Abstract
Anthropogenic activity has affected nearly every environment on the planet. The changes that have occurred as a consequence of human activities have altered aquatic habitats by exacerbating already existing extreme environments and by introducing novel stressors. In some cases, particularly adjacent to heavily industrialized areas, these changes have introduced sufficient novel selective pressures to drive resident populations to genetically adapt in order to survive in the altered habitats, while species that were unable to adapt have been extirpated from these extreme environments. In this chapter, we aim to explore the effects of natural and novel stressors, resulting from anthropogenic activity, on fish populations. We will provide an overview of the possible multi-generational outcomes of anthropogenic contamination, as well as explore documented examples of population-wide changes that have occurred. We present case studies, including population responses to UV light, radionuclides, and metals contamination, as well as adaptational responses to persistent organic pollutants. Through this examination, we aim to not only give an overview of the existing evolutionary changes in fish populations in response to anthropogenic contamination, but also identify future areas of research on the impacts, long-term persistence, and ecological significance of these effects.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Fitness Cost
- Aryl Hydrocarbon Receptor
- Aryl Hydrocarbon Receptor Pathway
- Radionuclide Contamination
- Gulf Killifish
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Anthropogenic Changes to Aquatic Environments
There is great natural variability in aquatic environments, which has provided the grounds for the phenomenal diversification and adaptation of fish species to fill available ecological niches. In addition to adaptation along natural environmental gradients, some fish possess unique phenotypes, which allow them to survive under the most extreme environmental conditions. Modern extreme environments include many aquatic habitats that have been so thoroughly contaminated by human activities that many aquatic species are unable to survive or reproduce in them. These are generally located in heavily industrialized areas or in waterways downstream of industrial activity, and are characterized by dramatic reductions in species diversity. Anthropogenic changes to the climate and environment are increasingly altering freshwater and marine habitats, and will require adaptive responses from resident fish populations to allow for their persistence (Moe et al. 2013; Rosenzweig and Neofotis 2013; Anderegg et al. 2010). As adaptation involves allele frequency changes over many generations, species with shorter generation times are likely to adapt more rapidly to ongoing anthropogenic environmental alterations. Thus, human-induced evolutionary change has been observed more often in simple unicellular or invertebrate species (Palumbi 2001; Medina et al. 2007).
Human-induced environmental changes can act to exacerbate preexisting extreme environments by increasing natural stressors, which include, but are not limited to, salinity/conductivity, temperature, metals, UV radiation, and pH balance (Kaushal et al. 2005; Poloczanska et al. 2013; Core Writing Team et al. 2007; Dijkstra et al. 2013; Veron 2008). In addition to augmenting existing extreme environments, anthropogenic activities have caused compounds with low natural occurrences to reach concentrations high enough to cause toxicity, thus creating new selective pressures shaping evolutionary change in natural populations. These new selective pressures will be referred to hereafter as novel stressors. Some pollutants, such as synthetic estrogenic compounds, pharmaceuticals, polychlorinated dibenzo dioxins/furans (PCDD/Fs), polychlorinated biphenyls (PCBs), and polycyclic aromatic hydrocarbons (PAHs), have known modes of action affecting the behavior, reproduction, and survival of fish (Knecht et al. 2013; Incardona et al. 2004, 2011; Clark et al. 2010; Matson et al. 2009; Wassenberg and Di Giulio 2004a; Nebert et al. 2004; Mennigen et al. 2011). Sources for these contaminants include heavy industrial activity, which leads to the production of many persistent organic pollutants (POPs) (Wenning et al. 1993; Walker et al. 2005), and urban water use, including the release of pharmaceuticals into freshwater and marine environments (Camacho-Munoz et al. 2014). Many of these compounds have been shown to adversely affect developmental processes (Wassenberg et al. 2002), survival (Nacci et al. 2010), and reproduction (Valenti et al. 2012) at environmentally relevant concentrations. As a result, environments with high concentrations of contamination pose a threat to resident aquatic biota (Wassenberg et al. 2002). Environmentally relevant concentrations of estrogenic compounds have been tested under realistic exposure conditions and have been shown to be detrimental to populations of fish (Kidd et al. 2007), whereas some persistent pollutants have already led to evolutionary adaptation in fish populations in the wild (Wirgin et al. 2011; Nacci et al. 2010). By focusing on the population-level responses of aquatic organisms to anthropogenic pollution, we account for the cumulative impacts of these novel extreme environments on organismal survival and reproduction. As discovery of human-induced phenotypic and genetic change has increased, attention to the developing field of evolutionary toxicology has also expanded and is likely to continue to grow, as new anthropogenic stressors are unleashed upon the environment (Bickham et al. 2000).
The effects of environmental stressors on populations of fish depend largely on the magnitude, duration, and frequency of the exposure. Even short-term exposures, such as transient oil spills or temporary encounters of a contaminated environment by a migratory species, can lead to trans- or multi-generational effects in organisms (Dubansky et al. 2013; Everett et al. 2012; Gao et al. 2011). Chronic exposure of sufficient magnitude to affect reproductive success or the integrity of genetic material in individuals can lead to evolutionary adaptation, making individuals more fit for those environments (Bickham 2011). Evolutionary theory would predict rapid spread and fixation of preexisting alleles (even when initially rare), if they conferred a selective advantage in responding to a new anthropogenic stressor (Bickham 2011; Bickham et al. 2000), while alternatively, the likelihood of a beneficial de novo mutation becoming common within a fish population in a century or less is less likely. Either way, we can only evaluate the adaptive changes after many generations, which makes evolutionary studies difficult when we are considering anthropogenic stressors introduced into the environment generally less than a century ago.
2 Organismal Responses to Stressors
When attempting to observe the effects of a stressor on populations of fish, we need to consider not only the characteristics of the event itself but also the various ways by which organisms are able to respond. Acclimation refers to a response in which exposed individuals are capable of dealing with the stressor by altering gene expression, protein levels or activity, or other physiological processes. In this case, the organisms can tolerate the stressful event depending on their genetic makeup and the degree of plasticity of relevant traits. Such changes in expression or activity can have trans-generational effects, but they are not genetically heritable. Thus, in the absence of the stressor, the changes in gene expression or physiology are likely to disappear within two generations. Mounting a physiological response can have various consequences, including higher energy expenditure, and long-term costs often depend on the severity and duration of the response.
There exists a middle ground between acclimation and adaptation that may result in long-term or multi-generational effects of stressors on populations of fish. The group of responses that encompass non-genetic, yet heritable, changes that affect the expression or behavior of genes are called epigenetic alterations (Laird 2003). Epigenetic events are often environmentally driven and include, but are not limited to, DNA methylation on cytosine nucleotides, acetylation on lysine residues of histones, and differential microRNA expression (Wolffe and Matzke 1999). Events such as the methylation of cytosine have been observed to increase mutation rates in those specific genomic regions and provide one of the connections between short-term exposures and resulting DNA alterations (Fryxell and Moon 2005; Gonzalgo and Jones 1997). Thus, with epigenetics in mind, even short-term environmental events can result in heritable multi-generational outcomes, including altered phenotypes resulting from differential gene expression (Guerrero-Bosagna and Skinner 2012; Pfennig and Servedio 2013). The field of epigenetics is very young, but researchers are making major strides in the understanding of this bridge between acclimation and adaptation. Even though we have a limited understanding of the impacts of these heritable non-genetic changes, they provide a potentially very important mechanism by which populations can respond to new stressors more rapidly than through more traditional evolutionary processes. Including epigenetic processes within the field of evolutionary toxicology or human-induced evolution radically expands the number of populations that could potentially be studied.
To study adaptive responses to anthropogenic contaminants, a model organism is needed that has sufficient genetic variability to provide the capacity for rapid adaptation to varying selective pressures. Teleost fishes are a unique and rich system to study novel gene function, partially because of the multiple gene duplications that have occurred in some lineages, resulting in as many as eight gene copies of some genes (Pittman et al. 2013). The availability of multiple gene copies reduces the selective disadvantage normally associated with new mutations. It also increases the opportunity for differential gene regulation, alternative splicing, or other post-translational modifications (Pittman et al. 2013). In addition to gene duplications, the potential for rapid environmental changes in aquatic systems has also selected for species with the capacity to acclimate and adapt to fluctuating environmental conditions. One example of this would be the highly variable nature of estuarine environments favoring euryhaline fish species, which are able to survive dramatic swings in salinity. Such conditions seem to produce robust species that have the genetic capability to respond relatively quickly to changes in environmental stressors (Pittman et al. 2013). Some euryhaline teleosts have previously been studied for their capability to tolerate high variability in temperature, salinity, and pollutants (Crawford and Powers 1992; Whitehead et al. 2011; Nacci et al. 1999). In the case of thermal adaptation, it has been shown that both acclimation and genetic alterations contribute to the physiological adaptation in Atlantic killifish (Fundulus heteroclitus) from different sites (Crawford and Powers 1989). Multiple similar studies suggest that estuarine teleosts may be valuable model organisms for identifying genetic and physiological responses to novel stressors. Their adaptability, ubiquitous presence, and short generation times allow for the investigation of the evolutionary effects of contaminants over relatively short time frames.
A previous summary focused on the mechanistic details of specific examples of adaptation to contaminants in North American fish populations (Wirgin and Waldman 2004). In this chapter, we aim to give an overview of anthropogenic alterations to the environment that are likely to or have already driven evolutionary adaptations in fish. We will discuss two major categories of anthropogenic environmental alterations: alteration of existing natural stressors and the introduction of novel stressors. In order to more comprehensively cover the possible genetic influences, we will discuss both short-term responses with multi-generational effects as well as mutations and shifts in allele frequencies that have led to evolutionary adaptation in fish species.
3 Altered Natural Stressors
3.1 UV Light
One of the many natural stressors that has been altered by anthropogenic activity is UV light penetration into aquatic environments. Factors such as the thickness of the ozone layer, amount of colored dissolved organic matter, and cloud density dictate the intensity and the amount of UV light that reaches aquatic organisms (Hader et al. 2011). Among the well-studied anthropogenic contributions to the increase of both UV-A (315–400 nm) and UV-B (280–315 nm) radiation is ozone depletion (Hader et al. 2011; Core Writing Team et al. 2007). The mechanisms of UV toxicity have been well documented to include dimerizations of thymine DNA bases, increase of reactive oxygen species (ROS) production, and an increased production of the adduct 8-hydroxyguanosine—a biomarker of oxidative stress (Durbeej and Eriksson 2002; Zhang et al. 2004). In addition, UV light has been known to exasperate the toxicity of some PAHs in various organisms because of common modes of toxicity or photoactivation of these compounds (Marquis et al. 2009; Nikkila et al. 1999; Shemer and Linden 2007; Wei et al. 2007). The synergistic increase in toxicity, however, is also accompanied by higher rates of degradation of PAHs due to UV light (Xu and Li 2014; Buth et al. 2010; Peachey 2005). Given these possible adverse effects of UV light, aquatic organisms that are dependent on light, such as algae, have developed methods to minimize UV exposure via mycosporine-like amino acids or absorptive surface pigments (Hader et al. 2011). On the other hand, some fish spend sensitive portions of their development exposed to sunlight in shallow waters, which leaves them vulnerable to the harmful effects of UV radiation (Hakkinen et al. 2004).
Since UV exposure can alter diverse molecular pathways, the effects of this stressor have been studied in various organisms. Increased mortality in aquatic invertebrates and fish has been observed as an effect of UV exposure, which suggests that it may be the driver behind evolutionary change (Nikkila et al. 1999; Jokinen et al. 2011). An example is the intertidal fish Girella laevifrons, which has been observed to spend large portions of its development in waters exposed to high levels of UV radiation (Carrasco-Malio et al. 2014). Since Chilean waters accommodate a multitude of migratory and resident species, biomonitoring efforts are in place to follow the effects of increasing UV. However, recent investigations suggest that, despite being one of the few resident species, G. laevifrons may be an unsuitable species for biomonitoring, due to an apparent resistance to UV-induced mortality (Carrasco-Malio et al. 2014). Even though increased enzymatic activity against ROS was uncovered (including superoxide dismutase, catalase, and lipid peroxidation) along with an increase in DNA damage in the liver, the researchers did not observe the previously reported mortality in G. laevifrons in response to larval UV exposure in the laboratory (Carrasco-Malio et al. 2014). Unfortunately, the results of this experiment are not enough to allow discrimination between acclimation, epigenetic mechanisms, or genetic causes of this apparent protection. A mechanistic explanation or a comparison with a similar species could allow an in-depth interpretation of the results, but the current evidence is promising in terms of documenting adaptation to UV stress. The hypothesized explanation for the lack of mortality in G. laevifrons is that increasing UV radiation in this southern hemisphere intertidal zone acted as a selective pressure to drive this species to adapt to the potentially lethal effects of high UV exposures (Carrasco-Malio et al. 2014).
3.2 Radionuclides
Another source of radiation pollution, which has increased dramatically through human activity, is radionuclide contamination. Natural sources of radioactivity exist and have been shown to have genotoxic effects on human populations resident in naturally radioactive areas (Forster et al. 2002). On the other hand, anthropogenic radionuclide contamination has been widely studied and often stems from nuclear power plant waste or in a few cases from significant disasters, like the Chernobyl and Fukushima nuclear disasters from 1986 and 2011, respectively (Steinhauser et al. 2014). As with radiation-exposed human populations, radionuclides have genotoxic effects on contaminated aquatic biota, with the study focus mainly being DNA damage (Theodorakis et al. 1997).
An investigation of channel catfish (Ictalurus punctatus) populations from radiocesium-contaminated nuclear reactor cooling ponds at Chernobyl showed that fish appear to exhibit higher levels of double-stranded DNA damage, but not an increase in micronuclei (Sugg et al. 1996). The study was unable to determine whether adaptation or acclimation was the cause of the lower apparent micronucleus formation, but it confirmed that radiation contamination could have effects on fish populations at the genetic level (Sugg et al. 1996). A second study focused on the decades-old radionuclide contamination of ponds at the U.S. Department of Energy’s Savannah River Site. Theodorakis and Shugart (1998) investigated populations of mosquitofishes, Gambusia affinis and G. holbrooki, to determine whether long-term exposure in contaminated ponds had affected the genetic integrity of resident populations. These species have short generation times, and their populations were restricted to the study ponds. Researchers found that after multiple generations of exposure, populations of exposed mosquitofishes exhibit higher levels of DNA strand breakage as well as reduced fertility (Theodorakis and Shugart 1998). On the other hand, some individuals in the contaminated sites exhibited lower DNA strand breakage and their genotypes, as determined by random amplified polymorphic DNA (RAPD) assay, were different from the rest of the sampled fish, which suggested a genetic basis for the phenotypic differences (Theodorakis et al. 1999). These studies imply that there may be a possibility of different genotypes providing different protection from radionuclide contamination in mosquitofishes and that human-induced evolution has occurred (Theodorakis et al. 1999).
There are a multitude of existing selective pressures on the planet and anthropogenic events are intentionally or unintentionally contributing to their magnitude. The example studies included in this chapter suggest that the increases of some stressors have led to emerging selective pressures or to the amplification of existing pressures through human activity. Aquatic organisms faced with such challenges may or may not have the capacity to adapt, depending on the natural history of the organism, the availability of genetic resources, and/or the complexity of altered selective pressure. Anthropogenic activities have led to significant alterations in the selection landscape including the increased release of estrogenic compounds, changes in temperature, UV permeability, radionuclide contamination, salinity, dissolved oxygen, and pH. Unfortunately, it is currently not possible to accurately predict the evolutionary impacts of anthropogenic alterations to the existing stressors. However, we are rapidly gaining insight into the mechanisms through which selection may act. This process is even more difficult when considering the potential evolutionary impacts of emerging contaminants.
4 Novel Stressors
This section considers stressors that prior to anthropogenic influences may have been present in the environment, but not at levels likely to have had significant toxicological effects on fish. These are often chemicals that are persistent in the environment and are highly toxic to fish, allowing those pollutants to act as selective pressures for extended periods of time. We will discuss most of these compounds in terms of putative evolutionary adaptations in fish. While there is certainly interest in the short-term impacts of many of these classes of contaminants, we will only focus on observed and potential adaptation in fish populations in response to chronic exposures.
4.1 Metals
The concentrations and bioavailability of many toxic metals have increased dramatically in aquatic environments as a result of human activities. Various metals have toxic properties due to interference with biological pathways in fish (Strydom et al. 2006). There are multiple sources of metals, both natural and more importantly anthropogenic, and each of them produces unique compositions of metals pollution that aquatic organisms experience. Mining for coal and minerals is an environmental concern in many parts of the world. For example, acid mine drainage often leaves environments heavily contaminated with metals (Griffith et al. 2012; Pumure et al. 2010). Concern for the integrity of the impacted environments is substantial, which often leads to wetlands being constructed to attempt to contain the considerable contaminant loads being released, and reduce impacts on downstream habitats (Turker et al. 2014; Guittonny-Philippe et al. 2014). The biological implications of high metals concentrations have been investigated for many years, and some studies have suggested that adaptational processes may have led to lower retention rates of metals in fish populations chronically exposed to high metals concentrations for over 50 years (Jeffree et al. 2014). These studies are not conclusive regarding the causality or mechanisms of this adaptation, but the increasing contamination and frequency of acid mine drainage and mountaintop removal beget the study of the biological impacts of observed adaptation.
A large portion of evolutionary toxicology studies focus on finding adaptive responses in the field by comparing populations of contaminant-exposed fish to reference populations, assuming that reference sites represent ancestral populations prior to the impact of contamination (Klerks et al. 2011). Such assays carry high environmental relevance, but sometimes lack the ability to establish causality. Another set of investigations focus on establishing and quantifying the causal links between contamination and adaptation through artificial selection experiments in the laboratory (Klerks et al. 2011). One significant advantage of these studies is that a true control can be included (i.e., a subset of individuals is not exposed while another subset is exposed to the contaminant for multiple generations). This allows for the derivation of true quantitative knowledge about the causality and heritability of adaptive traits (Klerks et al. 2011). On the other hand, long generation turnover times and a simplified exposure scenario are challenges inherent within such experiments, particularly when using vertebrates (Klerks et al. 2011). An artificial selection experiment exposed a population of least killifish, Heterandria formosa, to LC50 values of cadmium for six consecutive generations, continuing exposures of each generation until at least 50 % of the generation died from Cd exposures (Xie and Klerks 2003). The surviving individuals (generally 15–25 %) were used to produce consecutive generations. The final generation (sixth) produced in this long-term selection study exhibited a threefold increase in Cd resistance, relative to control fish (Xie and Klerks 2003). The realized heritability of the resistance was relatively high (h 2 = 0.5), allowing it to be carried to further generations quite rapidly (Xie and Klerks 2003).
When observing evolutionary events, one also needs to consider the costs that may be associated with the newly acquired phenotype. In the case of least killifish, the cadmium-resistant populations had lower heat tolerance, smaller size, as well as lower lifetime fecundity, brood size, and female life span (Xie and Klerks 2003, 2004). These results demonstrate that the effects of adaptation can be complex and often unpredictable. Populations of adapted fish may be compromised unexpectedly, without visible mortality from the selective stressor, leading to a multitude of possible susceptibilities. In this case, an artificial selection experiment was able to quantify and characterize the ability of organisms to adapt to a single contaminant, while other environmental studies have to deal with complex mixtures and their possible adaptational effects.
A separate study identified populations of Atlantic killifish (F. heteroclitus), which were present at a site with high concentrations of dioxin and metals, to have developed resistance to methylmercury toxicity (Weis et al. 1981a, b). In addition to the protection from the early teratogenic effects of methylmercury, F1-larvae from contaminated site fish also retained higher prey capture efficiency when dosed with meHg, compared to larvae from reference sites (Zhou et al. 1996). These results suggest that adaptive responses to metals can be observed in field-exposed populations, supporting the idea that metals contamination can have evolutionary impacts on aquatic organisms.
4.2 Polycyclic Aromatic Hydrocarbons
Polycyclic aromatic hydrocarbons (PAHs) are a class of widely distributed contaminants, formed by human activities as a by-product of incomplete combustion. They can also be found naturally in oil, natural gas, and coal, and can be produced by forest fires and volcanoes (Walker et al. 2005). These compounds are always found as complex mixtures, and several PAHs are known to be carcinogenic to a wide variety of organisms, including fish and mammals (Schneider et al. 2002; Hermann 1981; Karahalil et al. 1998). Mixtures of PAHs have also been found to have immunotoxic effects (Reynaud and Deschaux 2006; Carlson et al. 2004) and to cause DNA adducts (Jung et al. 2009) and cardiac deformities in aquatic organisms (Clark et al. 2010). Wastewater runoff, in addition to other anthropogenic activities, has been shown to move complex mixtures of PAHs, allowing them to deposit in coastal regions and estuaries (Walker et al. 2005; Menzie et al. 2002; Daskalakis and Oconnor 1995) and causing them to concentrate in surface sediment (Viguri et al. 2002). Thus, the highly teratogenic properties of PAH mixtures to fish embryos can introduce new selective pressures for estuarine fish (Wassenberg and Di Giulio 2004b).
The Atlantic Wood Industries Superfund site (AWI) on the Elizabeth River in Virginia has been on the National Priorities List since 1990 (Fig. 1) (Landers 2006). The contamination at the AWI mainly consists of PAHs (Mitra et al. 1999). PAHs are generally readily biotransformed by fish, but this process involves the activation of the aryl hydrocarbon receptor (AHR) pathway and has been linked to cardiovascular deformities in developing fish embryos, a shared mode of toxicity with some dioxins and co-planar PCBs (Heinrich et al. 1986; Ashurst et al. 1983; Wassenberg and Di Giulio 2004b). The source of the PAH contamination at the AWI was a wood treatment facility releasing creosote, primarily made up of heavy pyrogenic PAHs, into the river and contaminating aquatic life from 1926 to 1992 (Mitra et al. 1999). The incomplete remediation efforts to reduce PAH concentrations at the AWI included a capping of the old wood treatment facility location in 2002 and a dredging campaign in 2007 (Landers 2006). Additional dredging efforts have been planned for the contaminated sediment including the construction of a steel wall to contain the sediment, but these efforts have not yet been completed.
The first indication that fish were being impacted by contamination in the Elizabeth River was the discovery that mummichog (F. heteroclitus) from the AWI had a higher occurrence of hepatic neoplasms (Van Veld et al. 1991; Vogelbein et al. 1990). The cause of those lesions was not found, but was attributed to the PAH mixtures found throughout the Elizabeth River, particularly the high concentrations found at the AWI. Following this discovery, the same investigators tested the activity of the main enzyme involved in the biotransformation of large PAHs, cytochrome P4501A (CYP1A). The expected response following PAH exposure is a significant induction of CYP1A, which is why CYP1A is used as a biomarker of PAH exposure (Nacci et al. 1998; Matson et al. 2009). An example dataset with Fundulus grandis shows the induction of CYP1A activity, measured through a standard ethoxyresorufin-O-deethylase (EROD) assay, with a chosen PCB agonist (Fig. 2). This activity is commonly seen to diminish with the onset of cardiac deformities in embryos, while the causes of this relationship are not fully understood (Fig. 2). Despite the expectation that CYP1A should be elevated in AWI, F. heteroclitus from this highly contaminated site had depressed levels of CYP1A (Van Veld and Westbrook 1995). These results were confirmed in F1-progeny (Wills et al. 2010). In addition, protection from cardiac, tail, and bladder abnormalities in embryos was discovered and linked to the reduced activity of CYP1A, and more specifically a recalcitrant AHR pathway (Meyer et al. 2002; Ownby et al. 2002; Clark et al. 2010). Further studies went on to confirm that altered AHR2 activity was the specific AHR gene alteration likely responsible for the observed resistance, and not simply reduced CYP1A activity (Clark et al. 2010; Matson et al. 2008). These findings were consistent with mechanistic studies previously done in zebrafish (Danio rerio; Carney et al. 2004). The control and the mechanisms by which reduced responsiveness of the AHR pathway leads to protection from PAH toxicity in development remain unknown. Since the generation turnover time for F. heteroclitus is close to a year, there have likely been more than 50 generations, and perhaps as many as 85, since the onset of pollution at the AWI. The identification of an adaptation in a vertebrate species to novel toxicants is a rare and interesting event.
Relationship between CYP1A activity levels and developmental cardiac deformities in response to a common AHR agonist, PCB 126. When F1 embryos from a reference F. grandis population are dosed with PCB 126, the CYP1A activity (black line) is induced as part of the AHR pathway response to the toxicant. At higher concentrations, CYP1A has lower activity levels, and embryos develop cardiac deformities (gray bars). The inverse relationship between the two is a well-documented occurrence, while the mechanistic connection is yet to be elucidated. A subset of these data was presented in Oziolor et al. (2014)
Results documenting PAH resistance in F1-mummichog embryos from the AWI were quite interesting, but failed to clarify whether they represent evolutionary adaptation or a trans-generational epigenetic response to heavy contamination. In order to distinguish between the two options, later generations reared under common garden conditions (i.e., no PAH exposure) needed to be tested. However, studies conducted to date differ somewhat in their findings regarding the heritability of protection to F2-progeny (Ownby et al. 2002; Meyer and Di Giulio 2002; Clark et al. 2013a). Ownby et al. (2002) found that the resistance was maintained completely in F2-progeny, whereas Meyer and Di Giulio (2002) concluded that, while there was still resistance, F2-progeny were less resistant than the F1-generation. Nonetheless, high levels of mortality in AWI fish in the study seem to suggest that the more resistant fish might have been eliminated from contributing to the tested F2-progeny because of fitness costs associated with the resistance, and thus, the protective phenotype may have only appeared to not be heritable (Meyer and Di Giulio 2002). In addition, it is possible that epigenetic effects could be interacting with the adaptive change. The most thorough investigation of the heritability of PAH resistance in AWI mummichog was performed by Clark et al. (2013a). They found an interesting disconnect between the recalcitrance of the molecular pathway believed to be responsible for observed resistance, the AHR pathway, and the actual embryo resistance to cardiovascular defects. The F2-progeny in their study regained partial AHR pathway responsiveness, yet fully retained their protection from embryotoxicity. They concluded that multiple pathways may be involved in PAH resistance, but that none of them seem to be fully genetically heritable (Clark et al. 2013a).
In addition to the physiological differences, which were shown to be at least partially heritable, F. heteroclitus from the AWI were genetically distant from geographically proximate populations (Mulvey et al. 2002). Combined, these results support the hypothesis that there has been a selective sweep caused by the high contamination at the location, causing these non-migratory fish to become resistant to PAH toxicity. As with most adaptations, fitness costs are to be expected, and a few have been found within the AWI population. Fluoranthene, a CYP1A-inhibiting PAH, which is also phototoxic, was found to affect AWI populations more severely in combination with UV light than observed for a reference population (Meyer and Di Giulio 2003). In addition, AWI larvae have been documented to have a reduced resistance to short-term hypoxia exposures (see chapter “Low-Oxygen Lifestyles”), as well as exhibiting decreased survival under common-garden laboratory conditions (see above), suggesting a general reduction in fitness (Meyer and Di Giulio 2003). While some fitness costs have been identified, several other attempts to identify further fitness costs have been unsuccessful. Glutathione expression is on average lower in polluted site fish, but sex and present exposure confound the significance of those results (Bacanskas et al. 2004). Some specific fitness costs were hypothesized as a result of the mechanistic basis for the PAH resistance; because of the lower CYP1A activity, F. heteroclitus from polluted sites were hypothesized to be more susceptible to pesticides deactivated by that enzyme (Clark and Di Giulio 2012). Contrary to this expectation, polluted site mummichogs were shown to be highly cross-resistant to the acute toxicity of two CYP1A-detoxified pesticides, a carbamate and a pyrethroid (Clark and Di Giulio 2012). In addition, even though the main contaminant load at the AWI stems from PAHs, F. heteroclitus from that site are also protected from the effects of PCBs (Clark et al. 2013b). Other research suggests that field-collected fish may have an impaired reproductive system and increased chromosomal damage compared to reference populations (Frederick et al. 2007; Jung et al. 2011). The issue with these studies is that they often have a difficulty differentiating between the effects of PAHs on fish in the field, versus the fitness costs driven by the adaptation of the fish. Thus, the potential adverse effects of PAHs in AWI mummichog may involve multiple physiological changes, some of which are caused by genetic alterations in the fish, while others could be caused simply by the exposure to a toxic mixture with very diverse toxicity pathways.
4.3 Polychlorinated Biphenyls
There are 209 known congeners of PCBs that can be produced to create mixtures of various properties, dependent on their chlorine content (Safe 1984). They were produced in the USA until the early 1970s with known uses in plastics, insulation, various dyes, carbonless paper, and transformers (ATSDR 2000). Because of their stability and resilience to chemical, thermal, and photo-degradation, PCBs are persistent in the environment (Buckman et al. 2004; ATSDR 2000). Nevertheless, they are still found in the production of certain dyes and paint (Hu and Hornbuckle 2010). Resembling 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), some co-planar PCB congeners can upregulate the aryl hydrocarbon receptor (AHR) pathway constitutively and cause cancer, apoptosis, and cardiac deformity in aquatic organisms and mammals (Zhang et al. 2012; Thackaberry et al. 2005; Gao et al. 2011; Jonsson et al. 2007; Antkiewicz et al. 2006; Carney et al. 2004). These properties of co-planar PCBs make them likely selective agents in heavily PCB-contaminated environments (U.S. Environmental Protection Agency 2004).
4.3.1 Atlantic Tomcod (Microgadus tomcod)
Heavy PCB contamination often occurs in urban estuarine regions. A well-known site with historic PCB contamination is the Hudson River (Fig. 1; Feng et al. 1998). Following 70 years of contamination, including the release of an estimated 1.3 million pounds of PCBs, a 200-mile stretch of the Hudson River was put on the National Priorities List as a Superfund site in 1984 (U.S. General Accounting Office 2000). Since then, the fate and distribution of these compounds have been modeled and studied extensively (Connolly et al. 2000). Accumulation and toxicity have been seen both in fish and migratory piscivorous birds in the area (Custer et al. 2012; Deshpande et al. 2013; Koenig et al. 2013; Fernandez et al. 2004). The historic contamination with highly toxic PCBs in the Hudson River resulted in multi-generational exposures and the subsequent evolutionary adaptation of Atlantic tomcod, providing resistance to many of the toxic effects associated with PCBs (Roy and Wirgin 1997). Despite the infamy of this site as a PCB-contaminated site, there have also been levels of TCDD found in juvenile fish collected from the Hudson River that may be among some of the highest levels of this contaminant found in natural populations (Fernandez et al. 2004). Thus, the contamination at this site is complicated by multiple highly toxic contaminants at very high concentrations, many of which have similar ecotoxicological properties.
Resistance to PCBs by Atlantic tomcod is one of the clearest and mechanistically best-elucidated investigations of human-induced evolution through contaminants (Wirgin et al. 2011). The extraordinary concentrations of PCBs in the Hudson River were predicted to be highly detrimental to aquatic life, including the resident Atlantic tomcod (Fernandez et al. 2004). Not unexpectedly, initial investigations identified increased levels of hepatic tumors and decreased life spans of populations living in the Hudson River (Dey et al. 1993). Since a primary molecular mechanism linked to PCB toxicity is the activation of the AHR pathway, research quickly focused on potential alterations to this important pathway. Cytochrome P450 1A (CYP1A) was already discussed as a downstream regulated enzyme responsible for PAH degradation, but its activity is also highly induced in aquatic organisms exposed to co-planar PCBs and dioxins (Nacci et al. 1998; Courtenay et al. 1999). Populations of tomcod collected from the Hudson River did not increase the expression of CYP1A after exposure to PCBs, in contrast to fish from a proximate reference site (Roy and Wirgin 1997). Both populations had similarly increased levels of AHR expression, a normal response to PAHs, but their CYP1A expression was recalcitrant (Roy and Wirgin 1997). The downregulation of the pathway could not be attributed to the AHR repressor (AHRR), which was also found to be downregulated in all tissues, similar to CYP1A (Roy et al. 2006). Following these results, researchers sequenced AHR2, the more active form in fish, for tomcod from four reference populations and two populations in the polluted Hudson River. The sequence data elucidated the mechanistic basis of the phenotypic adaptation of these fish (Wirgin et al. 2011). A six base pair deletion in exon 10 was discovered in Hudson River tomcod, and kinetic tests confirmed that the mutated AHR2 protein had more than sevenfold reduced binding to a model dioxin (Wirgin et al. 2011). This mutation was close to fixation in polluted site populations, whereas it was extremely rare and was always heterozygous in reference site tomcod (Wirgin et al. 2011). Mitochondrial genome diversity revealed that the adaptation in polluted sites did not lead to a decrease in haplotype diversity, confirmed gene flow between resistant populations, and indicated the lack of significant gene flow to reference sites (Wirgin et al. 2011). However, Wirgin et al. (2011) concluded that the variant AHR2 allele, which is also present in some reference populations at low frequencies, likely predates anthropogenic pollution.
The identification of a molecular mechanism and the attribution of this change to anthropogenic pollution is the ultimate goal of evolutionary toxicology (Bickham 2011). Research on Atlantic tomcod populations in the Hudson River has quickly reached that target. In this case, the PCB pollution, likely with help from TCDD, in the Hudson River was intense enough to cause mortality in the resident Atlantic tomcod populations. In addition, PCBs are persistent enough to remain in the environment for enough time such that many generations of tomcod were exposed to the stress of pollution. Therefore, it seems that when a beneficial mutant allele is available in the populations originally exposed to the contaminants, as in the Atlantic tomcod, novel selective pressures can allow for the rapid fixation of this trait and for the success of populations that would normally collapse under these extreme anthropogenic selective pressures (Wirgin et al. 2011).
Estuaries polluted with elevated levels of PCBs are present throughout the world, and they have provided the opportunity to examine the possibility for additional phenotypic adaptations to anthropogenic contaminants (Nelson and Bergen 2012; Lakshmanan et al. 2010). Two species of killifish in the USA, both in the genus Fundulus, have also been found to have repeatedly evolved an increased ability to survive in estuaries with high PCB contaminations (Oziolor et al. 2014; Nacci et al. 1999). Despite being genetically isolated, both species include multiple, disparate populations that have apparently evolved phenotypes resistant to PCB toxicity independently. Unfortunately, the exact molecular mechanism responsible for tolerance remains unknown in either species, but the rapid evolution that has occurred in both suggests that it results from selection on standing genetic variation present in ancestral populations, which—if it turns out to be similar in both species—could predate the Fundulus heteroclitus–F. grandis split (Whitehead et al. 2010; Oziolor et al. 2014). Research on F. heteroclitus and F. grandis reveals a diverse side of human-induced evolution, which has the potential to contribute greatly to our understanding of adaptational processes in response to anthropogenic pollution.
4.3.2 New Bedford Harbor Atlantic Killifish (Fundulus heteroclitus)
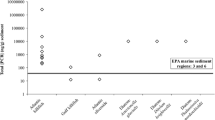
Another site on the Atlantic Coast of the United States, New Bedford Harbor (NBH), Massachusetts, was identified as a highly PCB-polluted estuary and placed on the National Priorities List in 1983 (Fig. 1; Nelson and Bergen 2012). Because of the high projected costs of remediating this site, it was placed on the EPA’s Long Term Monitoring program, which allowed for frequent tracking of the contamination at the site (Nelson and Bergen 2012). The first dredging event removed 14,000 cubic yards of sediment with concentrations of over 4,000 μg/g of PCBs in 1995 (Bergen et al. 2005). Through 2009, approximately 200,000 cubic yards of sediment had been dredged from NBH, leading to a significant decrease in PCB concentrations (Nelson and Bergen 2012).
Following 2009, the projected 20-year remediation process led to multiple investigations of toxicity to aquatic organisms at NBH (Nelson and Bergen 2012). To investigate potential population-level impacts, F. heteroclitus was used as a model for effects on fish, since it is a resident non-migratory species in NBH (Munns et al. 1997). Laboratory studies suggested that F. heteroclitus reproduction might be impaired by exposure to high PCB concentrations, signifying that the toxicants could act as a selective pressure in the polluted estuary (Black et al. 1998). Interest in the effects of chronic PCB contamination on F. heteroclitus finally led to the discovery that NBH populations had adapted to resist the developmental toxicity of PCBs (Nacci et al. 1999).
Populations of F. heteroclitus collected from NBH had lower embryonic and larval mortality in comparison with reference site populations (Nacci et al. 1999). This resistance extended to at least the F2-generation raised under common-garden conditions, confirming that the trait is genetic, rather than an example of physiological acclimation (Nacci et al. 2010). In addition, the resistant phenotype was also correlated with a reduced responsiveness of CYP1A activity, much like in the case of Atlantic tomcod from the Hudson River (Nacci et al. 1999). CYP1A has also been shown to be a sensitive biomarker of dioxin exposure and toxicity in F. heteroclitus (Toomey et al. 2001). In the process of studying the control of CYP1A by the AHR pathway, initial tests identified AHR1 to be more strongly and ubiquitously expressed in tissues of NBH fish, while not being responsive to dioxins or PCBs (Karchner et al. 1999). Adult fish and hepatocyte cell cultures also revealed that NBH populations were much less sensitive in inducing CYP1A mRNA expression or enzyme activity when exposed to dioxins and a model PAH (Bello et al. 2001). The AHR pathway was capable of being activated to the same magnitude, but it only occurred at much higher doses of pollutants (Bello et al. 2001). Results in zebrafish suggest that dioxin-mediated cardiovascular defects are AHR2 dependent, and that the heart deformities are prevented when AHR2, and thus its downstream genes, was knocked down (Carney et al. 2004). On the other hand, knocking down only CYP1A had no effect (Carney et al. 2004). These results support the hypothesis that the resistance of NBH populations of F. heteroclitus to PCB-mediated deformities is based on a recalcitrant AHR pathway and, by extension, its main inducible downstream enzymes (Toomey et al. 2001; Bello et al. 2001).
One factor that makes this case particularly interesting is that there are very few identified fitness costs associated with adaptation to PCBs in F. heteroclitus. Studies have identified the lower capacity of NBH killifish to deal with oxidative stress (Harbeitner et al. 2013). Resistant populations were also found to have lower hepatic, but higher intestinal, expression of P-glycoprotein, an enzyme responsible for the excretion of moderately hydrophobic compounds (Bard et al. 2002). This difference between populations diminished after they were placed in common-garden laboratory conditions for more than 8 days (Bard et al. 2002). Other studies dealing with retinoid depletion by PCB exposures have been unable to identify a difference between reference and resistant populations of F. heteroclitus (Nacci et al. 2001). In addition, it was found that the PCB sensitivity of populations of F. heteroclitus correlated with the concentrations of PCBs at the sites of their collection (Nacci et al. 2002). Further studies into that trend determined that there were several exceptions to the correlation between contamination levels and resistance, which may result from the vast variability of contaminants present at different locations (Nacci et al. 2010).
The lack of mechanistic evidence to explain the differential sensitivity to dioxins in NBH populations led investigators to explore genetic and epigenetic markers to better understand the possible shifts in genes or their control. The efforts began with the sequencing of the CYP1A promoter region, but after CYP1A knockdown was identified to not be protective of PCB-induced teratogenesis, efforts were shifted to the AHR (Powell et al. 2004). The methylation patterns of AHR1 and AHR2 were not observed to have an effect on the PCB sensitivity of F. heteroclitus, but rather to affect the gene expression patterns in the fish (Aluru et al. 2011). With the development of new technology, population resequencing studies have become more affordable, and investigations have begun into the genotypic differences between the AHR pathway genes of these fish (Reitzel et al. 2014). These studies identified a significant population genetic differentiation between resistant and reference populations (Reitzel et al. 2014). The results suggest that selection is possible for the tested genes (AHR1, AHR2, and AHRR), but with the current results, the study was unable to show strong significant differences that could identify the genetic basis of protection (Reitzel et al. 2014). Another exploration of the genetic variation between reference and resistant site fish through single-nucleotide polymorphism (SNP) analysis identified signs of positive selection in SNPs of AHR2 and CYP1A between these populations (Proestou et al. 2014). Such variation may suggest that the AHR pathway still represents a potential mechanism to explain part of the phenotypic differences in the responses of resistant killifish to contaminant-induced damage. Population genomics is a promising tool for the exploration of genetic effects on populations, and the F. heteroclitus populations at New Bedford Harbor are a promising start for this endeavor, but adaptation in killifish contains more complexity that still stands to be unraveled.
4.3.3 Newark Bay Atlantic Killifish (F. heteroclitus)
Shortly after the identification of the first adapted populations at NBH, another group was able to find a similar recalcitrant AHR phenotype in F. heteroclitus collected from Newark Bay (NB) (Fig. 1; Arzuaga and Elskus 2002). The NB estuary, part of the Passaic River in New Jersey, is a heavily contaminated area, which has been on the National Priorities List since 1984 as the “Diamond Alkali Superfund Site” (Crawford et al. 1995). In addition, the Passaic River runs parallel to the Hudson River discussed previously in terms of Atlantic tomcod adaptation. The PCB levels at the NB site are not as high as the ones at NBH, but the type of contamination is much more complex, involving high levels of polychlorinated dibenzo-dioxins and furans (PCDD/Fs), PCBs, and heavy metals (Crawford et al. 1995; Wenning et al. 1993; Armstrong et al. 2005). This has led to multiple fish consumption advisories for this area and to the identification of another population of contaminant-adapted F. heteroclitus (Pflugh et al. 2011; Arzuaga and Elskus 2002).
The initial studies identified a similar phenotype of resistance associated with protection from developmental toxicity and reduced CYP1A activity in F. heteroclitus populations collected from NB (Arzuaga et al. 2004; Arzuaga and Elskus 2002). In addition, de-methylating agents failed to revert either of those phenotypes, suggesting that epigenetics may not play a strong role in the observed resistance (Arzuaga et al. 2004). Similar to other adapted populations, NB fish were resistant not only to cardiovascular teratogenesis, but also to the induction of ROS by a PCB-simulating compound (Arzuaga and Elskus 2010; Arzuaga et al. 2006). These findings point to a similar phenotype of protection and shared fitness costs with F. heteroclitus populations from NBH. Additional studies on NB fish confirmed that complex contaminant exposures impose selection on populations by reducing the reproductive fitness of chronically exposed individuals (Bugel et al. 2010, 2011).
The discovery of three, geographically distant, populations of F. heteroclitus that have adapted to chronic contamination in a physiologically similar manner distinguishes this system from Atlantic tomcod. The tomcod population fixed a rare mutation that allowed it to survive in conditions of high contamination. Conversely, F. heteroclitus seems to have been able to adapt at multiple locations, and gene flow among adapted populations is extremely unlikely. In addition, the genetic basis of this adaptation or the full extent of the adaptation has still not been fully determined.
One of the most fascinating findings about F. heteroclitus is that they seem to have adapted to heavy PCB pollution in a very similar manner to the way they have adapted to PAHs, including recalcitrance in AHR pathway enzymes and protection from cardiac deformities (Arzuaga and Elskus 2002; Van Veld and Westbrook 1995; Roy and Wirgin 1997). The vast geographic separation has been shown to prevent gene flow among these distant adapted populations, as would be expected for this species (Duvernell et al. 2008). In addition, it has been shown that selection at NBH and AWI has had an influence on the population genetic differentiation between adapted and non-adapted populations (Duvernell et al. 2008). Other populations of F. heteroclitus have also been shown to exhibit resistance, with levels of pollution similar to those previously described at NBH and NB (Nacci et al. 2010). The vast genetic and geographic distance between these locations and the similar mode of adaptation between sites with varying pollution led to the hypothesis that standing genetic variation in ancestral populations is responsible for the rapid evolution of protection in F. heteroclitus from contaminated habitats (Whitehead et al. 2012). Populations from various locations were compared in terms of their resistance and their transcriptomic profiles, revealing that distant resistant populations had profiles more similar to each other than to their most geographically proximate reference sites (Whitehead et al. 2012). This could allow for the rapid fixation of the trait and the possibility for multiple populations to acquire this resistance, despite low migration rates in F. heteroclitus.
4.3.4 Houston Ship Channel Gulf Killifish (Fundulus grandis)
While the F. heteroclitus case is fascinating at its current intricacy, there is another level of complexity—F. grandis. Gulf killifish are the sister species of F. heteroclitus and are found primarily along the US Gulf Coast (Fig. 1; Gonzalez et al. 2009). These two species have been shown to overlap and hybridize along a short portion of the northeastern Florida Atlantic coast (Gonzalez et al. 2009). Fundulus grandis is found ubiquitously along the Gulf of Mexico coast and has been studied as a relevant environmental model for hypoxia exposures, osmoregulation in euryhaline fishes, as well as physiological and toxicological responses to the Deepwater Horizon Oil Spill (Dubansky et al. 2013; Landry et al. 2003; Love and Rees 2002; Virani and Rees 2000). As an ecologically important coastal fish in the Gulf of Mexico, it was of particular interest that populations of F. grandis were identified to be resident, and in fact quite common, in the Houston Ship Channel (HSC) (Fig. 1; Oziolor et al. 2014).
The HSC is a heavily industrialized commercial waterway, heavily polluted with a mixture of PCBs, dioxins, and PAHs (Lakshmanan et al. 2010). These compounds have been observed to accumulate in catfish (Ictalurus punctatus), reaching total PCB concentrations of 37 pg/g (Subedi and Usenko 2012). The spatial distributions of PCBs and PCDD/Fs have been studied intensely and were found to diminish as the channel continues through Galveston Bay (Howell et al. 2008, 2011). In addition, these levels of contamination have been observed historically over the last four decades allowing for chronic contamination of aquatic organisms over many generations (Yeager et al. 2007).
Recently, populations of F. grandis, collected from heavily contaminated areas in the HSC, were shown to exhibit a similar phenotypic resistance to PCB- and PAH-induced cardiovascular teratogenesis (Fig. 3; Oziolor et al. 2014). Cardiovascular teratogenesis occurs in embryos, if they come in contact with a toxicant at sensitive developmental stages. Often, these deformities are scored qualitatively from a normal, two-chambered heart (0) to severely deformed string-heart (2) (Fig. 4). The protection from PCBs in HSC F. grandis was of much higher magnitude than that observed for PAHs, which correlated well with the toxicity equivalency of these classes of compounds in the HSC (Oziolor et al. 2014). The protection from cardiovascular teratogenesis in F. grandis population also correlated highly with a recalcitrant AHR pathway, as measured via CYP1A activity, suggesting a similar mode of action as seen in adapted F. heteroclitus populations (Oziolor et al. 2014). The different levels of resistance to PCBs and PAHs, even when evaluating a known AHR-mediated toxicity endpoint, suggest that there may be additional complexity to the observed adaptation, beyond a simple AHR recalcitrance. Biparental crosses of reference and resistant populations suggest that each parent contributes equally to the resistant phenotype, suggesting a genetic basis of this adaptation (Oziolor et al. 2014). More recently, a gradient of this adaptation has been found, where populations from the HSC with lower predicted contaminant exposure exhibit lower levels of protection and lower levels of AHR recalcitrance (Fig. 5).
Sampling sites from two Superfund sites within the industrialized portion of the Houston Ship Channel, Vince Bayou (VB), and Patrick Bayou (PB). A site with predicted intermediate contamination levels, Cedar Bayou (CB), was collected from a portion proximate to the HSC, while a reference population, Gangs Bayou (GB), was collected far from the high contamination regions
Populations of F. grandis from chronically contaminated sites exhibit protection from cardiovascular deformities in a gradient-dependent manner. F1 embryos from the reference site, GB, show a normal dose–response curve for cardiovascular deformities in response to PCB 126, a known AHR agonist. The population from a site with predicted intermediate contamination, CB, develops deformities at higher concentrations of contaminant, showing significant levels of protection. On the other hand, sites within the industrialized portion of the HSC show no significant cardiac defects in response to PCB 126, revealing a >1,000× protection from contaminant induced deformities. A subset of these data was presented in Oziolor et al. (2014)
The identification of a sister species that has undergone a phenotypically similar genetic adaptation to human-induced pollution suggests that the hypothesis of adaptation through preexisting genetic variation would now extend to their shared common ancestor. Thus, the alleles responsible for this adaptation may have been carried through populations and generations of Fundulus even before the split of the two species. Such an extended timeframe begets multiple questions, including the question of why these alleles persisted through time, the nature of the fitness costs associated with them, and the natural stressors that may have led to their evolution.
Expanding adaptation to human-induced novel selective pressures in Fundulus to multiple species has the potential to answer questions extending from toxicology to the basics of evolutionary biology. Through these adaptations, we can study not only the effect of current toxicants in the environment but also about the nature of evolutionary processes that have led to the continuous selection of alleles that would provide some protection from xenobiotics. This should lead to a better understanding of how natural biological systems may respond to current anthropogenic pollutants and how they cross-react with historical biological selective pressures. The study of these two species is at the forefront of evolutionary toxicology and the discovery of the molecular mechanisms and history of these adaptations will significantly improve our understanding of the evolutionary effects of anthropogenic contamination.
5 Conclusions and Outlook
While research has identified some long-term effects of legacy contaminants following chronic exposures, there are many chemicals that have not been investigated, and novel stressors being released every year. For most systems, we are far from having a comprehensive understanding of how organisms will respond to the changing selective landscape in their natural environments. A crucial misconception with finding resistance to high concentrations of contaminants is that the public may interpret this to mean that contamination is acceptable or less of a problem, because organisms can adapt to it. Unfortunately, this is a misinterpretation of such results for two primary reasons. First, the fact that some species are able to adapt to pollution does not imply that it will happen in every organism, population, or pollution scenario. Second, the resistant population often has been changed in terms of its phenotypic response to more than just the compound it adapted to; in other words, adaptation to anthropogenic stressors almost certainly has fitness costs. Whether and how fish will respond to rising salinities in freshwater bodies and whether they will cope with higher radiation or temperature increases are questions stemming from already existing sources of human-induced alterations of natural selective regimes. Along with current levels of toxicity from pesticides or persistent pollutants that have already been introduced in the environment, a new direction of research will be to study the evolutionary consequences of constantly increasing levels of pharmaceuticals in aquatic environments. The toxicity of pharmaceuticals in the environment is being widely studied; but in terms of population adaptation, the effects of these compounds are still entirely unknown. There are multiple classes and types of anthropogenic effects on the environment, but our knowledge of how they shape fish populations is often limited to short exposure durations. In addition, as most of these compounds are found in very complex mixtures, it is of interest to understand how they interact with existing environmental stressors to change the selective landscape of resident organisms at varying locations. Future extremophile fish may evolve in response to already existing levels of alteration in the selective landscape within aquatic environments, and if the populations have sufficient standing genetic variation to adapt to these changes.
References
Aluru N, Karchner SI, Hahn ME (2011) Role of DNA methylation of AHR1 and AHR2 promoters in differential sensitivity to PCBs in Atlantic Killifish, Fundulus heteroclitus. Aquat Toxicol 101:288–294
Anderegg WRL, Prall JW, Harold J, Schneider SH (2010) Expert credibility in climate change. Proc Natl Acad Sci 107:12107–12109
Antkiewicz DS, Peterson RE, Heideman W (2006) Blocking expression of AHR2 and ARNT1 in zebrafish larvae protects against cardiac toxicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol Sci 94:175–182
Armstrong TN, Iannuzzi TJ, Thelen JB, Ludwig DF, Firstenberg CE (2005) Characterization of chemical contamination in shallow-water estuarine habitats of an industrialized river. Part II. Metals. Soil Sediment Contam 14:35–52
Arzuaga X, Elskus A (2002) Evidence for resistance to benzo a pyrene and 3,4,3′,4′-tetrachlorobiphenyl in a chronically polluted Fundulus heteroclitus population. Mar Environ Res 54:247–251
Arzuaga X, Elskus A (2010) Polluted-site killifish (Fundulus heteroclitus) embryos are resistant to organic pollutant-mediated induction of CYP1A activity, reactive oxygen species, and heart deformities. Environ Toxicol Chem/SETAC 29:676–682
Arzuaga X, Calcano W, Elskus A (2004) The DNA de-methylating agent 5-azacytidine does not restore CYP1A induction in PCB resistant Newark Bay killifish (Fundulus heteroclitus). Mar Environ Res 58:517–520
Arzuaga X, Wassenberg D, Di Giulio R, Elskus A (2006) The chlorinated AHR ligand 3,3′,4,4′,5-pentachlorobiphenyl (PCB 126) promotes reactive oxygen species (ROS) production during embryonic development in the killifish (Fundulus heteroclitus). Aquat Toxicol 76:13–23
Ashurst SW, Cohen GM, Nesnow S, Digiovanni J, Slaga TJ (1983) Formation of benzo[a]pyrene DNA adducts and their relationship to tumor initiation in mouse epidermis. Cancer Res 43:1024–1029
ATSDR (2000) Toxicological profile for polychlorinated biphenyls (PCBs). Department of Health and Human Services, Public Health Service Agency for Toxic Substances and Disease Registry (ATSDR)
Bacanskas LR, Whitaker J, Di Giulio RT (2004) Oxidative stress in two populations of killifish (Fundulus heteroclitus) with differing contaminant exposure histories. Mar Environ Res 58:597–601
Bard SM, Bello SM, Hahn ME, Stegeman JJ (2002) Expression of P-glycoprotein in killifish (Fundulus heteroclitus) exposed to environmental xenobiotics. Aquat Toxicol 59:237–251
Bello SM, Franks DG, Stegeman JJ, Hahn ME (2001) Acquired resistance to Ah receptor agonists in a population of Atlantic killifish (Fundulus heteroclitus) inhabiting a marine superfund site: In vivo and in vitro studies on the inducibility of xenobiotic metabolizing enzymes. Toxicol Sci 60:77–91
Bergen BJ, Nelson WG, Mackay J, Dickerson D, Jayaraman S (2005) Environmental monitoring of remedial dredging at the New Bedford Harbor, MA, Superfund site. Environ Monit Assess 111:257–275
Bickham JW (2011) The four cornerstones of evolutionary toxicology. Ecotoxicology 20:497–502
Bickham JW, Sandhu S, Hebert PDN, Chikhi L, Athwal R (2000) Effects of chemical contaminants an genetic diversity in natural populations: implications for biomonitoring and ecotoxicology. Mutat Res Rev Mutat Res 463:33–51
Black DE, Gutjahr-Gobell R, Pruell RJ, Bergen B, McElroy AE (1998) Effects of a mixture of non-ortho-and mono-ortho-polychlorinated biphenyls on reproduction in Fundulus heteroclitus (Linnaeus). Environ Toxicol Chem 17:1396–1404
Buckman AH, Norstrom RJ, Hobson KA, Karnovsky NJ, Duffe J, Fisk AT (2004) Organochlorine contaminants in seven species of Arctic seabirds from northern Baffin Bay. Environ Pollut 128:327–338
Bugel SM, White LA, Cooper KR (2010) Impaired reproductive health of killifish (Fundulus heteroclitus) inhabiting Newark Bay, NJ, a chronically contaminated estuary. Aquat Toxicol 96:182–193
Bugel SM, White LA, Cooper KR (2011) Decreased vitellogenin inducibility and 17 beta-estradiol levels correlated with reduced egg production in killifish (Fundulus heteroclitus) from Newark Bay, NJ. Aquat Toxicol 105:1–12
Buth JM, Steen PO, Sueper C, Blumentritt D, Vikesland PJ, Arnold WA, McNeill K (2010) Dioxin photoproducts of triclosan and its chlorinated derivatives in sediment cores. Environ Sci Technol 44:4545–4551
Camacho-Munoz D, Martin J, Santos JL, Aparicio I, Alonso E (2014) Concentration evolution of pharmaceutically active compounds in raw urban and industrial wastewater. Chemosphere 111:70–79
Carlson EA, Li Y, Zelikoff JT (2004) Benzo a pyrene-induced immunotoxicity in Japanese medaka (Oryzias latipes): relationship between lymphoid CYP1A activity and humoral immune suppression. Toxicol Appl Pharmacol 201:40–52
Carney SA, Peterson RE, Heideman W (2004) 2,3,7,8-tetrachlorodibenzo-p-dioxin activation of the aryl hydrocarbon receptor/aryl hydrocarbon receptor nuclear translocator pathway causes developmental toxicity through a CYP1A-independent mechanism in zebrafish. Mol Pharmacol 66:512–521
Carrasco-Malio A, Diaz M, Mella M, Montoya MJ, Miranda A, Landaeta MF, Sanchez G, Hidalgo ME (2014) Are the intertidal fish highly resistant to UV-B radiation? A study based on oxidative stress in Girella laevifrons (Kyphosidae). Ecotoxicol Environ Saf 100:93–98
Clark BW, Di Giulio RT (2012) Fundulus heteroclitus adapted to PAHs are cross-resistant to multiple insecticides. Ecotoxicology 21:465–474
Clark BW, Matson CW, Jung D, Di Giulio RT (2010) AHR2 mediates cardiac teratogenesis of polycyclic aromatic hydrocarbons and PCB-126 in Atlantic killifish (Fundulus heteroclitus). Aquat Toxicol 99:232–240
Clark BW, Bone AJ, Giulio RT (2013a) Resistance to teratogenesis by F1 and F2 embryos of PAH-adapted Fundulus heteroclitus is strongly inherited despite reduced recalcitrance of the AHR pathway. Environ Sci Pollut Res Int 21:13898–13908
Clark BW, Cooper EM, Stapleton HM, Di Giulio RT (2013b) Compound- and mixture-specific differences in resistance to polycyclic aromatic hydrocarbons and PCB-126 among Fundulus heteroclitus subpopulations throughout the Elizabeth River Estuary (Virginia, USA). Environ Sci Tech 47:10556–10566
Connolly JP, Zahakos HA, Benaman J, Ziegler CK, Rhea JR, Russell K (2000) A model of PCB fate in the Upper Hudson River. Environ Sci Technol 34:4076–4087
Core Writing Team, Pachauri RK, Reisinger A (eds) (2007) IPCC fourth assessment report: climate change 2007. IPCC, Geneva, Switzerland
Courtenay SC, Grunwald CM, Kreamer GL, Fairchild WL, Arsenault JT, Ikonomou M, Wirgin II (1999) A comparison of the dose and time response of CYP1A1 mRNA induction in chemically treated Atlantic tomcod from two populations. Aquat Toxicol 47:43–69
Crawford DL, Powers DA (1989) Molecular basis of evolutionary adaptation at the lactate dehydrogenase-B locus in the fish Fundulus heteroclitus. Proc Natl Acad Sci U S A 86:9365–9369
Crawford DL, Powers DA (1992) Evolutionary adaptation to different thermal environments via transcriptional regulation. Mol Biol Evol 9:806–813
Crawford DW, Bonnevie NL, Wenning RJ (1995) Sources of pollution and sediment contamination in Newark Bay, New Jersey. Ecotoxicol Environ Saf 30:85–100
Custer CM, Custer TW, Hines JE (2012) Adult tree swallow survival on the polychlorinated biphenyl-contaminated Hudson River, New York, USA, between 2006 and 2010. Environ Toxicol Chem 31:1788–1792
Daskalakis KD, Oconnor TP (1995) Distribution of chemical concentrations in US coastal and estuarine environments. Mar Environ Res 40:381–398
Deshpande AD, Dockum BW, Cleary T, Farrington C, Wieczorek D (2013) Bioaccumulation of polychlorinated biphenyls and organochlorine pesticides in young-of-the-year bluefish (Pomatomus saltatrix) in the vicinity of a Superfund Site in New Bedford Harbor, Massachusetts, and in the adjacent waters. Mar Pollut Bull 72:146–164
Dey WP, Peck TH, Smith CE, Kreamer GL (1993) Epizoology of hepatic neoplasia in Atlantic tomcod (Microgadus tomcod) from the Hudson River estuary. Can J Fish Aquat Sci 50:1897–1907
Dijkstra JA, Buckman KL, Ward D, Evans DW, Dionne M, Chen CY (2013) Experimental and natural warming elevates mercury concentrations in estuarine fish. PLoS One 8:e58401
Dubansky B, Whitehead A, Miller JT, Rice CD, Galvez F (2013) Multitissue molecular, genomic, and developmental effects of the deepwater horizon oil spill on resident gulf killifish (Fundulus grandis). Environ Sci Technol 47:5074–5082
Durbeej B, Eriksson LA (2002) Reaction mechanism of thymine dimer formation in DNA induced by UV light. J Photochem Photobiol A Chem 152:95–101
Duvernell DD, Lindmeier JB, Faust KE, Whitehead A (2008) Relative influences of historical and contemporary forces shaping the distribution of genetic variation in the Atlantic killifish, Fundulus heteroclitus. Mol Ecol 17:1344–1360
Everett MV, Antal CE, Crawford DL (2012) The effect of short-term hypoxic exposure on metabolic gene expression. J Exp Zool A Ecol Genet Physiol 317A:9–23
Feng H, Cochran JK, Lwiza H, Brownawell BJ, Hirschberg DJ (1998) Distribution of heavy metal and PCB contaminants in the sediments of an urban estuary: The Hudson River. Mar Environ Res 45:69–88
Fernandez MP, Ikonomou MG, Courtenay SC, Wirgin II (2004) Spatial variation in hepatic levels and patterns of PCBs and PCDD/Fs among young-of-the-year and adult Atlantic tomcod (Microgadus tomcod) in the Hudson River estuary. Environ Sci Technol 38:976–983
Forster L, Forster P, Lutz-Bonengel S, Willkomm H, Brinkmann B (2002) Natural radioactivity and human mitochondrial DNA mutations. Proc Natl Acad Sci U S A 99:13950–13954
Frederick LA, Van Veld PA, Rice CD (2007) Bioindicators of immune function in creosote-adapted estuarine killifish, Fundulus heteroclitus. J Toxicol Environ Health A 70:1433–1442
Fryxell KJ, Moon WJ (2005) CpG mutation rates in the human genome are highly dependent on local GC content. Mol Biol Evol 22:650–658
Gao K, Brandt I, Goldstone JV, Jonsson ME (2011) Cytochrome P450 1A, 1B, and 1C mRNA induction patterns in three-spined stickleback exposed to a transient and a persistent inducer. Comp Biochem Physiol C Toxicol Pharmacol 154:42–55
Gonzalez I, Levin M, Jermanus S, Watson B, Gilg MR (2009) Genetic assessment of species ranges in Fundulus heteroclitus and F. grandis in northeastern Florida salt marshes. Southeast Nat 8:227–244
Gonzalgo ML, Jones PA (1997) Mutagenic and epigenetic effects of DNA methylation. Mutat Res Rev Mutat Res 386:107–118
Griffith MB, Norton SB, Alexander LC, Pollard AI, LeDuc SD (2012) The effects of mountaintop mines and valley fills on the physicochemical quality of stream ecosystems in the central Appalachians: a review. Sci Total Environ 417:1–12
Guerrero-Bosagna C, Skinner MK (2012) Environmentally induced epigenetic transgenerational inheritance of phenotype and disease. Mol Cell Endocrinol 354:3–8
Guittonny-Philippe A, Masotti V, Hohener P, Boudenne JL, Viglione J, Laffont-Schwob I (2014) Constructed wetlands to reduce metal pollution from industrial catchments in aquatic Mediterranean ecosystems: A review to overcome obstacles and suggest potential solutions. Environ Int 64:1–16
Hader DP, Helbling EW, Williamson CE, Worrest RC (2011) Effects of UV radiation on aquatic ecosystems and interactions with climate change. Photochem Photobiol Sci 10:242–260
Hakkinen J, Vehniainen E, Oikari A (2004) High sensitivity of northern pike larvae to UV-B but no UV-photoinduced toxicity of retene. Aquat Toxicol 66:393–404
Harbeitner RC, Hahn ME, Timme-Laragy AR (2013) Differential sensitivity to pro-oxidant exposure in two populations of killifish (Fundulus heteroclitus). Ecotoxicology 22:387–401
Heinrich U, Pott F, Mohr U, Fuhst R, Konig J (1986) Lung tomors in rats and mice after inhalation of PAH rich emissions. Exp Pathol 29:29–34
Hermann M (1981) Synergistic effects of individual polycyclic aromatic hydrocarbons on the mutagenicity of their mixtures. Mutat Res 90:399–409
Howell NL, Suarez MP, Rifai HS, Koenig L (2008) Concentrations of polychlorinated biphenyls (PCBs) in water, sediment, and aquatic biota in the Houston Ship Channel, Texas. Chemosphere 70:593–606
Howell NL, Rifai HS, Koenig L (2011) Comparative distribution, sourcing, and chemical behavior of PCDD/Fs and PCBs in an estuary environment. Chemosphere 83:873–881
Hu DF, Hornbuckle KC (2010) Inadvertent polychlorinated biphenyls in commercial paint pigments. Environ Sci Technol 44:2822–2827
Incardona JP, Collier TK, Scholz NL (2004) Defects in cardiac function precede morphological abnormalities in fish embryos exposed to polycyclic aromatic hydrocarbons. Toxicol Appl Pharmacol 196:191–205
Incardona JP, Linbo TL, Scholz NL (2011) Cardiac toxicity of 5-ring polycyclic aromatic hydrocarbons is differentially dependent on the aryl hydrocarbon receptor 2 isoform during zebrafish development. Toxicol Appl Pharmacol 257:242–249
Jeffree RA, Markich SJ, Twining JR (2014) Diminished metal accumulation in riverine fishes exposed to acid mine drainage over five decades. PLoS One 9:e91371
Jokinen IE, Salo HM, Markkula E, Rikalainen K, Arts MT, Browman HI (2011) Additive effects of enhanced ambient ultraviolet B radiation and increased temperature on immune function, growth and physiological condition of juvenile (parr) Atlantic Salmon, Salmo salar. Fish Shellfish Immunol 30:102–108
Jonsson ME, Jenny MJ, Woodin BR, Hahn ME, Stegeman JJ (2007) Role of AHR2 in the expression of novel cytochrome p450 1 family genes, cell cycle genes, and morphological defects in developing zebrafish exposed to 3,3′,4,4′,5-pentachlorobiphenyl or 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol Sci 100:180–193
Jung D, Cho Y, Collins LB, Swenberg JA, Di Giulio RT (2009) Effects of benzo a pyrene on mitochondrial and nuclear DNA damage in Atlantic killifish (Fundulus heteroclitus) from a creosote-contaminated and reference site. Aquat Toxicol 95:44–51
Jung DW, Matson CW, Collins LB, Laban G, Stapleton HM, Bickham JW, Swenberg JA, Di Giulio RT (2011) Genotoxicity in Atlantic killifish (Fundulus heteroclitus) from a PAH-contaminated Superfund site on the Elizabeth River, Virginia. Ecotoxicology 20:1890–1899
Karahalil B, Burgaz S, Fisek G, Karakaya AE (1998) Biological monitoring of young workers exposed to polycyclic aromatic hydrocarbons in engine repair workshops. Mutat Res Genet Toxicol Environ Mutagen 412:261–269
Karchner SI, Powell WH, Hahn ME (1999) Identification and functional characterization of two highly divergent aryl hydrocarbon receptors (AHR1 and AHR2) in the teleost Fundulus heteroclitus – evidence for a novel subfamily of ligand-binding basic helix loop helix-Per-ARNT-Sim (bHLH-PAS) factors. J Biol Chem 274:33814–33824
Kaushal SS, Groffman PM, Likens GE, Belt KT, Stack WP, Kelly VR, Band LE, Fisher GT (2005) Increased salinization of fresh water in the northeastern United States. Proc Natl Acad Sci U S A 102:13517–13520
Kidd KA, Blanchfield PJ, Mills KH, Palace VP, Evans RE, Lazorchak JM, Flick RW (2007) Collapse of a fish population after exposure to a synthetic estrogen. Proc Natl Acad Sci U S A 104:8897–8901
Klerks PL, Xie L, Levinton JS (2011) Quantitative genetics approaches to study evolutionary processes in ecotoxicology; a perspective from research on the evolution of resistance. Ecotoxicology 20:513–523
Knecht AL, Goodale BC, Truong L, Simonich MT, Swanson AJ, Matzke MM, Anderson KA, Waters KM, Tanguay RL (2013) Comparative developmental toxicity of environmentally relevant oxygenated PAHs. Toxicol Appl Pharmacol 271:266–275
Koenig S, Fernandez P, Company JB, Huertas D, Sole M (2013) Are deep-sea organisms dwelling within a submarine canyon more at risk from anthropogenic contamination than those from the adjacent open slope? A case study of Blanes canyon (NW Mediterranean). Prog Oceanogr 118:249–259
Laird PW (2003) The power and the promise of DNA methylation markers. Nat Rev Cancer 3:253–266
Lakshmanan D, Howell NL, Rifai HS, Koenig L (2010) Spatial and temporal variation of polychlorinated biphenyls in the Houston Ship Channel. Chemosphere 80:100–112
Landers J (2006) Elizabeth River to be focus of major cleanup effort. Civil Eng 76:22–23
Landry CA, Manning S, Cheek AO (2003) Hypoxia affects reproduction in gulf killifish, Fundulus grandis. Integr Comp Biol 43:812
Love JW, Rees BB (2002) Seasonal differences in hypoxia tolerance in gulf killifish, Fundulus grandis (Fundulidae). Environ Biol Fishes 63:103–115
Marquis O, Miaud C, Ficetola GF, Bocher A, Mouchet F, Guittonneau S, Devaux A (2009) Variation in genotoxic stress tolerance among frog populations exposed to UV and pollutant gradients. Aquat Toxicol 95:152–161
Matson CW, Clark BW, Jenny MJ, Fleming CR, Hahn ME, Di Giulio RT (2008) Development of the morpholino gene knockdown technique in Fundulus heteroclitus: a tool for studying molecular mechanisms in an established environmental model. Aquat Toxicol 87:289–295
Matson CW, Gillespie AM, McCarthy C, McDonald TJ, Bickham JW, Sullivan R, Donnelly KC (2009) Wildlife toxicology: biomarkers of genotoxic exposures at a hazardous waste site. Ecotoxicology 18:886–898
Medina MH, Correa JA, Barata C (2007) Micro-evolution due to pollution: Possible consequences for ecosystem responses to toxic stress. Chemosphere 67:2105–2114
Mennigen JA, Stroud P, Zamora JM, Moon TW, Trudeau VL (2011) Pharmaceuticals as neuroendocrine disruptors: lessons learned from fish on prozac. J Toxicol Environ Health B Crit Rev 14:387–412
Menzie CA, Hoeppner SS, Cura JJ, Freshman JS, LaFrey EN (2002) Urban and suburban storm water runoff as a source of polycyclic aromatic hydrocarbons (PAHs) to Massachusetts estuarine and coastal environments. Estuaries 25:165–176
Meyer J, Di Giulio R (2002) Patterns of heritability of decreased EROD activity and resistance to PCB 126-induced teratogenesis in laboratory-reared offspring of killifish (Fundulus heteroclitus) from a creosote-contaminated site in the Elizabeth River, VA, USA. Mar Environ Res 54:621–626
Meyer JN, Di Giulio RT (2003) Heritable adaptation and fitness costs in killifish (Fundulus beteroclitus) inhabiting a polluted estuary. Ecol Appl 13:490–503
Meyer JN, Nacci DE, Di Giulio RT (2002) Cytochrome P4501A (CYP1A) in killifish (Fundulus heteroclitus): heritability of altered expression and relationship to survival in contaminated sediments. Toxicol Sci 68:69–81
Mitra S, Dickhut RM, Kuehl SA, Kimbrough KL (1999) Polycyclic aromatic hydrocarbon (PAH) source, sediment deposition patterns, and particle geochemistry as factors influencing PAH distribution coefficients in sediments of the Elizabeth River, VA, USA. Mar Chem 66:113–127
Moe SJ, De Schamphelaere K, Clements WH, Sorensen MT, Van den Brink PJ, Liess M (2013) Combined and interactive effects of global climate change and toxicants on populations and communities. Environ Toxicol Chem 32:49–61
Mulvey M, Newman MC, Vogelbein W, Unger MA (2002) Genetic structure of Fundulus heteroclitus from PAH-contaminated and neighboring sites in the Elizabeth and York Rivers. Aquat Toxicol 61:195–209
Munns WR, Black DE, Gleason TR, Salomon K, Bengtson D, GutjahrGobell R (1997) Evaluation of the effects of dioxin and PCBs on Fundulus heteroclitus populations using a modeling approach. Environ Toxicol Chem 16:1074–1081
Nacci D, Coiro L, Kuhn A, Champlin D, Munns W, Specker J, Cooper K (1998) Nondestructive indicator of ethoxyresorufin-O-deethylase activity in embryonic fish. Environ Toxicol Chem 17:2481–2486
Nacci D, Coiro L, Champlin D, Jayaraman S, McKinney R, Gleason TR, Munns WR, Specker JL, Cooper KR (1999) Adaptations of wild populations of the estuarine fish Fundulus heteroclitus to persistent environmental contaminants. Mar Biol 134:9–17
Nacci D, Jayaraman S, Specker J (2001) Stored retinoids in populations of the estuarine fish Fundulus heteroclitus indigenous to PCB-contaminated and reference sites. Arch Environ Contam Toxicol 40:511–518
Nacci DE, Champlin D, Coiro L, McKinney R, Jayaraman S (2002) Predicting the occurrence of genetic adaptation to dioxinlike compounds in populations of the estuarine fish Fundulus heteroclitus. Environ Toxicol Chem 21:1525–1532
Nacci DE, Champlin D, Jayaraman S (2010) Adaptation of the Estuarine Fish Fundulus heteroclitus (Atlantic Killifish) to Polychlorinated Biphenyls (PCBs). Estuaries Coasts 33:853–864
Nebert DW, Dalton TP, Okey AB, Gonzalez FJ (2004) Role of aryl hydrocarbon receptor-mediated induction of the CYP1 enzymes in environmental toxicity and cancer. J Biol Chem 279:23847–23850
Nelson WG, Bergen BJ (2012) The New Bedford Harbor Superfund site long-term monitoring program (1993–2009). Environ Monit Assess 184:7531–7550
Nikkila A, Penttinen S, Kukkonen JVK (1999) UV-B-induced acute toxicity of pyrene to the waterflea Daphnia magna in natural freshwaters. Ecotoxicol Environ Saf 44:271–279
Ownby DR, Newman MC, Mulvey M, Vogelbein WK, Unger MA, Arzayus LF (2002) Fish (Fundulus heteroclitus) populations with different exposure histories differ in tolerance of creosote-contaminated sediments. Environ Toxicol Chem 21:1897–1902
Oziolor EM, Bigorgne E, Aguilar L, Usenko S, Matson CW (2014) Evolved resistance to PCB- and PAH-induced cardiac teratogenesis, and reduced CYP1A activity in Gulf killifish (Fundulus grandis) populations from the Houston Ship Channel, Texas. Aquat Toxicol 150:210–219
Palumbi SR (2001) Evolution – humans as the world’s greatest evolutionary force. Science 293:1786–1790
Peachey RBJ (2005) The synergism between hydrocarbon pollutants and UV radiation: a potential link between coastal pollution and larval mortality. J Exp Mar Biol Ecol 315:103–114
Pfennig DW, Servedio MR (2013) The role of transgenerational epigenetic inheritance in diversification and speciation. Nongenet Inheritance 1:17–26
Pflugh KK, Stern AH, Nesposudny L, Lurig L, Ruppel B, Buchanan GA (2011) Consumption patterns and risk assessment of crab consumers from the Newark Bay Complex, New Jersey, USA. Sci Total Environ 409:4536–4544
Pittman K, Yufera M, Pavlidis M, Geffen AJ, Koven W, Ribeiro L, Zambonino-Infante JL, Tandler A (2013) Fantastically plastic: fish larvae equipped for a new world. Rev Aquaculture 5:S224–S267
Poloczanska ES, Brown CJ, Sydeman WJ, Kiessling W, Schoeman DS, Moore PJ, Brander K, Bruno JF, Buckley LB, Burrows MT, Duarte CM, Halpern BS, Holding J, Kappel CV, O'Connor MI, Pandolfi JM, Parmesan C, Schwing F, Thompson SA, Richardson AJ (2013) Global imprint of climate change on marine life. Nat Clim Change 3:919–925
Powell WH, Morrison HG, Weil EJ, Karchner SI, Sogin ML, Stegeman JJ, Hahn ME (2004) Cloning and analysis of the CYP1A promoter from the atlantic killifish (Fundulus heteroclitus). Mar Environ Res 58:119–124
Proestou DA, Flight P, Champlin D, Nacci D (2014) Targeted approach to identify genetic loci associated with evolved dioxin tolerance in Atlantic Killifish (Fundulus heteroclitus). BMC Evol Biol 14:17
Pumure I, Renton JJ, Smart RB (2010) Ultrasonic extraction of arsenic and selenium from rocks associated with mountaintop removal/valley fills coal mining: Estimation of bioaccessible concentrations. Chemosphere 78:1295–1300
Reitzel AM, Karchner SI, Franks DG, Evans BR, Nacci D, Champlin D, Vieira VM, Hahn ME (2014) Genetic variation at aryl hydrocarbon receptor (AHR) loci in populations of Atlantic killifish (Fundulus heteroclitus) inhabiting polluted and reference habitats. BMC Evol Biol 14:6
Reynaud S, Deschaux P (2006) The effects of polycyclic aromatic hydrocarbons on the immune system of fish: a review. Aquat Toxicol 77:229–238
Rosenzweig C, Neofotis P (2013) Detection and attribution of anthropogenic climate change impacts. Wiley Interdiscip Rev Clim Change 4:121–150
Roy NK, Wirgin I (1997) Characterization of the aromatic hydrocarbon receptor gene and its expression in Atlantic tomcod. Arch Biochem Biophys 344:373–386
Roy NK, Courtenay SC, Chambers RC, Wirgin II (2006) Characterization of the aryl hydrocarbon receptor repressor and a comparison of its expression in atlantic tomcod from resistant and sensitive populations. Environ Toxicol Chem 25:560–571
Safe S (1984) Polychlorinated biphenyls (PCBs) and polybrominated biphenyls (PBBs) – biochemistry, toxicology and mechanism of action. CRC Crit Rev Toxicol 13:319–395
Schneider K, Roller M, Kalberlah F, Schuhmacher-Wolz U (2002) Cancer risk assessment for oral exposure to PAH mixtures. J Appl Toxicol 22:73–83
Shemer H, Linden KG (2007) Photolysis, oxidation and subsequent toxicity of a mixture of polycyclic aromatic hydrocarbons in natural waters. J Photochem Photobiol A Chem 187:186–195
Steinhauser G, Brandl A, Johnson TE (2014) Comparison of the Chernobyl and Fukushima nuclear accidents: a review of the environmental impacts. Sci Total Environ 470:800–817
Strydom C, Robinson C, Pretorius E, Whitcutt JM, Marx J, Bornman MS (2006) The effect of selected metals on the central metabolic pathways in biology: a review. Water SA 32:543–554
Subedi B, Usenko S (2012) Enhanced pressurized liquid extraction technique capable of analyzing polychlorodibenzo-p-dioxins, polychlorodibenzofurans, and polychlorobiphenyls in fish tissue. J Chromatogr 1238:30–37
Sugg DW, Bickham JW, Brooks JA, Lomakin MD, Jagoe CH, Dallas CE, Smith MH, Baker RJ, Chesser RK (1996) DNA damage and radiocesium in channel catfish from Chernobyl. Environ Toxicol Chem 15:1057–1063
Thackaberry EA, Nunez BA, Ivnitski-Steele ID, Friggins M, Walker MK (2005) Effect of 2,3,7,8-tetrachlorodibenzo-p-dioxin on murine heart development: alteration in fetal and postnatal cardiac growth, and postnatal cardiac chronotropy. Toxicol Sci 88:242–249
Theodorakis CW, Shugart LR (1998) Genetic ecotoxicology III: the relationship between DNA strand breaks and genotype in mosquito fish exposed to radiation. Ecotoxicology 7:227–236
Theodorakis CW, Blaylock BG, Shugart LR (1997) Genetic ecotoxicology I: DNA integrity and reproduction in mosquitofish exposed in situ to radionuclides. Ecotoxicology 6:205–218
Theodorakis CW, Elbl T, Shugart LR (1999) Genetic ecotoxicology IV: survival and DNA strand breakage is dependent on genotype in radionuclide-exposed mosquitofish. Aquat Toxicol 45:279–291
Toomey BH, Bello S, Hahn ME, Cantrell S, Wright P, Tillitt DE, Di Giulio RT (2001) 2,3,7,8-Tetrachlorodibenzo-p-dioxin induces apoptotic cell death and cytochrome P4501A expression in developing Fundulus heteroclitus embryos. Aquat Toxicol 53:127–138
Turker OC, Vymazal J, Ture C (2014) Constructed wetlands for boron removal: a review. Ecol Eng 64:350–359
U.S. Environmental Protection Agency (2004) Exposure and human health reassessment of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) and related compounds. National Academy Sciences (External Review Draft), EPA/600/P-00/001Cb. U.S. Environmental Protection Agency, Washington, DC
U.S. General Accounting Office (2000) Superfund: information regarding EPA’s cleanup decision process on the Hudson River site, GAO/RCED-00-193. U.S. General Accounting Office, Washington, DC
Valenti TW, Gould GG, Berninger JP, Connors KA, Keele NB, Prosser KN, Brooks BW (2012) Human therapeutic plasma levels of the selective serotonin reuptake inhibitor (SSRI) sertraline decrease serotonin reuptake transporter binding and shelter-seeking behavior in adult male fathead minnows. Environ Sci Technol 46:2427–2435
Van Veld PA, Westbrook DJ (1995) Evidence for depression of cytochrome P4501A in population of chemically resistant mummichog (Fundulus heteroclitus). Environ Sci 3:221–234
Van Veld PA, Ko UC, Vogelbein WK, Westbrook DJ (1991) Glutathione-S-transferase in intestine, liver and hepatic lesions of mummichog (Fundulus heteroclitus) from a creosote-contaminated environment. Fish Physiol Biochem 9:369–376
Veron JEN (2008) Mass extinctions and ocean acidification: biological constraints on geological dilemmas. Coral Reefs 27:459–472
Viguri J, Verde J, Irabien A (2002) Environmental assessment of polycyclic aromatic hydrocarbons (PAHs) in surface sediments of the Santander Bay, Northern Spain. Chemosphere 48:157–165
Virani NA, Rees BB (2000) Oxygen consumption, blood lactate and inter-individual variation in the gulf killifish, Fundulus grandis, during hypoxia and recovery. Comp Biochem Physiol A Mol Integr Physiol 126:397–405
Vogelbein WK, Fournie JW, Van Veld PA, Huggett RJ (1990) Hepatic neoplasms in the mummichog Fundulus heteroclitus from a creosote-contaminated site. Cancer Res 50:5978–5986
Walker SE, Dickhut RM, Chisholm-Brause C, Sylva S, Reddy CM (2005) Molecular and isotopic identification of PAH sources in a highly industrialized urban estuary. Org Geochem 36:619–632
Wassenberg DM, Di Giulio RT (2004a) Synergistic embryotoxicity of polycyclic aromatic hydrocarbon aryl hydrocarbon receptor agonists with cytochrome P4501A inhibitors in Fundulus heteroclitus. Environ Health Perspect 112:1658–1664
Wassenberg DM, Di Giulio RT (2004b) Teratogenesis in Fundulus heteroclitus embryos exposed to a creosote-contaminated sediment extract and CYP1A inhibitors. Mar Environ Res 58:163–168
Wassenberg DM, Swails EE, Di Giulio RT (2002) Effects of single and combined exposures to benzo(a)pyrene and 3,3′,4,4′,5-pentachlorobiphenyl on EROD activity and development in Fundulus heteroclitus. Mar Environ Res 54:279–283
Wei M, Wang LP, Zheng BH (2007) Photoinduced toxicity single and binary mixtures of four polycyclic aromatic hydrocarbons to the marine diatom Skeletonema costatum. Acta Oceanol Sin 26:41–50
Weis JS, Weis P, Heber M, Vaidya S (1981a) Methylmercury tolerance of killifish (Fundulus heteroclitus) embryos from a polluted vs non-polluted environment. Mar Biol 65:283–287
Weis P, Weis JS, Heber MA (1981b) Variation in teratogenic response to methylmercury by killifish (Fundulus heteroclitus) embryos – differences within and between populations. Anat Rec 199:A270–A271
Wenning R, Paustenbach D, Johnson G, Ehrlich R, Harris M, Bedbury H (1993) Chemometric analysis of potential sources of polychlorinated dibenzo-p-dioxins and dibenzofurans in surficial sediments from Newark Bay, New-Jersey. Chemosphere 27:55–64
Whitehead A, Triant DA, Champlin D, Nacci D (2010) Comparative transcriptomics implicates mechanisms of evolved pollution tolerance in a killifish population. Mol Ecol 19:5186–5203
Whitehead A, Roach JL, Zhang SJ, Galvez F (2011) Genomic mechanisms of evolved physiological plasticity in killifish distributed along an environmental salinity gradient. Proc Natl Acad Sci U S A 108:6193–6198
Whitehead A, Pilcher W, Champlin D, Nacci D (2012) Common mechanism underlies repeated evolution of extreme pollution tolerance. Proc Roy Soc B Biol Sci 279:427–433
Wills LP, Matson CW, Landon CD, Di Giulio RT (2010) Characterization of the recalcitrant CYP1 phenotype found in Atlantic killifish (Fundulus heteroclitus) inhabiting a Superfund site on the Elizabeth River, VA. Aquat Toxicol 99:33–41
Wirgin I, Waldman JR (2004) Resistance to contaminants in North American fish populations. Mutat Res Fundam Mol Mech Mutag 552:73–100
Wirgin I, Roy NK, Loftus M, Chambers RC, Franks DG, Hahn ME (2011) Mechanistic basis of resistance to PCBs in Atlantic Tomcod from the Hudson River. Science 331:1322–1325
Wolffe AP, Matzke MA (1999) Epigenetics: regulation through repression. Science 286:481–486
Xie L, Klerks PL (2003) Responses to selection for cadmium resistance in the least killifish, Heterandria formosa. Environ Toxicol Chem/SETAC 22:313–320
Xie LT, Klerks PL (2004) Fitness cost of resistance to cadmium in the least killifish (Heterandria formosa). Environ Toxicol Chem 23:1499–1503
Xu X, Li XG (2014) QSAR for photodegradation activity of polycyclic aromatic hydrocarbons in aqueous systems. J Ocean Univ 13:66–72
Yeager KM, Santschi PH, Rifai HS, Suarez MP, Brinkmeyer R, Hung CC, Schindler KJ, Andres MJ, Weaver EA (2007) Dioxin chronology and fluxes in sediments of the Houston Ship Channel, Texas: influences of non-steady-state sediment transport and total organic carbon. Environ Sci Tech 41:5291–5298
Zhang XW, Wu RSS, Fu WY, Xu LH, Lam PKS (2004) Production of reactive oxygen species and 8-hydroxy-2′ deoxyguanosine in KB cells co-exposed to benzo a pyrene and UV-A radiation. Chemosphere 55:1303–1308
Zhang WS, Sargis RM, Volden PA, Carmean CM, Sun XJ, Brady MJ (2012) PCB 126 and other dioxin-like PCBs specifically suppress hepatic PEPCK expression via the aryl hydrocarbon receptor. PLoS One 7:e37103
Zhou T, Scali R, Weis P, Weis JS (1996) Behavioral effects in mummichog larvae (Fundulus heteroclitus) following embryonic exposure to methylmercury. Mar Environ Res 42:45–49
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Oziolor, E.M., Matson, C.W. (2015). Evolutionary Toxicology: Population Adaptation in Response to Anthropogenic Pollution. In: Riesch, R., Tobler, M., Plath, M. (eds) Extremophile Fishes. Springer, Cham. https://doi.org/10.1007/978-3-319-13362-1_11
Download citation
DOI: https://doi.org/10.1007/978-3-319-13362-1_11
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-13361-4
Online ISBN: 978-3-319-13362-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)