Abstract
About 20 years ago, various diseases caused by bacteria Rickettsia, Orientia, Ehrlichia, Anaplasma, Coxiella, and Bartonella were all denominated as rickettsial infections. Nowadays, based on genetic analysis, these bacteria are reorganized and considered as distinct entities. The order Rickettsiales includes at the moment two families: Anaplasmataceae and Rickettsiaceae, and the family Bartonellaceae, as well as Coxiella burnetii, are no more considered rickettsiae. Diseases caused by bacteria from the Anaplasmataceae family are presented in Chap. 15, and here diseases caused by Rickettsia and Orientia are presented. Although Bartonella spp. and Coxiella spp. are not anymore considered rickettsial disease, they are often still studied within the field of rickettsiology and thus are also presented here.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Rickettsial Diseases (Rickettsioses)
1.1 Introduction
Rickettsial diseases (rickettsioses) are diseases caused by bacteria from the genera Rickettsia and Orientia. Currently, there are 26 species within the genus Rickettsia with validated and published names. However, some species are divided into subspecies, and more than 70 strains remain unidentified or incompletely described pathogens. Moreover, each year still new species or stains are being discovered. The name of the genus Rickettsia was initially given by Brazilian infectologist Henrique da Rocha Lima (1879–1956) to intracellular microorganisms found in lice, Rickettsia prowazekii , the agent of epidemic typhus, in honor of American pathologist Howard Taylor Ricketts (1871–1910) and Czech zoologist and parasitologist Stanislaus Josef Mathias von Prowazek (1875–1915), who both died of typhus while studying the disease (see Chap. 3). Ricketts is remembered for discovering the causative organism and the transmission route of Rocky Mountain spotted fever, while von Prowazek and Rocha Lima discovered that typhus was transmitted to humans by the feces of lice, and the last described the bacteria. The genus Orientia on the other hand is represented only by one species, Orientia tsutsugamushi , the causative agent of scrub typhus. The name comes from the Orient, the area where the organism is widely distributed, and from “tsutsugamushi” that means in Japanese “dangerous mite ”.
1.2 Characterization
They are obligate intracellular bacteria which means that they can reproduce only within animal cells. The members of Rickettsiaceae family are maintained in nature through complex cycles involving reservoir in mammals and arthropod vectors such as ticks, mites, and lice. Except for R. prowazekii, humans are only accidental hosts for rickettsiae and usually get infected through bites or feces of infected arthropods. Some of these diseases are benign for humans, while others may be fatal. In some cases different isolates of the same species vary in virulence for the same host. Some rickettsioses, such as epidemic typhus caused by R. prowazekii, have been described since the sixteenth century, while others have been discovered recently. Many other species exist in arthropods without presently being associated with human disease .
1.3 Taxonomy and Life Cycle
The rickettsiae are rod-shaped , small (0.3–0.5 μm by 0.8–2.0 μm), Gram-negative, non-spore-forming bacteria. The genus Rickettsia has been divided into four major groups based on their antigenic and genetic characteristics: (1) the spotted fever group (SFG), (2) the typhus group (TG), (3) the Rickettsia bellii group, and (4) the Rickettsia canadensis group. However, this classification is changing as new data are available. The Rickettsia with medical importance belongs to the SFG and the TG. The SFG rickettsiae are mainly associated with mites and ticks while the TG rickettsiae with insects such as fleas and lice.
1.3.1 Typhus Group Rickettsia
The typhus group includes Rickettsia prowazekii and Rickettsia typhi .
-
Rickettsia prowazekii has been found worldwide and causes epidemic typhus, known also as louse-borne typhus fever , typhus exanthematicus , classical typhus fever , European typhus , or jail fever . Due to the difficulty of making a precise clinical diagnosis of epidemic typhus and the similarity of its symptomatology to other fevers prevalent in the past, its origins remain uncertain; however, it is possibly one of the oldest pestilential diseases of mankind and responsible for millions of deaths worldwide. In most parts of the world, humans are the only reservoir host for R. prowazekii. The primary vector in person-to-person transmission is the human body louse ( Pediculus humanus ). Lice become infected when they feed on the blood of infected patients and later infected lice inoculate R. prowazekii via bite into healthy humans. The pathogen is also present and able to survive in the feces of infected lice for weeks; thus, the transmission may occur by inhalation or contact of infected feces or even by crushing lice when the pathogen enters into a contact with the mucous membranes of the mouth and eyes. Infections can become latent and later recrudesce (referred to as Brill-Zinsser disease ). Humans with recrudescent typhus are capable of infecting lice and spreading the disease. Epidemics of typhus usually occur where louse populations are high; therefore, infections are typically seen not just in populations living in unsanitary, crowded conditions and prisons and refugee camps but also in the homeless louse-infested population in developed countries, and outbreaks are often associated with wars, famines, floods, and other disasters. Most epidemics occur during the colder months. In North America R. prowazekii has been recently found in flying squirrels ( Glaucomys volans ), and the disease (referred as indigenous epidemic typhus or sylvatic typhus ) may be acquired from flying squirrel-parasitizing arthropods .
-
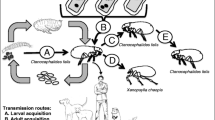
Rickettsia typhi causes murine typhus , known also as endemic typhus , Mexican typhus , or flea-borne typhus . Murine typhus usually occurs sporadically rather than in epidemics and is widely distributed, especially throughout the tropics and subtropics. Rats (Rattus norvegicus and R. rattus) are the animal reservoirs. The infection is spread when fleas, mainly the oriental rat flea ( Xenopsylla cheopis ), feed on infected rats. Rickettsia typhi infects the gut epithelial cells and is excreted in the feces. Transmission to humans occurs when the skin, respiratory tract, or conjunctiva is contaminated with infected flea feces. Infection via inhalation of dust from rat-infested buildings may occur and also the organism can be transmitted by fleabites alone. Endemic areas are primarily urban settings associated with commensal rats and their fleas. Seaports and coastal areas are favored; however, rural areas may also be affected. People whose occupation or living conditions bring them into close contact with rats are primarily affected, especially in food-storage areas or granaries. Most human cases are acquired indoors where rats are present.
1.3.2 Spotted Fever Group Rickettsia
The spotted fever group (SFG) includes etiologic agents of various diseases such as Rocky Mountain spotted fever , Mediterranean spotted fever, tick typhus , or rickettsialpox . With the exception of etiologic agent of rickettsialpox (Rickettsia akari) which is transmitted by mites and Rickettsia felis transmitted by fleas, the SFG rickettsiae are transmitted to humans by the bite of infected hard ticks (Acari, Ixodidae). They share characteristic clinical features including fever, headache, rash, and sometimes eschar formation at the site of the bite. The arthropod vector species and associated human clinical diseases vary depending on the geographical locations (Table 12.1).
The typical life cycle of a SFG rickettsia is complex and involves arthropods and mammalian species and is influenced by environmental conditions. Bacteria infect and multiply in almost all organs of their arthropod hosts, mainly infecting ticks. When the ovaries and oocytes of an adult tick female tick are infected, rickettsiae may be transmitted transovarially (hereditary transmission) to at least some of its offspring (larvae). Thus, once an egg is infected, all subsequent life stages of the tick will be infected, and rickettsiae infecting the ticks’ salivary glands can be transmitted to vertebrate hosts during feeding. However, it is known that some bacteria, for example, R. rickettsii, are partially pathogenic to ticks; therefore, the infection rate drops in the tick population with each tick generation because mortality rates are higher among infected than among uninfected arthropods. Under these conditions , a so-called amplifier vertebrate host is required to maintain the bacterium active in nature. The amplifier hosts (usually small- and middle-sized mammals), when inoculated by infected ticks with pathogens, maintain the bacteria in their bloodstream for some days or weeks, when new, not infected ticks get infected with bacteria giving continuation for the rickettsial infection among the tick population.
The number of the main mammalian hosts has a direct effect on tick presence. In other words, the higher the host abundance, the higher the chances that ticks will find the hosts to complete their life cycles, increasing the ticks’ populations. If additionally this mammalian host is also a good amplifier host for rickettsiae, its presence influences the infection in these ticks and consequently increases the risk for humans being bitten by infected tick. If it also happens that these amplifier hosts are increasing its abundance in disturbed and modified environments, when other potential hosts for ticks disappear, the risk of acquiring disease by humans is increasing dramatically. For example, the higher risk for humans of acquiring R. rickettsii in Southern Brazil is strongly associated with the numerous and uncontrolled populations of capybaras ( Hydrochoerus hydrochaeris ) in recreation areas, such as city parks. These rodents, besides being the most important hosts for all parasitic stages of aggressive human biting tick Amblyomma sculptum (formerly A. cajennense Footnote 1), are efficient amplifier hosts of R. rickettsii for this tick. By maintaining and increasing the infected ticks’ populations in the proximity of humans, capybaras are playing a crucial role in the epidemiology of spotted fever in Brazil as humans spending time in city parks, where these mammals are present, are frequently attracted by ticks that may be infected with R. rickettsii. Consequently, the geographic distribution of R. rickettsii is determined by the incidence of its tick amplifier hosts , in this case, capybaras (Figs. 12.1, 12.2, and 12.3).
Tick Amblyomma aureolatum is a vector of Rickettsia rickettsii causing Brazilian spotted fever (BSF) in some regions of Brazil. Human cases associated to these ticks occur when domestic dogs are bitten by adult ticks during incursions into the rain forest and bring them back to human dwellings. Infected ticks drop off from the dog accidentally (scratching, picked by humans) and bite humans inoculating bacteria. Image: M. Ogrzewalska
Capybaras ( Hydrochoerus hydrochaeris ) are amplifier hosts of Rickettsia rickettsii for Amblyomma sculptum ticks. Capybaras are abundant rodents in anthropized areas in Brazil, close to human settlements in riparian forests and at habitats with water bodies such as urban and peri-urban parks and garden of condominiums. Image: M. Ogrzewalska. (a) capybaras close to humans settelments, (b) capybara (H. hydrochaeris)
The number of unnamed and noncultivated Rickettsia is growing. New rickettsial species are continuously isolated from ticks around the world, but their pathogenicity for humans remains to be determined; for example, R. asiatica, R. hoogstraalii, or R. montanensis have been only found in ticks. However, some rickettsiae previously thought to be nonpathogenic, later, even sometimes after decades, have been associated with human disease, as in the case of R. africae, R. parkeri, R. slovaca, or R. helvetica, which were first isolated from ticks and subsequently from a patient’s blood. Thus, it is believed that each Rickettsia has a pathogenic potential, provided that its reservoir arthropod is capable of biting humans .
1.4 Clinical Manifestations
1.4.1 Typhus Group Rickettsia
-
Rickettsia prowazekii (louse-borne epidemic typhus). The disease starts abruptly after 2 weeks of incubation with malaise and nonspecific symptoms as fever, headache, arthralgias, and chills. There is no eschar of inoculation at the site of lice biting. Rash, macular, maculopapular, or petechial, begins at the trunk and spreads to extremities, saving the face, palms, and soles, and is presented in 20–60 % of cases. The central nervous system is affected. At severe cases , multiorgan commitment may occur. Without treatment, the disease is fatal in 10–30 % of patients. Recrudescent typhus or Brill-Zinsser disease occurs in individuals, usually Europeans, who had the primary disease during the World War II. It can be triggered by factors inherent to the host as lowering immune response, determining a more benign clinical picture without rash and not fatal.
-
Rickettsia typhi (murine typhus ). The disease usually presents with an abrupt onset of symptoms after an incubation period of 1–2 weeks. Fever, headache, chills, and rash are the common symptoms presented. Rash appears at various intervals and is described as macular at most cases, frequently affecting the trunk, rarely seen at the palms, soles, or face. Liver and spleen enlargement and gastrointestinal symptoms can occur. Malaise, anorexia, myalgia, cough, conjunctivitis, nausea, lymphadenopathy, central nervous system abnormalities, gastrointestinal impairment, and enlargement of the liver and spleen are the less common symptoms. Clinical course is usually uncomplicated and fatalities are uncommon (1–5 % of nontreated cases). G6PD insufficiency can aggravate or potentiate symptoms, leading to a life-threatening situation, conducting to more severe forms .
1.4.2 Spotted Fever Group Rickettsia
The best known is the spotted fever caused by R. rickettsii (Rocky Mountain spotted fever , Brazilian spotted fever ). It is a systemic disease with variable clinical findings. After 1–2 weeks following the bite of an infected tick, an abrupt onset with the classic triad may appear including fever (typically high—39–41 °C), headache (severe frontal), and malaise (sometimes nausea, vomiting). However, acute rickettsial infection develops nonspecific symptoms and is difficult to specifically diagnose because it is similar to many other infectious diseases. The patient may present atypical forms without rash, viscerotropic and/or fulminant. Symptoms may vary with intestinal dysfunction, hepatic impairment, pulmonary involvement, and renal and neurological manifestations. Rash is considered the most important sign , generally absent until the third to fifth day of fever. In approximately 20 % of patients, such as children and elderly patients, the absence of rash complicates and delays the diagnosis thus determining a higher number of deaths. Some patients (mainly black patients) can show a fleeting, evanescent, or atypical distribution of rash. Macular, initially pinkish, can develop in 2–5 days for a maculopapular, petechial pattern, reddish, typically reaching at onset of disease, the ends, spreading hereinafter centrally to the trunk. A “classic” rash has a characteristic of petechial lesions and is considered when the distribution includes the palms and soles, although this scenario is not seen in all cases. The presence of petechial rash means that the patient is often severely ill. Large ecchymotic injury, formed by coalescence of petechiae as a consequence of microvascular damage , can cause necrosis /gangrene .
Clinical manifestations of other SFG Rickettsia are presented here in alphabetic order of species:
-
Rickettsia aeschlimannii : The patients present typical clinical signs of spotted fever: eschar, high fever, and generalized maculopapular rash.
-
Rickettsia africae (African tick bite fever): After 5–10 days of the tick bite, the clinical course comprises an abrupt onset of fever, headache, nausea, and myalgia. Eschar is present at most cases (sometimes multiple eschars), and the less common clinical sign is the vesicular or maculopapular cutaneous rash and arthralgia. The infection is symptomatic in less than 50 % of cases. Most of the patients develop a mild to moderate illness and the disease resolves spontaneously within 10 days. Complication is rare and no fatalities are reported.
-
Rickettsia akari (rickettsialpox ): After a mean incubation period of 7 days, a painless papule, reddish at mite bite site, appears. Some days after, it evolves to a vesicular form and ulcerates, forming the eschar. This eschar is formed 3–7 days before onset and persists by 2–3 weeks. Regional lymph nodes are often presented enlarged and tender. The onset of signs and symptoms is sudden with high hectic fever, myalgias (backache), chills, and headache (frontal and severe). Diaphoresis, anorexia, and photophobia may be present also. Within 2–3 days after onset, a generalized papulovesicular rash appears. The severity of rash does not reflect the gravity of the systemic manifestations. Less than 30 % of patients present enanthem.Footnote 2 The lesions usually heal by crusting. Generally the disease is described as mild, benign, and self-limited. Untreated illness resolves in 2–3 weeks and death is very rare.
-
Rickettsia australis (Queensland tick typhus ): Sudden onset with fever, headache, and myalgia. Within 10 days, maculopapular or vesicular rash appears. Eschar in identified in approximately 65 % and lymphadenopathy in 71 % of cases, respectively.
-
Rickettsia conorii (Mediterranean spotted fever , MSF) : After a mean incubation time of 6 days, abrupt onset appears with high fever, headache, chills, arthromyalgias, and eschar (rarely multiple) at tick bite site. Exanthem appears 4 days (median) following the onset of fever and is initially macular and scarce becoming maculopapular and disseminated. Rash always presents at the palms and soles (limbs) but seldom at the face and usually is associated to a severe form of MSF. Usually patients will recover within 10 days without sequelae. Severe forms may occur at 5–6 % of cases with neurological manifestations and different organs’ involvement.
-
Rickettsia conorii israelensis (Israeli spotted fever , ISF): After the incubation period of 7–8 days from the tick bite, fever and rash on hands and feet start and then extend to the rest of the skin. Eschar is absent. Up to one third of patients can manifest headache , vomiting, myalgia, and arthralgia. Fatal cases and severe forms may occur, especially in children or in patients with risk factor, as glucose-6-phosphate dehydrogenase (G6PD) deficiency .
-
Rickettsia conorii caspia (Astrakhan fever , AF): The disease is similar to MSF but eschar is rare (less than 25 % of patients).
-
Rickettsia conorii indica (Indian tick typhus , ITT): The rash frequently purpuric differs from MSF and eschar is rarely identified.
-
Rickettsia felis (flea-borne spotted fever ): Clinical features are often confused with other febrile illnesses and with those found on patients with murine typhus caused by R. typhi. After contact with infected flea feces on wounds or abraded skin or infected fleabites, fever, fatigue, headache, vomiting, anorexia, cough, pharyngitis, and rhinitis are some of the manifestations presented. Patients may develop a cutaneous lesion (eschar) in the site of inoculation, a general maculopapular rash, pneumonia, and neurological signs. However, clinical findings can be more complex and more severe, although there is limited information at scientific literature.
-
Rickettsia heilongjiangensis (Far-Eastern tick-borne rickettsiosis ): Clinical diagnosis is similar to other spotted fever rickettsioses. Present as mild febrile rash, unique eschar, regional lymphadenopathy, macular or maculopapular rash (faint in most cases) and conjunctivitis.
-
Rickettsia honei (Flinders Island spotted fever ): Sudden onset of fever, headache, arthromyalgias (joint swelling), and cough. A few days later, a maculopapular rash appears with no vesiculation. Eschar presented only in 25 % of patients.
-
Rickettsia japonica (Japanese spotted fever ): Similar to other spotted fever rickettsioses, after tick bite, an eschar is presented and an abrupt onset with high fever, headache, and chills occurs. Macular rash appears after 3–4 days and disappears in 2 weeks. Severe cases can happen including encephalitis, disseminated intravascular coagulopathy, multiorgan failure, and acute respiratory distress syndrome.
-
Rickettsia massiliae : Patients present fever, necrotic eschar, and maculopapular rash involving the palms and soles.
-
Rickettsia parkeri : With multiple inoculation eschars , the patient presents fever, headache, myalgias and arthralgias, and maculopapular rash on the trunk and extremities, including the palms and soles. Enlarged lymph nodes are observed near bite sites.
-
Rickettsia sibirica mongolitimonae (lymphangitis-associated rickettsiosis ): Mild disease characterized by a fever, a headache, a discrete rash (maculopapular), an inoculation eschar, a lymphangitis expanding from the inoculation eschar to draining node, and a painful satellite lymphadenopathy.
-
Rickettsia sibirica (Siberian tick typhus ): The incubation period is 4–7 days after the tick bite; eschar appears accompanied by regional lymphadenopathy. Onset is acute with high fever, headache, myalgia and typical maculopapular rash, conjunctivitis, and digestive disturbances. Symptoms are concomitant and can last 6–10 days without treatment. Clinical course is benign.
-
Rickettsia slovaca (tick-borne lymphadenopathy , TIBOLA, and Dermacentor-borne necrosis erythema-lymphadenopathy , DEBONEL): Mean incubation of 7 days, fever (low grade), headache, and rash are uncommon. Chronic fatigue and localized alopecia at the bite site are sequelae presented. Eschar on the scalp can occur, associated with regional painful lymphadenopathy .
1.4.3 Scrub Typhus
Orientia tsutsugamushi causes scrub typhus . Eschar is formed by the chigger bite site developing during incubation period (average 10 days). The eschar evolves from a small papule to a blackened crust lesion resembling a cigarette burn. Onset begins abruptly with fever, headache, myalgias, chills, and malaise. Perishable macular rash on the trunk, maculopapular, spreads to extremities and may appear at the end of the first week of disease. Conjunctival suffusion, hearing loss , and cough sometimes with infiltrates are common feature. Severe cases can occur with multiple organ dysfunctions and respiratory failure is the most common cause of death .
1.5 Diagnosis
The diagnosis of rickettsial diseases is based primarily on clinical and epidemiological characteristics. Confirmatory techniques provide information that retrospectively validates the accuracy of the clinical diagnosis. The diagnosis of spotted fever is usually serological and retrospective. Serologic assays are commonly available through commercial laboratories. The indirect immunofluorescence antibody assay (IFA) is considered the gold standard and the analysis is conducted with two paired serum samples and obtained within 2–4 weeks. The fourfold increase of the antibody IgM or IgG titer confirms the diagnosis. Serologic diagnosis can only identify genus, and thus the etiology is only possible using the characterization of the agent through molecular technique.
Amplification of specific DNA by polymerase chain reaction (PCR) in blood and/or tissue or even ticks recovered from the patient provides a rapid method for detecting rickettsial disease infection. Currently, molecular diagnostics (PCR sequencing) has been used more frequently and is considered the most suitable and ideal diagnostic tool for identifying severe cases and fulminant ones, where samples for serological pairing are not always available. New techniques (real-time PCR ) might offer the advantages of speed, reproducibility, quantitative capability, and low risk of contamination, compared with conventional PCR.
Immunohistochemical staining analysis using immunofluorescence or immunoperoxidase test , even in paraffin-embedded tissue, allows identifying the presence of specific rickettsiae for the spotted fever group. Immunostaining of skin biopsy, preferably a 3 mm fragment derived from a maculopapular rash containing petechial lesions, may aid in diagnosis, before the specific antibiotic therapy is complete within 24 h.
Rickettsial isolation in cell culture from blood, skin biopsy, and/or other organ fragments can provide difficulties in performing the technique, and the risk of infection refrains its use in routine laboratory. The same methodology can be used in tick vectors eventually recovered from patients. This technique is more labor intensive and time consuming than the assays described above. Culture is rarely used for diagnosis and the other methods are used to confirm infection .
1.6 Treatment and Prevention
Treatment of patients with potential rickettsioses must be started quickly and should not await confirmatory laboratory testing. Doxycycline is the first-line treatment for adults and children of all ages and should be initiated immediately whenever rickettsioses are suspected. This drug is the first-line treatment for adults and children of all ages (adults, 100 mg every 12 h; children under 45 kg (100 lbs), 2.2 mg/kg body weight given twice a day).
Other drugs such as chloramphenicol , azithromycin , fluoroquinolones , and rifampin may be alternatives. These antimicrobial agents, although they are bacteriostatic, lead to the reduction of toxemia and other clinical signs in 24–48 h and improvement in 2–3 days without complications.
The hospitalization is not necessary in all cases and the treatment should be continued for at least 7–10 days or until no fever for over 24 h. Antibiotics should be administered intravenously in patients with nausea and vomiting. In countries where intravenous doxycycline is not available, in severely ill patients, chloramphenicol should be given, although studies prove that tetracycline is superior. Rickettsia spp. are resistant to antibiotics of β-lactam class, aminoglycosides and trimethoprim-sulfamethoxazole , which should not be used.
1.7 Prophylaxis and Prevention Measures
There are no vaccines available for rickettsial infections . The only sure way is to prevent infection by avoiding contact with infected arthropods. In the case of lice and flea associated with Rickettsia, parasite populations should be reduced, using insecticides to prevent additional exposure. Insecticidal powders are available for body-louse control and treatment of clothing for those at high risk of exposure. Insecticide powders with residual action should be used on rat runs and burrows.
Rickettsia prowazekii is susceptible to 1 % sodium hypochlorite, 70 % ethanol, glutaraldehyde, and formaldehyde. It can also be inactivated by moist heat (121 °C for a minimum of 15 min) and dry heat (160–170 °C for a minimum of an hour). Rickettsia typhi is destroyed by formalin, phenol, and temperatures greater than 56 °C for 30 min.
Reducing exposure to ticks is the most effective way to limit the probability of spotted fever infections. In people exposed to tick-infested environments , prevention measures should be aimed at personal protection:
-
1.
Wear light-colored clothing to allow you to see ticks that are crawling on your clothing.
-
2.
Tuck your pant legs into your socks so that ticks cannot crawl up the inside of your pant legs.
-
3.
Apply repellents to discourage tick attachment. Those repellents containing permethrin can be sprayed on boots and clothing and will last for several days. Repellents containing DEET can be applied to the skin, but will last only a few hours before reapplication is necessary.
-
4.
Prompt careful inspection and removal of crawling or attached ticks upon return from potentially tick-infested areas by searching your entire body for ticks.
-
5.
Remove any tick you find on your body by fine-tipped tweezers by grasping the tick as close to the skin surface as possible and pull upward. Do not twist or jerk the tick; this may cause the mouthparts to break off and remain in the skin.
Ticks may be carried into the household on pets, especially on dogs; thus they should be treated by repellent products recommended by vet and carefully examined daily. Ticks may not only be dangerous by transmitting pathogens that are causing dangerous diseases in pets, but they may leave the animal and bite humans, putting in risk to those who did not suspect being parasitized by ticks during outdoor activities such as small children or elderly people .
2 Bartonella Infections
2.1 Introduction
Since its discovery, bartonellosis refers to the Carrión’s disease (CD) , a biphasic disease of the Andes region caused by Bartonella bacilliformis . There was a dramatic outbreak of fever and hemolytic anemia among workers based in the upper Rimac valley , during construction of the railway line that would link the capital, Lima, to the city of La Oroya, 4000 m above sea level in the Andes in 1871. During the course of a week, at least 4000 workers from Chile and other regions of Peru died in those episodes, and the disease became known as Oroya fever , although the agent of the syndrome remained elusive.
In 1885, a medical student named Daniel Carrión injected himself with exudate from a verruga lesion, developed Oroya fever, and died. The common etiology of Oroya fever and Peruvian verruga was established, and Carrión is one of the most heroic images in Peruvian medical history. In recognition of his sacrifice, South American bartonellosis is commonly known as Carrión’s disease. Finally, in 1905, Alberto Barton solved the riddle of the etiologic agent of CD when he observed intracellular bacteria in blood smears from Oroya fever patients.
Cat scratch disease , caused by Bartonella henselae , was firstly discovered in a boy in France, in 1931, associated to superficial wounds caused by cat claws, and trench fever, caused by B. quintana and transmitted by body lice, was very frequent in the World War I trenches (see Chap. 3), but became very rare after this, being found in homeless persons in France and the United States .
2.2 Taxonomy and Life Cycle
Currently, there are more than 33 recognized species and subspecies and several Candidatus spp. within this genus, among which, 14 are associated to human diseases with a worldwide distribution, and that causes an increasingly large number of infectious diseases in humans and animal.
However, bartonellosis is not a reportable disease in human populations in most countries, including those in South America. Therefore, sufficient information to determine the exact incidence or prevalence of Bartonella infection is not available. In the United States, it was estimated that 22,000–24,000 humans developed cat scratch disease (CSD) during 1992, of whom 2000 were hospitalized. The estimated annual healthcare cost of the disease was more than $12 million. In Connecticut, which is the only state where the disease is reportable in the United States, the incidence of the disease from 1992 to 1993 was estimated to be 3.7 cases/100,000 persons, whereas in the Netherlands, there was an estimated 2000 cases/years or 12.5 cases/100,000 persons. These observations suggest that several thousand cases of cat scratch disease may occur every year in most part of the world. Bartonella human infection seroprevalence varies from 1.4 % (intravenous drugs user , IVDU, from Baltimore, USA) to 77.5 % (children and adults in an area with an outbreak of Carrion disease, Peru) in selected populations in the last decade (1996–2007).
Since the early 1990s, there have been substantial advances in the understanding of the etiology, reservoir potential, vector transmission, and pathogenesis of Bartonella infection in a wide range of mammals. In 1993, Brenner and colleagues proposed to unify the Bartonella and Rochalimaea genera and renamed some species as B. quintana, B. henselae, B. vinsonii, and B. elizabethae. As a result of this unification, the transfer of all these organisms from the family Rickettsiaceae to the family Bartonellaceae was required and removed the last family from the order Rickettsiales, based on phylogenetic relationships and the absence of obligate intracellular pathogen between the bartonellas. Nowadays, they belong to the α2 subgroup of the class Proteobacteria, order Rhizobiales, and are closely related to the genera Brucella, Agrobacterium, and Rhizobium.
Microscopically, all Bartonella spp. are Gram-negative bacilli or coccobacilli. Some species, such as B. bacilliformis and B. clarridgeiae, have flagella, which in the case of B. bacilliformis facilitates erythrocyte invasion. B. henselae appear to lack flagella. Members of the genus Bartonella are small; pleomorphic; facultatively intracellular, fastidious, and aerobic; and catalase-, urease-, nitrate reductase-, and oxidase-negative bacteria.
Bartonella infections have been encountered in all species surveyed, which have extended to members of different orders of mammalian including carnivores, primates, ungulates, rodents, and bats. It is believed that the vector preference for certain hosts can influence the transmission of these organisms and that it is responsible for association of a given Bartonella sp. with a specific host, i.e., B. henselae, B. clarridgeiae, and B. koehlerae in cats, B. alsatica in wild rabbits, and B. bacilliformis and B. quintana in humans.
Several hematophagous insects have been implicated in Bartonella transmission, including sand flies ( Lutzomyia verrucarum , L. peruensis ), human body lice ( Pediculus humanus ), cat fleas ( Ctenocephalides felis ), and, potentially, ticks ( Ixodes pacificus ).
2.3 Clinical Manifestations
Bartonella have been recognized as agents causing human disease, and the clinical spectrum of Bartonella infection has continually expanded. Bartonella bacilliformis (agent of Oroya fever and verruga peruana ), B. henselae (agent of cat scratch disease, bacillary angiomatosis, bacillary peliosis, endocarditis), and B. quintana (agent of trench fever, bacillary angiomatosis, bacteremia, and endocarditis) are the best-known species causing human illness.
The Bartonella spp. infection can cause great diversity of clinical manifestations in humans as a fever of unknown origin and recurrent, malaise, fatigue, insomnia, loss of memory, psychiatric disorders, lymphadenopathy, splenomegaly, angiomatosis and bacillary peliosis, endocarditis, hepatitis, osteomyelitis, encephalitis, meningitis, and other neuroretinitides.
Bartonella spp. have pathogenic characteristics, such as to invade cells causing prolonged intraerythrocytic bacteremia in their hosts and to lyse red blood cells. In humans, the infection cycle of Bartonella spp. is initiated by colonization of the primary niche. Besides erythrocytes, the endothelial cells represent another target of Bartonella in their mammalian hosts. Current opinion is that these cells serve as a primary niche for Bartonella prior to its entry in the bloodstream. In this stage, the infection is usually controlled by the immune system, and clinical manifestations are characterized by local lymphadenopathy (i.e., associated with B. henselae, B. quintana, and B. alsatica). Bartonella spp. have rapidly cleared from the blood after the initial inoculation but can reappear in the bloodstream . After 4 or 5 days, the bacteria (primarily B. bacilliformis and B. quintana) are released into the bloodstream and then are entitled to adhere or invade to mature erythrocytes, where intracellular replication occurs. Bartonella spp. can subsequently colonize secondary foci, particularly vascularized tissues such as heart valves, the liver, and the spleen. Bartonella spp. can circulate in the blood for the remaining life span of the infected erythrocyte. As a result, bacteremia can last for several weeks to months .
2.4 Diagnosis
There is not a standard diagnostic laboratory for infections caused by Bartonella spp. Nowadays it is evident that several techniques must be used to avoid simultaneously false-negative results. Diagnostic techniques for infections with Bartonella spp. include serology by immunofluorescence (IFA) to detect antibodies in the patient’s serum; culture of the pathogen; histopathological examination of lymph nodes or tissue biopsy of the skin, liver, or other affected organs; and molecular biology assays, especially PCR, to amplify Bartonella spp. genes from patient’s tissue fragments or blood. The blood smear stained with Giemsa method is utilized in the diagnosis of Carrión disease by B. bacilliformis to assess the prognosis; even the finding of more coccoid bacteria indicates good evolution.
Serology is particularly important because it allows rapid identification of Bartonella infection and should be performed systematically when investigating culture negative or in seroepidemiological studies showing their value in alerting to dispersion of pathogens throughout the world. It is limited by cross-reactions between the different species of Bartonella and between genera such as Coxiella and Chlamydia. Many studies have shown that serological differentiation between B. henselae and B. quintana through IFA is impossible, since cross-reactivity between these species is very high (95 %). It is also necessary to take into consideration the heterogeneity among strains and genotypes of Bartonella spp., differences between the parameters of analysis laboratories, and subjectivity of the readings of the results of IFA, which would result in false positives. Despite these limitations, IFA is the gold standard for the diagnosis of infection, present or previous.
The diagnosis of Bartonella infection should be confirmed by culturing the organism from aseptically obtained patient samples, including blood, CSF, lymph nodes, or other tissue aspiration samples, ocular exudates, and from surgical biopsies. The culture liquid Bartonella spp. becomes necessary to increase the sensitivity of detection of bacteremia through methods of molecular and is one of the most used methods of diagnosis worldwide. The isolation of most species of Bartonella in blood agar plates requires a long incubation period (6–8 weeks) at 35 °C in a water-saturated atmosphere containing 5 % CO2. The development of a new liquid culture medium called Bartonella alpha-Proteobacteria growth medium (BAPGM) that allows the growth of at least seven species of Bartonella enabled the improvement of this method as diagnosis. It has been widely used as a pre-enrichment and combined with molecular methods increased the success and sensitivity of culture for diagnosis in both animals and humans. Likewise, for other methods diagnosis, a consensus of choosing primers neither the PCR conditions does not exist. Species-specific PCR has been useful, especially when a particular diagnosis is already suspected.
It is known that ocular manifestations occur in 5–10 % of patients with cat scratch disease (CSD) . Several imaging modalities can be used to assist in the diagnosis and management of ocular CSD. They include color fundus photography that allows the clinician to monitor the fundus changes in this disease, fluorescein angiography that demonstrates leakage at the optic nerve in CSD neuroretinitis, and optical coherence tomography that provides confirmation in early stages of neuroretinitis before the formation of a macula star .
2.5 Treatment and Prevention
There are few studies on the treatment of bartonellosis. The treatment is more effective for immunodeficient than for immunocompetent patients. Antimicrobials have been used widely in the treatment of bartonellosis. CSD responds poorly to antibiotic treatment. However, a study using azithromycin in CSD showed a benefit in lymph node regression during the first 30 days, as compared with placebo. Available date does not support the use of antibiotics for the treatment of CSD.
In patients with Bartonella spp., bacteremia should be treated with 3 mg/kg body weight of gentamicin once daily for 2 weeks in combination with 200 mg of doxycycline , daily for 4 weeks. In immunocompromised patients the antimicrobials clearly indicated are erythromycin, clarithromycin, azithromycin, and doxycycline, either coadministered or not with rifampin, and the period of treatment ranges from 4 to 6 weeks.
In acute febrile phase of Carrión’s disease , the preferred treatment has been chloramphenicol. An initial dose of 50 mg/kg/day chloramphenicol for the first 3 days and a subsequent dose of 25 mg/kg/day until the completion of 14 days of treatment have been proposed as the best regimen for the of B. bacilliformis bacteremia. Patients with Bartonella endocarditis have higher death rate and undergo valvular surgery more frequently than patients with endocarditis caused by other pathogens. Patients with suspected or confirmed Bartonella endocarditis should be treated with 3 mg/kg/day gentamicin for 2 weeks in combination with 200 mg of doxycycline daily for 6 weeks. Overall, the treatment of Bartonella infections must be adapted based on whether the disease is in the acute or chronic form and based on the infecting Bartonella spp.
3 Coxiellosis (Q Fever)
3.1 Introduction
Historically in 1933, disease of unknown etiology was first identified in slaughterhouse workers in Brisbane, Queensland , Australia. Patients had fever, headache, and malaise and all laboratory tests for the screening of a large number of pathogens were negative. Because it was a disease of unknown etiology, it became known as Q fever (from the word “query”) in 1937 by Edward Holbrook Derrick. MacFarlane Burnet and his partner Mavis Freeman, whom Derrick had sent sample of infectious material, isolated a fastidious intracellular bacterium of the animals inoculated. Derrick and his colleagues investigated the epidemiology of the disease, especially the potential role of arthropod vector, and concluded that wild animals were the natural reservoirs of Q fever, while domestic animals are the secondary reservoirs, and an agent in which the disease could be transmitted by ticks or other arthropods.
In 1935, ignoring Derrick’s work in Australia, Gordon Davis, in the Rocky Mountain Laboratory in Hamilton, Montana, USA, was investigating the ecology of spotted fever. He noted that ticks collected in Nine Mile, Montana, caused a febrile response in mice that were fed.
In addition, the disease could be transmitted to uninfected guinea pigs by intraperitoneal inoculation of blood collected from infected animals. Analysis of the inflammatory cells of infected mice revealed microorganisms “rickettsial-like,” although the disease in the animal would not be compatible to spotted fever. Thus, unlike what occurred in Queensland, although the infectious agent had been proven, the disease was unknown in the United States.
The connection between the groups in Montana and Brisbane arose when a laboratory-acquired Q fever infection occurred in the Rocky Mountain Laboratory in 1938. Rolla Eugene Dyer, Director of the National Institutes of Health, became infected with the organism that the laboratory was working with. A febrile illness was reproduced in guinea pigs inoculated with Dyer’s blood, and rickettsiae were identified in spleen samples from the infected animals. Also, cross-immunity was demonstrated between microorganisms isolated from Dyer’s blood and the Nine Mile agent. Dyer then established a definitive link between the Nine Mile agent and the Australian Q fever agent. The etiologic agent of Q fever was first named Rickettsia burnetii. However, in 1938, Cornelius B. Philip proposed the creation of a new genus called Coxiella and the renaming of the etiologic agent as C. burnetii, a name that honors both Cox and Burnet, who had identified the Q fever agent as a new rickettsial species .
3.2 Taxonomy and Life Cycle
Q fever is a cosmopolitan zoonosis caused by Coxiella burnetii , a small Gram-negative pleomorphic obligate intracellular bacterium. Q fever was first described in slaughterhouse workers in Queensland, Australia, and is considered a major public health problem in Europe (especially in the Netherlands and France), Australia, and, more recently, North America, where a growing number of cases have been reported in the last 5 years and around 3 % of the general adult population is serologically reactive.
Q fever is thought to occur in almost all countries of the world, except in New Zealand and in Antarctica. The noninclusion of Q fever in a list of reportable diseases in most countries has led to significant gaps in the knowledge of its epidemiology. As such, Q fever epidemiology has been largely characterized from defined outbreak investigations and serological surveys of human and animal populations conducted in the Northern Hemisphere and Australia. Human infection with C. burnetii has been typically considered an occupational disease associated with ruminants . However, there are at least two distinct cycles that enable the perpetuation of C. burnetii infection:
-
1)
The urban/rural cycle with the participation of domestic animals.
-
2)
A wild cycle , with the participation of animals such as marsupials, rodents, and lagomorphs, among others, as well as some ectoparasites , especially ticks. The relations between the two epidemiological cycles are outlined in Fig. 12.4.
The aerosol route is the primary mode of transmission in human and domestic animals. Aerosol contamination can occur directly from the parturition fluids from infected animals, infecting newborn animals, placenta, and wool. Coxiella burnetii is highly resistant to destruction in nature, can be dispersed in wind, and survives for several weeks in areas where animals are present. Thus, Q fever can occur in patients whose epidemiological history does not identify any obvious contact with animals.
Intake, especially the intake of raw milk, is a less likely route of C. burnetii transmission that remains a point of controversy, as well as the transmission from person to person, which is an extremely rare event; sporadic cases of human Q fever have been identified: (i) after contact with an infected patient (in an obstetrician after performing an abortion), (ii) transmission via the placenta resulting in congenital infections, (iii) during the autopsy, (iv) by intradermal inoculation, or (v) through blood transfusion. Although C. burnetii was isolated from arthropods, especially ticks, it is unlikely that the transmission of C. burnetii to humans by arthropods is significant.
3.3 Clinical Manifestations
Coxiella burnetii infection in humans can vary from asymptomatic infection to fatal disease, and symptomatic patients may present with a wide clinical spectrum, including pneumonia, hepatitis, encephalitis, myocarditis, and other manifestations. Four contributing factors can account for this variation in clinical presentation of Q fever: (i) the route of infection with C. burnetii, including aerosol or gastrointestinal tract; (ii) priming dose of C. burnetii; (iii) infectious variant of C. burnetii, which can have high potential virulence; and (iv) host factors, including the immune status of the infected patient.
The inoculation route of C. burnetii in humans can be partly determined by the dominant clinical manifestation. Pneumonia is most common when the source of transmission is via contaminated aerosols, while granulomatous hepatitis is the predominant clinical manifestations when transmission occurs through ingestion of raw milk. In humans, the severity of acute Q fever has also been associated with the dose of the infecting inoculum. Finally, host factors resulting in a state of immunosuppression or pregnancy may influence the course of infection, including developing chronic disease.
Infection with C. burnetii can present with acute or chronic clinical manifestations. However, nearly 60 % of Q fever cases are asymptomatic. Among the 40 % of symptomatic patients, most (38–40 %) will experience a mild disease without the need for hospitalization. Hospitalized patients account for only 2 % of infected individuals, and only one in ten (0.2 % of the total infected) develops chronic Q fever . These proportions correspond to the data obtained from analysis of confirmed cases in the south of France, where the incidences of Q fever and endocarditis are 50/100,000 and 0.1/100,000 inhabitants, respectively.
Symptomatic acute Q fever is primarily manifested as a self-limiting febrile illness, atypical pneumonia, or granulomatous hepatitis, while endocarditis is the most common form of chronic Q fever. However, considering the wide ranging and nonspecific clinical spectrum of Q fever, the disease should be systematically considered in febrile patients with recent contact with parturient animals.
3.3.1 Acute Q Fever
In acute Q fever cases, after a 2–3-week incubation period, the patient develops an acute infection characterized by high fever, fatigue, chills, and headache. The most common clinical manifestation of the acute form is a self-limiting febrile illness associated with severe headache. Atypical pneumonia is also a frequent clinical presentation, and the clinical picture may vary from clinically asymptomatic pneumonia diagnosed in chest X-ray to acute respiratory failure, although the latter continues to be extremely rare.
Hepatitis is another common manifestation of acute Q fever and is usually identified during laboratory investigation of patients with hepatic frame with increased levels of aspartate aminotransferase (AST) , alanine aminotransferase (ALT) , and alkaline phosphatase . Although jaundice is rare, hepatomegaly is sometimes clinically detectable.
In addition to the conditions described above and the typical complications of acute Q fever, infection with C. burnetii has also been reported to produce endocarditis, osteomyelitis, Q fever in childhood, neurological manifestations, rash, fever of unknown origin, thrombocytopenia , or reactive thrombocytosis.
3.3.2 Chronic Q Fever
The diagnosis of chronic Q fever can be established from the persistence of clinical manifestation for more than 6 months after the onset of symptoms. Chronic Q fever occurs in approximately 5 % of patients infected with C. burnetii and may develop insidiously over months to years after the acute disease. Endocarditis, vascular infections, osteoarticular infections, chronic hepatitis, chronic lung infections, chronic fatigue syndrome, chronicity favored by pregnancy and preterm delivery, and abortion are some of the clinical presentations that result from chronic infection .
3.4 Diagnosis
Given that clinical diagnosis is often difficult due to similarities with a number of infectious or noninfectious diseases, in most cases the diagnosis of Q fever is confirmed by serological testing. Although a variety of serological techniques are available, the indirect microimmunofluorescence test has become the reference technique (Fig. 12.5). Serologic diagnosis is easy to establish, although antibodies are usually only detected after 2–3 weeks of disease onset. Thus, serological tests should be matched with blood samples collected in the acute and convalescent phase, which are unavailable at early diagnosis . The serology test also allows the differentiation of acute and chronic infections of Q fever. Other methods that have been used include indirect immunofluorescence assay (IFA) , agglutination , complement fixation , radioimmunoassay , indirect hemolysis test , enzyme-linked immunosorbent assay (ELISA) , enzyme-linked immunofluorescent assay (ELIFA) , dot immunoblotting , and Western blotting . The most commonly used techniques include IFA, ELISA, complement fixation, and agglutination. However, only the first two methods are commercially available.
The IFA remains the gold standard technique for the diagnosis of Q fever and has the advantage of requiring only small amounts of antigen—C. burnetii phase I and phase II with Nine Mile strain. During acute Q fever, seroconversion is usually detected 7–15 days after the onset of symptoms and the antibodies are detected by the third week in 90 % of cases. A titer of anti-IgG antibodies phase II of ≥200 and an IgM antibody titer antiphase II of ≥50 are considered to be significant, but the choice of the negative cutoff titers depends on the amount of antigen stimulation in the study population and can vary from one area to another. Chronic Q fever is characterized by the presence of antiphase I antibody, and an IgG antiphase I antibody titer of ≥800 is considered highly predictive of Q fever endocarditis.
Cross-reactivity is a major source of confusion in the interpretation of serological results and this may vary according to the serological technique. Cross-reactions have been described between C. burnetii, Legionella pneumophila, L. micdadei, B. henselae, and B. quintana. Such cross-reactions should be considered for the etiology of atypical pneumonia and endocarditis with negative blood culture that can also be caused by Legionella and Bartonella, respectively. A differential diagnosis is easily established if quantitative data are determined for antibodies against both the C. burnetii antigens (i.e., antiphase I and antiphase II).
PCR has been successfully used to detect C. burnetii DNA in cell cultures and clinical specimens. The availability of oligonucleotides for amplifying specific C. burnetii genes has enabled a simple and reliable method for detecting these bacteria , even in tissue preserved in paraffin. In addition, PCR has been shown to be more sensitive than standard culture techniques for retrospective diagnosis of frozen samples and for monitoring patients treated for chronic Q fever . The detection of C. burnetii in tissues should also be performed, particularly in patients who are undergoing treatment for chronic Q fever. Samples can be tested fresh or after fixation in formalin and paraffin. Immunodetection is accomplished using immunoperoxidase or immunofluorescence techniques and specific polyclonal or monoclonal antibodies. Only the latter technique can be used on paraffin samples .
3.5 Treatment and Prevention
Doxycycline has the highest therapeutic efficacy against C. burnetii and is the treatment of choice for acute Q fever in adults and in children over 8 years of age and in children of all ages with severe disease. Doxycycline is to be administered orally for 14–21 days at a dosage of 100 mg twice daily for adults and 2.2 mg/kg body weight twice daily for children under 45 kg. If these antibiotics are contraindicated, other antibiotics, such as trimethoprim/sulfamethoxazole, macrolides, and fluoroquinolones, can be used (Table 12.2). The benefits of using doxycycline outweigh the potential risk of discoloration of the permanent teeth in children seriously ill or hospitalized less than 8 years of age with acute Q fever. Treatment should be given in the first 3 days of illness for maximum effectiveness and not be delayed pending the results of laboratory tests or because of negative initial laboratory results in the first week of illness.
Prophylactic treatment after a suspected exposure to C. burnetii is not recommended as it can prolong the incubation period and does not prevent the infection from occurring. Likewise, treatment of asymptomatic infections or resolved cases is not routinely recommended, although it can be considered in patients with risk factors for developing chronic infection of Q fever.
Healthy patients without identified risk factor for chronic Q fever development should be clinically evaluated and subjected to serological testing by IFA 6 months after the acute infection. Patients with heart or other risk factors for the development of chronic disease should be evaluated clinically and serologically tested by IFA at 3, 6, 12, 18, and 24 months after acute infection. Pregnant women should be evaluated clinically and serologically, using the IFA at 3, 6, 12, 18, and 24 months after delivery.
Patients with chronic Q fever should be referred to a specialist in infectious diseases for tracking, since they require long-term treatment with antibiotics (using a combination of doxycycline and hydroxychloroquine), periodic diagnosis, and long-term monitoring.
Special care should be taken when introducing a new animal into a herd that is free of Q fever. Simply ensuring that the new animal is not infected is insufficient because Q fever is principally transmitted through the air. During outbreaks of Q fever, animal infections and contamination of the environment can be avoided or decreased by destroying placentas and fetuses (in order to avoid ingestion by wild or domestic carnivores). If possible, births must be confined to a specific location and disinfected without inducing aerosols. As in all zoonotic diseases, control of disease in animals will influence the level of disease observed in humans. Appropriate strategies of tick control and good hygiene practices can reduce environmental contamination. Fluids and infected fetal membranes, aborted fetuses, and contaminated bedding materials must be incinerated or buried after disinfection. In addition, the manure should be treated with lime or calcium cyanide (0.4 %) before spreading on fields, which should not be done in the wind (to prevent the spread of the organism over long distances). Although expensive, infected animals should be removed from herds or placed in separate confinement at delivery. Animal industry workers should be fully informed about the risk factors of contracting Q fever, and laboratories should be provided with appropriate safety devices and equipment (Fig. 12.6).
Leakage of potentially infectious materials must be decontaminated immediately by the use of 70 % ethyl alcohol, 5 % peroxide, or phenolic-based solutions. Biohazardous waste should be decontaminated by autoclaving and equipment/instruments decontaminated by disinfectants, autoclaving, or boiling for 10 min. However, the spore-like shape of C. burnetii may be resistant to normal disinfectants (e.g., sodium hypochlorite and ultraviolet radiation), heat, drying, pressure, and oxidative and osmotic stress (Table 12.3).
Pasteurization at 72 °C for 15 s and sterilizing of milk of infected flocks are regularly recommended, even if an oral route is not considered a major mode of transmission. Worryingly, the suspension of C. burnetii in aqueous solutions of 0.5 % hypochlorite, 5 % Lysol, or 5 % formalin at does not completely inactivate the agent after 24 h at 24 °C.
Q-VAX is the only type of vaccine that provides human protection against Q fever and has been widely used in Australia. The vaccine causes only minor reactions, including edema and hyperemia and, more rarely, edema at the site of inoculation .
Notes
- 1.
Taxonomic status of Amblyomma cajennense was recently reviewed by Nava et al. (2014).
- 2.
Exanthem is a rash in the skin and enanthem is a rash in mucosae (CBM).
References
Angelakis E, Raoult D (2010) Review Q fever. Vet Microbiol 140:297–309
Arricau-Bouvery N, Rodolakis A (2005) Is Q fever an emerging or re-emerging zoonosis? Vet Res 36:327–349
Brenner DJ, O’Connor SP, Winkler HH et al (1993) Proposals to unify the genera Bartonella and Rochalimaea, with descriptions of Bartonella quintana comb. nov., Bartonella vinsonii comb. nov., Bartonella henselae comb. nov., B. elizabethae comb. nov., and to remove the Family Bartonellaceae from to order Rickettsiales. Int J Syst Microbiol 43:711–715
Breitschwerdt EB, Maggi RG, Chomel BB et al (2010) Bartonellosis: an emerging infectious disease of zoonotic importance to animals and human beings. J Vet Emerg Crit Care 20:8–30
Birtles R (2005) Bartonellae as elegant hemotropic parasites. Ann N Y Acad Sci 106:3270–3279
Billeter SA, Levy MG, Chomel BB et al (2008) Vector transmission of Bartonella species with emphasis on the potential for tick transmission. Med Vet Entomol 22:1–15
Carcopino X, Raoult D, Bretelle F et al (2007) Managing Q fever during pregnancy the benefits of long-term cotrimoxazole therapy. Clin Infect Dis 45:548–555
Cazorla C, Socolovschi C, Jensenius M et al (2008) Tick-borne diseases: tick-borne spotted fever rickettsioses in Africa. Infect Dis Clin North Am 22:531–544
CDC, Centers for Disease Control and Prevention (2015) Q fever. Access http://www.cdc.gov/qfever/symptoms/index.html
Chomel BB, Boulois HJ, Maruyama S et al (2006) Bartonella spp. in pets and effect on human health. Emerg Infect Dis 12:389–394
Ducan AW, Maggi RG, Breitschwerdt EB (2007) A combined approach for the enhanced detection and isolation of Bartonella species in dogs blood samples: pre-enrichment liquid culture followed by PCR and subculture onto agar plates. J Microbiol Methods 69:273–281
Dumler JS, Barbet AF, Bekker CP et al (2001) Reorganization of genera in the families Rickettsiaceae and Anaplasmataceae in the order Rickettsiales: unification of some species of Ehrlichia with Anaplasma, Cowdria with Ehrlichia and Ehrlichia with Neorickettsia, descriptions of six new species combinations and designation of Ehrlichia equi and ‘HGE agent’ as subjective synonyms of “Ehrlichia phagocytophila”. Int J Syst Evol Microbiol 51:2145–2165
Eremeeva M, Dasch GA (2015) Challenges posed by tick-borne rickettsiae: eco-epidemiology and public health implications. Front Public Health 3:55
Georgiev M, Afonso A, Neubauer H et al (2013) Q fever in humans and farm animals in four European countries, 1982 to 2010. Euro Surveill 18:20407
Grisoli D, Million M, Edouard S et al (2014) Latent Q fever endocarditis in patients undergoing routine valve surgery. J Heart Valve Dis 23:735–743
Harms A, Dehio C (2012) Intruders below the radar: molecular pathogenesis of Bartonella spp. Clin Microbiol Rev 25:42–78
Hogerwerf L, van den Brom R, Roest HIJ et al (2011) Reduction of Coxiella burnetii prevalence by vaccination of goats and sheep, the Netherlands. Emerg Infect Dis 17:379–386
Kosoy M, Hayman DTS, Chan KS (2012) Bartonella bacteria in nature: where does population variability end and a species start? Infect Genet Evol 12:894–904
Labruna MB (2003) Brazilian spotted fever: the role of capybaras. In: Moreira JR, Ferraz KMPMB, Herrera EA et al (eds) Capybara: biology, use and conservation of an exceptional Neotropical species. Springer Science + Business Media, New York, pp 371–383
Labruna MB (2009) Ecology of Rickettsia in South America. Ann N Y Acad Sci 1166:156–166
Lamas CCA, Boia MN, Lemos ERS (2008) Human bartonellosis: seroepidemiological and clinical features with an emphasis on data from Brazil – a review. Mem Inst Oswaldo Cruz 103:221–235
Lazzerini M, Tickell D (2011) Antibiotics in severely malnourished children: systematic review of efficacy, safety and pharmacokinetics. Bull World Health Organ 89:593–606
Madariaga MG, Rezai K, Trenholme GM et al (2003) Q fever: a biological weapon in your backyard. Lancet Infect Dis 3:709–721
Maggi RG, Mascarelli PE, Pultorak EL et al (2011) Bartonella spp. bacteremia in high-risk immunocompetent patients. Diagn Microbiol Infect Dis 71:430–437
Maurin M, Raoult D (1999) Q fever. Clin Microbiol Rev 12:518–553
McClintic J, Srivastava S (2012) Imaging in the diagnosis and management of ocular cat scratch disease. Int Ophthalmol Clin 4:155–161
Miceli MH, Veryser AK, Anderson AD et al (2010) A case of person-to-person transmission of Q fever from an active duty serviceman to his spouse. Vector Borne Zoonotic Dis 10:539–541
Minick MF, Andreson BE, Lima A et al (2014) Oroya fever and verruga peruana: bartonelloses unique to South America. PLoS Negl Trop Dis 8:1–19
Nava S, Beati L, Labruna MB et al (2014) Reassessment of the taxonomic status of Amblyomma cajennense (Fabricius, 1787) with the description of three new species, Amblyomma tonelliae n. sp., Amblyomma interandinum n. sp. and Amblyomma patinoi n. sp., and reinstatement of Amblyomma mixtum Koch, 1844, and Amblyomma sculptum Berlese, 1888 (Ixodida: Ixodidae). Ticks Tick Borne Dis 5(3):252–276. doi:10.1016/j.ttbdis.2013.11.004
Nourse C, Allworth A, Jones A (2004) Three cases of Q fever osteomyelitis in children and a review of the literature. Clin Infect Dis 39:61–66
O’Neill TJ, Sargeant JM, Poljak Z (2014) The effectiveness of Coxiella burnetii vaccines in occupationally exposed populations: a systematic review and meta-analysis. Zoonoses Public Health 61:81–96
Paddock CD, Finley RW, Wright CS et al (2008) Rickettsia parkeri rickettsiosis and its clinical distinction from Rocky Mountain spotted fever. Clin Infect Dis 47(9):1188–1196
Parola P, Labruna MB, Raoult D (2009) Tick-borne rickettsioses in America: unanswered questions and emerging diseases. Curr Infect Dis Rep 11:40–50
Parola P, Paddock CD, Socolovschi C et al (2013) Update on tick-borne rickettsioses around the world: a geographic approach. Clin Microbiol Rev 26:657–702
Raoult D, Marrie T, Mege J (2005) Natural history and pathophysiology of Q fever. Lancet Infect Dis 5:219–226
Rodolakis A (2009) Q Fever in dairy animals. Rickettsiology and rickettsial diseases-fifth international conference. Ann N Y Acad Sci 1166:90–93
Sander A, Berner R, Ruess M (2001) Serodiagnosis of cat-scratch disease: response to Bartonella henselae in children and a review of diagnostic methods. Eur J Clin Microbiol Infect Dis 20:392–401
Szabó MPJ, Pinter A, Labruna MB (2013) Ecology, biology and distribution of spotted-fever tick vectors in Brazil. Front Cell Infect Microbiol 3:1–9
Walker DH (2007) Rickettsiae and rickettsial infections: the current state of knowledge. Clin Infect Dis 45:S39–S44
Further Reading
Anderson AD, Kruszon-Moran D, Loftis AD et al (2015) Seroprevalence of Q fever in the United States, 2003–2004. Am J Trop Med Hyg 92:247–255
Angelakis E, Raoult D (2014) Pathogenicity and treatment of Bartonella infections. Intern J Antimicrob Agents 44:16–25
Breitschwerdt EB, Kordick DL (2000) Bartonella infection in animals: carriership, reservoir potential, pathogenicity, and zoonotic potential for human infection. Clin Microbiol Rev 13:428–438
Centers for Disease Control and Prevention (CDC) http://www.cdc.gov/travel/diseases/typhus.htm
Guptill L (2010) Bartonellosis. Veterinary Microbiol 140:347–359
National Association of State Public Health Veterinarians and National Assembly of State Animal Health Officials (2013) Prevention and control of Coxiella burnetii infection among humans and animals: guidance for a coordinated public health and animal health response. Available from http://www.nasphv.org/Documents/Q_Fever_2013.pdf
Oteo JA, Santiago N, Sousa R et al (2014) Guías Latinoamericanas de la RIICER para el diagnóstico de las rickettsiosis transmitidas por garrapatas/Latinamerican guidelines of RIICER for diagnosis of tick-borne rickettsioses. Rev Chil Infect 31:54–65
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Ogrzewalska, M., Rozental, T., Favacho, A.R.M., de Mello Mares-Guia, M.A.M. (2017). Rickettsial Infections, Bartonella Infections, and Coxiellosis. In: Marcondes, C. (eds) Arthropod Borne Diseases. Springer, Cham. https://doi.org/10.1007/978-3-319-13884-8_12
Download citation
DOI: https://doi.org/10.1007/978-3-319-13884-8_12
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-13883-1
Online ISBN: 978-3-319-13884-8
eBook Packages: MedicineMedicine (R0)