell line. Colloids Surf B 91:212–214
Jagtap UB, Bapat VA (2013) Green synthesis of silver nanoparticles using Artocarpus heterophyllus L. seed extract and its antibacterial activity. Ind Crops Prod 46:132–137
Jain D, Daima HK, Kachhwaha S et al (2009) Synthesis of plant-mediated silver nanoparticles using papaya fruit extract and evaluation of their antimicrobial activities. Dig J Nanomater Bios 4:557–563
Jancy ME, Inbathamizh L (2012) Green synthesis and characterization of nano silver using leaf extract of Morinda pubescens. Asian J Pharm Clin Res 5:159–162
Jha AK, Prasad K (2010) Green synthesis of silver nanoparticles using Cycas leaf. Int J Green Nanotechnol: Phys Chem 1(2):110–117
Jha AK, Prasad K, Kumar V et al (2009a) Biosynthesis of silver nanoparticles using Eclipta leaf. Biotechnol Prog 25:1476–1479
Jha AK, Prasad K, Prasad K et al (2009b) Plant system: nature’s nanofactory. Colloids Surf B 73:219–223
Jia L, Zhang Q, Li Q et al (2009) The biosynthesis of palladium nanoparticles by antioxidants in Gardenia jasminoides E.: long lifetime nanocatalysts for pnitrotoluene hydrogenation. Nanotechnology 20. doi:10.1088/0957-4484/20/38/385601
Joglekar S, Kodam K, Dhaygude M et al (2011) Novel route for rapid biosynthesis of lead nanoparticles using aqueous extract of Jatropha curcas L. latex. Mater Lett 5:3170–3172
Karlsson MNA, Deppert K, Wacaser BA et al (2005) Size-controlled nanoparticles by thermal cracking of iron pentacarbonyl. Appl Phys A Mater Sci Process 80:1579–1583
Kasthuri J, Kathiravan K, Rajendiran N (2009a) Phyllanthin-assisted biosynthesis of silver and gold nanoparticles: a novel biological approach. J Nanopart Res 11:1075–1085
Kasthuri J, Veerapandian S, Rajendiran N (2009b) Biological synthesis of silver and gold nanoparticles using apiin as reducing agent. Colloids Surf B 68:55–60
Kaviya S, Santhanalakshmi J, Viswanathan B (2011) Green synthesis of silver nanoparticles using Polyalthia longifolia leaf extract along with D-Sorbitol: study of antibacterial activity. J Nanotechnol. doi:10.1155/2011/152970
Kelly KL, Coronado E, Zhao LL et al (2003) The optical properties of metal nanoparticles: the influence of size, shape, and dielectric environment. J Phys Chem B 107:668
Kesharwani J, Yoon KY, Hwang J et al (2009) Phytofabrication of silver nanoparticles by leaf extract of Datura metel: hypothetical mechanism involved in synthesis. J Bionanosci 3:1–6
Khan M, Khan M, Kuniyil M et al (2014) Biogenic synthesis of palladium nanoparticles using Pulicaria glutinosa extract and their catalytic activity towards the Suzuki coupling reaction. Dalton Trans 43:9026–9031
Klaus-Joerger T, Joerger R, Olsson E et al (2001) Bacteria as workers in the living factory: metal-accumulating bacteria and their potential for materials science. Trends Biotechnol 19(1):15–20
Kora AJ, Arunachala J (2012) Green fabrication of silver nanoparticles by gum Tragacanth (Astragalus gummifer): a dual functional reductant and stabilizer. J Nanomater. doi:10.1155/2012/869765
Kora AJ, Sashidhar RB, Arunachalam J (2010) Gum kondagogu (Cochlospermum gossypium): a template for the green synthesis and stabilization of silver nanoparticles with antibacterial application. Carbohydr Polym 82:670–679
Kora AJ, Sashidhar RB, Arunachalam J (2012) Aqueous extract of gum olibanum (Boswellia serrata): a reductant and stabilizer for the biosynthesis of antibacterial silver nanoparticles. Process Biochem 47:1516–1520
Korbekandi H, Iravani S, Abbasi S (2009) Production of nanoparticles using organisms. Crit Rev Biotechnol 29:279–306
Krishnaraj C, Jagan EG, Rajasekar S et al (2010) Synthesis of silver nanoparticles using Acalypha indica leaf extracts and its antibacterial activity against water borne pathogens. Colloids Surf B 76:50–56
Kudle KR, Donda MR, Alwala J et al (2012) Biofabrication of silver nanoparticles using Cuminum cyminum through microwave irradiation. Int J Nanomater Biostruct 2:65–69
Kudle KR, Donda MR, Merugu R et al (2013) Microwave assisted green synthesis of silver nanoparticles using Stigmaphyllon littorale leaves, their characterization and anti microbial activity. Int J Nanomater Biostruct 3:13–16
Kuhn LT, Bojesen A, Timmermann L et al (2002) Structural and magnetic properties of core-shell iron-iron oxide nanoparticles. J Phys: Condens Matter 14:13551–13567
Kumar DA (2012) Rapid and green synthesis of silver nanoparticles using the leaf extracts of Parthenium hysterophorus: a novel biological approach. Int Res J Pharm 3:169–173
Kumar V, Yadav SC, Yadav SK (2010) Syzygium cumini leaf and seed extract mediated biosynthesis of silver nanoparticles and their characterization. J Chem Technol Biotechnol 85:1301–1309
Kumar KM, Sinha M, Mandal BK et al (2012a) Green synthesis of silver nanoparticles using Terminalia chebula extract at room temperature and their antimicrobial studies. Spectrochim Acta Part A 91:228–233
Kumar R, Roopan SM, Prabhakarn A et al (2012b) Agricultural waste Annona squamosa peel extract: biosynthesis of silver nanoparticles. Spectrochimica Acta Part A 90:173–176
Kumar DA, Palanichamy V, Roopan S (2014) Green synthesis of silver nanoparticles using Alternanthera dentata leaf extract at room temperature and their antimicrobial activity. Spectrochim Acta Part A Mol Biomol Spectrosc 127:168–171
Lee C, Kim JY, Lee WI et al (2008) Bactericidal effect zero-valent iron nano-scale particles on Escherichia coli. Environ Sci Technol 42:4927–4933
Lee H-J, Lee G, Jang NR et al (2011) Biological synthesis of copper nanoparticles using plant extract. Nanotechnology 1:371–374
Li S, Shen Y, Xie A et al (2007) Green synthesis of silver nanoparticles using Capsicum annuum L. extract. Green Chem 9:852–858
Logeswari P, Silambarasan S, Abraham J (2013) Ecofriendly synthesis of silver nanoparticles from commercially available plant powders and their antibacterial properties. Scientia Iranica F 20:1049–1054
Lukman AI, Gong B, Marjo CE et al (2011) Facile synthesis, stabilization, and anti-bacterial performance of discrete Ag nanoparticles using Medicago sativa seed exudates. J Colloid Interface Sci 353:433–444
Machado S, Pinto SL, Grosso JP et al (2013) Green production of zero-valent iron nanoparticles using tree leaf extracts. Sci Total Environ 445–446:1–8
Maensiri S, Laokul P, Klinkaewnarong J et al (2008) Indium oxide (In2O3) nanoparticles using Aloe vera plant extract: synthesis and optical properties. J Optoelectron Adv Mater 10:161–165
Maheswari RU, Prabha AL, Nandagopalan V et al (2012) Green synthesis of silver nanoparticles by using rhizome extract of Dioscorea oppositifolia L. and their anti microbial activity against human pathogens. IOSR J Pharm Biol Sci 1:38–42
Maliszewska I, Aniszkiewicz Ł, Sadowski Z (2009) Biological synthesis of gold nanostructures using the extract of Trichoderma koningii. Acta Phys Pol, A 116:163–165
Mallikarjuna K, Dillip GR, Narashima G et al (2012) Phyto-fabrication and characterization of silver nanoparticles from Piper betle broth. Res J Nanosci Nanotech 2:17–23
Mallikarjuna K, John Sushma N, Subba Reddy BV et al (2013) Palladium nanoparticles: single-step plant-mediated green chemical procedure using Piper betle leaves broth and their anti-fungal studies. Int J Chem Anal Sci 4:14–18
Marchiol L, Mattiello A, Pošćić F et al (2014) In vivo synthesis of nanomaterials in plants: location of silver nanoparticles and plant metabolism. Nanoscale Res Lett 9:101
Mariselvam R, Ranjitsingh AJA, Usha Raja Nanthini A et al (2014) Green synthesis of silver nanoparticles from the extract of the inflorescence of Cocos nucifera (Family: Arecaceae) for enhanced antibacterial activity. Spectrochim Acta Part A Mol Biomol Spectrosc 129:537–541
Mason C, Vivekanandhan S, Misra M et al (2012) Switchgrass (Panicum virgatum) extract mediated green synthesis of silver nanoparticles. World J Nano Sci Eng 2:47–52
Mock JJ, Barbic M, Smith DR et al (2002) Shape effects in plasmon resonance of individual colloidal silver nanoparticles. J Chem Phys 116:6755–6759
Mondal S, Roy N, Laskar RA et al (2011) Biogenic synthesis of Ag, Au and bimetallic Au/Ag alloy nanoparticles using aqueous extract of mahogany (Swietenia mahogani JACQ.) leaves. Colloids Surf B 82:497–504
Mude N, Ingle A, Gade A, Rai M (2009) Synthesis of silver nanoparticles using callus extract of Carica papaya-a first report. J Plant Biochem Biotechnol 18(1):83–86
Mukherjee S, Sushma V, Patra S et al (2012) Green chemistry approach for the synthesis and stabilization of biocompatible gold nanoparticles and their potential applications in cancer therapy. Nanotechnology 23:455103. doi:10.1088/0957-4484/23/45/455103
Nabikhan A, Kandasamy K, Raj A et al (2010) Synthesis of antimicrobial silver nanoparticles by callus and leaf extracts from saltmarsh plant, Sesuvium portulacastrum L. Colloids Surf B 79:488–493
Nadagouda MN, Varma RS (2008) Green synthesis of silver and palladium nanoparticles at room temperature using coffee and tea extract. Green Chem 10:859–862
Nadagouda MN, Castle AB, Murdock RC et al (2010) In vitro biocompatibility of nanoscale zerovalent iron particles (NZVI) synthesized using tea polyphenols. Green Chem 12:114–122
Nagajyothi PC, Minh An TN, Sreekanth TVM et al (2013) Green route biosynthesis: characterization and catalytic activity of ZnO nanoparticles. Mater Lett 108:160–163
Nagati VB, Koyyati R, Donda MR et al (2012) Green synthesis and characterization of silver nanoparticles from Cajanus cajan leaf extract and its antibacterial activity. Int J Nanomater Biostruct 2(3):39–43
Naik LS, Marx KP, Sree vennela P et al (2013) Green synthesis of silver nanoparticles using Strawberry leaf extract (Arbutus unedo) and evaluation of its antimicrobial activity-a Novel study. Int J Nanomater Biostruct 3:47–50
Nalawade P, Mukherjee P, Kapoor S (2014) Biosynthesis, characterization and antibacterial studies of silver nanoparticles using pods extract of Acacia auriculiformis. Spectrochim Acta Part A Mol Biomol Spectrosc 129:121–124
Narayanan KB, Sakthivel N (2011) Extracellular synthesis of silver nanoparticles using the leaf extract of Coleus amboinicus Lour. Mater Res Bull 46:1708–1713
Nellore J, Pauline PC, Amarnath K (2012) Biogenic synthesis by Sphearanthus amaranthoids; towards the efficient production of the biocompatible gold nanoparticles. Dig J Nanomater Biostruct 7:123–133
Nethradevi C, Sivakumar P, Renganathan S (2012) Green synthesis of silver nanoparticles using Datura metel flower extract and evaluation of their antimicrobial activity. Int J Nanomater Biostruct 2:16–21
Niraimathi KL, Sudha V, Lavanya R et al (2013) Biosynthesis of silver nanoparticles using Alternanthera sessilis (L.) extract and their antimicrobial, antioxidant activities. Colloids Surf B 102:288–291
Njagi EC, Huang H, Stafford L et al (2011) Biosynthesis of iron and silver nanoparticles at room temperature using aqueous Sorghum bran extracts. Langmuir 27:264–271
Parashar UK, Saxenaa PS, Srivastava A (2009a) Bioinspired synthesis of silver nanoparticles. Dig J Nanomater Biol 4:159–166
Parashar V, Parashar R, Sharma B et al (2009b) Parthenium leaf extract mediated synthesis of silver nanoparticles: a novel approach towards weed utilization. Dig J Nanomater Biostruct 4:45–50
Parida UK, Bindhani BK, Nayak P (2011) Green synthesis and characterization of gold nanoparticles using onion (Allium cepa) extract. World J Nanosci Eng 1:93–98
Patil DC, Patil VS, Borase PH et al (2012) Larvicidal activity of silver nanoparticles synthesized using Plumeria rubra plant latex against Aedes aegypti and Anopheles stephensi. Parasitol Res 110:1815–1822
Pavani KV, Swati T, Snehika V et al (2012) Phytofabrication of lead nanoparticles using Grape skin extract. Int J Eng Sci Tech 4:3376–3380
Pavani KV, Gayathramma K, Banerjee A et al (2013) Phyto-synthesis of silver nanoparticles using extracts of Ipomoea indica flowers. Am J Nanomater 1:5–8
Petla RK, Vivekanandhan S, Misra M et al (2012) Soybean (Glycine max) leaf extract based green synthesis of palladium nanoparticles. J Biomater Nanobiotechnol 3:14–19
Philip D (2009) Biosynthesis of Ag, Au and Au–Ag nanoparticles using edible mushrooms extracts. Spectrochim Acta A 73:374–381
Philip D (2010) Green synthesis of gold and silver nanoparticles using Hibiscus rosa sinensis. Physica E 42:1417–1424
Philip D, Unni C (2010) Extracellular biosynthesis of gold and silver nanoparticles using Krishna tulsi (Ocimum sanctum) leaf. Physica E. doi:10.1016/j.physe.2010.10.006
Philip D, Unni C, Aromal SA et al (2010) Murraya koenigii leaf-assisted rapid green synthesis of silver and gold nanoparticles. Spectrochim Acta Part A Mol Biomol Spectrosc. doi:10.1016/j.saa.2010.12.060
Prasad TNVKV, Elumalai E (2011) Biofabrication of Ag nanoparticles using Moringa oleifera leaf extract and their antimicrobial activity. Asian Pac J Trop Biomed 1(6):439–442
Prathna TC, Chandrasekaran N, Raichur AM et al (2011) Biomimetic synthesis of silver nanoparticles by Citrus limon (lemon) aqueous extract and theoretical prediction of particle size. Colloids Surf B 82:152–159
Qu J, Luo C, Hou J (2011a) Synthesis of ZnO nanoparticles from Zn-hyperaccumulator (Sedum alfredii Hance) plants. Micro Nano Lett IET 6:174–176
Qu J, Yuan X, Wang X et al (2011b) Zinc accumulation and synthesis of ZnO nanoparticles using Physalis alkekengi L. Environ Pollut 159:1783–1788
Raghunandan D, Basavaraja S, Mahesh B et al (2009) Biosynthesis of stable polyshaped gold nanoparticles from microwave-exposed aqueous extracellular anti-malignant guava (Psidium guajava) leaf extract. Nanobiotechnology 5:34–41
Rai A, Singh A, Ahmad A et al (2006) Role of halide ions and temperature on the morphology of biologically synthesized gold nanotriangles. Langmuir 22:736–741
Raja K, Saravanakumar A, Vijayakumar R (2012) Efficient synthesis of silver nanoparticles from Prosopis juliflora leaf extract and its antimicrobial activity using sewage. Spectrochim Acta Part A Mol Biomol Spectrosc 97:490–494
Rajakumar G, Rahuman AA, Priyamvada B et al (2012) Eclipta prostrata leaf aqueous extract mediated synthesis of titanium dioxide nanoparticles. Mater Lett 68:115–117
Rajesh WR, Jaya RL, Niranjan SK et al (2009) Phytosynthesis of silver nanoparticle using Gliricidia sepium (Jacq.). Curr Nanosci 5:117–122
Rakesh C, Ananda S, Gowda NM (2013) Synthesis of chromium (III) oxide nanoparticles by electrochemical method and Mukia maderaspatana plant extract, characterization, KMnO4 decomposition and antibacterial study. Mod Res Catal 2:127–135
Ramamurthy CH, Padma M, Daisy mariya samadanam I et al (2013) The extra cellular synthesis of gold and silver nanoparticles and their free radical scavenging and antibacterial properties. Colloids Surf B 102:808–815
Ramesh C, Mohan kumar K, Senthil M et al (2012) Antibacterial activity of Cr2O3 nanoparticles against E.coli; reduction of chromate ions by Arachis hypogaea leaves. Arch Appl Sci Res 4:1894–1900
Ramezani N, Ehsanfar Z, Shamsa F et al (2008) Screening of medicinal plant methanol extracts for the synthesis of gold nanoparticles by their reducing potential. Z Naturforsch 63b:903–908
Ramteke C, Chakrabarti T, Sarangi BK et al (2013) Synthesis of silver nanoparticles from the aqueous extract of leaves of Ocimum sanctum for enhanced antibacterial activity. J Chem. doi:10.1155/2013/278925
Ranjan P, Das MP, Kumar MS et al (2013) Green synthesis and characterization of silver nanoparticles from Nigella sativa and its application against UTI causing bacteria. J Acad Ind Res 2:45–49
Rastogi L, Arunachalam J (2012) Microwave-assisted green synthesis of small gold nanoparticles using aqueous garlic (Allium sativum) extract: their application as antibiotic carriers. Int J Green Nanotechnol 4:163–173
Ravindra S, Murali Mohan Y, Narayana Reddy N et al (2010) Fabrication of antibacterial cotton fibres loaded with silver nanoparticles via “green approach”. Colloids Surf A 367:31–40
Richardson A, Chan BC, Crouch RD et al (2006) Synthesis of silver nanoparticles: an undergraduate laboratory using green approach. Chem Educ 11:331–333
Rodríguez-León E, Iñiguez-Palomares R, Navarro RE et al (2013) Synthesis of silver nanoparticles using reducing agents obtained from natural sources (Rumex hymenosepalus extracts). Nanoscale Res Lett 8:318
Roopan SM, Bharathi A, Kumar R et al (2012a) Acaricidal, insecticidal, and larvicidal efficacy of aqueous extract of Annona squamosa L peel as biomaterial for the reduction of palladium salts into nanoparticles. Colloids Surf B 92:209–212
Roopan SM, Bharathi A, Prabhakarn A et al (2012b) Efficient phyto-synthesis and structural characterization of rutile TiO2 nanoparticles using Annona squamosa peel extract. Spectrochim Acta Part A Mol Biomol Spectrosc 98:86–90
Roopan SM, Madhumitha G, Abdul Rahuman A et al (2013) Low-cost and eco-friendly phyto-synthesis of silver nanoparticles using Cocos nucifera coir extract and its larvicidal activity. Ind Crops Prod 43:631–635
Roy N, Barik A (2010) Green synthesis of silver nanoparticles from the unexploited weed resources. Int J Nanotechnol Appl 4:95–101
Sable N, Gaikwad S, Bonde S et al (2012) Phytofabrication of silver nanoparticles by using aquatic plant Hydrilla verticilata. Nus Biosci 4:45–49
Sadowski Z, Maliszewska GB, Polowczyk I et al (2008) Synthesis of silver nanoparticles using microorganisms. Mater Sci Pol 26:419–425
Safaepour M, Shahverdi AR, Shahverdi HR et al (2009) Green synthesis of small silver nanoparticles using geraniol and its cytotoxicity against fibrosarcoma-wehi 164. Avicenna J Med Biotech 1:111–115
Sankar R, Karthik A, Prabu A et al (2013) Origanum vulgare mediated biosynthesis of silver nanoparticles for its antibacterial and anticancer activity. Colloids Surf B 108:80–84
Santhoshkumar T, Rahuman AA, Rajakumar G et al (2010) Synthesis of silver nanoparticles using Nelumbo nucifera leaf extract and its larvicidal activity against malaria and filariasis vectors. Parasitol Res 108:693–702
Santhoshkumar T, Rahuman AA, Bagavan A et al (2012) Evaluation of stem aqueous extract and synthesized silver nanoparticles using Cissus quadrangularis against Hippobosca maculata and Rhipicephalus (Boophilus) microplus. Exp Parasitol 132:156–165
Sathishkumar M, Sneha K, Seob Kwak In et al (2009a) Phyto-crystallization of palladium through reduction process using Cinnamom zeylanicum bark extract. J Hazard Mater 171:400–404
Sathishkumar M, Sneha K, Won SW et al (2009b) Cinnamon zeylanicum bark extract and powder mediated green synthesis of nano-crystalline silver particles and its bactericidal activity. Colloids Surf B 73:332–338
Sathishkumar M, Krishnamurthy S, Yun YS (2010) Immobilization of silver nanoparticles synthesized using the Curcuma longa tuber powder extract on cotton cloth for bactericidal activity. Biores Technol 101:7958–7965
Sathishkumar G, Gobinath C, Karpagam K et al (2012) Phyto-synthesis of silver nanoscale particles using Morinda citrifolia L. and its inhibitory activity against human pathogens. Colloids Surf B 95:235–240
Sathishkumar M, Sneha K, Yun YS (2009c) Palladium nanocrystals synthesis using Curcuma longa tuber extract. Int J Mater Sci 4:11–17
Sathiya CK, Akilandeswari S (2014) Fabrication and characterization of silver nanoparticles using Delonix elata leaf broth. Spectrochim Acta Part A Mol Biomol Spectrosc 128:337–341
Satyavani K, Ramanathan T, Gurudeeban S (2011) Plant mediated synthesis of biomedical silver nanoparticles by using leaf extract of Citrullus colocynthis. J Nanosci Nanotechnol 1:95–101
Schabes-Retchkiman PS, Canizal G, Herrera-Becerra R et al (2006) Biosynthesis and characterization of Ti/Ni bimetallic nanoparticles. Opt Mater 29:95–99
Senapati S (2005) Biosynthesis and immobilization of nanoparticles and their applications. University of Pune, Ph.D. thesis, India
Senthil M, Ramesh C (2012) Biogenic synthesis of Fe3O4 Nanoparticles using Tridax procumbens leaf extract and its antibacterial activity on Pseudomonas aeruginosa. Dig J Nanomater Biostruct 7:1655–1660
Shaligram NS, Bule M, Bhambure R et al (2009) Biosynthesis of silver nanoparticles using aqueous extract from the compactin producing fungal strain. Process Biochem 44:939–943
Shankar SS, Absar A, Murali S (2003a) Geranium leaf assisted biosynthesis of silver nanoparticles. Biotechnol Prog 19:1627–1631
Shankar SS, Ahmad A, Pasricha R et al (2003b) Bioreduction of chloroaurate ions by geranium leaves and its endophytic fungus yields gold nanoparticles of different shapes. Mater Chem 13:1822–1826
Shankar SS, Rai A, Ahmad A et al (2004a) Rapid synthesis of Au, Ag and bimetallic Au shell nanoparticles using Neem. J Colloid Interface Sci 275:496–502
Shankar SS, Rai A, Ankamwar B et al (2004b) Biological synthesis of triangular gold nanoprisms. Nat Mater 3:482–488
Sharma NC, Sahi SV, Nath S et al (2007) Synthesis of plant-mediated gold nanoparticles and catalytic role of biomatrix-embedded nanomaterials. Environ Sci Technol 41:5137–5142
Sheny DS, Mathew J, Philip D (2011) Phytosynthesis of Au, Ag and Au–Ag bimetallic nanoparticles using aqueous extract and dried leaf of Anacardium occidentale. Spectrochim Acta A Mol Biomol Spectrosc 79:254–262
Singh C, Sharma V, Naik KRP et al (2011) A green biogenic approach for synthesis of gold and silver nanoparticles using Zingiber officinale. Dig J Nanomater Bios 6:535–542
Singh S, Saikia JP, Buragohain AK (2013) A novel ‘green’ synthesis of colloidal silver nanoparticles (SNP) using Dillenia indica fruit extract. Colloids Surf B 102:83–85
Sivakumar P, Nethradevi C, Renganathan S (2012) Synthesis of silver nanoparticles using Lantana camara fruit extract and its effect on pathogens. Asian J Pharm Clin Res 5:97–101
Sivaraj R, Rahman PKSM, Rajiv P et al (2014) Biosynthesis and characterization of Acalypha indica mediated copper oxide nanoparticles and evaluation of its antimicrobial and anticancer activity. Spectrochim Acta Part A Mol Biomol Spectrosc 129:255–258
Song JY, Kim BS (2008a) Biological synthesis of bimetallic Au/Ag nanoparticles using persimmon (Diopyros kaki) leaf extract. Korean J Chem Eng 25:808–811
Song JY, Kim B (2008b) Rapid biological synthesis of silver nanoparticles using plant leaf extracts. Bioprocess Biosyst Eng 32:79–84
Song JY, Jang H-K, Kim B (2009) Biological synthesis of gold nanoparticles using Magnolia kobus and Diopyros kaki leaf extracts. Process Biochem 44:1133–1138
Song JY, Kwon EY, Kim BS (2010) Biological synthesis of platinum nanoparticles using Diopyros kaki leaf extract. Bioprocess Biosyst Eng 33:159–164
Soundarrajan C, Sankari A, Dhandapani P et al (2012) Rapid biological synthesis of platinum nanoparticles using Ocimum sanctum for water electrolysis applications. Bioprocess Biosyst Eng 35:827–833
Starnes D, Jayjain A, Sahi S (2010) In planta engineering of gold nanoparticles of desirable geometries by modulating growth conditions: an environment-friendly approach. Environ Sci Technol 44:7110–7115
Sulochana S, Krishnamoorthy P, Sivaranjani K (2012) Synthesis of silver nanoparticles using leaf extract of Andrographis paniculata. J Pharmacol Toxicol 7:251–258
Suman TY, Radhika Rajasree SR, Kanchana A et al (2013) Biosynthesis, characterization and cytotoxic effect of plant mediated silver nanoparticles using Morinda citrifolia root extract. Colloids Surf B 106:74–78
Sun Q, Caia X, Li J et al (2014) Green synthesis of silver nanoparticles using tea leaf extract and evaluation of their stability and antibacterial activity. Colloids Surf A 444:226–231
Sundaravadivelan C, Nalini M (2011) Biolarvicidal effect of phyto-synthesized silver nanoparticles using Pedilanthus tithymaloides (L.) poit stem extract against the dengue vector Aedes aegypti L. (Diptera; Culicidae). Asian Pac J Trop Biomed 2012:1–8
Sundrarajan M, Gowri S (2011) Green synthesis of titanium dioxide nanoparticles by Nyctanthes arbor-tristis leaves extract. Chalcogenide Lett 8:447–451
Suresh G, Gunasekar PH, Kokila D et al (2014) Green synthesis of silver nanoparticles using Delphinium denudatum root extract exhibits antibacterial and mosquito larvicidal activities. Spectrochim Acta Part A Mol Biomol Spectrosc 127:61–66
Thirumurugan A, Jiflin GJ, Rajagomathi G et al (2010) Biotechnological synthesis of gold nanoparticles of Azadirachta indica leaf extract. Int J Biol Tech 1:75–77
Thirumurugan A, Tomy NA, Kumar HP et al (2011) Biological synthesis of silver nanoparticles by Lantana camara leaf extracts. Int J Nanomater Biostruct 1:22–24
Umoren SA, Obot IB, Gasem Z-M (2014) Green synthesis and characterization of silver nanoparticles using red apple (Malus domestica) fruit extract at room temperature. J Mater Environ Sci 5:907–914
Valodkar M, Jadeja RN, Thounaojam MC et al (2011) Biocompatible synthesis of peptide capped copper nanoparticles and their biological effect on tumor cells. Mater Chem Phys 128:83–89
Vanaja M, Annadurai G (2012) Coleus aromaticus leaf extract mediated synthesis of silver nanoparticles and its bactericidal activity. Appl Nanosci. doi:10.1007/s13204-012-0121-9
Vankar PS, Bajpai D (2010) Preparation of gold nanoparticles from Mirabilis jalapa flowers. Indian J Biochem Biophys 47:157–160
Veerasamy R, Xin TZ, Gunasagaran S et al (2011) Biosynthesis of silver nanoparticles using mangosteen leaf extract and evaluation of their antimicrobial activities. J Saudi Chem Soc 15:113–120
Velmurugan P, Anbalagan K, Manosathyadevan M et al (2014) Green synthesis of silver and gold nanoparticles using Zingiber officinale root extract and antibacterial activity of silver nanoparticles against food pathogens. Bioprocess Biosyst Eng 37:1935–1943
Vidya C, Hiremath S, Chandraprabha MN et al (2013) Green synthesis of ZnO nanoparticles by Calotropis gigantea. Int J Curr Eng Technol :118–120. http://inpressco.com/category/ijce
Vignesh V, Anbarasi KF, Karthikeyeni S et al (2013) A superficial phyto-assisted synthesis of silver nanoparticles and their assessment on hematological and biochemical parameters in Labeorohita (Hamilton, 1822). Colloids Surf A 439:184–192
Vijayakumar M, Priya K, Nancy FT et al (2013) Biosynthesis, characterisation and anti-bacterial effect of plant-mediated silver nanoparticles using Artemisia nilagirica. Ind Crops Prod 41:235–240
Vijayan SR, Santhiyagu P, Singamuthu M et al (2014) Synthesis and characterization of silver and gold nanoparticles using aqueous extract of seaweed, Turbinaria conoides, and their antimicrofouling activity. Sci World J. http://dx.doi.org/10.1155/2014/938272
Vilchis-Nestor AR, Sánchez-Mendieta V, Camacho-López MA et al (2008) Solventless synthesis and optical properties of Au and Ag nanoparticles using Camellia sinensis extract. Mater Lett 62:3103–3105
Vimala K, Sundarraj S, Paulpandi M et al (2014) Green synthesized doxorubicin loaded zinc oxide nanoparticles regulates the Bax and Bcl-2 expression in breast and colon carcinoma. Process Biochem 49:160–172
Wang CB, Zhang WX (1997) Synthesizing nanoscale iron particles for rapid and complete dechlorination of TCE and PCBs. Environ Sci Technol 31:2154–2156
Wang Y, He X, Wang K et al (2009) Barbated Skullcup herb extract-mediated biosynthesis of gold nanoparticles and its primary application in electrochemistry. Colloids Surf B 73:75–79
White II GV, Kerscher P, Brown MR et al (2012) Green synthesis of robust, biocompatible silver nanoparticles using Garlic Extract. J Nanomater. doi:10.1155/2012/730746
Yallappa S, Manjanna J, Sindhe MA et al (2013) Microwave assisted rapid synthesis and biological evaluation of stable copper nanoparticles using T. arjuna bark extract. Spectrochim Acta Part A Mol Biomol Spectrosc 110:108–115
Yang X, Li Q, Wang H et al (2010) Green synthesis of palladium nanoparticles using broth of Cinnamomum camphora leaf. J Nanopart Res 12:1589–1598
Yuvakkumar R, Suresh J, Joseph Nathanael A et al (2014) Novel green synthetic strategy to prepare ZnO nanocrystals using rambutan (Nephelium lappaceum L.) peel extract and its antibacterial applications. Mater Sci Eng, C 41:17–27
Zahir AA, Rahuman A (2012) Evaluation of different extracts and synthesised silver nanoparticles from leaves of Euphorbia prostrata against Haemaphysalis bispinosa and Hippobosca maculata. Vet Parasitol 187:511–520
Zhan G, Huang J, Du M et al (2011) Green synthesis of Au–Pd bimetallic nanoparticles: single-step bioreduction method with plant extract. Mater Lett 65:2989–2991
Acknowledgments
This chapter was supported by Isfahan University of Medical Sciences.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Iravani, S., Korbekandi, H., Zolfaghari, B. (2015). Phytosynthesis of Nanoparticles. In: Siddiqui, M., Al-Whaibi, M., Mohammad, F. (eds) Nanotechnology and Plant Sciences. Springer, Cham. https://doi.org/10.1007/978-3-319-14502-0_11
Download citation
DOI: https://doi.org/10.1007/978-3-319-14502-0_11
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-14501-3
Online ISBN: 978-3-319-14502-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)