Abstract
Age-related hearing loss (ARHL) is an exacerbation in the auditory receptor of the accumulation of cellular damage, characteristic of the aging process. In the cochlea, aging damage is likely aggravated by exceptional metabolic requirements and lifelong exposure to noise and other environmental damaging factors, on a background of genetic susceptibility. Genomic damage and instability, along with impaired epigenetic regulation and protein homeostasis, likely are primary causes of cellular aging (Cell 153:1194–1217, 2013). Relative contributions to ARHL still are unclear, although mutations in the mitochondrial genome seem particularly relevant. Primary damage mechanisms trigger defensive responses whose exhaustion leads to failure in cell function associated with aging. Relevant to ARHL are signaling pathways adapting cell growth and metabolism to ongoing needs, including those derived from damage. Insulin-like growth factor-1 (IGF-1), part of a major anabolic regulatory pathway, declines greatly in ARHL. Low levels of sirtuins, enzymes involved in catabolic control by regulating NAD+ levels, are also linked to ARHL. Beneficial effects of caloric restriction in ARHL may be mediated through sirtuin regulation of antioxidation mechanisms. A second most relevant aging defense mechanism which may be central to ARHL is exhausted mitochondrial function. Dysfunctional mitochondria, in connection with mitochondrial genome mutations and high cochlear metabolic demands, leads to excessive free radical buildup and cellular damage and apoptosis. This is likely a major contributor to ARHL, although a primary causative link is still missing. Age-related mitochondrial dysfunction in different cochlear cell types may be at the origin of different ARHL histopathologies. Like in aging in general, the combination of primary causes of damage and exhausted defensive mechanisms leads to the final ARHL phenotype. In this regard, the involvement in ARHL of age-related changes in central auditory pathways, due to a complex combination of limited inputs from the aged cochlea and brain aging, is very relevant. It includes changes in connectivity, synapses, and neurotransmitter systems which add further complexities and challenges to the understanding and management of ARHL.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- ARHL
- Presbycusis
- mtDNA mutations
- Oxidative stress
- Epigenetic factors
- Protein homeostasis
- Heat shock factors
- IGF-1
- Caloric restriction
- Hair cells
- Stria vascularis
- Sensory presbycusis
- Neural presbycusis
- Reactive oxygen species (ROS)
- Free radicals
- Antioxidants
- Apoptosis
- Central presbycusis
- GABA
1 Introduction: Age-Related Hearing Loss in the Context of Aging
Age-related hearing loss (ARHL), also known as presbycusis, is a progressive, age-dependent deterioration of auditory function reflected in increased response thresholds in conventional auditory tests, particularly in the high-frequency range, along with degraded frequency discrimination and speech comprehension. It is part of the functional decline of aging, although it should not be regarded as an unavoidable consequence of it. ARHL has the potential for being prevented or treated.
ARHL affects approximately 30–40 % of people in their 60s, about 50 % in their early 70s, and more than 65 % from late 70s onwards (Ohlemiller and Frisina 2008; Gopinath et al. 2009; Lin et al. 2011; Yamasoba et al. 2013). This makes ARHL a major health and socioeconomic problem. Although statistical data are fragmentary, ARHL contributes heavily to the burden of hearing impairment, which now affects over 360 million people worldwide (World Health Organization 2014) with a global cost estimated at 2.5–3 % of the US gross national product (GNP) in 2000 (Ruben 2000). Whereas hearing impairment in the first five decades of life profoundly affects educational and job opportunities, productivity, and general life satisfaction, in the aged population it contributes to family and social isolation, depression, and even cognitive decline and dementia (Lin et al. 2013). This growing problem is spurring extensive multidisciplinary research aimed at understanding, controlling, and treating the causes and consequences of ARHL in humans. A major goal is to unravel the intricate network of cellular and molecular mechanisms at the origin of ARHL. The expectation is to identify key processes capable of being therapeutically targeted, to prevent, block, or reverse the progress of presbycusis in the broader context of extending healthy aging.
In this chapter we will first address cellular mechanisms that may be involved in the pathogenesis of ARHL in the broader context of systemic aging (for additional review, see Chap. 13 by Someya). This will be followed by a more detailed review of the histopathological and pathogenic basis that provides a mechanistic framework for the understanding of ARHL. We will specifically address the pivotal involvement of mitochondrial dysfunction in ARHL, likely due to the accumulation of mitochondrial DNA (mtDNA) mutations/deletions and the progressive increase of oxidative stress and apoptosis dysregulation, followed by possible therapeutic implications (for additional discussion, see Chap. 16 by Yamasoba). Finally, we will outline the relevance of central auditory changes in ARHL. Rather than providing a comprehensive review, this last section will try to convey the idea of the need of an integrated approach to ARHL that should take into consideration both peripheral and central aging.
1.1 Cellular Mechanisms of Aging and Age-Related Hearing Loss
Insofar as ARHL may be viewed as part of the aging process, many factors contributing to general aging (Fig. 15.1) also contribute to a greater or lesser extent to ARHL. In this regard, ARHL may be considered a particular case of accumulation of cellular damage with time, which is at the origin of the aging process (Kirkwood 2005; López-Otín et al. 2013). In the hearing organ (i.e., the cochlea), this accumulated damage is likely aggravated by the exceptional metabolic requirements (Marcus et al. 1978) needed to maintain the energy-intensive mechanotransduction processes.
It is beyond the scope of this chapter to discuss in detail the multiplicity of factors contributing to aging at the cellular level, their combinations and interactions, which have become known from the use of model organisms from yeasts and worms to primates (for excellent recent reviews, see Gems and Partridge 2013; López-Otín et al. 2013). In Fig. 15.1, an outline of these factors in relation to ARHL is provided in order to place the factors that may contribute to ARHL within the much larger and complex, multifactorial nature of general aging. The relative contributions of each of these factors and potential therapeutic implications for prevention of ARHL have not yet been explored in adequate detail and represent targets for future research.
Factors contributing to aging are interconnected in complex networks. However, identification of hierarchical levels may facilitate operative categorizations. At the top of the proposed “hierarchical network” are the cumulative, combined effects of genome damage and associated genomic instability, altered epigenetic regulation, and defective protein homeostasis (for detailed discussion of the genetics of ARHL, see Chap. 14 by RD Frisina and DR Frisina). An interesting proposal is that these genetic factors may be the main “primers” of the aging process as a whole (López-Otín et al. 2013) (Fig. 15.1).
Accumulation of lesions to the genome, both nuclear and mitochondrial, due to sustained exogenous and endogenous damage and mutations, leading to imbalance of DNA repair and/or maintenance mechanisms, and associated unstable DNA transcription and genomic regulation when these mechanisms fail (Fig. 15.1), is central to age-related cell death (López-Otín et al. 2013). Disruption of nuclear architecture due to mutations in nuclear lamin genes contributes to age-associated genome instability, and mitochondrial DNA damage and mutations are of central relevance in ARHL.
A related primary cause of cellular damage associated with aging is impaired epigenetic regulation (López-Otín et al. 2013) (Fig. 15.1), likely due to damage to the enzymatic systems involved. Altered DNA methylation, aberrant posttranslational modifications of histones, or chromatin remodeling, all may add to genomic instability through faulty transcription, aberrant RNA processing, or defective DNA repair, with profound impact in key cell regulatory pathways (López-Otín et al. 2013). Understanding of epigenetic regulatory mechanisms and their impairment with age in the auditory system is still in its infancy (Provenzano and Domann 2007; Friedman and Avraham 2009; Rossman and Klein 2011), although the possible relevance of alterations in the epigenetic modification of histones during auditory aging has been recently demonstrated with the finding of a switch from acetylated to dimethylated histone H3 in the cochlea of aged mice (Watanabe and Bloch 2013).
A third major, primary factor associated with aging is impaired protein homeostasis (Fig. 15.1) (López-Otín et al. 2013). Sustained endogenous and exogenous cellular stress alters normal protein folding, overriding the molecular chaperones necessary for quality control and repair to assure proper folding and ubiquitination, proteasomal degradation, and regulated autophagy necessary for the disposal of misfolded proteins. Impaired refolding of damaged proteins or altered degradation mechanisms may lead to accumulation of abnormal proteins and subsequent cellular damage. In this regard, the heat shock transcription factor HSF1, the key regulator of the stress response mediated by heat shock proteins (Hsp) involved in protein folding repair, is intensely expressed in the cochlea (Fairfield et al. 2002). There is evidence that HSF1 and Hsps regulated by this transcription factor, notably those acting as molecular chaperones in the regulation of protein polymerization and folding, like Hsp27, Hsp70, or Hsp90, are involved in the stress response associated with different insults to the cochlea (Altschuler et al. 2002; Sugahara et al. 2003; Fairfield et al. 2004, 2005; Gong et al. 2012). The precise role of these mechanisms in cell death and loss of function (i.e., ARHL) remains elusive however. Pharmacological induction of Hsp expression attenuates age-related progression of hearing loss in some mice strains, albeit in a limited fashion (Mikuriya et al. 2008). Consistent with this, dysregulated autophagic stress with diminished autophagy activity and impaired removal of misfolded, aberrant proteins may also participate in age-related cell damage in spiral ganglion neurons (SGN) (Menardo et al. 2012).
A second set of factors involved in age-related damage likely is limited or exhausted cellular homeostatic protective mechanisms active against cumulative, sustained damage (Fig. 15.1). Such loss of protective mechanisms results in dysregulation of pathways for sensing and adapting metabolism to ongoing cell needs, mitochondrial dysfunction, and altered regulation of cellular senescence (Fig. 15.1) (López-Otín et al. 2013), which are relevant for ARHL as well.
Several signaling pathways involved in the control of cell growth and metabolic and energetic state, aimed at adapting cell needs to changing environmental conditions, show age-dependent dysregulations. Insulin-like growth factor-1 (IGF-1) is a major regulator of cell growth and differentiation, mediating the effects of growth hormone. IGF-1 shares signaling pathways with insulin. Risking oversimplification, insulin/IGF-1 signaling may be seen as an “anabolic sensor” system that couples rates of cell metabolism and growth to nutrient (glucose) abundance. It has been proposed that under conditions favoring damage like those operating during aging, the observed downregulation of insulin/IGF-1 signaling with age may be an adaptation to diminish metabolism and cell growth, thus limiting the cellular consequences of such damage (Schumacher et al. 2008; Garinis et al. 2008; López-Otín et al. 2013) and allowing lifespan extension. Below a given limit, however, such downregulation no longer provides a defensive role in aging but becomes incompatible with cell homeostasis and life. This is an attractive hypothesis that needs further experimental investigation. The impact of IGF-1 signaling in ARHL is complex, but IGF-1 appears to be protective in both mice and humans (for reviews see Bao and Ohlemiller 2010; Varela-Nieto et al. 2013). Studies in IGF-1 knockout mice show that IGF-1 is needed for the maintenance of hearing and that age-related decreasing levels of this growth factor parallel the progression of ARHL (Riquelme et al. 2010).
Another “anabolic sensor” mechanism relevant to aging includes the mTOR (mammalian target of rapamycin) intracellular cascade. mTOR kinases are themselves involved in the cascade of IGF-1 intracellular signaling. However, one of its functional forms, mTORC1, also participates in adapting cell growth and metabolism to amino acid concentrations through a complex pathway involving the lysosomal membrane (Efeyan et al. 2012). Genetic manipulations or pharmacological inhibition of mTOR with rapamycin extends lifespan in many animal models (Harrison et al. 2009). Increased mTOR activity contributes to age-related obesity (Yang and Ming 2012). Therefore, it seems that intense trophic and anabolic activity signaled through the insulin/IGF-1 or mTORC1 pathways are major contributors to aging, although their specific roles in ARHL remain to be examined in detail.
Two other metabolic state-sensing pathways acting in opposition to the insulin/IGF-1 and mTOR pathways are also relevant to aging. A pathway involving AMPK (5′AMP-dependent protein kinase) signals low-energy availability in cells by detecting high AMP levels. Pathways involving sirtuins (see below) also signal low energy by detecting high NAD+ levels (Houtkooper et al. 2010, 2012). Therefore, both AMPK and sirtuins detect limited nutrient availability and they signal catabolism predominance in cells. Their upregulation extends lifespan and seems to favor healthy aging in several aging models (López-Otín et al. 2013). Actually, the long known benefits of dietary or caloric restriction (CR) in extending life- and health span in many aging organisms (for a recent review, see Szafranski and Mekhail 2014) seem to be linked to the upregulation of AMPK and sirtuins and downregulation of mTOR and the insulin/IGF-1 pathway, triggered by limitation of nutrient availability, with subsequent positive effects on genomic stability and mitochondrial function. However, negative effects of CR on life and health span also have been reported (Szafranski and Mekhail 2014). This may be related to exhaustion of these metabolic sensors leading to deleterious effects in cell survival with age.
Although a connection between the AMPK pathway and ARHL has not yet been investigated in detail, the role of sirtuins in ARHL has gained particular relevance in recent years. The sirtuins (“silent mating-information regulators,” after their role in yeast) belong to a protein family with deacetylase or ADP-ribosyltransferase activities (Houtkooper et al. 2012). As previously mentioned, sirtuin enzymatic activity is linked to the cell energy state through NAD levels so that deacetylation by sirtuins is coupled to NAD hydrolysis. Several of the seven known mammalian sirtuins (Sirt1 to Sirt7) improve aging in mice (Houtkooper et al. 2012). While their actions are complex, many depend on improving genomic stability by epigenetic modifications of histones. It is interesting that a distinct role has been recognized for sirtuins in ARHL (Someya et al. 2010; Han and Someya 2013). CR limits oxidative damage to DNA in the cochlea and slows the progression of ARHL in wild-type mice. However, mice lacking the Sirt3 gene develop ARHL signs even under CR. It seems that Sirt3 directly deacetylates and activates mitochondrial isocitrate dehydrogenase 2 (Idh2), leading to increased NADPH levels and ultimately increased ratio of reduced/oxidized glutathione in mitochondria, thus protecting the auditory receptor from oxidative stress-induced cell death. If the effects of CR in ARHL may be mimicked and more finely controlled by direct (pharmacological) stimulation of sirtuins, this will open the door to new therapeutic targets for ARHL (Han and Someya 2013).
Accumulation of cellular damage caused by the “primary” aging factors previously discussed, along with reduced protective anti-damage mechanisms, can surpass the regulatory homeostatic capabilities of the cell. This leads to the age-damaged phenotype (Fig. 15.1), when there is also a decline in the regenerative potential of tissues. While mammalian inner ear sensory and neural cells show no regenerative potential, many other tissues of the inner ear do regenerate, and these cells are essential for tissue homeostasis (Menardo et al. 2012), although many details remain to be elucidated.
The previous paragraphs describing the role of genome damage and instability, altered epigenetic regulation, defective protein homeostasis, and reduced protective mechanisms give an idea of the general cellular aging landscape in which ARHL is embedded. Added to these specific mechanisms, there is a complex combination of genetic/intrinsic (e.g., sex, race, particular genes, etc.) and environmental/extrinsic (e.g., history of noise exposure, ototoxic drugs, etc.) factors that also contribute to the development of ARHL (Cruickshanks et al. 1998a, b; Agrawal et al. 2008; Lin et al. 2012; Marlenga et al. 2012; for review, see Yamasoba et al. 2013). In other words, systemic aging is a necessary but not sufficient condition for the development of ARHL. Along with contributions from sex and race, genetic risk factors (Lin et al. 2012; Marlenga et al. 2012) and predisposition to ARHL are reviewed in detail elsewhere (for reviews, see Yamasoba et al. 2013; Chap. 14 by RD Frisina and DR Frisina). It does not come as a surprise that many identified genetic variants and mutations related to susceptibility to ARHL involve genes related to antioxidation (see below) and also to glutamatergic neurotransmission (Sergeyenko et al. 2013; Yamasoba et al. 2013) and excitotoxicity (Pujol et al. 1991; Juiz et al. 1989). As far as environmental factors are concerned, the association of noise exposure and hearing loss is well known (see Chap. 7 by Altschuler and Dolan). In addition to simple additive relationships, noise-induced hearing loss (NIHL) that synergistically accelerates ARHL has more recently been recognized (Kujawa and Liberman 2006). Treatment with ototoxic drugs also results in hearing loss (see Chap. 10 by Rybak and Brenner, and Chap. 11 by Laurell and Pierre). To add further complexity, both noise damage and drug damage to the ear are also subject to genetic susceptibility (see Chap. 8 by Yamashita, Chap. 17 by Kohrman, and Chap. 18 by Green and Raphael). Thus, ARHL is a complex multifactorial disorder in which a lifelong accumulation of insults to the ear on a background of complex genetic predispositions and systemic aging leads to cell damage and hearing impairment (Gates and Mills 2005).
1.2 Histopathology of Age-Related Hearing Loss as a Mechanistic Framework
From a histological standpoint, ARHL is characterized by a progressive degeneration of hair cells starting at the basal end or high-frequency coding regions of the cochlea, atrophy and degeneration of the stria vascularis, and a variable degree of degeneration of the primary afferent SGN (Schuknecht and Gacek 1993). Therefore, cell populations in the cochlea involved directly in mechanotransduction (hair cells), in providing the electrochemical driving force for mechanotransduction (cells in the stria vascularis) or in transmitting and propagating transduced signals from the SGN to the central auditory pathway, may all degenerate and decrease in number and contribute to ARHL (Ohlemiller and Frisina 2008). Whether these universally acknowledged histopathological changes reflect independent, interdependent, or concurrent pathogenic events is an important question with impact on therapeutic strategies. A favored operational concept is that age-associated pathologies of the sensory epithelium, primary sensory neurons, or stria vascularis may arise and evolve independently, although at some point they may merge either by pathophysiological dependency on each other or simply by concurrence. This concept was elaborated by Schuknecht by correlating human patterns of hearing loss with the location of tissue damage and cell pathology in postmortem temporal bones. This seminal work led to the proposal of four major types of human presbycusis, according to the predominant histopathological pattern in relation to changes in auditory thresholds (Schucknecht 1955, 1964; Schuknecht and Gacek 1993; Ohlemiller and Frisina 2008; Schmiedt 2010).
In sensory presbycusis, the sensory epithelium is the site of primary damage particularly in high-frequency regions. A variable degree of progressive secondary SGN degeneration follows damage to the sensory lamina (in contrast to primary neural presbycusis). Age-related pathology primarily affecting hair cells may overlap with prolonged accumulation of noise or chemical damage to the auditory receptor making it difficult to separate contributions from preexisting lesions and aging. Therefore, to some extent, there may be a “pathogenic continuum” between accumulation of cell lesions from environmental factors and “true” age-related damage to sensory structures, with important consequences for prevention and treatment strategies.
In neural presbycusis, a specific primary loss of SGN with no damage to hair cells was proposed (Schucknecht 1955, 1964; Schuknecht and Gacek 1993; for recent reviews, see Ohlemiller and Frisina 2008; Schmiedt 2010). However, complex trophic relationships between SGNs and their target sensory epithelium challenge the notion of “pure” neural presbycusis. Certainly, there is evidence that SGN may experience progressive degeneration without detectable hair cell loss in young animals, and, conversely, they may survive for extended time periods after hair cell loss (Kujawa and Liberman 2006 ). Thus, SGN survival or death can be independent of hair cell targets in adulthood. Taken together, there may be a “pure” loss of SGN in neural presbycusis primarily due to aging mechanisms as recently shown in mice (see Boettcher et al. 1995; Schmiedt et al. 1996; Sergeyenko et al. 2013), or it may represent, again, a lifetime accumulation of insults, leading progressively to auditory neuropathy and overlapped with, and indistinguishable of, auditory aging.
In strial or metabolic presbycusis, the stria vascularis becomes atrophic and degenerates. The involvement of the stria vascularis in the pathogenesis of ARHL has attracted considerable attention, particularly in recent years. Schuknecht developed the concept that degeneration in the stria vascularis is a major contributor to hearing loss associated with the aging process (Schuknecht and Gacek 1993; Gates and Mills 2005; Schmiedt 2010). Strial pathology seems to be a common feature of ARHL in both humans and animal models. Actually it is the earliest identified pathological sign in the age-damaged cochlea, first seen in the third decade of life in humans, and it has the highest heritability index (Gates and Mills 2005; Ohlemiller 2009; Fetoni et al. 2011; Yamasoba et al. 2013). However, hearing loss characterized clinically as strial presbycusis sometimes includes loss of hairs cells in the basal turn of the cochlea as the most visible pathology. Some postulate that the hair cell loss could be the result of specific insults such as noise exposure, whereas the strial pathology is purely related to aging (Gates and Mills 2005).
Aged gerbils show a prominent degeneration and atrophy of the stria vascularis (Fetoni et al. 2011). They exhibit a progressive pathology of marginal and intermediate cells and of the Na+K+ATPase-positive fibrocytes in the spiral ligament (Spicer and Schulte 2002, 2005; Gates and Mills 2005). This age-dependent degenerative process involves loss of the expression of the Na+K+ATPase in the lateral wall and stria vascularis (Schulte and Schmiedt 1992). This ATPase regulates K+ and Na+ transport through the lateral wall and, as a consequence, the endocochlear potential (EP) (Spicer and Schulte 2005; Schmiedt 2010; for detailed discussion see Chap. 3 by Ohlemiller). Indeed, aged gerbils exhibit a progressive decline in EP and a disruption of ion homeostasis in the cochlea (Schulte and Schmiedt 1992; Schmiedt 1996, 2010). Thus, in aged animals, degeneration of the stria vascularis can lead to a progressive decline of the EP and impairment of the cochlear amplifier (Gates and Mills 2005; Schmiedt 2010).
Aged-related alterations in the microcirculation of the stria vascularis have been described in several experimental models (Shi 2011). The metabolic rate is high in the stria vascularis (Gates and Mills 2005; Fetoni et al. 2011; Böttger and Schacht 2013). Aged gerbils show atrophy and loss of strial capillaries that gradually expands from the apical to the middle region of the cochlea (Gratton and Schulte 1995). Age-related reduction of cochlear blood flow is exhibited by old gerbils specifically in the lateral wall (Prazma et al. 1990), and the EP decline observed in old gerbils and described above correlates with atrophic capillaries in the stria vascularis (Gratton et al. 1996). Further studies demonstrate age-dependent thickened basal membrane (BM) in 65–85 % with an increase of immunoglobulin and laminin deposition in strial capillaries (Sakaguchi et al. 1997a, b; Thomopoulos et al. 1997), suggesting BM alterations and age-dependent permeability in these vessels. Similarly, aged Fischer 344 rats show decrease in the vascularization of the stria vascularis (Buckiova et al. 2006) accompanied by age-dependent reduction of red blood cell velocity and increased capillary permeability (Seidman et al. 1996). Finally, in 18-month-old C57BL/6 J mice, a decreased expression of the specific endothelial marker VEGF in this tissue is described (Picciotti et al. 2004). These microvascular alterations contribute to the age-related pathology of the stria vascularis and the progressive worsening of ARHL.
Mechanical or conductive presbycusis is characterized by “stiffening” of the basilar membrane, although this remains unproven and under discussion (Gates and Mills 2005; Ohlemiller and Frisina 2008; Schmiedt 2010). However, presbycusis does not present in an isolated form of one of those described above. Instead it comprises a mixture of alterations that likely reflect the summation aging effects in many cell types (Schuknecht and Gacek 1993; Ohlemiller and Frisina 2008). In this regard, Schuknecht included a frequent form of presbycusis named mixed to point out that ARHL is often characterized by a broad cochlear degeneration due to coincident independent causes (Schuknecht and Gacek 1993). Actually, it has been recently proposed (Engle et al. 2013) that in the rhesus monkey, the accumulating number of cochlear pathologies correlates better with audiometric patterns than single pathologies. Finally, Schuknecht established the indeterminate presbycusis for those cases where no significant changes were observed in any cochlear structure and its audiogram differed from the conductive one (Schuknecht and Gacek 1993).
It is clear from the above that Schuknecht’s histopathological framework is limited and under continuous review. However, it still provides a valid context within which we may view new knowledge of ARHL mechanisms (Ohlemiller and Frisina 2008).
1.3 Mitochondrial Mutations and Dysfunction and Its Association with Age-Related Hearing Loss
Mitochondrial dysfunction is central to aging (López-Otín et al. 2013). Actually, altered mitochondrial function is of utmost relevance in ARHL, and it could be at the origin of all the histopathological types of presbycusis described by Schuknecht.
Mitochondria play a central role in the physiology of the cell as the primary source of ATP and are key players in many cellular processes, including calcium signaling and regulation of apoptotic cell death. Mitochondrial metabolism produces free radicals, including reactive oxygen species (ROS) and reactive nitrogen species (RNS) (for detailed discussion, see Chap. 2 by Leeuwenburgh). Both ROS and RNS have signaling roles under normal conditions but are toxic to the cell in excessive concentrations, leading to oxidative stress damage (Fig. 15.2) (Sena and Chandel 2012; Böttger and Schacht 2013). In this regard, mitochondrial dysfunction contributes to a large number of human age-related neurodegenerative diseases such as Alzheimer’s and Parkinson’s diseases, amyotrophic lateral sclerosis, and Huntington’s disease (Lin and Beal 2006) and damage attributed to aging (Lenaz et al. 2006; Lee and Wei 2012; Bratic and Larsson 2013). While there is much evidence supporting the idea that mutations in mitochondrial genomic DNA (mtDNA) and oxidative stress both contribute to aging (Lin and Beal 2006), recent evidence supports a primary role for mtDNA mutations in mammalian aging (Bratic and Larsson 2013; Liochev 2013; Viña et al. 2013). Thus, genetically modified mice undergoing progressive accumulation of mtDNA mutations exhibit an accelerated aging phenotype (Trifunovic et al. 2004; Kujoth et al. 2005). Further research demonstrated that these “mtDNA mutator mice” showed mitochondrial dysfunction unaccompanied by increased ROS production (Trifunovic et al. 2005), suggesting a direct link from the mtDNA mutation to mitochondrial dysfunction and the aging process. We will address the possible association between the mtDNA mutations and ARHL and then the role of ROS generation and oxidative stress in presbycusis.
Mitochondrial dysfunction in the pathogenesis of ARHL. Mitochondria have a central role in the pathogenesis of ARHL. During the aging process, many insults damage the cell and are progressively accumulated. In this regard, mitochondrial DNA (mtDNA) mutations and deletions (mut/del) are generated due to the “primary” aging factors and gradually accumulate. This leads to mitochondrial dysfunction that in turn contributes to an oxidative stress situation due to the additive effect of increased toxic levels of ROS and the progressive decline of antioxidant (AntiOx) enzymes that are observed with age in the cochlea. Both processes, mitochondrial dysfunction and oxidative stress, activate apoptosis pathways leading to death of specific cell populations in the auditory receptor contributing to and worsening the aging phenotype. Thus, in the mitochondria of the cochlear cells, there is a progressive accumulation of mtDNA mut/del that significantly contributes to the gradual increase in the ROS levels. Once the toxic level of ROS is reached, oxidative stress ensues, which is also enhanced by the gradual decrease of the antioxidant defense of the cell with age. All these factors will finally activate cell death pathways. The ROS concentration sketch is modified from Glasauer and Chandel (2013)
Mitochondrial genomic alterations and ARHL. As stated above, mtDNA deletions and mutations increase with age and also contribute to age-related pathology (Bratic and Larsson 2013; López-Otín et al. 2013). mtDNA mutations are at the origin of a large number of human genetic diseases, many of which include hearing loss (Fischel-Ghodsian 2003; Schon and Przedborski 2011). This suggests a key role for mtDNA mutations specifically in ARHL (Yamasoba et al. 2007). In this regard, it has been estimated that nearly 70 % of patients with mitochondrial genomic disorders also display sensorineural hearing loss (Gold and Rapin 1994; Seidman et al. 2004). In an animal model, 9-month-old “mtDNA mutator mice” showed a significantly greater loss of outer hair cells (OHCs) and SGNs in the basal turn of the cochlea, along with increased ABR threshold shifts, compared to age-matched controls (Kujoth et al. 2005). This suggests that the accumulation of mtDNA mutations in the cochlea as a function of aging may directly lead to hearing impairment attributed to age.
Bai et al. (1997) examined 34 human temporal bone samples, 17 of which belonged to people with ARHL. They found a 4977 base pair (bp) “common aging deletion,” which was significantly more frequent in cochlear tissues of ARHL patients than patients without ARHL. A similar 4834 bp common aging deletion was also found to accumulate with age and to be associated with progressive lower hearing sensitivity in Fischer rats (Seidman et al. 1997). Mutations in the mitochondrial cytochrome oxidase II gene in temporal bones from five ARHL patients have also been described, further supporting the likelihood that sporadic mtDNA mutations also contribute to ARHL (Fischel-Ghodsian et al. 1997). These observations were confirmed in humans by Dai et al. (2004), who found a high incidence of the mtDNA4977 deletion in the temporal bones of ARHL patients (17/34) compared to ears in the age-matched control group (4/19) and those in the young and middle-aged control group (0/14). Perhaps most compelling, a quantitative correlation was established between the level of the mtDNA4977 deletion in human temporal bone samples from ARHL patients and the severity of the hearing loss (Markaryan et al. 2009). These authors also observed a decrease in the expression of the mitochondrial cytochrome c oxidase subunit 3 (COX3) gene in SGNs and an increase in deletions different from the mtDNA common deletion in COX3-deficient SGNs (Markaryan et al. 2010). Taken together, these results are consistent with a causative relationship between the accumulation of mitochondrial genomic alterations with age, mitochondrial dysfunction, and the pathogenesis of ARHL (Fig. 15.2).
1.4 Mitochondria, Oxidative Stress, and Age-Related Hearing Loss
Because of the primarily energetic function of mitochondria, ROS are continuously produced in this organelle during the biochemical reactions of the electron transport chain. Superoxide is the primary radical, from which the rest of reactive species are generated through the activity of the cell antioxidant and detoxifying systems (Murphy 2009; Dröse and Brandt 2012). Redox regulatory mechanisms involve several antioxidant enzymes: superoxide dismutases (Cu/Zn-SOD and Mn-SOD), glutathione peroxidase (GPx), glutathione S-transferase (GST), and catalase (Balaban et al. 2005; Kowaltowski et al. 2009; Sena and Chandel 2012). Recent findings demonstrate that ROS also play essential roles in the physiology of the cell by acting in many cell signaling events (Finkel 2012; Ray et al. 2012; Sena and Chandel 2012) related to cellular homeostasis and its adaptation to stress situations (Sena and Chandel 2012). A currently accepted view is that oxidative stress implies an excessive concentration of ROS/RNS due to an imbalance between their generation and the inefficient capacity of the antioxidant systems to scavenge them (Fig. 15.2) (Lee and Wei 2012; Glasauer and Chandel 2013). It has been hypothesized that excessive production accumulates over time, damaging key molecules and cell structures (nuclear and mitochondrial DNA, membranes, and proteins) and resulting in tissue dysfunction during aging (Lee and Wei 2012). Since mitochondria are the major source of ROS (Balaban et al. 2005; Kowaltowski et al. 2009; Brand 2010), the primary effect of oxidative stress we expect would impact on mitochondria, leading to mitochondrial dysfunction with age as seen in several neurodegenerative diseases and other pathologies (Lin and Beal 2006; Sena and Chandel 2012).
Presbycusis is believed to reflect the progressive accumulation of metabolic alterations due in part to ROS/RNS and the progressive inefficient functioning of mitochondria with age on a background of unusually high metabolic demands in the inner ear tissues (Someya and Prolla 2010; Yamasoba et al. 2013). Although the stria vascularis has the highest aerobic metabolic rate of all cochlear structures (Böttger and Schacht 2013), iOHCs are also vulnerable to oxidative damage because of their high metabolic activity and lower content of antioxidants, like glutathione (Sha et al. 2001).
Recent evidence lends further support to the notion of oxidative stress as a contributing factor in the pathogenesis of ARHL (Someya and Prolla 2010; Yamasoba et al. 2013). There was a significant increase in oxidative stress markers (glutathione-conjugated proteins and 4-hydroxynonenal and 3-nitrotyrosine) and a decrease of the antioxidant scavengers AIF and SOD2 in the cochlea of aged CBA/J mice (Jiang et al. 2007), indicating an imbalance in the redox status of the cochlea with age. On the other hand, Sod1 deficiency in mice increased the age-related loss of both inner hair cells (IHCs) and OHCs following a base-to-apex gradient, suggesting a correlation between the SOD1 deficiency and the survival of the cochlear sensory cells (McFadden et al. 1999b). Further experiments correlated these hair cell losses with hearing impairment (measured by ABR thresholds) and showed age-related loss of SGN in the 13-month-old homozygous knockout mice (McFadden et al. 1999a). A study with the Sod1–null mice showed severe degeneration of SGN at 7–9 months of age, elevated threshold shifts in 12-month-old animals, and thickening of the stria vascularis at 15 months of age, a pattern of results suggesting early ARHL in Sod1 deficient mice (Keithley et al. 2005). In addition, overexpression of mitochondrially targeted catalase in C57BL/6 J mice delayed the onset of ARHL in mice by delaying hair cell loss (Someya et al. 2009). Finally, a moderate age-related increase in GPx activity has been described in the spiral ligament and stria vascularis in 24-month-old Fisher 344 rats (Coling et al. 2009). Taken together, these results indicate that imbalances in the cellular antioxidant machinery lead to early onset of ARHL and point to oxidative stress as an important contributing factor in its pathogenesis (Fig. 15.2).
If oxidative stress plays a causative role in the pathogenesis of ARHL, it is likely that priming antioxidant mechanisms may reduce cochlear damage and delay onset of pathological changes. However, in contrast to noise-induced and drug-induced hearing loss, where a causal relationship between oxidative stress blocking and improvement in hearing impairment is reasonably well established, in the case of ARHL the link is weaker (Böttger and Schacht 2013). Although many studies have shown effects of antioxidant administration against ARHL, others have not. Seidman (2000) studied the independent effects of several antioxidants (vitamin E, vitamin C, melatonin, and lazaroid) in Fischer 344 rats maintained on lifelong (average, 25 months) supplemented diets. They observed improved auditory thresholds and fewer mtDNA deletions in antioxidant-treated animals, with some agents being more protective than others (see also Chap. 4 by Seidman and Shirwany). Similar results were obtained with the oral administration of lecithin to 18- to 20-month-old Fischer 344 rats for 6 months (Seidman et al. 2002). Heman-Ackah et al. (2010) fed C57BL/6 mice with a combination of six antioxidant agents (l-cysteine-glutathione mixed disulfide, ribose cysteine, NW-nitro-l-arginine methyl ester, folate, vitamin C, and vitamin B12) and observed a significant decrease in threshold shifts in 12-month-old antioxidant-treated animals. Moreover, in C57BL/6 mice supplemented with the mitochondrial antioxidants α-lipoic acid and coenzyme Q10, a reversal in ARHL was demonstrated, with lower hair cell and SGN cell death (Someya et al. 2009). However, 15-, 18-, or 24-month-old Fischer 344 rats treated with l-carnitine with different methods of administration did not show improvement in progression of ARHL (Bielefeld et al. 2008), and 10-month-old CBA/J mice supplemented with an antioxidant-enriched diet composed of α-lipoic acid, vitamin A, vitamin C, vitamin E, and l-carnitine showed no delay in the onset of presbycusis or loss of hair cells or SGN at 24 months of age (Sha et al. 2012). Additional discussion of therapeutics administered to animal subjects is found in Chap. 16 by Yamasoba.
Taken together, these findings suggest that although oxidative stress is a contributing factor to the pathogenesis of ARHL, it is currently difficult to establish it as a causative factor, without further investigation. In this regard, our lab is studying the otoprotective mechanisms of a combination of antioxidants (vitamin A, vitamin C, and vitamin E) plus magnesium (Mg++). Mg++ has been demonstrated to increase inner ear blood flow (Scheibe et al. 2000; Haupt and Scheibe 2002), and its efficacy in attenuating NIHL has been demonstrated (Sendowski 2006). The ACEMg treatment was administered to guinea pigs (Le Prell et al. 2007) and CBA/J mice (Le Prell et al. 2011) showing reduced permanent threshold shifts after NIHL and also reduced threshold shift after gentamicin ototoxicity (Le Prell et al. 2014). Studies in humans indicate that regular dietary intake of antioxidants correlates with lower risks of hearing loss (Durga et al. 2007; Shargorodsky et al. 2010; Choi et al. 2014). Chapter 6 by Spankovich discusses epidemiological data on the role of nutrients in hearing loss prevention in humans.
1.5 Apoptosis Regulation and Age-Related Hearing Loss
Several animal models have been used to study the role of apoptosis in the pathogenesis of ARHL (Someya and Prolla 2010; Fetoni et al. 2011). Experiments with old gerbils and mice show that in the aged cochlea, there are various cell types that undergo programmed cell death, such as IHCs and OHCs and supporting cells in the organ of Corti and fibrocytes in the spiral ligament (Usami et al. 1997; Spicer and Schulte 2002). Similarly, aged Fischer 344 rats exhibit increased TUNEL (terminal deoxynucleotidyl transferase dUTP nick end labeling)-positive cells in both the marginal and basal cell layers of the stria vascularis and cells of the spiral ligament. Apoptosis results in a detachment of both layers. This correlates with altered otoacoustic emissions (Buckiova et al. 2007).
The molecular mechanism involved in such apoptotic death has been extensively studied. Experiments with aging gerbils and rats show downregulation of the anti-apoptotic Bcl family proteins Bcl-2 and Bcl-xL and the activation of pro-apoptotic proteins Bax and Caspase 3 (Alam et al. 2001; Nevado et al. 2006) leading to the hypothesis that the intrinsic pathway of apoptosis may mediate this aged-related cell death. In Fischer 344 rats, Hu et al. (2008) established a temporal sequence of molecular events associated with cell death molecular. Bax expression, release of mitochondrial cytochrome c, and DNA fragmentation take place before the nuclear condensation, followed by activation of caspases 3 and 9, continuing to the degradation of F-actin. On the other hand, gene expression studies in mice demonstrate age-dependent changes of apoptosis-related genes in the cochlea of aging animals (Tadros et al. 2008) and the central role of the mitochondrial pathway of apoptosis via the Bak protein as the key player in this process (Someya et al. 2009). The role of these pathways to cell death is brought together by Sha et al. (2009) who have shown that multiple cell signaling and cell death pathways (both the intrinsic and extrinsic apoptosis and also necrosis) are progressively activated in the cochlea of CBA/J mice with age. Finally, the overexpression of the anti-apoptotic protein XIAP (X-linked inhibitor of apoptosis protein) delays the progression of ARHL and decreases hair cell death in C57BL/6 J mice (Wang et al. 2010).
1.6 Cellular Mechanisms of Age-Related Hearing Loss in the Central Auditory Pathways
An understanding of ARHL and possible intervention strategies is incomplete without consideration of cellular and molecular changes associated with auditory aging in the central auditory pathways and their functional consequences. There has been comparatively less attention given to central mechanisms contributing to ARHL, and we strongly believe there is a need for a more integrated view of ARHL, combining peripheral and central mechanisms. Such an approach will not only accelerate understanding of ARHL but will also lead to more efficient management and therapeutic strategies through timely targeting of peripheral and central pathogenic mechanisms (Parham et al. 2011; Gates 2012; Kim and Chung 2013).
Defective processing by central auditory neurons and circuits in ARHL has a dual origin. One is declining peripheral input from the auditory receptor with age. This leads to diminished activity in central auditory neurons which is visible in auditory brainstem response evoked potentials (Fig. 15.3), with consequences for signal propagation and transmission, as well as trophic maintenance of nerve cells. The second is the aging process in the brain itself, which also leads to altered neuronal circuit function, although probably not through identical mechanisms. Both may overlap considerably and even be part of a pathogenic continuum in ARHL. Thus, a major challenge for research on central mechanisms of ARHL is to distinguish between relative contributions and impact of age-declining peripheral input versus direct effects of aging on the central auditory system and to establish their pathogenic sequences and timelines. Sorting the dual origins of the central causes and consequences of ARHL and, equally important, understanding how they interact will have an impact on future therapeutic strategies.
Changes in auditory brainstem responses with age in the rat. An example of ABR recordings in Wistar rats at 6- to 8-month-old (green), 12- to 14-month-old (blue), and 18- to 20-month-old (red). Wave amplitudes decrease at the different frequencies evaluated 0.5 (A), 2 (B), 8 (C), or 32 (D) kHz, and latencies increase as the age of the rats increase. Arrows indicate the stimulus onset. Stimulus intensity = 80 dB SPL
Animal models, in particular rodent strains with various onset, severity, and patterns of hearing loss, have been very useful to dissect out the contributions to central ARHL mechanisms of age-diminished peripheral input versus central aging (see Ohlemiller and Frisina 2008 for review and their chapters in this book). There is evidence that the progression of age-related loss of synaptic input from the auditory receptor affects neuronal survival, trophic support, and signal propagation and transmission (Fig. 15.3), with greatest severity of structural changes in caudal nuclei of the auditory brainstem. Neurons in the cochlear nucleus (CN) are primary recipients of synaptic endings from auditory nerve axons, and therefore the first to “sense” diminished peripheral input. There is an overall reduction of neuron number, packing density, and reduced cell size, distributed tonotopically and consistent with the time course and progression rate of the peripheral hearing loss (Willott et al. 1987, 1992). Early loss of high-frequency hearing in the C57 mouse strain (Willott et al. 1987) leads to early and stable structural changes in the CN. Delayed hearing loss, as in the CBA mouse (Willott et al. 1987), corresponds with delayed structural changes in aged CN neurons of similar nature. It is interesting that these peripherally driven effects on neuronal structure do not affect uniformly all CN neuronal populations, with octopus cells in the posteroventral CN being particularly resilient to limited auditory nerve input (Willott and Bross 1990). The type of neuron and its pattern of synaptic inputs seem to determine neuronal survival and trophic maintenance in the presence of limited peripheral input with age. However, it is also interesting to note that mouse strains (Willott et al. 1987) or those humans having a significantly larger number of neurons in the CN (Hinojosa and Nelson 2011) seem to be more prone to neuronal loss attributable to presbycusis, suggesting an added genetic component to peripherally driven neuronal loss. In the auditory pathway rostral to the CN, neuronal loss attributable to auditory aging is much more limited or not present (Shim et al. 2012; Willott et al. 1994). Neuronal body size diminishes or remains stable “upstream” of the CN, at least in the inferior colliculus (IC) (Willott et al. 1994; Kazee et al. 1995). Therefore, it seems that trophic support or survival of many CN neuron types is severely compromised by age-dependent reduction in direct peripheral inputs carried by the auditory nerve. However, at more rostral levels of the auditory pathway, beyond the primary inputs, diminished peripheral activity has much more limited impact on net cell survival, supporting the view that trophic signals differ at different levels of the auditory pathway, with possible consequences for the aging process.
As a consequence of damage to neuronal cell bodies, particularly in the CN, axonal degeneration is expected. However, the extent and rate of age-related degeneration of axons traveling in auditory tracts is unclear. Axonal connections from the CN to the inferior colliculus (IC) are not significantly reduced in mice with early signs of ARHL at any age (C57, Willott et al. 1985). However, in mice with mild ARHL, connections from the CN to the IC are significantly reduced with age (CBA; Frisina and Walton 2001, 2006). The number of fibers in the human lateral lemniscus is also reduced at advanced ages (Ferraro and Minckler 1977, cited in Ohlemiller and Frisina 2008). Regardless the extent and rate of axonal degeneration, connections from the CN to more rostral auditory nuclei carry degraded signals (reviewed in Frisina and Walton 2006) as a consequence of aging (Fig. 15.3). Among other things, as a consequence of high-frequency loss, there are rearrangements in the tonotopic map of more rostral nuclei, like the IC (Willott et al. 1991), with high-frequency regions becoming more sensitive to lower-frequency sounds. The nature of this peripherally induced “rewiring” is unknown, but it could add to limited frequency discrimination. In the auditory cortex, plastic reorganizations of the tonotopic map also take place after age-related high-frequency hearing loss, with low frequencies expanding its representation to higher-frequency regions (Willott et al. 1993). The reorganization in connectivity underlying such age-related auditory cortex plasticity is not known. Such plastic reorganizations in the auditory midbrain and forebrain do not seem to take place in rodent models aging with good hearing preservation (Willott et al. 1993).
Directly related to altered neuronal trophic support and structural and functional connectivity are age-related modifications or adaptations of synaptic circuits, reflecting both peripheral and intrinsic central changes, and relevant to understanding and management of central mechanisms of ARHL (Frisina and Walton 2006; Caspary et al. 2008) (Figs. 15.4 and 15.5). Rearrangements of synaptic structure and size take place in the CN with aging (Keithley and Croskrey 1990; Helfert et al. 2003). However, there seems to be no net synaptic loss (Helfert et al. 2003), which might be a consequence of aberrant reactive synaptogenesis (Keithley and Croskrey 1990), due to loss of primary inputs from the aging cochlea. In the IC, however, loss of excitatory and inhibitory endings predominates (Helfert et al. 1999; Kazee et al. 1995). Considering that synaptic loss and synaptic functional abnormalities are hallmarks of brain aging (Shankar 2010; Bano et al. 2011), it is difficult to distinguish those reflecting age-related peripheral changes and intrinsic central changes. It has been reported that the structure and number of synapses is well preserved in the IC of the CBA mouse, which shows well-preserved hearing until late in life (Kazee and West 1999). This is in contrast with the C57 mouse and other rodent strains with earlier and/or more severe hearing loss in which synaptic loss in the IC is greater (Kazee et al. 1995; Helfert et al. 1999). This indicates that age-dependent auditory peripheral deficits influence central synaptic survival, although the contribution of brain aging itself remains to be fully explored (Caspary et al. 2008).
Changes in central excitatory neurotransmission with age in the rat. Vesicular glutamate transporter-1 (VGLUT1) immunoreactivity in the AVCN (A–C) and PVCN (D–G) in 6- to 8-month-old (A, D), 12- to 14-month-old (B, E) and 18- to 20-month-old (C, F and G) Wistar rats. Immunopositive profiles (arrows) in 6- to 8-month-old rats (A, D) seem more abundant than in 12- to 14-month-old (B, E) and 18- to 20-month-old (C, F and G) rats. Bar graphs indicate the mean gray level of VGLUT1 immunostaining in the AVCN (H) and PVCN (I). The mean gray level in the 18- to 20-month-old rats is lower compared to the 6- to 8-month-old rats. Asterisks in A–G indicate neurons. Scale bar represents 25 μm in C *p < 0.05
Changes in central inhibitory neurotransmission with age in the rat. Vesicular GABA transporter (VGAT) immunostaining in the AVCN (A–C) and PVCN (D–H) in 6- to 8-month-old (A, D–F), 12- to 14-month-old (B, G), and 18- to 20-month-old (C, H) rats. An apparent decrease in the immunostaining of large and small inhibitory synaptic endings is observed with age, with VGAT profiles (arrows) distributed more abundantly in the 6- to 8-month-old rats (A, D–F) than in both the 12- to 14-month-old (B, G) and 18- to 20-month-old (C, H) rats. The mean gray level in the AVCN (I) and PVCN (J) in 18- to 20-month-old rats is significantly lower compared to both the 6- to 8-month-old rats and the 12- to 14-month-old rats. Asterisks in A–H indicate neurons. Scale bar represents 25 μm in H. *p < 0.05
Age-driven structural rearrangements in the distribution and/or number of central auditory synapses may be connected with adaptive synaptic changes (Figs. 15.4 and 15.5), to restore a lost delicate balance between excitation and inhibition (reviewed in Caspary et al. 2008). In the CN, excitatory input from the auditory nerve is reduced during aging as a result of degeneration of SGN (reviewed in Bao and Ohlemiller 2010) or altered synaptic transmission with deficient synaptic glutamate handling (Fig. 15.4) (Alvarado et al. 2014), lower glutamate release, and changes in glutamate receptor composition and kinetics (Wang and Manis 2005). To maintain homeostasis inhibition is downregulated to maintain original levels of activity (Caspary et al. 2008; Alvarado et al. 2014). Glycine-mediated inhibition is particularly affected in the CN (reviewed in Caspary et al. 2008). Age-related decreases in glycine (Willott et al. 1997), inhibitory amino acid synaptic vesicle transporters (Fig. 15.5) (Alvarado et al. 2014), and synaptic release of glycine (Xie and Manis 2013) are mirrored by changes in postsynaptic GlyR subunit expression and composition and altered glycine binding to GlyR (Willott et al. 1997; Caspary et al. 2008; Wang et al. 2009). Briefly, at the synaptic level, glycinergic inhibition decreases with age (Xie and Manis 2013). As a result, discharge rate of CN neurons increases, and dynamic range and ability to code signals that change very fast in time is degraded (Caspary et al. 2005, 2008). Although gamma-aminobutyric acid (GABA) is relevant to CN neuronal inhibition and signal processing, GABAergic neurotransmission in the CN is more limited. Actually, GABAergic markers do not change significantly with age in the CN (Raza et al. 1994; Sharma et al. 2014). Such a selective downregulation of glycinergic neurotransmission in the CN during ARHL is interesting since GABA and glycine typically co-localize and co-release from inhibitory synaptic endings in the CN (Juiz et al. 1996; Lim et al. 2000). In addition to altered inhibitory neurotransmission, changes in intrinsic properties reported in some neuronal classes (bushy cells, Wang and Manis 2006) may also contribute to degraded excitability in the CN during aging. These observation are consistent with the view that the hallmarks of ARHL, i.e., reduced speech discrimination in noisy backgrounds (Anderson et al. 2012), begin with age-altered synaptic circuit properties at the very first central auditory relay stations of the auditory pathway.
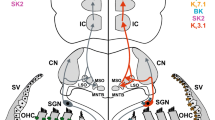
Excitatory-inhibitory synaptic imbalances with diminished inhibitory neurotransmission appear to be a constant feature of ARHL along the auditory pathway. In the superior olivary complex (SOC) which receives input from both CN and is involved in sound localization, inhibition also is impaired (Finlayson 1995; reviewed in Caspary et al. 2008). Reductions in GAD activity, the rate-limiting enzyme involved in GABA synthesis, have been reported in the nuclei of the lateral lemniscus (NLL) of aged Fischer 344 rats (Raza et al. 1994) although functional implications are unknown.
Age-related excitatory-inhibitory imbalances are especially well documented in the IC (Caspary et al. 2008; Ohlemiller and Frisina 2008). Virtually all available evidence points to a severe age-dependent decrease in inhibitory GABAergic function in the IC as a pivotal central mechanism of ARHL (Caspary et al. 2008), although interspecies variations may exist (Gleich et al. 2014). GABA and/or GAD levels measured in neurochemical assays or by immunocytochemistry show large age-related decreases in the IC (reviewed in Caspary et al. 2008 and Ohlemiller and Frisina 2008). Brain aging may contribute significantly to the decline of presynaptic markers of GABAergic function, but the share of peripheral and central contributions to diminished IC GABAergic inhibition is unknown (Caspary et al. 2008). GABA receptors also change their composition and binding properties with age in the IC, which may underlie compensatory mechanisms (Caspary et al. 2008). Gene and protein expression of subunits assembling ionotropic GABAA receptors responsible for “fast” inhibition have been reported to be downregulated to a greater or lesser extent in the IC with aging (Gutiérrez et al. 1994), with the notable exception of the gamma1 and alpha3 subunits which are upregulated (Milbrandt et al. 1997; Caspary et al. 1999). GABAA receptors enriched in gamma1 subunits may allow larger Cl influx, which may be part of a mechanism of compensation for impaired GABA release (Caspary et al. 2008) and as well as reduced receptor density (Gutiérrez et al. 1994). Changes with age in the binding properties of the picrotoxin site of the GABAA receptor also indicate modulation of GABAA responses with aging in the IC (Caspary et al. 2008). GABAB receptors, responsible for “slow” modulatory responses, have also been found to be diminished in the aging IC (Milbrandt et al. 1994). Taken together, this evidence supports a massive net downregulation of GABAergic inhibition in the IC with aging. Significant decreases in inhibitory responses of single IC units within their receptive fields, increases in the width of the response above threshold, and reduced precision in the temporal processing of AM and FM sounds (Caspary et al. 2008) may originate at least in part in defectively compensatory GABAergic downregulation. This could lead to degradation of temporal, spectral, and binaural processing in the IC with aging.
Evidence of excitatory-inhibitory imbalances, suggestive of maladaptive homeostatic plasticity during auditory aging, has also been found recently in the auditory thalamus of aged Fischer Brown Norway rats. An age-related reduction in GAD67 was found in the medial geniculate body (MGB) indicating reduced GABA synthesis. High-affinity GABAA receptors containing the delta subunit were reduced almost to half, and the membrane distribution of the high-affinity alpha4/delta GABAA receptor subunit combination was altered with age. This high-affinity GABAA receptor mediates long-lasting tonic inhibition which was found to be reduced with aging in the MGB, along with fast, phasic GABAergic inhibition (Richardson et al. 2013). Age-diminished tonic inhibition in the auditory thalamus may alter excitability, increasing temporal uncertainty and reducing coding fidelity.
Aging in the auditory cortex also involves maladaptive homeostatic synaptic plasticity, with inhibition imbalance, primarily involving GABAergic neurotransmission (Caspary et al. 2008). GAD is significantly decreased in the primary auditory cortex, actually more so than in other regions of the neocortex (Burianova et al. 2009; Ling et al. 2005). This likely involves downregulation of GABA synthesis rather than actual loss of GABAergic neurons in the cortex. Whether this represents an effect of aging, peripheral hearing, loss or both is unclear, although there seem to be no differences between aged rats from strains differing in progression of hearing loss (Burianova et al. 2009), indicating brain aging itself. Defective GABA synthesis may impact synaptic release, and this is mirrored by age-altered expression of GABAA receptors in neurons in the auditory cortex. The expression of protein subunits involved in the assembly of functional GABAA receptors in the cortex (alpha1, beta2, gamma2) has been recently found to be downregulated as a consequence of aging, with concomitant upregulation of the alpha3 subunit, suggesting compensatory changes in receptor composition and kinetics (Caspary et al. 2013), comparable in part to those found in the IC where the expression of the alpha3 subunit is also increased with age. However, compensatory GABA inhibition may include circuit-specific mechanisms, as increases in gamma1 subunit expression found in the IC (Caspary et al. 2008) do not seem to be present in the auditory cortex.
GABA downregulation with inadequate postsynaptic balancing results in profound age-altered inhibition at the level of the auditory cortex. This is likely reflected in age-related threshold increase, increases in spontaneous and evoked activity in the auditory cortex (Juarez-Salinas et al. 2010), modified receptive fields (Turner et al. 2005; Juarez-Salinas et al. 2010), and loss of spectral precision and directional selectivity. This decreases the ability to localize sounds and extract salient signals from complex acoustic backgrounds, resulting in degraded speech understanding (Caspary et al. 2008). There seems to be a direct association between age-related disruption in GABAergic neurotransmission and changes in signal coding at the level of the auditory cortex.
The critical role of Ca++ in the regulation of multiple basic cellular processes, including neuronal excitability, is well known, and Ca++ dysregulation is a central element of cellular aging (López-Otín et al. 2013). Neuronal Ca++ regulation is likely altered at all levels of the aging central auditory pathway. Calcium-binding proteins (CaBP) are essential to maintain intracellular Ca++ concentration within the narrow margins required for physiological functions. Major CaBP in the brain are parvalbumin, calbindin (calbindin D28k), and calretinin. There have been numerous reports of changes in the expression of these three CaBP in the aging auditory pathway (Burianova et al. 2009; see Ouda et al. 2008 and Ouda and Syka 2012 for review). A particularly relevant example is provided by parvalbumin, the most abundant CaBP in “core” areas of the auditory pathway, involved in fast auditory sensory processing. With few exceptions, parvalbumin expression increases with age in many neurons throughout the auditory pathway. This is probably a consequence of neuronal aging, aggravated and/or induced by diminished peripheral auditory inputs (Idrizbegovic et al. 2003; Ouda et al. 2008; Gray et al. 2014). Large increases in parvalbumin levels have been recently reported in the rhesus monkey during aging from the cochlear nucleus to the auditory thalamus (Gray et al. 2013, 2014; Engle et al. 2014). This highlights even more the relevance of altered neuronal Ca++ homeostasis in human ARHL. Increased levels of parvalbumin in neurons and/or increased numbers of neurons expressing parvalbumin during aging, along with concomitant changes in the expression of other CaBP, may be essential for buffering excessive intracellular free Ca++. Excessive neuronal accumulation of intracellular Ca++ during aging may originate from mechanisms such as mitochondrial dysfunction (see above) or unbalanced excitability with increased Ca++ influx (Cataldi 2013). Unusually high expression of parvalbumin in aging auditory neurons, actually higher than in other aging sensory systems, along with changes in other CaBP, may protect neurons from toxic excess of intracellular free Ca++. On the other hand, a very large number of GABAergic neurons, particularly in the IC and auditory cortex, also express parvalbumin. Increased parvalbumin expression in GABAergic neurons might alter their excitability through Ca++ buffering, thus contributing to inhibitory-excitatory imbalances during aging. However, regulatory compensation of Ca++ homeostasis in the aging auditory pathway may have other levels of complexity. The auditory cortex is unique in that changes in parvalbumin levels are not homogeneous, but rather field- and cortical-layer specific (Martin del Campo et al. 2012). Although parvalbumin expression is increased in deep cortical layers, it tends to diminish in GABAergic interneurons (Ouda and Syka 2012; Ouellet and de Villers-Sidani 2014), with no apparent neuronal loss. The varying age-related changes in auditory cortical inhibitory circuits may reflect a need-based plasticity. Thus, parvalbumin levels in GABAergic interneurons in the auditory cortex of the rat recover normal levels with training (de Villers-Sidani et al. 2010).
Besides adaptive changes in the expression of CaBP, other mechanisms also may contribute to Ca++ regulation with aging. It is interesting that primary excitatory synapses on spherical cells in the CN with age adaptively switch the normal subunit composition of their postsynaptic receptors from highly permeable to Ca++ to receptors impermeable to Ca++ (AMPA class of glutamate receptors enriched in the GluR2 subunit; see Wang and Manis 2005). This could be a very interesting general adaptive synaptic mechanism of aging which remains to be fully explored.
Central mechanisms of auditory aging arise from both defective synaptic input reflecting an aging auditory receptor and the effects of aging in the brain. Although both may overlap considerably, establishing the sequence and hierarchies of both mechanisms at different levels of the auditory pathway will impact future treatments of presbycusis. Central presbycusis may be partially seen as a synaptopathy with altered neuronal excitability that could be subject to pharmacological treatments. Behaviors and agents, e.g., antioxidants, that may prevent stress-induced peripheral pathology through life may provide the best strategy to protect the inner ear from age-related changes.
References
Agrawal Y, Platz EA, Niparko JK (2008) Prevalence of hearing loss and differences by demographic characteristics among US adults: data from the National Health and Nutrition Examination Survey, 1999–2004. Arch Intern Med 168:1522–1530
Alam SA, Oshima T, Suzuki M et al (2001) The expression of apoptosis-related proteins in the aged cochlea of Mongolian gerbils. Laryngoscope 111:528–534
Altschuler RA, Fairfield D, Cho Y et al (2002) Stress pathways in the rat cochlea and potential for protection from acquired deafness. Audiol Neurootol 7:152–156
Alvarado JC, Fuentes-Santamaría V, Gabaldón-Ull MC et al (2014) Wistar rats: a forgotten model of age-related hearing loss. Front Aging Neurosci 6:29
Anderson S, Parbery-Clark A, White-Schwoch T, Kraus N (2012) Aging affects neural precision of speech encoding. J Neurosci 32:14156–14164
Bai U, Seidman MD, Hinojosa R, Quirk WS (1997) Mitochondrial DNA deletions associated with aging and possibly presbycusis: a human archival temporal bone study. Am J Otol 18:449–453
Balaban RS, Nemoto S, Finkel T (2005) Mitochondria, oxidants, and aging. Cell 120:483–495
Bano D, Agostini M, Melino G, Nicotera P (2011) Ageing, neuronal connectivity and brain disorders: an unsolved ripple effect. Mol Neurobiol 43:124–130
Bao J, Ohlemiller KK (2010) Age-related loss of spiral ganglion neurons. Hear Res 264:93–97
Bielefeld EC, Coling D, Chen G-D, Henderson D (2008) Multiple dosing strategies with acetyl L-carnitine (ALCAR) fail to alter age-related hearing loss in the Fischer 344/NHsd rat. J Negat Results Biomed 7:4
Boettcher FA, White DR, Mills JH, Schmiedt BN (1995) Age-related changes in auditory evoked potentials of gerbils. III. Low-frequency responses and repetition rate effects. Hear Res 87:208–219
Böttger EC, Schacht J (2013) The mitochondrion: a perpetrator of acquired hearing loss. Hear Res 303:12–19
Brand MD (2010) The sites and topology of mitochondrial superoxide production. Exp Gerontol 45:466–472
Bratic A, Larsson N-G (2013) The role of mitochondria in aging. J Clin Invest 123:951–957
Buckiova D, Popelar J, Syka J (2006) Collagen changes in the cochlea of aged Fischer 344 rats. Exp Gerontol 41:296–302
Buckiova D, Popelar J, Syka J (2007) Aging cochleas in the F344 rat: morphological and functional changes. Exp Gerontol 42:629–638
Burianova J, Ouda L, Profant O, Syka J (2009) Age-related changes in GAD levels in the central auditory system of the rat. Exp Gerontol 44:161–169
Caspary DM, Holder TM, Hughes LF et al (1999) Age-related changes in GABA(A) receptor subunit composition and function in rat auditory system. Neuroscience 93:307–312
Caspary DM, Hughes LF, Ling LL (2013) Age-related GABAA receptor changes in rat auditory cortex. Neurobiol Aging 34:1486–1496
Caspary DM, Ling L, Turner JG, Hughes LF (2008) Inhibitory neurotransmission, plasticity and aging in the mammalian central auditory system. J Exp Biol 211:1781–1791
Caspary DM, Schatteman TA, Hughes LF (2005) Age-related changes in the inhibitory response properties of dorsal cochlear nucleus output neurons: role of inhibitory inputs. J Neurosci 25:10952–10959
Cataldi M (2013) The changing landscape of voltage-gated calcium channels in neurovascular disorders and in neurodegenerative diseases. Curr Neuropharmacol 11:276–297
Choi Y-H, Miller JM, Tucker KL et al (2014) Antioxidant vitamins and magnesium and the risk of hearing loss in the US general population. Am J Clin Nutr 99:148–155
Coling D, Chen S, Chi L-H et al (2009) Age-related changes in antioxidant enzymes related to hydrogen peroxide metabolism in rat inner ear. Neurosci Lett 464:22–25
Cruickshanks KJ, Klein R, Klein BE et al (1998a) Cigarette smoking and hearing loss: the epidemiology of hearing loss study. JAMA 279:1715–1719
Cruickshanks KJ, Wiley TL, Tweed TS et al (1998b) Prevalence of hearing loss in older adults in Beaver Dam Wisconsin. The epidemiology of hearing loss study. Am J Epidemiol 148:879–886
Dai P, Yang W, Jiang S et al (2004) Correlation of cochlear blood supply with mitochondrial DNA common deletion in presbycusis. Acta Otolaryngol (Stockh) 124:130–136
De Villers-Sidani E, Alzghoul L, Zhou X et al (2010) Recovery of functional and structural age-related changes in the rat primary auditory cortex with operant training. Proc Natl Acad Sci U S A 107:13900–13905
Dröse S, Brandt U (2012) Molecular mechanisms of superoxide production by the mitochondrial respiratory chain. Adv Exp Med Biol 748:145–169
Durga J, Verhoef P, Anteunis LJ, Schouten E, Kok FJ (2007) Effects of folic acid supplementation on hearing in older adults: a randomized, controlled trial. Ann Intern Med 146:1–9
Efeyan A, Zoncu R, Sabatini DM (2012) Amino acids and mTORC1: from lysosomes to disease. Trends Mol Med 18:524–533
Engle JR, Gray DT, Turner H et al (2014) Age-related neurochemical changes in the rhesus macaque inferior colliculus. Front Aging Neurosci 6:73
Engle JR, Tinling S, Recanzone GH (2013) Age-related hearing loss in rhesus monkeys is correlated with cochlear histopathologies. PLoS One 8:e55092
Fairfield DA, Kanicki AC, Lomax MI, Altschuler RA (2002) Expression and localization of heat shock factor (Hsf) 1 in the rodent cochlea. Hear Res 173:109–118
Fairfield DA, Kanicki AC, Lomax MI, Altschuler RA (2004) Induction of heat shock protein 32 (Hsp32) in the rat cochlea following hyperthermia. Hear Res 188:1–11
Fairfield DA, Lomax MI, Dootz GA et al (2005) Heat shock factor 1-deficient mice exhibit decreased recovery of hearing following noise overstimulation. J Neurosci Res 81:589–596
Ferraro JA, Minckler J (1977) The human lateral lemniscus and its nuclei. Brain Lang 4:156–164
Fetoni AR, Picciotti PM, Paludetti G, Troiani D (2011) Pathogenesis of presbycusis in animal models: a review. Exp Gerontol 46:413–425
Finkel T (2012) Signal transduction by mitochondrial oxidants. J Biol Chem 287:4434–4440
Finlayson PG (1995) Decreased inhibition to lateral superior olive neurons in young and aged Sprague-Dawley rats. Hear Res 87:84–95
Fischel-Ghodsian N (2003) Mitochondrial hearing impairment. Audiol Med 1:56–66
Fischel-Ghodsian N, Bykhovskaya Y, Taylor K et al (1997) Temporal bone analysis of patients with presbycusis reveals high frequency of mitochondrial mutations. Hear Res 110:147–154
Friedman LM, Avraham KB (2009) MicroRNAs and epigenetic regulation in the mammalian inner ear: implications for deafness. Mamm Genome 20:581–603
Frisina RD, Walton JP (2001) Aging of the mouse central auditory system. In: Willott JP (ed) From behavior to molecular biology. CRC, New York, pp 339–379
Frisina RD, Walton JP (2006) Age-related structural and functional changes in the cochlear nucleus. Hear Res 216–217:216–223
Garinis GA, van der Horst GTJ, Vijg J, Hoeijmakers JHJ (2008) DNA damage and ageing: new-age ideas for an age-old problem. Nat Cell Biol 10:1241–1247
Gates GA (2012) Central presbycusis: an emerging view. Otolaryngol Head Neck Surg 147:1–2
Gates GA, Mills JH (2005) Presbycusis. Lancet 366:1111–1120
Gems D, Partridge L (2013) Genetics of longevity in model organisms: debates and paradigm shifts. Annu Rev Physiol 75:621–644
Glasauer A, Chandel NS (2013) ROS. Curr Biol 23:R100–R102
Gleich O, Netz J, Strutz J (2014) Comparing the inferior colliculus of young and old gerbils (Meriones unguiculatus) with an emphasis on GABA. Exp Gerontol 57C:155–162
Gold M, Rapin I (1994) Non-Mendelian mitochondrial inheritance as a cause of progressive genetic sensorineural hearing loss. Int J Pediatr Otorhinolaryngol 30:91–104
Gong T-W, Fairfield DA, Fullarton L et al (2012) Induction of heat shock proteins by hyperthermia and noise overstimulation in hsf1 -/- mice. J Assoc Res Otolaryngol 13:29–37
Gopinath B, Rochtchina E, Wang JJ et al (2009) Prevalence of age-related hearing loss in older adults: Blue Mountains Study. Arch Intern Med 169:415–416
Gratton MA, Schmiedt RA, Schulte BA (1996) Age-related decreases in endocochlear potential are associated with vascular abnormalities in the stria vascularis. Hear Res 102:181–190
Gratton MA, Schulte BA (1995) Alterations in microvasculature are associated with atrophy of the stria vascularis in quiet-aged gerbils. Hear Res 82:44–52
Gray DT, Engle JR, Recanzone GH (2014) Age-related neurochemical changes in the rhesus macaque cochlear nucleus. J Comp Neurol 522:1527–1541
Gray DT, Rudolph ML, Engle JR, Recanzone GH (2013) Parvalbumin increases in the medial and lateral geniculate nuclei of aged rhesus macaques. Front Aging Neurosci 5:69
Gutiérrez A, Khan ZU, Morris SJ, De Blas AL (1994) Age-related decrease of GABAA receptor subunits and glutamic acid decarboxylase in the rat inferior colliculus. J Neurosci 14:7469–7477
Han C, Someya S (2013) Maintaining good hearing: calorie restriction, Sirt3, and glutathione. Exp Gerontol 48:1091–1095
Harrison DE, Strong R, Sharp ZD et al (2009) Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature 460:392–395
Haupt H, Scheibe F (2002) Preventive magnesium supplement protects the inner ear against noise-induced impairment of blood flow and oxygenation in the guinea pig. Magnes Res 15:17–25
Helfert RH, Krenning J, Wilson TS, Hughes LF (2003) Age-related synaptic changes in the anteroventral cochlear nucleus of Fischer-344 rats. Hear Res 183:18–28
Helfert RH, Sommer TJ, Meeks J et al (1999) Age-related synaptic changes in the central nucleus of the inferior colliculus of Fischer-344 rats. J Comp Neurol 406:285–298
Heman-Ackah SE, Juhn SK, Huang TC, Wiedmann TS (2010) A combination antioxidant therapy prevents age-related hearing loss in C57BL/6 mice. Otolaryngol Head Neck Surg 143:429–434
Hinojosa R, Nelson EG (2011) Cochlear nucleus neuron analysis in individuals with presbycusis. Laryngoscope 121:2641–2648
Houtkooper RH, Pirinen E, Auwerx J (2012) Sirtuins as regulators of metabolism and healthspan. Nat Rev Mol Cell Biol 13:225–238
Houtkooper RH, Williams RW, Auwerx J (2010) Metabolic networks of longevity. Cell 142:9–14
Hu BH, Yang W-P, Bielefeld EC et al (2008) Apoptotic outer hair cell death in the cochleae of aging Fischer 344/NHsd rats. Hear Res 245:48–57
Idrizbegovic E, Bogdanovic N, Viberg A, Canlon B (2003) Auditory peripheral influences on calcium binding protein immunoreactivity in the cochlear nucleus during aging in the C57BL/6J mouse. Hear Res 179:33–42
Jiang H, Talaska AE, Schacht J, Sha S-H (2007) Oxidative imbalance in the aging inner ear. Neurobiol Aging 28:1605–1612
Juarez-Salinas DL, Engle JR, Navarro XO, Recanzone GH (2010) Hierarchical and serial processing in the spatial auditory cortical pathway is degraded by natural aging. J Neurosci 30:14795–14804
Juiz JM, Helfert RH, Bonneau JM et al (1996) Three classes of inhibitory amino acid terminals in the cochlear nucleus of the guinea pig. J Comp Neurol 373:11–26
Juiz JM, Rueda J, Merchán JA, Sala ML (1989) The effects of kainic acid on the cochlear ganglion of the rat. Hear Res 40:65–74
Kazee AM, Han LY, Spongr VP et al (1995) Synaptic loss in the central nucleus of the inferior colliculus correlates with sensorineural hearing loss in the C57BL/6 mouse model of presbycusis. Hear Res 89:109–120
Kazee AM, West NR (1999) Preservation of synapses on principal cells of the central nucleus of the inferior colliculus with aging in the CBA mouse. Hear Res 133:98–106
Keithley EM, Canto C, Zheng QY et al (2005) Cu/Zn superoxide dismutase and age-related hearing loss. Hear Res 209:76–85
Keithley EM, Croskrey KL (1990) Spiral ganglion cell endings in the cochlear nucleus of young and old rats. Hear Res 49:169–177
Kim TS, Chung JW (2013) Evaluation of age-related hearing loss. Korean J Audiol 17:50–53
Kirkwood TBL (2005) Understanding the odd science of aging. Cell 120:437–447
Kowaltowski AJ, de Souza-Pinto NC, Castilho RF, Vercesi AE (2009) Mitochondria and reactive oxygen species. Free Radic Biol Med 47:333–343
Kujawa SG, Liberman MC (2006) Acceleration of age-related hearing loss by early noise exposure: evidence of a misspent youth. J Neurosci 26:2115–2123
Kujoth GC, Hiona A, Pugh TD et al (2005) Mitochondrial DNA mutations, oxidative stress, and apoptosis in mammalian aging. Science 309:481–484
Le Prell CG, Gagnon PM, Bennett DC, Ohlemiller KK (2011) Nutrient-enhanced diet reduces noise-induced damage to the inner ear and hearing loss. Transl Res 158:38–53
Le Prell CG, Hughes LF, Miller JM (2007) Free radical scavengers vitamins A, C, and E plus magnesium reduce noise trauma. Free Radic Biol Med 42:1454–1463
Le Prell CG, Ojano-Dirain C, Rudnick EW et al (2014) Assessment of nutrient supplement to reduce gentamicin-induced ototoxicity. J Assoc Res Otolaryngol 15:375–393
Lee H-C, Wei Y-H (2012) Mitochondria and aging. Adv Exp Med Biol 942:311–327
Lenaz G, Baracca A, Fato R et al (2006) New insights into structure and function of mitochondria and their role in aging and disease. Antioxid Redox Signal 8:417–437
Lim R, Alvarez FJ, Walmsley B (2000) GABA mediates presynaptic inhibition at glycinergic synapses in a rat auditory brainstem nucleus. J Physiol 525(Pt 2):447–459
Lin FR, Maas P, Chien W et al (2012) Association of skin color, race/ethnicity, and hearing loss among adults in the USA. J Assoc Res Otolaryngol 13:109–117
Lin FR, Thorpe R, Gordon-Salant S, Ferrucci L (2011) Hearing loss prevalence and risk factors among older adults in the United States. J Gerontol A Biol Sci Med Sci 66:582–590
Lin FR, Yaffe K, Xia J et al (2013) Hearing loss and cognitive decline in older adults. JAMA 173:293–299
Lin MT, Beal MF (2006) Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 443:787–795
Ling LL, Hughes LF, Caspary DM (2005) Age-related loss of the GABA synthetic enzyme glutamic acid decarboxylase in rat primary auditory cortex. Neuroscience 132:1103–1113
Liochev SI (2013) Reactive oxygen species and the free radical theory of aging. Free Radic Biol Med 60:1–4
López-Otín C, Blasco MA, Partridge L et al (2013) The hallmarks of aging. Cell 153:1194–1217
Marcus DC, Thalmann R, Marcus NY (1978) Respiratory rate and ATP content of stria vascularis of guinea pig in vitro. Laryngoscope 88:1825–1835
Markaryan A, Nelson EG, Hinojosa R (2009) Quantification of the mitochondrial DNA common deletion in presbycusis. Laryngoscope 119:1184–1189
Markaryan A, Nelson EG, Hinojosa R (2010) Major arc mitochondrial DNA deletions in cytochrome c oxidase-deficient human cochlear spiral ganglion cells. Acta Otolaryngol (Stockh) 130:780–787
Marlenga B, Berg RL, Linneman JG et al (2012) Determinants of early-stage hearing loss among a cohort of young workers with 16-year follow-up. Occup Environ Med 69:479–484
Martin del Campo HN, Measor KR, Razak KA (2012) Parvalbumin immunoreactivity in the auditory cortex of a mouse model of presbycusis. Hear Res 294:31–39
McFadden SL, Ding D, Burkard RF et al (1999a) Cu/Zn SOD deficiency potentiates hearing loss and cochlear pathology in aged 129, CD-1 mice. J Comp Neurol 413:101–112
McFadden SL, Ding D, Reaume AG et al (1999b) Age-related cochlear hair cell loss is enhanced in mice lacking copper/zinc superoxide dismutase. Neurobiol Aging 20:1–8
Menardo J, Tang Y, Ladrech S et al (2012) Oxidative stress, inflammation, and autophagic stress as the key mechanisms of premature age-related hearing loss in SAMP8 mouse Cochlea. Antioxid Redox Signal 16:263–274
Mikuriya T, Sugahara K, Sugimoto K et al (2008) Attenuation of progressive hearing loss in a model of age-related hearing loss by a heat shock protein inducer, geranylgeranylacetone. Brain Res 1212:9–17
Milbrandt JC, Albin RL, Caspary DM (1994) Age-related decrease in GABAB receptor binding in the Fischer 344 rat inferior colliculus. Neurobiol Aging 15:699–703
Milbrandt JC, Hunter C, Caspary DM (1997) Alterations of GABAA receptor subunit mRNA levels in the aging Fischer 344 rat inferior colliculus. J Comp Neurol 379:455–465
Murphy MP (2009) How mitochondria produce reactive oxygen species. Biochem J 417:1–13
Nevado J, Sanz R, Casqueiro JC et al (2006) Ageing evokes an intrinsic pro-apoptotic signalling pathway in rat cochlea. Acta Otolaryngol (Stockh) 126:1134–1139
Ohlemiller KK (2009) Mechanisms and genes in human strial presbycusis from animal models. Brain Res 1277:70–83
Ohlemiller KK, Frisina RD (2008) Age-related hearing loss and its cellular and molecular bases. In: Schacht J, Popper AN, Fay RR (eds) Auditory trauma, protection and repair. Springer, New York, pp 145–194
Ouda L, Druga R, Syka J (2008) Changes in parvalbumin immunoreactivity with aging in the central auditory system of the rat. Exp Gerontol 43:782–789
Ouda L, Syka J (2012) Immunocytochemical profiles of inferior colliculus neurons in the rat and their changes with aging. Front Neural Circuits 6:68
Ouellet L, de Villers-Sidani E (2014) Trajectory of the main GABAergic interneuron populations from early development to old age in the rat primary auditory cortex. Front Neuroanat 8:40
Parham K, McKinnon BJ, Eibling D, Gates GA (2011) Challenges and opportunities in presbycusis. Otolaryngol Head Neck Surg 144:491–495
Picciotti P, Torsello A, Wolf FI et al (2004) Age-dependent modifications of expression level of VEGF and its receptors in the inner ear. Exp Gerontol 39:1253–1258
Prazma J, Carrasco VN, Butler B et al (1990) Cochlear microcirculation in young and old gerbils. Arch Otolaryngol Head Neck Surg 116:932–936
Provenzano MJ, Domann FE (2007) A role for epigenetics in hearing: establishment and maintenance of auditory specific gene expression patterns. Hear Res 233:1–13
Pujol R, Rebillard G, Puel JL et al (1991) Glutamate neurotoxicity in the cochlea: a possible consequence of ischaemic or anoxic conditions occurring in ageing. Acta Otolaryngol (Stockh) Suppl 476:32–36
Ray PD, Huang B-W, Tsuji Y (2012) Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal 24:981–990
Raza A, Milbrandt JC, Arneric SP, Caspary DM (1994) Age-related changes in brainstem auditory neurotransmitters: measures of GABA and acetylcholine function. Hear Res 77:221–230
Richardson BD, Ling LL, Uteshev VV, Caspary DM (2013) Reduced GABA(A) receptor-mediated tonic inhibition in aged rat auditory thalamus. J Neurosci 33:1218–1227a
Riquelme R, Cediel R, Contreras J et al (2010) A comparative study of age-related hearing loss in wild type and insulin-like growth factor I deficient mice. Front Neuroanat 4:27
Rossman TG, Klein CB (2011) Genetic and epigenetic effects of environmental arsenicals. Met Integr Biometal Sci 3:1135–1141
Ruben RJ (2000) Redefining the survival of the fittest: communication disorders in the 21st century. Laryngoscope 110:241–245
Sakaguchi N, Spicer SS, Thomopoulos GN, Schulte BA (1997a) Increased laminin deposition in capillaries of the stria vascularis of quiet-aged gerbils. Hear Res 105:44–56
Sakaguchi N, Spicer SS, Thomopoulos GN, Schulte BA (1997b) Immunoglobulin deposition in thickened basement membranes of aging strial capillaries. Hear Res 109:83–91
Scheibe F, Haupt H, Vlastos GA (2000) Preventive magnesium supplement reduces ischemia-induced hearing loss and blood viscosity in the guinea pig. Eur Arch Otorhinolaryngol 257:355–361
Schmiedt RA, Mills JH, Boettcher FA (1996) Age-related loss of activity of auditory-nerve fibers. J Neurophysiol 76:2799–2803
Schmiedt RA (1996) Effects of aging on potassium homeostasis and the endocochlear potential in the gerbil cochlea. Hear Res 102:125–132
Schmiedt RA (2010) The physiology of cochlear presbycusis. In: Gordon-Salant S, Frisina RD, Popper AN, Fay RR (eds) Aging auditory system. Springer, New York, pp 9–38
Schon EA, Przedborski S (2011) Mitochondria: the next (neurode)generation. Neuron 70:1033–1053
Schucknecht HF (1955) Presbycusis. Laryngoscope 65:402–419
Schucknecht HF (1964) Further observations on the pathology of presbycusis. Arch Otolaryngol Chic Ill 80:369–382
Schuknecht HF, Gacek MR (1993) Cochlear pathology in presbycusis. Ann Otol Rhinol Laryngol 102:1–16
Schulte BA, Schmiedt RA (1992) Lateral wall Na, K-ATPase and endocochlear potentials decline with age in quiet-reared gerbils. Hear Res 61:35–46
Schumacher B, Garinis GA, Hoeijmakers JHJ (2008) Age to survive: DNA damage and aging. Trends Genet TIG 24:77–85
Seidman MD (2000) Effects of dietary restriction and antioxidants on presbyacusis. Laryngoscope 110:727–738
Seidman MD, Ahmad N, Joshi D et al (2004) Age-related hearing loss and its association with reactive oxygen species and mitochondrial DNA damage. Acta Oto-Laryngol Suppl 16–24
Seidman MD, Bai U, Khan MJ, Quirk WS (1997) Mitochondrial DNA deletions associated with aging and presbyacusis. Arch Otolaryngol Head Neck Surg 123:1039–1045
Seidman MD, Khan MJ, Dolan DF, Quirk WS (1996) Age-related differences in cochlear microcirculation and auditory brain stem response. Arch Otolaryngol Head Neck Surg 122:1221–1226
Seidman MD, Khan MJ, Tang WX, Quirk WS (2002) Influence of lecithin on mitochondrial DNA and age-related hearing loss. Otolaryngol Head Neck Surg 127:138–144
Sena LA, Chandel NS (2012) Physiological roles of mitochondrial reactive oxygen species. Mol Cell 48:158–167
Sendowski I (2006) Magnesium therapy in acoustic trauma. Magnes Res 19:244–254
Sergeyenko Y, Lall K, Liberman MC, Kujawa SG (2013) Age-related cochlear synaptopathy: an early-onset contributor to auditory functional decline. J Neurosci 33:13686–13694
Sha S-H, Chen F-Q, Schacht J (2009) Activation of cell death pathways in the inner ear of the aging CBA/J mouse. Hear Res 254:92–99
Sha S-H, Kanicki A, Halsey K et al (2012) Antioxidant-enriched diet does not delay the progression of age-related hearing loss. Neurobiol Aging 33:1010.e15–1010.e16
Sha SH, Taylor R, Forge A, Schacht J (2001) Differential vulnerability of basal and apical hair cells is based on intrinsic susceptibility to free radicals. Hear Res 155:1–8
Shankar SK (2010) Biology of aging brain. Indian J Pathol Microbiol 53:595–604
Shargorodsky J, Curhan SG, Eavey R, Curhan GC (2010) A prospective study of vitamin intake and the risk of hearing loss in men. Otolaryngol Head Neck Surg 142:231–236
Sharma S, Nag TC, Thakar A et al (2014) The aging human cochlear nucleus: changes in the glial fibrillary acidic protein, intracellular calcium regulatory proteins, GABA neurotransmitter and cholinergic receptor. J Chem Neuroanat 56:1–12
Shi X (2011) Physiopathology of the cochlear microcirculation. Hear Res 282:10–24
Shim HJ, Lee LH, Huh Y et al (2012) Age-related changes in the expression of NMDA, serotonin, and GAD in the central auditory system of the rat. Acta Otolaryngol (Stockh) 132:44–50
Someya S, Prolla TA (2010) Mitochondrial oxidative damage and apoptosis in age-related hearing loss. Mech Ageing Dev 131:480–486
Someya S, Xu J, Kondo K et al (2009) Age-related hearing loss in C57BL/6J mice is mediated by Bak-dependent mitochondrial apoptosis. Proc Natl Acad Sci U S A 106:19432–19437
Someya S, Yu W, Hallows WC et al (2010) Sirt3 mediates reduction of oxidative damage and prevention of age-related hearing loss under caloric restriction. Cell 143:802–812
Spicer SS, Schulte BA (2002) Spiral ligament pathology in quiet-aged gerbils. Hear Res 172:172–185
Spicer SS, Schulte BA (2005) Pathologic changes of presbycusis begin in secondary processes and spread to primary processes of strial marginal cells. Hear Res 205:225–240
Sugahara K, Inouye S, Izu H et al (2003) Heat shock transcription factor HSF1 is required for survival of sensory hair cells against acoustic overexposure. Hear Res 182:88–96
Szafranski K, Mekhail K (2014) The fine line between lifespan extension and shortening in response to caloric restriction. Nucl Austin Tex 5:56–65
Tadros SF, D’Souza M, Zhu X, Frisina RD (2008) Apoptosis-related genes change their expression with age and hearing loss in the mouse cochlea. Apoptosis Int J Program Cell Death 13:1303–1321
Thomopoulos GN, Spicer SS, Gratton MA, Schulte BA (1997) Age-related thickening of basement membrane in stria vascularis capillaries. Hear Res 111:31–41
Trifunovic A, Hansson A, Wredenberg A et al (2005) Somatic mtDNA mutations cause aging phenotypes without affecting reactive oxygen species production. Proc Natl Acad Sci U S A 102:17993–17998
Trifunovic A, Wredenberg A, Falkenberg M et al (2004) Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature 429:417–423
Turner JG, Hughes LF, Caspary DM (2005) Affects of aging on receptive fields in rat primary auditory cortex layer V neurons. J Neurophysiol 94:2738–2747
Usami S, Takumi Y, Fujita S et al (1997) Cell death in the inner ear associated with aging is apoptosis? Brain Res 747:147–150
Varela-Nieto I, Murillo-Cuesta S, Rodríguez-de la Rosa L et al (2013) IGF-I deficiency and hearing loss: molecular clues and clinical implications. Pediatr Endocrinol Rev 10:460–472
Viña J, Borras C, Abdelaziz KM et al (2013) The free radical theory of aging revisited: the cell signaling disruption theory of aging. Antioxid Redox Signal 19:779–787
Wang H, Turner JG, Ling L et al (2009) Age-related changes in glycine receptor subunit composition and binding in dorsal cochlear nucleus. Neuroscience 160:227–239
Wang J, Menchenton T, Yin S et al (2010) Over-expression of X-linked inhibitor of apoptosis protein slows presbycusis in C57BL/6J mice. Neurobiol Aging 31:1238–1249
Wang Y, Manis PB (2005) Synaptic transmission at the cochlear nucleus endbulb synapse during age-related hearing loss in mice. J Neurophysiol 94:1814–1824
Wang Y, Manis PB (2006) Temporal coding by cochlear nucleus bushy cells in DBA/2J mice with early onset hearing loss. J Assoc Res Otolaryngol 7:412–424
Watanabe K-I, Bloch W (2013) Histone methylation and acetylation indicates epigenetic change in the aged cochlea of mice. Eur Arch Oto Rhino Laryngol 270:1823–1830
Willott JF, Aitkin LM, McFadden SL (1993) Plasticity of auditory cortex associated with sensorineural hearing loss in adult C57BL/6J mice. J Comp Neurol 329:402–411
Willott JF, Bross LS (1990) Morphology of the octopus cell area of the cochlear nucleus in young and aging C57BL/6J and CBA/J mice. J Comp Neurol 300:61–81
Willott JF, Bross LS, McFadden SL (1992) Morphology of the dorsal cochlear nucleus in C57BL/6J and CBA/J mice across the life span. J Comp Neurol 321:666–678
Willott JF, Bross LS, McFadden SL (1994) Morphology of the inferior colliculus in C57BL/6J and CBA/J mice across the life span. Neurobiol Aging 15:175–183
Willott JF, Jackson LM, Hunter KP (1987) Morphometric study of the anteroventral cochlear nucleus of two mouse models of presbycusis. J Comp Neurol 260:472–480
Willott JF, Milbrandt JC, Bross LS, Caspary DM (1997) Glycine immunoreactivity and receptor binding in the cochlear nucleus of C57BL/6J and CBA/CaJ mice: effects of cochlear impairment and aging. J Comp Neurol 385:405–414
Willott JF, Pankow D, Hunter KP, Kordyban M (1985) Projections from the anterior ventral cochlear nucleus to the central nucleus of the inferior colliculus in young and aging C57BL/6 mice. J Comp Neurol 237:545–551
Willott JF, Parham K, Hunter KP (1991) Comparison of the auditory sensitivity of neurons in the cochlear nucleus and inferior colliculus of young and aging C57BL/6J and CBA/J mice. Hear Res 53:78–94
World Health Organization (2014) WHO|Deafness and hearing loss, Fact Sheet No 300. In: WHO. http://www.who.int/mediacentre/factsheets/fs300/en/. Accessed 6 May 2014
Xie R, Manis PB (2013) Glycinergic synaptic transmission in the cochlear nucleus of mice with normal hearing and age-related hearing loss. J Neurophysiol 110:1848–1859
Yamasoba T, Lin FR, Someya S et al (2013) Current concepts in age-related hearing loss: epidemiology and mechanistic pathways. Hear Res 303:30–38
Yamasoba T, Someya S, Yamada C et al (2007) Role of mitochondrial dysfunction and mitochondrial DNA mutations in age-related hearing loss. Hear Res 226:185–193
Yang Z, Ming X-F (2012) mTOR signalling: the molecular interface connecting metabolic stress, aging and cardiovascular diseases. Obes Rev 13(Suppl 2):58–68
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Melgar-Rojas, P., Alvarado, J.C., Fuentes-Santamaría, V., Juiz, J.M. (2015). Cellular Mechanisms of Age-Related Hearing Loss. In: Miller, J., Le Prell, C., Rybak, L. (eds) Free Radicals in ENT Pathology. Oxidative Stress in Applied Basic Research and Clinical Practice. Humana Press, Cham. https://doi.org/10.1007/978-3-319-13473-4_15
Download citation
DOI: https://doi.org/10.1007/978-3-319-13473-4_15
Publisher Name: Humana Press, Cham
Print ISBN: 978-3-319-13472-7
Online ISBN: 978-3-319-13473-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)