Abstract
Malaria is a preventable and curable but life-threatening disease caused by Plasmodium parasites that are transmitted to people through the bites of infected Anopheles mosquitoes. Approximately half of the world’s population is at risk although most malaria cases and deaths occur in sub-Saharan Africa. In 2013, 97 countries and territories had ongoing malaria transmission, including 21 countries from the region of the Americas. Early diagnosis and treatment reduces disease and transmission and prevents deaths. All cases of suspected malaria must be confirmed using parasite-based diagnostic testing before administering treatment. There are currently no licensed vaccines against malaria, and the risk of resistance to treatment remains a global threat, but effective antimalarial treatment combination options are available. In areas receptive and vulnerable to malaria, international travelers and migrants are recommended to take chemoprophylaxis. Vector control is a primary intervention that can reduce malaria transmission from very high levels. With the significant decline in malaria cases globally in recent years, and renewed global interest on effectively interrupting transmission, a number of countries with low and unstable transmission are currently working toward malaria elimination.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Malaria is an acute and chronic disease caused by Plasmodium parasites, a large genus of parasitic protozoa that currently has approximately 200 known species. The four species of Plasmodium that cause disease in humans are P. vivax, P. falciparum, P. malariae, and P. ovale. Plasmodium vivax and P. falciparum are the most common, with the latter also considered the most fatal. In recent years, simian parasites like P. knowlesi or P. cynomolgi, which are found in some forested areas of Southeast Asia, have been reported to likewise cause human cases of malaria.
The disease often manifests as a febrile illness which is caused by the asexual reproduction of parasites in erythrocytes. Resulting symptoms are variable, but the disease is usually characterized by paroxysms of fever and chills, anemia, sweating, and splenomegaly . Plasmodium falciparum is the most pathogenic parasite, can cause severe and complicated malaria, and can result in death. Humans infect female mosquitoes of the genus Anopheles which are the vectors that transmit parasites to other susceptible humans.
2 Life Cycle of the Parasite
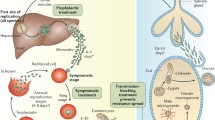
The life cycle consists of phases in both the human host and in the mosquito of the genus Anopheles, the disease vector (Fig. 20.1).
2.1 Phase in the Vector
When a female anopheline feeds on the blood of an infected human being, it can ingest gametocytes, the sexual stages of the parasite. The presence of both developing microgametocytes or male forms and the female macrogametocytes in the mosquito stomach results in the subsequent union of gametes in the stomach of the mosquito and the formation of motile ookinetes. The ookinetes penetrate the stomach wall and form oocysts in the outer wall of the stomach. Each oocyst that ruptures releases thousands of motile sporozoites in the mosquito’s body cavity that migrate to the mosquito salivary glands. Based on this, it can be noted that from one pair of gametocytes, several thousands of sporozoites may actually emerge. This process of sporozoite development, which begins with the binding of micro- and macrogametes up to the presence of sporozoites in the salivary glands, is known as sporogony and varies in terms of time between parasite species (Table 20.1).
Female anopheline mosquitoes with sporozoites in their salivary glands are infectious. Sporozoites accumulate in salivary glands in the course of their maturation and are injected with the mosquito’s saliva. The sporozoites may be injected even during probing so the ingestion of blood is not critical for a human to become infected.
2.2 Phases in the Human Being
2.2.1 Exoerythrocytic Phase
When an infected female Anopheles feeds on a human being, the sporozoites in its saliva are injected intradermally where some are destroyed by the local macrophages or may enter the lymphatic system, while others are able to access the subcutaneous blood vessels. The sporozoites which are able to enter the human circulatory system can reach the liver within 30 min to a few hours. Inside the liver cells, hepatocytes, each sporozoite develops into a schizont containing thousands of merozoites. The circumsporozoite protein of the parasite facilitates the environment for the growth and development of the parasite in the liver cells (Singh et al. 2007). This process, which includes the entry of the sporozoites, to the rupture of hepatocytes, and merozoite entry into erythrocytes, is known as the exoerythrocytic phase which may last 6–16 days, depending on the parasite species (Table 20.1).
The duration of the exoerythrocytic cycle between P. vivax and P. falciparum is similar and relatively longer compared with P. ovale and P. malariae. Also, the exoerythrocytic cycles of P. vivax and P. ovale usually have two cycles, one in which the merozoites develop and break for 6–9 days and another in which some merozoites, known as hypnozoites, go into a state of dormancy in the liver and may be undeveloped for a long time until they develop and rupture leading to erythrocyte infection and relapse. This does not occur with P. falciparum and P. malariae and therefore these parasites do not cause relapses (Table 20.1).
2.2.2 Erythrocytic Phase
When the merozoites are released after rupture of the hepatocyte, there is an exchange between substances in the merozoite surface and the erythrocytes, allowing its entry into the erythrocyte. This process takes place very quickly which minimizes the exposure of the antigens on the surface of the parasite, affording parasites protection from the host immune response (Greenwood et al. 2008). Molecular interactions between distinct ligands on the merozoite and host receptors on the erythrocyte membrane play a key role in the invasion of the merozoites into the red blood cells. Plasmodium vivax invades only the red blood cells that are Duffy blood group positive, using the Duffy-binding protein and the reticulocyte homology protein, found mostly on the reticulocytes. Plasmodium falciparum uses several different receptor families and alternate invasion pathways that are highly redundant, allowing it to invade any red cell (Weatherall et al. 2002).
The young forms of the parasite that develop in the erythrocytes subsequent to its invasion are known as rings and feed on hemoglobin. Within the erythrocytes, the parasite numbers expand rapidly. Despite the immunologic advantage provided by the erythrocytes to the growing parasite, the lack of standard biosynthetic pathways and intracellular organelles in the red cells tend to create obstacles for the fast-growing intracellular parasites.
As a result of the growth within the erythrocyte, they rupture and release merozoites that invade other erythrocytes, and the cycle is repeated each time with the invasion of previously uninfected erythrocytes. Each erythrocytic cycle occurs between about 2 and 3 days (Table 20.1). The cycles are repeated until the immunity of the person and/or antimalarial drugs slows the process. These merozoites are asexual but there are some which undergo differentiation and become sexual forms known as (macro- and micro)gametocytes. When the gametocytes are ingested from the human blood by female Anopheles mosquitoes, it becomes infected and capable of transmitting the disease .
3 Etiology and Pathophysiology
The pathophysiological changes observed among infected individuals are mainly caused by parasites in the erythrocytes and involve many organ systems. No pathophysiological changes are induced by sporozoites, gametocytes, or the exoerythrocytic phase. The presence of parasites in the erythrocytes results in erythrocyte rupture and sequestration, biochemical and electrolyte imbalances, and disorders of the hematological, gastrointestinal, pulmonary, neurological, and renal systems.
During the life cycle of the parasite in the blood, the destruction of erythrocytes results in anemia in conjunction with leukopenia and thrombocytopenia which are acute manifestations of malaria infections. When erythrocytes rupture, malaria pigment, other elements of parasites, and the erythrocyte membrane and intracellular contents enter the peripheral circulation. The circulating malaria pigments are taken up by macrophages leading to the production of pro-inflammatory cytokines, especially tumor necrosis factor and other pyrogenic cytokines, causing fever. Some of these factors may promote the coagulation cascade and result in disseminated intravascular coagulation.
Plasmodium falciparum is the most pathogenic of the parasites and is associated with severe morbidity and mortality resulting from other pathophysiological changes. When P. falciparum infects erythrocytes , it leads to expression of many surface proteins including P. falciparum erythrocyte membrane protein-1 (PfEMP-1) on the outer membrane of these infected erythrocytes making them more rigid and inflexible. When passing through microvessels in the tissues, they get stuck owing to adhesion to the endothelial cells, a process called cytoadherence. This cytoadhesion is largely due to the interaction of PfEMP-1 on infected erythrocytes with intercellular adhesion molecule 1 (ICAM-1) and CD36 acting as receptors on the endothelial membranes except in placental malaria. In the latter chondroitin sulfate A (CSA) is considered to be the cytoadhesion receptor for PfEMP-1 proteins. Accumulation of infected erythrocytes can lead to blockage of small vessels, limiting the oxygen delivery to tissues. However, this phenomenon of cytoadherence is apparently not uniform, and postmortem analyses of tissues in people who have succumbed to cerebral P. falciparum malaria have shown that the brain is preferentially the most parasite-infiltrated organ. Failure of the central nervous system is characteristic of cerebral malaria and may present with focal symptoms, such as seizures, deviated fixed gaze, or coma. Hypoglycemia, metabolic acidosis, and hyponatremia occur frequently especially in infections due to P. falciparum. Renal changes may also occur, resulting in the increase of urea, creatinine, and proteinuria. Acute renal failure is a complication of severe malaria that occurs more in adults. Pulmonary changes are also more common in adults than children and can result in adult respiratory distress syndrome and pulmonary edema.
3.1 Pathological Anatomy
The mortality associated with malaria is almost always due to infection with P. falciparum. However, malaria deaths caused by P. vivax, P. ovale, and P. malariae do occur, and those due to P. vivax are of particular importance due to the large number of infections of that species in some geographic regions of the world. The brain of patients who die from malaria typically presents with congestion, edema, and petechiae in the white matter. The veins and capillaries are usually full of parasitized erythrocytes and bleeding is observed. Bleeding and stroke are also possible conditions. The spleen is enlarged and has a gray color, with tensed vessels containing parasitized erythrocytes. The liver is also enlarged and has a gray color. The kidneys are slightly increased in size and congested. It may have bleeding, pigmentation in the glomeruli, and parasitized erythrocytes in the capillaries. In patients dying of hemoglobinuria, the kidneys are enlarged and exhibit edema and tubular necrosis. Often, the lungs are congested and with edema and with parasitized erythrocytes present in the lung capillaries. Minimal changes are observed in the heart with parasitized erythrocytes in the congested capillaries. Congestion and edema can also be observed in the gastrointestinal tract particularly in the intestinal mucosa with parasitized erythrocytes in vessels. In the placenta, parasitized erythrocytes are often seen in the maternal portion but almost never in the fetal portion.
3.2 Clinical Features and Manifestations
The host or the patient’s previous exposure or immunity to malaria affects the symptomatology and incubation period, but individuals with malaria typically become symptomatic a few weeks after infection. The typical incubation period also varies with each Plasmodium species (Table 20.1). Clinical symptoms may include the following: fever, chills, sweating, headache, malaise, back pain, muscle pain, dizziness, abdominal pain, nausea, vomiting, diarrhea, poor appetite, cough, and dyspnea. At the same time, the patient may also have postural hypotension, anemia, hepatosplenomegaly , jaundice, hypoglycemia, leukopenia, and thrombocytopenia.
The paroxysms of fever, chills, and sweats are also variable depending on species. Typically, they begin with a period of shivering and chills, lasting anywhere from 1 to 2 h and followed by a high fever. The patient then experiences excessive diaphoresis, and subsequently, the body temperature drops to normal or below normal. Several small fever spikes a day instead of the classic paroxysms may be reported by many patients early in the infection. Patients with long-standing infections are more likely to present with classic fever patterns. However, periodicity in febrile attacks is often not observed in P. falciparum infections so in general, the occurrence of periodicity of fever is not a reliable clue to the diagnosis of malaria.
As malaria symptoms are related to the maturation and rupture of infected erythrocytes, the clinical picture of infections with P. vivax and P. ovale parasitemias may last from days to a few months after the initial attack. The persistence of parasitemia from the initial infection can result to the recrudescence of symptoms within a short period of time. Also, without appropriate treatment, exoerythrocytic parasites that persist in the parenchymal cells of the liver or hypnozoites can cause relapses ranging from a few weeks up to 9 months. Relapses of P. vivax can last up to 3 or 4 years. Without treatment, P. malariae can persist up to 50 years (Table 20.1).
Infections caused by P. falciparum in patients without immunity to infection are very dangerous and can result in death. As the parasite has developed resistance to most commonly used antimalarials in almost all parts of the world, the patient’s condition may worsen and result in death unless an early diagnosis is made and the patient is treated in a timely and appropriate manner. Patients with complicated and severe malaria infections associated with P. falciparum can have very high fever, convulsions, delirium, coma, acute pulmonary distress syndrome, acute renal failure, liver failure, abdominal pain, nausea, vomiting, and diarrhea. Likewise, they may have severe anemia, hypoglycemia, and metabolic acidosis. Fortunately, P. falciparum infections do not result in the presence of hypnozoites in the liver and therefore do not relapse if the parasites are effectively eliminated in the blood during treatment. However, if the treatment was not successful in eliminating viable parasites in the blood, patients may experience recrudescence (renewed presence of parasites in circulation) within a short period of time.
Among children, congenital malaria may result from any of the four species of parasites during the first few months of life. In areas where the disease is endemic, infants receive maternal antibodies and may only have fever without other symptoms. Likewise, in these areas, it is quite difficult to make the differential diagnosis between congenital malaria and malaria transmitted by mosquitoes after the first 2 weeks of birth. In non-endemic areas, congenital malaria may present with fever, anemia, hepatomegaly, and splenomegaly , and patients may be anorexic and appear lethargic. Infection can also cause death in the first days of life.
3.3 Severe Malaria
Plasmodium falciparum accounts for the vast majority of severe malaria cases although P. vivax and P. knowlesi (Kantele and Jokiranta 2011; Daneshvar et al. 2009) can also cause severe disease. Uncomplicated malaria caused by these parasites can also result to severe form if effective treatment is delayed. Among children, severe P. falciparum malaria may develop so rapidly that early treatment may not even be possible.
As previously described, uncomplicated P. falciparum malaria is highly variable in clinical presentation among adults and children (Table 20.2) and mimics other diseases. Fever is common and often intermittent but may be absent in some cases. Young children may manifest with irritability, refusal to eat, and vomiting; and on physical examination, fever may be the only sign. If early diagnosis and management is missed, patients with falciparum malaria may deteriorate rapidly.
4 Diagnostic Approach
Many people with malaria infections do not present the typical symptoms of fever and chills with a well-defined periodicity. Persons living in non-endemic areas and returning from a trip to an endemic area can present with symptoms which can mimic any other febrile illness such as hepatitis, yellow fever, dengue, typhoid fever, leptospirosis, tuberculosis, trypanosomiasis, visceral leishmaniasis, or viral infections. Persons living in endemic areas have some degree of immunity and may have very mild symptoms or may even be asymptomatic. Malaria infection may also result from the transfusion of unscreened blood of an affected person. For these reasons, conducting a thorough clinical history is very important because malaria should be considered as a differential diagnosis for people who reside or come from an area where malaria transmission is possible, particularly for patients presenting clinical symptoms that may include fever, chills, sweating, headache, muscle pain, lack of appetite, malaise, anemia, drowsiness, or complications associated with the disease.
In many non-endemic areas, the disease is often overlooked, and in the absence of facilities for good quality laboratory diagnosis, the clinical picture may deteriorate rapidly resulting in serious consequences. As realities and contexts in various countries vary, national policies on the diagnosis and treatment of suspected and confirmed malaria cases should be taken into account. All cases of suspected malaria must be confirmed using parasite-based diagnostic testing before administering treatment, which suspicion should only be considered solely on the basis of clinical when a parasitological diagnosis is not accessible (World Health Organization 2010). In many countries, patients with confirmed P. vivax infection, potential glucose-6-phosphate dehydrogenase (G6PD) deficiency status, must also be borne in mind, as this condition has important implications in terms of treatment.
4.1 Microscopic Diagnosis
Microscopic examination of a blood smear is the gold standard for the diagnosis of the disease, and its use is particularly promoted and encouraged in the region of the Americas. The test may be extended to include a thick blood smear where detection and identification of parasites are easier and in which the erythrocytes are grouped and visualized more easily, but thin blood smears permit more accurate species identification. In general, blood smears are stained with Giemsa, but other dyes which have been used include Field, Leishman, Romanowsky, and Wright. Treatment regimens may vary according to parasite species, and it is therefore very important to identify and confirm the causative parasites of disease.
A large variation in parasite morphology can be observed in microscopic diagnosis for malaria. The different Plasmodium species exhibit characteristic morphological features in the various stages of their life cycle in the erythrocytes (Table 20.3). Most malaria infections (microscopic and submicroscopic) should be considered as potentially infectious and able to contribute to ongoing transmission. There is no need for routine detection of gametocytes using sensitive nucleic acid amplification methods in malaria surveys or clinical settings (World Health Organization 2014a, b, c, d, e).
4.2 Rapid Diagnostic Tests
In endemic and non-endemic areas where there is difficult or nonexistent access to the microscopic diagnosis, the use of rapid diagnostic tests (RDTs ) is recommended. Rapid tests detect parasite antigens in the peripheral blood. In general, RDTs detect the histidine-rich protein 2 which is specific to P. falciparum (HRP2), the protein lactate dehydrogenase (pLDH), and aldolase. These rapid tests allow the differentiation between P. falciparum and P. vivax infections.
As RDTs have varying indications, sensitivity, and specificity in terms of use, users are strongly advised to obtain guidance from the World Health Organization (WHO) RDT procurement recommendations and the results of the periodic RDT product testing (World Health Organization 2014a, b, c, d, e).
4.3 Other Diagnostic Methods
Nucleic acid amplification techniques are available and are more sensitive in detection of malaria compared to RDTs and microscopy. As these tests are expensive and require significant expertise, their use should be considered only in low transmission settings where there is already widespread implementation of malaria diagnostic testing and treatment and low parasite prevalence rates (e.g., <10 %). The use of nucleic acid amplification (NAA) -based methods should not divert resources away from malaria prevention and control interventions and strengthening of the healthcare services, including the surveillance system (World Health Organization 2014a, b, c, d, e).
5 Treatment
The treatment of malaria has different objectives, including the treatment of uncomplicated malaria to cure the infection, reduce the transmission, and prevent the emergence and spread of resistance to antimalarials. In people with severe malaria, the goal of treatment is to prevent death and prevention of neurological sequelae (World Health Organization 2015).
Different types of antimalarials act at different stages of the life cycle of the parasite. Those that destroy the asexual parasites (trophozoites) in erythrocytes are known as blood schizonticides and are used in patients with symptoms. Tissue schizonticides act against the exoerythrocytic stages (hypnozoites) caused by P. vivax and P. ovale in the liver. Gametocytocides destroy sexual stages (gametocytes), while sporontocides inhibit parasite development of oocysts in the anopheline vector.
According to structure, most antimalarials can be divided into the following groups:
-
Group 1.
Quinine, quinidine, and mefloquine
-
Group 2.
Chloroquine and amodiaquine
-
Group 3.
Pyrimethamine and proguanil
-
Group 4.
Sulphadoxine
-
Group 5.
Primaquine
-
Group 6.
Artemisinin and its derivatives
-
Group 7.
Atovaquone
-
Group 8.
Antibiotics with antimalarial effect as clindamycin, doxycycline, and tetracycline
Though they may have different modes of action, antimalarials of groups 1 and 2 have similar effects as both blood schizonticides and gametocytocides for Plasmodium parasites other than those of P. falciparum.
In infections with P. falciparum, a large variation in the number and size of trophozoites can be found about 10–12 days after infection in a primary attack; gametocytes appear 8–10 days after the primary attack which means that early diagnosis and timely and appropriate treatment will interrupt the development of gametocytes and the ability to infect the anopheline mosquito.
5.1 Treatment of Uncomplicated P. falciparum
WHO recommendations for the treatment of uncomplicated P. falciparum malaria are:
-
The treatment of choice for uncomplicated P. falciparum malaria is a combination of two or more antimalarial drugs with different mechanisms of action. The use of combination instead of single-drug therapies provides the benefit that combinations are more effective and prevents selection of mutant parasites resistant to a single drug as the partner drug kills it.
-
Artemisinin-based combination therapies (ACTs) are the recommended treatments for uncomplicated P. falciparum malaria. Some areas in the world continue to use chloroquine as the parasite species has still not developed resistance to this antimalarial. However, this is an exception worldwide rather than a rule and unless otherwise known, ACTs are preferred and recommended.
-
Artemisinin is a potent antimalarial but has a short half-life owing to which it is combined with other antimalarials with longer half-lives. Thus, the following ACTs are currently recommended for use:
-
Artemether plus lumefantrine
-
Artesunate plus amodiaquine
-
Artesunate plus mefloquine
-
Artesunate plus sulfadoxine-pyrimethamine
-
Dihydroartemisinin plus piperaquine
-
The choice of ACT in a country or region is based on the resistance level of artemisinin partner drug in the combination:
-
In areas of multidrug resistance (Southeast Asia): artesunate + mefloquine, artemether-lumefantrine, dihydroartemisinin + piperaquine.
-
In areas without multidrug resistance (Africa and other areas): artemether-lumefantrine, artesunate + amodiaquine, artesunate + sulfadoxine-pyrimethamine.
-
The artemisinin component of an ACT should be given at least 3 days for optimal effect, and artemether-lumefantrine should be used with a regimen of six doses.
-
Amodiaquine + sulfadoxine-pyrimethamine may be considered as an interim option in situations where ACTs may not be available.
-
Although artemisinin has a gametocytocidal effect, the current recommendation is to add a single dose of primaquine (0.25 mg/kg; 0.75 mg/kg is also acceptable and used in some countries) as a gametocytocide to the treatment regimen, usually given on the first day of treatment and irrespective of the presence or absence of gametocytes in the patient’s peripheral blood.
5.2 Treatment Regimens with Recommended ACTs
5.2.1 Artemether Plus Lumefantrine
Currently available as a fixed-dose co-formulated tablet containing 20 mg artemether and 120 mg lumefantrine, the recommended treatment regimen is artemether-lumefantrine tablets twice daily given for 3 days. A total therapeutic dose includes 1.4–4 mg/kg of artemether, 10–16 mg/kg of lumefantrine, and 0.75 mg/kg of primaquine. It should be given after a meal rich in fat as fat improves the absorption of lumefantrine (Table 20.4).
5.2.2 Artesunate Plus Amodiaquine
Fixed-dose formulations of the drug with tablets are available with 25/67.5 mg, 50/135 mg, or 100/270 mg of artesunate and amodiaquine, respectively. Full recommended treatment regimen is of 4 mg/kg/day of artesunate and 10 mg/kg/day of amodiaquine administered once daily for 3 days along with 0.75 mg/kg of primaquine given on the first day (Table 20.5).
5.2.3 Artesunate Plus Mefloquine
Separate tablets containing 50 mg of artesunate and 250 mg of mefloquine available, but not promoted, in order to reduce potential use of either as monotherapy. The recommended treatment is 4 mg/kg/day of artesunate administered once daily for 3 days, 25 mg/kg of total dose of mefloquine generally divided over 2 days (10 and 15 mg/kg/day) or 3 days (8.3 mg/kg/day), and 0.75 mg/kg of primaquine given on the first day. Mefloquine may cause nausea and vomiting among other side effects, and splitting the dose over a longer period (2–3 days) generally reduces the likelihood and severity of side effects. The use of mefloquine within 60 days of initial treatment could lead to increased risk of neuropsychiatric side effects, and thus its concomitant use within 60 days should be avoided. If two separate P. falciparum infections occur within the same patient within 60 days, the second episode should be treated with an ACT other than artesunate plus mefloquine if the first episode was also treated with it (Table 20.6).
5.2.4 Artesunate Plus Sulfadoxine-Pyrimethamine
The recommended treatment is a target dose of 4 mg/kg/day of artesunate given once a day for 3 days and sulfadoxine-pyrimethamine in a dose of 25/1.25 mg/kg on day 1. A 0.75 mg/kg dose of primaquine on day 1 should be added to this (Table 20.7).
5.2.5 Dihydroartemisinin Plus Piperaquine
A total dose of 4 mg/kg of dihydroartemisinin and 18 mg/kg/day of piperaquine once a day each for 3 days is recommended. Additionally primaquine (0.75 mg/kg) should be added on the first day of treatment.
5.3 Recommendations for Second-Line Antimalarial Treatment
It is possible that the same person may get infected with P. falciparum malaria more than once within the same month; however, when presenting fever and malaria parasites in peripheral blood after 14 days of start of treatment with ACT, the patient should be treated as new infection and treated accordingly with recommended ACTs. However, rarely some patients with uncomplicated P. falciparum malaria treated with ACTs might resolve, but parasites may reappear in peripheral blood within 14 days of start of treatment. In these cases it is recommended to use the following:
-
Another ACT known to be effective in the region.
-
Artesunate (2 mg/kg of body weight once a day) + tetracycline (4 mg/kg body weight four times a day) or doxycycline (3.5 mg/g body weight once daily) or clindamycin (10 mg/kg body weight twice daily). Any of these combinations should be given for at least 7 days.
-
Quinine (10 mg/kg body weight three times a day) + tetracycline or doxycycline or clindamycin can also be used. Any of these combinations should be given for at least 7 days.
5.4 Treatment in Specific Populations and Situations
5.4.1 Pregnant Women
Pregnant women with acute symptoms of malaria are a high-risk group and should receive efficacious antimalarial treatment. Malaria in pregnancy is associated with low birth weight, increased anemia, and, in areas of low transmission, an increased risk of severe malaria. The use of primaquine in pregnant women should be avoided as it may cause harm to the fetus. Recommendations for the treatment of uncomplicated P. falciparum malaria in pregnancy are:
-
First trimester: quinine and clindamycin for 7 days should be given. Quinine monotherapy can be given if clindamycin is unavailable. If this fails, artesunate and clindamycin should be used. Evidence on the effect of artemisinin and its partner drug in ACTs in first trimester pregnancy is presently limited, but no adverse effects have been seen. Thus ACTs are only recommended for use if they are the only treatment immediately available or treatment failure with previous regimens is seen.
-
Second and third trimesters: Any ACT known to be effective in the country/region except dihydroartemisinin and piperaquine, or artesunate plus clindamycin for 7 days, or quinine and clindamycin for 7 days .
If neither one of the above options nor clindamycin is available, monotherapy can be given.
5.4.2 Lactating Women
The use of primaquine in lactating women should be avoided unless the glucose-6-phosphate dehydrogenase (G6PD) deficiency status of the infant is known. Primaquine is known to cause hemolysis in G6PD-deficient individuals. Tetracycline is also contraindicated as it affects the bones and teeth of children. Any other antimalarial, preferably an ACT, should be used for the treatment of uncomplicated P. falciparum malaria in breastfeeding women.
5.4.3 Infants
Infants should receive standard antimalarial treatment (including ACTs) except for primaquine to be avoided in the first 6 months of infancy and tetracyclines at least until 8 years of age. As pediatric formulations are not available for most ACTs, appropriate care should be taken to provide a correct dose according to the body weight of the child. Infants are more likely to vomit or regurgitate antimalarial treatment than older children or adults, requiring repeat doses if necessary. Taste, volume, consistency, and gastrointestinal tolerability are important and crucial to treatment of infants. Artemisinin appears to be safe and well tolerated by young children, and so the choice of the ACT will be determined largely by the safety and tolerability of the partner drug. The limited information available does not indicate the specific difficulties ACT currently recommended in childhood.
5.4.4 Travelers
Travelers to malaria-endemic areas are often nonimmune adults , either from cities with little or no transmission in endemic countries, or visitors from non-endemic countries. Both tend to be at higher risk of contracting malaria and its consequences, as they have no immunity to malaria. When diagnosed with malaria in an endemic area, they should be treated as any other patient and according to malaria guidelines prevalent. If malaria is diagnosed on return to the patient’s home country (non-endemic), the following treatment is recommended :
-
Atovaquone-proguanil (15/6 mg/kg, the usual dose for adults, four tablets once daily for 3 days)
-
Artemether-lumefantrine (adult dose, four tablets twice daily for 3 days)
-
Quinine (10 mg salt/kg body weight every 8 h) + doxycycline (3.5 mg/kg body weight once daily) or clindamycin (10 mg/kg body weight twice a day), all drugs approved for 7 days
5.4.5 Coexisting Morbidities
5.4.5.1 HIV Infection
A growing number of people in malaria-endemic areas are living with HIV . It is increasingly clear that as HIV progresses and immunosuppression worsens, the manifestations of malaria also worsen. In pregnant women, adverse effects on birth weight increase. In patients with partial immunity to malaria, the severity of infection is greater. The treatment with sulfadoxine-pyrimethamine should not be administered to patients with co-trimoxazole, as they have probably a higher risk of sulfonamide-related adverse effects, and, in any case, since both drugs have antimalarial activity, malaria infection is likely to be resistant to sulfadoxine-pyrimethamine. Depending on the setting of the transmission of malaria, HIV-infected individuals have an increased risk of asymptomatic parasitemia, clinical malaria, or severe and complicated malaria. Therefore they have a greater need for control measures of malaria than people not infected with HIV. The following are the recommendations for treatment of uncomplicated P. falciparum malaria in patients with HIV infection :
-
Patients with HIV infection who develop malaria should receive standard ACT .
-
ACTs or intermittent preventive therapy with sulfadoxine-pyrimethamine should not be administered to HIV-infected patient receiving co-trimoxazole (trimethoprim and sulfamethoxazole).
-
ACTs with amodiaquine should be avoided in HIV-infected patients receiving zidovudine or efavirenz.
5.4.5.2 Severe Malnutrition
Malaria and malnutrition frequently coexist. Malnutrition in absorption may be reduced due to diarrhea, vomiting, intestinal transit, and rapid atrophy of the intestinal mucosa. The drug absorption by intramuscular and possibly rectal routes may be slower, and the decline in muscle mass may make it difficult to administer repeated intramuscular injections. Although there are many reasons for drug kinetics to be different in malnourished patients compared to others, changes in the current recommendations of dosage in mg/kg of body weight are not recommended.
5.4.6 Treatment of Uncomplicated P. vivax Malaria
The recommendations for treating uncomplicated P. vivax malaria are:
-
A total dose of 25 mg/kg of chloroquine divided over 3 days in combination with 0.25 mg/kg/day of primaquine taken once daily with food for 14 days is the treatment of choice for the chloroquine-sensitive infections. In Oceania and Southeast Asia, the dose of primaquine should be 0.5 mg/kg/day for 14 days.
-
ACTs with mefloquine, amodiaquine, or piperaquine along with primaquine should be used in P. vivax malaria resistant to chloroquine.
-
In moderate G6PD deficiency, primaquine 0.75 mg/kg should be administered once a week for 8 weeks. In severe G6PD deficiency, primaquine should not be given.
-
In pregnant or lactating women in whom primaquine is contraindicated, weekly chemoprophylaxis with chloroquine can be considered until such time that primaquine can be safely given .
5.4.7 Treatment of Complicated P. falciparum Malaria
Severe malaria is a medical emergency. After rapid clinical assessment and confirmation of the diagnosis, full doses of parenteral antimalarial treatment should be started without delay with any effective antimalarial first available:
-
For adults, artesunate 2.4 mg/kg BW IV or IM given on admission (time = 0), then at 12 and 24 h, and then once a day is the recommended treatment. Artemether, or quinine, is an acceptable alternative if parenteral artesunate is not available: artemether 3.2 mg/kg BW IM given on admission and then 1.6 mg/kg BW per day or quinine 20 mg salt/kg BW on admission (IV infusion or divided IM injection) and then 10 mg/kg BW every 8 h; infusion rate should not exceed 5 mg salt/kg BW per hour.
-
For children less than 20 kg in weight, artesunate 3 mg/kg BW IV or IM given on admission (time = 0), then at 12 and 24 h, and then once a day is the recommended treatment. Artemether, or quinine, is an acceptable alternative if parenteral artesunate is not available: artemether 3.2 mg/kg BW IM given on admission and then 1.6 mg/kg BW per day or quinine 20 mg salt/kg BW on admission (IV infusion or divided IM injection) and then 10 mg/kg BW every 8 h; infusion rate should not exceed 5 mg salt/kg BW per hour.
-
Whenever parenteral treatment is started, it should be continued for at least 24 h or till the patient can take treatment orally, whichever is later chosen.
-
Thereafter, treatment should be continued by giving a complete course of a recommended ACT:
-
Artemether plus lumefantrine
-
Artesunate plus amodiaquine
-
Dihydroartemisinin plus piperaquine
-
Artesunate plus sulfadoxine-pyrimethamine
-
Artesunate plus clindamycin or doxycycline (pregnancy)
-
Quinine plus clindamycin or doxycycline (pregnancy)
-
5.4.8 Treatment of Complicated P. vivax Malaria
Plasmodium vivax malaria may also cause a severe and debilitating febrile illness. It can lead to severe manifestations like cerebral malaria, severe anemia, severe thrombocytopenia and pancytopenia, jaundice, splenic rupture, acute renal failure, and acute respiratory distress syndrome. Treatment of severe P. vivax malaria is similar to that of severe P. falciparum malaria.
5.4.9 Treatment of Malaria Caused by P. ovale , P. malariae, and P. knowlesi
Resistance of P. ovale and P. malariae to antimalarials is not well characterized, and infections caused by these two species are generally considered sensitive to chloroquine.
The recommended treatment of malaria caused by P. ovale is the same as that for P. vivax, namely, chloroquine and primaquine. P. malariae and P. knowlesi should be treated with the standard regimen of chloroquine for malaria by P. vivax, but they do not require primaquine as hypnozoites are not formed in infections by these species.
Those with severe P. knowlesi malaria are treated as a severe P. falciparum malaria infection, except that the thresholds for severe malaria are changed to parasite density higher than 100,000 asexual forms/μL of whole blood or jaundice and parasite density higher than 20,000/μL.
5.5 Antimalarial Drug Resistance
Antimalarial drug resistance has been documented in P. falciparum, P. vivax, and P. malariae. Parasite resistance results in a delayed or incomplete clearance of parasites from the patient’s blood when the person is being treated with an antimalarial. The problem is compounded by cross-resistance, in which resistance to one drug confers resistance to other drugs that belong to the same chemical family or which have similar modes of action. Several antimalarials had to be removed from markets after the spread of parasite resistance in previous decades.
Enhanced surveillance of antimalarial drug efficacy is important to detect and respond to emerging drug resistance of treatment combinations or the individual partner drugs. Countries should monitor the efficacy of first-line malaria therapies, and treatment failure should prompt a change in national antimalarial treatment policy.
ACTs remain effective provided that the partner drugs remain efficacious. Unfortunately, P. falciparum resistance to artemisinin has emerged in five countries of the Greater Mekong Subregion—Cambodia, the Lao People’s Democratic Republic, Myanmar, Thailand, and Viet Nam. This is a serious public health concern that threatens the sustainability of the global effort to reduce and eliminate malaria. The global plan for artemisinin resistance containment (GPARC) emphasizes among others that a key priority for endemic countries and the global malaria community is protecting the efficacy of ACTs and developing new combinations .
The scale-up of all basic malaria interventions, including vector control, diagnostic testing, and quality-assured treatment, contributes to reducing the potential emergence of resistance. National regulatory authorities should regulate production, marketing authorization, export, import, and use of oral artemisinin-based monotherapies. Countries should also take regulatory action to remove fake and substandard drugs from health facilities to preserve the efficacy of ACTs (World Health Organization 2011).
5.6 Vaccines
As in other complex parasites, the development of a vaccine against the species of Plasmodium pathogenic to humans is a very challenging task. Currently, there is no licensed and commercially available malaria vaccine, although they are expected to be an important addition to the arsenal of tools against malaria in the coming years. A number of vaccine candidates are currently in various stages of development, with different modes of action, to prevent P. falciparum and P. vivax infections.
5.6.1 P. falciparum Vaccines
The most advanced P. falciparum malaria vaccine candidate is the RTS,S/AS01. The need for preventive measures such as the use of long-lasting insecticidal nets, confirmatory diagnosis either by microscopy or RDT, and treatment with ACTs will continue, even if RTS,S/AS01 becomes available and is used (World Health Organization 2013a, b, c).
5.6.2 P. vivax Vaccines
Funding for P. vivax vaccine candidates is relatively smaller compared with P. falciparum vaccine candidates. Much less is known about this species of the malaria parasite, but there is evolving interest in the light of its significance in malaria elimination efforts . In 1999, a P. vivax network to create, improve, and expand research on the parasite, cGMP6 production of candidate vaccines for human clinical trials, clinical testing site development, and funding was established in Asia (World Health Organization 1999). Currently, there are at least two ongoing P. vivax vaccine-related projects: ChAd63/MVA PvDBP and the experimental transmission of P. vivax to Anopheles (EVITA) which is enabling technology, not a vaccine (World Health Organization 2014a, b, c, d, e).
5.6.3 The Global Portfolio of Vaccines Against Malaria
WHO keeps track of the various malaria vaccine projects at advanced preclinical and clinical stages globally. Below is a table pertaining to ongoing projects from various research and pharmaceutical institutions that are currently contributing to the global malaria vaccine development efforts (Table 20.8).
6 Epidemiology
Malaria transmission involves the interaction between the malaria parasite, the human host, the Anopheles vector, and physical, biological, and socioeconomic factors that shape the environment. The intensity of transmission depends on the prevalence and incidence of different parasites in the human population; characteristics of the mosquito vectors such as its efficiency as a vector, density, feeding, and resting habits; susceptibility to infection and longevity that allows the development of sporozoites; human susceptibility to infection; and environmental factors that favor the presence of vector breeding population. Given the interaction of all these parameters, the disease is much focused, and in many areas where it is endemic, transmission varies with temperature, rainfall, and humidity. Lack of access to health services affecting diagnosis and treatment of the disease, poor housing conditions, and lack of measures to reduce the contact between man and vector as a direct result of poverty are major conditions associated with malaria transmission.
After the discovery of the residual activity of dichlorodiphenyltrichloroethane (DDT) and successes with its use for interruption of transmission over large areas, among others, in Italy and Greece in Europe, Venezuela and Guyana in the Americas, the World Health Organization (WHO) launched the global strategy of malaria eradication in 1955. This led to development and availability of tools and interventions and the scaling up of efforts to combat the disease. Interruption of malaria transmission was achieved in the former Soviet Union, most countries in Europe, Australia, many countries in Asia, North America, and all the Caribbean islands with the exception of the island of Hispaniola, shared by Haiti and the Dominican Republic.
However, the disease has persisted in many countries worldwide and even reemerged or has been reintroduced in many countries where it was greatly reduced and/or eliminated. Acknowledging that eradication would be difficult to achieve globally and given its high burden in Africa, where efforts at eradication had not been attempted, in 1992 the WHO decided to change the strategy to focus to reduce burden through prevention and control of the disease, an extremely important cause of childhood morbidity and mortality. Technically, the components of the focus were on early diagnosis and timely and appropriate treatment of cases; design and implementation of selective and sustainable preventive measures, including vector control; early detection, containment, or prevention of epidemics; and strengthening local capacities to enable and to promote regular analysis of the malaria situation in a country, including the analysis of the ecological, social, and economic determinants of disease research.
As of 2013, the World Health Organization (WHO) reported that 106 countries have ongoing malaria transmission (Fig. 20.2). Approximately 3.3 billion people are at risk of the disease, of which 1.2 billion are at high risk or living in areas where more than one malaria case occurs per 1000 population (World Health Organization 2014a, b, c, d, e). Estimates indicate that there were 198 million cases of malaria and 584,000 deaths in 2013 worldwide, with 90 % of all malaria deaths occurring in sub-Saharan Africa. Of the estimated deaths, 78 % were among children under 5 years of age. Outside of Africa, most of the malaria occurs in South and Southeast Asian and South American countries.
Global malaria map, based on total populations at risk (World Health Organization and Medicines for Malaria Venture 2014)
Most of the malaria is caused by P. falciparum especially in Africa, Papua New Guinea, and Hispaniola where it is almost the exclusive malaria parasite species. Plasmodium vivax predominates in Central American, South American, Central Asian, and Middle Eastern countries. Almost equal distribution of the two species is seen in South and Southeast Asian countries. Plasmodium ovale is restricted in its distribution to African countries and rarely found elsewhere, while P. malariae infections have been reported from tropical countries across the world. Recently, P. knowlesi human infections have been reported from areas adjacent to forests in Southeast Asian countries.
7 Vectors and Vector Control
About 60–70 of the approximate 490 species of Anopheles mosquitoes and sibling species worldwide can transmit malaria. Throughout the world, 30 of these vectors are of major importance (World Health Organization 2014a, b, c, d, e) in terms of transmitting malaria to humans (Fig. 20.3).
Global distribution of dominant or potentially important malaria vectors (Sinka et al. 2012)
Vector control is an essential component of all malaria control strategies and contributes in accelerating efforts toward malaria elimination. It has two core interventions—long-lasting insecticidal nets (LLINs) and indoor residual spraying (IRS)—which should be implemented on the basis of epidemiological and entomological surveillance data. Supplementary methods such as larval source management, improved housing including screens, and use of repellents and mosquito coils may also be appropriate in specific settings.
7.1 Long-Lasting Insecticidal Nets (LLINs )
LLINs provide both a physical barrier and an insecticidal effect that reduce human-mosquito contact. They last longer than conventional insecticide-treated bed nets, but still need to be replaced regularly. They also have an important community effect that leads to large-scale killing of mosquitoes so they should be provided in sufficient numbers to cover everyone exposed to transmission in target communities. This can be achieved through free LLIN mass campaigns and continuous distribution. Protection of risk groups, especially young children and pregnant women in high transmission areas, should be given preference in situations when there are temporary gaps in LLIN coverage. This can minimize deaths from malaria and should not replace the goal of universal coverage (World Health Organization 2013a, b, c).
7.2 Indoor Residual Spraying (IRS )
IRS involves spraying an effective dose of insecticide, once or twice per year, on indoor surfaces where malaria vectors are likely to rest after biting. It reduces malaria transmission by reducing the survival of mosquitoes that enter houses or sleeping units. IRS requires a high level of coverage in space and time to achieve its full effect as a method for community protection.
There are currently 12 insecticides recommended for IRS use by the WHO Pesticide Evaluation Scheme (WHOPES) (WHOPES 2013). They come from only four classes of insecticide: pyrethroids, organochlorines (dichlorodiphenyltrichloroethane, DDT), organophosphates, and carbamates. When implemented properly, IRS is highly effective and can provide protection to communities through a rapid mass effect on vector populations, reducing densities and longevity of vectors and their capacity to transmit malaria parasites. The quality of the spraying operation is also an important variable in determining IRS effectiveness, and at least 80 % of premises in target communities must be properly sprayed. IRS is effective usually for 3–6 months, but occasionally up to 9 months, depending on the insecticide used , the type of surface sprayed, and the seasonality of transmission.
7.3 Monitoring and Management of Insecticide Resistance
In 2012, the global plan for insecticide resistance management (GPIRM) in malaria vectors was launched which encouraged affected countries to develop and implement comprehensive insecticide resistance management strategies and have preemptive plans in place. To preserve the susceptibility of malaria vectors to currently available insecticides, the recommendations include: periodic changes in the class of insecticides used for indoor residual spraying and the use of multiple interventions in combination. The strategic pillars of GPIRM are the following: plan and implement insecticide resistance management strategies in malaria-endemic countries; ensure proper, timely entomological and resistance monitoring and effective data management; develop new, innovative vector control tools; fill gaps in knowledge on mechanisms of insecticide resistance and the impact of current insecticide resistance management strategies; and ensure that enabling mechanisms (advocacy, human and financial resources) are in place (World Health Organization 2012). Mosquito resistance is currently reported in 64 countries affecting all major vector species and all classes of insecticides (World Health Organization 2013a, b, c).
7.4 Malaria Elimination
Malaria elimination is the interruption of local mosquito-borne malaria transmission or the reduction to zero of the incidence of malaria infection in a defined geographical area. After elimination, continued measures are required to prevent reestablishment of transmission. Malaria eradication is defined as the permanent reduction to zero of the worldwide incidence of malaria infection caused by a particular malaria parasite species (World Health Organization 2014a, b, c, d, e).
In recent years, there have been significant reductions in malaria burden in the Americas and other regions including Africa particularly in some countries in south of the Sahara, where malaria incidence and mortality among children and adults have declined. These marked reductions in the levels of transmission are among the important driving forces behind the current global interest on malaria elimination and subsequent global eradication (Pan American Health Organization 2010).
While continuing to gain momentum and support for malaria elimination efforts is generally increasing, the path toward malaria-free status is clearly challenging and resource intensive. The malaria elimination process or continuum is characterized by four distinct program phases: control, pre-elimination, elimination, and prevention of reintroduction. A set of specific program interventions pertaining to prevention, treatment, surveillance, monitoring and evaluation, and health systems strengthening characterizes each of the program phases (Table 20.9).
In 2014, the WHO Global Malaria Programme initiated the process of consolidating the Post-2015 Global Technical Strategy (GTS) for Malaria which is the primary theme of accelerating malaria elimination. Efforts to reorient control programs toward elimination require an updating of strategies (PAHO 2010). Among malaria control programs, the overall objective is reducing malaria cases and deaths by providing access to preventive methods, diagnostic testing, and treatment to the entire population at risk. In the elimination phase, malaria cases have diminished and have become relatively focal. Programs at this stage should focus interventions on detecting all malaria cases, preventing onward transmission, managing malaria foci, and managing imported malaria cases. In order for a country to be certified and declared as malaria-free, it must demonstrate an effective malaria surveillance system and that there have been no cases of locally transmitted malaria for the past 3 years (Mendis et al. 2009; World Health Organization 2014a, b, c, d, e).
References
Daneshvar C, Davis TME, Cox-Singh J et al (2009) Clinical and laboratory features of human Plasmodium knowlesi infection. Clin Infect Dis 49:852–860
Giles HM, Warrell DA (1993) Bruce-Chwatt’s essential malariology, 3rd edn. Little Brown, Boston, MA
Greenwood BM, Fidock DA, Kyle DE et al (2008) Malaria: progress, perils, and prospects for eradication. J Clin Invest 118:1266–1276. doi:10.1172/JCI33996FullTextat, http://www.jci.org/articles/view/33996/files/pdf
Johns Hopkins Bloomberg School of Public Health. Life cycle of the malaria parasite. In: Epidemiology of infectious diseases. Johns Hopkins Bloomberg School of Public Health, Creative Commons BY-NC-SA. http://ocw.jhsph.edu
Kantele A, Jokiranta S (2011) Review of cases with the emerging fifth human malaria parasite, Plasmodium knowlesi. Clin Infect Dis 52:1356–1362
Mendis K, Rietveld A, Warsame M et al (2009) From control to malaria eradication: the WHO perspective. Trop Med Int Health 14(7):1–7
Pan American Health Organization (2010) Guide for the reorientation of malaria control programs with a view toward elimination of the disease. PAHO, Washington, DC, http://www2.paho.org/hq/dmdocuments/2011/Guide_Reor_Malaria%20Control.pdf
Pan American Health Organization (2014) Interactive malaria statistics. PAHO, Washington, DC, http://www.paho.org/hq/index.php?option=com_content&view=article&id=2632&Itemid=2130&lang=en
Singh AP, Buscaglia CA, Wang Q et al (2007) Plasmodium circumsporozoite protein promotes the development of the liver stages of the parasite. Cell 131:492–504
Sinka ME, Bangs MJ, Manguin S et al (2012) A global map of dominant malaria vectors. Parasit Vectors 5:69
Weatherall DJ, Miller LH, Baruch DI et al (2002) Malaria and the red cell. Haematology 1:35–57, http://asheducationbook.hematologylibrary.org/cgi/reprint/2002/1/35
WHO Pesticide Evaluation Scheme (2013) WHO recommended insecticides for indoor residual spraying against malaria vectors. WHO, Geneva, http://www.who.int/whopes/Insecticides_IRS_Malaria_25_Oct_2013.pdf
World Health Organization (1999) Meetings on Plasmodium vivax and Schistosoma japonicum in Asia. TDR News, WHO, Geneva
World Health Organization (2010) Basic malaria microscopy, 2nd edn. WHO, Geneva, http://www.who.int/entity/malaria/publications/atoz/9241547820/en/index.html
World Health Organization (2011) Global plan for artemisinin resistance containment. WHO, Geneva, http://apps.who.int/iris/bitstream/10665/44482/1/9789241500838_eng.pdf?ua=1
World Health Organization (2012) Management of severe malaria: a practical handbook, 3rd edn. WHO, Geneva, http://www.who.int/iris/bitstream/10665/79317/1/9789241548526_eng.pdf?ua=1
World Health Organization (2013a) Malaria entomology and vector control. WHO, Geneva, http://apps.who.int/iris/bitstream/10665/85890/1/9789241505819_eng.pdf
World Health Organization (2013b) Questions and answers on malaria vaccines. WHO, Geneva, http://www.who.int/immunization/research/development/WHO_malaria_vaccine_q_and_a_Oct2013.pdf?ua=1
World Health Organization (2013c) Recommendations for achieving universal coverage with long-lasting insecticidal nets in malaria control. WHO, Geneva, http://www.who.int/entity/malaria/publications/atoz/who_recommendations_universal_coverage_llins.pdf
World Health Organization (2014a) World malaria report 2014. WHO, Geneva, http://www.who.int/malaria/publications/world_malaria_report_2014/en/
World Health Organization (2014b) Malaria rapid diagnostic test performance. Results of WHO product testing of malaria RDTs: round 5 (2013). WHO, Geneva, http://apps.who.int/iris/bitstream/10665/128678/1/9789241507554_eng.pdf?ua=1&ua=1
World Health Organization (2014c) Policy recommendation on malaria diagnostics in low transmission settings. WHO, Geneva, http://www.who.int/malaria/publications/atoz/who-recommendation-diagnostics-low-transmission-settings-mar2014.pdf?ua=1
World Health Organization (2014d) Procedures for certification of malaria elimination. Wkly Epidemiol Rec 89(29):321–336, http://www.who.int/wer/2014/wer8929.pdf?ua=1
World Health Organization (2014e) Tables of malaria vaccine projects globally. WHO, Geneva, http://www.who.int/immunization/research/development/Rainbow_tables/en/
World Health Organization (2015) Guidelines for the treatment of malaria, 3rd edn. WHO, Geneva, http://www.who.int/malaria/publications/atoz/9789241549127/en/
World Health Organization and Medicines for Malaria Venture (2014) Global malaria mapper. WHO and MMV, Geneva, http://www.who.int/malaria/publications/world_malaria_report/global_malaria_mapper/en/
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Carter, K.H., Escalada, R.P., Singh, P. (2017). Malaria. In: Marcondes, C. (eds) Arthropod Borne Diseases. Springer, Cham. https://doi.org/10.1007/978-3-319-13884-8_20
Download citation
DOI: https://doi.org/10.1007/978-3-319-13884-8_20
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-13883-1
Online ISBN: 978-3-319-13884-8
eBook Packages: MedicineMedicine (R0)