Abstract
C. elegans provides a powerful in vivo model system in which to study epithelial apicobasal polarity during embryonic, larval, and adult development. Specifically, the assembly of adherens junctions and their role in tissue morphogenesis and organogenesis have been analyzed in great detail. In most C. elegans epithelia, junctional proteins localize to the multiplex apical junction (CeAJ), a single electron-dense structure that acts as a hub to integrate the barrier/fence and adhesive functions of different types of junctions found in vertebrates and Drosophila (e.g., tight and adherens junctions, desmosomes, septate junction).
Two core components of the CeAJ are the HMP-1/α-catenin–HMP-2/β-catenin–HMR-1/E-cadherin complex (CCC) and the DLG-1/Discs large–AJM-1 complex (DAC). The apically localized PAR-3–PAR-6–PKC-3 complex and the basolaterally localized regulator LET-413/Scribble both mediate the formation and maturation of the CeAJ, whereas LET-413 additionally maintains the polarization of C. elegans epithelia in the embryo. Starting in late embryogenesis and advancing in larval development, polarized trafficking and the lipid composition of the plasma membrane come more into focus with regard to the maintenance of epithelial cell polarity (e.g., in the intestine, a simple epithelial tube made of only 20 cells). Remarkably, the function of most embryonic epithelial polarity key players is still crucial for the de novo formation of epithelial tubes (e.g., the spermatheca) but seems dispensable for the maintenance of their apicobasal polarity during C. elegans postembryonic development.
The CeAJ promotes robust adhesion between epithelial cells and thus provides mechanical resistance for physical strains. However, in contrast to vertebrates and Drosophila, the CCC is not essential for general cell adhesion. In the C. elegans embryonic intestine, at least two adhesion systems, including HMR-1/E-cadherin and SAX-7/L1CAM, associated linker proteins (e.g., the DAC), and cytoskeletal organizers (e.g., ERM-1/ezrin–radixin–moesin, IFO-1, also referred to as TTM-4), act redundantly to mediate adhesion at the intestinal CeAJ.
In this chapter, we will first focus on the general aspects of intestinal development in C. elegans including specification, cell proliferation, and basic anatomy. We then discuss the establishment of the apicoluminal membrane domain (ALMD), the assembly of the CeAJ, and the formation of the lumen and the brush border. Next, we look at adhesion systems and cytoskeletal organizers that operate at the CeAJ and in the subapical cytoplasm to equip the lumen with a high degree of mechanical resilience and to ensure the integrity of the intestinal tube. Finally, we consider mechanisms that drive the expansion of the ALMD and maintain the apicobasal polarity of the intestine during the C. elegans’ life cycle.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- C. elegans intestine anatomy
- Junctional assembly
- Lumen/brush border formation
- Polarity establishment
- Polarity maintenance
- Proliferation
1 General Aspects of Intestinal Development in C. elegans
Nematodes are an extremely diverse and species-rich phylum. Roundworms inhabit virtually all available habitats on earth. The assumption that embryogenesis shows little variation within the phylum Nematoda is based on the observation that the early cell lineage in C. elegans (Sulston et al. 1983) is similar to the pattern found in Ascaris and other nematodes. In both species, five somatic founder cells (AB, E, MS, C, D; Fig. 2.1a–c) and a primordial germ cell (P4, Fig. 2.1c) are born through a series of stem cell-like asymmetric divisions. However, the analysis of a larger variety of species from different clades of the phylogenetic tree (Blaxter 2011) demonstrated that prominent variations in the crucial steps of embryogenesis exist among representatives of this phylum (Schierenberg 2006). While different cell patterns also exist to form an intestine in nematodes (Houthoofd et al. 2006), these evolutionary modifications seem to have no effect on the ultimate design of the embryonic intestine, a bilateral, symmetric, epithelial tube of only 20 cells (Fig. 2.2a). The intestine is one of the few cell lineages in C. elegans (Fig. 2.1j) where a reasonable transcriptional regulatory hierarchy can be proposed that controls development throughout the life cycle (Table 2.1), beginning with maternally derived factors in the cytoplasm of the zygote (e.g., SKN-1/Nrf and POP-1/TCF/LEF), progressing through a small number of zygotic GATA-type transcription factors (END-1 and END-3), and ending with a further set of GATA-type transcription factors (ELT-2, Fig. 2.2a, b, and ELT-7) that drive differentiation and function (Kormish et al. 2010; Maduro 2010; McGhee 2007; McGhee 2013; Maduro 2009).
C. elegans embryogenesis and development of the intestine (E lineage, the midgut, or endoderm). (a–c) Generation of five somatic founder cells (AB, MS, E, C, and D) and the primordial germ cell P4. (a) 4-cell stage; (b) 12-cell stage: the intestinal precursor cell, the E cell, is born at 35 min (Table 2.1) past the 2-cell stage; and (c) 26-cell stage/gastrulation (60 min): the two intestinal precursor cells (E2) migrate into the embryo. (d–f) Four (E4, 110 min), eight (E8, 160 min), and sixteen (E16, 260 min) intestinal precursor cells are born. (g) Morphogenesis phase (420 min): “tadpole” stage (E20 intestine). (h, i) Ultrastructure of the E20 intestine. Electron micrograph of the cross section through one intestinal ring (nuclei of E cells marked) in a “comma” embryo (Table 2.1). Microvilli (boxed area zoomed in) project into the nascent lumen (white asterisk), which is sealed by the CeAJ (white arrowheads). (j) Adult C. elegans hermaphrodite crawling on agar plate with E. coli as food source. Nomarski DIC optics (a–g), TEM micrographs (h, i; chitinase treated, osmium only; photo courtesy of Richard Durbin), and micrograph taken from dissecting scope (j, Nikon AZ100M, Canon EOS 6D). Black arrowheads (e–g) and white asterisks (g, i) indicate the anterior and posterior borders of the intestinal primordium and the developing lumen, respectively. The pharynx and hindgut (g) are to the left of the anterior and the posterior intestinal borders, respectively. Orientation (a–g, j): anterior (left), dorsal (top). Bars: 10 μm (a), 2 μm (h, i), 50 μm (j)
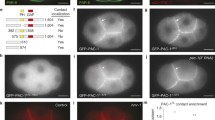
Distribution of intestinal markers during C. elegans embryogenesis. (a, b) Wild-type embryo (fourfold stage, Table 2.1) and apr-1(RNAi) embryo (threefold stage) stained against the nuclear ELT-2 GATA-factor (anti-GFP fluorescence) and the junctional DLG-1/Discs large–AJM-1 complex (DAC, merged anti-DLG-1 and anti-AJM-1 fluorescences). The GFP fluorescence and corresponding numbers (a) indicate the position of intestinal nuclei within crescent-shaped cells forming nine rings (the so-called ints: int1, four cells, and ints 2–9, each two cells). Note the increased number of intestinal cells (~40) after RNAi by feeding against the apr-1 gene (b). (c) In the wild-type embryo (fourfold stage), phosphotyrosine (PY) epitopes become enriched at the C. elegans apical junction (CeAJ), which is consistent with the results of studies in vertebrates and insects (Müller and Wieschaus 1996; Takata and Singer 1988). (d–g) In mid-morphogenesis (“comma” stage, Table 2.1), the PAR-3–PAR-6–aPKC complex (green) and the Crumbs protein (CRB-1, red) localize at the apicoluminal membrane domain (ALMD), which is sealed by the CeAJ (anti-AJM-1 (d–f, red) and anti-DLG-1 (g, green) fluorescences of the DAC), to separate from the basolateral membrane. (h–j) Junctional distribution of the DAC (green, anti-DLG-1 fluorescence), the HMP-1/α-catenin–HMP-2/β-catenin–HMR-1/E-cadherin complex (CCC, red, anti-HMP-1 fluorescence), and phosphorylated SAX-7/L1CAM (threefold stage). (k, l) Localization of cortical LET-413/Scribble (green, anti-GFP fluorescence) and the DAC (red, anti-AJM-1 fluorescence). In the “lima bean” stage (k), both proteins colocate at the ALMD of the intestine (note orange color). In the threefold stage (l), LET-413 is predominantly expressed basolaterally in C. elegans epithelia. (a–l) Immunofluorescence images showing confocal projections of the developing alimentary tract of C. elegans embryos after MeOH/acetone fixation. White arrowheads and white asterisks (c–l) indicate the anterior and posterior borders of the intestinal primordium and the developing lumen, respectively. The pharynx and hindgut (d–j) are to the left of the anterior and posterior intestinal borders, respectively. Orientation: anterior (left), dorsal (top). Bar: 10 μm
1.1 Basic Anatomy and Development of the Intestine
The C. elegans digestive tract is composed of a variety of tissues and cell types (Altun and Hall 2009c; White 1988; Bird and Bird 1991; Kormish et al. 2010). It forms an epithelial tube running inside the cylindrical body wall, is placed parallel to the gonad, and is separated from both by the pseudocoelom, a fluid-filled body cavity. The alimentary system can be subdivided into the foregut (stomodeum; buccal cavity and pharynx; Altun and Hall 2009d; Mango 2007), the midgut (intestine; Altun and Hall 2009b; McGhee 2007), and the hindgut (proctodeum; Altun and Hall 2009a) and is composed of only 127 cells (Sulston et al. 1983; Schnabel et al. 1997). In comparison to human digestive tracts, it lacks both an intestine-sheathing innervated muscle layer and a regenerating stem cell population. In C. elegans, the ingested E. coli bacteria flow through the digestive tract by the muscular pumping of the pharynx at the anterior end (Albertson and Thomson 1976; Mango 2007) (Mango 2009), and the waste material is discarded (Zhao and Schafer 2013; Wang et al. 2013; defecation cycle: Rae et al. 2012) through the opening of the anus at the posterior end by a coordinated action between body wall muscles and the muscles controlling the anus. Despite apparent differences in comparison to Drosophila and vertebrates, the basic biochemistry and cell biology of C. elegans intestinal cells have many of the same fundamental features as intestinal cells in these more complex systems: a striking apicobasal polarity; a prominent apical junctional belt; apical microvilli with rootlets extending into the terminal web region, both absorption and secretion; and a shared function as the place of the primary response to environmental stress.
Developmentally, the midgut/intestine derives clonally from the E lineage (Fig. 2.1), whereas the foregut and hindgut have a mixed lineage from ectodermal and mesodermal origins. The cell division sequence of the E blastomere has been described previously (Deppe et al. 1978; Schnabel et al. 1997; Leung et al. 1999; Sulston et al. 1983). We refer to the E cells collectively as the intestine (E lineage, the midgut, or endoderm) and indicate specific stages of the intestine according to the number of E cells present: E2, E4, E8, E16, E18, or E20 (Fig. 2.1). The E cell is born on the ventral surface of the 8-cell embryo where it divides along the a/p axis (Fig. 2.1b, c). During gastrulation, the E2 cells migrate into the interior of the embryo, where they divide l/r (Fig. 2.1c, d). The E4 and E8 cells (Fig. 2.1d, e) mostly divide a/p and some d/v. Hence, the E16 intestine (Fig. 2.1f) is made up of a dorsal layer of 10 cells (5 × 2 l/r pairs) and ventral layer of 6 cells (3 × 2 l/r pairs). The anterior- and posteriormost pairs undergo an additional d/v and a/p division, respectively, to finally generate the E20 intestine (Fig. 2.1g). In two distinct intercalation events, one in the E16 intestine and another in the E20 intestine, cell pairs of the ventral layer intercalate into the dorsal layer (Hoffmann et al. 2010; Leung et al. 1999). Thus, the basic anatomy of the E20 intestine can be represented as an a/p sequence of nine rings of intestinal cells (Fig. 2.2a) (the so-called ints: int1, four cells, and ints 2–9, each two cells; Sulston et al. 1983).
Each int forms part of the intestinal lumen at its apical pole and contains a basal lamina at its basal pole, whose constituents are either made by the intestine itself (laminin α and β, nidogen/entactin) or by the muscle and somatic gonad (type IV collagen) (Kramer 2005; Page and Johnstone 2007). The conserved extracellular matrix protein hemicentin stably affixes the anterior- and posteriormost ints to the body wall, hence facilitating passive movement or gliding of the remaining ints during feeding and locomotion and allowing the lumen to fill and empty freely while remaining attached to the body wall (Vogel and Hedgecock 2001; Vogel et al. 2006).
Many microvilli extend into the lumen from the apical surface (Fig. 2.1h, i), forming a brush border. The microvilli are anchored into a cytoskeletal network of cytoplasmic intermediate filaments (IFs) and actin filaments (AFs) at their base (Fig. 2.3d–i), called the terminal web (Hüsken et al. 2008; MacQueen et al. 2005; Bossinger et al. 2004; Carberry et al. 2009; Troemel et al. 2008). The core of each microvillus has a bundle of AFs that connects to this web (MacQueen et al. 2005). Each intestinal cell is sealed laterally to its neighbors by large CeAJs (Figs. 2.1i and 2.2c) (Labouesse 2006; Cox and Hardin 2004; Knust and Bossinger 2002; Pásti and Labouesse 2014) and connects to the neighboring intestinal cells via gap junctions on the lateral sides (Bossinger and Schierenberg 1992a; Altun et al. 2009; Guo et al. 2008).
Distribution of the three major cytoskeletal networks during development of the C. elegans intestine. (a–c) Tubulin-based microtubules (MTs, red, anti-α-tubulin fluorescence, mab4A1; Piperno and Fuller (1985)). (d–f) Actin-based microfilaments (AFs, green, phalloidin staining). (g–i) Intermediate filament (IF) protein-based IFs (green, anti-IFB2 fluorescence). Anti-DLG-1/Discs large fluorescence (a–c, green; g–i, red) specifies the CeAJ. (a–f) Immunofluorescence images showing confocal projections of the developing alimentary tract in C. elegans embryos (a, d, and g, “lima bean” stage; b, e, and h, “comma” stage; c, f, and i, “plum” stage; for timing see Table 2.1) after MeOH/acetone (a–c, g–i) or paraformaldehyde fixation (d–f). White arrowheads and white asterisks (c–l) indicate the anterior and posterior borders of the intestinal primordium and the developing lumen, respectively. The pharynx and hindgut (h, c, f, i) are to the left of the anterior and posterior intestinal borders, respectively. Orientation: anterior (left), dorsal (top). Bar: 10 μm
Transmission electron microscopy of epithelia identifies three electron-dense junctions in Drosophila and vertebrates, whereas the C. elegans embryo only possesses a single electron-dense junction (Knust and Bossinger 2002), commonly referred to as the C. elegans apical junction (Fig. 2.1i) (CeAJ; McMahon et al. 2001). Nevertheless, genetic and cellular analyses have demonstrated that epithelial cells in C. elegans do contain proteins of molecularly and functionally distinct junctional complexes that appear in tight junctions (e.g., CLC-1 to CLC-4/claudins) (Asano et al. 2003) and adherens junctions (e.g., the CCC) (Kwiatkowski et al. 2010; Maiden et al. 2013; Cox-Paulson et al. 2012), desmosomes (e.g., IFB-2/intermediate filament protein) (Bossinger et al. 2004), and septate junctions (e.g., DLG-1/Discs large) (Lockwood et al. 2008b) in other systems (Müller and Bossinger 2003; Cox and Hardin 2004; Armenti and Nance 2012; Labouesse 2006; Pásti and Labouesse 2014).
In the C. elegans intestine, the acquisition of apicobasal polarity, the formation of the CeAJ, and the generation of a central lumen are closely connected to each other. The CeAJ aligns in and between each successive a/p pair of intestinal cells (Fig. 2.2a) and together with the terminal web borders the lumen, making both ideal candidates to limit the width of the lumen that is remarkably uniform throughout the entire length of the intestine. The intestine can change in shape and function dramatically during the C. elegans’ life cycle. For instance, in the nonfeeding dauer larvae, the lumen becomes shrunken and the size and number of microvilli are greatly reduced. When the animal emerges from the dauer state, these changes are reversed in the new L4 larva (Albert and Riddle 1988; Popham and Webster 1979). Age-related changes in the intestine include the loss of E cell nuclei; the degradation of intestinal microvilli and changes in size, shape, and cytoplasmic contents of intestinal cells; and the increase of autofluorescent granules (McGee et al. 2011). A reassessment of blue autofluorescence in the C. elegans intestine led to the discovery of the phenomenon of death fluorescence, a burst of anthranilate fluorescence that indicates organismal death in C. elegans (Coburn et al. 2013; Coburn and Gems 2013).
1.2 Proliferation of Intestinal Cells
C. elegans intestinal cells can alter their cell cycle from mitotic cell divisions during embryogenesis to karyokinesis and then endoreplication, which are necessary to promote growth during larval and adult development (Table 2.1, Fig. 2.1j) (Ouellet and Roy 2007). In the L1 larval stage (Byerly et al. 1976), most intestinal nuclei (Fig. 2.2a) undergo karyokinesis (binucleation), resulting in an intestine still composed of 20 E cells but with a total of 30–34 nuclei that have increased their ploidy to 32n. Cells of int1 (see above) and int2 (usually) never binucleate, whereas cells of int3 to int7 always binucleate and cells of int8 and int9 may or may not binucleate (Hedgecock and White 1985; Sulston and Horvitz 1977). Postembryonic karyokinesis and endoreplication are not under control of the general cell cycle regulators in C. elegans (van den Heuvel 2005; van den Heuvel and Kipreos 2012), like the p21/p27-like cyclin-dependent kinase inhibitor CKI-1 or the positive S-phase regulator CDC-25.1, which are critical to control intestinal cell divisions during embryogenesis (Hong et al. 1998; Kostic and Roy 2002).
CDC25 phosphatase promotes progression through the eukaryotic cell cycle by dephosphorylation of cyclin-dependent kinase (Johnson and Kornbluth 2012). In C. elegans, cdc-25.1 is one of four homologues (Ashcroft et al. 1998). Clucas et al. (2002) and Kostic and Roy (2002) identified the mutant gain-of-function (gf) alleles of the cdc-25.1 gene. Despite the abnormal persistence of the gf mutant CDC-25.1 protein in all embryonic cells (Hebeisen and Roy 2008), hyperplasia is only inducible in the intestine at a specific time after the E8 stage (Fig. 2.1e), whereas other aspects of intestinal differentiation are retained. In cdc-25.1 gf mutants, between 30 and 45 intestinal cells are produced during embryogenesis. Because the C. elegans intestine consists of 20 cells, all cells cannot arise from the E cell by an identical pattern of cell divisions. In the E16 stage (Table. 2.1, Fig. 2.1f), at ~300 min of embryogenesis, only four E cells undergo further cell divisions. Hence, there is an asymmetry within the E cell lineage that must involve the differential regulation of the cell cycle in the intestine.
One regulator of cdc-25.1 (gf)-induced intestinal hyperplasia is LIN-23 (Segref et al. 2010; Hebeisen and Roy 2008), the C. elegans orthologue of the β-transducin repeat-containing protein (β-TrCP), a component of the Skp1/Cul1/F-box (SCF) ubiquitin ligase that, in cultured mammalian cells, has been shown to control cell cycle fluctuations and DNA damage response through the abundance of CDC25A and CDC25B via DSG and DDG motifs, respectively (Busino et al. 2003; Jin et al. 2003; Donzelli et al. 2002). Another regulator which suppresses the cdc-25.1(gf) embryonic phenotype in the intestine is a subset of splicing factors comprising U2- and U5-specific snRNPs (Hebeisen et al. 2008). Since knockdown of maternal cdc-25.1 or cyclin E (cye-1) can suppress the cdc-25.1(gf)-induced hyperplasia (Kostic and Roy 2002), it appears plausible that the suppression by a subset of splicing factors depends on the reduction of these two important cell cycle regulators.
The cdc-25.1(gf) mutations are causing an amino acid substitution (S46F or G47D) within a putative DSG phosphorylation site of CDC-25.1 (Clucas et al. 2002; Kostic and Roy 2002) that is also a consensus glycogen synthase kinase (GSK)3β phosphorylation site. A multiprotein complex containing axin, adenomatous polyposis coli tumor suppressor protein (APC), and GSK3β promotes phosphorylation of the DSG motif of mouse β-catenin to target its β-TrCP-dependent degradation (Kitagawa et al. 1999; Kikuchi et al. 2006). Mutations in APC or the β-catenin DSG motif are associated with colorectal cancer in humans (Karim and Huso 2013). In C. elegans, RNAi (Fire et al. 1998; Timmons and Fire 1998; Grishok 2013) against the APC orthologue APR (Hoier et al. 2000; Rocheleau et al. 1997) induces hyperproliferation of E cells in the majority of wild-type embryos (Fig. 2.2b) (our unpublished data; Segref et al. 2010; Putzke and Rothman 2010). To test whether apr-1 has a function mediated through CDC-25.1 controlling the intestinal cell cycle, Segref et al. (2010) repeated RNAi in a cdc-25.1(gf) background. They observed a significantly increased number of intestinal cells, indicating that apr-1 is synergistic with cdc-25.1(gf) and hence does not function through the same pathway as the gf mutant CDC-25.1 protein.
The role of APR-1 is puzzling because at the 4-cell stage of early embryogenesis, the protein is also involved in the correct specification of E cell fate by the Wnt/β-catenin asymmetry pathway. Wnt and Src signaling act together to regulate the asymmetry of the EMS blastomere (Fig. 2.1a) that produces the anterior MS and posterior E daughters (Fig. 2.1b), which generate mesoderm and endoderm, respectively (Mizumoto and Sawa 2007; McGhee 2013; Han 1997; Bei et al. 2002; Kim et al. 2013; Sugioka et al. 2011). How can depletion of APR-1 by RNAi cause a complete lack of E cells in ~23 % of embryos (Rocheleau et al. 1997; Segref et al. 2010; Bei et al. 2002), when the majority of embryos show intestinal hyperplasia (see above)?
The first observation can be easily interpreted by the redundancy of the Wnt and Src pathways because only interfering with both signals completely abolishes intestinal differentiation in C. elegans embryos (Bei et al. 2002). The second observation is more complex and to interpret it one has to keep in mind the dual nature of Wnt signaling. For example, hyperactivation of the Wnt pathway, caused by inactivating mutations in APC or activating mutations in β-catenin, is associated with various forms of cancer (Bienz and Clevers 2000; Polakis 2000), and decreased Wnt signaling can lead to increased invasiveness of tumor cells. In case of Wnt signaling in the C. elegans embryo, APR-1 acts either negatively on intestine induction early or positively on intestinal cell proliferation late. How can this contradictory observation be explained? The recent work by Putzke and Rothman (2010) suggests that removal of APR-1 (or Fer-type nonreceptor tyrosine kinase FRK-1) results in re-localization of cortical/junctional HMP-2/β-catenin to the nucleus and allows it to substitute for WRM-1, the nuclear β-catenin that normally transduces the Wnt signal during early endoderm induction. In C. elegans, HMP-2/β-catenin generally functions in cell adhesion (Costa et al. 1998; Grana et al. 2010; Segbert et al. 2004) and binds to HMP-1/α-catenin and HMR-1/cadherin (Kwiatkowski et al. 2010; Korswagen et al. 2000). So far HMP-2 has not been shown to activate Wnt reporters in tissue culture cells (Korswagen et al. 2000), and intestinal hyperproliferation resulting from excess nuclear HMP-2 appears to occur in the absence of POP-1(TCF/LEF) (Putzke and Rothman 2010), the central transcription factor in the separation of EMS into E and MS cell fates (Fig. 2.1a, b) (Lin et al. 1998; Lin et al. 1995; Yang et al. 2011). However, POP-1 asymmetry in sister cells at each a/p division of the E lineage is intriguing (Lin et al. 1998; Hermann et al. 2000; Schroeder and McGhee 1998) and together with the LIN-12/Notch signaling pathway is necessary for cells in the anterior intestine to undergo reproducible movements that lead to an invariant twist in the embryonic and larval intestine, probably allowing the adult intestine (Fig. 2.1j) to better coil with the developing gonad (Hermann et al. 2000; Neves et al. 2007; Neves and Priess 2005; Priess 2005). In other systems, the Notch signaling pathway is also involved in the development of colorectal tumors (Noah and Shroyer 2013). Notch and WNT signals cooperate to trigger intestinal tumorigenesis (Fre et al. 2009; Kim et al. 2012). In Apc Min mice, the continuous expression of Wnt target genes leads to the development of adenomas. However, inhibition of Notch signaling turned adenoma cells into goblet cells (van Es et al. 2005), whereas activation of Notch signaling in Apc mutant mice resulted in an increase in the number of adenoma cells (Fre et al. 2005). Concerning the apr-1(RNAi)-induced intestinal hyperplasia in C. elegans, the role of Notch, if any, still awaits to be investigated.
2 Defining the Apicoluminal Membrane Domain of the Intestine
2.1 Early Polarization Events
The principal requirement for a biological tube in general is that a lumen must form and the lumen must be sealed (Bryant and Mostov 2008). In the C. elegans intestine, the cell surface coating the future lumen of the epithelial tube develops as the ALMD with a prominent microvillar brush border and is sealed by the CeAJ (Fig. 2.1i) to separate from the basolateral membrane domain and to achieve its barrier function.
During polarization of the intestine in the E16 stage (Table 2.1, Fig. 2.1f), the centrally located intestinal nuclei and their centrosomes migrate toward the future apical pole, displacing the cytoplasm to the basal pole as seen by light microscopy (Fig. 2.1f). Although not explicitly described as cytoplasmic polarization, this initial asymmetry in the intestine was already observed by Sulston and coworkers (1983) and further elaborated in great detail by Leung et al. (1999).
Although 12 of the 16 E cells stop dividing, their centrosomes undergo one additional duplication or split to form centrosome pairs each containing two centrioles (Leung et al. 1999; Feldman and Priess 2012). The centrosomes and nuclei then move toward the lateral membrane. During this migration, associated microtubules (MTs) and pericentriolar material (PCM) carrying MT-organizing center (MTOC) activities, such as the MT nucleators γ-tubulin and its interacting protein CeGrip (=GIP-1), are first stripped from the centrosome and then become localized to the lateral membrane near the foci of the polarity proteins PAR-3 and PAR-6. Finally, these proteins move apically, thus defining the ALMD of intestinal cells. E16 cells treated with the MT inhibitor nocodazole show a strong delay in the apical localization of PAR-3 and γ-tubulin (Feldman and Priess 2012). Laser ablation studies and depletion of maternal and zygotic (m/z) PAR-3 suggest that both centrosomal and PAR-3 (but not PAR-6) functions are mutually dependent on each other and critical for the progression in MTOC function from centrosomes to the ALMD.
PAR-3, PAR-6, and PKC-3 are present at the ALMD (Fig. 2.2d–f) of the intestine (Bossinger et al. 2001; Köppen et al. 2001; Leung et al. 1999; McMahon et al. 2001; Wu et al. 1998). Deciphering the function of PAR proteins during intestinal polarization involved a sophisticated strategy to rescue their early need in the C. elegans zygote by tagging these proteins with the PIE-1 Zn-finger that mediates PIE-1 degradation in the soma (Nance et al. 2003). In par-3(m/z)-depleted embryos, the ALMD does not become polarized. Many proteins investigated so far (e.g., γ-tubulin, CeGrip, PAR-6, PKC-3, HMR-1, HMP-1, DLG-1, EAT-20, IFB-2) show a significant delay in the arrival at the ALMD and finally localize in aberrant patches (Achilleos et al. 2010; Feldman and Priess 2012; Totong et al. 2007). Hence, PAR-3 is required for the apical clustering and accumulation of polarity and junction proteins. RNAi feeding during C. elegans postembryonic development (Table 2.1) also established that PAR-3 is required to specify the ALMD and to assemble the CeAJ in the spermatheca, another epithelial tube (Aono et al. 2004). PAR-6 does not play a similar role, but instead, as in the epidermis, is essential to consolidate DAC and CCC puncta into a mature apical junctional belt (Totong et al. 2007). PAR-6 and PAR-3 functions appear dispensable to specify the ALMD in the epidermis (Achilleos et al. 2010; Totong et al. 2007). As in other species, the establishment of cell polarity in tubular organs and flat epithelial sheets appears to involve different processes (Nelson 2003; Datta et al. 2011). A role for PKC-3 in C. elegans embryonic epithelia (Fig. 2.2f), if any, awaits investigation.
After polarization of the intestine, the MT cytoskeleton appears to emerge in a fountain-like array from the ALMD and extends along the lateral surfaces of intestinal cells (Fig. 2.3a–c) (Leung et al. 1999). Its role during intestinal development is difficult to assess by genetic means. There are nine α-tubulins (TBA-1 to TBA-9) and six β-tubulins (TBB-1 to TBB-6) in the C. elegans genome (Table 2.1; wormbase.org). An important issue concerning the function of MTs (Fig. 2.3a–c) in the early intestinal polarization process is the question of additional signals that might participate either by direct release from the MTOC (as postulated for the C. elegans 1-cell embryo; Bienkowska and Cowan 2012) or by MT-based transport to the ALMD (as demonstrated during the polarization of the pharynx; Portereiko et al. 2004).
The two other main components of the cytoskeleton, AFs (Fig. 2.3d–f) and cytoplasmic IFs (Fig. 2.3g–i), start to localize at the ALMD around the same time (E16 stage) as the MTOC (Bossinger et al. 2004; Leung et al. 1999; van Fürden et al. 2004). The abundance of genes in both families again makes a genetic analysis difficult. The C. elegans genome (Table 2.1; wormbase.org) encodes 5 AFs (ACT-1 to ACT-5) and 11 cytoplasmic IFs (IFA-1 to IFA-4, IFB-1 to IFB-2, IFC-1 to -IFC2, IFD-1 to IFD-2, IFP-1). Nevertheless, the treatment of E16-stage embryos with the AF inhibitor latrunculin A does not affect the apical localization of PAR-3 (Fig. 2.2d) and γ-tubulin (Feldman and Priess 2012). Along the same line, interfering with individual gene functions of several intestine-specific IFs (Fig. 2.3g–i) or the intestinal filament organizer IFO-1 (also referred to as TTM-4) seems not to perturb the establishment of the ALMD in the intestine (Carberry et al. 2012; Hüsken et al. 2008; Karabinos et al. 2001; Bossinger et al. 2004). While AFs and IFs (Fig. 2.3d–i) seem dispensable for the early polarization of the C. elegans intestine, both filament systems and their regulators contribute to junction assembly and lumen morphogenesis.
2.2 Assembly of the Apical Junctional Belt
During the last decades, various approaches have been used to identify junctional proteins in C. elegans. This field was pioneered by Francis and Waterston. After raising monoclonal antibodies against insoluble membrane-associated embryonic extracts (Francis and Waterston 1991; Francis and Waterston 1985), some of these antibodies (e.g., MH27 or MH33) turned out to recognize proteins of the CeAJ (AJM-1/coiled-coil protein or IFB-2) by immunofluorescence and immunogold staining and provided an excellent platform to investigate the junction assembly and disassembly in C. elegans (Bossinger et al. 2004; Köppen et al. 2001; Podbilewicz and White 1994; Priess and Hirsh 1986; Hresko et al. 1994; Williams-Masson et al. 1997; MacQueen et al. 2005). Since then, many genes encoding junctional proteins have been identified by classical forward and reverse genetic means in screens for embryonic elongation defects (e.g., hmp-1, hmp-2, hmr-1, apr-1, vab-9; Costa et al. 1998; Hoier et al. 2000; Simske et al. 2003), enhancer screens (e.g., zoo-1, magi-1, jac-1; Lockwood et al. 2008a; Pettitt et al. 2003; Lynch et al. 2012), chromosomal deficiency screens (Labouesse 1997; e.g. let-413; Chanal and Labouesse 1997; Legouis et al. 2000), promoter trapping screens (e.g., eat-20; Shibata et al. 2000), and screens with stable transgenic strains (e.g., ifo-1; Carberry et al. 2012) or simply by analyzing the functions of homologous proteins after RNAi (e.g., CRB-1, DLG-1, IFC-2, CLC-1 to CLC-4; Bossinger et al. 2001; McMahon et al. 2001; Firestein and Rongo 2001; Asano et al. 2003; Hüsken et al. 2008) or targeted protein degradation (Nance et al. 2003; Totong et al. 2007; e.g., PAR-3, PAR-6; Achilleos et al. 2010). Some components were identified through protein–protein interaction screens (e.g., DLG-1, VANG-1; Köppen et al. 2001; Hoffmann et al. 2010). Tissue-specific RNAi (Bossinger and Cowan 2012; Qadota et al. 2007) and fluorescent protein fusions (Sarov et al. 2012) are now increasingly being used to identify new components of the CeAJ and to study their subcellular localization and kinetics.
In Drosophila, the interaction between several protein scaffolds—apically the Bazooka/DmPAR-6/DaPKC and Crumbs/Stardust/Patj complexes and basolaterally the Scribble/Discs large/Lethal giant larvae and Yurt/Coracle complexes—specifies apicobasal polarity and junction assembly (Laprise and Tepass 2011; Knust and Bossinger 2002; Nelson 2003). In C. elegans, epithelial polarization may rely on slightly divergent mechanisms and depends upon multiple, probably redundant, cues. For example, loss of HMR-1/cadherin affects neither apicobasal polarity nor cell adhesion as in other systems, and HMR-1/cadherin functions redundantly with SAX-7/L1CAM (Fig. 2.2j, Table 2.2) during C. elegans embryogenesis (Grana et al. 2010; Costa et al. 1998).
The polarization of the intestine clearly relies on the function of PAR-3/Bazooka (see above) but not PAR-6 (Fig. 2.2d, e). In par-6(m/z)-deficient embryos, apical junction proteins, PAR-3, and basolateral LET-413/Scribble (Fig. 2.2l) can become positioned asymmetrically, but apical junction proteins and PAR-3 require PAR-6 for their coalescence into belt-like structures, encircling the apex of intestinal cells (Totong et al. 2007). How PAR-6 achieves coalescence is not known. Recent results suggest that DmPAR-6 together with the small GTPase Cdc42 control trafficking events of junctional proteins in Drosophila epithelia (Balklava et al. 2007; Harris and Tepass 2010). PAR-6 seems not to function redundantly with the DAC, CCC (Fig. 2.2h, i), or LET-413 (Fig. 2.2k, l) to establish the apicobasal polarity of intestinal epithelial cells (Totong et al. 2007). However, PAR-6 may regulate apicobasal polarity through more redundant interactions, e.g., with the Crumbs/Stardust/Patj complex. Homologues of Crb, Stardust, and Patj exist in the C. elegans genome (Table 2.1; wormbase.org). In addition, CRB-1 (Fig. 2.2g), EAT-20, and CRB-3, the three Crumbs homologues, are present at the ALMD of the intestine. However, their absence alone or after double depletions seems not to affect apicobasal polarity (Bossinger et al. 2001; Shibata et al. 2000; our unpublished data). A potential contributory role in apicobasal polarity is revealed by the simultaneous knockdown of CRB-1, HMP-1/α-catenin, and LET-413 (Segbert et al. 2004).
In Drosophila, the basolaterally expressed Scribble and Discs large proteins oppose the activity of the apical polarity complexes, thus defining the basolateral position of adherens and septate junctions during epithelial polarization (Elsum et al. 2012). The C. elegans homologues LET-413 and DLG-1 have related functions but are not crucial to establish the initial apicobasal polarity (McMahon et al. 2001; Legouis et al. 2000; Bossinger et al. 2001; Firestein and Rongo 2001). Instead, both proteins—like PAR-6—promote compaction of the CeAJ (Fig. 2.4a, e–g) (McMahon et al. 2001; Köppen et al. 2001; Totong et al. 2007; Bossinger et al. 2001). An important future issue will be to determine whether LET-413, DLG-1, and PAR-6 act in the same or parallel pathways. The divergence of compaction defects and its enhancement in LET-413- and DLG-1-depleted embryos (Köppen et al. 2001) argue for the latter possibility.
Distribution of junctional and apicoluminal markers in C. elegans RNAi and mutant embryos. (a, b) RNAi against the DAC (DLG-1/Discs large–AJM-1 complex). Note that AJM-1 puncta do not consolidate into a mature apical junctional belt (a, compare to Fig. 2.2c), whereas DLG-1 spreads to the lateral membrane domain (LMD, black arrows) of intestinal cells (b). (c) Distribution of AJM-1 in a HMP-1/α-catenin mutant embryo. (d) Depletion of ezrin–radixin–moesin/ERM-1 yields a narrowing of the developing lumen (asterisk), as indicated by junctional constrictions (compare to Fig. 2.2c). (e, f) Double staining against the DAC (e) and the CCC (HMP-1/α-catenin–HMP-2/β-catenin–HMR-1/E-cadherin complex, f) in a let-413 mutant embryo. (g, h) Double staining against the DAC (g) and the intermediate filament protein IFB-2 (h) after depletion of LET-413/Scribble. Note the spreading of IFB-2 to the LMD (black arrows). (i, j) Double depletion of the DAC (dlg-1(RNAi)) and the CCC (hmp-1(RNAi)) induces rupture of the ALMD (straight black lines) as indicated by the double immunofluorescence of AJM-1 and PKC-3. (k, l) Double depletion of ERM-1 (erm-1(RNAi)) and the CCC (hmp-1(RNAi)) induces rupture of the ALMD (straight black lines) as indicated by double immunofluorescence of DLG-1 and PKC-3. (a–l) Inverted immunofluorescence images (antibody staining indicated in the top right corner) showing confocal projections of the developing alimentary tract in C. elegans embryos (“tadpole” and “plum” stages, for timing see Table 2.1) after MeOH/acetone fixation. Black arrowheads and white asterisks (c–l) indicate the anterior and posterior borders of the intestinal primordium and the developing lumen, respectively. The pharynx and hindgut are to the left of the anterior and posterior intestinal borders, respectively. Black arrows (b, h) indicate spreading of intestinal markers to the LMD. Straight black lines (i–l) indicate rupture of the ALMD. Orientation: anterior (left), dorsal (top). Bar: 10 μm
In addition, in LET-413- and DLG-1-deficient embryos, junctional proteins reach their subapical position less efficiently (Bossinger et al. 2001; Köppen et al. 2001; McMahon et al. 2001). Moreover, after depletion of LET-413, the ALMD progressively spreads into the lateral and basal membrane domains of intestinal cells (Fig. 2.4h), suggesting that LET-413 function is a prerequisite to maintain the apicobasal polarity during C. elegans embryogenesis (Bossinger et al. 2004; McMahon et al. 2001). How LET-413 acts at the molecular level is unknown. With regard to the process of junction compaction, an unexpected cue recently emerged from the observation that loss of the inositol-triphosphate receptor ITR-1 or loss of the inositol polyphosphate 5-phosphatase IPP-5 can partially compensate the knockdown of LET-413 by RNAi, suggesting that it might be Ca2+ sensitive (Pilipiuk et al. 2009). Intriguingly, ITR-1 interacts with myosin II (Walker et al. 2002), raising the possibility that myosin II is involved in junction compaction in C. elegans. Indeed, recent studies demonstrated the need for myosin II in the development of adherens junctions in cell culture (Yonemura et al. 2010).
2.3 Formation of the Lumen
Lumen formation in general enables essential functions such as nutrient uptake, gas exchange, and circulation. The reduced function of the ALMD or perturbation of the finely balanced control of lumen diameter is often fatal. Investigating the molecular mechanisms controlling the formation and maintenance of the lumina is key to better understand common human diseases (Datta et al. 2011). For instance, hyperdilated tubules associated with renal dysfunction occur in polycystic kidney diseases (Wilson 2011; Nagao et al. 2012), and reduction of lumen size is associated with vascular diseases such as hypertension (Iruela-Arispe and Davis 2009). Furthermore, early stages of many epithelial cancers display luminal filling, such as in ductal carcinomas in situ (Hebner et al. 2008).
The ALMD of the C. elegans intestine is bordered, in general, by only two cells. However, in the E16 stage, the nascent ALMD becomes established mostly between pairs of four radially symmetrical cells, two from the dorsal and two from the ventral layer of E cells (Leung et al. 1999). Remarkably, the intercalation of ventral intestinal cells into the dorsal layer does (see above) not define a new ALMD, and its rather ventral position matches with the location of the future lumen (Leung et al. 1999). Hence, intestinal cells must undergo a complex cytoskeletal rearrangement to take on a crescent shape and to form the lumen centrally.
The C. elegans intestinal lumen seems to form by cord hollowing (Lubarsky and Krasnow 2003). In cord hollowing, intracellular vesicles are thought to contain fluid that is taken up by endocytosis, trans-Golgi-derived material, and apical proteins. Their movement and delivery to the cell surface at a coordinated point between closely opposed cells creates a luminal space de novo (Bryant and Mostov 2008). In the E16 intestine, vesicles appear continuously and remain concentrated near the ALMD. If these apical vesicles are exocytosed, they might contribute to both apical membrane biogenesis and initial lumen formation (Leung et al. 1999).
In the developing zebrafish and mouse intestines, fusion of multiple rudimentary lumina into a single lumen occurs in a PKC- and ezrin-dependent manner respectively (Horne-Badovinac et al. 2001; Saotome et al. 2004). In C. elegans, multiple microlumina appear at the E16 to E20 stage (Leung et al. 1999), and loss of the C. elegans ezrin–radixin–moesin homologue ERM-1 yields luminal obstructions (van Fürden et al. 2004), suggesting that fusion is critical to form a central lumen in the intestine.
ERM-1 and SMA-1/βH-spectrin act as scaffolding proteins to connect AFs (Fig. 2.3d–f) to the luminal membranes of intestinal cells. Both proteins are involved in lumen formation and the organization of the brush border (Brown and McKnight 2010; Praitis et al. 2005; McKeown et al. 1998; van Fürden et al. 2004; Göbel et al. 2004; Saotome et al. 2004). ERM-1 is required along with the branched actin nucleator Arp2/3 and one of its activators (WAVE/SCAR, GEX-2/Sra1/p140/PIR121 and GEX-3/NAP1/HEM2/Kette, but not WASP) for apical F-actin enrichment in the embryonic intestine. Intestines developing with reduced ERM-1, Arp2/3, or WAVE/SCAR accumulate less apical F-actin and show altered lumen morphogenesis (Bernadskaya et al. 2011; van Fürden et al. 2004; Patel et al. 2008). Along the same line, depletion of formins, which promote linear actin formation, or C. elegans members of the TOCA family (TOCA-1, TOCA-2), which control actin dynamics through their interactions with actin remodeling factors (WAVE/SCAR, WASP), also leads to lower levels of phalloidin at the ALMD (Giuliani et al. 2009). Phalloidin staining also becomes reduced in ifo-1, which encodes a novel, histidine-rich, polyproline tract-containing nematode protein and interferes with the localization of intestine-specific IFs (Fig. 2.3g–i) (Carberry et al. 2012). Finally, apical enrichments of F-actin (Fig. 2.3d–f) and DLG-1 (Fig. 2.3a–c) (but not HMR-1/E-cadherin) are mutually dependent on each other (Bernadskaya et al. 2011).
The reduction of apical F-actin in the embryonic intestine has opposite effects on the width of the lumen. While the absence of TOCA and Arp2/3 complex proteins causes the lumen to become wider, the loss of ERM-1 yields extreme narrowing of the lumen and the reduction of IFO-1 and IFs seems to generate a rather wild-type-like lumen (Carberry et al. 2012; Bossinger et al. 2004; Hüsken et al. 2008). TOCA and Arp2/3 complex proteins seem to maintain lumen morphogenesis in controlling early endocytosis and the morphology of early endosomes (Patel and Soto 2013; Giuliani et al. 2009). Of note, endocytosis mutants, including chc-1/clathrin heavy chain, dyn-1/dynamin GTPase, and rab-5/Rab5 GTPase, show similar intestinal lumen expansion as observed after depletion of GEX-3 (Patel and Soto 2013). In mature epithelial cells of rat small intestine, immunogold localization of ezrin shows that most gold particles are associated with the microvilli. However, a low level of staining is also seen in the terminal web region, whereas no staining is seen in the region of adherens junctions (Berryman et al. 1993). Ezrin was initially believed to laterally tether the microvilli core bundle to the membrane (Takeuchi et al. 1994; Berryman et al. 1995; Crepaldi et al. 1997; Bonilha et al. 1999). However, this hypothesis was questioned in a recent work by Brown and McKnight (2010). Instead, as demonstrated by its knockout in mice, ezrin is believed to be important in maintaining a connection between the terminal web and the ALMD (Saotome et al. 2004). Ezrin is not absolutely required for the formation of brush border microvilli in mice and C. elegans (Saotome et al. 2004; Göbel et al. 2004).
Arp2/3–ERM-1 and IFO-1–IFs affect each other’s protein levels. Depletion of GEX-3 or IFO-1 leads to an increased junctional accumulation of ERM-1 or IFs respectively (Bernadskaya et al. 2011; Carberry et al. 2012). This supports a role for Apr2/3 and IFO-1 in maintaining the levels of ERM-1 and IFs in the terminal web and downregulating their levels at the CeAJ. F-actin, either nucleated by Arp2/3 and formins or enriched by ERM-1 and IFO-1, could provide stiffness to the lumen. The junctional enrichment of ERM-1 and IFs as seen in gex-3 and ifo-1 mutants may indicate that WAVE/SCAR proteins and IFO-1 prevent excessive flexibility of the lumen by upregulating ERM-1 and IFs in the terminal web.
erm-1 interacts genetically with ifo-1. An enhanced phenotype is observed for apical F-actin and anti-IFB-2 signals in the intestine, which are significantly more reduced in erm-1–ifo-1 mutant embryos (Carberry et al. 2012). Remarkably, a novel luminal defect becomes obvious. In contrast to the respective single mutants, the DLG-1-positive CeAJ and the junctional IFB-2 meshwork are discontinuous, indicative of luminal rupture in these embryos. In addition, erm-1 (Fig. 2.4k, l) and ifo-1 also genetically interact with the components of the CCC and DAC, respectively. During morphogenesis of the C. elegans intestine, only double knockdowns of ERM-1 and HMR-1/E-cadherin or IFO-1 and DLG-1/Discs large but not IFO-1 and HMR-1 or ERM-1 and DLG-1 (Carberry et al. 2012; van Fürden et al. 2004) generate a similar phenotype as that observed after depletion of ERM-1 and IFO-1. These genetic data suggest two parallel pathways (Table 2.2), ERM-1 + DAC and IFO-1 + CCC, which are both necessary to ensure luminal and junctional integrity, presumably by promoting cell adhesion (Fig. 2.4i, j). In the case of the ERM-1/DAC pathway, the L1CAM SAX-7 (Fig. 2.2j), a single-pass transmembrane cell adhesion receptor belonging to the immunoglobulin superfamily, has the potential to interact with ERM-1 and DLG-1 (Chen and Zhou 2010; Zhou et al. 2008; Chen et al. 2001). Although the loss of SAX-7 seems not to interfere with the junctional localization of the DAC (Bernadskaya et al. 2011), depletion of the DAC disturbs junctional localization of phosphorylated SAX-7 in the embryonic intestine (our unpublished data). Very recently, it has been demonstrated that SAX-7/L1CAM and HMR-1/E-cadherin also function redundantly in blastomere compaction and non-muscle myosin accumulation during C. elegans gastrulation (Fig. 2.1c) (Grana et al. 2010). Of note, during morphogenesis of the C. elegans epidermis, SAX-7 interacts with MAGI-1/MAGUK and its adapter protein AFD-1/afadin to maintain a stable, spatially ordered CeAJ (Lynch et al. 2012).
2.4 Formation of the Brush Border
The surface of most animal cells lining the intestinal lumen is characterized by a brush border. It consists of regularly spaced and evenly shaped microvilli that are anchored to the cytoskeleton-rich, organelle-free cytoplasmic terminal web and its associated apical junctions. Microvilli increase the absorptive and resorptive surface areas of the intestine and are characterized by a core of membrane-attached longitudinal F-actin filament bundles whose rootlets extend into the subapical terminal web region. The terminal web has been investigated at the ultrastructural level (Hirokawa et al. 1982; Bement and Mooseker 1996), and the principal components are known to be AFs (Fig. 2.3d–f), IFs (Fig. 2.3g–i), myosin, spectrin, and an assortment of actin-binding proteins (Fath and Burgess 1995; Ku et al. 1999; Mooseker 1985; Thomas 2001; Drenckhahn and Dermietzel 1988).
The intestinal terminal web in many nematodes contains a discrete and prominent substructure termed the endotube (Munn and Greenwood 1984). In C. elegans, the reactivity of actin proteins and the IF protein IFB-2, as detected by immunoelectron microscopy, decorates the endotube and continues into the region where the endotube joins the electron-dense structure of the intestinal CeAJ (MacQueen et al. 2005; Bossinger et al. 2004). Electron microscopy reveals a discontinuous endotube with large intermittent gaps in worms whose intestinal cells were infected with microsporidia (Troemel et al. 2008). A complete loss of the endotube and disordered but still intact microvilli are observed in ifo-1 animals (Carberry et al. 2012).
Within the C. elegans intestinal brush border (Fig. 2.1h, i), AFs, probably built by association of ACT-5 monomers, form long bundles. These bundles are capped at their barbed end by EPS-8A, the long isoform of the C. elegans homologue of the epidermal growth factor receptor substrate Eps8, which is localized at the tips of the brush border intestinal microvilli (MacQueen et al. 2005; Croce et al. 2004). act-5 seems not to encode the only actin in the embryonic intestine because in act-5 loss-of-function mutants, E cells are able to divide and terminally differentiate into polarized epithelial cells. Nevertheless, sequence differences between ACT-5 and ACT-1 to ACT-4 most likely render ACT-5 functionally distinct and specialized for microvilli formation. Ultrastructural analysis of animals grown on act-5(RNAi) reveals a complete loss of intestinal microvilli. The lumen is frequently round instead of ellipsoid and associated with an abnormally thick terminal web structure (MacQueen et al. 2005). In eps-8A(RNAi) L4 larvae, microvilli form an irregular layer, with an overall lower microvillar density and total absence of microvilli in some areas. Many microvilli are longer than in wild-type animals, indicating a lack of termination of microvilli elongation. In addition, the terminal web seems to detach from the microvillar layer (Croce et al. 2004).
How establishment of apicobasal polarity in the C. elegans intestine (see above) leads to the subsequent formation of the brush border (Fig. 2.1i) and how the distribution/density of microvilli in the ALMD is regulated are not understood. In human intestinal epithelial cell lines LKB1, the homologue of the PAR-4 polarity protein can induce complete apicobasal polarity in a cell-autonomous fashion in single isolated colon cells after activation by its specific adapter protein STRAD. Furthermore, upon LKB1 activation, single cells rapidly remodel their AFs to form an apical brush border and junctional proteins reallocate in a belt peripheral to the brush border (Baas et al. 2004). In this system, apicobasal polarity is translated directly into the acquisition of a brush border through a small G protein (Rap2A) signaling module whose action is positioned by a cortical lipid cue and finally executed by activated ezrin (Gloerich et al. 2012). During intestinal brush border formation, this signaling pathway from Rap2A to ezrin seems to be evolutionarily conserved. In C. elegans, immunostaining of wild-type L3 larvae for ERM-1 phosphorylated at its activating threonine (Thr 544) revealed its strong enrichment at the ALMD of the intestine. After depletion of the C. elegans Rap2 homologue, the level of anti-phospho-ERM-1(Thr544) staining becomes substantially decreased (Gloerich et al. 2012). Of course, the molecular details concerning microvilli morphogenesis in C. elegans (Fig. 2.1i) still await investigation.
3 Expansion and Maintenance of Intestinal Membrane Domains During the C. elegans Life Cycle
From late embryogenesis through larval and adult development (Table 2.1, Fig. 2.1g, j), the intestine, comprising roughly one third of the total somatic mass of C. elegans (McGhee 2007), expands by growth alone without further cell divisions. For instance, the volume of intestinal cells roughly doubles during embryogenesis, presumably by the internalization of yolk proteins, which are secreted from most blastomeres (Bossinger and Schierenberg 1992b; Yu et al. 2006; Bossinger et al. 1996).
The expanding C. elegans intestine has become an attractive in vivo model for the analysis of polarized membrane biogenesis. Because the conversion of polarized membrane domains and the formation of ectopic intestinal lumen can be easily followed during the C. elegans life cycle, a recent work has revealed that Lats kinase, glycosphingolipids (GSLs), clathrin heavy chain (CHC) and its AP-1 adapter, and RAB-11 recycling endosomes (REs) are important for sorting to the apical membrane and the maintenance of epithelial cell polarity (Zhang et al. 2012; Zhang et al. 2011; Shafaq-Zadah et al. 2012; Kang et al. 2009; Winter et al. 2012).
The warts (wts) gene, encoding a Lats kinase homologue in Drosophila, was first identified in genetic studies (Justice et al. 1995; Xu et al. 1995). In Drosophila and mammals, wts acts in the conserved Hippo pathway that promotes inhibition of apoptosis and drives cell proliferation (Enderle and McNeill 2013; Hergovich 2013). Surprisingly, wts-1 function in C. elegans primarily maintains the integrity of the intestinal ALMD but is not involved in the establishment of apicobasal polarity (Kang et al. 2009). In wts-1 homozygous L1 larvae, ACT-5::GFP, the CCC, and the DAC (Fig. 2.2i, h) gradually spread to the lateral membrane domain, and finally lumen-like structures, sealed by the CeAJ and containing a brush border, develop. Dependent on the function of the exocyst complex, which is known to be important for targeting proteins to the basolateral membrane (Grindstaff et al. 1998), only newly synthesized ACT-5::GFP becomes ectopically enriched (Kang et al. 2009). The exocyst is an evolutionarily conserved multisubunit protein complex implicated in tethering secretory vesicles to the plasma membrane. It localizes to restricted regions of the plasma membrane, where it mediates the delivery of proteins and lipids necessary for polarized membrane expansion (Heider and Munson 2012). From the phenotype caused by the wts-1 mutation in C. elegans, it seems plausible that WTS-1 function normally ensures that AFs (Fig. 2.3d–f) and CeAJ protein are properly transported and maintained near the ALMD to preserve normal expansion of the ALMD (Kang et al. 2009).
Several genes encoding enzymes of the GSL biosynthetic pathway, as well as CHC-1/AP-1, act as mediators of polarized transport to the ALMD in C. elegans late embryonic and larval intestines. Surprisingly, depletion of these genes does not affect the initial establishment of apicobasal polarity in the intestine (see above), but induces the mislocalization of apical molecules to lateral membrane domains, and thus promoting the formation of additional ectopic lumens exclusively during late embryonic (Zhang et al. 2012; Shafaq-Zadah et al. 2012) or larval development (Zhang et al. 2011) of intestinal cells (Table 2.1). Because the reduction-of-function phenotypes of GSLs and CHC-1/AP-1 produce strong synergistic effects, Zhang et al. (2013) proposed that both pathways contribute to the same or a parallel apical sorting function during biogenesis of the intestinal ALMD.
In epithelial cells, the apical and basolateral plasma membranes are generally enriched in GSLs/sphingomyelin and phosphatidylcholine, respectively, to form the so-called membrane/lipid rafts that are required in vivo for trafficking pathways and can act as hubs for many molecular scaffolds (Simons and Ikonen 1997; Head et al. 2014). In the C. elegans intestine, GSLs are the common apical polarity-affecting lipid species, and exogenous lipids supplied by food, including GSL, can partially rescue germline mutations in fatty acid biosynthetic enzymes. For instance, in let-767 larvae ectopic lateral lumina become closed, the central lumen is rebuilt, and the growth arrest and lethality are rescued (Zhang et al. 2011).
The functions of clathrin and AP1B in mammalian epithelial cell culture so far are both implicated in basolateral sorting, and neither clathrin nor AP1B seem to be required for the overall epithelial polarity maintenance (Weisz and Rodriguez-Boulan 2009; Gonzalez and Rodriguez-Boulan 2009; Fölsch et al. 1999). In contrast, in the C. elegans intestine, AP-1 is required to apically enrich RHO GTPase CDC-42 and RAB-11 recycling endosomes (REs), suggesting that AP-1 might function at the level of this compartment (Zhang et al. 2012; Shafaq-Zadah et al. 2012). Interestingly, another study in C. elegans found that PAR-5/14-3-3 protein and RAB-11–REs play a central role in maintaining the apicobasal polarity of the adult intestine. After depletion of PAR-5, RAB-11–REs become mispositioned basally along with patches of AFs in a process that depends on the kinesin-1 orthologue UNC-116 and AF modulators, such as ADF/cofilin and profilin (Winter et al. 2012).
In summary, during postembryonic development (Table 2.1) of the C. elegans intestine, GSL raft-dependent trafficking, clathrin/AP-1-dependent pathways, and the PAR-5 regulatory hub seem to intersect on the RAB-11–REs to control the expansion of the ALMD and to preserve the identity of the basolateral membrane domain (BMD). Whether the exocyst complex is a requirement for the mislocalization of apicoluminal membrane components to the BMD, as demonstrated in the case of C. elegans Lats kinase mutations (see above, Kang et al. 2009), remains to be investigated.
4 Future Perspectives
Despite the considerable progress in uncovering the basic mechanisms that are involved in the maintenance of cell polarity through trafficking during late embryonic, larval, and adult development of the C. elegans intestine, future progress should address the issue of how the vesicle trafficking machinery participates in the establishment of the apicoluminal membrane domain (including microvilli and lumen formation) and how a cross talk with the MT and F-actin networks is regulated. In addition, the molecular mechanism of LET-413/Scribble function is still a challenge in the early polarization events. We have probably reached a plateau in terms of describing the function of key molecules of the C. elegans apical junction in the embryonic intestine. Future progress should now approach the still mysterious issue of how the epithelial junctional belt and cytoskeletal filaments are organized and regulated during larval and adult development to support the intestine’s major roles in the response of C. elegans to environmental (e.g., toxins or infections) and mechanical stresses.
References
Achilleos A, Wehman AM, Nance J (2010) PAR-3 mediates the initial clustering and apical localization of junction and polarity proteins during C. elegans intestinal epithelial cell polarization. Development 137(11):1833–1842
Albert PS, Riddle DL (1988) Mutants of Caenorhabditis elegans that form dauer-like larvae. Dev Biol 126(2):270–293
Albertson DG, Thomson JN (1976) The pharynx of Caenorhabditis elegans. Philos Trans R Soc Lond B Biol Sci 275(938):299–325
Altun ZF, Chen B, Wang ZW, Hall DH (2009) High resolution map of Caenorhabditis elegans gap junction proteins. Dev Dyn 238(8):1936–1950
Altun ZF, Hall DH (2009a) Alimentary system, rectum and anus. WormAtlas. http://dx.doi.org/doi:10.3908/wormatlas.1.5
Altun ZF, Hall DH (2009b) Alimentary system, intestine. WormAtlas. http://dx.doi.org/doi:10.3908/wormatlas.1.4
Altun ZF, Hall DH (2009c) Alimentary system, overview. Wormatlas. http://dx.doi.org/doi:10.3908/wormatlas.1.2
Altun ZF, Hall DH (2009d) Alimentary System, Pharynx. WormAtlas. http://dx.doi.org/doi:10.3908/wormatlas.1.3
Altun ZF, Hall DH (2009e) Introduction. Wormatlas. http://dx.doi.org/doi:10.3908/wormatlas.1.1
Aono S, Legouis R, Hoose WA, Kemphues KJ (2004) PAR-3 is required for epithelial cell polarity in the distal spermatheca of C. elegans. Development 131(12):2865–2874
Armenti ST, Nance J (2012) Adherens junctions in C. elegans embryonic morphogenesis. Subcell Biochem 60:279–299
Asano A, Asano K, Sasaki H, Furuse M, Tsukita S (2003) Claudins in Caenorhabditis elegans: their distribution and barrier function in the epithelium. Curr Biol 13(12):1042–1046
Ashcroft NR, Kosinski ME, Wickramasinghe D, Donovan PJ, Golden A (1998) The four cdc25 genes from the nematode Caenorhabditis elegans. Gene 214(1–2):59–66
Baas AF, Kuipers J, van der Wel NN, Batlle E, Koerten HK, Peters PJ, Clevers HC (2004) Complete polarization of single intestinal epithelial cells upon activation of LKB1 by STRAD. Cell 116(3):457–466
Balklava Z, Pant S, Fares H, Grant BD (2007) Genome-wide analysis identifies a general requirement for polarity proteins in endocytic traffic. Nat Cell Biol 9(9):1066–1073
Bei Y, Hogan J, Berkowitz LA, Soto M, Rocheleau CE, Pang KM, Collins J, Mello CC (2002) SRC-1 and Wnt signaling act together to specify endoderm and to control cleavage orientation in early C. elegans embryos. Dev Cell 3(1):113–125
Bembenek JN, Richie CT, Squirrell JM, Campbell JM, Eliceiri KW, Poteryaev D, Spang A, Golden A, White JG (2007) Cortical granule exocytosis in C. elegans is regulated by cell cycle components including separase. Development 134(21):3837–3848
Bement WM, Mooseker MS (1996) The cytoskeleton of the intestinal epithelium: components, assembly, and dynamic rearrangements. In: John EH, Ian FP (eds) The cytoskeleton: a multi-volume treatise, vol 3. JAI, Greenwich, CT, pp 359–404
Benenati G, Penkov S, Muller-Reichert T, Entchev EV, Kurzchalia TV (2009) Two cytochrome P450s in Caenorhabditis elegans are essential for the organization of eggshell, correct execution of meiosis and the polarization of embryo. Mech Dev 126(5–6):382–393
Bernadskaya YY, Patel FB, Hsu HT, Soto MC (2011) Arp2/3 promotes junction formation and maintenance in the Caenorhabditis elegans intestine by regulating membrane association of apical proteins. Mol Biol Cell 22(16):2886–2899
Berryman M, Franck Z, Bretscher A (1993) Ezrin is concentrated in the apical microvilli of a wide variety of epithelial cells whereas moesin is found primarily in endothelial cells. J Cell Sci 105(Pt 4):1025–1043
Berryman M, Gary R, Bretscher A (1995) Ezrin oligomers are major cytoskeletal components of placental microvilli: a proposal for their involvement in cortical morphogenesis. J Cell Biol 131(5):1231–1242
Bienkowska D, Cowan CR (2012) Centrosomes can initiate a polarity axis from any position within one-cell C. elegans embryos. Curr Biol 22(7):583–589
Bienz M, Clevers H (2000) Linking colorectal cancer to Wnt signaling. Cell 103(2):311–320
Bird AF, Bird J (1991) The structure of nematodes. Academic, San Jose, CA
Blaxter M (2011) Nematodes: the worm and its relatives. PLoS Biol 9(4):e1001050
Bonilha VL, Finnemann SC, Rodriguez-Boulan E (1999) Ezrin promotes morphogenesis of apical microvilli and basal infoldings in retinal pigment epithelium. J Cell Biol 147(7):1533–1548
Bossinger O, Cowan CR (2012) Methods in cell biology: analysis of cell polarity in C. elegans embryos. In: Rothman J, Singson A (eds) Caenorhabditis elegans: cell biology and physiology, vol 107, 2nd edn. Academic, New York, NY, pp 207–238
Bossinger O, Fukushige T, Claeys M, Borgonie G, McGhee JD (2004) The apical disposition of the Caenorhabditis elegans intestinal terminal web is maintained by LET-413. Dev Biol 268(2):448–456
Bossinger O, Klebes A, Segbert C, Theres C, Knust E (2001) Zonula adherens formation in Caenorhabditis elegans requires dlg-1, the homologue of the Drosophila gene discs large. Dev Biol 230(1):29–42
Bossinger O, Schierenberg E (1992a) Cell-cell communication in the embryo of Caenorhabditis elegans. Dev Biol 151(2):401–409
Bossinger O, Schierenberg E (1992b) Transfer and tissue-specific accumulation of cytoplasmic components in embryos of C. elegans and R. dolichura: in vivo analysis with a low-cost signal enhancement device. Development 114:317–330
Bossinger O, Wiegner O, Schierenberg E (1996) Embryonic gut differentiation in nematodes: endocytosis of macromolecules and its experimental inhibition. Roux Arch Dev Biol 205:494–497
Brown JW, McKnight CJ (2010) Molecular model of the microvillar cytoskeleton and organization of the brush border. PLoS One 5(2):e9406
Bryant DM, Mostov KE (2008) From cells to organs: building polarized tissue. Nat Rev Mol Cell Biol 9(11):887–901
Busino L, Donzelli M, Chiesa M, Guardavaccaro D, Ganoth D, Dorrello NV, Hershko A, Pagano M, Draetta GF (2003) Degradation of Cdc25A by beta-TrCP during S phase and in response to DNA damage. Nature 426(6962):87–91
Byerly L, Cassada RC, Russell RL (1976) The life cycle of the nematode Caenorhabditis elegans. I. Wild-type growth and reproduction. Dev Biol 51(1):23–33
Carberry K, Wiesenfahrt T, Geisler F, Stöcker S, Gerhardus H, Ueberbach D, Davis W, Jorgensen E, Leube RE, Bossinger O (2012) The novel intestinal filament organizer IFO-1 contributes to epithelial integrity in concert with ERM-1 and DLG-1. Development 139:1851–1862
Carberry K, Wiesenfahrt T, Windoffer R, Bossinger O, Leube RE (2009) Intermediate filaments in Caenorhabditis elegans. Cell Motil Cytoskeleton 66(10):852–864
Chanal P, Labouesse M (1997) A screen for genetic loci required for hypodermal cell and glial-like cell development during Caenorhabditis elegans embryogenesis. Genetics 146(1):207–226
Chen L, Ong B, Bennett V (2001) LAD-1, the Caenorhabditis elegans L1CAM homologue, participates in embryonic and gonadal morphogenesis and is a substrate for fibroblast growth factor receptor pathway-dependent phosphotyrosine-based signaling. J Cell Biol 154(4):841–855
Chen L, Zhou S (2010) “CRASH”ing with the worm: insights into L1CAM functions and mechanisms. Dev Dyn 239(5):1490–1501
Clucas C, Cabello J, Bussing I, Schnabel R, Johnstone IL (2002) Oncogenic potential of a C. elegans cdc25 gene is demonstrated by a gain-of-function allele. EMBO J 21(4):665–674
Coburn C, Allman E, Mahanti P, Benedetto A, Cabreiro F, Pincus Z, Matthijssens F, Araiz C, Mandel A, Vlachos M, Edwards SA, Fischer G, Davidson A, Pryor RE, Stevens A, Slack FJ, Tavernarakis N, Braeckman BP, Schroeder FC, Nehrke K, Gems D (2013) Anthranilate fluorescence marks a calcium-propagated necrotic wave that promotes organismal death in C. elegans. PLoS Biol 11(7):e1001613
Coburn C, Gems D (2013) The mysterious case of the C. elegans gut granule: death fluorescence, anthranilic acid and the kynurenine pathway. Front Genet 4:151
Costa M, Raich W, Agbunag C, Leung B, Hardin J, Priess J (1998) A putative catenin-cadherin system mediates morphogenesis of the Caenorhabditis elegans embryo. J Cell Biol 141(1):297–308
Cox EA, Hardin J (2004) Sticky worms: adhesion complexes in C. elegans. J Cell Sci 117(Pt 10):1885–1897
Cox-Paulson EA, Walck-Shannon E, Lynch AM, Yamashiro S, Zaidel-Bar R, Eno CC, Ono S, Hardin J (2012) Tropomodulin protects alpha-catenin-dependent junctional-actin networks under stress during epithelial morphogenesis. Curr Biol 22(16):1500–1505
Crepaldi T, Gautreau A, Comoglio P, Louvard D, Arpin M (1997) Ezrin is an effector of hepatocyte growth factor-mediated migration and morphogenesis in epithelial cells. J Cell Biol 138(2):423–434
Croce A, Cassata G, Disanza A, Gagliani MC, Tacchetti C, Malabarba MG, Carlier MF, Scita G, Baumeister R, Di Fiore PP (2004) A novel actin barbed-end-capping activity in EPS-8 regulates apical morphogenesis in intestinal cells of Caenorhabditis elegans. Nat Cell Biol 6(12):1173–1179
Datta A, Bryant DM, Mostov KE (2011) Molecular regulation of lumen morphogenesis. Curr Biol 21(3):R126–R136
Deppe U, Schierenberg E, Cole T, Krieg C, Schmitt D, Yoder B, Vonehrenstein G (1978) Cell lineages of embryo of nematode Caenorhabditis-elegans. Proc Natl Acad Sci U S A 75(1):376–380
Donzelli M, Squatrito M, Ganoth D, Hershko A, Pagano M, Draetta GF (2002) Dual mode of degradation of Cdc25 A phosphatase. EMBO J 21(18):4875–4884
Drenckhahn D, Dermietzel R (1988) Organization of the actin filament cytoskeleton in the intestinal brush border: a quantitative and qualitative immunoelectron microscope study. J Cell Biol 107(3):1037–1048
Elsum I, Yates L, Humbert PO, Richardson HE (2012) The Scribble-Dlg-Lgl polarity module in development and cancer: from flies to man. Essays Biochem 53:141–168
Enderle L, McNeill H (2013) Hippo gains weight: added insights and complexity to pathway control. Sci Signal 6(296):re7
Fath KR, Burgess DR (1995) Microvillus assembly. Not actin alone. Curr Biol 5(6):591–593
Feldman JL, Priess JR (2012) A role for the centrosome and PAR-3 in the hand-off of MTOC function during epithelial polarization. Curr Biol 22(7):575–582
Fire A, Xu S, Montgomery M, Kostas S, Driver S, Mello C (1998) Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 391(6669):806–811
Firestein BL, Rongo C (2001) DLG-1 is a MAGUK similar to SAP97 and is required for adherens junction formation. Mol Biol Cell 12(11):3465–3475
Fölsch H, Ohno H, Bonifacino JS, Mellman I (1999) A novel clathrin adaptor complex mediates basolateral targeting in polarized epithelial cells. Cell 99(2):189–198
Francis G, Waterston R (1985) Muscle organization in Caenorhabditis elegans: localization of proteins implicated in thin filament attachment and I-band organization. J Cell Biol 101(4):1532–1549
Francis R, Waterston R (1991) Muscle cell attachment in Caenorhabditis elegans. J Cell Biol 114(3):465–479
Fre S, Huyghe M, Mourikis P, Robine S, Louvard D, Artavanis-Tsakonas S (2005) Notch signals control the fate of immature progenitor cells in the intestine. Nature 435(7044):964–968
Fre S, Pallavi SK, Huyghe M, Lae M, Janssen KP, Robine S, Artavanis-Tsakonas S, Louvard D (2009) Notch and Wnt signals cooperatively control cell proliferation and tumorigenesis in the intestine. Proc Natl Acad Sci U S A 106(15):6309–6314
Giuliani C, Troglio F, Bai Z, Patel FB, Zucconi A, Malabarba MG, Disanza A, Stradal TB, Cassata G, Confalonieri S, Hardin JD, Soto MC, Grant BD, Scita G (2009) Requirements for F-BAR proteins TOCA-1 and TOCA-2 in actin dynamics and membrane trafficking during Caenorhabditis elegans oocyte growth and embryonic epidermal morphogenesis. PLoS Genet 5(10):e1000675
Gloerich M, Ten Klooster JP, Vliem MJ, Koorman T, Zwartkruis FJ, Clevers H, Bos JL (2012) Rap2A links intestinal cell polarity to brush border formation. Nat Cell Biol 14:793
Göbel V, Barrett PL, Hall DH, Fleming JT (2004) Lumen morphogenesis in C. elegans requires the membrane-cytoskeleton linker erm-1. Dev Cell 6(6):865–873
Gonzalez A, Rodriguez-Boulan E (2009) Clathrin and AP1B: key roles in basolateral trafficking through trans-endosomal routes. FEBS Lett 583(23):3784–3795
Grana TM, Cox EA, Lynch AM, Hardin J (2010) SAX-7/L1CAM and HMR-1/cadherin function redundantly in blastomere compaction and non-muscle myosin accumulation during Caenorhabditis elegans gastrulation. Dev Biol 344(2):731–744
Grindstaff K, Yeaman C, Anandasabapathy N, Hsu S, Rodriguez-Boulan E, Scheller R, Nelson W (1998) Sec6/8 complex is recruited to cell-cell contacts and specifies transport vesicle delivery to the basal-lateral membrane in epithelial cells. Cell 93(5):731–740
Grishok A (2013) Biology and mechanisms of short RNAs in Caenorhabditis elegans. Adv Genet 83:1–69
Guo YM, Chen S, Shetty P, Zheng G, Lin R, Li WH (2008) Imaging dynamic cell-cell junctional coupling in vivo using Trojan-LAMP. Nat Methods 5(9):835–841
Han M (1997) Gut reaction to Wnt signaling in worms. Cell 90(4):581–584
Harris KP, Tepass U (2010) Cdc42 and vesicle trafficking in polarized cells. Traffic 11(10):1272–1279
Head BP, Patel HH, Insel PA (2014) Interaction of membrane/lipid rafts with the cytoskeleton: impact on signaling and function: membrane/lipid rafts, mediators of cytoskeletal arrangement and cell signaling. Biochim Biophys Acta 1838:532
Hebeisen M, Drysdale J, Roy R (2008) Suppressors of the cdc-25.1(gf)-associated intestinal hyperplasia reveal important maternal roles for prp-8 and a subset of splicing factors in C. elegans. RNA 14:2618
Hebeisen M, Roy R (2008) CDC-25.1 stability is regulated by distinct domains to restrict cell division during embryogenesis in C. elegans. Development 135(7):1259–1269
Hebner C, Weaver VM, Debnath J (2008) Modeling morphogenesis and oncogenesis in three-dimensional breast epithelial cultures. Annu Rev Pathol 3:313–339
Hedgecock EM, White JG (1985) Polyploid tissues in the nematode Caenorhabditis elegans. Dev Biol 107(1):128–133
Heider MR, Munson M (2012) Exorcising the exocyst complex. Traffic 13(7):898–907
Hergovich A (2013) Regulation and functions of mammalian LATS/NDR kinases: looking beyond canonical Hippo signalling. Cell Biosci 3(1):32
Hermann GJ, Leung B, Priess JR (2000) Left-right asymmetry in C. elegans intestine organogenesis involves a LIN-12/Notch signaling pathway. Development 127(16):3429–3440
Hirokawa N, Tilney LG, Fujiwara K, Heuser JE (1982) Organization of actin, myosin, and intermediate filaments in the brush border of intestinal epithelial cells. J Cell Biol 94(2):425–443
Hoffmann M, Segbert C, Helbig G, Bossinger O (2010) Intestinal tube formation in Caenorhabditis elegans requires vang-1 and egl-15 signaling. Dev Biol 339:268–279
Hoier EF, Mohler WA, Kim SH, Hajnal A (2000) The Caenorhabditis elegans APC-related gene apr-1 is required for epithelial cell migration and Hox gene expression. Genes Dev 14(7):874–886
Hong Y, Roy R, Ambros V (1998) Developmental regulation of a cyclin-dependent kinase inhibitor controls postembryonic cell cycle progression in Caenorhabditis elegans. Development 125(18):3585–3597
Horne-Badovinac S, Lin D, Waldron S, Schwarz M, Mbamalu G, Pawson T, Jan YN, Stainier DY, Abdelilah-Seyfried S (2001) Positional cloning of heart and soul reveals multiple roles for PKC lambda in zebrafish organogenesis. Curr Biol 11(19):1492–1502
Houthoofd W, Willems M, Vangestel S, Mertens C, Bert W, Borgonie G (2006) Different roads to form the same gut in nematodes. Evol Dev 8(4):362–369
Hresko MC, Williams BD, Waterston RH (1994) Assembly of body wall muscle and muscle cell attachment structures in Caenorhabditis elegans. J Cell Biol 124(4):491–506
Hüsken K, Wiesenfahrt T, Abraham C, Windoffer R, Bossinger O, Leube R (2008) Maintenance of the intestinal tube in Caenorhabditis elegans: the role of the intermediate filament protein IFC-2. Differentiation 76:881–896
Iruela-Arispe ML, Davis GE (2009) Cellular and molecular mechanisms of vascular lumen formation. Dev Cell 16(2):222–231
Jin J, Shirogane T, Xu L, Nalepa G, Qin J, Elledge SJ, Harper JW (2003) SCFbeta-TRCP links Chk1 signaling to degradation of the Cdc25A protein phosphatase. Genes Dev 17(24):3062–3074
Johnson ES, Kornbluth S (2012) Phosphatases driving mitosis: pushing the gas and lifting the brakes. Prog Mol Biol Transl Sci 106:327–341
Justice RW, Zilian O, Woods DF, Noll M, Bryant PJ (1995) The Drosophila tumor suppressor gene warts encodes a homolog of human myotonic dystrophy kinase and is required for the control of cell shape and proliferation. Genes Dev 9(5):534–546
Kang J, Shin D, Yu JR, Lee J (2009) Lats kinase is involved in the intestinal apical membrane integrity in the nematode Caenorhabditis elegans. Development 136(16):2705–2715
Karabinos A, Schmidt H, Harborth J, Schnabel R, Weber K (2001) Essential roles for four cytoplasmic intermediate filament proteins in Caenorhabditis elegans development. Proc Natl Acad Sci U S A 98(14):7863–7868
Karim BO, Huso DL (2013) Mouse models for colorectal cancer. Am J Cancer Res 3(3):240–250
Kikuchi A, Kishida S, Yamamoto H (2006) Regulation of Wnt signaling by protein-protein interaction and post-translational modifications. Exp Mol Med 38(1):1–10
Kim HA, Koo BK, Cho JH, Kim YY, Seong J, Chang HJ, Oh YM, Stange DE, Park JG, Hwang D, Kong YY (2012) Notch1 counteracts WNT/beta-catenin signaling through chromatin modification in colorectal cancer. J Clin Invest 122(9):3248–3259
Kim S, Ishidate T, Sharma R, Soto MC, Conte D Jr, Mello CC, Shirayama M (2013) Wnt and CDK-1 regulate cortical release of WRM-1/beta-catenin to control cell division orientation in early Caenorhabditis elegans embryos. Proc Natl Acad Sci U S A 110(10):E918–E927
Kitagawa M, Hatakeyama S, Shirane M, Matsumoto M, Ishida N, Hattori K, Nakamichi I, Kikuchi A, Nakayama K, Nakayama K (1999) An F-box protein, FWD1, mediates ubiquitin-dependent proteolysis of beta-catenin. EMBO J 18(9):2401–2410
Knust E, Bossinger O (2002) Composition and formation of intercellular junctions in epithelial cells. Science 298(5600):1955–1959
Köppen M, Simske JS, Sims PA, Firestein BL, Hall DH, Radice AD, Rongo C, Hardin JD (2001) Cooperative regulation of AJM-1 controls junctional integrity in Caenorhabditis elegans epithelia. Nat Cell Biol 3(11):983–991
Kormish JD, Gaudet J, McGhee JD (2010) Development of the C. elegans digestive tract. Curr Opin Genet Dev 20(4):346–354
Korswagen HC, Herman MA, Clevers HC (2000) Distinct beta-catenins mediate adhesion and signalling functions in C. elegans. Nature 406:527–532
Kostic I, Roy R (2002) Organ-specific cell division abnormalities caused by mutation in a general cell cycle regulator in C. elegans. Development 129(9):2155–2165
Kramer JM (2005) Basement membranes (September 1, 2005). WormBook, ed The C elegans Research Community Wormbook, http://dx.doi.org/doi:10.1895/wormbook.1.16.1, http://www.wormbook.org
Ku NO, Zhou X, Toivola DM, Omary MB (1999) The cytoskeleton of digestive epithelia in health and disease. Am J Physiol 277(6 Pt 1):G1108–G1137
Kwiatkowski AV, Maiden SL, Pokutta S, Choi HJ, Benjamin JM, Lynch AM, Nelson WJ, Weis WI, Hardin J (2010) In vitro and in vivo reconstitution of the cadherin-catenin-actin complex from Caenorhabditis elegans. Proc Natl Acad Sci U S A 107(33):14591–14596
Labouesse M (1997) Deficiency screen based on the monoclonal antibody MH27 to identify genetic loci required for morphogenesis of the Caenorhabditis elegans embryo. Dev Dyn 210(1):19–32
Labouesse M (2006) Epithelial junctions and attachments (January 13, 2006). WormBook, ed The C elegans Research Community Wormbook, http://dx.doi.org/doi:10.1895/wormbook.1.56.1, http://www.wormbook.org
Laprise P, Tepass U (2011) Novel insights into epithelial polarity proteins in Drosophila. Trends Cell Biol 21(7):401–408
Legouis R, Gansmuller A, Sookhareea S, Bosher JM, Baillie DL, Labouesse M (2000) LET-413 is a basolateral protein required for the assembly of adherens junctions in Caenorhabditis elegans. Nat Cell Biol 2(7):415–422
Leung B, Hermann GJ, Priess JR (1999) Organogenesis of the Caenorhabditis elegans intestine. Dev Biol 216(1):114–134
Lin R, Hill R, Priess J (1998) POP-1 and anterior-posterior fate decisions in C. elegans embryos. Cell 92(2):229–239
Lin R, Thompson S, Priess JR (1995) pop-1 encodes an HMG box protein required for the specification of a mesoderm precursor in early C. elegans embryos. Cell 83(4):599–609
Lockwood C, Zaidel-Bar R, Hardin J (2008a) The C. elegans zonula occludens ortholog cooperates with the cadherin complex to recruit actin during morphogenesis. Curr Biol 18(17):1333–1337
Lockwood CA, Lynch AM, Hardin J (2008b) Dynamic analysis identifies novel roles for DLG-1 subdomains in AJM-1 recruitment and LET-413-dependent apical focusing. J Cell Sci 121(Pt 9):1477–1487. doi:10.1242/jcs.017137
Lubarsky B, Krasnow MA (2003) Tube morphogenesis: making and shaping biological tubes. Cell 112(1):19–28
Lynch AM, Grana T, Cox-Paulson E, Couthier A, Cameron M, Chin-Sang I, Pettitt J, Hardin J (2012) A genome-wide functional screen shows MAGI-1 is an L1CAM-dependent stabilizer of apical junctions in C. elegans. Curr Biol 22(20):1891–1899
MacQueen AJ, Baggett JJ, Perumov N, Bauer RA, Januszewski T, Schriefer L, Waddle JA (2005) ACT-5 is an essential Caenorhabditis elegans actin required for intestinal microvilli formation. Mol Biol Cell 16(7):3247–3259
Maduro MF (2009) Structure and evolution of the C. elegans embryonic endomesoderm network. Biochim Biophys Acta 1789(4):250–260
Maduro MF (2010) Cell fate specification in the C. elegans embryo. Dev Dyn 239(5):1315–1329
Maiden SL, Harrison N, Keegan J, Cain B, Lynch AM, Pettitt J, Hardin J (2013) Specific conserved C-terminal amino acids of Caenorhabditis elegans HMP-1/alpha-catenin modulate F-actin binding independently of vinculin. J Biol Chem 288(8):5694–5706
Mango SE (2007) The C. elegans pharynx: a model for organogenesis (January 22, 2007). WormBook, ed The C elegans Research Community Wormbook, http://dx.doi.org/doi:10.1895/wormbook.1.129.1, http://www.wormbook.org
Mango SE (2009) The molecular basis of organ formation: insights from the C. elegans foregut. Annu Rev Cell Dev Biol 25:597–628
Mansfield LS, Gamble HR, Fetterer RH (1992) Characterization of the eggshell of Haemonchus contortus–I. Structural components. Comp Biochem Physiol B 103(3):681–686
McCarter J, Bartlett B, Dang T, Schedl T (1999) On the control of oocyte meiotic maturation and ovulation in Caenorhabditis elegans. Dev Biol 205(1):111–128
McGee MD, Weber D, Day N, Vitelli C, Crippen D, Herndon LA, Hall DH, Melov S (2011) Loss of intestinal nuclei and intestinal integrity in aging C. elegans. Aging Cell 10:699–710
McGhee JD (2007) The C. elegans intestine (March 27, 2007). WormBook, ed The C elegans Research Community Wormbook, http://dx.doi.org/doi:10.1895/wormbook.1.133.1, http://www.wormbook.org
McGhee JD (2013) The Caenorhabditis elegans intestine. Wiley Interdiscip Rev Dev Biol 2(3):347–367
McKeown C, Praitis V, Austin J (1998) sma-1 encodes a betaH-spectrin homolog required for Caenorhabditis elegans morphogenesis. Development 125(11):2087–2098
McMahon L, Legouis R, Vonesch JL, Labouesse M (2001) Assembly of C. elegans apical junctions involves positioning and compaction by LET-413 and protein aggregation by the MAGUK protein DLG-1. J Cell Sci 114(Pt 12):2265–2277
Mizumoto K, Sawa H (2007) Two betas or not two betas: regulation of asymmetric division by beta-catenin. Trends Cell Biol 17(10):465–473
Mooseker MS (1985) Organization, chemistry, and assembly of the cytoskeletal apparatus of the intestinal brush border. Annu Rev Cell Biol 1:209–241
Müller H, Wieschaus E (1996) armadillo, bazooka, and stardust are critical for early stages in formation of the zonula adherens and maintenance of the polarized blastoderm epithelium in Drosophila. J Cell Biol 134(1):149–163
Müller HA, Bossinger O (2003) Molecular networks controlling epithelial cell polarity in development. Mech Dev 120(11):1231–1256
Munn EA, Greenwood CA (1984) The occurrence of submicrovillar endotube (Modified Terminal Web) and associated cytoskeletal structures in the intestinal epithelia of nematodes. Philos Trans R Soc Lond B Biol Sci 306:1–18
Nagao S, Kugita M, Yoshihara D, Yamaguchi T (2012) Animal models for human polycystic kidney disease. Exp Anim 61(5):477–488
Nance J, Munro EM, Priess JR (2003) C. elegans PAR-3 and PAR-6 are required for apicobasal asymmetries associated with cell adhesion and gastrulation. Development 130(22):5339–5350
Nelson WJ (2003) Adaptation of core mechanisms to generate cell polarity. Nature 422(6933):766–774
Neves A, English K, Priess JR (2007) Notch-GATA synergy promotes endoderm-specific expression of ref-1 in C. elegans. Development 134(24):4459–4468
Neves A, Priess JR (2005) The REF-1 family of bHLH transcription factors pattern C. elegans embryos through Notch-dependent and Notch-independent pathways. Dev Cell 8(6):867–879
Noah TK, Shroyer NF (2013) Notch in the intestine: regulation of homeostasis and pathogenesis. Annu Rev Physiol 75:263–288
Olson SK, Greenan G, Desai A, Muller-Reichert T, Oegema K (2012) Hierarchical assembly of the eggshell and permeability barrier in C. elegans. J Cell Biol 198(4):731–748
Ouellet J, Roy R (2007) The lin-35/Rb and RNAi pathways cooperate to regulate a key cell cycle transition in C. elegans. BMC Dev Biol 7:38
Page AP, Johnstone IL (2007) The cuticle (March 19, 2007). WormBook, ed The C elegans Research Community Wormbook, http://dx.doi.org/doi:10.1895/wormbook.1.138.1, http://www.wormbook.org
Pásti G, Labouesse M (2014) Epithelial junctions, cytoskeleton, and polarity (November 4, 2014). WormBook, ed The C elegans Research Community Wormbook, http://dx.doi.org/doi:10.1895/wormbook.1.56.2, http://www.wormbook.org
Patel FB, Bernadskaya YY, Chen E, Jobanputra A, Pooladi Z, Freeman KL, Gally C, Mohler WA, Soto MC (2008) The WAVE/SCAR complex promotes polarized cell movements and actin enrichment in epithelia during C. elegans embryogenesis. Dev Biol 324(2):297–309
Patel FB, Soto MC (2013) WAVE/SCAR promotes endocytosis and early endosome morphology in polarized C. elegans epithelia. Dev Biol 377(2):319–332
Pettitt J, Cox EA, Broadbent ID, Flett A, Hardin J (2003) The Caenorhabditis elegans p120 catenin homologue, JAC-1, modulates cadherin-catenin function during epidermal morphogenesis. J Cell Biol 162(1):15–22
Pilipiuk J, Lefebvre C, Wiesenfahrt T, Legouis R, Bossinger O (2009) Increased IP3/Ca2+ signaling compensates depletion of LET-413/DLG-1 in C. elegans epithelial junction assembly. Dev Biol 327(1):34–47
Piperno G, Fuller MT (1985) Monoclonal antibodies specific for an acetylated form of alpha-tubulin recognize the antigen in cilia and flagella from a variety of organisms. J Cell Biol 101(6):2085–2094
Podbilewicz B, White J (1994) Cell fusions in the developing epithelia of C. elegans. Dev Biol 161(2):408–424
Polakis P (2000) Wnt signaling and cancer. Genes Dev 14(15):1837–1851
Popham JD, Webster CM (1979) Aspects of the fine structure of the dauer larva of the nematode Caenorhabditis elegans. Cand J Zool 57(4):794–800
Portereiko MF, Saam J, Mango SE (2004) ZEN-4/MKLP1 is required to polarize the foregut epithelium. Curr Biol 14(11):932–941
Praitis V, Ciccone E, Austin J (2005) SMA-1 spectrin has essential roles in epithelial cell sheet morphogenesis in C. elegans. Dev Biol 283(1):157–170
Priess JR (2005) Notch signaling in the C. elegans embryo (June 25, 2005). WormBook, ed The C elegans Research Community Wormbook, http://dx.doi.org/doi:10.1895/wormbook.1.4.1, http://www.wormbook.org
Priess JR, Hirsh D (1986) Caenorhabditis elegans morphogenesis: the role of the cytoskeleton in elongation of the embryo. Dev Biol 117(1):156–173
Putzke AP, Rothman JH (2010) Repression of Wnt signaling by a Fer-type nonreceptor tyrosine kinase. Proc Natl Acad Sci U S A 107(37):16154–16159
Qadota H, Inoue M, Hikita T, Koppen M, Hardin JD, Amano M, Moerman DG, Kaibuchi K (2007) Establishment of a tissue-specific RNAi system in C. elegans. Gene 400(1–2):166–173
Rae R, Witte H, Rodelsperger C, Sommer RJ (2012) The importance of being regular: Caenorhabditis elegans and Pristionchus pacificus defecation mutants are hypersusceptible to bacterial pathogens. Int J Parasitol 42(8):747–753
Rappleye CA, Paredez AR, Smith CW, McDonald KL, Aroian RV (1999) The coronin-like protein POD-1 is required for anterior-posterior axis formation and cellular architecture in the nematode Caenorhabditis elegans. Genes Dev 13:2838–2851
Rocheleau C, Downs W, Lin R, Wittmann C, Bei Y, Cha Y, Ali M, Priess J, Mello C (1997) Wnt signaling and an APC-related gene specify endoderm in early C. elegans embryos. Cell 90(4):707–716
Saotome I, Curto M, McClatchey AI (2004) Ezrin is essential for epithelial organization and villus morphogenesis in the developing intestine. Dev Cell 6(6):855–864
Sarov M, Murray JI, Schanze K, Pozniakovski A, Niu W, Angermann K, Hasse S, Rupprecht M, Vinis E, Tinney M, Preston E, Zinke A, Enst S, Teichgraber T, Janette J, Reis K, Janosch S, Schloissnig S, Ejsmont RK, Slightam C, Xu X, Kim SK, Reinke V, Stewart AF, Snyder M, Waterston RH, Hyman AA (2012) A genome-scale resource for in vivo tag-based protein function exploration in C. elegans. Cell 150(4):855–866
Schierenberg E (2006) Embryological variation during nematode development (January 02, 2006). WormBook, ed The C elegans Research Community Wormbook, http://dx.doi.org/doi:10.1895/wormbook.1.55.1, http://www.wormbook.org
Schnabel R, Hutter H, Moerman DG, Schnabel H (1997) Assessing normal embryogenesis in Caenorhabditis elegans using a 4D microscope: variability of development and regional specification. Dev Biol 184(2):234–265
Schroeder D, McGhee J (1998) Anterior-posterior patterning within the Caenorhabditis elegans endoderm. Development 125(24):4877–4887
Segbert C, Johnson K, Theres C, van Fürden D, Bossinger O (2004) Molecular and functional analysis of apical junction formation in the gut epithelium of Caenorhabditis elegans. Dev Biol 266(1):17–26
Segref A, Cabello J, Clucas C, Schnabel R, Johnstone IL (2010) Fate specification and tissue-specific cell cycle control of the Caenorhabditis elegans intestine. Mol Biol Cell 21(5):725–738
Shafaq-Zadah M, Brocard L, Solari F, Michaux G (2012) AP-1 is required for the maintenance of apico-basal polarity in the C. elegans intestine. Development 139:2061
Shibata Y, Fujii T, Dent JA, Fujisawa H, Takagi S (2000) EAT-20, a novel transmembrane protein with EGF motifs, is required for efficient feeding in Caenorhabditis elegans. Genetics 154(2):635–646
Simons K, Ikonen E (1997) Functional rafts in cell membranes. Nature 387(6633):569–572
Simske JS, Köppen M, Sims P, Hodgkin J, Yonkof A, Hardin J (2003) The cell junction protein VAB-9 regulates adhesion and epidermal morphology in C. elegans. Nat Cell Biol 5(7):619–625
Sugioka K, Mizumoto K, Sawa H (2011) Wnt regulates spindle asymmetry to generate asymmetric nuclear beta-catenin in C. elegans. Cell 146(6):942–954
Sulston J, Horvitz H (1977) Post-embryonic cell lineages of the nematode, Caenorhabditis elegans. Dev Biol 56(1):110–156
Sulston J, Schierenberg E, White J, Thomson J (1983) The embryonic cell lineage of the nematode Caenorhabditis elegans. Dev Biol 100(1):64–119
Takata K, Singer SJ (1988) Phosphotyrosine-modified proteins are concentrated at the membranes of epithelial and endothelial cells during tissue development in chick embryos. J Cell Biol 106(5):1757–1764
Takeuchi K, Sato N, Kasahara H, Funayama N, Nagafuchi A, Yonemura S, Tsukita S (1994) Perturbation of cell adhesion and microvilli formation by antisense oligonucleotides to ERM family members. J Cell Biol 125(6):1371–1384
The_C_elegans_Sequencing_Consortium (1998) Genome sequence of the nematode C. elegans: a platform for investigating biology. Science 282(5396):2012–2018
Thomas GH (2001) Spectrin: the ghost in the machine. Bioessays 23(2):152–160
Timmons L, Fire A (1998) Specific interference by ingested dsRNA. Nature 395(6705):854
Totong R, Achilleos A, Nance J (2007) PAR-6 is required for junction formation but not apicobasal polarization in C. elegans embryonic epithelial cells. Development 134(7):1259–1268
Troemel ER, Felix MA, Whiteman NK, Barriere A, Ausubel FM (2008) Microsporidia are natural intracellular parasites of the nematode Caenorhabditis elegans. PLoS Biol 6(12):2736–2752
van den Heuvel S (2005) Cell-cycle regulation (September 21, 2005). WormBook, ed The C elegans Research Community Wormbook, http://dx.doi.org/doi:10.1895/wormbook.1.28.1, http://www.wormbook.org
van den Heuvel S, Kipreos ET (2012) C. elegans cell cycle analysis. Methods Cell Biol 107:265–294
van Es J, van Gijn M, Riccio O, Van Den Born M, Vooijs M, Begthel H, Cozijnsen M, Robine S, Winton D, Radtke F, Clevers H (2005) Notch/γ-secretase inhibition turns proliferative cells in intestinal crypts and adenomas into goblet cells. Nature 435(7044):959–963
van Fürden D, Johnson K, Segbert C, Bossinger O (2004) The C. elegans ezrin-radixin-moesin protein ERM-1 is necessary for apical junction remodelling and tubulogenesis in the intestine. Dev Biol 272(1):262–276
Vogel BE, Hedgecock EM (2001) Hemicentin, a conserved extracellular member of the immunoglobulin superfamily, organizes epithelial and other cell attachments into oriented line-shaped junctions. Development 128(6):883–894
Vogel BE, Muriel JM, Dong C, Xu X (2006) Hemicentins: what have we learned from worms? Cell Res 16(11):872–878
Walker DS, Ly S, Lockwood KC, Baylis HA (2002) A direct interaction between IP(3) receptors and myosin II regulates IP(3) signaling in C. elegans. Curr Biol 12(11):951–956
Wang H, Girskis K, Janssen T, Chan JP, Dasgupta K, Knowles JA, Schoofs L, Sieburth D (2013) Neuropeptide secreted from a pacemaker activates neurons to control a rhythmic behavior. Curr Biol 23(9):746–754
Weisz OA, Rodriguez-Boulan E (2009) Apical trafficking in epithelial cells: signals, clusters and motors. J Cell Sci 122(Pt 23):4253–4266
Wharton D (1980) Nematode egg-shells. Parasitology 81(2):447–463
White J (1988) The anatomy. In: Wood WB (ed) The nematode C. elegans. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, pp 81–122
Williams-Masson E, Malik A, Hardin J (1997) An actin-mediated two-step mechanism is required for ventral enclosure of the C. elegans hypodermis. Development 124(15):2889–2901
Wilson PD (2011) Apico-basal polarity in polycystic kidney disease epithelia. Biochim Biophys Acta 1812(10):1239–1248
Winter JF, Hopfner S, Korn K, Farnung BO, Bradshaw CR, Marsico G, Volkmer M, Habermann B, Zerial M (2012) Caenorhabditis elegans screen reveals role of PAR-5 in RAB-11-recycling endosome positioning and apicobasal cell polarity. Nat Cell Biol 14:666
Wu SL, Staudinger J, Olson EN, Rubin CS (1998) Structure, expression, and properties of an atypical protein kinase C (PKC3) from Caenorhabditis elegans. PKC3 is required for the normal progression of embryogenesis and viability of the organism. J Biol Chem 273(2):1130–1143
Xu T, Wang W, Zhang S, Stewart RA, Yu W (1995) Identifying tumor suppressors in genetic mosaics: the Drosophila lats gene encodes a putative protein kinase. Development 121(4):1053–1063
Yang XD, Huang S, Lo MC, Mizumoto K, Sawa H, Xu W, Robertson S, Lin R (2011) Distinct and mutually inhibitory binding by two divergent {beta}-catenins coordinates TCF levels and activity in C. elegans. Development 138(19):4255–4265
Yonemura S, Wada Y, Watanabe T, Nagafuchi A, Shibata M (2010) alpha-Catenin as a tension transducer that induces adherens junction development. Nat Cell Biol 12(6):533–542
Yu X, Odera S, Chuang CH, Lu N, Zhou Z (2006) C. elegans Dynamin mediates the signaling of phagocytic receptor CED-1 for the engulfment and degradation of apoptotic cells. Dev Cell 10(6):743–757
Zhang H, Abraham N, Khan LA, Hall DH, Fleming JT, Gobel V (2011) Apicobasal domain identities of expanding tubular membranes depend on glycosphingolipid biosynthesis. Nat Cell Biol 13:1189
Zhang H, Kim A, Abraham N, Khan LA, Göbel V (2013) Vesicular sorting controls the polarity of expanding membranes in the C. elegans intestine. Worm 2(1):e23702
Zhang H, Kim A, Abraham N, Khan LA, Hall DH, Fleming JT, Göbel V (2012) Clathrin and AP-1 regulate apical polarity and lumen formation during C. elegans tubulogenesis. Development 139:2071
Zhao B, Schafer WR (2013) Neuropeptide signaling: from the gut. Curr Biol 23(11):R481–R483
Zhou S, Opperman K, Wang X, Chen L (2008) unc-44 Ankyrin and stn-2 gamma-syntrophin regulate sax-7 L1CAM function in maintaining neuronal positioning in Caenorhabditis elegans. Genetics 180(3):1429–1443
Acknowledgments
We thank R. Durbin for kindly providing electron micrographs (Fig. 2.1h, i); L. Chen, M. Fuller, J. McGhee, K. Kemphues, and J. Priess for providing antibodies and strains; and R. Leube, A. Dineen, B. Lancaster, and members of the Bossinger lab for the critical reading of the manuscript. T. W. is supported by the Canadian Institutes of Health Research operating grant to J. D. McGhee. Work in the laboratory of O. B. is funded by the DFG and Bayer Crop Science. Some nematode strains used in this work were provided by the Caenorhabditis Genetics Center, which is funded by the National Institutes of Health National Center for Research Resources (NCRR). The monoclonal antibodies were obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by the University of Iowa, Department of Biology, Iowa City, IA 52242, USA.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Bossinger, O., Wiesenfahrt, T., Hoffmann, M. (2015). Establishment and Maintenance of Cell Polarity in the C. elegans Intestine. In: Ebnet, K. (eds) Cell Polarity 2. Springer, Cham. https://doi.org/10.1007/978-3-319-14466-5_2
Download citation
DOI: https://doi.org/10.1007/978-3-319-14466-5_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-14465-8
Online ISBN: 978-3-319-14466-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)