Abstract
Despite its remarkable capacity to undergo self-repair, bone tissue cannot regenerate across critical-sized defects, and their successful reconstruction remains a major clinical challenge. Current treatment options are limited and often associated with a high incidence of complications, which may result in non-union or re-fracture. There is a great and growing need for alternative techniques to replace, restore or regenerate damaged or diseased bone. Biomaterials-based bone tissue engineering via the use of synthetic bone substitutes represents a particularly promising alternative, which circumvents the drawbacks of conventional treatments. To achieve successful reconstructive outcomes, synthetic bone substitutes need to be biocompatible and provide necessary signals to osteoprogenitor cells to control downstream cell responses including adhesion, migration, proliferation and differentiation into osteoblasts. One feasible approach to develop synthetic bone substitutes with such biological properties is to mimic the innate physical and/or chemical properties of bone. In this chapter, we discuss the design aspects of bone-biomimetic biomaterials that provide the signals necessary for bone regeneration, and the underlying mechanisms by which bone-biomimetic biomaterials determine the fate of mesenchymal stem cells/osteoprogenitor cells. Protein adsorption to biomaterial surfaces and their subsequent influence on cell adhesion and intracellular signal transduction will be discussed in detail, with particular emphasis on the key molecules and signalling pathways involved in directing the osteogenic development of cells.
Lu ZuFu and Li Jiao Jiao are equally contributed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Bone tissue has innate regenerative ability and undergoes constant remodelling throughout life. However, the repair and regeneration of critical-sized bone defects requires clinical intervention and remains an unresolved challenge. Accordingly, bone is the second most common transplanted tissue, and more than 500,000 bone-grafting procedures are performed annually in the United States alone (Baroli 2009; Amini et al. 2012). The current gold standard for the treatment of critical-sized bone defects is autologous bone grafting, but this procedure faces major drawbacks including limited availability and second site surgery, which leads to donor site morbidity in 20–30 % of cases (Schwartz et al. 2009). Allogeneic bone grafts may be used as an alternative if insufficient autologous bone graft material can be harvested, which is often the case for extensive bone defects, but these are complicated by variable bioactivity and the risk of immune rejection or disease transmission (Laurie et al. 1984; Quarto et al. 2001; Lord et al. 1988; Mankin et al. 1996). Therefore, there is a growing unmet clinical need for new and effective alternatives to circumvent the drawbacks of autologous and allogeneic bone grafting in bone repair and regeneration (Crowley et al. 2013; Kosuge et al. 2013). To address this need, a promising approach is to develop synthetic bone substitutes composed of one or more biomaterials.
Successful regeneration of critical-sized bone defects in load-bearing applications requires the use of a scaffolding material that has the mechanical strength to support bridging of the defect under load, has highly porous and interconnected architecture to promote new bone growth across the entire defect and is biodegradable at a controlled rate that is coupled to the rate of new bone formation with no release of toxic or inhibitory products. More importantly, the scaffolding material must also be biocompatible and capable of providing signals to direct the recruitment and differentiation of mesenchymal stem cells (MSCs) and osteoprogenitor cells, including adhesion, migration, proliferation and differentiation into osteoblasts. Recently, there has been increasing research focused on the development of bone-biomimetic biomaterials which mimic the physical and chemical characteristics of bone (Drevelle and Faucheux 2013; McMahon et al. 2013; Holzwarth and Ma 2011; Liu et al. 2009; Roohani-Esfahani et al. 2010, 2011, 2013). By mimicking the native bone microenvironment, these novel bone-biomimetic biomaterials are designed to provide signals for the recruitment and differentiation of local and systemic osteoprogenitor populations in order to achieve successful defect reconstruction.
In this chapter, we will discuss two main aspects of cell fate determination using bone-biomimetic biomaterials: (i) design aspects of bone-biomimetic biomaterials that provide the signals necessary for bone regeneration and (ii) the underlying mechanisms by which bone-biomimetic biomaterials determine the lineage commitment and differentiation of MSCs/osteoprogenitor cells into osteoblasts, with focus on the key molecules and necessary signalling pathways involved.
2 Biomaterial Design for Bone Regeneration
The minimal essential requirements for a biomaterial scaffold for bone regeneration across a critical-sized defect, is its ability to act as a filler to bridge the defect and as a carrier or guide through which cells can migrate to heal the defect. An ideal bone scaffold possesses both osteoconductive and osteoinductive properties. An osteoconductive scaffold allows the attachment, growth and extracellular matrix formation of bone-related cells on its surface and pores, while an osteoinductive scaffold can actively induce new bone formation via biomolecular signalling and recruitment of osteoprogenitor cells (Albrektsson and Johansson 2001). Optimal bone regeneration relies on the ability of the biomaterial scaffold to communicate with osteoprogenitor cells and direct their migration, differentiation and osteogenic activity. To achieve this aim, biomaterials have been developed using design strategies to mimic both the physical and chemical characteristics of bone.
2.1 Designs to Mimic Physical Characteristics of Bone
Bone has unique physical characteristics in terms of architecture, topography and mechanical properties, which fulfil its function and serve as important design targets for scaffold-based bone regeneration.
2.1.1 Designs to Mimic Architecture of Bone
A number of architectural characteristics including porosity, pore size and pore interconnectivity of the scaffold make a significant contribution to bone regeneration outcomes. Critical-sized bone defects often require regeneration of large amounts of cancellous bone, which is an interconnected network of small bone trabeculae containing vasculature and bone marrow with 50–90 % porosity (Sikavitsas et al. 2001). Scaffolds designed for bone regeneration generally attempt to match the porosity of cancellous bone (Karageorgiou and Kaplan 2005), and pore sizes within the range of 100–500 µm are considered as optimal for encouraging cell attachment, migration and ingrowth throughout the scaffold (Ikada 2006). In vitro and in vivo studies investigating osteogenic outcomes in polymer scaffolds with a range of different pore sizes have established optimal pore sizes of around 300 µm for bone regeneration (Murphy et al. 2010; Oh et al. 2007). Numerical and experimental studies have also underlined the importance of scaffold porosity and interconnectivity in bone regeneration, which determines the spatial distribution of new bone formation (Mastrogiacomo et al. 2006; Sanz-Herrera et al. 2010). In vivo, higher porosity and pore sizes generally result in greater bone ingrowth as the processes of bone formation and remodelling are intimately linked to vascularisation. Scaffold architecture can therefore influence the progression of osteogenesis, as small pores introduce hypoxic conditions which tend to induce the formation of osteochondral tissue before osteogenesis occurs. In contrast, larger pores promote rapid vascularisation leading to direct osteogenesis, as higher oxygen tension favours the differentiation of MSCs into the osteoblast lineage (Santos and Reis 2010).
Recently, several studies have indicated that scaffolds with multi-scale porosity, consisting of both macropores (>100 µm) and micropores (0.1–10 µm), can significantly improve bone regeneration in vivo due to their microstructural imitation of cancellous bone (Woodard et al. 2007; Pek et al. 2008; Lan Levengood et al. 2010). The mechanism of enhanced bone regeneration in the presence of scaffold micropores has been elucidated as increased surface area for cellular interaction in hydroxyapatite scaffolds with 2–8 µm micropores (Woodard et al. 2007), multi-scale osteointegration with micropores filled by osteogenic cells which proceed to form osteoid and mineralised matrix in biphasic calcium phosphate scaffolds with 1–10 µm micropores (Levengood et al. 2010) and improved protein adhesion and interfacial dynamics inducing osteoblastic differentiation of MSCs in collagen-apatite nanocomposite scaffolds with 50–100 nm ultrafine pores (Pek et al. 2008). Control of both macroporosity and microporosity is becoming a new paradigm in the architectural design of bone scaffolds.
2.1.2 Designs to Mimic Topography of Bone
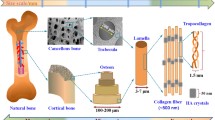
Bone has a nanocomposite structure consisting of an organic matrix (30 wt%) mainly composed of collagen fibrils which are around 15 µm in length and 40–70 nm in diameter, and inorganic hydroxyapatite nanocrystals (70 wt%) which are typically 20–80 nm long and 2–5 nm thick (Rogel et al. 2008; Zhang and Webster 2009). Scaffold design for bone regeneration has aimed at mimicking the nanoscale topography of bone, as the nanostructured extracellular matrix (ECM) closely surrounds bone-related cells and is believed to play an important role in regulating cell attachment, proliferation and differentiation. A range of nanofibrous polymer scaffolds have been investigated for their efficacy in promoting bone regeneration (Chen et al. 2006; Tuzlakoglu et al. 2005; Woo et al. 2007a), with the expectation that they would mimic the morphological function of collagen fibrils to create a more favourable microenvironment for osteogenesis. Compared to control scaffolds without nanofibrous structure, nanofibrous scaffolds were shown to significantly enhance the manifestation of osteogenic markers in osteoprogenitor cells, including both early markers such as alkaline phosphatase activity and runx2 mRNA expression (Tuzlakoglu et al. 2005; Woo et al. 2007a), and late markers such as bone sialoprotein and osteocalcin mRNA expression (Chen et al. 2006; Woo et al. 2007a). Nanofibrous scaffolds also promoted a greater extent of mineralisation and more uniform distribution throughout the scaffold (Chen et al. 2006; Woo et al. 2007a). Furthermore, one study has noted that osteoprogenitor cells cultured on nanofibrous scaffolds exhibited increased expression of integrins associated with collagen, fibronectin and vitronectin (Woo et al. 2007a). Coupled with the observation that nanofibrous scaffolds can selectively enhance protein adsorption including fibronectin and vitronectin (Woo et al. 2003), the nanofibrous structure may encourage the adhesion of osteoprogenitor cells and provide these cells with an ECM which more closely resembles in vivo conditions, thereby inducing increased bone formation. Other than mimicking the organic component of bone, some scaffold designs have aimed at mimicking the mineral component by the incorporation of hydroxyapatite nanocrystals, either dispersed in a polymer matrix (Thein-Han and Misra 2009) or deposited on a ceramic scaffold as part of a coating (Roohani-Esfahani et al. 2010). Significant improvements in the attachment and proliferation of osteoprogenitor cells and their differentiation into osteoblasts were observed, which were attributed to the hydroxyapatite nanocrystals providing a larger specific surface area for cell interactions, as well as causing changes in cell morphology which induced osteoconductive signals.
Recently, some studies have explored the effect of precisely controlled nanotopographies produced by lithography on the activities of osteoprogenitor cells (Biggs et al. 2009; Dalby et al. 2006, 2007). Nano-sized surface pits and grooves several hundred nanometres in depth have been found to profoundly affect cell-surface interactions and modulate osteoprogenitor cell activities and functions. Various nanotopographies were shown to direct cytoskeletal changes and allow control of cell adhesion, growth and production of osteoblast markers including osteocalcin and osteopontin (Biggs et al. 2009; Dalby et al. 2006). By producing nanotopographies with differentiation of osteoprogenitor cellstures, it was also possible to induce in vitro osteogenic differentiation of MSCs with mineral production in absence of osteogenic supplements (Dalby et al. 2007). These studies represent an important step in the topographical design of bone scaffolds to direct in vivo bone regeneration outcomes.
2.1.3 Designs to Mimic Mechanical Properties of Bone
The mechanical properties of cancellous bone vary widely with density, with midrange values of 5–10 MPa for strength and 50–500 MPa for modulus which can serve as design goals for biomaterials for bone regeneration (Yaszemski et al. 1996; Rezwan et al. 2006). The major challenge in designing biomaterial scaffolds for bone regeneration lies in matching the mechanical properties of bone to satisfy the initial mechanical requirements of the bone defect (often load-bearing) but without excessive mechanical properties sufficient to cause stress shielding, while incorporating other necessary properties such as bioactivity, sufficient porosity and adequate rate of degradation. Matching the mechanical properties of bone with monolithic polymer or ceramic scaffolds has proven to be difficult. Polymer scaffolds generally lack mechanical competence for load-bearing applications, while ceramic scaffolds suffer from high brittleness and low flexural strength, with the drawbacks of each exacerbated by the highly porous architecture required for bone regeneration (Mistry and Mikos 2005). To improve the mechanical properties and also low bioactivity of polymer scaffolds, attempts have been made to reinforce the polymer matrix with various fillers including hydroxyapatite particles (Shor et al. 2007; Bhumiratana et al. 2011) and nanoparticles (Kim et al. 2006), bioactive glass particles (Boccaccini and Maquet 2003) and nanoparticles (Hong et al. 2008), carbon nanotubes (Shi et al. 2007) and polymer particles (Rockwood et al. 2011). Mechanical properties of these reinforced polymer scaffolds were significantly improved compared to the unreinforced controls, but were generally still less than that of cancellous bone. For ceramic scaffolds, attempts have been made to reduce brittleness and enhance mechanical performance mainly by reinforcement with coating layers of polymers and/or ceramics. Several biocompatible and biodegradable polymers have been used to coat ceramic scaffolds, including poly(lactic-co-glycolic acid) (PLGA) (Miao et al. 2007, 2008), poly(D,L-lactic acid) (PDLLA) (Chen and Boccaccini 2006; Tian et al. 2008; Lu et al. 2008b; Zhao et al. 2009), polycaprolactone (PCL) (Kim et al. 2004; Zhao et al. 2008; Roohani-Esfahani et al. 2011), poly(3-hydroxybutyrate) (PHB) (Bretcanu et al. 2009) and silk fibroin (Wu et al. 2010; Roohani-Esfahani et al. 2012; Li et al. 2013b). Some of these polymer coatings have an additional ceramic component in the form of powder or nanoparticles for bioactivity and further strength enhancement (Miao et al. 2007; Roohani-Esfahani et al. 2011). Polymer-coated ceramic scaffolds generally showed significant improvements in mechanical properties, particularly in terms of strength and toughness. These improvements can be attributed to a micron-scale crack bridging mechanism (Pezzotti and Asmus 2001), where the polymer fills existing cracks in the ceramic microstructure and lowers the chance of crack propagation under load. The use of silk fibroin as a coating material for ceramic scaffolds in bone regeneration is a recent advancement, and as a natural polymer, offers the additional benefit of imparting some bioactivity to the coating.
Recently, some studies have explored the microstructural design of ceramic scaffolds to produce more solid scaffold struts with few cracks and defects, which results in high-strength ceramic scaffolds suitable for bone regeneration at load-bearing sites without need for further modification. In one study, high strut densification and reduction in microporosity greatly increased the compressive strength of glass–ceramic scaffolds (Vitale-Brovarone et al. 2009). Another study produced a unique ceramic microstructure and composition consisting of bioactive grains reinforced by a glass phase wetting the grain boundaries, with dispersed submicron crystals which function in crack deflection (Fig. 1). The result is a high-strength ceramic scaffold with improved toughness compared to conventional ceramic scaffolds, and mechanical properties comparable to cancellous bone even at 80–90 % porosity (Roohani-Esfahani et al. 2013). Such microstructural design strategies hold promise for the development of biomaterial scaffolds which satisfy the mechanical requirements for load-bearing bone regeneration without compromising bioactivity and porous architecture.
a Unique microstructure of a high-strength Sr-HT-Gahnite ceramic scaffold, b compressive strength of Sr-HT-Gahnite ceramic scaffolds match cancellous bone at 85 % porosity (Roohani-Esfahani et al. 2013)
2.2 Designs to Mimic Chemical Composition of Bone
The extracellular matrix of bone consists of an organic phase comprising collagen fibres and non-collagenous proteins, and a mineral phase comprising hydroxyapatite crystals. Many scaffold design strategies for the regeneration of bone have focussed on mimicking the chemical composition of its two phases.
2.2.1 Designs to Mimic Organic Phase of Bone
The organic phase of the bone extracellular matrix is composed primarily of type I collagen fibrils. Collagen molecules are secreted by osteoblasts and self-assemble into fibrils with a specific tertiary structure. The organic matrix also contains small amounts of non-collagenous proteins which may function to regulate mineralisation, including osteopontin, bone sialoprotein, osteonectin and osteocalcin (Rho et al. 1998). Biomimetic scaffold design strategies have explored the utilisation and/or incorporation of the organic matrix components of bone to improve regenerative outcomes.
Collagen has been extensively studied as a scaffold material for bone regeneration as it is the main component of the extracellular matrix. Collagen substrates can provide a native surface for cell attachment, and may influence the morphology, migration and even differentiation of cells (Kleinman et al. 1981). Collagen matrices used for bone tissue engineering in the form of gels or sponges were able to induce favourable osteoblastic differentiation in vitro and bone formation in vivo, particularly when coupled with mechanical stimulation or growth factor release (Ueda et al. 2002; Ignatius et al. 2005). However, a major drawback of using pure collagen as a biomaterial for tissue repair is its high degradation rate, which leads to rapid loss of mechanical properties (Puppi et al. 2010). To overcome this problem, various materials have been combined with collagen substrates both for stabilisation and to improve scaffold properties for bone regeneration, including natural polymers, synthetic polymers, mineral crystals, or combinations of these. The collagen–glycosaminoglycan scaffold consists of natural polymers and represents a biomimetic structure which supported the growth of osteoprogenitor cells and could direct the osteogenic differentiation of MSCs with subsequent mineralisation (Farrell et al. 2006; Murphy et al. 2010). A collagen–PLGA scaffold represents a natural–synthetic polymer hybrid and provided surface properties which promoted the adhesion and proliferation of embryonic stem cells and osteoblasts (Lee et al. 2006). Collagen–apatite scaffolds consist of a collagen matrix mineralised with calcium phosphate crystals, giving a biomimetic system that resembles the composition of native bone matrix. These scaffolds were osteoinductive and showed ability to heal critical-sized defects in mouse calvaria and pig tibia over 4 weeks and 6 months, respectively (Pek et al. 2008a; Xia et al. 2013). More complex systems consisting of collagen combined with hydroxyapatite and a synthetic biocompatible polymer were able to encourage the attachment, proliferation and osteogenic activity of osteoprogenitor cells (Akkouch et al. 2011; Liao et al. 2004), as well as bridge a radial segmental defect in the rabbit over 12 weeks (Liao et al. 2004). The ability of collagen to directly affect cell behaviour in bone regeneration is demonstrated by the binding of its specific motif, GFOGER, to α2β1 integrin which is involved in osteogenesis (Knight et al. 2000). Following on from this, the collagen-mimetic peptide GFOGER has been used to coat synthetic scaffolds to promote bone formation in a critical-sized segmental defect in rats (Wojtowicz et al. 2010).
Other strategies aimed at mimicking the organic matrix of bone have explored surface modification of scaffolds using biomimetic peptides to enhance cell adhesion and osteogenesis. Arg-Gly-Asp (RGD) is the most effective and frequently employed peptide sequence for stimulating cell adhesion on synthetic scaffold surfaces. It is present in many ECM proteins and promotes integrin-mediated cell adhesion in multiple cell types, which in turn activates cell-ECM signal transduction to influence cell behaviour including migration, proliferation, differentiation, apoptosis and survival (Ruoslahti 1996; Takada et al. 2007). RGD sequences immobilised on a variety of polymer scaffolds including poly(lactic acid) (PLA) (Hu et al. 2003), silk (Chen et al. 2003) and PCL (Zhang et al. 2009) enhanced the attachment of MSCs and osteoprogenitor cells, leading to increased cell survival and growth. Improved bone formation was also observed in some cases (Chen et al. 2003; Hu et al. 2003). Considering that RGD peptides interact with multiple cell types, peptide sequences that elicit more specific responses from selected cell types for bone regeneration have been identified. For example, hydrogels modified with an osteopontin-derived peptide were able to modulate osteoblast proliferation and migration, and the extent of modulation was dependent on peptide concentration (Shin et al. 2004). Other than biomimetic peptides, a recent study extracted non-collagenous proteins directly from the long bones of rats and integrated them with nanofibrous gelatin scaffolds (Sun et al. 2013). The mixture of non-collagenous proteins included bone sialoprotein, osteopontin and osteonectin, and their incorporation into the scaffold led to significantly enhanced osteoblast gene expression and mineralisation by osteoblasts, as well as improved reconstruction of a rat calvarial defect.
2.2.2 Designs to Mimic Mineral Phase of Bone
The mineral phase of bone consists of plate-like hydroxyapatite crystals which occupy discrete spaces within the matrix of collagen fibrils (Rho et al. 1998). Due to their chemical similarity to the composition of bone mineral, calcium phosphate ceramics have had a long history of application in bone regeneration. Calcium phosphate-based scaffolds are inherently bioactive, and can encourage bone formation in vivo via the formation of a hydroxyl carbonated apatite (HCA) layer at the bone-scaffold interface (LeGeros 2002). This is thought to be caused by a cell-mediated dissolution and precipitation process, where calcium and phosphate ions are released from the ceramic into the microenvironment and encourages the precipitation of HCA microcrystals. The extracellular matrix surrounding the scaffold therefore becomes richly mineralised and creates a favourable environment for bone formation. Furthermore, the high concentration of calcium ions adjacent to the scaffold may exert a chemotactic effect on osteoblasts, while phosphate is believed to play a critical role in bone matrix mineralisation (Chai et al. 2012). The most commonly used calcium phosphate-based ceramics for bone regeneration are synthetic hydroxyapatite, β-tricalcium phosphate (β-TCP) and biphasic calcium phosphate (BCP).
Synthetic hydroxyapatite (Ca10(PO4)6(OH)2) has a calcium to phosphate ratio of 1.67 and is the closest in composition to bone mineral (Samavedi et al. 2013). Early uses of hydroxyapatite as a bone graft substitute showed good functional recovery over long-term follow-up (Heise et al. 1990; Kitsugi et al. 1993). However, synthetic hydroxyapatite has very low solubility, exemplified by little degradation after more than 5 years of implantation in the long bone segmental defects of three patients (Quarto et al. 2001; Mastrogiacomo et al. 2005). Persisting hydroxyapatite at the implantation site interferes with bone formation and is prone to mechanical failure. Furthermore, synthetic hydroxyapatite is osteoconductive but not osteoinductive (Habibovic et al. 2008). In comparison, β-TCP possesses both osteoconductive and osteoinductive properties. β-TCP is the low temperature phase of tricalcium phosphate (Ca3(PO4)2) and has high degradability, which allows rapid precipitation of a surface HCA layer in physiological solution (Samavedi et al. 2013). A range of studies have demonstrated the ability of β-TCP scaffolds to promote bone formation in vivo, both in animal models (Dong et al. 2002; Kondo et al. 2005) and in patients (Gaasbeek et al. 2005; Galois et al. 2002) with good short- and long-term regenerative outcomes. However, the rapid degradation of β-TCP scaffolds in vivo accompanied by loss of scaffold integrity may hinder bone formation (Hing et al. 2007). One study reported less than 5 % of β-TCP scaffolds remaining after being implanted for 24 weeks in the cancellous bone of sheep (von Doernberg et al. 2006). Excessive solubility may lead to decoupling of scaffold degradation and bone formation, resulting in net bone loss at the defect site due to imbalances in bone remodelling (Okuda et al. 2007). BCP is a two-phase ceramic containing hydroxyapatite and β-TCP phases (obtained by sintering calcium-deficient apatite at high temperatures), which combines the low solubility and osteoconductivity of hydroxyapatite with the high solubility and osteoinductivity of β-TCP (Samavedi et al. 2013). The result is an osteoconductive and osteoinductive ceramic with HA/β-TCP ratios typically adjusted within 20/80 to 40/60 for optimal degradation to match the rate of bone formation (LeGeros et al. 2003). BCP scaffolds have induced superior in vivo bone formation in a range of animal models compared to hydroxyapatite or β-TCP scaffolds (Arinzeh et al. 2005; Bodde et al. 2007; Habibovic et al. 2008; Yuan et al. 2010). The osteoinductive properties of BCP ceramics have been demonstrated by in vitro and in vivo studies investigating the interactions between BCP and MSCs. An in vitro study showed that BCP surfaces were able to stimulate development of osteoblast features in MSCs in expansion medium without osteogenic supplements (Müller et al. 2008). An in vivo study in a canine model also provided evidence for the ability of BCP to induce the homing of MSCs from circulation to participate in ectopic bone formation at the implant site without growth factor delivery (Song et al. 2013).
An interesting set of design strategies to more closely mirror the chemical composition of bone mineral is by making atomic substitutions in the structure of hydroxyapatite, which also leads to improvements in bioactivity and degradability. Cationic substitutions for calcium include zinc (Zn-HA), strontium (Sr-HA) and magnesium (Mg-HA), while anionic substitutions for phosphate include silicate (Si-HA). These ions represent essential trace elements in the human body with ability to stimulate bone formation and/or reduce bone resorption (Shepherd et al. 2012). Zn-HA showed enhanced osteoblast proliferation and differentiation as a coating on porous titanium surfaces (Yang et al. 2012), as well as antimicrobial activity (Stanić et al. 2010). Sr-HA promoted osteogenic activity and mineralisation in osteoblasts and inhibited the proliferation of osteoclasts, and these effects were more prominent at higher strontium contents (Capuccini et al. 2009; Ni et al. 2011). Mg-HA demonstrated improved osteoconductivity and resorption compared to stoichiometric hydroxyapatite as bone fillers in a rabbit model (Landi et al. 2008). Si-HA was found to influence the differentiation of osteoblasts in vitro depending on the level of silicon substitution (Botelho et al. 2006), and promote bone remodelling at the bone-implant interface in an ovine model with apposition of organised collagen fibrils and apatite crystals (Porter et al. 2004). These modified hydroxyapatite materials hold potential for use in bone reconstruction as bioactive scaffolds imitating the mineral phase of natural bone.
3 Mechanisms of Cell Fate Determination by Bone-Biomimetic Biomaterial
One essential goal in the design of bone-biomimetic biomaterials is to provide osteoconductive and/or osteoinductive signals to osteoprogenitor cells and control their activity and fate to favour bone formation. Optimal bone regeneration outcomes therefore rely on the ability of the biomaterial to communicate with osteoprogenitor cells. Figure 2 illustrates the process by which a biomaterial substrate can communicate with cells and subsequently determine cell fate. First, there is rapid protein adsorption on the biomaterial surface once the biomaterial comes into contact with serum-containing culture medium or body fluids such as blood after being implanted into the body. Second, the adsorbed proteins selectively bind to cellular receptors on the cell membrane. Third, the binding of extracellular proteins to cell receptors specifically activates a cascade of signalling events. Finally, the activated signals determine cell fate.
3.1 Protein Adsorption
Blood proteins have long been regarded as key factors in determining the in vivo acceptance of implants (Rosengren et al. 2002; Horbett 1982). Upon in vivo implantation, the biomaterial surface is almost immediately coated with various proteins such as fibronectin from blood before cells sense the surface and attach to it (Shin et al. 2012). This rapid protein adsorption implies that the cell–biomaterial interaction might actually occur between cells and the adsorbed protein layer rather than directly with the material itself (Horbett 1982; Wilson et al. 2005). Thus it is critical to understand the relation between nature of the biomaterial and protein adsorption on its surface, which subsequently modulates cell behaviour including cell attachment, growth, migration and differentiation. Much research effort has been directed towards chemically and/or physically modifying biomaterial surface properties such as surface roughness (Deligianni et al. 2005; Wang et al. 2013), wettability (Wei et al. 2009), and surface energy (Zhao et al. 2005; Michiardi et al. 2007), to facilitate the adsorption of specific proteins that will determine cell adhesion and signal transduction ultimately leading to control of cell fate (Samavedi et al. 2013; Baxter et al. 2010; Wilson et al. 2005).
There are dozens of different proteins in blood, including albumin, globulins, fibrinogen, vitronectin and fibronectin. Among these, fibronectin and vitronectin are of particular interest, as they are also found in bone extracellular matrix, and can induce the reorganisation of actin microfilaments and promote cell adhesion and spreading, which in turn modulates cell behaviour such as cell shape and migration (Scotchford et al. 2003; Howlett et al. 1994). As a result, many studies have attempted to increase the deposition of fibronectin and vitronectin to improve the attachment, growth and osteoblastic differentiation of osteoprogenitor cells (Tran et al. 2012; Brun et al. 2013; Alves et al. 2008; Woo et al. 2007b). For example, nanoporous titanium surfaces have been designed to specifically increase the adsorption of fibronectin and vitronectin, which promoted osteoblast attachment and proliferation (Rivera-Chacon et al. 2013). Hydroxyapatite coated with iron oxide nanoparticles also resulted in enhanced osteoblast proliferation and differentiation by increasing fibronectin adsorption on the surface (Tran et al. 2012).
Apart from efforts on modulating the composition of adsorbed proteins, protein conformation is another important aspect that researchers have been attempting to address. Protein conformation includes the secondary, tertiary and quaternary structures, which dramatically affect protein interaction with receptors on the cell membrane leading to changes of material bioactivity. A number of studies have attempted to alter the bioactivity of biomaterials by changing the conformation of adsorbed surface proteins (Binazadeh et al. 2013, Depan and Misra 2013; Assal et al. 2013; Vasita and Katti 2012). One study investigated the influence of protein conformation adsorbed onto the surface of amorphous and crystallised bioactive glass on stem cell adhesion and spreading (Buchanan and EI-Ghannam 2010). It was found that the surface of amorphous bioactive glass led to significant expression of unordered secondary structure in the conformation of fibronectin, which increased cell adhesion and spreading. In contrast, the surface of crystallised bioactive glass resulted in exposure of the stable beta-sheet structure and alpha-helix conformation of fibronectin, which limited cell adhesion and spreading.
3.2 Integrin Signalling
Cell adhesion to biomaterials normally occurs via binding of cellular receptors to the ligands of the proteins adsorbed to the biomaterial surface. The adhesive processes can then trigger a cascade of intracellular signalling events leading to changes in cellular behaviour, such as migration, growth and differentiation. Integrins, the most important and extensively studied cell adhesion molecules, are a family of receptors characterised by transmembrane molecules composed of α and β chains that assemble noncovalently as heterodimers. Currently, 8 β and 18 α subunits have been identified, which form 24 distinct αβ integrin combinations each with unique binding property. These combinations possess dual functionalities of “outside-in” and “inside-out”, which are transducing signals in both directions through the cell membrane. “Inside-out” signalling occurs when integrins are activated by intracellular signals, which leads to conformational changes and promotes their binding affinity for extracellular ligands (Humphries et al. 2004). On the other hand, “outside-in” signalling takes place when extracellular ligands bind to integrins and initiate integrin clustering, cell adhesion and downstream intracellular signalling pathways (Cabodi et al. 2010; Schneider and Engelman 2004).
Over the past decades, a large number of integrin members have been identified in osteoprogenitor cells and osteoblasts, and their roles in mediating bone formation are highly appreciated (Marie 2013). Among these, β1 integrins are the most abundantly expressed by osteoprogenitor cells and serve as the predominant mediators for cell adhesion to bone ECM molecules including type I collagen and fibronectin (Marie 2013). The critical role of β1 integrins in bone formation is evidenced by a genetic functional study, which demonstrated that transgenic mice with a dominant-negative β1 integrin subunit have reduced bone mass, increased cortical porosity in long bones and thinner flat bones in the skull (Zimmerman et al. 2000). Within the β1 subfamily, α5β1 and α2β1 integrins have received considerable research attention for their roles in promoting osteoblast differentiation, which is attributed to their binding affinity for fibronectin and type I collagen (the predominant molecule in bone ECM). Functional studies demonstrated that the osteoblast-fibronectin interaction is a critical event in the differentiation of rat osteoblasts and involves the interaction between α5β1 and fibronectin (Moursi et al. 1997; Damsky 1999). These results are in agreement with studies demonstrating the roles of α5β1 in the osteogenic differentiation of human osteoblastic cells (Dedhar et al. 1987). The critical role of type I collagen-α2β1 integrin signalling in osteoblastic differentiation has also been demonstrated in several studies (Takeuchi et al. 1997; Schneider et al. 2001; Gronthos et al. 2001; Petrie et al. 2008). For instance, α2β1 integrin–collagen interaction is required for the induction of osteoblast-specific gene expression through a post-translational pathway (Xiao et al. 1998). In addition, other integrin members, including α1β1, α4β1 α11β1 and αvβ3 also participate in osteoblastogenesis (Marie 2013; Martino et al. 2009).
Consistent with the key role of integrins in bone formation, studies have found that the induction of relevant integrin signalling pathways is the underlying mechanism by which bone-biomimetic biomaterials promote osteogenic differentiation of osteoprogenitor cells (Lu et al. 2012; Lu and Zreiqat 2010a, b; Liu et al. 2013a; Woo et al. 2007a). We recently demonstrated that scaffolds coated with hydroxyapatite nanoparticles can promote the differentiation of MSCs into osteoblasts by inducing α2β1 integrin signalling (Lu et al. 2012). On the other hand, the critical roles of integrins for bone formation have inspired researchers to pre-design scaffolding materials with tailored integrin-mediated signals to promote bone repair and regeneration. When α2β1 integrin-specific collagen-mimetic peptide glycine-phenylalanine-hydroxyproline-glycine-glutamate-arginine was coated onto titanium surfaces, this specific integrin-targeted coating not only promoted in vitro osteoblast differentiation and mineral deposition in stem cells, but also significantly improved peri-implant bone regeneration and osseointegration in vivo (Reyes et al. 2007; Wojtowicz et al. 2010). α5β1 integrin signalling is another key target which can be employed for bone regeneration (Hamidouche et al. 2009; Martino et al. 2009; Keselowsky et al. 2005). The activation of endogenous α5β1 integrin using agonists such as a specific antibody has been shown to promote osteoblast differentiation and osteogenic capacity of MSCs (Hamidouche et al. 2009). The above findings demonstrate that control of integrin binding specificity to elicit desired cellular activities is of great value in the design of informative biomaterials for bone tissue repair and regeneration.
3.2.1 Integrin Downstream Signalling Pathways
Signal transduction occurs when an extracellular signalling molecule binds to cell surface receptors and initiates a cascade of intracellular responses, which can then be dramatically amplified. This signal amplifying process is well exemplified by integrin-mediated intracellular signalling pathways. In general, integrin binding to ECM proteins initiates integrin clustering, cell adhesion and activation of multiple downstream intracellular signalling pathways, which ultimately determines cell fate including migration, proliferation and differentiation. First, integrin clustering results in the binding of integrin cytoplasmic tails to a large complex of proteins such as focal adhesion kinase (FAK), talin, paxilin, vinculin and α-actinin, to form so-called focal adhesion complexes. Second, proteins in the focal adhesion complexes are activated by phosphorylation, which creates docking sites for the activation of other cytosolic protein kinases/phosphatases. Finally, focal adhesion complexes serve as a bridge to connect ECM molecules to their downstream intracellular signalling pathways.
FAK is one of the most important components of focal adhesion complexes which are recruited by integrin clustering. The dependence of FAK phosphorylation and activation on integrin binding to their extracellular ligands has been demonstrated in a variety of cell types (Schwartz et al. 1995). The binding of FAK to cytoplasmic domains of chimeric integrin receptors can automatically activate FAK via phosphorylation (Akiyama et al. 1994). In other words, information coded within the cytoplasmic domain of integrins is possibly sufficient for FAK activation. The activated FAK then phosphorylates and activates a variety of molecules, including Rho, Rac Src, phosphoinositide 3-kinase (PI3K), threonine–protein kinase (Akt) and mitogen-activated protein kinases (MAPK) (Marie 2013; Thompson et al. 2012; Li et al. 2013a), which exert their biological functions of regulating cytoskeletal organisation, cell migration, cell proliferation and differentiation (Wozniak et al. 2004). Here we will specifically discuss three signalling pathways: extracellular signal-regulated kinase (ERK/MAPK), Rho/Rock and PI3K-Akt (illustrated in Fig. 3), which play crucial roles in regulating cell behaviour involved in osteogenesis.
Integrin signalling modulation of cell fate. The binding of ECM ligands to integrins triggers a cascade of downstream signalling pathways, mainly involving FAK-Rho/Rock, FAK-PI3K/AKT and FAK-ERK/MAPK. Meanwhile, integrin signalling pathways promote the production of endogenous growth factors and coordinate with endogenous/exogenous growth factor-mediated signalling pathways to accomplish cell fate determination
3.2.1.1 ERK/MAPK Signalling Pathway
MAPK signal pathways, including ERK1/2, c-Jun N-terminal kinase (JNK) and p38 MAPK (p38), are regulated by a diverse group of extracellular stimuli and mediate a variety of cellular responses. In particular, ERK1/2 signalling has been shown to favour osteoblastic cell proliferation and differentiation (Binetruy et al. 2007; Geest and Coffer 2009). In bone, the ERK/MAPK pathway is a major conduit for conveying signals from the extracellular environment to the nucleus, and is also implicated in the response of bone to a variety of signals, including hormone/growth factor stimulation, extracellular matrix–integrin binding and mechanical loading (Zeng et al. 2013; Shi et al. 2012; Lu et al. 2008a; Lu and Zreiqat 2010a, b). A study in transgenic mice found that skeletal size and calvarial mineralisation are decreased in ERK1/2/MAPK knock-down mice but increased in ERK1/2/MAPK induced mice, and the process of endochondral ossification in diaphyseal regions of long bones is also drastically delayed with only early bone collar formation being visible in ERK1/2/MAPK knock-down mice (Ge et al. 2007). In agreement with the key roles of ERK1/2/MAPK signalling in bone development, different osteoconductive/osteoinductive components of bone ECM can induce differentiation of osteoblasts from MSCs by activating ERK1/2/MAPK associated signalling pathways, including type I collagen (Tsai et al. 2010), fibronectin (Ding et al. 2006) and bone sialoprotein (Gordon et al. 2009). We previously demonstrated that β-TCP promotes the differentiation of human osteoblasts by activating ERK1/2/MAPK signalling (Lu and Zreiqat 2010a).
3.2.1.2 Rho/ROCK Signalling Pathway
RhoA, a member of the large Rho-family of GTPases, has been widely implicated in integrin-mediated signalling (Schoenwaelder and Burridge 1999; Clark et al. 1998). RhoA can exert its biological function through one of its downstream effectors, the Rho-associated protein kinase or ROCK, to control cell migration and differentiation in response to different stimuli (Clark et al. 1998; Kalaji et al. 2012; Seo et al. 2011; Xu et al. 2012; Lu et al. 2008a, 2010). Cell migration is a key step in tissue repair and regeneration and involves recruitment of progenitor cells to injury sites. The involvement of Rho/Rock signalling pathway in mediating cell migration is evidenced during the development of various tissues including bone (Li et al. 2006; Breyer et al. 2012; Ichida et al. 2011; Montanez et al. 2009; Benoit et al. 2009), and Rho/ROCK signalling inhibition can increase cell movement into bone formation sites in a mouse model of ectopic bone formation (Ichida et al. 2011). In addition, a line of evidence suggests that Rho/Rock signalling plays a key role in directing osteoprogenitor cells into the osteoblast lineage in different models of osteoinduction (Shih et al. 2011; Santos et al. 2010; Khatiwala et al. 2009). Using micropatterned substrates to progressively restrict cell spreading and flattening, Rho/ROCK signalling has been shown to regulate BMP-induced signalling and osteoblast differentiation of MSCs (Wang et al. 2012), and matrix stiffness has also been shown to control the osteogenic phenotype of MSCs by affecting Rho/ROCK intracellular signalling (Shih et al. 2011). The mechanism by which the Rho/ROCK signalling pathway influences the differentiation of osteoblasts has been largely attributed to its ability of assembling actin fibres and regulating cell shape (Guilak et al. 2009; Mathieu and Loboa 2012). When MSCs are allowed to adhere, flatten and spread, they undergo differentiation into osteoblasts; in contrast, the unspread and round cells become adipocytes (McBeath et al. 2004).
3.2.1.3 PI3K-Akt Signalling Pathway
The PI3K-Akt signalling pathway can be activated by extracellular signals as well as growth factors, and regulates many fundamental cellular processes including cell growth, proliferation and survival (Cantrell 2001; Guntur and Rosen 2011). Following PI3K activation, the lipid product of phosphatidylinositol 3,4,5 triphosphate (PI3) recruits both Akt and PI-dependent kinase 1 (PDK1) to the plasma membrane. Akt is then phosphorylated on T308 by PDK1 and on S473 by mTORC2, leading to full activation (Guntur and Rosen 2011). Activated Akt, in turn, regulates several downstream pathways including Runx2, the master transcription factor for osteogenesis (Kita et al. 2008; Liu et al. 2013b). Recent studies revealed that Akt and its downstream targets are critical regulators of bone formation and remodelling (Peng et al. 2003; Ulici et al. 2009). In vivo, Akt1 knockout mice have shorter bones and delayed formation of secondary ossification centres (Ulici et al. 2009). In vitro, osteoblasts lacking the negative regulator of PI3K/AKT signalling have a strikingly decreased susceptibility to apoptosis and accelerated differentiation capacity in association with markedly increased levels of phosphorylated Akt (Vinals et al. 2002). The important role of the PI3K-Akt signalling pathway in bone formation is also reflected by biomaterial surface modification studies. For instance, collagen I surface treatment promotes the proliferation and osteogenesis of MSCs via activation of ERK and Akt pathways (Tsai et al. 2010). Altered surface microroughness and hydrophilicity also affects osteoblast proliferation and the early stage of osteoblast differentiation by activating the PI3K/Akt signalling pathway (Gu et al. 2013).
3.2.2 Crosstalk Between Integrin and Growth Factor Signalling
Integrins can determine cell fate by activating several signalling pathways independently as discussed above, but they are also frequently coupled with growth factor receptor-mediated signalling, including vascular endothelial growth factor (VEGF) receptor, transforming growth factor beta (TGF-β) receptor, insulin receptor, type 1 insulin-like growth factor (IGF) receptor, BMP-2 receptor and others (Fig. 3) (Schneller et al. 1997; Kisiel et al. 2013; Hudalla et al. 2011; Massuto et al. 2010; Rapraeger et al. 2013). One recent study reported that the combination of BMP-2 with a hydroxyapatite/fibronection hydrogel mediated integrin signalling resulted in the formation of twice as much bone with better organisation of collagen fibres, compared to delivering the growth factor in a non-functionalised HA hydrogel (Kisiel et al. 2013). Research in the field all points to the fact that integrin signalling pathways are able to modulate cell behaviour by inducing the production of endogenous growth factors, which then exert autocrine and/or paracrine effects (Lu and Zreiqat 2010b; Lu et al. 2011; Hudalla et al. 2011; Moyano et al. 2010; Liu et al. 2013a). We recently found that β-TCP scaffolds promote osteoblastic differentiation by increasing endogenous BMP-2 production through a process involving α2β1 integrin and MAPK/ERK signalling pathways (Lu and Zreiqat 2010b). Similar results were shown in another study which demonstrated that hydroxyapatite/chitosan scaffolds promote MSC adhesion, proliferation and osteoblast differentiations by activating integrin-mediated BMP signalling pathways (Liu et al. 2013a). Thus, the substrate-integrin-endogenous growth factor loop indicates the potential feasibility of designing a smart biomaterial for bone tissue regeneration while avoiding the use of exogenous growth factors.
4 Summary, Conclusion and Perspectives
To circumvent problems associated with current clinical methods of bone reconstruction, including autografting and allografting, the design and development of synthetic biomaterial scaffolds has been an area of great interest. By mimicking the physical and chemical characteristics of natural bone tissue, significant achievements have been made in designing biomaterials that meet the requirements for bone repair and regeneration. One of the key requirements for ideal bone scaffold materials is that they should have the properties to recruit MSCs and osteoprogenitor cells and direct their differentiation into osteoblasts, which require appropriate cell–biomaterial communication. Understanding and identifying the key molecules and signalling pathways involved in the cross-talk between biomaterials and osteoprogenitor cells will bring substantial benefit to the development of ideal bone scaffold materials with excellent bioactivity.
In this book chapter, we have summarised various design strategies which aim to optimise the osteoconductive and osteoinductive properties of bone scaffolds by mimicking the physical and chemical characteristics of bone, namely architecture, topography, mechanical properties and composition of its organic and mineral phases. This was followed by detailed discussion of the underlying mechanisms by which biomaterials determine cell fate. These include modulating protein adsorption on biomaterial surfaces, eliciting cell adhesion to biomaterials by binding to specific cellular receptors, and triggering a cascade of downstream intracellular signalling events. A range of key molecules (e.g. α2β1 integrin and α5β1 integrin) and signalling pathways (e.g. ERK/MAPK, Rho/Rock and PI3K-Akt) have been identified as being critical in the determination of cell fate when cells come into contact with a biomaterial intended for bone regeneration. The control of biomaterial binding specificity, such as binding to specific integrins to elicit desired cellular activities, has become a powerful tool in the design of bone informative scaffolding materials.
In the future, further exploration of developmental bone biology and the underlying mechanisms by which biomaterials communicate with relevant cells will continually contribute to biomaterial- and cell-based strategies for bone repair and regeneration. As increasing numbers of cell types, including osteoblasts, osteoclasts, MSCs, endothelial progenitor cells and macrophages, and their interactions have been shown to be critical for bone regeneration (Pirraco et al. 2010), a systematic methodology might be needed in order to assess the effect of biomaterial modulation on the behaviour of all of these cell types and their interactions. In addition, it is also imperative to identify the signals in each cell type that are spatially and temporally necessary for bone repair and regeneration, such that they can be incorporated into the design of biomaterial scaffolds to achieve optimal bone regeneration and thus functional repair of bone defects.
References
Akiyama SK, Yamada SS, Yamada KM, Laflamme SE (1994) Transmembrane signal transduction by integrin cytoplasmic domains expressed in single-subunit chimeras. J Biol Chem 269:15961–15964
Akkouch A, Zhang Z, Rouabhia M (2011) A novel collagen/hydroxyapatite/poly(lactide-co-ε-caprolactone) biodegradable and bioactive 3D porous scaffold for bone regeneration. J Biomed Mater Res Part A 96A:693–704
Albrektsson T, Johansson C (2001) Osteoinduction, osteoconduction and osseointegration. Eur Spine J 10:S96–S101
Alves CM, Yang Y, Marton D, Carnes DL, Ong JL, Sylvia VL, Dean DD, Reis RL, Agrawal CM (2008) Plasma surface modification of poly(D, L-lactic acid) as a tool to enhance protein adsorption and the attachment of different cell types. J Biomed Mater Res Part B-Appl Biomater 87B:59–66
Amini AR, Laurencin CT, Nukavarapu SP (2012) Bone tissue engineering: recent advances and challenges. Crit Rev Biomed Eng 40:363–408
Arinzeh TL, Tran T, McAlary J, Daculsi G (2005) A comparative study of biphasic calcium phosphate ceramics for human mesenchymal stem-cell-induced bone formation. Biomaterials 26:3631–3638
Assal Y, Mie M, Kobatake E (2013) The promotion of angiogenesis by growth factors integrated with ECM proteins through coiled-coil structures. Biomaterials 34:3315–3323
Baroli B (2009) From natural bone grafts to tissue engineering therapeutics: brainstorming on pharmaceutical formulative requirements and challenges. J Pharm Sci 98:1317–1375
Baxter FR, Bowen CR, Turner IG, Dent ACE (2010) Electrically active bioceramics: a review of interfacial responses. Ann Biomed Eng 38:2079–2092
Benoit YD, Lussier C, Ducharme PA, Sivret S, Schnapp LM, Basora N, Beaulieu JF (2009) Integrin alpha 8 beta 1 regulates adhesion, migration and proliferation of human intestinal crypt cells via a predominant RhoA/ROCK-dependent mechanism. Biol Cell 101:695–708
Bhumiratana S, Grayson WL, Castaneda A, Rockwood DN, Gil ES, Kaplan DL, Vunjak-Novakovic G (2011) Nucleation and growth of mineralized bone matrix on silk-hydroxyapatite composite scaffolds. Biomaterials 32:2812–2820
Biggs MJP, Richards RG, Gadegaard N, McMurray RJ, Affrossman S, Wilkinson CDW, Oreffo ROC, Dalby MJ (2009) Interactions with nanoscale topography: adhesion quantification and signal transduction in cells of osteogenic and multipotent lineage. J Biomed Mater Res Part A 91A:195–208
Binazadeh M, Zeng H, Unsworth LD (2013) Effect of peptide secondary structure on adsorption and adsorbed film properties. Acta Biomater 9:6403–6413
Binetruy B, Heasley L, Bost F, Caron L, Aouadi M (2007) Concise review: regulation of embryonic stem cell lineage commitment by mitogen-activated protein kinases. Stem Cells 25:1090–1095
Boccaccini AR, Maquet V (2003) Bioresorbable and bioactive polymer/bioglass® composites with tailored pore structure for tissue engineering applications. Compos Sci Technol 63:2417–2429
Bodde EWH, Wolke JGC, Kowalski RSZ, Jansen JA (2007) Bone regeneration of porous β-tricalcium phosphate (ConduitTM TCP) and of biphasic calcium phosphate ceramic (Biosel®) in trabecular defects in sheep. J Biomed Mater Res Part A 82A:711–722
Botelho CM, Brooks RA, Best SM, Lopes MA, Santos JD, Rushton N, Bonfield W (2006) Human osteoblast response to silicon-substituted hydroxyapatite. J Biomed Mater Res Part A 79A:723–730
Bretcanu O, Misra S, Roy I, Renghini C, Fiori F, Boccaccini AR, Salih V (2009) In vitro biocompatibility of 45S5 Bioglass®-derived glass–ceramic scaffolds coated with poly(3-hydroxybutyrate). J Tissue Eng Regenerative Med 3:139–148
Breyer J, Samarin J, Rehm M, Lautscham L, Fabry B, Goppelt-Struebe M (2012) Inhibition of Rho kinases increases directional motility of microvascular endothelial cells. Biochem Pharmacol 83:616–626
Brun P, Scorzeto M, Vassanelli S, Castagliuolo I, Palu G, Ghezzo F, Messina GML, Iucci G, Battaglia V, Sivolella S, Bagno A, Polzonetti G, Marietta G, Dettin M (2013) Mechanisms underlying the attachment and spreading of human osteoblasts: from transient interactions to focal adhesions on vitronectin-grafted bioactive surfaces. Acta Biomater 9:6105–6115
Buchanan LA, Ei-Ghannam A (2010) Effect of bioactive glass crystallization on the conformation and bioactivity of adsorbed proteins. J Biomed Mater Res Part A 93A:537–546
Cabodi S, di Stefano P, Leal MDC, Tinnirello A, Bisaro B, Morello V, Damiano L, Aramu S, Repetto D, Tornillo G, Defilippi P (2010) Integrins and signal transduction. Integrins Ion Channels Mol Complexes Signal 674:43–54
Cantrell DA (2001) Phosphoinositide 3-kinase signalling pathways. J Cell Sci 114:1439–1445
Capuccini C, Torricelli P, Boanini E, Gazzano M, Giardino R, Bigi A (2009) Interaction of Sr-doped hydroxyapatite nanocrystals with osteoclast and osteoblast-like cells. J Biomed Mater Res Part A 89A:594–600
Chai YC, Carlier A, Bolander J, Roberts SJ, Geris L, Schrooten J, van Oosterwyck H, Luyten FP (2012) Current views on calcium phosphate osteogenicity and the translation into effective bone regeneration strategies. Acta Biomater 8:3876–3887
Chen QZ, Boccaccini AR (2006) Poly(D, L-lactic acid) coated 45S5 Bioglass®-based scaffolds: processing and characterization. J Biomed Mater Res Part A 77A:445–457
Chen J, Altman GH, Karageorgiou V, Horan R, Collette A, Volloch V, Colabro T, Kaplan DL (2003) Human bone marrow stromal cell and ligament fibroblast responses on RGD-modified silk fibers. J Biomed Mater Res Part A 67A:559–570
Chen VJ, Smith LA, Ma PX (2006) Bone regeneration on computer-designed nano-fibrous scaffolds. Biomaterials 27:3973–3979
Clark EA, King WG, Brugge JS, Symons M, Hynes RO (1998) Integrin-mediated signals regulated by members of the rho family of GTPases. J Cell Biol 142:573–586
Crowley C, Wong JML, Fisher DM, Khan WS (2013) A systematic review on preclinical and clinical studies on the use of scaffolds for bone repair in skeletal defects. Curr Stem Cell Res Ther 8:243–252
Dalby MJ, McCloy D, Robertson M, Wilkinson CDW, Oreffo ROC (2006) Osteoprogenitor response to defined topographies with nanoscale depths. Biomaterials 27:1306–1315
Dalby MJ, Gadegaard N, Tare R, Andar A, Riehle MO, Herzyk P, Wilkinson CDW, Oreffo ROC (2007) The control of human mesenchymal cell differentiation using nanoscale symmetry and disorder. Nat Mater 6:997–1003
Damsky CH (1999) Extracellular matrix-integrin interactions in osteoblast function and tissue remodeling. Bone 25:95–96
Dedhar S, Argraves WS, Suzuki S, Ruoslahti E, Pierschbacher MD (1987) Human osteosarcoma cells resistant to detachment by an Arg-Gly-Asp-containing peptide overproduce the fibronectin receptor. J Cell Biol 105:1175–1182
Deligianni D, Korovessis P, Porte-Derrieu MC, Amedee J (2005) Fibronectin preadsorbed on hydroxyapatite together with rough surface structure increases osteoblasts’ adhesion “in vitro”: the theoretical usefulness of fibronectin preadsorption on hydroxyapatite to increase permanent stability and longevity in spine implants. J Spinal Disord Tech 18:257–262
Depan D, Misra RDK (2013) The interplay between nanostructured carbon-grafted chitosan scaffolds and protein adsorption on the cellular response of osteoblasts: structure-function property relationship. Acta Biomater 9:6084–6094
Ding HT, Wang CG, Zhang TL, Wang K (2006) Fibronectin enhances in vitro vascular calcification by promoting osteoblastic differentiation of vascular smooth muscle cells via ERK pathway. J Cell Biochem 99:1343–1352
Dong J, Uemura T, Shirasaki Y, Tateishi T (2002) Promotion of bone formation using highly pure porous β-TCP combined with bone marrow-derived osteoprogenitor cells. Biomaterials 23:4493–4502
Drevelle O, Faucheux N (2013) Biomimetic materials for controlling bone cell responses. Front Biosci (Schol Ed) 5:369–395
Farrell E, O’Brien FJ, Doyle P, Fischer J, Yannas I, Harley BA, O’Connell B, Prendergast PJ, Campbell VA (2006) A collagen-glycosaminoglycan scaffold supports adult rat mesenchymal stem cell differentiation along osteogenic and chondrogenic routes. Tissue Eng 12:459–468
Gaasbeek RDA, Toonen HG, van Heerwaarden RJ, Buma P (2005) Mechanism of bone incorporation of β-TCP bone substitute in open wedge tibial osteotomy in patients. Biomaterials 26:6713–6719
Galois L, Mainard D, Delagoutte JP (2002) Beta-tricalcium phosphate ceramic as a bone substitute in orthopaedic surgery. Int Orthop 26:109–115
Ge CX, Xiao GZ, Jiang D, Franceschi RT (2007) Critical role of the extracellular signal-regulated kinase-MAPK pathway in osteoblast differentiation and skeletal development. J Cell Biol 176:709–718
Geest CR, Coffer PJ (2009) MAPK signaling pathways in the regulation of hematopoiesis. J Leukoc Biol 86:237–250
Gordon JAR, Hunter GK, Goldberg HA (2009) Activation of the mitogen-activated protein kinase pathway by bone sialoprotein regulates osteoblast differentiation. Cells Tissues Organs 189:138–143
Gronthos S, Simmons PJ, Graves SE, Robey PG (2001) Integrin-mediated interactions between human bone marrow stromal precursor cells and the extracellular matrix. Bone 28:174–181
Gu YX, Du J, Si MS, Mo JJ, Qiao SC, Lai HC (2013) The roles of PI3K/Akt signaling pathway in regulating MC3T3-E1 preosteoblast proliferation and differentiation on SLA and SLActive titanium surfaces. J Biomed Mater Res Part A 101A:748–754
Guilak F, Cohen DM, Estes BT, Gimble JM, Liedtke W, Chen CS (2009) Control of stem cell fate by physical interactions with the extracellular matrix. Cell Stem Cell 5:17–26
Guntur AR, Rosen CJ (2011) The skeleton: a multi-functional complex organ. New insights into osteoblasts and their role in bone formation: the central role of PI3 Kinase. J Endocrinol 211:123–130
Habibovic P, Kruyt MC, Juhl MV, Clyens S, Martinetti R, Dolcini L, Theilgaard N, van Blitterswijk CA (2008) Comparative in vivo study of six hydroxyapatite-based bone graft substitutes. J Orthop Res 26:1363–1370
Hamidouche Z, Fromigue O, Ringe J, Haupl T, Vaudin P, Pages JC, Srouji S, Livne E, Marie PJ (2009) Priming integrin alpha5 promotes human mesenchymal stromal cell osteoblast differentiation and osteogenesis. Proc Natl Acad Sci USA 106:18587–18591
Heise U, Osborn JF, Duwe F (1990) Hydroxyapatite ceramic as a bone substitute. Int Orthop 14:329–338
Hing KA, Wilson LF, Buckland T (2007) Comparative performance of three ceramic bone graft substitutes. Spine J 7:475–490
Holzwarth JM, Ma PX (2011) Biomimetic nanofibrous scaffolds for bone tissue engineering. Biomaterials 32:9622–9629
Hong Z, Reis RL, Mano JF (2008) Preparation and in vitro characterization of scaffolds of poly(l-lactic acid) containing bioactive glass ceramic nanoparticles. Acta Biomater 4:1297–1306
Horbett TA (1982) Protein Adsorption on Biomaterials. Advances in Chemistry Series. Springer, Berlin, pp 233–244
Howlett CR, Evans MDM, Walsh WR, Johnson G, Steele JG (1994) Mechanism of initial attachment of cells derived from human bone to commonly used prosthetic materials during cell-culture. Biomaterials 15:213–222
Hu Y, Winn SR, Krajbich I, Hollinger JO (2003) Porous polymer scaffolds surface-modified with arginine-glycine-aspartic acid enhance bone cell attachment and differentiation in vitro. J Biomed Mater Res, Part A 64A:583–590
Hudalla GA, Kouris NA, Koepsel JT, Ogle BM, Murphy WL (2011) Harnessing endogenous growth factor activity modulates stem cell behavior. Integr Biol 3:832–842
Humphries MJ, Travis MA, Clark K, Mould AP (2004) Mechanisms of integration of cells and extracellular matrices by integrins. Biochem Soc Trans 32:822–825
Ichida M, Yui Y, Yoshioka K, Tanaka T, Wakamatsu T, Yoshikawa H, Itoh K (2011) Changes in cell migration of mesenchymal cells during osteogenic differentiation. FEBS Lett 585:4018–4024
Ignatius A, Blessing H, Liedert A, Schmidt C, Neidlinger-Wilke C, Kaspar D, Friemert B, Claes L (2005) Tissue engineering of bone: effects of mechanical strain on osteoblastic cells in type I collagen matrices. Biomaterials 26:311–318
Ikada Y (2006) Challenges in tissue engineering. J R Soc Interface 3:589–601
Kalaji R, Wheeler AP, Erasmus JC, Lee SY, Endres RG, Cramer LP, Braga VMM (2012) ROCK1 and ROCK2 regulate epithelial polarisation and geometric cell shape. Biol Cell 104:435–451
Karageorgiou V, Kaplan D (2005) Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 26:5474–5491
Keselowsky BG, Collard DM, Garcia AJ (2005) Integrin binding specificity regulates biomaterial surface chemistry effects on cell differentiation. Proc Natl Acad Sci USA 102:5953–5957
Khatiwala CB, Kim PD, Peyton SR, Putnam AJ (2009) ECM compliance regulates osteogenesis by influencing MAPK signaling downstream of RhoA and ROCK. J Bone Miner Res 24:886–898
Kim HW, Knowles JC, Kim HE (2004) Hydroxyapatite/poly(ε-caprolactone) composite coatings on hydroxyapatite porous bone scaffold for drug delivery. Biomaterials 25:1279–1287
Kim SS, Park MS, Jeon O, Choi CY, Kim BS (2006) Poly(lactide-co-glycolide)/hydroxyapatite composite scaffolds for bone tissue engineering. Biomaterials 27:1399–1409
Kisiel M, Martino MM, Ventura M, Hubbell JA, Hilborn J, Ossipov DA (2013) Improving the osteogenic potential of BMP-2 with hyaluronic acid hydrogel modified with integrin-specific fibronectin fragment. Biomaterials 34:704–712
Kita K, Kimura T, Nakamura N, Yoshikawa H, Nakano T (2008) PI3K/Akt signaling as a key regulatory pathway for chondrocyte terminal differentiation. Genes Cells 13:839–850
Kitsugi T, Yamamuro T, Nakamura T, Kotani S, Kokubo T, Takeuchi H (1993) Four calcium phosphate ceramics as bone substitutes for non-weight-bearing. Biomaterials 14:216–224
Kleinman HK, Klebe RJ, Martin GR (1981) Role of collagenous matrices in the adhesion and growth of cells. J Cell Biol 88:473–485
Knight CG, Morton LF, Peachey AR, Tuckwell DS, Farndale RW, Barnes MJ (2000) The collagen-binding A-domains of integrins α1β1 and α2β1 recognize the same specific amino acid sequence, GFOGER, in native (triple-helical) collagens. J Biol Chem 275:35–40
Kondo N, Ogose A, Tokunaga K, Ito T, Arai K, Kudo N, Inoue H, Irie H, Endo N (2005) Bone formation and resorption of highly purified β-tricalcium phosphate in the rat femoral condyle. Biomaterials 26:5600–5608
Kosuge D, Khan WS, Haddad B, Marsh D (2013) Biomaterials and scaffolds in bone and musculoskeletal engineering. Curr Stem Cell Res Ther 8:185–191
Lan Levengood SK, Polak SJ, Wheeler MB, Maki AJ, Clark SG, Jamison RD, Wagoner Johnson AJ (2010) Multiscale osteointegration as a new paradigm for the design of calcium phosphate scaffolds for bone regeneration. Biomaterials 31:3552–3563
Landi E, Logroscino G, Proietti L, Tampieri A, Sandri M, Sprio S (2008) Biomimetic Mg-substituted hydroxyapatite: from synthesis to in vivo behaviour. J Mater Sci Mater Med 19:239–247
Laurie SW, Kaban LB, Mulliken JB, Murray JE (1984) Donor-site morbidity after harvesting rib and iliac bone. Plast Reconstr Surg 73:933–938
Lee SJ, Lim GJ, Lee JW, Atala A, Yoo JJ (2006) In vitro evaluation of a poly(lactide-co-glycolide)-collagen composite scaffold for bone regeneration. Biomaterials 27:3466–3472
Legeros RZ (2002) Properties of osteoconductive biomaterials: calcium phosphates. Clin Orthop Relat Res 395:81–98
Legeros RZ, Lin S, Rohanizadeh R, Mijares D, Legeros JP (2003) Biphasic calcium phosphate bioceramics: preparation, properties and applications. J Mater Sci Mater Med 14:201–209
Li Z, Wang C, Prendergast GC, Pestell RG (2006) Cyclin D1 functions in cell migration. Cell Cycle 5:2440–2442
Li BJ, Moshfegh C, Lin Z, Albuschies J, Vogel V (2013a). Mesenchymal stem cells exploit extracellular matrix as mechanotransducer. Scientific reports, 3
Li JJ, Gil ES, Hayden RS, Li C, Roohani-Esfahani S-I, Kaplan DL, Zreiqat H (2013b) Multiple silk coatings on biphasic calcium phosphate scaffolds: effect on physical and mechanical properties and in vitro osteogenic response of human mesenchymal stem cells. Biomacromolecules 14:2179–2188
Liao SS, Cui FZ, Zhang W, Feng QL (2004) Hierarchically biomimetic bone scaffold materials: nano-HA/collagen/PLA composite. J Biomed Mater Res B Appl Biomater 69B:158–165
Liu X, Smith LA, Hu J, Ma PX (2009) Biomimetic nanofibrous gelatin/apatite composite scaffolds for bone tissue engineering. Biomaterials 30:2252–2258
Liu HH, Peng HJ, Wu Y, Zhang C, Cai YZ, Xu GW, Li Q, Chen X, Ji JF, Zhang YZ, Ouyang HW (2013a) The promotion of bone regeneration by nanofibrous hydroxyapatite/chitosan scaffolds by effects on integrin-BMP/Smad signaling pathway in BMSCs. Biomaterials 34:4404–4417
Liu JP, Chen L, Tao X, Tang KL (2013b) Phosphoinositide 3-kinase/Akt signaling is essential for prostaglandin E2-induced osteogenic differentiation of rat tendon stem cells. Biochem Biophys Res Commun 435:514–519
Lord CF, Gebhardt MC, Tomford WW, Mankin HJ (1988) Infection in bone allografts–incidence, nature, and treatment. J Bone Joint Surg-Am 70A:369–376
Lu ZF, Zreiqat H (2010a) Beta-tricalcium phosphate exerts osteoconductivity through alpha 2 beta 1 integrin and down-stream MAPK/ERK signaling pathway. Biochem Biophys Res Commun 394:323–329
Lu ZL, Zreiqat H (2010b) The osteoconductivity of biomaterials is regulated by bone morphogenetic protein 2 autocrine loop involving alpha 2 beta 1 integrin and mitogen-activated protein kinase/extracellular related kinase signaling pathways. Tissue Eng Part A 16:3075–3084
Lu ZF, Doulabi BZ, Huang CL, Bank RA, Helder MN (2008a) Beta 1 integrins regulate chondrogenesis and rock signaling in adipose stem cells. Biochemical Biophys Res Commun 372:547–552
Lu ZF, Zandieh Doulabi B, Wuisman PI, Bank RA, Helder MN (2008b) Influence of collagen type II and nucleus pulposus cells on aggregation and differentiation of adipose tissue-derived stem cells. J Cell Mol Med 12:2812–2822
Lu Z, Doulabi BZ, Huang C, Bank RA, Helder MN (2010) Collagen type II enhances chondrogenesis in adipose tissue-derived stem cells by affecting cell shape. Tissue Eng Part A 16:81–90
Lu ZF, Roohani-Esfahani SI, Kwok PCL, Zreiqat H (2011) Osteoblasts on rod shaped hydroxyapatite nanoparticles incorporated PCL film provide an optimal osteogenic niche for stem cell differentiation. Tissue Eng Part A 17:1651–1661
Lu ZF, Roohani-Esfahani SI, Wang GC, Zreiqat H (2012) Bone biomimetic microenvironment induces osteogenic differentiation of adipose tissue-derived mesenchymal stem cells. Nanomed-Nanotechnol Biol Med 8:507–515
Mankin HJ, Gebhardt MC, Jennings LC, Springfield DS, Tomford WW (1996) Long-term results of allograft replacement in the management of bone tumors. Clin Orthop Relat Res 324:86–97
Marie PJ (2013) Targeting integrins to promote bone formation and repair. Nat Rev Endocrinol 9:288–295
Martino MM, Mochizuki M, Rothenfluh DA, Rempel SA, Hubbell JA, Barker TH (2009) Controlling integrin specificity and stem cell differentiation in 2D and 3D environments through regulation of fibronectin domain stability. Biomaterials 30:1089–1097
Massuto DA, Kneese EC, Johnson GA, Burghardt RC, Hooper RN, Ing NH, Jaeger LA (2010) Transforming growth factor beta (TGFB) signaling is activated during porcine implantation: proposed role for latency-associated peptide interactions with integrins at the conceptus-maternal interface. Reproduction 139:465–478
Mastrogiacomo M, Muraglia A, Komlev V, Peyrin F, Rustichelli F, Crovace A, Cancedda R (2005) Tissue engineering of bone: search for a better scaffold. Orthod Craniofacial Res 8:277–284
Mastrogiacomo M, Scaglione S, Martinetti R, Dolcini L, Beltrame F, Cancedda R, Quarto R (2006) Role of scaffold internal structure on in vivo bone formation in macroporous calcium phosphate bioceramics. Biomaterials 27:3230–3237
Mathieu PS, Loboa EG (2012) Cytoskeletal and focal adhesion influences on mesenchymal stem cell shape, mechanical properties, and differentiation down osteogenic, adipogenic, and chondrogenic pathways. Tissue Eng Part B Rev 18:436–444
McBeath R, Pirone DM, Nelson CM, Bhadriraju K, Chen CS (2004) Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev Cell 6:483–495
McMahon RE, Wang LN, Skoracki R, Mathur AB (2013) Development of nanomaterials for bone repair and regeneration. J Biomed Mater Res Part B Appl Biomater 101B:387–397
Miao X, Tan LP, Tan LS, Huang X (2007) Porous calcium phosphate ceramics modified with PLGA-bioactive glass. Mater Sci Eng, C 27:274–279
Miao X, Tan DM, Li J, Xiao Y, Crawford R (2008) Mechanical and biological properties of hydroxyapatite/tricalcium phosphate scaffolds coated with poly(lactic-co-glycolic acid). Acta Biomater 4:638–645
Michiardi A, Aparicio C, Ratner BD, Planell JA, Gil J (2007) The influence of surface energy on competitive protein adsorption on oxidized NiTi surfaces. Biomaterials 28:586–594
Mistry AS, Mikos AG (2005) Tissue engineering strategies for bone regeneration. In: Yannas IV (ed) Regenerative medicine II. Springer, Berlin
Montanez E, Wickstrom SA, Altstatter J, Chu HY, Fassler R (2009) Alpha-parvin controls vascular mural cell recruitment to vessel wall by regulating RhoA/ROCK signalling. EMBO J 28:3132–3144
Moursi AM, Globus RK, Damsky CH (1997) Interactions between integrin receptors and fibronectin are required for calvarial osteoblast differentiation in vitro. J Cell Sci 110:2187–2196
Moyano JV, Greciano PG, Buschmann MM, Koch M, Matlin KS (2010) Autocrine transforming growth factor-beta 1 activation mediated by integrin alpha V beta 3 regulates transcriptional expression of Laminin-332 in Madin-Darby canine kidney epithelial cells. Mol Biol Cell 21:3654–3668
Müller P, Bulnheim U, Diener A, Lüthen F, Teller M, Klinkenberg E-D, Neumann H-G, Nebe B, Liebold A, Steinhoff G, Rychly J (2008) Calcium phosphate surfaces promote osteogenic differentiation of mesenchymal stem cells. J Cell Mol Med 12:281–291
Murphy CM, Haugh MG, O’Brien FJ (2010) The effect of mean pore size on cell attachment, proliferation and migration in collagen-glycosaminoglycan scaffolds for bone tissue engineering. Biomaterials 31:461–466
Ni G-X, Yao Z-P, Huang G-T, Liu W-G, Lu W (2011) The effect of strontium incorporation in hydroxyapatite on osteoblasts in vitro. J Mater Sci Mater Med 22:961–967
Oh SH, Park IK, Kim JM, Lee JH (2007) In vitro and in vivo characteristics of PCL scaffolds with pore size gradient fabricated by a centrifugation method. Biomaterials 28:1664–1671
Okuda T, Ioku K, Yonezawa I, Minagi H, Kawachi G, Gonda Y, Murayama H, Shibata Y, Minami S, Kamihira S, Kurosawa H, Ikeda T (2007) The effect of the microstructure of β-tricalcium phosphate on the metabolism of subsequently formed bone tissue. Biomaterials 28:2612–2621
Pek YS, Gao S, Arshad MSM, Leck KJ, Ying JY (2008) Porous collagen-apatite nanocomposite foams as bone regeneration scaffolds. Biomaterials 29:4300–4305
Peng XD, Xu PZ, Chen ML, Hahn-Windgassen A, Skeen J, Jacobs J, Sundararajan D, Chen WS, Crawford SE, Coleman KG, Hay N (2003) Dwarfism, impaired skin development, skeletal muscle atrophy, delayed bone development, and impeded adipogenesis in mice lacking Akt1 and Akt2. Genes Dev 17:1352–1365
Petrie TA, Raynor JE, Reyes CD, Burns KL, Collard DM, Garcia AJ (2008) The effect of integrin-specific bioactive coatings on tissue healing and implant osseointegration. Biomaterials 29:2849–2857
Pezzotti G, Asmus SMF (2001) Fracture behavior of hydroxyapatite/polymer interpenetrating network composites prepared by in situ polymerization process. Mater Sci Eng A 316:231–237
Pirraco RP, Marques AP, Reis RL (2010) Cell interactions in bone tissue engineering. J Cell Mol Med 14:93–102
Porter AE, Patel N, Skepper JN, Best SM, Bonfield W (2004) Effect of sintered silicate-substituted hydroxyapatite on remodelling processes at the bone–implant interface. Biomaterials 25:3303–3314
Puppi D, Chiellini F, Piras AM, Chiellini E (2010) Polymeric materials for bone and cartilage repair. Prog Polym Sci 35:403–440
Quarto R, Mastrogiacomo M, Cancedda R, Kutepov SM, Mukhachev V, Lavroukov A, Kon E, Marcacci M (2001) Repair of large bone defects with the use of autologous bone marrow stromal cells. N Engl J Med 344:385–386
Rapraeger AC, Ell BJ, Roy M, Li XH, Morrison OR, Thomas GM, Beauvais DM (2013) Vascular endothelial-cadherin stimulates syndecan-1-coupled insulin-like growth factor-1 receptor and cross-talk between alpha V beta 3 integrin and vascular endothelial growth factor receptor 2 at the onset of endothelial cell dissemination during angiogenesis. FEBS J 280:2194–2206
Reyes CD, Petrie TA, Burns KL, Schwartz Z, Garcia AJ (2007) Biomolecular surface coating to enhance orthopaedic tissue healing and integration. Biomaterials 28:3228–3235
Rezwan K, Chen QZ, Blaker JJ, Boccaccini AR (2006) Biodegradable and bioactive porous polymer/inorganic composite scaffolds for bone tissue engineering. Biomaterials 27:3413–3431
Rho JY, Kuhn-Spearing L, Zioupos P (1998) Mechanical properties and the hierarchical structure of bone. Med Eng Phys 20:92–102
Rivera-Chacon DM, Alvarado-Velez M, Acevedo-Morantes CY, Singh SP, Gultepe E, Nagesha D, Sridhar S, Ramirez-Vick JE (2013) Fibronectin and vitronectin promote human fetal osteoblast cell attachment and proliferation on nanoporous titanium surfaces. J Biomed Nanotechnol 9:1092–1097
Rockwood DN, Gil ES, Park SH, Kluge JA, Grayson W, Bhumiratana S, Rajkhowa R, Wang X, Kim SJ, Vunjak-Novakovic G, Kaplan DL (2011) Ingrowth of human mesenchymal stem cells into porous silk particle reinforced silk composite scaffolds: an in vitro study. Acta Biomater 7:144–151
Rogel MR, Qiu H, Ameer GA (2008) The role of nanocomposites in bone regeneration. J Mater Chem 18:4233–4241
Roohani-Esfahani SI, Nouri-Khorasani S, Lu Z, Appleyard R, Zreiqat H (2010) The influence hydroxyapatite nanoparticle shape and size on the properties of biphasic calcium phosphate scaffolds coated with hydroxyapatite-PCL composites. Biomaterials 31:5498–5509
Roohani-Esfahani SI, Nouri-Khorasani S, Lu ZF, Appleyard RC, Zreiqat H (2011) Effects of bioactive glass nanoparticles on the mechanical and biological behavior of composite coated scaffolds. Acta Biomater 7:1307–1318
Roohani-Esfahani SI, Lu ZF, Li JJ, Ellis-Behnke R, Kaplan DL, Zreiqat H (2012) Effect of self-assembled nanofibrous silk/polycaprolactone layer on the osteoconductivity and mechanical properties of biphasic calcium phosphate scaffolds. Acta Biomater 8:302–312
Roohani-Esfahani SI, Dunstan CR, Li JJ, Lu Z, Davies B, Pearce S, Field J, Williams R, Zreiqat H (2013) Unique microstructural design of ceramic scaffolds for bone regeneration under load. Acta Biomater 9:7014–7024
Rosengren A, Pavlovic E, Oscarsson S, Krajewski A, Ravaglioli A, Piancastelli A (2002) Plasma protein adsorption pattern on characterized ceramic biomaterials. Biomaterials 23:1237–1247
Ruoslahti E (1996) RGD and other recognition sequences for integrins. Annu Rev Cell Dev Biol 12:697–715
Samavedi S, Whittington AR, Goldstein AS (2013) Calcium phosphate ceramics in bone tissue engineering: a review of properties and their influence on cell behavior. Acta Biomater 9:8037–8045
Santos MI, Reis RL (2010) Vascularization in bone tissue engineering: physiology, current strategies, major hurdles and future challenges. Macromol Biosci 10:12–27
Santos A, Bakker AD, De Blieck-Hogervorst JMA, Klein-Nulend J (2010) WNT5A induces osteogenic differentiation of human adipose stem cells via rho-associated kinase Rock. Cytotherapy 12:924–932
Sanz-Herrera JA, Doblaré M, García-Aznar JM (2010) Scaffold microarchitecture determines internal bone directional growth structure: a numerical study. J Biomech 43:2480–2486
Schneider D, Engelman DM (2004) Involvement of transmembrane domain interactions in signal transduction by alpha/beta integrins. J Biol Chem 279:9840–9846
Schneider GB, Zaharias R, Stanford C (2001) Osteoblast integrin adhesion and signaling regulate mineralization. J Dent Res 80:1540–1544
Schneller M, Vuori K, Ruoslahti E (1997) αvβ3 integrin associates with activated insulin and PDGF beta receptors and potentiates the biological activity of PDGF. EMBO J 16:5600–5607
Schoenwaelder SM, Burridge K (1999) Bidirectional signaling between the cytoskeleton and integrins. Curr Opin Cell Biol 11:274–286
Schwartz MA, Schaller MD, Ginsberg MH (1995) Integrins: emerging paradigms of signal transduction. Annu Rev Cell Dev Biol 11:549–599
Schwartz CE, Martha JF, Kowalski P, Wang DA, Bode R, Li L, Kim DH (2009) Prospective evaluation of chronic pain associated with posterior autologous iliac crest bone graft harvest and its effect on postoperative outcome. Health Qual Life Outcomes 7(49):52
Scotchford CA, Ball M, Winkelmann M, Voros J, Csucs C, Brunette DM, Danuser G, Textor M (2003) Chemically patterned, metal-oxide-based surfaces produced by photolithographic techniques for studying protein- and cell-interactions. II: protein adsorption and early cell interactions. Biomaterials 24:1147–1158
Seo CH, Furukawa K, Montagne K, Jeong H, Ushida T (2011) The effect of substrate microtopography on focal adhesion maturation and actin organization via the RhoA/ROCK pathway. Biomaterials 32:9568–9575
Shepherd J, Shepherd D, Best S (2012) Substituted hydroxyapatites for bone repair. J Mater Sci Mater Med 23:2335–2347
Shi X, Sitharaman B, Pham QP, Liang F, Wu K, Edward Billups W, Wilson LJ, Mikos AG (2007) Fabrication of porous ultra-short single-walled carbon nanotube nanocomposite scaffolds for bone tissue engineering. Biomaterials 28(4078):4090
Shi Y, Xia YY, Wang L, Liu R, Khoo KS, Feng ZW (2012) Neural cell adhesion molecule modulates mesenchymal stromal cell migration via activation of MAPK/ERK signaling. Exp Cell Res 318:2257–2267
Shih YR, Tseng KF, Lai HY, Lin CH, Lee OK (2011) Matrix stiffness regulation of integrin-mediated mechanotransduction during osteogenic differentiation of human mesenchymal stem cells. J Bone Miner Res 26:730–738
Shin H, Zygourakis K, Farach-Carson MC, Yaszemski MJ, Mikos AG (2004) Attachment, proliferation, and migration of marrow stromal osteoblasts cultured on biomimetic hydrogels modified with an osteopontin-derived peptide. Biomaterials 25:895–906
Shin SH, Yoo JJ, Kim HN, Nam J, Kim HJ (2012) Enhanced cellular responses of human bone marrow stromal cells cultured on pretreated surface with allogenic platelet-rich plasma. Connect Tissue Res 53:318–326
Shor L, Güçeri S, Wen X, Gandhi M, Sun W (2007) Fabrication of three-dimensional polycaprolactone/hydroxyapatite tissue scaffolds and osteoblast-scaffold interactions in vitro. Biomaterials 28:5291–5297
Sikavitsas VI, Temenoff JS, Mikos AG (2001) Biomaterials and bone mechanotransduction. Biomaterials 22:2581–2593
Song G, Habibovic P, Bao C, Hu J, van Blitterswijk CA, Yuan H, Chen W, Xu HHK (2013) The homing of bone marrow MSCs to non-osseous sites for ectopic bone formation induced by osteoinductive calcium phosphate. Biomaterials 34:2167–2176
Stanić V, Dimitrijević S, Antić-Stanković J, Mitrić M, Jokić B, Plećaš IB, Raičević S (2010) Synthesis, characterization and antimicrobial activity of copper and zinc-doped hydroxyapatite nanopowders. Appl Surf Sci 256:6083–6089
Sun Y, Jiang Y, Liu Q, Gao T, Feng JQ, Dechow P, D’Souza RN, Qin C, Liu X (2013) Biomimetic engineering of nanofibrous gelatin scaffolds with noncollagenous proteins for enhanced bone regeneration. Tissue Eng Part A 19:1754–1763
Takada Y, Ye X, Simon S (2007) The integrins. Genome Biol 8:1–9
Takeuchi Y, Suzawa M, Kikuchi T, Nishida E, Fujita T, Matsumoto T (1997) Differentiation and transforming growth factor-beta receptor down-regulation by collagen-alpha 2 beta 1 integrin interaction is mediated by focal adhesion kinase and its downstream signals in murine osteoblastic cells. J Biol Chem 272:29309–29316
Thein-Han WW, Misra RDK (2009) Biomimetic chitosan–nanohydroxyapatite composite scaffolds for bone tissue engineering. Acta Biomater 5:1182–1197
Thompson WR, Rubin CT, Rubin J (2012) Mechanical regulation of signaling pathways in bone. Gene 503:179–193
Tian T, Jiang D, Zhang J, Lin Q (2008) Fabrication of bioactive composite by developing PLLA onto the framework of sintered HA scaffold. Mater Sci Eng C 28:51–56
Tran N, Hall D, Webster TJ (2012) Mechanisms of enhanced osteoblast gene expression in the presence of hydroxyapatite coated iron oxide magnetic nanoparticles. Nanotechnology 23:455104
Tsai KS, Kao SY, Wang CY, Wang YJ, Wang JP, Hung SC (2010) Type I collagen promotes proliferation and osteogenesis of human mesenchymal stem cells via activation of ERK and Akt pathways. J Biomed Mater Res A 94:673–682
Tuzlakoglu K, Bolgen N, Salgado AJ, Gomes ME, Piskin E, Reis RL (2005) Nano- and micro-fiber combined scaffolds: a new architecture for bone tissue engineering. J Mater Sci Mater Med 16:1099–1104
Ueda H, Hong L, Yamamoto M, Shigeno K, Inoue M, Toba T, Yoshitani M, Nakamura T, Tabata Y, Shimizu Y (2002) Use of collagen sponge incorporating transforming growth factor-β1 to promote bone repair in skull defects in rabbits. Biomaterials 23:1003–1010
Ulici V, Hoenselaar KD, Agoston H, McErlain DD, Umoh J, Chakrabarti S, Holdsworth DW, Beier F (2009) The role of Akt1 in terminal stages of endochondral bone formation: angiogenesis and ossification. Bone 45:1133–1145
Vasita R, Katti DS (2012) Structural and functional characterization of proteins adsorbed on hydrophilized polylactide-co-glycolide microfibers. Int J Nanomed 7:61–71
Vinals F, Lopez-Rovira T, Rosa JL, Ventura F (2002) Inhibition of PI3K/p70 S6K and p38 MAPK cascades increases osteoblastic differentiation induced by BMP-2. FEBS Lett 510:99–104
Vitale-Brovarone C, Baino F, Verné E (2009) High strength bioactive glass-ceramic scaffolds for bone regeneration. J Mater Sci Mater Med 20:643–653
von Doernberg MC, von Rechenberg B, Bohner M, Grünenfelder S, van Lenthe GH, Müller R, Gasser B, Mathys R, Baroud G, Auer J (2006) In vivo behavior of calcium phosphate scaffolds with four different pore sizes. Biomaterials 27:5186–5198
Wang YK, Yu X, Cohen DM, Wozniak MA, Yang MT, Gao L, Eyckmans J, Chen CS (2012) Bone morphogenetic protein-2-induced signaling and osteogenesis is regulated by cell shape, RhoA/ROCK, and cytoskeletal tension. Stem Cells Dev 21:1176–1186
Wang M, Cheng X, Zhu W, Holmes B, Keidar M, Zhang LG (2013) Design of biomimetic and bioactive cold plasma modified nanostructured scaffolds for enhanced osteogenic differentiation of bone marrow derived mesenchymal stem cells. Tissue Eng Part A 20(5–6):1060–1071
Wei J, Igarashi T, Okumori N, Maetani T, Liu B, Yoshinari M (2009) Influence of surface wettability on competitive protein adsorption and initial attachment of osteoblasts. Biomed Mater 4:045002
Wilson CJ, Clegg RE, Leavesley DI, Pearcy MJ (2005) Mediation of biomaterial-cell interactions by adsorbed proteins: a review. Tissue Eng 11:1–18
Wojtowicz AM, Shekaran A, Oest ME, Dupont KM, Templeman KL, Hutmacher DW, Guldberg RE, Garcia AJ (2010) Coating of biomaterial scaffolds with the collagen-mimetic peptide GFOGER for bone defect repair. Biomaterials 31:2574–2582
Woo KM, Chen VJ, Ma PX (2003) Nano-fibrous scaffolding architecture selectively enhances protein adsorption contributing to cell attachment. J Biomed Mater Res Part A 67A:531–537
Woo KM, Jun J-H, Chen VJ, Seo J, Baek J-H, Ryoo H-M, Kim G-S, Somerman MJ, Ma PX (2007a) Nano-fibrous scaffolding promotes osteoblast differentiation and biomineralization. Biomaterials 28:335–343
Woo KM, Seo J, Zhang RY, Ma PX (2007b) Suppression of apoptosis by enhanced protein adsorption on polymer/hydroxyapatite composite scaffolds. Biomaterials 28:2622–2630
Woodard JR, Hilldore AJ, Lan SK, Park CJ, Morgan AW, Eurell JAC, Clark SG, Wheeler MB, Jamison RD, Wagoner Johnson AJ (2007) The mechanical properties and osteoconductivity of hydroxyapatite bone scaffolds with multi-scale porosity. Biomaterials 28:45–54
Wozniak MA, Modzelewska K, Kwong L, Keely PJ (2004) Focal adhesion regulation of cell behavior. Biochimica Et Biophysica Acta-Molecular Cell Research 1692:103–119
Wu C, Zhang Y, Zhu Y, Friis T, Xiao Y (2010) Structure-property relationships of silk-modified mesoporous bioglass scaffolds. Biomaterials 31:3429–3438
Xia Z, Yu X, Jiang X, Brody HD, Rowe DW, Wei M (2013) Fabrication and characterization of biomimetic collagen–apatite scaffolds with tunable structures for bone tissue engineering. Acta Biomater 9:7308–7319
Xiao G, Wang D, Benson MD, Karsenty G, Franceschi RT (1998) Role of the alpha2-integrin in osteoblast-specific gene expression and activation of the Osf2 transcription factor. J Biol Chem 273:32988–32994
Xu BY, Song GB, Ju Y, Li X, Song YH, Watanabe S (2012) RhoA/ROCK, cytoskeletal dynamics, and focal adhesion kinase are required for mechanical stretch-induced tenogenic differentiation of human mesenchymal stem cells. J Cell Physiol 227:2722–2729
Yang F, Dong W-J, He F-M, Wang X-X, Zhao S-F, Yang G-L (2012) Osteoblast response to porous titanium surfaces coated with zinc-substituted hydroxyapatite. Oral Surg Oral Med Oral Pathol Oral Radiol 113:313–318
Yaszemski MJ, Payne RG, Hayes WC, Langer R, Mikos AG (1996) Evolution of bone transplantation: molecular, cellular and tissue strategies to engineer human bone. Biomaterials 17:175–185
Yuan H, Fernandes H, Habibovic P, de Boer J, Barradas AMC, de Ruiter A, Walsh WR, van Blitterswijk CA, de Bruijn JD (2010) Osteoinductive ceramics as a synthetic alternative to autologous bone grafting. Proc Natl Acad Sci 107:13614–13619
Zeng WF, Yan Y, Zhang FY, Zhang CL, Liang W (2013) Chrysin promotes osteogenic differentiation via ERK/MAPK activation. Protein Cell 4:539–547
Zhang L, Webster TJ (2009) Nanotechnology and nanomaterials: promises for improved tissue regeneration. Nano Today 4:66–80
Zhang H, Lin C-Y, Hollister SJ (2009) The interaction between bone marrow stromal cells and RGD-modified three-dimensional porous polycaprolactone scaffolds. Biomaterials 30:4063–4069
Zhao G, Schwartz Z, Wieland M, Rupp F, Geis-Gerstorfer J, Cochran DL, Boyan BD (2005) High surface energy enhances cell response to titanium substrate microstructure. J Biomed Mater Res, Part A 74A:49–58
Zhao J, Guo LY, Yang XB, Weng J (2008) Preparation of bioactive porous HA/PCL composite scaffolds. Appl Surf Sci 255:2942–2946
Zhao J, Lu X, Duan K, Guo LY, Zhou SB, Weng J (2009) Improving mechanical and biological properties of macroporous HA scaffolds through composite coatings. Colloids Surf B 74:159–166
Zimmerman D, Jin F, Leboy P, Hardy S, Damsky C (2000) Impaired bone formation in transgenic mice resulting from altered integrin function in osteoblasts. Dev Biol 220:2–15
Acknowledgment
The authors acknowledge the funding support from the Australia National Health and Medical Research Council (NHMRC).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Lu, Z., Li, J.J., Zreiqat, H. (2015). Bone-Biomimetic Biomaterial and Cell Fate Determination. In: Zreiqat, H., Dunstan, C., Rosen, V. (eds) A Tissue Regeneration Approach to Bone and Cartilage Repair. Mechanical Engineering Series. Springer, Cham. https://doi.org/10.1007/978-3-319-13266-2_8
Download citation
DOI: https://doi.org/10.1007/978-3-319-13266-2_8
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-13265-5
Online ISBN: 978-3-319-13266-2
eBook Packages: EngineeringEngineering (R0)