Abstract
Neural injury is a fortunately rare occurrence during pregnancy or childbirth. The incidence of intrapartum nerve injury is thought to be 0.92 %. Lateral femoral cutaneous neuropathy (meralgia paresthetica) is the most common neuropathy seen during both pregnancy and delivery. The femoral nerve, lumbosacral plexus, sciatic nerve, obturator nerve, and common peroneal nerve are other possible sites of injury. The pudendal nerves are thought to be very commonly injured during vaginal delivery—some studies estimate that up to 80 % of vaginal births can be complicated by pudendal neuropathy, which may contribute to the development of postpartum urinary and fecal incontinence. Neuropathies during pregnancy and childbirth are most often caused by traction or compression; focal demyelination is more common than axonal injury. Cesarean sections can also be complicated by neural injury, either due to malpositioning or poor retractor placement. Most neuropathies of pregnancy and delivery resolve spontaneously within 2–6 months’ time. The diagnosis of neuropathy is largely clinical in the acute timeframe after childbirth, although for women with symptoms that persist longer than 3 weeks, electrodiagnosis can play a significant role in the localization of the lesion and determination of prognosis. Treatment of neuropathies during pregnancy after childbirth can include physical therapy, supportive orthoses, medication management, injections, or surgical neurolysis or neurectomy for intractable cases.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Pregnancy
- Childbirth
- Neuropathy
- Meralgia paresthetica
- Lumbosacral plexopathy
- Nerve injury
- Pudendal neuropathy
Introduction
Neural injury is thankfully a rare occurrence in pregnant and parturient patients. When such a complication does occur, however, it can create significant pain and functional deficits. This chapter will address neuropathy arising from the lumbosacral plexus and its terminal branches. Radiculopathy will be covered in Chap. 7 and upper extremity neuropathies (including carpal tunnel syndrome) will be discussed in Chap. 9.
Anatomy of the Lumbosacral Plexus
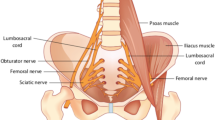
The lumbosacral plexus is made up of branches derived from the L1-S5 nerve roots [1]. The lumbar portion of the plexus originates from L1 to L4, and the sacral portion is typically considered to derive from L4-S5. Table 6.1 lists the major branches of the lumbosacral plexus with their innervations. Figure 6.1 shows the lumbosacral plexus and its relation to bony and ligamentous anatomy.
Mechanism of Neural Injury
Neural injury in pregnant and parturient women is most commonly due to nerve compression or traction [2]. The nerves in certain anatomic locations are more susceptible to compression injury. The lumbosacral plexus, for example, is susceptible to pressure from the descending fetal head as it courses along the lateral pelvic sidewall. Compression injury can also easily occur in superficial nerves such as the common peroneal nerve at the fibular head. Traction neuropathies result from an overstretch injury, which can occur either as a result of the body’s physiologic changes during pregnancy or as a result of labor and delivery positioning. During delivery, there is added potential for nerve injury via laceration (such as during a cesarean birth), ischemia, or due to the use of instrumentation such as forceps. Factors thought to be associated with the development of pregnancy-related neuropathies include excessive weight gain, hypermobility, and increased edema [2–4]. Neural injury during childbirth is thought to be related to nulliparity, prolonged second stage of labor, cephalopelvic disproportion, the use of thigh-hyperflexion pushing position, and assisted (forceps or vacuum) vaginal deliveries [3, 5]. Intrapartum neural injury has not been shown to be associated with maternal or fetal weight or mode of delivery [6]. There is conflicting data at present as to whether neuraxial anesthesia/analgesia is associated with increased incidence of nerve injury [3, 6, 7]. Neuraxial anesthesia may indirectly contribute to the development of neural injury as it is associated with a longer second stage of labor [8]. Women with neuraxial anesthesia-induced sensory blockade may also not recognize symptoms of impending nerve injury and may fail to shift their position in order to relieve nerve compression [6].
The most common type of neural pathology seen in both pregnant and postpartum patients is focal demyelination, with or without conduction block (also referred to as neuropraxia) [2]. This type of nerve injury is generally short-lived and patients can expect a good recovery within days to weeks. More severe nerve damage can result in axonal loss with Wallerian degeneration (also called axonotmesis). In these cases, a more prolonged recovery course would generally be expected, with full recovery on the order of months to a year. Severe crush injuries or nerve transection injuries (collectively referred to as neurotmesis) often involve loss of the nerve stroma and disruption of nerve continuity. With such injuries full recovery is not possible without surgical intervention. Luckily such severe nerve injuries are exceedingly rare in the pregnant/postpartum population [6].
Incidence of Neural Injury
Most of the published literature regarding neural injury in this patient population is in the form of case reports. There have been a handful of retrospective and prospective studies, specifically looking at incidence of intrapartum nerve injury producing lower extremity symptoms. There is no good data on the incidence of pregnancy-related neuropathies.
Looking at these studies in aggregate, the reported incidence of postpartum lower extremity motor and sensory dysfunction due to neurologic injury is thought to be between 0.008 and 0.92 % [3, 7, 9–12]. Study methodology seems to be related in large part to the wide variation in reported incidences, with studies which utilized individual patient follow-up reporting a higher incidence than either retrospective or prospective survey studies [6]. In addition, reported incidence seems to be inversely related to the sample size. For most of the published literature, the localization of nerve injury is determined solely based on history and physical examination—nerve conduction studies, EMG, and other types of diagnostic testing are rarely used. Therefore, the reported location of the injury within the plexus cannot always be assumed to be accurate.
The highest quality study to date is a prospective study by Wong et al. [3] in 2003, which estimated incidence of intrapartum nerve injury to be 0.92 %. This number was far higher than previously reported. The study looked at all women who delivered a live-born infant over a 1-year period of time at the Prentice Women’s Hospital in Chicago. Over 6,000 women included in the study were asked if they had any leg numbness or weakness on the day after delivery, and diagnosis was made with physical examination alone. This study found that the lateral femoral cutaneous nerve was the most commonly injured, followed by the femoral nerve, common peroneal nerve, lumbosacral plexus, obturator nerve, and sciatic nerve. The study did not evaluate for injury to abdominopelvic nerves such as the pudendal or ilioinguinal.
A prospective, case-controlled study of 3,341 parturients who received regional analgesia or anesthesia for labor and delivery reported symptoms of nerve injury in 0.58 % of study participants [7]. Two prospective survey studies from the 1990s of 467,491 and 48,066 deliveries found rates of nerve injury to be 0.01 % and 0.04 %, respectively [10, 12]. A retrospective review of 23,827 deliveries over a 9-year period found the incidence of paresthesias and motor dysfunction to be 0.189 % [9]. A second retrospective review of 143,019 deliveries over a 16-year period reported an incidence of 0.008 % [11].
Neuropathies which have been reported during pregnancy include that of the lateral femoral cutaneous, femoral, lumbosacral plexus, sciatic, and abdominal cutaneous nerves (iliohypogastric and thoracic lateral cutaneous). Intrapartum nerve injury during spontaneous vaginal delivery has been reported to the lateral femoral cutaneous, femoral, lumbosacral plexus, sciatic, obturator, common peroneal, ilioinguinal, and pudendal nerves [3]. Injury has been reported during cesarean delivery (or other surgeries with low transverse Pfannenstiel incisions) to the lateral femoral cutaneous, femoral, lumbosacral plexus, sciatic, common peroneal, iliohypogastric, ilioinguinal, and genitofemoral nerves [13].
Lateral Femoral Cutaneous Neuropathy
Otherwise known as meralgia paresthetica, neuropathy of the lateral femoral cutaneous nerve is the most common lower extremity nerve injury in both pregnant and postpartum patients [3, 4]. Symptoms include numbness and pain of the anterolateral thigh without motor weakness. Symptoms are unilateral in a vast majority of cases, but bilateral injury has been described [14, 15].
The nerve is typically injured via compression or traction at the anterior superior iliac spine or in the region of the inguinal ligament. Anatomic variation can play a role, as the nerve may bifurcate around the inguinal ligament, which makes it more susceptible to traction or compression by the posterior fascicle of the ligament [16]. In pregnancy, increased abdominal girth and lumbar lordosis are thought to be predisposing factors for the development of meralgia paresthetica [3]. Other risk factors can include obesity, excessive pregnancy weight gain, carrying a large fetus, concurrent diabetes, wearing tight clothing, or prolonged hip flexion [17, 18]. Carrying an older child on the ipsilateral hip can also exacerbate symptoms [2]. During delivery, the nerve may be injured during prolonged thigh flexion during the pushing phase of labor [3]. It has been proposed that the elastic belts used to hold monitors in place over the lower abdomen during delivery may also contribute to compression injury of the lateral femoral cutaneous nerve [3]. It also can be infrequently damaged during cesarean section delivery via stretch injury or with an excessively wide incision or poor retractor placement [19–21].
In a case-controlled study of general practitioners, the incidence rate of meralgia paresthetica in the general population was 4.3 per 10,000 person years, and was found to be 12 times more likely to occur in pregnant women compared with nonpregnant patients [22]. Wong et al. [3] found that the lateral femoral cutaneous nerve was the most commonly injured during labor and delivery, comprising 38 % of all nerve palsies identified. The overall incidence of new meralgia paresthetica in postpartum women was 0.4 %. In this study, one third of postpartum women with meralgia paresthetica actually reported having symptoms that initially started during pregnancy. Four out of the 24 women with new onset meralgia paresthetica after delivery underwent cesarean section before the second stage of labor.
Femoral Neuropathy
The femoral nerve is the second most common lower extremity nerve injured during childbirth, and it has also been reported infrequently during pregnancy. Patients with a femoral neuropathy can have a pure sensory deficit or combined sensory and motor loss [3]. Sensory loss is typically in the anterior thigh, although with a severe axonal injury to the femoral nerve, there could also be sensory abnormalities in the distribution of the saphenous nerve (medial lower leg and foot). Knee extension weakness is the most common motor finding, and knee buckling with attempts at standing or ambulation can occur with more severe injuries. Ascending and descending stairs and performing transitional movements such as rising from a seated position can be difficult. The femoral nerve innervates the iliopsoas muscle proximal to the inguinal ligament; if hip flexion weakness is also present, a more proximal femoral neuropathy should be suspected. There can also be diminished or absent patellar reflexes on physical examination.
Risk factors for the development of femoral neuropathy in pregnancy and childbirth are likely similar to those mentioned above for meralgia paresthetica, as the nerves are both located outside of the true pelvis, and therefore are unlikely to be injured via direct compression from the fetal head [3]. The femoral nerve is most likely injured during delivery due to compression or traction at the inguinal ligament during prolonged thigh flexion, external rotation, and abduction [3]. The intrapelvic portion of the femoral nerve is thought to be poorly vascularized, making the nerve more susceptible to stretch-induced ischemia with typical modern childbirth posturing in the semi-Fowler-lithotomy position [2, 23]. There has been one case report of femoral neuropathy associated with symphyseal separation as a complication of the McRoberts’ maneuver, done for the management of shoulder dystocia [24]. A split femoral nerve is a recognized anatomic variant, with bifurcation around slips of the psoas or iliacus muscles, and such anatomy could hypothetically make the nerve more prone to traction or compression injury [3, 25]. There have been multiple case reports of femoral neuropathy following lower abdominal surgery using a Pfannenstiel incision, although none of these reports involved a cesarean delivery [26–28]. In most cases, the etiologic factor seemed to be poorly placed self-retaining retractors. Femoral nerve injury has also been described after cesarean delivery complicated by a retroperitoneal hematoma [29].
The incidence of femoral neuropathy in the early twentieth century was reported as 3.2–4.7 % of all parturients, and 25 % of cases were bilateral [5, 11]. Femoral neuropathy is certainly much less common in modern times, perhaps due to changes in labor and delivery methods, decreased duration of labor, and increased use of cesarean delivery [11]. In the study by Wong et al. [3], femoral neuropathy was found to be the cause of 30 % of postpartum neuropathic symptoms (22 out of 63 patients), giving an overall incidence for postpartum femoral neuropathy of 0.36 %. Eight patients had unilateral sensory deficits, 13 patients had unilateral sensory loss combined with motor weakness, and one patient had bilateral sensory and motor deficits. All 14 patients with motor deficit presented with hip flexion weakness as well as loss of knee extensor strength, indicating injury proximal to the inguinal ligament. Femoral neuropathy in pregnancy is not common, but there have been at least two case reports, both of which indicated bilateral involvement [30, 31]. Both of these patients required cesarean section because of leg weakness and severe pain, and one delivery was performed early at 32 weeks gestation due to severity of symptoms.
Lumbosacral Plexopathy
A lumbosacral plexopathy can have varying clinical presentations, depending on severity and which portions of the plexus are involved. The part of the plexus originating at the L4 and L5 nerve roots seems to be the most often injured as it crosses anterior to the sacral ala and sacroiliac joint. Clinically, this makes intrapartum lumbosacral plexopathy hard to distinguish from a sciatic neuropathy. Foot drop is a common clinical manifestation, with dorsiflexion, eversion, and great toe extension weakness out of proportion to plantarflexion weakness (because L4 and L5 are more involved than the sacral portions of the plexus). There can be sensory loss below the knee, particularly of the anterolateral leg and foot dorsum. It is important to remember that a postpartum foot drop should not be automatically attributed to a lumbosacral plexopathy, as a sciatic neuropathy, common peroneal neuropathy, or radiculopathy could also cause similar clinical findings. A careful physical examination can often aid in distinguishing the etiology, although further diagnostic testing may ultimately be necessary and will be discussed later in this chapter.
Lumbosacral plexus lesions typically occur due to compression of the lumbosacral trunks against the pelvic brim by the fetal head [32]. Lumbosacral plexopathy has been reported to occur both in the late third trimester of pregnancy and during the second stage of labor [4, 32, 33]. Risk factors for the development of plexopathy include short stature, primiparity, increased fetal size, cephalopelvic disproportion, malpresentation (such as occiput posterior), and an arrested second stage of labor [4, 16, 32, 34]. Specific pelvic anatomic features may also play a predisposing role, such as a straight sacrum, a flat and wide posterior pelvis, posterior displacement of the transverse diameter of the inlet, wide sacroiliac notches, and prominent ischial spines [6, 16]. There is conflicting evidence as to whether the use of forceps is an independent variable leading to the development of intrapartum lumbosacral plexopathy, particularly because forceps are often used in cases of cephalopelvic disproportion and prolonged second stage of labor which are themselves known risk factors [32].
Most of what we know about intrapartum lumbosacral plexopathy is through individual case reports and case series [32, 34–36]. It seems to be predominantly demyelinating in origin with proximal conduction block, based on one series of seven patients which presented detailed nerve conduction study (NCS) and electromyography (EMG) data [32]. Wong et al. [3] reported that 3 out of their 63 patients with symptoms of postpartum nerve injury had a lumbosacral plexopathy. Seven additional patients, however, were described as having symptoms of either a sciatic neuropathy or a radiculopathy. No electrodiagnosis was done to differentiate between these clinically similar etiologies. It is certainly possible that all ten of these patients actually had a lumbosacral plexopathy, given that lumbosacral plexopathy is thought to be much more common in this patient population than either sciatic neuropathy or lumbar radiculopathy [6].
Lumbosacral plexopathy has been rarely reported as a complication of late pregnancy [33, 35, 37, 38]. In all of these cases, the symptoms began in the middle to late third trimester. Low back pain, foot drop, and sensory loss in the lateral lower leg were the most common clinical findings. Most of these cases were presented with associated electrodiagnostic data confirming the plexus as the origin of the symptoms. It is important to note that most cases of pregnancy-related low back pain which radiates down the leg are attributable to a pelvic girdle etiology and not to lumbosacral plexopathy [2].
Sciatic Neuropathy
Because the clinical presentation of lumbosacral plexopathy so closely mirrors sciatic neuropathy, it can be very difficult to tell the two apart clinically. On physical exam, sciatic neuropathy can differ from lumbosacral plexopathy in that sensation to the posterior thigh is usually intact (as this is innervated by the posterior femoral cutaneous nerve which comes off the plexus just inferior to the sciatic nerve). The peroneal portion of the sciatic is often injured more significantly than the tibial, leading to relative preservation of plantarflexion compared to dorsiflexion strength [4].
Mechanism of injury to the sciatic nerve apart from the rest of the plexus could be due to stretch injury during prolonged second stage of labor, particularly in the lithotomy or “tailor” positions [16, 39]. There have been several case reports of sciatic neuropathy associated with piriformis muscle spasm or other pathology, and this etiology is a reasonable one to consider as a cause of sciatic neuropathy both in pregnancy and in postpartum patients [40–42]. Wong et al. [3] reported one patient with symptoms of sciatic neuropathy that started during pregnancy in addition to two patients with new symptoms after delivery. There have been a few case reports of sciatic neuropathy presenting as foot drop after cesarean delivery [43, 44]. The proposed mechanism in each case was that the left lateral tilt position used during surgery caused compression of the left gluteal structures and ultimately the sciatic nerve.
Obturator Neuropathy
Obturator neuropathy has been rarely reported as a potential intrapartum injury. Clinically, this lesion presents as pain and numbness along the medial thigh along with adductor weakness. Obturator lesions are uncommon because the nerve is relatively protected within the deep pelvis and the medial thigh [45]. Both unilateral and bilateral neuropathies have been described in case reports [45–49]. Contributing factors to the development of intrapartum obturator neuropathy include compression by the fetal head or forceps as the nerve crosses the pelvic brim and prolonged time in the lithotomy position [3, 4]. The lithotomy position worsens the angulation of the nerve as it exits the obturator foramen [16]. Obturator neuropathies have also been described after cesarean delivery, and suggested mechanisms of nerve injury include stretching, compression by a retractor, or development of a hematoma [49]. One case has been reported of obturator neuropathy related to the development of a hematoma after an obstetric pudendal nerve block [5]. In the study by Wong et al. [3], only 3 out of 63 patients had symptoms of obturator neuropathy.
Common Peroneal Neuropathy
The common peroneal nerve is typically injured as it crosses superficially behind the fibular head. Symptoms of common peroneal neuropathy include ankle dorsiflexion and eversion weakness with numbness of the lateral lower leg and foot dorsum. The resultant gait is often described as a “slapping gait” as the foot hits the ground with an audible sound due to loss of dorsiflexion control. Plantarflexion of the ankle is preserved. The common peroneal nerve is most often injured during delivery via direct external compression, either by inappropriate leg positioning in stirrups or during hyperflexion of the knees with the mother’s hand on the lateral, upper aspect of the leg [3, 4, 50–52]. It has also been described secondary to squatting during childbirth, a practice which is common in some parts of the world [53, 54]. The compression time required to cause nerve injury is variable and can be as short as a few minutes, therefore patients need to be encouraged to change position frequently, and hand placement during the second stage of labor needs to be monitored [4, 54]. Wong et al. [3] identified just 3 patients out of 63 who had symptoms consistent with common peroneal neuropathy.
Abdominal Wall and Groin Neuropathies
There is one reported case of thoracic lateral cutaneous neuropathy in pregnancy, which clinically caused severe disabling lower abdominal wall pain [55]. Iliohypogastric neuropathy in pregnancy has also been described, with symptoms of severe lower abdominal and groin pain [56]. Associated regional numbness is also possible. It has been proposed that the rapidly expanding abdominal wall causes a traction on the nerves as they exit between the planes of abdominal wall musculature [56]. Spontaneous iliohypogastric nerve entrapment has been estimated to occur in 1 out of every 3,000 to 1 out of every 5,000 pregnancies [57]. Ilioinguinal and genitofemoral neuralgia have not been explicitly described in pregnancy, but it is reasonable to assume they could occur via a similar mechanism.
Ilioinguinal, iliohypogastric, and genitofemoral neuropathies have been described in postpartum patients as well [57, 58]. The ilioinguinal and iliohypogastric nerves are particularly susceptible to injury if a Pfannenstiel or low transverse incision is dissected too far laterally beyond the edge of the rectus abdominis muscles [13, 58]. Damage can occur from direct injury to the nerves, incorporation during the fascial closure, suture entrapment, or as a result of scar tissue formation after the surgery [13, 58]. Neuroma formation is common after such nerve damage and can be a source of chronic pain [59]. Compression of the genitofemoral nerve can be caused by poor placement of self-retaining retractors [13]. The Pfannenstiel incision is a common source of chronic pain, with 12.3–33 % of all postsurgical patients reporting symptoms [58–60]. A study by Loos et al. [59] noted that one third of almost 900 patients with a Pfannenstiel incision after cesarean section reported chronic incisional pain 2 years later. Eight percentage of the patients in that study rated their pain as moderate or severe, leading to limitations in daily functioning. Ilioinguinal and/or iliohypogastric nerve entrapment was found in 53 % of the patients reporting moderate-to-severe pain. Risk factors for the development of ilioinguinal and iliohypogastric neuropathy after cesarean section include a wide incision beyond the borders of the rectus abdominis muscle, emergency cesarean delivery, and recurrent surgeries with Pfannenstiel incisions [59]. Overall incidence of ilioinguinal and/or iliohypogastric nerve injury after a Pfannenstiel incision has been estimated at 2–4 % [58, 59, 61].
Pudendal Neuropathy
Injury to the pudendal nerves during vaginal delivery has been well-reported in the literature, and pudendal neuropathy has been implicated as a possible contributing factor to new onset postpartum urinary and fecal incontinence [62, 63]. Pudendal neuropathy can also present with symptoms of sexual dysfunction, dyspareunia, and pelvic pain [64, 65]. The pudendal nerve and its terminal branches (the inferior rectal nerve, the perineal nerve, and the dorsal nerve to the clitoris) are vulnerable to stretch or compression injury by the descending fetal head [65]. The distal terminal branches can also be injured as a result of perineal lacerations. Using 3D computer modeling, Lien et al. [66] looked at maximum nerve strains for the terminal pudendal branches, defined as (final length minus original length/original length) × 100. They demonstrated that the inferior rectal branch which innervates the external anal sphincter is the most affected, typically stretching well beyond the 15 % strain threshold known to cause permanent damage in appendicular peripheral nerves. They also found that the degree of perineal descent during the second stage of labor influences the strain on the pudendal nerve.
This modeling correlates well with what others have found regarding denervation injury to the sphincter and pelvic floor after childbirth. Allen et al. [67] recruited a group of 75 women who agreed to pudendal nerve terminal motor latency testing and needle EMG of the external anal sphincter at 36 weeks gestation and again at 2 months postpartum. While pregnant, pudendal neurophysiology testing was normal, but EMG evidence of pelvic floor reinnervation potentials were seen in 80 % of the postpartum women. Women who had prolonged second stage of labor and larger babies were noted to have the most EMG evidence of nerve damage. Forceps delivery and perineal tears did not seem to affect the amount of damage seen. There was a correlation between the most significant EMG findings and the immediate postpartum development of urinary and/or fecal incontinence. Women who had elective cesarean section delivery had EMG findings comparable to antenatal values, but those who underwent cesarean section after a failed trial of labor had EMG evidence of reinnervation, implying that labor itself rather than delivery, per se, may play a role in the denervation damage sustained. Multiple other studies have also demonstrated high incidence of pelvic floor denervation injury after vaginal delivery, and have shown correlates to the development of postpartum urinary and fecal incontinence [63, 64, 68–70]. It has been hypothesized that pudendal nerve injury during childbirth may be one of many etiologic factors leading to the development of pelvic floor disorders (including pelvic organ prolapse and incontinence) later in life [62, 71, 72].
Prognosis for Recovery from Neural Injury
By and large, most pregnant and postpartum patients with symptoms of lower extremity nerve injury will recover without treatment within a relatively short period of time after delivery. This is largely due to the fact that most of these injuries are predominantly demyelinating in nature, regardless of whether they are caused by compression, traction, or a combination of the two [3]. Wong et al. [3] reported that the median duration of symptoms in their study was 2 months, with a range from 1 week to greater than 14 months (in 2 out of their 63 injured patients). Ong et al. [9] reported resolution within 72 h for a majority of the 45 patients in their study, and Dar et al. [7] found that symptoms usually resolve within 6 months time. Recovery of most cesarean-related lower extremity nerve injuries has also been shown to follow a similar time course. One study of neuropathies associated with gynecologic surgery reported that symptoms had resolved in 93 % of patients within 6 months [73].
There is not a lot of data as to whether pudendal, ilioinguinal, and iliohypogastric injuries recover at similarly rapid rates, in part because it can be clinically more difficult to determine whether these nerves have fully healed. Postpartum patients may experience weeks to months of abdominopelvic pain and numbness regardless of whether a nerve injury occurred due to myofascial trauma and episiotomy and cesarean incisions. However, some of the studies reported earlier in this chapter seem to indicate the potential for these nerves to not heal as quickly or completely as injuries to nerves in the rest of the lumbosacral plexus. The ilioinguinal and iliohypogastric nerves can be injured via transection during cesarean section or become entrapped in scar tissue, which would more likely lead to higher degree of axonal involvement [58, 59]. The pudendal nerves can also become entrapped in scar, and the smaller, distal terminal branches can be transected in situations where there is significant high-grade perineal tearing. Certainly, most of the published postpartum pudendal nerve electrodiagnostic studies have indicated significant axonal (as well as demyelinating) neural injury, indicating less potential for swift recovery [67, 68].
Diagnosis of Neural Injury
Because most symptoms resolve fairly quickly after delivery, the diagnosis of neural injury is largely clinical and should be based on history and physical examination. Any patient with postpartum complaints of lower extremity weakness, numbness, or pain should be thoroughly evaluated. Important aspects of the history include delivery details such as duration of the second stage of labor, pushing position, mode of delivery, the use of neuraxial anesthesia, and degree of perineal laceration [6]. It is important to note whether any of the symptoms were present during pregnancy, as certain neuropathies like meralgia paresthetica may be present in mild form in pregnancy but then worsen considerably after delivery. Progression of symptoms is important to ascertain, because the symptoms of intrapartum injuries should be stable or improving over the initial hours to days after delivery. If symptoms are worsening, the patient may need to be evaluated emergently for infection, hemorrhage, or other obstetric comorbidities [6]. A thorough neurologic and musculoskeletal examination should be performed. It may be wise to consider obtaining XR imaging of the pelvis to rule out pubic symphysis or sacroiliac joint separation, coccyx fracture, or stress fracture in patients with significant postpartum pelvic or hip pain in weight bearing, as the symptoms from these musculoskeletal complications can sometimes mimic neural injury.
If symptoms persist for longer than 3 weeks after delivery, NCS and EMG can be conducted to attempt to localize the lesion, determine degree of axonal involvement and extent of denervation, and to look for signs of early reinnervation. NCS/EMG can be an important prognostic tool. Electrophysiologic studies cannot be conducted prior to 3 weeks postpartum because Wallerian degeneration will take time to progress to the point where abnormalities can be seen using the needle electrode at the level of the muscle [74]. If the patient has profound weakness immediately postpartum and axonal injury is suspected, it may be a good idea to obtain NCS/EMG within a few days of delivery to establish the patient’s baseline neural function (as any abnormalities seen on such testing would be indicative of problems the patient had prior to delivery). NCS/EMG is considered safe in pregnancy.
In addition to the standard NCS/EMG studies typically conducted in the lower extremities, the pudendal nerve can be evaluated electrophysiologically via a number of different methods. Pudendal nerve terminal motor latency (PNTML) can be obtained through the use of a St. Mark’s electrode, with nerve stimulation at the ischial spine and recording of muscle contraction response at the external anal sphincter (see Fig. 6.2) [62]. The usefulness of PNTML has been questioned, as it has been shown to have a high rate of interobserver and intraobserver variability [75]. Needle EMG of the external anal sphincter or bulbospongiosus muscles can be performed, either with concentric needle electrodes or with single-fiber electrodiagnostic technique [67]. The bulbocavernosus reflex latency (BCRL) can also be obtained by stimulating at the clitoris [64]. Electrodiagnostic testing for pudendal neuropathy may be less well-tolerated than standard NCS/EMG of the extremities.
A St. Mark’s electrode, used for PNTML testing. With kind permission from Springer Science +Business Media: Vaginal Surgery for Incontinence and Prolapse, Neurophysiologic Testing, 2006, p 68, Kenton K., Fig. 6.2
NCS/EMG has minimal diagnostic value for predominantly sensory neuropathies (lateral femoral cutaneous, genitofemoral, ilioinguinal, iliohypogastric), because EMG testing is only available for motor nerves, and the NCS responses for these sensory nerves are often extremely difficult to obtain. Diagnostic nerve blocks are a potentially good option for the diagnosis of painful sensory neuropathies. A positive response to infiltration of a local anesthetic around a purely sensory nerve is thought to be a reliable indicator of etiologic correlation, and techniques for performing diagnostic blocks of the lateral femoral cutaneous, ilioinguinal, iliohypogastric, and genitofemoral nerves have all been described [76–78]. It is always preferable to use ultrasound, pulsed radiofrequency, or CT guidance for better accuracy when performing these diagnostic injections. Diagnostic pudendal nerve blocks have also been described, but it is less clear that a positive response is definitively correlated with true pudendal pathology [79]. Pudendal nerve blocks should always been done under CT guidance for accuracy [75].
Imaging of neural injury will also be discussed in Chap. 3 of this text. Neuromuscular ultrasound is one possible imaging modality that can be used. Nerve injury most typically appears as focal enlargement of the nerve, often just proximal to the site of entrapment if such an entrapment exists [80]. Sonographic evaluation of neuropathy has been described for the common peroneal nerve at the fibular head, the lateral femoral cutaneous nerve, and the sciatic nerve, among others [80–82]. Ultrasound, in general, is not particularly useful for evaluating nerve injuries about the hip and pelvis, because these nerves are typically too deep to allow for long segment exploration and good visualization [83].
Traditional MRI sequence protocols are not especially sensitive for neural injury, but with appropriate spatial resolution certain types of nerve pathology, particularly involving the larger nerves, can be readily seen [83]. MR neurography technology, however, is rapidly becoming recognized as one of the most effective diagnostic tools for nerve injury, and is thought to be far superior for nerve visualization than standard MRI [42, 84]. MR neurography of the lumbosacral plexus is especially valuable because it is able to show injury to the small nerves within the abdominal wall and deep pelvis for which there are few reliable electrophysiologic testing options available. MR neurography can also readily demonstrate a proximal demyelinating lesion within the lumbosacral plexus, which would likely have normal or minimally abnormal NCS/EMG findings. Another advantage of MR neurography is that abnormal appearance of the pathologic nerve can be visible within hours of injury. Figure 6.3 is an axial MR neurography image of an axonal left sciatic neuropathy in a patient who is 3 months postpartum.
Arrow on left. Normal right sciatic nerve. Isointense and without prominent visible fascicles. Arrow on right. Abnormally enlarged left sciatic nerve, which appears hyperintense. Note the nerve fascicles which are clearly visible. Image courtesy of Dr. Avneesh Chhabra of UT Southwestern Medical Center
Treatment of Neural Injury During Pregnancy and in the Postpartum Period
Patients with neural injuries during pregnancy and postpartum can be reasonably assured that their expected prognosis and long-term functional outcomes should be quite good. Most patients with mild symptoms will not require any treatment. However, for a pregnant woman or a new mother with an infant to care for, even a few months of significant neurologic deficit and pain can be a real challenge. Supportive treatments can provide comfort and increase safety until nerve recovery has been achieved.
Physical therapy should be a mainstay of treatment for any pregnancy or postpartum neuropathy with motor involvement [2]. As with any neuropathic injury, the focus of therapy will likely include increasing strength, endurance, and flexibility, improving balance and coordination, and ensuring that the patient understands the appropriate way to biomechanically compensate for their neurologic deficits until recovery can be attained. Some patients may benefit from assistive devices or orthotics to help them to ambulate safely as healing progresses. Any patient with significant foot drop should be evaluated for an ankle-foot orthosis (AFO) to decrease risk of falls (see Fig. 6.4a) [6]. Patients with femoral neuropathies and lumbosacral plexopathies may also have weakness of the quadriceps which can result in knee buckling during ambulation. These patients may benefit from a supportive knee brace or even a knee-ankle-foot orthosis (KAFO) in extreme cases (see Fig. 6.4b). Some patients may have to use a cane or a walker to ambulate safely until strength returns. The physical therapist can help the patient to learn to use the adaptive equipment effectively.
The specifics of medication prescription for pregnant and lactating women are discussed in Chap. 14. For pregnant women with neuropathic pain (most often due to meralgia paresthetica), there are limited options for effective pain control. Tylenol and topical lidocaine patches or creams, and capsaicin can be tried as they are all pregnancy class B. Neuropathic pain medications are typically pregnancy class C or D. These should be used with caution and only with the expressed approval of the patient’s obstetrician for a patient with severe symptoms. Opioid medications should generally be avoided. Corticosteroids are pregnancy class C, but are routinely given to hasten fetal lung maturity in patients at risk for preterm labor [85]. A short course of low dose oral steroids may be helpful for severe pain symptoms, but again this needs to be discussed with the patient’s obstetrician. Most pregnant women with meralgia paresthetica are comforted simply by being told that the symptoms should resolve after delivery and will not desire any treatment.
For postpartum patients, there are many neuropathic pain medications available such as gabapentin, pregabalin, duloxetine, venlafaxine, amitriptyline, and nortriptyline. Lactating mothers may want to use caution in deciding whether to treat their pain with these medications, because potential risks to the infant have not been well established for most of these medications. Compounded neuropathic pain creams are being prescribed more frequently in recent years. These creams often consist of a mixture of various neuropathic medications (gabapentin, amitriptyline), but the key ingredient is typically ketamine at a concentration of 5–10 % [86, 87]. Other additives to the creams may include muscle relaxers such as baclofen or cyclobenzaprine and local analgesics like tetracaine. There is minimal data on the effectiveness of neuropathic pain creams—of the two randomized, placebo-controlled, double-blind trials which have been conducted, one showed a benefit and the other did not [88, 89]. Systemic absorption is thought to be low and side effects are typically minimal. A short course of oral corticosteroids (such as a tapered dosing of methylprednisolone) may be an option to consider for severe pain. It is important to remember that steroids can impair wound healing and affect the immune system and hypothalamic-pituitary-adrenal (HPA) and hypothalamic-pituitary-gonadal (HPG) axes [90].
There have been a variety of interventional treatments described for painful sensory neuropathies derived from the lumbosacral plexus. Most of what has been reported has been in the form of isolated case reports or retrospective case series; there have been very few prospective studies to date. Therapeutic injections of corticosteroid mixed with local anesthetic, delivered either as a single intervention or as an injection series, have been reported to be helpful for lateral femoral cutaneous, ilioinguinal, iliohypogastric, genitofemoral, thoracic lateral cutaneous, and pudendal neuropathies [75–78, 91]. As with diagnostic injections, therapeutic injections should ideally be performed under ultrasound or CT guidance. Some such injections have even been reported as successful and low risk in pregnant patients, when done by an experienced practitioner with proper ultrasound guidance and with the consent of the patient’s obstetrician [55, 56, 92]. Sciatic neuropathy has been reportedly treated with perisciatic injections, transsacral blocks, or piriformis muscle trigger point or botox injections [93–96]. Radiofrequency ablation and pulsed radiofrequency treatments for some of these nerves have also been described [97–99]. There is one case report of alcohol denaturation of the lateral femoral cutaneous nerve [100]. There have been a few descriptions of successful treatment of ilioinguinal or pudendal neuropathic pain via neuromodulation either at the level of the spinal cord, sacral plexus, or of the individual nerves themselves, but at this time neuromodulation has not been studied extensively enough to recommend its use in this patient population [101–104]. A therapeutic trial should always be conducted to assess for effectiveness before proceeding with the implantation of a neurostimulator.
Surgery can be an effective solution in some cases, particularly for chronic lateral femoral cutaneous, ilioinguinal, iliohypogastric, and genitofemoral neuralgia [58, 76, 105–107]. Two main surgical approaches have been described. Neurolysis involves the release of the nerve sheath and the breaking up of perineural adhesions while leaving the nerve itself intact. Neurectomy is also known as nerve resection or transection. Some have reported that neurectomy is preferable to neurolysis for the treatment of the cutaneous sensory nerves listed above, as the risk of long-term recurrence is lessened [105]. Rates of complete or moderate pain relief after neurolysis or neurectomy for the lateral femoral cutaneous, ilioinguinal, iliohypogastric, and genitofemoral nerves have been reported in the range of 66–100 % of patients [58, 105–107]. Surgical exploration and neurolysis has also been described for the sciatic, femoral, and common peroneal nerves with good treatment outcomes in terms of improved pain control as well as improved motor function and sensation [108–111].
Various approaches have been described for decompression of the pudendal nerve in cases of entrapment [75]. Outcomes for pudendal decompression surgeries have not been uniformly good. Short-term improvement of some degree has been seen in 50–70 % of patients after 3–12 months, but 50–66 % of all patients undergoing surgery have no long-term benefit [112, 113]. Appropriate patient selection and a high level of surgeon experience seem to be the keys to successful outcomes with higher satisfaction rates [65]. Hibner et al. [65] anecdotally reported that 70 % of their pudendal neuropathy patients have improvement of neuropathic symptoms after transgluteal decompression, although they also stated that many of these patients are still left with pelvic floor myofascial pain after surgery. There are many etiologies of pelvic pain which can mimic the symptoms of pudendal neuropathic pain, including inferior cluneal neuralgia, pelvic floor myofascial pain, and primary urologic, gynecologic, and anorectal pathologies. Patients with these conditions, with or without comorbid pudendal neuropathy, might not be expected to do as well with surgical decompression of the pudendal nerve.
Conclusion
Neural injury to the lumbosacral plexus and its terminal branches during pregnancy and childbirth is an infrequent complication, with the exception of pudendal neuropathy which seems to be quite common after vaginal delivery. More research is needed to clarify ways to further reduce the incidence of these injuries. Some data suggest that potential benefit might be derived by reducing the amount of time spent in the second stage of labor and specifically in the semi-Fowler lithotomy position, limiting the extent of perineal descent during the pushing phase, reducing the incidence of instrumented deliveries, and using care with surgical technique during cesarean delivery [3]. Maternal neuropathies typically improve significantly within months of delivery, and prognosis is generally very good. Diagnosis and treatment options are available for those patients with more severe neural injury.
References
Henry G. Anatomy of the human body. Philadelphia: Lea & Febiger; 1918. Bartleby.com, 2000. www.bartleby.com/107/.
Borg-Stein J, Dugan S. Musculoskeletal disorders of pregnancy, delivery, and postpartum. Phys Med Rehabil Clin N Am. 2007;18(3):459–76.
Wong C, Scavone B, Dugan S, et al. Incidence of postpartum lumbosacral spine and lower extremity nerve injuries. Obstet Gynecol. 2003;101(2):279–88.
Sax T, Rosebaum R. Neuromuscular disorders in pregnancy. Muscle Nerve. 2006;34(5):559–71.
Donaldson J. Neurology of pregnancy. Philadelphia: WB Saunders; 1989.
Wong C. Nerve injuries after neuraxial anaesthesia and their medicolegal implications. Best Pract Res Clin Obstet Gynaecol. 2010;24(3):367–81.
Dar A, Robinson A, Lyons G. Postpartum neurologic symptoms following regional blockade: a prospective study with case controls. Int J Obstet Anesth. 2002;11:85–90.
Sharma S, McIntire D, Wiley J. Labor analgesia and cesarean delivery: an individual patient meta-analysis of nulliparous women. Anesthesiology. 2004;100:142–8.
Ong B, Cohen M, Esmail A, et al. Paresthesias and motor dysfunction after labor and delivery. Anesth Analg. 1987;66:18–22.
Scott DB, Tunstall ME. Serious complications associated with epidural/spinal blockade in obstetrics: a two-year prospective study. Int J Obstet Anesth. 1995;4:133–9.
Vargo M, Robinson L, Nicholas J, et al. Postpartum femoral neuropathy: relic of an earlier era? Arch Phys Med Rehabil. 1990;71(8):591–6.
Holdcroft A, Gibberd FB, Hargrove RL, et al. Neurological complications associated with pregnancy. Br J Anesth. 1995;75:522–6.
Bradshaw A, Advincula A. Postoperative neuropathy in gynecologic surgery. Obstet Gynecol Clin North Am. 2010;37(3):451–9.
Tsen L. Neurologic complications of labor analgesia and anesthesia. Int Anesthesiol Clin. 2002;40:67–88.
Paul F, Zipp F. Bilateral meralgia paresthetica after cesarian section with epidural analgesia. J Peripher Nerv Syst. 2006;11(1):98–9.
Aminoff M. Neurological disorders and pregnancy. Am J Obstet Gynecol. 1978;12:1–5.
Kein A. Peripheral nerve disease in pregnancy. Clin Obstet Gynecol. 2013;56(2):382–8.
Van Diver T, Camann W. Meralgia paresthetica in the parturient. Int J Obstet Anesth. 1995;4(2):109–12.
Redick L. Maternal perinatal nerve palsies. Postgrad Obstet Gynecol. 1992;12:1–5.
Peters G, Larner AJ. Meralgia paresthetica following gynecologic and obstetric surgery. Int J Gynecol Obstet. 2006;95(1):42–3.
Yanaru T, Katori K, Higa K, et al. Unilateral temporary meralgia paresthetica after caesarian section: report of a case. Masui. 2012;61(10):1099–101.
van Slobbe AM, Bohnen AM, Bernsen RM, et al. Incidence rates and determinants in meralgia paresthetica in general practice. J Neurol. 2004;251(3):294–7.
al Hakim M, Katirji B. Femoral mononeuropathy induced by the lithotomy position: a report of 5 cases with a review of literature. Muscle Nerve. 1993;16(9):891–5.
Gherman R, Ouzounian J, Incerpi M, et al. Symphyseal separation and transient femoral neuropathy associated with the McRoberts’ maneuver. Am J Obstet Gynecol. 1998;178(3):609–10.
Spratt J, Logan B, Abrahams P. Variant slips of psoas and iliacus muscles, with splitting of the femoral nerve. Clin Anat. 1996;9:401–4.
Brasch R, Bufo A, Kreienberg P, et al. Femoral neuropathy secondary to the use of self-retaining retractor. Dis Colon Rectum. 1995;38:1115–8.
Al-Ajmi A, Rousseff RT, Khuraibet AJ. Iatrogenic femoral neuropathy: two cases and literature update. J Clin Neuromuscul Dis. 2010;12(2):66–75.
Huang W, Lin P, Yeh C, et al. Iatrogenic femoral neuropathy following pelvic surgery: a rare and often overlooked complication—four case reports and literature review. Chang Gung Med J. 2007;30(4):374–9.
Chao A, Chao A, Wang CJ, Chao AS. Femoral neuropathy: a rare complication of retroperitoneal hematoma caused by cesarean section. Arch Gynecol Obstet. 2013;287(3):609–11.
Kofler M, Kronenberg MF. Bilateral femoral neuropathy during pregnancy. Muscle Nerve. 1985;21(8):1106.
Pildner von Steinburg S, Kuhler A, Herrmann N, et al. Pregnancy-associated femoral nerve affection. Zentralbl Gynakol. 2004;126(5):328–30.
Katirji B, Wilbourn A, Scarberry S, et al. Intrapartum maternal lumbosacral plexopathy. Muscle Nerve. 2002;26(3):340–7.
Delarue MW, Vles JS, Hasaart TH. Lumbosacral plexopathy in the third trimester of pregnancy: a report of three cases. Eur J Obstet Gynecol Reprod Biol. 1994;53(1):67–8.
Bademosi O, Osuntokun B, Van de Werd H, et al. Obstetric neuropraxia in the Nigerian African. Int J Gynecol Obstet. 1980;17(6):611–4.
Brusse E, Visser LH. Footdrop during pregnancy or labor due to obstetric lumbosacral plexopathy. Ned Tijdschr Geneeskd. 2002;146(1):31–4.
Rageth J, Saurenmann E, Waespe W. Postpartum footdrop due to compression of the lumbosacral trunk. Gynakol Geburtshilfliche Rundsch. 2000;40(2):68–70.
Turgut F, Turgut M, Menteş E. Lumbosacral plexus compression by fetus: an unusual cause of radiculopathy during teenage pregnancy. Eur J Obstet Gynecol Reprod Biol. 1997;73(2):203–4.
Yoshimoto M, Kawaguchi S, Takebayashi T, et al. Diagnostic features of sciatica without lumbar nerve root compression. J Spinal Disord Tech. 2009;22(5):328–33.
Ley L, Ikhouane M, Staiti G, et al. Neurological complication after the “tailor posture” during labour with epidural anesthesia. Ann Fr Anesth Reanim. 2007;26(7–8):666–9.
Vallejo M, Mariano D, Kaul B, et al. Piriformis syndrome in a patient after cesarean section under spinal anesthesia. Reg Anesth Pain Med. 2004;29(4):364–7.
Kinahan A, Douglas M. Piriformis pyomyositis mimicking epidural abscess in a parturient. Can J Anaesth. 1995;42(3):240–5.
Petchprapa C, Rosenberg Z, Sconfienza L, et al. MR imaging of entrapment neuropathies of the lower extremity. Part 1. The pelvis and hip. RadioGraphics. 2010;30:983–1000.
Roy S, Levine A, Herbison G, et al. Intraoperative positioning during cesarean as a cause of sciatic neuropathy. Obstet Gynecol. 2002;99(4):652–3.
Postaci A, Karabeyoglu I, Erdogan G, et al. A case of sciatic neuropathy after cesarean section under spinal anaesthesia. Int J Obstet Anesth. 2006;15(4):317–9.
Nogajski J, Shnier R, Zagamim A. Postpartum obturator neuropathy. Neurology. 2004;63(12):2450–1.
Haas D, Meadows R, Cottrell R, et al. Postpartum obturator neurapraxia. A case report. J Reprod Med. 2003;48(6):469–70.
Lindner A, Schulte-Mattler W, Zierz S. Postpartum obturator nerve syndrome: case report and review of the nerve compression syndrome during pregnancy and delivery. Zentralbl Gynakol. 1997;119(3):93–9.
Hakoiwa S, Hoshi T, Tanaka M, et al. Case of bilateral obturator neuropathy after caesarean section. Masui. 2011;60(6):721–3.
Hong B, Ko Y, Kim H, et al. Intrapartum obturator neuropathy diagnosed after cesarean delivery. Arch Gynecol Obstet. 2010;282(3):349–50.
Colachis Iii SC, Pease WS, Johnson EW. A preventable cause of foot drop during childbirth. Am J Obstet Gynecol. 1994;171(1):270–2.
Sahai-Srivastava S, Amezcua L. Compressive neuropathies complicating normal childbirth: case report and literature review. Birth. 2007;34(2):173–5.
Radawski M, Strakowski J, Johnson E. Acute common peroneal neuropathy due to hand positioning in normal labor and delivery. Obstet Gyneco. 2011;118(2):421–3.
Reif M. Bilateral common peroneal nerve palsy secondary to prolonged squatting in natural childbirth. Birth. 1988;15:100–2.
Babayev M, Bodack M, Creatura C. Common peroneal neuropathy secondary to squatting during childbirth. Obstet Gynecol. 1998;91(5):830–2.
Peleg R, Gohar J, Koretz M, et al. Abdominal wall pain in pregnant women caused by thoracic lateral cutaneous nerve entrapment. Eur J Obstet Gynecol Reprod Biol. 1997;74(2):169–71.
Carter B, Racz G. Iliohypogastric nerve entrapment in pregnancy: diagnosis and treatment. Anesth Analg. 1994;79(6):1193–4.
Racz G, Hagstrom D. Iliohypogastric and ilioinguinal nerve entrapment: diagnosis and treatment. Pain Dig. 1992;2:43–8.
Loos M, Scheltinga M, Roumen R. Surgical management of inguinal neuralgia after a low transverse Pfannenstiel incision. Ann Surg. 2008;248(5):880–5.
Loos M, Scheltinga M, Mulders L, et al. The Pfannenstiel incision as a source of chronic pain. Obstet Gynecol. 2008;111(4):839–46.
Nikolajsen L, Sorensen H, Jensen TS, et al. Chronic pain following Caesarean section. Acta Anaesthesiol Scand. 2004;48:111–6.
Luijendijk R, Jeekel J, Storm R, et al. The low transverse Pfannenstiel incision and the prevalence of incisional hernia and nerve entrapment. Ann Surg. 1997;225:365–9.
Connolly A, Thorp J. Childbirth-related perineal trauma: clinical significance and prevention. Clin Obstet Gynecol. 1999;42(4):820–35.
Fynes M, Donnelly V, Behan M, et al. Effect of second vaginal delivery on anorectal physiology and faecal continence: a prospective study. Lancet. 1999;354(9183):983–6.
Ismael SS, Amarenco G, Bayle B, et al. Postpartum lumbosacral plexopathy limited to autonomic and perineal manifestations: clinical and electrophysiological study of 19 patients. J Neurol Neurosurg Psychiatry. 2000;68(6):771–3.
Hibner M, Castellanos M, Desai N, Balducci J. Glob. Libr. Women’s Med. (ISSN: 1756-2228) 2011; DOI:10.3843/GLOWM.10468
Lien K-C, Morgan DM, Delancey JOL, et al. Pudendal nerve stretch during vaginal birth: a 3D computer simulation. Am J Obstet Gynecol. 2005;192(5):1669–76.
Allen RE, Hosker GL, Smith AR, et al. Pelvic floor damage and childbirth: a neurophysiological study. Br J Obstet Gynaecol. 1990;97:770–9.
Snooks S, Setchell M, Swash M, et al. Injury to innervation of pelvic floor sphincter musculature in childbirth. Lancet. 1984;2(8402):546–50.
Tetzschner T, Sorensen M, Lose G, et al. Pudendal nerve function during pregnancy and after delivery. Int Urogynecol J. 1997;8(2):66–8.
Thorp J, Jones L, Bowes W, et al. Electromyography with acrylic plug surface electrodes after delivery. Am J Perinatol. 1995;12:125–8.
Pla-Martí V, Moro-Valdezate D, Alos-Company R, et al. The effect of surgery on quality of life in patients with faecal incontinence of obstetric origin. Colorectal Dis. 2007;9(1):90–5.
Oberwalder M, Dinnewitzer A, Baig M, et al. The association between late-onset fecal incontinence and obstetric anal sphincter defects. Arch Surg. 2004;139(4):429–32.
Warner M, Warner D, Harper M, et al. Lower extremity neuropathies associated with lithotomy positions. Anesthesiology. 2000;93:938–42.
Dumitru D, Amato A, Zwarts M. Electrodiagnostic medicine. Philadelphia: Hanley & Belfus; 2002.
Hibner M, Desai N, Robertson L, et al. Pudendal neuralgia. J Minim Invasive Gynecol. 2010;17:148–53.
Starling J, Harms B. Diagnosis and treatment of genitofemoral and ilioinguinal neuralgia. World J Surg. 1989;13(5):586–91.
Suresh S, Patel A, Porfyris S, et al. Ultrasound-guided serial ilioinguinal nerve blocks for management of chronic groin pain secondary to ilioinguinal neuralgia in adolescents. Paediatr Anaesth. 2008;18(8):775–8.
Tagliafico A, Serafini G, Lacelli F, et al. Ultrasound-guided treatment of meralgia paresthetica (lateral femoral cutaneous neuropathy): technical description and results of treatment in 20 consecutive patients. J Ultrasound Med. 2011;30(10):1341–6.
Labat J, Riant T, Robert R, et al. Diagnostic criteria for pudendal neuralgia by pudendal nerve entrapment (Nantes criteria). NeurourolUrodyn. 2008;27(4):306–10.
Cartwright M, Walker F. Neuromuscular ultrasound in common entrapment neuropathies. Muscle Nerve. 2013;48(5):696–704.
Aravindakannan T, Wilder-Smith E. High-resolution ultrasonography in the assessment of meralgia paresthetica. Muscle Nerve. 2012;45(3):434–5.
Kara M, Ozcakar L, Tiftik T, et al. Sonographic evaluation of sciatic nerves in patients with unilateral sciatica. Arch Phys Med Rehabil. 2012;93(9):1598–602.
Martinoli C, Miguel-Perez M, Padua L, et al. Imaging of neuropathies about the hip. Eur J Radiol. 2013;82(1):17–26.
Soldatos T, Andreisek G, Thawait G, et al. High-resolution 3-T MR neurography of the lumbosacral plexus. RadioGraphics. 2013;33:967–87.
Surbek D, Drack G, Irion O, et al. Antenatal corticosteroids for fetal lung maturation in threatened preterm delivery: indications and administration. Arch Gynecol Obstet. 2012;286(2):277–81.
Quan D, Wellish M, Gilden D. Topical ketamine treatment of postherpetic neuralgia. Neurology. 2003;60(8):1391–2.
Poterucha T, Murphy S, Rho R, et al. Topical amitriptyline-ketamine for treatment of rectal, genital, and perineal pain and discomfort. Pain Physician. 2012;15(6):485–8.
Mahoney J, Vardaxis V, Moore J, et al. Topical ketamine cream in the treatment of painful diabetic neuropathy: a randomized, placebo-controlled, double-blind initial study. J Am Podiatr Med Assoc. 2012;102(3):178–83.
Finch P, Knudsen L, Drummond P. Reduction of allodynia in patients with complex regional pain syndrome: a double-blind placebo-controlled trial of topical ketamine. Pain. 2009;146(1–2):18–25.
Melillo N, Corrado A, Quarta L, et al. Corticosteroids, a review. Panminerva Med. 2007;49(1):29–33.
Vancaillie T, Eggermont J, Armstrong G, et al. Response to pudendal nerve block in women with pudendal neuralgia. Pain Med. 2012;13(4):596–603.
Harney D, Patijn J. Meralgia paresthetica: diagnosis and management strategies. Pain Med. 2007;8(8):667–77.
Reus M, de Dios BJ, Vázquez V, et al. Piriformis syndrome: a simple technique for US-guided infiltration of the perisciatic nerve—preliminary results. Eur Radiol. 2008;18(3):616–20.
Childers M, Wilson D, Gnatz S, et al. Botulinum toxin type a use in piriformis muscle syndrome: a pilot study. Am J Phys Med Rehabil. 2002;81(10):751–9.
Eker H, Cok O, Aribogan A. A treatment option for post-injection sciatic neuropathy: transsacral block with methylprednisolone. Pain Physician. 2010;13(5):451–6.
Naja Z, Al-Tannir M, El-Rajab M, et al. The effectiveness of clonidine-bupivacaine repeated nerve stimulator-guided injection in piriformis syndrome. Clin J Pain. 2009;25(3):199–205.
Rhame E, Levey K, Gharibo C. Successful treatment of refractory pudendal neuralgia with pulsed radiofrequency. Pain Physician. 2009;12(3):633–8.
Fowler I, Tucker A, Mendez R. Treatment of meralgia paresthetica with ultrasound-guided pulsed radiofrequency ablation of the lateral femoral cutaneous nerve. Pain Pract. 2012;12(5):394–8.
Rozen D, Ahn J. Pulsed radiofrequency for the treatment of ilioinguinal neuralgia after inguinal herniorrhaphy. Mt Sinai J Med. 2006;73(4):716–8.
Chen CK, Phui VE, Saman MA. Alcohol neurolysis of lateral femoral cutaneous nerve for recurrent meralgia paresthetica. Agri. 2012;24(1):42–4.
Carmel M, Lebel M, Tu L. Pudendal nerve neuromodulation with neurophysiology guidance: a potential treatment option for refractory chronic pelvi-perineal pain. Int Urogynecol J. 2010;21:613–6.
Rigoard P, Delmotte A, Moles A, et al. Successful treatment of pudendal neuralgia with tricolumn spinal cord stimulation: case report. Neurosurgery. 2012;71(3):E757–63.
Heinze K, Nehiba M, van Ophoven A. Neuralgia of the pudendal nerve following violent trauma: analgesia by pudendal neuromodulation. Urologe. 2012;51(8):1106–8.
Rauchwerger J, Giordano J, Rozen D, et al. On the therapeutic viability of peripheral nerve stimulation for ilioinguinal neuralgia: putative mechanisms and possible utility. Pain Pract. 2008;8(2):138–43.
Emamhadi M. Surgery for meralgia paresthetica: neurolysis versus nerve resection. Turk Neurosurg. 2012;22(6):758–62.
Zacest A, Magill S, Anderson V, et al. Long-term outcome following ilioinguinal neurectomy for chronic pain. J Neurosurg. 2010;112(4):784–9.
Benezis I, Boutaud B, Leclerc J, et al. Lateral femoral cutaneous neuropathy and its surgical treatment: a report of 167 cases. Muscle Nerve. 2007;36(5):659–63.
Kyriacou S, Pastides P, Singh V, et al. Exploration and neurolysis for the treatment of neuropathic pain in patients with a sciatic nerve palsy after total hip replacement. Bone Joint J. 2013;95-B(1):20–2.
Martin H, Shears S, Johnson J, et al. The endoscopic treatment of sciatic nerve entrapment/deep gluteal syndrome. Arthroscopy. 2011;27(2):172–81.
Ducic I, Dellon L, Larson E. Treatment concepts for idiopathic and iatrogenic femoral nerve mononeuropathy. Ann Plast Surg. 2005;55(4):397–401.
Ramanan M, Chandran K. Common peroneal nerve decompression. ANZ J Surg. 2011;81(10):707–12.
Robert R, Labat J, Bensignor M, et al. Decompression and transposition of the pudendal nerve in pudendal neuralgia: a randomized controlled trial and long-term evaluation. Eur Urol. 2005;47(3):403–8.
Mauillon J, Thoumas D, Leroi A, et al. Results of pudendal nerve neurolysis-transposition in twelve patients suffering from pudendal neuralgia. Dis Colon Rectum. 1999;42(2):186–92.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Scott, K.M. (2015). Neural Injury During Pregnancy and Childbirth. In: Fitzgerald, C., Segal, N. (eds) Musculoskeletal Health in Pregnancy and Postpartum. Springer, Cham. https://doi.org/10.1007/978-3-319-14319-4_6
Download citation
DOI: https://doi.org/10.1007/978-3-319-14319-4_6
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-14318-7
Online ISBN: 978-3-319-14319-4
eBook Packages: MedicineMedicine (R0)