Abstract
Defining the specific role of nitric oxide (NO) in the regulation of the immune response against cancer is not a simple task. Despite of being extensively studied, NO, reactive nitrogen species (RNS) and reactive oxygen species (ROS) still maintain their reputation of “double-edge-swords”. However, by examining key issues related to their sources, concentrations and chemical nature and, their locations and neighboring molecules that potentially will be reacting with them, we will have a more precise interpretation of the functional aspects of NO and related RNS in the context of the immune response to tumor cells and pathogenesis of cancer. Variations in the local cellular concentration of the same reactive intermediates induce different outcomes of the immune response. NO and related reactive species trigger defined signal transduction pathways in cancer, and immune-related cells in a concentration-dependent manner. NO bioavailability and NO-dependent responses are strictly functions of the reactivity of ROS with NO-forming RNS. In this chapter, we will examine the basic biology of NO and related species in the context of the immune response to cancer in both their potential role in the pathogenesis of malignancies and also in the control and modulation of the immune response against tumor cells. We will discuss the potential use of NO and related species in the induction of specific anti-cancer activity by the immune system and the modulation of resistance or tolerogenic factors derived from the protective mechanism acquired by the tumor cells in order to evade the anti-tumor immune response .
In loving Memory of Professor Hermes Augusto Garbán Mendoza (August 28, 1936–October 08, 2013)
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Immunosensitization
- Immune response
- Apoptosis
- Immunomo-dulation
- Reactive oxygen species (ROS)
- Reactive nitrogen species (RNS)
- Immunotherapy
- Cancer therapy
- Tumor immunobiology
- Cancer pathogenesis
Introduction
Direct implications of nitric oxide (NO) or related species in the regulation of the immune response against malignancies have been addressed from various angles. However, when we refer to NO we are not addressing a single type of molecule, we are referring to a milieu of reactive molecules termed reactive nitrogen species (RNS) with different chemical and biochemical properties and significant diverse biological functions.
In order to understand the role of these RNS in the induction and regulation of the immune system against malignancies (cancer), it is always useful to consider their sources (e.g., endogenous or exogenous), their concentrations and chemical nature and, their locations and neighboring molecules that potentially will be reacting with them. Nevertheless, the role of RNS such as NO in cancer is not limited to the elimination or control of cancerous cells via activation or modulation of the immune system (directly or indirectly), NO may contribute with the pathogenesis of cancer as well .
NO has recently joined the clinical arena of cancer therapy. There is an increasing amount of preclinical data supporting the specific role of NO in the sensitization of resistant cancerous cells to radio-, chemo-, and immunotherapy . In addition, novel targeted immunotherapeutic alternatives have been developed based on nitroergic modifications of proteins in order to increase the antigenic determinant domains and revealing new immunological targets.
NO can also act in the modulation of the immune system by the enhancement of tumor-specific immune response and the sensitization of resistant tumor cells to immune-related effector mechanisms by regulating the expression of immune response-related genes including those bellowing to the tumor necrosis factor (TNF) receptor family and, inflammatory cytokines and chemokines.
Despite its importance, the specific role of NO signaling in immunity and cancer has remained elusive and many controversial data found in the literature contributes to the difficulty in understanding the specific role of NO against cancer. A broad spectrum of activities has been assigned to either the physiology or the patho-physiology of NO in tumor cells.
Approximately half of the scientific literature will support the general role of NO on the pathogenesis of cancer and the other half will support the role of NO and related species as anti-cancer molecules. This functional dichotomy of NO in cancer could be settled by examining these studies under the criteria abovementioned: sources, concentration and chemical nature and, location of neighboring molecules to react. Understanding this functional landscape of NO and related species, immunity and cancer will contribute to the better design of preventive means and more specific therapeutic alternatives in oncology.
Herein, we will examine the basic biology of NO and related species in the context of the immune response to cancer, in both their potential role in the pathogenesis of malignancies and also in the control and modulation of the immune response against cancer. We will discuss the potential use of NO and related species in the induction of specific anti-cancer activity by the immune system and the modulation of resistance or tolerogenic factors derived from the protective mechanisms acquired by the tumor cells in order to evade the anti-tumor immune response.
Nitric Oxide: Basic Concepts
Nitric oxide is a diatomic molecule that plays important roles as the smallest pleiotropic signaling messenger in mammalian cells [1]. The free radical, NO•, is an uncharged molecule containing an unpaired electron, enabling it to undergo several reactions functioning either as a weak oxidant or as an anti-oxidant. NO• is able to react with other inorganic molecules (e.g., oxygen, superoxide or transition metals), nucleic acids (e.g., pyrimidine bases), prosthetic groups (e.g., heme) or with proteins leading to S-nitrosylation of thiol groups, nitration of tyrosine residues or disruption of metal−sulfide clusters such as zinc-finger domains or iron−sulfide complexes [2]. NO can function as an anti-oxidant against reactive oxygen species (ROS) such as hydrogen peroxide (H2O2) and superoxide (O2 −) by diffusing and concentrating into the hydrophobic core of lipids [3]. In addition, NO can react with O2 − to form peroxynitrite (ONOO−), a highly oxidizing and nitrating reactive nitrogen species (RNS) responsible for mediating protein oxidation reactions under physiological conditions [4]. Noteworthy, one mechanism of NO-related reactivity is through the addition of an NO group to the thiol side chain of cysteine residues within proteins and peptides, termed S-nitrosylation, which plays a significant role in the ubiquitous influence of NO on cellular signal transduction [5].
NO is biologically synthesized by nitric oxide synthases (NOS). NOS catalyze the oxidation of L-arginine resulting in the formation of NO and L-citrulline. NO is produced by three different NOS, two of which are generally constitutively expressed, primarily in neurons (nNOS or Type I) and endothelial cells (eNOS or Type III), respectively [6–8]. An inducible isoform (iNOS or Type II) can be upregulated considerably in immune cells and many other tissues [9, 10]. It has been shown that IFN-γ alone or in combination with TNF-α, interleukin 1β (IL-1β) and bacterial lipopolysaccharide (LPS) can induce the expression of iNOS in a wide variety of tissue organs and in some tumor cell lines [11–13]. The inducible type of nitric oxide synthase (iNOS) is considered to be a central protein in the regulation of the immune response against tumor cells [14, 15].
Nitric Oxide and Immunity
Nitric oxide is an important component of the immune system. Early studies have shown that a substance that was released by macrophages and exhibited a wide range of pathogen toxicity and antitumor activity also required arginine for its production (Hibbs and coworkers) [16, 17]. These data supported an earlier observation that plasma levels of nitrite and nitrates increased upon infection, suggesting an increase in endogenous production of NO [18]. Furthermore, a pivotal connection between NO and the immune response was the observation that IL-2-mediated immune activation increased NO levels in patients and promoted tumor eradication in mice [19, 20]. Moreover, significant evidence that macrophages made nitrite and nitrate, as well as nitrosamines, was reported by a number of groups [21–23]. Studies by Stuehr and Nathan [24] have shown that NO generated by macrophages could kill leukemia cells. In addition, it has been demonstrated the formation of iron-NO complexes within activated macrophages [25]. Although some of these studies are referring to the direct toxicity of NO on infectious pathogens or their cellular components, the large majority of these studies have demonstrated an active NO-related anti-tumor immune response .
The Ca2+/CaM-independent inducible isoform iNOS is found in various cell types including macrophages, dendritic cells , fibroblasts, chondrocytes, osteoclasts, astrocytes, epithelial cells, and a variety of cancer cells. iNOS is generally associated with the immune system and is stimulated and upregulated via induction by cytokines and/or microbial agents such as LPS and is responsible for generating large amounts of NO sustained over long periods of time for the host defense against pathogens [26].
NO produced by iNOS within the cell can range from 10 nM to µM amounts for several days [27]. This generation of high levels of NO can control various NO-modulated effects within a tissue, each with potentially different functions. Therefore, induction of iNOS is not only characterized by the generation of NO in high local amounts, it can also generate a wide range of NO for variable periods of time [28]. iNOS provides a unique flexible response to a variety of immunological challenges.
An additional level of immune regulation by iNOS is its capacity to generate products other than NO. These include N-hydroxyarginine (NOHA) and O2 −. The generation of NOHA by iNOS has been shown to inhibit arginase (AG) activity, affecting the pathways that mediate cell growth (ornithine to polyamines) or tissue matrices (ornithine to proline) [29]. This diversity of NOS activities can produce different temporal and concentration profiles of NO as well as other products to facilitate and broaden the functional versatility of these enzymes during the immune response [30].
Regulation of Immunological Apoptosis-related Genes: The NF-κB Case
The most relevant transcription factor participating in the regulation of genes involved in apoptosis and the immune response is the nuclear factor kappa B (NF-κB) promoting the expression of anti-apoptotic genes and regulating pro-inflammatory cytokine expression [31–34].
NF-κB transcription factors are assembled through the dimerization of five subunits: RelA (p65), c-Rel, RelB, p50/NF-κB1 and p52/NF-κB2 [35]. In resting –unstimulated– state, most NF-κB dimmers are sequestered in the cytoplasm by binding to specific inhibitors IκBs. Cell stimulation activates the IκB kinase (IKK) complex. Activated IKK phosphorylates NF-κB-bound IκB proteins and targets them for polyubiquitination and rapid proteasome-mediated degradation [36]. Freed NF-κB dimers translocate to the nucleus where they control the transcriptional activation of several target genes in concert with other transcription factors [37–39].
For many of the immune pathways that are regulated by NO and ROS, NF-κB is critical in orchestrating the innate immune response outcomes [40, 41]. NF-κB is an oxidative stress-responsive transcription factor activated by reactive oxygen species (e.g., H2O2, O• 2 −, etc.) generated as part of the signaling cascade triggered by many molecules such as TNF-α [42, 43]. ROS have been implicated in the signaling pathways initiated by TNF-α. Stimulation of mammalian cells with TNF-α triggers the generation of various ROS [44, 45]. Moreover, the use of antioxidants resulted in the inhibition of various TNF-α-related effects, such as the activation of transcription factors, gene expression, and cytotoxicity, and exogenous ROS mimic TNF-α biological activity [46]. In biological systems the most important ROS generated upon TNF-α stimulation are the result of enzymatic partial reduction of oxygen yielding O• 2 −, which is immediately disproportionated by superoxide dismutase (SOD) to H2O2 and O2 or rapidly reacts with NO generating ONOO− [47–49].
It has been shown that NO sensitizes malignant cells to TNF-α-mediated apoptosis through the specific disruption of the TNF-α-induced generation of H2O2 and the subsequent inhibition of the NF-κB-dependent expression of anti-apoptotic genes [50].
In addition, NF-κB can be regulated by NO or related molecules via inhibition of its activation. It was originally suggested that NO stabilized the NF-κB inhibitor, IκBα, by preventing its degradation from NF-κB. NO also increased the mRNA expression of IκBα, but not NF-κB subunits, p65 or p50, suggesting specific transcriptional induction of IκBα by NO [51]. Also, NF-κB can be inhibited directly by NO through S-nitrosylation (SNO) of the p50 subunit. This SNO modification of NF-κB has been shown to prevent binding to its target DNA site [52, 53].
NO can act directly or indirectly on the transcriptional machinery, orchestrating the expression of apoptosis/survival genes related to the immune response against cancer, either by affecting the signaling molecules that will activate or repress transcription factors or by directly modifying key transcription factors and their DNA binding activity. It can be also cGMP dependent or independent following the general principles of “small concentrations” of NO, in a tight cellular environment NO will tend to favor a cGMP-dependent mechanism of regulation, whereas “high concentrations” of NO will trigger a cGMP-independent set of actions.
Deregulation of the expression of genes involved in apoptosis and immune response has been shown to be a critical aspect in determining the development and progression of numerous cancer types. Therefore, understanding the molecular mechanism involved in the control of apoptosis-related gene expression might facilitate the development of targeted anti-tumor therapies.
The dynamic coordination of genetic factors plays a major role in the regulation of apoptosis-related gene expression associated to the immune system under physiological or pathophysiological conditions. Uncontrolled activation of several transcription factors regulating the expression of genes involved in either pro-apoptotic or anti-apoptotic pathways have been identified as key players in the acquisition of the resistant phenotype of tumor cells. Among these transcription factors, we have examined the specific role of NO on the activity of the NF-κB as one of the most important regulators of anti-apoptotic gene expression and immune response. Nevertheless, there are other important factors such as yin-yang 1 (YY1) as a novel regulator (transcriptional repressor) of pro-apoptotic receptors and immune regulator, p53 as a key modulator of cell cycle and pro-apoptosis pathways and FOXP3 as a novel tolerogenic and apoptosis-resistance regulator in tumor cells and immune related cells. Thus, specific targeting of these genetic factors by NO or related species regulating the tumor cell sensitivity to apoptosis represents a plausible therapeutic alternative that can be used alone or in combination with already established anti-cancer immunotherapy [54].
Nitric Oxide: Pathogenesis of Cancer
The specific roles of NO in the immune responses and immunotherapy do not escape from controversy. As we have stated in previous sections, there are confounding data that can mislead the possible role of NO in the control of the immune response against cancer. On one hand, we have the role of NO inducing suppression of the immune system by increasing the killing of tumor reactive T cells, activating suppressive mechanisms or inducing the proliferation of T regulatory cells [55–57].
NO is also involved in immunosuppression by regulating circulating immune cells. For example, myeloid-derived suppressor cells (MDSCs) can be activated by NO-mediated increases in cGMP, which in turn, facilitates their binding to Cytotoxic T lymphocytes (CTLs) and reduces T cell proliferation [58]. When cell-to-cell contacts are formed, the expression of AG and iNOS are required to induce apoptosis [59]. Increased iNOS activity is also found in mature dendritic cells (DCs), where NO is associated with suppression of T cell proliferation. Furthermore, when activated by IFN-γ, TNF-α, or IL-1α/β, MDSCs produce chemokines and iNOS, which lead to the immunosuppression of those T cells in the vicinity of the MDSCs [60]. The resulting increase in chemokines and iNOS leads to the attenuation of T cell responsiveness. In general, T cell responses are decreased by NO.
Nitric Oxide, RNS, ROS and Anti-Cancer Therapy
Oxidative stress, a major component of the immune response, is associated with infection, inflammation, aging, etc. Clinically, a milieu of conditions is associated with oxidative damage including chronic inflammatory and autoimmune diseases, cancer, and age-related disorders [61–65]. As mentioned above, oxidative stress is mediated in its majority by reactive oxygen species (ROS) and reactive nitrogen species (RNS) among others. ROS are oxygen-based molecules possessing high chemical reactivity. These include biologically-produced free radicals (superoxide and hydroxyl radical, NO, etc) and non-radical species such as hydrogen peroxide and peroxynitrite [66].
Free radicals are reactive chemical species containing one or more unpaired electrons occupying an outer orbital. They can arise either by the univalent pathway of oxygen reduction or as a consequence of enzymatic/non-enzymatic reactions. The superoxide anion radical O2 − is formed by the one electron reduction of O2. The two electron reduction product of O2 in the fully protonated form is H2O2 while the three electron reduction product of O2 is the hydroxyl radical (OH•). A number of enzymatic and non-enzymatic reactions reduces oxygen to the more reactive superoxide radical. Though hydrogen peroxide is not a free radical by itself, it can lead to the formation of the more dangerous hydroxyl radical via the Fenton type reaction [67].
Exposure of proteins to ROS and RNS alters their composite amino acids and structure thereby generating neo-antigens (a neo-antigen being typically defined as a previously unrecognized host-derived protein which becomes immunogenic usually due to new physical or genetic modifications). However, the oxidative damage to biomolecules is rarely specific and is dependent on the concentration of the protein, its cellular location with respect to cellular oxidant generating systems and the rate of modified protein clearance [66, 68].
While the direct role of free radicals in causing oxidative damage at the molecular level has been known for decades, the extent to which oxidative damage alters tissue/organ function is still under intense research. In immunology, oxidative damage has been implicated in several autoimmune diseases, including systemic lupus erythematosus (SLE) where aberrant immune responses against neo-antigens suggest impairment of immune tolerance mechanisms (Reviewed in [66]). Factors that induce the formation of neo-antigens include inflammation, infection, drugs, ROS, and environmental factors.
Initial results indicate that the adaptive immune response is indeed enhanced by oxidative processes. With regards to humoral immunity, co-administration of oxidized carbohydrates with antigen increases the secretion of antigen-specific immunoglobulins. Parallel studies of T cell-dependent immune responses demonstrate similar increases in responsiveness when using the Schiff base-forming agent tucaresol during immunization [69]. Furthermore, endogenous NO generation by cytokine induction in immune-related cells and exogenous NO (provided locally by NO-releasing compounds) have been demonstrated to be essential for the priming of the immune response (T cell priming) against specific antigens and some tumor associated antigens (TAAs) [57, 70].
From Autoimmunity to Cancer Therapy
Autoimmune disorders display a spectrum of severities and durations. On one end, improvements in treatment options have allowed patients to enjoy qualities and durations of life nearly identical to those observed in healthy individuals for some forms of autoimmunity. On the other end of the spectrum, certain autoimmune disorders are devastatingly aggressive, incurring intense periods of tissue destruction, pain, and the shortening of life expectancy to as little as 6 months post diagnosis. Research conducted over the past few decades has focused on identifying many of the environmental and genetic risk factors associated with autoimmunity. The identification of the T cell surface protein cytotoxic T lymphocyte antigen 4 (or CTLA-4) is one of the most interesting discoveries in this field. CTLA-4 serves to inhibit T cell immune responses and competes with the activator protein CD28 for the same ligands, CD80 and CD86 [71]. More recently, blockade of CTLA-4 in cancer patients using monoclonal antibodies has emerged as one of the last lines of therapy against chemotherapy-resistant tumors. The anti-cancer activity of CTLA-4 blockade is believed to arise from subsequent immunological recognition and response against previously “masked” cancer neo-antigens, illustrating the potential of neo-antigen-revealing immunotherapy in combating cancer [72, 73].
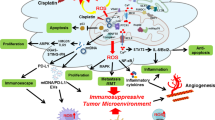
Final Remarks, Conclusions
Although extensively studied, the roles of NO and related species in the immunological outcome of cancer still remains as a debatable issue. In order to understand and sort the most realistic interpretation of the data and previous studies, we have to consider the broad spectrum of activities that have been assigned to either the physiology or the pathophysiology of NO in tumor cells (for a review, see [74]. First, we have to consider the amount and sources of NO, RNS and ROS generated. Low-output of NO has been correlated with increased blood flow and new blood vessels (angiogenesis) feeding the tumor area [75]. In addition, the generation of NO by tumor cells may inhibit the activation and proliferation or increase apoptosis of surrounding lymphocytes that can account for the immune suppression observed that accompanies tumor growth. Furthermore, high intratumoral-output of NO could inhibit the activation of caspases and therefore antagonizes the pro-apoptotic signals [76, 77]. However, the opposite effect also has been observed in many other systems whereby the generation of high-output of NO, either by iNOS induction or by the use of NO donors, inhibits tumor growth, metastasis and sensitize to immunotherapy [11, 16, 50, 78, 79]. Therefore, the final outcome of NO-mediated signaling will be determined by many factors including the local concentration and sources of NO in the tissue, and the presence of reactive molecules that might redirect the redox status in the cell with the potential of synergize with other anticancer therapeutic modalities and the development of innovative NO-based therapies.
Conflict of Interest
Dr. Hermes J. Garbán is currently the Head of Therapeutic Antibody Discovery and fully employed at NantBioscience, Inc. an affiliate of NantWorks, LLC.
Abbreviations
- AG:
-
Arginase
- Ca2+ :
-
Calcium
- CaM:
-
Calmodulin
- CD#:
-
Cluster of differentiation #
- cGMP:
-
Cyclic guanosine monophosphate
- CTL:
-
Cytotoxic T lymphocytes
- CTLA4:
-
Cytotoxic T lymphocyte antigen 4
- DC:
-
Dendritic cells
- eNOS:
-
Endothelial nos
- FOXP3:
-
Forkhead box P3
- H2O2 :
-
Hydrogen peroxide
- IFN-γ:
-
Interferon gamma
- IKB:
-
Inhibitors kappa B
- IKK:
-
IκB kinase
- IL-β:
-
Interleukin 1 beta
- iNOS:
-
Inducible nos
- LPS:
-
Lipopolysaccharide
- MDSC:
-
Myeloid derived suppressor cells
- NF-κB:
-
Nuclear factor kappa
- nNOS:
-
Neuronal NOS
- NO:
-
Nitric oxide
- NOHA:
-
N hydroxyarginine
- NOS:
-
Nitric oxide synthase
- O2 − :
-
Superoxide
- OH• :
-
Hydroxyl radical
- ONOO−,:
-
Peroxynitrite
- ROS:
-
Reactive oxygen species
- RNS:
-
Reactive nitrogen species
- SLE:
-
Systemic lupus erythematosus
- SNO:
-
S-nitrosylation
- SOD:
-
Superoxide dismutase
- TAA:
-
Tumor associated antigens
- TNF:
-
Tumor necrosis factor
- TNF-α:
-
TNF Qlpha
- YY1:
-
Yin-yang 1
References
Nathan C. Nitric oxide as a secretory product of mammalian cells. Faseb J. 1992;6(12):3051–64.
Bogdan C. Nitric oxide and the regulation of gene expression. Trends Cell Biol. 2001;11(2):66–75.
Benz D, Cadet P, Mantione K, Zhu W, Stefano G. Tonal nitric oxide and health–a free radical and a scavenger of free radicals. Med Sci Monit. 2002;8(1):RA1–4.
Tuteja N, Chandra M, Tuteja R, Misra MK. Nitric oxide as a unique bioactive signaling messenger in physiology and pathophysiology. J Biomed Biotechnol. 2004;2004(4):227–37.
Hess DT, Matsumoto A, Kim SO, Marshall HE, Stamler JS. Protein S-nitrosylation: purview and parameters. Nat Rev Mol Cell Biol. 2005;6(2):150–66.
Knowles RG, Moncada S. Nitric oxide synthases in mammals. Biochem J. 1994;298(Pt 2): 249–58.
Mayer B, Hemmens B. Biosynthesis and action of nitric oxide in mammalian cells [published erratum appears in Trends Biochem Sci 1998 Feb;23(2):87]. Trends Biochem Sci. 1997;22(12):477–81.
Nathan C, Xie QW. Regulation of biosynthesis of nitric oxide. J Biol Chem. 1994;269(19):13725–8.
Schmidt HH, Walter U. NO at work. Cell. 1994;78(6):919–25.
Xie Q, Nathan C. The high-output nitric oxide pathway: role and regulation. J Leukoc Biol. 1994;56(5):576–82.
Garban HJ, Bonavida B. Nitric oxide sensitizes ovarian tumor cells to Fas-induced apoptosis. Gynecol Oncol. 1999;73(2):257–64.
Geller DA, Nussler AK, Di Silvio M, Lowenstein CJ, Shapiro RA, Wang SC, Simmons RL, Billiar TR. Cytokines, endotoxin, and glucocorticoids regulate the expression of inducible nitric oxide synthase in hepatocytes. Proc Natl Acad Sci U S A. 1993;90(2):522–6.
Sherman PA, Laubach VE, Reep BR, Wood ER. Purification and cDNA sequence of an inducible nitric oxide synthase from a human tumor cell line. Biochemistry. 1993;32(43):11600–5.
Hokari A, Zeniya M, Esumi H. Cloning and functional expression of human inducible nitric oxide synthase (NOS) cDNA from a glioblastoma cell line A-172. J Biochem (Tokyo). 1994;116(3):575–81.
Langrehr JM, Hoffman RA, Lancaster JR Jr, Simmons RL. Nitric oxide–a new endogenous immunomodulator. Transplantation. 1993;55(6):1205–12.
Hibbs JB, Jr, Taintor RR, Vavrin Z. Macrophage cytotoxicity: role for L-arginine deiminase and imino nitrogen oxidation to nitrite. Science. 1987;235(4787):473–6.
Hibbs JB, Jr, Taintor RR, Vavrin Z, Rachlin EM. Nitric oxide: a cytotoxic activated macrophage effector molecule. Biochem Biophys Res Commun. 1988;157(1):87–94.
Green LC, Ruiz de Luzuriaga K, Wagner DA, R and W, Istfan N, Young VR, Tannenbaum SR. Nitrate biosynthesis in man. Proc Natl Acad Sci U S A. 1981;78(12):7764–8.
Hibbs JB, Jr, Westenfelder C, Taintor R, Vavrin Z, Kablitz C, Baranowski RL, Ward JH, Menlove RL, McMurry MP, Kushner JP, et al. Evidence for cytokine-inducible nitric oxide synthesis from L-arginine in patients receiving interleukin-2 therapy. J Clin Invest. 1992;89(3):867–77.
Yim CY, McGregor JR, Kwon OD, Bastian NR, Rees M, Mori M, Hibbs JB, Jr, Samlowski WE. Nitric oxide synthesis contributes to IL-2-induced antitumor responses against intraperitoneal Meth A tumor. J Immunol. 1995;155(9):4382–90.
Stuehr DJ, Marletta MA. Mammalian nitrate biosynthesis: mouse macrophages produce nitrite and nitrate in response to Escherichia coli lipopolysaccharide. Proc Natl Acad Sci U S A. 1985;82(22):7738–42.
Marletta MA. Mammalian synthesis of nitrite, nitrate, nitric oxide, and N-nitrosating agents. Chem Res Toxicol. 1988;1(5):249–57.
Kosaka H, Wishnok JS, Miwa M, Leaf CD, Tannenbaum SR. Nitrosation by stimulated macrophages. Inhibitors, enhancers and substrates. Carcinogenesis. 1989;10(3):563–6.
Stuehr DJ, Nathan CF. Nitric oxide. A macrophage product responsible for cytostasis and respiratory inhibition in tumor target cells. J Exp Med. 1989;169(5):1543–55.
Lancaster JR Jr, Hibbs JB Jr. EPR demonstration of iron-nitrosyl complex formation by cytotoxic activated macrophages. Proc Natl Acad Sci U S A. 1990;87(3):1223–7.
Blaise GA, Gauvin D, Gangal M, Authier S. Nitric oxide, cell signaling and cell death. Toxicology. 2005;208(2):177–92.
Thomas DD, Ridnour LA, Isenberg JS, Flores-Santana W, Switzer CH, Donzelli S, Hussain P, Vecoli C, Paolocci N, Ambs S, Colton CA, Harris CC, Roberts DD, Wink DA. The chemical biology of nitric oxide: implications in cellular signaling. Free Radic Biol Med. 2008;45(1):18–31.
Espey MG, Miranda KM, Pluta RM, Wink DA. Nitrosative capacity of macrophages is dependent on nitric-oxide synthase induction signals. J Biol Chem. 2000;275(15):11341–7.
Boucher JL, Custot J, Vadon S, Delaforge M, Lepoivre M, Tenu JP, Yapo A, Mansuy D. N omega-hydroxyl-L-arginine, an intermediate in the L-arginine to nitric oxide pathway, is a strong inhibitor of liver and macrophage arginase. Biochem Biophys Res Commun. 1994;203(3):1614–21.
Wink DA, Hines HB, Cheng RY, Switzer CH, Flores-Santana W, Vitek MP, Ridnour LA, Colton CA. Nitric oxide and redox mechanisms in the immune response. J Leukoc Biol. 2011;89(6):873–91.
Liu ZG, Hsu H, Goeddel DV, Karin M. Dissection of TNF receptor 1 effector functions: JNK activation is not linked to apoptosis while NF-kappaB activation prevents cell death. Cell. 1996;87(3):565–76.
Beg AA, Baltimore D. An Essential Role for NF-kB in Preventing TNF-alpha-Induc Cell Death. Science. 1996;274(1):782–4.
Van Antwerp DJ Martin SJ Kafri T Green DR Verma IM. Suppression of TNF-alpha-induced apoptosis by NF-kappaB. Science. 1996;274(5288):787–9.
Wang CY, Mayo MW, Baldwin AS Jr. TNF—and cancer therapy-induced apoptosis: potentiation by inhibition of NF-kappaB [see comments]. Science. 1996;274(5288):784–7.
Ghosh S, Karin M. Missing pieces in the NF-kappaB puzzle. Cell. 2002;109:S81–96.
Karin M, Ben-Neriah Y. Phosphorylation meets ubiquitination: the control of NF-[kappa]B activity. Annu Rev Immunol. 2000;18:621–63.
Werner SL, Barken D, Hoffmann A. Stimulus specificity of gene expression programs determined by temporal control of IKK activity. Science. 2005;309(5742):1857–61.
Park JM, Greten FR, Wong A, Westrick RJ, Arthur JS, Otsu K, Hoffmann A, Montminy M, Karin M. Signaling pathways and genes that inhibit pathogen-induced macrophage apoptosis–CREB and NF-kappaB as key regulators. Immunity. 2005;23(3):319–29.
Covert MW, Leung TH, Gaston JE, Baltimore D. Achieving stability of lipopolysaccharide-induc NF-kappaB activation. Science. 2005;309(5742):1854–7.
Baeuerle PA, Henkel T. Function and activation of NF-kappa B in the immune system. Annu Rev Immunol. 1994;12:141–79.
Gerondakis S, Fulford TS, Messina NL, Grumont RJ. NF-kappaB control of T cell development. Nat Immunol. 2014;15(1):15–25.
Hong YH, Peng HB, La Fata V, Liao JK. Hydrogen peroxide-mediated transcriptional induction of macrophage colony-stimulating factor by TGF-beta1. J Immunol. 1997;159(5): 2418–23.
Schreck R, Albermann K, Baeuerle PA. Nuclear factor kappa B: an oxidative stress-responsive transcription factor of eukaryotic cells (a review). Free Radic Res Commun. 1992;17(4): 221–37.
Garcia-Ruiz C, Colell A, Mari M, Morales A, Fernandez-Checa JC. Direct effect of ceramide on the mitochondrial electron transport chain leads to generation of reactive oxygen species. Role of mitochondrial glutathione. J Biol Chem. 1997;272(17):11369–77.
Hennet T, Richter C, Peterhans E. Tumour necrosis factor-alpha induces superoxide anion generation in mitochondria of L929 cells. Biochem J. 1993;289(Pt 2):587–92.
Suzuki YJ, Forman HJ, Sevanian A. Oxidants as stimulators of signal transduction. Free Radic Biol Med. 1997;22(1–2):269–85.
Huie RE, Padmaja S. The reaction of no with superoxide. Free Radic Res Commun. 1993;18(4):195–9.
Fukuto JM, Wink DA. Nitric oxide (NO): formation and biological roles in mammalian systems. Met Ions Biol Syst. 1999;36:547–95.
Szabo C, Ohshima H. DNA damage induced by peroxynitrite: subsequent biological effects. Nitric Oxide. 1997;1(5):373–85.
Garban HJ, Bonavida B. Nitric oxide disrupts H2O2-dependent activation of nuclear factor kappa B. Role in sensitization of human tumor cells to tumor necrosis factor-alpha -induced cytotoxicity. J Biol Chem. 2001;276(12):8918–23.
Peng HB, Libby P, Liao JK. Induction and stabilization of I kappa B alpha by nitric oxide mediates inhibition of NF-kappa B. J Biol Chem. 1995;270(23):14214–9.
DelaTorre A, Schroeder RA, Kuo PC. Alteration of NF-kappa B p50 DNA binding kinetics by S-nitrosylation. Biochem Biophys Res Commun. 1997;238(3):703–6.
Matthews JR, Botting CH, Panico M, Morris HR, Hay RT. Inhibition of NF-kappaB DNA binding by nitric oxide. Nucleic Acids Res. 1996;24(12):2236–42.
Olson SY, Garban HJ. Regulation of apoptosis-related genes by nitric oxide in cancer. Nitric Oxide. 2008;19(2):170–6.
Brito C, Naviliat M, Tiscornia AC, Vuillier F, Gualco G, Dighiero G, Radi R, Cayota AM. Peroxynitrite inhibits T lymphocyte activation and proliferation by promoting impairment of tyrosine phosphorylation and peroxynitrite-driven apoptotic death. J Immunol. 1999;162(6):3356–66.
Chen H, Li M, Campbell RA, Burkhardt K, Zhu D, Li SG, Lee HJ, Wang C, Zeng Z, Gordon MS, Bonavida B, Berenson JR. Interference with nuclear factor kappa B and c-Jun NH(2)-terminal kinase signaling by TRAF6C small interfering RNA inhibits myeloma cell proliferation and enhances apoptosis. Oncogene. 2006;25:6520–7.
Niedbala W, Wei XQ, Piedrafita D, Xu D, Liew FY. Effects of nitric oxide on the induction and differentiation of Th1 cells. Eur J Immunol. 1999;29(8):2498–505.
Serafini P, Meckel K, Kelso M, Noonan K, Califano J, Koch W, Dolcetti L, Bronte V, Borrello I. Phosphodiesterase-5 inhibition augments endogenous antitumor immunity by reducing myeloid-derived suppressor cell function. J Exp Med. 2006;203(12):2691–702.
Talmadge JE. Pathways mediating the expansion and immunosuppressive activity of myeloid-derived suppressor cells and their relevance to cancer therapy. Clin Cancer Res. 2007;13(18 Pt 1):5243–8.
Ren G, Zhang L, Zhao X, Xu G, Zhang Y, Roberts AI, Zhao RC, Shi Y. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell. 2008;2(2):141–50.
Csiszar A, Toth J, Peti-Peterdi J, Ungvari Z. The aging kidney: role of endothelial oxidative stress and inflammation. Acta Physiol Hung. 2007;94(1–2):107–15.
Federico A, Morgillo F, Tuccillo C, Ciardiello F, Loguercio C. Chronic inflammation and oxidative stress in human carcinogenesis. Int J Cancer. 2007;121(11):2381–6.
Hald A, Lotharius J. Oxidative stress and inflammation in Parkinson’s disease: is there a causal link? Exp Neurol. 2005;193(2):279–90.
Muller FL, Lustgarten MS, Jang Y, Richardson A, Van Remmen H. Trends in oxidative aging theories. Free Radic Biol Med. 2007;43(4):477–503.
Weiskopf D, Schwanninger A, Weinberger B, Almanzar G, Parson W, Buus S, Lindner H, Grubeck-Loebenstein B. Oxidative stress can alter the antigenicity of immunodominant peptides. J Leukoc Biol. 2010;87(1):165–72.
Kurien BT, Scofield RH. Autoimmunity and oxidatively modified autoantigens. Autoimmun Rev. 2008;7(7):567–73.
Cave AC, Brewer AC, Narayanapanicker A, Ray R, Grieve DJ, Walker S, Shah AM. NADPH oxidases in cardiovascular health and disease. Antioxid Redox Signal. 2006;8(5–6):691–728.
Griffiths HR. Is the generation of neo-antigenic determinants by free radicals central to the development of autoimmune rheumatoid disease? Autoimmun Rev. 2008;7(7):544–9.
Charo J, Lindencrona JA, Carlson LM, Hinkula J, Kiessling R. Protective efficacy of a DNA influenza virus vaccine is markedly increased by the coadministration of a Schiff base-forming drug. J Virol. 2004;78(20):11321–6.
Koncz A, Pasztoi M, Mazan M, Fazakas F, Buzas E, Falus A, Nagy G. Nitric oxide mediates T cell cytokine production and signal transduction in histidine decarboxylase knockout mice. J Immunol. 2007;179(10):6613–9.
Chambers CA, Kuhns MS, Egen JG, Allison JP. CTLA-4-mediated inhibition in regulation of T cell responses: mechanisms and manipulation in tumor immunotherapy. Annu Rev Immunol. 2001;19:565–94.
Naidoo J, Page DB, Wolchok JD. Immune modulation for cancer therapy. Br J Cancer. 2014;111:2214–9.
Wolchok JD, Hodi FS, Weber JS, Allison JP, Urba WJ, Robert C, O’Day SJ, Hoos A, Humphrey R, Berman DM, Lonberg N, Korman AJ. Development of ipilimumab: a novel immunotherapeutic approach for the treatment of advanced melanoma. Ann N Y Acad Sci. 2013;1291:1–13.
Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43(2):109–42.
Jenkins DC, Charles IG, Thomsen LL, Moss DW, Holmes LS, Baylis SA, Rhodes P, Westmore K, Emson PC, Moncada S. Roles of nitric oxide in tumor growth. Proc Natl Acad Sci U S A. 1995;92(10):4392–6.
Liu L, Stamler JS. NO: an inhibitor of cell death. Cell Death Differ. 1999;6(10):937–42.
Liu H, Sidiropoulos P, Song G, Pagliari LJ, Birrer MJ, Stein B, Anrather J, Pope RM. TNF-alpha gene expression in macrophages: regulation by NF-kappa B is independent of c-Jun or C/EBP beta. J Immunol. 2000;164(8):4277–85.
Shi Y, Lee JS, Galvin KM. Everything you have ever wanted to know about Yin Yang 1. Biochem Biophys Acta. 1997;1332(2):F49–66.
Garban HJ, Bonavida B. Nitric oxide inhibits the transcription repressor Yin-Yang 1 binding activity at the silencer region of the Fas promoter: a pivotal role for nitric oxide in the up-regulation of Fas gene expression in human tumor cells. J Immunol. 2001;167(1):75–81.
Acknowledgements
This study was partially supported by a private development grant given by California Capital Equity to H.J.G. (CCE-20131672) and an institutional support from the Division of Dermatology and the Department of Medicine of the University of California, Los Angeles (UCLA).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Conflict of Interest
Conflict of Interest
Dr. Hermes J. Garbán is currently the Head of Therapeutic Antibody Discovery and fully employed at NantBioscience, Inc. an affiliate of NantWorks, LLC.
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Garbán, H. (2015). Nitric Oxide, Immunity and Cancer: From Pathogenesis to Therapy. In: Bonavida, B. (eds) Nitric Oxide and Cancer: Pathogenesis and Therapy. Springer, Cham. https://doi.org/10.1007/978-3-319-13611-0_9
Download citation
DOI: https://doi.org/10.1007/978-3-319-13611-0_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-13610-3
Online ISBN: 978-3-319-13611-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)