Abstract
Efficient dermal wound care implies providing a healing environment at the site of injury. Current repair techniques, including polymeric dressings, are able to accelerate only the healing of epidermal and partial thickness acute wounds based on maintaining the area moist. However, these are not efficient in treatment of full-thickness and chronic wounds, which lack in inborn regenerative elements and are highly prone to infections. For this reason the research interest is nowadays shifted towards functional biomaterials to tackle severe skin deteriorations by providing a beneficiary for healing pro-active and pathogen-free environment. Recent advances in molecular biology and materials science together with better understanding of wound pathophysiology allowed for designing of new wound care approaches that rely on biochemical stimuli to promote wound closure. Biopolymers that couple intrinsic antimicrobial and wound repair properties with hydrophilicity appear as suitable dressing platforms. These can be further upgraded using various bio-entities (therapeutic molecules, cells) with the ability to address specific targets in the biochemical environment of wounds in order to stimulate the healing process. This chapter summarises the abundant experimental and clinical data on polymers in advanced wound dressings, scaffolds for dermal regeneration and platforms for drug delivery.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Wounds: Formation and Repair
Skin Integrity and Its Function
Skin, the largest organ in the human body composed of epidermis, dermis and hypodermis, has several important functions, including regulation of water, electrolyte balance and thermoregulation. The outer epidermis forms a protective barrier to keep the water in the body and prevent pathogens from entering in addition to helping the skin to regulate body temperature. This layer is a stratified scaly epithelium composed of proliferating basal and differentiated suprabasal keratinocytes. The stratum corneum is the outermost part of epidermis with specific barrier properties. It consists of non-viable cells and is very firm but pliable and wrinkled. The underlying dermis is composed of a very dense fibre network—extracellular matrix (ECM), dominating the mechanical behaviour of the total skin, and therefore providing structural support to the organ. This gel-like matrix, secreted locally by the cells that it surrounds, is composed of a variety of polysaccharides and proteins: collagen fibrils, microfibrils, and elastic fibres, embedded in proteoglycans. An intricate network of these macromolecules, assembled in close association with the surface of the cell that produced them, provides the unique structure of the ECM, exhibiting high tensile strength combined with the substantial elasticity and compressibility. The deepest skin layer, the hypodermis or subcutaneous adipose tissue, is composed of loose fatty connective tissue. All skin layers contain blood vessels, lymph vessels, nerve endings, sweat glands and hair follicles.
Acute Wounds and Healing Process
According to the Wound Healing Society (www.woundheal.org), a wound is the result of disruption of normal anatomic structure and function of a tissue. A dermal wound is defined as a break in the integrity of the skin, raised due to external or internal noxious stimulus, which leads to an inadequate performance of the skin functions. It is therefore vital to restore the skin integrity and consequently functions as soon as possible. A completely healed wound implies that the affected part of the skin has returned to its normal anatomical structure and function, and similar appearance within a reasonable period of time [1]. The primary causes of acute wounds include mechanical injuries due to external factors such as abrasion and tear, after a frictional contact between the skin and hard surfaces, including penetrating wounds caused by knives and firearm shots, surgical incisions, etc. Burns and chemical injuries arise from radiation, electricity, corrosive chemicals and thermal sources, among others. Another classification of wounds is based on the number of skin layers and area of skin affected. Based on this it is possible to distinguish between an epidermal (superficial) wound and an injury involving both the epidermis and dermis that in addition affects the blood vessels and is referred to as a partial thickness wound. Full thickness skin wounds occur when the underlying subcutaneous fat or deeper tissues are damaged in addition to the epidermis and dermal layers. Epidermal and partial thickness wounds can regenerate due to the self-healing capacity of the body for re-epithelisation. Conversely, in the full-thickness wounds regenerative elements that reside in dermis are completely destroyed and the epithelisation is possible only on the wound edges.
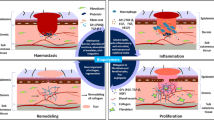
Normal wound healing involves a complex series of orchestrated events leading to the repair of injured tissues. These dynamic events occur as a sequential cascade of processes requiring the coordinated completion of a variety of cellular activities correlating with the appearance of different cell types in the wound during various stages of the healing process [2]. Acute wounds heal in a very orderly and efficient manner characterised by four overlapping phases: haemostasis, inflammation, cell migration/proliferation, and maturation (remodelling) phase. After injury the bleeding flush out bacteria and antigens from the injury site, also causing aggregation of the platelets now accumulated at the site that form a haemostatic plug and prevent an excessive loss of blood. In addition, platelets release growth factors and adhesive proteins to activate cells in the surrounding area. During the inflammatory phase neutrophils arrive at the site of the injury, liberating phagocytes and a variety of enzymes and reactive oxygen species (ROS), crucial for wound cleaning and the prevention of infections [3]. Phagocytes engulf dead cells (necrotic tissue) previously liquefied by a simultaneous enzymatic (protease) action to a yellowish slough with bad odour. The inflammatory phase occurs almost simultaneously with haemostasis and lasts for about 3 days in normal healing process. However, if the action of the inflammatory factors is prolonged or they are found in counts higher than normal, the process is harmful for healthy cells—the common situation found in chronic wounds [4]. The migration phase involves the movement of keratinocytes and fibroblasts to the injured area to replace damaged and lost tissue. These cells regenerate from the margins, rapidly growing over the wound to cause epithelial thickening. Accompanied proliferative phase is characterised by the in-growth of capillaries and lymphatic vessels into the wound and collagen synthesis by fibroblasts giving the skin strength and form. The newly formed granulation tissue and ECM consist mainly of glycosaminoglycans (GAGs), proteoglycans and collagen. During the angiogenesis (formation of new blood vessels) the fibroblasts transform into myofibroblasts that bring the margins of the wound together in order to reduce its size. The final phase of wound healing is the transformation of granulation tissue into a scar, characterised by a decreased inflammation. Myofibroblasts further undergo apoptosis and a new collagenous matrix replaces the provisional one [4]. The described cascade occurs in normal wound healing, with a reestablishment of the equilibrium between scar formation and scar remodelling, the longest part of the healing (up to two years) marking the end of the wound healing process.
Chronic Wounds and Impaired Healing
In contrast to acute wounds, abnormal response to an injury leads to formation of non-healing (chronic) wounds. Chronic wounds can affect many anatomical regions as a result of different underlying pathologies and accordingly are classified as venous leg ulcers, arterial ulcers, neuropathic ulcers, pressure ulcers (decubitus), vasculitis, etc. Except for serious burns that also cannot heal, all the conditions leading to chronic ulceration are more prevalent in the individuals with low mobility and elderly population. In addition, the increased occurrence and longevity of these ulcers are further compounded by the detrimental effects ageing has on the skin.
A chronic wound arises after disruption of the acute wound healing process at one or more of its phases, impairing the reestablishment of anatomical and functional integrity of the affected tissue in a physiologically appropriate length of time. Various factors can cause the failure of the normal healing process and trigger chronic inflammatory responses: foreign bodies introduced deep into the wound at the time of injury, inadequate management of already infected wounds, poor nutritional status of a patient, aged skin with reduced ability to fight infections, underlying diseases such as diabetes and anaemia that compromise the circulation resulting in poor delivery of blood cells and oxygen to the wound, etc. The disruption of the acute healing process is usually expressed in its inflammatory phase. The caused prolonged inflammation leads to an excessive matrix deposition and loss of tissue function [5].
Despite the different underlying aetiology, most chronic wounds show a similar behaviour and progress. This is because these ulcers share the specific biological markers which significantly differentiate from the environment of acute wounds. In acute wounds there is a balance between production and degradation of molecules (e.g. collagen); in chronic wounds this balance is disturbed in favour of degradation. Chronic ulcer fluids contain elevated level of neutrophils and neutrophil-derived enzymes compared with acute wound fluids due to their continuous influx during the prolonged inflammation [5]. Neutrophils undergo apoptosis at the site of injury and release oxidative enzyme myeloperoxidase (MPO). Under physiological conditions MPO catalyses the generation of hypochlorous acid (HClO), the most powerful ROS in human body, creating oxidative stress and impairing the healing process. The cytotoxicity of this reaction allows the killing of bacteria in the first line of defence. However, the HClO reacts with most biological molecules, including natural protease inhibitors. In healing wounds the production and activity of proteolytic enzymes, such as matrix metalloproteinases (MMPs) and serine proteases (e.g. elastase), are tightly regulated by counteraction of their natural inhibitors [6]. During granulation tissue formation levels of MMPs have been shown to be decreased compared to levels found in chronic wounds. In normal levels, these proteases play a role in cellular migration. In chronic wounds the generation of HClO induces disturbed ratio proteases/inhibitors [7], so that most of the enzymes are uninhibited [8]. In increased levels these proteases have detrimental effect on wound healing by uncontrolled digestion of the ECM and the growth factors [6].
Wound Management
Effective wound management depends on understanding a number of different factors such as the underlying mechanism of wound formation, the type of wound, the healing process and general condition of a patient in terms of health (e.g. diabetes, aged patient). First, in order to inspect a wound correctly for the proper treatment choice, it is indispensable to remove the necrotic tissue and foreign material from the areas around the wound. The removal of the dead tissue is known as wound debridement and could be carried out using several methods. Surgical removal with scalpel and/or scissors is the most effective and precise, however, can only be undertaken by highly skilled and trained practitioners. Wound irrigation, on the other hand, implies rehydration of necrotic tissue using e.g. hydrogel dressings and further its autolytic digestion by the enzymes accumulated in the wound site [9]. Here the outcome depends on many individual factors in the treated patient, and thus, little control over the process is granted. The more efficient alternative is the enzymatic removal of the necrotic tissue after liquefying using bacterial or animal derived collagenases. Despite being more controllable than the autolytic digestion, the biggest disadvantage of all enzymatic methods still remains much longer duration compared to the surgical debridement. Besides to assure establishing of the proper diagnosis of the wound, another objective of debridement is to provide a favourable environment at the surface of the wound in which healing could take place in combination with proper treatment strategy [10].
Depending on the wound type, the cause and its position on the body, nowadays the management involves one or the combination of the several available treatment options: topical application of antibiotics and/or antiseptics, multiple dressing changes per day and, recently, costly treatments involving the application of local mechanical stresses or energy to a wound—negative pressure wound therapy (NPWT) and mist ultrasound therapy.
Epicutaneous application of antimicrobial agents is, thus far, the most effective way to treat wound infections. However, such therapy is directed only to the ulcers with severe microorganism contamination. Moreover, the biggest concern is the overdoses due to the accumulation of immunoreactive compounds at the wound site [11].
NPWT consists of the controlled application of sub-atmospheric pressure to the local wound environment, using a sealed wound dressing connected to a vacuum pump. Studies suggested that NPWT assists in wound healing by providing a moist environment, stimulating circulation to the wound bed, decreasing bacterial colonisation and increasing the rate of granulation tissue formation [12]. However, the negative side of NPWT is a cost of the treatment and lack of an overall plan of care for the patients suffering different kind of ulcers. Although a consistent evidence of the benefit of NPWT in the treatment of diabetes-associated chronic leg ulcers together with safety of the treatment was demonstrated [13], its effectiveness compared to conventional/advances dressing treatments is still disputable [14, 15]. Moreover, the evaluation on “mixed wounds” was of poor quality and therefore requires better quality research to be conducted.
Another novel technology using non-contact, kilohertz-range ultrasound therapy is gaining popularity, especially in chronic wound management. Cells at the site of injury absorb the energy from the ultrasound wave and initiate signalling pathways with direct implications for the healing process. The therapy uses a low frequency, low intensity ultrasound to provide enough energy to stimulate healthy cells, but insufficient to damage them. The vibration of micron-sized bubbles, formed during sonication [16], within inter- and intracellular fluids causes changes in the cellular membrane potentials and cellular activities within the tissues. Ultrasound therapy has been shown to increase the healing rate in recalcitrant diabetic foot ulcers and other lower extremity wounds [17] however, it is rather cost-ineffective compared to standard wound care.
Compared to the above described physical methods for wound management the usage of modern polymer-based dressing materials is cheaper and more effective. Both synthetic polymers and biopolymers, e.g. chitosan, are easily processed into desired shape and design, and stabilised using different techniques for extended shelf-life [18]. Many of these macromolecules display beneficial features for the treatment of wounds, however, the variability in wound pathophysiology makes difficult to develop a dressing material that meets all the criteria for optimum healing. Much research is currently being undertaken to design wound dressings that can stimulate the healing taking into account all (or at least majority) of the factors impairing healing.
Polymer Dressings in Wound Treatment
Factors Influencing Dressing Choice
Many sophisticated dressings are nowadays available to the wound care practitioner, these made of a wide range of polymeric materials. Polymers may be used alone or in combinations thereof, being processed in different dressing designs such as films, foams, fibrous materials, beads, hydrogels, hydrocolloids or even pharmaceutical sprays comprising nano/micro-particulate systems. Depending on their composition and design, the polymeric dressings and formulations may be used to absorb exudate, provide and maintain a beneficial for wound healing moist environment, combat odour or infection, relieve pain, or promote debridement at the wound surface. Some dressings simply absorb exudate or wound fluid and may therefore be suitable for application to a variety of different wound types. Others have a more specific function and as such have a limited application being only suitable for the treatment of particular types of wounds or applied during one phase of the healing process. Due to the variations between pathophysiology of different wounds it is difficult to develop a dressing that meets all the criteria for optimum healing. Wound healing is a dynamic process and the performance requirements of a dressing can change as the wound progresses towards healing. For this reason, in most clinical cases a combination of individual component dressings that feature different functional properties is necessary in order to achieve effective wound healing in a reasonable time.
Nowadays, the design of a dressing is dominated by the hydrocolloids, useful for clean, granulating, epidermal wounds with low to medium exudate, but inefficient on infected and exuding wounds. Currently, polyurethane foams are promoted as an alternative to hydrocolloids or even used with them in combination carriers, without significant improvement in the healing rate of difficult to treat chronic ulcers. Nevertheless, the design of a dressing depends on the type of the wound, its thickness, position on the body, exudate volume, the stage of the healing process (early, late) and age of the affected individual. The modern dressings are most frequently found in the design of thin films/coatings, (hydro)gels and foam sheets (i.e. sponges) [10].
Thin transparent film dressings have low absorption capacity, thus are not useful for wounds with significant exudates. Films are mostly used for the later stage healing, when the wound exudates are minimised, or to simply cover the damaged area and protect the wound from external factors. Available in thickness ranging from μm to mm, these dressings are prepared by different methods (usually casting followed by evaporation) using one or more polymers. Sometimes films are used as top layers to keep other dressings in place, e.g. over ointment or hydrogel dressing. If applied alone, transparent films allow for on-site inspection of the healing progress without removal of the dressing. Thus, in advanced treatment strategies films/coatings bearing active functions could be an ideal solution for the combined wound treatment and monitoring of the wound status.
Hydrogels are highly absorbent water-insoluble networks of polymer chains with high degree of flexibility similar to that of the natural tissue. The hydrogels are normally classified according to the nature of network formation as physical and chemical hydrogels. The physical hydrogels are obtained by reversible electrostatic interactions (e.g. polyelectrolyte complexes) or through secondary interactions (e.g. hydrogen bonds), whereas the chemical hydrogels are covalently cross-linked. Hydrogels are useful for exudative wounds because of their high absorptive capacity, in addition to maintaining the moisture at the wound site and permitting the oxygen penetration. In addition, they provide excellent pain relief by reducing potential irritation and cooling the wound. Nevertheless, during application hydrogels should be covered by an outer layer of tape, netting or roll bandage. They were found particularly useful for the treatment of deep partial thickness wounds.
Hydrophilic foam dressings are permeable to oxygen and water vapour. They usually have a hydrophobic backing and some have an adhesive surface to make the application easier. Sponges are more easily saturated with wound exudates than hydrogels, hence their changing frequency is considerably higher, especially during early wound healing characterised with large amounts of exudates. Foam dressings are ideally suited for dry and semi-dry superficial and partial thickness wounds, in addition to chronic ulcers since they provide padding that can relieve pressure over bony prominences. From a technological point of view, the manufacturing of a foam dressing is commercially attractive consisting of an easy process of freeze-drying of a moulded suspension of the dressing components.
Synthetic Polymers as Dressing Materials
Besides the classification on their function and design, wound dressings can be categorised according to the type of material employed for their manufacturing: synthetic or naturally occurring polymers [19]. Synthetic polymers are mainly used as platforms for actuation and delivery of active agents, but also provide an optimal microenvironment for cell proliferation, migration and differentiation when used in biosynthetic skin grafts. Synthetic polymers used for permanent coverage (skin equivalents) should be fully biocompatible and with good mechanical properties. Biodegradability of synthetic polymers is the desired property when localised delivery of active compounds from temporary dressings/templates is required. Besides functionalisation with both organic and inorganic active agents, synthetic polymers are often used in a combination with biopolymers and further processed in different dressing designs [20]. Synthetic polymers show a superior mechanical strength compared to natural macromolecules, thus by their cross-linking or blending the mechanical properties of the latter are improved, whereas a bioactive component is added to the inert synthetic platform. Features of some of the most used synthetic polymers in wound healing are summarised below.
Polyurethanes are copolymers with repetitive urethane groups in their structures. This class of synthetic polymers have gained acceptance in the biomedical field due to their exceptional strength and biocompatibility. The physical properties of polyurethanes vary from brittle to very elastic. The biomedically acceptable polyurethanes are non-toxic and have elastomeric properties accompanied by good toughness, tear resistance and abrasion resistance. Such materials favour reepithelialisation during wound healing [21]. Polyurethane foams are designed to absorb large amounts of exudates and maintain a moist wound environment and as such are not used on low exudating ulcers as this would cause dryness of the wound site. Examples of polyurethane foams on the market are Smith&Nephew’s Allevyn® and Mölnlycke’s Lyofoam®.
Due to its low toxicity, low allergic properties and high biocompatibility with body tissues and blood, silicon is extensively used for preparation of biomedical materials [22]. Being also resistant to biodegradation, silicone is used in the preparation of permanent implant elastomers for soft tissue repair and in combination with biopolymers as support materials for wound treatment and diagnosis [23, 24]. Silicones aid to the wound treatment modalities as an occlusive contact medium, being generally accepted as the only materials able to manage hypertrophic (burn related) scarring without significant side effects [25]. In addition, silicon-based dressings on the market, e.g. Safetac®, are generally regarded as easily changeable and painless upon replacement.
Teflon, a polymer synthesised by polymerisation of tetrafluoroethylene at high temperature and pressure, is an inert non-carcinogenic material. It is easily processed into desired shapes and facile to apply to the injured area [26]. Teflon is frequently used in a combination with another polymeric dressing to lower the adherence of the latter at the wound site, aiding to painless bandage removal.
Biopolymers as Dressing Materials
Modern industries necessarily exploit renewable resources, promoting environmentally friendly and beneficial technologies. Constant improvement in quality of products and competitive technologies for their generation is a must in order to keep competitive market positions. Biopolymers, due to their intrinsically beneficial properties for a broad spectrum of applications, are gaining importance as raw materials in many industrial sectors. They comply with the requirements for the medical/pharmaceutical materials, being biodegradable, biocompatible and in most cases not causing immune response in organism, in addition to integration with a particular cell type/tissue upon application.
The biomaterials used in skin repair should display intrinsic biocompatibility and biodegradability at the ideal rate corresponding to the rate of new tissue formation [27]. Besides these biomaterials, their degradation products should also not be toxic, immunogenic and carcinogenic. Polysaccharides and proteins, being natural components of all living structures and displaying the above properties, are up to date the most suitable candidates for fabrication of wound dressing materials.
Chitin and its deacetylated derivative chitosan are suitable bioplatforms that can be further improved by targeted functionalisation for skin repair. For example, the unique biological properties of chitosan characterised with human cell biocompatibility, human serum biodegradability, non-toxicity, antibacterial and haemostatic properties justify the use of this biopolymer in skin repair processes. The haemostatic activity of chitosan is exploited in early treatment of wounds [28], especially in large injuries subjected to heavy bleeding [29]. Many haemostatic products on the market consist thus, fully or partially, of chitosan. Moreover, chitosan aids to a rapid closure of full-thickness wounds due to its supportive effect to the fast growing of new blood vessels (angiogenesis) in the injured tissue [30]. Chitin and chitosan can be prepared in a variety of forms targeting particular application, i.e. healing of a certain wound type or on different position on the body. Films, hydrogels, fibres, powders and micro-/nanoparticles, entirely or partially composed of chitosan, have been demonstrated and continue to be evaluated for their benefits in wound healing [18].
Glycosaminoglycans are important components of connective tissues where their chains are covalently linked to a protein to form proteoglycans. As highly hydrophilic structures GAGs possess especially high affinity for physiological fluids to promote swelling [31]. Chondroitin sulphate is an important structural component of cartilage and materials made from this GAG are biocompatible and non-immunogenic. Chondroitin sulphate acts as surrogate extracellular matrix, serving as a repository for cytokines and growth factors, important bioentities for the healing process [32], and providing structural frameworks for fibroblasts during the epithelial regeneration. Hyaluronic acid (HA) is an intracellular component of connective tissues such as the synovial fluid of joints and an important part of ECM. The most important property of HA is its effect on angiogenesis: whereas high molecular weight HA inhibits angiogenesis, HA oligosaccharide units are proangiogenic [33, 34]. HA readily interacts with proteins, growth factors and tissue components with a vital importance in acceleration of dermal tissue repair [35]. Regarding the HA-based biomaterials, cross-linked hydrogel films have been produced for the use as polymeric drug delivery platforms with improved exploitation characteristics [36]. Furthermore, the biological properties of HA have been also combined with other biomaterials for the production of tissue engineering scaffolds or membranes [37, 38].
Alginic acid, a natural polysaccharide derived from brown algae, is well-known absorbent which dressings regardless of design easily conform to the shape of a wound and adapt to a wound bed. In addition, alginic acid and its salts are haemostatic agents; hence their application in the treatment of large injuries and burns. They are applied in the form of a gel and sponges produced from calcium alginate. An example of a calcium alginate on the market is Suprasorb A from Lohmann&Rauscher, a dressing that creates a clean wound environment for heavily exuding wounds due to its high absorbency. This dressing absorbs large exudate volumes and rapidly forms a gel through the exchange of calcium ions from the wound dressing with the sodium ions in the wound exudate. The gel binds to the exudate, trapping bacteria and tissue debris. When the dressing is changed, the exudate, bacteria and tissue debris are removed with the alginate fibres. Some studies also indicated that calcium alginate increases cellular activity properties during the healing process of diabetic foot ulcers [39].
Among proteins, collagen and derived hydrolysates (i.e. gelatines) showed vast potential for efficient wound treatment as dressings and drug delivery systems. Collagen is a major constituent of connective tissue and a principal structural protein of many organs. In living beings collagen is produced by fibroblasts and stimulates the wound healing cellular and molecular cascade, development of new tissue and wound debridement. Materials made from collagen thus provide both structural and biological support for the various cells involved in dermal tissue regeneration, and as cell scaffolds are expected to replace native collagen-based ECM [40]. Collagen-based pharmaceutical formulations related to skin conditions include suspensions for dermal and wound topical injection, collagen suture and catguts, sponges for coating of affected joints in full-thickness ulcers, and wound dressing materials in various designs such as sponges, resorbable membranes, films, hydrogels, nano- and micro-fibre scaffolds for cell support [41]. Furthermore, both collagen and gelatine matrices can be medicated, thus serving as reservoirs for drug delivery. Their use for delivery of different antibiotics has been discussed extensively [42]. These biopolymers are also frequently conjugated with other biomaterials and even with animal skin substitutes. For example, Promogran®—a blend of collagen and regenerated cellulose, is the only clinically proved advanced dressing that promotes chronic wound healing. Promogran® inhibits MMPs by: (i) sequestering these cationic enzymes in the negatively charged regenerated cellulose dressing component, and (ii) deviating the enzymes from the ECM hydrolysis using the collagen component as a decoy MMP substrate [43, 44].
Other proteins are recently being evaluated for the treatment of many types of wounds. Fibrin, keratin, silk fibroin (SF), bovine serum albumin (BSA), and combinations thereof and with other biomaterials, interact with the wound environment to promote/accelerate healing rates. These biomaterials are processed in many ways to achieve natural-based wound dressings, templates for delivery of bioactive agents and scaffolds to support cellular growth. Fibrin-like haemostatic materials are useful for cleaning and treatment of full-thickness skin lesions [45], keratin-based matrices showed potential as chronic wound dressings because of their interaction with the proteolytic wound environment to facilitate the healing process [46], SF-containing films and sponges are being extensively investigated in animal models for both superficial and full-thickness skin defects healing [47–49], whereas electrospun nanofibres made entirely from a globular BSA are intended for suturing and acceleration of wound closure wound [50].
Although beneficiary for healing of many types of wounds, biopolymers are efficient in the tissue repair processes only to a certain extent, being limited in interaction at molecular level with wound pathogens. These natural macromolecules attracted much attention as dressing materials due to their biodegradability, where material degradation and the new tissue formation should be parallel processes, normally the case in the treatment of acute wounds. The situation with chronic wounds is more complicated due to very low stability of these materials in contact with the fluids containing elevated levels of hydrolytic enzymes: e.g. lysozyme cleaves chitosan-based materials [51], whereas collagen is a natural substrate of several MMPs. Moreover, the use of biopolymers in wound management has not yet been clearly translated into a platform for widespread clinical use, as the existing studies only offer evidences for beneficial properties of the biopolymers as dressing platforms and not as active agents.
Role of Polymers in Advanced Wound Treatment
Over recent years, many new polymeric dressings, but few new dressing types appeared on the market. The healing concept of most dressings relies on maintaining of a moisture environment, without any active agent involved. Regardless of the polymer component and the bandage design, a major focus of advanced wound care in recent years has been the development of new dressings able to accelerate wound healing by erasing the chronicity factors, combining as many as possible functional properties in one [10]. Managing chronic or non-healing ulcers especially requires a systematic multi-professional approach and a willingness to consider also the patient’s perspective to promote the most favourable conditions for healing. Despite all polymer-based materials promote wound repair to a certain extent, these materials exploit only the intrinsic properties of the matrix itself without any active agents to interact with the chronicity factors at the molecular level. The multifactorial nature of virtually all non-healing wounds requires biochemical stimuli to halter the events governing the ECM breakdown and impaired healing. Such effect can be expected only in case of controlled application of bioactive molecules, e.g. antimicrobials, enzyme inhibitors and growth factors.
From the point of view of a researcher, new bioactive wound dressings and formulations have been an area of tremendous growth following our understanding on the details of the wound repair. An orderly, predictable sequence of wound regeneration is driven by numerous cellular mediators, i.e. growth factors. The advanced wound products thus aim to accelerate repair by promoting/augmenting the activities of these mediators in non-healing conditions (Scheme 14.1). Although in many cases the role of polymers in such products is merely to provide a structural support, they are also crucial to maintain moisture environment, critical in the wound bed to promote growth factors, cytokines and migration of cells [52].
Accelerating Wound Healing with Active Agents—New Therapeutic Trends to be Combined with Polymers
Growth Factors and Cytokines
Growth factors are naturally occurring substances, secreted proteins and steroid hormones, capable of modulating cellular processes during tissue regeneration. They stimulate migration, infiltration, proliferation, and differentiation of mainly fibroblasts and keratinocytes by a complex signalling network. Accordingly, the capacity for wound repair can be augmented through the well-guided treatment involving these factors [53, 54]. The most promising growth factors that require clinical testing are vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF). The most known platelet-derived growth factor BB (PDGF-BB) is the only one successfully completed randomized double-blind clinical trials in conjunction with standard wound care [55], which led to FDA approval for treatment of diabetic ulcers in a gel form under the trade name Regranex®. Nevertheless, new trends are moving towards mimicking the natural ways of healing in order to bring to the injury site a set of biological supplements to accelerate the functional recovery of the tissue, instead of relying on single-agent therapies. For example, activated autologous platelet rich plasma (PRP), known as plasma rich in growth factors (PRGF-Endoret®), an autologous blood platelet concentrate product recently classified as a medication in Spain, which, when activated, releases a set of growth factors that stimulate the regenerative phase of wound healing [56].
Cytokines are small proteins that modulate the immune response to stimulate tissue remodelling. Cell signaling culminates in an inflammatory phase of healing, which large part is regulated by both pro-inflammatory and anti-inflammatory cytokines. Interleukin-1, which in vitro stimulates most of the human cells, was tested in pressure ulcer patients with inconclusive results [57]. Similarly, granulocyte macrophage colony stimulating factor (GM-CSF) has been also showed promising for wound treatment in vitro, mainly due to the stimulation of VEGF production [58]. Other studies have given encouraging results in clinical trials on patients with venous stasis ulcers [59] and diabetic-foot ulcers [60]. However, cytokines should not be considered individually, because in vivo they function in complex networks and cascades, frequently exhibiting antagonism (the effects of one cytokine may be inhibited by another cytokine). The overall outcome of a biological response during inflammation thus reflects the balance between pro-inflammatory and anti-inflammatory cytokines [61]. This makes a combined cytokine therapy, with optimal stimulatory factors for wound repair, an attractive intervention for chronic diseases in general [62]. Nevertheless, both in vitro and in vivo studies on optimal synergistic combinations, duration and additional adjuvant therapies are required to precede clinical trials in order to assess the full potential of cytokines as biologic wound supplements.
Antimicrobials and Antiseptics
As infections still remain a feared complication in wounds, the purpose of applying antimicrobial agents is mainly to prevent or combat microbial colonisation. Sustained delivery of antimicrobials, e.g. antibiotics and silver, to wound sites from polymeric dressings is a preferred option than topical administration that often results in toxic reactions due to overdoses. Regardless of the administration approach, these antimicrobials effectively reduce microbial bioburden in infected wounds. However, silver toxicity to mammalian systems is still not fully investigated [63], whereas the excessive use of both antibiotics and silver led to the emergence of bacterial resistance [64, 65]. Further widespread use of antibiotics/silver is not desirable because it can only further contribute to the risk of developing microbial resistance, ultimately weakening our ability to counteract infections.
Current alternatives to antibiotics are antiseptics, with different mechanisms of inhibition of bacterial colonisation and growth. Although not as efficient as antibiotics, antiseptics are less toxic, active against broader spectrum of microorganisms, whereas resistance to antiseptics occurs less frequently [66]. For example, polyhexamethylene biguanide (PHMB) is an antiseptic with very low risk to induce bacterial resistance. An example of PHMB in conjunction with polymers is Suprasorb X + PHMB dressing of Lohmann&Rauscher, a calcium alginate with physically entrapped antiseptic, released into the wound upon application due to swelling.
Enzyme Inhibitory Agents
Abnormal redox state during the prolonged inflammation in non-healing ulcers calls for the use of redox drugs. MMPs are a group of metalloenzymes, where the catalytic Zn2+ in the active centre is coordinated by a redox-sensitive cysteine residue. Displacement, e.g. upon oxidation, of the cysteine ligand leads to the activation of the enzyme [67]—a mechanism termed “cysteine switch”. The MMP activation/inhibition could be redox-regulated by e.g. thiol compounds affecting the sulfhydryl/disulphide state of the switch [68]. A non-specific regulation of MMPs activity could be achieved by zinc chelation. Since thiols combine metal chelating and redox functions [69], thiolated polymers are expected to control the activities of these enzymes via a combination of these two mechanisms. On the other hand, MPO is an oxidative enzyme able to produce HClO, overwhelming the natural shield of protease inhibitors, enabling their accumulation in the chronic wound site [70]. The prevention of MPO-derived HClO accumulation can be envisaged at two levels by: (i) using competitive amounts of substrates to avoid the enzyme chlorination activity and HClO production, and (ii) application of HClO scavengers. As thiols inhibit HClO production [71], the use of thiol-bearing compounds would be an integrated approach for attenuation of both oxidative and proteolytic enzyme activities.
Similar effects on both enzymes are expected using polyphenolic antioxidants. Plant polyphenolic extracts of varying structures from simple molecules to highly polymerised compounds are well-known for their antioxidant capacity and scavenging activity over free radical and non-radical reactive species [72], metal-chelating capability [73] and inhibitory activity over radical-generating enzymes [74]. Plant polyphenols also possess anti-inflammatory [75], antimicrobial [76] and wound healing promoting properties [77]. Some polyphenolic extracts are widely used in the therapy of skin conditions, skin damages such as burns, and as protective component in cosmetic formulations [78, 79]. Various polyphenolic extracts are reported as efficient inhibitors of both MPO [80] and MMPs [81].
Although majority of active agents are effective in preclinical models of dermal repair, most fail to demonstrate the healing improvements when applied topically or systemically in clinical settings. Their limited clinical success is attributed to short half-lives and lack of robust and approved delivery systems. Proper assembling of active agents with biocompatible delivery templates would ensure their stability during the application. For example, if the biopolymeric materials with intrinsic antimicrobial properties are upgraded with bioactive compounds to provide the biochemical stimuli in difficult to treat wounds, an integrated strategy for their efficient management could be achieved. Moreover, controllable enzymatic inhibition could be expected by tuning the degree of biopolymer functionalisation (e.g. biopolymer thiolation, dosed biopolymer impregnation with polyphenols). This would be a step forward towards the regulation of the optimal enzyme/inhibitors ratio necessary for healing. Additionally, if sustained delivery to targeted tissue compartments is achieved, prolonged effects may be expected with improved tissue repair outcomes. Currently, many novel systems based on synthetic and natural polymers are being developed and investigated as active agent delivery systems.
Next Generation Wound Dressings and Formulations Combining Polymers and Active Agents
Advances in biomaterials engineering and assembling/conjugation with biological agents allowed for application of novel wound healing therapies. Properly engineered hybrid biomaterials allow for accelerated recovery of damaged tissue by interfering with the wound healing process at the molecular level. Typically, two approaches for assembling active agents with biomaterials in wound repair are distinguished: (i) permanent immobilisation of the active agent onto polymeric matrix, and (ii) physical encapsulation of the active agent in the polymeric delivery system (matrix or template). The former approach involves chemical or enzymatic binding between the components where the active agent acts from the platform without being released to the wound. The advantage of this approach is the minimisation of the side effects due to the accumulation of immunoreactive compounds at the wound site, i.e. overdoses. The second approach is achieved by simple loading (impregnation) or encapsulation for programmed release of active agent [18]. If the delivery of an active in a consistent and sustained fashion over long periods of time is assured, the possibility of adverse effects and frequency of the dressing change also decrease.
Polymeric scaffolds that provide slow release of growth factors and cytokines have demonstrated the ability to attract cells through local signalling processes and stimulate the regenerative processes [82, 83]. Additionally, if these bioentities are integrated with biomaterials with beneficial for wound repair properties, enhanced wound healing properties to target more significant clinical utility are expected [84]. Among various biopolymers, gelatine, alginate, collagen and hyaluronic acid have been thus designed into gel matrices, porous sponges and microparticulate systems and used to deliver growth factors while maintaining their activity [85–89]. In one of the first studies of this type, a bilayer dressing comprising gelatine sponge and elastomeric synthetic polyurethane membrane used as the external layer was loaded with bFGF encapsulated in microparticles to achieve prolonged release [90]. The application of this hybrid wound dressing provided an optimum healing milieu for the proliferating cells and regenerating tissues in pig’s skin defect models. Actually, most of the growth factors and cytokines are proteins that easily interact with other biopolymers, which makes the choice of an appropriate (bio)material critical to achieve enhanced and sustained release, and thus its action at the wound site. In one unicentre randomised control trial the autologous PRP was evaluated in the combination with a protease modulating Promogran®. The results in 51 patients with diabetic foot ulcers (17 of whom received the combined therapy) showed that this combination reduced the ulcer area more than that when compared with the dressing or PRP alone, suggesting a synergistic interaction between these components [91]. Nevertheless, prior to the widespread clinical use, the integrated growth factors/active dressing therapies need to be optimised and further validated for management of different types of difficult to treat wounds, by assessing their potential in larger, multicentre clinical trials.
Both synthetic and natural polymers have been also investigated and continue to be evaluated as platforms for immobilisation or delivery of active agents. There are numerous examples of polymers that have been mixed with antimicrobial/antiseptic substances to develop antimicrobial dressings and enhance healing of many wound types: fibrous hydrocolloids, poyurethane foam films and silicone gels were combined with silver [92, 93]; antibiotics were impregnated onto various polymer matrices for their delivery in wounds such as gentamycin from collagen sponges [94], ofloxacin from silicone gel sheets [95, 96], and minocycline from chitosan [97] and chitosan-polyurethane film dressings [98]; whereas PHMB-incorporated alginate antiseptic dressing is already marketed under the trade name of Suprasorb X + PHMB [99–101]. Another concept to manage difficult to treat wounds, e.g. chronic ulcers, is to control the activities of oxidative and proteolytic enzymes in wound bed by bringing down their elevated levels into the ranges found in acute wounds to allow healing to progress. However, this task must be taken with precaution, as the total inhibition of these enzymes is not desirable because of their role in the reconstruction of the ECM and wound progression towards closure. In one attempt, an active dressing specifically targeted towards reducing local levels of collagenases in non-healing wounds was developed using two biopolymers, bovine collagen and oxidised regenerated cellulose [44]. When placed in the wound bed, the collagen component acts as a decoy substrate for the proteases, whereas the oxidised cellulose dislodges metal ions from the active centre of these enzymes. Although this composite is still a state-of-the-art dressing on the market meant specifically for chronic wound treatment, it addresses only attenuation of the activities of some proteases at the wound site. The concept is currently being complemented by addressing other common factors influencing non-healing nature of chronic wounds of various aetiologies. For example, in our previous works, collagen, gelatine, chitosan, hyaluronic acid, chondroitin sulphate were used individually or as composite platforms, further upgraded with different plant polyphenols and thiol compounds targeting attenuation of both proteolytic collagenases and oxidative MPO, in addition to inhibiting bacterial growth. The produced biopolymeric platforms were either impregnated or permanently modified with active agents using chemical or enzymatic methods. For example, collagen was cross-linked with naturally occurring genipin to improve its biostability in physiological fluids prior to be impregnated with polyphenolic extracts from Hamamelis virginiana [102]. These extracts were previously found to be efficient scavengers of radical and non-radical reactive species, act as MPO substrates to prevent the accumulation of ROS and irreversibly inhibit collagenase [103]. The loading of plant polyphenols on sponge-like collagen dressings has been achieved on the bases of their ability to interact with proteins and polysaccharides [104, 105]. These interactions determine the release patterns from the biopolymer platforms and the activity of the advanced dressing [106]. Accordingly, in the case of polymer composites, capacity of the attenuation and especially duration of the inhibition effect are tuneable by the biopolymer composition and selection of the polyphenolic compound, being lower for polysaccharide than for protein platforms for which the effect is maintained up to 5 days [107]. In another study, a multifunctional bioactive chitosan/gelatine hydrogel additionally stabilised with plant polyphenols was achieved using laccase-assisted gelation [108]. Whereas gelatine facilitated coupling reactions and gelation, chitosan was used as an antimicrobial dressing platform. The phenolic compounds were covalently bonded on the hydrogels and exerted both: (i) structural function stabilising the dressing, and (ii) bio-activity inhibiting deleterious wound enzymes to stimulate the wound healing process. Permanent immobilisation of active agents reduces risk from overdoses and adverse immune effects at the wound site. The modification of polymeric surfaces in such way is a key aspect in biotechnology nowadays, including development of substrates for regenerative medicine. By alteration of the surface functionality controlled biochemical interactions with body fluids can be achieved. Thiolated chitosan, a biodegradable conjugate obtained by different chemical coupling approaches, combines a series of interesting functions such as mucoadhesive [109], permeation-enhancing [110], in situ gelling and enzyme inhibition properties [111, 112]. This conjugate was further processed into functional nanoscale films/coatings built using a layer-by-layer approach for alternate deposition of oppositely charged polyelectrolytes [113]. Glycosaminoglycans, namely HA with different Mw and chondroitin sulphate, were used as counterions to cationic thiolated conjugates. The biopolymer thiolation degree was identified as a key factor to achieve control of the thickness/size of the multilayered films. In addition, tuneable inhibition/adsorption of the deleterious enzymes coupled to fibroblast attachment/proliferation was observed by ruling the biopolymer modification degree.
Polymer-Based Healing Solutions in the Market
Nowadays, more than 3,000 types of dressings overwhelm the wound management market. The characteristics of the various types of dressings depend on the intrinsic properties of the polymers employed for their preparation. The resulting products may be used individually or in combination to absorb exudate, combat odour and infection, relieve pain, promote autolytic debridement (wound cleansing) and/or provide and maintain a moist environment at the wound surface. An ideal marketable wound dressing should: (i) allow debridement, (ii), provide and maintain a moist wound environment, (iii) allow absorption, removal of blood and excess of wound exudate, (iv) permit gaseous exchange (water vapour and air), (v) prevent infection, (vi) provide thermal insulation, (vii) possess low adherence to allow non-painful dressing change, (viii) protect the wound from trauma, (ix) be cost effective, and (x) be biocompatible.
Taking into consideration the above properties, wide range of polymer-based materials are available to match particular wound requirements. Unfortunately, no single dressing can accomplish all these goals. Thus, the election of the appropriate dressing to a specific wound type is a difficult task and depends on factors related to the product itself, patient’s health status, wound type and location, and economic parameters, as summarised in Table 14.1.
Nowadays wound dressings frequently comprise the combination of polymeric layers with different functions that provide to the dressing particular characteristics. Table 14.2 presents an overview of various types of most frequent polymer-based wound dressings available in the market.
Conclusions
Polymers in the form of dressings and pharmaceutical formulations are already an integral part of modern wound care. Synthetic polymers have good mechanical properties, near-limitless supply, and are easy to process into suitable designs for wound repair, including appropriate pore size and scaffold geometry. However, these advantages are countered by their minimal intrinsic bioactive properties. Biopolymer dressings, on the other hand, interact with dermal tissue and cells to accelerate the acute healing process, but they have little effects on healing of complex wounds. In line with this, advanced wound repair is currently directed towards stimulation of physiological repair at molecular level. Combining synthetic and/or biopolymer dressing with the therapeutic potential of bioactive molecules has emerged as an exciting field of research for enhanced wound repair. The rationale for the development of these next generation composites lies in their superior efficacy in preclinical models relative to the application of their components alone. Many approaches for assembling polymers with therapeutically relevant compounds are established technologies with few of these already commercialised based on the available clinical evidences. Nevertheless, the large amount of research being conducted is likely to result in additional approvals, and more advanced polymer-based dressings will certainly attain market in the next few years, considering their current preclinical/clinical evaluation.
Future Perspectives
Polymer dressings are an important segment of the medical and pharmaceutical wound management market worldwide. Both low- and high-tech products ranging from traditional inert synthetic bandages to bioactive solutions for which preparation emergent technologies are employed find the place on this dynamic market. Despite the wide variety of products available, complex non-healing wounds are still a challenge to manage and accordingly attract a great deal of attention among the research community. Their treatment represents a huge health burden and drain on healthcare resources due to the intensive medical intervention required. Furthermore, as elderly individuals become the fastest-growing segment of the population, the complex wounds occurrence will have an even more pronounced economic impact in the future. Therefore, new solutions such as advanced dressings to facilitate chronic wound healing are needed.
The advanced wound care market is constantly growing and includes an array of competing technologies and solutions that can be classified according to the materials from which they are produced. Polymer-based hydrocolloids, hydrogels, thin films and foam sheets are its largest sector. They also include few bioactive dressings and skin substitutes on the market that combine polymers with various therapeutics, antimicrobials, enzyme inhibitors and/or biological supplements acting on specific molecular targets in wound environment. In near future it is expected that polymers will be exploited more efficiently since they can be easily modified via chemical or biochemical techniques. Permanent functionalisation of polymer matrices with bioactive agents will allow their acting from the polymer matrix, without being release to the wound and thus avoiding risk of overdoses and associated adverse effects.
One of the main reasons of the high cost of managing complex wounds, besides the cost of the materials, is the time of hospitalisation. The decision-making in their treatment is therefore crucial and is required to be at the same time fast and accurate. Currently, the clinicians often visually inspect the wound to evaluate the infection status or rely on time-consuming and costly biopsy analyses requiring special equipment. Among the most recent advances in this area are remote monitoring devices evaluating wound-related parameters such as margin, volume, depth and area or off-site diagnostic kits relying on different detection markers, but often requiring painful wound fluid collection [114]. In order to increase the patient’s comfort and at the same time provide reliable diagnosis, the wound care market should soon move from intuitive choices to proactive strategies for simultaneous healing and monitoring of the wound status. New point-of-care devices will be developed under the EU project InFact - Functional materials for fast diagnosis of wound infection (grant agreement nº FP7-609198), in order to implement a solution for fast infection diagnosis into an active wound dressing and thus complement the healing of wounds colonised with microorganisms. Such simple tool integrated into a next generation wound dressings will enable clinicians to assess the wound status rapidly avoiding painful wound fluid collection. The role of polymers in such devices will be equally important as in the nowadays modern dressings. Besides providing the structural support, various polymers will be employed to assemble the test components of a novel diagnostic tool, e.g. sample path (cellulose), conjugate pad (usually a polyester), assay matrix (nitro-cellulose membrane), absorbent pad (cellulose fibre sheets) and adhesive backing (polystyrene, vinyl or polyester) are employed in lateral flow devices.
Abbreviations
- bFGF:
-
Basic fibroblast growth factor
- BSA:
-
Bovine serum albumin
- ECM:
-
Extracellular matrix
- GAGs:
-
Glycosaminoglycans
- GM-CSF:
-
Granulocyte macrophage colony stimulating factor
- HA:
-
Hyaluronic acid
- HClO:
-
Hypochlorous acid
- MMPs:
-
Matrix metalloproteinases
- MPO:
-
Myeloperoxidase
- NPWT:
-
Negative pressure wound therapy
- PDGF-BB:
-
Platelet-derived growth factor BB
- PRP:
-
Platelet rich plasma
- PHMB:
-
Polyhexamethylene biguanide
- ROS:
-
Reactive oxygen species
- SF:
-
Silk fibroin
- VEGF:
-
Vascular endothelial growth factor
References
Lazarus, G.S., et al.: Definitions and guidelines for assessment of wounds and evaluation of healing. Wound Repair Regener. 2(3), 165–170 (1994)
Stadelmann, W.K., Digenis, A.G., Tobin, G.R.: Physiology and healing dynamics of chronic cutaneous wounds. Am. J. Surg. 176(2, Supplement 1): 26S–38S (1998)
Weiss, S.J.: Tissue destruction by neutrophils. N. Engl. J. Med. 320(6), 365–376 (1989)
Martin, P., Leibovich, S.J.: Inflammatory cells during wound repair: the good, the bad and the ugly. Trends Cell Biol. 15(11), 599–607 (2005)
Diegelmann, R.F.: Excessive neutrophils characterize chronic pressure ulcers. Wound Repair Regener. 11(6), 490–495 (2003)
Trengove, N.J., et al.: Analysis of the acute and chronic wound environments: the role of proteases and their inhibitors. Wound Repair Regener. 7(6), 442–452 (1999)
Wang, Y., et al.: Myeloperoxidase inactivates timp-1 by oxidizing its n-terminal cysteine residue. J. Biol. Chem. 282(44), 31826–31834 (2007)
Saarialho-Kere, U.K.: Patterns of matrix metalloproteinase and TIMP expression in chronic ulcers. Arch. Dermatol. Res. 290(1), S47–S54 (1998)
Falabella, A.F.: Debridement and wound bed preparation. Dermatol. Ther. 19(6), 317–325 (2006)
Boateng, J.S., et al.: Wound healing dressings and drug delivery systems: a review. J. Pharm. Sci. 97(8), 2892–2923 (2008)
Atiyeh, B.S., et al.: Effect of silver on burn wound infection control and healing: review of the literature. Burns 33(2), 139–148 (2007)
Gupta, S., et al.: Guidelines for managing pressure ulcers with negative pressure wound therapy. Adv. Skin Wound Care 17, 1–16 (2004)
Xie, X., McGregor, M., Dendukuri, N.: The clinical effectiveness of negative pressure wound therapy: a systematic review. J. wound care 19(11), 490–495 (2010)
Noble-Bell, G., Forbes, A.: A systematic review of the effectiveness of negative pressure wound therapy in the management of diabetes foot ulcers. Int. Wound J. 5(2), 233–242 (2008)
Ubbink Dirk, T., et al.: Topical negative pressure for treating chronic wounds. Cochrane Database Syst. Rev. 16 (2008). doi: CD001898
Suslick, K.S., Grinstaff, M.W.: Protein microencapsulation of nonaqueous liquids. J. Am. Chem. Soc. 112(21), 7807–7809 (1990)
Ennis, W.J., et al.: Evaluation of clinical effectiveness of mist ultrasound therapy for the healing of chronic wounds. Adv. Skin Wound Care: J. Prev. Healing 19(8), 437–446 (2006)
Francesko, A., Tzanov, T.: Chitin, chitosan and derivatives for wound healing and tissue engineering. In: Nyanhongo, G.S., Steiner, W., Gübitz, G. (eds.) Biofunctionalization of Polymers and their Applications, pp. 1–27. Springer, Berlin (2011)
Queen, D., et al.: A dressing history. Int. Wound J. 1(1), 59–77 (2004)
Mogoşanu, G.D., Grumezescu, A.M.: Natural and synthetic polymers for wounds and burns dressing. Int. J. Pharm. 463(2), 127–136 (2014)
Wright, K.A., et al.: Alternative delivery of keratinocytes using a polyurethane membrane and the implications for its use in the treatment of full-thickness burn injury. Burns 24(1), 7–17 (1998)
Jansson, E., Tengvall, P.: In vitro preparation and ellipsometric characterization of thin blood plasma clot films on silicon. Biomaterials 22(13), 1803–1808 (2001)
Losi, P., et al.: Luminal surface microgeometry affects platelet adhesion in small-diameter synthetic grafts. Biomaterials 25(18), 4447–4455 (2004)
Whelan, J.: Smart bandages diagnose wound infection. Drug Discov. Today 7(1), 9–10 (2002)
Van den Kerckhove, E., et al.: Silicones in the rehabilitation of burns: a review and overview. Burns 27(3), 205–214 (2001)
Raphael, K.G., et al.: Self-reported systemic, immune-mediated disorders in patients with and without proplast-teflon implants of the temporomandibular joint. J. Oral Maxillofac. Surg. 57(4), 364–370 (1999)
Kim, I.-Y., et al.: Chitosan and its derivatives for tissue engineering applications. Biotechnol. Adv. 26(1), 1–21 (2008)
Ueno, H., et al.: Accelerating effects of chitosan for healing at early phase of experimental open wound in dogs. Biomaterials 20(15), 1407–1414 (1999)
Wedmore, I., et al.: A special report on the chitosan-based hemostatic dressing: experience in current combat operations. J. Trauma Acute Care Surg. 60(3), 655–658 (2006)
Pietramaggiori, G., et al.: Effects of poly-N-acetyl glucosamine (pGlcNAc) patch on wound healing in db/db mouse. J. Trauma Acute Care Surg. 64(3), 803–808 (2008)
Dhanasingh, A., et al.: Tailored hyaluronic acid hydrogels through hydrophilic prepolymer cross-linkers. Soft Matter 6(3), 618–629 (2010)
Gilbert, M.E., et al.: Chondroitin sulfate hydrogel and wound healing in rabbit maxillary sinus mucosa. Laryngoscope 114(8), 1406–1409 (2004)
West, D., et al.: Angiogenesis induced by degradation products of hyaluronic acid. Science 228(4705), 1324–1326 (1985)
Perng, C.-K., et al.: In vivo angiogenesis effect of porous collagen scaffold with hyaluronic acid oligosaccharides. J. Surg. Res. 168(1), 9–15 (2011)
Park, S.-N., et al.: Biological characterization of EDC-crosslinked collagen–hyaluronic acid matrix in dermal tissue restoration. Biomaterials 24(9), 1631–1641 (2003)
Luo, Y., Kirker, K.R., Prestwich, G.D.: Cross-linked hyaluronic acid hydrogel films: new biomaterials for drug delivery. J. Controlled Release 69(1), 169–184 (2000)
Lin, Y.-C., et al.: Synthesis and characterization of collagen/hyaluronan/chitosan composite sponges for potential biomedical applications. Acta Biomater. 5(7), 2591–2600 (2009)
Chang, C.-H., et al.: Gelatin–chondroitin–hyaluronan tri-copolymer scaffold for cartilage tissue engineering. Biomaterials 24(26), 4853–4858 (2003)
Dumville, J., et al., Alginate dressings for healing diabetic foot ulcers. Cochrane Database Syst. Rev. 15(2) (2012)
Cen, L., et al.: Collagen tissue engineering: development of novel biomaterials and applications. Pediatr. Res. 63(5), 492–496 (2008)
Parenteau-Bareil, R., Gauvin, R., Berthod, F.: Collagen-based biomaterials for tissue engineering applications. Materials 3(3), 1863–1887 (2010)
Ruszczak, Z., Friess, W.: Collagen as a carrier for on-site delivery of antibacterial drugs. Adv. Drug Deliv. Rev. 55(12), 1679–1698 (2003)
Cullen, B., et al.: Mechanism of action of PROMOGRAN, a protease modulating matrix, for the treatment of diabetic foot ulcers. Wound Repair Regener. 10(1), 16–25 (2002)
Cullen, B., et al.: The role of oxidised regenerated cellulose/collagen in chronic wound repair and its potential mechanism of action. Int. J. Biochem. Cell Biol. 34(12), 1544–1556 (2002)
Rothwell, S., et al.: Wound healing and the immune response in swine treated with a hemostatic bandage composed of salmon thrombin and fibrinogen. J. Mater. Sci. Mater. Med. 20(10), 2155–2166 (2009)
Vasconcelos, A., Cavaco-Paulo, A.: Wound dressings for a proteolytic-rich environment. Appl. Microbiol. Biotechnol. 90(2), 445–460 (2011)
de Moraes, M.A., Beppu, M.M.: Biocomposite membranes of sodium alginate and silk fibroin fibers for biomedical applications. J. Appl. Polym. Sci. 130(5), 3451–3457 (2013)
Liu, T.-L., et al.: Cytocompatibility of regenerated silk fibroin film: a medical biomaterial applicable to wound healing. J. Zhejiang Univ. Sci. B 11(1), 10–16 (2010)
Zhu, X., et al.: Activation of T lymphocytes on local trauma after implantation of regenerated porous silk fibroin film. J. Clim. Rehabil. Tissue Eng. Res. 15, 7061–7065 (2011)
Dror, Y., et al.: Nanofibers made of globular proteins. Biomacromolecules 9(10), 2749–2754 (2008)
Jin, R., et al.: Injectable chitosan-based hydrogels for cartilage tissue engineering. Biomaterials 30(13), 2544–2551 (2009)
Sibbald, R.G., Woo, K.Y., Queen, D.: Wound bed preparation and oxygen balance—a new component? Int. Wound J. 4, 9–17 (2007)
Werner, S., Grose, R.: Regulation of wound healing by growth factors and cytokines. Physiol. Rev. 83(3), 835–870 (2003)
Barrientos, S., et al.: Perspective article: growth factors and cytokines in wound healing. Wound Repair Regener. 16(5), 585–601 (2008)
Steed, D.L., Study Group, t.D.U.*: Clinical evaluation of recombinant human platelet—derived growth factor for the treatment of lower extremity diabetic ulcers. J. Vasc. Surg. 21(1), 71–81 (1995)
Anitua, E., et al.: Platelet-rich plasma: preparation and formulation. Operative Tech. Orthop. 22(1), 25–32 (2012)
Vande Berg, J., Robson, M., Mikhail, R.: Extension of the life span of pressure ulcer fibroblasts with recombinant human interleukin-1 beta. Am. J. Pathol. 146(5), 1273–1282 (1995)
Cianfarani, F., et al.: Granulocyte/macrophage colony-stimulating factor treatment of human chronic ulcers promotes angiogenesis associated with de novo vascular endothelial growth factor transcription in the ulcer bed. Br. J. Dermatol. 154(1), 34–41 (2006)
Da Costa, R.M., et al.: Randomized, double-blind, placebo-controlled, dose- ranging study of granulocyte-macrophage colony stimulating factor in patients with chronic venous leg ulcers. Wound Repair Regener. 7(1), 17–25 (1999)
Cruciani, M., et al.: Are granulocyte colony-stimulating factors beneficial in treating diabetic foot infections?: a meta-analysis. Diabetes Care 28(2), 454–460 (2005)
Kavanaugh, A.: Combination cytokine therapy: the next generation of rheumatoid arthritis therapy? Arthritis Care Res. 47(1), 87–92 (2002)
Pellegrini, M., Mak, T., Ohashi, P.: Fighting cancers from within: augmenting tumor immunity with cytokine therapy. Trends Pharmacol. Sci. 31(8), 356–363 (2010)
Lansdown, A.B.G.: Silver 2: toxicity in mammals and how its products aid wound repair. J. Wound Care 11(5), 173–177 (2002)
Howell-Jones, R.S., et al.: A review of the microbiology, antibiotic usage and resistance in chronic skin wounds. J. Antimicrob. Chemother. 55(2), 143–149 (2005)
Landsdown, A., Williams, A.: Bacterial resistance to silver in wound care and medical devices. J Wound Care 16(1), 15–19 (2007)
Gilbert, P.: Avoiding the resistance pitfall in infection control. Does the use of antiseptic products contribute to the spread of antibiotic resistance? Ostomy Wound Manage 52(10 A Suppl): 1S–3S (2006)
Giles, N.M., et al.: Metal and redox modulation of cysteine protein function. Chem. Biol. 10(8), 677–693 (2003)
Gu, Z., et al.: S-nitrosylation of matrix metalloproteinases: signaling pathway to neuronal cell death. Science 297(5584), 1186–1190 (2002)
Andreassen, O.A., et al.: N-acetyl-L-cysteine improves survival and preserves motor performance in an animal model of familial amyotrophic lateral sclerosis. NeuroReport 11(11), 2491–2493 (2000)
Fu, X., et al.: Hypochlorous acid oxygenates the cysteine switch domain of pro-matrilysin (MMP-7). J. Biol. Chem. 276(44), 41279–41287 (2001)
Van Antwerpen, P., et al.: Thiol-containing molecules interact with the myeloperoxidase/H2O2/chloride system to inhibit LDL oxidation. Biochem. Biophys. Res. Commun. 337(1), 82–88 (2005)
Rice-Evans, C.A., Miller, N.J., Paganga, G.: Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 20(7), 933–956 (1996)
Mira, L., et al.: Interactions of flavonoids with iron and copper ions: a mechanism for their antioxidant activity. Free Radic. Res. 36(11), 1199–1208 (2002)
Pauff, J.M., Hille, R.: Inhibition studies of bovine xanthine oxidase by luteolin, silibinin, quercetin, and curcumin. J. Nat. Prod. 72(4), 725–731 (2009)
Ding, Z., et al.: Anti-inflammatory effects of scopoletin and underlying mechanisms. Pharm. Biol. 46(12), 854–860 (2008)
Geyid, A., et al.: Screening of some medicinal plants of Ethiopia for their anti-microbial properties and chemical profiles. J. Ethnopharmacol. 97(3), 421–427 (2005)
Kim, H., et al.: Enhanced wound healing by an epigallocatechin gallate-incorporated collagen sponge in diabetic mice. Wound Repair Regener. 16(5), 714–720 (2008)
Masaki, H., Atsumi, T., Sakurai, H.: Protective activity of hamamelitannin on cell damage of murine skin fibroblasts induced by UVB irradiation. J. Dermatol. Sci. 10(1), 25–34 (1995)
Deters, A., et al.: High molecular compounds (polysaccharides and proanthocyanidins) from Hamamelis virginiana bark: influence on human skin keratinocyte proliferation and differentiation and influence on irritated skin. Phytochemistry 58(6), 949–958 (2001)
Kato, Y., et al.: Inhibition of myeloperoxidase-catalyzed tyrosylation by phenolic antioxidants in vitro. Biosci. Biotechnol. Biochem. 67(5), 1136–1139 (2003)
Madhan, B., et al.: Role of green tea polyphenols in the inhibition of collagenolytic activity by collagenase. Int. J. Biol. Macromol. 41(1), 16–22 (2007)
Chen, R., Mooney, D.: Polymeric growth factor delivery strategies for tissue engineering. Pharm. Res. 20(8), 1103–1112 (2003)
Lee, K., Silva, E.A., Mooney, D.J.: Growth factor delivery-based tissue engineering: general approaches and a review of recent developments. J. R. Soc. Interface 8(55), 153–170 (2011)
Koria, P.: Delivery of growth factors for tissue regeneration and wound healing. BioDrugs 26(3), 163–175 (2012)
Ulubayram, K., et al.: EGF containing gelatin-based wound dressings. Biomaterials 22(11), 1345–1356 (2001)
Kolambkar, Y.M., et al.: An alginate-based hybrid system for growth factor delivery in the functional repair of large bone defects. Biomaterials 32(1), 65–74 (2011)
Değim, Z.: Use of microparticulate systems to accelerate skin wound healing. J. Drug Target. 16(6), 437–448 (2008)
Takemoto, S., et al.: Preparation of collagen/gelatin sponge scaffold for sustained release of bFGF. Tissue Eng. Part A 14(10), 1629–1638 (2008)
Matsumoto, Y., Kuroyanagi, Y.: Development of a wound dressing composed of hyaluronic acid sponge containing arginine and epidermal growth factor. J. Biomater. Sci. Polym. Ed. 21(6), 126–715 (2010)
Huang, S., et al.: Wound dressings containing bFGF-impregnated microspheres. J. Microencapsul. 23(3), 277–290 (2006)
Kakagia, D.D., et al.: Synergistic action of protease-modulating matrix and autologous growth factors in healing of diabetic foot ulcers. A prospective randomized trial. J. Diabetes Complications 21(6), 387–391 (2007)
Leaper, D.J.: Silver dressings: their role in wound management. Int. Wound J. 3(4), 282–294 (2006)
Leaper, D.: Silver dressings: their role in wound management. Int Wound J. 3(4), 282–294 (2006)
Fong, J., Wood, F.: Nanocrystalline silver dressings in wound management: a review. Int. J. Nanomed. 1(4), 441–449 (2006)
Rutten, H., Nijhuis, P.: Prevention of wound infection in elective colorectal surgery by local application of a gentamicin-containing collagen sponge. Eur. J. Surg. Suppl. 578, 31–35 (1997)
Sawada, Y., et al.: Treatment of dermal depth burn wounds with an antimicrobial agent-releasing silicone gel sheet. Burns 16(5), 347–352 (1990)
Sawada, Y., et al.: An evaluation of a new lactic acid polymer drug delivery system: a preliminary report. Br. J. Plast. Surg. 47(3), 158–161 (1994)
Galandiuk, S., et al.: Absorbable, delayed-release antibiotic beads reduce surgical wound infection. Am. Surg. 63(9), 831–835 (1997)
Aoyagi, S., Onishi, H., Machida, Y.: Novel chitosan wound dressing loaded with minocycline for the treatment of severe burn wounds. Int. J. Pharm. 330(1–2), 138–145 (2007)
Kingsley, A., et al.: Suprasorb X + PHMB: antimicrobial and HydroBalance action in a new wound dressing. Wounds UK 5(1), 72–77 (2009)
Glover, D., Wicks, G.: Suprasorb X + PHMB: the clinical evidence. J. wound care. ACTIVA healthcare Supplement, 15–21 (2009)
Elzinga, G., et al.: Clinical evaluation of a PHMB-impregnated biocellulose dressing on paediatric lacerations. J Wound Care 20(6), 280–284 (2011)
Antonio, F., et al.: Cross-linked collagen sponges loaded with plant polyphenols with inhibitory activity towards chronic wound enzymes. Biotechnol. J. 6(10), 1208–1218 (2011)
Díaz-GonzáLez, M., et al.: Inhibition of deleterious chronic wound enzymes with plant polyphenols. Biocatal. Biotransform. 30(1), 102–110 (2012)
Haslam, E.: natural polyphenols (vegetable tannins) as drugs: possible modes of action. J. Nat. Prod. 59(2), 205–215 (1996)
Madhan, B., et al.: Stabilization of collagen using plant polyphenol: role of catechin. Int. J. Biol. Macromol. 37(1–2), 47–53 (2005)
Tang, H.R., Covington, A.D., Hancock, R.A.: Structure–activity relationships in the hydrophobic interactions of polyphenols with cellulose and collagen. Biopolymers 70(3), 403–413 (2003)
Francesko, A., et al.: Functional biopolymer-based matrices for modulation of chronic wound enzyme activities. Acta Biomater. 9(2), 5216–5225 (2013)
Rocasalbas, G., et al.: Laccase-assisted formation of bioactive chitosan/gelatin hydrogel stabilized with plant polyphenols. Carbohydr. Polym. 92(2), 989–996 (2013)
Roldo, M., et al.: Mucoadhesive thiolated chitosans as platforms for oral controlled drug delivery: synthesis and in vitro evaluation. Eur. J. Pharm. Biopharm. 57(1), 115–121 (2004)
Föger, F., Schmitz, T., Bernkop-Schnürch, A.: In vivo evaluation of an oral delivery system for P-gp substrates based on thiolated chitosan. Biomaterials 27(23), 4250–4255 (2006)
Kast, C.E., et al.: Chitosan-thioglycolic acid conjugate: a new scaffold material for tissue engineering? Int. J. Pharm. 256(1–2), 183–189 (2003)
Kast, C.E., Bernkop-Schnürch, A.: Thiolated polymers—thiomers: development and in vitro evaluation of chitosan–thioglycolic acid conjugates. Biomaterials 22(17), 2345–2352 (2001)
Francesko, A., et al.: GAGs-thiolated chitosan assemblies for chronic wounds treatment: control of enzyme activity and cell attachment. J. Mater. Chem. 22(37), 19438–19446 (2012)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Francesko, A., Fernandes, M.M., Rocasalbas, G., Gautier, S., Tzanov, T. (2015). Polymers in Wound Repair. In: Puoci, F. (eds) Advanced Polymers in Medicine. Springer, Cham. https://doi.org/10.1007/978-3-319-12478-0_14
Download citation
DOI: https://doi.org/10.1007/978-3-319-12478-0_14
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-12477-3
Online ISBN: 978-3-319-12478-0
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)