Abstract
High salt concentration represents one of the most significant abiotic constraints, affecting all life forms including plants and cyanobacteria. Soil salinity curtails plant growth by way of osmotic, ionic and oxidative stresses resulting in multiple inhibitory effects on various physiological processes such as growth, photosynthesis, respiration and cellular metabolism. In order to combat high salinity, various adaptive strategies employed include ion homeostasis achieved by ion transport and compartmentalization of injurious ions, osmotic homeostasis by accumulation of compatible solutes/osmolytes and upregulation of antioxidant defence mechanism. The aforesaid processes are executed through SOS and MAPK signalling pathways leading to modulation of gene expression. Salt stress signal transduction pathways initiate through sensing extracellular Na+ ions causing modification of constitutively expressed transcription factors. This modification is responsible for expression of early transcriptional activators such as CBF/DREB gene family which eventually activate stress tolerance effector genes such as osmolyte biosynthesis genes, detoxification enzymes, and chaperones. Various genes/cDNAs encoding proteins involved in these adaptive mechanisms have been isolated and identified. Bioinformatic predictions through docking revealed interaction of salt across the species at conserved domains and motifs as a possible mechanism for response of a particular protein under salt stress. In this chapter, major aspects of salt stress are reviewed with emphasis on its detrimental consequences and biochemical and molecular mechanisms of signal transduction in plants and cyanobacteria under high salinity.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
4.1 Introduction
Salinity is one of the most widespread abiotic constraints curtailing plant growth and productivity. The source of soil salinization may be primary (natural) or secondary (anthropogenic). Nature-driven salinity may predominantly be due to (i) intrusion of highly salinized water in coastal or continental regions, (ii) deposition of wind- or rain-borne oceanic salts and (iii) weathering of parental rocks. In contrast, agricultural practices, e.g. fertigation (fertilizer application through irrigation) and irrigation with poor drainage, are considered as the major contributor to secondary salinization especially in arid and semiarid regions where higher rates of evapotranspiration cause solutes from the irrigation water to accumulate and eventually reach levels that have an adverse affect on plant growth. Current estimates indicate that 6 % of the world’s land and nearly 30 % of all irrigated lands are affected by salinity (UWP 2007).
The effect of salinity is widespread affecting all life forms including plants and cyanobacteria. Study suggests that salinity not only influences survival but also the distribution of cyanobacteria. While low salinity favours the presence of heterocystous cyanobacteria, high salinity mainly supports the survival of non-heterocystous genera (Srivastava et al. 2009). In plants also almost all aspects of developments including seed germination and vegetative and reproductive growth are adversely affected by high salt. On the basis of plant’s response to salt, they can be divided into two groups: halophytes and glycophytes. Halophytes are native to the saline habitat equipped with various adaptive mechanisms to thrive under that condition. However, a majority of cultivated plants including horticultural and cereal crops are glycophytes, relatively susceptible to excessive salt concentration.
Under saline environment, organisms employ a variety of mechanisms to maintain their osmotic status and ionic balance. Typically, NaCl is the most abundant salt, and in most cases the negative impact of soil salinity on different physiological processes of an organism is attributed to the increase in Na+ and Cl− concentration. In this review, effort has been made to collate the effects of salt, particularly of NaCl on various aspects of growth, mechanisms of salt signalling and variety of strategies employed by plants to cope with salinity stress. In view of close homology between cyanobacteria and plants and their relevance in agriculture, it was decided to include them in the present discussion. In view of the above, we also describe cyanobacterial responses to salt stress and compare with plants.
4.2 Effects of Salinity
Salinity-induced physiological aberrations are mainly because of two reasons: (i) elevated salt causes osmotic effect by reducing soil water potential, influences water and nutrient uptake and leads to cellular dehydration; (ii) a continuous flow of inorganic ions into the living cells occurs due to its high concentration in the surrounding environment, thus presenting ionic stress (Munns and Termaat 1986; Zhu 2001). 0.1 M of Na+ concentration is cytotoxic as it directly affects specific biochemical and physiological processes (Serrano 1996). Cl− accumulation 4–7 mg/g dry weight (DW) is critically toxic for Cl−-sensitive species and 15–50 mg/g DW for Cl−-tolerant species (Xu et al. 2000). Salt stress not only disturbs osmotic and ionic balance within the cell but also exerts some secondary effects (e.g. oxidative stress). Osmotic, ionic and oxidative stresses negatively affecting cell turgor, photosynthesis and transpiration, membrane constitution, and cellular metabolisms are reviewed under separate headings.
4.2.1 Cell Turgor and Water Relation
High salt content of rhizosphere instigates dramatic impact on osmotic relation between the cell interior and the surrounding medium resulting in reduced water uptake but enhanced efflux from the cell (Erdmann and Hagemann 2001). Water loss lowers the leaf water potential (ψ w) and consequently the leaf turgor potential (ψ p). Turgor loss is usually the earliest cellular response to water stress since even a decrease of 5–15 % of ψ w can cause large decrease in plant ψ p (Hsiao 1973; Turner 1979). Turgor reduction subsequently affects the turgor-dependent activities such as leaf expansion, root elongation and stomatal conductance which may correspond to reduction in transpiration, CO2 assimilation, plant water uptake and ultimately plant growth. These perturbations are aggravated in environments with high transpirational demands. During the latter phase of water stress, plant always tries to escape from dehydration by reducing osmotic potential (ψ s) and adjusting with osmolytes so as to maintain positive turgor despite low water potential associated with high rhizosphere salt concentration (Bolaños and Longstreth 1984). Maintaining cell turgor is crucial for proliferation of cyanobacteria which could be achieved by controlled accumulation of sucrose as its high concentration causes damaging effects (Ladas and Papageorgiou 2000). Thus, restoration of cell turgor or cell volume is one of the most important mechanisms to sustain growth and metabolism.
4.2.2 Nutrient Imbalance
Enhanced concentration of NaCl exhibits toxic effect on cell by promoting nutritional deficiencies. Nutritional disturbance may probably be due to (i) displacement of essential ions such as K+ and Ca2+ by Na+ from the cell surface, (ii) competitive Na+ uptake through non-specific ion channels and transporters and (iii) altered ion transport or partitioning within the cell (Erdmann and Hagemann 2001; Grattan and Grieve 1999). Mounting evidence points towards the reduced availability and uptake of K+, Ca2+, Mg2+, PO4 3− and NO3 − in salt-stressed organisms exposed to high salinity. The antagonistic effect of excess Na+ on Ca2+ and K+ uptake resulted in increase in Na+/Ca2+ and Na+/K+ ratio in salt-stressed plants and cyanobacterial species that adversely affect cellular metabolism and balanced ion relations.
4.2.3 Membranes
Biomembranes have always been the first to realize environmental fluctuations and respond accordingly by changing their lipid and protein composition. Membrane lipids such as sterols, phospholipids and fatty acids not only have profound role in regulating membrane fluidity and permeability but also responsible for the activity of membrane-associated channels/transporters (e.g. aquaporin) and enzymes (e.g. H+ ATPase). There are many examples highlighting importance of elevated sterol to phospholipid ratio in lipid bilayer to deal with the hypersaline condition which causes membrane rigidity and reduces NaCl permeability (Wu et al. 2005; Kerkeb et al. 2001; Alvarez-Pizarro et al. 2009). Free sterols, particularly the more planer ones (e.g. cholesterol, campesterol), play pivotal role in controlling membrane permeability (Kerkeb et al. 2001; Douglas and Walker 1984), and their increased level has been reported in many salt-tolerant plant species following exposure to high salinity (Douglas and Walker 1984; Yahya et al. 1995).
A relative compositional change in membrane phospholipids has been witnessed in response to salinity. A salt-tolerant plant generally has an increased ratio of phosphatidylcholine (PC) to phosphatidylethanolamine (PE) (Wu et al. 2005) while reverse is true for the sensitive plant (Norberg and Liljenberg 1991; Mansour et al. 2002). This is in accordance with the fact that PE in contrast to PC readily attains a non-lamellar-inverted hexagonal structure that may introduce hydrophilic water channels throughout the biological membrane (Caffrey 1985) altering its stability and permeability thereby making plants more vulnerable to salt stress.
Saturation level of membrane fatty acids generally increases with increasing salinity (Wu et al. 2005; Mansour et al. 2002). Though increased fatty acid saturation indicates reduced membrane fluidity and increased leakage, it cannot be considered as an absolute measure of salt sensitivity as changes in fatty acid saturation has been encountered in both halophytes and glycophytes. Alteration in membrane permeability owing to changes in fatty acid saturation was also observed in cyanobacteria. In some species such as Synechocystis (Huang et al. 2006) and Anacystis nidulans (Molitor et al. 1990), increased proportion of long-chain saturated fatty acids was observed. Whereas in an extreme halophyte alga Dunaliella salina when exposed to high salt, a considerable high ratio of unsaturated to saturated fatty acid was reported, thus indicating the protective role of unsaturated fatty acid in salt tolerance (Azachi et al. 2002). The role of unsaturation of fatty acids in membrane lipids in increasing resistance for photosynthetic machinery to salt-induced damage and in repair of Na+/H+ antiport system has also been acknowledged (Allakhverdiev et al. 2001).
4.2.4 Photosynthesis
Salinity evokes multiple inhibitory effects on photosynthesis which include alteration in photosynthetic pigments (Chl a, Chl b and carotenoids), photosystem efficiency, photophosphorylation and CO2 fixation. In both plants and cyanobacteria, similar effects on chlorophyll content and carotenoids observed were, namely, reduction in chlorophyll contents in salt-sensitive (Singh and Kshatriya 2002; Srivastava et al. 2005) and increase in salt-tolerant species (Saleh 2012; Lu and Vonshak 1999). Thus, chlorophyll content could be used as a parameter for selection of tolerant varieties of crop plants (Eryilmaz 2007). In many plants such as Zea mays, Carthamus tinctorius, bean and Paulownia imperialis, salt-induced reduction in chlorophyll was due to weakening of protein–pigment–lipid complex or increased chlorophyllase enzyme activity (Reddy and Vora 1986; Turan et al. 2007; Rahdari et al. 2012). In contrast, increment in pigment content in some rice cultivar (Doganlar et al. 2010) and purslane (Rahdari et al. 2012) was also reported. These may be due to an increase in the number of chloroplast in the salt-stressed plant leaves (Chaum and Kirdmanee 2009).
Phycobiliproteins (PBPs) specific to cyanobacteria serve as the accessory light-harvesting antenna for PSII and PSI. Salt stress has inhibitory effect on PBPs’ content and results in suppressed energy transfer from PBPs to PSII reaction centre (Lu and Vonshak 2002; Zhang et al. 2010).
Apart from the other photosynthetic pigments, carotenoids important for absorption, dissipation and transfer of light energy for photosynthesis show significant increase in salt-stressed plants (Borghesi et al. 2011) and cyanobacteria (Schubert et al. 1993). However, in some cases decreased carotenoids were also observed and correlated with decreased expression of carotenoid biosynthetic genes (Babu et al. 2011) and increased salt sensitivity.
The effect of salinity on photosynthetic electron transport and PSII activity remains a matter of debate in both cyanobacteria and higher plants. Numerous studies have reported severe impairment in PSII activity (Bongi and Loreto 1989; Everard et al. 1994; Rai et al. 2014) while some others indicated that photosynthetic electron transport is relatively insensitive to salt (Jeanjean et al. 1993; Abadia et al. 1999). The reduced PSII activity was supposedly due to the inhibition in electron transport from QA to QB at the acceptor side (Jafarinia and Shariati 2012) and oxygen-evolving complex at donor side of PSII (Lu and Vonshak 2002). Inhibition in synthesis of D1 protein of PSII (Allakhverdiev et al. 2002) in cyanobacteria and dissociation of 23 kDa polypeptide extrinsically bound to PSII in higher plant (Murata et al. 1992) could also be a cause of salinity-induced damage to PSII. Many studies indicate that salinity blocks PSII but enhances PSI activity (Zhang et al. 2010; Stepien and Johnson 2009) probably promoting cyclic electron flow through PSI.
Furthermore, salt-induced inhibition in photosynthesis partly attributes to stomatal closure that reduces stomatal conductance and partly due to non-stomatal factors involving direct effect of salt ions (Na+ and Cl−) on PSII-supported electron transport and photophosphorylation activity. In Lycium barbarum, initial photosynthesis inhibition was attributed to temporary stomatal limitation whereas non-stomatal limitation contributes to reduction in photosynthesis during prolonged salt exposure (Hui et al. 2004).
Dark reaction of photosynthesis (CO2 fixation) is also equally affected by high salinity. In Sesbania, salt stress enhances the oxygenase activity while curtails carboxylase activity of Rubisco (Sivakumar et al. 2000). Similar results were also observed in the cyanobacterium Anabaena doliolum (Srivastava et al. 2008). In Sorghum vulgare leaves, PEPC catalysing the first step of CO2 assimilation in C4 plants significantly increased during salt stress (García-Mauriño et al. 2003).
4.2.5 Cellular Metabolism
Cellular respiration is one of the common phenomena enhanced in both plants (Livne and Levin 1967; Begcy et al. 2011) and cyanobacteria (Molitor et al. 1990; Rai et al. 2014; Jeanjean et al. 1993; Srivastava et al. 2008) when exposed to salt stress. There is a line of evidence supporting the above fact, and this has been linked in some way with higher energy requirement of salt-affected cells in order to maintain turgor, ion homeostasis and production of more osmolytes. However, in some species decreased respiration was also noticed (Flowers 1972). The most dramatic effect of salinity was reported on protein synthesis and nitrogen metabolism. In general, protein synthesis is severely affected by high salt concentration due to inhibition of enzyme activity (Flowers 1972; Greenway and Osmond 1972). Hall and Flowers (1973) observed that microsomal amino acid incorporation fraction from halophilic Suaeda maritima is equally sensitive to added salt as salt-sensitive plants. This presumably indicates that salt tolerance in halophytes is related to the spatial separation of salt from cytoplasmic components either by salt exclusion or sequestration in vacuole that eventually lowers cytoplasmic Na+ concentration.
Alteration in nitrate absorption and nitrogen metabolism is also reported in hypersaline condition (Mansour 2000), and the resulting nitrate deficiency may correspond to the reduction in nitrate reductase activity as in the case of Cucumis sativus (Sacala et al. 2008). NaCl-induced reduction in ammonia and nitrate has also been reported in a halophyte Arthrocnemum fruticosum (Eddin and Doddema 1986). Plant’s response to salt not only varies with crop varieties or cultivars but also with the age. For example, in rice older leaves are reported to accumulate higher nitrate content than the younger ones probably because of an upregulated nitrate transporter OsNRT1;2 (Wang et al. 2012). Since many cyanobacterial species are endowed with the N2-fixing ability, it is relevant to study the effect of high salt on N2 fixation in general and nitrogenase in particular. Though Na+ has been recognized to be essential for nitrogenase (Thomas and Apte 1984) and nitrate/nitrite reductase activities (Brownell and Nicholas 1967), its excess is inhibitory for N2 fixation in many cyanobacteria (Page-Sharp et al. 1998; Fernandes et al. 1993). However, the inhibition of nitrogenase activity is not universal as many inhabitants of salty estuaries and Baltic Sea such as Nodularia, Anabaenopsis and Anabaena sp. displayed no significant difference in nitrogenase activity when exposed to high salt concentration (Moisnder et al. 2002). Further, the deleterious effect of salinity on N2 fixation was found to be a consequence of ionic component rather than the osmotic component of salt since nitrogenase activity was found to be completely insensitive to osmotic stress (Fernandes et al. 1993).
The most striking difference was observed in case of ice plant Mesembryanthemum crystallinum displaying metabolic shift from C3 to CAM when subjected to water deficit or saline condition. This metabolic shift requires accumulation of numerous enzymes such as phosphoenolpyruvate carboxylase (PVPC), pyruvate orthophosphate dikinase and NADP-malic enzyme (Cushman 2001). CAM metabolism enables plants to improve water-use efficiency, thus having a competitive advantage in day environment.
4.2.6 Growth and Development
Salinity-induced reduction in plant growth and development depends upon many factors including the species, genotype, plant age, ionic strength and composition of the salinizing solution and the organ in question. Major effects of salinity on crop plants can be described by two-phase model proposed by Munns et al. (1995)—phase 1 includes osmotic effect of salt which immediately reduces plant water uptake resulting in significant reduction in shoot growth whereas phase 2 corresponds to the salt-specific or ion excess effect which is due to the penetration of ions in transpiration stream resulting in death of transpiring leaves and reduction in the total photosynthetic leaf area. Thus, in a situation of reduced supply of photosynthate to the plant, the overall carbon balance gets affected thus reducing plant’s growth further.
Shoots growth is generally more sensitive to salt stress because elevated salinity leads to reduction in leaf area ratio which in turn decreases water-use efficiency of plants, enabling them to conserve soil moisture and prevent salt build-up in soil (Munns and Tester 2008). Whereas reduced uptake of important mineral nutrients, such as K+ and Ca2+, are largely responsible for root growth suppression, particularly root tip expansion (Larcher 1980). Reduction in plant growth as a result of salt stress has been reported in several plant species, but greater inhibition was observed in different tolerant genotypes relative to sensitive ones suggesting growth reduction possibly promotes salt tolerance enabling the tolerant plants to save energy for the maintenance of the processes (Mansour et al. 2005).
4.3 Salt Adaptation Mechanisms
Several investigators have demonstrated salt tolerance mechanisms based on factors such as ion accumulation, ion exclusion, accumulation of toxic ions like Na+ in older leaves, compatible solute production and toxic radical scavenging. Like plants, cyanobacteria possess similar mechanisms for salt tolerance which include Na+ pumps for active Na+ extrusion, transport systems for K+ and osmotically active organic molecules and enzymes creditworthy for their synthesis and qualitative and quantitative modifications of metabolic pathways. The common salt stress coping strategies adopted by these photoautotrophs have been reviewed here.
4.3.1 Intracellular Ion Homeostatic Processes
Compartmentalization of Na+ and Cl− into the vacuole, organized Na+ influx and homeostasis of K+, Ca2+, NO3 − and Pi are the phenomenon involved in intracellular homeostasis (Tester and Davenport 2003; Zhu 2003). Plant cell can tolerate sodium ion concentration less than 100 mM, but the basal activity of the cell gets interrupted when the cytosolic Na+ concentrations increase above 100 mM (Serrano et al. 1999). There are mainly three mechanisms involved in the prevention of excessive Na+ ion accumulation in cytosol which involves restriction of Na+ with selective ion uptake, storage of Na+ in vacuole and exportation of Na+ back to the apoplastic space (Zhu 2001).
4.3.1.1 Na+ Influx/Efflux and Limitation of K+ Loss
Na+ and K+ have good similarities between their physicochemical properties and lead to Na+ competition at transport sites for K+ entry into the symplast which may result in K+ deficiency and inhibition of metabolic processes that essentially depend on K+. Hence, plants tolerance to salt stress strongly depends on the status of their cytosolic K+–Na+ ratio which is further promoted by the cooperative action of transport systems located at plasma and vacuolar membranes and probably involves K+ and Na+ selective and non-selective pathways (Maathuis and Amtmann 1999). Under normal physiological conditions, cell tries to maintain relatively high K+ (100–200 mM) and low Na+ concentrations (1–10 mM) (Binzel et al. 1988).
Plants can deal with external K+ concentrations ranging from low μM to tens of mM. In general, cellular role of K+ is to act as (i) counterion for the large excess of negative charge on proteins and nucleic acids hence charge balancing in the cytoplasm; (ii) activator of crucial enzymatic reactions; (iii) endurer of non-lignified plant cells with structural rigidity by contributing to the osmotic pressure of the vacuole and cell turgor. In case of barley roots, it has been reported that the magnitude of the K+ efflux induced by salt inversely correlates with the productivity of 62 out of 69 cultivars contrasting in their sensitivity to salt stress (Chen et al. 2007). Unlike K+, Na+ as a macronutrient is only required for the translocation of pyruvate across the chloroplast envelope in some C4 species (Maathuis and Amtmann 1999). Active transport of both the ions occurs via the action of the K+–Na+ ATPase that moves K+ into the cell and extrudes Na+. Ion transport proteins in the membranes involve three classes of transport proteins, such as ‘pumps’ with turnover rate of around 102 per second fuelled by metabolic energy and able to transport substrates against an electrochemical gradient, ‘carriers’ with turnover rate of around 102–103 per second that undergo specific conformational changes during substrate transport are energized via coupling to an electrochemical gradient and ‘channels’ with turnover rate of around 106–108 per second catalyse the rapid ‘downhill’ dissipation of transmembrane ionic gradients and are under control of membrane potential (Maathuis and Amtmann 1999). Role played by K+ and non-selective cation channels (NSCCs) towards salinity stress is to induce Na+/K+ exchange which may be tissue and species specific. For example, in the pea mesophyll membrane, voltage-independent cation channel (VICs) arbitrates both Na+ influx and K+ efflux (Shabala et al. 2007). High-affinity K+ with low-affinity Na+ channels include inward-rectifying K+ channels (KIRCs) like AKT1, K+outward-rectifying channels (KORCs) and the KUP/HAK gene family of K+/H+ symporters (Maathuis and Amtmann 1999; Blumwald et al. 2000; Schachtman 2000). The high-affinity K+ transporter (HKT1), low-affinity cation transporter (LCT1) and NSCCs are among the transport systems that mediate Na+-specific cellular influx (Zhu 2003). NORC (non-selective outward-rectifying conductance) does not discriminate between cations and is activated by increased cytosolic Ca2+ concentrations (Wegner and De Boer 1997). Plasma membrane H+-ATPase restricts salt-induced membrane depolarization and its related K+ efflux. Also, it fuels the Na+/H+ antiport for energy-dependent Na+ efflux further improving the cytosolic Na+/K+ ratio. Salt-tolerant genotypes of barley possess intrinsically higher plasma membrane H+ pump activity, despite having the same level of H+-ATPase expression (Chen et al. 2007).
Cyanobacteria do not accumulate Na+ but extrude with the help of Na+ pumps with energy expenditure and maintain turgor with accumulation of K+ which lowers later with increase in the level of osmotic solutes. In Synechocystis PCC 6803, Nodularia harveyana and Aphanothece halophytica, salt tolerance mechanism involved maintenance of a high K+–Na+ ratio (Warr et al. 1985).
4.3.1.2 Ion Compartmentalization
Due to the disturbance in ion homeostasis, uptake of ions and their compartmentalization are essential for growth under saline condition which occurs at cellular as well as whole plant level. Plant compartmentalizes Na+ and Cl− in the vacuole or in different tissues for facilitating metabolic function, which is essential to minimize cytotoxicity. Na+/H+ antiporter in tonoplast plays a key role in Na+ transport from cytosol to vacuole under salt stress. The activity of such antiporter is controlled by electrochemical H+ gradient across the tonoplast generated by V-H+ATPase and vacuolar type pyrophosphatase (V-H+PPase). V-ATPase activity is found to be higher than V-H+PPase as former is required for energizing the tonoplast for ion uptake into the vacuole while the latter plays minor role as supported by the findings of Wang et al. (2001) on halophyte Suaeda salsa. Therefore, both V-H+ATPase and the tonoplast Na+/H+ antiporter play significant role in Na+ compartmentalization. Moreover, in the vacuole, NaCl helps in maintaining osmotic potential hence driving water under salt stress. Salt-sensitive plants involve exclusion of Na+ across plasma membrane while salt-tolerant plants prefer to accumulate Na+ in the vacuole (Munns and Tester 2008). Exception to this concept represented by Thellungiella halophila, a halophyte, has potential of a good excluder (Gong et al. 2005) whereas some glycophytes can accumulate salt to different degrees on the basis of their capacity to interchange Na+ for K+. In a study, concentration of Na+ and Cl− was analysed via X-ray microanalysis within mature root cortical cells of Suaeda maritima L. Dum. grown in 200 mM NaCl, and it was found to be fourfold higher in vacuoles as compared to cytoplasm or cell wall (Hajibagheri and Flowers 1989). Among the tissues, Na+ accumulation was found to be highest in the endodermis, followed by those in the exodermis, and stellar tissues in Salicornia europaea under salt stress (Lv et al. 2012). In addition, Ferreira et al. (2001) found the highest accumulation of Na+ and Cl− in guava leaves followed by the roots, while K+ and Mg2+ level decreased in leaves and the Ca2+ showed inverse relationship with Na+ in the roots. Also Cl− exclusion is an important mechanism in providing salt tolerance among legumes (Teakle and Tyerman 2010).
4.3.2 Osmotic Homeostasis
Osmotically active compounds known as osmolytes such as proline, glycine betaine, soluble sugars, free amino acids, and polyamines are responsible for osmotic adjustment in plants subjected to salt stress. These compounds show minimal affect on pH or ionic balance of the cytosol or luminal compartments of organelles. They not only raise osmotic pressure in the cytoplasm but act as low molecular weight chaperons by replacing water at the surface of proteins or membranes (Hasegawa and Bressan 2000). An early response to salt stress is the accumulation of proline which balances the water potential of the cytosol with the apoplast and vacuolar lumen hence helps in turgor maintenance of cells. The glutamate and ornithine pathways are the proline biosynthetic pathways in higher plants. Under salt stress, the former is responsible for proline biosynthesis (up to 80 % of amino acid pool under stress as compared to only 5 % under normal condition) as evidenced with the accumulation of pyrroline-5-carboxylate synthetase (P5CS) enzyme and P5C reductase (P5CR) in the chloroplast while the latter is involved in seedling development (Székely et al. 2008; Huang et al. 2013). Proline under salt stress not only acts as osmoprotectant but protects cellular macromolecules, scavenges free radicals and recycles NADPH+ H+ via its synthesis from the glutamate pathway and in redox signalling in all plants, including algae (Hare and Cress 1997). By restoring the pool of the terminal electron acceptor of the photosynthetic electron transport chain, proline may provide protection against photo-inhibition under stress (Szabados and Savoure 2009).
Glycine betaine, trigonelline, stachydrine and homostachydrine are aliphatic quaternary ammonium compounds reported to be accumulated under salt stress to serve as intercellular osmoticum. Increased accumulation of glycine betaine in chloroplasts with an increase in the activity of two enzymes, choline monooxygenase and betaine aldehyde dehydrogenase, therein occurs in response to salt stress. Some roles played by glycine betaine under salt stress include protection of membrane and macromolecules, promoting transcription and replication, which might accelerate protein synthesis de novo during recovery from stress. Even at low accumulation level betaine is compartmentalized at certain sites within cells to provide substantive protection against salt stress (Matoh et al. 1987).
The non-protein amino acid γ-aminobutyric acid (GABA) also gets accumulated with increased activity of enzyme involved in GABA metabolism under high salt stress (Renault et al. 2010). Carbon–nitrogen balance and ROS scavenging have been associated with GABA metabolism (Liu et al. 2011).
Polyamines the positively charged small aliphatic molecules in cellular pH mostly include putrescine, spermidine and spermine that help in protecting membrane and alleviating oxidative stress (Hussain et al. 2011). Plants deficient in spermine synthase are hypersensitive to salinity (Yamaguchi et al. 2006).
Salt stress leads to hydrolysis of starch by the β-amolytic pathway thence accumulation of soluble sugars in leaves (Kempa et al. 2008). Soluble sugars like sucrose, trehalose, etc. are involved in several metabolic events and behave as molecular signals regulating different genes involved in photosynthesis, sucrose metabolism and osmolyte synthesis.
Under high salt, these soluble sugars not only act as osmoregulators (Siringam et al. 2012) but prevent protein denaturation by interacting with proteins and membranes through hydrogen bonding. The other prominent roles of soluble sugars are in vitrification, which is the formation of a biological glass in the cytoplasm of dehydrated cells, hence helps in hindering diffusion of reactive compounds in the cell, decreases molecular movements and maintains structural and functional integrity of macromolecules. They also help in chelating Na+ with starch granules, hence facilitating detoxification (Kanai et al. 2007). In Synechocystis sp. PCC 6803, sucrose has been found to transduce a specific signalling pathway in response to salinity at early phase of stress (Desplats et al. 2005). Chemically inert osmolyte trehalose, a nonreducing disaccharide, has been found to accumulate under salt stress and helps in stabilizing membrane and proteins (Paul et al. 2008). Externally applied 5 mM (low concentration) trehalose reduced Na+ accumulation and inhibited growth while 10 mM (high concentrations) prevented chlorophyll loss in leaf blades and preserved root integrity (Garcia et al. 1997). Cyanobacteria use specific transporters to uptake their compatible solutes diffused in the periplasm, for example, a sucrose-, trehalose- and glucosylglycerol-specific transporter has been discovered for the first time in Synechocystis (Mikkat et al. 1996).
Another class of osmoprotectants in plant cell is raffinose family oligosaccharides (RFO) such as raffinose, stachyose and verbascose which accumulate during salt stress in leaves. Galactinol and raffinose also serve as scavengers of ROS and membrane protection. High level of galactinol and raffinose is accumulated by drought- and salinity-tolerantArabidopsis GolS1 and GolS2 (Nishizawa et al. 2008).
Enhanced accumulation of polyols, mannitol and sorbitol has been reported to provide tolerance to salt stress in several plant species. Over-expression of sorbitol-6-phosphate dehydrogenase (S6PDH) from apple prevents photosystem II from salinity impacts in persimmon trees (Gao et al. 2001). The cyclic polyols myo-inositol, and its methylated derivatives d-ononitol and d-pinitol, have also been found with higher level of accumulation under salt stress in several plant species (Sengupta et al. 2008). In halotolerant plants, over-expression of l-myo-inositol-1-phosphate synthase and inositol O-methyltransferase results in increased level of cyclic polyols providing salt stress tolerance in tobacco (Patra et al. 2010).
Reduced content of organic acids and TCA cycle intermediates in glycophytes under salt stress are involved in compensating ionic imbalance (Sanchez et al. 2008). Apart from plant, cyanobacterial adaptations to salt stress generally include accumulation of inorganic ions to balance osmotic potential and of osmoprotectants like sucrose, trehalose, glucosylglycerol (2-0-(a-d-glucopyranosy1)-glycerol), glycine betaine, proline and glutamate to prevent denaturation of macromolecules. Among these, sucrose and trehalose are the dominant osmolyte in Anabaena strain, glucosylglycerol in Synechocystis PCC 6803 and glycine betaine in Synechococcus strain. Different osmolytes show difference in their potential to cope with salt stress as the cyanobacteria having major accumulation of glycine betaine show higher salt tolerance (high salt tolerance maximum of 3.0 M NaCl) when compared to those accumulating glucosylglycerol and other polyols (moderate salt tolerance maximum of 1.8 M NaCl) showing better response than those having sucrose and trehalose as their major osmolytes (low salt tolerance, maximum of 0.7 M NaCl) (Reed 1986).
4.3.3 Antioxidant Defence System
Antioxidative defence system includes both enzymatic and non-enzymatic antioxidant systems and their cooperative effort protects the damage generated by salt stress in the plant tissue. Reactive oxygen species (ROS), partially reduced forms of atmospheric oxygen, are generally regarded as the main source of damage to cells under biotic and abiotic stresses. ROS are generated at the plasma membrane level or extracellular in the apoplast; they react with the unsaturated fatty acids of essential membrane lipids in plasma lemma or intracellular organelles. This contributes to the leakage of cellular contents, desiccation and cell death. Intracellular mitochondrial and chloroplast membrane damage causes hindrance to respiratory activity and results in pigment breakdown and loss of the carbon fixing ability, respectively (Scandalios 1993). ROS of polyunsaturated fatty acid generates aldehydic lipid breakdown product called malondialdehyde (MDA) which is used as a marker of oxidative lipid injury under salt stress (Moller et al. 2007). Lower MDA depicts higher antioxidative ability pondering higher resistance to salt stress.
Carotenoids de novo synthesized by all photosynthetic and many non-photosynthetic organisms are divided into the hydrocarbon carotenes, β-carotene and xanthophylls. β-Carotene bound to the core complexes of PSI and PSII provides protection against damaging effect of ROS further maintaining photochemical processes. Salt-tolerant species show an increase in carotenoids–chlorophyll ratio as well as enhanced anthocyanin, to protect from oxidative damage (Kytridis et al. 2008).
α-Tocopherol, a lipophilic antioxidant located primarily in thylakoid membranes, protects membranes from ROS and lipid radicals and hence helps in membrane stability. Three methyl groups in the molecular structure of α-tocopherol help it in possessing the highest antioxidative activity.
Ascorbate (ASC) has the capacity to directly eradicate ROS including singlet oxygen, superoxide, hydroxyl, peroxyl and alkoxyl radicals. It also helps in maintaining the membrane-bound antioxidant α-tocopherol in the reduced form by reducing α-chromoxyl radical and indirectly eradicates H2O2 through the activity of ascorbate peroxidase. Glutathione (GSH, g-glutamyl–cysteinyl–glycine) a thiol metabolite possesses strong reductant capacity that can scavenge toxic reactive oxygen species (ROS) such as 1O2, O2 ·− and OH– directly or in cooperation with other antioxidants like ascorbate and ROS-scavenging enzymes under high salt. H2O2 is a stable oxidant; at low concentration, it acts as a signal molecule involved in signal triggering and tolerance to salt stress, and at high concentration, it leads to programmed cell death. It has been reported that 50 % of photosynthesis is inhibited if H2O2 is present in chloroplast at a concentration of 10 μM (Kaiser 1979). Excess accumulation of H2O2 in the cell can also stimulate Haber–Weiss/Fenton reaction, yielding hydroxyl radicals (•OH) resulting in lipid peroxidation.
Antioxidative enzymes the major part of complex antioxidative defence system developed by living organisms include superoxide dismutase (SOD), catalase (CAT), guaiacol peroxidase (GPX), ascorbate peroxidase (APX) and glutathione reductase (GR). Other enzymes such as monodehydro ascorbate reductase (MDHAR) and dehydroascorbate reductase (DHAR) also help in ROS scavenging under salt stress. Their activity level under salt stress is taken as an indicator of stress tolerance.
SODs found in various cell compartments catalyse the dismutation of superoxide generated into oxygen and hydrogen peroxide. CAT, a tetrameric heme-containing enzyme, and peroxidases such as APX and GPX scavenge hydrogen peroxide to produce H2O and O2. CAT having one of the highest turnover rates among all enzymes can convert six million molecules of H2O2 to H2O and O2 per minute.
APX uses ascorbate as electron donor and possesses higher affinity for H2O2 (μM range) as compared to CAT and POD (mM range) and provides tolerance against salinity. Its involvement in ascorbate–glutathione antioxidant pathway results in reduction of hydrogen peroxide.
GR a flavo-protein oxidoreductase localized predominantly in chloroplast converts oxidized glutathione (GSSG) to reduced glutathione (GSH) for maintaining GSH pool (Rao and Reddy 2008), regulates GSH/GSSG ratio and supplies GSH for GPX and DHAR, which further convert hydrogen peroxide to water and reduce oxidized ascorbate, respectively, thus protect against oxidative stress by maintaining the ASH pool.
Although the activity of the above-mentioned enzymes generally increases under salt stress, nevertheless, different plant species show alteration in activity of their enzymes at varying salt concentrations. For example, in Glycine max, increased CAT activity level was almost similar both in shoot and root, i.e. about 150 and 352 %, 597 and 188 %, 740 and 547 % under 33, 66 and 99 mM NaCl treatment, respectively, as compared to control (Weisany et al. 2012). While in B. parviflora, APX, GPX, GR and SOD activity increased but CAT activity decreased (Parida et al. 2004).
4.3.4 Metabolic Rearrangement and Ionomics
Metabolite fingerprinting and profiling help to understand mechanism underlying stress physiology in plants hence in developing improved breeding strategies towards stress-tolerant crops as the dynamic alteration in metabolite pool or fluxes may decide the phenotype of the organism (Ratcliffe and Shachar-Hill2006). Species adapted to saline environment may be metabolically pre-adapted to salinity hence help in developing salt-tolerant genotypes (Gong et al. 2005; Sanchez et al. 2008).
The ionome represents the composition of inorganic component such as mineral nutrient and trace element of cellular and organismal systems. Quantitative and simultaneous measurement of alteration in elemental composition in response to physiological processes under differing environment helps us to understand the mechanisms of salt tolerance. On the basis of differential rearrangement of shoot nutrient levels, ionomics was applied using inductively coupled plasma-atomic/optical emission spectrometry (ICP-AES) on the extremophile Lotus creticus and two glycophytes Lotus corniculatus and Lotus tenuis upon exposure to salt stress, and it was found that Cl− exclusion from the shoot tissue was a vital phenomenon in providing tolerance against salinity in L. creticus (Sanchez et al. 2011).
4.4 Molecular and Omics Approach Towards Salt Stress
Almost all aspects of life are engineered at the molecular level, and without understanding molecules one can only have a very sketchy understanding of life itself. Molecular analysis to dissect the signal transduction pathways mediating the adaptive strategies employed by plants or cyanobacteria under various environmental stresses is imperative. Basically, organisms respond to various stresses by modulating gene expression which ultimately causes restoration of cellular homeostasis, detoxification, damage repair and growth recovery. This subsection encompasses molecular aspects of various salt-responsive genes/proteins and associated pathways in plants as well as in cyanobacteria.
4.4.1 Perception and Salt Stress Signal Transduction in Plants
Water deficit due to high salinity initially poses an ionic, osmotic or even a mechanical impact on the cell. It is plausible that all these signals have their own cognate receptors which operate either independently or cooperatively to initiate downstream signalling events. Sensing of salt by plants is an enigma. There is little knowledge about how sodium is sensed in any cellular system. Sodium ion can be sensed either before or after entry into the cell or both. Thus, there could be two different perception mechanisms employed by the cell in order to sense salt stress—direct and indirect. Direct perception includes sensing extracellular sodium ion by a membrane receptor or a putative direct osmosensor, and indirect perception is sensing intracellular sodium ion by sodium ion-sensitive enzymes (induced due to osmotic changes in cell volume, turgor pressure, membrane stability, individual solute concentration, ionic strength and accumulation of macromolecules in cytoplasm). Histidine kinases are among the best candidates for salt and osmotic stress receptors and defined as osmosensors in prokaryotes and yeast; however, these have not been characterized in plants yet. AtHK1 a histidine kinase from Arabidopsis structurally related to yeast histidine kinase osmosensor (SLN1) can rescue the salt sensitivity of SLN1 and SHO1 (another transmembrane osmosensor) deleted yeast mutant implying that AtHK1 might have a similar function in plants. In plants SOS1, a plasma membrane Na+/H+ antiporter has been proposed to sense Na+ ions and thus functions as sensor (Chinnusamy et al. 2006). Another potential candidate sensor is a Na+–K+co-transporter of Eucalyptus camaldulensis (Chinnusamy et al. 2006).
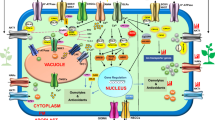
The major routes in salt stress signal transduction consist of pathways for ionic and osmotic homeostasis signalling, ROS (reactive oxygen species) detoxification (i.e. damage control and repair) and growth regulation. SOS (salt overly sensitive) signalling pathway encompasses the entire ionic aspect of salt stress whereas MAPK (mitogen-activated protein kinases) signalling pathway mediates the osmotic homeostasis and/or detoxification responses. All signalling cascades initially modify constitutively expressed transcription factors, causing expression of early response transcriptional activators which ultimately activates downstream delayed responsive stress tolerance effector genes (Fig. 4.1). Early response genes have a transient and quick expression whereas delayed response genes are activated slowly with sustained expression. Several examples of early response genes are CBF/DREB (C-repeat binding proteins/dehydration-responsive-element binding proteins) gene family, RD22BP, etc. Delayed response genes include large number of stress-responsive genes such as osmolyte biosynthesis genes, LEA (late embryogenesis abundant)/dehydrin-type genes, detoxification enzymes, and chaperones. (Fig. 4.1).
4.4.1.1 Acute Signalling Pathways
The first response of cells to combat hyperosmolarity is the flux of water and ions across various membranes after which osmolyte accumulation occurs when all the injuries become evident. Therefore, maintenance of ion homeostasis could be an acute response. SOS pathway in Arabidopsis represents some aspects of acute adaptive response and is responsible for Na+ homeostasis and salt tolerance (Zhu 2000). This pathway incorporates three components, SOS1, SOS2 and SOS3. SOS3 is a Ca+-binding protein which senses the cytosolic calcium ion signal elicited by salt stress (Liu and Zhu 1998). SOS2 is a serine/threonine protein kinase. Approximately, 270 amino acids present at N-terminal of SOS2 comprise the kinase catalytic domain which is inhibited by a 21-amino acid-long FISL motif present in C-terminal regulatory domain of SOS2. SOS2 is normally inactive, presumably because of an intramolecular interaction between the catalytic domain and the autoinhibitory regulatory domain. Binding of SOS3 to SOS2 at FISL motif removes its inhibitory effect and is determined to be crucial to bind SOS3. Interaction of SOS3 with SOS2 activates the substrate phosphorylation ability of SOS2 in the presence of Ca2+. The activated SOS3–SOS2 kinase complex phosphorylates and activates SOS1, a Na+/H+ antiporter on the plasma membrane through which ion homeostasis is maintained (Fig. 4.1). Similarly, for increased expression of other transporter genes activated SOS3–SOS2 kinase complex is perhaps necessary.
4.4.1.2 ROS Signalling Pathways
Salt stress-induced accumulation of ROS is not properly understood in plants. ROS particularly H2O2 triggers MAPK cascades, and since osmotic stress signalling also uses some of the MAPK modules it is plausible that there exists a crosstalk between salt stress and oxidative stress signalling at these modules ( Xiong and Zhu 2002). It is proposed that Ca2+ channels in guard cells are activated by exogenous and ABA-induced H2O2 which through activation of MAPK cascade mediates stomatal closure (Fig. 4.1). In Arabidopsis, it is reported that MAP kinase kinase ANP1 and downstream genes such as GSTG, HSP 18-2 and GH3 are activated by H2O2. Interestingly, tobacco plants overexpressing NPK1, an ANP1 ortholog, exhibit an increased tolerance to salt stress. ROS regulates expression of many genes including ROS scavengers (e.g. superoxide dismutase and catalases) or antioxidant (glutathione, thioredoxins). Transcription factors that bind to the cis elements in these gene promoters are well studied in yeast. These include Yap1 group b-Zip transcription factors, having conserved cysteine residues which may act as sensors for the redox status of the cell. Surprisingly, in spite of the presence of all these genes in plants, Yap-1-like transcription factors are missing from Arabidopsis genome (Xiong and Zhu 2002).
4.4.1.3 Signalling for Osmolyte Synthesis
Researchers have been attracted to study the underlying mechanism of increased synthesis of osmolytes during hyperosmolarity. In yeast, HOG1 (high osmotic glycerol 1) pathway is the best studied osmolarity signalling cascade, but in case of glycophytes osmolytes do not accumulate to a high level; therefore, the mechanism is not well understood. However, a signalling pathway similar to that of yeast MAPK–HOG pathway may be involved in regulation of osmolyte biosynthesis in plants. At low osmolarity, active form of Arabidopsis osmosensor AtHK1 inactivates a response regulator by phosphorylation. Whereas high osmolarity inactivates AtHK1 leading to accumulation of active nonphosphorylated response regulator which in turn activates osmolyte biosynthesis by activating MAPK pathway in plants. Further studies are required for better understanding of osmolyte signalling pathway.
4.4.2 Perception and Salt Stress Signal Transduction in Cyanobacteria
In living cells, perception of environmental stresses and the subsequent transduction of stress signals are primary events in the acclimation process. Cyanobacteria have several features that make them particularly suitable for the study of stress responses at the molecular level. Homologues of salt-induced genes in cyanobacteria (Synechocystis) are also regulated by salt stress in higher plants. Thus, cyanobacteria may serve as a good model system for discerning the molecular mechanism of the stress responses and acclimation of stresses in the plants (Bohnert et al. 2001). In general, sensing salt stress and subsequent signal transduction pathway are not properly understood. In the model cyanobacterium Synechocystis 6803, the two component system consists of histidine kinase (Hik) and response regulator (Rre).
The Hik perceives environmental changes by its sensory domain, forms homodimer and gets autophosphorylated at a histidine residue within histidine kinase domain (Stock et al. 2000). The phosphorylated group is transferred from the Hik to a conserved aspartate residue in the transducer, Rre. Phosphorylation leads to the conformational changes and activation of Rre. The activated Rre binds to the promoter regions of many salt-responsive genes such as those involved in ion homeostasis, osmolyte biosynthesis and transport processes (Fig. 4.2).
Upregulation of 38 genes including Hik/Rre pairs Hik33/Rre31, Hik34/Rre1, Hik2/Rre1, Hik16/Hik41/Rre17 and Hik10/Rre3 has been reported after 30 min of addition of 0.5 M NaCl that were particularly involved in perception and transduction of salt stress signal. Hik41, Hik2 and Hik34 are soluble proteins present in cytosol, and Hik33, Hik10 and Hik16 are transmembrane proteins (Hagemann 2011).
4.4.3 Genomic and Proteomic Aspects of Salt Stress
The main response amenable to molecular analysis are metabolic adaptations to salt stress and have led to the identification of a large number of genes induced by salt. Several such novel proteins and polypeptides were identified by both one- and two-dimensional gel electrophoresis. To understand plant osmotic stress at the molecular level, analysis of these genes is required. Based on their predicted physiologic or metabolic functions, these genes can be classified into functional groups (Sairam and Tyagi 2004). Some genes induced in different plant species under high salinity are listed in Table 4.1.
4.4.3.1 Genes Encoding Proteins Involved in Cellular Protection
Late embryogenesis abundance (LEA) proteins, induced by salinity and water deficit, belong to this functional category. These proteins were originally thought to be associated with desiccation tolerance during seed maturation but are also involved in protection of cellular structure and components from the harmful effects of water deficit during salt stress. Based on sequence and expression kinetics, LEA proteins are categorized in six subgroups.
-
1.
Group 1 LEA proteins: The predicted role of this subgroup is water binding, thus providing a protective aqueous environment for cellular components. The expression of members of this group of genes in vegetative tissues is induced by salt (Bostock and Quatrano 1992).
-
2.
Group 2 LEA proteins: The predicted function of these proteins is preventing denaturation and maintenance of the solvation of structural surfaces.
-
3.
Group 3 and 5 LEA proteins: The possible role of these proteins in salt-stressed cells is ion sequestration as these proteins exist as dimers and the polar face of dimerized helices might expose and bind ions via formation of salt bridges. There are reports of induction of group-3 LEA proteins in soybean and barley and also a gene encoding a group-5 LEA homologue of the salt-tolerant line Shamuti orange in response to salt and water deficit (Naot et al. 1995).
-
4.
Group 4 LEA proteins: These proteins may serve as reverse chaperones and possess water-binding properties; due to this, they can stabilize the surface of membranes and proteins by binding water and functioning as solvation film. In vegetative tissue, expression of these proteins occurs in response to salinity, drought, low temperature and ABA.
-
5.
LEA D95: Being hydrophobic in nature, this protein is unusual. It shows homology to cDNA pcC 27-45 from Craterostigma plantagineum and expresses in callus tissue in response to salt stress (Piatkowski et al. 1990).
4.4.3.2 Transporter Genes
Exclusion and ion compartmentation of Na+ ions via membrane transport is necessary for survival in saline environments. Several ATPase genes of plasma membrane as well as tonoplast are induced by high salt stress. Many of the salinity-induced proteins share sequence similarities with water channels. Salt-induced alteration of the tonoplast H+-pumping V-ATPase and H+-pyrophosphatase has been evaluated in hypocotyls of Vigna unguiculata seedlings (Otoch et al. 2001). The Arabidopsis thaliana AtNHX1 gene encodes a vacuolar Na+/H+ antiporter that is important in salt tolerance, and its expression is regulated by salt stress (Shi and Zhu 2002). In Synechocystis 6803, six different genes were annotated as Na+/H+ antiporters (Kaneko et al. 1996). In other completely sequenced cyanobacterial genomes, also multiple genes for Na+/H+ antiporters are present. Over-expression of cyanobacterial genes in defined E. coli mutants revealed that at least three antiporters from Synechocystis 6803—NhaS1, NhaS3 and NhaS4—are true antiporters among them NhaS3 shows the highest transport activity. NhaS1 shows similarities to the SOS1 Na+/H+ antiporter from Arabidopsis thaliana and is expressed in E. coli mutant defective in Na+/H+ antiporter activity which leads to the restoration of Na+ tolerance (Hamada et al. 2001). Thus, NhaS1 seems to be the most active Na+/H+ antiporter in Synechocystis 6803, and the gene encoding it (sll0689) was the most highly expressed of the Na+/H+ antiporter genes. Similar studies were performed in Aphanothece halophytica which revealed multiple Na+/H+ antiporter genes (Waditee et al. 2001; Wutipraditkul et al. 2005). Out of multiple genes, ApNhaP is related to NhaS1 from Synechocystis 6803. K+ ion is also present in high amount in control as well as salt-loaded cells, in contrast to Na+, and is also crucial for salt acclimation. Three types of transporters exist in E. coli: high-affinity ATP-dependent Kdp system and the low-affinity Trk and Kup systems. The potential candidates for K+ uptake in cyanobacteria are Kdp subunits, proteins related to Trk but named Ktr and different putative K+ channels. All cyanobacteria possess the structural genes for a functional ATP-dependent K+ transport system consisting of the Kdp ABC subunits. The KdpA subunit is the K+ permease, the KdpB subunit is a typical P-type ATPase that provides the energy and KdpC is involved in the assembly of the transport system.
4.4.3.3 Osmolyte Biosynthetic Genes
To prevent water loss and to re-establish turgor in order to expand, cells accumulate solutes during osmotic stress. Major organic solutes proline, betaine and ions such as K+, Na+ and Cl− maintain osmotic adjustment. Delta pyroline-5-carboxylate synthetase, a bifunctional enzyme involved in proline biosynthesis (Hu et al. 1992), was induced by high salt stress and dehydration (Delauney and Verma 1993). Similarly, genes involved in pinitol synthesis encoding myo-inositolO-methyltransferase were isolated from the ice plant (Vernon and Bohnert 1992) and found to be exclusive in salinity stress. In sugar beet, spinach and barley induced expression of genes and cDNAs encoding choline monooxygenases and betaine aldehyde dehydrogenase involved in conversion of choline to glycine betaine has been reported in response to salinity (Rathinasabapathi et al. 1997; Ishitani et al. 1995). Myo-inositol phosphate synthase, which encodes a precursor for pinitol synthesis, showed sixfold upregulation under salt stress. One more gene mannitol dehydrogenase downregulated by salt stress maintains the high concentration of mannitol in stressed cells in order to function as an osmoprotectant in rice (Williamson et al. 1995; Sairam and Tyagi 2004).
Similar to plants, in cyanobacteria also a large number of genes responsible for compatible solute accumulation are induced. One such compatible solute is sucrose. Salt-induced sucrose biosynthesis is achieved by sucrose phosphate synthase (SPS). Expression of the Synechocystis spsA transiently increased after salt shock which corresponds well to the transient sucrose accumulation in Synechocystis 6803 (Marin et al. 2004). Glucosyl glycerol (GG) represents another typical compatible solute of cyanobacteria. GgpS (GG-phosphate synthase) as well as GgpP (GG-phosphate phosphatase) involved in GG biosynthesis pathway became activated in crude protein extracts at 100 mM NaCl (Hagemann et al. 1996). The salt stimulation of GgpS and GgpP clearly explains the initial activation of GG synthesis in salt-shocked cyanobacterial cells characterized by transiently high ion contents.
4.4.3.4 Genes Encoding Proteins Involved in General Defence
Screening of salt-induced cDNA libraries resulted in a number of cDNAs associated with plant defence against pathogen or wounding damage which include PRP (pathogenesis-related protein) and β-glucanase from rice and endochitinase from tomato (Umeda et al. 1994; Chen et al. 1994). Osmotin a polypeptide related to a family of PRP is reported to have association with salinity adaptation. These are the most abundant proteins in salt-adapted tobacco cells (Singh et al. 1985). APX and GPX are the enzymes involved in controlling oxidative stress; genes encoding these enzymes are reported to be induced in salt stress. Methylglyoxal detoxifying enzyme glyoxalase is also induced by salinity stress (Holland et al. 1993; Espartero et al. 1995).
Similar to plants, several cyanobacterial genes involved in defence were found to be induced in high salinity. Nine proteins of defence pathway upregulated under salt stress in Anabaena include peroxidase, Alr3090 (similar to catalase), superoxide dismutase (SOD A and SOD B), glutathione reductase and AhpC (alkyl hydroperoxide reductase)/TSA family proteins (Rai et al. 2014).
4.4.3.5 Genes Encoding Proteins Involved in Metabolism
Induced expression of glyceraldehyde-3-phosphate dehydrogenase and phosphoglyceromutase, PEPcase, NADP-malate dehydrogenase and NADP-malic enzyme in Mesembryanthemum crystallinum has been reported in response to high salt stress (Cushman et al. 1989; Umeda et al. 1994; Foresthofel et al. 1995). Enhanced mRNA accumulation of both nuclear- and chloroplast-encoded transcripts of photosynthesis-related genes is known in salt-adapted cell cultures (Winicov and Button 1991; Locy et al. 1996).
In response to salt stress, proteins of purine and pyrimidine metabolism were upregulated in Anabaena. Some proteins of energy metabolism such as phosphoglycerate kinase, transketolase and FBPase (fructose 1,6-bisphosphatase) registered two–threefold upregulation (Rai et al. 2014). A clear accumulation of transaldolase, ribulose-phosphate 3-epimerase, phosphoglucomutase, transketolase, glycogen phosphorylase, phosphoglycerate kinase and fructose-1,6-bisphosphatase was found in Synechocystis sp. strain PCC 6803 in response to high salinity (Fulda et al. 2006).
4.4.3.6 Genes Encoding Proteins Involved in Protein Synthesis, Processing and Degradation
Salt-adapted tobacco cells dramatically accumulate elongation factor 1-alpha, one of the essential components of protein synthesis (Zhu et al. 1997). Protease inhibitors, normally induced upon insect attack, are also induced by salt stress (Downing et al. 1992; Lopez et al. 1994). In Arabidopsis, two different cysteine proteinases accumulate in response to salt stress. Several heat-shock proteins which act as chaperons to prevent denaturation and help denatured proteins to regain their native conformation are also expressed under salt stress. In Atriplex nummularia, ANJ1, member of the DnaJ family of HSPs, was induced in cell by high salt stress that had been adapted to high salinity but not in normal unadopted cells (Zhu et al. 1993b).
Salt stress in N2-fixing cyanobacteria Anabaena is known to increase protein synthesis and refolding of denatured proteins. Proteins showing remarkable changes include subunits of ribosome assembly (30S RPs1 in A. doliolum and Anabaena 7120; 30S RPs6 in Anabaena 7120), elongation factors (EfTu in Anabaena L31; EfTs in A. doliolum and Anabaena 7120), posttranscriptional regulators (RNA-binding proteins D and E in Anabaena 7120 and Anabaena L31) and molecular chaperons HSP1, DnaK and GroEL (Rai et al. 2014). In Synechocystis also, GroEL1, DnaK2 and GrpE were found among the accumulated proteins under salt stress (Fulda et al. 2006).
4.4.3.7 Genes Encoding Proteins Involved in Regulating Gene Expression
Salt-responsive regulatory genes, involved in regulation of other salt-responsive genes, are mostly transacting factors and protein kinases. In A. thaliana, a receptor-like protein kinase gene and gene encoding components of signal transduction (MAPK) have been reported to be expressed under salt stress. Expression of some other genes like MAPKK and a ribosomal 36 kinase which functions in the MAPK cascade also increases under salinity stress (Sairam and Tyagi 2004). An myb homologue in Arabidopsis plant regulated at transcriptional level by salt stress may bind to promoter of osmotic stress-regulated genes and regulate their transcription in response to osmotic stress (Zhu et al. 1997). A receptor protein kinase cDNA is also reported in rice (Naot et al. 1995).
4.5 Bioinformatic Predictions of Molecular Targets Under Salt Stress
Though a lot of work has been done on salt toxicity and salinity-induced effects on enzymes and proteins, studies regarding interaction of salt and proteins are still lacking. For proper understanding of salinity-induced loss of enzyme and protein activity, determination of active (functional) sites on proteins and how they interact with ions and salt is necessary. These favourable binding sites relate to locations where a putative ligand could bind. Recently, computational methods for the detection and characterization of functional sites on proteins have increasingly become an area of interest (Campbell et al. 2003). Molecular docking is one such method which simulates the molecular recognition process. The aim of molecular docking is to achieve an optimized conformation for both the proteins and the ligands and their relative orientation such that the free energy of the overall system is minimized. A binding interaction between a small molecule ligand and an enzyme protein may result in activation or inhibition of the enzyme. If the protein is a receptor, ligand binding may result in agonism or antagonism. In order to understand the protein–salt interaction, docking study was performed with certain commonly upregulated and downregulated proteins/enzymes in cyanobacteria and higher plants under salt stress.
The RNA-binding proteins (RBPs) are proteins that bind to the double- or single-stranded RNA in cells and participate in forming ribonucleoprotein complexes. RBPs have crucial roles in various cellular processes including posttranscriptional RNA maturation, transport, localization and gene regulation. Unfortunately, however, these proteins seem to be salt sensitive not only in plants (Pang et al. 2010) but also in cyanobacteria (Pandhal et al. 2008). The docking study was performed between the selected, downregulated RBP and the ligand NaCl to predict the possible binding site, and interestingly the amino acids which get affected under salt toxicity were found to be common in both higher plants and in cyanobacteria.
The docking study reveals that NaCl successfully docked with the three conserved amino acid residues Val, Ala and Gly present at RRM2 domain of RNA-binding proteins with the calculated binding energy of −52.31 and −47.56 for Arabidopsis thaliana and Anabaena RBP, respectively. Since RRMs (RNA recognition motifs) are crucial for RNA binding and recognition of specific RNA sequences, its interaction with NaCl could be the plausible explanation as why these proteins are uniformly sensitive under salt stress in both higher plants and cyanobacteria.
During the study, another protein called nitrate reductase of Nostoc sp. PCC7120 (Frias et al. 1997) and Zea mays (Baki et al. 2000) was found uniformly sensitive to high salt concentrations. The PDB structure of this protein was taken for docking and functional site analysis. The binding energy of salt with nitrate reductase of Nostoc sp. PCC7120 and Zea mays was found to be −31.75 and −29.75, respectively. The residues that were present in the conserved motif as well as at the active site of the protein, namely, Cys, Phe, Trp, Gly, Thr and Glu, were identified as the targets of NaCl thereby conferring its uniform toxicity on the nitrate reductases family of proteins.
Similarly, photosystem II light-harvesting proteins, the intrinsic transmembrane proteins CP43 (PsbC) and CP47 (PsbB) occurring in the reaction centre of photosystem II were commonly downregulated in cyanobacteria as well as in higher plants. In order to trace out the reasons behind salt toxicity at the molecular level, docking study was conducted taking the PDB structures of photosystem II proteins from Zea mays and Synechocystis sp. PCC 6803 as both have been reported to be highly salt sensitive (Jeanjean et al. 1993; Zörb et al. 2009). Here also the results were almost similar, i.e. the binding of the salt at the active site of the protein which is a part of the conserved motif of the protein’s functional domain determining its function. The residues that were found to be involved in the interaction were Met, Ala and Leu in Zea mays and Glu, Leu, Ser and Phe in Synechocystis sp. PCC 6803. These were the sites of interaction for the salt to confer its toxicity.
Furthermore, proteins commonly upregulated under salt stress in both cyanobacteria and higher plants were subjected to docking analysis. The selected proteins include superoxide dismutases [Oryza sativa (Fadzilla et al. 1997) and Nostoc sp. PCC7120 (Rai et al. 2014)], glutathione S-transferase (GST) [Oryza sativa (Chitteti and Peng 2007) and Nostoc sp. PCC7120 (Rai et al. 2014)] and heat-shock protein70 [Oryza sativa (Chitteti and Peng 2007) and Nostoc sp. PCC7120 (Rai et al. 2014)]. To study the effect of NaCl on SOD activity in terms of their binding affinity with each other, proteins from Oryza sativa (Fadzilla et al. 1997) and Nostoc sp. PCC7120 (Rai et al. 2014) were taken for docking calculations.
The interaction between salt and SOD in plant and cyanobacteria with a binding energy of −56.39 and −51.89 predicts a stable complex although only few residues were found common between them like His, Asp and Trp, but the point that needs to be noted here is that though these common residues are conserved, still there were residues that showed interaction with the salt but they are neither conserve nor present at the active site of these proteins thereby solubilizing the toxic effect of salt stress on superoxide dismutases and the interaction at the sod domain enhances the expression of this protein under salt stress.
Another protein GSTs comprise a family of eukaryotic and prokaryotic phase II metabolic isozymes best known for their ability to catalyse the conjugation of the reduced form of glutathione (GSH) to xenobiotic substrates for the purpose of detoxification. GST contains a C-terminal structural domain and an N-terminal catalytic domain. The glutathione molecule binds in a cleft between N- and C-terminal domains. Under salt stress, this protein was found to show interaction with the N-terminal domain with Val and Ser being common among both the higher plants and cyanobacteria with equal binding energy of −54.53 for both. In this case too, while these residues are conserved at the N-terminal they are not catalytically important as they do not constitute the functional active site of this protein and therefore, their interaction with the salt does not hamper the protein functioning under salt stress.
The 70 kDa heat-shock proteins (Hsp70s) are a family of conserved ubiquitously expressed proteins. The Hsp70s are an important part of the cell’s machinery for protein folding and help to protect cells from stress. Members of the Hsp70 family are strongly upregulated by heat stress and toxic chemicals, particularly heavy metals such as arsenic, cadmium, copper, and mercury. The docking study conducted between the HSP70 and salt depicted good interaction with a binding energy of −46.12 for Nostoc and −53.78 for Oryza sativa. But this interaction was neither at the conserved motif nor at the functional or catalytic site of this protein. The interacting residues were also found to be entirely different for both of them. Thus, the expression of this protein enhances on interaction with the salt stress making it a salt-tolerant one.
Docking study revealed that salt-interacting residues of some of the salt-responsive enzymes (RBPs, nitrate reductase) are conserved among cyanobacteria as well as higher plants; thus, similar mechanism might be involved in salt-induced toxicity to these enzymes. Since salt-interacting residues of photosystem II light-harvesting proteins, CP43 (PsbC) and CP47 (PsbB), were specific for cyanobacteria and higher plants, these organisms might possess specific mechanisms for salt toxicity. On the other hand, the salt-tolerant proteins showed good interaction with salt with almost equal binding energies as salt-sensitive proteins, but the interacting residues were quite distinct for higher plant and cyanobacteria. Only few conserved interacting residues existed but not located at the active site of the proteins. Moreover, these proteins had domains that help in their survival under stress. Interaction of salt with these domains triggers their function and thus expression of the protein under stress. One important point emerging from this study was the lack of significant homology between the selected proteins from cyanobacteria and higher plant at the sequence level, but some domains were common across the species unique for each group of proteins. Thus, the interaction of salt at these conserved domains and motifs decides the response of that particular protein under salt stress.
4.6 Conclusion/Future Perspectives
Despite substantial research, we are still far from a proper understanding of salt stress tolerance in plants and/or cyanobacteria. There are some apparent gaps which need attention—first, one cannot draw a clear cut line to identify the real players governing salt sensitivity; second, the molecular pathways for salt stress signal perception and transduction are still to be resolved. Furthermore, since many salt-responsive pathways and associated genes such as LEA, SOD, Prx, and Hsp are found to be common in other stresses, it would be rather interesting if such genes are exploited for development of transgenics tolerant to multiple abiotic stresses and properly tested at field level. In the recent years, bioinformatics has emerged as indispensable for the analysis of genes and proteins. There is a need to create interaction network of genes, proteins and metabolites participating in stress-regulated biological processes.
References
Abadia J, Morales F, Abadia A (1999) Photosystem II efficiency in low chlorophyll, iron-deficient leaves. Plant Soil 215:183–192
Allakhverdiev SI, Kinoshita M, Inaba M et al (2001) Unsaturated fatty acids in membrane lipids protect the photosynthetic machinery against salt-induced damage in Synechococcus. Plant Physiol 125(4):1842–1853
Allakhverdiev SI, Nishiyama Y, Miyairi S et al (2002) Salt stress inhibits the repair of photodamaged photosystem II by suppressing the transcription and translation of psbA genes in Synechocystis. Plant Physiol 130:1443–1453
Alvarez-Pizarro A, Gomes-Filho E, De Lacerda C et al (2009) Salt-induced changes on H+-ATPase activity, sterol and phospholipid content and lipid peroxidation of root plasma membrane from dwarf-cashew (Anacardium occidentale L.) seedlings. Plant Growth Regul 59:125–135
Azachi M, Sadka A, Fisher M et al (2002) Salt induction of fatty acid elongase and membrane lipid modifications in the extreme halotolerant alga Dunaliella salina. Plant Physiol 129(3):1320–1329
Babu MA, Singh D, Gothandam KM (2011) Effect of salt stress on expression of carotenoid pathway genes in tomato. J Stress Physiol Biochem 7(3):87–94
Baki GK, Siefritz F, Man HM et al (2000) Nitrate reductase in Zea mays L. under salinity. Plant Cell Environ 23:515–521
Begcy K, Mariano ED, Mattiello L et al (2011) An Arabidopsis mitochondrial uncoupling protein confers tolerance to drought and salt stress in transgenic tobacco plants. PLoS One 6(8):e23776
Benhayyim G, Faltin Z, Gepstein S et al (1993) Isolation and characterization of salt-associated protein in Citrus. Plant Sci 88:129–140
Binzel ML, Hess FD, Bressan RA et al (1988) Intracellular compartmentation of ions in salt adapted tobacco cells. Plant Physiol 86(2):607–614
Blumwald E, Aharon GS, Apse M (2000) Sodium transport in plant cells. Biochim Biophys Acta 1465:140–151
Bohnert HJ, Ayoubi P, Borchert C et al (2001) A genomics approach towards salt stress tolerance. Plant Physiol Biochem 39:295–311
Bolaños JA, Longstreth DJ (1984) Salinity effects on water potential components and bulk elastic modulus of Alternanthera philoxeroides (Mart.). Griseb Plant Physiol 75(2):281–284
Bongi G, Loreto F (1989) Gas-exchange properties of salt-stressed olive (Olea europaea L.) leaves. Plant Physiol 90:1408–1416
Borghesi E, González-Miret ML, Escudero-Gilete ML et al (2011) Effects of salinity stress on carotenoids, anthocyanins, and color of diverse tomato genotypes. J Agric Food Chem 59(21):11676–11682
Bostock RM, Quatrano RS (1992) Regulation of Em gene expression in Rice: interaction between osmotic stress and ABA. Plant Physiol 98:1356–1363
Brownell PF, Nicholas DJ (1967) Some effects of sodium on nitrate assimilation and N2 fixation in Anabaena cylindrica. Plant Physiol 42(7):915–921
Caffrey M (1985) Kinetics and mechanism of lamellar gel/lamellar liquid-crystal and lamellar/inverted hexagonal phase transition in phosphatidylethanolamine: a real-time X-ray diffraction study using synchrotron radiation. Biochemistry 24:4826–4844
Campbell SJ, Gold ND, Jackson RM et al (2003) Ligand binding: functional site location, similarity and docking. Curr Opin Struct Biol 13:389–395
Chaum S, Kirdmanee C (2009) Effect of salt stress on proline accumulation, photosynthetic ability and growth characters in two Maize cultivars. Pak J Bot 41(1):87–98
Chen RD, Yu LX, Greer AF et al (1994) Isolation of an osmotic stress and abscisic acid-induced gene encoding an acidic endochitinase from Lycopersicon chilense. Mol Gen Genet 245:195–202
Chen Z, Zhou M, Newman I et al (2007) Potassium and sodium relations in salinised barley tissues as a basis of differential salt tolerance. Funct Plant Biol 34:150–162
Chinnusamy V, Zhu J, Zhu J (2006) Salt stress signaling and mechanisms of plant salt tolerance. Annu Rev Genet Eng 27:141–177
Chitteti B, Peng Z (2007) Proteome and phosphoproteome differential expression under salinity stress in rice (Oryza sativa) roots. J Proteome Res 6:1718–1727
Cushman JC (2001) Crassulacean acid metabolism: a plastic photosynthesis adaptation to arid environment. Plant Physiol 127:1439–1448
Cushman JC, Meyer G, Michalowski CB et al (1989) Salt stress leads to differential expression of two isogenes of PEPCase during CAM induction in the common Ice plant. Plant Cell 1:715–725
Delauney AJ, Verma DPS (1993) Proline biosynthesis and osmoregulation in plants. Plant J 4:215–223
Desplats P, Folco E, Salerno GL (2005) Sucrose may play an additional role to that of an osmolyte in Synechocystis sp. PCC 6803 salt-shocked cells. Plant Physiol Biochem 43:133–138
Doganlar ZB, Demir K, Basak H et al (2010) Effects of salt stress on pigment and total soluble protein contents of the three different Tomato cultivars. Afr J Agric 5(15):2056–2065
Douglas TJ, Walker RP (1984) Phospholipids, free sterols and adenosine triphosphatase of plasma membrane-enriched preparations from roots of citrus genotypes differing in chloride exclusion ability. Plant Physiol 62:51–58
Downing WL, Mauxion F, Fauvarque MO et al (1992) A Brassica napus transcript en coding a protein related to the Kunitz protease inhibitors family accumulates upon water stress in leaves, not in seeds. Plant J 2:685–693
Eddin RS, Doddema H (1986) Effects of NaCl on the nitrogen metabolism of the halophyte Arthrocnemum fruticosum (L.) Moq. grown in a greenhouse. Plant Soil 92:373–385
Erdmann N, Hagemann M (2001) Salt acclimation of algae and cyanobacteria: a comparison. In: Rai LC et al (eds) Algal adaptation to environmental stresses. Springer, Heidelberg
Eryilmaz F (2007) The relationships between salt stress and anthocyanin content in higher plants. Biotechnol J 20(1):47–52
Espartero J, Sanchez-Aguayo I, Pardo JM (1995) Molecular characterization of glyoxalase-1 from a higher plant: upregulation by stress. Plant Mol Biol 29:1223–1233
Everard JD, Gucci R, Kann SC et al (1994) Gas exchange and carbon partitioning in the leaves of celery (Apium graveolens L.) at various levels of root zone salinity. Plant Physiol 106:281–292
Fadzilla NM, Finch PR, Burdon RH (1997) Salinity, oxidative stress and antioxidant responses in shoot culture of rice. J Exp Bot 48(307):325–331
Fernandes TA, Iyer V, Apte SK (1993) Differential responses of nitrogen-fixing cyanobacteria to salinity and osmotic stresses. Appl Environ Microbiol 59:899
Ferreira A, O’Byrne CP, Boor KJ (2001) Role of σB in heat, ethanol, acid, and oxidative stress resistance and during carbon starvation in Listeria monocytogenes. Appl Environ Microbiol 67:4454–4457
Flowers TJ (1972) Salt tolerance in Suaeda maritima (L.) Dum. The effect of sodium chloride on growth, respiration, and soluble enzymes in a comparative study with Pisum sativum L. J Exp Bot 23(2):310–321
Foresthofel NR, Cushman MAF, Cushman JC et al (1995) A salinity induced gene from the halophyte M. crystallinum encodes a glycolytic enzyme, cofactor-independent phosphoglyceromutase. Plant Mol Biol 29:213–216
Frias JE, Flores E, Herrero A (1997) Nitrate assimilation gene cluster from the heterocyst-forming cyanobacterium Anabaena sp. strain PCC 7120. J Bacteriol 179:477–486
Fulda S, Mikkat S, Huang F et al (2006) Proteome analysis of salt stress response in the cyanobacterium Synechocystis sp. strain PCC 6803. Proteomics 6:2733–2745
Gao M, Tao R, Miura K et al (2001) Transformation of Japanese persimmon (Diospyros kaki Thunb.) with apple cDNA encoding NADP-dependent sorbitol-6-phosphate dehydrogenase. Plant Sci 160:837–845
Garcia A-B, de Almeida EJ, Iyer S et al (1997) Effects of osmoprotectants upon NaCl in rice. Plant Physiol 115:159–169
García-Mauriño S, Monreal JA, Alvarez R et al (2003) Characterization of salt stress-enhanced phosphoenolpyruvate carboxylase kinase activity in leaves of Sorghum vulgare: independence from osmotic stress, involvement of ion toxicity and significance of dark phosphorylation. Planta 216(4):648–655
Gong Q, Li P, Ma S et al (2005) Salinity stress adaptation competence in the extremophile Thellungiella halophila in comparison with its relative Arabidopsis thaliana. Plant Physiol 44:826–839
Grattan SR, Grieve CM (1999) Salinity-mineral nutrient relations in horticultural crops. Sci Hortic 78:127–157
Greenway H, Osmond CB (1972) Salt responses of enzymes from species differing in salt tolerance. Plant Physiol 49:256–259
Hagemann M (2011) Molecular biology of cyanobacterial salt acclimation. FEMS Microbiol Rev 35(1):87–123
Hagemann M, Schoor A, Erdmann N (1996) NaCl acts as a direct modulator in the salt adaptive response: salt-dependent activation of glucosylglycerol synthesis in vivo and in vitro. J Plant Physiol 149:746–752
Hajibagheri MA, Flowers TJ (1989) X-ray microanalysis of ion distribution within root cortical cells of the halophyte Suaeda maritima (L.) Dum. Planta 177:131–134
Hall JL, Flowers TJ (1973) The effect of salt on protein synthesis in the halophyte Suaeda maritime. Planta (Berl) 110:361–368
Hamada A, Hibino T, Nakamura T et al (2001) Na+/H+ antiporter from Synechocystis sp. PCC 6803, homologous to SOS1, contains an aspartic residue and long C-terminal tail important for the carrier activity. Plant Physiol 125:437–446
Hare PD, Cress WA (1997) Metabolic implications of stress induced proline accumulation in plants. Plant Growth Regul 21:79–102
Hasegawa PM, Bressan RA (2000) Plant cellular and molecular responses to high salinity. Annu Rev Plant Physiol Plant Mol Biol 51:463–499
Holland D, Hayyim GB, Faltin Z et al (1993) Molecular characterization of a salt stress associated protein in citrus and cDNA sequence homology to mammalian glutathione peroxidases. Plant Mol Biol 21:923–927
Hsiao TC (1973) Plant responses to water stress. Annu Rev Plant Physiol 24:519–570
Hu C, Delauney AJ, Verma DPS (1992) A bifunctional enzyme (1-pyrroline-5-carboxylate synthetase) catalyzes the first two steps in proline biosynthesis in plants. Proc Natl Acad Sci U S A 89:9354–9358
Huang F, Fulda S, Hagemann M et al (2006) Proteomic screening of salt-stress-induced changes in plasma membranes of Synechocystis sp. strain PCC 6803. Proteomics 6:910
Huang Z, Zhao L, Chen D et al (2013) Salt stress encourages proline accumulation by regulating proline biosynthesis and degradation in Jerusalem Artichoke plantlets. PLoS One 8(4):e62085
Hui H, Xu X, Li S (2004) Possible mechanism of inhibition on photosynthesis of Lycium barbarum under salt stress. Chin J Ecol 23:5–9
Hussain SS, Ali M, Ahmad M et al (2011) Polyamines: natural and engineered abiotic and biotic stress tolerance in plants. Biotechnol Adv 29:300–311
Ishitani M, Nakamura T, Han SY et al (1995) Expression of the betaine aldehyde dehydrogenase gene in barley in response to osmotic stress and abscisic acid. Plant Mol Biol 27:307–315
Ishitani M, Majumder AL, Bornhouser A et al (1996) Coordinate transcriptional induction of myo-inositol metabolism during environmental stress. Plant J 9:537–548
Jafarinia M, Shariati M (2012) Effects of salt stress on photosystem II of canola plant (Brassica napus L.) probing by chlorophyll a fluorescence measurements. Iran J Sci Technol AI:71–76
Jeanjean R, Matthijs HCP, Onana B et al (1993) Exposure of cyanobacterium Synechocystis PCC6803 to salt stress induces concerted changes in respiration and photosynthesis. Plant Cell Physiol 34:1073–1079
Kaiser WM (1979) Reversible inhibition of the Calvin cycle and activation of oxidative pentose phosphate cycle in isolated intact chloroplast by hydrogen peroxide. Planta 145:377–382
Kanai M, Higuchi K, Hagihara T et al (2007) Common reed produces starch granules at the shoot base in response to salt stress. New Phytol 176:572–580
Kaneko T, Sato S, Kotani H (1996) Sequence analysis of the genome of the unicellular cyanobacterium Synechocystis sp. strain PCC 6803. II. Sequence determination of the entire genome and assignment of potential protein-coding regions. DNA Res 3:109–136
Kempa S, Krasensky J, Dal Santo S et al (2008) Central role of abscisic acid in stress-regulated carbohydrate metabolism. PLoS One 3:e3935
Kerkeb L, Donaire JP, Rodriguez-Rosales MP (2001) Plasma membrane H+-ATPase activity is involved in adaptation of tomato to NaCl. Plant Physiol 111:483–490
Kytridis VP, Karageorgou P, Levizou E et al (2008) Intra-species variation in transient accumulation of leaf anthocyanins in Cistus creticus during winter: evidence that anthocyanins may compensate for an inherent photosynthetic and photoprotective inferiority of the red-leaf phenotype. J Plant Physiol 165:952–959
Ladas NP, Papageorgiou GC (2000) Cell turgor: a critical factor for the proliferation of cyanobacteria at unfavorable salinity. Photosynth Res 65(2):155–164
Larcher W (1980) Physiological plant ecology, 2nd edn. Springer, Berlin
Liu J, Zhu JK (1998) A calcium sensor homolog required for plant salt tolerance. Science 280:1943–1945
Liu C, Zhao L, Yu G (2011) The dominant glutamic acid metabolic flux to produce gamma-amino butyric acid over proline in Nicotiana tabacum leaves under water stress relates to its significant role in antioxidant activity. J Integr Plant Biol 53:608–618
Livne A, Levin N (1967) Tissue respiration and mitochondrial oxidative phosphorylation of NaCl-treated pea seedlings. Plant Physiol 42(3):407–414
Locy RD, Chang CC, Nielsen BL et al (1996) Photosynthesis in salt adapted heterotrophic tobacco cells and regenerated plants. Plant Physiol 110(1):321–328
Lopez F, Yansuyt G, Fourcroy P et al (1994) Accumulation of a 22 kDa protein and its mRNA in the leaves of Raphanus sativus in response to salt stress or water deficit. Plant Physiol 91:605–614
Lu C, Vonshak A (1999) Characterization of PSII photochemistry in salt-adapted cells of cyanobacterium Spirulina platensis. New Phytol 141:231–239
Lu C, Vonshak A (2002) Effects of salinity stress on photosystem II function in cyanobacteria Spirulina platensis cells. Plant Physiol 114:405–413
Lv S, Jiang P, Chen X et al (2012) Multiple compartmentalization of sodium conferred salt tolerance in Salicornia europaea. Plant Physiol Biochem 51:47–52
Maathuis JMF, Amtmann A (1999) K+ nutrition and Na+ toxicity: the basis of cellular K+/Na+ ratios. Ann Bot 84:123–133
Mansour MMF (2000) Nitrogen containing compounds and adaptation of plants to salinity stress. Plant Biol 43:491–500
Mansour MMF, Al-Mutawa MM, Salama KHA et al (2002) Effect of NaCl and polyamines on plasma membrane lipids of wheat roots. Plant Biol 45:235–239
Mansour MMF, Salama KHA, Ali FZM et al (2005) Cell and plant responses to NaCl in Zea mays L. cultivars differing in salt tolerance. Gen Appl Plant Physiol 31:29–41
Marin K, Kanesaki Y, Los DA (2004) Gene expression profiling reflects physiological processes in salt acclimation of Synechocystis sp. strain PCC 6803. Plant Physiol 136:3290–3300
Matoh T, Watanabe J, Takahashi E (1987) Sodium, potassium, chloride, and betaine concentrations in isolated vacuoles from salt-grownAtriplex gmelini leaves. Plant Physiol 84:173–177
Mikkat S, Hagemann M, Schoor A (1996) Active transport of glucosylglycerol is involved in salt adaptation of the cyanobacterium Synechocystis sp. strain PCC 6803. Microbiology 142(7):1725–1732
Moisnder PH, McClinton E, Paer HW (2002) Salinity effects on growth, photosynthetic parameters, and nitrogenase activity in estuarine planktonic cyanobacteria. Microb Ecol 43:432–442
Molitor V, Trnka M, Erber W et al (1990) Impact of salt adaptation on esterified fatty acids and cytochrome oxidase in plasma and thylakoid membranes from the cyanobacterium Anacystis nidulans. Arch Microbiol 154:112–119
Moller IM, Jensen PE, Hansson A (2007) Oxidative modification to cellular components in plants. Annu Rev Plant Biol 58:480–481
Mundy J, Yamaguchi-Shinozaki K, Chua NH (1990) Nuclear proteins bind conserved elements in the abscisic acid responsive promoter of a rice RAB gene. Proc Natl Acad Sci U S A 87:1406–1410
Munns R, Termaat A (1986) Whole plant responses to salinity. Aust J Plant Physiol 13:143–160
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Annu Rev Plant Biol 59:651–681
Munns R, Schachtman D, Condon A (1995) The significance of a two-phase growth response to salinity in wheat and barley. Funct Plant Biol 22(4):561–569
Murata N, Mohanty PS, Hayashi H et al (1992) Glycine betaine stabilizes the association of extrinsic proteins with the photosynthetic oxygen evolving complex. FEBS Lett 296:187–189
Naot D, Ben-Hayyim G, Eshdat Y et al (1995) Drought, heat, and salt stress induce the expression of a citrus homologue of an atypical late embryogenesis Lea5 gene. Plant Mol Biol 27:619–622
Nishizawa A, Yabuta Y, Shigeoka S (2008) Galactinol and raffinose constitute a novel function to protect plants from oxidative damage. Plant Physiol 147:1251–1263
Norberg P, Liljenberg C (1991) Lipids of plasma membranes prepared from oat root cells: effects of induced water-deficit tolerance. Plant Physiol 96:1136–1141
Otoch MDL, Sobreira ACM, deAragao MEF et al (2001) Salt modulation of vacuolar H+-ATPase and H+-pyrophosphatase activities in Vigna unguiculata. J Plant Physiol 158:545–551
Page-Sharp M, Behm CA, Smith GD (1998) Cyanophycin and glycogen synthesis in a cyanobacterial Scytonema species in response to salt stress. FEMS Microbiol Lett 160:11–15
Pandhal J, Wright PC, Biggs CA (2008) Proteomics with a pinch of salt: a cyanobacterial perspective. Saline Systems 4:1. doi:10.1186/1746-1448-4-1
Pang Q, Chen S, Dai S et al (2010) Comparative proteomics of salt tolerance in Arabidopsis thaliana and Thellungiella halophila. J Proteome Res 9(5):2584–2599
Parida AK, Das AB, Mohanty P (2004) Defense potentials to NaCl in a mangrove, Bruguiera parviflora: differential changes of isoforms of some antioxidative enzymes. J Plant Physiol 161(5):531–542
Patra B, Ray S, Richter A et al (2010) Enhanced salt tolerance of transgenic tobacco plants by co-expression of PcINO1 oxidase enhances freezing tolerance of plants. Plant J 22:449–453
Paul MJ, Primavesi LF, Jhurreea D et al (2008) Trehalose metabolism and signaling. Annu Rev Plant Biol 59:417–441
Piatkowski D, Schneider K, Salamini F et al (1990) Characterization of five abscisic acid responsive cDNA clones isolated from the desiccation tolerant plant Craterostigma plantagineum and their relationship to other water stress genes. Plant Physiol 94:447–451
Quintero FJ, Garciadeblas B, Rodriguez-Navarro A (1996) The SAL1 gene of Arabidopsis, encoding an enzyme with 3′(2′),5′-bisphosphate nucleotidase and inositol polyphosphate 1-phosphatase activities, increases salt tolerance in yeast. Plant Cell 8:529–537
Rahdari P, Tavakoli S, Hosseini SM (2012) Studying of salinity stress effect on germination, proline, sugar, protein, lipid and chlorophyll content in Purslane (Portulaca oleracea L.) leaves. J Stress Physiol Biochem 8(1):182–193
Rai S, Agrawal C, Shrivastava AK et al (2014) Comparative proteomics unveils cross species variations in Anabaena under salt stress. J Proteomics 98:254–270
Rao CASV, Reddy AR (2008) Glutathione reductase: a putative redox regulatory system in plant cells. In: Khan NA, Singh S, Umar S (eds) Sulfur assimilation and abiotic stresses in plants. Springer, Dordrecht, pp 111–147
Ratcliffe RG, Shachar-Hill Y (2006) Measuring multiple fluxes through plant metabolic networks. Plant J 45:490–511
Rathinasabapathi B, Brunet M, Russell BL et al (1997) Choline monooxygenase, an unusual iron sulfur enzyme catalyzing the first step of glycine betaine synthesis in plants: prosthetic group characterization and cDNA cloning. Proc Natl Acad Sci U S A 94:3454–3458
Reddy MP, Vora AB (1986) Changes in pigment composition, Hill reaction activity and saccharides metabolism in Bajra (Pennisetum typhoides S & H) leaves under NaCl salinity. Photosynthetica 20:50–55
Reed RH (1986) Halotolerant and halophilic microbes. In: Herbert RA, Codd GA (eds) Microbes in extreme environment. Academic, London, pp 55–82
Renault H, Roussel V, El Amrani A et al (2010) The Arabidopsis pop2-1 mutant reveals the involvement of GABA transaminase in salt stress tolerance. BMC Plant Biol 10:20
Reviron MP, Vartanian N, Sallantin M et al (1992) Characterization of a novel protein induced by progressive or rapid drought and salinity in Brassica napus leaves. Plant Physiol 100:1486–1493
Sacala E, Demczuk A, Grzys E et al (2008) Effect of salt and water stresses on growth, nitrogen and phosphorous metabolism in Cucumis sativus L. seedlings. Acta Soc Bot Pol 77:23–28
Sadaka A, Himmelhoch S, Zamir A (1991) A 150 kilodalton cell surface protein is induced by salt in the halotolerant green alga Dunaliella salina. Plant Physiol 95:822–831
Sairam RK, Tyagi A (2004) Physiology and molecular biology of salinity stress tolerance in plants. Curr Sci 86:407–421
Saleh B (2012) Salt stress alters physiological indicators in cotton (Gossypium hirsutum L.). Soil Environ 31(2):113–118
Sanchez DH, Siahpoosh MR, Roessner U et al (2008) Plant metabolomics reveals conserved and divergent metabolic responses to salinity. Physiol Plant 132:209–219
Sanchez DH, Pieckenstain FL, Escaray F et al (2011) Comparative ionomics and metabolomics in extremophile and glycophytic Lotus species under salt stress challenge the metabolic pre-adaptation hypothesis. Plant Cell Environ 34:605–617
Scandalios JG (1993) Oxygen stress and superoxide dismutase. Plant Physiol 101:712–726
Schachtman DP (2000) Molecular insights into the structure and function of plant K+ transport mechanisms. Biochim Biophys Acta 1465:127–139
Schubert H, Fluda S, Hagemann M (1993) Effects of adaptation to different salt concentrations on photosynthesis and pigmentation of the cyanobacterium Synechocystis sp. PCC6803. J Plant Physiol 142:291–295
Sengupta S, Patra B, Ray S et al (2008) Inositol methyl tranferase from a halophytic wild rice, Porteresia coarctata Roxb. (Tateoka): regulation of pinitol synthesis under abiotic stress. Plant Cell Environ 31:1442–1459
Serrano R (1996) Salt tolerance in plants and microorganisms: toxicity targets and defense responses. Int Rev Cytol 165:1–52
Serrano R, Mulet JM, Ríos G et al (1999) A glimpse of the mechanism of ion homeostasis during salt stress. J Exp Bot 50:1023–1036
Shabala S, Cuin TA, Prismall L et al (2007) Expression of animal CED-9 anti-apoptotic gene in tobacco modifies plasma membrane ion fluxes in response to salinity and oxidative stress. Planta 227:189–197
Shi HZ, Zhu JK (2002) Regulation of expression of the vacuolar Na+/H+ antiporter gene AtNHX1 by salt stress and abscisic acid. Plant Mol Biol 50:543–550
Singh DP, Kshatriya K (2002) Characterization of salinity-tolerant mutant of Anabaena doliolum exhibiting multiple stress tolerance. Curr Microbiol 45:165–170
Singh NK, Handa AK, Hasegawa PM et al (1985) Proteins associated with adaptation of cultured tobacco cells to NaCl. Plant Physiol 79:126–137
Singh NK, Bracker CA, Hasegawa PM et al (1987) Characterization of osmotin Athumatin-like protein associated with osmotic adaptation in plant cells. Plant Physiol 85(79):126–137
Siringam K, Juntawong N, Cha-um S et al (2012) Salt tolerance enhancement in indica rice (Oryza sativa L. spp. indica) seedlings using exogenous sucrose supplementation. Plant Omics J 5:52–59
Sivakumar P, Sharmila P, Pardha Saradhi P (2000) Proline alleviates salt-stress-induced enhancement in ribulose-1,5-bisphosphate oxygenase activity. Biochem Biophys Res Commun 279(2):512–515
Srivastava AK, Bhargava P, Rai LC (2005) Salinity and copper-induced oxidative damage and changes in the antioxidative defence systems of Anabaena doliolum. World J Microbiol Biotechnol 21:1291
Srivastava AK, Bhargava P, Thapar R et al (2008) Salinity-induced physiological and proteomic changes in Anabaena doliolum. Environ Exp Bot 64:49
Srivastava AK, Bhargava P, Kumar A et al (2009) Molecular characterization and the effect of salinity on cyanobacterial diversity in the rice fields of Eastern Uttar Pradesh, India. Saline Systems 5:4
Stepien P, Johnson GN (2009) Contrasting responses of photosynthesis to salt stress in the glycophyte Arabidopsis and the halophyte Thellungiella: role of the plastid terminal oxidase as an alternative electron sink. Plant Physiol 149(2):1154–1165
Stock AM, Robinson VL, Goudreau PN (2000) Two-component signal transduction. Annu Rev Biochem 69:183–215
Szabados L, Savoure A (2009) Proline: a multifunctional amino acid. Trends Plant Sci 2:89–97
Székely G, Abrahám E, Cséplo A et al (2008) Duplicated P5CS genes of Arabidopsis play distinct roles in stress regulation and developmental control of proline biosynthesis. Plant J 53(1):11–28
Teakle NL, Tyerman SD (2010) Mechanisms of Cl(−) transport contributing to salt tolerance. Plant Cell Environ 33:566–589
Tester M, Davenport R (2003) Na+ tolerance and Na+ transport in higher plants. Ann Bot 91:503–527
Thomas J, Apte SK (1984) Sodium requirement and metabolism in nitrogen-fixing cyanobacteria. J Biosci 6(5):771–794
Torres-Schumann S, Godoy JA, Pintor-Toro JA (1992) A probable lipid transfer protein gene is induced by NaCl stress in tomato plants. Plant Mol Biol 18:749–757
Turan MA, Katkat V, Taban S (2007) Variations in proline, chlorophyll and mineral elements contents of Wheat plants grown under salinity stress. Agron J 6(1):137–141
Turner NC (1979) Drought resistance and adaptation to water deficits in crop plants. In: Muel H, Staps RC (eds) Stress physiology of crop plants. Wiley, New York, pp 343–372
Umeda M, Hara C, Matsubayashi Y et al (1994) Expressed sequence tags from cultured cells of rice (Oryza sativa L.) under stressed conditions: analysis of transcripts of genes engaged in ATP-generating pathways. Plant Mol Biol 25:469–478
UNESCO Water Portal (2007) http://www.unesco.org/water
Vernon DM, Bohnert HJ (1992) A novel methyltransferase induced by osmotic stress in the facultative halophyte Mesembryanthemum crystallinum. EMBO J 11:2077–2085
Waditee R, Hibino T, Tanaka Y et al (2001) Halotolerant cyanobacterium Aphanothece halophytica contains an Na+/H+ antiporter, homologous to eukaryotic ones, with novel ion specificity affected by C terminal tail. J Biol Chem 276:36931–36938
Wang BS, Luttge U, Ratajczak R (2001) Effects of salt treatment and osmotic stress on V-ATPase and V-PPase in leaves of the halophyte Suaeda salsa. J Exp Bot 52:2355–2365
Wang H, Wu Z, Han J et al (2012) Comparison of ion balance and nitrogen metabolism in old and young leaves of alkali-stressed rice plants. PLoS One 7(5):e37817
Warr SRC, Reed RH, Chudek JA et al (1985) Osmotic adjustment in Spirulina platensis. Planta 163:424–429
Wegner LH, De Boer AH (1997) Properties of two outward-rectifying channels in root xylem parenchyma cells suggest a role in K1 homeostasis and long-distance signaling. Plant Physiol 115:1707–1719
Weisany W, Sohrabi Y, Heidari G et al (2012) Changes in antioxidant enzymes activity and plant performance by salinity stress and zinc application in soybean (Glycine max L.). Plant Omics J 5(2):60–67
Williamson JD, Stoop JMH, Massel MO et al (1995) Sequence analysis of a mannitol dehydrogenase cDNA from plants reveals a function for the pathogenesis-related protein EL13. Proc Natl Acad Sci U S A 92:7148–7152
Winicov I, Button JD (1991) Accumulation of photosynthesis gene transcripts in response to sodium chloride by salt-tolerant alfalfa cells. Planta 183:478–483
Wu J, Seliskar DM, Gallagher JL (2005) The response of plasma membrane lipid composition in callus of the halophyte, Spartina patens, to salinity stress. Am J Bot 92:852–858
Wutipraditkul N, Waditee R, Incharoensakdi A et al (2005) Halotolerant cyanobacterium Aphanothece halophytica contains NapA-type Na+/H+ antiporters with novel ion specificity that are involved in salt tolerance at alkaline pH. Appl Environ Microbiol 71:4176–4184
Xiong L, Zhu JK (2002) Molecular and genetic aspects of plant responses to osmotic stress. Plant Cell Environ 25:131–139
Xu G, Magen H, Tarchitzky J et al (2000) Advances in chloride nutrition of plants. Adv Agron 68:97–150
Yahya A, Liljenberg C, Nilsson R et al (1995) Effects of pH and minerals nutrition supply on lipid composition and protein pattern of plasma membranes from sugar beet roots. J Plant Physiol 146:81–87
Yamaguchi K, Takahashi Y, Berberich T et al (2006) The polyamine spermine protects against high salt stress in Arabidopsis thaliana. FEBS Lett 580:6783–6788
Zhang T, Gong H, Wen X et al (2010) Salt stress induces a decrease in excitation energy transfer from phycobilisomes to photosystem II but an increase to photosystem I in the cyanobacterium Spirulina platensis. J Plant Physiol 167(12):951–958
Zhu JK (2000) Genetic analysis of plant salt tolerance using Arabidopsis thaliana. Plant Physiol 124:941–948
Zhu JK (2001) Plant salt tolerance. Trends Plant Sci 6:66–71
Zhu JK (2003) Regulation of ion homeostasis under salt stress. Curr Opin Plant Biol 6:441–445
Zhu JK, Shi J, Bressan RA et al (1993a) Expression of an Atriplex nummularia gene encoding a protein homologous to the bacterial molecular chaperone DnaJ. Plant Cell 5:341–349
Zhu JK, Shi J, Singh U (1993b) Enrichment of vitronectin and fibronectin like proteins in NaCl-adapted plant cells and evidence for their involvement in plasma membrane-cell wall adhesion. Plant J 3:637–646
Zhu J, Hasegawa PM, Bressan RA (1997) Molecular aspects of osmotic stress in plants. Crit Rev Plant Sci 16(3):253–277
Zörb C, Herbst R, Forreiter C et al (2009) Short-term effects of salt exposure on the maize chloroplast protein pattern. Proteomics 9(17):4209–4220
Acknowledgements
L.C. Rai is thankful to DST for project and J.C. Bose National Fellowship. Chhavi Agrawal is thankful to DBT for SRF, Sonia Sen to CSIR for SRF, Shivam Yadav to UGC for JRF and Antra Chatterjee and Shweta Rai to DST and Shilpi Singh to DST for WOSA for financial support.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Agrawal, C. et al. (2015). Signal Perception and Mechanism of Salt Toxicity/Tolerance in Photosynthetic Organisms: Cyanobacteria to Plants. In: Tripathi, B., Müller, M. (eds) Stress Responses in Plants. Springer, Cham. https://doi.org/10.1007/978-3-319-13368-3_4
Download citation
DOI: https://doi.org/10.1007/978-3-319-13368-3_4
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-13367-6
Online ISBN: 978-3-319-13368-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)